Abstract
Rodent models for human diseases contribute significantly to the understanding of human physiology and pathophysiology. However, with the accelerating pace of drug development, there is a crucial need for in vivo preclinical models of human biology and pathology. The humanized mouse is one tool to bridge the gap between traditional animal models and the clinic. The development of immunodeficient mouse strains with a high level engraftment of normal and diseased human immune-hematopoietic cells has made in vivo functional characterization possible. As a patient-derived xenograft (PDX) model, humanized mice functionally correlate putative mechanisms with in vivo behavior and help reveal pathogenic mechanisms. Combined with single cell genomics, humanized mice can facilitate functional precision medicine such as risk stratification and individually optimized therapeutic approaches.
Keywords: Humanized mouse, human hematopoiesis, xenograft, human immunity, leukemia
The humanized mouse as an in vivo model of human hematopoiesis
In vivo repopulation of human hematopoiesis in laboratory animals has been technically difficult due to significant species-specific hematological and immunological barriers preventing efficient xenogeneic (see Glossary) engraftment. Humanized mice or HIS (Human immune system) mice evolved with the development of a series of highly immunodeficient mice as sensitive and efficient recipients of human hematopoietic and immune cell populations[1, 2]. Humanized mice are now a widely used tool for studying in vivo kinetics and function of human hematopoietic and immune systems. Here, we describe the development of humanized mice as an in vivo model of normal and malignant human hematopoiesis and discuss the contribution of new humanized mouse models to basic and translational research. Humanized mice can allow in vivo functional characterization of human hematopoiesis, and following engraftment with patient-derived xenografts (PDX), this model supports analyses of pathogenic mechanisms directly linked to individual patients’ clinical conditions (Box 1). We posit that in combination with single cell genomics, the use of humanized mice may enable risk stratification and the development of individually-tailored therapeutic approaches.
Box 1: Clinician’s Corner.
Leukemias are a biologically heterogeneous disease with complex genetic abnormalities. In particular, long-term outcomes in AML are poor.
Even with the accelerated development of molecular targeting drugs, currently available targeted therapies are not curative.
Leukemia humanized mice, created by transplantation of patient-derived leukemia-initiating cells, enable our understanding of human leukemia biology in vivo, and can also be used for in vivo therapeutic testing.
As a platform for in vivo functional testing, humanized mice can facilitate functional precision medicine, optimizing treatment strategies tailored to individual patients with complex and heterogeneous diseases such as leukemia.
In vivo human hematopoiesis and immunity in the humanized mouse
The discovery of the severe combined immunodeficiency mutation (Prkdcscid or scid) in CB17 mice in 1983 followed by the development of NOD-scid mice in 1995, paved the way to modeling human hematopoiesis in mice by transplantation of human hematopoietic stem/progenitor cells (HSPCs) [3–8]. The scid mutation results in defective DNA-dependent protein kinase activity leading to faulty T and B cell receptor recombination and a loss of adaptive immunity in CB17-scid mice [9]. The NOD strain background contributes deficiencies in innate immunity, particularly NK cells [7]. In addition, a polymorphism in the signal regulatory protein alpha (Sirpa) gene in the NOD strain allows interactions between mouse myeloid cells, including macrophages, with human CD47-expressing hematopoietic cells to provide a “do not eat me” signal, improving human hematopoietic engraftment [10, 11]. However, these early models suffered from low and inconsistent levels of engraftment due to genetic limitations inherent to the strain background and residual innate mouse immunity[12]. While the NOD-scid recipients showed improved engraftment of human cells compared with CB17-scid, NOD-scid mice were still unable to support robust human T cell development, limiting their utility for studying human immune systems[12].
In the early 2000s, a significant advancement in the field was achieved: the development of an immunodeficient mouse strain bearing a targeted mutation in the IL-2 receptor common gamma chain gene (IL2rg) [13–16]. As reviewed elsewhere [1, 17–20], the mouse strains used for current humanized mouse models such as NOD.Cg-PrkdcscidIL2rgtm1Wjl (NSG) [21], NOD.Cg-PrkdcscidIL2rgtm1Sug (NOG) [22], NOD.Cg-Rag1tm1MomIL2rgtm1Wjl (NRG) [23] or C.129S4-Rag2tm1Fwa1IL2rgtm1Sug (BRG) [24] harbor Il2rg mutations as well as the Prkdcscid, Rag1null or Rag2null mutations[25, 26]. In these highly immunosuppressed strains, xenotransplantation of human HSPCs led to improved human leukocyte engraftment and multi-lineage repopulation in the bone marrow (BM), spleen, and peripheral blood (PB) compared to the predecessor strains, although the NSG and NRG strains supported higher levels of engraftment than the BRG strain deriving from the BALB/c background [27]. This was largely due to the xenogenic polymorphism of Sirp-α in BALB/c mice resulting in an insufficient interaction with human CD47 to create a do not eat me signal. Although the scid mutation results in increased radiosensitivity, neither the Rag1 or Rag2 knockouts show normal responses to x-irradiation.[28, 29] To develop a more permissive Sirpa microenvironment, the generation of BRG mice expressing either the NOD Sirpa allele or the human SIRPA transgene, as well as the development of C57BL/6-Rag2 null CD47 null IL2rgnull mice led to improved human hematopoietic cell engraftment[30–32]. An overview of the published strains of immunodeficient IL2rgnull mice and their availability is extensively reviewed elsewhere [33] [34]. Moreover, humanized mice applications in infectious diseases and immunity [35] and in inflammation [36] have also been reviewed elsewhere.
Immunodeficient mice engrafted with functional human immuno-hematopoietic systems are referred to as “humanized mice” [37]. Humanized mice, using highly immunodeficient mouse strains, support in vivo development of various populations of human innate and acquired immune cells (T-, B-,and myeloid cells, and their subpopulations) following engraftment of human HSPCs [38]. In particular, consistent human T cell development is achieved when newborn mouse recipients are used [38]. This may reflect the requirement for human HSPCs to migrate into the mouse thymus before the mouse thymic tissues become atrophic [39]. NSG mice, engrafted with human HSPCs, support in vivo human monocyte differentiation, and less efficiently, neutrophil differentiation in the myeloid lineage[40]. Development of mature and functional human myeloid subsets occurs in hematopoietic stem cell (HSC)-engrafted NSG mice and BRG mice. [24, 40] Development of conventional and plasmacytoid dendritic cells (DC) have also been confirmed in multiple humanized mouse models [38]. Although human HSPC engrafted NSG mice support in vivo human hematopoiesis, there is incomplete replication of native human hematopoiesis. For instance, human T cells developing in humanized mice are educated on murine MHC, and are Histocompatibility system 2 (H2)-restricted, resulting in MHC mismatched interactions with HLA-expressing human antigen-presenting cells (APCs) [41]. Moreover, frequencies of human myeloid lineage cells are lower in the humanized mouse BM than in human BM, leading to higher proportions of human B cells in the humanized mouse BM environment than in human BM [42]. Within innate immune cell populations, human NK cell development is also reduced in humanized mice relative to humans[43, 44]. Of note, erythroid and megakaryocyte/platelet differentiation from injected human HSPCs remains suboptimal in current humanized mouse models. [45] Thus, achieving improved native human hematopoiesis in emerging humanized mouse models constitutes a major goal in the field.
Further ‘humanization’ of humanized mice
To achieve the goal of increasing the ‘humanization’ of existing humanized mice, several strategies have been explored, aiming to increase the efficiency of recipients in supporting human hematopoiesis and immune system development, including: i) Engraftment of human organoids through co-implantation of human fetal thymus with syngeneic fetal liver HSPCs[46–48] or through implantation of human ossicles with intravenous injection of human cord blood HSPCs[49, 50], ii) transgenic expression of HLA molecules [51–53] and iii) expression of human cytokines. [42, 54–57] Engraftment of NSG mice with human fetal liver and thymus (BLT model) results in robust human immune systems [58, 59]. BLT mice have been applied to studies of HLA-restricted human T cell response and development of therapeutic strategies in variety of viruses. [46, 60, 61] Specifically, in one study, an ossicle was created as a humanized niche and subcutaneously implanted in NSG humanized mice to support engraftment of normal and diseased human hematopoiesis [49]. Human myeloid lineage developed efficiently from human HSPCs in the ossicle. In malignant hematopoiesis, it became feasible to identify disease-initiating stem cells in acute promyelocytic leukemia and myelofibrosis which do not otherwise engraft well in immunocompromised mice. To create strains with HLA or human cytokine expression on the NOD-scid background, extensive backcrossing of mice was initially required. Recipients with transgenic HLA-expression on NSG and certain other backgrounds enabled the development of human HLA-restricted T cell responses[12, 18–20, 62–65]. In addition, HLA expression through adeno-associated virus (AAV)-based vectors has been used to rapidly induce HLA-expression without needing to create new transgenic mice[66, 67]. To overcome the limitation of insufficient production of human cytokines in humanized mice, a number of strains with transgenic /knock-in expression of human cytokines on NSG and other backgrounds have been created. Specifically, to better support myeloid development, cytokines such as SCF, IL-3, GM-CSF, G-CSF, IL6 and others, have been knocked-in (KI) or transgenically expressed in the NSG and other backgrounds [68–70]. BRG mice expressing human M-CSF, IL-3, SIRPA, and TPO (MISTRG) were found to support engraftment and maintenance of human HSCs and myeloid lineage populations more efficiently than BRG mice without human cytokine expression [54]. Cytokine-expressing Rag1null and Rag2null-based immune-compromised mouse strains can provide a benefit due to their radiation resistance and their non-leakiness (i.e. the development of murine lymphocytes in older mice) compared with scid-based mice [54, 71]. Recent reports described NOD.Cg-PrkdcscidIl2rgtm1WjlTg(CMV-IL3,CSF2,KITL)1Eav/MloySzJ mice expressing hSCF, IL3, and GM-CSF (NSG-SGM3) which supported increased normal and malignant human myeloid cell engraftment, as well as heightened development of regulatory T cells following injection of human HSPCs [72–74]. Long-term maintenance of circulating human erythrocytes and platelets in humanized mice has been more difficult due to the rapid clearance of human red blood cells and platelets by reticuloendothelial cells in mouse spleen and liver[1, 12]. However, treatment of NSG mice with liposome-encapsulated clodronate to reduce host macrophage numbers in the short-term has permitted analyses of circulating human erythrocytes[75] and furthermore, humanized mice are expected to contribute to the development of anti-malarial drugs[76].
By taking advantage of the strengths of these humanized mouse models, investigators have made significant progress in understanding in vivo human hematopoiesis. We now focus our discussion to three approaches to using the humanized system: i) developmental hierarchy in human hematopoiesis, ii) how normal hematopoiesis is transformed into malignant hematopoiesis, and iii) human immune cell interactions with malignant cells.
Understanding the complexity and heterogeneity of human hematopoiesis using Humanized Mice
In understanding normal human hematopoietic hierarchy, combined investigations of humanized mice with single cell genomics have paved an important path. In humanized mice, less than 1,000 human CD34+CD38−CD45RA− HSPCs are sufficient to engraft and generate human myeloid, B and T cells in the murine BM and spleen, leading to the differentiation of HSCs into multiple hematopoietic subsets in vivo. [77, 78] To increase the sensitivity of humanized mice in supporting engraftment of human hematopoietic cells and to improve the efficiency of secondary engraftment, humanized mice with mutations at the mouse W/c-kit locus were developed [54]. Along with enhanced self-renewal of human HSCs, this model supported improved reconstitution of erythroid and megakaryocyte lineages from human HSPCs relative to humanized mice with wildtype (WT) mouse c-Kit [79]. In vivo cell fates of human hematopoietic progenitors were also assessed using immuno-compromised mice, namely, NSG mice expressing human IL-3 and GM-CSF along with human SCF; indeed, c-Kit+, CD66b+ neutrophil-restricted progenitor cells were identified among CD34+ cells, furthering our understanding regarding the heterogeneity of immature human CD34+ cells [80]. Indeed, humanization of the BM microenvironment has increased our knowledge of immature human CD34+ cell heterogeneity. The mutated mouse c-Kit was also introduced in BRG mice [81], enabling investigators to identify megakaryocyte-specific human hematopoietic progenitor cells [82]. Moreover, the capacity to generate B/T/NK lymphocyte in sub-fractions of human CD34+ cells was assessed by transplanting isolated cell populations based on the expression of cell surface markers such as CD127 (IL-7RA), CD10, CD7, CD62L or certain integrins [83]. In particular, distinct engraftment patterns by CD127+ early lymphoid progenitors (ELPs) and CD127− ELPs were found in NSG mice. Moreover, the production of myeloid/erythroid/megakaryocyte lineages by distinct populations of progenitor cells has also been carefully evaluated in vivo, and in vivo short-term (2 weeks post-transplantation) engraftment of human erythroid cells by sub-fractionated CD34+CD38+CD10−CD45RA−CD135− cells based on the expression of CD71 and BAH-1 were detected [84]. These studies successfully defined distinct functions of rare HSPC subpopulations by connecting their transcriptional programs to in vivo cell fates. Moreover, humanized mice have been used to examine cell homing, in vivo cell fates, as well as the functions of human HSPCs through chemical manipulation and genome editing of specific molecules [85, 86]. Single cell colony-forming assays further revealed that single cells isolated from the HSC population could generate either erythroid colonies exclusively, myeloid colonies exclusively, or mixed colonies in vitro, demonstrating functional heterogeneity even within human CD34+CD38−CD45RA−CD90+CD49f+ HSCs [77]. Thus, single cells with CD34+CD38−CD45RA− surface phenotype possess uni-, oligo- or multi-lineage differentiation potential. To address this intra-population functional heterogeneity, single cell lineage tracing using genetic barcodes has been used[87]. For example, lentivirus-based cellular barcoding were used to determine that HSC-derived progeny of distinct lineages were of the same stem cell origin in multiple hematopoietic organs including the thymus [88]. As another example of studying functional heterogeneity of hematopoietic progenitor cells, CD164 was proposed by single cell RNAseq as a cell surface antigen which might be helpful in segregating distinct HSPC fractions by fluorescence-activated cell sorting. In this study, NSG mice expressing mutated mouse c-Kit and expressing human SCF, were used to show that CD164high CD34+ cells were potent in enabling hematopoietic short-term and long-term BM reconstitution [89]. The authors also found short-term and long-term engraftment only in CD34+CD164high cells, but not in CD34+CD164low cells concluding that the stem and progenitor activities of CD34+ cells were dependent upon those of the CD164high fraction [89]. From another angle, DEGS1, an enzyme mediating sphingolipid biosynthesis, serves as a functional molecule for HSCs to maintain self-renewal capacity through enhanced autophagy [90]. Transplantation of HSPCs with pharmacological inhibition of DEGS1 resulted in altered hematopoietic differentiation in NSG mice [90]. Thus, using these humanized mice, it might be possible to further unravel the metabolic regulation of human HSPC maintenance of stemness and differentiation. A continuum from multi-lineage to uni-lineage differentiation potential in human HSPCs has been previously proposed [91–94]. Recently, a study suggested that myeloid and lymphoid lineages are determined within phenotypically-defined HSCs [95]. Moreover, single cell RNA sequencing of human HSCs and progenitors, have demonstrated a correlation between gene expression programs, and in vivo and in vitro cell fates.[91, 96, 97] Indeed, Integrative analysis of humanized mice and single cell sequencing may extend our understanding of immune reconstitution in patients who undergo stem cell transplantation [97]. As introduced in these studies, merging single cell RNA sequencing with humanized mouse research is improving our understanding of the complexity and heterogeneity of normal human hematopoiesis.
Understanding malignant transformation in human hematopoiesis using humanized mice
One study pioneered the xenotransplantation of primary human leukemia cells in CB17-scid mouse recipients [98]. NSG and NRG strains also support the growth of hematological cancers that do not grow in CB17-scid or NOD-scid mice [99]. Since the introduction of these strains as recipients, patient-derived xenografts (PDXs) using acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL) and other hematologic malignancies have become increasingly utilized to study the in vivo biology of these malignancies. In these strains, which are highly permissive for human engraftment, it has been possible to recapitulate human primary leukemia progression through engraftment of leukemia stem cells (LSCs) or leukemia-initiating cells (LICs)present in variable frequencies in patient samples[100, 101]. The NSG PDX system replicates characteristics of primary leukemia such as clinical aggressiveness [102]. One study first reported the normal hematopoietic reconstitution of NSG mice with AML patient-derived CD34+ cell pre-leukemic stem cell subpopulations harboring somatic mutations [103].
Although isolation and functional analyses of pre-leukemic stem cells and leukemia-initiating stem cells were executed using humanized mice, the extent to which humanized mice recapitulate the complexity and heterogeneity of human hematologic malignancies has not been precisely determined. Taking advantage of the sensitivity of the humanized mouse system, investigators explored the transition of human hematopoiesis from functionally normal stem cells to malignant leukemia initiating cells. After CD34+ subpopulations were functionally identified as pre-leukemic stem cells or as leukemia-initiating cells, investigators performed DNA sequencing to identify distinct mutational profiles between functionally normal, and malignant stem cells [103–105]. The majority of AML-associated mutations that occurred in genes such as DNMT3A, TET2, NPM1, WT1, IDH1/2, BRAS and FLT3 were detected in humanized mice engrafted with patient leukemic cells.[103–105] With bulk [103, 104] and single cell [105] DNA sequencing, it then became possible to determine the order in which mutations were acquired. In addition, by using the humanized mouse as a functional assay for malignant transformation, it became possible to identify mutations with high malignant potential [104–106]. Therefore, successfully-engrafted humanized mice for AML bear significant potential as in vivo models for pre-clinical drug testing and for functionally predictions of optimal, targeted, therapeutic strategies to treat human hematologic malignancies in the presence of multiple somatic mutations. Investigators have also used humanized mice to further identify the contributions of genetically-distinct AML clones to enabling in vivo leukemia propagation, and to identify new “stemness” antigens/genes. [107, 108] The humanized mouse system is not only used for quantifying frequencies and identifying cell surface phenotype of leukemia-initiating cells but also for detecting stem cells in pre-AML disorders such as myelodysplastic syndrome (MDS). [109].
One of the current limitations of humanized mice is in assessing the functional and genomic distinction between pre-leukemic and leukemia-initiating stem cells, since pre-leukemic stem cells are a relatively rare subpopulation within the BM of leukemia patients in aggressive clinical status.[110–112] The number of functionally normal pre-leukemic stem cells obtained from a patient may not be sufficient for successful xenogeneic transplantation. Even in cases where pre-leukemic stem cells successfully engraft, the levels of engraftment are low and repopulation following serial transplantation is inefficient. [103, 105, 113] Therefore, in the majority of transplantation experiments using patient-derived cell populations containing both pre-leukemic stem cells and AML-initiating stem cells, increased outgrowth of LIC-derived malignant clones can result compared with normal hematopoietic engraftment from pre-leukemic stem cells. [112] Accordingly, discrepancies in variant allele frequencies between patient blood or BM, and humanized mouse BM have been noted [114]. Further assessments comparing primary patient specimens with humanized mouse-engrafted leukemia cells are needed to determine the extent of replication of the clonal heterogeneity of human leukemia in humanized mice. Currently available humanized mouse models have made possible the detection and identification of rare primary human pre-leukemic stem cells. Further improvements in the model will be required to assess the self-renewal capacity of pre-leukemic stem cells and in vivo progression of pre-leukemic to leukemic stem cells on a population level as well as at a clonal level. [115]
Interactions between human immune cells and malignant cells in humanized mice
Recently, clinical success using immune checkpoint blockade for certain malignancies has encouraged both immunologists and oncologists to investigate interactions between the immune system and malignant diseases. However, disease-to-disease and patient-to-patient heterogeneity render approaches to predict and optimize outcomes, challenging. In addition to the successful engraftment of immortalized or patient-derived tumor cells, recent humanized mouse systems can allow the simultaneous in vivo replication of normal human immunity and disease, and may enable studying interactions between normal and malignant cell populations (Table 1 [43, 54, 116–138]). Among various human immune subsets, T cells play central roles in immune surveillance against HLA-expressing tumor cells. To enable immunologic interactions between T cells and target cells in vivo, an NSG strain expressing HLA-A0201, the most common HLA-A allele in the Caucasian population was developed [52, 53]. Using this strain, antigen-specific HLA-restricted human CD8+ T cell responses against Epstein Barr Virus (EBV)-associated tumors were assessed [139]. In this study, human CD8+ T cells matured in the context of the mouse thymic microenvironment with HLA expression and were found to exert effector-to-target ratio-dependent cytotoxicity against EBV-infected lymphoblastoid cell lines. These reports suggest that exogenous expression of HLA in murine thymus may enable HLA-restricted thymic T cell maturation. Along with humanization of immune microenvironment using genetic modification, implantation of human lung tissue in BM/liver/thymus humanized mice resulted in antigen-specific humoral and cellular responses against cytomegalovirus (CMV) infection. These approaches may lead to a better understanding of orchestrated B/T/myeloid cell responses against malignant and non-malignant cells, and potentially, to the future development of vaccine treatments for certain conditions[140]. Taking a different strategy, investigators have also aimed to examine human T cell immunity in human peripheral blood lymphocyte (PBL)-engrafted tumor-bearing NOG mice devoid of mouse MHC expression [141]. Due to the lack of mouse MHC expression, transferred human T cells do not cause xenogeneic graft-versus-host disease (GVHD). Instead, investigators can identify specific CD8+ T cell clones that are able to directly attack human tumors in an HLA-restricted manner.[141, 142] To facilitate differentiation and maturation of human NK cells from human HSPCs and to enhance maintenance of PB-derived human NK cells in the mouse microenvironment, human IL-15-expressing SIRPA knock-in, BRG (SRG), NOG and NSG mice were created [43, 143, 144]. Using hIL-15 KI SRG mice, human NK cell infiltration in inoculated Burkitt lymphomas was demonstrated and antibody-dependent cellular cytotoxicity (ADCC) of anti-CD20 antibody against RAJI lymphoma cell lines was observed.[43] In addition, human innate lymphoid cell activity against non-hematologic tumors was examined in an NSG ovarian cancer xenograft model [145]. In this model, intraperitoneal injection of in vitro generated human CD34+ cell-derived NK cells reduced tumor progression in NSG mice inoculated with ovarian cancer spheroids, demonstrating the potential utility of humanized mice in examining immune cell-cancer cell interactions, and in developing putative immune-mediated anti-cancer treatments. Moreover, in vivo transfer of human DCs into Sirpa-KI Flk2-null BRGS mice, led to the development of human innate lymphoid subsets in liver, lung, and hematopoietic organs [146]. In this study, intraperitoneal injection of FLT3 ligand into BRGS mice accelerated differentiation of human DCs from CD34+ HSPCs. Further treatment of the engrafted BRGS mice with poly I:C increased the number of human NK cells in the recipient spleen and human lineage-CD7+CD127+ innate lymphoid cells, indicating that the co-treatment with FLT3 ligand and poly I:C facilitated in vivo interactions between human DCs and innate lymphoid cells in Flk2-null BRGS mice. Humanized mouse systems that are able to support a more diverse and potent T cell-specific anti-tumor immunity are necessary for an actual in vivo replication of such anti-tumor immunity. One approach has been the creation of new strains of immunocompromised BRG, NOG, and NSG recipients carrying HLA class I and II molecules, and those that lack mouse MHC class I and II molecules to achieve HLA-restricted human T cell responses without hyper-activation of T cells recognizing xenogeneic antigens (summarized in [33]). Another goal has been the creation of humanized mice that can recreate physiologically immune cell interactions that occur in the lymph node.[33] However, one of the limitations of using IL2rγ-deficient mouse models for instance, has been that this deficiency results in suboptimal lymphatic architecture, as well as suboptimal lymph node development and organization[12, 18, 19, 147]. By contrast, enhanced expression of IL-7 in the presence of transcription factor RORγ results in increased number of IL-7RA+ lymphoid tissue inducer (LTi) cells and VCAM+ lymphoid tissue organizer (LTo) cells in the fetal gut relative to humanized mice without hIL-7 expression [148, 149]. Consistent with these findings, the absence of the IL2rγ-chain and loss of the high-affinity IL7 receptor in recent strains of immuno-compromised mice appear to be responsible for the suboptimal development of lymphoid tissue-inducer (LTi) cells and lymph node anlage in mice [148, 149].Related to a scenario of insufficiently developed LN structure, robust human immune responses are not elicited in the absence of follicular helper T cells (Tfh) and follicular dendritic cells (FDCs), upon various stimuli and antigen recognition. Humanized mice with lymph node structure that allow development of inducer and organizer cells with sufficient function in adequate numbers are expected to elicit more potent human response upon stimulation with disease-associated antigens or vaccine treatment. To overcome the limitation in humanized mouse models, IL2rγ-deficient mouse recipients have been generated in which mouse LTi function has been restored by transgenic expression of i) γc molecule specifically and exclusively on LTi cells [150] or ii) thymic stromal lymphopoietin (TSLP), which signals independently of the IL2rγ-chain, but plays an important role in LN development [13, 151–153]. In fact, IL-21 producing Tfh cells have been detected in TLSP-expressing, Sirpa-KI, BRG (BRGST) mice [153]. Based on the improvement of lymphoid tissue organization and lymphocyte development in TSLP-expressing humanized mice, we await further advances in our understanding of immuno-oncology and in clarifying the mechanisms underlying clinical responses of checkpoint blockade [92] in order to improve patient outcomes [154].
Table 1. In vivo assessment of immune responses against tumors in humanized mice.
Examples of the cellular interactions between tumors and immune cells are listed. Donor cells for the reconstitution of human immunity as well as specific immune cells interacting with tumor cells are shown. Ab: anitbody; Ag: antigen; CTL: cytotoxic T cells; MSC: mesenchymal stem cells; TIL: tumor infiltrating lymphocytes; MDSC: myeloid derived suppressor cells; DC: dendritic cells; Treg: regulatory T cells; PB: peripheral blood; PBMC: peripheral blood mononuclear cells; TAM: tumor associated macrophages; MSC: mesenchymal stem cells; CB: cord blood cells; AML: acute myeloid leukemia.
| Transplanted/injected human cells | Human immune cells recognizing tumor or being recognized by tumor | Innoculated/injected tumor | Reported tumor-immunity interaction | Ref |
|---|---|---|---|---|
| CD34+ CB cells | T cells | melanoma cell line (A2058) | Recruitment of CTL to A2058 tumor sites induced by ONCOS-102 | 98 |
| DC-activated lymphocytes | CTL | ovarian cancer cell line (A2780) | Efficient splenic production of IFNγ | 10 8 |
| CXCR2 transduced T cells | CXCR2 transduced T cells | malignant melanoma cell lines | Improved homing of CXCR2-transduced human T cells to melanoma | 95 |
| Tumor infiltrating T cells (TILs) | autologous T cells | malignant melanoma cell lines | Tumor regression by transfer of tumor-infiltrating lymphocytes and supply of hIL-2 | 96 |
| autologous EBV-specific T cells | autologous EBV-specific T cells | EBV-transformed LCL | Enhanced cytotoxic activity of EBV-specific human T cells by anti-hCD39 Ab | 10 1 |
| CD34+ CB cells | T cell, NK cell, MDSC, macrophages | primary hepatocellular carcinoma | Multiple innate immune cells (NK, macrophages, MDSC) in tumors | 11 0 |
| CD34+ CB cells | T cell, NK cell, MDSC, DC | primary and immortalized non-small cell lung cancer | Reduction of tumor size and activation of CD8+ T cells by Permbrolizumab | 10 2 |
| CD34+ CB cells | T cells | primary AML | Suboptimal human CB HSPC-derived T cell response against AML | 10 5 |
| CD34+ CB cells | T cells | breast cancer cell line (MDA-MB-231) | Effect of chemotherapy and anti-ICOS Ab on tumor growth and on Treg cells | 90 |
| CD34+ CB cells | monocytes, DC | breast cancer cell line (MDA-MB-231) | Detection of PD-L1 in tumor cells by micro-SPECT/CT imaging | 93 |
| CD34+ CB cells | T cell, B cell (in vivo treatment with either iEBVs-Rab27a siRNA or iEBVs-NC siRNA) | colon cancer cell line (Lovo cell) | Inhibition of tumor growth and increase of CD8+ T cells by EBV-mediated in vivo silencing of Rab27a | 10 9 |
| PBMC | CD8+/CD4+/Fox p3+ T cells | primary EML4-ALK variant NSCLC, NSCLC cell line (H3122: EML4-AKL variant 1) | Distribution of TILs in tumor nest and stroma | 10 4 |
| PBMC | T cells | bladder cancer cell line (VMCUB1) | Alteration of chemokines and integrins in tumor microenvironment by inhibition of PI3K pathway | 89 |
| PBMC | T cells | melanoma cell line (C8161) | Targeting PD-L1+ tumor cells by CD3xPD-L1 bi-specific T cell engager (BiTE) | 94 |
| TAMs | TAMs | primary colon cancer | Role of homeobox protein, VentX, in TAMs | 10 0 |
| CD56+NKp44+ NK cells | CD56+NKp44+ NK cells | primary head and neck squamous cell carcinoma | NKp44-associated immune checkpoint blockade by monoclonal antibody to PCNA | 97 |
| DC-activated lymphocytes | CTL, NK cells | lung cancer cell line (A549/A2.1) | CTL induction by engineered human DCs | 10 7 |
| PB γδ T cells | γδ T cells | neuroblastoma cell lines | Regression of neuroblastoma with combination treatment using γδ T cell and molecular-targeting drugs. | 11 1 |
| BM MSCs from neuroblastoma patients, NK cells and monocytes from | NK cells, MSC, monocytes | neuroblastoma cell lines | Immune-suppressive effect by MSCs and monocytes in neuroblastoma | 10 6 |
| PBMC | ||||
| PB CTL | CTL | primary AML, breast cancer cell line (SKBR3) | Homing of Ag-specific CTLs by fucosylation | 88 |
| CD34+ CB cells, CAR-T | CD14+ monocytes | ALL cell line (ALL-CM) | Mechanism of cytokine release syndrome in CAR-T treatment | 10 3 |
| CD34+ CB cells | CD8+ T cells, B cells, myeloid cells | primary adrenocortical carcinoma | Effect of anti-PD1 Ab on human immune cells | 99 |
| CD34+ CB cells | T cells, B cells, myeloid cells | triple negative breast cancer cell line (MDA-MB-231) | Effect of anti-PD1 Ab on human immune cells | 91 |
| CD34+ CB cells | NK cells | Burkit lymphoma cell line (Raji) | Human NK cell biology in IL-15 expressing SRG mice | 92 |
| CD34+ CB cells | macrophages | melanoma cell line (Me290) | Infiltration of a specific subtype of human macrophages in MISTRG mice | 48 |
Concluding remarks
Undoubtedly, an increased understanding of the in vivo dynamics of normal and malignant stem cells would be one of the greatest advantages of utilizing humanized mouse models in biomedical research. However, a lack of human environmental factors remains an important shortcoming of the system. On the one hand, genetic introduction of human cytokines and adhesion molecules in “new generation humanized mice” can allow us to better understand human hematopoiesis and immunity. On the other hand, precisely determining the nature of the interactions between human immune cells and malignant cells, as well as the clonal heterogeneity of human hematologic and solid tumors, remains a challenge, but is of crucial importance. These questions speak directly to future clinical directions, such as personalized precision medicine (Box 1), targeting pre-malignant and malignant stem cells, reversing tumor-driven immunosuppression, and ideally, potentiating human immune responses (see Oustanding Questions).
Outstanding Questions.
Can engraftment and differentiation of certain lineages and cell types (e.g. erythroid lineage, megakaryocytes and platelets, certain T cell subsets) be further improved in humanized mice to approach physiological levels? How might this be achieved?
In patients with hematological malignancies, normal immune and hematopoietic cells coexist and interact with malignant cells; Can these interactions be replicated in humanized mice?
Humanized mice engrafted with human hematopoietic malignancies can be used for in vivo therapeutic testing. Can the results correlate with responses in patients? Can preclinical therapeutic testing using humanized mice lead to the development of more predictive and effective treatment options?
How can the humanized mouse model be expanded for human non-hematopoietic systems?
To functionally recapitulate positive and negative selection of human T cells in the humanized mouse thymus, homology of pertinent human and mouse proteins remains an important issue. [155] Moreover, among diverse human diseases and disease-associated antigens, future experiments should aim to address which human diseases or which antigen-specific human immune responses can be best feasibly investigated in humanized mice (Box 2). These questions are relevant as humanized mouse research is likely to make major contributions in precision/personalized medicine for heterogenous human diseases. Investigators have begun to address these questions for potential translation into the clinic. For instance, engraftment efficacy and proliferative properties of human tumors in humanized mice have correlated well with clinical outcomes, such as disease relapse in patients [156]. PDX-models using humanized mice can enable the in vivo treatment of multiple mice engrafted with individual patient cells and allow the creation of in vivo models of human diseases with defined genetic abnormalities.[157] By conducting pre-clinical in vivo therapeutic experiments for an MDM2 inhibitor, researchers have identified a B-ALL subtype with good sensitivity to the compound. Pre-clinical studies in PDX-models using the humanized mice might facilitate safe and efficient clinical trials in the future. Moreover, stemness score, as demonstrated by xenogeneic transplantation, has been proven to be helpful in predicting the risk of acute leukemia in the clinic [158]. These findings highlight the potential of using humanized mice as PDXs in predicting clinical outcomes of patients with heterogenous malignant diseases, and in determining potential treatment strategies for individual patients.
Box 2. Examples of Future Directions for Studying Cellular Interactions within Tumors in Humanized Mice.
To discuss future directions of humanized mouse research, we need to consider the tumor-specific environment of leukemia and solid tumors. While some cancer cells promote growth of fibroblasts (cancer-associated fibroblasts), mesenchymal cells or stromal components are generally of mouse origin in humanized mice or xenograft models.[19, 159] One of the promising ways of analyzing the interactions among tumor cells, immune cells, and mesenchymal cells is to engraft human tissues in humanized mice without promoting graft-versus-host disease (Figure 1). In hematological tumors, in vivo injection of leukemia-initiating cells results in homing of the cells and proliferation of malignant hematopoietic cells in the bone marrow (BM) followed by systemic circulation of the leukemic cells.[100, 160, 161] Co-injection of patient-derived pre-leukemic and leukemic stem cells or HLA-matched normal and leukemic stem cells into immuno-compromised mice expressing niche-associated molecules along with HLA expression in the thymic microenvironment may further enable studies assessing the interactions between leukemic cells and the BM niche, as well as between leukemic cells and immune cells (Figure 1).
Although it took decades to establish humanized mouse models that support engraftment of normal and malignant human stem cells, they have now become a widely available in vivo tool for scientists to clarify previously unknown aspects of malignant and non-malignant human diseases, as well as generally, human biology and physiology. We expect that such efforts by investigators will globally translate to achieve a better understanding and more efficacious treatment options for difficult-to-treat human diseases.
Figure 1. In vivo analysis of heterogenous cellular interactions in hematologic and solid tumors in Humanized mice.
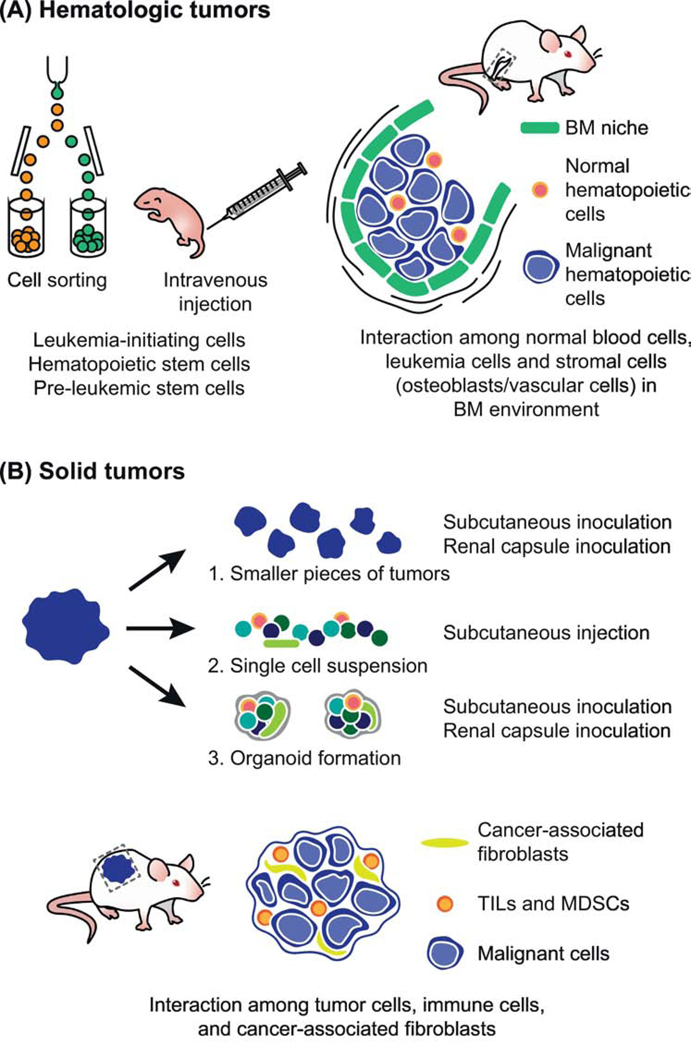
A. Hematologic tumors: Intravenous injection of human leukemia-initiating stem cells into immune-compromised mice results in engraftment of human leukemic cells in the bone marrow (BM). By co-injecting hematopoietic stem cells or pre-leukemic stem cells as well as leukemia-initiating stem cells, normal hematopoietic and immune systems can also be reconstituted. Currently, by using transgenic or knock-in approaches, humanized BM microenvironments have been created. Interactions between the BM niche, immune cells, and leukemia cells awaits further study with the improved models of humanized mice.
B. Solid tumors: Tumor cells are prepared in three distinct ways for making xenografts, i)smaller pieces for renal/subcutaneous inoculation, ii) single cell suspensions for intravenous/subcutaneous injection, or iii) organoid formation for renal/subcutaneous inoculation. Interactions between malignant cells, cancer-associated fibroblasts (CAFs), and immune cells (either tumor infiltrating lymphocytes (TILs) or myeloid derived suppressor cells (MDSCs)) awaits more study with further development of humanized mice.
Box 3. Creation of PDX-models for hematologic and non-hematologic malignancies using Humanized Mice.
To create PDX-models of human hematologic malignancies, FACS-based purification of normal, pre-malignant, and leukemic stem cells is followed by transplantation experiments using Il2rg deficient immuno-compromised mice as recipients. Co-engraftment of normal/pre-malignant immune cells and leukemic cells can be achieved by injecting normal and malignant stem cells and by creating a BM microenvironment supporting the interactions between stem cells and the niche. In addition, in the thymic microenvironment, T cell selection, and selection of autologous pre-leukemic stem cells or allogeneic normal HSCs are vital for the analysis of the crosstalk between normal human hematopoiesis, immunity and malignancy. For solid tumors, patient-derived tissues can be processed for xenografting in three ways: i) cutting into smaller pieces for subcutaneous or renal capsular inoculation, ii) making single cell suspensions for intravenous or subcutaneous injections, or iii) making organoids for inoculation under skin or renal capsule. Varying degrees of cancer-associated fibroblasts or immune cells attacking tumors or even fostering tumor progression can be present in a xenograft. Graft-versus-host reactions by tumor infiltrating T lymphocytes (TILs) has been an event used to monitor immune reactions against tumors in vivo.
Highlights.
New generations of humanized mice replicate much of the complexity and heterogeneity of both normal and malignant human hematopoiesis.
Humanized mice can allow the in vivo characterization of the engraftment and differentiation capacities of hematopoietic stem/progenitor cell subpopulations, as well as the functional discrimination of normal and malignant stem cells.
Humanized mice can support a functional correlation of genomic data with the in vivo characteristics of hematopoietic cells; Through combination with single cell genomics, the genetic heterogeneity within subpopulations of normal or malignant human hematopoietic cells can be linked to in vivo functions and can reveal pathogenetic mechanisms.
Acknowledgments
We thank Drs. Haruhiko Koseki, Osamu Ohara, Shuichi Taniguchi, Dale Greiner and Michael Brehm for collaboration in humanized mouse research. Humanized mouse research for normal and malignant hematopoiesis is supported by RIKEN Presidential discretionary fund. and by NIH grants OD026440, AI132963, DK104218 and CA237307.
Glossary
- Adeno-associated virus (AAV)-based vector
used to introduce human genes into experimental animals or in gene therapy applications.
- Antibody-dependent cellular cytotoxicity (ADCC)
binding of antibodies to a target cell through a specific antibody-antigen interaction leads to target cell lysis by effector cells, typically NK cells, in a complement independent manner.
- BRG mice
immunodeficient mice (C.129-Rag2tm1FwaIL2rgtm1Sug) on a BALB/c background lacking both IL2rg and Rag2.
- Follicular helper T (Tfh) cells
specialized subset of CD4+ T cells located in the B cell zone of secondary lymphoid organs. Tfh cells induce the formation of germinal centers during an ongoing immune response.
- Genetic barcodes
Artificial DNA tags used to label and trace individual cells within a proliferating cell population.
- Graft-versus-host disease (GVHD)
condition associated with transplantation where immune cells of a donor origin recognize host cells as foreign and attack the latter.
- Histocompatibility system 2 (H2)
Major histocompatibility complex (MHC) in mice.
- Humanized mice
engrafted with functional human cells or tissues or expressing human transgenes. In the majority of humanized mice, hematopoietic and immune systems are humanized.
- Immune checkpoint blockade (ICB)
Inhibition of immune checkpoint molecules (these modulate adaptive immune responses and are required for self-tolerance and suppression of autoimmunity). CTLA4 and PD-L1, when expressed on tumor cells, can inhibit T cell activation, leading to immune escape. ICB can restore anti-tumor T cell immunity.
- Leukemia-initiating cells or Leukemic stem cells
Stem-like cells with in vivo leukemogenic capacity. In leukemia, developmental hierarchy analogous to normal hematopoiesis has been proposed, where mature leukemia cells or blasts are thought to originate from quiescent stem-like cells that have undergone malignant transformation.
- Lymph node anlage
Rudimentary precursor of the lymph node present during embryogenesis; thought to develop through interactions between mesenchymal cells and LTi cells.
- Lymphoid tissue inducer (LTi) cells
type of innate lymphoid cells whose functions include formation of lymph nodes and Peyer’s patches, embryonic development of the thymus and survival of memory T cells within secondary lymphoid organs.
- Lymphoid tissue organizer (LTo) cells
Thought to be precursors of stromal cells in secondary lymphoid organs that arise through interaction with LTi cells during embryonic development.
- Mutations at the mouse W/c-kit locus
Murine proto-oncogene c-kit encodes a transmembrane receptor tyrosine kinase and maps to the mouse dominant white spotting (W) locus. Mutations in the W/c-kit locus lead to defects in stem cells of the hematopoietic lineage, in addition to those in the melanocytic and germ cell lineages, leading to a competitive advantage for human hematopoietic stem and progenitor cells and improving xenotransplantation efficiency.
- NOG mice
immuno-deficient mice on the NOD-scid background (NOD.Cg-PrkdcscidIL2rgtm1Sug) with partial deletion of IL2rγ. Lack mature T, B and NK cells.
- NRG mice
immuno-deficient mice on the NOD-Rag1null background (NOD.Cg-Rag1tm1MomIL2rgtm1Wjl) lacking both IL2rγ and Rag1. Lack mature T, B and NK cells.
- NSG mice
immuno-deficient mice on NOD-scid background (NOD.Cg-PrkdcscidIL2rgtm1Wjl) with complete null mutation of IL2rγ. Lack mature T, B and NK cells.
- Organoid
miniaturized in vitro model of an organ.
- Ossicle
bone organoid mimicking the human BM microenvironment in vivo in model animals.
- Patient-derived xenografts (PDXs)
Models of human diseases where cells or tissues from patients are transplanted or implanted into immunodeficient host animals.
- Pre-leukemic (hematopoietic) stem cells
bear leukemia-associated mutations with a capacity for normal hematopoiesis.
- Prkdcscid
Autosomal recessive mutation mapped to the centromeric end of chromosome 16. SCID (severe combined immunodeficiency) mice are homozygous for the Prkdcscid allele, and lack functional B and T lymphocytes due to impaired VDJ rearrangement.
- Regulatory T cells
specialized subset of CD4+ T cells that regulate immune responses by suppressing the activation, proliferation and cytokine production of CD4+ and CD8+ T cells. They are also thought to suppress B cells and dendritic cells.
- Stemness
A set of universal properties and characteristics defining stem cells. Includes the ability to undergo lineage differentiation, propagate progeny and self-renew. Both intrinsic (genetic, epigenetic) and extrinsic (environmental interactions) underly these properties.
- Syngeneic
Of the same genetic background.
- Xenogeneic
Of different genetic background, such as those of different species.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shultz LD. et al. (2007) Humanized mice in translational biomedical research. Nat Rev Immunol 7 (2), 118–30. [DOI] [PubMed] [Google Scholar]
- 2.Manz MG. and Di Santo JP. (2009) Renaissance for mouse models of human hematopoiesis and immunobiology. Nat Immunol 10 (10), 1039–42. [DOI] [PubMed] [Google Scholar]
- 3.Bosma GC. et al. (1983) A severe combined immunodeficiency mutation in the mouse. Nature 301 (5900), 527–30. [DOI] [PubMed] [Google Scholar]
- 4.Kamel-Reid S. and Dick JE. (1988) Engraftment of immune-deficient mice with human hematopoietic stem cells. Science 242 (4886), 1706–9. [DOI] [PubMed] [Google Scholar]
- 5.McCune JM. et al. (1988) The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241 (4873), 1632–9. [DOI] [PubMed] [Google Scholar]
- 6.Mosier DE. et al. (1988) Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 335 (6187), 256–9. [DOI] [PubMed] [Google Scholar]
- 7.Shultz LD. et al. (1995) Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol 154 (1), 180–91. [PubMed] [Google Scholar]
- 8.Koyanagi Y. et al. (1997) High levels of viremia in hu-PBL-NOD-scid mice with HIV-1 infection. Leukemia 11 Suppl 3, 109–12. [PubMed] [Google Scholar]
- 9.Blunt T. et al. (1996) Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc Natl Acad Sci U S A 93 (19), 10285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown EJ. and Frazier WA. (2001) Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 11 (3), 130–5. [DOI] [PubMed] [Google Scholar]
- 11.Barclay AN. and Brown MH. (2006) The SIRP family of receptors and immune regulation. Nat Rev Immunol 6 (6), 457–64. [DOI] [PubMed] [Google Scholar]
- 12.Shultz LD. et al. (2012) Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 12 (11), 786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao X. et al. (1995) Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 2 (3), 223–38. [DOI] [PubMed] [Google Scholar]
- 14.DiSanto JP. et al. (1995) Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci U S A 92 (2), 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohbo K. et al. (1996) Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood 87 (3), 956–67. [PubMed] [Google Scholar]
- 16.Jacobs H. et al. (1999) PIM1 reconstitutes thymus cellularity in interleukin 7- and common gamma chain-mutant mice and permits thymocyte maturation in Rag- but not CD3gamma-deficient mice. J Exp Med 190 (8), 1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shultz LD. et al. (2011) Humanized mice as a preclinical tool for infectious disease and biomedical research. Ann N Y Acad Sci 1245, 50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenney LL. et al. (2016) Humanized Mouse Models for Transplant Immunology. Am J Transplant 16 (2), 389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theocharides AP. et al. (2016) Humanized hemato-lymphoid system mice. Haematologica 101 (1), 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh NC. et al. (2017) Humanized Mouse Models of Clinical Disease. Annu Rev Pathol 12, 187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shultz LD. et al. (2005) Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 174 (10), 6477–89. [DOI] [PubMed] [Google Scholar]
- 22.Ito M. et al. (2002) NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100 (9), 3175–82. [DOI] [PubMed] [Google Scholar]
- 23.Pearson T. et al. (2008) Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin Exp Immunol 154 (2), 270–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traggiai E. et al. (2004) Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304 (5667), 104–7. [DOI] [PubMed] [Google Scholar]
- 25.Mombaerts P. et al. (1992) RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68 (5), 869–77. [DOI] [PubMed] [Google Scholar]
- 26.Shinkai Y. et al. (1992) RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68 (5), 855–67. [DOI] [PubMed] [Google Scholar]
- 27.Brehm MA. et al. (2010) Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes 17 (2), 120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolas N. et al. (1998) A human severe combined immunodeficiency (SCID) condition with increased sensitivity to ionizing radiations and impaired V(D)J rearrangements defines a new DNA recombination/repair deficiency. J Exp Med 188 (4), 627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wunderlich M. et al. (2019) Improved chemotherapy modeling with RAG-based immune deficient mice. PLoS One 14 (11), e0225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legrand N. et al. (2011) Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc Natl Acad Sci U S A 108 (32), 13224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strowig T. et al. (2011) Transgenic expression of human signal regulatory protein alpha in Rag2−/−gamma(c)−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A 108 (32), 13218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamauchi T. et al. (2013) Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood 121 (8), 1316–25. [DOI] [PubMed] [Google Scholar]
- 33.Shultz LD. et al. (2019) Humanized mouse models of immunological diseases and precision medicine. Mamm Genome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito R. et al. (2018) Humanized mouse models: Application to human diseases. J Cell Physiol 233 (5), 3723–3728. [DOI] [PubMed] [Google Scholar]
- 35.Masse-Ranson G. et al. (2018) Humanized mouse models to study pathophysiology and treatment of HIV infection. Curr Opin HIV AIDS 13 (2), 143–151. [DOI] [PubMed] [Google Scholar]
- 36.Boettcher S. and Manz MG. (2017) Regulation of Inflammation- and Infection-Driven Hematopoiesis. Trends Immunol 38 (5), 345–357. [DOI] [PubMed] [Google Scholar]
- 37.Macchiarini F. et al. (2005) Humanized mice: are we there yet? J Exp Med 202 (10), 1307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishikawa F. et al. (2005) Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106 (5), 1565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanker A. (2004) Is thymus redundant after adulthood? Immunol Lett 91 (2–3), 79–86. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka S. et al. (2012) Development of mature and functional human myeloid subsets in hematopoietic stem cell-engrafted NOD/SCID/IL2rgammaKO mice. J Immunol 188 (12), 6145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe M. et al. (2009) Immunological aspects of REIC/Dkk-3 in monocyte differentiation and tumor regression. Int J Oncol 34 (3), 657–63. [DOI] [PubMed] [Google Scholar]
- 42.Wunderlich M. et al. (2018) Improved multilineage human hematopoietic reconstitution and function in NSGS mice. PLoS One 13 (12), e0209034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herndler-Brandstetter D. et al. (2017) Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc Natl Acad Sci U S A 114 (45), E9626–E9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huntington ND. et al. (2009) IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med 206 (1), 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazurier F. et al. (2003) Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med 9 (7), 959–63. [DOI] [PubMed] [Google Scholar]
- 46.Nixon CC. et al. (2020) Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 578 (7793), 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melkus MW. et al. (2006) Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 12 (11), 1316–22. [DOI] [PubMed] [Google Scholar]
- 48.Honeycutt JB. et al. (2018) T cells establish and maintain CNS viral infection in HIV-infected humanized mice. J Clin Invest 128 (7), 2862–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reinisch A. et al. (2016) A humanized bone marrow ossicle xenotransplantation model enables improved engraftment of healthy and leukemic human hematopoietic cells. Nat Med 22 (7), 812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reinisch A. et al. (2017) Generation and use of a humanized bone-marrow-ossicle niche for hematopoietic xenotransplantation into mice. Nat Protoc 12 (10), 2169–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki M. et al. (2012) Induction of human humoral immune responses in a novel HLA-DR-expressing transgenic NOD/Shi-scid/γcnull mouse. Int Immunol 24 (4), 243–52. [DOI] [PubMed] [Google Scholar]
- 52.Strowig T. et al. (2009) Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med 206 (6), 1423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shultz LD. et al. (2010) Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A 107 (29), 13022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rongvaux A. et al. (2014) Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol 32 (4), 364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kametani Y. et al. (2017) NOG-hIL-4-Tg, a new humanized mouse model for producing tumor antigen-specific IgG antibody by peptide vaccination. PLoS One 12 (6), e0179239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshimi A. et al. (2017) Robust patient-derived xenografts of MDS/MPN overlap syndromes capture the unique characteristics of CMML and JMML. Blood 130 (4), 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song Y. et al. (2019) A highly efficient and faithful MDS patient-derived xenotransplantation model for pre-clinical studies. Nat Commun 10 (1), 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denton PW. et al. (2012) IL-2 receptor gamma-chain molecule is critical for intestinal T-cell reconstitution in humanized mice. Mucosal Immunol 5 (5), 555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalscheuer H. et al. (2012) A model for personalized in vivo analysis of human immune responsiveness. Sci Transl Med 4 (125), 125ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovarova M. et al. (2018) Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat Commun 9 (1), 4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lavender KJ. et al. (2018) Pathogenicity of Ebola and Marburg Viruses Is Associated With Differential Activation of the Myeloid Compartment in Humanized Triple Knockout-Bone Marrow, Liver, and Thymus Mice. J Infect Dis 218 (suppl_5), S409–S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Legrand N. et al. (2009) Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host Microbe 6 (1), 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brehm MA. et al. (2013) Overcoming current limitations in humanized mouse research. J Infect Dis 208 Suppl 2, S125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brehm MA. et al. (2014) Generation of improved humanized mouse models for human infectious diseases. J Immunol Methods 410, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akkina R. et al. (2016) Improvements and Limitations of Humanized Mouse Models for HIV Research: NIH/NIAID “Meet the Experts” 2015 Workshop Summary. AIDS Res Hum Retroviruses 32 (2), 109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang J. et al. (2014) An AAV vector-mediated gene delivery approach facilitates reconstitution of functional human CD8+ T cells in mice. PLoS One 9 (2), e88205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perrin GQ. et al. (2016) Dynamics of antigen presentation to transgene product-specific CD4(+) T cells and of Treg induction upon hepatic AAV gene transfer. Mol Ther Methods Clin Dev 3, 16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brehm MA. et al. (2012) Engraftment of human HSCs in nonirradiated newborn NOD-scid IL2rgamma null mice is enhanced by transgenic expression of membrane-bound human SCF. Blood 119 (12), 2778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takagi S. et al. (2012) Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood 119 (12), 2768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller PH. et al. (2013) Enhanced normal short-term human myelopoiesis in mice engineered to express human-specific myeloid growth factors. Blood 121 (5), e1–4. [DOI] [PubMed] [Google Scholar]
- 71.Barve A. et al. (2018) Comparative utility of NRG and NRGS mice for the study of normal hematopoiesis, leukemogenesis, and therapeutic response. Exp Hematol 67, 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wunderlich M. et al. (2010) AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 24 (10), 1785–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Billerbeck E. et al. (2011) Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rgamma(null) humanized mice. Blood 117 (11), 3076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coughlan AM. et al. (2016) Myeloid Engraftment in Humanized Mice: Impact of Granulocyte-Colony Stimulating Factor Treatment and Transgenic Mouse Strain. Stem Cells Dev 25 (7), 530–41. [DOI] [PubMed] [Google Scholar]
- 75.Thompson HL. et al. (2016) F4/80(+) Host Macrophages Are a Barrier to Murine Embryonic Stem Cell-Derived Hematopoietic Progenitor Engraftment In Vivo. J Immunol Res 2016, 2414906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Favuzza P. et al. (2020) Dual Plasmepsin-Targeting Antimalarial Agents Disrupt Multiple Stages of the Malaria Parasite Life Cycle. Cell Host Microbe 27 (4), 642–658.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Notta F. et al. (2011) Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333 (6039), 218–21. [DOI] [PubMed] [Google Scholar]
- 78.Majeti R. et al. (2007) Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 1 (6), 635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahmig S. et al. (2016) Improved Human Erythropoiesis and Platelet Formation in Humanized NSGW41 Mice. Stem Cell Reports 7 (4), 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu YP. et al. (2018) Identification of an Early Unipotent Neutrophil Progenitor with Pro-tumoral Activity in Mouse and Human Bone Marrow. Cell Rep 24 (9), 2329–2341 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yurino A. et al. (2016) Enhanced Reconstitution of Human Erythropoiesis and Thrombopoiesis in an Immunodeficient Mouse Model with Kit(Wv) Mutations. Stem Cell Reports 7 (3), 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyawaki K. et al. (2017) Identification of unipotent megakaryocyte progenitors in human hematopoiesis. Blood 129 (25), 3332–3343. [DOI] [PubMed] [Google Scholar]
- 83.Alhaj Hussen K. et al. (2017) Molecular and Functional Characterization of Lymphoid Progenitor Subsets Reveals a Bipartite Architecture of Human Lymphopoiesis. Immunity 47 (4), 680–696 e8. [DOI] [PubMed] [Google Scholar]
- 84.Notta F. et al. (2016) Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 351 (6269), aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang X. et al. (2018) Neutralizing negative epigenetic regulation by HDAC5 enhances human haematopoietic stem cell homing and engraftment. Nat Commun 9 (1), 2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park SH. et al. (2019) Highly efficient editing of the beta-globin gene in patient-derived hematopoietic stem and progenitor cells to treat sickle cell disease. Nucleic Acids Res 47 (15), 7955–7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu R. et al. (2011) Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat Biotechnol 29 (10), 928–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brugman MH. et al. (2015) Development of a diverse human T-cell repertoire despite stringent restriction of hematopoietic clonality in the thymus. Proc Natl Acad Sci U S A 112 (44), E6020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pellin D. et al. (2019) A comprehensive single cell transcriptional landscape of human hematopoietic progenitors. Nat Commun 10 (1), 2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie SZ. et al. (2019) Sphingolipid Modulation Activates Proteostasis Programs to Govern Human Hematopoietic Stem Cell Self-Renewal. Cell Stem Cell 25 (5), 639–653 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Velten L. et al. (2017) Human haematopoietic stem cell lineage commitment is a continuous process. Nat Cell Biol 19 (4), 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buenrostro JD. et al. (2018) Integrated Single-Cell Analysis Maps the Continuous Regulatory Landscape of Human Hematopoietic Differentiation. Cell 173 (6), 1535–1548 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giladi A. et al. (2018) Single-cell characterization of haematopoietic progenitors and their trajectories in homeostasis and perturbed haematopoiesis. Nat Cell Biol 20 (7), 836–846. [DOI] [PubMed] [Google Scholar]
- 94.Karamitros D. et al. (2018) Single-cell analysis reveals the continuum of human lympho-myeloid progenitor cells. Nat Immunol 19 (1), 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Belluschi S. et al. (2018) Myelo-lymphoid lineage restriction occurs in the human haematopoietic stem cell compartment before lymphoid-primed multipotent progenitors. Nat Commun 9 (1), 4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watson CJ. et al. (2020) The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 367 (6485), 1449–1454. [DOI] [PubMed] [Google Scholar]
- 97.Jacobsen SEW. and Nerlov C. (2019) Haematopoiesis in the era of advanced single-cell technologies. Nat Cell Biol 21 (1), 2–8. [DOI] [PubMed] [Google Scholar]
- 98.Lapidot T. et al. (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367 (6464), 645–8. [DOI] [PubMed] [Google Scholar]
- 99.Shultz LD. et al. (2014) Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc 2014 (7), 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ishikawa F. et al. (2007) Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 25 (11), 1315–21. [DOI] [PubMed] [Google Scholar]
- 101.Ninomiya M. et al. (2007) Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia 21 (1), 136–42. [DOI] [PubMed] [Google Scholar]
- 102.Morisot S. et al. (2010) High frequencies of leukemia stem cells in poor-outcome childhood precursor-B acute lymphoblastic leukemias. Leukemia 24 (11), 1859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corces-Zimmerman MR. et al. (2014) Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci U S A 111 (7), 2548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shlush LI. et al. (2017) Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 547 (7661), 104–108. [DOI] [PubMed] [Google Scholar]
- 105.Saito Y. et al. (2017) Overcoming mutational complexity in acute myeloid leukemia by inhibition of critical pathways. Sci Transl Med 9 (413). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Labuhn M. et al. (2019) Mechanisms of Progression of Myeloid Preleukemia to Transformed Myeloid Leukemia in Children with Down Syndrome. Cancer Cell 36 (2), 123–138 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Boer B. et al. (2018) Prospective Isolation and Characterization of Genetically and Functionally Distinct AML Subclones. Cancer Cell 34 (4), 674–689 e8. [DOI] [PubMed] [Google Scholar]
- 108.Quek L. et al. (2016) Genetically distinct leukemic stem cells in human CD34-acute myeloid leukemia are arrested at a hemopoietic precursor-like stage. J Exp Med 213 (8), 1513–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Woll PS. et al. (2014) Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell 25 (6), 794–808. [DOI] [PubMed] [Google Scholar]
- 110.Sykes SM. et al. (2015) Clonal evolution of preleukemic hematopoietic stem cells in acute myeloid leukemia. Exp Hematol 43 (12), 989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jan M. et al. (2012) Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med 4 (149), 149ra118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shlush LI. et al. (2014) Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506 (7488), 328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shlush LI. et al. (2014) Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506 (7488), 328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Potter N. et al. (2019) Single cell analysis of clonal architecture in acute myeloid leukaemia. Leukemia 33 (5), 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steensma DP. and Ebert BL. (2020) Clonal hematopoiesis as a model for premalignant changes during aging. Exp Hematol 83, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alatrash G. et al. (2019) Fucosylation Enhances the Efficacy of Adoptively Transferred Antigen-Specific Cytotoxic T Lymphocytes. Clin Cancer Res 25 (8), 2610–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Borcoman E. et al. (2019) Inhibition of PI3K pathway increases immune infiltrate in muscle-invasive bladder cancer. Oncoimmunology 8 (5), e1581556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burlion A. et al. (2019) A novel combination of chemotherapy and immunotherapy controls tumor growth in mice with a human immune system. Oncoimmunology 8 (7), 1596005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Capasso A. et al. (2019) Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts. J Immunother Cancer 7 (1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heskamp S. et al. (2019) PD-L1 microSPECT/CT Imaging for Longitudinal Monitoring of PD-L1 Expression in Syngeneic and Humanized Mouse Models for Cancer. Cancer Immunol Res 7 (1), 150–161. [DOI] [PubMed] [Google Scholar]
- 121.Horn LA. et al. (2017) CD3xPDL1 bi-specific T cell engager (BiTE) simultaneously activates T cells and NKT cells, kills PDL1(+) tumor cells, and extends the survival of tumor-bearing humanized mice. Oncotarget 8 (35), 57964–57980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Idorn M. et al. (2018) Chemokine receptor engineering of T cells with CXCR2 improves homing towards subcutaneous human melanomas in xenograft mouse model. Oncoimmunology 7 (8), e1450715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jespersen H. et al. (2017) Clinical responses to adoptive T-cell transfer can be modeled in an autologous immune-humanized mouse model. Nat Commun 8 (1), 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kundu K. et al. (2019) Inhibition of the NKp44-PCNA Immune Checkpoint Using a mAb to PCNA. Cancer Immunol Res 7 (7), 1120–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuryk L. et al. (2019) Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. Oncoimmunology 8 (2), e1532763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lang J. et al. (2020) Development of an Adrenocortical Cancer Humanized Mouse Model to Characterize Anti-PD1 Effects on Tumor Microenvironment. J Clin Endocrinol Metab 105 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Le Y. et al. (2018) The homeobox protein VentX reverts immune suppression in the tumor microenvironment. Nat Commun 9 (1), 2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li XY. et al. (2019) Targeting CD39 in Cancer Reveals an Extracellular ATP- and Inflammasome-Driven Tumor Immunity. Cancer Discov 9 (12), 1754–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meraz IM. et al. (2019) An Improved Patient-Derived Xenograft Humanized Mouse Model for Evaluation of Lung Cancer Immune Responses. Cancer Immunol Res 7 (8), 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Norelli M. et al. (2018) Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 24 (6), 739–748. [DOI] [PubMed] [Google Scholar]
- 131.Pyo KH. et al. (2019) Promising preclinical platform for evaluation of immuno-oncology drugs using Hu-PBL-NSG lung cancer models. Lung Cancer 127, 112–121. [DOI] [PubMed] [Google Scholar]
- 132.Tanaskovic O. et al. (2019) Human cord blood (hCB)-CD34+ humanized mice fail to reject human acute myeloid leukemia cells. PLoS One 14 (9), e0217345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu HW. et al. (2019) Anti-CD105 Antibody Eliminates Tumor Microenvironment Cells and Enhances Anti-GD2 Antibody Immunotherapy of Neuroblastoma with Activated Natural Killer Cells. Clin Cancer Res 25 (15), 4761–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu L. et al. (2018) Induction of antitumor cytotoxic lymphocytes using engineered human primary blood dendritic cells. Proc Natl Acad Sci U S A 115 (19), E4453–E4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu W. et al. (2017) MtHsp70-CLIC1-pulsed dendritic cells enhance the immune response against ovarian cancer. Biochem Biophys Res Commun 494 (1–2), 13–19. [DOI] [PubMed] [Google Scholar]
- 136.Zhang F. et al. (2019) Specific Decrease in B-Cell-Derived Extracellular Vesicles Enhances Post-Chemotherapeutic CD8(+) T Cell Responses. Immunity 50 (3), 738–750 e7. [DOI] [PubMed] [Google Scholar]
- 137.Zhao Y. et al. (2018) Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut 67 (10), 1845–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zoine JT. et al. (2019) Ex vivo expanded patient-derived gammadelta T-cell immunotherapy enhances neuroblastoma tumor regression in a murine model. Oncoimmunology 8 (8), 1593804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ruhl J. et al. (2019) Heterologous prime-boost vaccination protects against EBV antigen-expressing lymphomas. J Clin Invest 129 (5), 2071–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wahl A. et al. (2019) Precision mouse models with expanded tropism for human pathogens. Nat Biotechnol 37 (10), 1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ashizawa T. et al. (2017) Antitumor Effect of Programmed Death-1 (PD-1) Blockade in Humanized the NOG-MHC Double Knockout Mouse. Clin Cancer Res 23 (1), 149–158. [DOI] [PubMed] [Google Scholar]
- 142.Brehm MA. et al. (2019) Lack of acute xenogeneic graft-versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB J 33 (3), 3137–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Katano I. et al. (2017) Long-term maintenance of peripheral blood derived human NK cells in a novel human IL-15-transgenic NOG mouse. Sci Rep 7 (1), 17230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Matsuda M. et al. (2019) Human NK cell development in hIL-7 and hIL-15 knockin NOD/SCID/IL2rgKO mice. Life Sci Alliance 2 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hoogstad-van Evert JS. et al. (2017) Umbilical cord blood CD34(+) progenitor-derived NK cells efficiently kill ovarian cancer spheroids and intraperitoneal tumors in NOD/SCID/IL2Rg(null) mice. Oncoimmunology 6 (8), e1320630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lopez-Lastra S. et al. (2017) A functional DC cross talk promotes human ILC homeostasis in humanized mice. Blood Adv 1 (10), 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rongvaux A. et al. (2013) Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol 31, 635–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meier D. et al. (2007) Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 26 (5), 643–54. [DOI] [PubMed] [Google Scholar]
- 149.Onder L. and Ludewig B. (2018) A Fresh View on Lymph Node Organogenesis. Trends Immunol 39 (10), 775–787. [DOI] [PubMed] [Google Scholar]
- 150.Takahashi T. et al. (2017) Enhanced Antibody Responses in a Novel NOG Transgenic Mouse with Restored Lymph Node Organogenesis. Front Immunol 8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Adachi Y. et al. (1998) Effects of administration of monoclonal antibodies (anti-CD4 or anti-CD8) on the development of autoimmune diseases in (NZW x BXSB)F1 mice. Immunobiology 198 (4), 451–64. [DOI] [PubMed] [Google Scholar]
- 152.Chappaz S. and Finke D. (2010) The IL-7 signaling pathway regulates lymph node development independent of peripheral lymphocytes. J Immunol 184 (7), 3562–9. [DOI] [PubMed] [Google Scholar]
- 153.Li Y. et al. (2018) A human immune system mouse model with robust lymph node development. Nat Methods 15 (8), 623–630. [DOI] [PubMed] [Google Scholar]
- 154.Liu D. et al. (2019) Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med 25 (12), 1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li Y. et al. (2019) Humanized Mice Reveal New Insights Into the Thymic Selection of Human Autoreactive CD8(+) T Cells. Front Immunol 10, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Izumchenko E. et al. (2017) Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann Oncol 28 (10), 2595–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Townsend EC. et al. (2016) The Public Repository of Xenografts Enables Discovery and Randomized Phase II-like Trials in Mice. Cancer Cell 29 (4), 574–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ng SW. et al. (2016) A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 540 (7633), 433–437. [DOI] [PubMed] [Google Scholar]
- 159.Akkina R. (2013) Human immune responses and potential for vaccine assessment in humanized mice. Curr Opin Immunol 25 (3), 403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bhatia M. et al. (1998) A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med 4 (9), 1038–45. [DOI] [PubMed] [Google Scholar]
- 161.Dick JE. and Lapidot T. (2005) Biology of normal and acute myeloid leukemia stem cells. Int J Hematol 82 (5), 389–96. [DOI] [PubMed] [Google Scholar]


