Abstract
Idiopathic inflammatory myopathies (IIM) represent a heterogeneous group of autoimmune diseases whose treatment is often a challenge. Many patients, even after immunosuppressive therapy, do not respond to treatment, so new alternatives have been sought for this. Therefore, other signaling pathways that could contribute to the pathogenesis of myositis have been investigated, such as the expression of myokines in skeletal muscle in response to the inflammatory process. In this review, we will refer to these muscle cytokines that are overexpressed or downregulated in skeletal muscle in patients with various forms of IIM, thus being able to contribute to the maintenance of the autoimmune process. Some muscle cytokines, through their antagonistic action, may be a helpful contributor to the disease modulation, and thus, they could represent personalized treatment targets. Here, we consider the main myokines involved in the pathogenesis of myositis, expressing our view on the possibility of using them as potential therapeutic targets: interleukins IL-6, IL-15, and IL-18; chemokines CXCL10, CCL2, CCL3, CCL4, CCL5, and CCL20; myostatin; follistatin; decorin; osteonectin; and insulin-like 6. An interesting topic regarding the complex connection between myokines and noninflammatory pathways implied in IIM has also been briefly described, because it is an important scientific approach to the pathogenesis of IIM and can be a therapeutic alternative to be considered, especially for the patients who do not respond to immunosuppressive treatment.
1. Introduction
This review aims to examine the most recent studies regarding the presence and role of myokines in inflammatory myopathies. There are few reviews concerning the activity and role of myokines in normal muscle and in other muscular pathologies, but from our knowledge, there is no recent review specifically dedicated to myokines in myositis. In addition, we would like to focus the attention on myokines as possible therapeutic targets in idiopathic inflammatory myopathies (IIM), as there are still difficulties in treatment approaches that do not have the expected results yet. In this complex group of diseases, there are overlapped clinical diagnostics, nonresponder patients, or a complicated pathogenesis not elucidated so far. Having a summary of the recent studies and an overview of possible further research, readers can easily draw conclusions on the results already achieved and some starting points for investigations to be made in this field.
Myositis or IIM represent a heterogeneous group of autoimmune diseases characterized by muscle weakness, the presence of inflammatory muscle infiltrates, as well as overexpression of MHC class I in muscle fibers sarcolemma. IIM are traditionally divided into 5 subtypes: polymyositis (PM), dermatomyositis (DM), inclusion body myositis (IBM), autoimmune necrotizing myopathy, myositis overlap-syndromes, e.g., those associated with cancers or other systemic autoimmune diseases [1]. The myositis classification is constantly changed. The classification criteria have been a subject of debate for many years, but the Bohan and Peter criteria have remained the basics [2, 3], valid today, but to which other categories have been added over the years. Most of the modifications refer to the clinico-serological criteria by the discovery of new myositis-specific and myositis-associated autoantibodies. A recent review regarding the new classification criteria of myositis is that of Leclair and Lundberg [4]. In these conditions, sometimes it is difficult to put a diagnosis because of heterogeneity presented by this group of diseases, and also because of overlapping syndromes. In addition to clinical examination, laboratory tests, MRI investigation, muscle and skin biopsy, and electromyography are performed.
Inflammation is constantly present, but there are clear differences between the IIM forms: macrophages, CD8+ T-cells, mainly involved in PM and IBM, and CD4+ T-cells and B-cells, mainly involved in DM. In DM, inflammatory infiltrates are found especially around blood vessels, while in PM and IBM, it is an inflammatory cell invasion of nonnecrotic fibers.
Other morphological changes in skeletal muscles are muscular atrophy, the presence of necrotic fibers, collagen proliferation, and rimmed vacuoles (in IBM). An overexpression of MHC class I and MHC class II in sarcolemma are present in all types of IIM [5–7]. We mentioned all these pathological changes observed in the muscles, because they are related to the subject presented in this review.
Besides the fact that the diagnosis is sometimes difficult to establish, it is also noted that anti-inflammatory treatment often does not give the expected results, and muscle weakness persists.
In recent years, a special attention has begun to be given to skeletal muscle cytokines called myokines. They are involved in the inflammatory process triggered by the immune system, aggravating or ameliorating inflammatory pathology. Thus, myokines may become important therapeutic targets for patients with IIM. Furthermore, the presence of myokines in muscle biopsy or in blood samples of IIM patients could be an indication for a specific and personalized diagnosis.
Given the fact that myositis is such a heterogeneous group of autoimmune diseases, it is very important to highlight new data on this pathology, both for a correct diagnosis and for finding new methods of treatment. In this review, we aim to draw attention to the role of myokines in IIM pathology, as well as to the existence of complex interconnected cellular signaling pathways in which muscle cytokines play an increasingly important role.
2. Myokines Definition
Myokines are cytokines, chemokines, or peptides produced and released by muscle cells [8] under the action of contractile activity [9] or as an effect of other stimuli, just like an endocrine organ, as described in recent years [10]. Cytokines are a large group of small proteins whose primary role is associated with immune response. They may have proinflammatory and anti-inflammatory functions, depending on the initial stimuli, target cell, or additional cytokines released in conjunction [11].
Myokines have begun to be more and more studied in connection with muscular pathology such as cancer cachexia [12], muscular dystrophy [13–15], and myositis [16]. There are myokines that have positive effects, others that have negative effects, and some that have both positive and negative effects, depending on muscle pathology or metabolic conditions.
3. Role of Myokines in Idiopathic Inflammatory Myopathies
There are studies that have shown that myokines can have a pathogenic effect in the skeletal muscles, can induce muscle weakness, and decrease muscle mass in myopathies, both in muscular dystrophies and myositis as well as in muscular cachexia from cancers.
IIM are autoimmune diseases, cytokines released by inflammatory infiltrate cells in muscle tissue inducing the production and releasing of myokines and overexpression of MHC I [17–19]. Myoblasts, in addition to the production of chemokines, may be involved themselves in the recruitment of leukocytes [17, 20]. Once released, myokines, interacting with the endoplasmic reticulum stress pathways, can activate the production of inflammatory cytokines, helping to maintain inflammation on the one hand, and maintaining muscle weakness in the absence of inflammation on the other hand [21, 22] (Figure 1). Contrariwise, there are studies that have clearly shown that endurance exercise, causing the release of myokines, is a successful therapeutic intervention in IIM [23].
Figure 1.
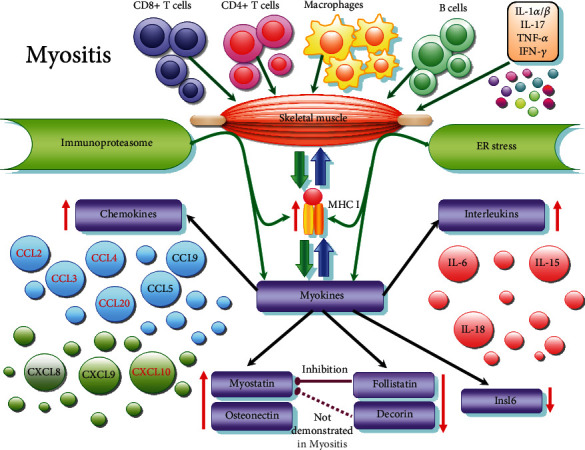
Schematic representation of myokines released in myositis. Skeletal muscle inflammation is induced by invading immune cells (thin dark green arrows): macrophages, T-cells (CD4 + T-cells, CD8 + T-cells), B cells which release proinflammatory cytokines (IL: interleukin; IFN: interferon; TNF: tumour necrosis factor). Most important cytokines related to myokines releasing are IL-1α/β, IL17, TNF-α, and INF-γ. In response to inflammation, MHC class I is overexpressed on the sarcolemma, contributing to muscle injury. Moreover, MHC I activates ER stress response and inflammasome activation. Immunoproteasome and endoplasmic reticulum (ER) stress contribute, in turn, to MHC I overexpression (thin green arrows). Under these conditions, muscle cells secrete myokines (thick green arrows), such as interleukins—IL-6, IL-15, IL-18; chemokines—CCL2, CCL3, CCL4, CCL5, CCL9, CCL20, CXCL8, CXCL9, CXCL10 (the most important in red); myostatin, follistatin, osteonectin, decorin (probably present, but uninvestigated in myositis), and insulin-like 6 (Insl6). Myokines, in turn, attract inflammatory cells, maintaining, to a certain extent, inflammation (thick blue arrows).
In this review, we will refer to the role of myokines in myositis (Table 1). It is important to know their mode of action in certain physiological or pathological conditions, so that the modulation of their expression could become a therapeutic model. Controlling myokines in myositis may become an important factor in finding an appropriate or personalized treatment.
Table 1.
Myokines present in idiopathic inflammatory myopathies. In this table, we present the main role and activity of the most important myokines in myositis.
| Myokines | Role in myositis |
|---|---|
| IL-6 | (i) Controversial: proinflammatory as cytokine, anti-inflammatory as myokine. (ii) High level in IIM patients and in vitro studies; harmful [46, 48]. (iii) Mediator of innate and adaptive immune response [27, 28]. (iv) Its production induced by inflammatory cytokines TNF-α, IL-17, IL-1β in myoblasts, in vitro [18, 46], not in IIM, but with possible same effects in IIM. (v) Blocking IL-6 receptors, positive effect, in vivo study on PM model [45]. (vi) Possible biomarker for DM patients [43]. |
|
| |
| IL-15 | (i) Upregulated in myositis—muscle and serum (DM, PM patients) [61, 62]. (ii) Causes muscle weakness—DM, PM patients [64]. (iii) Closely connected with CD163 macrophages—PM patients and PM in vitro model [65]. (iv) Upregulates MMP-9 expression in PM [65]. (v) Possible promotor of autoimmune inflammation in vivo [67]. |
|
| |
| IL-18 | (i) Implicated in autoimmune diseases [75]. (ii) Localised in inflammatory cells and capillaries in IIM patients [76]. (iii) High serum levels in DM/DM with interstitial lung disease [77, 78] and PM [77]. |
|
| |
| CXCL10 | (i) High level in myositis. His CXCR3 receptor also—in vivo [85]; in sIBM patients [86]; in PM, sIBM, DM patients [87]; in juvenile DM [88]. (ii) Possible promotor of autoimmune inflammation through initiation and maintenance of type 1 T-helper cells [84]. (iii) Possible therapeutic target in adult myositis patients [90]. |
|
| |
| CCL2, CCL3, CCL4, CCL5 | (i) MHC I overexpression leads to CCLs release, in vitro [91]. (ii) CCL2 increased levels in IIM patients [48, 82], including juvenile DM [92]. (iii) CCL3, CCL4—upregulated in IIM [93, 94]. (iv) CCL5—low expression in few inflammatory cells in IIM [93]. (v) CCR1 receptor—in macrophages and endothelial cells in sIBM [93]. (vi) CCR5 receptor—in inflammatory cells invading nonnecrotic muscle fibers in IIM [93]. (vii) CCL2 and CCR2 receptor, high levels in IIM patients [95] |
|
| |
| CCL20 | (i) Upregulated in the presence of IL-17 and IL-1β in muscle cells—in vitro study and in DM, PM muscular biopsies [45]. |
|
| |
| Myostatin | (i) Accumulates and associates with aggregates containing Aβ in sIBM patients [108]. (ii) Upregulated in IIM [109]. (iii) Myostatin gene expression attenuated, inhibitors gene expression (follistatin) upregulated in resistance training in IBM [110, 111]. |
|
| |
| Follistatin | (i) Upregulated in inflammatory diseases [120]. Proposed as an inhibitor of myostatin. (ii) Follistatin gene transfer to sIBM patients—clinical trial, resulted in an improvement in muscle regeneration [117]. (iii) Follistatin high serum levels concomitant with decreased serum myostatin levels in IIM patients [118]. |
|
| |
| Decorin | Although studies have been performed on its role as opposed to myostatin in skeletal muscle, there are no studies on IIM pathology regarding decorin. |
|
| |
| Osteonectin | (i) Upregulated in IIM and in muscular dystrophies [134]. |
|
| |
| Insl6 | (i) Protect the muscle against IIM development; downregulated in IIM—in vivo study [137]. (ii) Insl6 deficiency resulted in a worsened myositis phenotype—in vivo [137]. (iii) When it is downregulated, inflammatory cytokines expression is increased [137]. (iv) Inhibits the proliferation/activation of T-cells [137]. (v) Reduced in PM and DM patients (Insl6 transcript expression) [137]. |
Among the myokines that have been cited in the literature as having a possible role in modulating the pathogenesis of myositis, we will address the following: interleukins (IL-6, IL-15, IL-18), chemokines (CXCL10, CCL2, CCL4, CCL5, CCL20), myostatin, follistatin, decorin, osteonectin, and insulin-like 6.
4. Interleukins
Interleukins (ILs) are cytokines that were first seen to be expressed by leukocytes, hence the name. But later, they have been found to be produced by many other types of cells. ILs are key factors in the functioning of the immune system, in activation and differentiation of immune cells by binding to high-affinity receptors on cell surfaces. They stimulate the development and differentiation of T and B lymphocytes and also of haematopoietic stem cells [24, 25]. ILs are involved in systemic inflammation and immune system modulation, so they play important roles in fighting infectious diseases or cancer.
There have been identified around 40 different ILs [26]. They are implied in multiple signaling pathways, exhibit diverse roles in immune regulation and cellular networks, and target numerous proteins that regulate biological responses. Identification of ILs antagonists (antibodies and receptor antagonists), as treatment option for autoimmune disorders and other inflammatory diseases, is a target in current and future research.
The best-known interleukins that are secreted by the skeletal muscle are IL-6, IL-15, and IL-18 which are presented below.
4.1. IL-6
IL-6, a glycoprotein of 21-28 kDa, is a pleiotropic prototypical cytokine (four-helix bundle cytokine) that activates an acute immune response in infections and in muscle tissue injuries [27, 28]. It is the most studied myokine in IIM. It acts as a mediator of innate and adaptive immune responses [27, 28]. IL-6 exerts both an endocrine and a metabolic function in several organs, such as the pancreas, gut, fat, liver, and skeletal muscle.
IL-6 was among the first myokines seen upregulated after intense exercise [29], up to 100-fold in relation to other cytokines such as TNF-α or IL-1β [30–32]. IL-6 upregulates antioxidant defenses as response to oxidative stress, as shown by Sacheck et al. [33].
The number of IL-6 receptors also increases after exercise [34]. Serrano et al. in an in vitro study on mice lacking IL-6 gene in skeletal muscle [35] have shown that this myokine is an important regulator of muscular hypertrophy mediated by the satellite cells in normal muscle.
Several studies have revealed that IL-6 has a dual role in muscle, in some pathological cases inducing atrophy [36, 37], in other cases supporting regeneration by increasing myoblasts proliferation [38]. A review showing the pivotal role of IL-6 between skeletal muscle wasting and renewal belongs to Belizario et al. [39]. A surprising fact was observed concerning this dual effect of IL-6; although, this cytokine has a proinflammatory role in rheumatic diseases, for example [32], when released from muscle it has an anti-inflammatory role [40]. It was shown that the role of IL-6 is predominantly played in metabolism rather than in inflammation. IL-6 regulates energy metabolism. We consider that these results could have similar connotations in IIM, and further research is needed to define the role of IL-6 in this pathology.
Given the dual role of IL-6, in cachexia high plasma levels of this myokine cause a decrease in muscle weight [41], cause muscle atrophy [42] and an increased cathepsin level. Studies on a transgenic mouse model have shown that IL-6 is involved in the regulation of muscle protein degradation [36]. Some authors consider that the significance of IL-6 overexpression in skeletal muscle in cachexia [12] is the same as in the muscle with inflammatory pathology, increasing the disease progression.
Bilgic et al. [43] observed in patients with DM that there is a correlation between IL-6 serum levels and the disease activity, being a candidate biomarker for adult and juvenile DM.
An in vivo research on a murine model of PM showed that a treatment with anti-IL-6 receptors antibodies had a positive effect [44]. Experimental studies on human muscle cell cultures showed that IL-6 production in human myoblasts is induced by the presence of TNF-α, IL-17, and IL-1β [18, 45]. Thus, the muscle inflammation on isolated myoblasts was induced by incubation with these exogenous cytokines arguing for an increase in IL-6 levels in response to inflammation. As a result, blocking IL-6 signaling pathway could be a potential therapy in IIM. In fact, this blockage has been approved for treatment in rheumatoid arthritis [46].
Scuderi et al. showed on a transgenic mouse IL-6-/- treated with rabbit myosin to induce IIM, which there was no inflammation in the muscle fibers, a complete absence of necrosis and leukocyte infiltrates, as well as the absence of myofiber regeneration. Muscle infiltrates present in muscle were macrophages. Their conclusion was that the absence of IL-6 prevents the infiltration of muscle with monocytes, due to a chemotaxic inhibition [47].
A study on patients with IIM, made by Loaiza-Felix et al. [48] to assess whether levels of serum adipokines may be disease markers, showed higher levels of IL-6 in patients compared with healthy individuals. It is interesting that in this study IL-6 is considered as adipokine, cytokine secreted by adipose tissue. High serum IL-6 levels were also found in IIM patients with interstitial lung disease [49]. Given that IL-6 is both adipokine and myokine, it can be called adipo-myokine. Even though their function has not yet been completely clarified, adipo-myokines seem to have a double effect, beneficial or adverse, depending on the extent and kinetics of these molecules in serum [50, 51].
In conclusion, the role of IL-6 in myositis is not yet fully understood, and further studies on this cytokine as a therapeutic target should be considered, taking into account both the role of IL-6 as myokine, but also as a serum cytokine, or its dual role played in metabolism.
4.2. IL-15
IL-15 is a 14-15 kDa molecule, member of the 4α-helix bundle cytokine family. It is another myokine released by the muscle after exercise.
IL-15 and its receptor, IL-15Rα, have widespread expression [52, 53] at the transcriptional level in many normal human tissues and cells including skeletal muscle [52], but they are rarely detected at the protein level under normal conditions [54].
Initial research concerning the functionality of IL-15 appeared to indicate a role in skeletal muscle hypertrophy [55]. However, more recent studies have indicated a potential alternative role in muscle oxidative and fatigability properties [56, 57]. IL-15 stimulates myosin-heavy chain accumulation in differentiated myocytes [55, 58].
Concerning the IL-15 expression in response to different resistance training exercises, previous literature has reported a variability in its serum levels. Riechman et al. [59] observed a statistically significant increase in plasma IL-15 following a resistance training regimen. In contrast, Nielsen et al. did not observe any change in plasma IL-15 following a resistance exercise protocol [60].
In myositis, a report based on patients with PM and DM [61, 62] showed an upregulation of IL-15 in muscle fibers and serum, in correlation with disease severity. It was suggested a possible role of IL-15 in the PM and DM mechanisms. IL-15 production is stimulated by IL-1α/β, TNF-α, IFN-γ. and LPS, and also by CD40 ligation. In turn, IL-15 stimulates the dendritic cells, NK cells, and effector T-cells [63].
Zhong et al. investigated the IL-15 and IL-15Rα expression in skeletal muscle of patients with PM and DM after treatment [64], and they observed that IL-15/IL-15Rα expression was correlated with clinical findings: patients with a high number of IL-15-expressing cells in muscle tissue after immunosuppressive treatment had less improvement in muscle performance, which might indicate that IL-15 has a role in causing muscle weakness.
Another research on PM patients and experimentally, on a PM rat model [65], reported that the levels of CD163 macrophages were dramatically reduced after the treatment with the antibody anti-IL-15, indicating that IL-15 is closely connected to CD163 macrophages and has a significant influence upon the pathogenesis of idiopathic myositis. The authors showed also that being involved in the NF-κB signaling pathway, IL-15 can upregulate the expression levels of matrix metallopeptidase 9 (MMP-9). MMP-9 has been proved to be involved in the inflammatory process of muscle degeneration, and there may be an association between MMP-9 and the emergence of PM [66]. The researchers' opinion was that IL-15, as a key regulator in PM, is likely to become a promising therapeutic target and a possible treatment for multiple myositis.
Po-Lin et al. [67] hypothesized that IL-15 contributes to the development of autoimmune inflammation in the myositis by promoting autoreactive CD8+ T-cell function, constituting a potential therapeutic target.
However, the exact role of IL-15 in myositis is not yet known enough, and further studies are needed to bring new data on this. However, it appears that elevated levels are harmful and help maintain inflammation in myositis.
4.3. IL-18
IL-18 is an immunoregulatory cytokine which induces interferon-gamma (IFN-γ) [68]. As a consequence, the first name of IL-18 was IFN-γ-inducing factor, changing in IL-18 after the molecular cloning [69]. The primary sources for active IL-18 are macrophages and dendritic cells, but its precursors are present in epithelial cells [70].
IL-18 is a proinflammatory cytokine and together with IL-12, induces cell-mediated immunity. As myokine, IL-18 plasma levels were found normally increased after acute exercise [71].
A study concerning the involvement of IL-18 in muscle pain suggests that increased neutrophil numbers and IL-18 secretion from neutrophils produce mechanical hyperalgesia induced by repeated excessive muscle contraction [72]. This research was based on the observations that neutrophils are a source of IL-18 [73, 74].
The activity of IL-18 is balanced by the naturally occurring IL-18 binding protein (IL-18BP), as a study has shown in humans [75], and local IL-18BP administration attenuated hyperalgesia caused by excessive muscle contraction [72].
IL-18 has been implicated in numerous disorders, including autoimmune diseases [75]. Helmers et al. reported that IL-18 expression was predominantly localized to inflammatory cells and capillaries in patients with myositis compared with healthy controls where IL-18 was found mostly in capillaries. They observed also that the total IL-18 expression appeared lower in biopsies from patients receiving and improving with immunosuppressive treatment [76].
Gono et al. [77] observed that serum IL-18 level in patients with DM and PM was higher compared with healthy controls. Moreover, patients with DM had higher levels than PM patients, and DM patients with interstitial lung disease (ILD) had higher IL-18 serum levels than DM patients without ILD. In conclusion, serum IL-18 was strikingly elevated in DM patients, associated particularly with disease activity and ILD complications. A similar study was conducted by Yang et al. [78], showing the same results for DM–DM with ILD; however, no higher serum IL-18 levels were observed in patients with PM compared with controls.
The studies on IL-18 expression in IIM are few, but the important results obtained so far necessitate future investigations. It is clear that elevated levels of IL-18 in IIM may be a diagnostic marker and may be a therapeutic target.
5. Chemokines Family
Chemokines are small cytokines with a chemoattractant role, which guide the migration of leukocytes into the area of inflammation and regulate the homeostatic trafficking of lymphocytes and dendritic cells. Functionally, they are divided into 2 groups: homeostatic (responsible for basal leukocytes migration) [79] and proinflammatory [80]. The latter are produced in pathological conditions, under the action of proinflammatory stimuli, such as IL-1, TNF-α, and INF-γ, active in myositis. The chemokines detected in IIM muscle are CCL2, CCL3, CCL5, CCL9, CXCL8, CXCL9, and CXCL10 [81–83].
Accumulation of chemokines in muscle may enhance the activation and migration of leukocytes, maintaining autoimmune attack. There are in vitro studies, on myoblast cultures, that have shown that proinflammatory stimuli can induce the synthesis of chemokines in muscle cells, thus helping to perpetuate inflammation [20].
5.1. CXCL10 (C-X-C Motif Chemokine 10)
CXCL10, known also as interferon gamma-induced protein 10, has been defined as a chemokine that plays a role in the immunopathogenesis of autoimmune diseases, so in IIM, through the initiation and maintenance of type 1 T-helper cells (Th1) response [84]. It is an 8.7 kDa protein belonging to CXC chemokine family.
In a CIM (C-protein-induced myositis) mouse model, Jinhyun et al. [85] have shown that the expression of CXCL10 and its receptor CXCR3 (expressed on Th1 cells) was increased. Its blocking with anti-CXCL10 antibody or anti-RVG1 (rotavirus IgG1) suppressed muscle inflammation.
A study on patients with sporadic IBM (sIBM) revealed increased levels of CXCL10, and also CXCL9, in serum and muscle lysates [86]. However, the authors have shown that these results are not specific for sIBM and may be valid for other types of myositis also.
De Paepe et al. [87], in a study on patients with different forms of myositis, reported an abundant expression of CXCL10 both in T-cells and macrophages infiltrates in endomysium, in PM and sIBM, and also in T-cell aggregates present in perimysium, in DM. Concerning the CXCL10 receptor, CXCR3, the authors found it abundantly expressed in IIM, especially in PM and DM, on Th1 cells.
A research paper demonstrated that the levels of CXCL10 together with tumour necrosis factor receptor type II (TNFRII) and galectin 9 were increased in patients with active juvenile DM [88]. The results of this study revealed the CXCL10 as an important chemoattractant for monocytes, dendritic cells, and T-cells, which are present in the biopsy specimens of myositis patients and in their plasma also. These outcomes suggested that CXCL10 might also be a therapeutic target in patients with juvenile DM. In connection with these data, a human anti-CXCL10 antibody (MDX1100) was tested in clinical trials for ulcerative colitis and rheumatoid arthritis [89]. Other studies have suggested also the CXCL10 to be a therapeutic target in adult myositis patients [90].
Given these reports, which all showed an abundant expression of this chemokine and its receptor, we consider CXCL10 and CXCR3 receptor to be potential targets for a selective therapy in some forms of myositis.
5.2. CCL2, CCL3, CCL4, CCL5 (Chemokine C-C Motif Ligand 2, -3, -4, -5)
CCL2, CCL3, CCL4, and CCL5 are chemokines implied in IIM as chemotactic factors that coordinate the recruitment of leukocytes. It has been observed that MHC I overexpression in the muscle leads to the release of CCL chemokines. Thus, Lightfoot et al. [91] reported in an in vitro study on C2C12 myotubes transfected with a MHC I (H-2 kb) overexpression vector, using Lipofectamine2000, the release of CCL2, CCL4, and CCL5, together with IL-6, via the ER stress pathway.
A study on patients with IIM [48] have shown that serum CCL2 titres correlate strongly with CK levels, being expressed in macrophages and T-cells which actively invade muscle fibers [82]. An increased serum CCL2 levels were demonstrated in patients with juvenile DM, their concentrations being correlated with disease duration but not with disease activity [92].
Civatte et al. [93] have reported a strong expression of CCL3 and CCL4 in all IIM subtypes, revealed by RT-PCR and immunohistochemistry techniques. CCL4 was present in all vessels in DM, but in PM and sIMB, it was restricted to vessels in the vicinity of inflammatory exudates. CCL5 had a low expression in a few inflammatory cells. The CCR1 receptor expression was restricted to macrophages and s-IBM endothelial cells, whereas CCR5 receptor expression was observed in inflammatory cells invading nonnecrotic muscle fibers. The conclusion was that the upregulation of these myokines and some of their receptors may contribute to chronic inflammation in IIM. These results are confirmed by other authors too [81, 82, 94].
The CCL2 receptor, CCR2, was also found upregulated in IIM [95].
Therefore, it is likely that a better understanding of the molecular events leading to the formation of inflammatory infiltrates in IIM as well as a better description of various beta chemokines, and receptors will offer hope for a more selective immunotherapy in the future.
5.3. CCL20 (Chemokine C-C Motif Ligand 20)
It is a small cytokine of CC chemokine family, heaving chemotactic action for lymphocytes, named also MIP-3a (macrophage inflammatory protein-3a).
CCL20/MIP-3ais a chemokine involved also in dendritic cells migration [96]. There are studies that have shown that CCL20 induced migration of immature DC, of natural killer cells and T-cells also, but not of monocytes [97, 98].
Chevrel et al. [45] have revealed in an in vitro study (on primary cell culture from normal human muscle incubated with T-cell-derived cytokine IL-17 and monocyte-derived cytokine IL-1β) that low levels of a combination of IL-17 and IL-1β in muscle cells (human myoblasts) can trigger the expression/production of inflammatory factors, as IL-6 and the upregulation of CCL20/MIP-3a. They showed that IL-17 and CCL20 were immunohistochemically located in T-lymphocyte-rich areas in biopsies from patients with DM and PM. In addition to the attention paid to CCL20, the authors of the study considered IL-17 produced by lymphocytes in the myositis muscle (in PM and DM) as a possible therapeutic target.
Taking into account these reports, we can say that CCL20, along with other chemokines and interleukins with which it interacts, could represent a possible target in the IIM therapeutic strategies.
6. Myostatin
Myostatin, also called growth differentiation factor 8/GDF8, is a protein belonging to the TGF-β (transforming growth factor-beta) superfamily that negatively regulates muscle growth during development and muscle mass in adulthood [99]. In knocking out mouse models for myostatin gene, the muscle mass is larger [99].
Myostatin is synthesized as a precursor protein, and the processing action from myostatin precursor protein to myostatin has been proposed to occur intracellularly, possibly through the action of furin [100]. Outside the muscle fiber, myostatin is inactivated by its binding to follistatin, which thus inhibits its binding to cellular receptors on muscle fibers [99, 101].
A mode of action of myostatin has been proposed as follows: myostatin and activin A bind type 2 activin receptors. After dimerization, they bind activin receptor type 1 (ALK4/5) and signal through a TGF-β signaling pathway involving Smad2 and 3 phosphorylation [102]. Phosphorylation of Smad2 and 3 results in the downregulation of genes associated with muscle differentiation and inhibits AKT (protein kinase B) signaling. This AKT pathway is normally activated during muscle hypertrophy and often inhibited over muscle atrophy [103].
Myostatin inhibition has been shown to be beneficial in increasing of muscle mass [104, 105]. During the past 14 years in many clinical trials, attempts have been made to improve muscle function and muscle mass by inhibiting myostatin signaling pathway, targeting pathologies such as different muscular dystrophies or IBM. The results were not always what it was expected [106]. Preventing or reversing muscle atrophy remains an unmet medical need. Most of the preclinical or clinical studies for muscle atrophy therapy include myostatin inhibitors. The data obtained by Pirruccello-Straub et al. demonstrated a novel approach to myostatin inhibition, targeting the prodomain to modulate the activation of the mature myostatin rather than by blocking receptor-ligand interactions which may develop cross-reactivity with other TGF-β growth factors [107].
There are not many studies focusing on myostatin in IIM, but the interest in this field is growing. Wojcik et al. demonstrated for the first time that within sIBM muscle fibers, myostatin/myostatin precursor accumulates and associates with aggregates containing Amyloid-beta (Aβ). The expressions of myostatin precursor protein and myostatin dimer are increased, and myostatin precursor protein binds Aβ [108]. Given these results, a therapeutic attempt in s-IBM may be the decreasing myostatin/myostatin precursor.
Myostatin signaling pathway was reported to be upregulated in IBM [109] and an assay to block it was performed through gene therapy using follistatin as a myostatin inhibitor.
Another study showed that resistance training with vascular occlusion in a patient with IBM led to an increase in muscle mass and strength [110, 111], the myostatin playing an essential role in this mechanism. The myostatin gene expression was attenuated, while the gene expression of myostatin endogenous inhibitors, as follistatin, follistatin-like 3, and SMAD-7, was upregulated.
Decorin, such as follistatin, is another myostatin inhibitor. Although not studied in IIM, it is known to be overexpressed after exercise in normal muscle [112]. Here, we just want to draw attention to it as a possible modulator of myostatin. Future research is needed to evaluate the role of decorin and follistatin in IIM and their use in therapy by modulating myostatin expression.
7. Follistatin
Follistatin is a glycoprotein expressed in all tissues in variable concentrations [113]. It is an inhibitor of myostatin, promoting muscle growth by binding to ActRIIB (activin A bound to the extracellular domain of a type II receptor). In this way, follistatin neutralises the effect of various members of TGF-β (transforming growth factor-β) superfamily, including myostatin and activin–inhibin complex [114, 115].
Being an inhibitor of myostatin, follistatin may be a therapeutically target in IIM. Myostatin inhibition through follistatin gene intervention was proposed. Thus, a single administration of a vector carrying the follistatin gene resulted in an increase in muscle mass and strength, with reduced fibrosis. The experimental in vivo research was done on a mouse model with muscular dystrophy [116]. We consider that it is also an approachable subject for further studies on therapy in the IIM.
A clinical trial of follistatin gene transfer to 6 patients with sIBM has already been done. The patients received an intramuscular injection in quadriceps muscles of both legs with an isoform of follistatin (FS344) using AAV1 vectors, in combination with exercise. Six months later, there was observed an improvement in muscle regeneration and a reduction in fibrosis [117].
Vernerova et al. reported higher follistatin and lower myostatin levels in circulation and attenuated expression of myostatin pathway signaling components in skeletal muscle in IIM patients compared with healthy controls [118].
There is little information regarding the potential pathophysiological role of the activin A–myostatin–follistatin system in modulating disease progression or response to therapy in patients with IIM; although, it has proved its importance in recent years. McConnell et al. showed also that follistatin is synthesized in many tissues usually induced by activin stimulation [119]. The circulating levels of activin A and follistatin may be associated with the inflammatory condition in IIM. The overproduction of follistatin in response to inflammation-induced muscle damage is presumed that could serve as a compensatory mechanism leading to lower levels of myostatin and its signaling attenuation [120].
In conclusion, follistatin can be modulated as a therapeutic factor in the IIM, inhibiting myostatin to maintain muscle mass at a normal level.
8. Decorin
There are very few studies regarding the proteoglycan decorin in skeletal muscle and even fewer regarding its presence in IIM. However, it is an important myokine that binds myostatin. It is released from contracting human myotubes, and its levels are increased in the response to acute resistance exercise and chronic training in humans. Decorin is part of the extracellular matrix [121, 122].
In an in vivo experimental study, it was shown that overexpression of decorin in murine skeletal muscle promoted expression of Mighty gene [123], negatively regulated by myostatin, and an increased response of myogenic factor Myod1 [124] and follistatin [112]. It is known that decorin has an antagonistic action with myostatin [125], which has been shown also on C2C12 myoblasts, decorin enhancing proliferation and differentiation of the cells by suppressing myostatin activity [126]. Another in vitro research reported that decorin binds to myostatin [127] reducing the myostatin inhibitory effect.
Goetsch et al. showed in an in vitro study on C2C12 cells, which decorin binds TGF-β2, modifying the inhibition effect of this growth factor on cell migration and promoting the motility of myoblasts, positively influencing skeletal muscle regeneration [128]. Decorin has an antifibrotic effect also by forming the complex decorin/TGF-β [129, 130]. When decorin was injected into a traumatised muscle, in vivo, it could induce the regeneration of murine muscle, with minimal fibrotic scar tissue formation [131, 132].
Given the studies cited above and the fact that myostatin is upregulated in IBM, we can speculate that decorin may be downregulated in IIM. This myokine could be considered in the future a possible therapeutic target in myositis, in order to stimulate muscle regeneration.
9. Osteonectin
Osteonectin, named also SPARC (secreted protein acidic rich in cysteine), is a myokine that plays an important role in cell-matrix interactions, collagen binding, and bone mineralization. Osteonectin was found to be involved also in tumorigenesis inhibition by enhancing apoptosis in colon cancer cells [133]. It was observed an increased expression of SPARC in skeletal muscle after exercise, in mice and humans [133].
A study on muscular dystrophies such as Duchenne/Becker muscular dystrophy and congenital muscular dystrophy, and on IIM such as IBM and PM, have shown that SPARC is upregulated in muscle wasting [134].
In muscle progenitor cell line C2C12, osteonectin overexpression almost completely abolished myogenic differentiation [135]. Jorgensen et al. showed that SPARC-positive cells were present both in fetal and neonatal muscle [134]. Osteonectin protein was detected in mononuclear cells of which few were pax7 positive, in myotubes, and regenerating myofibers. The osteonectin expression-degree seemed to reflect the severity of the lesion. Primary human-derived satellite cells in vitro were found to express SPARC both during proliferation and differentiation.
Thus, it appears that SPARC plays a role in muscle cell regeneration. However, very limited studies are associated with the SPARC function in muscle development and pathology.
10. Insulin-Like 6
There is a small number of studies on Insl6. Insulin-like 6 (Insl6) is a myokine recently approached in myositis studies, being a member of insulin-like/relaxin family. It has been observed that Insl6 is overexpressed after the acute skeletal muscle injury [136].
Based on an experimental model of autoimmune myositis (EAM), induced in mice with human myosin-binding protein C, deficiency in Insl6 resulted in a worsened myositis phenotype with infiltration of CD4 and CD8 T-cells as well with an increased expression of inflammatory cytokines [137]. Muscle-specific Insl6 overexpression protects the muscle against the development of myositis and results in reduced lymphocyte infiltration in muscle tissue, decreased expression of inflammatory cytokines, and an improvement in motor function. The same study showed that an improvement in inflammatory conditions was produced in vitro by an acute hydrodynamic release of a plasmid encoding murine Isl6. The authors have established that Insl6 inhibits the proliferation and activation of T-cells. Furthermore, in a cohort of patients with PM and DM, Insl6 transcript expression in muscle was reduced. The authors of this study suggested that Insl6 could become a target for the treatment of myositis.
11. Myokines–MHC I–ER Stress—Proteasome Dialogue
An interesting toping regarding the complex connection between myokines and noninflammatory pathways implied in IIM has been attracting the attention of researchers in recent years.
Due to the ineffectiveness of the immunosuppressive treatment in some patients with myositis, research has turned in last years to new directions, such as the role of endoplasmic reticulum stress (ERs) in inflammatory pathology or, more recently, the role of myokines in maintaining this condition. The therapeutic approach in connection with these new features may lead to new more effective methods of treatment.
Regarding the role of ERs, many studies have been done and many significant results have emerged. Thus, permanent overexpression of MHC I in IIM can lead to ERs, with the accumulation of misfolded glycoproteins and activation of NF-κB [138, 139]. Inversely, MHC I overexpression can be induced and sustained by ER stress, and this has been observed in both patients with myositis and in in vivo experimental studies on MHC I transgenic mice [140].
In order to move towards the role of myokines in the modulation of myositis pathology, we mention an in vitro study that demonstrated that overexpression of MHC I in C2C12 cell line myotubes led to the release of inflammatory chemokines CCL2 and CCL5 via the ER stress pathway [91] (Figure 1).
This nonimmune mechanism, ERs, can upregulate the expression of some cytokines as IL-1β and TNF-α [21], thus contributing to maintaining muscle weakness [141]. In sIBM, it was observed that ERs and myostatin deposition have been implicated in disease pathology [142]. Myostatin signaling pathway is an important target for the treatment of muscle atrophy. In Sachdev et al. study an analysis of the presence of myostatin precursors in a human muscle cell line was performed, and they found that increased levels of these precursors induce ERs. Metabolites of myostatin precursors were retained within the endoplasmic reticulum. Importantly to mention, ERs also impaired the secretion of mature myostatin. The authors speculate that reduced circulating myostatin growth factor could be one explanation for the poor clinical efficacy of drugs targeting the myostatin pathway in sIBM [142].
The complex pathway of protein degradation, from proteasome to endoplasmic reticulum, and the MHC I implication (Figure 1) was reviewed by Vigneron and Van den Eynde [143]. The ubiquitin-proteasome system (UPS) is the major ATP-dependent protein degradation system in cells, and it is considered essential to maintain cellular protein homeostasis and ensure the elimination of misfolded proteins [144, 145]. Proteasome-dependent proteolysis is also essential to regulate other cellular processes, such as cell differentiation, cell-cycle progression, or apoptosis. Proteins degraded by proteasome are ubiquitin tagged to be recognised by the components of proteasome. Upon proteasomal degradation, small peptides are further degraded by cytosolic peptidases to recycle the pool of amino acids. A fraction of the peptides released by proteasomal degradation system is transferred into the lumen of the endoplasmic reticulum where is further trimmed. Some of peptides associate then with MHC I and peptide-MHC I complexes are finally displayed at the cell surface for potential recognition by cytolytic T-lymphocytes which are major sentinels poised to rapidly recognize and destroy cells expressing mutant, infectious or tumoral proteins. Thus, the proteasome is a major regulator of protein homeostasis in cells.
An in vitro study on primary human muscle cell cultures from myositis patients showed that immunoproteasome is involved in the maintenance of myokines (IL-6, IL-1β, CXCL9, and CXCL10) production and in MHC I overexpression [16]. Its inhibition or administration of selective drugs increases the expression of myokines in myoblasts during inflammatory conditions. The immunoproteasome is actively upregulated in myofibers and responsible for MHC-I expression in IIM. But immunoproteasome has also nonimmune regulating function in inflammatory condition being involved in the degradation of inflammatory response mediators. The results of this study revealed that the expression of immunoproteasome is important to maintain the myokines homeostasis and myokines mediated attraction of immune cells in muscle tissue [16].
This complex system, once disrupted, can cause many cellular alterations in signaling pathways, and we can speculate that in the pathology of IIM these aspects must be investigated, especially if patients do not respond to normal anti-inflammatory treatment. Not only ERs, which is already a complicated system, intervene in these cases but also its interrelation with the proteasome and myokines can complicate the pathogenesis of myositis, so that in the future studies should look at this connection as well.
12. Conclusion
Analysing the expression of myokines in the pathological conditions of myositis, we realized that their careful modulation could contribute to the improvement of treatment for this group of diseases. After immunosuppressant therapy, especially in nonresponder patients, stimulating or inhibiting of the production of these muscle cytokines could be the missing link in therapy. As this group of diseases is heterogeneous, they have many determinants that are triggered by the autoimmune process, so the treatment should be complex and focus more than inflammation. Unfortunately, for now, the therapeutic options for patients with IIM are limited.
We have seen that some of myokines, as follistatin and Insl6, have a low expression in myositis, intense exercise being already a good modulator, so as to induce their overexpression for the benefit of muscle tissue. Most myokines are upregulated in IIM, as interleukins, chemokines, myostatin, and osteonectin. They could be modulated not only by exercise but also by blocking them in different ways as we have shown above.
The role of myokines in myositis is not yet fully elucidated, but it is obvious that the development of new drugs based on future studies will be beneficial and could contribute to a more effective therapy for patients with inflammatory myopathies.
Acknowledgments
The present study will be integrated in the original part of the PhD thesis of author PhD student Vlad Mageriu. This work was supported by the Ministry of Education and Research in Romania, under grant: Program 1—The improvement of the National System of Research and Development, Subprogram 1.2—Institutional Excellence—Projects of Excellence Funding in RDI, contract no. 7PFE/16.10.2018; grant PN 16.22.02.04; and by UEFISCDI, grant: project PN-III-P1-1.2-PCCDI-2017-0341.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Authors' Contributions
Vlad Mageriu and Emilia Manole have an equal contribution to this article.
References
- 1.Dalakas M. C., Pongratz D. Inflammatory Myopathies. In: Brandt T., Caplan L., Dichgans J., Diener C. H., Kennard C., editors. Neurological Disorders. Course and Treatment. UK: Academic Press; 2003. pp. 1363–1368. [Google Scholar]
- 2.Bohan A., Peter J. B. Polymyositis and dermatomyositis. The New England Journal of Medicine. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Bohan A., Peter J. B. Polymyositis and dermatomyositis (second of two parts) The New England Journal of Medicine. 1975;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 4.Leclair V., Lundberg I. E. New myositis classification criteria - what we have learned since Bohan and Peter. Current Rheumatology Reports. 2018;20(4):p. 18. doi: 10.1007/s11926-018-0726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalakas M. C. Polymyositis, dermatomyositis and inclusion-body myositis. The New England Journal of Medicine. 1991;325(21):1487–1498. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- 6.Nagaraju K., Raben N., Loeffler L., et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(16):9209–9214. doi: 10.1073/pnas.97.16.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das L., Blumbergs P. C., Manavis J., Limaye V. S. Major histocompatibility complex class I and II expression in idiopathic inflammatory myopathy. Applied Immunohistochemistry & Molecular Morphology. 2013;21(6):539–542. doi: 10.1097/PAI.0b013e31827d7f16. [DOI] [PubMed] [Google Scholar]
- 8.Schnyder S., Handschin C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone. 2015;80:115–125. doi: 10.1016/j.bone.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen B. K., Febbraio M. A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiological Reviews. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen B. K., Febbraio M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature Reviews. Endocrinology. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 11.Peake J., Della Gatta P., Suzuki K., Nieman D. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exercise Immunology Review. 2015;21:8–25. [PubMed] [Google Scholar]
- 12.Manole E., Ceafalan L. C., Popescu B. O., Dumitru C., Bastian A. E. Myokines as possible therapeutic targets in cancer cachexia. Journal of Immunology Research. 2018;2018:9. doi: 10.1155/2018/8260742.8260742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou S., Qian B., Wang L., Zhang C., Hogan M. V., Li H. Altered bone-regulating myokine expression in skeletal muscle of Duchenne muscular dystrophy mouse models. Muscle & Nerve. 2018;58(4):573–582. doi: 10.1002/mus.26195. [DOI] [PubMed] [Google Scholar]
- 14.Lee J. H., Jun H.-S. Role of myokines in regulating skeletal muscle mass and function. Frontiers in Physiology. 2019;10 doi: 10.3389/fphys.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamori M., Hamanaka K., Thomas J. D., et al. Aberrant myokine Signaling in congenital myotonic dystrophy. Cell Reports. 2017;21(5):1240–1252. doi: 10.1016/j.celrep.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattarai S., Ghannam K., Krause S., et al. The immunoproteasomes are key to regulate myokines and MHC class I expression in idiopathic inflammatory myopathies. Journal of Autoimmunity. 2016;75:118–129. doi: 10.1016/j.jaut.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Nagaraju K., Raben N., Merritt G., Loeffler L., Kirk K., Plotz P. A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clinical and Experimental Immunology. 1998;113(3):407–414. doi: 10.1046/j.1365-2249.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevrel G., Granet C., Miossec P. Contribution of tumour necrosis factor alpha and interleukin (IL) 1beta to IL6 production, NF-kappaB nuclear translocation, and class I MHC expression in muscle cells: in vitro regulation with specific cytokine inhibitors. Annals of the Rheumatic Diseases. 2005;64(9):1257–1262. doi: 10.1136/ard.2004.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lightfoot A. P., Sakellariou G. K., Nye G. A., et al. SS-31 attenuates TNF-α induced cytokine release from C2C12 myotubes. Redox Biology. 2015;6:253–259. doi: 10.1016/j.redox.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rossi M., Bernasconi P., Baggi F., de Waal Malefyt R., Mantegazza R. Cytokines and chemokines are both expressed by human myoblasts: possible relevance for the immune pathogenesis of muscle inflammation. International Immunology. 2000;12(9):1329–1335. doi: 10.1093/intimm/12.9.1329. [DOI] [PubMed] [Google Scholar]
- 21.Kim S., Joe Y., Kim H. J., et al. Endoplasmic reticulum stress-induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production. The Journal of Immunology. 2015;194(9):4498–4506. doi: 10.4049/jimmunol.1401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coley W., Rayavarapu S., Pandey G. S., et al. The molecular basis of skeletal muscle weakness in a mouse model of inflammatory myopathy. Arthritis and Rheumatism. 2012;64(11):3750–3759. doi: 10.1002/art.34625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lightfoot A. P., Cooper R. G. Editorial: endurance exercise: an important therapeutic adjuvant in the overall treatment of myositis? Arthritis & Rheumatology. 2016;68(7):1578–1581. doi: 10.1002/art.39615. [DOI] [PubMed] [Google Scholar]
- 24.Paul W. E., Seder R. A. Lymphocyte responses and cytokines. Cell. 1994;76(2):241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 25.Robb L. Cytokine receptors and hematopoietic differentiation. Oncogene. 2007;26(47):6715–6723. doi: 10.1038/sj.onc.1210756. [DOI] [PubMed] [Google Scholar]
- 26.Catalan-Dibene J., McIntyre L. L., Zlotnik A. Interleukin 30 to Interleukin 40. Journal of Interferon & Cytokine Research. 2018;38(10):423–439. doi: 10.1089/jir.2018.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal M., Febbraio M. A., Whitham M. From cytokine to myokine: the emerging role of interleukin-6 in metabolic regulation. Immunology and Cell Biology. 2014;92(4):331–339. doi: 10.1038/icb.2014.16. [DOI] [PubMed] [Google Scholar]
- 28.Hunter C. A., Jones S. A. IL-6 as a keystone cytokine in health and disease. Nature Immunology. 2015;16(5):448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 29.Ostrowski K., Rohde T., Zacho M., Asp S., Pedersen B. K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. The Journal of Physiology. 1998;508(3):949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen B. K. Exercise and cytokines. Immunology and Cell Biology. 2000;78(5):532–535. doi: 10.1111/j.1440-1711.2000.t01-11-.x. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen B. K., Steensberg A., Fischer C., et al. The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor? The Proceedings of the Nutrition Society. 2004;63(2):263–267. doi: 10.1079/PNS2004338. [DOI] [PubMed] [Google Scholar]
- 32.Benatti F. B., Pedersen B. K. Exercise as an anti-inflammatory therapy for rheumatic diseases--myokine regulation. Nature Reviews Rheumatology. 2015;11(2):86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 33.Sacheck J. M., Cannon J. G., Hamada K., Vannier E., Blumberg J. B., Roubenoff R. Age-related loss of associations between acute exercise-induced IL-6 and oxidative stress. American Journal of Physiology-Endocrinology and Metabolism. 2006;291(2):E340–E349. doi: 10.1152/ajpendo.00052.2005. [DOI] [PubMed] [Google Scholar]
- 34.Keller P., Penkowa M., Keller C., et al. Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. The FASEB Journal. 2005;19(9):1181–1183. doi: 10.1096/fj.04-3278fje. [DOI] [PubMed] [Google Scholar]
- 35.Serrano A. L., Baeza-Raja B., Perdiguero E., Jardí M., Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metabolism. 2008;7(1):33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Tsujinaka T., Fujita J., Ebisui C., et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. The Journal of Clinical Investigation. 1996;97(1):244–249. doi: 10.1172/JCI118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baltgalvis K. A., Berger F. G., Pena M. M. O., Davis J. M., Muga S. J., Carson J. A. Interleukin-6 and cachexia inApcMin/+mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2008;294(2):R393–R401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 38.Cantini M., Massimino M. L., Rapizzi E., et al. Human Satellite Cell-Proliferation _in Vitro_ Is Regulated by Autocrine Secretion of IL-6 Stimulated by a Soluble Factor(s) Released by Activated Monocytes. Biochemical and Biophysical Research Communications. 1995;216(1):49–53. doi: 10.1006/bbrc.1995.2590. [DOI] [PubMed] [Google Scholar]
- 39.Belizário J. E., Fontes-Oliveira C. C., Borges J. P., Kashiabara J. A., Vannier E. Skeletal muscle wasting and renewal: a pivotal role of myokine IL-6. Springerplus. 2016;5(1) doi: 10.1186/s40064-016-2197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muñoz-Cánoves P., Scheele C., Pedersen B. K., Serrano A. L. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? The FEBS Journal. 2013;280(17):4131–4148. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou W., Jiang Z. W., Tian J., Jiang J., Li N., Li J. S. Role of NF-kappaB and cytokine in experimental cancer cachexia. World Journal of Gastroenterology. 2003;9(7):1567–1570. doi: 10.3748/wjg.v9.i7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haddad F., Zaldivar F., Cooper D. M., Adams G. R. IL-6-induced skeletal muscle atrophy. Journal of Applied Physiology. 2005;98(3):911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 43.Bilgic H., Ytterberg S. R., Amin S., et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis and Rheumatism. 2009;60(11):3436–3446. doi: 10.1002/art.24936. [DOI] [PubMed] [Google Scholar]
- 44.Okiyama N., Sugihara T., Iwakura Y., Yokozeki H., Miyasaka N., Kohsaka H. Therapeutic effects of interleukin-6 blockade in a murine model of polymyositis that does not require interleukin-17A. Arthritis and Rheumatism. 2009;60(8):2505–2512. doi: 10.1002/art.24689. [DOI] [PubMed] [Google Scholar]
- 45.Chevrel G., Page G., Granet C., Streichenberger N., Varennes A., Miossec P. Interleukin-17 increases the effects of IL-1 beta on muscle cells: arguments for the role of T cells in the pathogenesis of myositis. Journal of Neuroimmunology. 2003;137(1-2):125–133. doi: 10.1016/S0165-5728(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 46.Mima T., Nishimoto N. Clinical value of blocking IL-6 receptor. Current Opinion in Rheumatology. 2009;21(3):224–230. doi: 10.1097/BOR.0b013e3283295fec. [DOI] [PubMed] [Google Scholar]
- 47.Scuderi F., Mannella F., Marino M., Provenzano C., Bartoccioni E. IL-6-deficient mice show impaired inflammatory response in a model of myosin-induced experimental myositis. Journal of Neuroimmunology. 2006;176(1-2):9–15. doi: 10.1016/j.jneuroim.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 48.Loaiza-Félix J., Moreno-Ramírez M., Pérez-García F. L., Jiménez-Rojas V., Sánchez-Muñoz F., Amezcua-Guerra M. L. Serum levels of adipokines in patients with idiopathic inflammatory myopathies: a pilot study. Rheumatology International. 2017;37(8):1341–1345. doi: 10.1007/s00296-017-3752-z. [DOI] [PubMed] [Google Scholar]
- 49.Gono T., Kaneko H., Kawaguchi Y., et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology. 2014;53(12):2196–2203. doi: 10.1093/rheumatology/keu258. [DOI] [PubMed] [Google Scholar]
- 50.Raschke S., Eckel J. Adipo-myokines: two sides of the same coin—mediators of inflammation and mediators of exercise. Mediators of Inflammation. 2013;2013:16. doi: 10.1155/2013/320724.320724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li F., Li Y., Duan Y., Hu C.-A. A., Tang Y., Yin Y. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine & Growth Factor Reviews. 2017;33:73–82. doi: 10.1016/j.cytogfr.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Fehniger T. A., Caligiuri M. A. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. doi: 10.1182/blood.V97.1.14. [DOI] [PubMed] [Google Scholar]
- 53.Schluns K. S., Stoklasek T., Lefrançois L. The roles of interleukin-15 receptor α: Trans-presentation, receptor component, or both? The International Journal of Biochemistry & Cell Biology. 2005;37(8):1567–1571. doi: 10.1016/j.biocel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Tagaya Y., Bamford R. N., DeFilippis A. P., Waldmann T. A. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4(4):329–336. doi: 10.1016/S1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 55.Quinn L. S., Haugk K. L., Grabstein K. H. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995;136(8):3669–3672. doi: 10.1210/endo.136.8.7628408. [DOI] [PubMed] [Google Scholar]
- 56.Pistilli E. E., Quinn L. B. S. From Anabolic to Oxidative. Exercise and Sport Sciences Reviews. 2013;41(2):100–106. doi: 10.1097/JES.0b013e318275d230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye J. Beneficial metabolic activities of inflammatory cytokine interleukin 15 in obesity and type 2 diabetes. Frontiers in Medicine. 2015;9(2):139–145. doi: 10.1007/s11684-015-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furmanczyk P. S., Quinn L. S. Interleukin-15 increases myosin accretion in human skeletal myogenic cultures. Cell Biology International. 2003;27(10):845–851. doi: 10.1016/S1065-6995(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 59.Riechman S. E., Balasekaran G., Roth S. M., Ferrell R. E. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. Journal of Applied Physiology. 2004;97(6):2214–2219. doi: 10.1152/japplphysiol.00491.2004. [DOI] [PubMed] [Google Scholar]
- 60.Nielsen A. R., Mounier R., Plomgaard P., et al. Expression of interleukin-15 in human skeletal muscle—effect of exercise and muscle fibre type composition. The Journal of Physiology. 2007;584(1):305–312. doi: 10.1113/jphysiol.2007.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sugiura T., Harigai M., Kawaguchi Y., et al. Increased IL-15 production of muscle cells in polymyositis and dermatomyositis. International Immunology. 2002;14(8):917–924. doi: 10.1093/intimm/dxf062. [DOI] [PubMed] [Google Scholar]
- 62.Sugiura T., Kawaguchi Y., Harigai M., et al. Increased CD40 expression on muscle cells of polymyositis and dermatomyositis: role of CD40-CD40 ligand interaction in IL-6, IL-8, IL-15, and monocyte chemoattractant protein-1 production. Journal of Immunology. 2000;164(12):6593–6600. doi: 10.4049/jimmunol.164.12.6593. [DOI] [PubMed] [Google Scholar]
- 63.Abadie V., Jabri B. IL-15: a central regulator of celiac disease immunopathology. Immunological Reviews. 2014;260(1):221–234. doi: 10.1111/imr.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zong M., Loell I., Lindroos E., et al. Effects of immunosuppressive treatment on interleukin-15 and interleukin-15 receptor α expression in muscle tissue of patients with polymyositis or dermatomyositis. Annals of the Rheumatic Diseases. 2012;71(6):1055–1063. doi: 10.1136/annrheumdis-2011-200495. [DOI] [PubMed] [Google Scholar]
- 65.Yan W., Fan W., Chen C., et al. IL-15 up-regulates the MMP-9 expression levels and induces inflammatory infiltration of macrophages in polymyositis through regulating the NF-kB pathway. Gene. 2016;591(1):137–147. doi: 10.1016/j.gene.2016.06.055. [DOI] [PubMed] [Google Scholar]
- 66.Fukushima K., Nakamura A., Ueda H., et al. Activation and localization of matrix metalloproteinase-2 and -9 in the skeletal muscle of the muscular dystrophy dog (CXMDJ) BMC Musculoskeletal Disorders. 2007;8(1):p. 54. doi: 10.1186/1471-2474-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang P.-L., Hou M.-S., Wang S.-W., Chang C.-L., Liou Y.-H., Liao N.-S. Skeletal muscle interleukin 15 promotes CD8+ T-cell function and autoimmune myositis. Skeletal Muscle. 2015;5(1):p. 33. doi: 10.1186/s13395-015-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura K., Okamura H., Wada M., Nagata K., Tamura T. Endotoxin induced serum factor that stimulates gamma interferon production. Infection and Immunity. 1989;57(2):590–595. doi: 10.1128/IAI.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okamura H., Tsutsui H., Komatsu T., et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 70.Dinarello C. A. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. The American Journal of Clinical Nutrition. 2006;83(2):447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- 71.Garneau L., Parsons S. A., Smith S. R., Mulvihill E. E., Sparks L. M., Aguer C. Plasma myokine concentrations after acute exercise in non-obese and obese sedentary women. Frontiers in Physiology. 2020;11:p. 18. doi: 10.3389/fphys.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshida S., Hagiwara Y., Tsuchiya M., et al. Involvement of neutrophils and interleukin-18 in nociception in a mouse model of muscle pain. Molecular Pain. 2018;14 doi: 10.1177/1744806918757286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fortin C. F., Ear T., McDonald P. P. Autocrine role of endogenous interleukin-18 on inflammatory cytokine generation by human neutrophils. The FASEB Journal. 2008;23:194–203. doi: 10.1096/fj.08-110213. [DOI] [PubMed] [Google Scholar]
- 74.Robertson S. . E., Young J. . D., Kitson S., et al. Expression and alternative Processing of IL-18 inhuman neutrophils. European Journal of Immunology. 2006;36(3):722–731. doi: 10.1002/eji.200535402. [DOI] [PubMed] [Google Scholar]
- 75.Dinarello C. A., Novick D., Kim S., Kaplanski G. Interleukin-18 and IL-18 binding protein. Frontiers in Immunology. 2013;4 doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Helmers S. B., Bruton M., Loell I., et al. Expression of interleukin-18 in muscle tissue of patients with polymyositis or dermatomyositis and effects of conventional immunosuppressive treatment. Rheumatology. 2018;57(12):2149–2157. doi: 10.1093/rheumatology/key222. [DOI] [PubMed] [Google Scholar]
- 77.Gono T., Kawaguchi Y., Sugiura T., et al. Interleukin-18 is a key mediator in dermatomyositis: potential contribution to development of interstitial lung disease. Rheumatology. 2010;49(10):1878–11881. doi: 10.1093/rheumatology/keq196. [DOI] [PubMed] [Google Scholar]
- 78.Yang Y., Yin G., Hao J., Xie Q., Liu Y. Serum interleukin-18 level is associated with disease activity and interstitial lung disease in patients with dermatomyositis. Arch. Rheumatol. 2017;32(3):181–188. doi: 10.5606/ArchRheumatol.2017.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zlotnik A., Burkhardt A. M., Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nature Reviews. Immunology. 2011;11(9):597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 80.Zlotnik A., Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Confalonieri P., Bernasconi P., Megna P., Galbiati S., Cornelio F., Mantegazza R. Increased expression of β-chemokines in muscle of patients with inflammatory myopathies. Journal of Neuropathology and Experimental Neurology. 2000;59(2):164–169. doi: 10.1093/jnen/59.2.164. [DOI] [PubMed] [Google Scholar]
- 82.De Bleecker J. L., Paepe B. D., Vanwalleghem I. E., Schröder J. M. Differential expression of chemokines in inflammatory myopathies. Neurology. 2002;58(12):1779–1785. doi: 10.1212/WNL.58.12.1779. [DOI] [PubMed] [Google Scholar]
- 83.Raju R., Vasconcelos O., Granger R., Dalakas M. C. Expression of IFN-γ-inducible chemokines in inclusion body myositis. Journal of Neuroimmunology. 2003;141(1-2):125–131. doi: 10.1016/S0165-5728(03)00218-2. [DOI] [PubMed] [Google Scholar]
- 84.Scolletta S., Colletti M., Di Luigi L., Crescioli C. Vitamin D receptor agonists target CXCL10: new therapeutic tools for resolution of inflammation. Mediators of Inflammation. 2013;2013:11. doi: 10.1155/2013/876319.876319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim J., Choi J., Park S. H., et al. Therapeutic effect of anti-C-X-C motif chemokine 10 (CXCL10) antibody on C protein-induced myositis mouse. Arthritis Res Ther. 2014;16(3):p. R126. doi: 10.1186/ar4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Allenbach Y., Chaara W., Rosenzwajg M., et al. Th1 response and systemic treg deficiency in inclusion body myositis. PLoS One. 2014;9(3):p. e88788. doi: 10.1371/journal.pone.0088788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Paepe B., De Keyzer K., Martin J.-J., De Bleecker J. L. Alpha-chemokine receptors CXCR1-3 and their ligands in idiopathic inflammatory myopathies. Acta Neuropathologica. 2005;109(6):576–582. doi: 10.1007/s00401-005-0989-5. [DOI] [PubMed] [Google Scholar]
- 88.Bellutti Enders F., van Wijk F., Scholman R., et al. Correlation of CXCL10, tumor necrosis factor receptor type II, and galectin 9 with disease activity in juvenile dermatomyositis. Arthritis & Rhematology. 2014;66(8):2281–2289. doi: 10.1002/art.38676. [DOI] [PubMed] [Google Scholar]
- 89.Yellin M., Paliienko I., Balanescu A., et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis and Rheumatism. 2012;64(6):1730–1739. doi: 10.1002/art.34330. [DOI] [PubMed] [Google Scholar]
- 90.Crescioli C., Sottili M., Bonini P., et al. Inflammatory response in human skeletal muscle cells: CXCL10 as a potential therapeutic target. European Journal of Cell Biology. 2012;91(2):139–149. doi: 10.1016/j.ejcb.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 91.Lightfoot A. P., Goljanek-Whysall K., Cotton C. V., Earl K. E., McArdle A., Cooper R. G. O42. Is muscle a chemotactic organ in the idiopathic inflammatory myopathies (IIM)? Overexpression of MHC I (H-2Kb) in C2C12 myotubes results in release of pro-inflammatory cytokines. Rheumatology. 2015;54 doi: 10.1093/rheumatology/kev085.006. [DOI] [Google Scholar]
- 92.Sanner H., Schwartz T., Flatø B., Vistnes M., Christensen G., Sjaastad I. Increased levels of eotaxin and MCP-1 in juvenile dermatomyositis median 16.8 years after disease onset; associations with disease activity, duration and organ damage. PLoS One. 2014;9(3):p. e92171. doi: 10.1371/journal.pone.0092171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Civatte M., Bartoli C., Schleinitz N., Chetaille B., Pellissier J. F., Figarella-Branger D. Expression of the beta chemokines CCL3, CCL4, CCL5 and their receptors in idiopathic inflammatory myopathies. Neuropathology and Applied Neurobiology. 2005;31(1):70–79. doi: 10.1111/j.1365-2990.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- 94.Liprandi A., Bartoli C., Figarella-Branger D., Pellissier J. F., Lepidi H. Local expression of monocyte chemoattractant protein-1 (MCP-1) in idiopathic inflammatory myopathies. Acta Neuropathologica. 1999;97(6):642–648. doi: 10.1007/s004010051041. [DOI] [PubMed] [Google Scholar]
- 95.Bartoli C., Civatte M., Pellissier J. F., Figarella-Branger D. CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol. 2001;102(4):385–392. doi: 10.1007/s004010100394. [DOI] [PubMed] [Google Scholar]
- 96.Dieu M. C., Vanbervliet B., Vicari A., et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. The Journal of Experimental Medicine. 1998;188(2):373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Power C. A., Church D. J., Meyer A., et al. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3α from lung dendritic cells. The Journal of Experimental Medicine. 1997;186(6):825–835. doi: 10.1084/jem.186.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dieu-Nosjean M. C., Massacrier C., Homey B., et al. Macrophage inflammatory protein 3α is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. The Journal of Experimental Medicine. 2000;192(5):705–718. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez-Cadavid N. F., Bhasin S. Role of myostatin in metabolism. Current Opinion in Clinical Nutrition and Metabolic Care. 2004;7(4):451–457. doi: 10.1097/01.mco.0000134365.99523.7f. [DOI] [PubMed] [Google Scholar]
- 100.Jin H. J., Dunn M. A., Borthakur D., Kim Y. S. Refolding and purification of unprocessed porcine myostatin expressed in Escherichia coli. Protein Expression and Purification. 2004;35(1):1–10. doi: 10.1016/j.pep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 101.McNally E. M. Powerful genes—myostatin regulation of human muscle mass. The New England Journal of Medicine. 2004;350(26):2642–2644. doi: 10.1056/NEJMp048124. [DOI] [PubMed] [Google Scholar]
- 102.Sartori R., Milan G., Patron M., et al. Smad2 and 3 transcription factors control muscle mass in adulthood. American Journal of Physiology. Cell Physiology. 2009;296(6):C1248–C1257. doi: 10.1152/ajpcell.00104.2009. [DOI] [PubMed] [Google Scholar]
- 103.Bodine S. C., Stitt T. N., Gonzalez M., et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Rheumatology (Oxford, England) 2004;43:49–54. [Google Scholar]
- 104.Lee S.-J., McPherron A. C. Regulation of myostatin activity and muscle growth. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(16):9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pirottin D., Grobet L., Adamantidis A., et al. Transgenic engineering of male-specific muscular hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(18):6413–6418. doi: 10.1073/pnas.0502426102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mariot V., Joubert R., Hourdé C., et al. Downregulation of myostatin pathway in neuromuscular diseases may explain challenges of anti-myostatin therapeutic approaches. Nature Communications. 2017;8(1):p. 1859. doi: 10.1038/s41467-017-01486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pirruccello-Straub M., Jackson J., Wawersik S., et al. Blocking extracellular activation of myostatin as a strategy for treating muscle wasting. Scientific Reports. 2018;8(1):p. 2292. doi: 10.1038/s41598-018-20524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wójcik S., Engel W. K., McFerrin J., Askanas V. Myostatin is increased and complexes with amyloid-β within sporadic inclusion-body myositis muscle fibers. Acta Neuropathol. 2005;110(2):173–177. doi: 10.1007/s00401-005-1035-3. [DOI] [PubMed] [Google Scholar]
- 109.Amato A. A., Sivakumar K., Goyal N., et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology. 2014;83(24):2239–2246. doi: 10.1212/WNL.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gualano B., Neves M., Lima F. R., et al. Resistance training with vascular occlusion in inclusion body Myositis. Medicine and Science in Sports and Exercise. 2010;42(2):250–254. doi: 10.1249/MSS.0b013e3181b18fb8. [DOI] [PubMed] [Google Scholar]
- 111.Santos A., Manuel Neves B. G., Jr., Laurentino G., Antonio Lancha C. U., Jr., Lima F., Aoki M. Blood flow restricted resistance training attenuates Myostatin gene expression in a patient with inclusion body myositis. Biology of Sport. 2014;31(2):121–124. doi: 10.5604/20831862.1097479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kanzleiter T., Rath M., Görgens S. W., et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochemical and Biophysical Research Communications. 2014;450(2):1089–1094. doi: 10.1016/j.bbrc.2014.06.123. [DOI] [PubMed] [Google Scholar]
- 113.Al-Zaidy S. A., Sahenk Z., Rodino-Klapac L. R., Kaspar B., Mendell J. R. Follistatin gene therapy improves ambulation in Becker muscular dystrophy. J Neuromuscul Dis. 2015;2(3):185–192. doi: 10.3233/JND-150083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thompson T. B., Lerch T. F., Cook R. W., Woodruff T. K., Jardetzky T. S. The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Developmental Cell. 2005;9(4):535–543. doi: 10.1016/j.devcel.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 115.Amthor H., Nicholas G., McKinnell I., et al. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Developmental Biology. 2004;270(1):19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 116.Haidet A. M., Rizo L., Handy C., et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4318–4322. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mendell J. R., Sahenk Z., Al-Zaidy S., et al. Follistatin Gene Therapy for Sporadic Inclusion Body Myositis Improves Functional Outcomes. Molecular Therapy. 2017;25(4):870–879. doi: 10.1016/j.ymthe.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vernerová L., Horváthová V., Kropáčková T., et al. Alterations in activin A–myostatin–follistatin system associate with disease activity in inflammatory myopathies. Rheumatology. 2020:1–11. doi: 10.1093/rheumatology/kez651. [DOI] [PubMed] [Google Scholar]
- 119.McConnell D. S., Wang Q., Sluss P. M., et al. A two-site chemiluminescent assay for activin-free follistatin reveals that most follistatin circulating in men and normal cycling women is in an activin-bound state. The Journal of Clinical Endocrinology and Metabolism. 1998;83(3):851–858. doi: 10.1210/jcem.83.3.4651. [DOI] [PubMed] [Google Scholar]
- 120.Yaden B. C., Croy J. E., Wang Y., et al. Follistatin: a novel therapeutic for the improvement of muscle regeneration. The Journal of Pharmacology and Experimental Therapeutics. 2014;349(2):355–371. doi: 10.1124/jpet.113.211169. [DOI] [PubMed] [Google Scholar]
- 121.Henningsen J., Rigbolt K. T. G., Blagoev B., Pedersen B. K., Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol.Cell Proteomics. 2010;9(11):2482–2496. doi: 10.1074/mcp.M110.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brandan E., Fuentes M. E., Andrade W. The proteoglycan decorin is synthesized and secreted by differentiated myotubes. Eur.J.Cell Biol. 1991;55(2):209–216. [PubMed] [Google Scholar]
- 123.Marshall A., Salerno M. S., Thomas M., et al. Mighty is a novel promyogenic factor in skeletal myogenesis. Experimental Cell Research. 2008;314(5):1013–1029. doi: 10.1016/j.yexcr.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 124.Weintraub H., Davis R., Tapscott S., et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 125.Miura T., Kishioka Y., Wakamatsu J.-i., et al. Decorin binds myostatin and modulates its activity to muscle cells. Biochemical and Biophysical Research Communications. 2006;340(2):675–680. doi: 10.1016/j.bbrc.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 126.Kishioka Y., Thomas M., Wakamatsu J., et al. Decorin enhances the proliferation and differentiation of myogenic cells through suppressing myostatin activity. Journal of Cellular Physiology. 2008;215(3):856–867. doi: 10.1002/jcp.21371. [DOI] [PubMed] [Google Scholar]
- 127.Lee S.-J. Regulation of muscle mass by myostatin. Annual Review of Cell and Developmental Biology. 2004;20(1):61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- 128.Goetsch K. P., Niesler C. U. The extracellular matrix regulates the effect of decorin and transforming growth factor beta-2 (TGF-β2) on myoblast migration. Biochemical and Biophysical Research Communications. 2016;479(2):351–357. doi: 10.1016/j.bbrc.2016.09.079. [DOI] [PubMed] [Google Scholar]
- 129.Riquelme C., Larraín J., Schönherr E., Henriquez J. P., Kresse H., Brandan E. Antisense inhibition of decorin expression in myoblasts decreases cell responsiveness to transforming growth factor β and accelerates skeletal muscle differentiation. The Journal of Biological Chemistry. 2001;276(5):3589–e3596. doi: 10.1074/jbc.M004602200. [DOI] [PubMed] [Google Scholar]
- 130.Cabello-Verrugio C., Santander C., Cofré C., Acuña M. J., Melo F., Brandan E. The internal region leucine-rich repeat 6 of decorin interacts with low density lipoprotein receptor-related protein-1, modulates transforming growth factor (TGF)-β-dependent signaling, and inhibits TGF-β-dependent fibrotic response in skeletal muscles. The Journal of Biological Chemistry. 2012;287(9):6773–e6787. doi: 10.1074/jbc.M111.312488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fukushima K., Badlani N., Usas A., et al. The use of an antifibrosis agent to improve muscle recovery after laceration. The American Journal of Sports Medicine. 2017;29:394–e402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- 132.Fukushima H., Ohsawa M., Ikura Y., et al. Mast cells in diffuse large B-cell lymphoma; their role in fibrosis. Histopathology. 2006;49(5):498–e505. doi: 10.1111/j.1365-2559.2006.02534.x. [DOI] [PubMed] [Google Scholar]
- 133.Aoi W., Naito Y., Takagi T., et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62(6):882–889. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 134.Jørgensen L. H., Petersson S. J., Sellathurai J., et al. Secreted protein acidic and rich in cysteine (SPARC) in human skeletal muscle. The Journal of Histochemistry and Cytochemistry. 2008;57(1):29–39. doi: 10.1369/jhc.2008.951954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Petersson S. J., Jorgensen L. H., Andersen D. C., Norgaard R. C., Jensen C. H., Schroder H. D., et al. SPARC is up-regulated during skeletal muscle regeneration and inhibits myoblast differentiation. Histol Histopatho. 2013;28:1451–1460. doi: 10.14670/HH-28.1451. [DOI] [PubMed] [Google Scholar]
- 136.Zeng L., Akasaki Y., Sato K., Ouchi N., Izumiya Y., Walsh K. Insulin-like 6 is induced by muscle injury and functions as a regenerative factor. The Journal of Biological Chemistry. 2010;285(46):36060–36069. doi: 10.1074/jbc.M110.160879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zeng L., Maruyama S., Nakamura K., et al. The injury-induced myokine insulin-like 6 is protective in experimental autoimmune myositis. Skeletal Muscle. 2014;4(1):p. 16. doi: 10.1186/2044-5040-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fréret M., Drouot L., Obry A., et al. Overexpression of MHC class I in muscle of lymphocyte-deficient mice causes a severe myopathy with induction of the unfolded protein response. The American Journal of Pathology. 2013;183(3):893–904. doi: 10.1016/j.ajpath.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 139.Manole E., Bastian A. E., Butoianu N., Goebel H. H. Myositis non-inflammatory mechanisms: an up-dated review. Journal of Immunoassay and Immunochemistry. 2017;38(2):115–126. doi: 10.1080/15321819.2017.1298525. [DOI] [PubMed] [Google Scholar]
- 140.Nagaraju K., Casciola-Rosen L., Lundberg I., et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis and Rheumatism. 2005;52(6):1824–1835. doi: 10.1002/art.21103. [DOI] [PubMed] [Google Scholar]
- 141.Lightfoot A. P., Nagaraju K., McArdle A., Cooper R. G. Understanding the origin of non-immune cell-mediated weakness in the idiopathic inflammatory myopathies – potential role of ER stress pathways. Curr Opin Rheumatol. 2015;27(6):580–585. doi: 10.1097/BOR.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 142.Sachdev R., Kappes-Horn K., Paulsen L., et al. Endoplasmic reticulum stress induces Myostatin high molecular weight aggregates and impairs mature Myostatin secretion. Molecular Neurobiology. 2018;55(11):8355–8373. doi: 10.1007/s12035-018-0997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vigneron N., Van den Eynde B. J. Proteasome subtypes and regulators in the processing of antigenic peptides presented by class I molecules of the major histocompatibility complex. Biomolecules. 2014;4(4):994–1025. doi: 10.3390/biom4040994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rock K. L., Goldberg A. L. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annual Review of Immunology. 1999;17(1):739–779. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- 145.Tomko R. J., Jr., Hochstrasser M. Molecular architecture and assembly of the eukaryotic proteasome. Annual Review of Biochemistry. 2013;82(1):415–445. doi: 10.1146/annurev-biochem-060410-150257. [DOI] [PMC free article] [PubMed] [Google Scholar]


