Abstract
Prior studies have shown that patients suffering from chronic Low Back Pain (cLBP) have impaired somatosensory processing including reduced tactile acuity, i.e. reduced ability to resolve fine spatial details with the perception of touch. The central mechanism(s) underlying reduced tactile acuity are unknown but may include changes in specific brain circuitries (e.g. neuroplasticity in the primary somatosensory cortex, S1). Furthermore, little is known about the linkage between changes in tactile acuity and the amelioration of cLBP by somatically-directed therapeutic interventions, such as acupuncture. In this longitudinal neuroimaging study, we evaluated healthy control adults (HC, N = 50) and a large sample of cLBP patients (N = 102) with structural brain imaging (T1-weighted MRI for Voxel-Based Morphometry, VBM; Diffusion Tensor Imaging, DTI) and tactile acuity testing using two-point discrimination threshold (2PDT) over the lower back (site of pain) and finger (control) locations. Patients were evaluated at baseline and following a 4-week course of acupuncture, with patients randomized to either verum acupuncture, two different forms of sham acupuncture (designed with or without somatosensory afference), or no-intervention usual care control. At baseline, cLBP patients demonstrated reduced acuity (greater 2PDT, P = 0.01) over the low back, but not finger (P = 0.29) locations compared to HC, suggesting that chronic pain affects tactile acuity specifically at body regions encoding the experience of clinical pain. At baseline, Gray Matter Volume (GMV) was elevated and Fractional Anisotropy (FA) was reduced, respectively, in the S1-back region of cLBP patients compared to controls (P < 0.05). GMV in cLBP correlated with greater 2PDT-back scores (ρ = 0.27, P = 0.02). Following verum acupuncture, tactile acuity over the back was improved (reduced 2PDT) and greater improvements were associated with reduced S1-back GMV (ρ = 0.52, P = 0.03) and increased S1-back adjacent white matter FA (ρ = 0.56, P = 0.01). These associations were not seen for non-verum control interventions. Thus, S1 neuroplasticity in cLBP is linked with deficits in tactile acuity and, following acupuncture therapy, may represent early mechanistic changes in somatosensory processing that track with improved tactile acuity.
Keywords: Low back pain, Tactile acuity, Two-point discrimination threshold, Primary sensory cortex, Acupuncture
1. Introduction
Chronic low back pain (cLBP) is a leading cause of disability and continues to be a major burden for many public health systems (Balague et al., 2012). Multiple studies have characterized altered sensory processing in cLBP patients (Meints et al., 2019), including reduced tactile acuity (Moseley et al., 2008; Wand et al., 2010; Luomajoki and Moseley, 2011; Adamczyk et al., 2018). While cortical reorganization is hypothesized to be a mechanism supporting reduced tactile acuity in cLBP, this has not been empirically demonstrated to date.
Tactile acuity is typically assessed via behavioral perceptual testing, such as with two-point discrimination threshold (2PDT), defined as the distance between two tactile stimulation sources at which the subject perceives two stimuli instead of one (Catley et al., 2014; Godde et al., 2000). Greater 2PDT is a proxy for reduced tactile acuity. In cLBP patients, impaired tactile acuity may present alongside preserved tactile sensory threshold (Wand et al., 2010). Although tactile acuity is partially dependent on peripheral nervous system and spinal cord organization (e.g. innervation density and receptive field size) (Vallbo and Johansson, 1984), it is also linked with cortical representations in the brain’s primary somatosensory cortex (S1) (Moseley et al., 2008; Wand et al., 2013; Catley et al., 2014). Interestingly, increased 2PDT, particularly over the lower back, has been linked with both clinical low back pain and the fidelity of cortical representation maps within S1 (Lotze and Moseley, 2007; Wand et al., 2010). Furthermore, a recent meta-analysis across 19 studies, with varying 2PDT protocols, demonstrated that lumbar tactile acuity was reduced in cLBP patients (Adamczyk et al., 2018).
Impaired tactile acuity in chronic pain patients might be improved following targeted interventions that relieve clinical pain, for instance following tactile discrimination training (Pleger et al., 2005; Moseley et al., 2008). In such training, the participant is asked to report the frequency, location, or type of stimulation, and feedback is given. Another somatosensory-based intervention has been training-independent sensory learning, wherein repetitive tactile stimuli are delivered to multiple regions with variable synchrony and frequency (Kalisch et al., 2007; Ragert et al., 2008). Both methods have been suggested to improve tactile acuity via cortical reorganization (Flor et al., 2001; Hoffken et al., 2007; Freyer et al., 2012).
In fact, non-pharmacological therapies have been gaining increasing attention for cLBP due to the mounting costs of the opioid epidemic in countries such as the United States (Volkow and McLellan, 2016) and elsewhere, and the evidence base for therapies such as acupuncture, is growing, particularly for cLBP (Cherkin et al., 2009; Berman et al., 2010; Liu et al., 2015). The mechanisms by which acupuncture reduces LBP and modulates sensory processing in cLBP patients remains unknown. While multiple brain-based mechanisms of action for acupuncture have been proposed, a possible mechanism for cLBP may involve neuroplasticity in somatosensory pathways, and manifest in improvements in tactile acuity. Interestingly, acupuncture coupled with needle-based sensory discrimination training has been found to enhance back pain reduction in cLBP (Wand et al., 2013). However, it is not known if acupuncture itself, as training-independent somatosensory stimulation, can similarly modulate tactile acuity and S1 organization or microstructure in cLBP.
Importantly, S1 neuroplasticity following acupuncture has been documented for other chronic pain disorders using non-invasive neuro-imaging techniques. For instance, carpal tunnel syndrome patients demonstrate altered S1 cortical representations and S1-adjacent white matter microstructure in the hand representation region (Napadow et al., 2006; Dhond et al., 2012; Maeda et al., 2014). Previous neuroimaging studies have shown that other chronic pain disorders also demonstrate altered brain structure in S1, as well as other pain-related brain areas (As-Sanie et al., 2012; Kong et al., 2013; Kregel et al., 2015; Ung et al., 2014). Following acupuncture, such maladaptive neuroplasticity showed improvement, and was linked with improved pain and/or peripheral nerve conduction latency (Napadow et al., 2007; Maeda et al., 2017). Although acupuncture-induced neuroplasticity had been reported for neuropathic pain disorders such as carpal tunnel syndrome, similar outcomes have not been reported for cLBP.
In this longitudinal neuroimaging study, we evaluated healthy control (HC) adults and cLBP patients with structural brain imaging and tactile acuity testing. Patients were evaluated at baseline and following a 4-week course of acupuncture therapy, with patients randomized to either verum, two different forms of sham acupuncture (with or without somatosensory afference), or no-intervention usual care control. We hypothesized that cLBP patients would demonstrate reduced tactile acuity and altered S1 structure compared to HC, and that 2PDT would be correlated with white- and gray-matter structural metrics for cLBP. We also hypothesized that verum, but not sham, acupuncture would improve tactile acuity, and that post-therapy structural S1 neuroplasticity would be associated with improvements in tactile acuity.
2. Materials and methods
We completed a single-center, single-blinded, placebo controlled, randomized parallel-group longitudinal neuroimaging study, preregistered with ClinicalTrials.gov (NCT01598974). The study took place at the Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital (MGH), in Boston, MA from January 2012 to October 2017. All study protocols were approved by MGH and Partners Human Research Committee and all subjects provided written informed consent. All procedures were in accordance with the Helsinki Declaration of 1975, as revised in 2008.
2.1. Subjects
Our study recruited cLBP patients for a randomized controlled trial with the following inclusion criteria: 1) aged 18–60 years, 2) cLBP meeting Quebec Task Force Classification System categories I-II (i.e., patients were unlikely to have significant nerve root involvement, stenosis, or mechanical instability (Abenhaim et al., 2000; Loisel et al., 2002)) as confirmed by the study physician and/or review of medical records with the use of previous x-ray reports, 3) duration of low back pain longer than 6 months, and 4) severity of low back pain averaging at least 4 on a 0–10 pain intensity scale (0: no pain, 10: most pain imaginable) over the past two weeks. Exclusion criteria for cLBP patients were as follows: 1) back pain due to cancer, fracture, or infection, 2) constant radicular pain radiating below the knee, 3) complicated chronic back syndromes (e.g., prior back surgery, ongoing medicolegal issues), 4) active self-reported substance abuse disorder in the past two years, 5) major systemic or neuropsychiatric disease that may present as a confound (e.g., severe fibromyalgia, rheumatoid arthritis, major psychiatric disorders, psychosis, seizure disorder, severe cardiorespiratory or nervous system diseases, etc.) 6) use of prescription opioids exceeding 60 mg morphine equivalents per day or steroids for pain, 7) acupuncture contraindications (e.g., coagulopathy) or history of acupuncture treatment, and 8) presence of typical contraindications for MRI scanning. Healthy Controls (HC) aged 18–60 demographically matched to cLBP patients were also enrolled, with exclusion criteria as for cLBP above, in addition to any low back or other acute/chronic pain disorder.
2.2. Study Design
This study randomized cLBP patients to one of four parallel arms: 1) verum acupuncture with acupuncture needles traditionally used in clinical settings, 2) sham acupuncture with Streitberger needles (i.e., non-penetrating acupuncture needles), 3) mock laser acupuncture as a form of sham acupuncture control devoid of any somatosensory afference, and 4) no-treatment, usual care control (Supplementary Fig. 1). Behavioral and MRI assessment was completed by eligible subjects at baseline and following the intervention/usual care period (duration between assessments was 7.0 ± 2.7 weeks). Both experimenters and subjects were blinded as to group allocation for the three intervention groups. HC subjects were similarly evaluated with behavioral and MRI assessments for baseline comparisons. Data analyses used automated methods, blinded to group allocation on the subject level.
For the three acupuncture intervention groups, subjects received 6 treatments over 4 weeks using a tapering schedule common to the clinic: two treatments/week for 2 weeks; one treatment/week for 2 weeks. The licensed and MGH-credentialed acupuncturists (J.G., and K.W., at least 3 years of clinical experience) were informed of group allocation at the first treatment visit, and were not aware of details of behavioral and MRI procedures completed by the patient. For the no-treatment control group, cLBP subjects did not come in for study visits and continued their usual care throughout the 4 weeks of the intervention period.
Subjects across all intervention groups lay prone on the acupuncture table, as instructed by the acupuncturist. As visual input is known to strongly modify pain (Longo et al., 2009), lying prone allowed patients to receive no visual information of what the therapy consisted of, thus accounting for confounds of different needles/devices used across the intervention arms (described below).
For the verum acupuncture group, acupoint locations were selected based on the standardized acupuncture protocol for cLBP (Cherkin et al., 2009). These included seven acupoints commonly used for cLBP (GV-3, BL-23 bilateral, BL-40 bilateral, KI-3 bilateral, see Supplementary Fig. 2), combined with 2–3 bilateral ah-shi (tender by palpation) points over the lower back/buttocks. All verum acupuncture needles (0.20–0.25 mm diameter, 25–50 mm length, stainless steel; Asiamed) were inserted 10–40 mm deep, depending on location, and retained for 20 min, with manual needle stimulation (2 Hz) via twirling at 10 min and again just before needle removal.
For subjects randomized to the sham acupuncture group, the same acupoints were used as for the verum group, thereby varying only one aspect of treatment – skin penetration. Non-inserted Streitberger sham needles were used (i.e. stimulation restricted to cutaneous levels).
For subjects randomized to the mock laser acupuncture group, a deactivated laser acupuncture device (Vita-Laser 650, Lhasa OMS) was held by the acupuncturist and waved over all of the previously defined acupoints for at least 15 s per point, as the acupuncturist walked around the prone-lying subjects. The subjects were introduced to the real laser-acupuncture device and shown that although a laser light shines on the skin it does not produce any sensation (therefore subjects did not expect strong somatosensation during treatments). The goal of this intervention arm was to include the ritualistic components of acupuncture without any somatosensory afference to the body (Irnich et al., 2010). See Supplementary Methods for more detail.
2.3. Clinical and behavioral outcomes
Eligible subjects participated in a baseline clinical/behavioral assessment session prior to MRI sessions. The primary clinical outcome in this study was low back pain bothersomeness over the past week; secondary clinical outcomes included the Patient-Reported Outcomes Measurement Information System (PROMIS-29) scale (e.g., pain interference) (Cella et al., 2010).
In order to assess tactile acuity, we performed the two-point discrimination threshold (2PDT) test for both HC and cLBP subjects. We used a two-point aesthesiometer (Mitutoyo Digital Caliper, Mitutoyo), applied to the right lower back (over the erector spinae muscles, medial to posterior superior iliac spine, and level with the lumbar vertebrae) followed by testing at a non-painful control site, the right index finger (middle phalanx and palmar surface). Subjects’ eyes were closed throughout 2PDT testing. As in previous studies (Moseley et al., 2008; Wand et al., 2010; Luomajoki and Moseley, 2011), subjects completed a series of ascending (in which the two points of the aesthesiometer are initially adjacent) and descending (in which the two points of the aesthesiometer are initially far apart) trials in which they indicate whether they “feel one or two points” when the stimulus is applied. The results of ascending and descending trials were then averaged to calculate the two-point discrimination threshold (2PDT). See our previous publication (Meints et al., 2019) for more detail.
2.4. MRI outcomes
2.4.1. S1 fMRI localization for ROI definition
For Region of Interest (ROI) analyses, we needed to localize the S1 representation for the low back and finger. We conducted an event-related fMRI localizer scan on a 3.0 T Skyra (Siemens Medical, Erlangen, Germany) equipped with 32-channel head coil at the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital. BOLD fMRI data were collected with the following parameters: TR/TE = 3000/30 ms; 44 tilted axial slices; voxel size = 2.6 × 2.6 × 3.1 mm; 80 volumes (4 min) per scan run. Nociceptive afference was provided by electrical stimulation delivered to the right lower back and right fingers (2nd and 3rd digits) using MR-compatible Ag/AgCl electrodes. For each fMRI scan, electrical stimuli at 25 Hz were applied in randomized order for 13 2-s duration trials with individually-tailored current intensity to evoke 40/100 pain (applied current intensity 3.5 ± 2.9 mA for a rating of 40 out of 100 [i.e. p40], 0 = “no pain”, 100 = “most pain imaginable”) and 13 identical-duration blocks individually-tailored to evoke 7/10 moderate but not painful sensation (1.5 ± 1.4 mA for a rating of 7 out of 10 [i.e. not painful, p0], 0 = “no sensation”, 10 = “on the verge of pain”), with inter-trial intervals ranging from 6 to 12 s. Two 4-min fMRI scan runs for each body region were completed and separate group activation maps for right low back and finger stimulation were acquired (see Supplementary Methods, and our previous publications (Kim et al., 2019; Lee et al., 2019) for more detail). Localization of S1 representations of the low back and finger (S1-back/finger mask) was then determined by the intersection of group maps (z > 2.3), and the Juelich probabilistic atlas for the 3b/1 areas (>25%) (Eickhoff et al., 2005). Previous somatotopy fMRI studies demonstrated that for S1, the 3b/1 area shows higher selectivity for different digits (Martuzzi et al., 2014; Nelson and Chen, 2008). Thus, we used a mask of the S1 3b/1 area for focusing our analyses of the brain structural correlates associated with tactile acuity. These masks were used as ROIs for VBM analyses, and masks were dilated by 1 voxel (fslmaths, FSL) to project onto the S1-adjacent white matter skeleton for DTI analyses (see below).
2.4.2. Structural MRI acquisition
Structural MRI was performed on the same MRI scanner as above, using a T1-weighted three-dimensional (3D) MEMPRAGE pulse sequence with the following parameters: TR/TE1/TE2/TE3/TE4 = 2530/1.69/3.5/5.36/7.22 ms; flip angle = 7°; voxel size = 1 mm isotropic. For DTI analyses, diffusion-weighted images were also=obtained using a spin-echo echo-planar imaging (EPI) sequence with the following parameters: TR/TE = 10300/85 ms; voxel size = 2 mm isotropic. The DTI pulse sequence used diffusion-sensitizing gradients with two different b-values - 600 s/mm2 and 1200 s/mm2, applied along 30 non-collinear directions.
2.4.3. Structural image processing
Longitudinal VBM analyses used T1-weighted images, processed with the Computational Anatomy Toolbox (CAT12 (Gaser and Schlaug, 2003)) and SPM12 (Ashburner and Friston, 2005). First, the initial alignment between T1-weighted images at baseline and post-treatment time points were conducted to yield the mean subject image, which was employed as the reference image for bias-correction. Subsequently, the aligned images were segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) after signal inhomogeneities correction with regard to the reference mean image. DARTEL was used with nonlinear registration to standard space, and intensity modulation was conducted using the linear and non-linear components of the Jacobian determinant derived from the deformation fields. The intracranial volume was calculated by summing the volumes of the segmented gray matter, white matter, and cerebrospinal fluid compartments. Finally, mean GM volume for the S1-back and finger areas was calculated using the S1-back and finger ROI mask defined above, within which the modulated GM intensity was calculated.
For DTI, diffusion-weighted images were aligned to the b0 image using affine registration, which serves to correct distortion due to eddy currents. After removal of non-brain tissue, DTI metric maps (fractional anisotropy, and mean, radial and axial diffusivity) were computed from the diffusion-weighted images using FMRIB’s DiffusionToolbox (FDT, FSL), which fits a diffusion tensor model to each voxel. We adopted a longitudinal diffusion processing scheme based on TBSS similar to previous analyses (Douaud et al., 2009; Engvig et al., 2012). First, the initial alignment between brain-extracted images at baseline and post-treatment time points were conducted using FMRIB’s Linear Image Registration Tool (FLIRT, degrees of freedom = 6), and both images were resampled to a common space halfway between the two (Jenkinson et al., 2002), which only requires a single registration per volume and thus minimizes registration bias towards one of the two time points. Next, we averaged the two registered fractional anisotropy maps to generate a subject-wise mid-space template, and images were aligned to the FMRIB58_FA template using FMRIB’s Nonlinear Registration Tool (Jenkinson et al., 2012). The mean fractional anisotropy map was thinned and thresholded at fractional anisotropy >0.2 to generate a white matter tract skeleton representing the center of the tracts common to all subjects (Smith et al., 2006). Each image was warped to the standard MNI space using these transformations, and skeletonized after spatial smoothing (full-width at half-maximum = 4 mm). The S1-adjacent white matter skeleton for back/finger cortical representations was defined using the projection of the dilated S1-back/finger mask defined above onto the mean fractional anisotropy (FA) skeleton. Notably, this mask did not include the superior longitudinal fasciculus, located medial to S1-adjacent U-fibers, as it is difficult to interpret fractional anisotropy in areas with high crossing-fiber density (Douaud et al., 2011).
2.5. Statistical analyses
To compare data between cLBP and HC, we used Student’s t-tests. To investigate associations between variables, Spearman’s correlation was used, while comparisons between correlation strengths were assessed using the Cocor tool (R Statistical Package, R-Core-Team, 2012, based on Steiger’s procedure and Fisher’s r-to-z transformation (Steiger, 1980; Diedenhofen and Musch, 2015)).
To test for baseline differences across intervention arms in LBP bothersomeness, PROMIS-29 pain interference, and 2PDT, ANOVA was used. For assessment of post-therapy change in LBP bothersomeness, PROMIS-29 pain interference, and 2PDT, an ANOVA or ANCOVA was performed, where the baseline values served as a covariate if significant group baseline differences existed. As previous studies have found that %-change scores, which effectively normalize difference scores by baseline value, are less sensitive to baseline differences than absolute difference scores (Farrar et al., 2001; Jensen et al., 2003; Hanley et al., 2006), group difference analyses used %-change scores for 2PDT and pain. We also used a one-sample t-test to evaluate whether each group independently improved on the tactile acuity measure (2PDT), as group differences may exist without a significant change from nil for any individual group.
In order to assess change of GM volume or FA in S1-back and S1-finger regions, repeated measures ANOVA was performed over time points and two hemispheres and across intervention arms, using the Greenhouse-Geisser correction for sphericity when appropriate. Previous lesion studies of tactile perception and spatial attention showed that hemispheric dominance or asymmetry can affect cognitive processing, which may have played a role in 2PDT test results. For instance, prior research has demonstrated that deficits in right hemisphere affected tactile perception and spatial attention more than deficits in left hemisphere (Benton et al., 1973; Mapstone et al., 2003). Thus, we also considered the left/right hemisphere as a factor in these analyses. For GM regional volume and FA analyses, age-, sex-, and intracranial volume-adjusted volumes, and age- and sex-adjusted FA values were used. To ensure that our findings (i.e. structural difference between groups) were robust, we conducted voxel-wise whole brain comparisons, which gave similar results (see Supplementary Fig. 3). Also, for confirmation, we compared tactile acuity and brain measures (GM volume and FA) between a reduced (N = 50) cLBP and age- and sex-matched HC group (also N = 50).
Significance was set at alpha = 0.05. All the statistical tests, except the comparison of correlation strength, were performed using the Statistical Package for Social Sciences 17.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Demographic and clinical/behavioral assessments
A total of 103 chronic LBP participants and 50 age-matched HC were enrolled and 102 patients performed baseline clinical/behavioral assessments. One subject was excluded due to inability to comply with 2PDT testing instructions. Twenty patients dropped out prior to the baseline MRI scan, while 4 dropped out prior to interventional arm randomization. Once randomized, 3 patients dropped out from the mock laser acupuncture arm, while 4 dropped out from the usual care/no intervention arm prior to the post-treatment behavioral evaluation, and one more prior to post-treatment MRI.
Tactile acuity scores for the back (2PDT-back) were significantly greater (i.e. worse) (t-test, P = 0.01, Table 1, Fig. 1a), for LBP (4.21 ± 1.63 cm, mean ± SD) compared to HC (3.37 ± 1.54 cm) while acuity scores for the finger (2PDT-finger) did not differ between groups (P = 0.29).
Table 1.
Demographic and clinical/behavioral data.
| Healthy controls (N = 50) | Low back pain patients (N = 102) | P-valuea | |
|---|---|---|---|
| Age (years) | 41.4 ± 12.3 | 41.2 ± 12.0 | 0.92 |
| Sex (F/M) | 34/16 | 58/44 | 0.17 |
| 2PDT-backb (cm) | 3.37 ± 1.54 | 4.21 ± 1.63 | 0.01** |
| 2PDT-fingerb (mm) | 3.18 ± 1.51 | 3.48 ± 1.37 | 0.29 |
| LBP duration (years) | N/A | 8.6 ± 8.3 | N/A |
| LBP bothersomeness | N/A | 5.4 ± 2.1 | N/A |
| PROMIS-Pain interferencec | 41.6 ± 0.0 | 58.6 ± 5.7 | <0.0001** |
| PROMIS-Physical functionc | 56.4 ± 2.7 | 42.9 ± 5.6 | <0.0001** |
| PROMIS-Anxietyc | 45.9 ± 6.3 | 49.7 ± 8.5 | 0.02* |
| PROMIS-Depressionc | 43.3 ± 5.1 | 45.2 ± 6.3 | 0.12 |
| PROMIS-Fatiguec | 39.9 ± 6.1 | 50.9 ± 9.0 | <0.0001** |
| PROMIS-Sleep disturbancec | 41.3 ± 6.0 | 52.2 ± 8.2 | <0.0001** |
| PROMIS-Social satisfactionc | 69.5 ± 5.1 | 57.2 ± 10.2 | <0.0001** |
| Beck Depression Inventoryc | 1.7 ± 3.6 | 5.4 ± 5.9 | 0.001** |
| Pain Catastrophizing Scalec | 3.2 ± 5.3 | 11.1 ± 8.5 | <0.0001** |
Data are shown as mean ± SD.
P < 0.05;
P < 0.01.
Student’s t-tests were used for the comparison between groups except the comparison for sex distribution, where χ2 test was used.
Data available for subset of subjects (HC, N = 34; LBP, N = 100).
Data available for subset of subjects (HC, N = 35; LBP, N = 102).
Fig. 1.
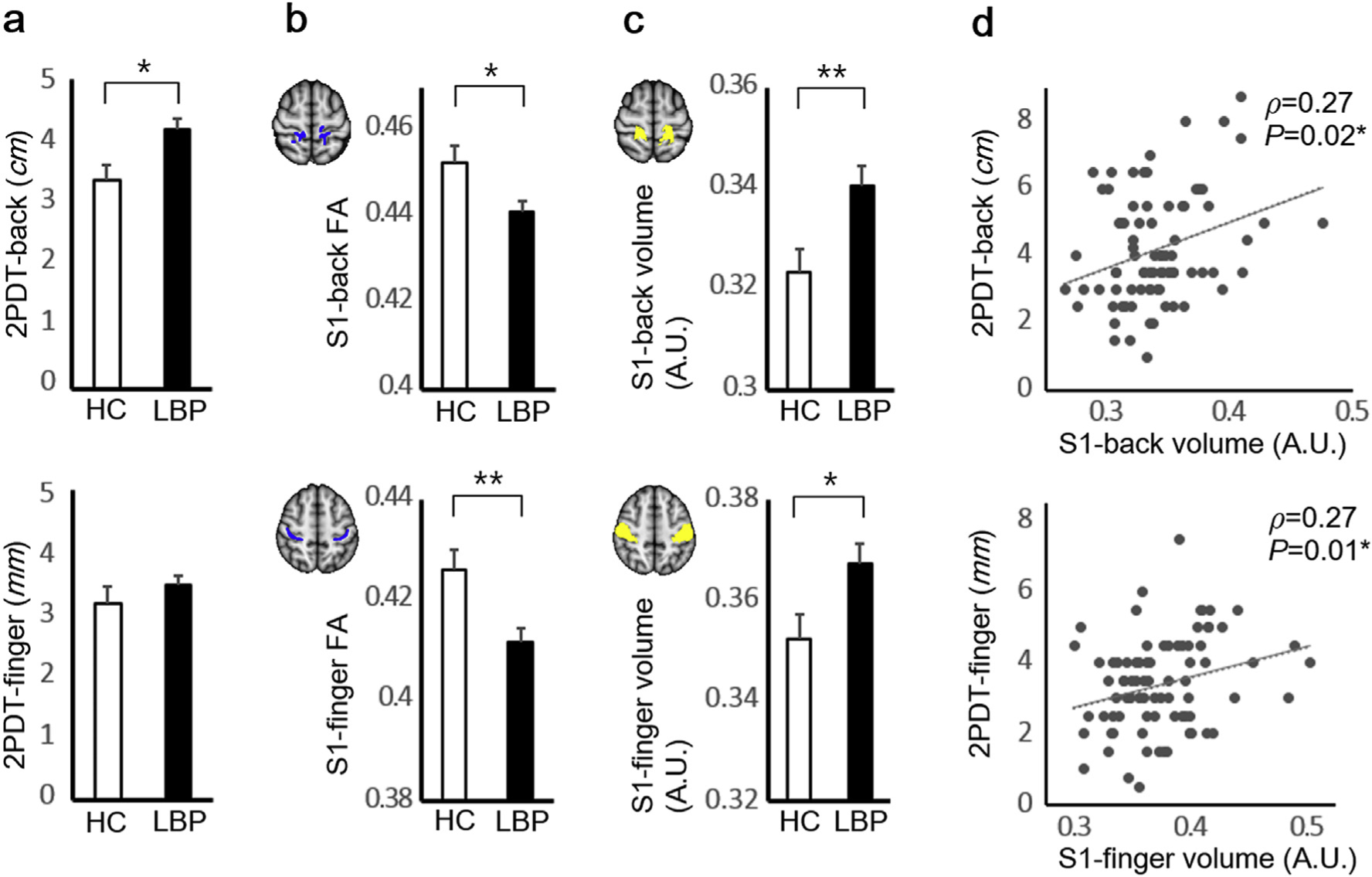
(a) The 2PDT-back but not 2PDT-finger scores were greater (worse tactile acuity) for cLBP compared to HC. (b) Mean FA in white matter adjacent to back and finger ROIs (blue color for white matter skeleton) was reduced in cLBP compared to HC. (c) S1 gray matter volume was increased for cLBP in both back (z = 60 mm) and finger (z = 53 mm) S1 cortical representation ROIs (yellow color) (d) For cLBP, worse 2PDT scores for back and finger locations were associated with greater gray matter volume in back and finger S1 representation ROIs, respectively. No significant associations were found for HC. *P < 0.05, **P < 0.01. Error bars represent SEM. Abbreviations: 2PDT = two-point discrimination threshold, A.U. = arbitrary unit, cLBP = chronic low back pain patients, HC = healthy controls, FA = fractional anisotropy, S1 = primary somatosensory cortex.
3.2. FA on S1-adjacent white matter skeleton
Based on the results of an fMRI localizer scan, we defined S1-adjacent white matter skeleton, and compared mean FA on the skeleton. Specifically, we used a repeated measures ANOVA over 2 hemispheres (left/right) and 2 ROIs (back/finger). There was a significant group effect (F [1,124] = 9.35, P = 0.003), indicating that cLBP patients showed smaller S1-back and S1-finger FA compared to HC. There were significant hemisphere × group (cLBP/HC) and hemisphere × ROI interactions (P = 0.04; P < 0.0001, respectively). Thus, further analyses evaluated mean FA of each hemisphere and each region separately.
Mean FA was significantly reduced for cLBP compared to HC in both left and right S1-back ROI, and left S1-finger ROIs (P = 0.01; P = 0.04; P < 0.0001, respectively). Mean FA difference of the right S1-finger ROIs between groups (cLBP/HC) was marginally significant (P = 0.08). For presentation purposes, left and right FA for each ROI were merged (Fig. 1b).
3.3. S1 GM volume
Based on the results of an fMRI localizer scan, we defined S1 ROIs and performed an ROI analysis comparing mean gray matter volume between cLBP and HC. We used a repeated measures ANOVA over 2 hemispheres (left/right) and 2 ROIs (back/finger). We found a significant group effect (F[1,130] = 7.59, P = 0.007), which indicated that cLBP patients demonstrated greater S1-back and S1-finger volume compared to HC, as was evident in the post hoc analysis (Fig. 1c, independent t-test for age, sex, and intracranial volume-adjusted volume, P = 0.008 for S1-back; P = 0.02 for S1-finger). There was no significant ROI × group (cLBP/HC), hemisphere × group, and ROI × hemisphere × group interaction (P = 0.66; P = 0.58; P = 0.20, respectively).
Finally, as different group sample sizes may have influenced our results, we also compared tactile acuity and brain metrics (GM volume and FA) between HC and a reduced N, age- and sex-matched cLBP group (both groups, N = 50). This subgroup analysis produced similar results as for the full analysis, suggesting that unequal sample sizes did not adversely influence our reported outcomes (see Supplementary Table 1).
Subsequently, we evaluated associations between S1 gray matter volume and tactile acuity. As the correlations for left and right S1 volume with 2PDT were not significantly different for both back and finger ROIs (P = 0.67; P = 1.00, respectively), we used mean S1 gray matter volume across both left and right hemispheres for further analyses. We found that for cLBP, S1-back gray matter volume was significantly correlated with 2PDT-back (Fig. 1d, Spearman’s correlation, ρ = 0.27; P = 0.02), but not with 2PDT-finger (ρ = 0.08, P = 0.48). However, S1-finger volume showed significant correlation with 2PDT-finger (ρ = 0.27; P = 0.01), and trending-level correlation with 2PDT-back (ρ = 0.21, P = 0.07). Thus, larger S1 gray matter volume was associated with worse 2PDT scores, with evidence of body-region to S1-subregion specificity. For HC, no significant correlations were found between either ROI for S1 gray matter volume and 2PDT scores (all −0.2<ρ<0.1, P’s>0.3).
3.4. Acupuncture treatment effects
A total of 78 cLBP patients (42 female, age = 40.9 ± 11.8 years) were randomized to four study arms, verum acupuncture (N = 18, 11 female, age = 41.3 ± 14.0 years), sham acupuncture (N = 18, 7 female, age = 41.8 ± 12.2 years), mock laser acupuncture (N = 19, 11 female, age = 41.7 ± 12.3 years), and no treatment/usual care (n 23, 13 female, age = 39.1 ± 9.8 years). There was no significant difference in age or female/male distribution between groups (F[3,74] = 0.24, P = 0.87 Chi-square test, P = 0.53). Symptom duration also did not differ significantly between groups (F[3,74] = 1.18, P = 0.32; verum acupuncture = 7.6 ± 7.2 years, sham acupuncture 10.6 ± 10.8 years, mock laser = 6.0 ± 5.4 years, and no treatment/usual care = 9.2 ± 7.6 years). Drop outs were mostly due to scheduling difficulties, and a total of 70 cLBP subjects completed their treatment protocol and post-therapy MRI evaluation. No significant difference was found between the four study arms for subjects’ expectation of pain relief at baseline (F[3,74] = 0.44, P = 0.73), nor after the final treatment session for verum, sham, and mock laser acupuncture arms (F[2,48] = 0.94, P = 0.40).
3.5. Acupuncture effects on clinical outcomes
The primary clinical outcome for symptom severity was LBP bothersomeness. There was a significant baseline difference (F[3,66] 3.01, P = 0.04) across the 4 intervention groups (verum acupuncture, sham acupuncture, mock laser acupuncture and usual care). There was no significant difference in %-change (F[3,64] = 0.26, P = 0.86) across the 4 groups after controlling for baseline symptom severity.
PROMIS-29 pain interference was used as a functional scale for measuring pain-related interference with one’s life. There was no significant baseline difference (F[3,66] = 1.79, P 0.16) across the 4 intervention groups, and no significant difference=in %-change (F[3,66] = 1.62, P = 0.19) across the 4 groups after intervention. As an exploratory analysis, we also compared %-change of PROMIS-29 pain interference between verum acupuncture and a merged control group (non-verum acupuncture groups including sham acupuncture, mock laser acupuncture, and usual care). At baseline, pain interference was significantly different between verum acupuncture and control groups (F[1,68] = 4.06, P = 0.048). The post-intervention %-change in pain interference was significantly different between verum acupuncture and control interventions after controlling for baseline pain interference (F[1,67] = 4.09, P = 0.047), which indicated that verum acupuncture improved pain interference more than controls although both verum and control showed improved pain interference (mean ± SD, verum = −11.0 ± 10.6%, P = 0.0004; control = −4.6 ± 11.7%, P = 0.007).
3.6. Acupuncture effects on two-point discrimination threshold (2PDT)
There was no significant baseline difference for 2PDT-back or 2PDT-finger (F[3,66] = 1.09, P = 0.36; F[3,66] = 1.66, P = 0.19, respectively) across the 4 intervention groups. There was a significant difference in %-change for 2PDT-back (F[3,66] = 4.23, P = 0.009), but no significant difference in %-change for 2PDT-finger (F[3,66] = 0.22, P = 0.88) across the 4 groups after the intervention.
As a post-hoc analysis, we compared %-change of 2PDT between verum acupuncture and all other controls in a merged group (i.e. sham acupuncture, mock laser acupuncture, and usual care). At baseline, neither 2PDT-back nor 2PDT-finger showed significant differences between verum acupuncture and merged controls (F[1,68] = 1.89, P = 0.17 for back; F[1,68] = 2.01, P = 0.16 for finger, respectively). Post- treatment %-change for 2PDT-back was significantly different between verum acupuncture and controls (Fig. 2a, verum acupuncture = −18.5 ± 33.0%, control 4.9 ± 40.7%, F[1,68] = 4.84, P = 0.03). We found no group differences for 2PDT-finger (Fig. 2b, verum acupuncture = −7.2 ± 26.1%, control = 1.5 ± 61.9%, F[1,68] = 0.33, P = 0.57). For individual groups, verum acupuncture improved tactile acuity for the back (one- sample t-test, P = 0.03), but not finger (P = 0.26) regions. Control groups did not change tactile acuity for either back or finger regions (P = 0.39; P = 0.86,= respectively).
Fig. 2.
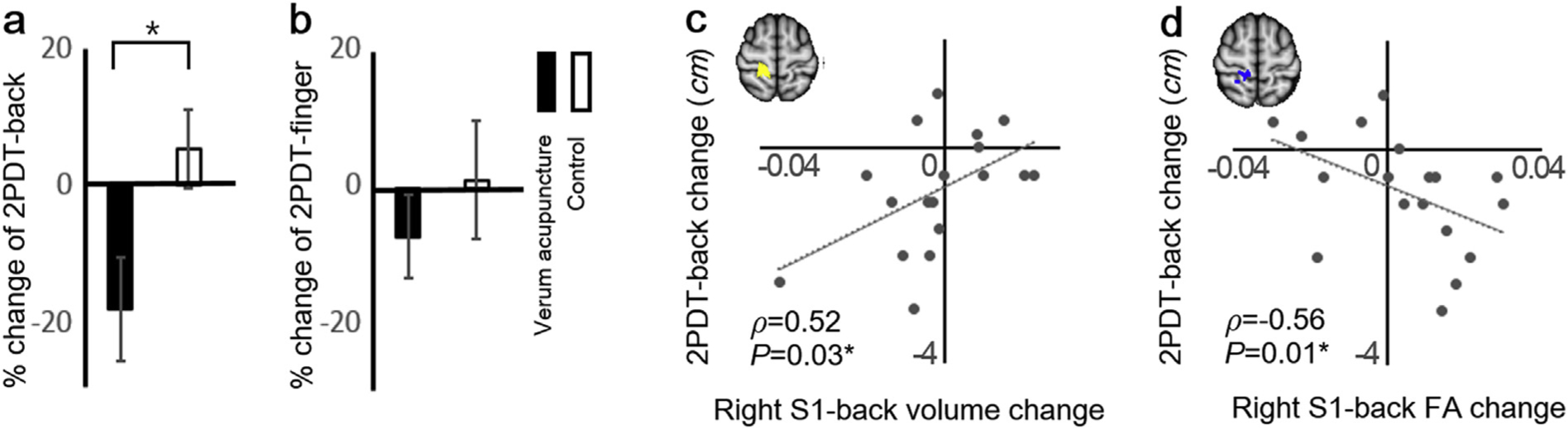
Verum acupuncture, but not control, improved tactile acuity (2PDT) for the back (a), but not finger regions (b), and improvement for 2PDT-back was associated with greater right S1-back GM volume decrease (c) and the adjacent white matter FA increase following verum acupuncture (d). *P < 0.05. Error bars represent SEM. Abbreviations: 2PDT = two-point discrimination threshold, FA = fractional anisotropy, S1 = primary somatosensory cortex.
3.7. Association between the changes in tactile acuity and S1-back structure after verum acupuncture
We found that right S1-back GM volume change showed significant correlation with change in 2PDT-back after verum acupuncture (Fig. 2c, Spearman’s correlation, ρ = 0.52, P = 0.03), but not after control interventions (ρ = 0.03, P = 0.82 for merged control; ρ = 0.02, P = 0.94 for sham acupuncture; ρ=−0.11, P = 0.69 for mock laser; ρ = 0.11, P = 0.67 for usual care, respectively). The difference of the correlation strength between verum and control demonstrated trending significance (P = 0.06). Left S1-back GM volume change did not show significant correlation after either verum acupuncture or control interventions (ρ = 0.39; P = 0.11 for verum acupuncture; ρ = 0.0 P = 0.99 for merged control; ρ = 0.07, P = 0.79 for sham acupuncture; ρ = −0.19, P = 0.48 for mock laser; ρ = 0.09, P = 0.74 for usual care, respectively). The strength of the correlation was also not significantly different between verum and control (P = 0.16). Neither left nor right S1-back GM volume change showed significant correlation with 2PDT-finger change after verum acupuncture or control interventions (all P’s > 0.45). Neither left nor right S1-finger GM volume change showed significant correlation with 2PDT-back change after verum acupuncture or control interventions (all P’s > 0.24).
As for microstructural changes in S1-adjacent white matter, we found that FA change in right S1-back adjacent region was correlated with 2PDT-back change after verum acupuncture (Fig. 2d, ρ = −0.56, P = 0.01), but not after control interventions (ρ = −0.10, P = 0.47 for merged control; ρ=−0.37, P = 0.15 for sham acupuncture; ρ = 0.20, P = 0.46 for mock laser; ρ = 0.04, P = 0.89 for usual care, respectively). The difference of the correlation strength between verum and control demonstrated trending significance (P = 0.07). FA change in left S1-back adjacent white matter did not show significant correlation after verum acupuncture or control interventions (ρ = −0.06, P = 0.81 for verum acupuncture; ρ = −0.12, P = 0.40 for merged control; ρ = −0.38, P = 0.13 for sham acupuncture; ρ = −0.05, P = 0.86 for mock laser; ρ = 0.08, P = 0.76 for usual care, respectively). The strength of the correlation between verum and control was not significantly different (P = 0.84). Neither FA change of left nor right S1-back adjacent white matter showed significant correlation with 2PDT-finger change after verum acupuncture or control interventions (all P’s > 0.29).
3.8. Association between the changes in pain with S1-back structure and tactile acuity after verum acupuncture
There were no significant correlations between post-intervention changes in pain bothersomeness/pain interference and changes in S1-back microstructure (GM volume/FA in adjacent white matter) after verum acupuncture (all P’s > 0.20). Furthermore, neither FA change nor GM volume change in S1-back showed significant correlation with pain change after control interventions (all P’s > 0.21). There were also no significant correlations between the change in pain bothersomeness/pain interference and changes in tactile acuity after verum acupuncture or control interventions (all P’s > 0.20).
3.9. Acupuncture effects on S1-back and S1-finger gray matter volume
For the assessment of GM volume change, a repeated measures ANOVA was performed over 2 time points (Time; before/after 4 weeks of therapy), 2 ROIs (Region; back/finger), and 2 hemispheres (Hemisphere; left/right).
There were no significant baseline differences for left/right S1-finger GM volume (all P’s > 0.43) across groups (verum acupuncture, sham acupuncture, mock laser acupuncture, and usual care). However, baseline differences for left/right S1-back GM volume approached significance in some cases (all P’s > 0.07). There were no significant Time × Group, Time × Hemisphere × Group, Time × Region × Group, and Time × Region × Hemisphere × Group interactions (all Ps > 0.25) .Any change of volume for left/right S1-back or S1-finger GM (i.e. volume difference between before/after 4-week treatment) was not significantly different across groups after controlling for baseline GM volume (all P’s > 0.29).
3.10. Acupuncture effects on S1-adjacent white matter FA change
For DTI assessment of change in white matter FA after therapy, a repeated measures ANOVA was performed over 2 time points (before/after 4 weeks of therapy), 2 ROIs (back/finger), and 2 hemispheres (left/right).
There was no significant baseline difference for left/right S1-back or S1-finger FA (all P’s > 0.5) across groups (verum acupuncture, sham acupuncture, mock laser acupuncture, and usual care). There was no significant Time × Group, Time × Hemisphere × Group, Time × Region × Group, and Time × Region × Hemisphere × Group interaction (all P’s > 0.31).
4. Discussion
Our results confirmed previous reports of reduced tactile acuity in cLBP (Adamczyk et al., 2018). Compared to HC, cLBP patients demonstrated reduced acuity over the low back (greater 2PDT-back), but not at the finger, suggesting that chronic pain affected tactile acuity selectively, at body regions where patients experience persistent pain. Furthermore, we found that gray matter volume in the primary somatosensory cortical representation of the back, which was elevated in cLBP, was correlated with greater 2PDT-back scores. DTI assessment of FA in S1-adjacent white matter found that cLBP patients demonstrate reduced FA in S1-back regions. Following 4-weeks of acupuncture therapy, tactile acuity was improved (reduced 2PDT scores over the back) and greater improvements were associated with reduced S1-back gray matter volume and increased S1-back adjacent white matter FA. These associations were not seen for control acupuncture interventions or usual care group designed to impart reduced or absent somatosensory afference from deep tissue receptors. However, neuroanatomical and tactile acuity changes were not linked with clinical outcomes after 4-weeks of therapy, and S1 neuroplasticity may represent early mechanistic changes in somatosensory processing that ultimately lead to longer term improvements in clinical pain severity following successful therapy.
Tactile acuity is the ability to resolve fine spatial details with the perception of touch. Impaired tactile acuity in chronic pain is not solely a result of deficits in peripheral nervous system processing (Wand et al., 2010), and previous studies have demonstrated that altered tactile acuity was accompanied by cortical reorganization in S1 (Maihofner et al., 2004; Lissek et al., 2009; Catley et al., 2014). Disruption of tactile acuity may closely link with chronic pain and may thus be a target for non-pharmacological, somatically-directed interventions. Previous studies showed that sensory discrimination learning or repetitive sensory stimuli could improve the tactile perception, which subsequently could reduce low back and limb pain (Moseley et al., 2008; Wand et al., 2013). This was first demonstrated for amputees with phantom limb pain (Flor et al., 2001). In fact, repetitive somatosensory stimulation by acupuncture was also found to relieve pain and normalize tactile perception in amputees (Bradbrook, 2004).
Similar to our findings, previous VBM neuroimaging studies in idiopathic LBP (Kong et al., 2013; Ung et al., 2014) also showed increased S1 volumes in cLBP patients compared to healthy adults. In fact, our previous study found that even within a defined chronic neuropathic pain population, patients for whom pain is predominant over paresthesia demonstrate greater S1-hand cortical thickness, with greater thickness correlated with greater pain severity (Maeda et al., 2016). Furthermore, our current cLBP study found elevated gray matter volume and reduced FA in both S1-back and S1-finger areas for cLBP patients. Thus, structural alterations extend beyond the cortical representations of the body area to which persistent pain is attributed. This is consistent with our previous study, which found that hand-affected neuropathic pain (i.e. carpal tunnel syndrome) patients demonstrate altered FA not only in the S1-hand area, but also S1-leg, and -face areas (Maeda et al., 2017). However, at least for cLBP patients, behavioral deficits stemming from altered gray matter or white matter microstructure in S1 may follow distinct somatotopic organization, as we demonstrated here for tactile acuity. Specifically, inter-subject differences in S1-back gray matter volume were correlated with 2PDT-back (and not 2PDT-finger), while S1-finger volume was correlated with 2PDT-finger (and not 2PDT-back). Moreover, these associations were not found for healthy adults, suggesting that S1 microstructure is linked with tactile acuity most directly for chronic pain patients (Catley et al., 2014), possibly when these metrics fluctuate outside of the normal physiological range.
Our longitudinal neuroimaging study then found that tactile acuity over the back (but not finger) was improved following 4-weeks of verum acupuncture. Control acupuncture interventions with reduced or absent somatosensory afference did not improve tactile acuity. Furthermore, improvements in tactile acuity over the back following verum acupuncture were associated with reduced S1-back gray matter volume and increased FA in the white matter adjacent to the S1-back (and not S1-finger) area. The exact cellular and molecular mechanisms inducing changes in GM volume and FA measured by MRI are not fully understood. GM volume changes may reflect changes in neuronal morphology, such as synaptogenesis and remodeling of neuronal processes (Lerch et al., 2011; Sumiyoshi et al., 2014), or non-neuronal changes such as angio-genesis, microglial proliferation, and astrogenesis (Taubert et al., 2012; Sumiyoshi et al., 2014). Indeed, our group recently reported elevated S1 PET PBR28 ligand binding (evidence of glial activation) in cLBP (Loggia et al., 2015). WM FA changes may reflect fiber myelination, axonal morphology, as well as oligodendritic structure (Beaulieu, 2002; Taubert et al., 2012). Furthermore, changes in tactile acuity and pain may reflect the balance of excitatory and inhibitory neurotransmitter systems involving glutamate and GABA, respectively, and induce morphological changes in synaptic transmission via long-term potentiation or long-term depression (Dinse et al., 2003; Ji et al., 2003; Draganski and May 2008). Such changes following acupuncture may contribute to microstructural MRI signal changes, though the exact mechanism remains unclear.
The verum acupuncture procedure included touch palpation, needle insertion targeting deep tissue receptors, manual stimulation by twirling the inserted needles, leading to needling-induced perceptions such as numbness, soreness, distention, heaviness, and dull pain (Kong et al., 2007; Napadow et al., 2009; Wand et al., 2013). Such somatosensory afference may serve to guide S1 neuroplasticity following manual acupuncture. Furthermore, as the acupuncture ritual may focus attention toward the location of the needling, acupuncture may share mechanisms with more conventional sensory discrimination trainings in terms of improving tactile acuity and producing S1 neuroplasticity. In fact, previous studies have demonstrated that both sensory training and training-independent sensory stimulation can induced changes in tactile perception via S1 reorganization (Flor et al., 2001; Beste and Dinse, 2013; Wand et al., 2013). For instance, simultaneous and repetitive tactile co-stimulation of multiple body regions can drive S1 neuroplasticity (Beste and Dinse, 2013).
In our study, verum acupuncture included repetitive sensory stimulation at 9–10 acupoints, mainly over the lower back and buttocks, applied over 6 sessions across 4 weeks. While most previous studies of training-independent sensory stimulation adopted short-duration experimental protocols (<3 days (Godde et al., 2000)), our study demonstrated that less intensive somatosensory stimulation spread over a longer time frame can also improve tactile performance, concomitant with S1 neuroplasticity. As verum acupuncture targets deeper receptors, the induced sensations of soreness, numbness, heaviness, and distention (MacPherson and Asghar, 2006) are unusual, are more salient to the patient, potentially leading to increased arousal and attention directed to the needling procedure, contributing to its impact on tactile acuity and neuroplasticity. Future studies should assess whether the targeting of deeper receptors located in fascia and/or muscle tissue and eliciting sensory qualities and brain responses that differ from the stimulation of cutaneous receptors by sham acupuncture (Napadow et al., 2009), plays a role in the differences in behavioral outcomes and S1 neuroplasticity for verum versus sham acupuncture. In fact, a previous study showed that cutaneous stimulation using a cork probe also had no effect on tactile acuity (Moseley et al., 2008), suggesting that cutaneous receptor targeting may not be sufficient for driving change in tactile acuity.
While our study did not find a link between clinical pain reduction and tactile acuity improvements or S1 neuroplasticity following verum or sham acupuncture, such links have been suggested by prior studies. Bradbrook et al. found that acupuncture both relieved phantom limb pain and restored tactile sensation in amputees (Bradbrook, 2004), however a direct linkage between somatosensory recovery and pain relief was not demonstrated. Our previous study in carpal tunnel syndrome found that adaptive S1 neuroplasticity following 8-week acupuncture therapy predicted long-term (3-month post therapy) improvements in pain and paresthesia (Maeda et al., 2017). It’s possible that our current study in cLBP was not able to link improvements in S1 microstructure and tactile acuity with clinical pain due to the shorter duration of therapy used. Clinical pain reduction following shorter durations of acupuncture therapy may be better reflected in reorganization of functional brain networks (Tu et al., 2019); in fact, our previous study in fibromyalgia showed that a 4-week acupuncture therapy reduced heightened cross-network functional connectivity of the default mode network and the insula, and the degree of reduction correlated with the level of clinical pain reduction (Napadow et al., 2012).
Limitations to our study should also be noted. As there was variability in laterality of back pain between subjects and even within-patient visit to visit variability, we evaluated 2PDT-back at a single site on all subjects. It’s possible that unilateral low back pain lateralized to the testing side or opposite side, may introduce variability in tactile acuity outcome. However, associations between tactile acuity and VBM outcomes were bilateral at baseline, suggesting that this result was not sensitive to laterality in testing. Another limitation, as noted above, was the short duration of therapy (4 weeks), which was long enough to elicit reduction in pain interference and tactile acuity but was perhaps too brief to allow for robust neuroimaging-sensitive changes in S1 microstructure – something we have previously shown following 8 weeks of therapy in neuropathic pain patients (Maeda et al., 2017). Future studies should incorporate more treatment sessions over a longer course of treatment. Our study demonstrated that repetitive tactile stimuli by acupuncture needles improved tactile acuity and pain-related interference in cLBP patients. Historically, the effects of acupuncture are also theorized to be mediated by the stimulation of specifically targeted acupoints along the body. The current study focused on the somatosensory aspect of acupuncture stimulation and not acupoint specificity, and thus we did not include a control group with verum acupuncture at non-acupoint locations. However, our study did demonstrate that a standardized manual acupuncture protocol with repeated deep receptor somatosensory afference could produce pain relief and improve tactile acuity over the lower back.
In conclusion, compared to HC, cLBP patients demonstrated impaired tactile acuity, which was associated with increased gray matter volume in the S1 representation for the low back. Patients also demonstrated reduced FA in the white matter adjacent to this S1 subregion. Following 4-weeks of verum acupuncture therapy, tactile acuity over the back (but not finger) was improved, and improvements were associated with reduced S1-back gray matter volume and increased FA in the white matter adjacent to the S1-back subregion. These changes and associations were not present following control acupuncture interventions designed to have reduced or absent somatosensory afference. Our study suggests that acupuncture may improve tactile acuity over pain-affected regions in cLBP via somatotopically-specific structural S1 neuroplasticity.
Supplementary Material
Acknowledgments
Funding
This project was supported by the National Institutes of Health, National Center for Complementary and Integrative Health (United States, P01-AT006663, R01-AT007550, R61-AT009306 to VN and BRR), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (United States, R01-AR064367 to VN and RRE), National Institute of Neurological Disorders and Stroke (United States, R01NS095937, R01NS094306 to MLL), and the National Center for Research Resources (United States, P41RR14075, S10RR021110, S10RR023043 to BRR). Support was also generously provided by the Korean Institute of Oriental Medicine (South Korea, KSN2013240 to VN, JK, and HK) and by the Traditional Korean Medicine R&D program funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (South Korea, HI17C2212 to HK).
Abbreviations:
- cLBP
chronic low back pain
- DTI
diffusion tensor imaging
- GM
gray matter
- S1
primary somatosensory cortex
- 2PDT
two-point discrimination threshold
Footnotes
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Declaration of competing interest
The authors declare no competing financial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2020.116899.
References
- Ashburner J, Friston KJ, 2005. Unified segmentation. Neuroimage 26 (3), 839–51. [DOI] [PubMed] [Google Scholar]
- Cherkin DC, Sherman KJ, Avins AL, Erro JH, Ichikawa L, Barlow WE, et al. , 2009. A randomized trial comparing acupuncture, simulated acupuncture, and usual care for chronic low back pain. Arch. Intern. Med 169 (9), 858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, et al. , 2011. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. Neuroimage 55 (3), 880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Schlaug G, 2003. Brain structures differ between musicians and non-musicians. J. Neurosci 23 (27), 9240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17 (2), 825–41. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. Fsl. Neuroimage 62 (2), 782–90. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. , 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31 (4), 1487–505. [DOI] [PubMed] [Google Scholar]
- Abenhaim L, Rossignol M, Valat JP, Nordin M, Avouac B, Blotman F, et al. , 2000. The role of activity in the therapeutic management of back pain. Report of the International Paris Task Force on Back Pain. Spine (Phila Pa 1976 25 (4 Suppl. l), 1S–33S. [DOI] [PubMed] [Google Scholar]
- Adamczyk W, Luedtke K, Saulicz E, 2018. Lumbar tactile acuity in patients with low back pain and healthy controls: systematic review and meta-analysis. Clin. J. Pain 34(1), 82–94. [DOI] [PubMed] [Google Scholar]
- As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, et al. , 2012. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain 153 (5), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balague F, Mannion AF, Pellise F, Cedraschi C, 2012. Non-specific low back pain. Lancet 379 (9814), 482–491. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, 2002. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 15 (7–8), 435–455. [DOI] [PubMed] [Google Scholar]
- Benton AL, Levin HS, Varney NR, 1973. Tactile perception of direction in normal subjects: implications for hemispheric cerebral dominance. Neurology 23 (11), 1248–1250. [DOI] [PubMed] [Google Scholar]
- Berman BM, Langevin HM, Witt C, Dubner R, 2010. Acupuncture for chronic low back pain. N. Engl. J. Med 363, 454–461. [DOI] [PubMed] [Google Scholar]
- Beste C, Dinse HR, 2013. Learning without training. Curr. Biol 23 (11), R489–R499. [DOI] [PubMed] [Google Scholar]
- Bradbrook D, 2004. Acupuncture treatment of phantom limb pain and phantom limb sensation in amputees. Acupunct. Med 22 (2), 93–97. [DOI] [PubMed] [Google Scholar]
- Catley MJ, O’Connell NE, Berryman C, Ayhan FF, Moseley GL, 2014. Is tactile acuity altered in people with chronic pain? a systematic review and meta-analysis. J. Pain 15 (10), 985–1000. [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. , 2010. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol 63 (11), 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond R, Ruzich E, Witzel T, Maeda Y, Malatesta C, Morse L, et al. , 2012. Spatiotemporal mapping cortical neuroplasticity in carpal tunnel syndrome. Brain 135 (Pt 10), 3062–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedenhofen B, 2015. Musch J. cocor: a comprehensive solution for the statistical comparison of correlations. PloS One 10 (3), e0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M, 2003. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science 301 (5629), 91–94. [DOI] [PubMed] [Google Scholar]
- Douaud G, Mackay C, Andersson J, James S, Quested D, Ray MK, et al. , 2009. Schizophrenia delays and alters maturation of the brain in adolescence. Brain 132 (Pt 9), 2437–2448. [DOI] [PubMed] [Google Scholar]
- Draganski B, May A, 2008. Training-induced structural changes in the adult human brain. Behav. Brain Res 192 (1), 137–142. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. , 2005. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25 (4), 1325–1335. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, et al. , 2012. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum. Brain Mapp 33 (10), 2390–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM, 2001. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94 (2), 149–158. [DOI] [PubMed] [Google Scholar]
- Flor H, Denke C, Schaefer M, Grusser S, 2001. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet 357 (9270), 1763–1764. [DOI] [PubMed] [Google Scholar]
- Freyer F, Reinacher M, Nolte G, Dinse HR, Ritter P, 2012. Repetitive tactile stimulation changes resting-state functional connectivity-implications for treatment of sensorimotor decline. Front. Hum. Neurosci 6, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde B, Stauffenberg B, Spengler F, Dinse HR, 2000. Tactile coactivation-induced changes in spatial discrimination performance. J. Neurosci 20 (4), 1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley MA, Jensen MP, Ehde DM, Robinson LR, Cardenas DD, Turner JA, et al. , 2006. Clinically significant change in pain intensity ratings in persons with spinal cord injury or amputation. Clin. J. Pain 22 (1), 25–31. [DOI] [PubMed] [Google Scholar]
- Hoffken O, Veit M, Knossalla F, Lissek S, Bliem B, Ragert P, et al. , 2007. Sustained increase of somatosensory cortex excitability by tactile coactivation studied by paired median nerve stimulation in humans correlates with perceptual gain. J. Physiol 584 (Pt 2), 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irnich D, Salih N, Offenbacher M, Fleckenstein J, 2010. Is sham laser a valid control for acupuncture trials? eCAM (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Chen C, Brugger AM, 2003. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J. Pain 4 (7), 407–414. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ, 2003. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26 (12), 696–705. [DOI] [PubMed] [Google Scholar]
- Kalisch T, Tegenthoff M, Dinse HR, 2007. Differential effects of synchronous and asynchronous multifinger coactivation on human tactile performance. BMC Neurosci. 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Mawla I, Kong J, Lee J, Gerber J, Ortiz A, et al. , 2019. Somatotopicallyspecific Primary Somatosensory Connectivity to Salience and Default Mode Networks Encodes Clinical Pain. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub R, Huang T, Polich G, Napadow V, Hui K, et al. , 2007. Acupuncture de qi, from qualitative history to quantitative measurement. J. Alternative Compl. Med 13 (10), 1059–1070. [DOI] [PubMed] [Google Scholar]
- Kong J, Spaeth RB, Wey HY, Cheetham A, Cook AH, Jensen K, et al. , 2013. S1 is associated with chronic low back pain: a functional and structural MRI study. Mol. Pain 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel J, Meeus M, Malfliet A, Dolphens M, Danneels L, Nijs J, Cagnie B, 2015. Structural and functional brain abnormalities in chronic low back pain: a systematic review. In: Seminars in Arthritis and Rheumatism, vol. 45, pp. 229–237, 2. [DOI] [PubMed] [Google Scholar]
- Lee J, Mawla I, Kim J, Loggia ML, Ortiz A, Jung C, et al. , 2019. Machine learning-based prediction of clinical pain using multimodal neuroimaging and autonomic metrics. Pain 160 (3), 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Yiu AP, Martinez-Canabal A, Pekar T, Bohbot VD, Frankland PW, et al. , 2011. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage 54 (3), 2086–2095. [DOI] [PubMed] [Google Scholar]
- Lissek S, Wilimzig C, Stude P, Pleger B, Kalisch T, Maier C, et al. , 2009. Immobilization impairs tactile perception and shrinks somatosensory cortical maps. Curr. Biol 19 (10), 837–842. [DOI] [PubMed] [Google Scholar]
- Liu L, Skinner M, McDonough S, Mabire L, Baxter GD, 2015. Acupuncture for low back pain: an overview of systematic reviews. Evid Based Complement Alternat Med 2015, 328196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, et al. , 2015. March 1 Evidence for brain glial activation in chronic pain patients. Brain 38 (3), 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel P, Vachon B, Lemaire J, Durand MJ, Poitras S, Stock S, et al. , 2002. Discriminative and predictive validity assessment of the quebec task force classification. Spine (Phila Pa 1976 27 (8), 851–857. [DOI] [PubMed] [Google Scholar]
- Longo MR, Betti V, Aglioti SM, Haggard P, 2009. Visually induced analgesia: seeing the body reduces pain. J. Neurosci 29 (39), 12125–12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Moseley GL, 2007. Role of distorted body image in pain. Curr. Rheumatol. Rep 9 (6), 488–496. [DOI] [PubMed] [Google Scholar]
- Luomajoki H, Moseley GL, 2011. Tactile acuity and lumbopelvic motor control in patients with back pain and healthy controls. Br. J. Sports Med 45 (5), 437–440. 10.1136/bjsm.2009.060731. Epub 2009 Jun 23. [DOI] [PubMed] [Google Scholar]
- MacPherson H, Asghar A, 2006. Acupuncture needle sensations associated with De Qi: a classification based on experts’ ratings. J. Alternative Compl. Med 12 (7), 633–637. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Kettner N, Holden J, Lee J, Kim J, Cina S, et al. , 2014. Functional deficits in carpal tunnel syndrome reflect reorganization of primary somatosensory cortex. Brain 137 (Pt 6), 1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Kettner N, Kim J, Kim H, Cina S, Malatesta C, et al. , 2016. Primary somatosensory/motor cortical thickness distinguishes paresthesia-dominant from pain-dominant carpal tunnel syndrome. Pain 157 (5), 1085–1093. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Kim H, Kettner N, Kim J, Cina S, Malatesta C, et al. , 2017. Rewiring the primary somatosensory cortex in carpal tunnel syndrome with acupuncture. Brain 140 (4), 914–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Neundorfer B, Birklein F, 2004. Cortical reorganization during recovery from complex regional pain syndrome. Neurology 63 (4), 693–701. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Weintraub S, Nowinski C, Kaptanoglu G, Gitelman DR, Mesulam MM, 2003. Cerebral hemispheric specialization for spatial attention: spatial distribution of search-related eye fixations in the absence of neglect. Neuropsychologia 41 (10), 1396–1409. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, van der Zwaag W, Farthouat J, Gruetter R, Blanke O, 2014. Human finger somatotopy in areas 3b, 1, and 2: a 7T fMRI study using a natural stimulus. Hum. Brain Mapp 35 (1), 213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meints SM, Mawla I, Napadow V, Kong J, Gerber J, Chan ST, et al. , 2019. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. Pain 160 (4), 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley GL, Zalucki NM, Wiech K, 2008. Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain 137 (3), 600–608. [DOI] [PubMed] [Google Scholar]
- Napadow V, Kettner N, Ryan A, Kwong KK, Audette J, Hui KK, 2006. Somatosensory cortical plasticity in carpal tunnel syndrome–a cross-sectional fMRI evaluation. Neuroimage 31 (2), 520–530. [DOI] [PubMed] [Google Scholar]
- Napadow V, Liu J, Li M, Kettner N, Ryan A, Kwong KK, et al. , 2007. Somatosensory cortical plasticity in carpal tunnel syndrome treated by acupuncture. Hum. Brain Mapp 28 (3), 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Dhond RP, Kim J, LaCount L, Vangel M, Harris RE, et al. , 2009. Brain encoding of acupuncture sensation–coupling online rating with fMRI. Neuroimage 47 (3), 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Kim J, Clauw DJ, Harris RE, 2012. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 64 (7), 2398–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJ, Chen R, 2008. Digit somatotopy within cortical areas of the postcentral gyrus in humans. Cerebr. Cortex 18 (10), 2341–2351. [DOI] [PubMed] [Google Scholar]
- Pleger B, Tegenthoff M, Ragert P, Forster AF, Dinse HR, Schwenkreis P, et al. , 2005. Sensorimotor returning in complex regional pain syndrome parallels pain reduction. Ann. Neurol 57 (3), 425–429. [DOI] [PubMed] [Google Scholar]
- Ragert P, Kalisch T, Bliem B, Franzkowiak S, Dinse HR, 2008. Differential effects of tactile high- and low-frequency stimulation on tactile discrimination in human subjects. BMC Neurosci. 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH, 1980. Tests for comparing elements of a correlation matrix. Psychol. Bull 87, 245–251. [Google Scholar]
- Sumiyoshi A, Taki Y, Nonaka H, Takeuchi H, Kawashima R, 2014. Regional gray matter volume increases following 7days of voluntary wheel running exercise: a longitudinal VBM study in rats. Neuroimage 98, 82–90. [DOI] [PubMed] [Google Scholar]
- Taubert M, Villringer A, Ragert P, 2012. Learning-related gray and white matter changes in humans: an update. Neuroscientist 18 (4), 320–325. [DOI] [PubMed] [Google Scholar]
- Tu Y, Jung M, Gollub RL, Napadow V, Gerber J, Ortiz A, et al. , 2019. Abnormal medial prefrontal cortex functional connectivity and its association with clinical symptoms in chronic low back pain. Pain 160 (6), 1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S, 2014. Multivariate classification of structural MRI data detects chronic low back pain. Cerebr. Cortex 24 (4), 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Johansson RS, 1984. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum. Neurobiol 3 (1), 3–14. [PubMed] [Google Scholar]
- Volkow ND, McLellan AT, 2016. Opioid abuse in chronic pain–misconceptions and mitigation strategies. N. Engl. J. Med 374 (13), 1253–1263. [DOI] [PubMed] [Google Scholar]
- Wand BM, Di Pietro F, George P, O’Connell NE, 2010. Tactile thresholds are preserved yet complex sensory function is impaired over the lumbar spine of chronic non-specific low back pain patients: a preliminary investigation. Physiotherapy 96 (4), 317–323. [DOI] [PubMed] [Google Scholar]
- Wand BM, Abbaszadeh S, Smith AJ, Catley MJ, Moseley GL, 2013. Acupuncture applied as a sensory discrimination training tool decreases movement-related pain in patients with chronic low back pain more than acupuncture alone: a randomised cross-over experiment. Br. J. Sports Med 47 (17), 1085–1089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


