Abstract
Mollusc shells are an abundant resource that have been long used to predict the structures of ancient ecological communities, examine evolutionary processes, reconstruct paleoenvironmental conditions, track and predict responses to climatic change, and explore the movement of hominids across the globe. Despite the ubiquity of mollusc shell in many environments, it remains relatively unexplored as a substrate for molecular genetic analysis. Here we undertook a series of experiments using the New Zealand endemic greenshell mussel, Perna canaliculus, to explore the utility of fresh, aged, beach-cast and cooked mollusc shell for molecular genetic analyses. We find that reasonable quantities of DNA (0.002–21.48 ng/mg shell) can be derived from aged, beach-cast and cooked mussel shell and that this can routinely provide enough material to undertake PCR analyses of mitochondrial and nuclear gene fragments. Mitochondrial PCR amplification had an average success rate of 96.5% from shell tissue extracted thirteen months after the animal’s death. A success rate of 93.75% was obtained for cooked shells. Amplification of nuclear DNA (chitin synthase gene) was less successful (80% success from fresh shells, decreasing to 10% with time, and 75% from cooked shells). Our results demonstrate the promise of mollusc shell as a substrate for genetic analyses targeting both mitochondrial and nuclear genes.
Keywords: Perna canaliculus, Mollusc shell, DNA analysis
Introduction
Molluscs are the most diverse of the marine phyla, with some 85,000 described species (Chapman, 2009) that include animals such as clams, slugs and octopuses (Appeltans et al., 2012). The characteristic mollusc shell, common to much of the phylum, consists mainly of calcium-carbonate (Furuhashi et al., 2009), is highly resilient, and can persist in the environment long after the animal has died (Vendrasco et al., 2016). In fossil assemblages, for instance, shell material can be a predictor of ancient community composition (Kidwell, 2001), and harbour a rich source of information for exploring mollusc evolution (Vendrasco et al., 2016; Der Sarkissian et al., 2017; Coutellec, 2017) and undertaking paleoenvironmental reconstructions (Rhoads, 1970; Krantz, Williams & Jones, 1987; Coutellec, 2017; Der Sarkissian et al., 2017). Likewise, the almost ubiquitous exploitation of marine mollusc as a coastal food source in many early human communities provides a rich source of information on the lives of those people and the ecology and climate in which they lived (Balbo et al., 2011; Barsh & Murphy, 2008; Colonese et al., 2011).
Whether from natural samples or from samples collected and used by humans, analysis of mollusc shell remains provide important insights into our past. The application of techniques such as radiocarbon dating and the recently developed Amino Acid Racemization (AAR) technique (Demarchi et al., 2011) give samples of mollusc shell chronological meaning, while stable isotope analysis enables quantification of nutrient flows and may provide some insights into food web dynamics (Zanden & Rasmussen, 2001). Finally, taxonomic analysis of mollusc shell remains provides knowledge of species abundance and community structure, and when coupled with accurate chronology can lead to significant insights into how species, communities and ecosystems respond to a variety of anthropogenic pressures and environmental changes (Balbo et al., 2011; Estevez et al., 2001).
Species identification based on shell valves often relies on specialised taxonomic knowledge, a scientific skill in continuous decline since the 1950’s (Tautz et al., 2003; Hebert & Gregory, 2005; Kim & Byrne, 2006). Unlike DNA from soft tissues, which degrades relatively quickly following the death of the organism, DNA molecules incorporated within mineral matrix such as bone and teeth is largely protected from enzymatic and microbial degradation (Pääbo et al., 2004; Higgins et al., 2015). The development of techniques that enabled the extraction of trace amounts of DNA from ancient mineralized tissue samples such as bone and teeth (Kalmár et al., 2000; Höss & Pääbo, 1993; Rohland & Hofreiter, 2007) sparked interest in whether other mineralized tissues, such as mollusc shell, might also have utility for DNA analyses (Doherty, Gosling & Was, 2007; Barsh & Murphy, 2008; Geist, Wunderlich & Kuehn, 2008; Wang et al., 2012; Der Sarkissian et al., 2017).
In bivalves, a group of specialised hematocyte cells called refractive granulocytes has been shown to be involved in the secretion, and active remodelling of calcium carbonate crystals for the formation of the shell (Mount et al., 2004; Li et al., 2016; Ivanina et al., 2017). It is likely that during this process, these cells might be trapped and absorbed in the growing shell, leaving traces of DNA (Doherty, Gosling & Was, 2007; Wang et al., 2012; Barsh & Murphy, 2008; Der Sarkissian et al., 2017). These DNA traces provide an opportunity to genetically analyse recently dead individuals, historical specimens, samples from shell middens and even ancient shell samples (Der Sarkissian et al., 2017).
Moreover, because living molluscs can quickly repair shell damage (Fleury et al., 2008), shell sampling techniques that avoid damaging the underlying mantle epithelia enable non-lethal sampling of valuable animals, such as aquaculture broodstock or those of endangered species (Wang et al., 2012).
Despite its broad appeal, the use of mollusc shell as a source of DNA for molecular analysis remains incipient. Here we undertook a series of experiments, using shell remains from the widespread and commercially important bivalve mollusc, the New Zealand greenshell mussel (Perna canaliculus (Gmelin 1791)), to develop an effective and reliable method for extracting genomic DNA from mollusc shells. We test the effects of shell age and treatment (e.g., cooking and weathering) on DNA quality and suitability for downstream analysis by PCR using mitochondrial and nuclear primers.
Materials & Methods
For all experiments, live greenshell mussels were acquired from commercial sources in Dunedin, New Zealand. Animals were completely removed from their shells, which were then cleaned with at least three rinses of distilled water before being dried in an incubator at 20 °C overnight. One of the valves was subsequently roughly smashed and a 0.5 g shell sample taken for DNA extraction.
Ageing shells
For each of 10 individuals, shell samples were taken from the same valve at monthly intervals over 13 months (130 samples in total). DNA extraction was performed immediately after each collection. During the sampling period, shells were individually wrapped in foil and stored at room temperature in a plastic bag to ensure minimal airflow.
Cooked mussel shells
Sixteen live whole greenshell mussels were divided into four treatment groups, and cooked either by steaming in salted water or cooking over firewood embers, two common ways to cook shellfish, for either five or ten minutes (four mussels per treatment). Shells of cooked mussels were thoroughly cleaned to remove soft tissue, before being rinsed and dried as described above. After drying, large amounts of the periostracum –the outermost layer of the shell - peeled off, particularly around the edges. Shell samples were collected from each animal and immediately processed for DNA extraction.
Beach-cast shells
Eleven shells (single valves) of P. canaliculus were collected from two locations (Dunedin, New Zealand), cleaned and dried as described previously. For each valve there was an attempt to collect samples from (1) the outermost ventral part of the shell; (2) within the area of the pallial sinus, and (3) the umbo of each shell, which would include ligament tissue if present (Fig. 1). Some valves were found broken, and did not allow sampling of all three areas. Given the low DNA yield expected for such shells, we assessed the potential risk of cross-contamination of mussel DNA within our laboratory. An additional sample was collected from 8 of the beach-cast shell valves and used for DNA extraction and amplification in a different research facility (different lab in another building of the University of Otago, Dunedin campus) following the extraction and amplification methods described here, so that the risk of contamination in our own facility could be assessed. Once collected, samples were processed for DNA extraction immediately after collection.
Figure 1. P. canaliculus shell regions sampled in the beach-cast valves.
(1) ventral region; (2) pallial sinus; (3) umbo. Image credit: Sara Ferreira.
DNA extraction method
Shell samples were broken into smaller pieces using a mortar and pestle, re-weighed, and placed in a SafeLock two mL tube (Eppendorf) with a stainless steel five mm bead (Qiagen). All samples and two blank tubes (steal bead but no shell) were processed using a TissueLyser II machine (Qiagen) at the maximum frequency setting (30 Hz) for five minutes. DNA was extracted following a salting-out method (Gemmell & Akayama, 1996), with these modifications: (1) lysis incubation was performed overnight; (2) the resulting DNA was resuspended in 100 µL low EDTA TE buffer (10 mM Tris-HCl, 0.1 mM EDTA, pH 8). DNA yields were determined using a Qubit® 2.0 Fluorometer (ThermoFisher Scientific), using the dsDNA HS Assay (ThermoFisher Scientific). DNA (ng)/shell (mg) sample ratios were calculated for each sample. Shell valves from beach-stranded specimens were very brittle, and were processed solely with mortar and pestle. DNA from these samples was eluted in 40 µL low EDTA TE buffer.
DNA quality assessment by PCR - mtDNA
We designed a set of two mitochondrial DNA (mtDNA) PCR primers to produce amplicons in the range of < 200 to 300 base pairs (bp) (Table 1) using Primer3Plus (Untergasser et al., 2012), under the expectation that DNA extracted from bivalve shell material would be degraded and of low yield (Taberlet, Waits & Luikart, 1999). Primers were designed using published sequences from the work of Blair et al. (2006), with the exception of one primer which was originally designed by these same authors (Blair et al., 2006) (primer COX1 Fwd, see Table 1). Targeted mitochondrial regions were the COX1 gene (primers COX1 Fwd and COX1 Rev, specific to Perna spp.) and the NADH4/ ATP8 gene region (primers Pcan Fwd and Pcan Rev, specific to P. canaliculus alone) (Table 1). PCR reactions were carried out in a total volume of 30 µL, containing 1x Bioline NH4 Reaction Buffer, 0.1U Bioline BIOTAQ™ DNA Polymerase, 2.75 mM MgCl2 solution, 0.25 µM of each primer, 0.25 µM dNTP’s and 1.5 µL DNA. Bovine Serum Albumin (BSA) was also added at a concentration of 10 mM BSA in the PCR using Pcan primers, and 40 mM in the COX1 PCR. Reactions were run in an MJ Research Tetrad PTC-225 Thermal Cycler.
Table 1. Primers used to assess DNA quality from shell tissue extracts.
| Primer | Sequence | Amplicon (bp) | Target Region |
|---|---|---|---|
| Pcan Fwd | ACAGACTTCACTTATACACAACAAC | 305 | NADH4 –ATP8 gene region |
| Pcan Rev | AGGGCTCTAACTCAATATAACCCC | ||
| COXI Fwda | GRGATCCTGTTTTATTTCAGCAYGT | 191 | COX1 gene |
| COXI Rev | TGCCCAAACAACACACCCTA | ||
| Chitin S .Fwd | CCGTGTCTGTAATGTTGGTCTA | 198 | Chitin Synthase nuclear gene |
| Chitin S. Rev | TCTTTGCCATTCGTTCACAC |
Notes.
Similar to primer Pernacox1F used by Blair et al. (2006).
The PCR program for mtDNA primers was 1 min at 96 °C, followed by 35 cycles of 1 min at 96 °C, 30 s at 57 °C and 30 s at 72 °C, with a final elongation step of 5 min at 72 °C. Positive and negative (no template) controls were always included. The positive control was a 1:100 dilution of DNA (1.5 ng/µL) extracted from fresh mantle tissue of one individual sacrificed for the ageing shell experiment, using the DNA extraction method described by Gemmell & Akayama (1996). PCR product (20 µL) was visualised through a 1.5% agarose gel stained with 1× GelRed (Biotum), together with 5 µL of EasyLadder I DNA ladder (Bioline).
Samples not producing a visible band were re-amplified using template dilutions of 1:5 and 1:10 in water, with the thought that PCR inhibitors compounds might have been co-extracted with biomineralized tissue sourced DNA, and preventing amplification.
Failure to observe a visible band in all PCR attempts led us to designate that particular sample as unsuccessful.
To verify the species specificity of our mitochondrial PCRs, we tested the Perna canaliculus DNA positive control template alongside DNA templates from Perna viridis (Florida, USA) and Perna perna (Haga Haga, South Africa).
DNA quality assessment by PCR - gDNA
The chitin synthase (CS1) gene previously identified from Mytilus galloprovincialis (Weiss et al., 2006), was used to test the tractability of obtaining genomic DNA (gDNA) from our shell samples. Using genomic and transcriptomic data already collected by our group for the New Zealand greenshell mussel, a homologue of the chitin synthase gene was identified and its sequence registered on GenBank (Benson et al., 2012) with accession reference MT305043. Primers were designed to amplify a 198 bp region within the coding sequence of the P. canaliculus chitin synthase gene sequence (Fig. 2). Our analyses suggest this gene is well conserved and apparently single-copy in molluscs. PCRs were performed as described earlier but without BSA, and using an annealing temperature of 53 °C.
Figure 2. Alignment of the chitin synthase gene in Perna canaliculus (Ashby et al unpublished) and Mytilus galloprovincialis (Benson et al., 2012) (GenBank accession number: EF535882.1).
Primer sequences highlighted in grey, base differences between P. canaliculus and M. galloprovincialis in red.
To have a sense of the availability of nuclear gDNA throughout the ageing experiment, the chitin synthase PCR was tested with the 0, 1st, 6th and 13th month collections. These primers were also tested in the cooked and beach-cast shell samples. Sample success or failure was explored as described for the mitochondrial amplicons.
DNA quantification by qPCR
To confirm amplification of mDNA specific to our target species in our extracts, qPCR was also performed in the ageing shell experiment collections at 0, 1, 6 and 13 months, cooked shell experiment and on a subset of the beach-cast shell extracts. qPCR reactions (25 µL) were carried out in triplicates using the SensiMix SYBR Low-Rox kit (Bioline), 1 µl shell extract sample and Pcan Fwd and Pcan Rev primers (Table 1) (250 nM final concentration). Reactions were run in a QuantStudio 3 Real-Time PCR System (ThermoFisher Scientific), using the following program: initial denaturation with 10 min at 95 °C, followed by 40 cycles of 15 s. at 95 °C, 15 s. at 57 °C and 15 s. at 72 °C, and a melt curve cycle with 15 s. at 95 °C, 1 min at 60 °C and 1 s. at 95 °C. Data collection points were set at every annealing and elongation steps of the cycling program, and at the end of the melt curve cycle. A standard dilution series (undiluted DNA sample and six 1:10 serial dilutions) was made using P. canaliculus DNA extracted from fresh mantle tissue of one individual using the DNA extraction method described by (Gemmell & Akayama, 1996). DNA amount of each the standard dilution was quantified with a Qubit® 2.0 Fluorometer (ThermoFisher Scientific), using the dsDNA HS Assay (ThermoFisher Scientific). All six standard dilutions, in addition to undiluted P. canaliculus DNA sample and a negative (no template) control, were included in each qPCR run as duplicates. Amplification results were visualised and acquired through the ThermoFisher Connect™ software. A linear regression for the serial dilution series was made for every qPCR run and used correlate amplification results with P. canaliculus-specific DNA concentration for every sample tested. Amplification for each sample was determined through its triplicate set results; any triplicate result inconsistent with the other two replicates was discarded. Results with the outcomes “No amplification”, or “Undetermined” due to no amplification were quantified as having 0 ng/µl of P. canaliculus DNA. Melting curve results for each sample were used to control for the specificity of the amplified products.
DNA amplicon sequencing and sequence analysis
PCR products of samples showing a visible band of expected size in agarose gels were purified using an AcroPrep™ Advance filter plate (PALL) following the manufacturer’s instructions. Purified products were quantified with a NanoDrop 2000 spectrophotometer, and sequenced using an ABI 3730xL DNA Analyser (Applied Biosystems) through the Genetic Analysis Services (GAS) at Otago University. Sequences were checked for taxonomic congruence using BLASTn http://blast.ncbi.nlm.nih.gov (Benson et al., 2012) and the results visualised using the BLAST Tree View function. Amplicon sequences were subsequently aligned with the Geneious software against their respective references. Sequences with poor quality (low signal, short length or failed reaction) were re-submitted to GAS, or re-amplified if they were again of poor quality.
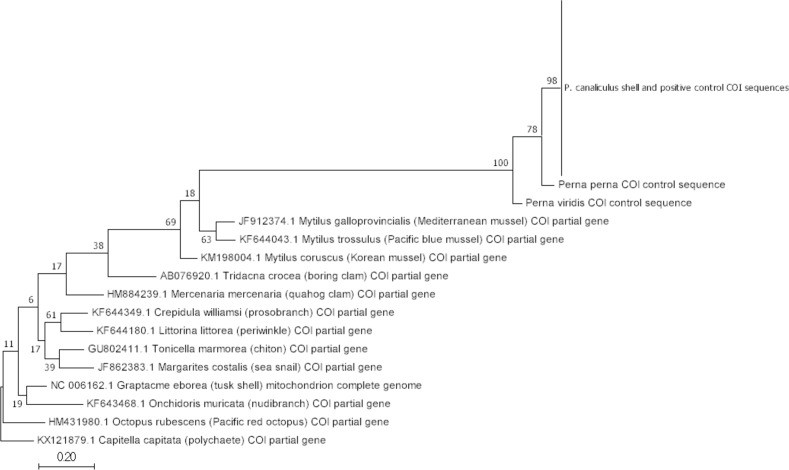
Additionally for the COX1 amplicons, all P. canaliculus shell sequences were also aligned using MEGA version 7 (Kumar, Stecher & Tamura, 2016) together with COX1 sequences from P. perna and P. viridis we obtained from our COX1 control PCR, and other published COX1 sequences of representative molluscs (AB076920.1 Tridacna crocea; AY260822.1 Antalis sp.; GU802411.1 Tonicella marmorea; HM431980.1 Octopus rubescens; HM862494.1 Limacina helicina; HM884239.1 Mercenaria mercenaria; HM884246.1 Modiolus modiolus; JF862383.1 Margarites costalis; JF912374.1 Mytillus galloprovincialis; KF643468.1 Onchidoris muricata; KF644043.1 Mytilus trossulus; KF644180.1 Littorina littorea; KF644349.1 Crepidula williamsi; KM198004.1 Mytilus coruscus). A phylogenetic tree was constructed using Maximum Likelihood approaches with MEGA version 7 (Kumar, Stecher & Tamura, 2016) using KX121879.1 Capitella capitata (annelid) as outgroup (Fig. 3).
Figure 3. Molecular phylogenetic analysis of P. canaliculus COX1 shell sequences and positive control sequence, together with other COX1 mollusc species sequences.
Maximum Likelihood method based on the Tamura-Nei model (Tamura & Nei, 1993). Sequence KX121879.1 Capitella capitata was selected as outgroup. The tree with the highest log likelihood (−1371.62) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbour-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved a total of 130 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated. There was a total of 110 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar, Stecher & Tamura, 2016).
Results
DNA extraction and quantification
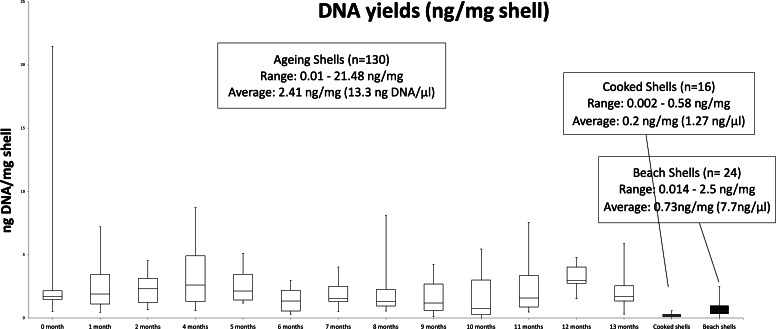
Qubit measured DNA yields ranged from 0.01 to 88.4 ng DNA/µl (0.002 to 21.48ng DNA/mg shell) (Fig. 4), with an overall average of 13.3 ng/µl. In the shell ageing experiment, DNA yields were highly variable in the first collection at 0 months (Fig. 4), and included the sample with the highest yield (88.4ng/µl or 21.48 ng/ mg shell). Cooked shells included the sample with the lowest yield (0.01ng/µl or 0.002 ng DNA/mg shell) (Fig. 4). No statistical difference between the four treatments was detected when comparing Qubit measured DNA yields (one-way ANOVA, F3,12 = 0.35, P = 0.79, MS Excel (2016)).
Figure 4. BoxPlot of average DNA yields.
Yields as ng DNA/mg shell weight ratio. Values through time in shell ageing experiment, and total average DNA yields from cooked and beach-cast shells (black filled box plot samples).
Beach-cast shells had on average some three-fold higher Qubit measured DNA yields than cooked shells (Fig. 4). There was no significant difference (one-way ANOVA, F2,21 = 1.95, P = 0.17, MS Excel (2016)) found between the Qubit measured DNA yields from each of the three locations in the shells (ventral, palial sinus and umbo regions).
DNA quality assessment by PCR
Successful PCR amplification for each primer set is depicted in Table 2. A total of 168 samples were tested with each of the mitochondrial primer sets. Pcan primers were used to successfully amplify, sequence and identify as P. canaliculus 85% of the samples. The COXI primer set was successful in 91% of the samples. The Chitin Synthase nuclear primer set was tested with a total of 80 samples, and successfully amplified, sequenced and identified as P. canaliculus in 40% of these.
Table 2. Samples successfully identified as P. canaliculus in each experiment.
Numbers in parenthesis relate to number of samples tested, and are followed by the number of samples that were successfully amplified.
| Mitochondrial Amplicons | Nuclear Amplicon | ||||
|---|---|---|---|---|---|
| Collection (month) | Pcan(305 bp) | qPCR Pcan(305 bp) | COX1(191 bp) | Chitin Synthase(198 bp) | |
| Aged shells | 0 (n = 10) | 8 | (n = 10) 9 | 9 | 8 |
| 1 (n = 10) | 10 | (n = 9) 9 | 10 | 7 | |
| 2 (n = 10) | 9 | 9 | – | ||
| 4 (n = 10) | 7 | 9 | – | ||
| 5 (n = 8) | 8 | 8 | – | ||
| 6 (n = 10) | 10 | (n = 9) 9 | 10 | 1 | |
| 7 (n = 10) | 10 | 10 | – | ||
| 8 (n = 10) | 10 | 10 | – | ||
| 9 (n = 10) | 10 | 10 | – | ||
| 10 (n = 10) | 10 | 10 | – | ||
| 11 (n = 10) | 10 | 10 | – | ||
| 12 (n = 10) | 10 | 10 | – | ||
| 13 (n = 10) | 10 | (n = 8) 8 | 10 | 3 | |
| N total = 128 | Average= 95.3% | Average= 97.2% | Average= 97.7% | Average= 47.5% | |
Between the three standard PCR primer sets used, a total of 416 reactions were tested, and of those, 253 (60.8%) were successfully amplified, sequenced and identified without template dilution. Template dilution in the remaining samples was helpful to a further 76 reactions, but had no effect in amplification success of the remaining 87 (20.9%). Most reactions that failed amplification despite template dilution used the chitin synthase nuclear primers. A total of 46 samples could not be amplified with this primer pair. Across all three primer pairs, the majority of failed reactions (52 samples) used beach-cast shell templates.
Elution of replicate DNA extracts in either TE buffer with low EDTA (0.1 mM) or deionized water did not improve amplification success.
Due to a combination of use of template in optimization trials, and evaporation in the microcentrifuge tube, two individual shell samples from the 5th month collection of the aged shell experiment did not have enough template for PCR analysis with any of our PCRs (Table 2).
Beach-cast shell samples, independently extracted in another laboratory, showed similar amplification successes to our own extractions, confirming the unlikelihood of source of contamination in our own research facility.
PCR using the Pcan primers did not amplify with either P. viridis or P. perna templates, confirming species-specificity for this assay. As expected, the COX1 PCR amplified equally well in all three Perna species.
DNA quantification by qPCR
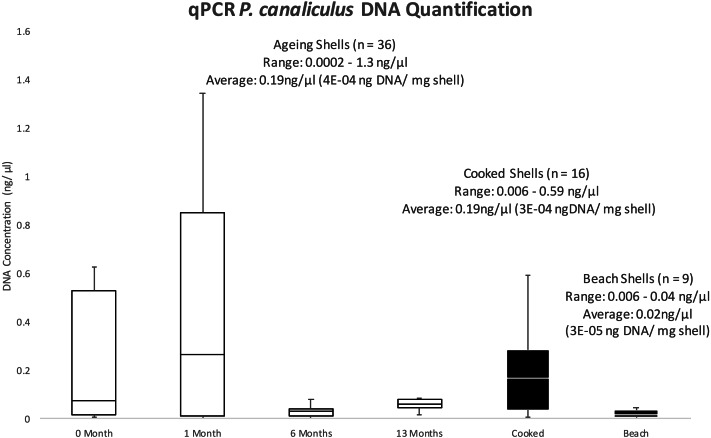
A total of 61 samples were tested with our qPCR assay (Table 2), with an average yield of 0.19 ng/µl for both ageing shells and cooked shells, and 0.02 ng/µl in beach-cast shells tested (Fig. 5). Yields in ng DNA/ mg shell were 4E–04, 3E–04 and 3E–05 for each of the treatments, respectively (Fig. 5). For every sample tested, qPCR determined DNA concentration (ng//µL) was lower than the corresponding Qubit measured concentration. However, qPCR determined yields in the aged shells showed a similar pattern of P. canaliculus DNA availability (Fig. 5) as the Qubit measured yields (Fig. 4) in the months that were tested.
Figure 5. BoxPlot of DNA yields (ng/µl) determined by qPCR.
Showing the selected samples from the shell ageing, cooked shells and beach-cast shell collections.
qPCR was able to amplify three samples that had previously failed on all attempts of amplification using standard PCR with the same primers (Pcan primers) (Table 2).
DNA amplicon sequencing and sequence analysis
BLAST results for amplicon sequences derived from the Pcan primer set had highest sequence similarity to GenBank Accession Ref. DQ343605.1 reporting the NADH4 mitochondrial gene for P. canaliculus (Blair et al., 2006) (average BLAST query cover (QC) and percent identity (Id) 89% and 98%, respectively). COX1 amplicon sequences generally aligned as most similar to either the P. canaliculus COX1 sequence with the GenBank Accession Ref. DQ343592.1 (Blair et al., 2006) (average BLAST QC 87% and Id 98%) or to GenBank Accession Ref. MG766134.1 (Ranjard et al., 2018), describing the P. canaliculus complete mitochondrion sequence (average BLAST QC 82% and Id 98%), with overall BLAST top results of QC 84% and Id 98%.
All chitin synthase amplicon sequences derived from our primers aligned solely with our GenBank ref. MT305043 for the P. canaliculus chitin synthase gene (partial, Fig. 2), with an average BLAST QC of 89% and Id of 99%.
Aged shells
Mitochondrial amplification and subsequent identification by standard PCR of samples was 100% successful in all individuals of all but three collections (Table 2). Standard PCR using Pcan primers was overall slightly less successful than with using COX1 primers (95.3% amplification and identification compared to 97.7%) (Table 2).
Best results for the nuclear chitin synthase primer were achieved on samples from shells of freshly killed animals (month 0), and in the month 1 sampling (Table 2). The two other collections tested (6 and 13 months after death) had much lower rates of identification (10% and 30%, respectively) (Table 2).
qPCR with Pcan primers in the ageing shell sample subset successfully amplified all but one sample tested, including a sample on the 0 month collection that had not previously been amplified with standard PCR using the same primers (Table 2).
Both ng of DNA/ mg shell tissue ratio and qPCR calculated DNA yields show a similar pattern in the collections tested (months 0, 1, 6 and 13), in that there was an overall increase in yields from month 0 to month 1, followed by a decrease in the following months (Figs. 2 and 4).
Cooked shells
93.8% of samples were identified with each of the mitochondrial primer sets, while the nuclear chitin synthase primers identified 75% of samples (Table 2). The sample with the lowest DNA yield of cooked shells, which was also the lowest value from all samples extracted for this work, failed to give results with all three amplicons with standard PCR, and also in the qPCR assay (Table 2).
To test our method even further, we collected and extracted replicate DNA samples from shell areas that were more highly charred in two different individuals in the five minute ember treatment (data not shown). There was no statistical difference detected (paired T-test, p > 0.05, MS Excel (2016)) in DNA yield values (ng DNA/ mg shell), however one of the charred samples failed to amplify with any of the PCRs, despite its uncharred paired replicate amplifying successfully in all of them (data not shown).
Beach-cast shells
Despite higher Qubit measured DNA yields than the cooked shells (Fig. 4), this group of shell samples showed the lowest levels of successful amplification and identification with standard PCR. A total of 54.2% of samples were identified with the COX1 amplicon, and 25% with the Pcan amplicon overall (Table 2). Out of the 24 samples tested, only one amplified successfully and was sequenced with nuclear primers (Table 2). qPCR was successful in 55.5% of the subset of samples tested (Table 2), and calculated yields had an average of 0.02 ng/µl of P. canaliculus DNA in these samples (Fig. 5).
Discussion
Our results show that the carbonate shell matrix of P. canaliculus is a source of genetic material. The DNA yield from mussel shells likely originates from shell remodeling cells trapped and embedded in the matrix, which appears to provide good protection from enzymatic and chemical degradation that would otherwise degrade nucleic acid molecules in soft tissues. We have demonstrated that DNA can be extracted from P. canaliculus shells whether they are fresh, aged or cooked, and subsequently applied to amplify mitochondrial and nuclear regions of the genome.
Our DNA extraction method (Gemmell & Akayama, 1996) avoids the use of phenol-based solutions and extraction columns, and thus is a cheap, but effective alternative to commercial kits. Provided that the sample is at least 0.5 g in weight, and that primer design takes into account the need for short-length amplicons (Taberlet, Waits & Luikart, 1999), our data demonstrates the suitability of this method for extracting PCR-amplifiable DNA from bivalve shells for up to thirteen months, and in fresh samples prepared as they would be for human consumption.
We propose that the amount and quality of DNA that can be extracted and amplified from shell material relies on a balance between fine and coarsely grounded shell tissue, and shell integrity, particularly of inner shell layers. We observed that disruption of shell samples of similar age showed variability of DNA yields (Fig. 4), as also observed by Geist, Wunderlich & Kuehn (2008) in fresh pearl mussel shells.
Well-weathered samples such as those from the beach-cast set had low shell integrity as they were very brittle, presented areas lacking the periostracum layer, breakage, and evidence of boring trematode parasites, likely due to a combination of age and environmental exposure causing DNA degradation, which translated as the group of samples with lowest DNA yields tested (Fig. 5). This relationship was also observed by Der Sarkissian et al. (2017) when extracting DNA from ancient shell samples. Loss of the periostracum layer during cooking of fresh shells decreased DNA extraction yields, but did not have an effect on DNA analysis success in this group of samples, even in those collected from charred areas.
qPCR DNA quantification showed a similar trend in the amounts of DNA extracted through time as with quantifying using a Qubit® 2.0 fluorometer. It also identified significant foreign DNA contamination in the beach-cast shell samples, which allied with the low yields of species-specific DNA, possibly highly degraded, explains the poorest amplification results of all sample groups. We were therefore not able to establish a connection between shell sample area (ventral, palial or umbo) and DNA extraction success.
Co-extraction of PCR inhibitors from samples collected from the environment, such as with forensic or ancient samples, is a well-known issue (Alaeddini, 2012). Dilution of DNA extracts with DNase-free water is a common method to circumvent PCR inhibition (Kemp, Monroe & Smith, 2006), and appears to have been moderately successful in our experiment.
Both mitochondrial primer sets were successfully used to amplify shell extracts. As with (Frantzen et al., 1998), our COX1 primers amplifying a fragment ∼100 bp shorter that the Pcan amplicon (Table 1), were the most successful.
A slightly lower amplification success with mitochondrial primers in the earliest collections of the aged shell experiment (Table 2) could be linked to the presence of foreign DNA from biofilm or shell parasite organisms in the sample’s surface. This DNA would be expected to degrade with time, as it is not imbedded in the shell’s tissue matrix.
The sharp drop in nuclear DNA identification success subsequent to the first month of sampling could indicate that reliable extraction and amplification of nuclear DNA from aged Perna canaliculus shell may require specialized protocols. Other studies amplifying mitochondrial and nuclear DNA from shell extracts also reported poorer results with nuclear amplicons (Barsh & Murphy, 2008; Geist, Wunderlich & Kuehn, 2008). The disparity between mitochondrial and nuclear results is likely a reflection of higher cell copy numbers of mitochondrial DNA molecules compared to nuclear molecules and the inherent protection afforded by a circular genome. If, as suggested by Mount et al. (2004), DNA in shells has its origin in occasionally trapped cells, the higher copy number of mitochondrial DNA molecules would enable amplification of mitochondrial sequences, but not necessarily of nuclear sequences. Geist, Wunderlich & Kuehn (2008) reported 89% success genotyping fresh shells of freshwater pearl mussels with nine microsatellite markers in. Our nuclear amplification success with fresh shells in the ageing experiment was 80%, which is very comparable.
Both nuclear and mitochondrial DNA analyses are feasible in shells of bivalves prepared as they would be for human consumption by exposure to high heat, possibly even from charred shell regions. This is of particular significance for the analysis of shell midden material, which is usually characterized by stratified deposits of consumed shelled animals such as bivalve molluscs, and indicates that DNA imbedded in bivalve shell matrix is well protected against degradation from high temperatures such as those used for cooking food.
Mitochondrial DNA extraction and amplification from mollusc shells has been reported in a variety of species, from fresh shells of freshwater pearl mussels (Geist, Wunderlich & Kuehn, 2008) and Pacific oyster (Wang et al., 2012), up to 50 year old snail shells (Andree & López, 2013; Caldeira et al., 2004; Villanea, Parent & Kemp, 2016), 70 year old abalone shells (Hawk, 2010) and even 125 year old oyster shells (Barsh & Murphy, 2008). In fact, Der Sarkissian et al. (2017) recently established that shell DNA extraction from a variety of marine mollusc species aged up to 7,000 years BP is possible and can be used to retrieve not only mollusc endogenous DNA, but also that of the natural microbiome, which could include pathogenic organisms, symbiotic to living molluscs. It is therefore very likely that mitochondrial DNA analysis can be successful in P. canaliculus shells for periods longer than the ones tested here.
Our findings could be significant in a number of areas, such as genotyping of historical collections, bioprospection of invasive species, and potentially shell midden research. In the greenshell mussel aquaculture industry, engraving the shell of animals with an identification number is a common technique (Camara & Symonds, 2014) that could be coupled with DNA extraction using the shell chips produced as a way of genotyping valuable aquaculture specimens, or endangered mollusc species, without the need to kill the animals.
Ancient shells DNA analysis techniques for shells such as those described by Der Sarkissian et al. (2017) are exciting new developments in this field, demonstrating the ability of mollusc shell matrices of entrapping and providing protection against nucleic acid degradation post-mortem, potentially for thousands of years.
Bivalve molluscs have been widely used for coastal monitoring anthropogenic aquatic contaminants such as heavy metals (Goldberg et al., 1978; Azizi et al., 2018), micro plastics (Ward, Rosa & Shumway, 2019) and human pathogen transmission (Gyawali et al., 2019; Razafimahefa, Ludwig-Begall & Thiry, 2019) together with seasonal ecotoxicological problems such as algal blooms (Gibble, Peacock & Kudela, 2016; Hinder et al., 2011). Their shells have also been used for almost four decades as a proxy to determine exposure to heavy metal concentrations in modern samples (Yap et al., 2002; Koide, Lee & Goldberg, 1982), historic (Wing et al., 2019) and archaeological samples (Binkowski et al., 2019). Along with being a source of genetical material not just of the individual itself, but pathogens and conditions that they’ve been exposed to makes mollusc shells a valuable resource to assess the environment in present and past times.
Supplemental Information
DNA yields (ng/mg shell) for each sample were calculated using the Qubit measured amount of the DNA extract divided it by the amount as weight (in mg) of shell used when extracting.
Data shown for all subset samples tested.
Funding Statement
This work was funded by the University of Otago, New Zealand. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Sara Ferreira conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Rachael Ashby and Kim Rutherford analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Gert-Jan Jeunen conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Catherine Collins performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Erica V. Todd analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Neil J. Gemmell conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The Perna canaliculus chitin synthase gene region used to design nuclear primers and amplify nuclear DNA from our samples is avaialble at GenBank: MT305043.
Data Availability
References
- Alaeddini (2012).Alaeddini R. Forensic implications of PCR inhibition–a review. Forensic Science International: Genetics. 2012;6:297–305. doi: 10.1016/j.fsigen.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Andree & López (2013).Andree KB, López MA. Species identification from archived snail shells via genetic analysis: a method for DNA extraction from empty shells. Molluscan Research. 2013;33:1–5. doi: 10.1080/13235818.2012.754141. [DOI] [Google Scholar]
- Appeltans et al. (2012).Appeltans W, Ahyong ST, Anderson G, Angel MV, Artois T, Bailly N, Bamber R, Barber A, Bartsch I, Berta A, Błażewicz-Paszkowycz M, Bock P, Boxshall G, Boyko CB, Brandão SN, Bray RA, Bruce NL, Cairns SD, Chan TY, Cheng L, Collins AG, Cribb T, Curini-Galletti M, Dahdouh-Guebas F, Davie PJ, Dawson MN, De Clerck O, Decock W, De Grave S, De Voogd NJ, Domning DP, Emig CC, Erséus C, Eschmeyer W, Fauchald K, Fautin DG, Feist SW, Fransen CH, Furuya H, Garcia-Alvarez O, Gerken S, Gibson D, Gittenberger A, Gofas S, Gomez-Daglio L, Gordon DP, Guiry MD, Hernandez F, Hoeksema BW, Hopcroft RR, Jaume D, Kirk P, Koedam N, Koenemann S, Kolb JB, Kristensen RM, Kroh A, Lambert G, Lazarus DB, Lemaitre R, Longshaw M, Lowry J, Macpherson E, Madin LP, Mah C, Mapstone G, Mclaughlin PA, Mees J, Meland K, Messing CG, Mills CE, Molodtsova TN, Mooi R, Neuhaus B, Ng PK, Nielsen C, Norenburg J, Opresko DM, Osawa M, Paulay G, Perrin W, Pilger JF, Poore GC, Pugh P, Read GB, Reimer JD, Rius M, Rocha RM, Saiz-Salinas JI, Scarabino V, Schierwater B, Schmidt-Rhaesa A, Schnabel KE, Schotte M, Schuchert P, Schwabe E, Segers H, Self-Sullivan C, Shenkar N, Siegel V, Sterrer W, Stöhr S, Swalla B, Tasker ML, Thuesen EV, Timm T, Todaro MA, Turon X, Tyler S, Uetz P, Der Land J, Vanhoorne B, Van Ofwegen LP, Van Soest RWM, Vanaverbeke J, Walker-Smith G, Walter TC, Warren A, Williams GC, Wilson SP, Costello MJ. The magnitude of global marine species diversity. Current Biology. 2012;22:2189–2202. doi: 10.1016/j.cub.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Azizi et al. (2018).Azizi G, Akodad M, Baghour M, Layachi M, Moumen A. The use of Mytilus spp. mussels as bioindicators of heavy metal pollution in the coastal environment. A review. Journal of Materials and Environmental Sciences. 2018;9:1170–1181. [Google Scholar]
- Balbo et al. (2011).Balbo A, Madella M, Godino IB, Álvarez M. Shell midden research: an interdisciplinary agenda for the Quaternary and Social Sciences. Quaternary International. 2011;239:147–152. doi: 10.1016/j.quaint.2011.03.032. [DOI] [Google Scholar]
- Barsh & Murphy (2008).Barsh R, Murphy M. West coast native oyster restoration: 2007 workshop proceedings. US Department of Commerce, NOAA Restoration Centre; 2008. Opportunities for reconstruction of pre-contact native oyster distribution and population structure in north Puget Sound. 86 pp. [Google Scholar]
- Benson et al. (2012).Benson DA, Karsch-Mizrachi I, Clark K, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Research. 2012;40:D48–D53. doi: 10.1093/nar/gkr1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkowski et al. (2019).Binkowski LJ, Blaszczyk M, Przystupinska A, Ozgo M, Massanyi P. Metal concentrations in archaeological and contemporary mussel shells (Unionidae): reconstruction of past environmental conditions and the present state. Chemosphere. 2019;228:756–761. doi: 10.1016/j.chemosphere.2019.04.190. [DOI] [PubMed] [Google Scholar]
- Blair et al. (2006).Blair D, Waycott M, Byrne L, Dunshea G, Smith-Keune C, Neil KM. Molecular discrimination of Perna (Mollusca: Bivalvia) species using the polymerase chain reaction and species-specific mitochondrial primers. Marine Biotechnology. 2006;8:380–385. doi: 10.1007/s10126-005-6121-y. [DOI] [PubMed] [Google Scholar]
- Caldeira et al. (2004).Caldeira RL, Jannotti-Passos LK, Lira PM, Carvalho OS. Diagnostic of Biomphalaria snails and Schistosoma mansoni: DNA obtained from traces of shell organic materials. Memorial Institute Oswaldo Cruz. 2004;99:499–502. doi: 10.1590/s0074-02762004000500007. [DOI] [PubMed] [Google Scholar]
- Camara & Symonds (2014).Camara MD, Symonds JE. Genetic improvement of New Zealand aquaculture species: programmes, progress and prospects. New Zealand Journal of Marine and Freshwater Research. 2014;48:466–491. doi: 10.1080/00288330.2014.932291. [DOI] [Google Scholar]
- Chapman (2009).Chapman AD. Numbers of Living Species in Australia and the World, 2nd ed. Report for the Australian Biological Resources Study, Canberra. https://www.environment.gov.au/system/files/pages/2ee3f4a1-f130-465b-9c7a-79373680a067/files/nlsaw-2nd-complete.pdf. 2009. https://www.environment.gov.au/system/files/pages/2ee3f4a1-f130-465b-9c7a-79373680a067/files/nlsaw-2nd-complete.pdf
- Colonese et al. (2011).Colonese AC, Mannino MA, Bar-Yosef Mayer DE, Fa DA, Finlayson JC, Lubell D, Stiner MC. Marine mollusc exploitation in Mediterranean prehistory: an overview. Quaternary International. 2011;239:86–103. doi: 10.1016/j.quaint.2010.09.001. [DOI] [Google Scholar]
- Coutellec (2017).Coutellec MA. Mollusc shells as metagenomic archives: the true treasure is the chest itself. Molecular Ecology Resources. 2017;17:854–857. doi: 10.1111/1755-0998.12716. [DOI] [PubMed] [Google Scholar]
- Demarchi et al. (2011).Demarchi B, Williams MG, Milner N, Russell N, Bailey G, Penkman K. Amino acid racemization dating of marine shells: a mound of possibilities. Quaternary International. 2011;239:114–124. doi: 10.1016/j.quaint.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der Sarkissian et al. (2017).Der Sarkissian C, Pichereau V, Dupont C, Ilsoe PC, Perrigault M, Butler P, Chauvaud L, Eiriksson J, Scourse J, Paillard C, Orlando L. Ancient DNA analysis identifies marine mollusc shells as new metagenomic archives of the past. Molecular Ecology Resources. 2017;17:835–853. doi: 10.1111/1755-0998.12679. [DOI] [PubMed] [Google Scholar]
- Doherty, Gosling & Was (2007).Doherty S, Gosling E, Was A. Bivalve ligament—a new source of DNA for historical studies. Aquatic Biology. 2007;1:161–165. doi: 10.3354/ab00020. [DOI] [Google Scholar]
- Estevez et al. (2001).Estevez J, Piana E, Schiavini A, Juan-Muns N. Archaeological analysis of shell middens in the Beagle Channel, Tierra del Fuego Island. International Journal of Osteoarchaeology. 2001;11:24–33. doi: 10.1002/oa.543. [DOI] [Google Scholar]
- Fleury et al. (2008).Fleury C, Marin F, Marie B, Luquet G, Thomas J, Josse C, Serpentini A, Lebel JM. Shell repair process in the green ormer Haliotis tuberculata: a histological and microstructural study. Tissue and Cell. 2008;40:207–218. doi: 10.1016/j.tice.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Frantzen et al. (1998).Frantzen MAJ, Silk JB, Ferguson JWH, Wayne RK, Kohn MH. Empirical evaluation of preservation methods for faecal DNA. Molecular Ecology. 1998;7:1423–1428. doi: 10.1046/j.1365-294x.1998.00449.x. [DOI] [PubMed] [Google Scholar]
- Furuhashi et al. (2009).Furuhashi T, Schwarzinger C, Miksik I, Smrz M, Beran A. Molluscan shell evolution with review of shell calcification hypothesis. Comparative Biochemistry and Physiology B. 2009;154:351–371. doi: 10.1016/j.cbpb.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Geist, Wunderlich & Kuehn (2008).Geist J, Wunderlich H, Kuehn R. Use of mollusc shells for DNA-based molecular analyses. Journal of Molluscan Studies. 2008;74:337–343. doi: 10.1093/mollus/eyn025. [DOI] [Google Scholar]
- Gemmell & Akayama (1996).Gemmell NJ, Akayama S. An efficient method for the extraction of DNA from vertebrate tissues. Trends in Genetics. 1996;12:338–339. doi: 10.1016/S0168-9525(96)80005-9. [DOI] [PubMed] [Google Scholar]
- Gibble, Peacock & Kudela (2016).Gibble CM, Peacock MB, Kudela RM. Evidence of freshwater algal toxins in marine shellfish: implications for human and aquatic health. Harmful Algae. 2016;59:59–66. doi: 10.1016/j.hal.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Goldberg et al. (1978).Goldberg ED, Bowen VT, Farrington JW, Harvey G, Martin JH, Parker PL, Risebrough RW, Roberston W, Schneider E, Gamble E. The mussel watch. Environmental Conservation. 1978;5:101–125. doi: 10.1017/S03768929000005555. [DOI] [Google Scholar]
- Gyawali et al. (2019).Gyawali P, Croucher D, Ahmed W, Devane M, Hewitt J. Evaluation of pepper mild mottle virus as an indicator of human faecal pollution in shellfish and growing waters. Water Research. 2019;154:370–376. doi: 10.1016/j.watres.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Hawk (2010).Hawk H. Historic genetic diversity of the endangered white abalone (Haliotis sorenseni). San Diego: California Sea Grant College Program. 2010. http://escholarship.org/uc/item/0d93d9nz http://escholarship.org/uc/item/0d93d9nz
- Hebert & Gregory (2005).Hebert PD, Gregory TR. The promise of DNA barcoding for taxonomy. Systematic Biology. 2005;54:852–859. doi: 10.1080/10635150500354886. [DOI] [PubMed] [Google Scholar]
- Higgins et al. (2015).Higgins D, Rohrlach AB, Kaidonis J, Townsend G, Austin JJ. Differential nuclear and mitochondrial DNA preservation in post-mortem teeth with implications for forensic and ancient DNA studies. PLOS ONE. 2015;10:e0126935. doi: 10.1371/journal.pone.0126935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder et al. (2011).Hinder SL, Hays GC, Brooks CJ, Davies AP, Edwards M, Walne AW, Gravenor MB. Toxic marine microalgae and shellfish poisoning in the British Isles: history, review of epidemiology, and future implications. Environmental Health. 2011;10:54. doi: 10.1186/1476-069X-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höss & Pääbo (1993).Höss M, Pääbo S. DNA extraction from Pleistocene bones by a silica-based purification method. Nucleic Acids Research. 1993;21:3913–3914. doi: 10.1093/nar/21.16.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanina et al. (2017).Ivanina AV, Falfushynska HI, Beniash E, Piontkivska H, Sokolova IM. Biomineralization-related specialization of hemocytes and mantle tissues of the Pacific oyster Crassostrea gigas. Journal of Experimental Biology. 2017;220:3209–3221. doi: 10.1242/jeb.160861. [DOI] [PubMed] [Google Scholar]
- Kalmár et al. (2000).Kalmár T, Bachrati C, Marcsik A, Raskó I. A simple and efficient method for PCR amplifiable DNA extraction from ancient bones. Nucleic Acids Research. 2000;28:E67. doi: 10.1093/nar/28.12.e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, Monroe & Smith (2006).Kemp BM, Monroe C, Smith DG. Repeat silica extraction: a simple technique for the removal of PCR inhibitors from DNA extracts. Journal of Archaeological Science. 2006;33:1680–1689. doi: 10.1016/j.jas.2006.02.015. [DOI] [Google Scholar]
- Kidwell (2001).Kidwell SM. Preservation of species abundance in marine death assemblages. Science. 2001;294 doi: 10.1126/science.1064539. [DOI] [PubMed] [Google Scholar]
- Kim & Byrne (2006).Kim KC, Byrne LB. Biodiversity loss and the taxonomic bottleneck: emerging biodiversity science. Ecological Research. 2006;21:794–810. doi: 10.1007/s11284-006-0035-7. [DOI] [Google Scholar]
- Koide, Lee & Goldberg (1982).Koide M, Lee DS, Goldberg ED. Metal and transuranic records in mussel shells, Byssal threads and tissues. Estuarine, Coastal and Shelf Science. 1982;15:679–695. doi: 10.1016/0272-7714(82)90079-8. [DOI] [Google Scholar]
- Krantz, Williams & Jones (1987).Krantz DE, Williams DF, Jones DS. Ecological and paleoenvironmental information using stable isotope profiles from living and fossil molluscs. Palaeogeography, Palaeoclimatology, Palaeoecology. 1987;58:249–266. doi: 10.1016/0031-0182(87)90064-2. [DOI] [Google Scholar]
- Kumar, Stecher & Tamura (2016).Kumar S, Stecher G, Tamura K. Mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2016).Li S, Liu Y, Liu C, Huang J, Zheng G, Xie L, Zhang R. Hemocytes participate in calcium carbonate crystal formation, transportation and shell regeneration in the pearl oyster Pinctada fucata. Fish and Shellfish Immunology. 2016;51:263–270. doi: 10.1016/j.fsi.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Mount et al. (2004).Mount AS, Wheeler AP, Parakdar RP, Snider D. Hemocyte-mediated shell mineralization in the Eastern Oyster. Science Reports. 2004;304:297–300. doi: 10.1126/science.1090506. [DOI] [PubMed] [Google Scholar]
- Pääbo et al. (2004).Pääbo S, Poinar H, Serre D, Jaenicke-Despres V, Hebler J, Rohland N, Kuch M, Krause J, Vigilant L, Hofreiter M. Genetic analyses from ancient DNA. Annual Review of Genetics. 2004;38:645–679. doi: 10.1146/annurev.genet.37.110801.143214. [DOI] [PubMed] [Google Scholar]
- Ranjard et al. (2018).Ranjard L, Wong TKF, KÜLheim C, Rodrigo AG, Ragg NLC, Patel S, Dunphy BJ. Complete mitochondrial genome of the green-lipped mussel, Perna canaliculus (Mollusca: Mytiloidea), from long nanopore sequencing reads. Mitochondrial DNA Part B. 2018;3:175–176. doi: 10.1080/23802359.2018.1437810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafimahefa, Ludwig-Begall & Thiry (2019).Razafimahefa RM, Ludwig-Begall LF, Thiry E. Cockles and mussels, alive, alive, oh-The role of bivalve molluscs as transmission vehicles for human norovirus infections. Transboundary and Emerging Diseases. 2019 doi: 10.1111/tbed.13165. Epub ahead of print 2019 24 June. [DOI] [PubMed] [Google Scholar]
- Rhoads (1970).Rhoads DCPG. The use of molluscan shell growth patterns in ecology and palaeoecology. Lethaia. 1970;3:143–161. doi: 10.1111/j.1502-3931.1970.tb01854.x. [DOI] [Google Scholar]
- Rohland & Hofreiter (2007).Rohland N, Hofreiter M. Ancient DNA extraction from bones and teeth. Nature Protocols. 2007;2:1756–1762. doi: 10.1038/nprot.2007.247. [DOI] [PubMed] [Google Scholar]
- Taberlet, Waits & Luikart (1999).Taberlet P, Waits LP, Luikart G. Noninvasive genetic sampling: look before you leap. Trends in Ecology and Evolution. 1999;14:323–327. doi: 10.1016/S0169-5347(99)01637-7. [DOI] [PubMed] [Google Scholar]
- Tamura & Nei (1993).Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tautz et al. (2003).Tautz D, Arctander P, Minelli A, H TR, Vogler A. A plea for DNA taxonomy. Trends in Ecology and Evolution. 2003;18:70–75. doi: 10.1016/S0169-5347(02)00041-1. [DOI] [Google Scholar]
- Untergasser et al. (2012).Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3–new capabilities and interfaces. Nucleic Acids Research. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrasco et al. (2016).Vendrasco MJ, Rodríguez-Navarro AB, Checa AG, Devaere L, Porter SM. To infer the early evolution of mollusc shell microstructures. Key Engineering Materials. 2016;672:113–133. doi: 10.4028/www.scientific.net/KEM.672.113. [DOI] [Google Scholar]
- Villanea, Parent & Kemp (2016).Villanea FA, Parent CE, Kemp BM. Reviving galápagos snails: ancient DNA extraction and amplification from shells of probably extinct endemic land snails. Journal of Molluscan Studies. 2016;82:449–456. doi: 10.1093/mollus/eyw011. [DOI] [Google Scholar]
- Wang et al. (2012).Wang X, Song X, Li L, G Zhang. An improved method for DNA extraction from the shell of the Pacific oyster. Crassostrea gigas. http://hdl.handle.net/10524/31832 The Israeli Journal of Aquaculture. 2012;64 [Google Scholar]
- Ward, Rosa & Shumway (2019).Ward JE, Rosa M, Shumway SE. Capture, ingestion, and egestion of microplastics by suspension-feeding bivalves: a 40-year history. Anthropocene Coasts. 2019;2:39–49. doi: 10.1139/anc-2018-0027. [DOI] [Google Scholar]
- Weiss et al. (2006).Weiss IM, Schonitzer V, Eichner N, Sumper M. The chitin synthase involved in marine bivalve mollusk shell formation contains a myosin domain. FEBS Letter. 2006;580:1846–52. doi: 10.1016/j.febslet.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Wing et al. (2019).Wing SR, O’Connell-Milne SA, Wing LC, Reid MR. Trace metals in Antarctic clam shells record the chemical dynamics of changing sea ice conditions. Limnology and Oceanography. 2019;65:504–514. doi: 10.1002/lno.11318. [DOI] [Google Scholar]
- Yap et al. (2002).Yap CK, Ismail A, Tan SG, Rahim IAbdul. Can the shell of the green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia be a potential biomonitoring material for Cd, Pb and Zn? Estuarine, Coastal and Shelf Science. 2002;57:623–630. doi: 10.1016/S0272-7714(02)00401-8. [DOI] [Google Scholar]
- Zanden & Rasmussen (2001).Zanden MJV, Rasmussen JB. Variation in δ15N and δ13N trophic fractionation: implications for aquatic food web studies. Limnology and Oceanography. 2001;46:2061–2066. doi: 10.4319/lo.2001.46.8.2061. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DNA yields (ng/mg shell) for each sample were calculated using the Qubit measured amount of the DNA extract divided it by the amount as weight (in mg) of shell used when extracting.
Data shown for all subset samples tested.
Data Availability Statement
The following information was supplied regarding data availability:
DNA yields (ng DNA/ mg shell) for all experiments are available in File S1. qPCR calculated DNA yields (ng/ul) are available in File S2.