Abstract
Traditional diagnostic tests for dry eye disease (DED), such as fluorescein tear film break-up time and the Schirmer test, are often associated with poor reproducibility and reliability, which make the diagnosis, follow-up, and management of the disease challenging. Advances in ocular imaging technology enables objective and reproducible measurement of changes in the ocular surface, tear film, and optical quality associated with DED. In this review, the authors will discuss the application of various imaging techniques, such as, noninvasive tear break-up time, anterior segment optical coherence tomography, in vivo confocal microscopy, meibography, interferometry, aberrometry, thermometry, and tear film imager in DED. Many studies have shown these devices to correlate with clinical symptoms and signs of DED, suggesting the potential of these imaging modalities as alternative tests for diagnosis and monitoring of the condition.
1. Introduction
Dry eye disease (DED) is a multifactorial disease characterized by a loss of homeostasis of the tear film [1]. It a highly prevalent disease that can affect up to one-third of the people worldwide [2, 3]. The disease is associated with symptoms such as ocular discomfort, dryness, pain, foreign body sensation, and visual disturbance [4, 5], which significantly interfere with daily activities including reading, driving, watching TV, and using mobile devices or computer [2, 3]. In clinical practice, the diagnosis and management of DED is often difficult because of its multifactorial nature, as well as discrepancy between dry eye signs and symptoms [2, 6–8]. Moreover, conventional diagnostic tests for DED, such as the fluorescein tear film break-up time (TBUT) and the Schirmer test, often show unsatisfactory reliability and reproducibility, which also renders the diagnosis and monitoring of the condition challenging [9].
Advances in technology led to the development of various imaging devices that have enabled visualization and evaluation of the tear film and ocular surface, such as noninvasive tear break-up time measurements, anterior segment optical coherence tomography, confocal microscopy, meibography, interferometry, aberrometry, thermography, and tear film imager [10].
In this review, we aim to provide an overview of imaging devices for DED and discuss the application of the modalities for clinical practice and research for DED.
2. Noninvasive Tear Break-Up Time (NITBUT)
The TBUT reflects the stability and quality of the tear film, which is crucial for maintenance of ocular surface integrity and clear vision [1, 11]. Measurement of the TBUT has generally been performed after fluorescein dye instillation [10]. However, the variability of the concentration and amount of the dye leads to reduced reliability and reproducibility [12]. Reflex tearing induced by fluorescein instillation can also lead to decreased accuracy [12]. Moreover, the fluorescein TBUT is unable to simultaneously evaluate the tear break-up across the entire corneal surface [10].
The noninvasive TBUT (NIBUT) was developed to overcome these limitations [10, 11]. Instead of using fluorescein dye instillation, the NIBUT measurement involves application of topographic systems to evaluate changes in a regularly patterned image projected onto the tear film that reflects compromised tear film integrity [11, 13]. Changes in reflected videokeratographic mires or grids from an illuminated placido disc are observed to detect tear film disruption (Figure 1) [13].
Figure 1.
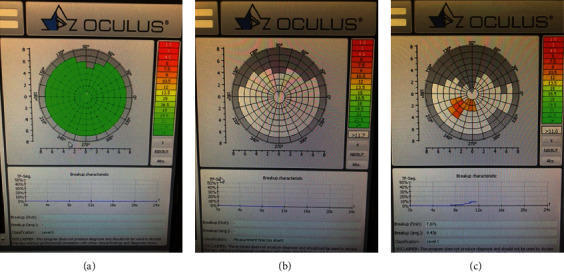
Noninvasive tear film break-up time (NIBUT) using the Oculus Keratograph 5 M (Oculus Optikgeräte GmbH, Wetzlar, Germany) presented as a tear film break-up color-code map. (a) No tear film break-up by 22–23 sec. (b) Immediately after tear film break-up. (c) At 7 sec after blinking.
The NIBUT was shown to have a correlation with the dry eye symptom score and a good diagnostic value for DED [14, 15]. Dry Eye Workshop II (DEWS II) suggested the NIBUT with a cutoff value of ≤10 seconds as an indicator for diagnosis of DED with 82% to 84% sensitivity and 76% to 94% specificity [16]. The NIBUT was also revealed to be useful for monitoring of treatment response in DED [15].
Bandlitz et al. [17] recently reported that objective measurement of the NIBUT using the Keratograph 5 M (Oculus Optikgeräte GmbH, Wetzlar, Germany) showed good repeatability and reasonable agreement with subjective NIBUT measurement using the Tearscope Plus (Keeler, Windsor, UK), Polaris (bon Optic, Lübeck, Germany), and EasyTear Viewplus (Easytear, Rovereto, Italy), which also support viability of the NIBUT in the diagnosis and treatment of DED [15].
However, measurement of the NIBUT using two different topography platforms, the Keratograph 5 M and RT-7000 Auto Refractor-Keratometer (Tomey, Nagoya, Japan), showed poor agreement [18], suggesting that measured values using different topographers with different algorithms should not be assessed interchangeably [10].
Although the NIBUT has a correlation with the fluorescein TBUT, its results should be carefully interpreted as it actually evaluates the thinning of the tear film, not the break-up of the full-thickness of the tear film [19].
3. Anterior Segment Optical Coherence Tomography
Anterior segment optical coherence tomography (AS-OCT) produces cross-sectional images of anterior segment structures by low-coherence interferometry [10, 20]. The technique enables measurement of tear meniscus parameters important for the diagnosis and monitoring of DED [16, 21–26], such as the tear meniscus height (TMH) and tear meniscus area (TMA) [10, 20, 27], without reflex tearing due to its noncontact nature and rapid image acquisition [11, 27].
Ibrahim et al. [22] showed that the TMH measured by time-domain (TD) OCT was correlated with strip meniscometry, corneal staining scores, and the Schirmer score. Spectral-domain (SD) OCT enabled higher resolution and faster image acquisition compared to TD-OCT, resulting in improved image quality with minimal artifact, as well as enhanced repeatability [27–29]. SD-OCT allowed improved sensitivity and specificity for the TMH and TMA as diagnostic biomarkers for DED compared to TD-OCT [11, 22]. SD-OCT findings also showed a close correlation with dry eye symptoms and the Schirmer score [24]. Qiu et al. [30] reported that diagnostic accuracy of SD-OCT was the highest for Sjögren's syndrome, moderately acceptable for non-Sjögren's aqueous-deficient DED, and the lowest for evaporative DED, suggesting the SD-OCT can be a viable option in the diagnosis of aqueous-deficient DED [30]. AS-OCT was also shown to be able to accurately measure the thickness of the overall tear film [31, 32]. Sher et al. [33] demonstrated that AS-OCT may also be helpful for the quantitative evaluation of corneal epithelial erosion.
AS-OCT is also useful for monitoring of treatment responses in DED [34–36]. Measurement of the TMH using TD-OCT might be effective in monitoring tear meniscus changes after punctal occlusion [35]. SD-OCT was useful for quantifying the sequential changes of tear meniscus parameters after artificial tear instillation [37]. Nagahara et al. [38] showed that the TMH decreased with CL wear and increased after the instillation of diquafosol sodium. Although the TMH and TMA measured using AS-OCT may be valuable biomarkers for DED [11], factors including the time-from-blink, palpebral aperture, presence of conjunctivochalasis, and lid length should be considered in the interpretation of these tear meniscus measurements [39].
Swept source OCT (SS-OCT) enables acquisition of three-dimensional images, as well as an enhanced scanning speed and greater imaging depth compared to TD and SD-OCT [40], which allows the measurement of the tear meniscus volume (TMV) in addition to the TMH and TMA [41]. The TMH, TMV, and TMA measured using SS-OCT showed a correlation with the corneal staining score, BUT, and Schirmer score [25]. All three parameters showed the strongest correlation with the Schirmer score, suggesting that the tear meniscus parameters mostly reflect the quantity of tear fluid [25]. SS-OCT was also useful for evaluation of increased tear meniscus parameters after installation of eye drops, such as sodium hyaluronate, diquafosol, and rebamipide [34].
En-face OCT may be useful for observation of changes in the ocular surface, particularly due to its noncontact nature and large scan width [42]. Ghouali et al. [43] evaluated the palisade of Vogt using this device to determine the changes in the limbal anatomy in DED patients and showed that the visibility score of the palisades was lower in DED patients with a decreased score in accordance with the severity of DED [43].
AS-OCT is expected to be useful for the diagnosis of meibomian gland dysfunction (MGD), one of the most common causes of DED [44]. Hwang et al. [45] introduced a method of developing 3D images of meibomian glands (MGs) by reconstructing a series of tomograms of MGs captured by high-speed SD-OCT. SS-OCT can provide 3D high-resolution images of MG acini and ducts that cannot be observed by infrared meibography [46]. OCT meibography showed a decreased MG length and width in obstructive MGD, which correlated with ocular surface symptoms and signs [47]. However, so far, there are only preliminary data; thus, further studies are needed for clinical application of MG imaging using OCT [48].
4. In Vivo Confocal Microscopy
In vivo confocal microscopy (IVCM) a noninvasive method that provides real-time imaging of the ocular surface at the histologic level, which enables the evaluation of changes in cells reflecting ocular surface damage and inflammation, such as corneal epithelial cells, keratocytes, and dendritic cells [1, 10, 49, 50]. IVCM also allows for observation of changes in the corneal nerve associated with DED [49].
In 2003, Tuominen et al. [51] reported that IVCM demonstrated reduced central corneal thickness, patchy alterations or irregularities in corneal epithelial cells, activated keratocytes reflected by abnormal hyper-reflectivity, and abnormal morphology of sub-basal nerve fiber bundles resembling nerve sprouting, suggesting active neural regeneration in Sjögren's syndrome [51]. Villani et al. [52] showed that IVCM revealed reduced density of the superficial epithelial cells, as well as decreased central corneal thickness in Sjögren's syndrome [52]. Other studies also showed decreased cell densities in the superficial corneal epithelial layer in both Sjögren's syndrome and non- Sjögren's DED [53–55].
Several studies showed decreased sub-basal nerve density, as well as increased number of beadings and nerve tortuosity in both Sjögren's syndrome and non-Sjögren's DED [50, 52, 54–57]. These changes in corneal nerves had correlation with corneal sensitivity, dry eye symptoms, and the Schirmer score [55–57]. By contrast, Zhang et al. [58] reported that IVCM showed increased corneal nerve density in Sjögren's syndrome. However, they also reported increased tortuosity in corneal nerves in Sjögren's syndrome [58]. Morphologic changes including beading and tortuosity may reflect an attempted regeneration of the corneal nerve [51, 52, 55] and suggested to be indices of metabolic activity of the sub-basal nerve plexus [57].
Dendritic cells are antigen-presenting cells that play an important role in ocular surface immunology [59]. In DED, dessication stress causes proinflammatory stimulation on the ocular surface, which conceivably promotes migration and maturation of dendritic cells (Figure 2) [59]. Lin et al. [60] demonstrated increased dendritic cells in the central cornea in both Sjögren's syndrome and non-Sjögren's DED [60]. They also revealed an increased number of “activated” dendritic cells characterized by the increased dendrites [60]. Kheirkhah et al. [61] demonstrated marked increase in dendritic cell density at the sub-basal epithelial region in aqueous-deficient immunologic DED, such as Sjögren's syndrome and graft versus host disease (GVHD), compared with the aqueous-deficient nonimmunologic DED, evaporative DED, and controls [61].
Figure 2.
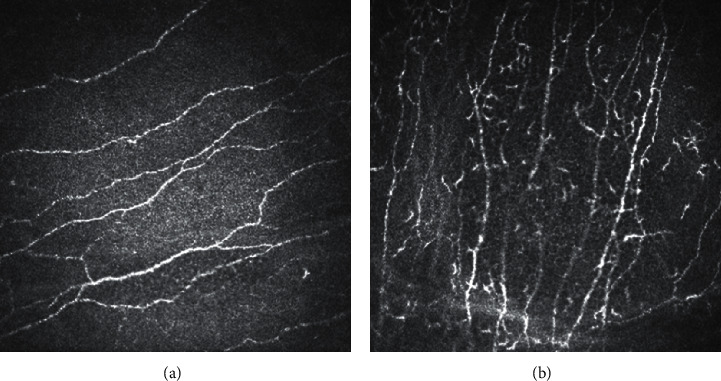
In vivo confocal microscopy. (a) Normal sub-basal nerve plexus. (b) Increased dendritic cells in dry eye disease.
IVCM was shown to be useful for the monitoring of treatment responses in DED [59]. Using an in vivo laser-scanning confocal microscope (Heidelberg Retina Tomograph, Rostock Corneal Module (HRT-RCM); Heidelberg Engineering GmgH, Heidelberg, Germany), Villani et al. [62] demonstrated that the density of sub-basal dendritic cells and activated keratocytes significantly decreased after 4 weeks of treatment with topical steroid. Iaccheri et al. [63] reported increased cell density of the corneal intermediate epithelium, decreased activated keratocytes, and reduced tortuosity of corneal nerve fibers after 6 months of treatment with 0.05% topical cyclosporine in DED [63]. Levy et al. [64] also reported an increase in corneal sub-basal nerves and a decrease in dendritic cell density after 6 months of treatment with topical 0.05% cyclosporine in Sjögren's syndrome. IVCM also demonstrated decreased corneal basal epithelial cell density and reduced numbers of nerve beadings after treatment with autologous serum eye drops [65, 66].
IVCM can also provide high-resolution imaging of the MGs; thus, it can be useful for the evaluation of the MG morphology at a cellular level, which is important for the diagnosis of MGD (Figure 3) [10, 67]. IVCM enabled determination of novel MG parameters, e.g., meibomian gland acinar unit density (MGAUD), shortest diameter (MGASD) and longest diameter (MGALD), and inflammatory cell density [67, 68]. These parameters have a significant correlation with tear film parameters, ocular surface signs, and MG expressibility [67, 68].
Figure 3.
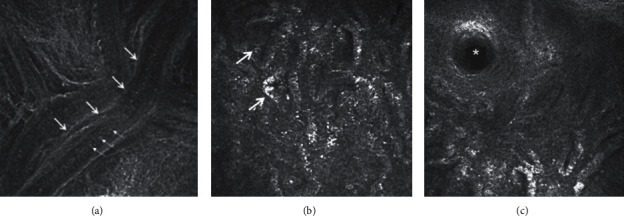
(a) In vivo confocal microscopy showing the meibomian gland duct of one of the meibomian glands of the upper eyelid. Large arrows show the wall of the terminal duct and small arrows show the meibum within. (b) The multiple irregular globular structures (arrows) indicate the acini of meibomian glands. (c) The orifice of a single meibomian gland is shown by the asterix.
IVCM with HRT-RCM demonstrated morphologic alterations in MGD, such as enlargement of glandular acinar units, extensive periglandular inflammatory cell infiltration, and hyperkeratinization of the ductal epithelium [67–69]. In severe MGD, atrophy in MGs with extensive periglandular fibrosis was observed [70].
Ibrahim et al. [68] revealed that MGD was associated with lower MGAUD, larger MGASD and MGALD, and higher inflammatory cell density. Matsumoto et al. [70] also reported similar findings and showed the acinar unit density and diameters had association with the severity of MG dropout and expressibility. They also showed a significant reduction in the inflammatory cell density of MGs after treatment with lid hygiene, topical 0.5% levofloxacin and 0.1% fluorometholone, and oral 100 mg minocycline [70]. Ban et al. [71] demonstrated that patients with DED/GVHD had significantly lower MGAUD, shorter MGASD and MGALD, and a higher fibrosis grade compared to those with non-DED/non-GVHD. Villani et al. [72] revealed that patients with Sjögren's syndrome had greater acinar density, shorter diameters, higher density of periglandular inflammatory cells, and lower secretion reflectivity compared with those with MGD.
However, IVCM has a limitation that it is not capable of cellular and tissue phenotyping, and analysis is only based on morphology and reflectivity [48]. The small field of view (<0.25 mm [2]) is also a major limitation of the device [48].
5. Infrared Meibography
Noncontact infrared meibography based on combined transillumination with infrared photography provides two-dimensional silhouette of MGs [10, 73]. The technique has been widely used for the evaluation of MG dropout since its introduction in 2008, as it can provide improved image quality with a short acquisition time and minimal patient discomfort (Figure 4) [74]. In meibography, healthy meibum is visualized as a light area due to its autofluorescence [11, 73]. Dark areas in the MG conceivably indicate loss of acinar tissue or an altered meibum condition, which is determined as MG dropout [73, 75].
Figure 4.
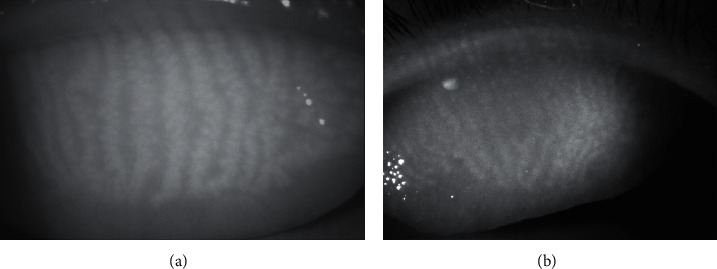
(a) Infrared meibography showing relatively normal meibomian glands in the upper eyelid. Brighter areas indicate glandular areas, whereas darker areas indicate intergland tissue. (b) Meibography showing slight atrophy of the meibomian glands in the proximal margin of the tarsal plate. The abnormal area is greyish without typical whitish tracks that represent the glands.
Among various grading scales proposed for MG dropout [76], the Gestalt grading scale and meiboscale were recommended by the MGD Workshop [74, 75]. In the Gestalt grading scale, grading of each lid is performed on a scale from 1 to 4, based on the ratio of partial glands as follows [11]: grade 1 = no partial glands; grade 2 = less than 25% partial glands; grade 3 = 25% to 75% partial glands; and grade 4 = greater than 75% partial glands [11]. In the meiboscale, each lid is graded based on the ratio of MG dropout: grade 0 = no loss of MGs; grade 1 = area loss ≤ 25%; grade 2 = area loss ≤ 50%; grade 3 = area loss ≤ 75%; and grade 4: area loss ≤ 100% [77].
Recently, continuous grading scales using semiautomated software that automatically calculates the ratio of the area of MG loss to the total area of the eyelid have been developed [11, 78]. As the ratio of MG dropout is expressed as numeric values ranging from 0 to 100, the continuous scales may be advantageous for evaluating subtle changes that may not be detected using categorical grading scales and can facilitate the determination of efficacy of treatment, such as intraductal probing and eyelid warming [11, 79, 80].
The area of MG dropout showed a positive correlation with the meibum grade [81, 82]. Both the severity of MGD and the percentage of MG dropout had a correlation with the TBUT, dry eye symptom score, and corneal stain score [83]. The ductal length and acini area measured by meibography were correlated with the tear film, corneal stain score, and meibum level [84]. Arita et al. [85] demonstrated the correlation between MG dropout and Schirmer's score in MGD, suggesting that tear fluid production may increase to compensate for tear film instability due to MGD. MG dropout measured using infrared meibography has proven to be useful for differential diagnosis of MGD and aqueous deficiency DED [86].
However, infrared meibography has a limitation that it cannot provide three-dimensional images of the deeper structures [10]. Hence, the MG dropout evaluated by infrared meibography should be carefully interpreted [10]. Additional information using AS-OCT or IVCM might be helpful for the diagnosis and monitoring of MGD.
6. Interferometry
Tear interferometry is a noninvasive method for the investigation of the tear lipid layer by visualization of the reflection of light at the lipid-aqueous interface of the tear film [10, 87, 88]. Interferometry allows objective evaluation of tear film properties, such as lipid layer thickness, break-up characteristics and changes in thickness of the tear film, its distribution, and wetting patterns with sequential blinking [81, 89, 90].
Yokoi et al. [91] showed that grading of the lipid layer interference pattern had a significant correlation with the corneal staining score and TBUT. Goto et al. [92] developed a tear interference color chart for the DR-1 α interferometer (Kowa, Nagoya, Japan), which can be useful for converting tear interference color information to the lipid layer thickness (LLT) [92]. Subsequently, Arita et al. [93] showed that the DR-1α interferometer could measure the TMH as reliably as SS-OCT and showed that the interferometric TMH had correlation with Schirmer's score. Arita et al. [88] also described a difference in interferometric patterns among aqueous-deficient DED, MGD, and normal control on the DR-1α interferometry, suggesting that the device can be helpful for differential diagnosis of subtypes of DED.
The DR-1α interferometer is also capable of kinetic analysis of spread and stability of the lipid layer [94]. MGD was associated with significantly increased lipid spread time [94]. The kinetic analysis can also be helpful for evaluation of the improvement of the lipid layer after treatment, e.g., low-dose lipid application on the lid margin or punctal occlusion [95, 96].
The LipiView II (LVII) ocular surface interferometer (TearScience, Johnson and Johnson Vision, Jacksonville, FL, USA) is capable of providing quantitative information of the LLT, as well as images of MGs using a patented Lid Everter and infrared diodes for eversion and illumination of the eyelid [10, 97]. Ji et al. [81] showed that LLT measured using the LVII had a significant correlation with the TBUT, MGD grade, and MG dropout, suggesting that it can be an alternative to traditional dry eye tests. Eom et al. [98] also revealed that LLT measured with LVII was negatively correlated to MG loss and obstructive MGD was associated with lower LLT [98]. LLT may reflect the changes in meibum secretion and be helpful for differential diagnosis of MGD [10]; LLT would be increased in hypersecrectory MGD and decreased in obstructive MGD [99]. However, the LLT should be carefully interpreted as it can be affected by factors, such as age, sex, and ocular surgical history [100].
7. Wavefront and Double-Pass Aberrometry
DED is often associated with complaints including blurred vision, glare, and fluctuating vision with blinking, which is difficult to measure by conventional visual acuity testing [19, 101]. As the air-tear film interface forms the first refractive component of the eye, irregularity of the tear film in DED may cause decreased optical quality [10].
Tear film instability increases higher-order aberrations (HOAs) and ocular forward light scattering, resulting in “fluctuating vision with blinking” and “glare,” respectively [101]. Damage in the central cornea, i.e., the overlying optical zone, is associated with increased HOAs and corneal backward light scattering, leading to “blurred vision” [101].
Quantification of HOA using wavefront sensors, such as the Shack–Hartmann aberrometer, allows evaluation of optical aberrations associated with DED [102–104]. Wavefront sensing has shown that break-up of the tear film was associated with increased HOAs both in photopic and scotopic conditions [102]. Sequential measurement of HOAs demonstrated that superficial punctate keratopathy may aggravate both baseline HOAs and sequential postblink changes in HOAs in patients with DED [105]. Sequential wavefront measurements in eyes with a short TBUT have shown that a prolonged blink interval leads to increased HOAs with a marked upward curve after blinking, suggesting that suppressed blinking, such as while working with a video display terminal, can result in reduced optical quality [106]. Denoyer et al. [107] also reported a progressive increase in postblink HOAs in patients with DED and the correlation of the changes in HOAs with the OSDI score and TBUT [107].
Evaluation of the objective scatter index (OSI) obtained using the double-pass image of a point source projected on the retina enables the quantification of the ocular light scattering that cannot be measured using conventional wavefront sensors [104, 108, 109]. Using the double-pass aberrometer, Tan et al. [110] showed an increase in the OSI in patients with DED and the correlation between the OSI change and severity of DED. The rate of change in the OSI was correlated to the corneal staining score [111]. Koh et al. [112] revealed that DED was associated with greater ocular forward light scattering and corneal backward light scattering. Superficial punctate keratopathy overlying the optical zone was related to increased corneal backward light scattering [112].
An improvement in HOA and light scattering was reported after instilling artificial tear drops in patients with DED [113]. Serial examinations showed increased HOAs and forward light scattering immediately after the instillation of a highly viscous 0.3% sodium hyaluronate solution and the instillation of 2% rebamipide suspension, respectively, accounting for a temporal reduction in optical quality after instillation of eye drops with high viscosity or suspensibility [114].
8. Thermography
Infrared thermography is used to measure the amount of infrared radiation emitted from the ocular surface using an infrared thermal camera; thus, it allows a noninvasive evaluation of the changes in the ocular surface temperature (OST) caused by tear fluid evaporation [10, 115]. Decrease in the OST had a correlation with the TBUT and NIBUT [116, 117], conceivably, because tear film instability facilitates tear fluid evaporation and heat loss [116]. Su et al. [115] demonstrated that the OST difference 3 seconds after blinking was correlated with the TMH and Schirmer score.
Using a thermographic device (Ocular Surface Thermographer; OST, Tomey, Nagoya, Japan), Kamao et al. [117] reported that DED was associated with a greater decrease in the OST at 10 seconds after eye opening and suggested that changes in the OST could be an indicator for DED. Tan et al. [118] also showed that the temperature of the extreme nasal conjunctiva at 5 and 10 seconds after eye opening was a good detector for DED. Arita et al. [119] revealed that obstructive MGD was associated with lower surface temperature of the tarsal conjunctiva, which might increase the viscosity of the meibum and result in obstruction of the glands [119].
9. Tear Film Imager
The tear film imager (TFI) is a new technology that provides real-time images of the mucoaqueous and lipid tear layers [120]. Using spectral interference technology, this instrument enables noninvasive measurement of parameters including the thickness of mucoaqueous and lipid layers, thickness change rate, and the break-up time with a large field of view and nanometer axial resolution [120, 121].
Segev et al. [121] recently revealed that the device can reproducibly measure the mucoaqueous thickness which correlates with the Schirmer score. Lipid break-up time measurement with the TFI had a correlation with the TBUT [121]. DED was associated with lower mucoaqueous thickness and shorter lipid break-up time [121]. Cohen et al. [122] demonstrated that the TFI was capable of creating detailed maps of the lipid layer thickness and quantifying the lipid map uniformity due to its nanometer thickness resolution, which could be helpful for the diagnosis and treatment of DED.
10. Conclusions
Advances in ocular imaging technology have enabled objective and reproducible evaluation of ocular surface change, tear film parameters, and optical quality associated with DED; thus, they can be useful for diagnosis and management of the disease, as well as elucidation of its pathogenesis [123].
Studies have indicated the efficacy of various imaging modalities, i.e., the NIBUT for evaluation of the TBUT, AS-OCT for quantification of tear meniscus parameters, IVCM for high-resolution visualization of the ocular surface, infrared meibography for evaluation of MG dropout, interferometry for the measurement of the tear lipid layer, wavefront aberrometry and the OSI for the quantification of optical quality, thermography for the detection of changes in the OST, and the TFI for the evaluation of mucoaqueous and lipid layers [10].
With technological developments, these imaging devices are expected to provide more precise and accurate information on structural and functional changes associated with DED. Further studies are, therefore, warranted for clinical application of these devices and establishment of guidelines for the use of the modalities for diagnosis and management of DED.
Acknowledgments
This study was conducted with the support of a research grant of Kangwon Institute for Unification Studies, Kangwon National University, in 2019, and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant no. NRF-2017R1D1A1B03029983).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Craig J. P., Nichols K. K., Akpek E. K., et al. TFOS DEWS II definition and classification report. The Ocular Surface. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Han S. B., Hyon J. Y., Woo S. J., et al. Prevalence of dry eye disease in an elderly Korean population. Archives of Ophthalmology. 2011;129(5):633–638. doi: 10.1001/archophthalmol.2011.78. [DOI] [PubMed] [Google Scholar]
- 3.Lin P.-Y., Tsai S.-Y., Cheng C.-Y., Liu J.-H., Chou P., Hsu W.-M. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai eye study. Ophthalmology. 2003;110(6):1096–1101. doi: 10.1016/s0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 4.Miljanovic B., Trivedi K. A., Dana M. R., et al. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. The American Journal of Clinical Nutrition. 2005;82(4):887–893. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchino M., Schaumberg D. A., Dogru M., et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008;115(11):1982–1988. doi: 10.1016/j.ophtha.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Baudouin C., Irkec M., Messmer E. M., et al. Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmologica. 2017;96(2):111–109. doi: 10.1111/aos.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han S. B., Yang H. K., Hyon J. Y., Wee W. R. Association of dry eye disease with psychiatric or neurological disorders in elderly patients. Clinical Interventions in Aging. 2017;12:785–792. doi: 10.2147/cia.s137580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vehof J., Kozareva D., Hysi P. G., Hammond C. J. Prevalence and risk factors of dry eye disease in a British female cohort. British Journal of Ophthalmology. 2014;98(12):1712–1717. doi: 10.1136/bjophthalmol-2014-305201. [DOI] [PubMed] [Google Scholar]
- 9.Nichols K. K., Mitchell G. L., Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23(3):272–285. doi: 10.1097/00003226-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Chan T. C. Y., Wan K. H., Shih K. C., Jhanji V. Advances in dry eye imaging: the present and beyond. British Journal of Ophthalmology. 2018;102(3):295–301. doi: 10.1136/bjophthalmol-2017-310759. [DOI] [PubMed] [Google Scholar]
- 11.Binotti W. W., Bayraktutar B., Ozmen M. C., Cox S. M., Hamrah P. A review of imaging biomarkers of the ocular surface. Eye Contact Lens. 2019;46(2):84–105. doi: 10.1097/icl.0000000000000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson M. E., Murphy P. J. The Effect of instilled fluorescein solution volume on the values and repeatability of TBUT measurements. Cornea. 2005;24(7):811–817. doi: 10.1097/01.ico.0000154378.67495.40. [DOI] [PubMed] [Google Scholar]
- 13.Goto T., Zheng X., Okamoto S., Ohashi Y. Tear film stability analysis system: introducing a new application for videokeratography. Cornea. 2004;23(8):S65–S70. doi: 10.1097/01.ico.0000136667.63870.51. [DOI] [PubMed] [Google Scholar]
- 14.Fuller D. G., Potts K., Kim J. Noninvasive tear breakup times and ocular surface disease. Optometry and Vision Science. 2013;90(10):1086–1091. doi: 10.1097/opx.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 15.Prabhasawat P., Tesavibul N., Mahawong W. A randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea. 2012;31(12):1386–1393. doi: 10.1097/ico.0b013e31823cc098. [DOI] [PubMed] [Google Scholar]
- 16.Wolffsohn J. S., Arita R., Chalmers R., et al. TFOS DEWS II diagnostic methodology report. The Ocular Surface. 2017;15(3):539–574. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Bandlitz S., Peter B., Pflugi T., et al. Agreement and repeatability of four different devices to measure non-invasive tear breakup time (NIBUT) Contact Lens and Anterior Eye. 2020;42(6):p. e3. doi: 10.1016/j.clae.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Lee R., Yeo S., Aung H. T., Tong L. Agreement of noninvasive tear break-up time measurement between Tomey RT-7000 Auto refractor-keratometer and Oculus Keratograph 5 M. Clinical Ophthalmology. 2016;10:1785–1790. doi: 10.2147/opth.s110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima T., Dogru M., Kawashima M., Nakamura S., Tsubota K. Advances in the diagnosis and treatment of dry eye. Progress in Retinal and Eye Research. 2020:p. 100842. doi: 10.1016/j.preteyeres.2020.100842. In press. [DOI] [PubMed] [Google Scholar]
- 20.Huang D., Swanson E., Lin C., et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mainstone J. C., Bruce A. S., Golding T. R. Tear meniscus measurement in the diagnosis of dry eye. Current Eye Research. 1996;15(6):653–661. doi: 10.3109/02713689609008906. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim O. M. A., Dogru M., Takano Y., et al. Application of visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology. 2010;117(10):1923–1929. doi: 10.1016/j.ophtha.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 23.Zhou S., Li Y., Lu A. T.-H., et al. Reproducibility of tear meniscus measurement by Fourier-domain optical coherence tomography: a pilot study. Ophthalmic Surgery, Lasers, and Imaging. 2009;40(5):442–447. doi: 10.3928/15428877-20090901-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen P., Huang D., Li Y., et al. Correlation between optical coherence tomography-derived assessments of lower tear meniscus parameters and clinical features of dry eye disease. Cornea. 2012;31(6):680–685. doi: 10.1097/ico.0b013e3182261577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akiyama R., Usui T., Yamagami S. Diagnosis of dry eye by tear meniscus measurements using anterior segment swept source optical coherence tomography. Cornea. 2015;34(11):S115–S120. doi: 10.1097/ico.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 26.Qiu X., Gong L., Sun X., Jin H. Age-related variations of human tear meniscus and diagnosis of dry eye with Fourier-domain anterior segment optical coherence tomography. Cornea. 2011;30(5):543–549. doi: 10.1097/ico.0b013e3181fb84ea. [DOI] [PubMed] [Google Scholar]
- 27.Ramos J. L. B., Li Y., Huang D. Clinical and research applications of anterior segment optical coherence tomography - a review. Clinical & Experimental Ophthalmology. 2009;37(1):81–89. doi: 10.1111/j.1442-9071.2008.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan H. H., Zhao Y., Tun T. A., Tong L. Repeatability of tear meniscus evaluation using spectral-domain Cirrus HD-OCT and time-domain Visante OCT. Contact Lens and Anterior Eye. 2015;38(5):368–372. doi: 10.1016/j.clae.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Canan H., Altan-Yaycioglu R., Ulas B., Sizmaz S., Coban-Karatas M. Interexaminer reproducibility of optical coherence tomography for measuring the tear film meniscus. Current Eye Research. 2014;39(12):1145–1150. doi: 10.3109/02713683.2014.898311. [DOI] [PubMed] [Google Scholar]
- 30.Qiu X., Gong L., Lu Y., Jin H., Robitaille M. The diagnostic significance of Fourier-domain optical coherence tomography in Sjögren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmologica. 2012;90(5):e359–e366. doi: 10.1111/j.1755-3768.2012.02413.x. [DOI] [PubMed] [Google Scholar]
- 31.Bai Y., Ngo W., Gu B., Zhang Y., Nichols J. J. An imaging system integrating optical coherence tomography and interferometry for in vivo measurement of the thickness and dynamics of the tear film. BioMedical Engineering OnLine. 2018;17(1):p. 164. doi: 10.1186/s12938-018-0597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szegedi S., Scheschy U., Schmidl D., et al. Effect of single instillation of two hyaluronic acid-based topical lubricants on tear film thickness in patients with dry eye syndrome. Journal of Ocular Pharmacology and Therapeutics. 2018;34(9):605–611. doi: 10.1089/jop.2018.0069. [DOI] [PubMed] [Google Scholar]
- 33.Sher I., Tzameret A., Szalapak A. M., et al. Multimodal assessment of corneal erosions using optical coherence tomography and automated grading of fluorescein staining in a rabbit dry eye model. Translational Vision Science & Technology. 2019;8(1):p. 27. doi: 10.1167/tvst.8.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akiyama-Fukuda R., Usui T., Yoshida T., Yamagami S. Evaluation of tear meniscus dynamics using anterior segment swept-source optical coherence tomography after topical solution instillation for dry eye. Cornea. 2016;35(5):654–658. doi: 10.1097/ico.0000000000000807. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim O. M. A., Dogru M., Kojima T., et al. OCT assessment of tear meniscus after punctal occlusion in dry eye disease. Optometry and Vision Science. 2012;89(5):E770–E776. doi: 10.1097/opx.0b013e31824eeb07. [DOI] [PubMed] [Google Scholar]
- 36.Napoli P. E., Satta G. M., Coronella F., Fossarello M. Spectral-domain optical coherence tomography study on dynamic changes of human tears after instillation of artificial tears. Investigative Opthalmology & Visual Science. 2014;55(7):4533–4540. doi: 10.1167/iovs.14-14666. [DOI] [PubMed] [Google Scholar]
- 37.Bujak M. C., Yiu S., Zhang X., Li Y., Huang D. Serial measurement of tear meniscus by FD-OCT after instillation of artificial tears in patients with dry eyes. Ophthalmic Surgery, Lasers, and Imaging. 2011;42(4):308–313. doi: 10.3928/15428877-20110603-02. [DOI] [PubMed] [Google Scholar]
- 38.Nagahara Y., Koh S., Maeda N., Nishida K., Watanabe H. Prominent decrease of tear meniscus height with contact lens wear and efficacy of eye drop instillation. Eye & Contact Lens: Science & Clinical Practice. 2015;41(5):318–322. doi: 10.1097/icl.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 39.Chan T. C., Ye C., Ng P. K., Li E. Y. M., Yuen H. K. L., Jhanji V. Change in tear film lipid layer thickness, corneal thickness, volume and topography after superficial cauterization for conjunctivochalasis. Scientific Reports. 2015;5(1):p. 12239. doi: 10.1038/srep12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leite M. T., Rao H. L., Zangwill L. M., Weinreb R. N, Medeiros F. A. Comparison of the diagnostic accuracies of the spectralis, cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology. 2011;118(7):1334–1339. doi: 10.1016/j.ophtha.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuda R., Usui T., Miyai T., Yamagami S., Amano S. Tear meniscus evaluation by anterior segment swept-source optical coherence tomography. American Journal of Ophthalmology. 2013;155(4):620–624. doi: 10.1016/j.ajo.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Tahiri Joutei Hassani R., Liang H., El Sanharawi M., et al. En-face optical coherence tomography as a novel tool for exploring the ocular surface: a pilot comparative study to conventional B-scans and in vivo confocal microscopy. The Ocular Surface. 2014;12(4):285–306. doi: 10.1016/j.jtos.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Ghouali W., Tahiri Joutei Hassani R., Djerada Z., et al. In vivo imaging of palisades of Vogt in dry eye versus normal subjects using en-face spectral-domain optical coherence tomography. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187864.e0187864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baudouin C., Messmer E. M., Aragona P., et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. British Journal of Ophthalmology. 2016;100(3):300–306. doi: 10.1136/bjophthalmol-2015-307415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang H. S., Shin J. G., Lee B. H., et al. In Vivo 3D meibography of the human eyelid using real time imaging fourier-domain OCT. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0067143.e67143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo Y.-S., Na K.-S., Kim D. Y., Yang S.-W., Joo C.-K. Morphological evaluation for diagnosis of dry eye related to meibomian gland dysfunction. Experimental Eye Research. 2017;163:72–77. doi: 10.1016/j.exer.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Liang Q., Pan Z., Zhou M., et al. Evaluation of optical coherence tomography meibography in patients with obstructive meibomian gland dysfunction. Cornea. 2015;34(10):1193–1199. doi: 10.1097/ico.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 48.Villani E., Arita R. Imaging of meibomian glands: from bench to bedside and back. Eye. 2019;33(5):695–697. doi: 10.1038/s41433-019-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villani E., Bonsignore F., Cantalamessa E., Serafino M., Nucci P. Imaging biomarkers for dry eye disease. Eye & Contact Lens: Science & Clinical Practice. 2019;46:141–145. doi: 10.1097/icl.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 50.Alhatem A., Cavalcanti B., Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Seminars in Ophthalmology. 2012;27(5-6):138–148. doi: 10.3109/08820538.2012.711416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuominen I. S. J., Konttinen Y. T., Vesaluoma M. H., Moilanen J. A. O., Helinto¨ M., Tervo T. M. T. Corneal innervation and morphology in primary Sjögren’s syndrome. Investigative Opthalmology & Visual Science. 2003;44(6):2545–2549. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 52.Villani E., Galimberti D., Viola F., Mapelli C., Ratiglia R. The cornea in Sjögren’s syndrome: an in vivo confocal study. Investigative Opthalmology & Visual Science. 2007;48(5):2017–2022. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X., Chen Q., Chen W., Cui L., Ma H., Lu F. Tear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eye. Ophthalmology. 2011;118(5):902–907. doi: 10.1016/j.ophtha.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 54.Erdélyi B., Kraak R., Zhivov A., Guthoff R., Németh J. In vivo confocal laser scanning microscopy of the cornea in dry eye. Graefe’s archive for clinical and experimental ophthalmology. 2007;245(1):39–44. doi: 10.1007/s00417-006-0375-6. [DOI] [PubMed] [Google Scholar]
- 55.del Castillo J. M. B. T., Wasfy M. A. S., Fernandez C., Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Investigative Opthalmology & Visual Science. 2004;45(9):3030–3035. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 56.Villani E., Magnani F., Viola F., et al. In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optometry and Vision Science. 2013;90(6):576–586. doi: 10.1097/opx.0b013e318294c184. [DOI] [PubMed] [Google Scholar]
- 57.Benitez-Del-Castillo J. M., Acosta M. C., Wassfi M. A., et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Investigative Opthalmology & Visual Science. 2007;48(1):173–181. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 58.Zhang M., Chen J., Luo L., Xiao Q., Sun M., Liu Z. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea. 2005;24(7):818–824. doi: 10.1097/01.ico.0000154402.01710.95. [DOI] [PubMed] [Google Scholar]
- 59.Matsumoto Y., Ibrahim O. M. A. Application of in vivo confocal microscopy in dry eye disease. Investigative Opthalmology & Visual Science. 2018;59(14):p. 41. doi: 10.1167/iovs.17-23602. [DOI] [PubMed] [Google Scholar]
- 60.Lin H., Li W., Dong N., et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Investigative Opthalmology & Visual Science. 2010;51(1):122–128. doi: 10.1167/iovs.09-3629. [DOI] [PubMed] [Google Scholar]
- 61.Kheirkhah A., Rahimi Darabad R., Cruzat A., et al. Corneal epithelial immune dendritic cell alterations in subtypes of dry eye disease: a pilot in vivo confocal microscopic study. Investigative Opthalmology & Visual Science. 2015;56(12):7179–7185. doi: 10.1167/iovs.15-17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villani E., Garoli E., Termine V., Pichi F., Ratiglia R., Nucci P. Corneal confocal microscopy in dry eye treated with corticosteroids. Optometry and Vision Science. 2015;92(9):e290–e295. doi: 10.1097/opx.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 63.Iaccheri B., Torroni G., Cagini C., et al. Corneal confocal scanning laser microscopy in patients with dry eye disease treated with topical cyclosporine. Eye. 2017;31(5):788–794. doi: 10.1038/eye.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy O., Labbé A., Borderie V., et al. Increased corneal sub-basal nerve density in patients with Sjögren syndrome treated with topical cyclosporine A. Clinical & Experimental Ophthalmology. 2017;45(5):455–463. doi: 10.1111/ceo.12898. [DOI] [PubMed] [Google Scholar]
- 65.Mahelkova G., Jirsova K., Seidler Stangova P., et al. Using corneal confocal microscopy to track changes in the corneal layers of dry eye patients after autologous serum treatment. Clinical and Experimental Optometry. 2017;100(3):243–249. doi: 10.1111/cxo.12455. [DOI] [PubMed] [Google Scholar]
- 66.Semeraro F., Forbice E., Nascimbeni G., et al. Effect of autologous serum eye drops in patients with Sjögren syndrome-related dry eye: clinical and in vivo confocal microscopy evaluation of the ocular surface. In Vivo. 2016;30(6):931–938. doi: 10.21873/invivo.11016. [DOI] [PubMed] [Google Scholar]
- 67.Matsumoto Y., Sato E. A., Ibrahim O. M., Dogru M., Tsubota K. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Molecular Vision. 2008;14:1263–1271. [PMC free article] [PubMed] [Google Scholar]
- 68.Ibrahim O. M. A., Matsumoto Y., Dogru M., et al. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117(4):665–672. doi: 10.1016/j.ophtha.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 69.Villani E., Ceresara G., Beretta S., Magnani F., Viola F., Ratiglia R. In vivo confocal microscopy of meibomian glands in contact lens wearers. Investigative Opthalmology & Visual Science. 2011;52(8):5215–5219. doi: 10.1167/iovs.11-7427. [DOI] [PubMed] [Google Scholar]
- 70.Matsumoto Y., Shigeno Y., Sato E. A., et al. The evaluation of the treatment response in obstructive meibomian gland disease by in vivo laser confocal microscopy. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2009;247(6):821–829. doi: 10.1007/s00417-008-1017-y. [DOI] [PubMed] [Google Scholar]
- 71.Ban Y., Ogawa Y., Ibrahim O. M., et al. Morphologic evaluation of meibomian glands in chronic graft-versus-host disease using in vivo laser confocal microscopy. Molecular Vision. 2011;17:2533–2543. [PMC free article] [PubMed] [Google Scholar]
- 72.Villani E., Beretta S., De Capitani M., Galimberti D., Viola F., Ratiglia R. In vivo confocal microscopy of meibomian glands in Sjögren’s syndrome. Investigative Opthalmology & Visual Science. 2011;52(2):933–939. doi: 10.1167/iovs.10-5995. [DOI] [PubMed] [Google Scholar]
- 73.Arita R. Meibography: a Japanese perspective. Investigative Opthalmology & Visual Science. 2018;59(14):DES48–DES55. doi: 10.1167/iovs.17-23631. [DOI] [PubMed] [Google Scholar]
- 74.Arita R., Itoh K., Inoue K., Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115(5):911–915. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 75.Geerling G., Tauber J., Baudouin C., et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Investigative Opthalmology & Visual Science. 2011;52(4):2050–2064. doi: 10.1167/iovs.10-6997g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nichols J. J., Berntsen D. A., Mitchell G. L., Nichols K. K. An assessment of grading scales for meibography images. Cornea. 2005;24(4):382–388. doi: 10.1097/01.ico.0000148291.38076.59. [DOI] [PubMed] [Google Scholar]
- 77.Pult H., Riede-Pult B. Comparison of subjective grading and objective assessment in meibography. Contact Lens and Anterior Eye. 2013;36(1):22–27. doi: 10.1016/j.clae.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 78.Tomlinson A., Bron A. J., Korb D. R., et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Investigative Opthalmology & Visual Science. 2011;52(4):2006–2049. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maskin S. L. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea. 2010;29(10):1145–1152. doi: 10.1097/ico.0b013e3181d836f3. [DOI] [PubMed] [Google Scholar]
- 80.Arita R., Morishige N., Shirakawa R., Sato Y., Amano S. Effects of eyelid warming devices on tear film parameters in normal subjects and patients with meibomian gland dysfunction. The Ocular Surface. 2015;13(4):321–330. doi: 10.1016/j.jtos.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 81.Ji Y. W., Lee J., Lee H., Seo K. Y., Kim E. K., Kim T.-I. Automated measurement of tear film dynamics and lipid layer thickness for assessment of non-Sjögren dry eye syndrome with meibomian gland dysfunction. Cornea. 2017;36(2):176–182. doi: 10.1097/ico.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 82.Kim H. M., Eom Y., Song J. S. The relationship between morphology and function of the meibomian glands. Eye & Contact Lens: Science & Clinical Practice. 2018;44(1):1–5. doi: 10.1097/icl.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 83.Gulmez Sevim D., Gumus K., Unlu M. Reliable, noncontact imaging tool for the evaluation of meibomian gland function: sirius meibography. Eye & Contact Lens: Science & Clinical Practice. 2020;46:135–140. doi: 10.1097/icl.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 84.Ban Y., Shimazaki-Den S., Tsubota K., Shimazaki J. Morphological evaluation of meibomian glands using noncontact infrared meibography. The Ocular Surface. 2013;11(1):47–53. doi: 10.1016/j.jtos.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Arita R., Morishige N., Koh S., et al. Increased tear fluid production as a compensatory response to meibomian gland loss: a multicenter cross-sectional Study. Ophthalmology. 2015;122(5):925–933. doi: 10.1016/j.ophtha.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 86.Arita R., Itoh K., Maeda S., Maeda K., Tomidokoro A., Amano S. Efficacy of diagnostic criteria for the differential diagnosis between obstructive meibomian gland dysfunction and aqueous deficiency dry eye. Japanese Journal of Ophthalmology. 2010;54(5):387–391. doi: 10.1007/s10384-010-0858-1. [DOI] [PubMed] [Google Scholar]
- 87.McDonald J. E. Surface phenomena of tear films. Transactions of the American Ophthalmological Society. 1968;66:905–939. [PMC free article] [PubMed] [Google Scholar]
- 88.Arita R., Morishige N., Fujii T., et al. Tear interferometric patterns reflect clinical tear dynamics in dry eye patients. Investigative Opthalmology & Visual Science. 2016;57(8):3928–3934. doi: 10.1167/iovs.16-19788. [DOI] [PubMed] [Google Scholar]
- 89.Finis D., Pischel N., Schrader S., Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea. 2013;32(12):1549–1553. doi: 10.1097/ico.0b013e3182a7f3e1. [DOI] [PubMed] [Google Scholar]
- 90.Doane M. G. An instrument for in vivo tear film interferometry. Optometry and Vision Science. 1989;66(6):383–388. doi: 10.1097/00006324-198906000-00008. [DOI] [PubMed] [Google Scholar]
- 91.Yokoi N., Takehisa Y., Kinoshita S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. American Journal of Ophthalmology. 1996;122(6):818–824. doi: 10.1016/s0002-9394(14)70378-2. [DOI] [PubMed] [Google Scholar]
- 92.Goto E., Dogru M., Kojima T., Tsubota K. Computer-synthesis of an interference color chart of human tear lipid layer, by a colorimetric approach. Investigative Opthalmology & Visual Science. 2003;44(11):4693–4697. doi: 10.1167/iovs.03-0260. [DOI] [PubMed] [Google Scholar]
- 93.Arita R., Yabusaki K., Hirono T., et al. Automated measurement of tear meniscus height with the kowa DR-1α tear interferometer in both healthy subjects and dry eye patients. Investigative Opthalmology & Visual Science. 2019;60(6):2092–2101. doi: 10.1167/iovs.18-24850. [DOI] [PubMed] [Google Scholar]
- 94.Goto E., Tseng S. C. Differentiation of lipid tear deficiency dry eye by kinetic analysis of tear interference images. Archives of Ophthalmology. 2003;121(2):173–180. doi: 10.1001/archopht.121.2.173. [DOI] [PubMed] [Google Scholar]
- 95.Goto E., Dogru M., Fukagawa K., et al. Successful tear lipid layer treatment for refractory dry eye in office workers by low-dose lipid application on the full-length eyelid margin. American Journal of Ophthalmology. 2006;142(2):264–270. doi: 10.1016/j.ajo.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 96.Goto E., Tseng S. C. G. Kinetic analysis of tear interference images in aqueous tear deficiency dry eye before and after punctal occlusion. Investigative Opthalmology & Visual Science. 2003;44(5):1897–1905. doi: 10.1167/iovs.02-0818. [DOI] [PubMed] [Google Scholar]
- 97.Wong S., Srinivasan S., Murphy P. J., Jones L. Comparison of meibomian gland dropout using two infrared imaging devices. Contact Lens and Anterior Eye. 2019;42(3):311–317. doi: 10.1016/j.clae.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 98.Eom Y., Lee J.-S., Kang S.-Y., Kim H. M., Song J.-S. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. American Journal of Ophthalmology. 2013;155(6):1104–1110. doi: 10.1016/j.ajo.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 99.Knop E., Knop N., Millar T., Obata H., Sullivan D. A. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investigative Opthalmology & Visual Science. 2011;52(4):1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jung J. W., Park S. Y., Kim J. S., Kim E. K., Seo K. Y., Kim T.-I. Analysis of factors associated with the tear film lipid layer thickness in normal eyes and patients with dry eye syndrome. Investigative Opthalmology & Visual Science. 2016;57(10):4076–4083. doi: 10.1167/iovs.16-19251. [DOI] [PubMed] [Google Scholar]
- 101.Koh S. Mechanisms of visual disturbance in dry eye. Cornea. 2016;35(1):S83–S88. doi: 10.1097/ico.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 102.Koh S., Maeda N., Kuroda T., et al. Effect of tear film break-up on higher-order aberrations measured with wavefront sensor. American Journal of Ophthalmology. 2002;134(1):115–117. doi: 10.1016/s0002-9394(02)01430-7. [DOI] [PubMed] [Google Scholar]
- 103.Maeda N., Fujikado T., Kuroda T., et al. Wavefront aberrations measured with Hartmann-Shack sensor in patients with keratoconus. Ophthalmology. 2002;109(11):1996–2003. doi: 10.1016/s0161-6420(02)01279-4. [DOI] [PubMed] [Google Scholar]
- 104.Elhusseiny A. M., Khalil A. A., El Sheikh R. H., et al. New approaches for diagnosis of dry eye disease. International Journal of Ophthalmology. 2019;12(10):1618–1628. doi: 10.18240/ijo.2019.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koh S., Maeda N., Hirohara Y., et al. Serial measurements of higher-order aberrations after blinking in patients with dry eye. Investigative Opthalmology & Visual Science. 2008;49(1):133–138. doi: 10.1167/iovs.07-0762. [DOI] [PubMed] [Google Scholar]
- 106.Koh S., Maeda N., Hori Y., et al. Effects of suppression of blinking on quality of vision in borderline cases of evaporative dry eye. Cornea. 2008;27(3):275–278. doi: 10.1097/ico.0b013e31815be9c8. [DOI] [PubMed] [Google Scholar]
- 107.Denoyer A., Rabut G., Baudouin C. Tear film aberration dynamics and vision-related quality of life in patients with dry eye disease. Ophthalmology. 2012;119(9):1811–1818. doi: 10.1016/j.ophtha.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Güell J. L., Pujol J., Arjona M., Diaz-Douton F., Artal P. Optical quality analysis system; instrument for objective clinical evaluation of ocular optical quality. Journal of Cataract & Refractive Surgery. 2004;30(7):1598–1599. doi: 10.1016/j.jcrs.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 109.Artal P., Benito A., Perez G. M., et al. An objective scatter index based on double-pass retinal images of a point source to classify cataracts. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0016823.e16823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan C.-H., Labbé A., Liang Q., et al. Dynamic change of optical quality in patients with dry eye disease. Investigative Opthalmology & Visual Science. 2015;56(5):2848–2854. doi: 10.1167/iovs.14-15757. [DOI] [PubMed] [Google Scholar]
- 111.Benito A., Pérez G. M., Mirabet S., et al. Objective optical assessment of tear-film quality dynamics in normal and mildly symptomatic dry eyes. Journal of Cataract & Refractive Surgery. 2011;37(8):1481–1487. doi: 10.1016/j.jcrs.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 112.Koh S., Maeda N., Ikeda C., et al. Ocular forward light scattering and corneal backward light scattering in patients with dry eye. Investigative Opthalmology & Visual Science. 2014;55(10):6601–6606. doi: 10.1167/iovs.14-15125. [DOI] [PubMed] [Google Scholar]
- 113.Diaz-Valle D., Arriola-Villalobos P., García-Vidal S. E., et al. Effect of lubricating eyedrops on ocular light scattering as a measure of vision quality in patients with dry eye. Journal of Cataract & Refractive Surgery. 2012;38(7):1192–1197. doi: 10.1016/j.jcrs.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 114.Koh S., Maeda N., Ikeda C., et al. Effect of instillation of eyedrops for dry eye on optical quality. Investigative Opthalmology & Visual Science. 2013;54(7):4927–4933. doi: 10.1167/iovs.13-12409. [DOI] [PubMed] [Google Scholar]
- 115.Su T. Y., Ho W. T., Lu C. Y., Chang S. W., Chiang H. K. Correlations among ocular surface temperature difference value, the tear meniscus height, Schirmer’s test and fluorescein tear film break up time. British Journal of Ophthalmology. 2015;99(4):482–487. doi: 10.1136/bjophthalmol-2014-305183. [DOI] [PubMed] [Google Scholar]
- 116.Purslow C., Wolffsohn J. The relation between physical properties of the anterior eye and ocular surface temperature. Optometry and Vision Science. 2007;84(3):197–201. doi: 10.1097/opx.0b013e3180339f6e. [DOI] [PubMed] [Google Scholar]
- 117.Kamao T., Yamaguchi M., Kawasaki S., Mizoue S., Shiraishi A., Ohashi Y. Screening for dry eye with newly developed ocular surface thermographer. American Journal of Ophthalmology. 2011;151(5):782–791. doi: 10.1016/j.ajo.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 118.Tan L. L., Sanjay S., Morgan P. B. Screening for dry eye disease using infrared ocular thermography. Contact Lens and Anterior Eye. 2016;39(6):442–449. doi: 10.1016/j.clae.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 119.Arita R., Shirakawa R., Maeda S., Yamaguchi M., Ohashi Y., Amano S. Decreased surface temperature of tarsal conjunctiva in patients with meibomian gland dysfunction. JAMA Ophthalmology. 2013;131(6):818–819. doi: 10.1001/jamaophthalmol.2013.1895. [DOI] [PubMed] [Google Scholar]
- 120.Cohen Y., Epshtein S., Harris A., Gefen R., Kagemann L., Arieli Y. Tear film imager for dynamic mapping of the human tear film. Applied Optics. 2019;58(29):7987–7995. doi: 10.1364/ao.58.007987. [DOI] [PubMed] [Google Scholar]
- 121.Segev F., Geffen N., Galor A., et al. Dynamic assessment of the tear film muco-aqueous and lipid layers using a novel tear film imager (TFI) British Journal of Ophthalmology. 2020;104(1):136–141. doi: 10.1136/bjophthalmol-2018-313379. [DOI] [PubMed] [Google Scholar]
- 122.Cohen Y., Trokel S., Arieli Y., Epshtien S., Gefen R., Harris A. Mapping the lipid layer of the human tear film. Cornea. 2020;39(1):132–135. doi: 10.1097/ico.0000000000002101. [DOI] [PubMed] [Google Scholar]
- 123.Chan T. C. Y., Chow S. S. W., Wan K. H. N., Yuen H. K. L. Update on the association between dry eye disease and meibomian gland dysfunction. Hong Kong Medical Journal. 2019;25(1):38–47. doi: 10.12809/hkmj187331. [DOI] [PubMed] [Google Scholar]


