Abstract
Donor-reactive memory T cells generated via heterologous immunity represent a potent barrier to long-term graft survival following transplantation because of their increased precursor frequency, rapid effector function, altered trafficking patterns, and reduced reliance on costimulation signals for activation. Thus, the identification of pathways that control memory T cell survival and secondary recall potential may provide new opportunities for therapeutic intervention. Here, we discovered that donor-specific effector/memory CD8+ T cell populations generated via exposure to acute vs. latent vs. chronic infections contain differential frequencies of CD8+ T cells expressing the inhibitory Fc receptor FcγRIIB. Results indicated that frequencies of FcγRIIB-expressing CD8+ donor-reactive memory T cells inversely correlated with allograft rejection. Further, adoptive T cell transfer of Fcgr2b−/− CD8+ T cells resulted in an accumulation of donor-specific CD8+ memory T cells and enhanced recall responses, indicating that FcγRIIB functions intrinsically to limit T cell CD8+ survival in vivo. Lastly, we show that deletion of FcγRIIB on donor-specific CD8+ memory T cells precipitated costimulation blockade-resistant rejection. These data therefore identify a novel cell-intrinsic inhibitory pathway that functions to limit the risk of memory T cell-mediated rejection following transplantation and suggest that therapeutic manipulation of this pathway could improve outcomes in sensitized patients.
1. Introduction
T cell-mediated rejection is a major risk that limits long-term allograft survival and can necessitate re-transplanation. Due to their rapid ability to exhibit effector function after reencountering antigen, graft-specific memory T cells in particular can pose a dangerous threat to transplanted organs. Seminal studies have shown that the quantity of donor-reactive alloreactive memory T cells present prior to transplantation are associated with risk of T cell-mediated rejection and are a barrier to tolerance induction [1,2]. In addition, we have previously shown that the quality of memory T cells, including cell-surface phenotype and differentiation status, is a determinant of the relative risk posed by memory T cell populations [3,4]. Donor-specific memory T cells can be elicited via past transfusion, transplant, or pregnancy, and via the process of heterologous immunity, in which pathogen-elicited memory T cells cross-react with alloantigens and cause damage to the graft [5–7]. Novel pathways that modulate memory T cell survival or functionality may represent useful potential therapeutic targets to mitigate memory T cell-mediated acute rejection episodes.
Belatacept, a recently FDA-approved novel biologic that targets the CD28 costimulatory pathway, confers a reduced risk of certain comorbidities, such as diabetes and cardiovascular events, relative to CNI-based immunosuppression regimens [8,9]. However, belatacept is associated with increased incidence and severity of acute rejection episodes [10,11]. It has been proposed that the early and severe acute rejection episodes observed in belatacept-treated individuals may be mediated by effector and memory CD8+ T cells that pose a barrier to costimulation blockade therapy due to their reduced requirements for costimulatory signals [1,2,12]. Published reports demonstrate that, owing to the reliance of memory T cells on integrins for trafficking into sites of inflammation, antagonism of either the LFA-1 or VLA-4 integrins can overcome this costimulation blockade-resistant rejection and even result in long-term graft survival in recipients containing donor-reactive memory T cells [13–16]. However, the impact of memory T cell quality on the efficacy of combined costimulation/ integrin antagonism remains to be fully elucidated.
FcγRIIB is a conserved inhibitory Fc receptor in mice and humans that is best known for its inhibitory function on B cells, macrophage and dendritic cell (DC) lineages, and granulocytes, thereby regulating aspects of humoral immunity, innate immunity, and antigen presentation [17]. In these cell types, intracellular tyrosine-based inhibitory motifs (ITIM) within the cytoplasmic domain allow ligation of FcγRIIB to disrupt internal signaling processes through the recruitment of inhibitory phosphatases [18–20]. The consensus in the field for the past few decades has been that T cells do not express FcγRIIB [17]. However, emerging evidence suggests that effector CD8+ T cells can express FcγRIIB [21]. Work from our group has further shown that not only can effector CD8+ T cells express FcγRIIB, but that this receptor modulates T cell survival in a cell intrinsic manner during primary immune responses in models of transplantation and melanoma [22].
Here we aimed to determine what role, if any, FcγRIIB plays on microbially-elicited memory T cells in mediating costimulation/integrin blockade-resistant allograft rejection. To do this, we utilized a model in which mice are infected with pathogens expressing the OVA protein and subsequently rechallenged with an OVA-expressing skin graft, modeling an extreme version of heterologous immunity in which memory T cells are not merely cross-reactive to donor antigen but indeed recognize their cognate antigen on donor tissues. We have previously shown that rejection in this model is mediated by memory T cells, in that adoptive transfer of sorted memory T cells conferred costimulation blockade-resistant rejection [23], and depletion of graft-specific memory T cells eliminated costimulation blockade-resistant rejection [13]. Here, we employed this model of memory T cell-mediated rejection to interrogate the role of FcγRIIB on memory CD8+ T cells. Results show that type of infection (acute bacterial vs. latent viral vs. chronic viral) induced differential frequencies of FcγRIIB+ memory CD8+ T cells, and that the loss of FcγRIIB specifically on graft-specific memory CD8+ T cells results in costimulation/integrin blockade-resistant rejection.
2. Materials and Methods
2.1. Mice
Male C57BL/6 (H-2b) and B6/Ly5.2 mice aged 6–8 weeks were obtained from the National Cancer Institute (Frederick, MD). OT-I [24] transgenic mice were purchased from Taconic Farms (Germantown, NY) and bred to Thy1.1+ background at Emory University. OVA-expressing mice (C57BL/6 background, H-2b) [25] were a generous gift from Dr. Marc Jenkins (University of Minnesota, Minneapolis, MN). EM:06078 Fcgr2b Fcgr2bB6null B6(Cg)-Fcgr2btm12Sjv/Cnbc (Fcgr2b−/−) mice were made by Dr. J.S. Verbeek [26] (Academisch Siekenhuis Leiden/Leiden University Medical Center) and obtained via the European Mutant Mouse Archive. Fcgr2b−/− mice were bred to OT-I transgenic mice at Emory University (Fcgr2b−/− OT-I). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. The protocol (PROTO201700558) was approved by the Institutional Animal Care and Use Committee of Emory University. All surgery was performed under general anesthesia with maximum efforts made to minimize suffering. All animals were housed in specific pathogen-free animal facilities at Emory University.
2.2. Adoptive cell transfers
To monitor antigen-specific donor-reactive CD8+ T cell responses, we used our previously described system in which OVA-specific transgenic T cells are adoptively transferred prior to skin transplantation with OVA-expressing skin or infection with OVA-expressing pathogens [13,25]. For the adoptive transfer, WT OT-I (Thy1.1+, CD45.2+) or Fcgr2b−/− OT-I (CD45.2+) transgenic cells were harvested from the spleen and processed into a single cell suspension. Cells were counted using a Nexcelom Cellometer Auto T4 (Nexecelom Bioscience, Lawrence, MA) and stained with CD8- BV785, CD4- PacBlue, CD45.2-PE-Dazzle, Thy1.1- PerCP, Vα2- FITC, and Vβ5- PE (Biolegend). Frequency of OT-I T cells was determined via Vα2 and Vβ5 TCR co-expression. Cells were resuspended in 1X PBS, and 104 CD45.2+ OT-I T cells adoptively transferred into naïve CD45.1 hosts 24 hours prior to infection.
2.3. Infections and transplantation
Animals were infected with Listeria monocytogenes (Listera), murine γ-herpesvirus-68 (Herpes), or mouse polyomavirus (Polyoma) engineered to express the OVA epitope [27–29]. For Listeria infection, 1×104 cfu were injected IP; for Herpes, 2×103 pfu were injected IP, and for Polyoma, 1×105 pfu were injected IP. Where indicated, mice were transplanted with full thickness tail, ear, or trunk skins on the dorsal thorax and wrapped with adhesive bandages as previously described [12]. Where indicated, mice were treated with 250ug CTLA-4Ig (abatacept, Bristol Myers-Squibb), 250ug anti-CD154 (MR-1, BioXCell, West Lebanon, NJ), and 250ug VLA-4 or LFA-1 (BioXCell, West Lebanon, NJ) on days 0, 2, 4, and 6. Grafts were considered rejected when less than 10% of viable graft remained.
2.4. Quantitative cultures of spleen
For quantitative cultures of spleen, weighed tissue specimens were ground in 200μl of sterile 0.85% saline using 15 mL sterile disposable tissue grinders (Covidien, Mansfield, MA). Serial 10-fold dilutions of the ground specimen from 10−1 to 10−3 were prepared and 100 μl aliquots were plated on blood agar plates (Remel, Lenexa, KS) and incubated at 35°C in a 5% CO2 atmosphere for 24 hours. Colony counts were obtained from plates containing fewer than 300 colonies. The number of colony forming units per gram of original sample was determined by multiplying the number of colonies by the reciprocal of the dilution counted and adjusted for the volume of sample plated. The organisms recovered were identified as Listeria using MALDI-TOF mass spectrometry (bioMerieux, Durham, NC).
2.5. Lymphocyte isolation from liver and skin grafts
Prior to organ harvesting, the portal vein was perfused with 10 mL cold PBS. Livers were homogenized manually and filtered, and then spun lightly at 300rpm to pellet the hepatocytes. The supernatant was resuspended in a 40% percoll solution and overlaid on 60% percoll, and then spun at 2000rpm for 20 minutes. The buffy coat was isolated, washed, and stained with antibodies for flow cytometry. Skin grafts were cleaned and defatted, chopped, and digested with 2mg/mL Collagenase P (Sigma-Aldrich) in HBSS (with calcium and magnesium) for 30 minutes at 37⁰C. Digested grafts were then filtered, washed, and stained with antibodies for flow cytometry.
2.6. Flow cytometry and intracellular cytokine staining
Peripheral blood was processed using High Yield Lysis buffer (Thermofisher) and stained with biotinylated CD16/CD32 (2.4G2, BD Biosciences), biotinylated isotype control (IgG2b κ isotype, BD Biosciences), and CD4-PacBlue, CD8- BV785, CD19-BV510, CD44- APC-Cy7, CD62L- PE-Cy7,Thy1.1- PerCP, CD45.1- BV605, CD45.2- PE-Dazzle, and streptavidin-APC (all from Biolegend). For intracellular cytokine staining, splenocytes were ex vivo stimulated at 37⁰C with 30nm OVA257–264 (SIINFEKL) peptide and 10ug/mL GolgiPlug (BD Biosciences). After 4 hours, cells were processed and stained using an intracellular cytokine staining kit (BD Biosciences) with TNF- PE-Cy7 and IFNγ- Alexafluor700 (all from Biolegend). Flow cytometry samples were acquired on an LSR II flow cytometer (BD Biosciences) and data were analyzed using FlowJo (Tree Star, San Carlos, CA) and Prism (GraphPad Software). Absolute cell numbers were calculated using CountBright Beads (Life Technologies) according to manufacturer’s instructions.
2.7. Statistics
Mann-Whitney U tests were performed to compare two groups. One-way ANOVA was performed when comparing one variable over multiple groups, two-way ANOVA was performed when comparing two variables over multiple groups. Survival data were plotted on Kaplan-Meier curves, and a log-rank (Mantel-Cox) test was performed. All analyses were done using Prism (GraphPad Software). In all legends and figures, mean + SEM is shown, and *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
3. Results
3.1. Pathogen stimulation history modulates frequency of FcγRIIB-expressing CD8+ effector and memory T cells
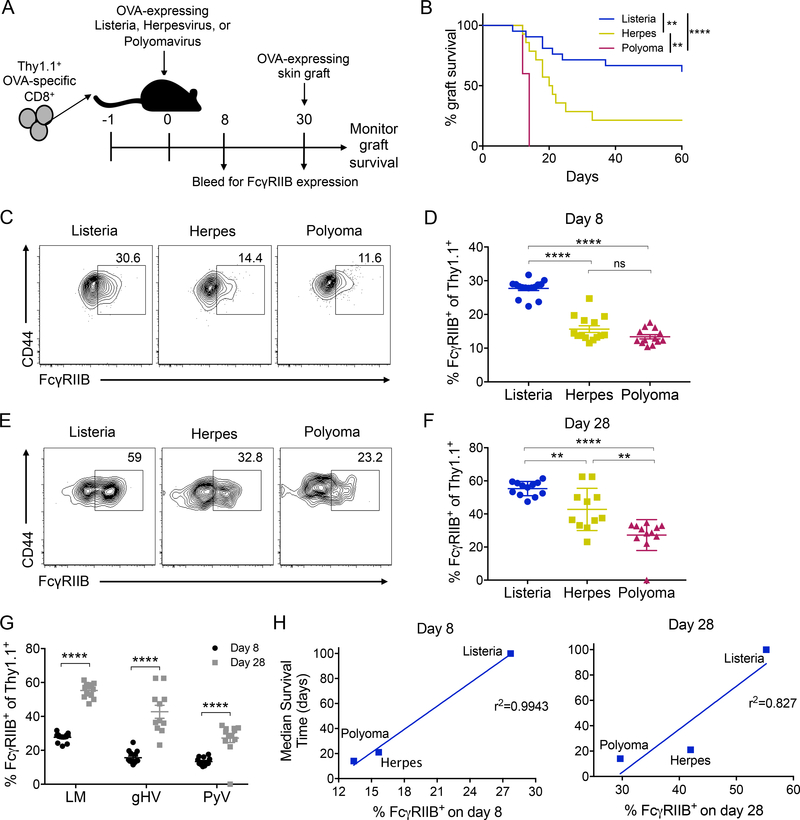
Because heterologous immunity can pose a potent barrier to transplantation, we developed a model to test how the quality of memory T cells elicited by three distinct pathogens correlates with risk of rejection [13]. In this model, we use a transgenic system in which we adoptively transferred OVA-specific TCR transgenic CD8+ OT-I T cells into naïve recipients that were then infected with OVA-expressing pathogens: Listeria monocytogenes (Listeria) [27], an acutely cleared bacterial infection; murine γ-herpesvirus-68 (Herpes) [28], a murine EBV homolog and latent viral infection; and mouse polyomavirus (polyoma) [29], a murine BK homolog and persistent infection. To determine the memory T cell barrier elicited by each infection, we infected mice with either Listeria, Herpes, or Polyoma. At day 30 post-infection, following the generation of OVA-specific CD8+ memory T cells, mice were grafted with OVA-expressing skin grafts and treated with CTLA-4Ig, anti-CD154, and anti-VLA-4 (Figure 1A). Multiple groups have shown that integrin antagonism is a strategy to control costimulation blockade-resistant memory T cells [15,23]. We previously assessed the efficacy of the combined costimulation and integrin blockade against memory T cells of differential quality as elicited by acute, latent, and chronic infections [13]. Results indicated that memory T cells pose a differential barrier to graft survival in that mice with donor-reactive memory T cells elicited from an acute infection were most susceptible to immunosuppression and had a low risk of allograft rejection (MST undefined), whereas donor-reactive memory T cells elicited from a latent infection had a moderately increased risk for allograft rejection (MST 20.5 days), and donor-reactive memory T cells elicited from a chronic infection were least susceptible to immunosuppression and had a severe increased risk for allograft rejection (MST 14 days) [13] (Figure 1B). However, the mechanisms underlying these differential barriers are not known.
Figure 1: Pathogen stimulation history modulates frequency of FcγRIIB-expressing cells and is correlated with prolonged graft survival.
OVA-specific Thy1.1+ CD8+ T cells (OT-I) were harvested from the spleen and adoptively transferred into WT B6 hosts that were then either infected with Listeria, Herpes, or Polyoma engineered to express OVA. Mice were bled on days 8 and 28. On day 30, mice were grafted with an OVA-expressing skin graft and treated with 250ug CTLA-4Ig, anti-CD154 (MR-1), and anti-VLA-4 on days 0, 2, 4 and 6 and graft survival was monitored. A) Schematic of experimental design. B) Graft survival, data from ref 13 Figure 3B–D, animals treated with CoB + anti- VLA4. Pooled data from two independent experiments, n=7 per group. A log-rank (Mantel-Cox) test was performed to compare groups **p<0.01, ****p<0.0001. C-D) Representative flow plots and summary data of the frequency of FcγRIIB-expressing cells on day 8 post infection in the peripheral blood. Pooled data from 2 independent experiments, n=7–8 mice per group. One-way ANOVA was performed with multiple comparisons, ****p<0.0001. E-F) Representative flow plots and summary data of the expression of FcγRIIB on day 28 post infection in the peripheral blood. Pooled data from 2 independent experiments, n=7–8 mice per group. One-way ANOVA was performed with multiple comparisons ****p<0.0001. G) Summary data of D and F comparing day 8 and 28 frequencies of FcγRIIB. Two-way ANOVA was performed with multiple comparisons, ****p<0.0001. H) Linear regression of the frequency of FcγRIIB-expressing OT-I T cells on day 8 and 28 post infection and the median survival time.
Emerging evidence has demonstrated that FcγRIIB is expressed on a subset of polyfunctional CD44hi CD8+ T cells [21,22]. To understand whether pathogen stimulation history affects the expression of the inhibitory Fcγ receptor FcγRIIB on memory CD8+ T cells, we probed CD44hi Thy1.1+ donor-specific CD8+ T cells elicited by the different infections for expression of FcγRIIB on days 8 and 28. Interestingly, we found that the donor-specific CD8+ CD44hi effector T cell population elicited via an acute infection contained significantly higher frequencies of FcγRIIB+ cells compared to CD8+ CD44hi populations elicited via either the latent or chronic viral infection (Figure 1C–D) on day 8 post-infection. A similar result was observed at day 28 post-infection (Figure 1E–G). Further, donor-specific CD8+ CD44hi effector T cells elicited via a chronic infection exhibited lower frequencies of FcγRIIB+ cells as compared to the CD8+ CD44hi effector T cells elicited via a latent viral infection. Importantly, we found that the frequency of FcγRIIB+ expressing cells among the CD8+ CD44hi population present at both day 8 (r2= 0.9943) and day 28 (r2= 0.827) directly correlated with the median graft survival time (shown in Figure 1B) for each infection (Figure 1H). These results indicated that mice containing memory T cells with high frequencies of the FcγRIIB coinhibitory molecule were more susceptible to combined costimulation/integrin blockade.
3.2. FcγRIIB functions intrinsically on CD8+ T cells to alter the T cell response
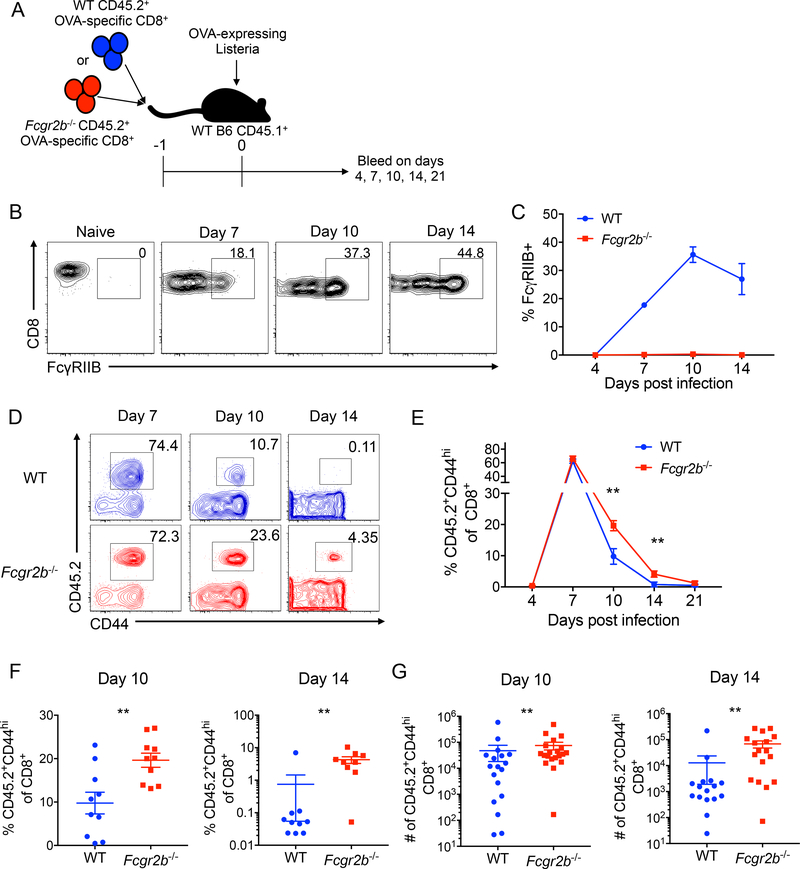
The above results demonstrated an association between reduced FcγRIIB expression and memory T cell resistance to costimulation/integrin blockade. Next, we sought to determine the causal relationship between these findings. We utilized a system in which we adoptively transfer Fcgr2b−/− donor-reactive TCR transgenic OT-I CD8+ T cells. In this model, WT or Fcgr2b−/− donor-reactive CD8+ T cells were adoptively transferred into animals, which were subsequently infected with Listeria (Figure 2A). WT donor-reactive CD8+ T cells expressed little FcγRIIB early after infection, but by day 10, ~30% of WT donor-reactive CD8+ T cells expressed FcγRIIB and this frequency remained elevated on day 14 (Figure 2B–C). Compared to WT donor-reactive CD8+ T cells, the frequency and numbers of Fcgr2b−/− donor-reactive CD45.2+ CD8+ T cells were significantly increased on days 10 and 14 relative to WT donor-reactive CD45.2+ CD8+ T cells (Figure 2D–G), indicating that FcγRIIB functions intrinsically on CD8+ T cells to inhibit their survival and/or accumulation. Of note, recipients of Fcgr2b−/− OT-I T cells possessed significantly fewer CD44hi CD45.1+ endogenous non-OT-I effectors relative to recipients of WT OT-I on day 10, and exhibited a trend toward fewer on day 14 (Supplemental Figure 1). These data suggest that the absence of FcγRIIB on the transferred graft-specific cells confers a competitive advantage relative to endogenous CD8+ T cells responding to the allograft. Splenic bacterial load on d5 post-infection was not different between recipients of WT or Fcgr2b−/− OT-I T cells, revealing that FcγRIIB on CD8+ T cells does not impact the clearance of Listeria (Supplemental Figure 2).
Figure 2: FcγRIIB functions intrinsically to inhibit CD8+ T cell responses to Listeria.
WT OVA-specific CD45.2+ CD8+ T cells (WT OT-I) or Fcgr2b−/− OVA-specific CD45.2+ CD8+ T cells (Fcgr2b−/− OT-I) were harvested from the spleen and adoptively transferred into WT CD45.1+ C57BL/6 hosts that were then infected with Listeria and bled on days 4, 7, 10, 14, and 21. A) Schematic of experimental design. B-C) Representative flow plots and summary data of the frequency of FcγRIIB-expressing naïve and WT OT-I T cells on days 7, 10, and 14. Due to low cell number, FcγRIIB frequency could not be assessed on day 21. Representative data from two independent experiments, n=5 mice per group. D) Representative flow plots of the frequency of WT and Fcgr2b−/− OT-I T cells on days 7, 10, and 14. E) Summary data of the frequency of WT and FcγRIIB−/− OT-I T cells in the blood on days 4, 7, 10, 14 and 21. Representative data from two independent experiments, n=5 mice per group. Two-way ANOVA was performed, **p<0.01. F-G) Summary data of the frequency and absolute numbers of WT and Fcgr2b−/− OT-I T cells in the blood on days 10 and 14. Pooled data from 2–3 independent experiments, n=5 mice per group. Mann-Whitney t test was performed, **p<0.01.
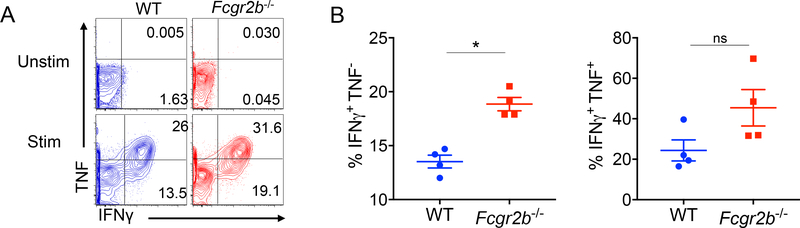
We next assessed the cytokine effector function of CD8+ T cells genetically deficient in FcγRIIB. Splenocytes were harvested from mice containing either WT or Fcgr2b−/− donor-reactive CD8+ T cell populations on day 10 post-infection. Results indicated that WT and Fcgr2b−/− donor-reactive CD8+ T cell populations contained similar frequencies of IFNγ+TNF+ cytokine-producing cells. However, Fcgr2b−/− donor-reactive CD8+ T cells exhibited an increased frequency of IFNγ+ single producers (Figure 3A–B).
Figure 3: Donor-specific T cells deficient of FcγRIIB maintain ability to produce effector cytokines.
WT OVA-specific CD45.2+ CD8+ T cells (WT OT-I) or Fcgr2b−/− OVA-specific CD45.2+ CD8+ T cells (Fcgr2b−/− OT-I) were harvested from the spleen and adoptively transferred into WT CD45.1+ C57BL/6 hosts that were then infected with Listeria. Animals were sacrificed and spleens were harvested on day 10 to assess cytokine response of the OT-I T cells. A) Representative flow plots of IFNγ- and TNF-producing OT-I T cells (splenocytes) on day 10 post adoptive transfer of either WT or Fcgr2b−/− OT-I T cells and subsequent infection with Listeria. B) Summary data of the frequency of IFNγ+TNF+ double-producing OT-I T cells and the frequency of single-producing IFNγ+TNF− OT-I T cells. Representative data from two independent experiments, n=4–5 mice per group. Mann-Whitney t test was performed, *p<0.05, ns=nonsignficant.
3.3. FcγRIIB expression on memory CD8+ T cells modulates costimulation and integrin blockade resistance
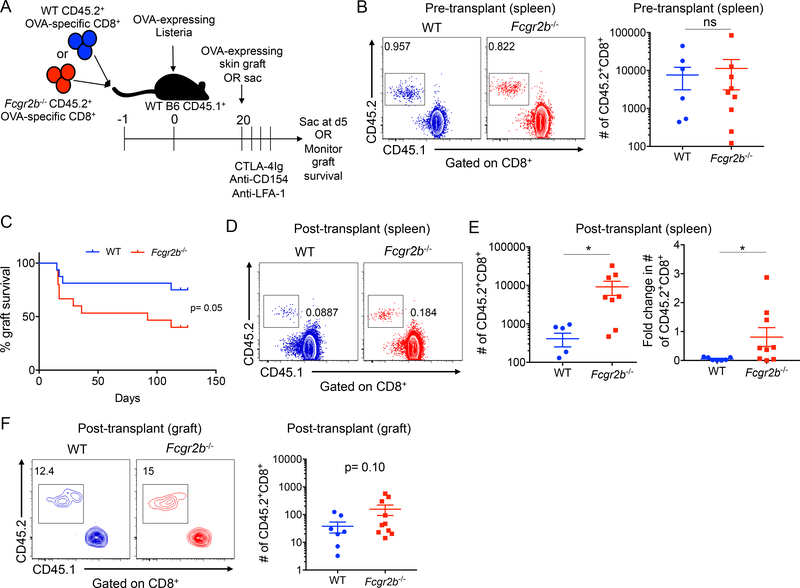
Because these data indicated that FcγRIIB is induced on T cells following infection and functions to inhibit donor-reactive CD8+ T cells, we asked whether expression of FcγRIIB on memory donor-reactive CD8+ T cells modulates graft survival following treatment with the costimulation and integrin blockade regimen. To first assess whether FcγRIIB alters the number of memory CD8+ T cells at steady state after the resolution of infection, WT or Fcgr2b−/− donor-reactive CD8+ were transferred into naïve hosts, which were subsequently infected with Listeria and sacrificed on day 22 (Figure 4A). Analysis of the spleen indicated no significant differences in the number of WT vs. Fcgr2b−/− CD8+ T cells at this timepoint (Figure 4B). We further assessed frequencies of memory CD8+ T cells in the blood, bone marrow, liver, and mesenteric lymph nodes and also found no significant differences in number of WT vs. Fcgr2b−/− CD8+ T cells in these compartments, indicating that FcγRIIB does not function to modulate the number of memory T cells at steady state (Supplemental Figure 3). To then determine if the quality or magnitude of the recall response of memory WT vs. Fcgr2b−/− CD8+ T cells altered graft survival, Listeria-infected mice possessing WT vs. Fcgr2b−/− CD8+ T cells were grafted with OVA-expressing skin approximately 20 days following infection and treated with CTLA-4Ig, anti-CD154, and anti-LFA-1 and monitored for graft survival.
Figure 4: FcγRIIB expression on memory CD8+ T cells modulates costimulation and integrin blockade resistance.
WT OVA-specific CD45.2+ CD8+ T cells (WT OT-I) or Fcgr2b−/− OVA-specific CD45.2+ CD8+ T cells (Fcgr2b−/− OT-I) were harvested from the spleen and adoptively transferred into WT CD45.1+ C57BL/6 hosts that were then infected with Listeria. A) Schematic of experimental design. B) Representative flow plots of WT and Fcgr2b−/− OT-I T cells (gated on CD8+) in the spleen on day 22 post infection. Summary data is also shown (due to logarithmic axis, zero values are not shown: 3 for WT, 1 for Fcgr2b−/−). Pooled data from two independent experiments, n=4–5 mice per group. Mann-Whitney T test was performed, ns=nonsignificant. C) On day 20, mice were grafted with OVA-expressing skin grafts and treated with 250ug CTLA-4Ig, anti-CD154 (MR-1), and anti-LFA-1 on days 0, 2, 4 and 6 and graft survival was monitored. Log-rank (Mantel-Cox) test was performed, p=0.05. D-F: On day 20, mice were grafted with OVA-expressing skin grafts and then sacrificed on day 5 and spleen and skin grafts were harvested. D) Representative flow plots of WT and Fcgr2b−/− CD45.2+ OT-I (gated on CD8+ T cells) from the spleen on day 5 post grafting. E) Summary data of the absolute cell number on day 5 and the fold change from day 22 in (B) to day 5 of WT and Fcgr2b−/− CD45.2+ OT-I from the spleen post grafting. Due to logarithmic axis, zero values are not shown: 2 for WT, 1 for Fcgr2b−/−. Pooled data from two independent experiments, n=4–5 mice per group. Mann-Whitney T test was performed, *p<0.05. F) Representative flow plots of WT and Fcgr2b−/− CD45.2+ OT-I (gated on CD8+ T cells) from the skin graft on day 5 post grafting. Summary data is also shown of the absolute cell number of WT and Fcgr2b−/− CD45.2+ OT-I from the skin graft on day 5 post grafting. Due to logarithmic axis, zero values are not shown: 1 for WT. Pooled data from two independent experiments, n=4–5 mice per group. Mann-Whitney t test was performed, p=0.10.
Importantly, we observed that animals containing Fcgr2b−/− donor-reactive memory CD8+ T cells exhibited accelerated graft survival compared to animals receiving WT donor-reactive memory CD8+ T cells (Figure 4C), suggesting that FcγRIIB expression on donor-reactive memory CD8+ T cells modulates the susceptibility of secondary recall responses to costimulation and integrin blockade-based immunosuppression. As such, we next directly assessed the secondary recall response in recipients of WT vs. Fcgr2b−/− donor-reactive CD8+ T cells, and found that animals containing Fcgr2b−/− donor-reactive CD8+ T cells possessed significantly increased frequencies of graft-specific CD8+ effector cells in the spleen at day 5 following challenge with a skin graft relative to mice containing WT donor-specific CD8+ T cells (Figure 4D–E). Further, the fold change in cell number from pre-transplant (Figure 4B) to post-transplant day 5 was significantly greater in the Fcgr2b−/− donor-reactive CD8+ T cell population relative to the WT donor-reactive CD8+ T cell population (Figure 4E). Finally, to assess whether FcγRIIB alters migration of CD8+ T cells into the graft, we analyzed the skin grafts on day 5 post transplant for donor-reactive graft-infiltrating CD8+ T cells. Results indicated that recipients of donor-reactive Fcgr2b−/− CD8+ T cells exhibited a trend toward a greater number of graft-infiltrating CD45.2+ donor-reactive CD8+ T cells relative to recipients of WT CD45.2+ donor-reactive CD8+ T cells (p=0.10), suggesting that the increased number of Fcgr2b−/− CD8+ T cells in the spleen may feed an increased number of Fcgr2b−/− CD8+ T cells migrating into the skin graft (Figure 4F).
Discussion
Memory T cells present a defined risk to allograft survival [1,2,7], highlighting the need to identify novel pathways to target memory T cell survival and/or function during transplantation. Here, we show that memory CD8+ T cells elicited via three distinct infections (acute, latent, or chronic) express the inhibitory Fc receptor FcγRIIB, and that the presence of greater frequencies of FcγRIIB-expressing donor-reactive CD8+ memory T cells correlates with longer graft survival. Mechanistically, CD8+ T cell-specific deletion of FcγRIIB results in increased accumulation of donor-reactive CD8+ T cells following allograft rechallenge, indicating that it has a cell-intrinsic role in mitigating donor-reactive CD8+ memory T cell responses. Finally, animals with Fcgr2b−/− donor-specific CD8+ memory T cells exhibit accelerated costimulation/integrin blockade-resistant rejection. The data presented here therefore implicate FcγRIIB as a crucial mitigator of pathogenicity of donor-reactive memory CD8+ T cell responses following transplantation.
First, our data highlight a cell intrinsic role for FcγRIIB in regulating CD8+ T cells during transplantation. FcγRIIB has been previously most well-appreciated for its role on B cells and APCs and is known to dampen alloreactive B cell responses in the setting of transplantation. For example, FcγRIIB was shown to function on APCs was found to be implicated in a rat model of graft tolerance, likely by inhibiting the IgM to IgG class switch [30]. Further, Callaghan et al. found that chronic rejection was accelerated in Fcgr2b−/− animals, and that the overexpression of FcγRIIB on B cells and macrophages significantly prolonged graft survival [31]. Although these studies and others suggested a role for FcγRIIB on B cells, the data described here uncover a novel intrinsic, inhibitory function of FcγRIIB on CD8+ memory T cells and its contribution to transplant outcome in a model of memory T cell mediated graft rejection. What are the mechanisms by which FcγRIIB functions to limit memory CD8+ T cell accumulation? We recently showed in models of primary CD8+ T cell immunity to transplant and tumor antigens that FcγRIIB functions in a cell-intrinsic fashion on CD8+ T cells to increase caspase 3/7-mediated apoptosis[22]. Of note, ligation of FcγRIIB by IgG antibody was not required for FcγRIIB-mediated inhibition of CD8+ T cell responses. Instead, ligation with the suppressive cytokine fibrinogen-like 2 (Fgl2) resulted in FcγRIIB-mediated CD8+ T cell apoptosis. Fgl2 is an anti-inflammatory cytokine secreted by many cell types (including CD8+ T cells themselves) as a counter-regulatory mechanism in response to inflammation[32]. We and others have shown that Fgl2 is increased systemically during allograft rejection[22] and microbial infection [33,34]. Thus, we postulate that the differences observed here in accumulation/ survival of Fcgr2b−/− vs. WT CD8+ T cells during both the priming (Listeria infection) and recall (graft rechallenge) phases of the response are dependent on the presence of Fgl2. The fact that these differences in Fcgr2b−/− vs. WT CD8+ T cell numbers are not observed during times of quiescence (Fig. 4) may be indicative of the absence of Fgl2 during these timepoints.
The data presented here demonstrating that a chronic viral infection induces low frequencies of FcγRIIB+ memory CD8+ T cells are in line with a recently published study from Zehn and colleagues, in which the authors found that Fcgr2b was downregulated in virus-specific CD8+ T cells isolated from chronically infected mice [35]. We further show that this reduced FcγRIIB expression is associated with accelerated graft rejection in the context of costimulation/ integrin blockade-based immunosuppression. These data are consistent with reports that patients with chronic viral infections have worse graft outcomes on immunosuppression [36]. Future studies directed at assessing whether the differential upregulation of FcγRIIB on CD8+ T cells may underlie these observations are warranted.
Finally, we show that animals with memory CD8+ T cells that lack FcγRIIB exhibit increased costimulation/integrin blockade-resistant rejection. We posit that the ability to upregulate FcγRIIB on CD8+ T cells limits accumulation of the alloaggressive CD8+ T cell response and facilitates prolonged graft survival. In line with these findings, we recently showed that FCGRIIB mRNA was upregulated in the PBMC of renal transplant recipients that were stable following weaning from tacrolimus immunosuppression as part of the CTOT-09 clinical trial, as compared to patients that experienced acute rejection following weaning from tacrolimus [22]. Moreover, bioinformatic CellCODE analysis of gene expression signatures revealed that the increased FCGRIIB expression was much more tightly associated with CD8+ T cells than any other immune cell lineage. Together these data argue that CD8+ T cell-expressed FcγRIIB represents an additional physiologic mechanism by which donor-specific T cells are inhibited following transplantation, potentially reducing the memory T cell barrier such that costimulation/integrin blockade is now effective, or that patients can effectively be weaned from immunosuppression. In sum, the identification of the FcγRIIB inhibitory pathway on CD8+ T cells, which can be influenced by infection history, warrants further investigation into the utility of therapeutic manuipulation of this pathway to limit alloimmunity and improve outcomes in transplantation.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Aron Lukacher (Pennsylvania State College of Medicine) for the contribution of polyomavirus-expressing OVA, Dr. Samuel Speck (Emory University) for the contribution of γ-herpesvirus-68-expressing OVA, and Dr. Michael Bevan (University of Washington) for the contribution of Listeria monocytogenes-expressing OVA. The authors would also like to thank Dr. Eileen Burd for quantitative Listeria cultures. The study was funded by NIH awards AI073707 and AI104699 to MLF.
Abbreviations
- DC
dendritic cell
- gHV
γ-herpesvirus-OVA
- ITIM
inositol tyrosine-based inhibitory motif
- LM
Listeria monocytogenes-OVA
- OVA
ovalbumin
- PyV
polyomavirus-OVA
- TCR
T cell receptor
- LFA-1
leukocyte functional antigen-1
- VLA-4
very late antigen-4
Footnotes
Disclosure
The authors of this manuscript have no conflicts to disclose as described by the American Journal of Transplantation.
Data sharing statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M: Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol 1999, 163:2267–2275. [PubMed] [Google Scholar]
- 2.Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, Sachs DH, Allan J, Madsen JC, Kawai T, et al. : Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med 2011, 3:86ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krummey SM, Floyd TL, Liu D, Wagener ME, Song M, Ford ML: Candida-elicited murine Th17 cells express high Ctla-4 compared with Th1 cells and are resistant to costimulation blockade. J Immunol 2014, 192:2495–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortes-Cerisuelo M, Laurie SJ, Mathews DV, Winterberg PD, Larsen CP, Adams AB, Ford ML: Increased Pretransplant Frequency of CD28(+) CD4(+) TEM Predicts Belatacept-Resistant Rejection in Human Renal Transplant Recipients. Am J Transplant 2017, 17:2350–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM: Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J Immunol 2003, 170:4077–4086. [DOI] [PubMed] [Google Scholar]
- 6.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A: T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol 2002, 169:3686–3693. [DOI] [PubMed] [Google Scholar]
- 7.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, et al. : Heterologous immunity provides a potent barrier to transplantation tolerance. Journal of Clinical Investigation 2003, 111:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, Anderson D, Cowan S, Price K, Naemura J, et al. : Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant 2005, 5:443–453. [DOI] [PubMed] [Google Scholar]
- 9.Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, Rial Mdel C, Florman S, Block A, Di Russo G, et al. : A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 2010, 10:547–557. [DOI] [PubMed] [Google Scholar]
- 10.Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, Lang P, Urrea EM, Massari P, Mondragon-Ramirez G, et al. : Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant 2012, 12:210–217. [DOI] [PubMed] [Google Scholar]
- 11.Wen X, Casey MJ, Santos AH, Hartzema A, Womer KL: Comparison of Utilization and Clinical Outcomes for Belatacept- and Tacrolimus-Based Immunosuppression in Renal Transplant Recipients. Am J Transplant 2016, 16:3202–3211. [DOI] [PubMed] [Google Scholar]
- 12.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, et al. : Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest 1999, 104:1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badell IR, Kitchens WH, Wagener ME, Lukacher AE, Larsen CP, Ford ML: Pathogen Stimulation History Impacts Donor-Specific CD8(+) T Cell Susceptibility to Costimulation/Integrin Blockade-Based Therapy. Am J Transplant 2015, 15:3081–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, Avila JG, Cano JA, Johnson BE, Song M, et al. : LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest 2010, 120:4520–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setoguchi K, Schenk AD, Ishii D, Hattori Y, Baldwin WM, 3rd, Tanabe K, Fairchild RL: LFA-1 Antagonism Inhibits Early Infiltration of Endogenous Memory CD8 T Cells into Cardiac Allografts and Donor-Reactive T Cell Priming. Am J Transplant 2011, 11:923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reisman NM, Floyd TL, Wagener ME, Kirk AD, Larsen CP, Ford ML: LFA-1 blockade induces effector and regulatory T-cell enrichment in lymph nodes and synergizes with CTLA-4Ig to inhibit effector function. Blood 2011, 118:5851–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimmerjahn F, Ravetch JV: Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008, 8:34–47. [DOI] [PubMed] [Google Scholar]
- 18.Smith KG, Clatworthy MR: FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol 2010, 10:328–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearse RN, Kawabe T, Bolland S, Guinamard R, Kurosaki T, Ravetch JV: SHIP recruitment attenuates Fc gamma RIIB-induced B cell apoptosis. Immunity 1999, 10:753–760. [DOI] [PubMed] [Google Scholar]
- 20.Ono M, Bolland S, Tempst P, Ravetch JV: Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature 1996, 383:263–266. [DOI] [PubMed] [Google Scholar]
- 21.Starbeck-Miller GR, Badovinac VP, Barber DL, Harty JT: Cutting edge: Expression of FcgammaRIIB tempers memory CD8 T cell function in vivo. J Immunol 2014, 192:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris AB, Farley CR, Pinelli DF, Adams LE, Cragg MS, Boss JM, Scharer CD, Fribourg M, Cravedi P, Heeger PS, et al. : Signaling through the Inhibitory Fc Receptor FcgammaRIIB Induces CD8(+) T Cell Apoptosis to Limit T Cell Immunity. Immunity 2020, 52:136–150 e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitchens WH, Haridas D, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML: Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am J Transplant 2012, 12:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR: T cell receptor antagonist peptides induce positive selection. Cell 1994, 76:17–27. [DOI] [PubMed] [Google Scholar]
- 25.Ehst BD, Ingulli E, Jenkins MK: Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant 2003, 3:1355–1362. [DOI] [PubMed] [Google Scholar]
- 26.Boross P, Arandhara VL, Martin-Ramirez J, Santiago-Raber ML, Carlucci F, Flierman R, van der Kaa J, Breukel C, Claassens JW, Camps M, et al. : The inhibiting Fc receptor for IgG, FcgammaRIIB, is a modifier of autoimmune susceptibility. J Immunol 2011, 187:1304–1313. [DOI] [PubMed] [Google Scholar]
- 27.Dudani R, Chapdelaine Y, Faassen Hv H, Smith DK, Shen H, Krishnan L, Sad S: Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol 2002, 168:5737–5745. [DOI] [PubMed] [Google Scholar]
- 28.Braaten DC, Sparks-Thissen RL, Kreher S, Speck SH, Virgin HWt: An optimized CD8+ T-cell response controls productive and latent gammaherpesvirus infection. J Virol 2005, 79:2573–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews NP, Pack CD, Lukacher AE: Generation of antiviral major histocompatibility complex class I-restricted T cells in the absence of CD8 coreceptors. J Virol 2008, 82:4697–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Texier L, Thebault P, Lavault A, Usal C, Merieau E, Quillard T, Charreau B, Soulillou JP, Cuturi MC, Brouard S, et al. : Long-term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant 2011, 11:429–438. [DOI] [PubMed] [Google Scholar]
- 31.Callaghan CJ, Win TS, Motallebzadeh R, Conlon TM, Chhabra M, Harper I, Sivaganesh S, Bolton EM, Bradley JA, Brownlie RJ, et al. : Regulation of allograft survival by inhibitory FcgammaRIIb signaling. J Immunol 2012, 189:5694–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu J, Yan J, Rao G, Latha K, Overwijk WW, Heimberger AB, Li S: The Duality of Fgl2 - Secreted Immune Checkpoint Regulator Versus Membrane-Associated Procoagulant: Therapeutic Potential and Implications. Int Rev Immunol 2016, 35:325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foerster K, Helmy A, Zhu Y, Khattar R, Adeyi OA, Wong KM, Shalev I, Clark DA, Wong PY, Heathcote EJ, et al. : The novel immunoregulatory molecule FGL2: a potential biomarker for severity of chronic hepatitis C virus infection. J Hepatol 2010, 53:608–615. [DOI] [PubMed] [Google Scholar]
- 34.Khattar R, Luft O, Yavorska N, Shalev I, Phillips MJ, Adeyi O, Gao D, Bartczak A, Urbanellis P, Shyu W, et al. : Targeted deletion of FGL2 leads to increased early viral replication and enhanced adaptive immunity in a murine model of acute viral hepatitis caused by LCMV WE. PLoS One 2013, 8:e72309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y, et al. : TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 2019, 571:265–269. [DOI] [PubMed] [Google Scholar]
- 36.Egli A, Binggeli S, Bodaghi S, Dumoulin A, Funk GA, Khanna N, Leuenberger D, Gosert R, Hirsch HH: Cytomegalovirus and polyomavirus BK posttransplant. Nephrol Dial Transplant 2007, 22 Suppl 8:viii72–viii82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.