Abstract
Introduction
The effect of anti-infective agents in COVID-19 is unclear. The impact of changes in practice on prognosis over time has not been evaluated.
Methods
Single center, retrospective study in adults hospitalized in a medicine ward for COVID-19 from March 5th to April 25th 2020. Patient characteristics were compared between two periods (before/after March 19th) considering French guidelines. The aim of the study was to evaluate how medical care impacted unfavorable outcome, namely admission to intensive care unit (ICU) and/or death.
Results
A total of 132 patients were admitted: mean age 59.0±16.3 years; mean C-reactive protein (CRP) level 84.0±71.1 mg/L; 46% had a lymphocyte count <1000/mm3. Prescribed anti-infective agents were lopinavir-ritonavir (n=12), azithromycin (AZI) (n=28) and AZI combined with hydroxychloroquine (HCQ) (n=52). There was a significant decrease in ICU admission, from 43% to 12%, between the two periods (P<0.0001). Delays until transfer to ICU were similar between periods (P=0.86). Pulmonary computerized tomography (CT)-scans were performed significantly more often with time (from 50% to 90%, P<0.0001), and oxygen-dependency (53% vs 80%, P=0.001) and prescription of AZI±HCQ (from 25% to 76%, P<0.0001) were also greater over time. Multivariate analyses showed a reduction of unfavorable outcome in patients receiving AZI±HCQ (hazard ratio [HR]=0.45, 95% confidence interval [CI: 0.21-0.97], P=0.04), particularly among an identified category of individuals (lymphocyte ≥1000/mm3 or CRP ≥100 mg/L).
Conclusion
The present study showed a significant decrease in admission to ICU over time, which was probably related to multiple factors, including a better indication of pulmonary CT-scan, oxygen therapy, and a suitable prescription of anti-infective agents.
Keywords: azithromycin, hydroxychloroquine, Covid-19, pneumonia
1. Introduction
Management and medical care of COVID-19 pneumonia in hospitalized patients is currently still debated, particularly as data regarding an emerging pathogen are constantly evolving over time and across countries. Numerous therapies, including oxygen, anti-infective agents and corticosteroids, have been proposed.
Gautret et al. [1,2] and Million et al. [3] observed in Marseille (France) that combination therapy using hydroxychloroquine (HCQ) and azithromycin (AZI) could potentially reduce viral shedding and the incidence of COVID-19 pneumonia. Concomitantly, an observational study conducted by Mahevas et al. [4] evaluating HCQ alone prescribed in an in-hospital setting showed no impact of HCQ on the transfer rate to the intensive care unit (ICU) and/or death. This study is concordant with a publication issued in the United States by Geleris et al. [5], who concluded that HCQ administration was not associated with a greatly lowered risk of intubation or death.
Interestingly, although corticosteroids were considered potentially harmful in the early care of COVID-19-infected patients [6], the RECOVERY trial (NCT04381936) stated that dexamethasone could reduce mortality rate by up to 30% in severely ill patients admitted for COVID-19 pneumonia, and revealed no interest of HCQ (data not published), meanwhile the AZI arm is still being investigated. A recent multicenter study in the United States reopened the debate concerning the efficacy of HCQ with or without AZI [7]. Furthermore, antiviral therapies, notably lopinavir–ritonavir, showed no benefit compared to standard-of-care in a large, randomized trial [8], whereas remdesivir showed a reduction in time to clinical improvement in two trials but no significant impact on mortality [9,10].
Overall, those reports have raised concerns about the true interest of anti-infective agents in COVID-19 pneumonia in the context of heterogeneous medical practices that have evolved over time. In the absence of a clear recommendation for treatment initiation, it is difficult to assume or to invalidate the effect of anti-infective agents on prognosis of patients with COVID-19.
To our knowledge, the impact of changes in practice, including use of anti-infective agents, on prognosis of patients admitted to a medical ward for COVID-19 pneumonia over time has not been evaluated. In this study, potential factors associated with an unfavorable outcome, namely admission to ICU and/or death, during this first wave of the epidemic were retrospectively evaluated.
2. Methods
2.1. Setting
This was a single center, retrospective study comprising adults admitted to the medicine wards in a tertiary university hospital, Hôpital Raymond Poincaré (AP-HP), Garches, France, from March 5th to April 25th 2020.
All adults admitted to the medicine wards for a COVID-19 infection confirmed by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) reverse transcriptase-polymerase chain reaction (RT-PCR) and/or a compatible pulmonary computerized tomography (CT)-scan were included. Exclusion criteria were: i) patients directly admitted to ICU; ii) patients discharged from ICU to a medicine ward; iii) patient opposition to data collection.
2.2. Data collection
The following data were collected from patient medical charts:
-
-
Patient characteristics: age, sex, diabetes, cardiovascular risk factors, smoking habits, obesity, chronic pulmonary disease, Charlson comorbidity index (CCI) [11],
-
-
Infection characteristics: delay between onset of symptoms and admission, presence of super-infection, C-reactive protein (CRP) and white blood cell count (WBC) at admission, percentage of lung injuries on CT-scan, if applicable, positive PCR amplifying the betacoronavirus E gene and the SARS-CoV-2 RdRp gene on nasopharyngeal swab or sputum,
-
-
Treatment characteristics: requiring ICU support with invasive ventilation and associated therapeutic strategies (e.g. oxygen, anti-infective agents),
-
-
Endpoint was defined as unfavorable outcome assessed by the requirement of a transfer to ICU for invasive ventilation and/or death within 30 days,
-
-
Patients were followed-up until hospital discharge. After discharge, patients were monitored for 30 days by telemedicine through the French covidom platform [12],
-
-
Derived variables: moderate lymphocytopenia was based on a lymphocyte count with a threshold at 1000/mm3 and high systemic inflammation was defined as a CRP threshold ≥100 mg/L.
2.3. Treatment strategies
All patients who required oxygen received systematic beta-lactam (preferably ceftriaxone or cefotaxime) for at least 5 days to treat a potential super-infection.
Patients were eligible to receive a supposedly effective anti-infective agent against COVID-19 (HCQ, AZI, lopinavir-ritonavir), independently of biological abnormalities and considering the following indications: i) patient presenting a clinical pneumonia confirmed by SARS-CoV-2 PCR, requiring oxygen therapy (independently of the CT scan findings); and ii) high suspicion of COVID-19 pneumonia considering the clinical presentation and/or pulmonary CT-scan showing ground-glass opacity affecting ≥10% of the whole parenchyma.
Patients were categorized as receiving an anti-infective agent once they received at least one dose. Patients who received lopinavir-ritonavir were categorized in a no treatment group, as this antiviral drug did not show any benefit for the treatment of COVID-19 [7].
Before initiation of HCQ or AZI, patients systematically underwent an electrocardiogram (ECG) to evaluate corrected QT interval using the Framingham formula, and were monitored twice per week during the whole treatment. Serum potassium levels were also monitored. A loading dose at day 1 with 800 mg/day was administered followed by a maintenance dose of 400 mg/day up to 600 mg/day in case of obesity (body mass index [BMI]) >30 kg/m2) for a total 10 days. In addition, 500 mg of AZI was prescribed the first day, followed by 250 mg for 4 days. Patients were informed that HCQ and lopinavir-ritonavir were currently off-label for the treatment of COVID-19 pneumonia until March 25th 2020 in France, where the ministerial decree #2020-314 authorized the in-hospital prescription of HCQ in this particular indication. In cases where the patient refused the prescription of HCQ or the latter was contraindicated (by ECG or drug interactions), this was noted on the patient medical chart and the patient did not receive HCQ.
2.4. Objective
The aim of the study was to describe the medical care over time (oxygen therapy, anti-infective agents, pulmonary CT-scan) and to determine whether potential factors were related to an unfavorable outcome (transfer to ICU and/or death).
2.5. Statistical analysis
Descriptive statistics are presented as counts and percentages, or means and standard deviations, with skewed continuous data summarized as medians and interquartile ranges.
Two periods were defined: the first two weeks (March 5th to March 19th) and the weeks thereafter (March 20th to April 25th), where practices became more standardized considering the French COVID-19 guidelines on the management of patients in ICU [13]. Patients were grouped according to these two periods and compared. A Student test (equal variance) or Welche Satterthwaite t-test (unequal variance) was used to analyse quantitative variables, a Mantel-Haenszel Chi-Square test was used to analyse qualitative variables and Fisher's exact test was used when the sample sizes were small (n<5).
Moving averages over 15 days were plotted to describe the evolution of care management over time using the following formula:
Time to endpoint was calculated from the date of hospitalization to the date of unfavorable outcome or hospital discharge. Two Cox proportional-hazards models were used to estimate hazard ratios (HR) for unfavorable outcome associated with medical care, after adjustment on risk factors and one biological parameter (one included lymphocyte count and the other included CRP level). Potential factors included were CCI (including age), obesity, oxygen flow and treatment. Interactions between treatment and lymphocyte count or CRP level were tested, and Kaplan-Meier curves were plotted to assess unfavorable outcome from admission depending on these biological parameters.
Statistical significance was set at 0.05 (two-tailed test). All statistical calculations were performed using R software version 4.2.0.
2.6. Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study passed the CESREES/Health Data Hub regarding ethics committee approval (MR1811190620) and is registered on ClinicalTrials.gov (NCT04453501). As part of an anonymous and retrospective study, a non-opposition and information letter was sent to participants afterwards.
3. Results
3.1. Description of the population
Between March 5th and April 25th 2020, 132 patients with COVID-19 were hospitalized. At baseline, mean age was 59.0±16.3 years with 64% male. Among them, 11% were obese (BMI >30), 22% were smokers, 23% had CCI >5 and 46% had lymphocyte count <1000/mm3. Mean CRP level was 84.0±71.1 mg/L with 46% >100 mg/L. Seventy-two percent of patients were oxygen-dependent at admission, with 8% of patients having oxygen flow therapy >5 L/min. Among the patients who underwent a pulmonary CT scan, 83% had lung injuries compatible with COVID-19 of >10% of the whole parenchyma. SARS-CoV-2 RT-PCR was positive in 95.5% (n=126) of cases.
3.2. Treatment strategies
Overall, 92 (70%) patients received one anti-infective agent. Among them, 12 (13%) received lopinavir-ritonavir, 28 (29%) azithromycin (AZI) and 52 (55%) AZI combined with HCQ (Table S1 in Supplementary Data). Mean delay from admission to treatment initiation was 0.7±1.5 days. Moreover, delay before treatment initiation was similar in the first and second period (1.3±1.9 days vs. 0.8±1.1 days, P=0.46). Of note, only one patient in the no treatment group received a short course of oral corticosteroids after 14 days of hospitalization.
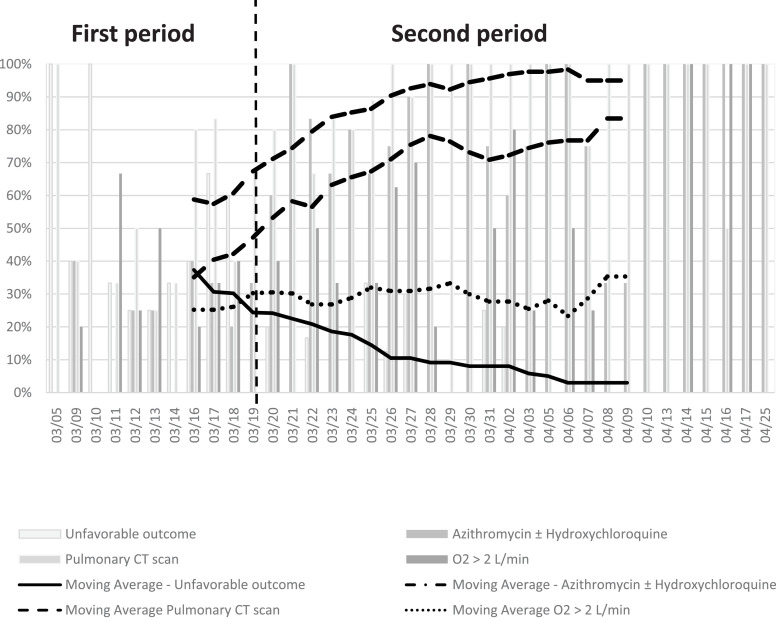
During the first period, 40 (30%) patients were hospitalized whereas 92 (70%) were admitted thereafter. There were significantly more oxygen-dependent patients hospitalized during the second period than the first (80% vs. 53%, P=0.001). Also, a significantly higher number of pulmonary CT scans were performed over time, with 50% in the first period and 90% in the second (P<0.0001), independently of CT-scan severity (Table 1 ). Prescription of AZI (with or without HCQ) increased over time, from 25% to 76% between the two periods (P<0.0001) (Fig. 1 ).
Table 1.
Baseline characteristics of patients with COVID-19 according to periods of hospitalization.
| Characteristics at baseline | In first period † | In second period ‡ | P-value |
|---|---|---|---|
| N= 40 | N= 92 | ||
| Age (year) — mean ± SD | 62.17 ± 15.24 | 57.59 ± 16.64 | 0.13 |
| Sex (M) — no. (%) | 26 (58) | 59 (64) | 0.99 |
| Obesity — no. (%) | 2 (4) | 13 (14) | 0.22 |
| Smoking (yes) — no. (%) | 13 (29) | 16 (17) | 0.09 |
| CCI* — no. (%) | |||
| 0 | 4 (10) | 20 (22) | 0.38 |
| 1-2 | 14 (35) | 33 (36) | |
| 3-4 | 11 (28) | 20 (22) | |
| ≥5 | 11 (28) | 19 (21) | |
| Pulmonary CT scan — no. (%) | 20 (50) | 83 (90) | <0.0001 |
| Normal | 2 (10) | 5 (6) | 0.46 |
| Limited | 6 (30) | 11 (13) | |
| Mild | 0 (0) | 24 (29) | |
| Moderate | 9 (45) | 32 (39) | |
| Severe | 3 (15) | 11 (13) | |
| Lymphocyte count <1000/mm3 — no. (%) | 17 (42) | 54 (59) | 0.13 |
| PMN count >8000/mm3 | 5 (13) | 9 (10) | 0.64 |
| CRP mg/L — mean ± SD | 84.59 ± 70.31 | 83.70 ± 71.86 | 0.95 |
| Oxygen (yes) — no. (%) | 21 (53) | 74 (80) | 0.001 |
| ≤2L/min | 10 (48) | 38 (51) | 0.55 |
| 2 – 5 L/min | 10 (48) | 27 (36) | |
| >5 L/min | 1 (5) | 9 (12) | |
| Treatment strategies — no. (%) | |||
| No treatment | 30 (75) | 22 (24) | <0.0001 |
| AZI ± HCQ | 10 (25) | 70 (76) |
In first period is defined as 03/05 to 03/19
In second period is defined as 03/20 to 04/25; AZI, Azithromycin; HCQ, Hydroxychloroquine; N, number; %, percent; SD, standard deviation; M, men; Obesity with body mass index ≥30 kg/m²
CCI, Charlson Comorbidity Index; PMN, polymorphonuclear leukocyte; CRP, C-reactive protein; CT, computerized tomography; pulmonary CT scan category normal 0%, limited <10%, mild 10–25%, moderate 25–50%, severe >50%; a Student test (equal variance) or Welche-Satterthwaite t test (unequal variance) was used to analyse quantitative variables, a Mantel-Haenszel Chi-Square test was used to analyse the qualitative variables and the exact test of Fisher was used when the sample sizes were small (<5). Test significant (P<0.05).
Fig. 1.
Evolution of medical care for COVID-19 patients from March 5th to April 25th.
Among the patients who did not receive HCQ, 5 had cardiac contraindication and 2 refused to be treated with this molecule. During combined treatment with AZI and HCQ, only 1 patient presented an adverse event (prolonged QT interval on ECG without clinical event) that led to discontinuation of HCQ within 48 h, and was switched to AZI alone.
3.3. Unfavorable outcome (ICU admission or death)
A total of 28 (21%) patients had an unfavorable outcome; 26 (93%) were transferred to ICU and 2 (7%) died without being transferred to ICU. Mean delay between hospitalization and admission to ICU was 2.45±1.45 days (2.4±1.5 days during the first period vs. 2.4±1.6 days during the second period, P=0.86). A trend towards a lower frequency of admission to ICU was observed, from 43% in the first period to 12% in the second period (P<0.0001) (Fig. 1).
3.4. Potential factors associated with unfavorable outcome
Overall, the risk of death or admission to ICU was significantly related to oxygen flow (P<0.001) and to lymphocyte count in a first model (i.e. lymphocyte count <1000/mm3) (HR=4.90, 95% confidence interval [CI: 1.95–12.3], P=0.0007) or to high systemic inflammation in a second model (i.e. CRP ≥100 mg/L) (HR=2.78, 95% CI [1.00–5.23], P=0.05). In addition, a relationship was observed between favorable outcome and use of AZI, whether or not it was combined with HCQ, compared with patients without any treatment (P=0.04) (Table 2 ).
Table 2.
Potential factors associated with unfavorable outcome: Cox model regression.
| Variables |
n/N |
Univariate model |
Multivariate model 1 |
Multivariate model 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR [IC95%] | P-value | HR [IC95%] | P-value | HR [IC95%] | P-value | |||||
| |
|
|||||||||
| Adjusted on ICC, obesity, O2, lymphocyte count and treatments | Adjusted on ICC, obesity, O2 CRP and treatments | |||||||||
| Characteristics at baseline | ||||||||||
| Age (years) | 132/132 | 1.02 [1.00 – 1.05] | 0.07 | - | - | - | - | |||
| Sex (M) | 85/132 | 0.86 [0.40 – 1.85] | 0.71 | - | - | - | - | |||
| Obesity (yes) | 15/132 | 0.27 [0.04 – 1.98] | 0.20 | 0.47 [0.06- 3.63] | 0.47 | 0.44 [0.06 – 3.45] | 0.43 | |||
| Smoking (yes) | 29/132 | 1.00 [0.41 - 2.48] | 0.99 | - | - | - | - | |||
| CCI* | ||||||||||
| 0 | 24/132 | 1* | - | 1* | - | 1 | - | |||
| 1-2 | 47/132 | 0.88 [0.26 - 3.00] | 0.83 | 1.05 [0.29 – 3.87] | 0.47 | 1.10 [0.31 – 3.92] | 0.89 | |||
| 3-4 | 31/132 | 1.88 [0.58 – 6.12] | 0.29 | 0.39 | 1.30 [0.37 – 4.54] | 0.68 | 0.97 | 1.74 [0.52 – 5.81] | 0.37 | 0.73 |
| ≥5 | 30/132 | 1.63 [0.49 – 5.43] | 0.42 | 1.10 [0.32 – 3.75] | 0.87 | 1.08 [0.32 – 3.71] | 0.90 | |||
| PMN count ≥8000/mm3 | 14/132 | 1.42 [0.49 – 4.10] | 0.52 | - | - | - | - | |||
| Lymphocyte count <1000/mm3 | 71/132 | 4.91 [1.99 – 12.1] | 0.0006 | 4.90 [1.95 – 12.3] | 0.0007 | - | - | |||
| CRP ≥100 mg/L | 85/132 | 2.86 [1.35 – 6.05] | 0.006 | - | - | 2.78 [1.00 – 5.23] | 0.05 | |||
| Treatment strategies | ||||||||||
| Oxygen (L/min) | 1.20 [1.10 - 1.31] | <0.0001 | 1.25 [1.13 – 1.38] | <0.0001 | 1.20 [1.08 - 1.32] | 0.0005 | ||||
| No treatment and | 52/132 | 1* | - | 1* | - | 1* | - | |||
| AZI ± HCQ | 80/132 | 0.63 [0.30 – 1.23] | 0.23 | 0.45 [0.21 – 0.97] | 0.04 | 0.42 [0.18 – 0.95] | 0.04 | |||
n/N number/total; 1* indicates the reference category; HR, Hazard ratio; CI, confidence interval; NS, not significant (P>0.05); PMN, polymorphonuclear; *CCI, The Charlson Comorbidity Index; CRP, C-Reactive protein; AZI, Azithromycin; HCQ, Hydroxychloroquine; No treatment defined as patients who have had no treatment or lopinavir-ritonavir; Multivariate Cox model regression was used to identify the potential factors associated with unfavorable outcome (ICU admission or death after ICU), adjusted on CCI (including age), obesity, oxygen and treatment strategies groups according to CRP.
3.5. Unfavorable outcome according to biological parameters (Kaplan-Meier curves)
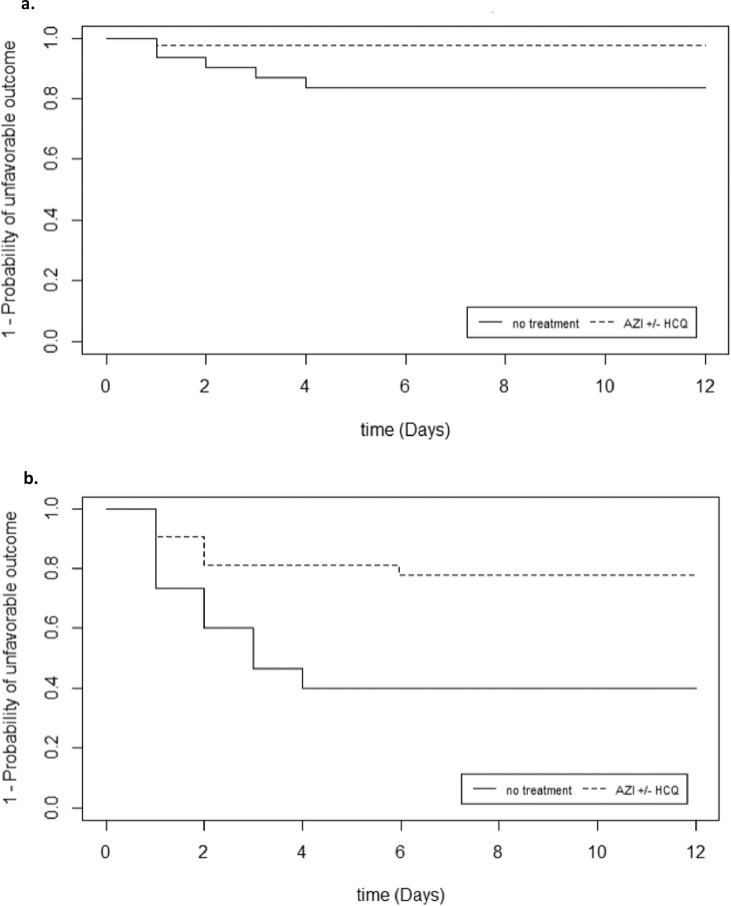
There was a statistically significant interaction between treatment and CRP level (P=0.02) and an interaction at the limit of statistical significance for lymphocyte count (P=0.06) supporting a subgroup analysis. In univariate analysis, patients who benefited from AZI±HCQ with a lymphocyte count ≥1000/mm3 were less likely to have an unfavorable outcome compared with patients with no treatment (P=0.04) (Fig. 2 a). Patients who benefited from AZI±HCQ with CRP ≥100 mg/L were less likely to have an unfavorable outcome compared with patients with no treatment (P=0.009) (Fig. 2b). However, these results were not reproducible in patients with lymphocyte count <1000/mm3 (P=0.80) or with CRP <100 mg/L (P=0.50) (Figure S3a, S3b in Supplementary Data).
Fig. 2.
a Kaplan-Meier survival curve for patients with an unfavorable outcome in function of treatment according to lymphocyte count ≥1000/mm3 (Log-Rank, P = 0.04).
b Kaplan-Meier survival curve for patients with an unfavorable outcome in function of treatment according to CRP ≥100 mg/L (Log-Rank, P = 0.009).
4. Discussion
In this study, unfavorable outcome (transfer to ICU and/or death) decreased over time during management of the first wave of the epidemic and was associated with an increased realization of pulmonary CT-scan and prescription of anti-infective agents, despite an increased need of oxygen therapy at admission. This indicates that medical care of COVID-19 patients improved over time in our hospital.
The results indicate that patients were admitted later in the second period than during the first period of the epidemic because of lockdown and this might explain why patients in the second period required more oxygen therapy at baseline. In case of a second wave, it could be relevant to introduce telemedicine monitoring of vital signs, including pulse oximetry, at home. Indeed, oxygen therapy at home, as proposed by the French covidom platform in patients discharged from hospital during the first wave of the epidemic, was of interest [12].
In multivariate analyses, models adjusted on lymphocyte count or CRP showed that patients who benefited from AZI±HCQ were 2.2 and 2.4 times less likely to have an unfavorable outcome than patients without treatment (P=0.04), respectively. This finding indicates that lymphocyte count, which is already known to be closely related to COVID-19 disease severity [14,15], could also be a predictive factor of anti-infective therapy response. Indeed, patients with lymphocyte count ≥1000/mm3 might be at an early stage of COVID-19, arguing for the earliest initiation of anti-infective agents, as previously demonstrated with oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1) [16]. However, any relationship between lymphocyte count and delay from first onset of symptoms to admission was not studied because this variable is declarative and thus not reliable. Likewise, AZI±HCQ was of interest in hospitalized patients with high systemic inflammation (CRP ≥100 mg/L), known as the “cytokine storm”. This is one argument for a possible immune-modulator effect of treatment, as previously described by Zhao et al. [17].
The present findings are concordant with a recent multicenter, retrospective, observational study conducted in the United States by Arshad et al. [7], who concluded that treatment with HCQ alone and in combination with AZI was associated with reduced COVID-19-associated mortality in hospitalized patients. Another study by Lagier et al. [18], partly composed of ambulatory care patients, showed a favorable outcome and decreased virological shedding using the combination of AZI with HCQ in a large sample size (n>3000) in a majority of patients with mild lymphocytopenia (≥1000/mm3). Mahevas et al. [4] also observed 15/15 favorable outcomes in a subgroup of patients receiving AZI with HCQ.
Interestingly, the present study focused on the potential interest of treatment with AZI, whether or not it was combined depending on certain biological parameters. Indeed, the potential antiviral activity of AZI is concordant with previous in vitro studies regarding SARS-CoV-2 [19] or H1N1-pdm09 [20] and one randomized clinical trial in in the prevention of children respiratory infections [21]. In addition, a recent publication emphasized the role of AZI against COVID-19 through the stem cell CD147 receptor [22]. Moreover, a study by Rosenberg et al. [23] highlighted a potential trend to decreased mortality in patients receiving AZI vs. HCQ or standard-of-care despite being non-statistically significant (P=0.14). Moreover, the authors discussed that the rapidity with which patients entered the ICU (within 48 h) might have underestimated the treatment efficacy. Also, as AZI is commonly prescribed for bronchitis and authorized in ambulatory care, a study conducted among general practitioners could be relevant to evaluate early indication of this single therapy for the treatment of COVID-19 in fragile outpatients.
In addition, our experience does not report any serious side effect of this combination therapy as long as we take the necessary caution and perform follow-up ECG using a conventional dose of HCQ, as proposed by Borba et al. [24].
The present study has several limitations. The was a single center study and describing the experience of a unique center might not be generalizable. However, the study was carried out in a hospital specialized for decades in the treatment of infectious diseases, ICU and rehabilitation. Since the beginning of the COVID-19 epidemic, an entire building has been entirely dedicated to admitting only COVID-19-positive patients. During the peak of the epidemic, we had a maximum capacity of 85 beds in medicine wards and 32 beds in ICU.
A better favorable outcome was observed over time related to an increased number of pulmonary CT-scans performed (these were not recommended at the beginning of the epidemic in our hospital) and, therefore, a more relevant prescription of anti-infective agents. Nevertheless, other confounding factors might have played a role as this was an unpredictable epidemic, with the need to constantly update guidelines about ICU admission, notably recommending to keep patients longer in medicine wards with high oxygen flow (>6 L/min) during the second period of the epidemic. Nevertheless, delay between admission and transfer to ICU were similar between the two time periods, which minimizes this confounding factor.
Considering the inherent limitation of a descriptive study with a limited sample size (n=132), causality in the association between the use of AZI±HCQ and the ameliorated prognosis in COVID-19 patients could not be inferred. Besides, some unforeseen confounders (e.g. pre-hospital medication and delay to admission) may still potentially alter the magnitude of AZI effects on the outcome of COVID-19 pneumonia. Also, choices in anti-infective agents differed between the first and second periods, notably because prior to March 25th, HCQ was not authorized by the French Minister of Health and this partly explained the common use of lopinavir-ritonavir during this period.
Finally, a multivariate model was chosen rather than a propensity score because the aim of this study was not to evaluate the effect of AZI±HCQ on prognosis but to evaluate all factors that could have impacted on medical care.
In conclusion, findings from this study showed that rate of admission to ICU decreased from 43% during the first period (from March 5th to March 19th) to 12% during the second period (from March 20th to April 25th).
Numerous factors might be involved in the improvement of care, including the implementation of routine pulmonary CT-scans, better management of oxygen therapy in the medicine ward and possibly use of anti-infective agents. Indeed, the present study results indicate that AZI±HCQ might have impacted COVID-19 outcome in a subpopulation of patients (lymphocyte count ≥1000/mm3 or CRP ≥100 mg/L), raising the question of optimal timing of treatment interventions. A larger and randomized controlled study is necessary to explore the profiles of patients responding to this therapeutic strategy and to confirm the potential interest of biological parameters for treatment initiation.
Acknowledgments
Contributors’ Statement
BD, PDT and CP conceptualized and designed the study, carried out the initial analyses, coordinated and supervised data collection, drafted the initial manuscript, and reviewed the manuscript.
BD, FB, PDT and TL designed the data collection instruments, collected data and reviewed and revised the manuscript. VP, DA, PM and AL participated in patient enrollment.
GB and IV were in charge of the statistical analyses and contributed to the final version of the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
Authors would like to thank Pr Xavier Paoletti for his proofreading of the manuscript and his particular attention to the statistical analyses.
Funding: The authors have no financial relationships relevant to this article to disclose.
Competing Interests: BD has received consulting fees or travel grants from ViiV Healthcare and Gilead Sc. PdT has received consulting fees or travel grants from ViiV Healthcare, M.S.D and Gilead Sc. The remaining authors have no specific conflict of interest.
Ethical Approval: Not required.
Randomized Controlled Trial: NCT04453501.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2020.106129.
Appendix
List of Collaborators
Department of Intensive Care
Djillali Annane, MD, PhD (1,2,5)
Xavier Ambrosi, MD (4)
Suzanne Amthor, MD (1)
Rania Bounab, MD (1,2)
Ryme Chentouh, MD (1)
Bernard Clair, MD (1)
Abdallah Fayssoil, MD (1,2,5)
Diane Friedman, MD (1)
Nicholas Heming, MD, PhD (1,2,5)
Virginie Maxime, MD, (1)
Pierre Moine, MD, PhD (1,2,5)
Myriam Niel Duriez, MD (1)
David Orlikowski, MD, PhD (1,2,5,8)
Francesca Santi, MD (1,2)
Pharmacy
Frédérique Bouchand, PharmD (1)
Muriel Farcy-Afif, PharmD (1)
Hugues Michelon, PharmD, MSc (1)
Maryvonne Villart, PharmD (1)
Research Staff
Isabelle Bossard (8)
Tiphaine Barbarin Nicolier (1)
Stanislas Grassin Delyle, MCUPH (2,3,5)
Elodie Lamy (2,5)
Camille Roquencourt, MD (5)
Gabriel Saffroy (2)
Etienne Thevenot (5)
Department of Intensive Care Interns
Baptiste Abbar (1)
Steven Bennington (1)
Juliah Dray (1)
Pierre Gay (1)
Elias Kochbati (1)
Majistor Raj Luxman Maglorius Renkilaraj (1)
Myriam Moucachen (1)
Alice Pascault (1)
Juan Tamayo (1)
Justine Zini (1)
Department of Anesthesia, Perioperative Care, and Pain
Marie Boutros, MD (1)
Anne Lyse Bron, MD (11)
Denys Coester, MD (12)
Etiennette Defouchecour, MD (11)
Brigitte Dosne Blachier, MD (11)
Léa Guichard, MD (1)
Damien Hamon Pietrin, MD, PhD (1)
Hakim Khiter, MD (1)
Valéria Martinez, MD, PhD (1,2,6)
Simone Meuleye, MD (1)
Suzanne Reysz, MD (1)
Sebastien Schitter, MD (1)
Chawki Trabelsi, MD (1)
Pediatric Critical Care Unit
Helge Amthor, MD, PhD (1,2,7)
Jean Bergounioux MD (1,2,5)
Maud Guillon, MD (1)
Amal Omar, MD (1)
Laboratory of Physiology
Frédéric Lofaso, MD, PhD (1,2,7,10)
Helene Prigent, MD, PhD (1,2,7,10)
Department of Rehabilitation and Physical Medicine
Djamel Bensmail, MD, PhD (1,2,7,10)
Pierre Denys, MD, PhD (1,2,7,10)
Charles Joussain, MD, PhD (1)
Lauren Kagane, MD (1)
Thibaut Lansaman, MD (1)
Hélène Le Liepvre, MD (1)
Antoine Leotard, MD, MS (1)
Jonathan Levy, MD, MS (1,2,7,10)
Claire Malot, MD (1)
Julie Paquereau, MD (1)
Celia Rech, MD (1)
Department of Rehabilitation Interns
Florence Angioni (1)
Elsa Chkron (1)
Céline Karabulut (1)
Jérôme Lemoine (1)
Noémie Trystram (1)
Julien Vibert (1)
Department of Infectious Diseases
Pascal Crenn, MD, PhD (1,2,7)
Benjamin Davido, MD, MS (1)
Stéphanie Landowski, MD (1)
Christian Perronne, MD, PhD (1,2)
Véronique Perronne, MD (1)
Pierre de Truchis, MD, MS (1)
Department of Infectious Diseases Interns
Marc Hobeika (1)
Louis Jacob (1)
Nicolas Kiavue (1)
Aymeric Lanore (1)
Aurélie Le Gal (1)
Julia Nguyen Van Thang (1)
Department of Microbiology and Innovative Biomarkers Platform
Coralie Favier (1)
Jean Louis Gaillard, MD, PhD (1,2,5)
Elyanne Gault, MD, PhD (1,2,5)
Jean-Louis Herrmann, PharmD, PhD (1,2,5)
Christine Lawrence, PharmD (1)
Virginie Lebidois, PharmD (1)
Latifa Noussair, MD (1)
Martin Rottman, MD, PhD (1,2,5)
Anne-Laure Roux, PharmD, PhD (1,2,5)
Sophie Tocqueville (1)
Marie-Anne Welti, MD, PhD (1,2,5)
And the non-medical staff of the Department
Department of Laboratory Medicine and Pharmacology
Jean Claude Alvarez, MD, PhD (1,2,5)
Mehdi Djebrani, PharmD (1)
Pierre-Alexandre Emmanuelli (1)
Firas Jabbour, PharmD (1)
Lotfi Lahjomri, MD (1)
Mathilde Parent, MD (1)
And the non-medical staff of the Department
Department of Radiology
Amine Ammar, MD (1)
Najete Berradja, MD (1)
Robert-Yves Carlier, MD, MS (1,2,7,14)
Annaelle Chetrit, MD (1,2)
Caroline Diffre, MD (1,2)
Myriam Edjlali, MD, PhD (1,15)
Zaki El Baz, MD (1,14)
Adrien Felter, MD (1)
Catherine Girardot, MD (1,13)
Ahmed Mekki, MD, MS (1,2)
Dominique Mompoint, MD (1)
Dominique Safa, MD (1)
Tristan Thiry, MD (1)
Department of Radiology Interns
Margot Armani (1)
Olivier de Barry (1)
Antoine Kirchner (1)
Jeffery Zhou (1)
Department of Forensic Medicine
Geoffroy Lorin de La Grandmaison MD, PhD (1)
Department of Forensic Medicine Intern
Kevin Mahe (1)
Affiliations
1. Hôpital Raymond Poincaré, GHU APHP, Université Paris Saclay, Garches, France
2. Faculté Simone Veil Santé, Université Versailles Saint Quentin en Yvelines, Université Paris Saclay, Montigny-le-Bretonneux, France
3. Hôpital Foch, Suresnes, France
4. Centre Hospitalier Universitaire de Nantes, Nantes, France
5. Université de Versailles Saint-Quentin-en-Yvelines/INSERM, Laboratory of Infection & Inflammation–U-1173, Montigny-le-Bretonneux, France
6. Université de Versailles Saint-Quentin-en-Yvelines/INSERM, Centre d'Evaluation et de Traitement de la Douleur–U-987, Boulogne-Billancourt, France
7. Université de Versailles Saint-Quentin-en-Yvelines/INSERM, Handicap Neuromusculaire–U-1179, Montigny-le-Bretonneux, France
8. Centre d'Investigation Clinique, Garches, France
9. Commissariat à l'Energie Atomique, CEA Paris Saclay, Gif-sur-Yvette, France
10. Fondation Garches, Garches, France
11. Clinique Jouvenet, Ramsay Santé, Paris, France
12. Clinique de la Muette, Ramsay Santé, Paris, France
13. Polyclinique Mantaise, Mantes-La-Jolie, France
14. Centre Hospitalier Intercommunal Poissy/Saint-Germain, GHT Yvelines Nord, Poissy, France
15. IMA-BRAIN/INSERM–UMR-1266, DHU-Neurovasc, Centre Hospitalier Sainte-Anne, Paris, France
Appendix. Supplementary materials
References
- 1.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Sevestre J., et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Million M., Lagier J.-C., Gautret P., Colson P., Fournier P.-E., Amrane S., et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahévas M., Tran V.-T., Roumier M., Chabrol A., Paule R., Guillaud C., et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/nejmoa2007764. [DOI] [PubMed] [Google Scholar]
- 11.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Group T.C.-A. Assistance Publique–Hôpitaux de Paris’ response to the COVID-19 pandemic. Lancet. 2020;395(10239):1760–1761. doi: 10.1016/S0140-6736(20)31210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SRLF-SFAR-SFMU-GFRUP-SPILF-SPLF. Recommandations d'experts portant sur la prise en charge en réanimation des patients en période d’épidémie à SARS-CoV22020:29. https://www.srlf.org/wp-content/uploads/2020/03/RFE-COVID_V3_FINAL-1.pdf.
- 14.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Segentanis T.N., Politou M., et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/AJH.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A. Early versus late oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1): speed is life. J Antimicrob Chemother. 2011;66(5):959–963. doi: 10.1093/jac/dkr090. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020;55(6) doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagier J.-C., Million M., Gautret P., Colson P., Cortaredona S., Giraud-Gatineau A., et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med Infect Dis. 2020;36 doi: 10.1016/J.TMAID.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Touret F., Gilles M., Barral K., Nougairède A., Decroly E., Lamballerie X. de, et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. BioRxiv 2020:2020.04.03.023846. doi: 10.1101/2020.04.03.023846. [DOI] [PMC free article] [PubMed]
- 20.Tran D.H., Sugamata R., Hirose T., Suzuki S., Noguchi Y., Sugawara A., et al. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J Antibiot (Tokyo) 2019;72(10):759–768. doi: 10.1038/S41429-019-0204-X. [DOI] [PubMed] [Google Scholar]
- 21.Bacharier L.B., Guilbert T.W., Mauger D.T., Boehmer S., Biegelman A., Fitzpatrick A.M., et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034–2044. doi: 10.1001/JAMA.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulrich H., Pillat M.M. CD147 as a target for COVID-19 Treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. 2020;16(3):434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., et al. Effect of High vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.