Abstract
Background
The use of waterpipe tobacco smoking (WTS) is on the rise throughout the world, especially among young people and even athletes. There is a belief among consumers that exercise prevents the harmful effects of hookah smoke on the body. We examined this belief by evaluation of lung injury following to concurrent WTS and swimming endurance training in male Wistar rats.
Methods
Animals were randomly divided to sedentary control (CTL) group, exercise training group (Ex group), sedentary WTS (S) group, and exercise plus WTS (S + Ex) group.
Findings
8 weeks of WTS was associated with significant increase in serum level of cotinine, lung damage, reduction in alveolar number AN/SA (mm2) and increase in malondialdehyde (MDA) level of lung tissue. Combination of exercise with WTS significantly decreased these negative effects; however, it could not fully protect the lung from smoking damage. Waterpipe smoking (WPS) also significantly increased the pro-inflammatory cytokines of lung tissue such as tumor necrosis factor alpha (TNF-α) (P < 0.001), interleukin 1 beta (IL-1β) (P < 0.010), and IL-6 (P < 0.050) in comparison with CTL group. Exercise training to some degree reduced the levels of pro-inflammatory cytokines and increased the level of IL-10 as an anti-inflammatory IL and glutathione peroxidase (GPX) activity in animals exposed to WTS.
Conclusion
It is suggested that combination of mild to moderate exercise with WTS may attenuate the hookah smoking-induced lung damage. This effect partly is mediated through balancing of pro/anti-inflammatory and redox systems.
Keywords: Water pipe smoking, Lung injury, Swimming, Interleukins, Antioxidants
Introduction
It is reported that tobacco consumption kills up to half of its users and is responsible for the death of 8 million people in each year.1 Hookah (shisha, ghalyan, narghile, hubble bubble) tobacco smoking is a traditional and non-cigarette form of tobacco use becoming more prevalent worldwide, especially among youth.2 In waterpipe tobacco smoking (WTS) or waterpipe smoking (WPS) method, tobacco is burned by hot charcoal and their combined smoke is passed from a bowl before inhalation by user. The details were explained previously.3 Despite the existing evidence in relation to more severe complications of WTS than smoking cigarettes, unfortunately because of the lack of negative social norm against this type of smoking and also exotic, social, and group nature of this behavior, the WTS is growing fast among the youth compared to cigarette smoking.4 The prevalence of hookah smoking consumption in Middle Eastern societies is estimated at 6.0% to 43.8% of their population, especially among adolescents.5-7 It is reported that in general, the prevalence among adults was highest in the Eastern Mediterranean, and among youth was about equal between Eastern Mediterranean and European regions.8 WTS is associated with pulmonary inflammation, oxidative stress, chronic pulmonary dysfunction, cardiovascular and hematological impairments, and lung, gastrointestinal (GI), and bladder malignancies.4
Some users believe that using hookah with exercise can prevent its negative effects. Unfortunately, based on this unconfirmed belief and despite the lack of adequate scientific information regarding the difference of hookah side effects in athletes compared to non-athletes, we are witnessing an increase in hookah smoke consumption in the athletes' community.9 To examine this attitude, we investigated the effects of concurrent hookah smoking and endurance training on lung histology and also some cytokines and antioxidants levels of lung tissue in rat.
Methods
Study was conducted with considering the principles of the national guideline for using laboratory animals and protocol was approved by the Ethics Committee of Kerman University of Medical Sciences, Kerman, Iran (permission no. EC/96-10/KNRC). Materials were purchased from different companies as below: Cotinine kit (Sigma-Aldrich, UK), thiopental sodium (Sandoz, Austria), double apple flavor tobacco (Al Fakher, Ajman, UAE), lipid peroxidation assay kit (Navand, Iran), rat interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), IL-10, and IL-1β enzyme-linked immunosorbent assay (ELISA) kits (Hangzhou Eastbiopharm Co., LTD, China), rat superoxide dismutase (SOD) and glutathione peroxidase (GPX) ELISA kits (Shanghai Crystal Day Biotech Co., China).
Twenty-four male Wistar rats in the weight range of 200-250 g were randomly divided to sedentary control (CTL) group, exercise training group (Ex group) who practiced swimming for 8 weeks, sedentary smoking (S) group which was exposed to WPS (30 minute/day) during study, and exercise plus WPS (S + Ex) group which received smoking plus exercise training. The animals had free access to water and food, and 12-hour light/12-hour dark cycles were applied during study.
Smoking protocol: A chamber with dimensions of 50 (length) * 30 (width) * 12 (height) cm was prepared in order to put the animals there for smoking inhalation. The details of smoking machine operation method was described previously.3 The cycle for WPS ventilation was two thirty consecutive seconds, which included 30 seconds release of smoke into the chamber, then smoke valve was closed and fresh air flowed to wash the chamber and the animals inhaled the fresh air for 30 seconds. This cycle was repeated 30 times (30 times × 1 minute exposure = 30 minutes exposure session) for S and S + Ex groups daily. The level of carbon monoxide (CO) concentration was maintained at similar levels [mean ± standard deviation (SD): 886 ± 103 parts per million (ppm)] during all of the exposure sessions by Testo 310 (Germany) CO measurement device. Sedentary and Ex groups also were placed in the chamber for 30 minutes to simulate the environment stress.3
Exercise training: The exercise model was swimming and was performed for 5 days/week with low to moderate severity based on previous studies.3,10 In brief, training exercise was done in a pool with 50 (depth) and 120 (length) cm containing warm water (30-32 °C). It began with 20 minutes on the first day and was enhanced to 10 more minutes every following day, so the final period was 60 minutes at the end of first week. During the next weeks, the exercise duration was constant (60 minutes/day); however, animal was carrying a caudal dumbbell equivalent to 2% of its body weight when swimming. The dumbbell weight gradually increased to 5% of body weight on the sixth week and then the weight remained constant until the end of the study. The simulation of the water stress also was imposed for sedentary rats.11
Blood sampling and cotinine measurement: 24 hours after the end of the smoking and exercise program, under deep anesthesia by using sodium thiopental [50 mg/kg, intraperitoneal (i.p.)], blood sampling was done. Then the serum was separated and cotinine level was measured by ELISA kit according to its manufacturer’s instructions.
Measurement of cytokines, malondialdehyde (MDA), and antioxidants: A part of left lung was removed and rinsed with ice-cold phosphate-buffered saline (PBS) (pH: 7.4) for molecular measurement. For assessments of TNF-α and ILs (IL-1β, IL-10, and IL-6), 40 mg of lung tissue was homogenized on ice and centrifuged at 3000 revolutions per minute (rpm) for 20 minutes at 4 °C and 60 μl of the supernatants were utilized for additional analysis. The concentration of total protein was measured by Bradford method. Quantitative assessments of cytokines concentrations were measured using their ELISA kits based on manufacturer’s instructions. SOD and GPX activity was measured according to the manufacturer’s instructions. Briefly, 50 mg of lung tissue was homogenized on ice in proper amount of PBS (pH = 7.4). The sample was then centrifuged at 3000 rpm for 20 minutes at 4 °C. 40 μl of the supernatant was used to measure the activity of enzymes. The absorbance of each sample was read under 450 nm wavelengths. The level of lung MDA, as an index of lipid peroxidation, was measured based on the concentration of thiobarbituric acid reactive substances (TBARS). 50-100 mg of lung sample was homogenized using 1.5% potassium chloride solution. The homogenate was centrifuged at 1200 rpm for 10 minutes. 250 μl of the supernatant was used to measure it according to the kit's instructions.
Lung histopathological scoring and alveolar number (AN): The lung of each animal was harvested and fixed in 10% neutral formalin for 24 hours. Then, under dehydration by increasing concentrations of ethanol, the lung tissue was cleared with xylene and embedded in paraffin. 5-μm sections were prepared from paraffin blocks and stained with haematoxylin and eosin (H&E). The stained sections were blindly evaluated by the pathologist using light microscopy. H&E-stained slides were scored absent (0), minimal (1), mild (2), moderate (3), and severe (4) lesions for the following parameters: peribronchial inflammation, inflammatory cell infiltration (ICI), expansion of the alveolar interstitial space, enlargement of airway, destruction of septum of alveoli, congestion, and fibrosis. The total lung score was expressed as the sum of the scores for each parameter. Mean AN (MAN) as an indicator for density of alveoli was calculated by MAN = AN/surface area (SA) (mm2) formula according to AN in each field of view and SA of the field.12
The normality of data was assessed using the Shapiro-Wilk test and the homoscedasticity between groups was determined using Levene's test. The differences among quantitative data were assessed using one-way analysis of variance (ANOVA) and Tukey post-hoc tests. Differences of pathological scores were analyzed by Kruskal-Wallis test and Mann-Whitney test. P < 0.050 was considered statistically significant.
Results
Cotinine: Serum cotinine level as a reliable marker for smoking status significantly increased in WTS groups (P < 0.001 vs. non-smoking groups). Smoking along with exercise training was associated with lower level of serum cotinine (P < 0.010 vs. S group) but still was higher in comparison with non-smoking groups (P < 0.001) (Figure 1).
Figure 1.
The values of serum cotinine in different animal groups Values are presented as mean ± standard error (SE), n = 6-7 in each group, ***P < 0.001 versus control and exercise groups; **P < 0.010 versus waterpipe smoking (WPS) groups; #P < 001 versus other groups CTL: Control group; S: Sedentary waterpipe tobacco smoking group; Ex: Exercise training group, S + Ex: Waterpipe tobacco smoking + exercise training group
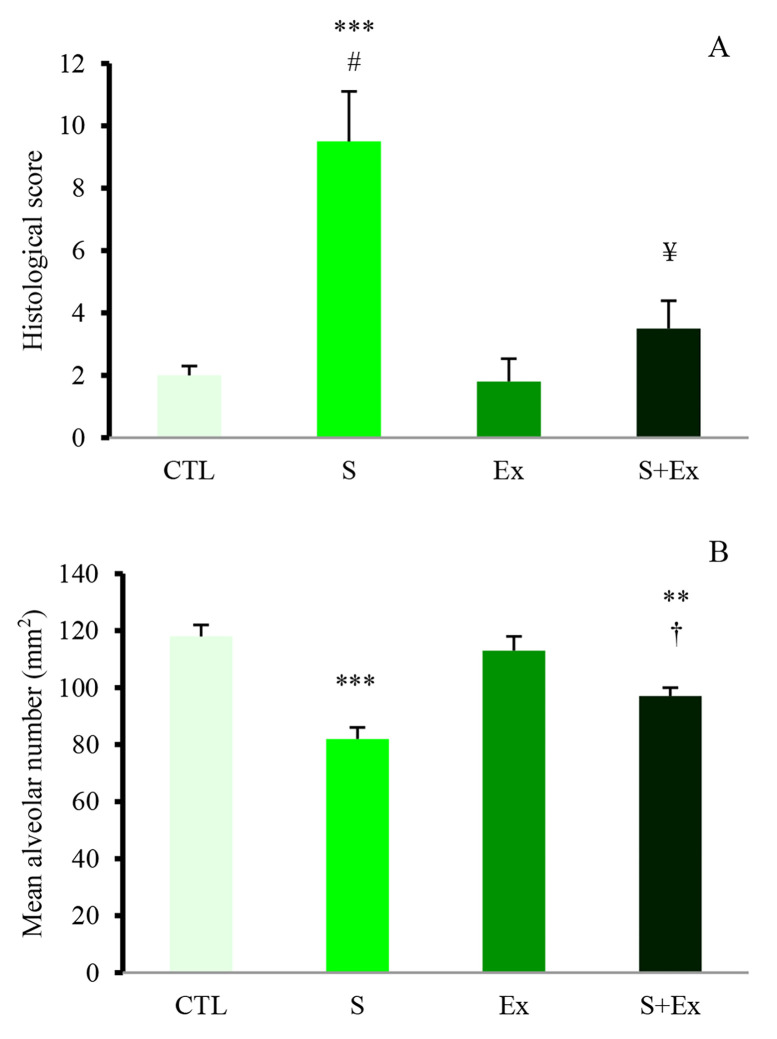
Histopathology: semi-quantitative histological scoring of the lungs demonstrated moderate to severe inflammation, interstitial leukocytes infiltration, congestion, destruction of septum of alveoli, expansion of the alveolar interstitial space, and fibrosis in WPS group in comparison with CTL and Ex groups (P < 0.001). Combination of exercise with WPS significantly decreased the negative effect of smoking (P < 0.010); however, there was still some degree of damage in S + Ex group (P < 0.050 vs. CTL group) (Figures 2, A and 3).
Figure 2.
Lung histopathological score in different experimental groups The total score was expressed as the sum of the scores for peribronchial inflammation, inflammatory cell infiltration (ICI), interalveolar septal thickness, enlargement of airway, destruction of septum of alveoli, congestion, and fibrosis. Values are presented as mean ± standard error (SE), n = 5-6 in each group CTL: Control group; S: Sedentary waterpipe tobacco smoking group; Ex: Exercise training group; S + Ex: Waterpipe tobacco smoking + exercise training group; ***P < 0.001 vs. control and exercise groups; #P < 0.010 vs. Ex + S group; ¥P < 0.050 vs. control and exercise groups (A); Mean alveolar number (MAN) as an indicator for density of alveoli in each microscopic field of view; ***P < 0.001 vs. control and exercise groups; **P < 0.010 vs. control and exercise groups; †P < 0.050 vs. S group (B)
Figure 3.
Microscopic fields of lung tissue in different groups at the end of study a) The normal lung tissue in control group, b) The lung tissue of waterpipe tobacco smoking (WTS) group. Severe extensive intercellular and interalveolar leukocytes infiltration, decreasing in alveolar number (AN), increasing in alveolar septal thickness and expansion of the alveolar interstitial space and congestion are obvious, c) The field of lung tissue of exercise training (Ex) group with mild congestion but unremarkable change, d) Lung tissue with congestion, mild expansion of interstitial space with focal mild to moderate inflammatory cell infiltration (ICI) in group exposed to both waterpipe smoking (WPS) and exercise training. Histopathologic examination was performed under a light microscopy at a magnification of × 400.
Alveolar density was estimated by calculating MAN in a microscopic vision of the lung tissue. WPS was associated with reduction in AN/SA (mm2) when compared with CTL and Ex groups (P < 0.001) and exercise training attenuated this effect (P < 0.050 vs. S + Ex group). However, there was a significant difference in MAN between CTL and S + Ex groups of rats (P < 0.010) (Figures 2, b and 3).
Cytokines: WPS significantly increased the pro-inflammatory cytokines of lung tissue including TNF-α (P < 0.001), IL-1β (P < 0.010), and IL-6 (P < 0.050) in comparison with CTL group. Exercise training mitigates the levels of pro-inflammatory cytokines in animals exposed to WTS. However, the levels of TNF-α and IL-1β still were higher in S + Ex group than CTL group (P < 0.010 and P < 0.050, respectively) (Figures 4, A, B, C). On the other hand, exercise alone and along with smoking increased the level of IL-10 as an anti-inflammatory interleukin (P < 0.059 vs. CTL and S groups, respectively) (Figure 4, D).
Figure 4.
The effect of 8 weeks of waterpipe smoking (WPS) and/or swimming exercise on the levels of tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), IL-6, and IL-10 in animal groups. Values are presented as mean ± standard error (SE), n = 6 in each group CTL: Control group; S: Sedentary waterpipe tobacco smoking group; Ex: Exercise training group; S + Ex: Waterpipe tobacco smoking + exercise training group. A) ***P < 0.001; **P < 0.010; *P < 0.050 vs. CTL group. B) **P < 0.010; *P < 0.050 vs. CTL group. C) *P < 0.050 vs. CTL and Ex groups. D) *P < 0.050 vs. CTL and S groups; ¥P < 0.050 vs. S group
MDA and antioxidants: Smoking or exercise alone had no significant effect on GPX and SOD activities of lung tissue; however, their combination increased the GPX activity in S + Ex group (P < 0.050 vs. CTL group) (Figures 5, A, B). On the other hand, MDA significantly increased in animals exposed to hookah smoke (P < 0.050). Concomitant exercise with WTS prevented the increasing effect of smoking on MDA level of lung tissue in S + Ex group (Figure 5, C).
Figure 5.
The effect of 8 weeks of water pipe smoking (WPS) and/or swimming exercise on the (A) superoxide dismutase (SOD) and (B) glutathione peroxidase (GPX) activities and the level of (C) malondialdehyde (MDA) in animal groups. Values are presented as mean ± standard error (SE), n = 6 in each group CTL: Control group; S: Sedentary waterpipe tobacco smoking group; Ex: Exercise training group; S + Ex: Waterpipe tobacco smoking + exercise training group. B) *P < 0.050 vs. CTL group; C) *P < 0.050 vs. all other groups
Discussion
Present study was conducted to examine whether exercise has any influence on the harmful effects of WTS on the lung tissue. The results indicated that 4 weeks of shisha smoking significantly increased the serum level of cotinine and induced lung lesions as decreasing in ANs, increasing congestion, inflammation, leukocytes infiltration, expansion of the alveolar interstitial space, fibrosis, and other injuries in rats. Smoking increased the pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 and decreased the IL-10, an anti-inflammatory interleukin, and also increased the MDA, a lipid peroxidation index in lung tissue.
Cotinine is an alkaloid of tobacco and is the predominant metabolic of nicotine.13 It is a biomarker for exposure to tobacco smoke and its increasing in smoking groups in present study confirmed the method.
Consistent with our findings, there is sufficient evidence that confirms the damaging effect of WPS on lung tissue. WTS contains similar toxins to cigarettes toxins; however, it nearly has a 4-fold more CO exposure and 56-fold more inhaled smoke volume than cigarette smoking.10 WPS can suppress the pulmonary function which can lead to an increasing risk of chronic obstructive pulmonary diseases (COPD).14 Compared with non-smokers, Waterpipe smokers have more coughing with sputum expectoration, lower lung diffusing capacity, abnormal epithelial lining fluid metabolome profile, reduced amounts of small airway epithelial ciliated and basal cells, and raised levels of apoptotic endothelial cell microparticles.15 WPS decreases the proliferation of alveolar epithelial cells and leads to their cell cycle arrest,16 which may be associated with increase in oxidative stress. In line with our findings, previous studies reported that mice which were exposed to chronic WPS showed significant increase in the number of airway inflammatory cells,17 interstitial ICI, and airway resistance.18 Also, the results are in agree with another study which indicated that WPS increased the oxidative stress as a significant increase in lipid peroxidation markers, MDA, and nitric oxide (NO) levels in the lungs and liver of mice in the test group compared with the control group.19 In addition, inhaling the smoke of waterpipe increased the total white blood cell (WBC) count and enhanced the expression of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α and decreased IL-10 in the bronchoalveolar lavage (BAL) fluid and lung tissue.20
We also demonstrated that the destructive effects of WTS decreased but were not fully prevented in animals exposed to WTS which participated in swimming exercise training program. Results also showed that exercise training significantly attenuated the negative oxidant and pro-inflammatory effects of smoking in lung as decreasing MDA, IL-6, IL-1β, and TNF-α and increasing GPX and IL-10. With the best of our knowledge, only limited studies have focused on the effects of exercise on WTS-induced lung dysfunction. Aerobic physical training with moderate intensity can attenuate the development of pulmonary emphysema and lung elastance induced by chronic cigarette smoking.21 In agree with our findings, recently Nemmar et al. reported that WTS-exposed C57BL/6 mice showed focal moderate interstitial ICI and treadmill exercise training significantly reduced these effects and also decreased the WTS-induced increase of TNF-α and IL-6 concentrations in lung tissue. They suggested that this positive effect was mediated through inhibiting nuclear factor kappa B (NF-κB) and activating nuclear factor erythroid 2-related factor 2 (NRF2) signaling pathways.18 It is indicated that mild to moderate exercise leads to activation of NRF2/antioxidant response element (ARE) signaling and subsequent enhancement of antioxidant defense pathways.22,23 Previous studies showed the role of pro-inflammatory cytokines especially IL-6 and TNF-α in induction and continuation of cigarette- and WPS-induced inflammation.18,24,25 We showed that swimming exercise training significantly reduced the increase of TNF-α, IL-1β, and IL-6 caused by WPS which is in agreement with previous findings which showed that exercise training prevented the WTS and also exhausted particle-induced increase of pro-inflammatory cytokines in the lung.18,26
Conclusion
Our findings indicated that WTS disrupted the normal physiological equilibrium between the generation of free radicals/antioxidant defense and pro-inflammatory/anti-inflammatory mechanisms and hence, led to lung injury. Combination of 8 weeks of mild to moderate swimming exercise training with WTS significantly attenuated the WTS-induced lung injury partly through balancing of pro/anti-inflammatory and redox systems. Further studies are needed to assess the other involved mechanisms and generalize these findings to the clinic.
Acknowledgments
The authors are thankful to the Institute of Neuropharmacology and Vice Chancellor of Research of Kerman University of Medical Sciences for financial support.
Conflicts of Interest
The authors have no conflict of interest.
Authors’ Contribution
SJ and MRN devised the main conceptual ideas and along with MRZ and NN designed the study. MRN and MN treated and trained animals. SJ, YMA and MRN collected the samples and YMA MRN measured the cotinine and biochemical parameters. MI and SJ prepared the slides and examined the morphological changes. The statistical analysis was done by NN and SJ. Also SJ supervised the all part of study and prepared the paper
REFERENCES
- 1.World Health Organization. Tobacco [Online]. 2019. Available from: URL: https://www.who.int/news-room/fact-sheets/detail/tobacco.
- 2.Maziak W, Taleb ZB, Bahelah R, Islam F, Jaber R, Auf R, et al. The global epidemiology of waterpipe smoking. Tob Control. 2015;24(Suppl 1):i3–i12. doi: 10.1136/tobaccocontrol-2014-051903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakhaee MR, Joukar S, Zolfaghari MR, Rostamzadeh F, Masoumi-Ardakani Y, Iranpour M, et al. Effects of endurance exercise training on cardiac dysfunction induced by waterpipe tobacco smoking. Addict Health. 2019;11(2):100–9. doi: 10.22122/ahj.v11i2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav S, Rawal G. Waterpipe tobacco smoking: A mini-review. J Transl Int Med. 2018;6(4):173–5. doi: 10.1515/jtim-2016-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danaei M, Jabbarinejad-Kermani A, Mohebbi E, Momeni M. Waterpipe tobacco smoking prevalence and associated factors in the southeast of Iran. Addict Health. 2017;9(2):72–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Haddad L, Kelly DL, Weglicki LS, Barnett TE, Ferrell AV, Ghadban R. A systematic review of effects of waterpipe smoking on cardiovascular and respiratory health outcomes. Tob Use Insights. 2016;9:13–28. doi: 10.4137/TUI.S39873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu F, Chen Y, Parvez F, Segers S, Argos M, Islam T, et al. A prospective study of tobacco smoking and mortality in Bangladesh. PLoS One. 2013;8(3):e58516. doi: 10.1371/journal.pone.0058516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jawad M, Charide R, Waziry R, Darzi A, Ballout RA, Akl EA. The prevalence and trends of waterpipe tobacco smoking: A systematic review. PLoS One. 2018;13(2):e0192191. doi: 10.1371/journal.pone.0192191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaabane Z, Murlasits Z, Mahfoud Z, Goebel R. Tobacco use and its health effects among professional athletes in Qatar. Can Respir J. 2016;2016:2684090. doi: 10.1155/2016/2684090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobb CO, Shihadeh A, Weaver MF, Eissenberg T. Waterpipe tobacco smoking and cigarette smoking: A direct comparison of toxicant exposure and subjective effects. Nicotine Tob Res. 2011;13(2):78–87. doi: 10.1093/ntr/ntq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binayi F, Joukar S, Najafipour H, Karimi A, Abdollahi F, Masumi Y. The effects of nandrolone decanoate along with prolonged low-intensity exercise on susceptibility to ventricular arrhythmias. Cardiovasc Toxicol. 2016;16(1):23–33. doi: 10.1007/s12012-015-9313-3. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Jiang X, Zhang L, Wang L, Li Z, Sun W. Simvastatin mitigates functional and structural impairment of lung and right ventricle in a rat model of cigarette smoke-induced COPD. Int J Clin Exp Pathol. 2014;7(12):8553–62. [PMC free article] [PubMed] [Google Scholar]
- 13.Dwoskin LP, Teng L, Buxton ST, Crooks PA. (S)-(-)-Cotinine, the major brain metabolite of nicotine, stimulates nicotinic receptors to evoke [3H] dopamine release from rat striatal slices in a calcium-dependent manner. J Pharmacol Exp Ther. 1999;288(3):905–11. [PubMed] [Google Scholar]
- 14.Al-Fayez SF, Salleh M, Ardawi M, Zahran FM. Effects of sheesha and cigarette smoking on pulmonary function of Saudi males and females. Trop Geogr Med. 1988;40(2):115–23. [PubMed] [Google Scholar]
- 15.Strulovici-Barel Y, Shaykhiev R, Salit J, Deeb RS, Krause A, Kaner RJ, et al. Pulmonary abnormalities in young, light-use waterpipe (Hookah) smokers. Am J Respir Crit Care Med. 2016;194(5):587–95. doi: 10.1164/rccm.201512-2470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shihadeh A, Eissenberg T, Rammah M, Salman R, Jaroudi E, El-Sabban M. Comparison of tobacco-containing and tobacco-free waterpipe products: Effects on human alveolar cells. Nicotine Tob Res. 2014;16(4):496–9. doi: 10.1093/ntr/ntt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Sawalha NA, Migdadi AM, Alzoubi KH, Khabour OF, Qinna NA. Effect of waterpipe tobacco smoking on airway inflammation in murine model of asthma. Inhal Toxicol. 2017;29(2):46–52. doi: 10.1080/08958378.2017.1280105. [DOI] [PubMed] [Google Scholar]
- 18.Nemmar A, Al-Salam S, Yuvaraju P, Beegam S, Ali BH. Exercise training mitigates water pipe smoke exposure-induced pulmonary impairment via inhibiting NF-kappaB and activating Nrf2 signalling pathways. Oxid Med Cell Longev. 2018;2018:7459612. doi: 10.1155/2018/7459612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charab MA, Abouzeinab NS, Moustafa ME. The protective effect of selenium on oxidative stress induced by waterpipe (Narghile) smoke in lungs and liver of mice. Biol Trace Elem Res. 2016;174(2):392–401. doi: 10.1007/s12011-016-0737-9. [DOI] [PubMed] [Google Scholar]
- 20.Khabour OF, Alzoubi KH, Al-Sawalha N, Ahmad MB, Shihadeh A, Eissenberg T. The effect of chronic exposure to waterpipe tobacco smoke on airway inflammation in mice. Life Sci. 2018;200:110–4. doi: 10.1016/j.lfs.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 21.Toledo AC, Magalhaes RM, Hizume DC, Vieira RP, Biselli PJ, Moriya HT, et al. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur Respir J. 2012;39(2):254–64. doi: 10.1183/09031936.00003411. [DOI] [PubMed] [Google Scholar]
- 22.Muthusamy VR, Kannan S, Sadhaasivam K, Gounder SS, Davidson CJ, Boeheme C, et al. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic Biol Med. 2012;52(2):366–76. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thirupathi A, Pinho RA. Effects of reactive oxygen species and interplay of antioxidants during physical exercise in skeletal muscles. J Physiol Biochem. 2018;74(3):359–67. doi: 10.1007/s13105-018-0633-1. [DOI] [PubMed] [Google Scholar]
- 24.Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med. 2004;170(5):492–8. doi: 10.1164/rccm.200404-511OC. [DOI] [PubMed] [Google Scholar]
- 25.Mineo D, Ambrogi V, Cufari ME, Gambardella S, Pignotti L, Pompeo E, et al. Variations of inflammatory mediators and alpha1-antitrypsin levels after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med. 2010;181(8):806–14. doi: 10.1164/rccm.200910-1476OC. [DOI] [PubMed] [Google Scholar]
- 26.Vieira RP, Toledo AC, Silva LB, Almeida FM, Damaceno-Rodrigues NR, Caldini EG, et al. Anti-inflammatory effects of aerobic exercise in mice exposed to air pollution. Med Sci Sports Exerc. 2012;44(7):1227–34. doi: 10.1249/MSS.0b013e31824b2877. [DOI] [PubMed] [Google Scholar]