Abstract
Hydroponic experiments were conducted to investigate the effects of different concentrations of sodium selenate (Na2SeO4) and sodium selenite (Na2SeO3) on durum wheat seed germination and seedling growth under salt stress. The treatments used were 0 and 50 mM NaCl solutions, each supplemented with Na2SeO4 or Na2SeO3 at 0, 0.1, 1, 2, 4, 8, or 10 μM. Salt alone significantly inhibited seed germination and reduced seedling growth. Addition of low concentrations (0.1–4 μM) of Na2SeO4 or Na2SeO3 mitigated the adverse effects of salt stress on seed germination, biomass accumulation, and other physiological attributes. Among them, 1 μM Na2SeO4 was most effective at restoring seed germination rate, germination energy, and germination index, significantly increasing these parameters by about 12.35, 24.17, and 11.42%, respectively, compared to salt-stress conditions. Adding low concentrations of Na2SeO4 or Na2SeO3 to the salt solution also had positive effects on chlorophyll fluorescence indices, decreased the concentrations of free proline and malondialdehyde, as well as electrolyte leakage, and increased catalase, superoxide dismutase, and peroxidase activities in roots and shoots. However, high concentrations (8–10 μM) of Na2SeO4 or Na2SeO3 disrupted seed germination and seedling growth, with damage caused by Na2SeO3 being more severe than that by Na2SeO4. It is thus clear that exogenous selenium can improve the adaptability of processing wheat to salt stress and maintain higher photosynthetic rate by decreasing the accumulation of reactive oxygen species and alleviating the degree of membrane lipid peroxidation. Na2SeO4 was more effective than Na2SeO3 at all given concentrations.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02358-3) contains supplementary material, which is available to authorized users.
Keywords: Durum wheat, Sodium selenate, Sodium selenite, Seed germination, Seedling growth, Salt stress
Introduction
Salinity is one of the most harmful abiotic stresses limiting crop growth and productivity. High salinity can cause water scarcity and ion disequilibrium that reduces plant growth by disrupting physiological processes. Symptoms of salt stress are expressed as both stomatal and non-stomatal limitations (Sarabi et al. 2019). Under stomatal limitation, the plant reduces the stomatal aperture to prevent injury, leading to a decrease in CO2 availability and net photosynthesis (Pilon et al. 2018). Most of the reduction in photon flux energy used for photochemistry can be explained as an increase in non-photochemical dissipation of excitation energy, which seems to be associated with the photosystem II (PSII) complex (Cengiz et al. 2019; José et al. 2015). The chlorophyll fluorescence yield of PSII, including potential efficiency of PSII (Fv/Fo) and minimal fluorescence yield (Fo), can indicate stress to photosynthesis and damage to the photosynthetic apparatus (Wu et al. 2019). Non-stomatal limitations may affect plant photosynthetic efficiency, as has been reported for mulberry (Huihui et al. 2020) and potato (Li et al. 2017). Photochemical activity is impaired in plants exposed to environmental stresses, leading to a relatively higher intercellular CO2 concentration with lower stomatal conductance (Miner et al. 2017). Salt stress can also cause the inhibition of ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO) activity, the degradation of pigment–protein complexes and the destruction of fine chloroplast structure (Ahanger et al. 2020). The chloroplast, the main site for photosynthesis, is the most sensitive organelle to the environment (Wang et al. 2015). It is also the major source of reactive oxygen species (ROS) production. Metabolic imbalances caused by salt stress may also lead to secondary stresses, such as oxidative stress triggered by the accumulation of ROS. ROS can cause serious damage to the membranes and other important macro-molecules, such as lipids, nucleic acids, and photosynthetic pigments. Plants respond to salt stress via an efficient antioxidant defense system network that maintains ROS balance and protects against peroxidative reactions. Studies have shown that superoxide dismutase (SOD) is an integral part of this antioxidant defense system network, converting the harmful superoxide radical (O2−) to hydrogen peroxide (H2O2), which is then converted by catalase (CAT) and/or peroxidase (POD) to water (Harpreet et al. 2018; Kong et al. 2005). Another mechanism by which plants can alleviate some negative effects of salt stress is through nutrient enrichment, toward osmotic adjustment. However, most of the aforementioned studies focused on macronutrients, such as phosphorus (Liu et al. 2017), potassium (Wu et al. 2018), and calcium (Feng et al. 2018).
Selenium (Se), a rare and dispersed element in the earth’s crust, is recognized as an essential micronutrient for humans and other animals, as well as some species of microorganisms (Handa et al. 2018b; Yan et al. 2018). Se has two predominant inorganic forms in the soil, selenate (SeO42−), and selenite (SeO32−), which show completely different adsorption properties: SeO32− adsorption increases with decreasing pH and increasing concentration and is greater than SeO42− adsorption (Balistrieri and Chao 1990). Although Se (SeO42− and SeO32−) has not been identified as an essential element for higher plants, both chemical species can be absorbed by the plants, thereby entering the food chain. The absorption potential for Se in plants is highly correlated with differences in genetic traits that affect Se uptake and migration in plant materials (Meetu and Shikha 2016). SeO42− competes with sulfate for absorption by plants, involved in sulfur assimilation, and is then transported to the plants through the sulfur transport pathway in chloroplasts. Numerous studies have shown that Se is able to promote plants’ growth by improving their antioxidant capacity, and their tolerance to the detrimental effects of diverse environmental stressors (Ahmad et al. 2016; D’Amato et al. 2018; Handa et al. 2018a; Hawrylak-Nowak et al. 2018; Jawad Hassan et al. 2020). POD and CAT activity in leaves has been found to be the most sensitive indicator for plants exposed to SeO42− and SeO32− application, respectively (Yu and Gu 2007). However, the specific physiological and molecular mechanisms that underlie the positive effects of Se in response to environmental stresses need to be further clarified.
Wheat (Triticum spp.), one of the oldest cereals, is the most important crop in the world. With approximately 620 million tons produced annually worldwide, wheat provides about 20% of the calories consumed by humans (Zvi et al. 2009). Wild emmer wheat is the tetraploid (2n = 4x = 28; genome BBAA) progenitor of both domesticated tetraploid durum wheat [T. turgidum ssp. durum (Desf.) MacKey] and hexaploid (2 = 6x = 42; BBAADD) bread wheat (T. aestivum L.) (Peleg et al. 2008). It harbors a broad repertoire of resistance genes to diseases, drought, and salt (Peleg et al. 2010). This study investigated the responses of durum wheat grown in hydroponic solution spiked with SeO42− or SeO32−, with an emphasis on the correlation between seed germination, seedling growth, and Se application under salt stress. In addition, we compared the effects of SeO42− and SeO32− on chlorophyll fluorescence and the antioxidant system in salt-stressed durum wheat seedlings to ascertain the effect induced by different forms and concentrations of Se. The present study might serve to better understand the potential mechanisms by which different forms of Se enhance tolerance to salt stress and protect photochemical efficiency, by upregulating antioxidant enzyme activity and accumulating osmolytes against salt-induced damage of wheat seeds and seedlings.
Materials and methods
Plant material and growth conditions
The test plant material used in this study consisted of five F7 recombinant inbred lines (RILs, durum wheat) derived from a cross between durum wheat (female) cv. Langdon and wild emmer wheat (male) accession G18-16. The five F7 RILs, currently planted in a greenhouse at Chengdu University, China, were supplied by the Institute of Evolution, University of Haifa, Israel. Uniform seeds were disinfected using 3.6% sodium hypochlorite for 10 min and then rinsed with deionized water. Seventy healthy seeds were selected for each treatment and transferred to petri dishes (120 mm diameter) on filter paper for germination. Salt and two types of Se were provided in the deionized water as NaCl, sodium selenate (Na2SeO4), and sodium selenite (Na2SeO3), respectively (Table 1). The concentrations of Na2SeO3 and Na2SeO4 were decided by further refining the Se concentration mentioned in the literature (Elkelish et al. 2019; Qin et al. 2018), while for NaCl, the concentration with the most stimulatingly response to different concentrations of Se as observed from preliminary studies was used. Each treatment was replicated five times. The solutions were replenished every 2 days. Seeds were germinated in a growth chamber under a day/night temperature of 25/20 °C, 16-h photoperiod, with 60–70% relative humidity and 300 µmol m−2 s−1 irradiance. The number of sprouted seeds was recorded daily and no fertilizer was added during the experiment. After 10 days, ten seedlings from each petri dish were harvested to calculate and analyze different parameters.
Table 1.
Summary of the experiments conducted in this study, including Se and salt treatments, forms of applied Se and genotypes used
| Treatments | Salt stress NaCl (mM) | Se supply (μM) | Se application forms | Extreme grain Se content genotypes from RILs |
|---|---|---|---|---|
| Control | 0 | 0 | – |
R6, R113, R126, R128, R171 |
| S0 | 50 | 0 | – | |
| S1 | 50 | 0.1 | Na2SeO4 or Na2SeO3 | |
| S2 | 50 | 1 | Na2SeO4 or Na2SeO3 | |
| S3 | 50 | 2 | Na2SeO4 or Na2SeO3 | |
| S4 | 50 | 4 | Na2SeO4 or Na2SeO3 | |
| S5 | 50 | 8 | Na2SeO4 or Na2SeO3 | |
| S6 | 50 | 10 | Na2SeO4 or Na2SeO3 |
Seed germination and growth measurements
Seed germination rate (GR, %) was recorded by measuring the percentage of seed germination over 7 days, and germination energy (GE, %) was measured for 3 days according to the method described by Wang et al. (2012). Germination index (GI) was calculated using Eq. (1). After 10 days of treatment, the shoots and roots of ten seedlings were harvested separately and their corresponding lengths and fresh weight (FW) were measured:
| 1 |
Here, Gt is the number of seeds germinating on day and Dt is the number of days of germination.
Collection and measurement of antioxidative enzymes
SOD, POD, and CAT contents in the roots and shoots were determined using an ultraviolet–visible spectrophotometer (U-T6, Yipu Instrument Manufacturing, Inc., China). For enzyme extraction, fresh root or shoot tissues (0.2 g) were extracted in ice-cold potassium phosphate buffer (50 mM, pH 7.8) containing 1% (w/v) polyvinyl pyrrolidone using a pre-chilled mortar and pestle. The homogenate was centrifuged at 12,000×g for 25 min at 4 °C, and the supernatant was used as the crude enzyme extract.
The activity of CAT in roots and shoots was determined by measuring the decrease in H2O2 at 240 nm using the above crude enzyme extract to initiate the reaction in 3 mL of a reaction mixture containing 50 mM phosphate buffer (pH 7.0) and 10 mM H2O2 (Abei 1984). SOD activity in roots and shoots was assayed by measuring its capacity to inhibit the photochemical reduction of nitroblue tetrazolium, as previously described by Beauchamp et al. (1971). POD activity in roots and shoots was measured using guaiacol substrates, with one unit of enzyme activity expressed as an increase of 0.01 absorbance units at 470 nm (Upadhyaya et al. 1985).
Chlorophyll fluorescence measurements
Chlorophyll fluorescence was measured using a PAM chlorophyll fluorometer (PAM-2500, WALZ, Inc., Germany) as described by Liu et al. (2018). The leaves of 10-day-old seedlings were stored in the dark for 20 min, and the maximal and minimal fluorescence yields (Fm and Fo, respectively) and maximal PSII photochemical efficiency (Fv/Fm) were then measured. Photochemical quantum yield [Y(II)], non-photochemical quenching (NPQ), variable fluorescence (Fv), and Fv/Fo were then measured on the same leaves in the light-adapted state.
Estimation of free proline and malondialdehyde (MDA) contents, and electrolyte leakage
For the estimation of proline, 0.5 g of fresh leaf sample was homogenized using a pestle and mortar with 5 ml of 3% (w/v) sulfosalicylic acid. After centrifugation (5 min at 20,000×g), 0.5 ml of the supernatant was incubated at 100 °C for 60 min with 0.5 ml of glacial acetic acid and 0.5 ml of ninhydrin reagent. After cooling, 1 ml of toluene was added to the mixture, and the proline content was measured by colorimetric method at 520 nm using toluene as a blank (Bates et al. 1973; Handa. et al. 2018a, b).
MDA content was determined using the thiobarbituric acid method as described by Heath and Packer (1968). Briefly, leaves (0.5 g) were homogenized in 5 mL of 20% (w/v) trichloroacetic acid. The homogenate was centrifuged at 10,000×g for 20 min. The supernatant (1 mL) was mixed with 4 mL of 20% trichloroacetic acid containing 0.5% (w/v) thiobarbituric acid, and the mixture was boiled at 95 °C for 30 min. After cooling in an ice bath, the samples were recentrifuged for 10 min and absorbance of the resulting supernatant was read at 532 and 600 nm.
Electrolyte leakage from leaves was measured by the method described by Alyemeni et al. (2018a) with some modifications. Ten seedling leaves from each treatment were floated on 10 mL distilled water, and then, initial electrical conductivity (EC0) was measured using an EC meter (DDSJ-308A, INESA Scientific Instrument Co., Ltd., China) at 25 °C. The same samples were then boiled at 30 °C for 20 min and 120 °C for 10 min to measure the respective EC values (EC1 and EC2). Electrolyte leakage was calculated as:
| 2 |
Statistical analysis
All results were expressed as an average of five F7 RILs values. Data were analyzed using JMP® ver. 6.0 software (SAS Institute) and one-way analysis of variance (ANOVA). Tukey–Kramer’s honestly significant difference (HSD) test was used to compare means of all pairs (significance level, 5%). SigmaPlot® ver. 12.0 software was used to draw histograms and line graphs, and images were edited with Photoshop CS4. Correlation network analysis was carried out by Pearson correlation coefficients. A correlation matrix was obtained using the R language statistical package together with Cytoscape® ver. 2.7.0 software.
Results
Seed germination
Salt stress significantly decreased GR, GE, and GI by about 16.92, 17.91, and 13.68%, respectively, compared to non-salt-stressed controls (Table 2). However, application of 0.1, 1, 2, and 4 μM Na2SeO4 or Na2SeO3 under salt-stressed conditions increased these parameters. The increment in germination parameters from 0.1 to 4 μM treatment concentration was higher for Na2SeO4 than Na2SeO3. However, treatment with 8 and 10 μM Na2SeO4 or Na2SeO3 resulted in a significant decrease in these parameters. Na2SeO3 caused more of a decrease in GR, GE, and GI than Na2SeO4 at the same concentration, for both 8 and 10 μM. Overall, Na2SeO4 had a more pronounced effect on the germination parameters of durum wheat than equal strengths of Na2SeO3. In terms of restoring germination parameters, 1 μM Na2SeO4 was the most effective, as compared to both other concentrations of Na2SeO4 and all concentrations of Na2SeO3. Seeds treated with 1 μM Na2SeO4 showed a significant improvement in GR, GE, and GI, by approximately 12.35, 24.17, and 11.42%, respectively, compared to non-Se-treated seeds subjected to salt stress.
Table 2.
Effects of application of Na2SeO4 or Na2SeO3 at different concentrations on germination parameters of durum wheat seeds exposed to salt stress
| Salt stress NaCl (mM) |
Se supply (μM) | Na2SeO4 | Na2SeO3 | ||||
|---|---|---|---|---|---|---|---|
| GR (%) | GE (%) | GI | GR (%) | GE (%) | GI | ||
| 0 | 0 | 75.14 ± 11.57a | 51.86 ± 5.12b | 17.25 ± 8.00a | 75.14 ± 11.57a | 51.86 ± 5.12b | 17.25 ± 8.00a |
| 50 | 0 | 62.43 ± 8.26bc | 42.57 ± 5.60 cd | 14.89 ± 8.13ab | 62.43 ± 8.26b | 42.57 ± 5.60d | 14.89 ± 8.13ab |
| 50 | 0.1 | 63.78 ± 13.01bc | 48.57 ± 7.56c | 15.10 ± 9.60ab | 62.57 ± 8.94b | 43.57 ± 5.65d | 14.99 ± 8.21ab |
| 50 | 1 | 70.14 ± 15.00a | 58.86 ± 7.79a | 16.59 ± 9.91a | 68.29 ± 18.39ab | 53.86 ± 6.78a | 16.21 ± 8.84a |
| 50 | 2 | 68.29 ± 19.70ab | 54.00 ± 8.42b | 16.19 ± 10.44a | 66.14 ± 13.50b | 46.01 ± 6.21 cd | 15.65 ± 8.01ab |
| 50 | 4 | 65.29 ± 18.59abc | 51.57 ± 6.65b | 15.21 ± 8.41ab | 65.14 ± 12.47b | 49.71 ± 6.38bc | 15.35 ± 8.33ab |
| 50 | 8 | 55.29 ± 13.12 cd | 42.11 ± 4.59 cd | 14.20 ± 7.27ab | 52.86 ± 15.24c | 42.06 ± 6.59d | 12.44 ± 7.99ab |
| 50 | 10 | 46.42 ± 22.07d | 40.43 ± 4.72d | 11.96 ± 6.85b | 44.86 ± 15.44c | 35.00 ± 5.77e | 11.41 ± 7.24b |
Data are mean ± SD of five replicates. Different letters in a column indicate significant differences at P < 0.05
GR seed germination rate, GE Germination energy, GI Germination index
Plant growth
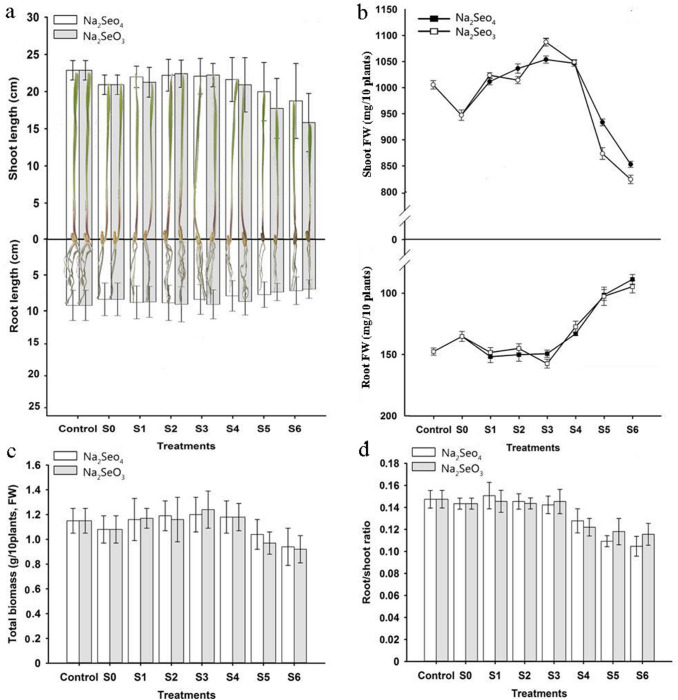
The effects of salinity and Se supplementation on growth of durum wheat seedling are summarized in Fig. 1. Root and shoot lengths, root and shoot FW, total biomass, and root/shoot ratio of salt-treated seedlings were lower than those of the control seedlings, indicating that the seedlings were affected by salt stress. Exogenous application of Na2SeO4 or Na2SeO3 to the salt-stressed seedlings had positive effects on seedling growth. The seedling growth parameters increased with low concentrations of Na2SeO4 or Na2SeO3, and decreased at high Na2SeO4 or Na2SeO3 concentrations. Seedlings treated with 0.1, 1, 2, and 4 μM Na2SeO4 or Na2SeO3 showed increases in shoot length, shoot FW, and total biomass. The changes in root length, root FW, and root/shoot ratio of seedlings in response to 0.1, 1, and 2 μM Na2SeO4 or Na2SeO3 showed similar trends. Thus, both Na2SeO4 and Na2SeO3 had stimulatory effects on durum wheat seedling development under salt stress. However, the growth parameters following treatment with high concentrations of Na2SeO4 or Na2SeO3 (8 and 10 µM) decreased more drastically than under salt stress alone, demonstrating that high Na2SeO4 or Na2SeO3 concentrations cause more damage than salt stress. Moreover, 8 and 10 μM Na2SeO3 caused a greater decrease in growth parameters than equal concentrations of Na2SeO4.
Fig. 1.
Effects of application of Na2SeO4 or Na2SeO3 at different concentrations on growth parameters of durum wheat seedlings exposed to salt stress. Values are means of five replicates ± SD. Different letters indicate significant difference at P < 0.05. FW fresh weight. Control 0 mM NaCl+0 μM Se (only deionized water), S0 50 mM NaCl + 0 μM Na2SeO4 (or Na2SeO3), S1 50 mM NaCl + 0.1 μM Na2SeO4 (or Na2SeO3), S2 50 mM NaCl + 1 μM Na2SeO4 (or Na2SeO3), S3 50 mM NaCl + 2 μM Na2SeO4 (or Na2SeO3), S4 50 mM NaCl + 4 μM Na2SeO4 (or Na2SeO3), S5 50 mM NaCl + 8 μM Na2SeO4 (or Na2SeO3), S6 50 mM NaCl + 10 μM Na2SeO4 (or Na2SeO3)
Chlorophyll fluorescence parameters
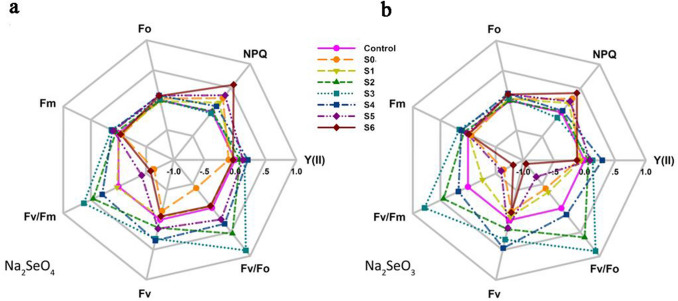
The effects of Se and salt stress on chlorophyll fluorescence of durum wheat seedlings are shown as radar plots (Fig. 2) according to JIP test. Salt stress had negative effects on the chlorophyll fluorescence parameters. The first observed symptom of salt stress was a decrease in Fm. This was followed by significant decreases in Y(II), Fv/Fm, Fv, and Fv/Fo, and notable increases in NPQ and Fo compared to the controls (Fig. 2, Table S1). Addition of Na2SeO4 or Na2SeO3 to the salt solution had positive effects on fluorescence indices. Y(II), Fm and Fv increased significantly with increasing Na2SeO4 concentrations from 0.1 to 4 μM, and then decreased as the Na2SeO4 concentration rose from 4 to 10 μM (Fig. 2a, Table S1). Increasing the Na2SeO4 concentrations from 0.1 to 2 μM resulted in significant increases in Fv/Fm and Fv/Fo, as well as significant decreases in NPQ and Fo. However, Fv/Fm and Fv/Fo decreased, and NPQ and Fo increased when Na2SeO4 was increased from 2 to 10 μM. Salt-stressed seedlings grown with the addition of Na2SeO3 showed trends similar to the Na2SeO4 treatment for all chlorophyll fluorescence parameters (Fig. 2b, Table S1). However, leaf Y(II) and Fv peaked at 4 μM Na2SeO3, and leaf Fm, Fv/Fm and Fv/Fo peaked at 2 μM Na2SeO3. In addition, Fo and NPQ dropped to their lowest values with 1 and 2 μM Na2SeO3, respectively. It should be noted that the increases in Y(II), Fm, Fv/Fm, Fv and Fv/Fo, and the decreases in NPQ and Fo, caused by addition of a low concentration (0.1 μM) of Na2SeO4 were more pronounced than with 0.1 μM Na2SeO3. However, the higher Na2SeO4 or Na2SeO3 treatment (10 μM) caused a drastic reduction in Y(II), Fm, Fv/Fm, Fv, and Fv/Fo, and a drastic increase in NPQ and Fo, with more pronounced changes in the presence of 10 μM Na2SeO3 than with Na2SeO4.
Fig. 2.
Effects of application of Na2SeO4 (a) or Na2SeO3 (b) at different concentrations on chlorophyll fluorescence parameters in seedlings exposed to salt stress. Each value is expressed as a mean of the ratio: (treated sample−control sample)/control sample. The value of the control (non-salt-stressed) sample is used as a reference and set to zero. Control 0 mM NaCl + 0 μM Se (only deionized water), S0 50 mM NaCl + 0 μM Na2SeO4 (or Na2SeO3), S1 50 mM NaCl + 0.1 μM Na2SeO4 (or Na2SeO3), S2 50 mM NaCl + 1 μM Na2SeO4 (or Na2SeO3), S3 50 mM NaCl + 2 μM Na2SeO4 (or Na2SeO3), S4 50 mM NaCl + 4 μM Na2SeO4 (or Na2SeO3), S5 50 mM NaCl + 8 μM Na2SeO4 (or Na2SeO3), S6 50 mM NaCl + 10 μM Na2SeO4 (or Na2SeO3)
Oxidative damage to shoots
As a reflection of membrane damage, free proline and MDA contents and electrolyte leakage (Table 3) increased significantly, by about 72.2, 87.50 and 195.33%, respectively, in the leaves of salt-stressed plants. Leaf contents of free proline and MDA, and electrolyte leakage exhibited similar changes in response to exogenous Na2SeO4 and Na2SeO3. At low Na2SeO4 and Na2SeO3 concentrations (0.1–4 μM), leakage tended to decrease; notably, at 4 μM Na2SeO4 and Na2SeO3, free proline and MDA contents, and electrolyte leakage decreased to a minimum, with 4 μM Na2SeO3 resulting in dramatic decreases of 37.76, 38.90, and 55.00%, respectively, relative to salt-stressed seedlings grown without Se addition. The decrement in membrane damage was higher for the Na2SeO4 vs. Na2SeO3 treatment. Increasing the concentrations of Na2SeO4 and Na2SeO3 to 8 and 10 μM enhanced the production of free proline and caused a significant increase in MDA content and electrolyte leakage compared to the 4 µM Se treatments. In salt-stressed seedlings grown with 10 μM Se, the values of the membrane-damage parameters were similar to those in the salt-only treatments. However, these effects varied with the type of Se. Na2SeO4 at 10 μM significantly increased free proline, MDA, and electrolyte leakage by 57.57, 70.07, and 118.30%, respectively, compared to 4 μM Na2SeO4; even bigger changes in free proline and MDA contents and electrolyte leakage were caused by Na2SeO3 at equivalent concentrations.
Table 3.
Effects of application of Na2SeO4 or Na2SeO3 at different concentrations on free proline and MDA contents, and electrolyte leakage in durum wheat shoots exposed to salt stress
| Salt stress NaCl (mM) |
Se supply (μM) | Na2SeO4 | Na2SeO3 | ||||
|---|---|---|---|---|---|---|---|
| Free proline (µg g FW−1) | MDA (mmol g FW−1) | Electrolyte leakage (%) | Free proline (µg g FW−1) | MDA (mmol g FW−1) | Electrolyte leakage (%) | ||
| 0 | 0 | 42.24 ± 2.16d | 9.68 ± 0.54d | 8.36 ± 0.96d | 42.24 ± 2.16d | 9.68 ± 0.54c | 8.36 ± 0.96c |
| 50 | 0 | 73.59 ± 3.01a | 18.15 ± 0.36a | 24.69 ± 1.12a | 73.59 ± 3.01a | 18.15 ± 0.36a | 24.69 ± 1.12a |
| 50 | 0.1 | 69.82 ± 2.59a | 17.11 ± 1.05ab | 23.56 ± 1.01a | 70.53 ± 2.54ab | 17.86 ± 0.96a | 23.97 ± 1.21a |
| 50 | 1 | 62.40 ± 2.39b | 15.14 ± 1.23bc | 19.24 ± 0.87b | 63.69 ± 1.59bc | 16.00 ± 0.54ab | 20.04 ± 1.18b |
| 50 | 2 | 54.72 ± 1.69c | 13.35 ± 1.37c | 16.36 ± 0.60c | 56.72 ± 2.31c | 13.95 ± 0.91b | 17.57 ± 0.96b |
| 50 | 4 | 44.76 ± 2.21d | 10.09 ± 0.64d | 10.22 ± 0.88d | 45.80 ± 1.36d | 11.09 ± 1.01c | 11.11 ± 1.00c |
| 50 | 8 | 63.43 ± 2.00b | 16.59 ± 0.78ab | 16.06 ± 1.03c | 65.29 ± 1.54b | 17.69 ± 0.90a | 18.46 ± 0.96b |
| 50 | 10 | 70.53 ± 1.26a | 17.16 ± 1.11ab | 22.31 ± 1.20a | 73.50 ± 2.68a | 18.15 ± 1.21a | 24.36 ± 1.10a |
Data are mean ± SD of five replicates. Different letters in a column indicate significant differences at P < 0.05
Activities of antioxidant enzymes in roots and shoots
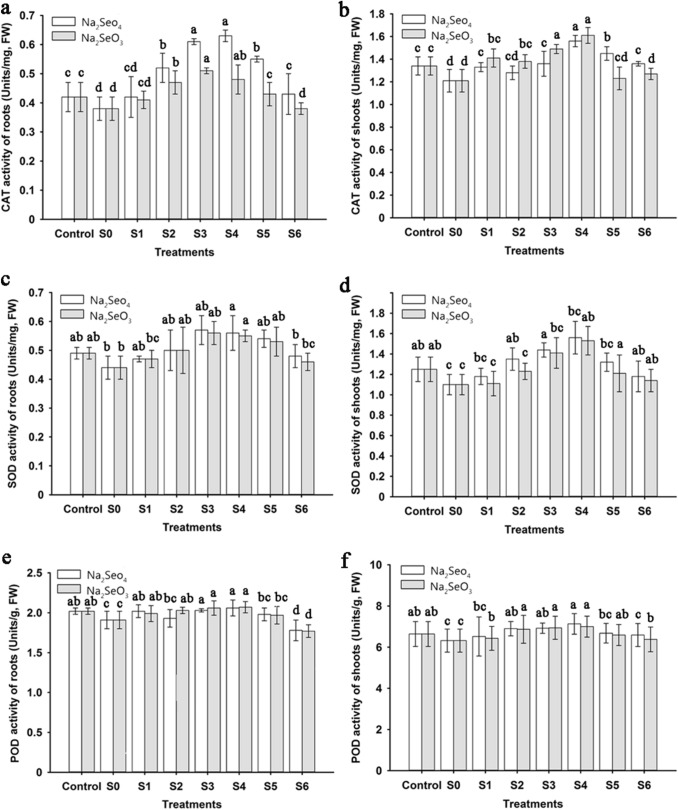
To avoid oxidative damage, plants initiate complex antioxidant defense mechanisms which regulate the contents and activities of ROS in response to stress. As shown in Fig. 3, the activities of CAT, SOD, and POD were significantly decreased in salt-stressed seedlings compared to controls: by 9.52, 10.20, and 5.45%, respectively, in the roots, and by 9.70, 12.00, and 4.82%, respectively, in the shoots. The effects of Se on these antioxidant enzymes’ activities in the salt-stressed seedlings were both type- and concentration-dependent. Addition of Na2SeO4 or Na2SeO3 generally enhanced CAT, SOD, and POD activities in the roots and shoots, while the positive effects of high Na2SeO4 or Na2SeO3 concentration (8 and 10 μM) on these enzyme activities declined somewhat relative to the low-concentration additions. Compared to salt stress alone, CAT, SOD, and POD activities in the roots and shoots increased dramatically with increasing Na2SeO4 from 0.1 to 4 μM. However, their activities gradually decreased when the rate of Na2SeO4 was increased from 4 to 10 μM. In the salt-stressed seedlings treated with Na2SeO3, these enzymes’ activities showed trends similar to those with Na2SeO4 addition. However, their activities in roots and shoots peaked at 2 μM and 4 μM Na2SeO3, respectively. It is worth noting that the increase in CAT, SOD, and POD activities caused by treatments with Na2SeO4 was higher than that with addition of Na2SeO3, except for CAT activity in the shoots.
Fig. 3.
Effects of application of Na2SeO4 or Na2SeO3 at different concentrations on activities of CAT, SOD, and POD in durum wheat roots and shoots exposed to salt stress. Values are means of five replicates ± SD. Different letters indicate significant differences at P < 0.05. FW fresh weight, CAT catalase, SOD superoxide dismutase, POD peroxidase. Control 0 mM NaCl + 0 μM Se (only deionized water), S0 50 mM NaCl + 0 μM Na2SeO4 (or Na2SeO3), S1 50 mM NaCl + 0.1 μM Na2SeO4 (or Na2SeO3), S250 mM NaCl + 1 μM Na2SeO4 (or Na2SeO3), S3 50 mM NaCl + 2 μM Na2SeO4 (or Na2SeO3), S4 50 mM NaCl + 4 μM Na2SeO4 (or Na2SeO3), S5 50 mM NaCl + 8 μM Na2SeO4 (or Na2SeO3), S6 50 mM NaCl + 10 μM Na2SeO4 (orNa2SeO3)
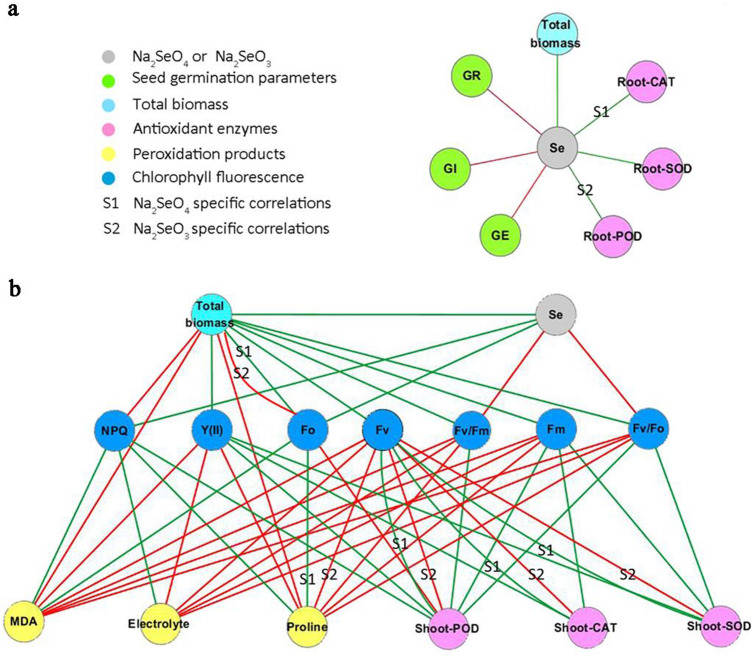
Correlation network analysis
Correlation network analysis can reveal relationships between antioxidative indicators (Huang et al. 2018). To investigate the relationships between the different forms of Se, chlorophyll fluorescence, and antioxidant enzyme activities, we performed a pairwise correlation analysis. Of these correlations, 7 and 52 significant correlations (P < 0.05) were identified in Fig. 4a, b, respectively. In Fig. 4a, both Na2SeO4 and Na2SeO3 were found to be negatively correlated with GR, GE, and GI, and positively correlated with root SOD activity and total biomass. Na2SeO4 was positively correlated with root CAT activity, but showed no correlation with root POD activity, whereas Na2SeO3 showed the opposite. In Fig. 4b, most of the correlations were of total biomass–chlorophyll fluorescence parameters, antioxidative enzyme activities–chlorophyll fluorescence parameters, and membrane-damage parameters–chlorophyll fluorescence parameters. Among them, the correlation between Fv and CAT, SOD, and POD activities in the shoots differed with the different forms of Se. Fv was positively correlated with CAT, SOD, and POD activities in the shoots of seedlings treated with Na2SeO4, whereas it was negatively correlated with these antioxidant enzyme activities in the shoots of seedlings treated with Na2SeO3. This was also the situation for Fo and total biomass correlations under Na2SeO4 and Na2SeO3 treatment. Furthermore, both Na2SeO4 and Na2SeO3 were significantly correlated with antioxidant enzymes in the roots, but not in the shoots.
Fig. 4.
Correlations among Se, seed germination parameters, total biomass, antioxidant enzyme activities, peroxidation products, and chlorophyll fluorescence parameters under exposure to salt stress. Edges between nodes indicate positive and negative correlations (green and red lines, respectively). All correlations were significant (P < 0.05) in the network. Se selenium, GR seed germination rate, GI Germination index, GE Germination energy, NPQ non-photochemical quenching, Y(II) photochemical quantum yield, Fo minimal fluorescence yield, Fv variable fluorescence, Fv/Fm maximal PSII photochemical efficiency, Fm maximal fluorescence yield, Fv/Fo potential efficiency of PSII, PSII photosystem II, MDA malondialdehyde, CAT catalase, SOD superoxide dismutase, POD peroxidase
Discussion
The most important stages in the ontogeny of higher plants are seed germination and seedling growth (Foti et al. 2018). Here, salt stress caused a drastic reduction in durum wheat seed germination parameters (Table 2). However, addition of low concentrations (1–4 μM) of Na2SeO4 or Na2SeO3 to the salt-containing medium led to a significant increase in GR, GE, and GI compared to the salt-stressed controls. At high concentrations (8–10 μM), the beneficial effects of Na2SeO4 and Na2SeO3 declined. The observed effects of Se are in agreement with recent reports by Moulick et al. (2016), who observed that at a low concentration (0.8 mg L−1), Se significantly promotes the GR of abiotically stressed rice seeds, whereas at a higher concentration (1 mg L−1), it has an adverse effect on seed germination ability. Se is an essential component of glutathione peroxidase. Glutathione peroxidase can scavenge free radicals produced by lipid hydroperoxides and reduce peroxidation damage in the seed cell membrane. Therefore, low concentrations of Se can maintain the antioxidant system, promote growth and metabolism, alleviate stress damage, and enhance stress resistance in seeds. However, adding a high concentration of Se to the growth substrate can reduce antioxidant activity and α-amylase activity in the seeds, and have a toxic effect. Under salt-stress conditions, the effects of Se on seedling growth were similar to those on seed germination. Application of Na2SeO4 or Na2SeO3 at low concentrations promoted the growth of salt-stressed durum wheat seedlings, whereas at high concentrations, they had an inhibitory effect on biomass accumulation (Fig. 1). The two forms of Se differed in the concentration at which they exerted their maximum positive effect on seed germination and growth parameters: 1 μM for Na2SeO4 and 2 μM for Na2SeO3. At the same time, correlation network analysis showed that both Na2SeO4 and Na2SeO3 are negatively correlated with germination parameters, and positively correlated with total biomass of seedlings (Fig. 4a). These results indicated that the seeds are more sensitive to exogenous Se than seedlings, possibly because germinating seeds are the first interface of material exchange between the growing plant and the environment. In addition, the increment in germination parameters was higher for Na2SeO4 vs. Na2SeO3 treatment. This indicates that the specific effect depends not only on the dosage, but also the form of the Se application.
In addition to its inhibitory effects on seed germination and plant growth, salinity stress provokes a decrease in photosynthesis (Nassar et al. 2020). Chlorophyll fluorescence reflects the primary reactions of photosynthesis (Pleban et al. 2020), and its measurement is frequently used to monitor the photosynthetic process in plants because of its sensitivity to abiotic stress and its relatively small damage to plant samples (Ni et al. 2019). Reductions in Fv, Fv/Fo, Fv/Fm, and Y(II) indicate inhibition of photosynthesis and a decline in the photochemical activity of PSII (Ya-wei et al. 2019). In the present study, Fv, Fv/Fo, Fv/Fm, and Y(II) were significantly reduced under salt stress (Fig. 2 and Table S1), indicating that salinity induces a reduction in PSII photochemical activity and electron transport. Similar results were obtained by Sui et al. (2018) and Zhang et al. (2018). Fv, Fv/Fo, Fv/Fm, and Y(II) were increased in the salt-stressed durum wheat seedlings treated with low concentrations (0.1–2 μM) of Na2SeO4 or Na2SeO3, showing improvements in PSII photochemical activity and conversion efficiency under salt stress. However, at high concentrations (8–10 μM) of Na2SeO4 or Na2SeO3, the damage intensified, possibly related to increased Se accumulation in the leaves. The high concentration of Se in the leaves might inhibit the enzyme kinetics or electron transport chain of photosynthesis through the replacement of sulfur, thereby affecting the photosynthesis. The tertiary structure of most proteins depends on the formation of disulfide bonds (S–S). When cysteine is replaced by selenocysteine, a new diselenide bond (Se–Se) or selenium–sulfide bond (Se–S) is formed. This damages the structure of the PSII complex in the chloroplast and exerts a strong inhibitory effect on photosynthetic electron transfer. In addition, replacement of the sulfur atom by Se in the key enzymes of chlorophyll synthesis destroys their configuration and reduces their activity, seriously hindering chlorophyll synthesis. The NPQ value represents the plant’s photo-protective capacity, i.e., its ability to prevent damage from excess light energy. Fo reflects the initial fluorescence yield, when the electron acceptor quencher A in PSII is maximally oxidized. The Fm value indicates the fluorescence level when quencher A is maximally reduced. In this study, we also observed that low concentrations of Na2SeO4 or Na2SeO3 significantly decrease NPQ, Fo, and Fm in the salt-stressed durum wheat, whereas these parameters increased with increasing Na2SeO4 or Na2SeO3 concentration (Fig. 2). In addition, network correlation analysis revealed that the total biomass of wheat is positively correlated with multiple chlorophyll fluorescence parameters (Fig. 4b). These results suggest that a suitable level of Se prevents excessive excitation of PSII via regulation of the photo-protective mechanism under salt stress. However, a high level of Se may cause the loss of thylakoid membrane integrity and damage plant photosynthesis (Fig. 5). Moreover, the lower Na2SeO4 concentration (0.1 μM) resulted in a more positive effect on chlorophyll fluorescence parameters than 0.1 μM Na2SeO3. However, at the highest Se concentration (10 μM), the negative effect on these parameters was more pronounced for Na2SeO3 than for Na2SeO4. This might be due to the different uptake and biotransformation pathways of Na2SeO4 and Na2SeO3 (Fig. 5). Although both chemical species of Se were absorbed by plants from the growth media, biotransformation of Na2SeO4 and Na2SeO3 in the chloroplast relies heavily on the availability of specifically designated enzymes in the plant tissue (Hawrylak-Nowak 2009). In addition to passive absorption, the absorption of Na2SeO3 by plants is also closely related to root metabolic processes, whereas Na2SeO4 absorption by plant roots is dominated by active absorption. This could produce the different responses of the chlorophyll fluorescence parameters to increasing Se concentrations observed in this study.
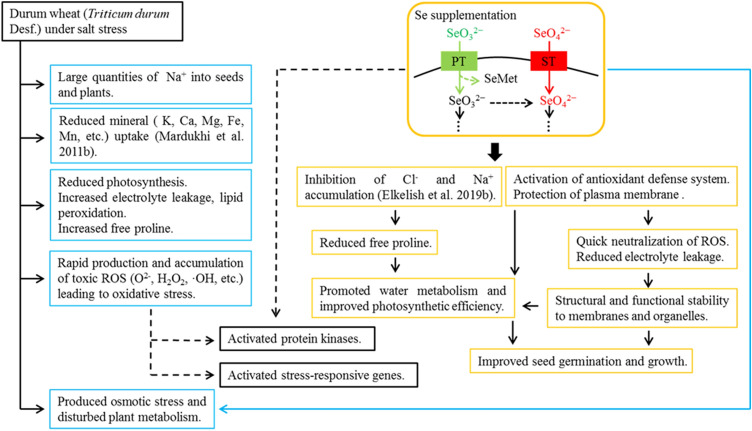
Fig. 5.
Possible mechanism of Se-induced salt tolerance in durum wheat. Salt accumulation triggers oxidative damage by increasing the production of ROS. Increased electrolyte leakage and lipid peroxidation, as well as reduced mineral absorption, damage the biomembrane in salt-stressed plants leading to a considerable decline in seed germination ability and growth retardation. Se supplementation strengthens the endogenous tolerance mechanisms by inhibiting the accumulation of Cl− and Na+, and inducing enzymes (antioxidants) involved in protecting the metabolic and assimilatory pathways. Se-induced upregulation of the antioxidant system leads to rapid elimination of ROS, thereby protecting membranes, while the reduced accumulation of Cl− and Na+ absorption improves water metabolism, resulting in significant enhancement of seed germination, photosynthesis, and growth. In addition, SeO42− and SeO32− have different active sites in plant roots and different transport mechanisms in plants, leading to differences in their bioavailability and toxicity. High concentrations of Se can damage seed germination and plant growth. Black dotted lines indicate the probable involvement of protein kinases and stress-responsive genes (modified after (Alyemeni et al. (2018b)). ST high-affinity sulfate transporters, PT high-affinity phosphate transporters, SeMet selenomethionine
As the site for photosynthesis, chloroplasts are also the main source of ROS (Khorobrykh et al. 2020). Increased accumulation of ROS in leaves has been shown to destroy photosynthetic pigments and enhance membrane damage (Bukhat et al. 2019). By monitoring the changes in free proline and MDA contents and degree of electrolyte leakage, we demonstrated the effects of Na2SeO4 and Na2SeO3 on the oxidative stress imposed by salt stress in the leaves. For example, applying low concentrations (0.1–4 μM) of Na2SeO4 or Na2SeO3 clearly inhibited electrolyte leakage, and the decrease in free proline and MDA accumulation in salt-treated wheat leaves (Table 3). Gong et al. (2012) believe that maintenance of the structural and functional integrity of plant membranes under osmotic stress is primarily related to free proline and MDA content. Our study also showed that Se supplementation prevents the leakage of important cellular components, in agreement with Wei et al. (2018). However, these positive effects declined with increasing Se concentration. Thus, the observed growth-enhancing effect of Se can be partially related to low levels of membrane damage, as demonstrated by diminished lipid peroxidation, whereas it can cause membrane damage at high concentrations (Fig. 5).
Plants have enzymatic and non-enzymatic antioxidant defense systems against ROS (Ahmad et al. 2010). Under normal conditions, plants use the ROS-scavenging system to remove harmful ROS, and protect photosynthetic organs, membranes, and functional biological molecules (Biswojit et al. 2018). SOD, CAT, and POD are important antioxidant enzymes. Se application enhanced the activities of these enzymes in wheat roots and shoots under salt stress (Fig. 3), counteracting the adverse effects of salt stress on their growth. Similar results have been found by Jiang et al. (2017) for maize. Under salt stress, the plant absorbs excess salt ions, and their toxic effect lies in their destruction of the dynamic balance of the ROS-metabolism system, resulting in the accumulation of free radicals. Se plays an important role in the plant’s non-enzymatic antioxidant system. The application of exogenous Se improved the activity of CAT, SOD, and POD in the plant, removed accumulated superoxide free radicals, and improved the plant’s overall antioxidant capacity and adaptability to salt stress. However, we also observed that the effect of Se addition under salt stress on SOD, CAT, and POD activities in the roots and shoots depended on both Se concentration and Se form. These three antioxidant enzymes’ activities showed trends similar with the increase of Se. Namely, as the Se content increased, their activities first increased and then decreased. However, the increase in CAT, SOD, and POD activities following addition of Na2SeO4 was more pronounced than after addition of Na2SeO3, except for CAT activity in the shoots. Correlation analysis revealed that CAT and POD activities in the roots are significantly positively correlated with Na2SeO4 and Na2SeO3, respectively (Fig. 4a). This further confirmed that CAT and POD activities in the roots are the most sensitive indexes of exposure to SeO42− and SeO32−, respectively (Xiao-Zhang and Ji-Dong 2007). The correlation analysis further showed that the three antioxidant enzymes’ activities in the shoots are significantly correlated with multiple chlorophyll fluorescence parameters (Fig. 4b). This may be due to the Se-induced increase in antioxidant enzyme activity, thereby protecting photosynthetic electron transport from salt-induced oxidative damage by maintaining a suitable nicotinamide adenine dinucleotide phosphate/reduced nicotinamide adenine dinucleotide phosphate (NADP/NADPH) ratio. Fv was positively correlated with CAT, SOD, and POD activities in shoots treated with Na2SeO4, whereas it was negatively correlated with these antioxidant enzymes’ activities in shoots treated with Na2SeO3. This might be because biotransformation of Na2SeO4 and Na2SeO3 in the chloroplast relies heavily on the specific enzymes that are available in the plant tissue; further research into this issue is warranted.
In conclusion, the present study suggests that durum wheat seeds are more sensitive to Na2SeO4 or Na2SeO3 concentrations than seedlings, and that Se’s alleviation of the effects of salt stress in wheat depends on its concentration and form. Low concentrations of Na2SeO4 or Na2SeO3 added to salt-stressed durum wheat seedlings increased the photosynthetic capacity in their leaves, alleviated the salt-induced oxidative damage in their chloroplasts, and restored cell wall integrity, thereby reducing the effects of salt stress on wheat growth. This most likely occurs through regulation of the antioxidant enzyme systems, resulting in an increase in the photochemical efficiency of PSII in salt-stressed plants, thereby increasing photosynthesis and promoting plant growth. However, high dosages of Na2SeO4 or Na2SeO3 can damage seed germination and seedling growth. Na2SeO4 was found to be more beneficial to wheat seed germination and seedling growth than Na2SeO3 at the same concentration. Although further studies should explore this phenomenon in greater depth, application of exogenous Na2SeO4 or Na2SeO3, at concentrations that have a positive effect on plants, is suggested as a remediation strategy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Science Foundation of China (31560578), the cultivation Project of Sichuan Science and Technology Innovation Seedling Program (2019101), Sichuan International Science and Technology Cooperation and Exchange Research and Development Project (2018HH0116), and China–Israel cooperation program grants from the Ministry of Science and Technology in China (2013DFA32200).
Abbreviations
- CAT
Catalase
- EC
Electrical conductivity
- GE
Germination energy
- GI
Germination index
- GR
Germination rate
- Fm
Maximal fluorescence yield
- Fo
Minimal fluorescence yield
- Fv
Variable fluorescence
- Fv/Fo
Potential efficiency of PSII
- Fv/Fm
Maximal PSII photochemical efficiency
- FW
Fresh weight
- MDA
Malondialdehyde
- Na2SeO3
Sodium selenite
- Na2SeO4
Sodium selenate
- NPQ
Non-photochemical quenching
- POD
Peroxidase
- PSII
Photosystem
- RILs
Recombinant inbred lines
- ROS
Reactive oxygen species
- RuBisCO
Ribulose-1, 5-bisphosphate carboxylase/oxygenase
- Se
Selenium
- SeO32−
Selenite
- SeO42−
Selenate
- SOD
Superoxide dismutase
- Y(II)
Photochemical quantum yield
Author contributions
Yong Liang and Jun Yan contributed to the study conception and design. Reagents, materials, and analysis tools were contributed by Jianping Cheng, Gang Zhao, Tzion Fahima, and Jun Yan. The draft of the manuscript was written by Yong Liang, Daqing Li, and Yuexing Chen. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
All the authors declare no conflict of interest with respect to this paper.
References
- Abei H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahanger MA, Aziz U, Alsahli AA, Alyemeni MN, Ahmad P. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed vigna angularis. Biomolecules. 2020;10(1):42–58. doi: 10.3390/biom10010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol. 2010;30(3):161–175. doi: 10.3109/07388550903524243. [DOI] [PubMed] [Google Scholar]
- Ahmad P, Allah EF, Hashem A, Sarwat M, Gucel S. Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J Plant Growth Regul. 2016;35(4):936–950. doi: 10.1007/s00344-016-9592-3. [DOI] [Google Scholar]
- Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma. 2018;255(3):459–469. doi: 10.1007/s00709-017-1162-4. [DOI] [PubMed] [Google Scholar]
- Balistrieri LS, Chao TT. Adsorption of selenium by amorphous iron oxyhydroxide and manganese dioxide. Geochim Cosmochim Acta. 1990;54(3):739–751. doi: 10.1016/0016-7037(90)90369-v. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Bioanal Chem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Biswojit D, Mubasher H, Muhammad I, Sangeeta M, Min L, Shuang L, Qiu D. Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molecules. 2018;23:388–398. doi: 10.3390/molecules23020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhat S, Manzoor H, H-u-R Athar, Zafar ZU, Rasul S. Salicylic acid induced photosynthetic adaptability of raphanus sativus to salt Stress is associated with antioxidant capacity. J Plant Growth Regul. 2019;2019:1–14. doi: 10.1007/s00344-019-10024-z. [DOI] [Google Scholar]
- Cengiz K, David H, Muhammad A, Mohammed N, Parvaiz A. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol Plant. 2019;168(2):256–277. doi: 10.1111/ppl.12976. [DOI] [PubMed] [Google Scholar]
- D’Amato R. The selenium supplementation influences olive tree production and oil stability against oxidation and can alleviate the water deficiency effects. Front Plant Sci. 2018;9:1191–1199. doi: 10.3389/fpls.2018.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem. 2019;137:1–8. doi: 10.1016/j.plaphy.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Feng W. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr Biol. 2018;28(5):666–675. doi: 10.1016/j.cub.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti C, Khah EM, Pavli OI. Germination profiling of lentil genotypes subjected to salinity stress. Plant Biol. 2018;21(3):480–486. doi: 10.1111/plb.12714. [DOI] [PubMed] [Google Scholar]
- Gong X, Yu H, Chen J, Han B. Cell surface properties of Lactobacillus salivarius under osmotic stress. Eur Food Res Technol. 2012;234(4):671–678. doi: 10.1007/s00217-012-1677-z. [DOI] [Google Scholar]
- Handa N, Kohli SK, Sharma A, Thukral AK, Ahmad P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. Plants Environ Exp Bot. 2018;161:180–192. doi: 10.1016/j.envexpbot.2018.11.009. [DOI] [Google Scholar]
- Handa N, Kohli SK, Thukral AK, Bhardwaj R, Ahmad P. Protective role of selenium against chromium stress involving metabolites and essential elements in Brassica juncea L. seedlings. Biotech. 2018;8(1):66–75. doi: 10.1007/s13205-018-1087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa N, Sukhmeen KK, Anket S, Ashwani KT, Renu B, Mohammed N, Leonard W, Parvaiz A. Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. South Afr J Bot. 2018;119:1–10. doi: 10.1016/j.sajb.2018.08.003. [DOI] [Google Scholar]
- Harpreet K, Geetika S, Renu B, Alyemeni MN, Siddique KHM, Parvaiz A. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in Brassica juncea. Sci Rep. 2018;8:8735–8746. doi: 10.1038/s41598-018-27032-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylak-Nowak B. Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol Trace Elem Res. 2009;132(1–3):259–269. doi: 10.1007/s12011-009-8402-1. [DOI] [PubMed] [Google Scholar]
- Hawrylak-Nowak B, Dresler S, Rubinowska K, Matraszek-Gawron R, Woch W, Hasanuzzaman M. Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol Biochem. 2018;S0981–9428(18):30176. doi: 10.1016/j.plaphy.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Huang B, et al. Oxidative damage and antioxidative indicators in 48h germinated rice embryos during the vitrification–cryopreservation procedure. Plant Cell Rep. 2018;37(9):1325–1342. doi: 10.1007/s00299-018-2315-4. [DOI] [PubMed] [Google Scholar]
- Huihui Z, et al. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotox Environ Saf. 2020;195:110–119. doi: 10.1016/j.ecoenv.2020.110469. [DOI] [PubMed] [Google Scholar]
- Jawad Hassan M, et al. Selenium and salt interactions in black gram (Vigna mungo L): ion uptake, antioxidant defense system, and photochemistry efficiency. Plants. 2020;9:467–476. doi: 10.3390/plants9040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep. 2017;7:420–429. doi: 10.1038/srep42039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- José PM, Acuña KI, Angela SA, Camila S, Alfredo S, Corcuera LJ, Bravo LA. Photosynthetic light responses may explain vertical distribution of hymenophyllaceae species in a temperate rainforest of southern chile. PLoS ONE. 2015;10(12):e0145475. doi: 10.1371/journal.pone.0145475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorobrykh S, Havurinne V, Mattila H, Tyystjärvi E. Oxygen and ROS in photosynthesis. Plants. 2020;9(1):91–100. doi: 10.3390/plants9010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Wang M, Bi D. Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul. 2005;45:155–163. doi: 10.1007/s10725-005-1893-7. [DOI] [Google Scholar]
- Liu H, Xia Y, Fan H, Xu Q, Du S, Fang Z, Xia H. Effect of imidazolium-based ionic liquids with varying carbon chain lengths on arabidopsis thaliana: response of growth and photosynthetic fluorescence parameters. J Hazard Mater. 2018;358(SEP.15):327–336. doi: 10.1016/j.jhazmat.2018.06.046. [DOI] [PubMed] [Google Scholar]
- Li J, Cang Z, Feng J, Bai X, Ding Z, Zhai R. Influence of drought stress on photosynthetic characteristics and protective enzymes of potato at seedling stage. J Saudi Soc Agric Sci. 2017;16(1):82–88. doi: 10.1016/j.jssas.2015.03.001. [DOI] [Google Scholar]
- Liu C, Dai Z, Sun H. Potential of duckweed (Lemna minor) for removal of nitrogen and phosphorus from water under salt stress. J Environ Manag. 2017;187(1):497–503. doi: 10.1016/j.jenvman.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Meetu G, Shikha G. An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci. 2016 doi: 10.3389/fpls.2016.02074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner GL, Bauerle WL, Baldocchi DD. Estimating the sensitivity of stomatal conductance to photosynthesis: a review. Plant Cell Environ. 2017;40(7):1214–1238. doi: 10.1111/pce.12871. [DOI] [PubMed] [Google Scholar]
- Moulick D, Ghosh D, Chandra SS. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol Biochem. 2016;109(12):571–578. doi: 10.1016/j.plaphy.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Nassar RMA, Kamel HA, Ghoniem AE, Alarcón JJ, Sekara A, Ulrichs C, Abdelhamid MT. Physiological and anatomical mechanisms in wheat to cope with salt Stress induced by seawater. Plants. 2020;9:237–246. doi: 10.3390/plants9020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Lu Q, Huo H, Zhang H. Estimation of chlorophyll fluorescence at different scales: a review. Sensors. 2019;19(13):3000–3009. doi: 10.3390/s19133000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg Z. High-density genetic map of durum wheat×wild emmer wheat based on SSR and DArT markers. Theor Appl Climatol. 2008;117(4):103–115. doi: 10.1007/s00122-008-0756-9. [DOI] [PubMed] [Google Scholar]
- Peleg Z, Fahima T, Krugman T, Abbo S, Yakir D, Korol AB, Saranga Y. Genomic dissection of drought resistance in durum wheat x wild emmer wheat recombinant inbreed line population. Plant Cell Environ. 2010;32(7):758–779. doi: 10.1111/j.1365-3040.2009.01956.x. [DOI] [PubMed] [Google Scholar]
- Pilon C, et al. Assessing stomatal and non-stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.) J Plant Physiol. 2018;231:124–134. doi: 10.1016/j.jplph.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Pleban J, Guadagno CR, Mackay D, Weinig C, Ewers B (2020) Rapid chlorophyll a fluorescence light response curves mechanistically inform photosynthesis modeling. Plant Physiol:pp.00375.02019. https://doi.org/10.1104/pp.19.00375 [DOI] [PMC free article] [PubMed]
- Qin X, Nie Z, Liu H, Zhao P, Qin S, Shi Z. Influence of selenium on root morphology and photosynthetic characteristics of winter wheat under cadmium stress. Environ Exp Bot. 2018;150:232–239. doi: 10.1016/j.envexpbot.2018.03.024. [DOI] [Google Scholar]
- Sarabi B. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Physiol Biochem. 2019;141:1–19. doi: 10.1016/j.plaphy.2019.05.010. [DOI] [PubMed] [Google Scholar]
- Sui N, Wang Y, Liu S, Yang Z, Wang F, Wan S. Transcriptomic and physiological evidence for the relationship between unsaturated fatty acid and salt stress in peanut. Front Plant Sci. 2018;9:7–15. doi: 10.3389/fpls.2018.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya A, Sankhla D, Davis TD, Sankhla N, Smith BN. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J Plant Physiol. 1985;121(5):453–461. doi: 10.1016/S0176-1617(85)80081-X. [DOI] [Google Scholar]
- Wang Y, Le L, Cui W, Sheng X, Shen W, Ren W. Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil. 2012;351(2):107–119. doi: 10.1007/s11104-011-0936-2. [DOI] [Google Scholar]
- Wang L, Pan D, Li J, Tan F, Hoffmannbenning S, Liang W, Chen W. Proteomic analysis of changes in the Kandelia candel chloroplast proteins reveals pathways associated with salt tolerance. Plant Sci. 2015;231:159–172. doi: 10.1016/j.plantsci.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Wei J, Hu C, Ming J, Zhao Y, Xin J, Sun X, Zhao X. Action of selenium against Sclerotinia sclerotiorum: Damaging membrane system and interfering with metabolism. Pestic Biochem Phys. 2018;150(2):10–16. doi: 10.1016/j.pestbp.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Wu YW. Effect of low-nitrogen stress on photosynthesis and chlorophyll fluorescence characteristics of maize cultivars with different low-nitrogen tolerances. J Integr Agric. 2019;18:1246–1256. doi: 10.1016/S2095-3119(18)62030-1. [DOI] [Google Scholar]
- Wu H, Zhang X, Giraldo JP, Shabala S. It is not all about sodium: revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil. 2018;431(1–3):1–17. doi: 10.1007/s11104-018-3770-y. [DOI] [Google Scholar]
- Xiao ZY, Guo JD. Metabolic responses of weeping willows to selenate and selenite. Environ Sci Pollut Res. 2007;14(7):510–517. doi: 10.1065/espr2007.04.407. [DOI] [PubMed] [Google Scholar]
- Ya WW. Effect of low-nitrogen stress on photosynthesis and chlorophyll fluorescence characteristics of maize cultivars with different low-nitrogen tolerances. J Integr Agric. 2019;18(9):1246–1256. doi: 10.1016/s2095-3119(18)62030-1. [DOI] [Google Scholar]
- Yan J, Xue W, Yang RZ, Qin HB, Zhao G, Fahima T, Cheng JP. Quantitative trait loci conferring grain selenium nutrient in durum wheat × wild emmer wheat RIL population. Czech J Genet Plant Breed. 2018;54(2):1–9. doi: 10.17221/112/2016-cjgpb. [DOI] [Google Scholar]
- Zhang H. Rootstock alleviates salt stress in grafted mulberry seedlings: physiological and PSII function responses. Front Plant Sci. 2018;9(12):1–12. doi: 10.3389/fpls.2018.01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvi P, Ismail C, Levent O, Atilla Y, Yan J, Hikmet B, Abraham B, Tzion F, Yehoshua S. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat x wild emmer wheat RIL population. Theor Appl Climatol. 2009;119(3):353–369. doi: 10.1007/s00122-009-1044-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.