Abstract
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, has been declared by the World Health Organization as an emerging public health problem of global importance and classified as a pandemic. SARS-CoV-2 infection can result in diverse, multiorgan pathology, the most significant being in the lungs (diffuse alveolar damage in its different phases, microthrombi, bronchopneumonia, necrotizing bronchiolitis, viral pneumonia), heart (lymphocytic myocarditis), kidney (acute tubular injury), central nervous system (microthrombi, ischemic necrosis, acute hemorrhagic infarction, congestion, and vascular edema), lymph nodes (hemophagocytosis and histiocytosis), bone marrow (hemophagocytosis), and vasculature (deep vein thrombosis). An understanding of the spectrum and frequency of histologic findings in COVID-19 is essential for gaining a better understanding of disease pathophysiology and its ongoing impact on public health. To this end, we conducted a systematic meta-analysis of histopathologic observations to date and review the reported findings.
Keywords: COVID-19, Histopathology, Autopsy, Pathology, SARS-CoV-2
1. Introduction
By late December 2019, several health-care centers in Wuhan, Hubei province, China, reported on several clusters of patients presenting with pneumonia of unknown cause, which were epidemiologically linked to a wholesale seafood market [1]. In response to this outbreak, the Chinese Center for Disease Control and Prevention sent a response team to accompany health authorities and conduct an epidemiological and etiological investigation. They concluded that the pneumonia was viral in origin [1]. The virus could not be contained, given the high flow of travelers from Wuhan to other cities in China and elsewhere in the world. At this time, a new human pathogen with a high zoonotic capacity, known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, was identified as the cause of coronavirus disease 2019 (COVID-19). It was declared by the World Health Organization (WHO) as an emerging public health problem of global importance and cataloged as a pandemic on March 11, 2020 [2,3].
As of July 22, 2020, there were 15 million confirmed cases and more than 617,000 deaths from COVID-19 in 215 countries [[2], [3], [4]]. The United States had confirmed more than 3.92 million cases and more than 142,000 deaths. In Latin America, Brazil has reported more than 2.16 million cases, being the second country worldwide in confirmed cases. The case fatality rate in COVID-19 ranges from 1% to 20% [4]. Given the current situation of the pandemic, we carried out a systematic review of the literature describing histopathological findings from samples received at surgical pathology laboratories and from postmortem studies.
Here, we summarize the histopathological findings of COVID-19 reported in publications currently available in the literature.
2. Methods
An electronic search was performed in databases such as PubMed/Medline, SciELO, Scopus, Google Scholar, and Web of Science. Search terms were as follows: “COVID-19 and histopathology”“COVID-19 and autopsy” “COVID-19 and Cytology”“COVID-19 and biopsies” “COVID-19 and Histology” “COVID-19 and the acute respiratory syndrome” ““COVID-19 and post mortem findings” The search ended on June 1, 2020. In addition, we reviewed other general sources of information. Studies in English, Spanish, Portuguese, and French were considered for inclusion.
3. Results
The literature search yielded 16,932 articles using the aforementioned search terms. After a careful review by two independent researchers, 27 articles were considered for full analysis and extraction of information for this meta-analysis. These articles combined a total of 226 autopsies and 9 biopsies reported (Table 1 ).
Table 1.
Summary of the main histopathological findings of COVID-19 in the studies currently published in the search databases.
| Author/country | Number of biopsies | Number of autopsies | Most important histopathological findings of organs |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung |
Heart |
Liver |
Kidney |
||||||||||||||||||||||||
| Diffuse alveolar damage |
Microthrombi/thrombi | Viral cytopathic changes | Viral inclusions | Heart attacks | Inflammatory changes | Pneumonia | Extensive pulmonary fibrosis | Not sampled | Chronic inflammatory infiltrate | Hypertrophy | No specific findings | Not sampled | Lymphocytic myocarditis | Steatosis | Inflammation | Necrosis | No specific finding | Not sampled | Acute tubular injury | Not sampled | Collapse of glomerular capillaries | No specific findings | |||||
| Exudative stage | Proliferative phase | Fibrotic phase | |||||||||||||||||||||||||
| Bryce et al. [15]/USA | 67 | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Carsana et al. [16]/Italy | 38 | X | X | X | X | X | X | X | |||||||||||||||||||
| Bradley et al. [17]/USA | 12 | X | X | X | X | ||||||||||||||||||||||
| Schaller et al. [18]/Germany | 12 | X | X | X | X | X | X | X | |||||||||||||||||||
| Remmelink et al. [19]/Belgium | 17 | X | X | X | X | X | X | X | X | X | |||||||||||||||||
| Nunes Duarte-Neto et al. [20]/Brazil | 10 | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Prilutskiy A et al. [21]/USA | 4 | X | X | X | X | ||||||||||||||||||||||
| Xu et al. [22]/China | 1 | X | X | X | X | X | X | ||||||||||||||||||||
| Konopka, et al. [23]/USA | 1 | X | X | X | X | X | X | X | |||||||||||||||||||
| Yan et al. [24]/USA | 1 | X | X | X | X | X | X | X | |||||||||||||||||||
| Fitzek et al. [25]/Germany | 1 | X | X | X | X | X | X | X | |||||||||||||||||||
| Autopsias [26]/Spain | 1 | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Luo et al. [27]/China | 1 | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Tian et al. [28]/China | 2 | X | X | X | X | X | X | X | |||||||||||||||||||
| Kuang et al. [29]/China | 1 | X | X | X | X | X | X | ||||||||||||||||||||
| Pernazza et al. [30]/Italy | 1 | X | X | X | X | X | |||||||||||||||||||||
| Barton et al. [31]/USA | 2 | X | X | X | X | X | |||||||||||||||||||||
| Tian et al. [32]/China | 4 | X | X | X | X | X | X | ||||||||||||||||||||
| Fox et al. [33]/USA | 4 | X | X | X | X | X | X | X | |||||||||||||||||||
| Zhang et al. [34]/China | 1 | X | X | X | X | X | |||||||||||||||||||||
| Yao et al. [35]/China | 3 | X | X | X | X | X | X | X | |||||||||||||||||||
| Li et al. [36]/USA | 1 | X | X | X | X | X | X | ||||||||||||||||||||
| Su et al. [37]/China | 26 | X | X | X | X | ||||||||||||||||||||||
| Rossi et al. [38]/Italy | 1 | X | X | X | X | ||||||||||||||||||||||
| Peleg et al. [39]/Africa | 1 | X | X | X | X | ||||||||||||||||||||||
| Wichmann et al. [40]/Germany | 12 | X | X | X | X | X | X | ||||||||||||||||||||
| Dolhnikoff et al. [43]/Brazil | 10 | X | X | X | X | X | X | ||||||||||||||||||||
| Total | 9 | 226 | 22 | 9 | 3 | 11 | 4 | 3 | 1 | 21 | 6 | 1 | 3 | 2 | 1 | 5 | 16 | 3 | 4 | 2 | 1 | 5 | 18 | 3 | 19 | 1 | 4 |
Abbreviations: COVID-19, coronavirus disease 2019.
3.1. Autopsy performance in COVID-19 deaths
Postmortem examination in COVID-19 deaths has been performed in the following ways: postmortem microbiological sampling without an autopsy, limited autopsy with examination/biopsy of organs of interest, and complete autopsy [5]. As per recommendations of the US Centers for Disease Control and Prevention (CDC), autopsies in suspected or confirmed COVID-19 cases are practicable as long as appropriate safety precautions for infectious disease cases are implemented. However, in many countries, such measures may not be possible owing to lack of adequate infrastructure and/or universal protective equipment availability for prosectors [6]. To lessen the risk of virus transmission between the cadaver and pathologist, organs should be examined in a way that minimizes aerosolization and fluid splash. In addition, to avoid contamination of the surrounding environment, the autopsy should be performed in a site with appropriate biosafety conditions such as negative-pressure ventilation and restricted access [7,8].
The US CDC recommends standard precautions, contact precautions, and airborne precautions with eye protection (goggles or face shields) during the autopsy. The following factors should be considered when deciding to perform an autopsy in a confirmed or suspected COVID-19 case: legal, medical jurisdiction; environmental monitoring facilities; availability of recommended personal protective equipment (PPE); family; and cultural wishes [9]. If an autopsy is performed on a suspected COVID-19 case, collection of the following postmortem specimens is recommended: postmortem swab specimens for SARS-CoV-2 testing (upper respiratory tract, nasopharynx, lower respiratory tract, and both lungs); separate swabs to analyze for other respiratory pathogens; and tissues fixed in formalin, as per autopsy protocol. If an autopsy is not performed for a suspected case of COVID-19, collection of the following postmortem specimens is recommended: nasopharyngeal swab specimen and separate swabs to test for other respiratory pathogens. If an autopsy is performed for a confirmed case of COVID-19, the following postmortem samples are recommended: separate swab samples to analyze for other respiratory pathogens and formalin-fixed tissues, as per autopsy protocol [10].
In the laboratory biosecurity manual, the WHO classifies the intrinsic biological characteristics of infectious agents into four risk groups (RGs). These range from level 1 (RG1), which includes microorganisms that are unlikely to cause disease in humans or in animals, up to level 4 (RG4), referring to those pathogens that cause severe illnesses and are easily transmissible from one individual to another. As per the international consensus on biosecurity, SARS-CoV-2 should be classified as a human pathogen of RG3 [10]. Laboratory biosecurity is classified into four levels (BSL-1 to BSL-4). These levels constitute a series of protections, including proper safeguards designed to protect laboratory personnel as well as the environment and the surrounding community. The level of biosecurity required in laboratories derives from the risk characterization and is not automatically derived from the RG to which the pathogen belongs [10].
Coronaviruses related to severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) are considered HG3 pathogens, whereas most of the other viruses in the Coronavirinae subfamily are considered RG2 pathogens. SARS-CoV-2 has recently been classified as an RG3 organism. Other viruses within RG3 include rabies virus; poliovirus; dengue virus; hepatitis B, C, D, and E viruses; and HIV 1 and 2, among others [10]. In general, performing an autopsy on a patient with suspected HG3 organisms requires four areas of attention: risk assessment, understanding of the pathology that can be found, universal standard precautions, and any standard operating procedures for specific HG3 pathogens. The effective use of universal precautions mitigates inaccurate or incomplete information used in risk assessment of individual cases [11].
3.2. Histopathology findings in biopsies and autopsies of COVID-19 cases
The histopathological features of COVID-19 closely resemble those seen in SARS and MERS [12]. SARS, which is a type of pneumonia caused by the SARS coronavirus (SARS-CoV), is highly contagious and can affect multiple organs. The SARS outbreak in 2003 prompted extensive studies of its histopathological features, with the discovery that the disease predominantly attacks the lung and the immune system [13]. According to Ding and Bian [14], the main histopathological changes can be summarized as lung disease, damage to the immune organs, systemic vasculitis, and differences in systemic toxicity and secondary infections [[13], [14]]. Injury to the lungs results in clinical acute respiratory distress syndrome, which corresponds to diffuse alveolar damage (DAD) histologically. MERS is caused by the Middle East respiratory syndrome coronavirus. The histopathologic features comprise three major patterns: DAD, multiple organ microvasculitis, and lymphocyte infiltration and changes in immune organs [13,14].
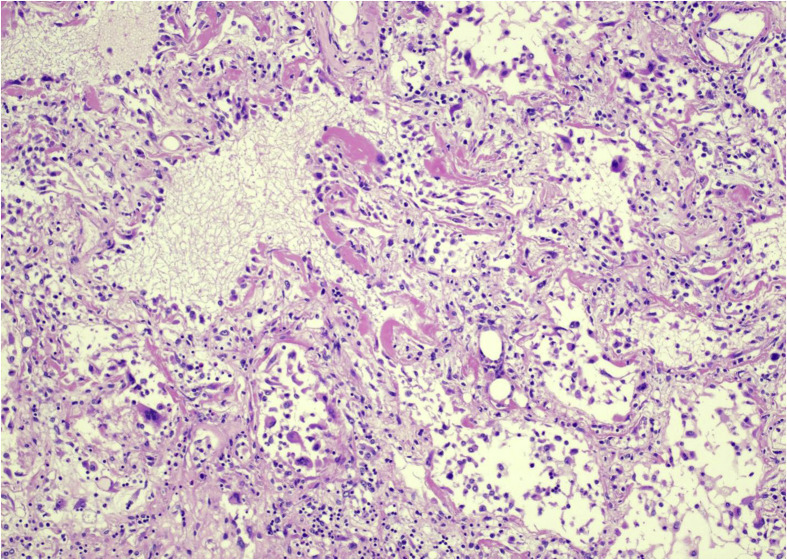
Like SARS-CoV and MERS-CoV, SARS-CoV-2 mainly attacks the lungs, causing DAD (Fig. 1 ), with edema and hyaline membrane formation, which is accompanied by macrophage and lymphocytic infiltration to varying degree. These findings are common to viral pneumonias in general; however, ongoing histopathological studies are determining the specific characteristics with more certainty [13,14]. Additional significant histopathological findings that have been found are described in several case series of surgical samples and autopsies performed in patients and decedents with COVID-19. These findings are summarized in Table 1.
Fig. 1.
Lung: diffuse alveolar damage with hyaline membranes (arrows) (H&E, ×10). Photos from Grimes, Bryce, and Paniz-Mondolfi. H&E, heamtoxylin and eosin.
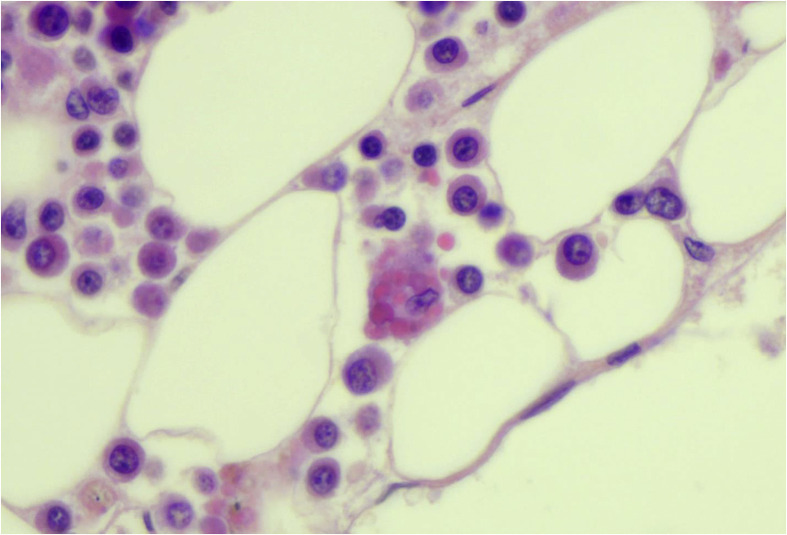
Bryce et al. performed 67 autopsies and described macroscopic diffusely consolidated lungs. The histology revealed DAD in the acute, exudative, and early proliferative phases in 22 of 25 cases evaluated. In addition, intranuclear inclusions suggestive of viral cytopathic effect, acute and necrotizing pneumonia, intravascular fibrin thrombi, and interstitial inflammatory infiltrate were seen. Other findings were in the liver, with cirrhosis, steatosis, necrosis, congestion, venous flow obstruction, and newly organized thrombi, and in the kidneys, with acute tubular injury. Thoracic lymph nodes showed sinus histiocytosis, with focal hemophagocytosis. In 15 of 25 cases, examination of the heart revealed an epicardial mononuclear infiltrate with a predominance of CD4+ T lymphocytes, and there were occasional small vessel thrombi in regions of epicardial inflammation. Hemophagocytosis was seen in 4 of 6 bone marrows (Fig. 2 ) and in the spleen (9/22 cases). Within the central nervous system, the prominent finding was widespread presence of microthrombi and acute infarction, observed in 6 of 20 cases. Based on these findings, the authors conclude that COVID-19 causes a hypercoagulable state, endothelial dysfunction, and an imbalance of innate and adaptive immune responses [15].
Fig. 2.
Bone marrow: hemophagocytosis—macrophages with ingested red cells (H&E, ×60). Photos from Grimes, Bryce, and Paniz-Mondolfi. H&E, heamtoxylin and eosin. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
A series from Italy discussed autopsies of patients with COVID-19, focusing on lung lesions. There were 38 cases, with 33 (86.84%) men and 5 (13.16%) women, with an average age of 69 years (range = 32–86 years); the hospitalization time was from 1 to 23 days (average = 6.87 days). A D-dimer level was available in 26 of 38 patients, with all having a high value (>10 times the upper reference limit). On gross examination, the lungs were congested, with marked edema. On microscopy, the most significant findings were DAD in the exudative and proliferative phases. The fibrotic phase was rarely observed owing to the short life span of the studied patients. Fibrin thrombi of small arterial vessels (diameter <1 mm) were found in 33 of 38 patients, half of them with >25% tissue involvement and associated with high levels of D-dimer in the blood. These histopathological findings could explain the severe hypoxemia that characterizes the SARS symptoms in patients with SARS-CoV-2 infection, in which they conclude that the data found strongly supports the hypothesis proposed by recent clinical studies that COVID-19 is strictly related to coagulopathy and thrombosis. In addition, detection of D-dimer values >1 μg/ml has been associated with a fatal outcome of COVID-19. For these reasons, the use of anticoagulants has recently been suggested as potentially beneficial in patients with severe COVID-19, although their efficacy and safety have not been demonstrated. Immunohistochemistry (IHC) studies were performed on selected cases (CD45, CD68, CD61, TTF1, p40, Ki67), and Masson's trichrome staining was performed to better characterize the inflammatory infiltrate, epithelial cells, and fibrosis [16].
A series of 12 cases studied in Washington had a similar age range, with the age range described in the autopsy series from Italy (70.4 years, range = 42–84 years). The hospital stay after the onset of symptoms until death ranged from 1 to 14 days, with an average of 7 days. The macroscopic findings are also similar to those of other studies, with edematous lungs having an average weight of 1804 g, without evidence of lung consolidation. One autopsy case had mild splenomegaly, 350 g. In another case, dotted subarachnoid hemorrhages were observed overlying the brain. Other findings were sinusoidal congestion in the liver (100%), hypertensive changes in the kidney (80%), and various degrees of atherosclerotic coronary artery disease (60%). Histopathological findings in the lungs consisted of DAD in 75% of cases. In one case, there was lymphocytic myocarditis. Kidney findings consisted of arterionephrosclerosis. Viral particles were detected by electron microscopy in type I and II pneumocytes [17]. The kidney showed viral particles in the tubular epithelium, endothelium, and podocytes, without significant inflammation. Viral particles were also observed in the trachea and large intestine. SARS-CoV-2 RNA was detected in the heart tissue of the patient with lymphocytic myocarditis [17].
Schaller et al. [18] performed ten autopsies, with a mean decedent age of 79 years (64–90 years), finding DAD in the lungs in the exudative and proliferative phases, with 1 of 10 cases having DAD in the fibrotic phase and with mild lymphocytic myocarditis. Remmelink et al. [19] performed 17 autopsies. In addition to DAD in the exudative phase and fibrotic phase, they observed pulmonary arterial microthrombi, acute pneumonia, and bronchopneumonia. SARS-CoV-2 was identified by IHC in the lungs in 11 of 17 autopsies. In the heart, chronic ischemic cardiomyopathy and acute myocardial infarction were identified. In the liver, steatosis, hepatitis, and lobular lymphocytic infiltrates were identified. Cerebral hemorrhage was present in 8 of 17 cases. Other findings in the brain were ischemic necrosis (3/17), edema, and diffuse vascular congestion, in 5 of 17 cases. SARS-CoV-2 RNA was detected in at least one organ of each patient (lung, heart, liver, intestine, spleen, kidney, and brain) [19].
Nunes Duarte-Neto et al. [20] performed ultrasound-guided minimally invasive autopsies on ten cadavers, collecting tissue from different organs. In the lung samples, DAD, as well as foci of squamous alveolar metaplasia, was found in the exudative and proliferative phase. Immunohistochemical Ki-67 expression in alveolar and bronchiolar cells indicated a high rate of epithelial cell proliferation. Other findings were fibrin thrombi in alveolar arterioles and suppurative pneumonia (Fig. 3, Fig. 4 ). The extrapulmonary findings were divided into three categories: those attributed to comorbidities such as hypertension and diabetes, those attributable to shock, and findings that had an uncertain etiology, that is, secondary to SARS-CoV-2 infection [20]. Prilutskiy et al. [21] studied four autopsies directed to the thoracic cavity and abdomen. They found DAD in the lungs in the exudative phase. A further important finding was present in 3 of 4 autopsies: hemophagocytosis in hilar and mediastinal lymph nodes, the spleen, the liver, and the bone marrow. The predominant form of hemophagocytosis identified was lymphophagocytosis, and these were clinically related [21].
Fig. 3.
Pulmonary thromboembolus. Photos from Grimes, Bryce, and Paniz-Mondolfi.
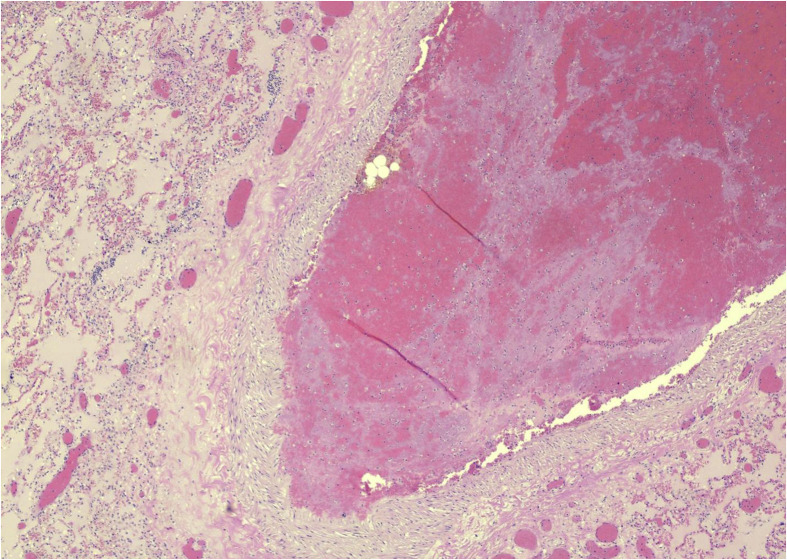
Fig. 4.
Lung: pulmonary thromboembolus (H&E, ×10). Photos from Grimes, Bryce, and Paniz-Mondolfi. H&E, heamtoxylin and eosin.
Some case reports have described bilateral DAD, with evidence of hyaline membrane formation and pneumocyte denudation in the right lung, pulmonary edema with hyaline membrane formation in the left lung, interstitial lymphocytic inflammatory infiltrates in both lungs, and cytopathic viral changes, as well as hepatic steatosis and mononuclear infiltrates in the heart [[22], [23], [24]]. Other case reports have revealed congestive cardiomyopathy, with detection of the SARS-CoV-2 viral RNA in pharyngeal mucosa and lung tissue [25]. The first autopsy findings reported from Spain consisted of proliferative and fibrotic phases of DAD in the lungs with cytopathic changes and an interstitial inflammatory infiltrate composed of CD8+ lymphocytes with neutrophils. Thrombi were found in the medium-sized and small blood vessels, the former showing expression of CD61 by IHC, indicating their platelet composition [26]. In a study describing the lung tissue of a 60-year-old patient with COVID-19, the gross findings were congestion of the lung parenchyma with a hemorrhagic and necrotic appearance. On histology, the main findings were interstitial change with hyaline degeneration, fibrosis, and hemorrhagic infarction, as well as the formation of microthrombi. There was an inflammatory infiltrate consisting mainly of lymphocytes and plasma cells. Necrotizing bronchiolitis and alveolitis was present, with intracytoplasmic viral inclusions in the alveoli and multinucleated giant cells; IHC showed a mixed inflammatory cell population, variously immunopositive for CD3, CD4, CD8, CD20, CD79a, CD5, CD38, and CD68 [27].
In patients who went for surgery owing to cancer with concurrent COVID-19, the histology of the lungs showed proliferative and exudative phases of DAD with accompanying edema and prominent proteinaceous and fibrinoid exudates, vascular congestion, and inflammatory cells including multinucleated giant cells. In addition, reactive alveolar hyperplasia was observed. Fibroblastic proliferation was present, indicative of early organization [28]. Similar findings were described in a report of surgical samples in patients with lung cancer, complicated by COVID-19. IHC revealed a sparse infiltration of CD3, CD20, and CD8 lymphocytes into the alveolar septum. In contrast, plasma cells and CD68-positive macrophages predominated [29]. Pernazza et al. [30], in a surgical sample of a patient with a history of lung cancer, found histological findings such as pneumocyte damage, alveolar hemorrhage, and clusters of macrophages and interstitial inflammatory infiltrates, characteristics similar to those previously described in SARS-CoV-2 infection and consistent with previously postulated mechanisms underlying the lung damage seen in SARS.
A team from Oklahoma, USA, performed two complete autopsies of patients with COVID-19, the first was a 77-year-old male patient, with a six-day history of chills and intermittent fever and a past medical history significant for hypertension, deep vein thrombosis, splenectomy, pancreatitis due to cholelithiasis, and osteoarthritis after total knee replacement. Among the critical findings, the lungs were heavy, weighing 2452 g, firm and diffusely edematous; histologically, there was DAD in the acute stage. IHC studies revealed a mixed inflammatory infiltrate with variable positivity for CD3, CD20, CD8, and CD4. The second case was a 42-year-old man with a history of fever, respiratory distress, and cough, in critical condition, and with a history of myotonic muscular dystrophy. At autopsy, the lungs weighed 1191 g combined and were edematous without effusion. Microscopy revealed acute bronchopneumonia with numerous CD68-positive macrophages in areas of inflammation [31].
Tian et al. [32] performed lung, heart, and liver puncture biopsies on four decedents. The ages of the patients were between 59 and 81 years, with a history of chronic lymphocytic leukemia, cirrhosis, and hypertension. The duration of the clinical course from the start of symptomatic COVID-19 until death ranged from 15 to 52 days. The significant histological finding was DAD in the lung. The heart histology revealed only mild interstitial fibrosis and benign myocardial hypertrophy secondary to the preexisting heart disease. Within the liver, centrilobular sinusoidal dilation and mild lymphocyte infiltration were observed [32].
A case series by Fox et al. [33] from New Orleans reported the first cardiopulmonary findings in four autopsies. The decedents' ages ranged from 44 to 26 years, with a history of chronic noncommunicable diseases such as diabetes. They presented with mild cough and fever. At autopsy, macroscopic features of the lungs were congestion, the lung weights ranging from 680 to 1030 g on the left (reference = 583 ± 216 g) and from 800 to 1050 g on the right (reference = 663 ± 239 g). In some cases, small, firm vascular thrombi were present in sections of peripheral parenchyma. In 3 of 4 cases, the heart was enlarged (cardiomegaly), with right ventricular dilation. Histopathologically, the lung sections showed bilateral DAD with a mild to moderate lymphocytic infiltrate, composed of a mixture of CD4+ and CD8+ lymphocytes, fibrin thrombi within small vessels, and distended capillaries [33].
Zhang et al. [34] described the histopathological findings of a 72-year-old patient who underwent a lung biopsy and reported DAD in the organization phase. Immunostaining of lung sections with an antibody against the Rp3 NP protein of SARS-CoV-2 revealed prominent expression in alveolar cells, including sloughed and damaged cells within the alveolar space [34]. Yao et al. [35], in their study of three minimally invasive postmortems with biopsy of lung tissue, also found that COVID-19 mainly involved the lungs, observing DAD in its different phases as well as hyaline thrombi in blood vessels [35]. Other authors such as Li et al. [36], in their examination of lung tissue taken from a patient with COVID-19, concluded that, in addition to the DAD, thrombotic microangiopathy and associated hemorrhage contributed significantly to death, finding evidence of megakaryocyte activation and formation of small vessel clots [36].
Su et al. [37] reviewed the postmortem histopathological findings of 26 patients with COVID-19. They focused their attention on kidney pathology because clinical observations of patients with COVID-19 revealed acute kidney injury with an incidence of 0.9–29% between different centers. Nineteen men and seven women were included, with an average age of 69 years (range = 39–87 years). Eleven of twenty-six patients had a history of hypertension or diabetes or both. Histopathological findings were acute tubular injury in all patients and acute pyelonephritis with multiple foci of bacteria and diffuse polymorphonuclear leukocyte tubular casts in 2 of 26 patients. Hemosiderin granules were present in the tubular epithelium of 4 of 26 patients [37]. Rossi et al. [38] recently published kidney biopsy findings from a patient with COVID-19. They reported extensive acute tubular injury, with marked degenerative changes and focal acute tubular necrosis [38]. In contrast, Peleg et al. [39] reported a case of a 46-year-old male patient from West Africa with a history of acute kidney injury who tested positive for COVID-19 and then underwent a kidney biopsy. The histopathology revealed collapsing variant of focal segmental glomerulosclerosis, also known as collapsing glomerulopathy, a finding that was not described in the series published by Peleg et al. [39] Among the histopathological findings of 12 autopsies reported by Wichmann et al. [40], there were 7 clinically unsuspected deep vein thromboses. Early-phase DAD, as well as microthrombi within small pulmonary arteries and an interstitial inflammatory infiltrate, was present in 8 of 12 cases. In 1 of 12 cases, there was lymphocytic myocarditis. Polymerase chain reaction (PCR) detected the highest concentration of SARS-CoV-2 RNA in the lung and pharyngeal tissue, and the authors concluded that the high incidence of thromboembolic events is related to COVID-19–induced coagulopathy; however, further study is needed to determine how the virus could disrupt coagulation pathways [40].
Hosier et al. [41] recently published a case of placental infection with SARS-CoV-2 from a 22-week pregnant woman with COVID-19. Macroscopically, the placenta had adherent marginal blood clot associated with a focal placental infarction, supporting the clinical diagnosis of placental abruption. On histology, diffuse intervillous fibrin was present, and there was an inflammatory infiltrate composed of T lymphocytes and macrophages, the latter evidenced by IHC for CD68. The maternal vessels did not show characteristics of decidual vasculopathy. SARS-CoV-2 was located predominantly in syncytiotrophoblast cells, as demonstrated by IHC for the SARS-CoV-2 protein and SARS-CoV-2 in situ RNA hybridization. Electron microscopic analysis of the placental region adjacent to the umbilical cord identified virus particles within the cytoplasm of placental cells. The virus particles had a diameter of 75–100 nm, which is consistent with the known size and appearance of SARS-CoV-2 [41]. Shanes et al. [42] performed a pathological study on 16 placentas from patients with COVID-19. The authors identified poor maternal vascular perfusion, in particular abnormal or injured maternal vessels, and intervillous thrombi. They conclude that these changes may reflect an inflammatory or hypercoagulable systemic state that adversely affects placental physiology [42].
Dolhnikoff et al. [43] have studied ten COVID-19 autopsy cases to date: five men and five women, with a mean age of 67.8 years (33–83 years). Overall, 7 of 10 decedents had comorbidities including high blood pressure, diabetes mellitus, ischemic heart disease, and chronic obstructive pulmonary disease. The average hospital stay was 5.4 days (0–15 days). Histologically, the lungs showed DAD in the exudative/proliferative phase and little lymphocytic infiltration. The authors also observed a variable number of fibrin thrombi in small pulmonary arterioles in areas of damaged lung parenchyma, concluding and supporting the current concept of a hypercoagulable state in critically ill patients and also demonstrating that the frequency of pulmonary microthrombosis is high [43]. A complex and still poorly understood relationship has been observed between COVID-19 and thrombogenesis, in which SARS-CoV-2 induces a cytokine storm in severe cases, ultimately leading to the activation of the coagulation cascade and causing thrombotic phenomena. However, because autopsy studies to date have a small sample size and frequently use limited sampling of organs, more comprehensive pathological studies are needed to confirm the presence and frequency of thrombi in the lungs and other organs in severe COVID-19 cases and to provide further justification for treatment. A survey of three autopsies was recently published in which the authors reported viral elements within endothelial cells and inflammatory cells; this finding suggests that SARS-CoV-2 infection facilitates the induction of endotheliitis in various organs as a direct consequence of viral participation during the host's inflammatory response. Endotheliitis could explain the systemic microcirculatory function in different vascular beds and its clinical sequelae in patients with COVID-19 [44]. Acute respiratory failure and systemic coagulopathy are critical aspects of morbidity and mortality that characterize severe infections with SARS-CoV-2. A microvascular injury syndrome, mediated by activation of complement pathways and an associated procoagulant state, is likely central to the devastating clinical course observed in these patients. This concept provides a basis for a better understanding of the pathophysiological importance of the complement in COVID-19 and could suggest targets for a specific intervention [45]. Understanding the mechanism of viral sepsis in COVID-19 is vital for optimizing clinical care in these patients.
4. Discussion
In summary, the organs most reported to be affected by COVID-19 to date included the lung, heart, vasculature, central nervous system, hemolymphatic system, and kidney. Of these, the most common histology findings are within the lungs: DAD at different stages (especially the exudative stage, reported in 22 of 27 studies), inflammatory changes (in 21 studies), and microthrombi/thrombi (in 11 studies) (Table 1).
Findings in other organs include the following: focal lymphocytic myocarditis within the heart, acute tubular injury in the kidney, central nervous system vascular abnormalities with microthrombi, ischemic necrosis, acute hemorrhagic infarction, congestion and vascular edema, hemophagocytosis within the lymph nodes and bone marrow, and deep vein thrombosis within the vasculature with associated pulmonary thromboembolism.
The extent of postmortem examination varies between the different studies, ranging from microbiological sampling without autopsy, limited examination of organs of interest, through full autopsy. Reasons for this variability have included lack of adequate infrastructure, availability of appropriate biosafety conditions and/or PPE, and attempts to minimize prosector exposure to the virus.
After multiple rounds of discussion among basic science researchers, pathologists, and clinicians working on COVID-19, Li et al. [46] have presented various hypotheses on the pathogenesis of SARS-CoV-2. Hypotheses for the pathogenesis of these findings at biopsy or autopsy include disordered immune response to the virus, with a hyperinflammatory state, and abnormal coagulation, which may be secondary to the immune response or related directly to viral injury of endothelial cells. Ongoing scientific research is needed to further explore SARS-CoV-2 and its impact on the human body and, by extension, global public health [46].
Because the WHO declared the COVID-19 pandemic, all health-care personnel, including pathologists, have been urged in a common effort of solidarity in the different disciplines in which they work, with an emphasis on prioritization of activities at this time of global health emergency, thus maintaining a high-level and optimal response time for routine diagnostic activity while concurrently investigating the COVID-19 process. Postmortem histopathological findings and biopsies could play an essential role in understanding the pathophysiology of SARS-CoV-2 infection. Indeed, using appropriate biosafety precautions, the US CDC and the WHO calls for action to perform full and detailed autopsies on patients who have suffered from SARS-CoV-2 infection, leading to COVID-19 [47,48]. Finally, further research using fresh, frozen, and formalin-fixed tissues obtained from autopsy or biopsy is needed to better understand the tropism of SARS-CoV-2 and the extent of its effects on different organs and tissues. For example, the central nervous system deserves in-depth analysis to reveal the impact of the virus on the brain and correlation with neurological manifestations and complications [[49], [50], [51]]. IHC, genomic, and PCR-based techniques performed on these tissues will be relevant for such investigations in the near future.
Footnotes
Competing interests: All authors declare no conflict of interest.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Morales A.J., Sánchez-Duque J.A., Hernández Botero S., Pérez-Díaz C.E., Villamil-Gómez W.E., Méndez C.A., et al. Preparación y control de la enfermedad por coronavirus 2019 (COVID-19) en América Latina. Acta Méd Peru. 2020;37:3–7. [Google Scholar]
- 3.Sánchez-Duque J.A., Arce-Villalobos L.R., Rodríguez-Morales A.J. Enfermedad por coronavirus 2019 (COVID-19) en América Latina: papel de la atención primaria en la preparación y respuesta. Atención Primaria. 2020;52:369–372. doi: 10.1016/j.aprim.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan American Health Organization/World Health Organization . OPS/OMS; Washington, D.C.: 2020. Epidemiological update: coronavirus disease (COVID-19). May 22, 2020. [Google Scholar]
- 5.Farkas C.B., Petrétei D., Babinszky G., Dudás G., Szabó G., Bognár C., et al. Elhunytakkal kapcsolatos teendők COVID–19-gyanús. valószínűsített és megerősített esetekben. 2020;161:713–722. doi: 10.1556/650.2020.31818. [DOI] [PubMed] [Google Scholar]
- 6.Fineschi V., Aprile A., Aquila I., Arcangeli M., Asmundo A., Bacci M., et al. Management of the corpse with suspect , probable or confirmed COVID-19 respiratory infection – Italian interim recommendations for personnel potentially exposed to material from corpses , including body fluids , in morgue structures and during autopsy pra. Pathologica. 2020;112:64–77. doi: 10.32074/1591-951X-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue Y., Lai L., Liu C., Niu Y., Zhao J. Perspectives on the death investigation during the COVID-19 pandemic. Forensic Sci Int: Synergy. 2020;2:126–128. doi: 10.1016/j.fsisyn.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao D.M., Zhou N., Zheng D., Yue J.C., Zhao Q.H., Guan D.W., et al. Guide to the forensic pathology practice on death cases related to corona virus disease 2019 (COVID-19. J Forensic Med. 2020;36:6–15. doi: 10.12116/j.issn.1004-5619.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Collection and submission of postmortem specimens from deceased persons with known or suspected COVID-19.
- 10.Barbareschi M., Ascoli V., Bonoldi E., Cavazza A., Colombari R., Cozzi I., et al. Biosafety in surgical pathology in the era of SARS-Cov2 pandemia. A statement of the Italian Society of Surgical Pathology and Cytology. Pathologica. 2020;1:1–5. doi: 10.32074/1591-951X-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanley B., Lucas S.B., Youd E., Swift B., Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020;73:239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q., Wang R.S., Qu G.Q., Wang Y.Y., Liu P., Zhu Y.Z., et al. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36:21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang H.J., Du S.H., Yue X., Chen C.X. Review and prospect of pathological features of corona virus disease. Fa Yi Xue Za Zhi. 2020;36:16–20. doi: 10.12116/j.issn.1004-5619.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Ding Y.Q., Bian X.W. [Analysis of coronavirus disease-19 (COVID-19) based on SARS autopsy] Zhonghua bing li xue za zhi = Chinese J Pathol. 2020;49:291–293. doi: 10.3760/cma.j.cn112151-20200211-00114. [DOI] [PubMed] [Google Scholar]
- 15.Bryce C., Grimes Z., Pujadas E., Ahuja S., Beth M., Albrecht R., et al. Pathophysiology of SARS-CoV-2 : targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. Mount Sinai COVID-19 Autopsy Experience. medRxiv. 2020 [Google Scholar]
- 16.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Diseases. Published online June 8, 2020. [DOI] [PMC free article] [PubMed]
- 17.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections. medRxiv. 2020 doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaller T., Hirschbühl K., Burkhardt K., Braun G., Trepel M., Märkl B., et al. Postmortem examination of patients with COVID-19. J Am Med Assoc. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907. [published online ahead of print, 2020 May 21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remmelink M., De Mendoca R., D'Haene N., De Clercq S., Verocq C., Lebrun L., et al. Unspecific post-mortem findings despite multiorgan 1 viral spread in COVID-19 patients. medRxiv. 2020 doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunes Duarte-Neto A., de Almeida Monteiro R.A., da Silva L.F.F., Malheiros D.M.A.C., de Oliveira E.P., Theodoro Filho J., et al. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology. 2020 doi: 10.1111/his.14160. [published online ahead of print, 2020 May 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prilutskiy A., Kritselis M., Shevtsov A., Yambayev I., Vadlamudi C., Zhao Q., et al. SARS-CoV-2 Infection Associated Hemophagocytic Lymphohistiocytosis: an autopsy series with clinical and laboratory correlation. medRxiv. 2020 doi: 10.1093/ajcp/aqaa124. 2020.05.07.20094888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konopka K.E., Wilson A., Myers J.L. Postmortem lung findings in a patient with asthma and coronavirus disease 2019. Chest. 2020 doi: 10.1016/j.chest.2020.04.032. [published online ahead of print, 2020 Apr 28] S0012-3692(20)30775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan L., Mir M., Sanchez P., Beg M., Peters J., Enriquez O., et al. Archives of Pathology & Laboratory Medicine; 2020 May 18. COVID-19 in a hispanic woman: autopsy report with clinical pathological correlation. [DOI] [PubMed] [Google Scholar]
- 25.Fitzek A., Sperhake J., Edler C., Schröder A.S., Heinemann A., Heinrich F., et al. Evidence for systematic autopsies in COVID-19 positive deceased. Rechtsmedizin. 2020;30:184–189. doi: 10.1007/s00194-020-00401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Autopsia de COVID-19 La primera autopsia de COVID-19 en España realizada durante las primeras etapas de la pandemia. Rev Esp Patol. 2020;53:182–187. Dirección electrónica: anapat.hrc@salud.madrid.org. [Google Scholar]
- 27.Luo W., Yu H., Gou J., Li X., Sun Y., Li J., et al. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19) Preprints. 2020:2020020407. [Google Scholar]
- 28.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.-Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuang D., Xu S.P., Hu Y., Liu C., Duan Y.Q., Wang G.P. [The pathological changes and related studies of novel coronavirus infected surgical specimen] Zhonghua bing li xue za zhi = Chinese J Pathol. 2020;49:E008. doi: 10.3760/cma.j.cn112151-20200315-00205. [DOI] [PubMed] [Google Scholar]
- 30.Pernazza A., Mancini M., Rullo E., Bassi M., De Giacomo T., Rocca C.D., et al. Early histologic findings of pulmonary SARS-CoV-2 infection detected in a surgical specimen. Virchows Arch. 2020:1–6. doi: 10.1007/s00428-020-02829-1. [published online ahead of print, 2020 Apr 30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M., et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respiratory Med. 2020 Jul;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Zhou P., Wei Y., Yue H., Wang Y., Hu M., et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., et al. [A pathological report of three COVID-19 cases by minimal invasive autopsies] Zhonghua Bing li xue za zhi = Chinese J Pathol. 2020 May;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 36.Li G., Fox S.E., Summa B., Wenk C., Akmatbekov A., Harbert J.L., et al. Multiscale 3-dimensional pathology findings of COVID-19 diseased lung using high-resolution cleared tissue microscopy. bioRxiv. 2020 Apr 11 [Google Scholar]
- 37.Su H., Yang M., Wan C., Yi L.-X., Tang F., Zhu H.-Y., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi G.M., Delsante M., Pilato F.P., Gnetti L., Gabrielli L., Rossini G., et al. Kidney biopsy findings in a critically ill COVID-19 patient with dialysis-dependent acute kidney injury: a case against “SARS-CoV-2 Nephropathy”. Kidney International Reports. 2020 Jul 1;5 doi: 10.1016/j.ekir.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peleg Y., Kudose S., D'Agati V., Siddall E., Ahmad S., Nickolas T., et al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Inter Reports. 2020;5:940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosier H., Farhadian S., Morotti R., Deshmukh U., Lu-Culligan A., Campbell K., et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020 doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolhnikoff M., Duarte-Neto A.N., de Almeida Monteiro R.A., da Silva L.F.F., de Oliveira E.P., Saldiva P.H.N., et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemostasis. 2020;18:1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Enothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbareschi M., Facchetti F., Fraggetta F., Sapino A. What are the priorities of pathologists' activities during COVID-19 emergency? Pathologica. 2020:1–2. doi: 10.32074/1591-951X-15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barth R.F., Xu X., Buja L.M. A call to action: the need for autopsies to determine the full extent of organ involvement associated with COVID-19. Chest. 2020;158:43–44. doi: 10.1016/j.chest.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimes Z., Bryce C., Sordillo E., Gordon R., Reidy J., Paniz-Mondolfi A.E., et al. Fatal pulmonary thromboembolism in SARS-CoV-2-infection. Cardiovasc Pathol. 2020;48:107227. doi: 10.1016/j.carpath.2020.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., et al. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. 2020;33 doi: 10.1128/CMR.00028-20. e00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]