Abstract
Endocrine disrupting chemicals (EDCs) are chemicals that can interfere with normal endocrine signals. Human exposure to EDCs is particularly concerning during vulnerable periods of life, such as pregnancy. However, often overlooked is the effect that EDCs may pose to the placenta. The abundance of hormone receptors makes the placenta highly sensitive to EDCs. We have reviewed the most recent advances in our understanding of EDC exposures on the development and function of the placenta such as steroidogenesis, spiral artery remodeling, drug-transporter expression, implantation and cellular invasion, fusion, and proliferation. EDCs reviewed include those ubiquitous in the environment with available human biomonitoring data. This review also identifies critical gaps in knowledge to drive future research in the field.
Keywords: endocrine disrupting chemicals, placental development, trophoblast dysfunction
1. Introduction
1.a. Environmental chemicals and pregnancy outcomes
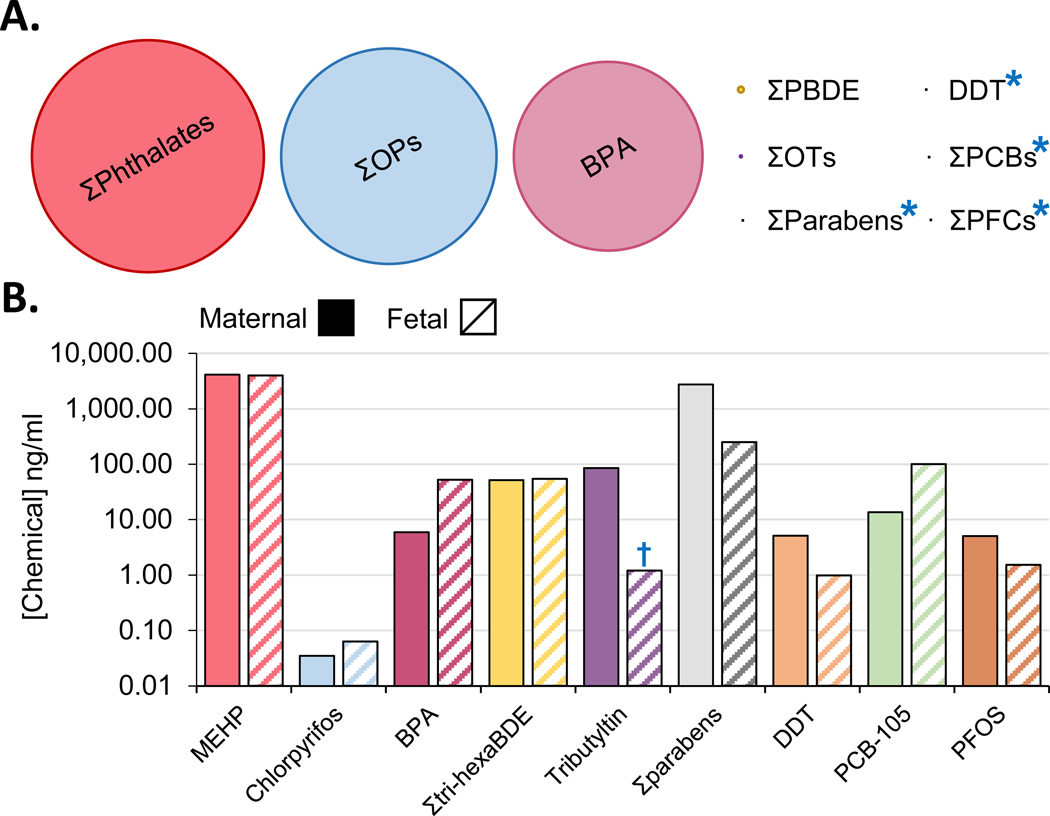
Over 86,000 chemicals are registered with the EPA through the Toxic Substances Control Act [1], and many of which are considered endocrine disrupting chemicals (EDCs) (see Glossary) as their exposure can alter normal endocrine function. Growing evidence supports the notion that these chemicals pose a risk to human health. Particularly concerning are exposures that occur during pregnancy whose effects on the developing fetus lead to long-term postnatal pathologies [2]. Discrepancies between the volume of chemical produced annually and its detection in humans through biomonitoring studies (Figure 1A) [3–11] highlights the fact that higher production does not necessarily translate to a higher human exposure. Chemicals used in everyday personal care products and plastics, such as phthalates, parabens, and bisphenols are detected in human circulation at the highest levels (Figure 1B) [12–20] compared to other high-volume production chemicals, like organophosphates, that are not as prevalent in human circulation.
Figure 1.
(A) Graphical representation of worldwide endocrine disrupting chemicals (EDC) production. (B) Paired maternal (solid bars) blood or urine and umbilical cord blood (hatched bars) EDCs concentrations (B). EDCs included here have been demonstrated to alter placental function (see text for details and Figures 2 and 3 for a graphical summary). BPA: bisphenol A, Σtri-hexaBDE: sum of tri - hexa brominated diphenyl ethers, DDT: dichlorodiphenyltrichloroethane, PCB-105: polychlorinated biphenyl congener #105, PFOS: perfluorooctane sulfonate. Blue asterisk means that the size of the dot is the smallest visible size and thus larger than the total production volume, † means that concentration was taken from placental tissue due to lack of available maternal-fetal paired-matched data.
During pregnancy, women can be exposed to over 50 different chemicals in combination [21, 22], stressing the need to understand the combined effects of the total chemical body burden during pregnancy. Epidemiological studies have begun to associate environmental chemical exposure to pathological pregnancy outcomes on birth weight, placental weight, and more recently pregnancy complications [23–25]. Whether this increased prevalence is related to the ever-increasing exposure to environmental chemicals [26] is a highly active area of research. Epidemiological evidence linking chemical exposures to hypertensive pregnancy disorders prevalence [27] and pregnancy outcomes [28] has been reviewed elsewhere. However, the evidence of chemical exposure outcomes in the context of placental dysfunction is at its infancy, which is the focus of this review.
1.b. The placenta, a transient, vulnerable organ
Pregnancy is a vulnerable period for fetal and maternal health due to the dynamic nature of the developmental and tissue remodeling processes. Pregnancy complications occur in ~19% of pregnancies [29], and include disorders like gestational diabetes, gestational hypertension, preeclampsia, eclampsia, preterm birth, and placenta percreta spectrum disorders. The prevalence of pregnancy complications such as hypertension and postpartum hemorrhaging have steadily increased over the past few decades [30], pointing to environmental exposures as one of the potential contributors to this increasing prevalence [31].
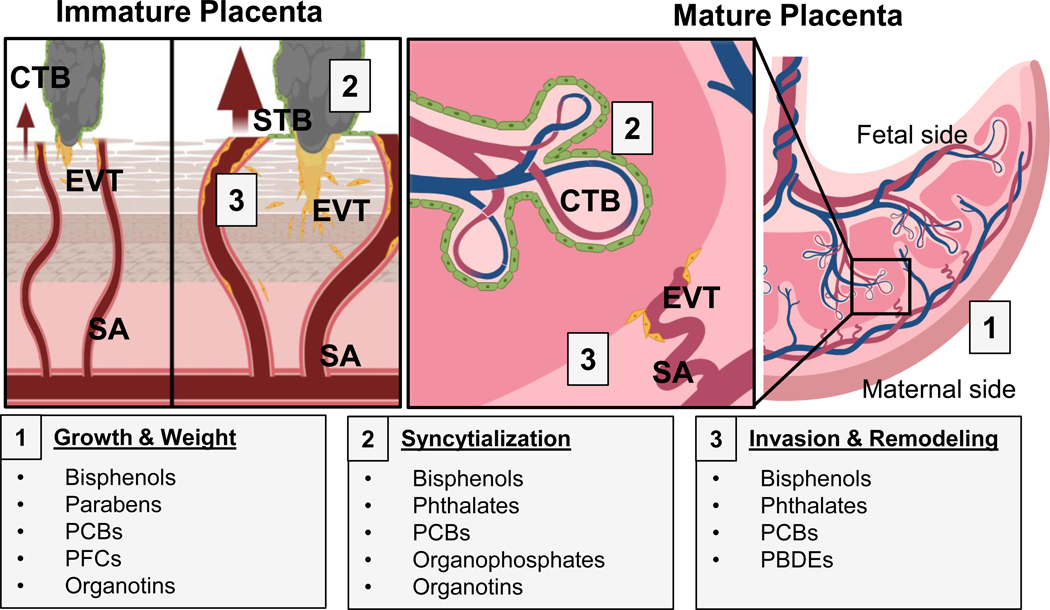
The placenta is a transient multifunctional organ necessary for fetal development that facilitates cholesterol and steroid biosynthesis, and chemical metabolism and transport. Multiple factors including nutrition, stress, and maternal diseases can result in inadequate placental development [32], causing harmful long-lasting effects to the fetus, including cardiovascular and metabolic diseases [33, 34]. Of fetal origin, the placenta develops from the trophectoderm layer of the blastocyst, comprised of stem cells known as cytotrophoblast cells (CTBs). In humans, around day 10 of pregnancy, CTBs begin to differentiate into two functionally different paths, invasion or syncytialization [35]. What determines CTB differentiation to either pathway is still not fully understood, but may be due to gene regulation, epigenetic changes [36], secretory peptides [37], or distinct stem cell populations [38]. Invasive CTBs, known as extravillous trophoblasts (EVTs), migrate away from the primary trophectoderm bundle, forming an anchoring villus (Figure 2). EVTs embed themselves in the maternal uterine lining and upon invasion, act by widening the spiral arteries to increase blood flow to the endometrial space where implantation occurred. As gestation progresses, endometrial vessel remodeling is required to perfuse the main body of the placenta and the developing fetus with maternal blood (Figure 2). Dysregulation in EVT invasion can result in placental defects such as placenta accreta, increta, or percreta, which may result in miscarriage and postpartum hemorrhaging [39]. Concurrently, CTBs begin to terminally fuse, forming a multinucleated syncytium of cells known as syncytiotrophoblasts (STBs) located above the CTB layer, which continue to proliferate and fuse. STBs line the placental villi and act as a barrier for direct maternal blood exposure to the fetus. Functionally, STBs secrete progesterone, human chorionic gonadotropin (hCG) and other proteins [40–42]. Abnormal or poor syncytialization can impair pregnancy through the loss of progesterone production, and has been implicated in abnormal birth pathologies such as preeclampsia and intrauterine growth restriction [43]. Importantly, the syncytium facilitates gas and nutrient exchange between mother and fetus, serving as a semi-permeable barrier for fetal chemical exposures [32].
Figure 2.
Overview of developmental, anatomical, and histopathological sites in the placenta affected by endocrine disrupting chemical (EDC) exposure. In the immature placenta (left), cytotrophoblasts (CTB; grey) may differentiate into two distinct lineages: invasive extravillous trophoblasts (EVT; yellow), and barrier syncytiotrophoblasts (STB; green). EVTs invade into maternal tissues and allow increased maternal blood perfusion (arrows) towards the placenta through spiral artery (SA) remodeling. In the mature placenta (right), CTBs replenish the STB population, and EVT-remodeled SAs bathe the fetal villi in maternal blood. EDC-induced alterations include: placental gross mass/wet weight (grey box 1), CTB fusion/syncytialization (grey box 2), and EVT invasion and SA remodeling (grey box 3). PBDEs: polybrominated diphenyl ethers, PCBs: polychlorinated biphenyls, and PFCs: perfluorinated compounds.
2. Endocrine disrupting chemicals and the placenta
The high abundance of steroid hormone receptor expression in the placenta [32] make it especially vulnerable to endocrine disruption. Research focused to understand the effect of EDCs on placental development has steadily increased over the past 5 years and is presented here by chemical class. Chemical classes included in this review have 1) available biomonitoring data, 2) a reported chemical-induced placental defect and 3) reported congeners that are able to cross from maternal circulation - through the placenta - into fetal circulation [16, 44–52]. As data on heavy metal exposures, like with cadmium, on placenta-specific outcomes is extensive and has been previously summarized [53, 54], those compounds were excluded from this review. World-wide human exposure levels to these chemicals have been previously summarized [13]. Overviews of anatomical sites on the placenta affected by EDC exposure, and their subsequent mechanisms and outcomes are summarized in Figures 2, 3, and 4, respectively.
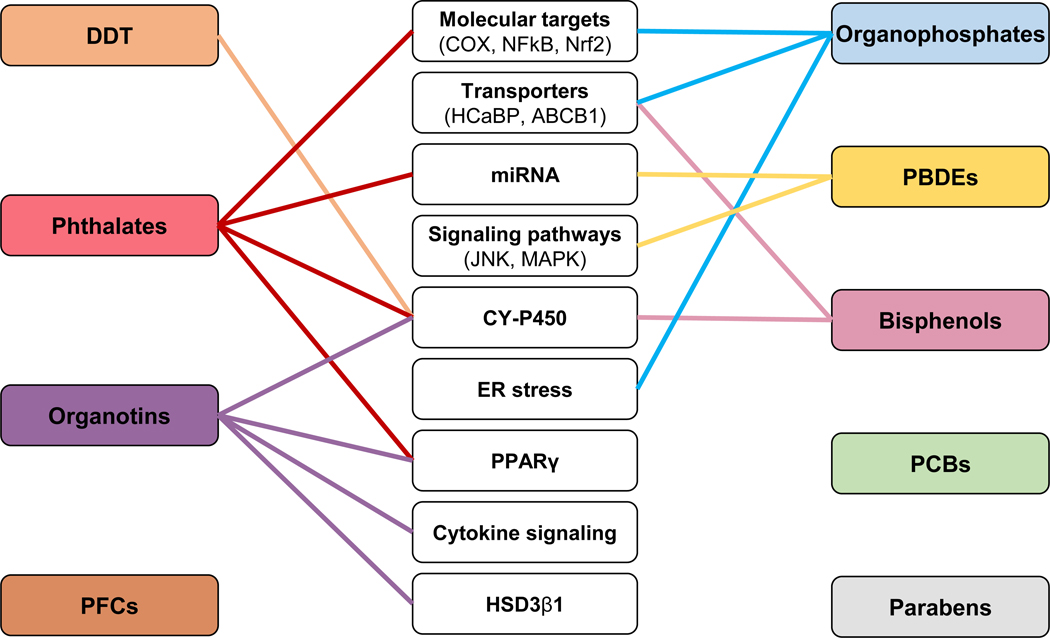
Figure 3.
Summary of mechanisms of action of endocrine disrupting chemicals (EDCs) on the placenta. EDCs reviewed are listed in left and right-side boxes. Potential effectors are shown in center boxes (transporters, signaling pathways, transcriptions factors, enzymes, endoplasmic reticulum stress, and microRNA). PFCs, PCBs, and parabens have no identified mechanisms of action in the context of placental outcomes. See text for additional details. ABCB1: ATP-binding cassette sub-family B member 1, COX: cyclooxygenase, CY-P450: cytochromes P450, DDT: dichlorodiphenyltrichloroethane, ER: endoplasmic reticulum, HCaBP: calcium-binding protein, HSD3β1: hydroxy-Δ-5-steroid dehydrogenase 3-β and steroid Δ-isomerase 1, JNK: c-Jun N terminal kinase, MAPK: p38/mitogen-activated protein kinase, miRNA: microRNA, NFκB: nuclear factor κ-light-chain-enhancer of activated B cells, Nrf2: nuclear factor erythroid 2-related factor 2, PBDEs: polybrominated diphenyl ethers, PCBs: polychlorinated biphenyl, PFCs: perfluorinated compounds, PPARγ: peroxisome proliferator-activated receptor γ.
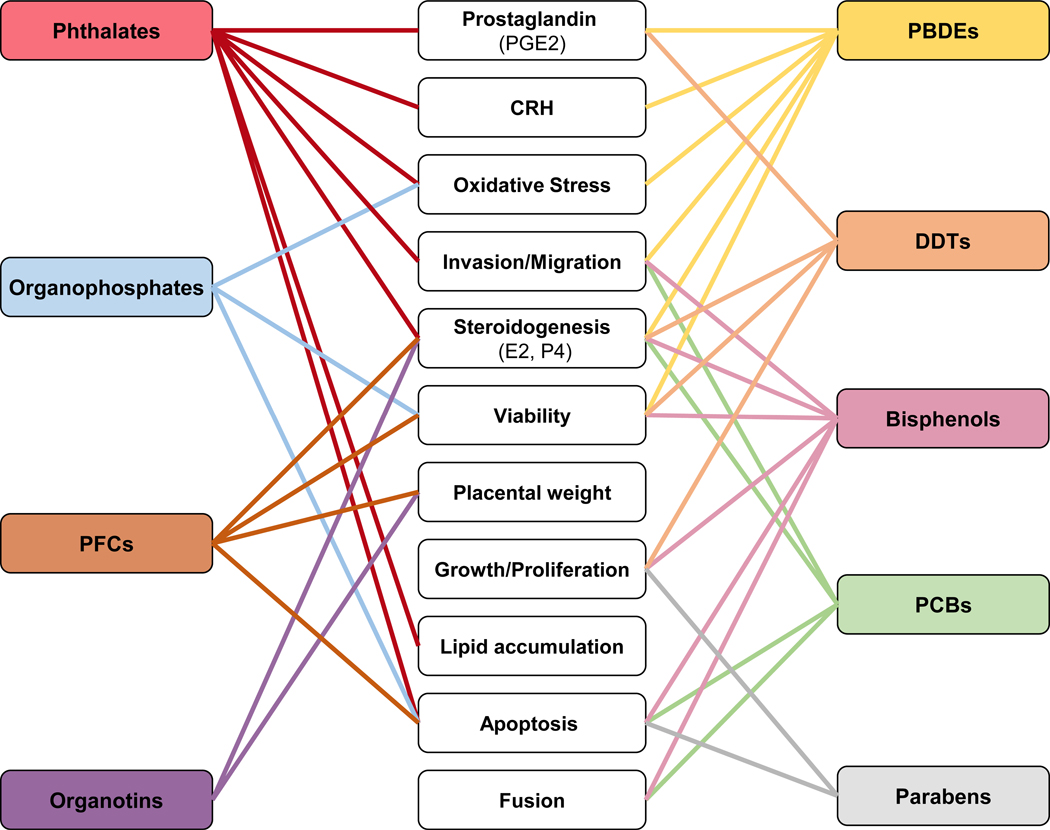
Figure 4.
Summary of functional placental disruptions linked to endocrine disrupting chemical (EDC) exposures on the placenta. EDCs reviewed are listed in left and right-side boxes Potential outcomes are shown in center boxes (hormones production, oxidative stress, invasion/migration, viability, placental weight, cell proliferation, lipid accumulation, apoptosis, and fusion). See text for additional details. DDT: dichlorodiphenyltrichloroethane, PBDEs: polybrominated diphenyl ethers, PCBs: polychlorinated biphenyl, PFCs: perfluorinated compounds, CRHs: corticotropin-releasing hormone, E2: estradiol, P4: progesterone, PGE2: prostaglandin E2.
2.a. Bisphenols
Bisphenols are man-made chemicals widely used in the production of polycarbonate plastics, epoxy resins, and thermal receipt paper [55, 56]. With the exception of bisphenol A (BPA), the effect of bisphenol exposure on the development of the placenta is not well established. Although BPA biomonitoring exposure levels may have been underestimated to date [57], epidemiological data demonstrate a positive association between total BPA concentration in the placenta and placental global methylation [58]. Lower birth weight has also been linked to a higher ratio of BPA concentration in the amniotic fluid vs. maternal plasma in pair-matched samples [44], suggesting that an individual’s placental permeability to bisphenols may be one of the defining factors driving exposure levels and subsequent outcomes.
In vivo exposure to BPA during pregnancy has been studied in doses ranging from 0.002 to 200 mg/kg/day across pregnancy. Much of these data have been already reviewed [59, 60], and highlights BPA’s impact on inducing placental cell apoptosis, labyrinth layer loss, and altered expression of nuclear hormone receptors. Importantly, both intrauterine growth restriction (IUGR)-like [61] and pre-eclampsia-like phenotypes [62] have been reported, and were hypothesized to result from aberrant spiral artery remodeling (Figure 2). At higher doses (BPA: 0.5 mg/kg/day), an IUGR-like phenotype accompanied by placental inflammatory changes has been recently reported in sheep [63]. Studies regarding placenta-specific outcomes following exposure to BPA-analogues, or “replacement” chemicals remain scarce. A single study has reported a placental defect following exposure to bisphenol S (BPS: 0.5 mg/kg/day) [64] with a reduction in binucleate cells, the sheep homologue of human STBs and hypothesized to occur through a cell fusion defect (downregulation of e-cadherin) [64]. Despite lower world-wide exposures to BPS than BPA [65], a recent toxicokinetic study reported a prolonged half-life in fetal circulation [66].
In vitro, BPA exposure at 1,000 μM can either reduce [67] or increase [68] cell proliferation in the choriocarcinoma cell line BeWo - an in vitro model of syncytialization. Functionally, BPA exposure in human metastatic choriocarcinoma-derived JEG-3 cell line (doses: 0.1 – 50 μM) reduced estrogen synthesis [69, 70] and altered cytochrome P450 (CYP) enzymatic activity [71] and protein expression (CYP11A1 [72]; CYP19 [73]; and CYP1A1 [70]) (Figure 3). These exposure conditions disrupted hormone signaling via a reduction in corticotropin gene expression [74]. Of the emerging BPA-analogue compounds [75], only BPS exposure has been studied for placenta-specific outcomes. BPS reduces the activity of the transport protein ATP-binding cassette transporter (ABC) B1 in CRL-1584 cells, a transformed placental epithelial cell line (0.5 nM) [76]. In contrast, BPA can directly stimulate ABCB1 expression in the choriocarcinoma cell line BeWo (10 μM) [77], leading to an increase in drug efflux. In vitro BPA’s effect on EVTs invasiveness and apoptosis has been reviewed [78]. However, the mechanism of action of BPA on EVT invasiveness has not been fully elucidated [78]. BPA has also been shown to have epigenetic effects, increasing microRNA expression at relatively high doses (25 ng/μl BPA) in the EVT cell-line HTR8-SVneo [79]. Despite this breadth of in vitro data demonstrating that bisphenols can have placenta-specific effects, epidemiological evidence supportive of such bisphenol-induced placental dysfunctions in humans remains lacking.
2.b. Phthalates
Phthalates are ubiquitous chemicals present in a myriad of consumer and personal care products, pesticides, and solvents. Found in ~100% of humans tested [45, 80, 81] phthalates tend to be higher in females compared to males [82]. Total maternal urinary metabolites for di-2-ethylhexyl phthalate (ΣDEHP) have been inversely associated with placental weight at term in U.S. and E.U. cohorts, suggestive of placental insufficiency [83, 84]. ΣDEHP urine concentrations are also higher in IUGR pregnancies [85], and associated with lower expression of trophoblast differentiation genes [86] and various long non-coding RNAs (lncRNAs) with unknown placental function [87]. Phthalate exposure during the first trimester has also been negatively associated with the expression and methylation of the epidermal growth factor receptor (EGFR) in placental tissue [25]. Given that EGFR is most abundant in placental tissue compared to any other tissue and the role of EGFR in human placental development [88], the implications of these epigenetic modifications on placental pathology should be investigated.
In vivo data on the effects of phthalate exposure on placental function is limited to rodents and oral exposure to the most historically produced phthalate [89], di-2-ethylhexyl phthalate (DEHP), although other phthalates such as dihexyl phthalate (DHP) and dicyclohexyl phthalate (DCHP) have also been investigated [90]. Phthalate exposure regimens span gestation, in most cases starting at vaginal plug detection, and use doses ranging from 20 to 1,500 mg DEHP/kg/day. Data from these studies has been previously reviewed [59, 60]. In brief, all histopathological observations reported a reduction in the placental labyrinth layer which is analogous to the human syncytium, containing spongiotrophoblast cells similar in function to human STBs [91]. Reduction in the STB population in human placentas has been linked to the pregnancy complication preeclampsia, and is a noted a defect in the placentas from IUGR pregnancies [43]. Gene expression changes in the placenta following DEHP exposure reflect altered fatty acid homeostasis, apoptosis, and angiogenesis which is accompanied by irregular vessel formation in the labyrinth resulting in an IUGR-like phenotype [92] (Figure 2). Importantly, dosing regimens in the above-mentioned studies exceed not only the estimated population daily intake [93], but both the U.S. Environmental Protection Agency’s (EPA) reference dose (RfD) and the E.U. Scientific Committee for Toxicity, Ecotoxicity and the Environment’s the tolerable daily intake for DEHP by, in some cases, over three orders of magnitude [93]. This highlights the need to conduct studies that better reflect environmentally relevant exposure levels in human pregnancies.
Although non-cytotoxic at doses up to 500 μM [94], in vitro studies for the DEHP metabolite monoethylhexyl phthalic acid (MEHP) can induce apoptosis, and increase reactive oxygen species production and DNA damage in HTR-8/SVneo cells [59] (Figure 4). Of note, H2O2-induced oxidative stress alters the expression levels of miRNAs and mRNA expression of genes involved in placental development [95], an effect also observed with miR-16 [96], which plays an important role in MEHP-induced trophoblast cell apoptosis by decreasing B-cell lymphoma 2 (BCL-2) expression [96]. Additionally, in human CTBs, MEHP exposure inhibited hCG production [97], but enhanced mRNA expression of corticotrophin releasing hormone (CRH) [98]. Marked accumulation of glycerolipids and glycerophospholipids in the rat trophoblast cell line HRP1 [99] coupled with altered lipid metabolism in JEG-3 cells [100] also points to a potential phthalate-induced placental lipid imbalance. Although the effect of an altered placental lipidome is not yet understood [100], glycerolipids and glycerophospholipids can inhibit receptor binding of progesterone and estrogen [101]. An enhanced inflammatory response following MEHP exposure was also noted in primary isolated placental macrophages (180 μM) [102] and human CTBs [98] through an increase in cyclooxygenase 2 (COX2) mRNA expression and protein abundance (Figure 3). Even though the mechanisms underlying most of these phenotypes remain elusive, MEHP-induced inflammatory responses appear to be driven through peroxisome proliferator-activated receptor γ (PPARγ) activation [86], of which MEHP has been shown to be a high affinity ligand [103]. Furthermore, it has been demonstrated that HTR-8/SVneo cell invasion is reduced upon MEHP exposure [59].
Similar to other EDCs, the occurrence of phthalates in humans is in a mixture [104]. Phthalates in mixture have been shown to act through PPARγ in a sex-specific manner, and are hypothesized to be PPARγ agonists in females and antagonists in males using primary isolated CTBs [104]. Although phthalates are among the best studied chemicals in the context of placental function, emerging chemicals in DEHP-free plasticizers, such as di(isononyl) cyclohexane 1,2-dicarboxylate (DINCH), di(2-propylheptyl) phthalate (DPHP), di(ethylhexyl) adipate (DEHA), and O-acetyl tributyl citrate (ATBC) [105] have yet to be evaluated.
2.c. Parabens
Generally recognized as safe by the U.S. Food and Drug Administration (FDA), parabens are used as antimicrobial agents in personal care products [106]. To our knowledge, a single epidemiologic study has evaluated placenta-specific outcomes, detecting a placental growth defect via a positive association between total maternal urinary paraben levels and placental weight [83] (Figure 2). In vivo pregnancy exposure studies are restricted to a pharmacokinetic study in pregnant rats where a 3-fold higher concentration of ethylparaben was observed in the placenta vs. the fetal liver [107], suggestive of placental accumulation. Additionally, in humans, the negative association observed between cord blood ethylparaben and testosterone concentrations points to a potential risk for prenatal development [46]. A recent in vitro study using HTR-8/SVneo cells reported that butylparaben exposure inhibits cell proliferation and induces both apoptosis and endoplasmic reticulum stress (200 μM) [108] (Figure 4). However, the specific molecular mechanism(s) of how paraben exposure results in these outcomes remains unexplored. As world-wide exposure to parabens during pregnancy is second most only to phthalates (see Figure 1B), the large discrepancy between known gestational exposure outcomes between EDC classes is likely driven by the fact that parabens are generally recognized as safe. This provides a gap in knowledge worth evaluating, especially in combination with other common chemical exposures.
2.d. Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are man - made organic chemicals used in the production of electrical equipment and building materials, and contain over 200 known congeners [109]. Despite being banned in the U.S. since 1979, many products manufactured earlier still contain PCBs and thus, exposures continue to occur to this day [110]. Because PCBs were used in the form of chemical mixtures (trademark examples include: Arochlor, Clorphen, or Phenochlor) that included several PCB congeners, PBCs were among the first chemicals evaluated as EDC mixtures [111]. The 10–15 years half-life estimated for PCBs results in long-term exposure in humans. Given that PCBs can cross the placental barrier [47] the developing fetus is at risk of PCB exposure. Epidemiologically, PCBs concentrations in the placentas from the Japan Environment and Children’s Study cohort have been associated with a decrease in syncytiotrophoblast volume in the placenta and elevated placental growth factor (PIGF) expression, which stimulates placental vessel branching and spiral artery remodeling [112]. Importantly, birth weight has been inversely correlated with placental PCB concentrations in a Chinese cohort [113], an effect that could be attributed to placental disruption.
Gestational exposure to over two magnitudes of the EPA’s RfD for PCBs ((20 ng/kg BW/day; [114]) disrupts the placental labyrinth layer in rats (20 μg/kg BW/day, PCB-126 [115]) and minks (0.65 mg/day in feed, Clophen A50 [116]). In minks, this effect was combined with altered spiral artery remodeling resulting in fetal growth retardation or demise [116]. However, despite the estrogenic, antiestrogenic, or androgenic effects of PCBs [117, 118], human studies have reported no association between PCB exposure and the risk of spontaneous abortion and/or stillbirth [119]. In vitro models using BeWo cells have demonstrated placental transfer of PCBs with transfer speeds differing across PCB congeners (i.e. PCB-180 transfers more rapidly than PCB-52) [47]. PCB mixtures can induce trophoblast cell apoptosis through upregulation of the adaptive immune response (PCB mixtures #77, #126, and #169: 40–120 μmol/l [120]), disrupt invasion in HTR-8/SVneo cells (10 μg/ml Aroclor 1254 [121]), and induce anti-angiogenic effects at the maternal-fetal interface (Aroclor 1254 [121]) (Figure 4). Bovine placental explants exposed to different doses of a PCB mixture (PCB-153, PCB-126, and PCB-77; 1–100 ng/ml) report increased connexin 43 (Cx43) and 32 (Cx32) expression [122]; of which Cx43 is involved in the intercellular communication required for placental cell fusion [123]. Most PCB congeners are formulated for use as a mixture. This, coupled with the fact that PCBs have an accumulative environmental persistence, identifying any placenta-specific mechanisms responsible for exposure outcomes remain amongst the most challenging.
2.e. Perfluorinated compounds
Produced since the 1950s, perfluorinated compounds (PFCs) are used in the production of antifouling paints, non-stick cookware, and waterproof clothing [124]. While perfluorooctonoic acid (PFOA) and perfluorooctane sulfonate (PFOS) are the most commonly studied PFCs, others persist in the environment [125]. Using pair-matched maternal blood, cord blood, breast milk, and placental samples, epidemiological studies have demonstrated that all PFCs examined (PFOS, PFOA, PFDA, and PFTrDA) are able to enter fetal circulation both prenatally through placental transport, and postnatally during lactation [48, 49]. Recently, umbilical cord blood levels of PFCs perfluorobutane sulfonate (PFBS), perfluorohexane sulfonate (PFHxS) and perfluoroundecanoic acid (PFUA) from a Chinese birth cohort have all been positively associated with preeclampsia [126]. Because of PFCs cumulative nature and long half-lives (~3.4 and ~2.7 years for PFOS and PFOA, respectively [127]), pregnancy exposures are of particular concern. Limited animal studies include mice [128] and rat [129] PFOS exposure through oral gavage (8 – 20 mg/kg/day) during mid-to-late gestation resulting in reduced fetal and placental weights accompanied with placental necrosis [128] (Figure 2), an increase in fetal serum corticosterone [129], and an inhibition of placental 11-β-hydroxysteroid dehydrogenase (HSD) activity [129]. However, doses used were 6 magnitudes higher than EPA’s RfD (20 ng/kg BW/day) for both PFOS and PFOA [130, 131].
In vitro, PFOS modulates steroid hormone signaling by suppressing aromatase production, estradiol secretion, and progesterone production in a concentration-dependent manner in primary isolated human CTBs with effects noted at doses as low as 0.001 μM [132] (Figure 4). Aromatase inhibition was also observed in JEG-3 cells after PFOS, PFOA and PFBS exposure (IC50: 57 – 80 μM [133]. PFOS additionally led to decreased cell viability [132, 133] and induction of apoptosis [132] in the same cell lines (Figure 4). A perfusion model using human placental explants has reported a negative correlation between the organic anion uptake transporter OAT4 and fetal PFOA transfer [134], demonstrating the protective potential of placental OAT4 against fetal PFC exposure. Most in vivo data use rodents, which have been shown to eliminate PFCs more rapidly than humans [135], making them a less than ideal animal model for gestational PFC exposures. Despite the concerns raised from in vitro experiments using human cell lines, studies that focus on lower, more physiologically relevant dosing strategies are necessary to further the toxicological evaluation of PFCs.
2.f. Organophosphate
Organophosphates are esters of phosphoric acid used as insecticides due to their direct interference with the neurotransmitter acetylcholinesterase (AChE), causing systemic muscle paralysis [136]. To our knowledge, no epidemiologic studies exist reporting placenta-specific outcomes in association with organophosphates exposure. However, the assessment of life-long or past organophosphate exposures appears to be a crucial limitation to epidemiologic studies because organophosphates are not persistent in the human body [137]. Despite the existence of over 40 organophosphates, placenta-specific outcomes evaluated are restricted to chlorpyrifos and methyl parathion. Limited mid-to-late gestation exposures (GD 9 – 21) in rats, high dose exposures (10 – 30 mg/kg) to either chlorpyrifos, methyl parathion, or a mixture of the two, inhibited placental AChE activity [138]. Gestational exposure to only methyl parathion (1 – 2 mg/kg) reduced the trophoblast giant cell population, and increased phagosome vacuoles in the labyrinth layer [139] (Figure 2). To note, these cytotoxic findings occurred at doses within two magnitudes of the EPA established RfD for human exposure to organophosphates (0.025 – 100 μg/kg/day [140, 141]).
Chlorpyrifos exposure, even at micromolar concentrations, is also cytotoxic in human placental choriocarcinoma cells (JEG-3 [142]) (Figure 4) and can induce apoptosis in JAR cells through tumor necrosis factor (TNF) modulation [143] (Figure 3). However, not all studies have reported cytotoxicity, even with the same cell line (JEG-3) [144]. Chlorpyrifos also altered expression of pregnancy maintenance markers such as the ABC transporter ABCG2, the transcription factor GCM1 (glial cells missing transcription factor 1) and hormone subunit β-hCG [144], but not progesterone or estradiol production [145]. Additionally, enhanced reactive oxygen species (ROS) production [146] and upregulation of endoplasmic reticulum (ER) stress-related proteins [147] occurred after chlorpyrifos exposure in JEG-3 cells. Attenuation of chlorpyrifos-induced oxidative stress [146] and ER stress [147] occurs through adaptive activation of the Nrf2-antioxidant response element signaling pathway.
One major limitation in the study of the effects of organophosphates exposures is that they are commonly found in mixtures, such as the flame-retardant mixture fire-master (FM) 550, for which the exact composition and RfD are not available. FM 550 accumulates in the placenta to a greater extent in males than females [148], but no association with developmental outcomes has yet been reported. Dosing regimens used in these studies (300 or 1,000 μg/kg/day FM 550 [148, 149]) are both within the RfD range for organophosphates. Due to the sex-specific accumulative nature of FM 550 in the placenta, outcomes following exposure to other organophosphates chemical mixtures as well as long-term fetal exposure outcomes should be further evaluated.
2.g. Dichlorodiphenyltrichloroethane
Dichlorodiphenyltrichloroethane (DDT) is an insecticide whose use has been banned in the U.S. since 1973 due to its environmental persistence (biological half-life: ~7 years). Despite concerns over its estrogenic properties, accumulation in adipose tissue, potential carcinogenicity, and developmental neurotoxicity [150], DDT continues to be used for malaria outbreaks in developing countries [151, 152]. DDT crosses the placenta and enters fetal circulation [51, 153]. Epidemiologically, exposure to DDT has been associated with maternal hypertensive disorders [154]. Associations of DDT and birth weight are cohort dependent with an inverse correlation in a Saudi Arabian cohort [155], and a positive [156] or no correlation [157] in two U.S. cohorts. Despite a breadth of knowledge on human health effects following DDT exposure [158, 159], to our knowledge, no available in vivo studies exploring gestational DDT exposure focus on placenta-specific outcomes. In vitro, DDT exposure results in decreased placenta cell viability at doses higher than 25 μM (HTR-8/SVneo [160]) but did not alter proliferation at lower doses (1 nM) [161] (Figure 4). In bovine placental explants, DDT increased the explant’s expression of prostaglandin E2 (PGE2) synthase, 3β-HSD, and CYP11A1 (doses: 1, 10 or 100 ng/ml [162]) (Figure 3). Alterations in enzyme expressions were accompanied by an increase in oxytocin, estradiol, and progesterone secretion [162]. These effects were also observed in human placental explants [163, 164] along with inhibition of aromatase activity [163]. Given the role of intercellular communication in placental syncytium formation [123], DDT exposure on connexin protein expression was tested, but no effect was observed [122]. Overall, DDT exposure reduces the secretory activity of the placenta, and given its carcinogenic effect [165] and continued commercial use [151], further understanding of its effect upon gestational exposure - specifically on the placenta - is warranted.
2.h. Polybrominated diphenyl ethers
Similar in chemical structure to PCBs, polybrominated diphenyl ethers (PBDEs) are persistent chemicals used as flame retardants in paints, plastics, electrical equipment, and textiles [166]. Of the 209 known PBDE congeners [167], less brominated congeners such as tetra- and penta-BDEs have a high affinity for lipids and tend to accumulate in animals, suggestive of a greater toxic potential [166]. PBDEs can be found in human breast milk, cord blood, and placental tissue [52] where they tend to bioaccumulate [168] and get transferred to the fetus [169, 170]. Interestingly, PBDE concentrations have been reported to be up to two-fold higher in the placentas of males than females [171]. Cord blood concentrations of PBDEs have been negatively correlated with birth weight [172], and inversely correlated with placental DNA methylation changes in human pair-matched samples [173, 174], with changes specific to PBDE congener and methylation site (tetraBDE-66, LINE1; hexaBDE-153, NR3C1 and IGF2; decaBDE-209, IGF2) [174]. Placenta PBDE concentrations have also been positively associated with changes in microRNA (miR)-188–5p and miR-1537 expression (decaBDE-209, [175]). Both miRs have unknown roles in the placenta, but miR-188–5p is abnormally upregulated in pre-eclamptic placentas [176], providing with a potential biomarker for early detection of pregnancy complications. Despite the fact that PBDE accumulates in the fetal portion of the placenta [177], no animal studies exploring placental effects of PBDEs are available. Gestational PBDE exposure in rats results in reduced weight at birth, and has been linked to a loss in maternal triiodothyronine production [177].
PBDE exposure is cytotoxic in second trimester human CTBs at doses over 10 mM (BDE-47 and BDE-99 [178]), significantly reducing cell viability and leading to apoptosis [178] (Figure 4). BDE-47 at the same dose also reduced the migration and invasion of CTBs, and altered lipid and cholesterol metabolism [178]. BDE-47 is the most studied PBDE in the context of placental function, with reported effects on oxidative stress [179] that result in an increase in PGE2 production in HTR-8/SVneo cells [128]. Exposure to PBDE mixtures (congeners: 47, 99, and 100) also resulted in higher PGE2 production in second trimester placental explants [180]. In JEG-3 cells, from doses as low as 0.5 nM and in a dose dependent manner, BDE-47 increased CRH production [181], which has been associated with premature delivery in humans. This same dose-dependent effect was observed in the mRNA expression of signal transduction proteins like protein kinase C-α subunit (PKCα), c-Jun N terminal kinase (JNK), and p38/mitogen-activated protein kinase (MAPK) phosphorylation [181] (Figure 3). Considering that only a fraction of >200 PBDEs have been tested for placenta-specific outcomes and that less studied hydroxylated metabolites of PBDEs (OH-PBDE) can inhibit CYP19 [182], research into the effect of PBDEs on reproductive and placenta-specific outcomes is merited.
2.i. Organotins
Organotin compounds are chemicals with a central tin (Sn) atom and hydrocarbon substituents that are commonly used as polyvinyl chloride stabilizers and biocides [183]. Organotins can cross the placental barrier, resulting in the accumulation of Sn in the conceptus and decidual mass in rat pregnancies [184]. Organotins have been shown to lead to embryonic lethality in non-human primates [185] and rats [186], but just recently have been shown to lead to fetal mortality, conceptus apoptosis and malformations, lower placental weight, thinner labyrinth and basal placental layers (dibutyltin (DBT) chloride, 20 mg/kg, [184]) (Figure 2). Although these in vivo studies were conducted with higher doses than those observed in human exposures, a prospective Danish cohort on cryptorchidism (58 male placental homogenates) found an inverse association between the sum of placental organotins and reverse 3,3’,5’-triiodothyronine (rT3), the third most common iodothyronine [50]. This association was more pronounced in samples with higher a tributyltin (TBT) concentration. However, these findings have yet to be reproduced in an animal study and other human cohorts. Out of the many organotins, only TBT and TPT have been studied extensively in the context of placental dysfunctions in vitro. TBT has been shown to either decrease in micromolar dosages (JEG-3, [187]) or increase at nanomolar concentrations (placental explants, [188]; JAR, [189]) progesterone production (Figure 4). This alteration in steroid hormone production was accompanied by a reduction in 3β-HSD activity (JEG-3, [187]) or an increase in 3β-HSD expression (JAR; [189]) (Figure 3). Higher hCG production was also observed in JAR and JEG-3 cells [190]. These endocrine changes have been shown to be mediated either by PPARγ [189] or retinoid X receptor (RXR) [191]. TBT exposure also resulted in gene expression changes associated with cytokine signaling in non-human primate trophoblast stem cells [192]. Importantly, TBT, a known obesogenic chemical [13], can also increase di- and tri-acylglycerol in JEG-3 cells [193]. Despite the global ban in TBT use for anti-fouling paints in 2008, the use of organotin chemicals continues to be widespread [183] and therefore, studies investigating the effects of organotins on placental function at environmentally relevant doses are needed.
3. Concluding Remarks
This review provides a holistic overview of the current knowledge on placental outcomes following EDC exposures by reviewing epidemiological, in vivo and in vitro data. Despite the seemingly large body of literature, there are many limitations to these studies which are summarized in Box 1. One of the main limitations is the use of supraphysiological dosing regimens, which are often magnitudes higher than human exposures and report directly cytotoxic outcomes. Human biomonitoring and in vivo pharmacokinetic data should be used to develop predictive physiologically-based toxicokinetic mathematical models to establish environmentally relevant dosing strategies. These doses should be coupled with in vitro models that better resemble the in vivo placental microenvironment, such as the use of 3-dimensional models that recreate cell-to-cell interactions and incorporate tissue shear stress and extracellular matrix components [194, 195] (see summary of Future Directions in Box 2). These alternative in vitro models should be used in combination with in vivo studies that integrate relevant animal models to capture more human-representative toxicokinetic profiles or anatomy. For instance, two well established models of studying the placenta for translatability into humans are guinea pig and sheep whose advantages in human placental translatability have been previously summarized [196]. However, few studies have adopted either as animal models.
Box 1: Limitations to current studies.
Most studies evaluating placental toxicity tend to use supraphysiological exposure conditions, thus reducing the relevance for human translatability.
Single chemical toxicity studies are not representative of complex human exposures. In these conditions, there can be both intra- and inter-class mixtures, further complicating data interpretation.
The fundamental lack of knowledge regarding human trophoblast commitment, differentiation, invasion, and specific trophoblast populations make it difficult to develop standardized placental cell isolation and purification processes for toxicological screening. This lack of procedural standardization can lead to a lack of reproducibility. In addition, contradicting in vitro results, as noted for BPA, make the process of in vitro to in vivo translatability challenging.
Currently, there are no available EPA guideline studies for placenta-specific outcomes. This creates not only a barrier for studies which may identify only placenta-specific outcomes from being used in regulatory decision processes, but also makes uniformity among studies difficult.
Placental studies routinely use altricial, litter bearing rodents. Confounding factors like uterine implantation site and fetal position in these species can lead to non-uniform chemical exposures.
The detection of sex-specific effects on placental development following chemical insult calls for the need of immortalized placental cell populations with known sex. However, the lack of non-cancer derived commercially available human trophoblast cell populations with known sex makes understanding sex-specific outcomes difficult. Despite the identification of sex-specific placental outcomes upon chemical exposures, very limited knowledge of such is available.
Box 2: Future directions for EDC-induced placental dysfunction research.
Epidemiologic and/or biomonitoring data should be used to identify emerging “replacement” chemicals. This knowledge will aid to drive in vitro and in vivo toxicological studies.
Use of low dosing strategies more relevant to world-wide human exposures can help strengthen the impact and translatability of placenta-specific toxicological findings.
The use of animal models more suitable for human translatability should be chosen, such as guinea pig, which are a precocial species and have a more comparable placentation to humans. Importantly, such animal studies should be design with enough power to detect sex-specific effects.
The use of a large animal model, like the sheep, would allow non-terminal collection of an intact placenta through natural birth. Because pregnancy would not need to be terminated to collect the placenta in this manner, the offspring can remain as part of a transgenerational study.
While in vivo studies allow for a more holistic and integrative picture of the chemical disruption, in vitro models allow for higher throughput. However, current “standard” in vitro placental cell culture techniques can be limiting.
The use of 3-dimensional culture systems with human cell lines that incorporate cell-cell interactions and shear stress can better mimic the in vivo environment and thus, have greater translational relevance. The placenta has a sex and therefore, in vitro studies should also begin to take into consideration sex as a biological variable.
Greater attention should be paid to the study of chemical mixtures, as they have been associated in complex pregnancy disorders like intrauterine growth restriction and preeclampsia. Identifying pharmacokinetic of pharmacodynamic interactions within mixtures will be critical when interpreting their effects. Additionally, determining if there are causal links between mixture exposures and pregnancy disorders should be a research priority.
Forming interdisciplinary teams to bridge the knowledge gaps between epidemiological and cause-and-effect studies could exponentially advance the already growing body of literature on placental toxicity outcomes.
The development of systematic analyses and standardized exposure protocols will help increase interstudy reproducibility and overall data confidence.
Additionally, to date, most placental studies focus on single chemical exposures with mixture studies predominately restricted to rodent models investigating organophosphate mixtures. Using relevant, complex mixtures in toxicology studies will improve our understanding of the potential pharmacokinetic and pharmacodynamic interactions between chemicals and their effects on placental development and function. Additional complexity to placental toxicological studies is fostered by the lack of EPA guidelines for placenta-specific outcomes. Another aspect that is often unrecognized is that, derived from the embryonic trophoblast layer, the placenta has a defined sex. However, sex-specific effects are most often not reported. In vitro studies are also limited by sex, as commercially available placental cell lines are derived from male pregnancies, or do not account for sex. Recent studies reporting sex-specific associations showing PBDE, organophosphate, and paraben accumulation in the male placenta, with no reported placenta-specific outcomes, highlight the need to further explore this gap in knowledge. Overall, significant advances in risk assessment of EDCs, particularly on understanding exposure effects on the development and function of the placenta, can be made by addressing these limitations and working towards the proposed future directions.
INTERACTIVE QUESTIONS
QUESTION #1
Question (max 240 characters)
-
1)
How many chemicals are women exposed to during pregnancy?
Check correct answer
-
a)
1
-
b)
10
-
c)
30
-
d)
over 50
Explain why this is the correct answer (max. 2500 chars):
Chemical body burden in pregnant women occurs as a mixture. In studies testing up to 60 different chemicals, over 50 chemicals have been detected in a single pregnant woman at any given time.
QUESTION #2
Question (max 240 characters)
-
2)
What are the 3 most common mechanisms that can be altered in placenta cells by endocrine disrupting chemicals (EDC)?
Check correct answer
-
a)
ER stress, transporters and cytokine signaling
-
b)
Cytokine signaling, miRNA and HSD-3β1
-
c)
CY-P450, signaling pathways and molecular targets
-
d)
Transporters, miRNA and ER stress
Explain why this is the correct answer (max. 2500 chars):
Most of the studies report changes in steroidogenesis through altered CY-P450 activity and abundance (bisphenols, organophosphates, PCBs, DDT, phthalates and organotins). Other commonly reporter changes include altered signaling pathways (like JNK and MAPK) by bisphenols, organophosphates, and PCBs, and molecular targets (like COX, NFkB, Nrf2) by PBDEs, organophosphates and phthalates.
QUESTION #3
Question (max 240 characters)
-
3)
What are the gaps in knowledge pertaining to the research of endocrine disrupting chemical (EDC) exposures and placenta development and function?
Check correct answer
-
a)
Studies with mixtures
-
b)
Environmentally relevant doses
-
c)
Sex-specific effects
-
d)
All of the above.
Explain why this is the correct answer (max. 2500 chars):
Chemicals in mixture can have both pharmacokinetic and pharmacodynamic effects on each other. This can include competition for shared receptors (competitive binding), or metabolic substrates. Additional studies are necessary to begin to understand the complexity of mixtures in the placental compartment.
Many studies investigating the effects of EDCs on placental function rely on relatively large or supraphysiological exposure doses to demonstrate a phenotype, but the use of more environmentally relevant exposure doses will better mimic real-world scenarios.
Despite evidence that some EDCs, such as phthalates, organophosphates and PBDEs can have sex-specific effects, this variable is often ignored in placental studies particularly in cell models where placental cell lines used are of unknown or male origin. This is important because sex can drive specific cellular responses.
QUESTION #4
Question (max 240 characters)
-
4)
Which of the following are specific placental functions affected by phthalates? Check correct answer
Check correct answer
-
a)
Invasion
-
b)
Apoptosis
-
c)
Fatty acid homeostasis
-
d)
All of the above
Explain why this is the correct answer (max. 2500 chars):
In vivo studies have demonstrated that gene changes in the placenta following gestational phthalate exposure results in altered fatty acid homeostasis and apoptosis. In vitro studies have shown that phthalates, such as the DEHP metabolite MEHP, can induce apoptosis and inhibit extravillous trophoblast invasion. Additionally, in vitro effects observed from phthalate exposure includes altered lipid metabolism in both human choriocarcinoma (JEG-3) and rat trophoblast (HRP1) cells.
Outstanding questions.
How can maternal chemical body burden be minimized, especially during vulnerable windows of exposure such as pregnancy?
What are the potential pharmacokinetic and pharmacodynamic interactions between chemicals in mixture? Do these interactions affect placental health?
If placental health is disrupted by exposure to an EDC, is the effect carried over transgenerationally?
What are the underlying mechanisms driving sex-specific effects on the placenta, including sex-specific chemical accumulation, such as for organophosphates?
What is the best approach to integrate epidemiologic associations and cause-and-effect animal studies on placenta-specific outcomes?
Are the effects of EDC exposure observed in mice and rats representative of more relevant placental animal models like guinea pigs or non-human primates?
Do EDC-induced effects on placental development observed following high exposure doses represent the effects observed at environmentally relevant doses?
Highlights.
This review covers placenta-specific outcomes associated with exposure to nine major classes of known endocrine disrupting chemicals that cross the placental barrier and are present in human biomonitoring studies.
Despite epidemiologic associations, direct cause and effect links between EDC exposure and the development of complex obstetric disorders, such as preeclampsia, remain a challenge in the field.
Mice are the predominate animal model used for placenta-specific outcomes. More translatable placental mammal models, like guinea pig and non-human primate are underused.
Both in vivo and in vitro toxicological studies focusing on placenta-specific outcomes often use supraphysiological doses.
A limited number of studies dose chemicals in mixture. Additionally, studies that dose with mixtures use intra-class mixtures, not necessarily reflective of the human condition where both intra- and extra-class mixtures occur.
In vitro studies are often limited by the use of commercially available cell lines, which originate from male or unknown sex placentas.
Acknowledgements:
Figure 2 was created with BioRender.com.
Funding sources: Research reported in this publication was supported the National Institute of Environmental Health Sciences of the National Institute of Health (R01ES027863 to A.V-L.), Michigan State University (MSU) General Funds, and MSU AgBioResearch. Funding for J.G. was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NICHD) under Award Number T32HD087166. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- Endocrine Disrupting Chemicals
Natural or man-made chemicals that can interfere with the endocrine system. This includes altered hormone production, secretion, and/or action
- Invasion
Defines the ability of cells to become motile and, through enzymatic secretions, infiltrate into the extracellular matrix within a tissue. During placental development, extravillous trophoblasts (EVT) utilize this process to infiltrate into and remodel maternal uterine arteries
- Placenta accreta
A pregnancy complication in which the placenta invades deeply into the maternal uterine wall and remains partially attached during parturition. This often results in severe post-partum hemorrhage
- Placenta barrier
The placental cell layer in direct contact with the maternal blood. It is semipermeable, which allows transfer of nutrients such as glucose and fatty acids and prevents transfer of larger molecules like insulin. Expressing drug efflux transporters such as the ATP-binding cassette transporter ABCA1, the syncytium serves as a selective membrane to substances passing from maternal to fetal blood and vice versa
- Pregnancy complications
Pathological processes associated with pregnancy. These can occur during or after pregnancy and range from minor discomfort to severe diseases that require medical intervention, such as preeclampsia, eclampsia, or placenta percreta
- Syncytialization
The process of cellular fusion in cytotrophoblasts. Fused cytotrophoblasts form the outer-most layer of the placental villi known as the syncytium. The syncytium is hormonally active and represents the site of maternal and fetal gases and nutrients exchange
Footnotes
Disclosure statement: The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.EPA (2019) TSCA Chemical Substance Inventory. https://www.epa.gov/tsca-inventory/about-tsca-chemical-substance-inventory, (accessed 11/6/2019).
- 2.Gore AC et al. (2015) Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36 (6), 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnbaum LS and Cohen Hubal EA (2006) Polybrominated diphenyl ethers: a case study for using biomonitoring data to address risk assessment questions. Environ Health Perspect 114 (11), 1770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grun F. (2014) The obesogen tributyltin. Vitam Horm 94, 277–325. [DOI] [PubMed] [Google Scholar]
- 5.van den Berg H. (2009) Global status of DDT and its alternatives for use in vector control to prevent disease. Environ Health Perspect 117 (11), 1656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IARC (2016) Polychlorinated Biphenyls and Polybrominated Biphenyls. IARC Monogr Eval Carcinog Risks Hum 107, 9–500. [PMC free article] [PubMed] [Google Scholar]
- 7.Lim TC et al. (2011) Emission inventory for PFOS in China: review of past methodologies and suggestions. ScientificWorldJournal 11, 1963–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grube A et al. , Pesticides Industry Sales and Usage, 2006 and 2007 Market Estimates, in: EPA; (Ed.) 2011. [Google Scholar]
- 9.Kelland K. (2010) Experts demand European action on plastics chemical. https://www.reuters.com/article/us-chemical-bpa-health/experts-demand-european-action-on-plastics-chemical-idUSTRE65L6JN20100622, (accessed 5 December 2019).
- 10.Hassanzadeh N. (2017) Histopathological evaluation of Zebrafish (Danio rerio) testes following exposure to methyl paraben. International Journal of Aquatic Biology 5, 71–78. [Google Scholar]
- 11.Petrovic M et al. (2007) Chemical Analysis of Contaminants in Sediments. Sustainable Management of Sediment Resources 1, 61–129. [Google Scholar]
- 12.Li LX et al. (2013) Exposure levels of environmental endocrine disruptors in mother-newborn pairs in China and their placental transfer characteristics. PLoS One 8 (5), e62526. [Google Scholar]
- 13.Veiga-Lopez A et al. (2018) Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps. Trends Endocrinol Metab 29 (9), 607–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frederiksen M et al. (2010) Polybrominated diphenyl ethers in paired samples of maternal and umbilical cord blood plasma and associations with house dust in a Danish cohort. Int J Hyg Environ Health 213 (4), 233–42. [DOI] [PubMed] [Google Scholar]
- 15.Pycke BF et al. (2015) Maternal and fetal exposure to parabens in a multiethnic urban U.S. population. Environ Int 84, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whyatt RM et al. (2003) Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect 111 (5), 749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rantakokko P et al. (2014) Association of placenta organotin concentrations with growth and ponderal index in 110 newborn boys from Finland during the first 18 months of life: a cohort study. Environ Health 13 (1), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L et al. (2016) Placental Transfer of Perfluoroalkyl Substances and Associations with Thyroid Hormones: Beijing Prenatal Exposure Study. Sci Rep 6, 21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X et al. (2018) Transplacental transfer characteristics of organochlorine pesticides in paired maternal and cord sera, and placentas and possible influencing factors. Environ Pollut 233, 446–454. [DOI] [PubMed] [Google Scholar]
- 20.Fisher M et al. (2016) Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study. Environ Health 15 (1), 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodruff TJ et al. (2011) Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119 (6), 878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johns LE et al. (2017) Urinary BPA and Phthalate Metabolite Concentrations and Plasma Vitamin D Levels in Pregnant Women: A Repeated Measures Analysis. Environ Health Perspect 125 (8), 087026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birks L et al. (2016) Occupational Exposure to Endocrine-Disrupting Chemicals and Birth Weight and Length of Gestation: A European Meta-Analysis. Environ Health Perspect 124 (11), 1785–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsit CJ (2016) Placental Epigenetics in Children’s Environmental Health. Semin Reprod Med 34 (1), 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grindler NM et al. (2018) Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women. Sci Rep 8 (1), 6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skakkebaek NE et al. (2011) The exposure of fetuses and children to endocrine disrupting chemicals: a European Society for Paediatric Endocrinology (ESPE) and Pediatric Endocrine Society (PES) call to action statement. J Clin Endocrinol Metab 96 (10), 3056–8. [DOI] [PubMed] [Google Scholar]
- 27.Kahn LG and Trasande L. (2018) Environmental Toxicant Exposure and Hypertensive Disorders of Pregnancy: Recent Findings. Curr Hypertens Rep 20 (10), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strakovsky RS and Schantz SL (2018) Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ Epigenet 4 (3), dvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavazos-Rehg PA et al. (2015) Maternal age and risk of labor and delivery complications. Matern Child Health J 19 (6), 1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC (2019) Data on Selected Pregnancy Complications in the United States. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications-data.htm, (accessed 11/6/2019).
- 31.Varshavsky J et al. (2019) Heightened susceptibility: A review of how pregnancy and chemical exposures influence maternal health. Reprod Toxicol. May 2. pii: S0890–6238(18)30434–9. doi: 10.1016/j.reprotox.2019.04.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fowden AL et al. (2015) Review: Endocrine regulation of placental phenotype. Placenta 36 Suppl 1, S50–9. [DOI] [PubMed] [Google Scholar]
- 33.Burton GJ et al. (2016) Placental Origins of Chronic Disease. Physiol Rev 96 (4), 1509–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marciniak A et al. (2017) Fetal programming of the metabolic syndrome. Taiwan J Obstet Gynecol 56 (2), 133–138. [DOI] [PubMed] [Google Scholar]
- 35.Cross JC et al. (1994) Implantation and the placenta: key pieces of the development puzzle. Science 266 (5190), 1508–18. [DOI] [PubMed] [Google Scholar]
- 36.Kwak YT et al. (2019) Human Trophoblast Differentiation Is Associated With Profound Gene Regulatory and Epigenetic Changes. Endocrinology 160 (9), 2189–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrish DW et al. (2001) Life and death in the placenta: new peptides and genes regulating human syncytiotrophoblast and extravillous cytotrophoblast lineage formation and renewal. Curr Protein Pept Sci 2 (3), 245–59. [DOI] [PubMed] [Google Scholar]
- 38.James JL et al. (2005) Cytotrophoblast differentiation in the first trimester of pregnancy: evidence for separate progenitors of extravillous trophoblasts and syncytiotrophoblast. Reproduction 130 (1), 95–103. [DOI] [PubMed] [Google Scholar]
- 39.Shamshirsaz AA et al. (2019) Coagulopathy in surgical management of placenta accreta spectrum. Eur J Obstet Gynecol Reprod Biol 237, 126–130. [DOI] [PubMed] [Google Scholar]
- 40.Yabe S et al. (2016) Comparison of syncytiotrophoblast generated from human embryonic stem cells and from term placentas. Proc Natl Acad Sci U S A 113 (19), E2598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar P and Magon N. (2012) Hormones in pregnancy. Niger Med J 53 (4), 179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guller S. (2009) Role of the syncytium in placenta-mediated complications of preeclampsia. Thromb Res 124 (4), 389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu F et al. (2015) Oxidative Stress in Placenta: Health and Diseases. Biomed Res Int 2015, 293271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zbucka-Kretowska M et al. (2019) Simultaneous analysis of bisphenol A fractions in maternal and fetal compartments in early second trimester of pregnancy. J Perinat Med 47 (7), 765–770. [DOI] [PubMed] [Google Scholar]
- 45.Hogberg J et al. (2008) Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect 116 (3), 334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolatorova L et al. (2018) Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ Res 163, 115–122. [DOI] [PubMed] [Google Scholar]
- 47.Correia Carreira S et al. (2011) Studying placental transfer of highly purified non-dioxin-like PCBs in two models of the placental barrier. Placenta 32 (3), 283–91. [DOI] [PubMed] [Google Scholar]
- 48.Chen F et al. (2017) Chlorinated Polyfluoroalkyl Ether Sulfonic Acids in Matched Maternal, Cord, and Placenta Samples: A Study of Transplacental Transfer. Environ Sci Technol 51 (11), 6387–6394. [DOI] [PubMed] [Google Scholar]
- 49.Liu J et al. (2011) Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environ Int 37 (7), 1206–12. [DOI] [PubMed] [Google Scholar]
- 50.Li ZM et al. (2018) Association of In Utero Persistent Organic Pollutant Exposure With Placental Thyroid Hormones. Endocrinology 159 (10), 3473–3481. [DOI] [PubMed] [Google Scholar]
- 51.Sapbamrer R et al. (2008) Placental transfer of DDT in mother-infant pairs from Northern Thailand. J Environ Sci Health B 43 (6), 484–9. [DOI] [PubMed] [Google Scholar]
- 52.Tang J and Zhai JX (2017) Distribution of polybrominated diphenyl ethers in breast milk, cord blood and placentas: a systematic review. Environ Sci Pollut Res Int 24 (27), 21548–21573. [DOI] [PubMed] [Google Scholar]
- 53.Caserta D et al. (2013) Heavy metals and placental fetal-maternal barrier: a mini-review on the major concerns. Eur Rev Med Pharmacol Sci 17 (16), 2198–206. [PubMed] [Google Scholar]
- 54.Geng HX and Wang L. (2019) Cadmium: Toxic effects on placental and embryonic development. Environ Toxicol Pharmacol 67, 102–107. [DOI] [PubMed] [Google Scholar]
- 55.Eckardt M and Simat TJ (2017) Bisphenol A and alternatives in thermal paper receipts - a German market analysis from 2015 to 2017. Chemosphere 186, 1016–1025. [DOI] [PubMed] [Google Scholar]
- 56.Konieczna A et al. (2015) Health risk of exposure to Bisphenol A (BPA). Rocz Panstw Zakl Hig 66 (1), 5–11. [PubMed] [Google Scholar]
- 57.Gerona R et al. (2019) BPA: have flawed analytical techniques compromised risk assessments? Lancet Diabetes Endocrinol. [DOI] [PubMed] [Google Scholar]
- 58.Nahar MS et al. (2015) In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere 124, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strakovsky RS and Schantz SL (2018) Using Experimental Models to Assess Effects of Bisphenol A (BPA) and Phthalates on the Placenta: Challenges and Perspectives. Toxicol Sci 166 (2), 250–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vrooman LA et al. (2016) Morphologic and molecular changes in the placenta: what we can learn from environmental exposures. Fertil Steril 106 (4), 930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller JE et al. (2018) Bisphenol A exposure during early pregnancy impairs uterine spiral artery remodeling and provokes intrauterine growth restriction in mice. Sci Rep 8 (1), 9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye Y et al. (2019) Bisphenol A exposure alters placentation and causes preeclampsia-like features in pregnant mice involved in reprogramming of DNA methylation of WNT2. FASEB J 33 (2), 2732–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song W et al. (2019) Developmental programming: Prenatal bisphenol A treatment disrupts mediators of placental function in sheep. Chemosphere 243, 125301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gingrich J et al. (2018) Gestational bisphenol S impairs placental endocrine function and the fusogenic trophoblast signaling pathway. Arch Toxicol 92 (5), 1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lehmler HJ et al. (2018) Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 3 (6), 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gingrich J et al. (2019) Toxicokinetics of bisphenol A, bisphenol S, and bisphenol F in a pregnancy sheep model. Chemosphere 220, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang ZY et al. (2015) Effect of Bisphenol A on invasion ability of human trophoblastic cell line BeWo. Int J Clin Exp Pathol 8 (11), 14355–64. [PMC free article] [PubMed] [Google Scholar]
- 68.Ponniah M et al. (2015) Bisphenol A increases BeWo trophoblast survival in stress-induced paradigms through regulation of oxidative stress and apoptosis. Chem Res Toxicol 28 (9), 1693–703. [DOI] [PubMed] [Google Scholar]
- 69.Huang H and Leung LK (2009) Bisphenol A downregulates CYP19 transcription in JEG-3 cells. Toxicol Lett 189 (3), 248–52. [DOI] [PubMed] [Google Scholar]
- 70.Xu H et al. (2019) Bisphenol A affects estradiol metabolism by targeting CYP1A1 and CYP19A1 in human placental JEG-3 cells. Toxicol In Vitro 61, 104615. [DOI] [PubMed] [Google Scholar]
- 71.Nativelle-Serpentini C et al. (2003) Aromatase activity modulation by lindane and bisphenol-A in human placental JEG-3 and transfected kidney E293 cells. Toxicol In Vitro 17 (4), 413–22. [DOI] [PubMed] [Google Scholar]
- 72.Chu PW et al. (2018) Low-dose bisphenol A activates the ERK signaling pathway and attenuates steroidogenic gene expression in human placental cells. Biol Reprod 98 (2), 250–258. [DOI] [PubMed] [Google Scholar]
- 73.Marqueno A et al. (2019) Toxic effects of bisphenol A diglycidyl ether and derivatives in human placental cells. Environ Pollut 244, 513–521. [DOI] [PubMed] [Google Scholar]
- 74.Basak S et al. (2018) Bisphenol-A impairs cellular function and alters DNA methylation of stress pathway genes in first trimester trophoblast cells. Reprod Toxicol 82, 72–79. [DOI] [PubMed] [Google Scholar]
- 75.Chen D et al. (2016) Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ Sci Technol 50 (11), 5438–53. [DOI] [PubMed] [Google Scholar]
- 76.Speidel JT et al. (2018) Bisphenol A (BPA) and bisphenol S (BPS) alter the promoter activity of the ABCB1 gene encoding P-glycoprotein in the human placenta in a haplotype-dependent manner. Toxicol Appl Pharmacol 359, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin H and Audus KL (2005) Effect of bisphenol A on drug efflux in BeWo, a human trophoblast-like cell line. Placenta 26 Suppl A, S96–S103. [DOI] [PubMed] [Google Scholar]
- 78.Yang C et al. (2019) A mechanism for the effect of endocrine disrupting chemicals on placentation. Chemosphere 231, 326–336. [DOI] [PubMed] [Google Scholar]
- 79.Avissar-Whiting M et al. (2010) Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol 29 (4), 401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva MJ et al. (2004) Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 112 (3), 331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marsee K et al. (2006) Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect 114 (6), 805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.James-Todd T et al. (2012) Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ Health Perspect 120 (9), 1307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Philippat C et al. (2019) Prenatal Exposure to Select Phthalates and Phenols and Associations with Fetal and Placental Weight among Male Births in the EDEN Cohort (France). Environ Health Perspect 127 (1), 17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mustieles V et al. (2019) Placental weight in relation to maternal and paternal preconception and prenatal urinary phthalate metabolite concentrations among subfertile couples. Environ Res 169, 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Y et al. (2015) Prenatal phthalate exposure, infant growth, and global DNA methylation of human placenta. Environ Mol Mutagen 56 (3), 286–92. [DOI] [PubMed] [Google Scholar]
- 86.Adibi JJ et al. (2010) Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure. Environ Health Perspect 118 (2), 291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Machtinger R et al. (2018) Placental lncRNA Expression Is Associated With Prenatal Phthalate Exposure. Toxicol Sci 163 (1), 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whigham CA et al. (2019) The untapped potential of placenta-enriched molecules for diagnostic and therapeutic development. Placenta 84, 28–31. [DOI] [PubMed] [Google Scholar]
- 89.vom Saal FS et al. (2008) Endocrine disruptors: effect in wildlife and laboratory animals In Encyclopedia of Ecology (1st edn) (Jorgensen SE and Fath BD eds), pp. 1261–1264, Oxford. [Google Scholar]
- 90.Ahbab MA et al. (2017) Comparative developmental toxicity evaluation of di- n-hexyl phthalate and dicyclohexyl phthalate in rats. Toxicol Ind Health 33 (9), 696–716. [DOI] [PubMed] [Google Scholar]
- 91.Bradbury J. (2008) Secrets of the placental labyrinth unlocked. Development 135 (12), e1201-e1201. [Google Scholar]
- 92.Yu Z et al. (2018) Gestational di-(2-ethylhexyl) phthalate exposure causes fetal intrauterine growth restriction through disturbing placental thyroid hormone receptor signaling. Toxicol Lett 294, 1–10. [DOI] [PubMed] [Google Scholar]
- 93.Koch HM et al. (2003) An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int J Hyg Environ Health 206 (2), 77–83. [DOI] [PubMed] [Google Scholar]
- 94.Perez-Albaladejo E et al. (2017) Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in JEG-3 human placental cells. Toxicol In Vitro 38, 41–48. [DOI] [PubMed] [Google Scholar]
- 95.Cross CE et al. (2015) Oxidative Stress Alters miRNA and Gene Expression Profiles in Villous First Trimester Trophoblasts. Biomed Res Int 2015, 257090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meruvu S et al. (2016) Mono-(2-ethylhexyl) Phthalate Increases Oxidative Stress Responsive miRNAs in First Trimester Placental Cell Line HTR8/SVneo. Chem Res Toxicol 29 (3), 430–5. [DOI] [PubMed] [Google Scholar]
- 97.Shoaito H et al. (2019) The Role of Peroxisome Proliferator-Activated Receptor Gamma (PPARgamma) in Mono(2-ethylhexyl) Phthalate (MEHP)-Mediated Cytotrophoblast Differentiation. Environ Health Perspect 127 (2), 27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang XK et al. (2016) Mono-(2-Ethylhexyl) Phthalate Promotes Pro-Labor Gene Expression in the Human Placenta. PLoS One 11 (1), e0147013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu Y et al. (2006) Effects of di-(2-ethylhexyl)-phthalate and its metabolites on the lipid profiling in rat HRP-1 trophoblast cells. Arch Toxicol 80 (5), 293–8. [DOI] [PubMed] [Google Scholar]
- 100.Petit J et al. (2018) Lipidome-wide disturbances of human placental JEG-3cells by the presence of MEHP. Biochimie 149, 1–8. [DOI] [PubMed] [Google Scholar]
- 101.Pulkkinen M and Hamalainen MM (1995) Myometrial estrogen and progesterone receptor binding in pregnancy: inhibition by the detergent action of phospholipids. J Steroid Biochem Mol Biol 52 (3), 287–94. [DOI] [PubMed] [Google Scholar]
- 102.Tetz LM et al. (2015) Mono-ethylhexyl phthalate stimulates prostaglandin secretion in human placental macrophages and THP-1 cells. Reprod Biol Endocrinol 13, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hurst CH and Waxman DJ (2003) Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci 74 (2), 297–308. [DOI] [PubMed] [Google Scholar]
- 104.Adibi JJ et al. (2017) An Investigation of the Single and Combined Phthalate Metabolite Effects on Human Chorionic Gonadotropin Expression in Placental Cells. Environ Health Perspect 125 (10), 107010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salthammer T. (2020) Emerging indoor pollutants. Int J Hyg Environ Health 224, 113423. [DOI] [PubMed] [Google Scholar]
- 106.Halla N et al. (2018) Cosmetics Preservation: A Review on Present Strategies. Molecules 23 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frederiksen H et al. (2008) Higher levels of ethyl paraben and butyl paraben in rat amniotic fluid than in maternal plasma after subcutaneous administration. Toxicol Sci 106 (2), 376–83. [DOI] [PubMed] [Google Scholar]
- 108.Yang C et al. (2018) Butyl paraben promotes apoptosis in human trophoblast cells through increased oxidative stress-induced endoplasmic reticulum stress. Environ Toxicol 33 (4), 436–445. [DOI] [PubMed] [Google Scholar]
- 109.EPA (2003) Table of Polychlorinated Biphenyl (PCB) Congeners. https://www.epa.gov/pcbs/table-polychlorinated-biphenyl-pcb-congeners, (accessed 12 November 2019).
- 110.Muller MHB et al. (2019) Prenatal exposure to persistent organic pollutants in Northern Tanzania and their distribution between breast milk, maternal blood, placenta and cord blood. Environ Res 170, 433–442. [DOI] [PubMed] [Google Scholar]
- 111.Baker FD et al. (1977) Toxicity and persistence of low-level PCB in adult wistar rats, fetuses, and young. Arch Environ Contam Toxicol 5 (2), 143–56. [DOI] [PubMed] [Google Scholar]
- 112.Tsuji M et al. (2013) Polychlorinated biphenyls (PCBs) decrease the placental syncytiotrophoblast volume and increase Placental Growth Factor (PlGF) in the placenta of normal pregnancy. Placenta 34 (7), 619–23. [DOI] [PubMed] [Google Scholar]
- 113.Wu K et al. (2011) In utero exposure to polychlorinated biphenyls and reduced neonatal physiological development from Guiyu, China. Ecotoxicol Environ Saf 74 (8), 2141–7. [DOI] [PubMed] [Google Scholar]
- 114.EPA (2017) Exposure Levels for Evaluating Polychlorinated Biphenyls (PCBs) in Indoor School Air. https://www.epa.gov/pcbs/exposure-levels-evaluating-polychlorinated-biphenyls-pcbs-indoor-school-air, (accessed 11/12/2019).
- 115.Ahmed RG et al. (2018) Gestational 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) exposure disrupts fetoplacental unit: Fetal thyroid-cytokines dysfunction. Life Sci 192, 213–220. [DOI] [PubMed] [Google Scholar]
- 116.Backlin BM et al. (1998) Polychlorinated biphenyl (PCB) exposure produces placental vascular and trophoblastic lesions in the mink (Mustela vison): a light and electron microscopic study. APMIS 106 (8), 785–99. [DOI] [PubMed] [Google Scholar]
- 117.Drenth HJ et al. (1998) Effects of some persistent halogenated environmental contaminants on aromatase (CYP19) activity in the human choriocarcinoma cell line JEG-3. Toxicol Appl Pharmacol 148 (1), 50–5. [DOI] [PubMed] [Google Scholar]
- 118.Svobodova K et al. (2009) Estrogenic and androgenic activity of PCBs, their chlorinated metabolites and other endocrine disruptors estimated with two in vitro yeast assays. Sci Total Environ 407 (22), 5921–5. [DOI] [PubMed] [Google Scholar]
- 119.Small CM et al. (2007) Risk of spontaneous abortion among women exposed to polybrominated biphenyls. Environ Res 105 (2), 247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gu PQ et al. (2012) Endocrine disruptors, polychlorinated biphenyls-induced gC1qR-dependent apoptosis in human trophoblast cell line HTR-8/SVneo. Reprod Sci 19 (2), 181–9. [DOI] [PubMed] [Google Scholar]
- 121.Kalkunte S et al. (2017) Polychlorinated biphenyls target Notch/Dll and VEGF R2 in the mouse placenta and human trophoblast cell lines for their anti-angiogenic effects. Sci Rep 7, 39885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wojciechowska A et al. (2018) The protein expression disorders of connexins (Cx26, Cx32 and Cx43)and keratin 8 in bovine placenta under the influence of DDT, DDE and PCBs. Pol J Vet Sci 21 (4), 721–729. [DOI] [PubMed] [Google Scholar]
- 123.Cronier L et al. (2003) Requirement of gap junctional intercellular communication for human villous trophoblast differentiation. Biol Reprod 69 (5), 1472–80. [DOI] [PubMed] [Google Scholar]
- 124.Corsini E et al. (2014) Perfluorinated compounds: emerging POPs with potential immunotoxicity. Toxicol Lett 230 (2), 263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dorman F RE (2012) Chapter 28 - Emerging and Persistent Environmental Compound Analysis, Elsevier. [Google Scholar]
- 126.Huang R et al. (2019) Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environ Health 18 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y et al. (2018) Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75 (1), 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee CK et al. (2015) Effects of perfluorooctane sulfuric acid on placental PRL-family hormone production and fetal growth retardation in mice. Mol Cell Endocrinol 401, 165–72. [DOI] [PubMed] [Google Scholar]
- 129.Li X et al. (2016) In utero perfluorooctane sulfonate exposure causes low body weights of fetal rats: A mechanism study. Placenta 39, 125–33. [DOI] [PubMed] [Google Scholar]
- 130.EPA (2016) Health Effects Support Document for PFOS. https://www.epa.gov/sites/production/files/2016-05/documents/pfos_hesd_final_508.pdf, (accessed 13 December 2019).
- 131.EPA (2016) Health Effects Support Document for PFOA. https://www.epa.gov/sites/production/files/2016-05/documents/pfoa_hesd_final_508.pdf, (accessed 11/6/2019).
- 132.Zhang N et al. (2015) Reduction of progesterone, estradiol and hCG secretion by perfluorooctane sulfonate via induction of apoptosis in human placental syncytiotrophoblasts. Placenta 36 (5), 575–80. [DOI] [PubMed] [Google Scholar]
- 133.Gorrochategui E et al. (2014) Perfluorinated chemicals: differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol Appl Pharmacol 277 (2), 124–30. [DOI] [PubMed] [Google Scholar]
- 134.Kummu M et al. (2015) Organic anion transporter 4 (OAT 4) modifies placental transfer of perfluorinated alkyl acids PFOS and PFOA in human placental ex vivo perfusion system. Placenta 36 (10), 1185–91. [DOI] [PubMed] [Google Scholar]
- 135.Filgo AJ et al. (2015) Perfluorooctanoic Acid (PFOA)-induced Liver Lesions in Two Strains of Mice Following Developmental Exposures: PPARalpha Is Not Required. Toxicol Pathol 43 (4), 558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Colovic MB et al. (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11 (3), 315–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Blanc-Lapierre A et al. (2013) Cognitive disorders and occupational exposure to organophosphates: results from the PHYTONER study. Am J Epidemiol 177 (10), 1086–96. [DOI] [PubMed] [Google Scholar]
- 138.Abu-Qare AW et al. (2001) Inhibition of cholinesterase enzymes following a single dermal dose of chlorpyrifos and methyl parathion, alone and in combination, in pregnant rats. J Toxicol Environ Health A 63 (3), 173–89. [DOI] [PubMed] [Google Scholar]
- 139.Levario-Carrillo M et al. (2004) Placental morphology of rats prenatally exposed to methyl parathion. Exp Toxicol Pathol 55 (6), 489–96. [DOI] [PubMed] [Google Scholar]
- 140.Clegg DJ and van Gemert M. (1999) Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. J Toxicol Environ Health B Crit Rev 2 (3), 211–55. [DOI] [PubMed] [Google Scholar]
- 141.NCBI Compound summary: methyl parathion, CID=4130. https://pubchem.ncbi.nlm.nih.gov/compound/Methyl-parathion, (accessed 11/12/2019).
- 142.Guinazu N et al. (2012) Effects of the organophosphate insecticides phosmet and chlorpyrifos on trophoblast JEG-3 cell death, proliferation and inflammatory molecule production. Toxicol In Vitro 26 (3), 406–13. [DOI] [PubMed] [Google Scholar]
- 143.Saulsbury MD et al. (2008) Characterization of chlorpyrifos-induced apoptosis in placental cells. Toxicology 244 (2–3), 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ridano ME et al. (2012) Chlorpyrifos modifies the expression of genes involved in human placental function. Reprod Toxicol 33 (3), 331–8. [DOI] [PubMed] [Google Scholar]
- 145.Rieke S et al. (2014) Combination effects of (tri)azole fungicides on hormone production and xenobiotic metabolism in a human placental cell line. Int J Environ Res Public Health 11 (9), 9660–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chiapella G et al. (2013) The organophosphate chlorpyrifos disturbs redox balance and triggers antioxidant defense mechanisms in JEG-3 cells. Placenta 34 (9), 792–8. [DOI] [PubMed] [Google Scholar]
- 147.Reyna L et al. (2017) Chlorpyrifos induces endoplasmic reticulum stress in JEG-3 cells. Toxicol In Vitro 40, 88–93. [DOI] [PubMed] [Google Scholar]
- 148.Baldwin KR et al. (2017) Sex Specific Placental Accumulation and Behavioral Effects of Developmental Firemaster 550 Exposure in Wistar Rats. Sci Rep 7 (1), 7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rock KD et al. (2018) EDC IMPACT: Molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain. Endocr Connect 7 (2), 305–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Turusov V et al. (2002) Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect 110 (2), 125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van den Berg H et al. (2017) Global trends in the production and use of DDT for control of malaria and other vector-borne diseases. Malar J 16 (1), 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bouwman H et al. (2011) DDT and malaria prevention: addressing the paradox. Environ Health Perspect 119 (6), 744–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ando M. (1978) Transfer of 2,4,5,2’,4’,5’-hexachlorobiphenyl and 2,2-bis-(p-chlorophenyl),1,1,1-trichloroethane (p,p’-DDT) from maternal to newborn and suckling rats. Arch Toxicol 41 (3), 179–86. [DOI] [PubMed] [Google Scholar]
- 154.Murray J et al. (2018) Exposure to DDT and hypertensive disorders of pregnancy among South African women from an indoor residual spraying region: The VHEMBE study. Environ Res 162, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Al-Saleh I et al. (2012) Levels of DDT and its metabolites in placenta, maternal and cord blood and their potential influence on neonatal anthropometric measures. Sci Total Environ 416, 62–74. [DOI] [PubMed] [Google Scholar]
- 156.Kezios KL et al. (2013) Dichlorodiphenyltrichloroethane (DDT), DDT metabolites and pregnancy outcomes. Reprod Toxicol 35, 156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Farhang L et al. (2005) Association of DDT and DDE with birth weight and length of gestation in the Child Health and Development Studies, 1959–1967. Am J Epidemiol 162 (8), 717–25. [DOI] [PubMed] [Google Scholar]
- 158.Longnecker MP et al. (1997) The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu Rev Public Health 18, 211–44. [DOI] [PubMed] [Google Scholar]
- 159.Merida-Ortega A et al. (2019) Polyunsaturated fatty acids and child neurodevelopment among a population exposed to DDT: a cohort study. Environ Health 18 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dominguez-Lopez P et al. (2012) Differential effect of DDT, DDE, and DDD on COX-2 expression in the human trophoblast derived HTR-8/SVneo cells. J Biochem Mol Toxicol 26 (11), 454–60. [DOI] [PubMed] [Google Scholar]
- 161.Derfoul A et al. (2003) Estrogenic endocrine disruptive components interfere with calcium handling and differentiation of human trophoblast cells. J Cell Biochem 89 (4), 755–70. [DOI] [PubMed] [Google Scholar]
- 162.Wojciechowska A et al. (2017) Changes in the mRNA expression of structural proteins, hormone synthesis and secretion from bovine placentome sections after DDT and DDE treatment. Toxicology 375, 1–9. [DOI] [PubMed] [Google Scholar]
- 163.Wojtowicz AK et al. (2007) DDT and its metabolite DDE alter steroid hormone secretion in human term placental explants by regulation of aromatase activity. Toxicol Lett 173 (1), 24–30. [DOI] [PubMed] [Google Scholar]
- 164.Wojtowicz AK et al. (2008) Time-dependent action of DDT (1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane) and its metabolite DDE (1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene) on human chorionic gonadotropin and progesterone secretion. Gynecol Endocrinol 24 (1), 54–8. [DOI] [PubMed] [Google Scholar]
- 165.Harada T et al. (2016) Toxicity and Carcinogenicity of Dichlorodiphenyltrichloroethane (DDT). Toxicol Res 32 (1), 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Siddiqi MA et al. (2003) Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases. Clin Med Res 1 (4), 281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Accustandard(2017)FlameRetardantStandardsGuide. https://www.accustandard.com/assets/Flame_Retardant_Guide.pdf, (accessed 11/12/2019).
- 168.Leonetti C et al. (2016) Concentrations of polybrominated diphenyl ethers (PBDEs) and 2,4,6-tribromophenol in human placental tissues. Environ Int 88, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhao Y et al. (2013) Polybrominated diphenyl ethers (PBDEs) in aborted human foetuses and placental transfer during the first trimester of pregnancy. Environ Sci Technol 47 (11), 5939–46. [DOI] [PubMed] [Google Scholar]
- 170.Frederiksen M et al. (2010) Placental transfer of the polybrominated diphenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: an experimental study. Environ Health 9, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leonetti C et al. (2016) Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environ Health 15 (1), 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhao X et al. (2017) Correlation between Prenatal Exposure to Polybrominated Diphenyl Ethers (PBDEs) and Infant Birth Outcomes: A Meta-Analysis and an Experimental Study. Int J Environ Res Public Health 14 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim S et al. (2018) Prenatal exposure to persistent organic pollutants and methylation of LINE-1 and imprinted genes in placenta: A CHECK cohort study. Environ Int 119, 398–406. [DOI] [PubMed] [Google Scholar]
- 174.Zhao Y et al. (2016) Umbilical cord blood PBDEs concentrations are associated with placental DNA methylation. Environ Int 97, 1–6. [DOI] [PubMed] [Google Scholar]
- 175.Li Q et al. (2015) Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the National Children’s Study (NCS). Epigenetics 10 (9), 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang S et al. (2015) Deregulated microRNA species in the plasma and placenta of patients with preeclampsia. Mol Med Rep 12 (1), 527–34. [DOI] [PubMed] [Google Scholar]
- 177.Ruis MT et al. (2019) PBDEs Concentrate in the Fetal Portion of the Placenta: Implications for Thyroid Hormone Dysregulation. Endocrinology 160 (11), 2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Robinson JF et al. (2019) Genomic Profiling of BDE-47 Effects on Human Placental Cytotrophoblasts. Toxicol Sci 167 (1), 211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Park HR and Loch-Caruso R. (2014) Protective effect of nuclear factor E2-related factor 2 on inflammatory cytokine response to brominated diphenyl ether-47 in the HTR-8/SVneo human first trimester extravillous trophoblast cell line. Toxicol Appl Pharmacol 281 (1), 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Peltier MR et al. (2012) Polybrominated diphenyl ethers enhance the production of proinflammatory cytokines by the placenta. Placenta 33 (9), 745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhu Y et al. (2017) The flame retardant 2,2’,4,4’-Tetrabromodiphenyl ether enhances the expression of corticotropin-releasing hormone in the placental cell model JEG-3. Chemosphere 174, 499–505. [DOI] [PubMed] [Google Scholar]
- 182.Canton RF et al. (2008) Inhibition of human placental aromatase activity by hydroxylated polybrominated diphenyl ethers (OH-PBDEs). Toxicol Appl Pharmacol 227 (1), 68–75. [DOI] [PubMed] [Google Scholar]
- 183.Sousa A et al. (2014) History on organotin compounds, from snails to humans. Environmental Chemistry Letters 12. [Google Scholar]
- 184.Furukawa S et al. (2017) Effect of dibutyltin on placental and fetal toxicity in rat. J Toxicol Sci 42 (6), 741–753. [DOI] [PubMed] [Google Scholar]
- 185.Ema M et al. (2009) Developmental toxicity of dibutyltin dichloride given on three consecutive days during organogenesis in cynomolgus monkeys. Drug Chem Toxicol 32 (2), 150–7. [DOI] [PubMed] [Google Scholar]
- 186.Ema M et al. (1992) Susceptible period for the teratogenicity of di-n-butyltin dichloride in rats. Toxicology 73 (1), 81–92. [DOI] [PubMed] [Google Scholar]
- 187.Cao S et al. (2017) The Effects of Fungicides on Human 3beta-Hydroxysteroid Dehydrogenase 1 and Aromatase in Human Placental Cell Line JEG-3. Pharmacology 100 (3–4), 139–147. [DOI] [PubMed] [Google Scholar]
- 188.Arita Y et al. (2018) Effects of tributyltin on placental cytokine production. J Perinat Med 46 (8), 867–875. [DOI] [PubMed] [Google Scholar]
- 189.Hiromori Y et al. (2016) Organotin compounds cause structure-dependent induction of progesterone in human choriocarcinoma Jar cells. J Steroid Biochem Mol Biol 155 (Pt B), 190–8. [DOI] [PubMed] [Google Scholar]
- 190.Nakanishi T et al. (2002) Trialkyltin compounds enhance human CG secretion and aromatase activity in human placental choriocarcinoma cells. J Clin Endocrinol Metab 87 (6), 2830–7. [DOI] [PubMed] [Google Scholar]
- 191.Nakanishi T et al. (2006) Organotin compounds enhance 17beta-hydroxysteroid dehydrogenase type I activity in human choriocarcinoma JAr cells: potential promotion of 17beta-estradiol biosynthesis in human placenta. Biochem Pharmacol 71 (9), 1349–57. [DOI] [PubMed] [Google Scholar]
- 192.Midic U et al. (2018) Changes in gene expression following long-term in vitro exposure of Macaca mulatta trophoblast stem cells to biologically relevant levels of endocrine disruptors. Reprod Toxicol 77, 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gorrochategui E et al. (2014) Characterization of complex lipid mixtures in contaminant exposed JEG-3 cells using liquid chromatography and high-resolution mass spectrometry. Environ Sci Pollut Res Int 21 (20), 11907–16. [DOI] [PubMed] [Google Scholar]
- 194.Blundell C et al. (2018) Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier. Adv Healthc Mater 7 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fry RC et al. (2019) Developing novel in vitro methods for the risk assessment of developmental and placental toxicants in the environment. Toxicol Appl Pharmacol 378, 114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Grigsby PL (2016) Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy. Semin Reprod Med 34 (1), 11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]