Abstract
Flue gas desulfurization gypsum (FGDG) is an industrial by-product generated during the flue gas desulfurization process in coal-fired power plants. Due to its abundance, chemical and physical properties, FGDG has been used in several beneficial applications. However, during the past decade, the rate of beneficially used FGDG has gradually decreased, while its production has drastically increased. The presence of hazardous elements such as arsenic, mercury, cadmium, lead, and selenium in FGDG has reduced its beneficial value. Nevertheless, due to the recent developments in flue gas desulfurization processes, the “modern” FGDG contains lesser amounts of these elements, thus increasing its beneficial value and appeal to be included in other products. Hence, there are novel and traditional FGDG applications in different reuse scenarios investigated recently that have been deemed to pose minimal environmental concern – these need to be better understood. This review summarizes beneficial FGDG applications that have been deemed to pose minimal environmental concern, emphasizing their principles, research gaps, and potential developments, with the aim of increasing the reuse rate of FGDG.
Keywords: Beneficial use, Coal combustion residues, FGDG, Materials management, Sustainability, Materials reuse
1. Introduction
According to the U.S. Energy Information Administration’s statistics, in the 2006–2016 period, approximately 42% of the electricity consumed in the U.S.A was produced by coal combustion (U.S. Energy Information Administration, 2018). The coal combustion process generates various types of residues such as fly ash, bottom ash, boiler slag, flue bed combustion ash, and flue gas desulfurization gypsum (FGDG), that are collectively known as coal combustion residues (CCR). According to the Cross-State Air Pollution Rule (CSAPR), finalized by the U.S. EPA in 2011, SO2 emissions from coal combustion needs to be reduced prior to being released into the atmosphere (U.S. EPA, 2016). This resulted in U.S. coal-fired power plants adopting flue gas desulfurization (FGD) technologies. Consequently, FGDG production rates have increased from ~11 million metric tons (MT) in 2006 to ~29 million MT in 2016 (American Coal Ash Association, 2016). However, this increase in FGDG generation has not been accompanied with a similar increase in its utilization, with utilization rates decreasing from 79% in 2006 to 57% in 2016 (Fig. 1). This has led to FGDG accumulation, creating the need for extra capacity in landfills. To reduce the amounts of disposed FGDG, it is necessary an increase in its utilization rate through new applications.
Figure 1.
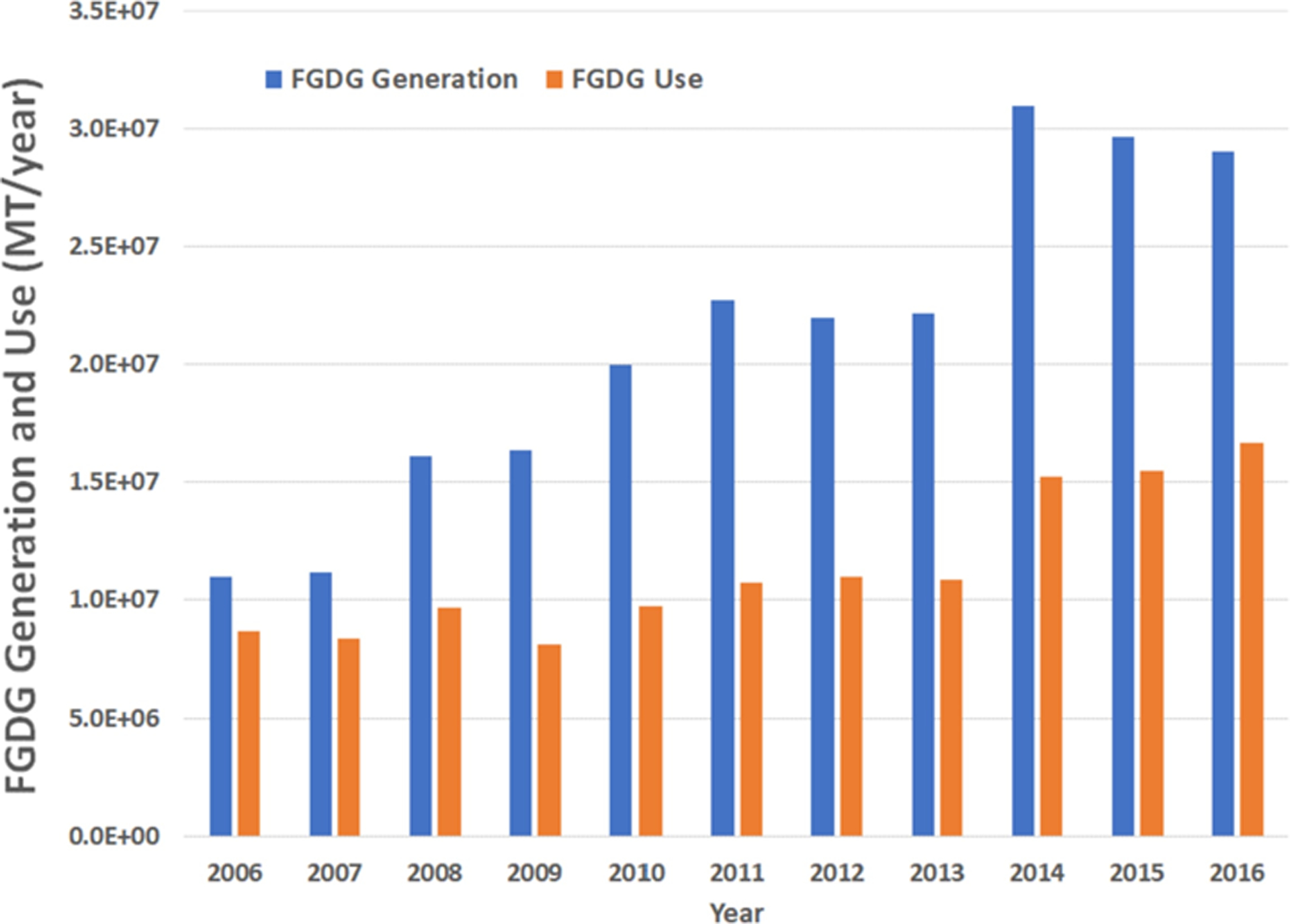
FGDG generation and utilization, data source: ACAA annual reports contained in (American Coal Ash Association, 2018).
Moreover, considering the current trends in power generation, to increase the use of renewable energy sources the future could bring a decrease in coal combustion, which in turn would reduce the FGDG generation and disposal. This would allow FGDG present in landfills and temporary storage locations to be perceived as a valuable source for gypsum for these newly identified applications. In the past, the presence of certain contaminants in FGDG has reduced its beneficial value; but, thanks to recent developments in FGDG technology, the “modern” FGDG is more environmentally friendly. In addition, the utilization of FGDG in a stabilized manner can drastically reduce contaminant transport. Therefore, the major objective of this review is to summarize the recent advances in FGDG processes and beneficial applications thought to have low environmental risk. Specifically, to cover recent research and developments that have modified conventional FGDG applications, such as gypsum panel production and other materials used in the construction industry, agricultural practices, and soil amendments. Novel applications of FGDG in water treatment and the underlying mechanisms are discussed. The gaps in current knowledge and applications are identified. Since there are plenty of studies presenting FGDG applications, we performed a selection based on the novelty of the applications of “modern” FGDG, particularly those that provide details about the environmental risks, and those that might have a potential to be used at industrial scale.
1.1. Flue gas desulfurization processes
The flue gas desulfurization (FGD) processes can be separated into two main categories: once-through and regenerable processes, depending on how the sorbent is treated after SO2 adsorption (Srivastava and Jozewicz, 2001). Once-through, or non-regenerable, processes utilize the sorbent as a beneficial by-product or dispose of it as waste. On the other hand, the regenerable processes release the sorbed SO2 to generate other products such as elemental S, H2SO4, or liquid SO2. Detailed descriptions of all these FGD processes can be found in Pandey et al. (2005) and Srivastava and Jozewicz (2001). Non-regenerable FGD processes, which can be divided into wet (aqueous solutions used to scrub SO2) and dry (dry sorbent injected into the flu gas stream) systems, are summarized in Table 1.
Table 1.
Flue gas desulfurization processes.
| Non-regenerable processes: wet methods | |||||
|---|---|---|---|---|---|
| Flue gas desulfurization process | Sorbent/additives | SO2 removal efficiency | Final product/s | Remarks | Reference |
| Limestone forced oxidation (LSFO) | Limestone | Up to 99% | CaSO4.2H2O | Prevent scaling. Complete oxidation of CaSO3 |
Srivastava et al., 2001, Srivastava, 2000 |
| Limestone inhibited oxidation (LSIO) | Limestone, sodium thiosulfate | ~95% | CaSO3 | Difficult to dewater the waste. Form large crystals of CaSO3 |
Srivastava et al., 2001, Srivastava, 2000 |
| Jet bubbling reactor | Limestone | 90% | CaSO4.2H2O | Provide large liquid/gas interface for SO2 sorption. Lower slurry pH (3.5–4.5), hence 100% of limestone utilization |
Zheng et al. (2003) |
| Lime and Mg enhanced lime | CaO, Mg salts | 99% | CaSO4.2H2O lighter in color (higher value), if desired Mg(OH)2 |
More expensive than limestone processes, but produces high quality gypsum | Srivastava (2000) |
| Dual alkali process | Sodium solution (Na2SO3) for scrubbing, lime | 90% | CaSO4/CaSO3 sludge | By-product contains Na salts, hence needs to be treated before disposal | Mo et al. (2006) |
| Sea water process | Natural sea water | >96% | SO2 dissolved in sea water | Discharged into oceans | Abrams et al. (1988) |
| NH3-based wet desulfurization process | (NH4)2SO3 | 50–60% of SO3 absorption | NH4HSO3 | Higher SO3 absorption | Huang et al. (2016) |
| Non-regenerable processes: dry methods | |||||
| Flue gas desulfurization process | Sorbent/additives | SO2 removal efficiency | Final product/s | Remarks | Reference |
| Lime spray drying | Lime slurry | Up to 98% | CaSO3 | SO2 absorption efficiency strongly depends on the ratio of the water evaporation rate to the absorption rate | Hill and Zank, 2000, Babcock and Wilcox, 2009 |
| In-furnace sorbent injection | Hydrated lime | 27–72% | CaSO4 | The furnace temperature and the residence time are important for proper SO2 sorption | Shemwell et al. (2000) |
| Limestone injection into furnace and activation of unreacted calcium (LIFAC) | Finely pulverized limestone | >80% | CaSO4 | Flue gas temperature, residence time in the furnace, droplet size of water, and residence time in the reactor affect SO2 removal efficiency | Srivastava and Jozewicz (2001) |
| Economizer sorbent injection | Lime | 80% | CaSO3 | Porosity of calcitic hydrate (Ca(OH)2) is important for an effective SO2 absorption | Muzio and Often (1987) |
| Duct sorbent injection | Finely dispersed hydrated lime, NaHCO3 |
50–60% with lime 80% with NaHCO3 85% with lime and NaOH as additive |
CaSO4 | Surface moisture content of lime is important for efficient SO2 removal | Stouffer et al. (1989) |
| Duct spray drying | Slaked lime slurry | >50% | CaSO3 and CaSO4 | Adequate mixing of reactant and resident time are significant for maximum SO2 removal | Murphy et al. (1986) |
| Circulating fluidized bed | Hydrated lime | – | CaSO3 | Semi-dry FGD process | Neathery (1996) |
| Hybrid pollution abatement system (HYPAS) sorbent injection | Dry mixture of lime and recycled solids | Near commercial, but not reported | CaSO4 | Byproducts and remaining fly ash collected in a pulse jet fabric filter | Srivastava and Jozewicz (2001) |
The limestone-based wet scrubbing is one of the most common FGDG methods. The chemical reactions involved can be summarized as follows (Córdoba, 2015):
1.1.1. SO2 in flue gas
SO2(g)↔SO2(aq) [dissolution of SO2]
SO2(aq) + H2O(aq)↔H2SO3(aq) [hydrolysis of SO2]
H2SO3(aq)↔H+(aq) + HSO3−(aq)
HSO3−(aq)↔H+(aq) + SO32−(aq) [acid dissociation]
1.1.2. Dissolution of limestone
CaCO3(s) + H+(aq)↔Ca2+(aq) + HCO3−(aq) [dissolution of limestone]
HCO3−(aq) + H+(aq) ↔ CO2(aq) + H2O(aq) [neutralization]
CO2(aq)↔CO2(g)
An overall chemical reaction of the above processes, which occurred in the FGD system is given below:
SO2(g) + CaCO3(s) + 2H2O(aq) = CaSO3·2H2O(aq) + CO2(g)
The produced calcium sulfite (CaSO3·2H2O) is then oxidized, resulting in FGDG.
CaSO3·2H2O(aq) + 1/2O2(g)↔CaSO4·2H2O(s)
Natural oxidation processes may occur in desulfurization systems, depending on the pH of the limestone slurry, and the SO2 and excess air content of the flue gas. In this case, a mixture of CaSO3·1/2H2O and CaSO4·.2H2O is produced as the by-product. But, under forced oxidation CaSO4.2H2O is dominant (>90%) (Córdoba, 2015).
The efficiency of the FGD process is determined by the SO2 sorption rate. Several additives, such as MgO, Na2CO3, and organic acids, are used to increase the SO2 removal efficiency (del Valle-Zermeño et al., 2015, Frandsen et al., 2001, Gao et al., 2011). The dissolution and adsorption of SO2 largely depend on the pH of the solution. At high pH, both the dissolution and the adsorption of SO2 increase and vice versa at low pH (Seo et al., 2015). The addition of MgO and Na2CO3 provides alkalinity to the limestone slurry increasing the liquid-phase mass transfer.
Usually, limestone (CaCO3) and lime (CaO) are used as Ca(OH)2 sources thanks to their abundance and low cost. Due to its low solubility, limestone needs to be pulverized in order to be used in the FGD process, adding cost and energy expenditure to the process. Kikkawa et al. (2002) tried to scrub SO2 from FGD without limestone size reduction. Beyond the energy savings, this maintained a large size difference between the resulting gypsum and limestone, making it easier to separate the two. However, the use of granular limestone made a neutralization column necessary to ensure the rubbing of the limestone particles in it. This kept the limestone surface fresh for reactions, maintaining a 90% SO2 removal efficiency, even in the presence of aluminum ions.
Liu et al. (2009) introduced a method to increase the dissolution rate of limestone, thereby increasing SO2 adsorption without any size reduction, using acetic acid in the system. They observed an increase of SO2 removal from 60.7% (without acetic acid) to 93.5% with 30 mmol/L of acetic acid, but the quality of the resulting gypsum with acetic acid addition was not reported.
Besides the wet-limestone FGD system, there are other desulfurization FGD systems that have been proposed and used: sea water, dual alkali, NH3, dry or semi-dry systems, spray dry, furnace sorbent injection, duct sorbent injection, and circulating fluid bed dry scrubber (Córdoba, 2015). In every case, the quality of the obtained FGDG dependens upon the utilized materials and the conditions of the chemical reactions taking place in each process.
1.2. Composition of FGDG
The major component of FGDG is CaSO4 but other elements, such as Mg, K, Cl, F, B, Al, Fe, Si, and Se, may be present. Furthermore, some toxic elements, such as As, Hg, Cd, and Pb, could be present in trace amounts. The quantities of these trace elements depend on the composition of the combusted coal and the limestone, lime, and other additives used in the FGD process. For example, Na salts used as additives can increase the Na content of the final FGDG. Natural limestone typically contains some impurities (e.g. Mg, Si, and Al) that affect the FGDG composition. Nevertheless, the concentration of hazardous elements in the coal itself greatly influences their concentrations within FGDG. In Table 2 we compared the reported composition of the “modern” FGDG with the “old” FGDG. The “modern” FGDG is represented by the composition of the FGDG generated by 8 power plants in the U.S. from 2007 to 2010 as reported by Kost et al. (2018). The “old” FGDG is represented by samples collected from 1991 to 1992 from 59 power plants in the U.S. found in Kost et al. (2005). The comparison showed that “modern” FGDG contained lower amounts of several elements considered as contaminants for beneficial use purposes, such as As, Cd, Co, Cr, Cu, and Pb.
Table 2.
Comparison of the elemental composition of “modern” and “old” FGDG found in literature.
| Element | “Modern” FGDGa | “Old” FGDGb | ||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| mg/kg | mg/kg | mg/kg | mg/kg | |
| Al | 653 | 140–2,120 | 13,700 | 6,000–28,000 |
| As | 2.17 | 1.35–2.99 | 74.7 | 5.4–213 |
| B | 11.1 | 2.24–42.4 | 145 | 68–302 |
| Ba | 58.6 | 6.91–123 | n.r. | n.r. |
| Ca | 198,000 | 160,000–243,000 | 312,000 | 241,000–412,000 |
| Cd | 0.40 | 0.08–1.12 | 2.3 | 0.5–3.9 |
| Co | 0.96 | 0.312–2.22 | 8.9 | 3.9–27.3 |
| Cr | 5.67 | 1.8–13.2 | 16.9 | 11.7–25.3 |
| Cu | 0.70 | <0.378–3.25 | 177 | 16.5–913 |
| Fe | 697 | 334–1,230 | 16,000 | 7,000–27,000 |
| Hg | 0.54 | 0.198–1.33 | <5 | <5 |
| K | 393 | 183–700 | 1,200 | 800–1,500 |
| Li | 54.8 | 9.18–268 | 11.4 | 5.2–20.0 |
| Mg | 2,060 | 600–7,430 | 91,800 | 3,800–162,000 |
| Mn | 31.3 | 0.97–160 | 207 | 63–625 |
| Mo | 1.65 | 0.59–5.58 | 8.6 | <0.02–25.3 |
| Na | 307 | 36–577 | n.r. | n.r. |
| Ni | 2.19 | 0.884–5.02 | 33 | 16.4–58.2 |
| P | 94.3 | 22.1–272 | 141 | 59–235 |
| Pb | 1.59 | 1.33–1.84 | 11 | 5.0–28.0 |
| S | 177,000 | 143,000–209,000 | 126,000 | 82,000–183,000 |
| Sb | 8.04 | 4.57–10.9 | n.r. | n.r. |
| Se | 8.25 | 2.92–18.8 | 4 | 2.3–4.6 |
| Si | 644 | 176–1,230 | 25,000 | 20,000–34,000 |
| Sr | 163 | 67–269 | 308 | 98–895 |
| Tl | <1.44 | <1.44–2.8 | n.r. | n.r. |
| V | 7.22 | 1.38–29.8 | 20 | 0.0–41.6 |
| Zn | 14.5 | 4.26–29 | 163 | 55–389 |
n.r.: nor reported.
Source: Kost et al. (2005) as reported by Koralegedara et al. (2017).
Source: Kost et al. (2018).
Several studies reported the partitioning of Hg, As, Se, B, and Cl in the coal combustion process followed by the FGD process (Cheng et al., 2009, Córdoba et al., 2012). According to these studies, the majority of Hg (90%) and a significant portion of Se (30%) are associated with FGDG; whereas the majority of As (88%) and Se (56%) are associated with electrostatic precipitator ash or with fly ash. The major portions of the more soluble B (>90%) and Cl (>90%) are usually accumulated in the FGD wastewater. Hg speciation in FGDG was found to contain Hg(0), Hg(I), and Hg–Fe compounds (Al-Abed et al., 2008, Beatty et al., 2012, Rallo et al., 2010, Sun et al., 2014). Al-Abed et al. (2008) reported the major Se species in FGDG was Se(IV) and the major fraction of As was As(V) associated with amorphous Fe and Mn oxides. Higher F leaching from CCRs, including FGDG, was reported by Álvarez-Ayuso and Querol (2007). In a separate study, Álvarez-Ayuso et al. (2006) used amorphous Al-oxides to reduce F leaching from FGDG after being disposed in landfills. Córdoba et al. (2012) reported that a large portion of F (74%) comes from the flue gas, with a significant fraction of it (16%) originated from limestone. A fraction of F (91%) in FGDG was found to be CaF2.
Since the composition of coal varies according to its region of origin (Falcon and Ham, 1988), the composition of the FGDG produced in different regions could be significantly different. Besides, the FGD process can impact the characteristics and the trace elements content of all associated CCRs (Skousen et al., 2012). For example, Liu et al. (2013) stated that a high Hg content and a low Cl− content are indicative of FGDG produced in China; while Skousen et al. (2012) reported that ashes from the Midwest and Western USA tend be alkaline.
2. Beneficial uses of FGDG
According to the annual report of the American Coal Ash Association (ACAA), a total of 29 million MT of FGDG were produced in 2016 (American Coal Ash Association, 2016). Due to its abundance, purity, chemical and physical properties, and similarities to natural gypsum, FGDG is being used in several beneficial applications in two different forms: encapsulated and non-encapsulated. The most common beneficial use of FGDG is in wallboard (i.e., drywall, consisting mainly of dihydrous calcium sulfate) production. FGDG can be used as an ingredient in cement, in the production of asphalt, and as a set retarder in concrete and grout. The primary land application of FGDG is its use as a soil amendment and as a fertilizer in agriculture. Furthermore, FGDG is used as a structural fill in embankment constructions. According to the cited American Coal Ash Association, 2016 annual report, 54% of the total FGDG was used in drywall production and 8% in cement/concrete/asphalt production (Fig. 2). Moreover, 17% of the total FGDG production was used in terrestrial applications such as structural filling, agriculture, and mine reclamation. Despite these beneficial applications, around 43% of the total FGDG production was disposed of in landfills. However, due to the limited space available in landfills nationally, it is of a great importance to identify novel re-use applications of FGDG to prevent its disposal and its eventual accumulation in landfills.
Figure 2.
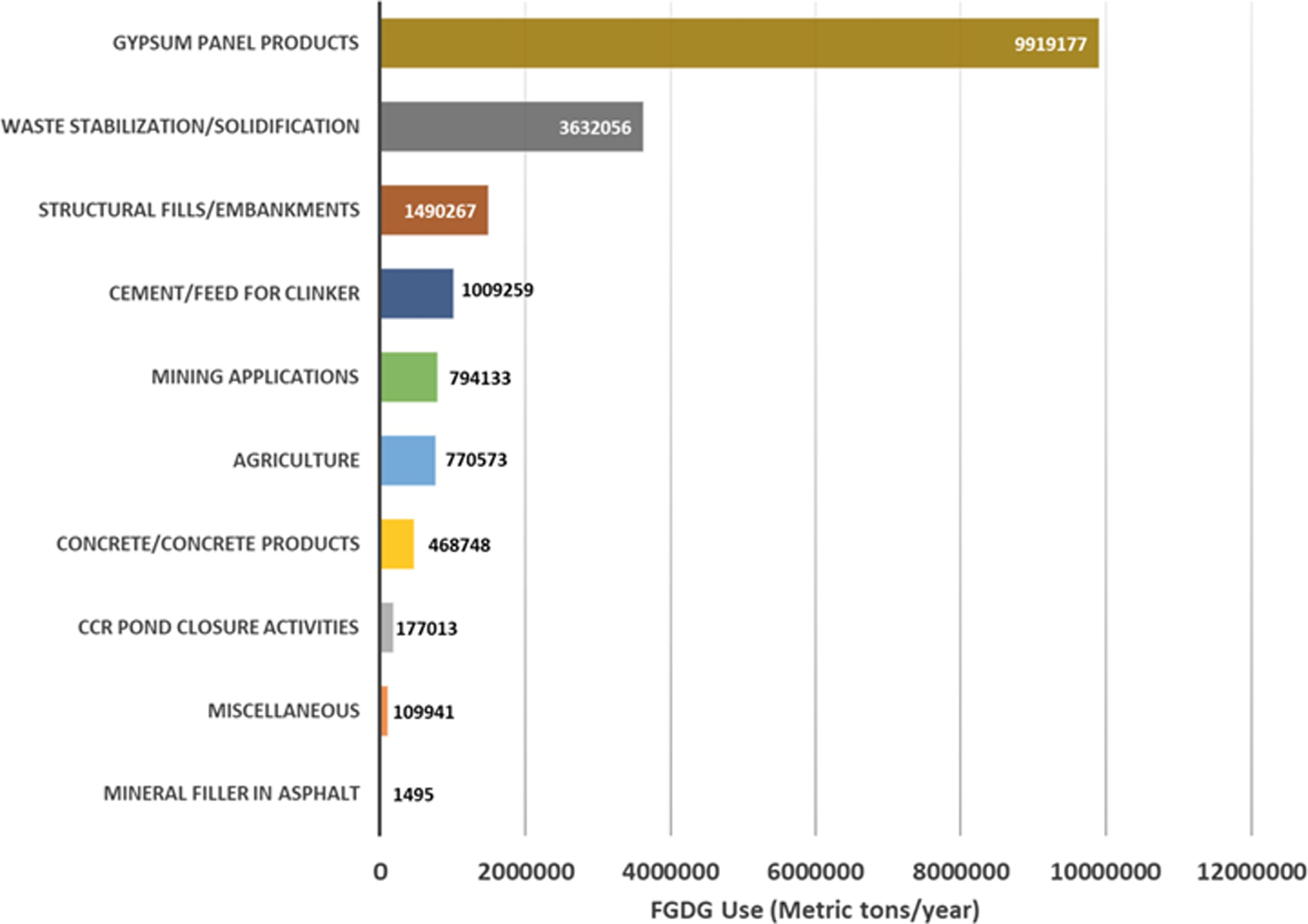
Most common applications of flue gas desulfurization gypsum (ACAA annual report 2016).
2.1. Recent advances in beneficial applications
2.1.1. Material synthesis
2.1.1.1. Wallboard production
Even though FGDG has been used in the wallboard production industry for decades, research aiming at improving the quality of the product, at the environmental impact of the use of FGDG in these boards, and on different methods to use the FGDG, is still in progress. Currently, for FGDG to be incorporated in wallboards, it must fulfill these basic requirements: 1) Over 92% of CaSO4·2H2O content, 2) Moisture content <10%, 3) CaSO4·.1/2H2O content should be less than 0.5%, 4) Cl− content <200 mg/kg, 5) the total content of K+, Na+ and Mg2+ should be less than 0.06% (Han et al., 2014).
Recent studies have mainly focused on producing gypsum boards with increased fire resistance, water resistance, and low density. The production of multi-layer thermal insulating boards using coal combustion by-products was patented in 2003 by Stache and Kahl (2003). Recently, Leiva et al. (2010) and Li et al. (2015a) have compared the physiochemical properties of gypsum boards with different compositions, including a 100% FGDG composition. According to Leiva et al. (2010), gypsum panels of 100% FGDG passed the European standards for physiochemical (density, moisture content, water adsorption capacity, resistance to sulfuric acid attack) and mechanical properties (compressive and bending strength, surface hardness and impact resistance) for commercial gypsum panels. Interestingly, data from this study revealed the heat insulating capacity of 100% FGDG panels is higher than that of the commercial gypsum panels. Meanwhile, Li et al. (2015a) reported higher insulating capacity of 100% FGDG panels compared to the gypsum panels manufactured with different proportions of fly ash and FGDG, attributing the difference to the high free water content of FGDG.
Gypsum blocks are another type of construction material in which FGDG is being used as a replacement of natural gypsum. In contrast with the FGDG boards, FGDG-containing blocks have poor water resistance, which reduces the benefit of using FGDG in the blocks. Nevertheless, recent investigations showed an increase in the water resistance of gypsum blocks made by FGDG using granulated blast furnace slag (Zhao et al., 2012), high calcium fly ash (Du et al., 2014, Zhao et al., 2012), and different water-resistant additives, such as organic emulsions (Li et al., 2013).
2.1.1.2. Concrete/cement and asphalt production
Natural gypsum can be used in concrete production as a replacement for calcium aluminate cement (CAC), opening the possibility of replacing the natural gypsum with FGDG in this application with success, even though some studies have reported a reduction of compressive strength and water solubility when compared to conventional cement mixtures (Wansom et al., 2019), other researchers have reported similar properties (Tzouvalas et al., 2004), while others used activators and epoxy resins to counteract these reductions (Liu et al., 2019). Tzouvalas et al. (2004) reported that cement produced from natural and synthetic gypsums showed similar compressive strengths, suggesting that FGDG can replace natural gypsum in concrete production, but found a 1-h delay in the initial setting time of the concrete with FGDG compared to that with natural gypsum. Still, Glasser and Zhang (2001) described several benefits of the addition of FGDG as a replacement of CAC in concrete production: cost reduction, early strength increment, prevention of strength decline at later ages, shrinkage reduction, and sulfate corrosion resistance improvement. Guan et al. (2009) tested different FGDG/CAC ratios and concluded that the addition of FGDG shortened the setting time, which is favorable in construction. Guo et al. (2009) combined FGDG with type-G slag powder and with ordinary slag powder to produce several concrete mixtures. The mixtures with the G-slag powder had higher compressive strength, resistance to chloride, and gas permeability than the mixtures produced with ordinary slag. Xu et al. (2017) tested the use of FGDG in the preparation of calcium sulfoaluminate cement resulting in higher strengths when the pastes were cured at 40 °C, but lower strengths at 0, 10, and 20 °C. It is relevant to point out that prior to its use in cement, FGDG needs to be dehydrated and crystalized and the heat involved in this dehydration process directly affects the cement properties. This effect was studied by Yue et al. (2012) and Yang et al. (2013). Both studies concluded that 1) concrete setting time decreases as the heat treatment temperature of FGDG increases and 2) cement produced with FGDG calcined at 200 °C and with a 3.5% content of SO3 showed the maximum strength.
The FGDG incorporated cement is not only used in building construction but in ecosystem conservation projects, as well. Collins et al. (1994), reported the use of FGDG in stabilized cement to build artificial coral reefs with the incorporation of the Coal Waste Artificial Reef Program (CWARP). In this program, several epifauna and flora developments on the artificial reefs constructed in Pool Bay on the south coast of England were observed and the authors reported no environmental concerns of toxic elements being released into the ecosystems. Liu et al. (2019) used FGDG, along with fly ash, Portland cement, steel slag, and a lightweight coarse aggregate to produce foamed concrete, finding that the final product had better flexural strength, compressive strength, heat preservation performance and water resistance when epoxy resin was added. Zhang et al. (2019) used ground blast furnace, basic oxygen furnace steel slag, and FGDG to make a cement mixture to stabilize arsenic from mine tailings, the performed leaching tests determined that the mixture was environmentally acceptable. The prevailing mechanisms for arsenic stabilization was solidification in double salt minerals, where some arsenic ions may enter the crystal lattice by ion exchange.
2.1.1.3. Other construction materials
There are several applications that aim at the improvement of existing construction materials with the use of FGDG and there are other novel construction materials that could use FGDG as a constituent. Wu et al. (2019) added silicate clinker to increase the strength and water resistance of FGDG blocks. The particle size and the amount of silicate clinker added affected the block properties. It was shown that FGDG, calcium silicate, and ettringite produced FGDG crystals through a hydration process improving the performance of the block as a construction material. Wu et al. (2018) used lightweight FGDG to add lateral stiffness and shear strength to cold-formed steel framing wall, reporting that the gypsum-filled specimens allowed an increase in load of 1.72–2.54 greater than the specimens unfilled. Zhong et al. (2012) produced a series of mortars using composite binder made with uncalcined FGDG, fly ash, and ground granulated furnace slag. The mortars containing FGDG had lower drying shrinkage (563–938 micro strain) than the mortars prepared without the FGDG.
2.1.1.4. CaCO3 production
Many different procedures have been used to produce CaCO3 from FGDG:
a) As a by-product of the formation of elemental sulfur from FGDG (de Beer et al., 2015):
CaSO4·2H2O (s) + 2C (s) → CaS (s) + 2 CO2 (g) + 2H2O (l) [thermal reduction process]
CaS (s) + H2O (l) + CO2(g) → H2S (g) + CaCO3 (s) [carbonation process]
2H2S (g) + O2(g) → 2S (s) + 2 H2O (l) [recovery of elemental sulfur]
Even though this process produced high quality elemental S, the produced CaCO3 reached a maximum purity of 90% (de Beer et al., 2014).
b) Using atmospheric CO2
Lee et al. (2012) showed the formation of CaCO3 using NH4OH and atmospheric CO2 according to the reaction:
CaSO4·2H2O (s) + CO2 (g) + 2NH4OH (l) → CaCO3 (s) + (NH4)2SO4 (aq)
The generated CaCO3 had purity >90% at room temperature and atmospheric pressure conditions. The observed carbonation rate was 95% with a CO2 supply of 4 L/min. It is important to note that vaterite, another polymorph of CaCO3, was produced. Since the reaction occurred under atmospheric conditions, this procedure could be performed on-site, where the FGDG is generated. The resulting CaCO3 can be reused in the flue gas desulfurization process, while (NH4)2SO4 could be used as fertilizer; thus, this technique could provide sustainable waste management for coal-fired plants. Ding et al. (2015) confirmed the formation of high purity CaCO3 (≥99%) from FGDG using atmospheric CO2, with a maximum CO2 sequestration of 373 kgCO2 tresidue−1. In addition to the formation of CaCO3 as a by-product of CO2 sequestration, the use of FGDG for the direct synthesis of (NH4)2SO4 was reported by Chou et al. (2005). Lee et al. (2012) found that the optimum temperature of the CO2/N2 mixture for maximum carbonation efficiency (96%) was 40 °C.
Beyond controlling the atmospheric CO2 level, the formation of CaCO3 increases the FGDG market value. Song et al. (2015) synthesized different CaCO3 polymorphs using FGDG under different reaction conditions. Under the CO2/NH3 stoichiometric ratio of 2 and in the presence of FGDG, calcite crystals with rhombohedral morphology formed; whereas under excess NH3 conditions, spherical vaterite crystals formed. With an excess of NH3 and 30–50% (v/v) ethanol, the formation of peanut-shaped (dual-lobe ellipsoid) aragonite crystals was reported. The formation of CaCO3 and its polymorphs using FGDG helps to reduce the use of natural CaO and CaCO3 ores in CaCO3 production. Wang et al. (2019) investigated the synthesis of CaCO3 injecting CO2 into a solution containing FGDG and ammonia. The generated CaCO3 was a mixture of vaterite (~60%) and calcite (~40%) concluding that the process could be feasible at larger scale, but there are still several variables to be further studied.
2.1.1.5. Production of calcium sulfate
The use of FGDG to produce calcium sulfate hemihydrate is a common practice and its applications include architecture and design, medicine, ceramics, molding and construction, often requiring high temperatures processes and further purification (Sumner, 1993). Lu et al. (2016) used sulfuric acid in a method called “atmospheric acid solution” to decolor gypsum by controlling the crystallization of the gypsum and the dissolution process. Calcium sulfate gypsum of 98% purity was obtained after three consecutive cycles of purification. Guan et al. (2011) prepared α-calcium sulfate hemihydrate (α-HH) from FGDG in pilot-scale and bench-scale tests obtaining α-HH with a purity of 95% taking 3.5–6.0 h at 94 °C. The authors concluded that the operation could be feasible at industrial scale. Calcium sulfate hemihydrate (CSH) whiskers, a different calcium sulfate product, have low solubility and superior workability as fillers or as reinforcing agents for construction, medicine, and papermaking applications. Miao et al. (2015) synthetized high purity CSH whiskers using a CaCl2 solution at low temperature and atmospheric pressure without applying any further purification. With increasing reaction time, the amorphous calcium sulfate dihydrate gradually changed to short rods, probably due to dissolution and reprecipitation. After 2.5 h, the whiskers started to form. The optimum conditions for the process were 102 °C, 10% of calcium sulfate dihydrate in solution, 1.5% concentration of H2SO4, and 3.5 h of reaction time. The impurities of the FGDG had little impact on the retention time and morphology of the formed whiskers.
2.1.2. Land applications
One of the most important and widely used land applications of FGDG is as a soil amendment. As such, FGDG is used to improve soil quality, water infiltration, porosity, and particle aggregation. Due to its chemical composition, FGDG is used also as fertilizer. Baligar et al. (2011) reviewed the land applications of FGDG, hence only the most recent advances of beneficial land applications are included in this review.
2.1.2.1. Soil amendment
The use of FGDG as a soil amendment is well known and has been investigated thoroughly over the past few decades (Baligar et al., 2011, Chi et al., 2012, Chun et al., 2001, Dick et al., 2006, Sakai et al., 2004). The most recent studies have mainly focused on amending the sodic soils, specifically in coastal areas (Huang et al., 2013, Li et al., 2015b, Torbert and Watts, 2014, Wang et al., 2013, Yu et al., 2014, Yu et al., 2015). Poor water drainage is the primary cause for soil salinization. However, in coastal areas, saline water intrusions by tidal currents naturally increase the soil salinity. The adsorption of Na in soil colloids depends on how the Na enters the soil. For example, soil colloids easily adsorb Na from NaHCO3 and Na2CO3 compounds but not from NaCl (Yu et al., 2014). Soil desalination can be achieved by irrigation leaching, phyto-desalination and subsurface pipe drainage systems (Li et al., 2015b). FGDG plays a major role in soil desalination due to its high Ca2+ content, which can easily replace the exchangeable Na+ in soil. The dissolution-exchange reaction in soil colloids can be expressed as:
NaX + CaSO4 → CaX + Na+ +SO42−
where X is the exchangeable form of Na compounds.
The ratio of Na:(Ca + Mg) in the soil solution is critical to the desalination process. Once the Na+ ions in the soil colloids are replaced with Ca2+, the resulting aggregation creates larger soil particles, increasing soil porosity and producing enhanced water infiltration (Baligar et al., 2011).
Even though the coastal soil Na+ content is relatively high compared to inland agricultural soils, recent research proved that FGDG can be successfully used to reclaim coastal plains. Li et al. (2015b) observed a 50% reduction of exchangeable Na+ in top soil in a tidal land after one year of the FGDG amendment. Huang et al. (2013) concluded that a combination of FGDG, humic acid, and polyacrylamide was effective in increasing Na leaching and enhanced plant growth as compared to non-treated soil. Nevertheless, this combination could be harmful because acrylamides have been deemed as neurotoxins (Lapin et al., 1982, Prasad and Muralidhara, 2018). Kost et al. (2018) compared the results on crop yields, chemistry of the soil, plant tissue properties and vadose water chemistry of the use of FGDG and mined gypsum as soil amendments in a total of 10 different studies from literature. Mined gypsum typically had higher content of K, Na, Mg, and Sr than FGDG. The soil content reported higher concentrations of Ca, S, and Sr on the amended soils, along with no significant changes in As, Se, and Hg. The vadose water contained higher concentrations of several elements with FGDG than with mined gypsum, but As and Se were reported below detection limits and Hg had the same concentration as the control without amendment. The authors also reported an increase in crop yields with the sustained application of the amendment for a minimum of three to five years and an environmental benefit due to the reduction of soluble P losses from the treated fields.
Srisomang et al. (2015) mixed leonardite, FGDG, clay, and sawdust to produce a ceramic mixture to be baked at 650 °C to serve as a ceramic product for plant growth, finding it environmentally safe and suitable for plant growth.
Phosphorus runoff from agricultural lands generates phosphorus contamination in soils and FGDG could help stabilizing them. The application of FGDG reduced the water-soluble phosphorus concentrations in soils with greater soil test (P = 0.0002) and lower cation exchange capacity (P = 0.0087). In other sites where the same technique was tested, the soluble phosphorus was not reduced (Sindelar and Wolkowski, 2019). Torbert et al. (2018) added different concentrations of FGDG and poultry litter to test the response of bermudagrass pasture, soil, and runoff to the amendment. The authors concluded that the application of FGDG may reduce the negative consequences of adding animal manure as amendment because the migration of toxic elements did not increase, while P, As, and Fe migration with the runoff was reduced even when poultry litter was applied to the soil.
2.1.2.2. Fertilizer
The main purpose of the use of FGDG as a fertilizer is to provide Ca and S for plant growth. In addition, Mg, K, and Se are available in FGDG in significant amounts. Even though excess amounts of Se can be toxic, it is considered a dietary essential (Ammerman and Miller, 1975). To prevent Se deficiency in ruminant animals, specifically cows, goats and sheep, fertilizers containing Se can be added to the food sources (grass fields) of these animals, particularly in dairy farms.
The effect of FGDG on crop growth and yield, including crops that require high Ca2+ and S, such as peanuts, tomatoes, cantaloupes, alfalfa and soybeans, have been studied by Baligar et al. (2011). Recently, DeSutter et al. (2014) grew wheat in FGDG amended soils and reported no significant impact on yield. Similarly, mixed effects of FGDG amendment on corn and hay yields were reported by Kost et al. (2014). According to their results, both corn and hay yields increased as the FGDG application rate increased (20 Mg/ha), but low (0.2 Mg/ha) and intermediate (2 Mg/ha) application rates had no significant difference with the control treatment (no FGDG).
2.1.2.3. Reduction of soil erosion and eutrophication
Surface water bodies and associated sediments are the destination for many contaminants present in the environment. Excess nutrients in surface waters generates excessive plant and algae density, resulting in a lack of oxygen known as “eutrophication”. This has become one of the major problems in surface water sources around the world. The increased use of fertilizers containing PO43− and NO3− and soil erosion due to anthropogenic activities have elevated the number of eutrophicated water sources. There has been extensive research reporting the efficacy of FGDG in reducing surface runoff, increasing infiltration, and decreasing sediment transportation by decreasing surface sealing/crusting (Endale et al., 2014, Norton, 2011, Torbert and Watts, 2014, Truman et al., 2010), which indirectly helps to prevent eutrophication. The elevated Ca2+ content in FGDG amended soil facilitates the flocculation of clay particles and, thereby, increases the porosity of the soil. The improved soil structure with higher porosity increases the water infiltration and reduces surface runoff and sediment transportation, hence preventing eutrophication.
Likewise, FGDG has been demonstrated to reduce the P and N release from soil. For instance, Seshadri et al. (2014) reported a reduction in P leaching in CCR-amended soil (including FGDG) mainly due to adsorption and precipitation. The peak efficiency in P retention was observed at low and neutral pH. The pH increment and the high Ca content in the CCR-amended soil are the main factors contributing to an effective P retention in soil allowing Ca3(PO4)2 to precipitate. Bryant et al. (2012) used FGDG to construct a ditch filter on a poultry farm to remove dissolved P from the drainage, concluding that bare Al and Fe from the CCRs acted as P sorbents. During a 3.6 year study period, they observed 22% removal of total dissolved P in the drainage with the FGDG filter. Recently, Chen et al. (2016) compared coarse and nano-sized gypsum P removal efficiency. Due to the higher surface area, greater solubility, and better contact with soil, nano-gypsum showed a higher P removal rate. The authors presumed the formation of calcium-phosphate complexes (Ca2HPO4(OH)2, CaHPO4.2H2O, Ca4H(PO4)3.3H2O), Ca3(PO4)2, Ca5(OH)(PO4)3) to be caused by the interaction between orthophosphate and the Ca2+ released from gypsum.
2.1.2.4. Mine reclamation
Abandoned coal mine lands are a worldwide environmental concern as the heavy metal leaching from these abandoned mines can greatly affect surface and groundwater quality. Mine soils typically have lower organic matter and nutrient content, higher Fe-oxides and toxic metals, lower pH, and poorer water holding capacity than native soils (Chi et al., 2012). The main processes involved in mine soil metal stabilization are: raising the pH of the soil, increasing its organic matter content, improving the soil structure, and increasing the water holding capacity. The usage of top soil and limestone to treat mine lands is a well established technique (Ziemkiewicz et al., 1997). In addition, composting provides organic matter and nutrients, which improves the physical and chemical properties of mine soil (Bagatto and Shorthouse, 2000, Sydnor and Redente, 2002). Several people have used coal combustion by-products in mine reclamation (Park et al., 2014, Skousen et al., 2012). The alkaline nature of CCRs neutralizes mine drainage and acidic soil, thereby decreasing metal solubility by precipitating metals as hydroxides. Fly ash, the CCR with the highest alkalinity, has been successfully used in mine reclamation (Gitari et al., 2008a, Gitari et al., 2008b, Ram and Masto, 2010). Even though FGDG is not as alkaline as fly ash, it has been investigated for mine soil reclamation in several studies (Chen et al., 2013a, Chen et al., 2013b, Chen et al., 2015a, Liu and Lal, 2013). Liu and Lal (2013) performed a comparison study using different materials (e.g. FGDG, fly ash, biosolids, and zeolites) to amend mine soil. According to their results, the FGDG amendment had the highest pH increment, whereas the highest water holding capacity was associated with the biosolid amendment. The highest seed germination and shoot elongation of lettuce was reported in soil with an FGDG amendment, indicating that FGDG is suitable for mine soil reclamation. The long-term effect of the application of FGDG, alone and mixed with compost, on coal mine reclamation was studied by Chen et al. (2013b). The chemical, physical, and biological properties of coal mine soil after FGDG treatment were evaluated in periods of 1–17 years after the amendment. According to this study, FGDG alone increased the mine soil pH from 3.1 to 6.9 after one year and maintained a ~6.4 pH after 15 years of the amendment. The authors reported a reduction of extractable Pb, P, and Ba in FGDG amended mine soil due to the formation of insoluble precipitates PbSO4, Ca3(PO4)2, and BaSO4, respectively. In a separate study, Chen et al. (2013a) reported increased bacterial population and diversity in mine soil after 16 years of the FGDG amendment. Recently, Chen et al. (2015a) reported the effect of FGDG addition on the runoff and tile flow water quality associated with a reclaimed mine soil. A sustained pH > 7 in surface runoff and a pH > 5 in tile flow drainage were observed in FGDG-added mine sites. The authors confirmed the presence of RCRA regulated elements in levels below the regulated limits in both surface runoff and tile flow water. In conclusion, FGDG alone, or mixed with compost, could be an effective material for long-term remediation of abandoned mines, improving re-vegetation and water quality of reclaimed areas.
2.1.2.5. Remediation of heavy metal contaminated soil
FGDG has been used for several remediation applications that were studied by Wang and Yang (2018) who presented the mechanisms and effects of the application of FGDG in degraded soils (Fig. 3). Some of the experiences of different applications and the remediation on the chemical and biological activities of soil can be found below.
Figure 3.
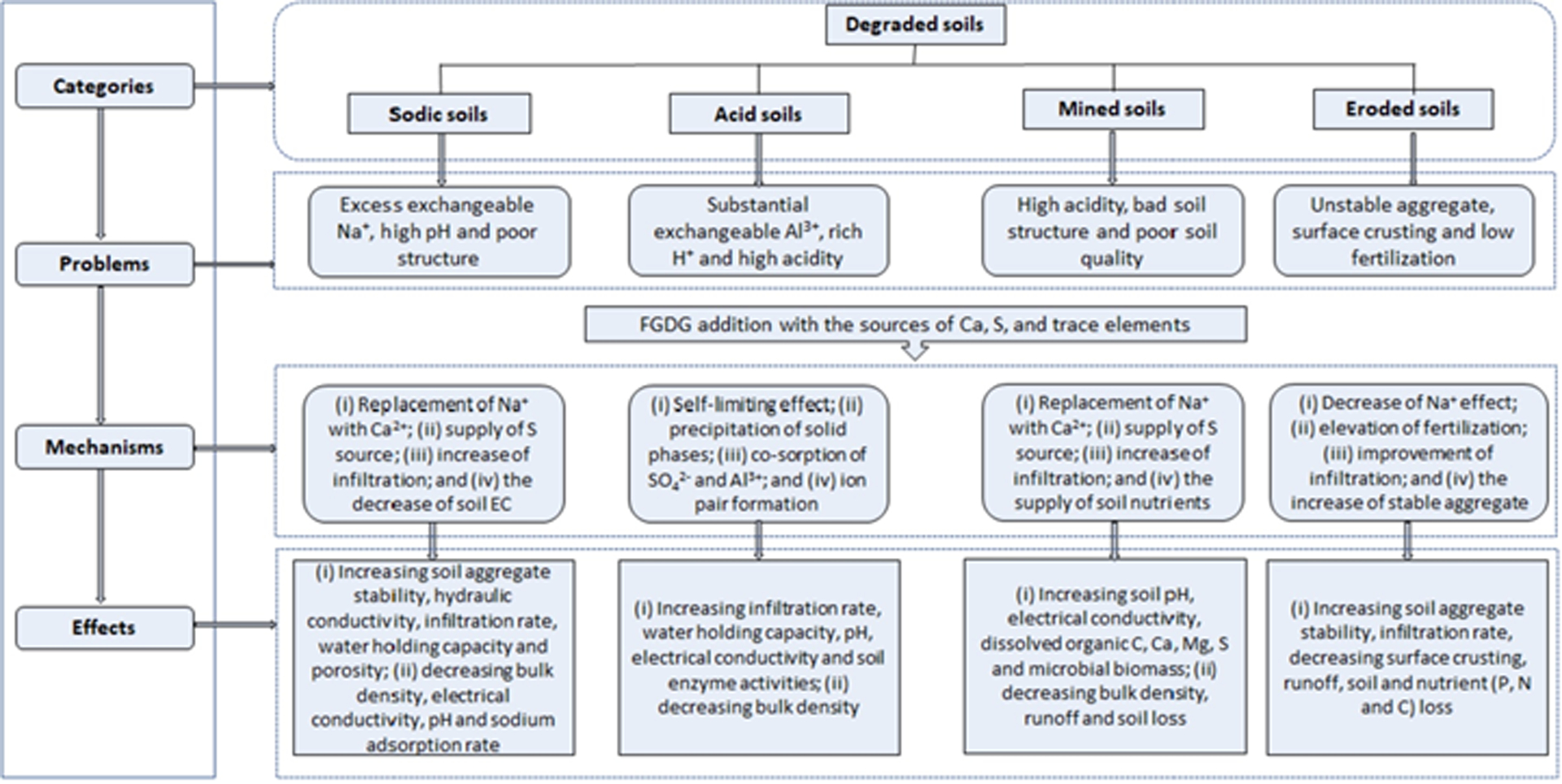
Mechanisms and effects of FGDG remediation in degraded soils, reproduced with modifications from Wang and Yang (2018).
a) Experiences with applications
Industrially contaminated soil is another significant environmental problem. Many industrial processes and anthropogenic activities contaminate soil with harmful substances, including toxic metals. Arsenic (As), lead (Pb), and cadmium (Cd) are some of the most common inorganic soil contaminants. The main mechanisms involved in stabilization of contaminated soils include precipitation, adsorption, and complexation. The increase of soil pH is a common remediation method as contaminant leaching mostly occurs under acidic pH. A novel trend for contaminated soil remediation is the use of waste materials to increase the soil pH, which reduces the amount of waste to be disposed. Fly ash and bottom ash have been used to stabilize contaminated soil (Dermatas and Meng, 2003, Gu et al., 2013, Houben et al., 2012, Querol et al., 2006). However, the use of FGDG in metal stabilization of contaminated soils is uncommon. Recently, González-Núñez et al. (2015) reported that a mixture of calcite and FGDG was successful in remediating soil contaminated with pyritic minerals. They found that the FGDG-calcite mixture increased both the soil pH from 2.5 to 6.9 and the acid neutralizing capacity (ANC) from −86 to 1,513 meq/kg, which, in turn, reduced the extractable concentration of As and metals. Interestingly, the authors reported that FGDG alone is not as effective as the FGDG-calcite mixture. This is probably due to the low pH of the soil, which required a material with a higher ANC than FGDG.
A recent study by Koralegedara et al. (2017) showed the effectiveness of FGDG in reducing Pb leaching under different scenarios. The formation of insoluble Pb minerals with the aid of the sulfate released from FGDG (such as anglesite (PbSO4) under acidic pH and leadhillite (Pb4SO4(CO3)2(OH)2) under neutral pH) was reported in this study. Moreover, it was found that Pb sorption increased in the presence of FGDG as the released sulfate released allowed Pb to sorb onto ferrihydrite ((Fe)2O3·1/2H2O) by the formation of ferrihydrite-Pb-SO4 ternary complexes. Furthermore, the decrease of Pb sorption onto humic acid in the presence of FGDG was verified using XAS analysis. Chen et al. (2015b) reported an important level of Pb stabilization in sodic soils after the FGDG addition. In conclusion, FGDG can reduce the contaminant leaching by increasing soil pH, forming insoluble precipitates, and enhancing sorption properties of other soil constituents, specifically iron(oxy)hydroxides. However, since these properties can vary depending on soil composition, it is important to perform tests with the specific contaminated soil being remediated with different soil to FGDG ratios or FGDG mixtures to warrant the effectiveness of the stabilization.
b) Consequences of FGDG remediation on chemical and biological activities of soil
Since FGDG is applied to soil for long periods, the leaching of toxic elements from FGDG has been one of the top concerns on its land applications (Dick et al., 2006, Yutdanaiyodthongdee et al., 2013). However, due to the advancements in FGDG production, the “modern” FGDG is very low in toxic element content, hence the leaching potential and the possible toxic effects on the environment are very low (Chaney et al., 2014, Koralegedara et al., 2017). Furthermore, several recent studies showed the chemical changes that occurred in the soil after the addition of FGDG have a positive effect in soil nutrient balance and agricultural productivity (Briggs et al., 2014, DeSutter et al., 2014, Kost et al., 2014, Watts and Dick, 2014). However, the FGDG application rate and its composition significantly impact the overall chemistry of the amended soils. Not only directly hazardous elements, but also the major elements may become harmful when added in excessive amounts. For example, excess Ca2+ from FGDG can outcompete other cations, such as K+ and Mg2+, for soil cation exchange sites, which may lead to K+ and Mg2+ release (DeSutter et al., 2014). Briggs et al. (2014) reported that Hg release into air or water from FGDG amended soil is low and comparable to the release of non-amended soil. Similarly, the Hg uptake by vegetation is comparable to the non-amended soil. The authors reported their experiments did not generate methyl-Hg in the amended soil, in spite of the SO42− from the addition of FGDG. However, the possibility of Hg methylation in FGDG amended soil under anoxic condition was not tested in this research and it deserves to be explored in the future.
Kost et al. (2014) compared the influence of FGDG and natural gypsum on soil chemistry. After one year at a gypsum rate of 20 Mg ha−1, soil treated with FGDG had pH levels lower than the untreated soil, and lower than the soils treated with natural gypsum, because natural gypsum has a higher CaCO3 content, hence a higher ANC. The authors reported relatively high Hg content in FGDG amended soil, but no significant difference was observed on the levels of essential plant nutrients (N, P, and K) resulting in similar crop yields.
As a low-density, highly porous material with high Ca content, FGDG can affect the soil’s physical properties. Buckley and Wolkowski (2014) studied the use of FGDG as an amendment of sodic soil, concluding that it improves its aggregate stability, water infiltration, penetrometer resistance, and decreases soil density. These authors reported seasonal changes in the physical properties of FGDG amended non-sodic soil. Some negative effects of FGDG application at high rates, such as weakened soil aggregates stability and increased bulk density were observed in this study. The use of non-sodic soil with a good soil structure and good chemical balance might be the reason for the observed negative effects of FGDG amendment. This suggests the need of further experiments on the effect of FGDG amendment on physical properties of different types of soil, whether sodic or non-sodic. Zhao et al. (2019) monitored the reclamation of sodic soil using FGD for 17 years. The authors observed a small decrease in soil pH, while Ca2+ and SO42− leached at higher concentrations from the treated soils and CO32− and HCO3− leached at lower concentrations. The metal content of the soil did not change; hence the reclamation was considered environmentally safe.
In addition to the chemistry and physical properties of the soil, the macro and microbial communities present in the soil are highly important for nutrients balance and agricultural productivity. Chen et al. (2014) evaluated the ability of mined gypsum and FGDG to impact the Hg, Se, and As content of earthworms and agricultural soil when amended with them. A significant decrease in earthworm population density (expressed in number of earthworms/m2) and biomass density (expressed as the dry weight of earthworms in g/m2) were reported in both FGDG and natural gypsum amended soil compared to the control soil. The authors suggested the decrease in earthworm density and biomass could be attributed to the increased soluble salt content of soil by gypsum application. There was a slight increase (statistically insignificant) in the Hg level in earthworms and soils exposed to the FGDG amendment. However, higher concentrations of Cd, Cu, Ni, Pb, and Zn are accumulated in earthworms living in gypsum amended soils compared to those in control soil.
The FGDG produced by forced oxidation mainly contained CaSO4. However, its incomplete oxidation may result in higher CaSO3 content. Nonetheless, as Lee et al. (2008) mentioned, in the presence of water and oxygen, a rapid conversion of SO32− to SO42− in the soil is possible. The authors evaluated the effect of FGD-CaSO3 addition on the soil’s microbial community by measuring its enzymatic activity. They reported no significant difference in the FGDG amended soil by measurements of p-nitrophenyl-β-glucopyranoside (used to measure β-glucosidase activity), p-nitrophenyl phosphate (for acid and alkaline phosphatase activity), and p-nitrophenyl sulfate (for arylsulfatase activity). A similar study performed by Alam et al. (2014) reported no significant differences of FGDG and FGD-CaSO3 amendments on soil enzymatic activities.
2.1.3. Water treatment processes
Due to the increase in the production of “modern” FGDG, the benefits of its use currently go beyond land applications. One of the important new applications is water treatment, which takes advantage of FGDG’s alkaline pH, and high Ca2+ and SO42− content. The beneficial uses of FGDG in water treatments, including those associated with agricultural drainage, surface water, industrial wastewater, and sewage sludge treatment, are summarized in Table 3 and discussed separately in detail.
Table 3.
Details of the application of FGDG in the treatment of different types of water.
| Application | Type of water | Mechanism | References |
|---|---|---|---|
| Heavy metal removal | Industrial wastewater | Precipitation, stable mineral formation, adsorption with the aid of SO42−, OH−, CO32−, Ca2+, and Fe–Al oxides | Jayaranjan and Annachhatre, 2013, Yan et al., 2014a, Yan et al., 2014b |
| Industrial wastewater | Forming hydroxyapatite | Yan et al. (2015) | |
| Cyanobacterial growth reduction and metabolites removal | Lake water | Charge neutralization by Ca2+ addition and promotes coagulation | Whangchai et al. (2016) |
| Dye removal | Industrial wastewater | Adsorption | Deniz and Saygideger (2010) |
| Dissolved organic carbon removal | Lake water, agricultural drainage | Ca2+ cross-linked organic matter with clay particle in sediment | Varcoe et al. (2010) |
| Reduce fecal bacteria contamination | Poultry litter drainage | Charge neutralization by Ca2+ addition and coagulation. Reduced bacterial growth by increasing pH |
Jenkins et al. (2014) |
2.1.3.1. Reduction of dissolved organic carbon
Varcoe et al. (2010) studied the efficiency of FGDG application on the reduction of dissolved organic carbon (DOC) from surface water used as a drinking water source in Australia. It was reported that Ca2+ was able to flocculate the natural organic matter (NOM) present in the water by cross-linking carboxylic and other functional groups within the organic matter and then with clay particles in the sediment. Using the same theory, the authors explained their observation of 50% DOC reduction in a reservoir with relatively high NOM. The same study compared the use of FGDG with alum treatment in conventional water treatment processes and reported similar efficacy for both treatments. These observations lead to the use of FGDG in drinking water treatment processes, especially to reduce the DOC in water, with the potential of being an alternate replacement for alum.
2.1.3.2. Reduction of fecal bacteria contamination in surface water
Poultry litter is commonly used as a source of plant nutrients in agricultural applications. Once the poultry litter is applied to the soil, it becomes a potential non-point source of eutrophication for the surface water. In addition, transportation of fecal matter and associated bacteria to the surface water with the runoff could cause some adverse health problems. The use of FGDG to reduce the eutrophication by decreasing N, P, and organic C was previously documented and discussed. The reduction of E-coli transportation by FGDG application was demonstrated in a field test over a three-year period by Jenkins et al. (2014). Even though the underlying mechanism was not explained in that study, the charge neutralization between the Ca2+ released from FGDG and with the E-coli may have reduced the E-coli transportation.
2.1.3.3. Metals removal
The use of FGDG to remove metals in aqueous phase was reported recently (Jayaranjan and Annachhatre, 2013, Yan et al., 2014a, Yan et al., 2014b). Other types of gypsums, such as phosphogypsum and natural gypsums, have been used to remove metals from water (Raii et al., 2014). Jayaranjan and Annachhatre (2013) used FGDG to remove metals from the leachates from coal ash dump sites, resembling a sustainable waste management practice. They attempted to generate S2− from FGDG with the aid of sulfur reducing bacteria and organic matter under anaerobic conditions as follows:
Organic matter
The generated HS− then reacts with metals in the leachate to form stable sulfidic metal precipitates.
Me2+ + HS− → MeS (s) + H+
Yan et al. (2014a) removed Pb and Cd from wastewater using hydroxyapatite (Ca10(PO4)6(OH)2) synthesized from FGDG, which was possible due to its high Ca content. For that purpose, FGDG was reacted with (NH4)2HPO4 at room temperature maintaining the pH between 10 and 11 with the addition of NH3.H2O and heated for 24 h at 150 °C. This is the first study reporting the synthesis of hydroxyapatite using FGDG. Due to its high adsorption capacity and stability under oxidative and reductive conditions, hydroxyapatite is an effective sorbent for wastewater treatment processes. The authors demonstrated a higher affinity of Pb than Cd with the FGDG-hydroxyapatite, with maximum adsorption capacities of 277.8 and 43.1 mg/g for Pb and Cd, respectively, as obtained from the Langmuir isotherms. The same FGDG-hydroxyapatite was used to remove Cu2+ in water by Yan et al. (2014b). The complete adsorption of Cu2+ from a 24.75 mg/g concentrated solution was observed with 3.11 g/L FGDG-hydroxyapatite load at pH 4.96. Song et al. (2011) used FGDG to generate calcite and utilized this calcite to remove Cd from a 100 mg/L solution reporting a maximum adsorption capacity of 7.99 mg/g with a sorbent load of 10 g/L in the pH range of 4–8 (at higher pH Cd would precipitate). A sorbent load of 20 g/L was needed to obtain a complete Cd removal from the solution.
In 2015, Yan et al. (2015) used plain FGDG to remove Pb and Cd from solutions, concluding that Pb removal by FGDG was caused by the formation of PbSO4, whereas Cd removal was attributed to ion exchanging: the Cd2+ in water is exchanged with Ca2+ present in the FGDG. Even though the pH effect (from pH 2 to 7) on Cd and Pb sorption was considered in this study, the underlying mechanism was not described in detail. As Pb formed multifarious mineral precipitates, mostly depending on pH, the Pb removal form FGDG was not necessarily considered an adsorption process. The Pb removal by FGDG can be correlated to the following arguments, based on the stability of Pb precipitates in Pb-FGDG aqueous systems at different pH (Escudero et al., 2013, Hem, 1976).
pH 2–4 – PbSO4 (s)
pH 5–7 – Pb4SO4(CO3)2(OH)2 (s)
pH 6–8 – PbCO3 (s)
pH 8–10 – Pb3(CO3)2(OH)2 (s)
pH > 10 – Pb(OH)2 (s)
2.1.3.4. Reduction of cyanobacterial growth and their metabolites
In addition to metal removal by precipitation, FGDG has been used for other water treatment processes. The removal of off-flavor metabolites, such as geosmin and 2- methylisoborneol (MIB), from water using FGDG was tested by Whangchai et al. (2016). About 63% of geosmin and 75% of MIB removal was reported with FGDG doses of 600 mg/L and 400 mg/L, respectively. The effect of the addition of FGDG on cyanobacterial growth was evaluated. Chlorophyll A, which is an indicator of algal biomass, was effectively removed by FGDG through a coagulation mechanism. Considering all the aforementioned factors, Whangchai et al. (2016) concluded that a 200 mg/L FGDG dose was the optimum to remove the particulate forms of geosmin and MIB in pond water. The neutralization of the colloidal charges accomplished by the release of Ca2+ from FGDG facilitated the coagulation of algal biomass. A maximum chlorophyll A removal of 1.26 μg chlorophyll A/mg FGDG was reported with a 200 mg/L FGDG addition. Higher releases of Ca2+ from FGDG could cause hardness in water, which could be utilized as source of water hardness in aquaculture.
2.1.3.5. Dye removal from industrial wastewater
Synthetic dyes, used in several industries, are prevalent water contaminants. The use of gypsum to remove Basic Red 46, a common dye used in textile industry, was investigated by Deniz and Saygideger (2010). A monolayer adsorption capacity of 39.2 mg/g was reported with the use of gypsum. Even though the underlying mechanism was described as adsorption, a detailed explanation was not given. Other researchers used coal fly ash to remove dye in aqueous phase, explaining that SiO2 and Fe2O3 present in the fly ash were involved in the dye adsorption process (Dizge et al., 2008, Kumar et al., 2005, Lin et al., 2008, Sun et al., 2010). Zhang et al. (2013) showed that SiO2 and Fe2O3 content of can be greater in FGDG than in natural gypsum, suggesting that a similar adsorption process could responsible for the successful dye removal using FGDG.
2.1.3.6. Fluoride removal
Kang et al. (2019) investigated the use of FGDG to precipitate fluoride as calcium fluoride from water obtaining a maximum removal efficiency of 93% and finding that the suitable pH range was 5–11.
2.1.3.7. Improving sludge characteristics
The presence of toxic elements, such as As, Hg and Cd, even in minute quantities, and the release of high Ca2+, which can cause hardness in water, may have limited the use of FGDG in drinking water treatment in the past. However, FGDG has been used to improve sludge dewatering in municipal wastewater treatment. In these cases, the use of organic polymers added during the water treatment process helped to increase the dewatering rate, but not its extent (Zhao, 2006). The flocs generated by organic polymers addition did not resist high pressure. Therefore, other options to obtain more resistant flocs in sludge dewatering have been suggested. Gypsum combined with different anionic organic polymers, such as Magnafloc LT25 and PW 85, was used to improve the dewatering of alum sludge (Zhao, 2002, Zhao, 2006, Zhao and Bache, 2001). Gypsum was proven to act as a skeleton builder in the process, even at high pressures, enhancing the interaction between the organic polymer and the sludge particles. The obtained more rigid lattice structure increased the sludge porosity and thereby the dewatering efficiency. Liang et al. (2014) used semi dry-FGDG residue to treat municipal sewage sludge. The authors reported increased settling velocity, decreased moisture content, and decreased specific resistance of sludge with the addition of semi dry-FGDG residues. Furthermore, the alkaline nature of the FGDG facilitated the reduction of harmful bacteria in the sludge cake.
The use of wet FGD or FGD wastewater as a SO32− source in SANI (Sulfate reduction, Autotrophic denitrification and Nitrification Integrated) process was reported by Jiang et al. (2013) and Qian et al. (2015). The new process was named “FGD-SANI.” The SO32− in FGD wastewater was reduced to sulfide and thiosulfate by the sulfur reducing bacteria (SRB) in sewage sludge under anaerobic conditions. The formed sulfide and thiosulfate acted as electron donors in the autotrophic denitrification process. Both authors confirmed that FGD-SANI process was an effective sludge-minimized sewage treatment method.
3. Conclusions
Recent advances in FGD processes have prompted the production of a modern, environmentally safer FGDG in coal power plants around the world. This FGDG is a great source of gypsum for several applications, but a good portion of this FGDG is currently being disposed of in landfills. This FGDG could be a more important source of gypsum in the future, considering the use of renewable energy would increase while coal combustion would decrease. Consequently, even the landfilled FGDG could be an important material to look for in several reuse scenarios, current and new. In this study we reviewed the Flue Gas Desulfurization processes and the different options to improve its efficiency as Non-regenerable Wet and Dry methods, the composition of the modern FGDG, and its beneficial applications. We also revised the beneficial applications of FGDG, covering recent advances for the synthesis of concrete, cement, asphalt, carbonate-products, calcium sulfate-hemihydrate, and other construction materials. We analyzed the main variables tested in these applications and the main conclusions about the optimum conditions for better product performance and maximum utilization of FGDG. We also discussed the use of FGDG as a fertilizer and soil amendment in agricultural applications and the stabilization of contaminants. Among all the beneficial use scenarios, water treatment is the most novel application, including heavy metal and dye removal from industrial wastewater and mining-impacted water, and metabolic inhibition of cyanobacteria in lake water. Nonetheless, there are important gaps that should be covered with future research. Among those, the potential use of FGDG in landfill leachate treatment, the consequences of increasing the water hardness created by Ca2+ in FGDG, the effects of other FGDG constituents on aquatic ecosystems should be explored. We also found that long-term effects of FGDG amendment on the physicochemical characteristics of soil constituents, such as humic substances, iron(oxy)hydroxides, clay minerals and other alumino-silicates have not been studied in detail.
It is relevant to point out that since the composition of FGDG is highly dependent on the coal composition and process conditions, the FGDG composition should be thoroughly analyzed prior to any beneficial application. As the elements leaching from the FGDG can vary based on the type of application and on the conditions of the reuse scenario, the risk of constituents release should be evaluated using appropriate leaching tests at similar conditions of the intended reuse scenario.
Highlights.
FGDG generation has increased in the U.S., but its reuse rate has decreased.
We reviewed advances in the FGD process that generate a “modern” FGDG.
“Modern” FGDG could be used in different applications with low environmental risk.
We analyzed the main variables and optimum conditions for safe FGDG reuse.
References
- Abrams JZ, Zaczek SJ, Benz AD, Awerbuch L, Haidinger J Use of seawater in flue gas desulfurization a new low-cost fgd system for special applications J. Air Pollut. Control Assoc, 38 (1988), pp. 969–974 [Google Scholar]
- Al-Abed SR, Jegadeesan G, Scheckel KG, Tolaymat Speciation T, characterization, and mobility of As, Se, and Hg in flue gas desulphurization residues Environ. Sci. Technol, 42 (2008), pp. 1693–1698 [DOI] [PubMed] [Google Scholar]
- Alam F, Bigham J, Dick WA, Slater B, Chen L, Lee YB Enzyme activities in soil treated with sulfite- or sulfate-based flue gas desulfurization products Biol. Fertil. Soils, 50 (2014), pp. 991–995 [Google Scholar]
- Álvarez-Ayuso E, Querol X Stabilization of FGD gypsum for its disposal in landfills using amorphous aluminium oxide as a fluoride retention additive Chemosphere, 69 (2007), pp. 295–302 [DOI] [PubMed] [Google Scholar]
- Álvarez-Ayuso E, Querol X, Tomás A Environmental impact of a coal combustion-desulphurisation plant: abatement capacity of desulphurisation process and environmental characterisation of combustion by-products Chemosphere, 65 (2006), pp. 2009–2017 [DOI] [PubMed] [Google Scholar]
- American Coal Ash Association Coal Combustion Product (CCP) Production and Use in Survey Report 2016 American Coal Ash Association, Farmington Hills, MI, USA: (2016) [Google Scholar]
- American Coal Ash Association Coal Combustion Products Production & Use Statistics, Production & Use Reports American Coal Ash Association, Farmington Hills, MI, USA: (2018) [Google Scholar]
- Ammerman CB, Miller SM Selenium in ruminant nutrition: a review J. Dairy Sci, 58 (1975), pp. 1561–1577 [DOI] [PubMed] [Google Scholar]
- Babcock and Wilcox T.B.W. Company (Ed.), Spray Dry Flue Gas Desulfurization Systems, Barberton, Ohio, United States: (2009) [Google Scholar]
- Bagatto G, Shorthouse JD Evaluation of municipal solid waste (MSW) compost as a soil amendment for acidic, metalliferous mine tailings Int. J. Surf. Min. Reclam. Environ, 14 (2000), pp. 205–214 [Google Scholar]
- Baligar VC, Clark RB, Korcak RF, Wright RJ Chapter two - flue gas desulfurization product use on agricultural land Sparks DL (Ed.), Advances in Agronomy, Academic Press; (2011), pp. 51–86 [Google Scholar]
- Beatty WL, Schroeder K, Beatty CLK Mineralogical associations of mercury in FGD products Energy Fuels, 26 (2012), pp. 3399–3406 [Google Scholar]
- Briggs CW, Fine R, Markee M, Gustin MS Investigation of the potential for mercury release from flue gas desulfurization solids applied as an agricultural amendment J. Environ. Qual, 43 (2014), pp. 253–262 [DOI] [PubMed] [Google Scholar]
- Bryant RB, Buda AR, Kleinman PJ, Church CD, Saporito LS, Folmar GJ, Bose S, Allen AL Using flue gas desulfurization gypsum to remove dissolved phosphorus from agricultural drainage waters J. Environ. Qual, 41 (2012), pp. 664–671 [DOI] [PubMed] [Google Scholar]
- Buckley ME, Wolkowski RP In-season effect of flue gas desulfurization gypsum on soil physical properties J. Environ. Qual, 43 (2014), pp. 322–327 [DOI] [PubMed] [Google Scholar]
- Chaney RL, Watts DB, Schomberg HH, Torbert HA Soluble Calcium Amendment: Minimizing Negative Environmental Impacts, Grand Challenges Great Solutions ASA, CSSA, & SSSA International Annual Meeting Soil Science Society of America, Long Beach, CA, United States: (2014) [Google Scholar]
- Chen L, Stehouwer R, Wu M, Kost D, Guo X, Bigham JM, Beeghly J, Dick WA Minesoil response to reclamation by using a flue gas desulfurization product Soil Sci. Soc. Am. J, 77 (2013), pp. 1744–1754 [Google Scholar]
- Chen L, Tian Y, Stehouwer R, Kost D, Guo X, Bigham JM, Beeghly J, Dick WA Surface coal mine land reclamation using a dry flue gas desulfurization product: long-term biological response Fuel, 105 (2013), pp. 258–265 [Google Scholar]
- Chen L, Kost D, Tian Y, Guo X, Watts D, Norton D, Wolkowski RP, Dick WA Effects of gypsum on trace metals in soils and earthworms J. Environ. Qual, 43 (2014), pp. 263–272 [DOI] [PubMed] [Google Scholar]
- Chen L, Stehouwer R, Tong X, Kost D, Bigham JM, Dick WA Surface coal mine land reclamation using a dry flue gas desulfurization product: short-term and long-term water responses Chemosphere, 134 (2015), pp. 459–465 [DOI] [PubMed] [Google Scholar]
- Chen Q, Wang S, Li Y, Zhang N, Zhao B, Zhuo Y, Chen C Influence of flue gas desulfurization gypsum amendments on heavy metal distribution in reclaimed sodic soils Environ. Eng. Sci, 32 (2015), pp. 470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Szostak P, Wei Z, Xiao R Reduction of orthophosphates loss in agricultural soil by nano calcium sulfate Sci. Total Environ, 539 (2016), pp. 381–387 [DOI] [PubMed] [Google Scholar]
- Cheng CM, Hack P, Chu P, Chang YN, Lin TY, Ko CS, Chiang PH, He CC, Lai YM, Pan WP Partitioning of mercury, arsenic, selenium, boron, and chloride in a full-scale coal combustion process equipped with selective catalytic reduction, electrostatic precipitation, and flue gas desulfurization systems Energy Fuels, 23 (2009), pp. 4805–4815 [Google Scholar]
- Chi CM, Zhao CW, Sun XJ, Wang ZC Reclamation of saline-sodic soil properties and improvement of rice (Oriza sativa L.) growth and yield using desulfurized gypsum in the west of Songnen Plain, northeast China Geoderma, 187–188 (2012), pp. 24–30 [Google Scholar]
- Chou MIM, Bruinius JA, Benig V, Chou SFJ, Carty RH Producing ammonium sulfate from flue gas desulfurization by-products Energy Sources, 27 (2005), pp. 1061–1071 [Google Scholar]
- Chun S, Nishiyama M, Matsumoto S Sodic soils reclaimed with by-product from flue gas desulfurization: corn production and soil quality Environ. Pollut, 114 (2001), pp. 453–459 [DOI] [PubMed] [Google Scholar]
- Collins KJ, Jensen AC, Lockwood APM, Turnpenny AWH Evaluation of stabilized coal-fired power station waste for artificial reef construction Bull. Mar. Sci, 55 (1994), pp. 1251–1262 [Google Scholar]
- Córdoba P Status of Flue Gas Desulphurisation (FGD) systems from coal-fired power plants: overview of the physic-chemical control processes of wet limestone FGDs Fuel, 144 (2015), pp. 274–286 [Google Scholar]
- Córdoba P, Ochoa-Gonzalez R, Font O, Izquierdo M, Querol X, Leiva C, López-Antón MA, Díaz-Somoano M, Rosa Martinez-Tarazona M, Fernandez C, Tomás A Partitioning of trace inorganic elements in a coal-fired power plant equipped with a wet Flue Gas Desulphurisation system Fuel, 92 (2012), pp. 145–157 [Google Scholar]
- de Beer M, Maree JP, Liebenberg L, Doucet FJ Conversion of calcium sulphide to calcium carbonate during the process of recovery of elemental sulphur from gypsum waste Waste Manag, 34 (2014), pp. 2373–2381 [DOI] [PubMed] [Google Scholar]
- de Beer M, Doucet FJ, Maree JP, Liebenberg L Synthesis of high-purity precipitated calcium carbonate during the process of recovery of elemental sulphur from gypsum waste Waste Manag, 46 (2015), pp. 619–627 [DOI] [PubMed] [Google Scholar]
- del Valle-Zermeño R, Niubó M, Formosa J, Guembe M, Aparicio JA, Chimenos JM Synergistic effect of the parameters affecting wet flue gas desulfurization using magnesium oxides by-products Chem. Eng. J, 262 (2015), pp. 268–277 [Google Scholar]
- Deniz F, Saygideger SD Investigation of adsorption characteristics of Basic Red 46 onto gypsum: equilibrium, kinetic and thermodynamic studies Desalination, 262 (2010), pp. 161–165 [Google Scholar]
- Dermatas D, Meng X Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils Eng. Geol, 70 (2003), pp. 377–394 [Google Scholar]
- DeSutter TM, Cihacek LJ, Rahman S Application of flue gas desulfurization gypsum and its impact on wheat grain and soil chemistry J. Environ. Qual, 43 (2014), pp. 303–311 [DOI] [PubMed] [Google Scholar]
- Dick WA, Kost DA, Chen L Beneficial land application uses of FGD products 23rd Annual International Pittsburgh Coal Conference, PCC - Coal-Energy, Environment and Sustainable Development (2006) [Google Scholar]
- Ding W, Yang H, Ouyang J Mineral carbonation of a desulfurization residue for CO2 sequestration RSC Adv, 5 (2015), pp. 67184–67194 [Google Scholar]
- Dizge N, Aydiner C, Demirbas E, Kobya M, Kara S Adsorption of reactive dyes from aqueous solutions by fly ash: kinetic and equilibrium studies J. Hazard Mater, 150 (2008), pp. 737–746 [DOI] [PubMed] [Google Scholar]
- Du CW, Lv Y, Li GZ Research on Preparation and Properties of New Type Desulfurization Gypsum Block Applied Mechanics and Materials (2014), pp. 217–220 [Google Scholar]
- Endale DM, Schomberg HH, Fisher DS, Franklin DH, Jenkins MB Flue gas desulfurization gypsum: implication for runoff and nutrient losses associated with broiler litter use on pastures on ultisols J. Environ. Qual, 43 (2014), pp. 281–289 [DOI] [PubMed] [Google Scholar]
- Escudero R, Espinoza E, Tavera FJ Precipitation of lead species in Pb-H2O systemRes. J. Recent Sci, 2 (2013), pp. 1–4 [Google Scholar]
- Falcon R, Ham AJ The characteristics of South African coals J. S. Afr. Inst. Min. Metall, 88 (1988), pp. 145–161 [Google Scholar]
- Frandsen JBW, Kiil S, Johnsson JE Optimisation of a wet FGD pilot plant using fine limestone and organic acids Chem. Eng. Sci, 56 (2001), pp. 3275–3287 [Google Scholar]
- Gao Y, Xie X, Zou P, Liang X, Yang Y, Li Z, Wang H, Xiao T, Cai W Study on Additives for Wet Limestone FGD, Asia-Pacific Power and Energy Engineering Conference APPEEC (2011)
- Gitari WM, Petrik LF, Etchebers O, Key DL, Iwuoha E, Okujeni C Passive neutralisation of acid mine drainage by fly ash and its derivatives: a column leaching study Fuel, 87 (2008), pp. 1637–1650 [Google Scholar]
- Gitari WM, Petrik LF, Etchebers O, Key DL, Okujeni C Utilization of fly ash for treatment of coal mines wastewater: solubility controls on major inorganic contaminants Fuel, 87 (2008), pp. 2450–2462 [Google Scholar]
- Glasser FP, Zhang L High-performance cement matrices based on calcium sulfoaluminate-belite compositions Cement Concr. Res, 31 (2001), pp. 1881–1886 [Google Scholar]
- González-Núñez R, Alba MD, Vidal M, Rigol A Viability of adding gypsum and calcite for remediation of metal-contaminated soil: laboratory and pilot plant scales Int. J. Environ. Sci. Technol, 12 (2015), pp. 2697–2710 [Google Scholar]
- Gu HH, Li FP, Guan X, Xu YL, Liu YJ, Chen XT, Wang XH, Wang Z Effects of fly ash on heavy metal uptake of rice growing on multi-metal contaminated acidic soil Adv. Mater. Res (2013), pp. 94–99 [Google Scholar]
- Guan B, Lou W, Ye Q, Fu H, Wu Z Calorimetric study of calcium aluminate cement blended with flue gas desulfurization gypsum J. Therm. Anal. Calorim, 98 (2009), pp. 737–742 [Google Scholar]
- Guan B, Yang L, Fu H, Kong B, Li T, Yang L α-calcium sulfate hemihydrate preparation from FGD gypsum in recycling mixed salt solutions Chem. Eng. J, 174 (2011), pp. 296–303 [Google Scholar]
- Guo XL, Shi HS, Liu HY Effects of a combined admixture of slag powder and thermally treated flue gas desulphurization (FGD) gypsum on the compressive strength and durability of concrete Materials and Structures/Materiaux et Constructions, 42 (2009), pp. 263–270 [Google Scholar]
- Han BJ, Yue T, Jing P, Zuo PL, Wang CL, Qi SF, Ding YH Study on Comprehensive Utilization Status and Technical Route of FGD Gypsum from Coal-Fired Power Plants Applied Mechanics and Materials (2014), pp. 713–717 [Google Scholar]
- Hem JD Inorganic chemistry of lead in water U.S.G. Survey (Ed.), Lead in the Environment, Lovering, T. G., Washington D.C: (1976) [Google Scholar]
- Hill FF, Zank J Flue gas desulphurization by spray dry absorption Chem. Eng. Process: Process Intensification, 39 (2000), pp. 45–52 [Google Scholar]
- Houben D, Pircar J, Sonnet P Heavy metal immobilization by cost-effective amendments in a contaminated soil: effects on metal leaching and phytoavailability J. Geochem. Explor, 123 (2012), pp. 87–94 [Google Scholar]
- Huang Z, Sun Z, Lu Z Effects of Soil Amendments on Coastal Saline-Alkali Soil Improvement and the Growth of Plants, Adv. Mater. Res (2013), pp. 152–159 [Google Scholar]
- Huang R, Yu R, Wu H, Pan D, Zhang Y, Yang L Investigation on the removal of SO3 in ammonia-based WFGD system Chem. Eng. J, 289 (2016), pp. 537–543 [Google Scholar]
- Jayaranjan MLD, Annachhatre AP Precipitation of heavy metals from coal ash leachate using biogenic hydrogen sulfide generated from FGD gypsum Water Sci. Technol, 67 (2013), pp. 311–318 [DOI] [PubMed] [Google Scholar]
- Jenkins MB, Schomberg HH, Endale DM, Franklin DH, Fisher DS Hydrologic transport of fecal bacteria attenuated by flue gas desulfurization gypsum J. Environ. Qual, 43 (2014), pp. 297–302 [DOI] [PubMed] [Google Scholar]
- Jiang F, Zhang L, Peng GL, Liang SY, Qian J, Wei L, Chen GH A novel approach to realize SANI process in freshwater sewage treatment - use of wet flue gas desulfurization waste streams as sulfur source Water Res, 47 (2013), pp. 5773–5782 [DOI] [PubMed] [Google Scholar]
- Kang J, Gou X, Hu Y, Sun W, Liu R, Gao Z, Guan Q Efficient utilisation of flue gas desulfurization gypsum as a potential material for fluoride removal Sci. Total Environ, 649 (2019), pp. 344–352 [DOI] [PubMed] [Google Scholar]
- Kikkawa H, Nakamoto T, Morishita M, Yamada K New wet FGD process using granular limestone Ind. Eng. Chem. Res, 41 (2002), pp. 3028–3036 [Google Scholar]
- Koralegedara NH, Al-Abed SR, Rodrigo SK, Karna RR, Scheckel KG, Dionysiou DD Alterations of lead speciation by sulfate from addition of flue gas desulfurization gypsum (FGDG) in two contaminated soils Sci. Total Environ, 575 (2017), pp. 1522–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost DA, Bigham JM, Stehouwer RC, Beeghly JH, Fowler R, Traina SJ, Wolfe WE, Dick Chemical WA and physical properties of dry flue gas desulfurization products J. Environ. Qual, 34 (2005), pp. 676–686 [DOI] [PubMed] [Google Scholar]
- Kost D, Chen L, Guo X, Tian Y, Ladwig K, Dick WA Effects of flue gas desulfurization and mined Gypsums on soil properties and on hay and corn growth in eastern Ohio J. Environ. Qual, 43 (2014), pp. 312–321 [DOI] [PubMed] [Google Scholar]
- Kost D, Ladwig KJ, Chen L, DeSutter TM, Espinoza L, Norton LD, Smeal D, Torbert HA, Watts DB, Wolkowski RP, Dick WA Meta-Analysis of gypsum effects on crop yields and chemistry of soils, plant tissues, and vadose water at various research sites in the USA J. Environ. Qual, 47 (2018), pp. 1284–1292 [DOI] [PubMed] [Google Scholar]
- Kumar KV, Ramamurthi V, Sivanesan S Modeling the mechanism involved during the sorption of methylene blue onto fly ash J. Colloid Interface Sci, 284 (2005), pp. 14–21 [DOI] [PubMed] [Google Scholar]
- Lapin EP, Weissbarth S, Maker HS, Lehrer GM The sensitivities of creatine and adenylate kinases to the neurotoxins acrylamide and methyl n-butyl ketone Environ. Res, 28 (1982), pp. 21–31 [DOI] [PubMed] [Google Scholar]
- Lee YB, Bigham JM, Dick WA, Kim PJ Impact of flue gas desulfurization-calcium sulfite and gypsum on soil microbial activity and Wheat growth Soil Sci, 173 (2008), pp. 534–543 [Google Scholar]
- Lee MG, Jang YN, Ryu KW, Kim W, Bang JH Mineral carbonation of flue gas desulfurization gypsum for CO2 sequestration Energy, 47 (2012), pp. 370–377 [Google Scholar]
- Leiva C, García Arenas C, Vilches LF, Vale J, Gimenez A, Ballesteros JC, Fernández-Pereira C Use of FGD gypsum in fire resistant panels Waste Manag, 30 (2010), pp. 1123–1129 [DOI] [PubMed] [Google Scholar]
- Li JQ, Gong MZ, Li GZ Research on a new kind of waterproof agent for desulphurization gypsum materials block Adv. Mater. Res (2013), pp. 268–271 [Google Scholar]
- Li J, Zhuang X, Leiva C, Cornejo A, Font O, Querol X, Moeno N, Arenas C, Fernández-Pereira C Potential utilization of FGD gypsum and fly ash from a Chinese power plant for manufacturing fire-resistant panels Constr. Build. Mater, 95 (2015), pp. 910–921 [Google Scholar]
- Li X, Mao Y, Liu X Flue gas desulfurization gypsum application for enhancing the desalination of reclaimed tidal lands Ecol. Eng, 82 (2015), pp. 566–570 [Google Scholar]
- Liang H, Shi L, Yang G Improving sludge dewaterability by adding the semi-dry flue gas desulfurization residue under microwave radiation Clean. - Soil, Air, Water, 42 (2014), pp. 1239–1247 [Google Scholar]
- Lin JX, Zhan SL, Fang MH, Qian XQ, Yang H Adsorption of basic dye from aqueous solution onto fly ash J. Environ. Manag, 87 (2008), pp. 193–200 [DOI] [PubMed] [Google Scholar]
- Liu R, Lal R A laboratory study on amending mine soil quality. Water, air & Soil Pollution, 224 (2013), p. 1679 [Google Scholar]
- Liu SY, Qu B, Gao J, Liu JY, Ye ZX, Xu CH New limestone-gypsum flue gas desulfuization technology 2009 International Conference on Energy and Environment Technology, ICEET 2009 (2009), pp. 78–81 [Google Scholar]
- Liu X, Wang S, Zhang L, Wu Y, Duan L, Hao J Speciation of mercury in FGD gypsum and mercury emission during the wallboard production in China Fuel, 111 (2013), pp. 621–627 [Google Scholar]
- Liu T, Shi G, Li G, Wang Z Study on properties of foamed concrete with EPS as coarse aggregate IOP Conference Series: Materials Science and Engineering (3 ed) (2019) [Google Scholar]
- Lu JZ, Zhao B, Chen XQ, Cao JL Preparation of the high-quality gypsum by dihydrate FGD Rengong Jingti Xuebao/J. Synthetic Crystals, 45 (2016), pp. 97–103 [Google Scholar]
- Miao M, Feng X, Wang G, Cao S, Shi W, Shi L Direct transformation of FGD gypsum to calcium sulfate hemihydrate whiskers: preparation, simulations, and process analysis Particuology, 19 (2015), pp. 53–59 [Google Scholar]
- Mo JS, Wu ZB, Cheng CJ, Li FC, Guan BH, Zhao Experimental WR and theoretical studies on desulfurization efficiency of dual-alkali FGD system in a RST scrubber Guocheng Gongcheng Xuebao/The Chinese J. Process Eng, 6 (2006), pp. 718–723 [Google Scholar]
- Murphy KR, Shilling NZ, Pennline H Status of the DOE/GEESI in-duct scrubbing pilot study J. Air Pollut. Control Assoc, 36 (1986), pp. 953–958 [Google Scholar]
- Muzio LJ, Often GR Assessment of dry sorbent emission control technologies Part I. Fundamental processes JAPCA, 37 (1987), pp. 642–654 [Google Scholar]
- Neathery JK Model for flue-gas desulfurization in a circulating dry scrubber AIChE J, 42 (1996), pp. 259–268 [Google Scholar]
- Norton LD Environmental evaluation of flue gas desulfurization gypsum as a BMP for erosion control ASABE - International Symposium on Erosion and Landscape Evolution 2011 (2011), pp. 421–429 [Google Scholar]
- Pandey RA, Biswas R, Chakrabarti T, Devotta S Flue gas desulfurization: physicochemical and biotechnological approaches Crit. Rev. Environ. Sci. Technol, 35 (2005), pp. 571–622 [Google Scholar]
- Park JH, Edraki M, Mulligan D, Jang HS The application of coal combustion by-products in mine site rehabilitation J. Clean. Prod, 84 (2014), pp. 761–762 [Google Scholar]
- Prasad SN, Muralidhara The vulnerability of diabetic rats to neurotoxin acrylamide: an interactive study Int. J. Res. Pharm. Sci, 9 (2018), pp. 940–949 [Google Scholar]
- Qian J, Lu H, Jiang F, Ekama GA, Chen GH Beneficial co-treatment of simple wet flue gas desulphurization wastes with freshwater sewage through development of mixed denitrification-SANI process Chem. Eng. J, 262 (2015), pp. 109–118 [Google Scholar]
- Querol X, Alastuey A, Moreno N, Alvarez-Ayuso E, García-Sánchez A, Cama J, Ayora C, Simón M Immobilization of heavy metals in polluted soils by the addition of zeolitic material synthesized from coal fly ash Chemosphere, 62 (2006), pp. 171–180 [DOI] [PubMed] [Google Scholar]
- Raii M, Minh DP, Sanz FJE, Nzihou Lead A and Cadmium Removal from Aqueous Solution Using an Industrial Gypsum By-Product Procedia Engineering (2014), pp. 415–422 [Google Scholar]
- Rallo M, Lopez-Anton MA, Perry R, Maroto-Valer MM Mercury speciation in gypsums produced from flue gas desulfurization by temperature programmed decomposition Fuel, 89 (2010), pp. 2157–2159 [Google Scholar]
- Ram LC, Masto RE An appraisal of the potential use of fly ash for reclaiming coal mine spoil J. Environ. Manag, 91 (2010), pp. 603–617 [DOI] [PubMed] [Google Scholar]
- Sakai Y, Matsumoto S, Sadakata M Alkali soil reclamation with flue gas desulfurization gypsum in China and assessment of metal content in corn grains Soil Sediment Contam, 13 (2004), pp. 65–80 [Google Scholar]
- Seo SK, Chu YS, Shim KB, Lee JK, Song H A study on the desulfurization efficiency of limestone sludge with various admixtures J. Korean Chem. Soc, 52 (2015), pp. 479–482 [Google Scholar]
- Seshadri B, Bolan NS, Kunhikrishnan A, Choppala G, Naidu R Effect of coal combustion products in reducing soluble phosphorus in soil II: leaching study Water Air Soil Pollut, 225 (2014) [Google Scholar]
- Shemwell B, Atal A, Levendis YA, Simons GA A laboratory investigation on combined in-furnace sorbent injection and hot flue-gas filtration to simultaneously capture SO2, NO(x), HCl, and particulate emissions Environ. Sci. Technol, 34 (2000), pp. 4855–4866 [Google Scholar]
- Sindelar ME, Wolkowski RP Reducing water-soluble phosphorus in soil through flue gas desulfurization gypsum application: year of application effects at multiple sites in Wisconsin J. Environ. Qual, 48 (2019), pp. 654–659 [DOI] [PubMed] [Google Scholar]
- Skousen J, Ziemkiewicz P, Yang JE Use of coal combustion by-products in mine reclamation: review of case studies in the USA Geosystem Eng, 15 (2012), pp. 71–83 [Google Scholar]
- Song K, Kim W, Ryu T, Ryu K-W, Bang J-H, Jang Y-N Adsorption of Cd(II) on waste calcite produced by the carbonation of flue gas desulfurization (FGD) gypsum Mater. Trans, 52 (2011), pp. 224–228 [Google Scholar]
- Song W Kim JH Bang S Park CW Jeon Polymorphs of pure calcium carbonate prepared by the mineral carbonation of flue gas desulfurization gypsum Mater. Des, 83 (2015), pp. 308–313 [Google Scholar]
- Srisomang R, Naksata W, Thiansem S, Sooksamiti P, O.-a. Arqueropanyo Utilization of leonardite, flue gas desulfurization gypsum and clay for production of ceramic plant growth material Environ. Earth Sci, 73 (2015), pp. 1621–1628 [Google Scholar]
- Srivastava RK EPA/600/R-00/093 U.S.E.P. Agency (Ed.), Controlling SO2 Emissions: A Review of Technologies, U.S. EPA, Washington, D.C: (2000) [Google Scholar]
- Srivastava RK, Jozewicz W Flue gas desulfurization: the state of the art J. Air Waste Manag. Assoc, 51 (2001), pp. 1676–1688 [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Jozewicz W, Singer C SO2 scrubbing technologies: a review Environ. Prog, 20 (2001), pp. 219–227 [Google Scholar]
- Stache L, Kahl D Procedure for the Production of a Multilayer Thermal-Insulating Structural Board Based on Fly Ash and Gypsum Mixture Biag Produktions & Co KG GmbH, Germany (2003)
- Stouffer MR, Yoon H, Burke FP An investigation of the mechanisms of flue gas desulfurization by in-duct dry sorbent injection Ind. Eng. Chem. Res, 28 (1989), pp. 20–27 [Google Scholar]
- Sumner ME (first ed.), Advances in Agronomy, 51, Academic Press, Inc., London, Englad: (1993) [Google Scholar]
- Sun D, Zhang X, Wu Y, Liu X Adsorption of anionic dyes from aqueous solution on fly ash J. Hazard Mater, 181 (2010), pp. 335–342 [DOI] [PubMed] [Google Scholar]
- Sun M, Hou J, Cheng G, Baig SA, Tan L, Xu X The relationship between speciation and release ability of mercury in flue gas desulfurization (FGD) gypsum Fuel, 125 (2014), pp. 66–72 [Google Scholar]
- Sydnor MEW, Redente EF Reclamation of high-elevation, acidic mine waste with organic amendments and topsoil J. Environ. Qual, 31 (2002), pp. 1528–1537 [DOI] [PubMed] [Google Scholar]
- Torbert HA, Watts DB Impact of flue gas desulfurization gypsum application on water quality in a coastal plain soil J. Environ. Qual, 43 (2014), pp. 273–280 [DOI] [PubMed] [Google Scholar]
- Torbert HA, Watts DB, Chaney RL Impact of flue gas desulfurization gypsum and manure application on transfer of potentially toxic elements to plants, soil, and runoff J. Environ. Qual, 47 (2018), pp. 865–872 [DOI] [PubMed] [Google Scholar]
- Truman CC, Nuti RC, Truman LR, Dean JD Feasibility of using FGD gypsum to conserve water and reduce erosion from an agricultural soil in Georgia Catena, 81 (2010), pp. 234–239 [Google Scholar]
- Tzouvalas G, Rantis G, Tsimas S Alternative calcium-sulfate-bearing materials as cement retarders: Part II. FGD gypsum Cement Concr. Res, 34 (2004), pp. 2119–2125 [Google Scholar]
- U.S. Energy Information Administration Energy, U.S.D.o (Ed.), Electric Power Annual 2016, U.S. Department of Energy, Washington, DC: (2018) [Google Scholar]
- U.S. EPA C.A.M. Division (Ed.), Cross-State Air Pollution Rule (CSAPR), U.S. EPA, Washington, D.C: (2016) [Google Scholar]
- Varcoe J, J.A.v. Leeuwen, D.J. Chittleborough, J.W. Cox, R.J. Smernik, A. Heitz Changes in water quality following gypsum application to catchment soils of the Mount Lofty Ranges, South Australia Org. Geochem, 41 (2010), pp. 116–123 [Google Scholar]
- Wang J, Yang P Potential flue gas desulfurization gypsum utilization in agriculture: a comprehensive review Renew. Sustain. Energy Rev, 82 (2018), pp. 1969–1978 [Google Scholar]
- Wang J, Bai Z, Yang P Effect of byproducts of flue gas desulfurization on the soluble salts composition and chemical properties of sodic soils PLoS One, 8 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Pan Z, Du Z, Cheng H, Cheng F Effect of impure components in flue gas desulfurization (FGD) gypsum on the generation of polymorph CaCO3 during carbonation reaction J. Hazard Mater, 369 (2019), pp. 236–243 [DOI] [PubMed] [Google Scholar]
- Wansom S, Chintasongkro P, Srijampan W Water resistant blended cements containing flue-gas desulfurization gypsum, Portland cement and fly ash for structural applications Cement Concr. Compos, 103 (2019), pp. 134–148 [Google Scholar]
- Watts DB, Dick WA Sustainable uses of FGD gypsum in agricultural systems: Introduction J. Environ. Qual, 43 (2014), pp. 246–252 [DOI] [PubMed] [Google Scholar]
- Whangchai N, Gutierrez R, Sompong U, Suwanpakdee S, Pimolrat P, Itayama T, Ariyadet C, Whangchai K Use of flue gas desulfurization gypsum for the removal of off-flavor compounds in fish pond water Int. J. GEOMATE, 11 (2016), pp. 2253–2258 [Google Scholar]
- Wu H, Chao S, Zhou T, Liu X Cold-formed steel framing walls with infilled lightweight FGD gypsum Part I: cyclic loading tests Thin-Walled Struct, 132 (2018), pp. 759–770 [Google Scholar]
- Wu Q, Ma H, Chen Q, Huang Z, Zhang C, Yang T Preparation of waterproof block by silicate clinker modified FGD gypsum Constr. Build. Mater, 214 (2019), pp. 318–325 [Google Scholar]
- Xu L, Wu K, Li N, Zhou X, Wang P Utilization of flue gas desulfurization gypsum for producing calcium sulfoaluminate cement J. Clean. Prod, 161 (2017), pp. 803–811 [Google Scholar]
- Yan Y, Dong X, Sun X, Sun X, Li J, Shen J, Han W, Liu X, Wang L Conversion of waste FGD gypsum into hydroxyapatite for removal of Pb2+ and Cd2+ from wastewater J. Colloid Interface Sci, 429 (2014), pp. 68–76 [DOI] [PubMed] [Google Scholar]
- Yan Y, Dong XL, Sun XL, Li J, Shen JY, Han WQ, Liu XD, Sun XY, Wang LJ Cu2+ Removal from Wastewater Using Hydroxyapatite Prepared from FGD Gypsum (2014)
- Yan Y, Li Q, Sun X, Ren Z, He F, Wang Y, Wang L Recycling Flue Gas Desulphurization (FGD) Gypsum for Removal of Pb(II) and Cd(II) from Wastewater (2015) [DOI] [PubMed]
- Yang ZY, Zhao S, Hu J, LuThe ZC Effects of FGD Gypsum on the Performance of Cement Applied Mechanics and Materials (2013), pp. 35–38 [Google Scholar]
- Yu H, Yang P, Lin H, Ren S, He X Effects of sodic soil reclamation using flue gas desulphurization gypsum on soil pore characteristics, bulk density, and saturated hydraulic conductivity Soil Sci. Soc. Am. J, 78 (2014), pp. 1201–1213 [Google Scholar]
- Yu HL, Gu W, Tao J, Huang JY, Lin HS Impact of addition of FGDB as a soil amendment on physical and chemical properties of an alkali soil and crop yield of maize in Northern China Coastal Plain J. Chem, 2015 (2015), Article 540604 [Google Scholar]
- Yue L, Jianhui W, Bin H, DaCheng L Heat Treatment Process of the Flue Gas Desulphurization Gypsum and its Influence on the Properties of Cement Applied Mechanics and Materials (2012), pp. 40–44 [Google Scholar]
- Yutdanaiyodthongdee P Sooksamiti J Jakmunee S Lapanantnoppakhun Uptake of nutrients in vegetables grown on FGD-gypsum-amended soils Orient. J. Chem, 29 (2013), pp. 1027–1032 [Google Scholar]
- Zhang JX, Duan PX, Zhang Y A Comparative Study on Preparation of High Strength Gypsum by Flue Gas Desulfurization Gypsum and Natural Gypsum Applied Mechanics and Materials (2013), pp. 641–645 [Google Scholar]
- Zhang Y, Zhang S, Ni W, Yan Q, Gao W, Li Y Immobilisation of high-arsenic-containing tailings by using metallurgical slag-cementing materials Chemosphere (2019), pp. 117–123 [DOI] [PubMed] [Google Scholar]
- Zhao YQ Enhancement of alum sludge dewatering capacity by using gypsum as skeleton builder Colloid. Surf. Physicochem. Eng. Asp, 211 (2002), pp. 205–212 [Google Scholar]
- Zhao YQ Involvement of gypsum (CaSO4 2H2O) in water treatment sludge dewatering: a potential benefit in disposal and reuse Separ. Sci. Technol, 41 (2006), pp. 2785–2794 [Google Scholar]
- Zhao YQ, Bache DH Conditioning of alum sludge with polymer and gypsum Colloid. Surf. Physicochem. Eng. Asp, 194 (2001), pp. 213–220 [Google Scholar]
- Zhao FQ, Liu HJ, Hao LX, Li Q Water resistant block from desulfurization gypsum Constr. Build. Mater, 27 (2012), pp. 531–533 [Google Scholar]
- Zhao Y, Wang S, Li Y, Zhuo Y, Liu J Sustainable effects of gypsum from desulphurization of flue gas on the reclamation of sodic soil after 17 years Eur. J. Soil Sci, 70 (5) (2019) [Google Scholar]
- Zheng Y, Kiil S, Johnsson JE Experimental investigation of a pilot-scale jet bubbling reactor for wet flue gas desulphurization Chem. Eng. Sci, 58 (2003), pp. 4695–4703 [Google Scholar]
- Zhong S, Ni K, Li J Properties of mortars made by uncalcined FGD gypsum-fly ash-ground granulated blast furnace slag composite binder Waste Manag, 32 (2012), pp. 1468–1472 [DOI] [PubMed] [Google Scholar]
- Ziemkiewicz PF, Skousen JG, Brant DL, Sterner PL, Lovett RJ Acid mine drainage treatment with armored limestone in open limestone channels J. Environ. Qual, 26 (1997), pp. 1017–1024 [Google Scholar]


