Abstract
The tooth has a unique configuration with respect to biomaterials that are used for its treatment. Cells inside of the dental pulp interface indirectly with biomaterials via a calcified permeable membrane, formed by the dentin matrix and several thousands of dentinal tubules (~2 μm in diameter). Although the cytotoxic response of the dental pulp to biomaterials has been extensively studied, there is a shortage of in vitro model systems that mimic the dentin–pulp interface and enable an improved understanding of the morphologic, metabolic and functional influence of biomaterials on live dental pulp cells. To address this shortage, here we developed an organ-on-a-chip model system which integrates cells cultured directly on a dentin wall within a microfluidic device that replicates some of the architecture and dynamics of the dentin–pulp interface. The tooth-on-a-chip is made out of molded polydimethylsiloxane (PDMS) with a design consisting of two chambers separated by a dentin fragment. To characterize pulp cell responses to dental materials on-chip, stem cells from the apical papilla (SCAPs) were cultured in odontogenic medium and seeded onto the dentin surface, and observed using live-cell microscopy. Next, to evaluate the tooth-on-a-chip as a platform for materials testing, standard dental materials used clinically (2-hydroxyethylmethacrylate – HEMA, phosphoric acid – PA, and Adper-Scotchbond – SB) were tested for cytotoxicity, cell morphology, and metabolic activity on-chip, and compared against standardized off-chip controls. All dental materials had cytotoxic effects in both on-chip and off-chip systems in the following order: HEMA > SB > PA (p < 0.05), and cells presented consistently higher metabolic activity on-chip than off-chip (p < 0.05). Furthermore, the tooth-on-a-chip enabled real-time tracking of gelatinolytic activity in a model hybrid layer (HL) formed in the microdevice, which suggests that dental pulp cells may contribute to the proteolytic activity in the HL more than endogenous proteases. In conclusion, the tooth-on-a-chip is a novel platform that replicates near-physiologic conditions of the pulp–dentin interface and enables live-cell imaging to study dental pulp cell response to biomaterials.
Introduction
Treatment of dental diseases, such as caries or sensitivity, requires the application of a biomaterial directly onto a cavity formed on the tooth surface. Typically, these involve the attachment of the biomaterial onto dentin – the calcified tissue underlying the outer dental enamel. Therefore, the structural configuration of the tooth results in the formation of a unique interface, where biomaterials contact the dentin matrix, and indirectly allow reaction by-products and leachates to diffuse into the underlying dental pulp, via dentinal tubules (~2 μm in diameter), which are homogeneously distributed across the dentin matrix, and house odontoblast cell processes that extend a significant length into the tubules. Therefore, together with the dental pulp, a restored tooth forms an intricate biomaterials/dentin/cell complex1,2 that is unique in the body. The biocompatibility and cytotoxicity of dental materials on pulp cells have been extensively studied. Existing model systems include cells cultured on plates,3–5 larger devices that require specialized equipment, such as the Hume model,6 the in vitro pulp chamber,7 dentin barrier tests,8 or ex vivo models such as the rodent slice culture,9 and entire human tooth culture.10 Despite their extensive usefulness, these models have limited ability for direct observation and control (i.e., live cell imaging) of the morphologic and metabolic events that occur as cells inside of the tooth are exposed to biomaterials overtime. Arguably, these events hold important information regarding the ability of the tooth to respond to different biomaterials and treatments.
Organs-on-a-chip integrate microengineered substrates with microfluidic technologies to replicate levels of tissue functionality that are difficult to achieve with conventional 2D or 3D cell culture models. Similarly, these miniaturized organ systems allow for straightforward experimental control over multifactorial questions that are too difficult to study controllably and systematically in vivo.11,12 Several organ-on-a-chip model systems have demonstrated outstanding ability to replicate the complex multicellular architecture, cell–cell/cell–matrix interactions, tissue mechanics, and fluid-flow conditions that are naturally present in complex tissues.13–15 For instance, the gut on-chip was engineered to replicate the human gut epithelial microvilli, vasculature, microflora, and peristalsis.14 Similar methods have also been used to engineer the liver-on-a-chip,16 lung-on-a-chip,17 bone-marrow-ona-chip,15 and disease models such as cancer metastasis,18 thrombosis,19 and other immune organs.20
More specific to craniofacial and dental research, it is challenging to develop a platform that emulates the interface between dental materials or biofilms with the tooth and the underlying dental pulp, and examples of such a platform have remained non-existent in the literature. Recent reports have described microfluidic platforms to engineer salivary gland tissue capable of long-term secretory function,21 devices to test dental biofilm growth,22 or the remodeling of oral mucosa on-a-chip.23 In another example, a microfluidic chip was developed to include 2 μm microchannels that resemble the structure and size of dentinal tubules, as to study the growth and differentiation of odontoblasts processes.24 Nevertheless, the interface of dentin with either the dental pulp cells or restorative materials remains to be addressed. Therefore, the field of organs-on-a-chip has remained largely underdeveloped in the scope of craniofacial and dental research.
Here we report the development, optimization, and testing of a novel microfluidic model of the pulp–dentin–biomaterials interface, which we refer to as the ‘tooth-on-a-chip’. We determine the real-time response of pulp-cells to various dental materials such as phosphoric acid, adhesive monomers, and dental adhesive systems. We then compare the cytotoxicity, metabolic activity, and morphologic changes of an engineered odontoblast-like cell monolayer interfaced with the native human dentin as it is exposed to these materials, in comparison to an ISO in vitro model. We show that the tooth-on-a-chip provides direct visualization of the complexity of the pulp–dentin–biomaterials interface, and enables real-time assessment of the response of pulp cells to dental materials on a level that was previously not possible.
Materials and methods
Microdevice fabrication
The device design was created using a Computer Aided Design (CAD) software (Autodesk Fusion 360, Autodesk Inc, San Rafael, CA, USA) and a positive template was laser-cut (Boss LS1416, Boss laser, Sandorf, FL, US) in a polymethylmethacrylate (PMMA) board. Next, the templates were attached to the base of an impression container, molded with PDMS (poly dimethyl siloxane, Dow Corning) pre-polymer (Fig. 1A), and cured at 80 °C overnight (Fig. 1B). The cured PDMS mold was removed from the template (Fig. 1C) and four reservoirs were prepared with an 8 mm punch (Fig. 1D). The device is comprised of two parallel channels, two perfusable chambers (300 μm W × 1 mm L × 1 mm H), and a central groove that holds a dentin fragment (Fig. 1E). Human dentin from third molars extracted for orthodontic reasons according to the institutional ethics committee guidelines was used. Teeth were sectioned into fragments of 500 μm W × 1 mm H × 4.5 mm L cut perpendicular to the dentin tubules using an low speed saw (Accutom 5) (ESI† Fig. S1). The PDMS piece and a coverslip were then plasma treated (Plasma Cleaner, PDC-32G, Harrick Plasma, Ithaca, NY, US), as to increase the silanol groups (−OH) at the surface of the PDMS to form covalent Si–O–Si bonds with the glass. Of note, we did not subject the dentin fragments to plasma treatment to prevent any chemical or structural change to the dentin matrix. Immediately after plasma treating the PDMS mold and coverslips, a dentin fragment was carefully inserted into each PDMS mold using tweezers, and the system was assembled onto the glass coverslip using slight pressure, forming a sealed and leak-proof microdevice (Fig. 1E and F) with two chambers separated by a semi-permeable membrane (dentin) creating distinct microenvironments for each chamber (Fig. 1G and H). Although the dentin fragments had4.5 mm in length, the borders were placed within the PDMS holders (Fig. 1G and H), thus the dentin area exposed to dental treatments and cell attachment in the device were about 2–2.5 mm L × 1 mm H and 0.5 mm W. Each tooth-on-a-chip was then filled with sterile water until use to prevent dentin dehydration.
Fig. 1.
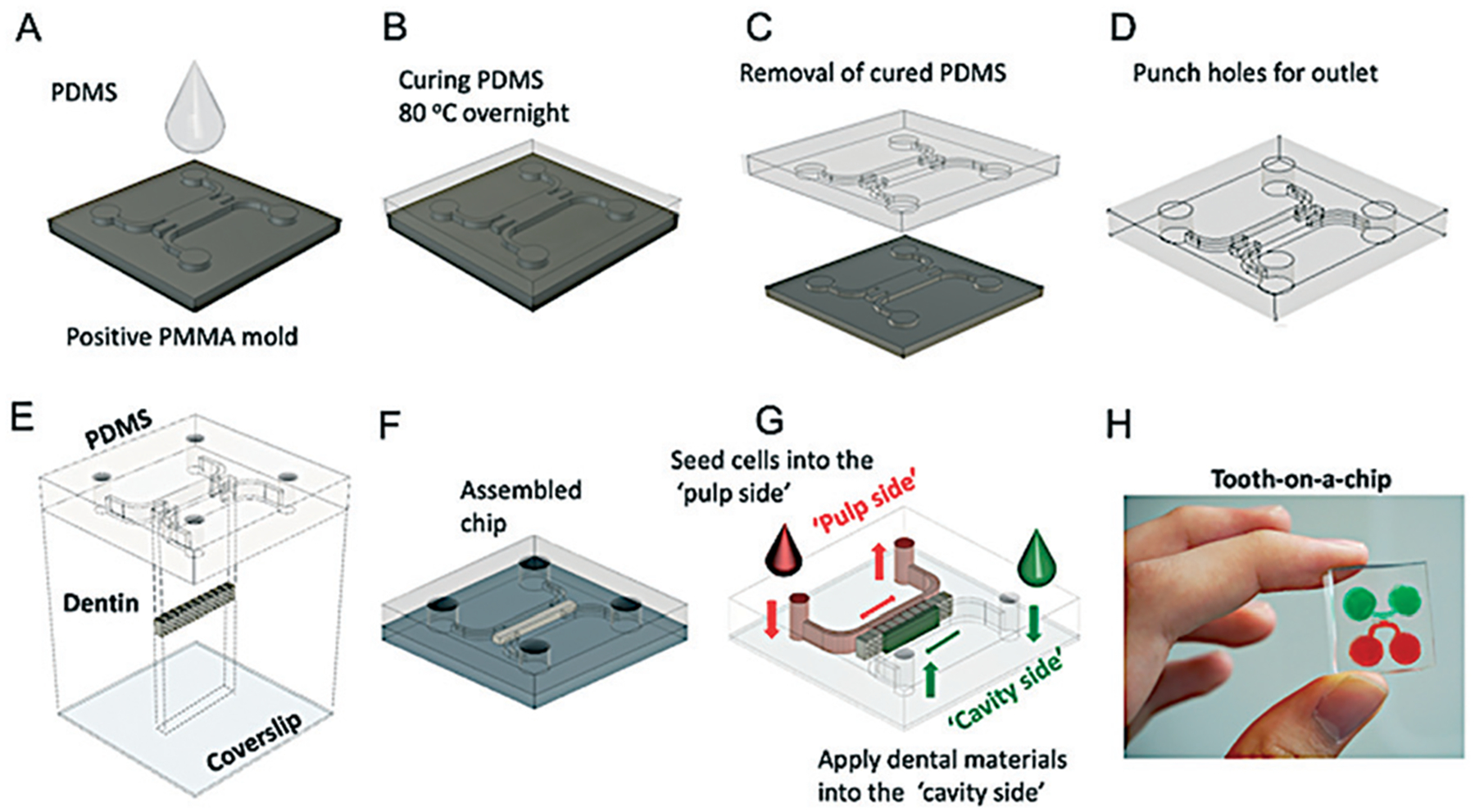
Fabrication of the tooth-on-a-chip. (A) PDMS prepolymer is poured onto a positive PMMA mold, and (B) cured overnight at 80 °C. Next, (C) the PDMS is released from the template, and (D) 8 mm holes are punched at the end of the channels to form reservoirs. (E) The PDMS and coverslips are plasma-treated, and a dentin fragment is placed in the center, between the two chambers and (F) the system is assembled. Two different chambers representing the ‘pulp side’ and the ‘cavity side’ are formed. (G) The assembled microdevice with dentin as a semipermeable membrane is shown in (H).
The fully assembled microdevice replicates the interface of dentin with the dental pulp on one side and the dental material with dentin on the other side, thus forming two accessible chambers representing the “pulp side” and the “cavity side”, respectively (Fig. 1G and H). The system was tested for leakage around the dentin fragments with a liquid dye, and our experiments suggest that the coloured medium would typically penetrate through the dentin tubules and intertubular matrix in the presence or absence of active flow (ESI† Fig. S2 and S3).
The microfluidic devices were then sterilized with ultraviolet light (EXFO Acticure 4000, 365 nm, at 8.5 cm distance, light density: 45 mW cm−2) for 40 min prior to use.
Cell culture
Stem cells from apical papilla (SCAPs) were cultured for 10 days in odontogenic medium (OM) (ESI†) to pre-differentiate cells into an odontoblast-like lineage. This is to more closely mimic the natural tooth anatomy where odontoblasts form a monolayer interfacing with the dentin. Dentin was treated with 17% EDTA for 45 seconds to remove the smear layer, thoroughly rinsed with water, and seeded with a suspension of 20 μL with 105 SCAP per ml on the ‘pulp side’ chamber. The device was incubated for 1 h to promote cell contact and attachment onto the dentin wall. Next, (Fig. 1H) the reservoirs were filled with 100 μL of cell medium and cells were cultured for 7 days with daily cell medium changes.
Live-cell imaging
To demonstrate that the tooth-on-a-chip enables live imaging of the cells in close contact with the dentin, a monolayer of odontogenically differentiated SCAP was imaged overnight, every 30 minutes using a spinning disk confocal microscope (ESI†). For analyses of cell morphology, on days 1 and 7, chips (n = 4) were fixed with 4% paraformaldehyde, stained for actin filaments and nuclei, and imaged using a confocal microscope (LSM 880 Zeiss) (ESI†). The whole monolayer in contact with dentin was photographed in 3 consecutive images and analyzed using ImageJ (Fiji, NIH, Maryland, USA).
Cytotoxicity
After the monolayer formation, three dental materials were tested: (a) HEMA dissolved in cell culture medium at a known cytotoxic concentration of 10 mM, (b) 37% phosphoric acid gel (PA) (Ultradent Products, South Jordan, UT, USA) used to etch the dentin for 15 s, and (c) 35% PA plus Adper Single Bond 2 (SB) (3M/ESPE, St Paul, MN, USA) applied per manufacturer recommendations (ESI†). The materials were introduced to the ‘cavity side’ of the device, thus forming an interface similar to the dentin–pulp complex in a restored tooth (n = 4). Live-cell images of SCAPs assembling as a monolayer on the dentin surface were obtained using a Yokogawa (Japan) CSU-X1 spinning disk field scanning confocal system. Cells were imaged using Nikon 10× objective lens (numerical aperture = 0.5). The temperature was maintained at 37 °C using an all-in-one stage incubator. To identify dead cells, SCAPs were incubated with 50 nM of Helix NP NIR (Biolegend, San Diego, CA) diluted in cell culture medium 10 minutes before imaging. The Helix NP dye does not require rinsing, so cells continued to be imaged to provide a baseline. After three minutes of imaging, 20 mM of HEMA was added to the opposite side of the dentin, and live-cell images were taken every 10 minutes for one hour.
We then compared on-chip experiments against experiments performed using the ISO-10993–1 (ref. 25) standard, which we refer to as the off-chip group. To that end, 104 SCAPs/well were seeded in 96-well plates, and after 24 h, cells were supplemented with the ISO recommended concentrations of HEMA, PA, and extracts of SB obtained by immersing the photopolymerized SB disks in culture media (n = 6) (ESI†). Next, cells were incubated for 24 h with the conditioned medium of each dental material, and the medium was replaced with the untreated medium for 7 days. Controls were samples cultured in standard culture medium not exposed to the dental materials. Cell metabolic activity was measured using Alamar Blue (ThermoFisher) on days 0, 1, 3, 5, and 7.
Gelatinolytic activity of hybrid layer on-a-chip
After determining the cytotoxicity of HEMA, PA, and SB, we evaluated the contribution of MMPs released by dental pulp cells in the degradation of the hybrid layer formed after SB treatment.
For proper bonding of resin restorations to the tooth, dentin needs to be conditioned with an acid to partially expose the collagen network present in the dentin organic matrix, and then an adhesive agent is applied to fabricate the hybrid layer, which consists of a hybrid system of collagen fibrils embedded with an adhesive resin. The final quality and longevity of the dental restoration depend significantly on the integrity of the hybrid layer, and it has been shown that matrix metalloproteinases (MMPs) contribute to the its degradation.26
We hypothesized that the immediate response of pulp cells to the acid attack and monomer exposure might stimulate cells to secrete substantial amounts of proteases, which may further promote the hybrid layer degradation in vivo – a phenomenon that cannot be measured using traditional off-chip gelatinolytic assays. To that end, we applied SB as previously described to the ‘cavity side’ of the chips fabricated with or without SCAPs (n = 4). The gelatinolytic activity at the hybrid layer was performed as detailed in ESI† using fluorescein-conjugated gelatin (DQ™ gelatin, EnzChek Gelatinase Assay Kit, ThermoFisher) incubated for at least 48 h, after which the proteolytic activity was imaged via confocal microscopy.
Statistics
Results were analyzed using either a Student’s t-test, one-way ANOVA or two-way ANOVA followed by Tukey post-hoc tests (α = 0.05) on GraphPad Prism 8.
Results
Morphogenesis of SCAP on dentin–pulp interface on-a-chip
The tooth-on-a-chip was fabricated with a 500 μm thick dentin barrier and a monolayer of differentiated SCAPs on the opposite side of the dentin wall to emulate a deep dental cavity.
Fig. 2A–D shows a time-lapse of the monolayer formation on the dentin wall (Fig. 2A) until the complete attachment to the dentin was visible (Fig. 2D) (Movie S1†). Only cells located up to 60 μm away from the dentin were considered as part of the odontoblast-like monolayer. We also tracked the cell death by incubating SCAPs with Helix NP NIR (Biolegend, San Diego, CA), a fluorescent dye that only binds to the DNA of non-viable cells (Fig. 2E–H) (Movie S2†), as the samples were treated with 20 mM of 2-hydroxyethyl methacrylate (HEMA) (Esstech, PA, USA).
Fig. 2.
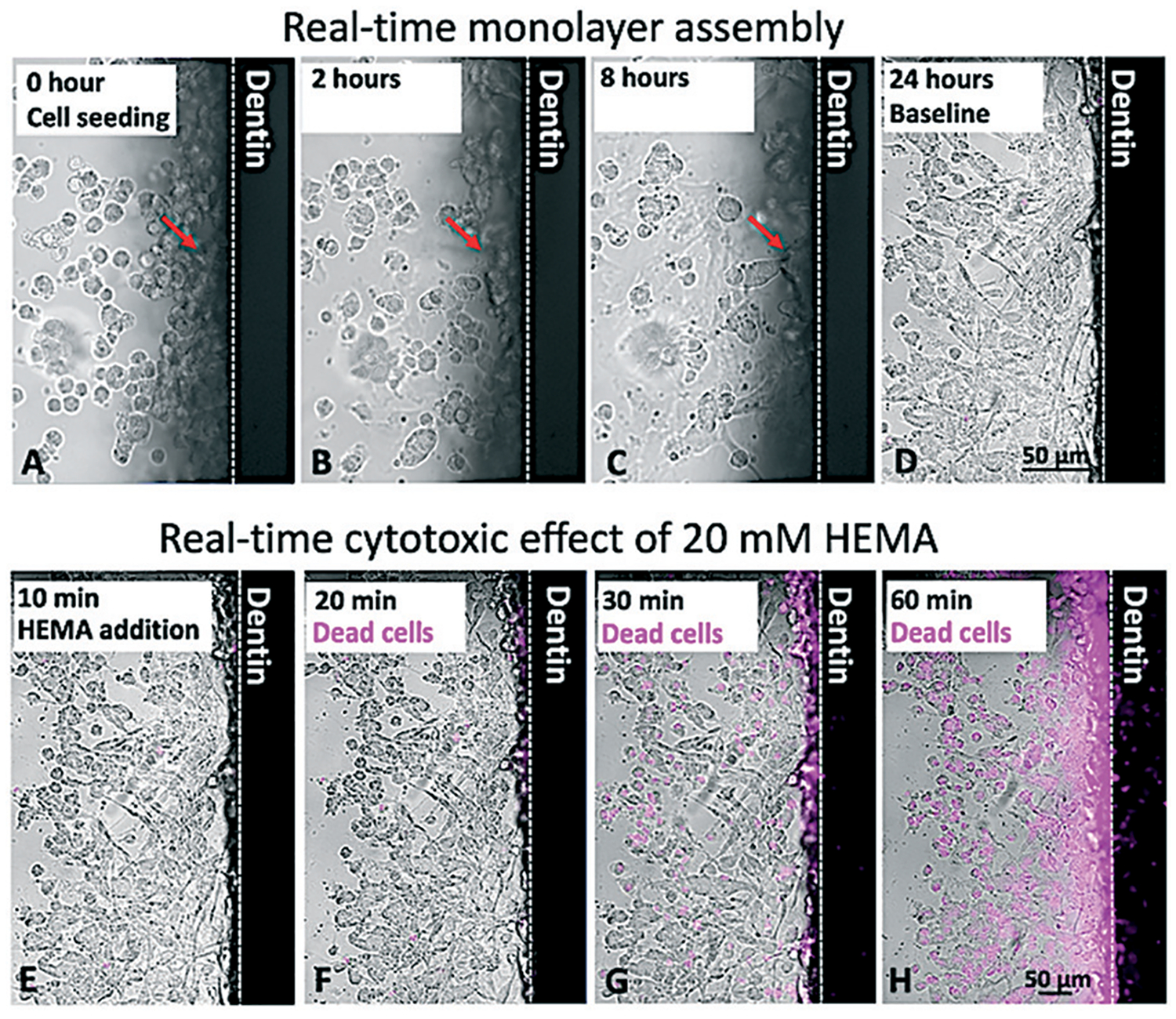
Live-cell imaging on-chip. 105 stem cells from apical papilla were seeded on-chip (A) and spread in 4 hours (B) to 8 hours (C). After 24 hours, the monolayer was completely formed (D). Arrows show the morphological changes of a single cell, which goes from being round and unattached to a spread morphology as it attaches to the dentin wall after 8 hours (A–C, arrow). Cells cultured on-chip after 24 hours were incubated with a DNA dye (Helix NP NIR) and imaged, demonstrating initial cell viability near 100% (E). Next, 20 mM HEMA was added to the ‘cavity side’ of the chip, and after 10 minutes of incubation, cells still showed high viability (F). After 30 minutes, almost 50% of cells had their nuclei stained, which is suggestive of cell death (G). After 60 minutes, nearly all cells were not viable (H).
While the effects of HEMA were visible on-chip, we also sought to demonstrate the feasibility of real-time observation of cell response to specific biomaterials in the device, as the material was being applied. Fig. 3 shows a sequence of images of the device as the dentin fragment and underlying cells were exposed to phosphoric acid. The interaction of the dentin with the acid results in the formation of several small bubbles, which appear to stem from the dentin tubules and intertubular dentin (Fig. 3A–D (Movie S3†). Moreover, on the pulp side of the device, cells in the monolayer appear to contract towards the dentin wall in response to the decreasing pH in the chamber (Fig. 3E–L) (Movie S4†). Of note, these results may not be translatable to current clinical protocols, as the time of exposure to phosphoric acid was substantially longer (86 seconds) than the current protocols, which are typically limited to 20 seconds or less. Nevertheless, these results provide proof-of-principle evidence for the capability of the tooth-on-a-chip to function as a new window of observation into the biological complexity of the tooth–biomaterials interface.
Fig. 3.
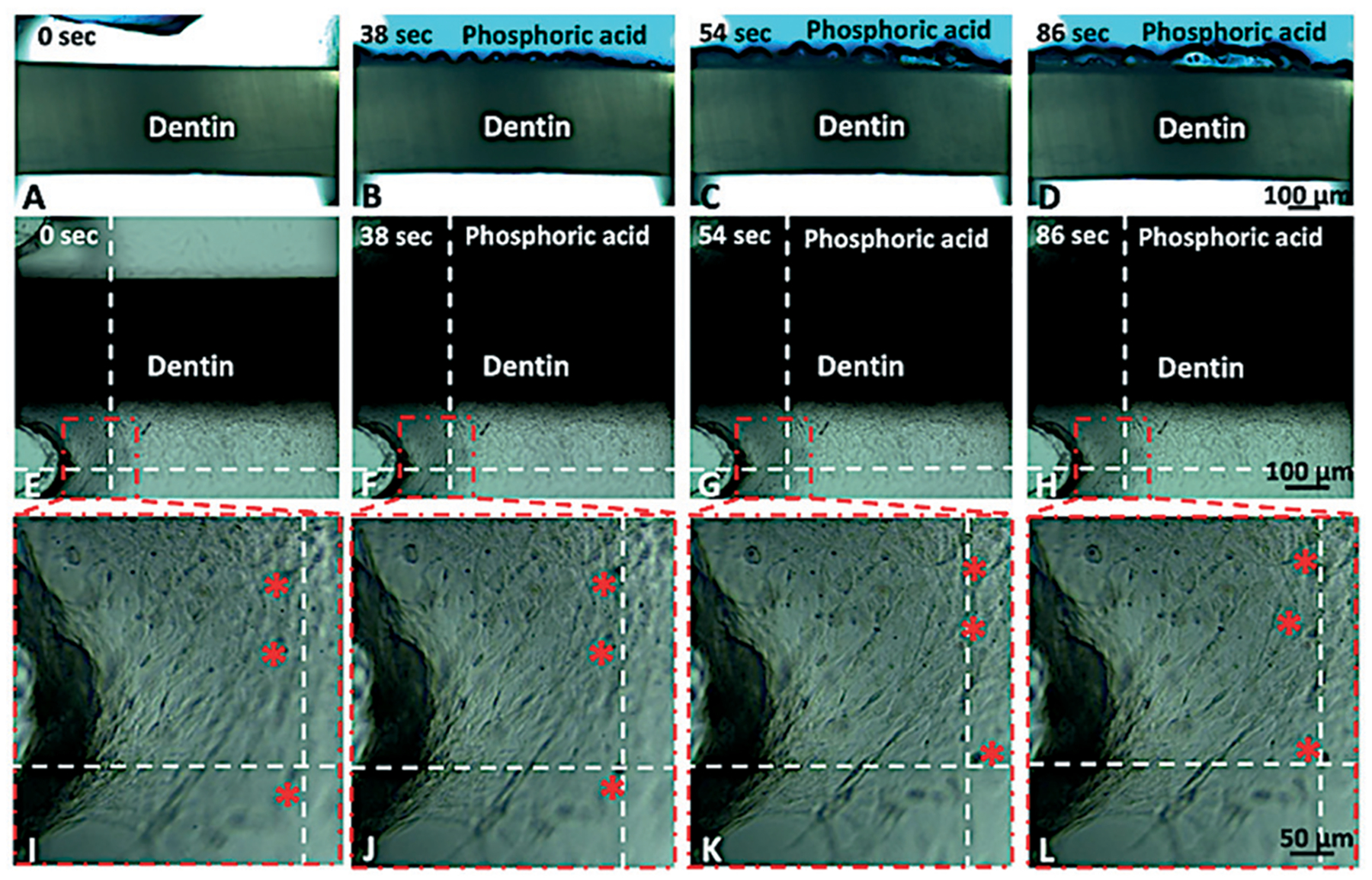
Time-lapse showing the process of dentin acid etching with 35% phosphoric acid for 86 seconds. Figures A–D show live imaging of the acid interacting with the dentin tissue (cells are not seen due to the intensity of light). Figures E–L present the same process with low light intensity to show the cell monolayer as the dentin is acid etched. Figures I–L show what appears to be the contraction of cells in the monolayer as a function of exposure to phosphoric acid. Red asterisks show cells that appear to move relative to the dotted lines, which are provided as reference. Using the scale bar as reference, cells appear to move approximately 50 μm over the course of 86 seconds (ESI,† Movie S4).
Effect of dental materials on SCAP morphology and proliferation
Each tested material elicited apparent cellular injury with as early as 24 h after treatment for both on-chip (Fig. 4A–D) and off-chip (Fig. 4E–H) groups. The monolayer in the HEMA group consisted of poorly connected round cells with pyknotic nuclei (Fig. 4B), while off-chip, HEMA (Fig. 4F) resulted in a visible reduction in cell number relative to the untreated controls. PA etching of the dentin on-chip caused more discrete monolayer disorganization and cytoplasmic injuries (Fig. 4C). Similar cytoplasmic changes were seen off-chip, but, overall, samples had a more visible effect (Fig. 4G). SB treatment on-chip also caused cytoplasmic changes characterized by a dim actin stain and increased intercellular spaces (Fig. 4D), while off-chip samples presented a visible decrease in cell numbers (Fig. 4H). After 7 days, there were striking differences between on-chip (Fig. 4I–L) and off-chip (Fig. 4M–P) samples regarding cell number and morphology, except for the untreated control samples (Fig. 4I and M). Untreated cells on-chip showed little change from day 1 (Fig. 4A and I), while samples treated with HEMA (Fig. 4J), PA (Fig. 4K) and SB (Fig. 4L) had visible morphological changes. Samples cultured off-chip (Fig. 4M) had confluent monolayers when left untreated for 7 days, but had very few cells with faint cytoplasms when treated with HEMA (Fig. 4N), PA (Fig. 4O), and SB (Fig. 4P).
Fig. 4.
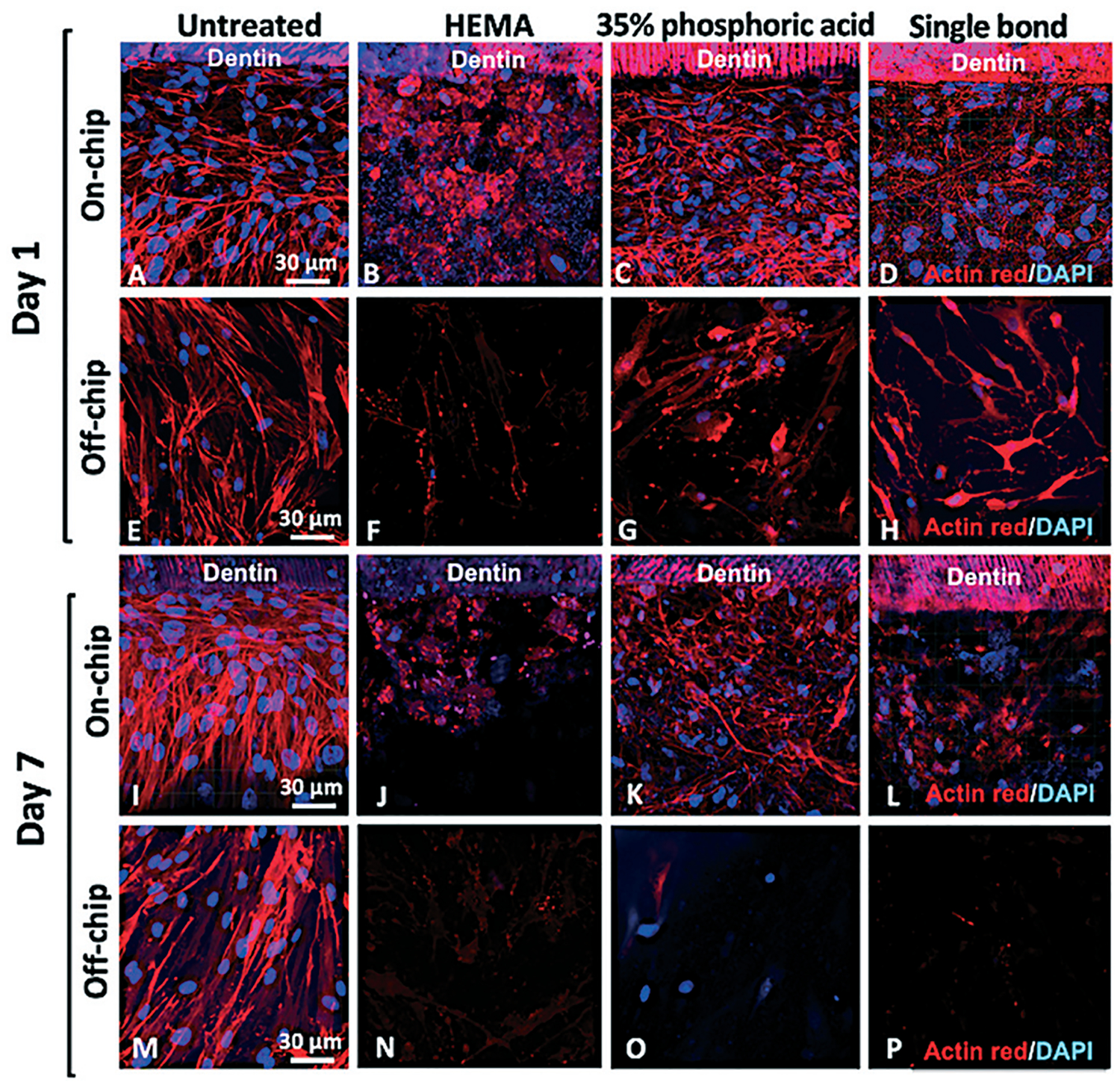
SCAP morphology after biomaterials treatment on-chip and off-chip. On day 1, untreated samples (A) had SCAP monolayers that were morphologically stable for at least 7 days, while (B) HEMA, (C) PA, and (D) SB groups showed significant cell morphology changes and decreased cell numbers on-chip. Cells cultured off-chip on day 1 showed polygonal morphology with oval nuclei in the control group (E), and almost no cells were visible after (F) HEMA treatment. Severe cytoplasmic changes and apparently fragmented nuclei were seen for (G) PA and (H) SB groups. On day 7, (I) untreated cells on-chip showed little change from day 1, while samples treated with (J) HEMA, (K) PA and (L) SB had visible morphological changes. Cells cultured off-chip that were not treated (M) showed confluent monolayers, whereas (N) HEMA, (O) PA, and (P) SB had very few cells with faint cytoplasmic staining.
Quantitatively, cell numbers were significantly higher on-chip compared to off-chip controls after 7 days for all treatments, with both HEMA and SB leading to a significant reduction in cell number relative to untreated controls on day 1 (Fig. 5A), and for all treatment groups on day 7 (Fig. 5B) (p < 0.05).
Fig. 5.
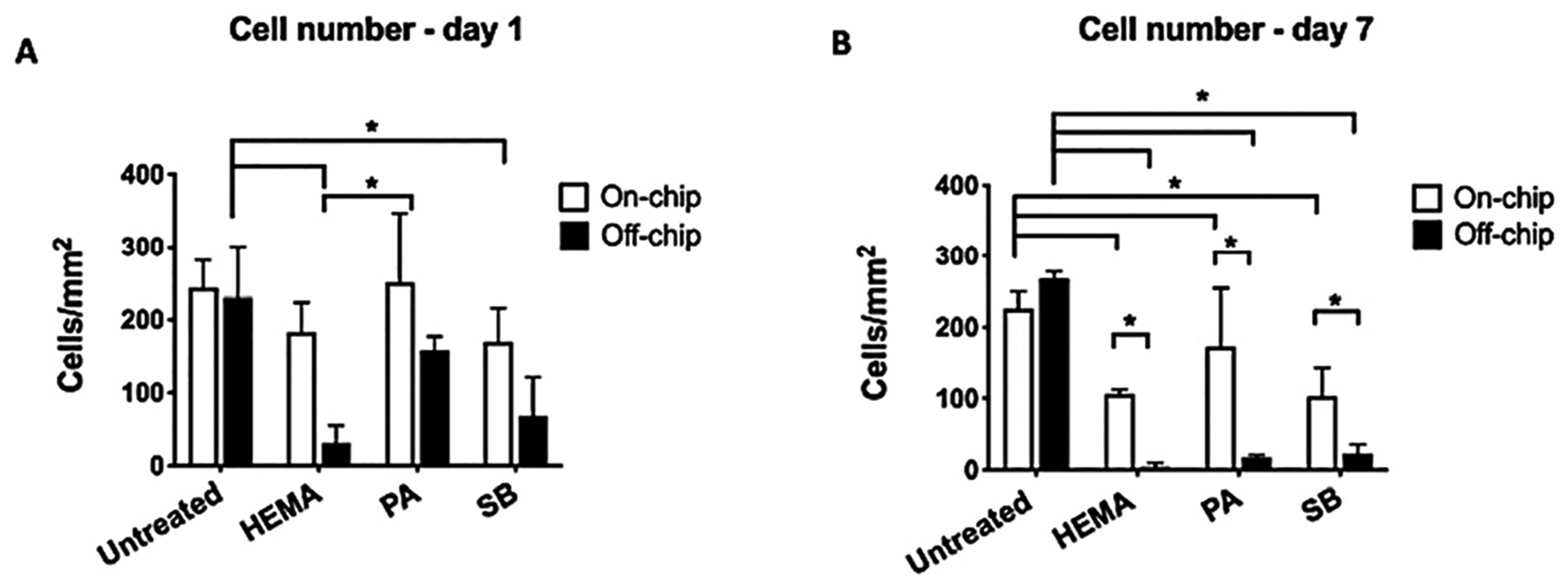
Cell count. (A) Day 1 and (B) day 7 cell count indicated more cells on-chip than off-chip after dental materials application (two-way ANOVA, *p < 0.05).
Metabolic activity
Cells cultured on-chip showed consistently higher metabolic activity than cells cultured off-chip (Fig. 6). On-chip, both untreated samples and samples treated with PA did not present a significant decrease in metabolic activity over time (Fig. 6A and C). Samples treated with HEMA and SB, on the other hand, had a significantly lower metabolic activity only after 7 days (p < 0.05). Off-chip, untreated cells presented a spontaneous decrease in cell metabolism as shortly as 24 h after treatment (Fig. 6A), with HEMA, PA, and SB showing a significant and continuous decrease in metabolic activity from day 1 to day 7 (Fig. 6B–D) (p < 0.05).
Fig. 6.
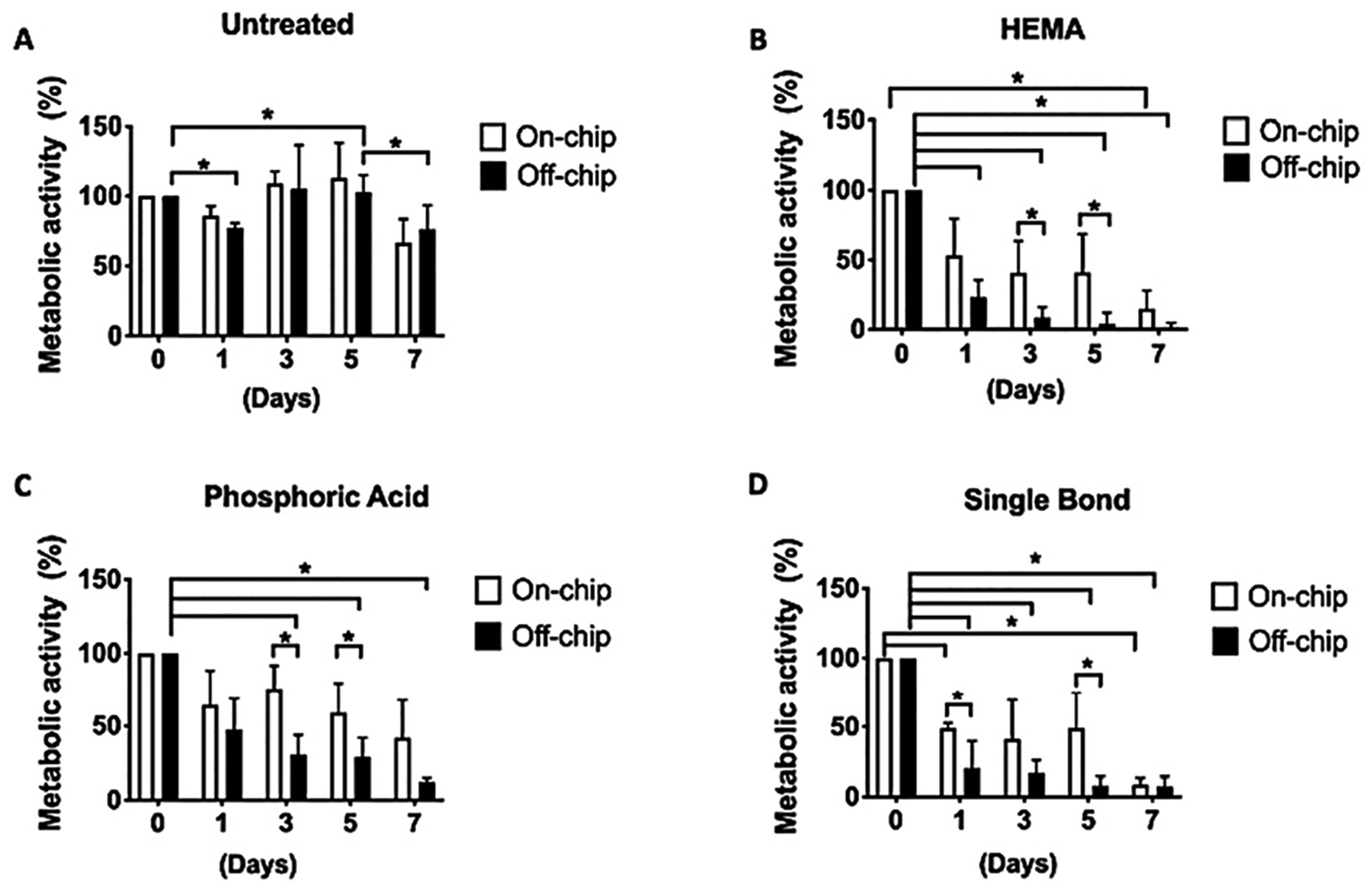
Comparison of metabolic activity between cultures on-chip and off-chip. There was no difference in the metabolic activity of untreated cells on-chip and off-chip (A). Cells cultured on-chip had higher metabolic activity than cells cultured off-chip using ISO standards. HEMA (B) was the most cytotoxic material followed by SB (D) and PA (C) (two-way ANOVA, *p < 0.05).
Gelatinolytic activity
To determine the contribution of cellular gelatinases to hybrid layer degradation, the gelatinolytic activity at the hybrid layer was measured with fluorescein-conjugated gelatin on devices cultured with and without cells. Green fluorescence, indicative of MMP activity, was visible after 24 h and peaked after 48 h for both groups (Fig. 7). Chips seeded with cells had visibly greater fluorescence than chips incubated without cells (Fig. 7E and F). Interestingly, an intense green fluorescence was detected in colocalization with cell cytoplasm, indicating that the conjugated gelatin was also hydrolyzed at the monolayer (Fig. 7H and J). Conversely, chips without cells presented discrete MMP activity at the hybrid layer and inside dentin tubules close to the adhesive (Fig. 7G).
Fig. 7.
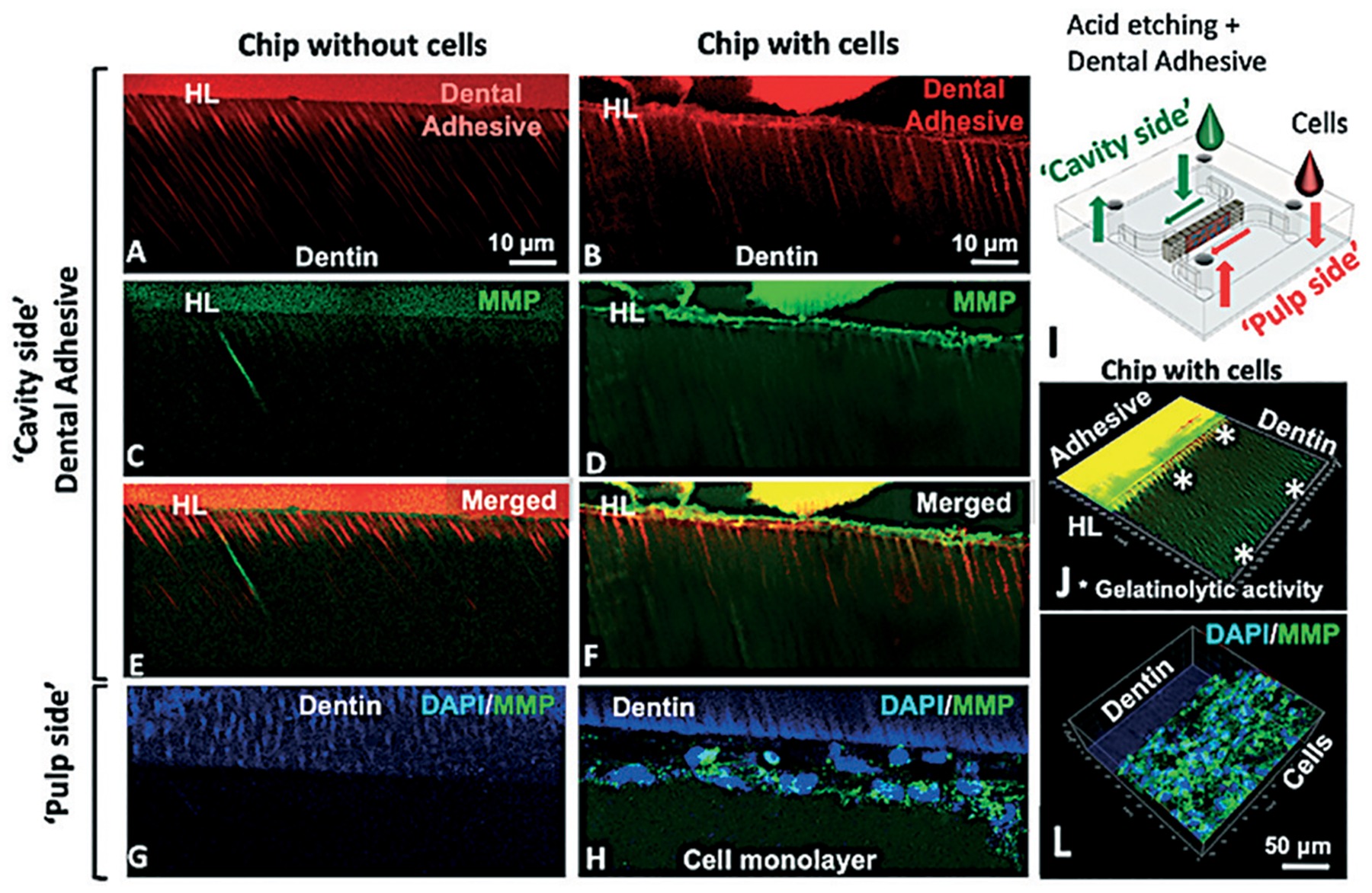
Gelatinolytic activity in the hybrid layer on-chip with and without cells after 48 h. Hybrid layer and resin tags present in chips without (A) and with cells (B). Fluorescein-conjugated gelatin showing gelatinolytic activity in the hybrid layer and dentin tubules (C–F). No evidence of gelatinolytic activity on the ‘pulp side’ for chips without cells (G) while for chips with cells, gelatinolytic activity was co-localized with cell cytoplasm (H). Schematic of the chip (I) and 3D orthogonal view of the adhesive side of a chip with cells (J) showing gelatinolytic activity in the hybrid layer inside dental tubules (*) and on the cell side (L), unquenched gelatin co-localized with cell cytoplasm.
Discussion
One of the key prerequisites for mimicking the dental pulp in vitro is the establishment of a stable cell–dentin interface comprised of an odontoblast-like cell monolayer that interfaces with the dentin wall and responds to stimuli. First, we tried to encapsulate the cells in collagen to promote a 3D environment on-chip; however, with this strategy, cells did not form monolayers adequately, given that the gels prevent homogenous cell–matrix interactions at the cell–tissue interface. We then seeded high cell densities on-chip and observed a complete monolayer formation within 24 hours. Early cell attachment to the dentin was promoted via EDTA pre-treatment, and pre-culturing SCAP in odontogenic medium for 7–10 days before seeding the cells on-chip. This allowed us to emulate the dentin–pulp interface by fabricating consistent monolayers in a short period of time (Fig. 2A–D).
We chose SCAPs because they have a high proliferation rate, potential to differentiate into odontogenic lineage cells in vitro,27,28 and to form a typical dentin–pulp like complex in vivo.29 With the tooth-on-a-chip experiments, the combination of odontogenic medium and the dentin matrix provided cells with a microenvironment comprised of biochemical signals, topography, and stiffness that is favorable to produce monolayers that are stable for several days.
We have extensively optimized the process of cell loading into our chips to enhance the formation of consistent monolayers against the dentin wall, with minimal interference from cells away from the native tissue. Despite these optimization steps, it is natural that cells proliferate and spread to occupy the empty regions of the chamber. As to reduce the influence of cells seeded on glass, our morphologic analyses only considered cells that were located up to 60 μm away from the dentin walls. Future iterations of the proposed microdevice will include both cells seeded in monolayer against the tissue and also cells three-dimensionally embedded within hydrogels adjacent to the cell monolayer, which forms the basis for future work.
We tested commonly used dental materials on-a-chip and compared their cytotoxic response against well-established ISO controls. Dental materials can interact with the dentin–pulp complex both mechanically, chemically, and indirectly via leachates that travel through the dentin tubules and diffuse through the porosity of the intertubular matrix. Therefore, for comprehensive cytotoxicity screenings, ISO determined various approaches of structuring the material–cell interface, such as direct cell contact, extract test, diffusion tests, dentin barrier, and tooth slice models.4,25,30 A key factor to be considered in the test choice is that dentin has a protective effect on dental pulp cells functioning as a source of growth factors,31,32 and as a semipermeable barrier, limiting diffusion of leachates to pulp cells.1,2,33 Thus, more sophisticated in vitro models, such as in vitro pulp chamber, dentin barrier tests, and tooth slices, are preferable.7,8,30,34 The tooth-on-a-chip is designed to include a dentin barrier (with a controllable thickness), while being compatible with direct visualization of biological phenomena in real-time, as well as virtually any standard analyses of molecular, metabolic and genetic function, such as PCR, immunostaining and metabolic activity assays like those performed here, as well as others.11 Considerable advantages of this microfluidic devices are the micro-volumes of reagents to maintain the cells in the system – each chip used 100–300 μL of cell culture medium while other systems using dentin disks use more than 1.5 ml per sample;7 the reduced dimensions of the chip, which enable a better use of human tooth since we could get around 12 dentin fragments from each tooth, being able to fabricate 12 chips with only one tooth, and most importantly the ability of real-time assessment with extensive experimental control.
On-chip tests with HEMA induced cell death, pyknosis, and cytoplasmic shrinkage within 24 h (Fig. 4B) without completely depleting the pulp-like tissue from viability and response, as it is expected clinically. Cellular responses to HEMA are characterized by an increase in reactive oxygen species and mitochondrial damage,35,36 which corroborate our morphological findings on-chip. However, off-chip cells were even more sensitive to HEMA, showing a 10-times decrease in cell number and metabolic activity. Potential explanations for this finding, in addition to the lack of dentin barrier function, are that cells cultured off-chip lack the cell–matrix contact with dentin making them more susceptible to external injuries.4
Etching dentin with 35% PA for 15 seconds proved to be harmful to the cell monolayer on-chip, but not enough to fully disrupt it in 24 h. Moreover, after 7 days, the monolayer was almost completely reconstituted (Fig. 4L). Again, this is more consistent with clinical outcomes, where acid-etching elicits the solubilization of dentin matrix components that are important for pulp regeneration31,32,37 instead of causing odontoblast death. Conversely, off-chip experiments showed that the same 15 s of acid application (as recommended by ISO), elicited 90% of cell death after 7 days (Fig. 5) and a decrease in metabolic activity (Fig. 6C), indicating that cells off-chip were far more sensitive to acidic conditions. It seems that results obtained off-chip overestimated PA cytotoxicity. We measured the pH on the cell side before and after adding the phosphoric acid on the cavity side, and we found out that the average pH of the cell medium dropped from 6.5 to 4.5 in as little as 30 s (ESI† Fig. S4), which may be the reason of the changes that we saw in cell morphology. Moreover, even though we have tested the chips for leakage before using them, and have consistently detected no leakage problems, we cannot rule out the fact that the acidic medium may have facilitated the movement of minute amounts of low pH medium around the corners of the dentin.
Next, we replicated the clinical application of a multipurpose dental adhesive system including acid etching, rising, drying the dentin, and photopolymerizing the adhesive, following the same steps and times recommended by the manufacturer. Our results indicate that in 24 h the monolayer is partially disrupted, showing visible cytoplasmic changes (Fig. 4), while on day 7 the monolayer had disappeared, and cell numbers had decreased to 20–30 cell per mm2, suggesting the potential toxic effect of leachates over time. Likewise, the on-chip morphological results denote different cellular cytotoxicity mechanisms for HEMA and Single Bond, partially explained by the fact that even though HEMA is a significant component of the Single Bond adhesive composition, HEMA is a freely soluble monomer with a fast action, while Single Bond adhesive is a monomer blend that was photopolymerized, thus releasing potentially toxic leachates much more slowly over time. Cell morphology changes as a consequence of these physical characteristics of the biomaterials, and in the context of the pulp–dentin interface, have remained elusive so far. Additionally, it is possible that other monomers in the composition of Single Bond, i.e., UDMA and Bis-GMA may be less soluble than HEMA, in which case they diffuse more slowly (or not at all) through the dentin tubules to affect the cells.1,2 This same finding could not be reproduced off-chip, and on day 1, Single Bond application proved to be highly cytotoxic to cells, leading to nuclei changes followed by more than 90% of cell death on day 7.
Lastly, we performed a functional assay with the tooth-on-a-chip by investigating the role of cellular MMPs in hybrid layer degradation. The release of endogenous dentin MMPs that are bound to collagen fibrils in mineralized dentin and become exposed after acid etching procedures has been implicated in the HL enzymatic degradation, eventually deteriorating the resin–dentin bonding interface and contributing to the failure of dental restorations.26,38,39 Studies conducted with quenched fluorescein-conjugated gelatin in dentin slices or dentin extracts show gelatinolytic activity in the hybrid layer.26,38,40 However, the cellular role in MMP production has been less explored. In 2009, Lehmann et al. provided evidence that self-etching adhesives can stimulate odontoblasts to produce MMP2.41 Our system is uniquely capable of culturing cells, and investigating gelatinolytic activity while simultaneously imaging the hybrid layer as formed by adhesive systems. This enables one to assess the collective effect of proteases in HL degradation, including both cell- and matrix-derived proteases, which is more meaningful to the proteolytic activity occurring in vivo. Here, we fabricated hybrid layers in devices with and without cells, simulating a clinical dental protocol, with all the intermediate steps observed in a clinical setting. Chips with cells presented more gelatinolytic activity in the HL, inside the intratubular dentin, and co-localized with the cell cytoplasm (Fig. 7), suggesting that cells actively participate in the degradation of the HL.
Organs-on-a-chip are bioengineering devices that attempt to reconstruct key functions of tissues and organs that cannot be modeled using other existing cell culture systems. The main challenge of an organ-on-chip is to recapitulate in vivo physiology of at least a subset of functions and then to progressively add additional functions over time to speed up the development of new materials, drugs, and disease models.42 Similarly to what has been done with other organ-on-a-chip models,14,43 the tooth on-a-chip opens a wide range of systematic investigations of the dentin–pulp tissue. One of the novelties of the chip is the possibility to treat the dentin using the same protocols used in the dental clinic and to image live-cell morphological and metabolic changes concomitantly. The time lapses of monolayer formation and disassembling due to HEMA solubilization through dentin tubules, as well as the dentin acid etching and hybrid layer formation, demonstrate the tooth-on-a-chip as a window to track pulp cellular and subcellular responses in an environment more consistent with the in vivo conditions. One limitation of this study, however, is that it is not feasible to cover all aspects of the microfluidic device at once. Future iterations of the device, which are currently being developed, include the addition of controllable flow, built-in biosensors and variable designs for different materials systems. Additionally, critical biological functionality will require the addition of functional capillaries, immune cells, and innervation on the ‘pulp side’, and microbiome and salivary flow on the ‘cavity side’. This forms the basis for future studies.
Conclusion
In conclusion, the tooth-on-a-chip is a novel platform that replicates near-physiologic conditions of the pulp–dentin interface and enables live-cell imaging to study dental pulp cell response to biomaterials.
Supplementary Material
Acknowledgements
We wish to acknowledge Dr. Anibal Diogenes (University of Texas) for the donation of SCAPs. We acknowledge expert technical assistance from Dr. Crystal Chaw in the Advanced Light Microscopy Core at the Jungers Center at Oregon Health & Science University. This project was supported by funding from the National Institute of Dental and Craniofacial Research (R01DE026170 and 3R01DE026170-03S1 to LEB), the Oregon Clinical & Translational Research Institute (OCTRI) – Biomedical Innovation Program (BIP), the Innovation in Oral Care Awards sponsored by GlaxoSmithKline (GSK), International Association for Dental Research (IADR), the Michigan-Pittsburgh-Wyss Resource Consortium – Regenerative Medicine Resource Center (MPW-RM), the OHSU Fellowship for Diversity and Inclusion in Research (OHSU-OFDIR to CMF).
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9lc00915a
Conflicts of interest
There are no conflicts to declare.
References
- 1.Bertassoni LE, Orgel JP, Antipova O and Swain MV, Acta Biomater, 2012, 8, 2419–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertassoni LE, Dent. Mater, 2017, 33, 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldas IP, Alves GG, Barbosa IB, Scelza P, de Noronha F and Scelza MZ, Dent. Mater, 2019, 35, 195–205. [DOI] [PubMed] [Google Scholar]
- 4.Schmalz G and Galler KM, Dent. Mater, 2017, 33, 382–393. [DOI] [PubMed] [Google Scholar]
- 5.Chaves CA, Machado AL, Vergani CE, de Souza RF and Giampaolo ET, J. Prosthet. Dent, 2012, 107, 114–127. [DOI] [PubMed] [Google Scholar]
- 6.Hume WR, J. Dent. Res, 1984, 63, 881–884. [DOI] [PubMed] [Google Scholar]
- 7.Hanks CT, Craig RG, Diehl ML and Pashley DH, J. Oral Pathol, 1988, 17, 396–403. [DOI] [PubMed] [Google Scholar]
- 8.Schmalz G, Schuster U, Nuetzel K and Schweikl H, J. Endod, 1999, 25, 24–29. [DOI] [PubMed] [Google Scholar]
- 9.Murray PE, Lumley PJ, Ross HF and Smith AJ, Biomaterials, 2000, 21, 1711–1721. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri J, Laurent P and About I, J. Endod, 2014, 40, 1846–1854. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia SN and Ingber DE, Nat. Biotechnol, 2014, 32, 760–772. [DOI] [PubMed] [Google Scholar]
- 12.Esch EW, Bahinski A and Huh D, Nat. Rev. Drug Discovery, 2015, 14, 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY and Ingber DE, Science, 2010, 328, 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HJ, Huh D, Hamilton G and Ingber DE, Lab Chip, 2012, 12, 2165–2174. [DOI] [PubMed] [Google Scholar]
- 15.Torisawa YS, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ and Ingber DE, Nat. Methods, 2014, 11, 663–669. [DOI] [PubMed] [Google Scholar]
- 16.Prot JM, Bunescu A, Elena-Herrmann B, Aninat C, Snouber LC, Griscom L, Razan F, Bois FY, Legallais C, Brochot C, Corlu A, Dumas ME and Leclerc E, Toxicol. Appl. Pharmacol, 2012, 259, 270–280. [DOI] [PubMed] [Google Scholar]
- 17.Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA and Ingber DE, Sci. Transl. Med, 2012, 4, 159ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin MK, Kim SK and Jung H, Lab Chip, 2011, 11, 3880–3887. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YS, Davoudi F, Walch P, Manbachi A, Luo X, Dell’Erba V, Miri AK, Albadawi H, Arneri A, Li X, Wang X, Dokmeci MR, Khademhosseini A and Oklu R, Lab Chip, 2016, 16, 4097–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun W, Luo Z, Lee J, Kim HJ, Lee K, Tebon P, Feng Y, Dokmeci MR, Sengupta S and Khademhosseini A, Adv. Healthcare Mater, 2019, 8, e1900754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song YS, Uchida A, Piraino H, Delouise L, Ovitt L and Benoit D, Engineered Salivary Gland Tissue Chips, IADR/AADR/CADR General Session, 2019, Available at: https://iadr.abstractarchives.com/abstract/19iags-3182992/engineered-salivary-gland-tissue-chips. [Google Scholar]
- 22.Lam RH, Cui X, Guo W and Thorsen T, Lab Chip, 2016, 16, 1652–1662. [DOI] [PubMed] [Google Scholar]
- 23.Rahimi C, Rahimi B, Padova D, Rooholghodos SA, Bienek DR, Luo X, Kaufman G and Raub CB, Biomicrofluidics, 2018, 12, 054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu L, Zhang H, Liu Y, Wang Y, Li A, Liu R, Zou R and Yang Q, ACS Biomater. Sci. Eng, 2019, 5(9), 4844–4851. [DOI] [PubMed] [Google Scholar]
- 25.ISO, in International Standard. 10993 - Biological Evaluation of Medical Devices, ed. ISO, ISO, Switzerland, 3rd edn, 2009, ch. 5. [Google Scholar]
- 26.Mazzoni A, Nascimento FD, Carrilho M, Tersariol I, Papa V, Tjaderhane L, Di Lenarda R, Tay FR, Pashley DH and Breschi L, J. Dent. Res, 2012, 91, 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AA, Takimoto K, Wealleans J and Diogenes A, J. Endod, 2018, 44, 599–603. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Wang J, Deng F, Huang E, Yan Z, Wang Z, Deng Y, Zhang Q, Zhang Z, Ye J, Qiao M, Li R, Wang J, Wei Q, Zhou G, Luu HH, Haydon RC, He TC and Deng F, Biomaterials, 2015, 39, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S and Shi S, PLoS One, 2006, 1, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ISO/ADA, in Dentistry - Evaluation of bioocompatibility of medical devices used in dentistry, ed. I. O. F. Standardization, ISO, Geneve, Switzerland, 2008. [Google Scholar]
- 31.Salehi S, Cooper P, Smith A and Ferracane J, Dent. Mater, 2016, 32, 334–342. [DOI] [PubMed] [Google Scholar]
- 32.Ferracane JL, Cooper PR and Smith AJ, J. Adhes. Dent, 2013, 15, 407–412. [DOI] [PubMed] [Google Scholar]
- 33.Hamid A and Hume WR, J. Oral Rehabil, 1997, 24, 20–25. [DOI] [PubMed] [Google Scholar]
- 34.Schmalz G, Schuster U, Thonemann B, Barth M and Esterbauer S, J. Endod, 2001, 27, 96–102. [DOI] [PubMed] [Google Scholar]
- 35.Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P and Geurtsen W, Dent. Mater, 2011, 27, 608–617. [DOI] [PubMed] [Google Scholar]
- 36.Spagnuolo G, D’Anto V, Valletta R, Strisciuglio C, Schmalz G, Schweikl H and Rengo S, J. Endod, 2008, 34, 684–688. [DOI] [PubMed] [Google Scholar]
- 37.Athirasala A, Lins F, Tahayeri A, Hinds M, Smith AJ, Sedgley C, Ferracane J and Bertassoni LE, Sci. Rep, 2017, 7, 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjaderhane L, Visintini E, Cadenaro M, Tay FR, De Stefano Dorigo E and Pashley DH, Dent. Mater, 2010, 26, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tjaderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho M, Carvalho RM, Tay FR and Pashley DH, Dent. Mater, 2013, 29, 999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu L, Mazzoni A, Gou Y, Pucci C, Breschi L, Pashley DH, Niu L and Tay FR, J. Dent. Res, 2018, 97, 409–415. [DOI] [PubMed] [Google Scholar]
- 41.Lehmann N, Debret R, Romeas A, Magloire H, Degrange M, Bleicher F, Sommer P and Seux D, J. Dent. Res, 2009, 88, 77–82. [DOI] [PubMed] [Google Scholar]
- 42.Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM and Ingber DE, Nat. Biomed. Eng, 2017, 1(5), 0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HJ, Li H, Collins JJ and Ingber DE, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, E7–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


