Abstract
The search for alternatives to allotransplants is driven by the shortage of corneal donors and is demanding because of the limitations of the alternatives. Indeed, current progress in genetically engineered (GE) pigs, the introduction of gene-editing technology by clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9, and advanced immunosuppressants have made xenotransplantation a possible option for a human trial. Porcine corneal xenotransplantation is considered applicable because the eye is regarded as an immune-privileged site. Furthermore, recent non-human primate studies have shown long-term survival of porcine xenotransplants in keratoplasty. Herein, corneal immune privilege is briefly introduced, and xenogeneic reactions are compared with allogeneic reactions in corneal transplantation. This review describes the current knowledge on special issues of xenotransplantation, xenogeneic rejection mechanisms, current immunosuppressive regimens of corneal xenotransplantation, preclinical efficacy and safety data of corneal xenotransplantation, and updates of the regulatory framework to conduct a clinical trial on corneal xenotransplantation. We also discuss barriers that might prevent xenotransplantation from becoming common practice, such as ethical dilemmas, public concerns on xenotransplantation, and the possible risk of xenozoonosis. Given that the legal definition of decellularized porcine cornea (DPC) lies somewhere between a medical device and a xenotransplant, the preclinical efficacy and clinical trial data using DPC are included. The review finally provides perspectives on the current standpoint of corneal xenotransplantation in the fields of regenerative medicine.
Keywords: Xenotransplantation, Xenozoonosis, Galα1-3Galβ1-4GlcNAc-R (αGal), Regulatory framework, Corneal transplantation, Porcine endogenous retrovirus (PERV)
1. Introduction
1.1. Background
The global blindness prevalence is to be estimated at 36 million per year, with one of the top five causes being corneal blindness (Bourne et al., 2017; Flaxman et al., 2017; Porth et al., 2019). Full-thickness, anterior or posterior lamellar keratoplasty is applied depending on the etiologies of corneal blindness (Tan et al., 2012). Currently, Fuchs' endothelial corneal dystrophy or endothelial failure are becoming the leading causes of keratoplasty in the USA and Europe, while keratitis is still the primary indication of keratoplasty in Asia, Africa and Middle East (Ayalew et al., 2017; Bigan et al., 2018; Flockerzi et al., 2018; Mathews et al., 2018; Matthaei et al., 2017) (Fig. 1 ). Unfortunately, the supply of donor corneas is insufficient due to cultural barriers, lack of education, or logistical problems related to procuring the donor tissue (Almeida et al., 2018; Gain et al., 2016; Hara and Cooper, 2011; Kim, 2017; Lamm et al., 2014; Lee et al., 2017a; Wong et al., 2017; York and Tinley, 2017) (Table 1 ). Furthermore, a global survey reported that 53% of the world's population did not have access to keratoplasty (Gain et al., 2016). The increased prevalence of infectious disease is expected to lead to an increased shortage of donor corneas (Stern et al., 2018).
Fig. 1.
Global reports on the distribution of indications for penetrating keratoplasty in the last 25 years. Black indicates keratitis or stromal opacity as an indication of penetrating keratoplasty, gray indicates endothelial disease as an indication of endothelial keratoplasty and white indicates keratoconus for lamellar keratoplasty. Most of the data were from the systematic review by Matthaei et al. (Matthaei et al., 2017), and data on Germany* was from a study by Flockerzi et al. (Flockerzi et al., 2018).
Table 1.
Reported numbers of Wait-lists in each country compared with the number of corneal transplanted cases.
| Countries | Numbers of patients in wait-list | Transplanted cases | Reported year ref |
|---|---|---|---|
| Australia | 0 | 1096 | 2008 (Hara and Cooper, 2011) |
| Brazil | 10,923 | 14,534 | 2018 (Almeida et al., 2018) |
| China | 2,000,000 | <8000 | 2017 (Wong et al., 2017) |
| India | 7,000,000 | 25,000 | 2017 (Wong et al., 2017) |
| Japan | 2769 | 1634 | 2008 (Hara and Cooper, 2011) |
| Korea | 2184 | 322 | 2018a |
| South Africa | 4300b | 89 | 2017 (York and Tinley, 2017) |
| United Kingdom | 500 | 2711 | 2008 (Hara and Cooper, 2011) |
| United States | 0 | 41,652 | 2008 (Hara and Cooper, 2011) |
Data from Korean Network for Organ Sharing © 2018 (11-1351155-000001-01).
Data from The Organ Donor Foundation of South Africa © 2019 (NPO/PBO Numbers 003–458 NPO PBO: 18/11/13/4).
Since organ and corneal tissue trafficking are global concerns, the Declaration of Istanbul and The Barcelona Principles have announced principles to alleviate organ or tissue trafficking (Martin et al., 2019; Steering Committee of the Istanbul, 2008; The Global Alliance of Eye Bank Associations Inc, 2018). Furthermore, allograft substitutes is another approach to resolve the trafficking problem (Kim, 2016, 2017; Kim and Hara, 2015; Treasure, 2007; Wang et al., 2019). Therefore, translational researches such as the use of stem cell-based therapy, bioengineered products, and xenotransplantation have been investigated to replace allografts (Carlsson et al., 2003; Cooper, 2003; Cooper et al., 2002; Mehta et al., 2019; Stern et al., 2018).
1.2. History of clinical experiences with xenotransplantation
The concept of xenotransplantation originated in the 17th century with the first sheep-to-human blood transfusion attempt by Jean Baptiste Denis in 1667 (Denis, 1667). After which, numerous attempts have been reported (Table 2 ) (Ekser et al., 2017). In 1824, Franz Reisinger was credited for formulating the concept of keratoplasty (Reisinger, 1824). He proposed to replace an opacified human cornea with a transparent animal cornea. The 1st real corneal transplantation in humans using a porcine graft occurred in 1838 by Richard Kissam (1844). This is a very historic report, for being the first human transplant in general. This occurred more than 50 years before the 1st corneal allo- and renal xeno-transplantation in 1905 (Hara and Cooper, 2011; Zirm, 1989). Thereafter, until the early 1970s, corneal xenotransplantation was anecdotally reported with xenotransplants from gibbons and fish in humans, (Haq, 1972; Hara and Cooper, 2011; Soomsawasdi et al., 1964). Among them, gibbon-to-human corneal xenotransplants showed more than 5 months of survival in 50% of the ten recipients (Soomsawasdi et al., 1964).
Table 2.
Historical milestone in clinical xenotransplantation attempted in human.
| Year | Donor | Organ/tissue/cell | Patient Survival | Doctor | References |
|---|---|---|---|---|---|
| 1667 | Sheep | Bloods | survived | Jean Baptiste Denis | (Denis, 1667; Ekser et al., 2017) |
| 1838 | Pig | Cornea | several weeksa | Richard Kissam | (Hara and Cooper, 2011; Kissam, 1844) |
| 1905 | Rabbit | Kidney slice | 16 days | Princeteau | (Lambrigts et al., 1998; Princeteau, 1905) |
| 1906 | Pig | Kidney | 3 days | Mathieu Jaboulay | (Jaboulay, 1906; Lambrigts et al., 1998) |
| 1963–1964 | Chimpanzee | Kidneys | Up to 9 months | Keith Reemtsma | (Michel et al., 2015; Reemtsma et al., 1964) |
| 1964 | Gibbon | Cornea | >5 monthsc | Soomsawasdi B | Soomsawasdi et al. (1964) |
| 1964 | Chimpanzee | Heart | 2 h | James Hardy | (Hardy et al., 1964; Michel et al., 2015) |
| 1984 | Baboon | Heart | 20 days | Leonard Bailey | (Bailey et al., 1985; Michel et al., 2015) |
| 1992 | Baboon | Livers | 70 days | Thomas Starzl | Starzl et al. (1993) |
| 2013–2017 | New born pig | Islets | NAb | Wei Wang | Wang et al. (2019) |
Indicates graft survival.
Not available about the survival data (No death was reported). Substantial improvement of the condition was reported.
50% of the patients showed more than 5 months graft survival in 10 patients.
Conversely, the first organ xenotransplant attempt was reported using rabbit or pig kidneys between 1905 and 1906 (Jaboulay, 1906; Princeteau, 1905). Furthermore, a clinical trial milestone was performed by Keith Reemtsma between 1963 and 1964 where a chimpanzee xenotransplant was transplanted on six patients with renal disease, one of whom survived for 9 months (Reemtsma et al., 1964). This was then followed by the first heart xenotransplant performed using a chimpanzee donor in 1964 by James Hardy (Hardy et al., 1964); a baboon heart secondly implanted into an infant who survived 20 days in 1984 (Bailey et al., 1985); the first liver xenotransplantation performed using baboon donors in 1992 by Thomas Starzl, of whom one survived for up to 70 days (Starzl et al., 1993).
Since the 1990s, xenotransplantation clinical trials have not been conducted in the United States and most European countries due to issues concerning efficacy, xenozoonosis, and debates regarding regulatory framework. However, anecdotal reports indicate that Russia and China have used xenotransplants to treat diabetes. Furthermore, between 2013 and 2017, newborn pig islets were transplanted into ten diabetes patients in China (Wang et al., 2019).
2. Prerequisites for xenotransplantation
2.1. Pigs as a donor source
Non-human primates (NHPs) are phylogenetically the most similar animals to humans. However, as a donor, the use of NHP has several drawbacks including ethical concerns, easy zoonotic transmission of infection, high costs and long breeding time, and lack of experience in genetic modification of NHP (Cooper et al., 2002).
Compared to NHPs and other animals, the use of pigs has several advantages (Table 3 ) (Cooper et al., 2002; Editors, 2016). Pigs are easy and less costly to breed, have multiple litters, can be genetically modified to overcome immune barriers with in-depth experiences, and can be produced in pathogen-free conditions. Ethical concerns are fewer since pigs are farmed as a human food. Therefore, using pig organs or tissues as a source of xenotransplantation is of considerable interest.
Table 3.
Advantages of the usage of pig as a donor compared with non-human primate.
| Pig | Non-human primate | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
2.2. Genetically engineered (GE) pigs
Unlike allogeneic rejection, xenogeneic response to the porcine organ includes a unique hyperacute rejection within hours that is mediated by natural anti-Galα1-3Galβ1-4GlcNAc-R (anti-αGal) antibodies (Abs), followed by an acute humoral and cellular rejection within days that is mediated by Abs, activated complements, coagulation system, and innate immune cells, and chronic cellular rejection that is mediated mainly by T cells and macrophages within several weeks (Sprangers et al., 2008). To overcome the xenogeneic rejection beyond immunosuppression, three major approaches were attempted (Institute of Medicine (US), 1996); (1) genetic alteration of the source animal, (2) development of bone marrow chimerism in the recipient, and (3) encapsulation of the xenogeneic cells or tissues.
Alpha-1,3-galactosyltransferase gene-knockout (GT-KO) pigs generated by somatic nuclear transfer technology in the early 2000s brought xenotransplantation a step closer to the clinics by reducing hyperacute rejection (Dai et al., 2002; Lai et al., 2002). Over 40 genes have currently been engineered: expression of human complement regulatory proteins [Cluster of differentiation (CD)55, CD46, CD59], human coagulation-regulatory proteins (thrombomodulin, CD39), human anti-inflammatory proteins (CD47, signal regulatory protein alpha (SIRPα), heme oxygenase 1, CD39), and natural killer (NK) cell modulatory molecules (human leukocyte antigen G (HLA-G), HLA-E, beta2 microglobulin) as well as reduction of the antigenic epitopes such as αGal, major histocompatibility complex (MHC) I and MHC II transactivator dominant-negative (CIITA-DN) (Table 4 ) (Ekser et al., 2012; Perkel, 2016; Sprangers et al., 2008).
Table 4.
Immunological target genes for a gene-editing in pig xenotransplants.
| Rejection period | Immune response | Rejection mechanism | Target gene editing |
|---|---|---|---|
| Hyperacute | Innate | Reduction of natural anti- αGal responses by deleting αGal epitope |
|
| Acute | Innate | Complement regulation |
|
| Coagulation/thrombosis reduction |
|
||
| Natural Killer cells regulation |
|
||
| Macrophage/monocyte regulation |
|
||
| Chronic | Adaptive | T cells |
|
| B cells |
|
AbbreviationsαGal: Galα1-3Galβ1-4GlcNAc-R, MHC: major histocompatibility complex.
Given that conventional technology through homologous recombination and somatic cell nuclear transfer takes 3 years to generate a pig homozygous with a single gene modification, the latest gene-editing technology using clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 enables multiple genetic alterations with high speed. Both immunological genes and all porcine endogenous retrovirus (PERV) genes can be disrupted with this new technology, therefore eliminating the risk of xenozoonosis (Editors, 2016; Perkel, 2016).
Currently, xenotransplants from available GE pigs still experience both humoral and cellular xenogeneic rejection in NHP models. Therefore, the optimal combination of immunological gene-editing for the least antigenicity in pig xenotransplants has not yet been determined.
2.3. Immune privilege of the eye
Since Peter B. Medawar discovered that skin allograft can survive longer in the anterior chamber than in conventional body sites (Medawar, 1948), plenty of evidence during the past 70 years has shown that both suppressive and active immunoregulatory mechanisms exist in corneal transplantation (Hori et al., 2019; Niederkorn and Larkin, 2010; Streilein, 2003). Mechanisms of the immune privilege in the corneal transplantation are summarized as follows (Fig. 2 ) (Hori et al., 2019; Streilein, 2003): (1) lymphangiogenic and hemangiogenic privilege, (2) cell surface or soluble immunomodulatory molecules in the cornea and anterior chamber, (3) regulatory T cells (Tregs) related to anterior chamber-associated immune deviation (ACAID), and (4) neural regulations on immune cell stimulation.
Fig. 2.
Mechanisms of corneal immune privilege. Lymphangiogenic and hemangiogenic privilege, cell surface, or soluble immunomodulatory molecules, regulatory T cells (Tregs), anterior chamber-associated immune deviation (ACAID), and neural regulations are involved in immune privilege (Modified from the studies by Hori et al. (Hori et al., 2019) and Streilein (Streilein, 2003)).
Abbreviations: α-MSH: alpha melanocyte-stimulating hormone, CD: Cluster of differentiation, PEDF: Pigment epithelium-derived factor, TGF: transforming growth factor, TNF: tumor necrosis factor, TRAIL: TNF-related apoptosis-inducing ligand, TSP: thrombospondin, sFlt-1: soluble fms-like tyrosine kinase 1, VEGFR: vascular endothelial growth factor receptor, VIP: vasoactive intestinal peptide.
The cornea is known to be regulated by anti-lymphangiogenic and anti-hemangiogenic factors. However, it can be breached by the molecules such as interleukin (IL)-1 & −8, monocyte chemotactic protein 1, vascular endothelial growth factor (VEGF), 12-hydroxy-5,8,14-eicosatrienoic acid or by epithelial cell dysfunction (Hori et al., 2019; Ma et al., 2006). The anti-hemangiogenic or anti-lymphangiogenic factors are secreted from corneal epithelial cells, epithelial basement, and endothelial cells or exist in the aqueous humor (Hori et al., 2019). As a check-point, the eye has a cell surface and soluble immunomodulatory factors to suppress effector T cells and other innate cells or induce Tregs (Hori et al., 2019; Streilein, 2003). Furthermore, CD46, CD55, and CD59 present on the corneal epithelial cells and stroma or in aqueous humor as soluble molecules act as complement modulators (Bora et al., 1993; Hori et al., 2019; Sohn et al., 2000b). Since functionally an active complement system is present in the aqueous humor, tears, and cornea, a tight regulation by the complement regulatory proteins is critical for the maintenance of the ocular immune privilege (Chandler et al., 1974; Mondino et al., 1980; Sohn et al., 2000a). Thus, the dysfunction or absence of complement regulatory molecules of the appropriate species on the corneal xenograft may augment more complement-mediated damage than in an allograft. Corneal endothelial cells constitutively express inhibitory costimulatory signaling molecules that mediate T cell apoptosis, Tregs induction, or ACAID induction, all of which result in a decrease of allograft rejection (Hori et al., 2019; Niederkorn and Larkin, 2010). ACAID has a systemic tolerance for alloantigens placed in the anterior chamber, where antigen-bearing antigen presenting cells (APCs) migrate through the bloodstream, preferentially to the spleen by the upregulation of Tregs (Medawar, 1948; Niederkorn and Larkin, 2010; Niederkorn and Mellon, 1996). CD8+ Tregs act in the eye as efferent regulators, whereas CD4+ Tregs act in the lymphoid organs as afferent regulators that contribute to corneal allograft survival (Chauhan et al., 2009; Niederkorn and Mellon, 1996).
Besides, the adrenergic nerve is identified as a regulator of leukocyte recruitment, while the sympathetic and sensory nerves influence the migration of APCs (Hori et al., 2019). Streilein JW reported that penetrating keratoplasty (PKP) abolished ACAID (Streilein et al., 1996, 2000), which may accelerate graft rejection (Yamaguchi et al., 2016). The modulation of neuropeptides can prolong corneal allograft survival (Hamrah et al., 2009; Paunicka et al., 2015).
In summary, despite the breach of the ocular immune privilege after keratoplasty (Paunicka et al., 2015), the corneal graft may be benefited from the ocular immune privilege as for a candidate of xenotransplantation, whereas renal or cardiac grafts undergo strong xenogeneic rejection.
2.4. Current standpoint on corneal xenotransplantation as a regenerative medicine
Scientific advances suggest that the new therapies could replace an allotransplant for a diseased cornea (Bobba et al., 2018; Chakrabarty et al., 2018; Griffith et al., 2016; Kim and Hara, 2015; Matthyssen et al., 2018; Nishida, 2003; Stern et al., 2018). The technical approaches can be grouped into three major categories: (1) stem cell therapy, (2) xenotransplantation, and (3) bioengineered products. These techniques are applied differently depending on the corneal layer to be replaced. These methods are still in the developing stage and need to be further improved before clinical usage. Herein, key challenges, benefits, position in clinical stages and ethical concerns are addressed in each approach and compared to each other (Table 5 ) (Bobba et al., 2018; Brunette et al., 2017; Chakrabarty et al., 2018; Griffith et al., 2016; Kim and Hara, 2015; Matthyssen et al., 2018; Nakamura et al., 2016; Nishida, 2003; Stern et al., 2018). Clinical outcomes of cultivated limbal or oral mucosal epithelial transplantation have been reported, autologous limbal epithelial stem cell therapy has been conditionally approved and clinical trials have been conducted using some biosynthetic analogs (Brunette et al., 2017; Griffith et al., 2016; Nakamura et al., 2016; Stern et al., 2018). Xenozoonosis and xenogeneic rejection are major obstacles to overcome for an application of xenotransplantation in human.
Table 5.
Comparative characteristics of each regenerative medical approach for a substitute of corneal or limbal allografts.
| Stem cell-based therapy | Xenotransplantation | Bioengineered product | |
|---|---|---|---|
| Indication |
|
|
|
| Main Sources |
|
|
|
| Benefits |
|
|
|
| Hurdles |
|
|
|
| Position in clinical pipeline |
|
|
|
| Ethical concern |
|
|
|
AbbreviationsCLET: cultivated limbal epithelial transplantation, COMET: cultivated oral mucosal epithelial transplantation, DPF: designated pathogen-free, GE: genetically-engineered, ES: embryonic stem cells, EU: European Union, iPS: induced pluripotent stem cells, LSCD: limbal stem cell deficiency, MSC: mesenchymal stem cells.
3. Characteristics of porcine cornea as a proper donor product
Characteristics of porcine corneal thickness, biomechanical, optical, and endothelial cell properties compared to the human cornea are shown in Table 6 .
Table 6.
Characteristics of the porcine cornea in comparison with the human.
| Breed of pig | Pig data | Pig Age (months) | Human data | Human Age (years) | ||||
|---|---|---|---|---|---|---|---|---|
| Central Thickness (μm) | Danish Landrace (WT) | 666 ± 68 | 3.5 | 527 ± 34 | 57.9 ± 10.8 | |||
| Wally Whippo (WT) | 722 ± 5 | 2–3.8 | ||||||
| 995 ± 10 | 42 | |||||||
| SNU miniature (WT) | 833 ± 66 | 41.7 ± 18.7 | ||||||
| GE (Revivicor) | 659 ± 31 | 1.5 | ||||||
| 914 ± 25 | 20–25 | |||||||
| Tensile strength (MPa) | 3.7 ± 0.2 | NA | 3.8 ± 0.4 | NA | ||||
| Median shear modulus (kPa)a | Danish Crown (WT) | 0.34 | NA | 2.0 | 53 ± 14 | |||
| Young's modulus (MPa) | WT | 0.22ζ | 4–6 | 0.29ζ | 50–64 | |||
| 0.30ζζ | 0.43ζζ | |||||||
| Stress-strain relationshipb | α | WT | 39.3 ± 11.0 | NA | 42.8 ± 11.7 | NA | ||
| β | 2.97 ± 0.2 | 2.97 ± 0.2 | ||||||
| Stress-relaxationc | P (X100) | WT | 64.6 ± 3.3 | NA | 85.6 ± 1.5 | NA | ||
| K (−) | 0.055 ± 0.007 | 0.017 ± 0.002 | ||||||
| Average stress reduction (%)d | WT | 49.2 ± 8.3 | 4–6 | 27.7 ± 5.6 | 76 ± 6 | |||
| The ratio of average creep strain (%) | WT | 42 | 4–6 | 49 | 58 ± 6 | |||
| Swell pressure (mmHg) | Danish Crown (WT) | 45e | NA | 84f | NA | |||
| Refractive Power (Diopter) | Sus scrofa domestica (WT) | 40.0 ± 2.3 | 6–8 | 43.7 ± 1.6 | 55 ± 11 | |||
| SNU miniature (WT) | 36.5 ± 1.8 | 41.7 ± 18.7 | ||||||
| GT-KO miniature (GE) | 43.2 ± 6.1 | 11.3 ± 3.4 | ||||||
| Endothelial cell density (/mm2) | Wally Whippo (WT) | 3093 ± 285 | 5–10 | 2720 ± 364g | 40–75 | |||
| GE (Revivicor) | 3022 ± 258 | 15 | ||||||
| SNU miniature (WT) | 2647 ± 32 | 32 ± 15 | ||||||
| Wally Whippo (WT) | 2130 ± 194 | 42 | ||||||
| GE (Revivicor) | 1714 ± 19 | 20–25 | ||||||
Abbreviations: GE: genetically-engineered, NA: not available, WT: wild-type.
The ratio of average creep strain at 300 and 1000 s between pig and human showed statistical significance (Elsheikh et al., 2008).
Young's modulus was measured by inflation test per 20 mm Hgζ or 40 mm Hgζζ (Elsheikh et al., 2008).
Median shear modulus (kPa) at 5% axial Compression with full-hickness porcine cornea and 0% axial compression with full-thickness human cornea (Sondergaard et al., 2013a).
α is scale factor and β is the exponent of the nonlinear relationship between stress and strain using the strip extensiometry method (Zeng et al., 2001).
P (X100) is the value of G(t) at the end of the stress-relaxation test, K is the slope of fitted G(t)-ln t line, and both data were statistically significant between pig and human (Zeng et al., 2001).
Average loss of initial stress after 400s in Stress-relaxation behavior (Elsheikh et al., 2008).
The swelling pressure in the full-thickness porcine corneas at 0% compression, at 760 μm (Sondergaard et al., 2013b).
The swelling pressure at a standard stromal thickness of 500 μm (Han et al., 2015; Olsen and Sperling, 1987).
In vivo human data with non-contact specular microscopy (Snellingen et al., 2001).
3.1. Biomechanical and optical properties of porcine cornea
The central porcine cornea is thicker (659–995 μm) compared to that of humans (Kim et al., 2016; Kim, 2017; Kim and Hara, 2015; Lee et al., 2014b). If the central thickness of the xenotransplant was greater than 900 μm, it may be unacceptable for a transplant. Unlike humans, the peripheral thickness in pigs is similar to the central thickness (Kim and Hara, 2015). Given that porcine corneal thickness depends on the age and the breed of the pig, the appropriate pig's age should be selected to match its corneal peripheral thickness with that of the recipient's as much as possible. Considering that the presence of an edema leads to a greater thickness measured in ex vivo evaluation than that an in vivo, a porcine corneal graft with a slightly greater central thickness than that of a human is considered applicable for transplantation.
With regard to biomechanics, the cornea shows both anisotropic elasticity and viscoelastic property. To characterize elasticity, Young's modulus (the ratio of longitudinal stress to strain) and shear modulus (the ratio of shear stress to shear strain) are commonly used (Hjortdal, 2018). Elasticity is an indicator of material stiffness (stress-strain relation) and tensile strength, as an intensive property of the material, is the maximum stress that a material can withstand while being stretched (Vellara and Patel, 2015), while viscoelastic behavior is the time-dependent response on strain rate, represented by the stress-relaxation and creep (Vellara and Patel, 2015). Stress-relaxation is defined as a change in the load applied to the material under a constant strain, and creep is defined as the deformational change in the strain of a material under a constant load (Vellara and Patel, 2015). The cornea also has a swelling property that affects its biomechanics.
Swelling pressure of the porcine cornea is reported to be lower than that of the human cornea, suggesting rapid swelling in the storage media (Olsen and Sperling, 1987; Sondergaard et al., 2013b). Although the tensile strength of the porcine cornea seems comparable to that of the human cornea, the stress–strain relationship indicates that the porcine cornea is less resistant to both longitudinal and shear stresses (Elsheikh et al., 2008; Sondergaard et al., 2013a; Zeng et al., 2001). Stress-relaxation behavior and creep test show that the porcine cornea appears to be less able to maintain its initial shape than the human cornea, which is statistically significant (Elsheikh et al., 2008; Zeng et al., 2001). Meanwhile, a report has shown similar stress-relaxation in the values of the spring stiffness constants and the time constants between the human and porcine corneas (Ahearne et al., 2007). In this study, we used two human donors (79 and 34 years old) with long storage time (2–3 months), which may affect the outcome of stress-relaxation. In summary, the fact that the porcine cornea is less stiff and more viscoelastic than the human cornea might be due to 1) species-specific structural differences of the stroma or 2) confounding factors such as the age-dependent natural cross-linking in older age in humans and 3) the quicker swelling of porcine cornea in storage solution (Hara and Cooper, 2011). So far, long-term mechanical maintenance of the grafts has been stable within normal ranges of intraocular pressure in NHP studies despite these biomechanical differences (Choi et al., 2015; Kim et al., 2018a). Given that the viscoelastic behavior is influenced by intraocular pressure (Perez et al., 2013), in vivo measurement of the hysteresis in porcine corneal graft should be further investigated with different intraocular pressures.
The refractive power of the wild-type (WT) porcine cornea (36.5–40.4 D) is flatter than that of the human cornea; however, it is steepened after keratoplasty when an oversized xenotransplant is used (Kim et al., 2016, 2018a; Kim and Hara, 2015). The refractive power of the GT-KO porcine cornea (43.2 ± 6.1 D) is similar to that of the human cornea (Yoon et al., 2020). Indeed, from a surgical point of view, the anatomical characteristics of a porcine cornea (WT or GT-KO) are comparable to those of a human cornea, when the selected donor has the appropriate age (Table 6) (Kim and Hara, 2015).
3.2. The characteristics of endothelial cells in porcine cornea
Corneal endothelial cells can keep the cornea transparent. Therefore, the functional potential of the endothelial cells in the porcine cornea should be similar to those of humans. Indeed, the proliferative potential of porcine corneal endothelial cells is comparable to that of humans, irrespective of WT or GT-KO pigs (Fujita et al., 2013; Kim et al., 2016).
Furthermore, porcine corneal endothelial cell density (CED) decreases with age, similarly to humans (Table 6) (Kim et al., 2016; Snellingen et al., 2001) and the age-dependent decrease of CED in GE pigs is higher than that in WT pigs (Table 6) (Kim and Hara, 2015). Given that more than 2200/mm2 of the CED is preferred for a donor graft, the pig's age may be limited in accordance with the CED. A previous study reported that the age of WT pigs may be limited to 72 months old or younger for suitable grafts (CED ≥2200 cells/mm2) or 48 months old or younger for qualified grafts (CED ≥2500 cells/mm2). The age limitation of GE pigs may be different from that of WT pigs. Indeed, preservation time-dependent decrease of CED is comparable to that in humans as a suitable graft, regardless of WT or GT-KO pigs (Kim et al., 2016; Yoon et al., 2020). In summary, the mechanical and biophysical properties of porcine cornea are comparable to those of humans when the selected donor is of an appropriate age.
4. Rejection mechanisms in corneal xenotransplantation
To understand the different xenogeneic rejections between the vascular organ and the cornea, we will briefly mention rejection responses in cardiac or renal pig-to-NHP xenotransplantation (Fig. 3 A) (Li et al., 2009; Sprangers et al., 2008); (1) hyperacute rejection (HAR) occurs within minutes or hours and it is induced by complement activation via preexisting natural Abs mostly against αGal on the vascular endothelium, (2) Delayed xenograft rejection (DXR) (i.e. acute vascular rejection) occurs within days or weeks and is mediated by anti-αGal, anti-non αGal, and T cell -dependent Abs; activated complements and the coagulation system; and innate immune cells such as natural killer (NK) cells, macrophages, and neutrophils and, (3) chronic rejection follows within weeks or months and is mediated mainly by CD4+ T cells and macrophages. However, the detailed mechanism of chronic rejection is not well known because acute humoral and cellular rejection has not been overcome in cardiac and renal xenotransplantation so far.
Fig. 3.
The rejection mechanism in organ xenotransplantation (A), and corneal allo-(B) and xeno-transplantation (C). A. hyperacute rejection (HAR) and delayed xenotransplant rejection (DXR), which is mediated by Abs against αGal and other antigens, activated complement and coagulation systems, and innate immune cells such as NK cells, macrophages, and neutrophils occur in organ xenotransplantation. B. Corneal allograft rejection is mediated primarily by CD4+ T cells. Under high-risk circumstances, allografts can be rejected by stimulating complement-dependent cytolytic Abs. C. In corneal xenotransplantation, HAR is not seen, but DXR and chronic rejections are evident through both αGal and non-αGal Ab-dependent complement-mediated cytolysis, and T cell-mediated cellular and Ab responses. NK cell-associated rejection is not evident. Abbreviations: Ab: antibody, αGal: Galα1-3Galβ1-4GlcNAc-R, CD: Cluster of differentiation, NK: natural killer.
To acknowledge the differences between xenogeneic and allogeneic responses in keratoplasty, allogeneic rejection is described hereafter. Although the eye is an immune-privileged site, allogeneic rejection still occurs due to the breach of immune-privilege (Fig. 3B). Allogeneic rejection is mediated primarily by CD4+ T cells (He et al., 1991; Qian and Dana, 2001). It has been debated whether CD8+ T cells mediate allogeneic rejection (Boisgerault et al., 2001; Niederkorn et al., 2006a, b; Qian and Dana, 2001). In early studies, CD8+ T cells do not contribute to allogeneic rejection (Boisgerault et al., 2001; Qian and Dana, 2001), however recent studies show CD8+ T cell-mediated rejection despite slow responses (Niederkorn et al., 2006a, b). Under high-risk circumstances, allotransplants can be rejected by stimulating complement-dependent cytolytic Abs (Hargrave et al., 2000; Niederkorn, 2007). It has been also reported that NK cells have a role in corneal allogeneic rejection (Claerhout et al., 2004; Schwartzkopff et al., 2010).
4.1. Xenogeneic rejection mechanism with variant donors in early studies
The immunological barriers in corneal xenotransplantation is less than in solid organ xenotransplantation, however it exceeds the barriers in corneal allotransplantation (Larkin and Williams, 1995). Early studies employ small animal models (i.e. rodent) to investigate the rejection mechanism, or medium to large animals (i.e. rabbit, cat, pig, NHP) to evaluate the efficacy as a preclinical trial with various donors (fish, rabbit, chicken, dog, pig, cow, sheep, NHP, human) (Hara and Cooper, 2010; Larkin and Williams, 1995; Ross et al., 1993). Herein, xenogeneic rejection mechanisms are shown in early studies with variant donors except pig donors (Table 7 ). The graft survival and xenogeneic rejection mechanism are affected depending on phylogenetic discordance, size of the donor, surgical and post-operative trauma, and different animal models (host immune system) (Hara and Cooper, 2010), In the rat models, Ross et al., 1993, 1994 and Larkin et el (Larkin and Williams, 1995) reported that there was 1) no hyperacute rejection, 2) infiltration of T cell, neutrophil and macrophage, and deposition of Ig G and Ig M in the graft, and 3) production of xenoreactive Ig M and Ig G post-transplantation or preformed xenoreactive Abs in the serum. It has been also reported that sensitization to xenoantigen accelerated corneal xenogeneic rejection (Ross et al., 1993) and similar rejection time was detected in athymic rats (3 days) compared to that in euthymic rats (3 days), suggesting an important role of the innate immune system (Larkin and Williams, 1995). Infiltration of eosinophils were also found in some rat model (Larkin and Williams, 1995). Taken together, acute humoral response with later cell-mediated response is the critical feature differentiating xenogeneic from allogeneic rejection.
Table 7.
Xenogeneic rejection mechanism in orthotopic corneal xenotransplantation using small to medium animal models with variant donors except pig.
| Recipient type | References | Type (PKP/lamella) | Donor | Recipient | Donor size (mm) | N | AST or MST | Histology of the rejected grafts/serum/DLN | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Rat | (Ross et al., 1993, 1994) | PKP | Guinea pig | Lewis ACI | 3.5 | 10 | 8 D |
|
||
| Lewis ACI (+xeno-skin graft) | 8 | 5 D |
|
|||||||
| Larkin and Williams (1995) | PKP | Guinea pig | Fischer 344 | 3.0 | NA | 3 D |
|
|||
| CBH-rnu/rnu (athymic) | NA | 3 D |
|
|||||||
| Chicken | Fischer 344 | NA | 2 D |
|
||||||
| Mouse | Tanaka et al. (2000) | PKP | Guinea pig | C.B-17SCID | 2.0 | 8 | >56 D†† | |||
| C57BL/6 | 15 | 10 D |
|
|||||||
| BALB/c | 10 | 16 D |
|
|||||||
| μ KO | 15 | 8 D | ||||||||
| C3 KO | 13 | 21 D†† | ||||||||
| β-2 KO | 14 | 9 D | ||||||||
| CD4 KO | 11 | 27 D†† | ||||||||
| Tanaka et al. (2001) | PKP | Guinea pig | Class II KO | 2.0 | 11 | 31 D†† | ||||
| Yamagami et al. (1997) | PKP | Rat (Lewis) | BALB/c (H2d) | 2.0 | 13 | 5.8 D |
|
|||
| BALB/c (H2d) (+FK506/anti-LFA-1 mAb) |
9 | 67.7 D††† |
|
|||||||
| Pindjakova et al. (2005) | PKP | Rat (Lewis) | BALB/c | 2.0 | 11 | 8 D | ||||
| BALB/c (+anti CD4 Ab) | 7 | 16.9 D††† | ||||||||
| BALB/c (+anti CD8 Ab) | 11 | 8.9 D | ||||||||
| Sedlakova et al. (2005) | PKP | Sprague-Dawley rats | BALB/c (+FTY720, 3 mg/kg/d) | 2.0 | 5 | 15.6 D††† |
|
|||
| Holan et al. (2005) | PKP | Rat (Lewis) | BALB/c (+donor-specific anti-sera) | 2.0 | 8 | 7.4 D* | ||||
| BALB/c (+anti CD4 Ab) | 10 | 44.1 D | ||||||||
| BALB/c (+donor-specific anti-sera/anti CD4 Ab) | 8 | 21.5 D** | ||||||||
| PKP | Guinea pig | CB.17 SCID (+Whole splenocytes) | 2.0 | 13 | 15 D | |||||
| CB.17 SCID (+CD4+ T cell-depleted splenocytes) | 27 D*** |
|
||||||||
| CB.17 SCID (+CD4+/CD8+ T cell-depleted splenocytes) | 49 D | |||||||||
| Medium | Babel and Bourquin (1952) | lamellar | ox, sheep, horse, pig, guinea-pig, rabbit, human | rabbit | 5 | 30 | 1 W-3.5 M |
|
||
| Faber et al. (2009) | PKP | human | pig | NA | 1 | 15 D |
|
|||
Abbreviations: Ab: antibody, AST: average survival time, c/w: compared with, D: days, DLN: drainage lymph node, KO: knockout, LFA: leukocyte function-associated antigen, M: months, mAb: monoclonal antibody, MST: median survival time, N: number of animals experimented, NA: not available, PBMC: peripheral blood mononuclear cell, W: weeks.
Significantly increased survival compared with that of wild-type control††, or with that of control†††
Significantly shortened survival compared with that of control (9.4 D)* or with that of anti CD4 Ab treated group (44.1 D)**.
Significantly shortened survival compared with that of SCID reconstituted with CD4+/CD8+ T cell-depleted splenocytes (49 D) *** and increased survival compared with that of SCID reconstituted with whole splenocytes (15 D)***.
In mice models, graft survival seems to be relatively longer than that in the rat model. Most mice studies support the importance of CD4+ T cell-(Holan et al., 2005; Pindjakova et al., 2005; Tanaka et al., 2000) or T cell-(Sedlakova et al., 2005; Yamagami et al., 1997) mediated xenogeneic rejection. Involvement of complement (Tanaka et al., 2000) or indirect pathway of T-cell activation through the recipient's MHCII+ antigen-presenting cells in xenogeneic rejection (Tanaka et al., 2001) has been also reported. Although Tanaka et al. demonstrated no role of B cells using μ KO mice (Tanaka et al., 2000), other three reports indicate the involvement of humoral response in xenogeneic rejection (Holan et al., 2005; Sedlakova et al., 2005; Yamagami et al., 1997). Meanwhile, mice studies did not indicate an involvement of CD8+T cells in xenogeneic rejection (Pindjakova et al., 2005; Tanaka et al., 2000), on the contrary, a rat study presented CD8+ T cells in xenotransplants (Larkin and Williams, 1995). Additionally, corneal xenotransplants in CD4+ T cell-depleted mice are susceptible to rejection by interferon (IFN)γ secreting CD8+ T cells not by cytotoxic CD8+ T cells (Higuchi and Streilein, 2003).
In rabbit or pig studies, eosinophil, lymphocytes, neutrophils, or macrophages were observed in the grafts, and increased CD4+ T cells and preformed Abs were observed in the blood (Babel and Bourquin, 1952; Faber et al., 2009). Currently, through small to medium animal studies, evidences have emerged that both cellular and humoral immune responses are involved in corneal xenogeneic rejection (Hara and Cooper, 2010). Taken together, the effect of the each immune cell or humoral factors on xenogeneic rejection are suggested in the following order; CD4+ T cells »» macrophages »> Abs, complement » neutrophils, eosinophils > CT8+ T cells (Table 7) (Hara and Cooper, 2010). It has been reported that NK/NK-T cells are not involved in corneal xenograft rejection (Tanaka et al., 2000).
4.2. Xenogeneic rejection mechanism with pig donors
Corneal pig-to-NHP xenotransplants survive longer (>933 days) than orthotopic cardiac (>195 days) or renal (310 days) pig-to-NHP xenotransplants (Choi et al., 2015; Cooper et al., 2014; Langin et al., 2018; Wijkstrom et al., 2017), suggesting less immunological barriers. HAR is not observed in porcine corneal xenotransplants unlike solid organs (Hara and Cooper, 2010). Given that both αGal and non-αGal (e.g. N-glycolylneuraminic acid (NeuGc)) are expressed in porcine corneal cells, acute humoral rejection (i.e. DXR) still occurs (Cohen et al., 2014; Kim et al., 2009; Lee et al., 2007). Although αGal-related immune response does not occur in pig-to-rodents or pig-to-rabbits due to the expression of αGal epitopes in host animals, huge disparities of the hierarchical discordance and size between pig and rodents/rabbits still cause the short survivals. Xenogeneic rejection mechanism in orthotopic porcine corneal transplantation using small or medium animals are shown in Table 8 .
Table 8.
Xenogeneic rejection mechanism of orthotopic corneal xenotransplantation in pig-to-small or medium sized animal model.
| Recipient type | References | Type (PKP/lamellar) | Donor pig breed | Recipient | Donor size (mm) | N | MST (Days) | Histology of the rejected grafts/plasma |
|---|---|---|---|---|---|---|---|---|
| Rat | Lee et al. (2007) | PLK | Landrace | rat | 6.0 | 21 | 8.8 |
|
| Lee et al. (2008) | PLK | Domestic | Sprague-Dawley rat | 6.0 | 21 | 9.3 |
|
|
| Sprague-Dawley rat (+ISa) | 70 | 9.1 | ||||||
| Oh et al. (2009b) | PLK | SNU miniature | Sprague–Dawley rat | 5.0 | 5 | 9.7 |
|
|
| Sprague–Dawley rat (+allogeneic rat MSC) | 5 | 10.5 | ||||||
| Lee et al. (2010) | IST | Domestic | Sprague–Dawley rat (+topical steroid) | 2.0 | 40 | 14.0 |
|
|
| Mouse | Oh et al. (2009a) | PLK | SNU miniature | BALB/c | 3.0 | 10 | 9.0 | |
| C57BL/6 | 12 | 9.0 |
|
|||||
| Athymic Nude | 10 | 16.0c |
|
|||||
| SCID | 12 | 16.4c |
|
|||||
| NOG | 5 | 16.9 |
|
|||||
| Oh et al. (2010) | PLK | Domestic | C57BL/6 | 3.0 | 18 | 9.4 |
|
|
| SCID | 18 | 16.4 |
|
|||||
| C57BL/6 (+CVF) | 18 | 15.5d |
|
|||||
| SCID (+CVF) | 18 | 20.5d |
|
|||||
| Choi et al. (2011a) | PLK | SNU miniature | C57BL/6 | 2.5 | 4 | 8 |
|
|
| GT-KO (H-2b) | 4 | 9 |
|
|||||
| medium | Oh et al. (2008) | ALK | SNU miniature | rabbit | 6.0 | 10 | 19-28b |
|
| Oh et al. (2009c) | ALK | SNU miniature | rabbit | 7.0 | 8 | 29.1e |
|
|
| PKP | 7.5 | 8 | 16.8 |
|
||||
| Oh et al. (2009d) | ALK | SNU miniature | rabbit | 8.0 | 7 | 28 |
|
Abbreviations: Ab: antibody, αGal: Galα1-3Galβ1-4GlcNAc-R, ALK: anterior lamellar keratoplasty, CD: cluster of differentiation, CVF: cobra venom factor, D: days, HPF: high power field, GT-KO: α1,3-galactosyltransferase gene-knockout, IS: Immunosuppressant, IST: intrastromal transplantation, M: months, MSC: mesenchymal stem cells, MST: median survival time, N: number of animals experimented, NK: natural killer, NOG: NOD/SCID/γcnull, PLK: posterior lamellar keratoplasty, PMN: polymorphonuclear, SCID: severe combined immunodeficiency, SNU: Seoul National University, WT: wild-type.
Variable combinations of Immunosuppressant applied based on as follows; systemic cyclosporin A 1.25–10.0 mg/kg, dexamethasone 1.0–2.0 mg/kg, Mycophenolate mofetil 1.25 mg/kg.
MST is not available.
Statistically significant longer survival compared with that of control.
Statistically significant longer survival compared with that of control (B6 vs B6+CVF; SCID vs SCID + CVF).
Statistically significant longer survival of ALK compared with that of PKP.
In the rat models, 1) infiltration of T cells and macrophages are evident in the graft, 2) neutrophils and monocytes were found, 3) CD8+ T cells were found more than CD4+ T cells unlike in mouse model, and 4) NK cells were rarely found (Lee et al., 2008, 2010; Oh et al., 2009b). Humoral response had not been investigated in a pig-to-rat corneal transplantation. Furthermore, the systemic administration of steroid, cyclosporin and mycophenolate mofetil (MMF) or the topical application of allogenic rat mesenchymal stem cells (MSCs) did not prolong graft survivals (Lee et al., 2008; Oh et al., 2009b).
In mice models, the median survival time (MST) of the grafts was significantly prolonged in nude (T cell-defective), severe combined immunodeficiency (SCID; T and B cell-defective), or NOD/SCID/γcnull (NOG; T, B, and NK cell-defective) mice compared to WT mice (Oh et al., 2009a). MST was not different among the nude, SCID, and NOG mice, suggesting that T cells are important for the rejection compared with B or NK cells (Oh et al., 2009a). The MST significantly increased in both WT and SCID mice when complement depleted by cobra venom factor (Oh et al., 2010). Histology also showed that 1) infiltration of CD4+ T cells and macrophages, and deposition of Ig M and Ig G are evident, 2) neutrophils and eosinophils were found in early period and monocytes were infiltrated later, 3) CD8+ T cells and NK cells were rarely found (Choi et al., 2011a; Oh et al., 2009a, 2010). Furthermore, a pig-to-GT-KO mouse corneal xenotransplantation study showed that a gradual increase of IgG αGal Ab in the plasma and deposition of anti- αGal Ig G and Ig M in the grafts (Choi et al., 2011a). In an in vitro study, the absence of αGal or NeuGc on corneal endothelial cells reduced human Ab binding (Lee et al., 2016). Hence, αGal or non-αGal might have a role in corneal xenotransplantation.
In pig-to-rabbit corneal xenotransplantation, T cells, monocytes, and eosinophils are found (Oh et al., 2008, 2009c, 2009d) and lamellar grafts survived longer than full thickness grafts (Oh et al., 2009c). Based on histological analysis in rejected grafts of NHPs (Table 9 ), CD4+ and CD8+ T cells, macrophages, B cells and deposition of Ig G and complement are observed, and neutrophils and eosinophils are sometimes found. Moreover, aqueous humor activated complement and CD8+IFNγ+ cells in the blood have been reported to be a predictive biomarker of xenogeneic rejection in NHPs (Yoon et al., 2019). Hence, the critical role of the complement in xenogeneic rejection is presumably related with the fact that the porcine equivalent complement-regulatory protein is less capable of providing protection from the activation of the human complement; this probably due to relatively species-specific complementary modulatory proteins (Larkin and Williams, 1995; Zhou et al., 2019). Involvement of CD8+IFNγ+ cells in NHPs corresponds with that of a previous SCID mouse study reconstituted with CD4+ T cell-depleted splenocytes (Higuchi and Streilein, 2003). Increased donor specific Ig G and anti-non-αGaI Ig G are consistently observed in most NHPs with rejected grafts, while anti-αGal Ig M/Ig G increased in rejected WT grafts (Table 9).
Table 9.
Preclinical efficacy data and accompanying xenogeneic rejection mechanism of pig-to-NHP corneal xenotransplantation.
| References | Type | Donor pig breed/GE | Graft size (mm)/Thickness (㎛) | Survival days | Immunosuppression | Histology, AH and blood changes in NHPs with the rejected grafts | |
|---|---|---|---|---|---|---|---|
| Amano et al. (2003) | ALK | Domestic/WT | 5.0/100-200 | >30,>30, >30, 75, 165, 180 |
None |
|
|
| Zhiqiang et al. (2007) | ALK | WZS miniature/WT | 6.0/anterior half | >90, >90, >90, >90 |
None |
|
|
| Li et al. (2011) | ALK | WZS miniature/WT | 7.0/anterior half | 180, 15, 180,180,180 |
None |
|
|
| Choi et al. (2011b) | ALK | SNU miniature/WT | 7.5/312.5–375 | >398, >194, 24.5, 24.5 |
Topical, subconjunctival, and systemic steroid |
|
|
| Vabres et al. (2014) | ALK | Large White/WT | 6.5/350-400 | 9,70,21,21 | Topical steroid |
|
|
| Kim et al. (2017) | DALK | SNU miniature/WT | 7.5/687.5–750 | >389, >382, >236, >201, >61 |
Topical, subconjunctival, and systemic steroid + IVIG + Anti-CD40 Ab |
|
|
| Vabres et al. (2014) | ALK | Large White/hCTLA4-Ig transgenic | 6.5/350-400 | 21,50, 90,120 |
Topical steroid |
|
|
| ALK | Sus scrofa/GT-KO + hCD39 + hCD55 + hCD59 + FT | 6.5/350-400 | 9,34 | ||||
| Zhiqiang et al. (2007) | PKP | WZS miniature/WT | 6.0/Full | 12, 16, 16, 16, 12, 18 |
None |
|
|
| PKP | WZS miniature/WT | 6.0/Full | 129, 276, 182, 144 |
Subconjunctival steroid |
|
||
| Jie et al. (2013) | PKP | WZS miniature/WT | 6.0/Full | 32, 42, 40, 34, 38, 30 |
Systemic cyclophosphamide + BMT |
|
|
| PKP | WZS miniature/WT | 6.0/Full | 12, 18, 16, 20, 20, 20 |
Systemic cyclophosphamide |
|
||
| Choi et al. (2015) | PKP | SNU miniature/WT | 8.0/Full | 21,28,29 | Topical, subconjunctival, and systemic steroid |
|
|
| >933, >243, 318, >192 |
Topical, subconjunctival, and systemic steroid + Anti-CD154 Ab |
|
|||||
| Dong et al. (2017) | PKP | Large White·Landrace/WT | 6.5/Full | 157, 28, 92, 33 |
Topical and subconjunctival steroid |
|
|
| Large White·Landrace/GT-KO + CD46 | 128, 57, 47, 171 |
|
|||||
| Lee et al. (2017b) | PKP | Large White/GT-KO + CD46 | 6.5/Full | >90, >90, >90,>90 | Topical, subconjunctival, and systemic steroid |
|
|
| Kim et al. (2018a) | PKP | SNU miniature/WT | 7.5/Full | 41, >196, >203, >273, >422 > 511 | Topical, subconjunctival, and systemic steroid + IVIG + Anti-CD40 Ab |
|
|
| 97>, 134, >184, >210, >260, 297, >470 | Topical, subconjunctival, and systemic steroid + Tacrolimusa + IVIG + Basiliximab + Anti-CD20 Abb | ||||||
| Yoon et al. (2019) | PKP | SNU miniature/WT | 7.5/Full | 56, 92, 162, >181, >182, >182,>198 |
Topical, subconjunctival, and systemic steroid + Tacrolimusc + IVIG + Basiliximab + Anti-CD20 Abd |
|
|
| 29, 149, 161 | Topical, subconjunctival, and systemic steroid + Tacrolimuse + IVIG + Basiliximab | ||||||
| Yoon et al. (2020) | PKP | White Yucatan/GK-KO or GT-KO + hCD39 | 7.5/Full | 37, 55, 72, 91, 165b |
Topical, subconjunctival, and systemic steroid + Tacrolimuse |
|
|
| White Yucatan/GT-KO or GT + CMAH + iGb3s triple KO | >83, >187, >187, >375 | Topical, subconjunctival, and systemic Steroid + Tacrolimusa + IVIG + Basiliximab + Anti-CD20 Abb |
|
||||
| Liu et al. (2019) | DSAEK | WZS miniature/WT | 6.0/posterior lamellara | <30, <30, >58, >180, >180, >270, >298 | Topical and subconjunctival steroid |
|
|
Abbreviations: Ab: antibody, αGal: Galα1-3Galβ1-4GlcNAc-R, AH: aqueous humor, ALK: anterior lamellar keratoplasty, BMT: bone marrow transplantation, C3c & C3a: component of complement fragment, CD: cluster of differentiation, CMAH: cytidine monophospho-N-acetylneuraminic acid hydroxylase, DALK: deep anterior lamellar keratoplasty, DSAEK: Descemet stripping automated endothelial keratoplasty, FT: fucosyltransferase, GE: genetically-engineered, GT: α1,3-galactosyltransferase, GT-KO: α1,3-galactosyltransferase gene-knockout, hCTLA4-Ig: human cytotoxic T-lymphocyte associated antigen 4-immunoglobulin, hCD39: human ectonucleoside triphosphate diphosphohydrolase-1, hCD46: human complementary regulatory protein, hCD55: human complement decay-accelerating factor, hCD59: human MAC-inhibitory protein, iGb3s: isoglobotrihexosylceramide 3 synthase, IVIG: intravenous immunoglobulin, IFNγ: interferon gamma, IL: interleukin, IP-10: interferon gamma-induced protein, PKP: penetrating keratoplasty, MCP: monocyte chemoattractant protein, M: months, RANTES: regulated upon activation normal T cell expressed and secreted, SNU: Seoul National University, TNFα: tumor necrosis factor-α, W: weeks, WZS: Wuzhishan, WT: wild-type.
Tacrolimus was intramuscularly administered twice daily at a dose of 0.05a or 0.035c mg/kg or at a dose of 0.05 mg/kg for 4 weeks followed by 0.035 mg/kge.
Anti-CD20 Ab was intravenously administered at a dose of 20 mg/kg on postoperative days 0 and 7, and every 2b or 3d months.
Posterior lamellar graft was made after removal of anterior lamellae by 510 ㎛.
One corneal graft that survived 165 days was derived from a pig of the crossbreeding Landrace with Chicago minipig.
Therefore, xenogeneic rejection mechanisms in porcine corneal xenotransplantation are summarized as follows (Fig. 3C): (1) HAR is not seen in all animal models, (2) acute and chronic rejections are evident through CD4+ T cell-mediated cellular, humoral, and complement-mediated innate responses, (3) involvement of CD8+ T cell is different depending on the animal models, and CD8+ T cell-associated rejection is evident in NHP model, and (4) NK cell-associated rejection is not seen in all the animal models.
A few studies on the immunogenicity of MHC in xenogeneic rejection have been reported. In corneal allotransplantation, studies published before the year 2000 have shown controversial outcomes on the effect of HLA matching allograft survival along with erroneous HLA typing results (van Essen et al., 2015). However, the technical advances in HLA typing methods after the year 2000 allowed recent studies to provide consistent evidence that HLA matching is beneficial to allograft survival in both low- and high-risk corneal allografts (van Essen et al., 2015). New evidences indicate that homologous sequences are identified between HLA and swine leukocyte antigens (SLA), and some anti-HLA antibodies are cross-reactive with SLA in sensitized individuals (Byrne, 2018; Mulder et al., 2010). Furthermore, an evidence indicates that porcine cells present SLA antigens directly to human T cells (Murray et al., 1994), suggesting that MHC antigen-mediated T cell response may have a role in xenotransplantation. Additionally, CIITA-DN transgenic pigs that reduce SLA class II expression also inhibit human anti-pig T cell responses (Hara et al., 2013). Moreover, SLA I was expressed in both pig corneal and aortic endothelial cells with in vitro human CD4+ T cell responses against them (Hara et al., 2011). Therefore, it is possible that SLA mediated T cell responses or recipient's MHC antibody mediated cross-responses in corneal xenogeneic rejection.
5. Immunomodulating agents in xenotransplantation
The principal goal of immunomodulating agent administration is to balance the benefit of rejection prevention and the risk of over-immunosuppression. To find the optimal combinations to prevent xenogeneic rejection, almost all immunosuppressants currently applied to allotransplantation, along with various biologic agents under investigation, have been tested in pig-to-NHP transplantation models, which are considered the optimal animal models to justify initiating a clinical trial (Hering et al., 2016; Kim et al., 2014). Table 10 shows various kinds of immunosuppressants and their mechanism of action in the immune system. Table 10 also includes effective drugs that enabled long-term survival of the porcine heart, kidney, islet and corneal grafts in NHP recipients for more than 6 months. Although targeted genetic manipulation of the donor pig has allowed to significantly reduce immunosuppression, potent systemic immunosuppressive strategies are still needed to overcome antigenic differences between species. Conversely, common protocols used for induction and maintenance therapy in current solid organ allotransplantation including heart, liver, lung, kidney and pancreas (Chang et al., 2014; Costa et al., 2017; Dhanasekaran, 2017; Kimelman and Brandacher, 2013; Lim et al., 2017) are less effective in xenotransplantation.
Table 10.
Immunomodulating agents applied to allotransplantation and potential candidates for xenotransplantation clinical trials.
| Immunomodulating agents | Mechanism of action | Specific use | pig-to-NHP Xenotransplantationa | |
|---|---|---|---|---|
| FDA approved for transplantation | ||||
| Induction | Anti-thymocyte globulin | Polyclonal Ab from horses or rabbits immunized with human thymocytes, T-cell depleting | ACR | Heart, islets |
| Basiliximab | Inhibition of IL-2 receptor (CD25), T-cell non-depleting | PKP, islets | ||
| Belatacept, abatacept | Prevent interaction between CD80 and CD86 receptors on the antigen presenting cell and CD28 on the T cell | Islets | ||
| Maintenance | Cyclosporine A | Inhibition of the enzyme calcineurin by binding of cytoplasmic cyclophilin A/D | ||
| Tacrolimus | Inhibition of the enzyme calcineurin by binding of FKBP-12 | PKP | ||
| Azathioprine | Inhibition of the cell cycle by antagonizing purine metabolism | |||
| Mycophenolic acids | Inhibition of the lymphocyte cycle by blocking inosine monophosphate dehydrogenase | Kidney, heart, islets | ||
| Sirolimus, everolimus | Inhibition of the enzyme mTOR by binding of FKBP-12 | Heart, islets | ||
| Belatacept, abatacept | Prevent interaction between CD80 and CD86 receptors on the antigen presenting cell and CD28 on the T cell | Islets | ||
| FDA non-approved, commercially available | ||||
| Corticosteroids | Binding of the glucocorticoid receptor | ACR, AMR | PKP, LKP, DSAEK, kidney, heart, islets | |
| Alemtuzumab | Binding of CD52, T-cell depleting | |||
| Intravenous immunoglobulin | Modulating antigen presenting cell activity and compliment activation | Desensitization, AMR | PKP | |
| Rituximab | Monoclonal Ab against CD20 | Desensitization, AMR | PKP, heart | |
| Bortezomib | Reversible inhibitor of the 26 S proteasome Suppression of Ab production from mature plasma cells |
Desensitization, AMR | ||
| Eculizumab | Blockade of the C5b-9 membrane attack complex | Desensitization, AMR | ||
| C1 esterase inhibitor | Inhibition of compliment system | Heart | ||
| Fingolimod | Sphingosine-1-phosphate receptor agonist | Islets | ||
| Efalizumab | Blockade of interaction between leukocyte function associated antigen 1 and intracellular adhesion molecule | Islets | ||
| Etanercept, adalimumab | Anti-tumor necrosis factor-alpha inhibitor | Heart, islets | ||
| IL-1 receptor antagonist | IL-1 inhibitor | Heart | ||
| IL-6 receptor antagonist | IL-6 inhibitor | Heart | ||
| Under investigation | ||||
| Anti-CD154 Ab | Blockade of CD40−CD154 costimulatory signal | PKP, kidney, heart, islets | ||
| Anti-CD40 Ab | Blockade of CD40−CD154 costimulatory signal | PKP, LKP, heart, islets | ||
| CD4R1 | Rhesus recombinant CDR-grafted anti-CD4 Ab | Kidney | ||
| M-T807R1 | Mouse/rhesus CDR-grafted form of the depleting anti-CD8a Ab | Kidney | ||
| Cobra venom factor | Inhibition of compliment system | Islets | ||
| Regulatory T cells | Induction and maintenance of peripheral tolerance | Islets | ||
| Regulatory B cells | Development of peripheral tolerance | |||
| Tolerogenic/regulatory dendritic cells | Development of peripheral tolerance | |||
| Mixed chimerism | Development of central and peripheral tolerance | |||
Abbreviations: Ab: antibody, ACR: acute cellular rejection, AMR: antibody-mediated rejection, CD: cluster of differentiation, CDR: complementarity determining region, DSAEK: Descemet stripping automated endothelial keratoplasty, FDA: Food and Drug Administration, FKBP: FK506-binding protein, IL: interleukin, LKP: lamellar keratoplasty, mTOR: mammalian target of rapamycin, NHP: non-human primate, PKP: penetrating keratoplasty.
Immunosuppressive drugs used in successful combinations that enabled long-term survival of porcine xenotransplants (more than 6 months) in non-human primate recipients.
5.1. Current immunomodulating regimen in corneal allotransplantation
Given the huge antigenic difference between pig and humans, the clinical application of a porcine corneal graft may need specific management of high-risk corneal allotransplantation. The following management is suggested for high-risk corneal allotransplantation. As preoperative management, a useful approach is to reduce the host corneal neovascularization with corticosteroids (CSs) (Kim et al., 2013a) or anti-VEGF Ab (Bock et al., 2013; Fasciani et al., 2015; Vassileva and Hergeldzhieva, 2009). In addition to CS, topical cyclosporine A (CsA) 2% (Belin et al., 1989; Inoue et al., 2000) or tacrolimus 0.03% (Dhaliwal et al., 2008; Magalhaes et al., 2013) are reported to be effective in high-risk keratoplasty as a selective T cell inhibitor. Regarding a systemic immunosuppressive regimen, CSs represents the key medication (Abud et al., 2017; Di Zazzo et al., 2017; Hos et al., 2019) and CsA has long been used to prevent rejection in high-risk corneal allotransplantation. However, there has been no consensus on its efficacy (Hill, 1994; Shimazaki et al., 2011). In summary, tacrolimus, MMF, and rapamycin have been reported to be administered in high-risk recipients (Birnbaum et al., 2006; Chatel and Larkin, 2010; Joseph et al., 2007).
5.2. Immunomodulating regimen in corneal xenotransplantation
Fig. 4 shows several immunosuppressive drugs that are applied in the xenotransplantation area and their targets. Certain combinations of the following drugs enabled long-term (>6 months) survival of porcine corneal grafts in NHP recipients, and thus, might be potential candidates for corneal xenotransplantation human clinical trials (Choi et al., 2015; Kim et al., 2018a).
Fig. 4.
Immunosuppressive drugs for corneal transplantation and their sites of action in the immune system. There are various kinds of immunosuppressive drugs that have been used in high-risk corneal allotransplantation or pig-to-non-human primate (NHP) corneal transplantation experiments. Most of these drugs target interactions between T cells and antigen-presenting cells or intracellular pathway involved in T cell activation. Drugs highlighted with a red-colored text are potential candidates for corneal xenotransplantation clinical trials, of which efficacy and safety have been proven in pig-to-NHP corneal transplantation studies. Abbreviations: AP-1: activating protein-1, APC: antigen presenting cell, CS: corticosteroid, CsA: cyclosporine A, CTLA: cytotoxic T lymphocyte antigen, FKBP: FK506-binding protein, G1: cell cycle gap phase 1, G2: cell cycle gap phase 2, IVIG: intravenous immunoglobulin, M: cell cycle mitosis phase, MHC: major histocompatibility complex, MPA: mycophenolic acid, mTOR: mammalian target of rapamycin, NFAT: nuclear factor of activated T cells, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, TCR: T cell receptor, S: cell cycle synthesis phase. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
5.2.1. Emerging biological agents for corneal xenotransplantation
5.2.1.1. Antibody-based non-costimulatory blockade agents
Basiliximab is a chimeric monoclonal antibody (mAb), which competitively inhibits the alpha subunit of the IL-2 receptor (CD25) (Rodrigues et al., 2009); it inhibits T-cell proliferation but does not cause depletion and is used in combination with other immunosuppressants with no increase in adverse effects (Henry and Rajab, 2002). Rituximab is also a chimeric mAb against the protein CD20 primarily on B cells. Both basiliximab and rituximab have been used for corneal pig-to-NHP studies and showed long-term survival of grafts (Kim et al., 2018a; Yoon et al., 2020). Intravenous immunoglobulin (IVIG) products are derived from pooled human plasma and suppress APC activity, Ab production, and complement activation. IVIG has emerged as an important component of desensitization protocols and for the treatment of antibody-mediated rejection (AMR) and pig-to-NHP corneal xenotransplantation (Choi et al., 2015; Jordan et al., 2011).
5.2.1.2. Antibody-based costimulatory blockade agents
Belatacept is a high-affinity variant of fusion protein composed of the Fc fragment of a human IgG1 immunoglobulin linked to the extracellular domain of cytotoxic T lymphocyte antigen (CTLA)-4, which has been used for long-term survival of porcine islet cells in NHP recipients (Cardona et al., 2006; Thompson et al., 2011). It can increase the risk of posttransplant lymphoproliferative disorder (PTLD) in Epstein-Barr virus (EBV)-seronegative recipients (Vincenti et al., 2005).
Anti-CD154 Ab is the one of the main therapy used in cardiac, renal, or corneal pig-to-NHP transplantation (Choi et al., 2015; Langin et al., 2018; Wijkstrom et al., 2017). Based on the preclinical efficacy of anti-CD154 treatment, a clinical trial was initiated using a humanized anti-CD154 mAb (ruplizumab/BG9588) in kidney transplantation (Kirk et al., 2001). However, the development of this drug was discontinued because of concerns related to thromboembolic complications (Kawai et al., 2000; Koyama et al., 2004). Anti-CD154 mAb can directly activate platelets since CD154 is expressed on the platelet surface (Xu et al., 2006). At least three anti-CD154 Abs (letolizumab/BMS-986004, dapirolizumab pegol/CDP7657, and VIB4920) have been developed to minimize thromboembolic complications and are undergoing human clinical trial for autoimmune diseases (Schroder et al., 2019), which are expected to be applied in the xenotransplantation field.
CD40 became an alternative therapeutic target to avoid thromboembolic complications in cardiac and corneal pig-to-NHP transplantation (Kim et al., 2018a; Langin et al., 2018). Bleselumab/ASKP1240 is a fully human IgG4 anti-CD40 mAb, and a recent phase II clinical trial for kidney transplantation demonstrated noninferiority to tacrolimus and MMF-based standard care (Harland et al., 2020). CFZ533 is an anti-CD40 mAb with a modified Fc domain (Ristov et al., 2018), and a phase I/II clinical trial for kidney transplantation showed efficacy. BI-655064, a humanized anti-CD40 mAb, is involved in an ongoing clinical trial to investigate the safety, efficacy, and therapeutic mechanism against autoimmune diseases (Visvanathan et al., 2019).
5.2.1.3. Cell-based therapies
Cell-based therapy is innovative and a possible strategy to minimize the use of immunosuppression and improve long-term graft survival (Cai and Chandraker, 2019; Forrester et al., 2013). Indeed, a marked prolongation of porcine renal graft survival has been reported in baboon recipients with co-transplanted vascularized thymic tissue (Yamada et al., 2005). Furthermore, Tregs have been extensively studied for its prevention of graft rejection. Indeed, at least 15 clinical trials in solid organ transplantation are ongoing using ex vivo expanded autologous Tregs (Romano et al., 2019). Moreover, regulatory B cells can modulate the differentiation of T cells and produce IL-10, transforming growth factor (TGF)-β, and IL-35 and early phase clinical trials of tolerogenic/regulatory dendritic cells (DCtols/DCregs) in renal or liver transplantation have begun (Ochando et al., 2020). A few clinical trials have used MSCs to investigate safety and feasibility, while ongoing clinical trials with MSCs are studying the minimization of immunosuppression in renal and liver transplantation (Reinders et al., 2018).
Mixed chimerism is defined as a state where donor and recipient hematopoietic cells coexist at levels sufficient to be detected by standard techniques. Mixed chimerism has been used for renal and corneal pig-to-NHP studies (Jie et al., 2013; Sachs, 2018). Sustained full chimerism can allow central deletional tolerance, while transient chimerism-based tolerance appear to initially depend on Tregs followed by gradual, peripheral deletion of donor-reactive T cells. At least three clinical trials are ongoing in living-donor kidney transplantation (Oura et al., 2017).
5.2.2. Clinically relevant immunomodulating regimens based on pig-to-NHP corneal xenotransplantation studies
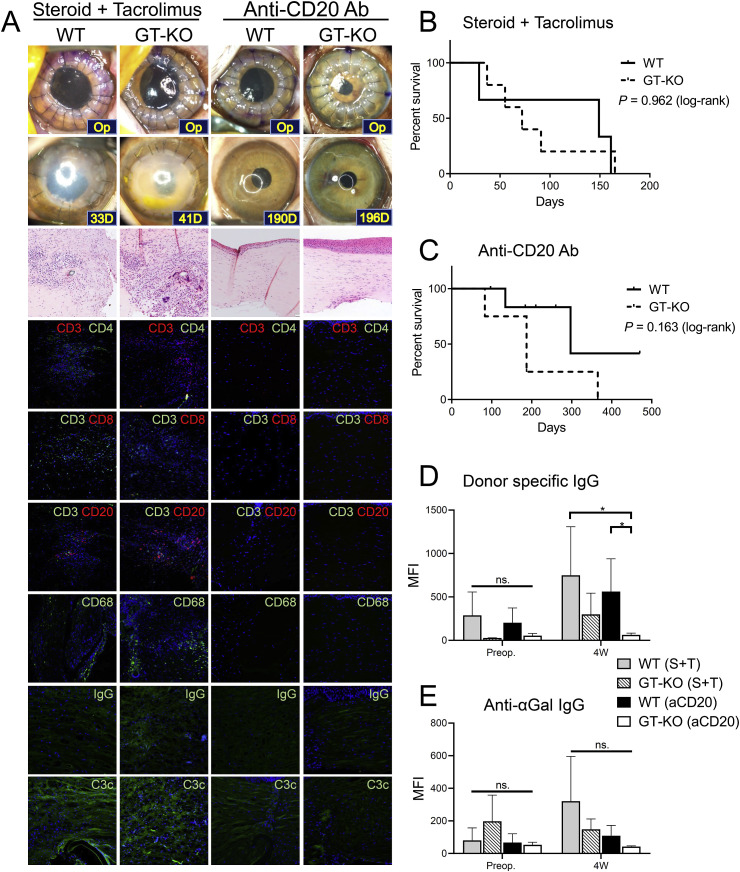
Although parts of pig-to-NHP studies showed CS-based regimens prolonged the survival of grafts, CS-only immunosuppression does not guarantee the long-term survival of grafts even in lamellar keratoplasty (LKP) models (Choi et al., 2011b; Vabres et al., 2014; Zhiqiang et al., 2007). The introduction of GE pigs or mixed chimerism did not show long-term survival of xenotransplants (Dong et al., 2017; Jie et al., 2013; Vabres et al., 2014). In contrast, both anti-CD154 mAb- and anti-CD40 Ab-based immunosuppression significantly prolonged the survival of WT corneal xenotransplants (Choi et al., 2015; Kim et al., 2017, 2018a). However, both of them are not commercially available. Nonetheless, it is noteworthy that a combination of commercially available drugs, including rituximab and tacrolimus, also demonstrated long-term survival of both WT and GT-KO full-thickness xenotransplants (Choi et al., 2018; Kim et al., 2018a; Yoon et al., 2020). Induction therapy and preventions of AMR seem to be crucial, and to date, induction using CS, basiliximab, IVIG, and rituximab and maintenance with tacrolimus and rituximab are a feasible option for human clinical trials based on pig-to-NHP corneal transplantation data (Choi et al., 2019b; Kim et al., 2018a). Since a recent pig-to-NHP endothelial keratoplasty (EK) study showed efficacy under subconjunctival injection of CSs (Liu et al., 2019), this implies that a regional CSs might be an option for patients with compromised endothelial cells.
Regarding general health status, NHP recipients with porcine corneal transplants maintained good appetite and physical activity during the long-term period of immunosuppression using CSs-, costimulatory blockade agents-, and rituximab/tacrolimus/basiliximab-based regimens, although costimulatory blockade agents and rituximab/tacrolimus/basiliximab induced early weight loss (Choi et al., 2015, 2018; Kim et al., 2017, 2018a). A case of asymptomatic tacrolimus-associated thrombotic microangiopathy, diagnosed via laboratory tests, was reportedly treated with a discontinuation of tacrolimus and administration of anticoagulant (Kim et al., 2018b). Long-term immunosuppression with the mentioned above immunosuppressive regimens have neither significantly affected white blood cell counts, liver enzyme levels, blood urea nitrogen/creatinine levels, and hematocrit nor caused electrolyte imbalance in rhesus recipients (Choi et al., 2018).
For the long-term survival of other xenografts including heart, kidney, and islet cells, treatment with costimulation blockade agents is also crucial for maintenance therapy in most pig-to-NHP transplantation experiments (Chan and Mohiuddin, 2017; Cooper et al., 2018; Higginbotham et al., 2015; Liu et al., 2017; Wijkstrom et al., 2017). Only two studies showed long-term survival of neonatal or embryonic islet xenotransplants with CD40/CD154 pathway-sparing regimens using rituximab, anti-thymocyte globulin (ATG), and belatacept as induction regimens; and abatacept, everolimus, and FTY720 as maintenance therapy (Hecht et al., 2009; Thompson et al., 2012).
6. Preclinical efficacy data of porcine corneal xenotransplantation in NHP studies
Corneal xenotransplant survival depends on the graft type, graft size, level of immunosuppression, and a hierarchical discordance between the donor and the recipient (Kim, 2017). Therefore, the survival of corneal xenotransplants in NHP studies differs between small and medium animal models. Given that porcine corneal matrix proteins showed higher suitability for humans compared to other studied species (Sharifi et al., 2019), the preclinical efficacy data of corneal xenotransplantation using pig donor are reviewed (Table 9).
The International Xenotransplantation Association (IXA) reached a consensus to set a threshold for the preclinical efficacy of NHP study sufficient to justify starting a clinical trial for corneal xenotransplantation (Kim et al., 2014). NHP data support a clinical trial if the porcine corneal xenotransplants survived for more than 6 months in five of eight consecutive NHPs; and ideally for 12 months in one or two successful cases (Kim et al., 2014). The preclinical efficacy of all NHP studies since 2003 are shown in Table 9.
6.1. Anterior lamellar keratoplasty
Since endothelial cells are not included, the immune response in ALK is less than in PKP. When the anterior lamellar (100 μm to anterior half thickness) grafts with a small diameter (5.0–7.0 mm) are transplanted, about 90–180 days of survival had been reported even without immunosuppression (Amano et al., 2003; Li et al., 2011; Zhiqiang et al., 2007). Meanwhile, although topical CS was used, thicker lamellar grafts (350–400 μm) were rejected within 21 days (Vabres et al., 2014). When anti-CD40 Ab was used, deep anterior lamellar keratoplasty (DALK) showed more than 180 days of xenotransplant survivals (Kim et al., 2017). One study used cornea from GE pigs. The human cytotoxic T-lymphocyte associated antigen4-immunoglobulin (hCTLA4-Ig) or GT-KO/hCD39/hCD55/hCD59/Fucosyltransferase transgenic pig corneas did not increase the survival of xenotransplant in ALK (Vabres et al., 2014).
6.2. Penetrating keratoplasty
PKP requires strong immunosuppressants to overcome xenogeneic rejection. Since corneal grafts of 7.5–8.0 mm size are commonly used in humans (Seitz et al., 2003), the outcome of NHP studies that used 7.5 mm or larger grafts is closer to the outcome of human PKP than those with smaller grafts.
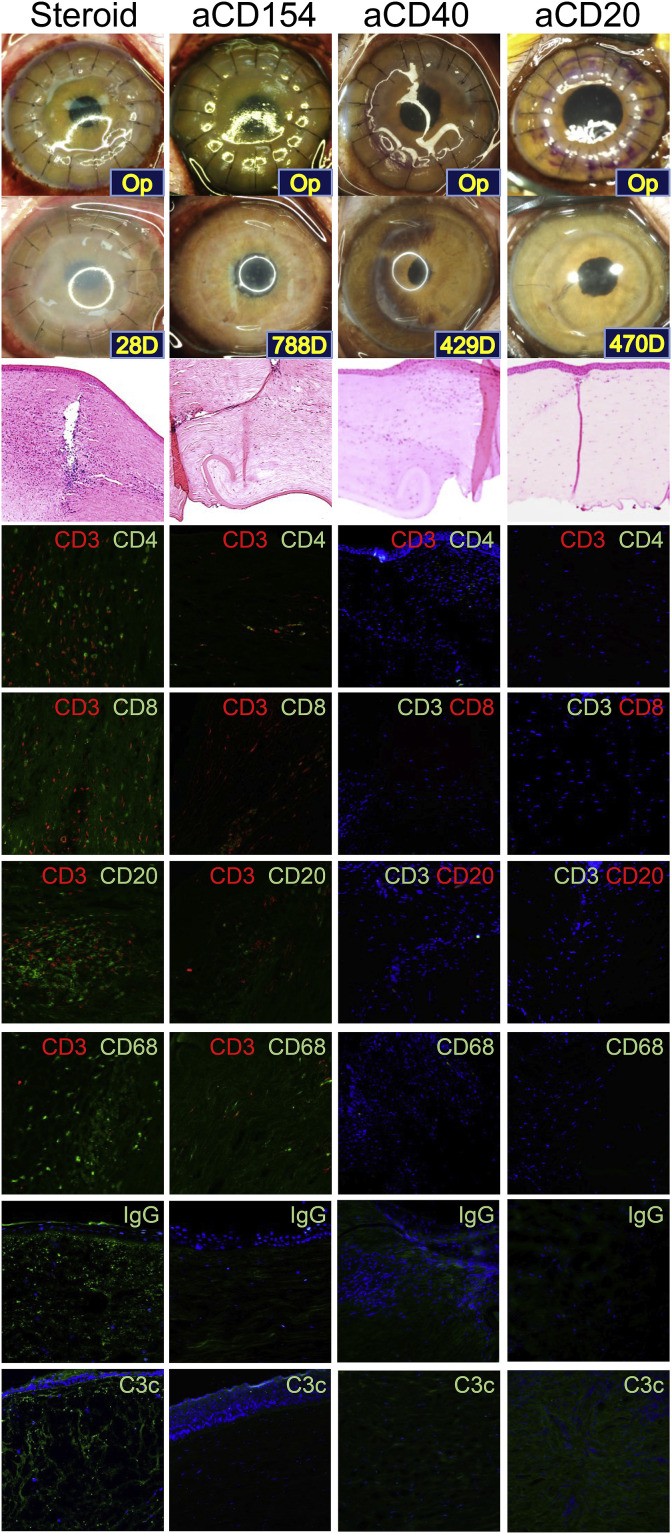
Five studies have reported graft survival for more than 6 months (Choi et al., 2015; Kim et al., 2018a; Yoon et al., 2019, 2020; Zhiqiang et al., 2007). Among these studies, one satisfied the threshold requirements for a clinical trial with anti-CD40 Ab- or anti-CD20 Ab/tacrolimus-based immunosuppressive regimen (Table 9) (Kim et al., 2018a). Conversely, studies where CS and tacrolimus were applied failed to achieve long-term graft survival and to reduce inflammatory biomarkers (Table 9 and Fig. 5 and Fig. 6 ) (Choi et al., 2015; Kim et al., 2018a; Yoon et al., 2019). In the rejected graft, a dense infiltration of CD4+ T and CD8+ T cells, B cells, and macrophages were observed, while most accepted grafts showed minimal infiltration of those cells (Table 9 and Fig. 5) (Choi et al., 2015; Kim et al., 2018a). In NHPs with rejected grafts, the complement component 3a (C3a) in aqueous humor increased and severe deposition of complement component 3c (C3c) was found in the grafts (Figs. 5 and 6) (Choi et al., 2015; Kim et al., 2018a; Yoon et al., 2019). Treatment with anti-CD40 Ab- or anti-CD20 Ab/tacrolimus-based immunosuppressive regimen inhibited inflammatory cell infiltration, IgG and complement deposition in the grafts and reduced aqueous activated complement, donor-specific IgG, anti-αGal IgG, or activated B cells as well (Figs. 5 and 6) (Choi et al., 2015; Kim et al., 2018a; Yoon et al., 2019).
Fig. 5.
Representative photographs, H&E, and immunofluorescence staining images in NHPs with wild-type porcine corneal grafts with different immunosuppressive regimens. All grafts were rejected in the steroid group within 4 weeks, while long-term graft survival (>6 months) was shown in anti-CD154 Ab (aCD154), anti-CD40 Ab (aCD40), and anti-CD20 Ab (aCD20) groups. Rejected grafts show densely infiltrated by CD3+CD4+ T, CD3+CD8+ T, and CD3−CD20+ B cells and CD68+ macrophages with dense depositions of IgG and C3c. Abbreviations: Ab: antibody, C3c: complement component 3c, CD: cluster of differentiation, H&E: hematoxylin and eosin, Ig: immunoglobulin, NHP: non-human primate, Note: Fluorescein color of CD8 and CD20 are green in Steroid and aCD154 groups, and red in aCD40 and aCD20 groups. All NHPs received methylprednisolone intramuscularly at an initial dose of 2 mg/kg/d and tapered over 5 weeks. aCD154 group: Recombinant anti-CD154 Ab (V-regions from mouse 5C8 clone; C-regions human IgG1k) was intravenously administered 15 to 19 times at a dose of 20 mg/kg aCD40 group: A mouse-rhesus chimeric monoclonal anti-CD40 Ab (2C10R4, NIH Non-human Primate Reagent Resource) was intravenously administered 15 times at a dose of 30–50 mg/kg aCD20 group: Anti-CD20 Ab (Rituximab; MabThera®, Hoffmann-La Roche, Basel, Switzerland) was intravenously administered at a dose of 20 mg/kg on postoperative days 0 and 7, and every 2 months. Tacrolimus (Prograf®; Astellas Pharma US, Deerfield, IL, USA) was intramuscularly administered twice daily at a dose of 0.05 mg/kg. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Comparative analysis of graft survival and inflammatory biomarkers in NHPs with wild-type porcine corneal grafts with different immunosuppressive regimens. (A–F) Kaplan-Meier survival curve shows longer median survival time (MST) in anti-CD20 Ab (aCD20) or Costimulation blockade (CoB) group than in steroid (S) or steroid + tacrolimus (S + T) group (log-rank test, P < 0.05), while no differences of MST were found between S and S + T group or between aCD20 and CoB group, respectively. (G) The postoperative level of C3a in the aqueous humor was significantly higher in S group than in aCD20 or CoB group, (H) level of donor-specific IgG was significantly higher in S group than in the aCD20 or CoB group as well, and (I) level of anti-αGal IgG was significantly higher in S group than in CoB group at 4 weeks. (J) The concentration of CD8+IFNγ+ cells at 2 weeks. (K) The concentration of activated B cells was significantly lower in the aCD20 group than in the S, S + T, or CoB groups. Statistical significance was tested by Kruskal-Wallis test followed by an uncorrected Dunn's test. Abbreviations: Ab: antibody, αGal: Galα1-3Galβ1-4GlcNAc-R, C3a: complement component 3a, CD: cluster of differentiation, Ig: Immunoglobulin, MFI: mean fluorescence intensity, NHP: non-human primate, ns: not significant, Preop: preoperative, W: weeks; Notes: All NHPs received methylprednisolone intramuscularly at an initial dose of 2 mg/kg/d and tapered over 5 weeks. Steroid group (S; n = 3) was received steroid only. Steroid combined with tacrolimus (S + T; n = 3) group: Tacrolimus (Prograf®; Astellas Pharma US, Deerfield, IL, USA) was intramuscularly administered twice daily at a dose of 0.035 mg/kg. CoB group includes both aCD154 and aCD40 groups. aCD154 group (aCD154; n = 4): Recombinant anti-CD154 Ab (V-regions from mouse 5C8 clone; C-regions human IgG1k) was intravenously administered 15 to 19 times at a dose of 20 mg/kg aCD40 group (aCD40; n = 6): A mouse-rhesus chimeric monoclonal anti-CD40 Ab (2C10R4, NIH Non-human Primate Reagent Resource) was intravenously administered 15 times at a dose of 30–50 mg/kg aCD20 group (aCD20; n = 7): Anti-CD20 Ab (Rituximab; MabThera®, Hoffmann-La Roche, Basel, Switzerland) was intravenously administered at a dose of 20 mg/kg on postoperative days 0 and 7, and every 2 months. Tacrolimus (Prograf®; Astellas Pharma US, Deerfield, IL, USA) was intramuscularly administered twice daily at a dose of 0.05 mg/kg.All data are described as mean ± standard error; *P < 0.05; **P < 0.01.
Regarding the GE porcine corneas, most PKP studies used various pigs including GT-KO. To date, GE porcine cornea did not show a significant increase in graft survival compared to WT porcine cornea (Dong et al., 2017; Yoon et al., 2020). Indeed, one study achieved long-term graft survival of GT-KO based GE porcine corneas, only when the recipients received anti-CD20 Ab/tacrolimus-based immunosuppressive regimen (Yoon et al., 2020). Furthermore, survival analysis showed no differences between WT and GE porcine cornea regardless of steroid/tacrolimus- or anti-CD20 Ab-based regimens (Fig. 7 ) (Yoon et al., 2019, 2020). In GT-KO/CD46 transgenic porcine corneal grafts, T cell infiltration is accompanied by an increased non-αGal Abs in NHPs (Dong et al., 2017). Moreover, T and B cells still infiltrate in hCTLA4-Ig transgenic porcine corneal grafts (Vabres et al., 2014). This indicates that T and B cell-mediated reactions are not exempted in GE pigs that had been available until now. Therefore, it remains unclear whether the absence of αGal expression on GE porcine cornea might have an advantage over WT porcine cornea.
Fig. 7.
Comparative analysis of graft survivals, histology, and inflammatory biomarkers in NHPs with α1,3-galactosyltransferase gene-knockout based (GT-KO) vs wild-type (WT) porcine corneal grafts with different immunosuppressive regimens. (A) Representative photographs, hematoxylin & eosin (H&E) and immunofluorescence staining show graft edema and inflammatory cell infiltration in NHPs that received systemic steroid and tacrolimus (S + T), while clear grafts without inflammatory cell infiltration with anti-CD20 Ab-based regimen regardless of donor pig types. (B, C) Kaplan-Meier survival curve shows no differences in graft survival between GT-KO and WT donor porcine cornea under the same regimen. (D) Level of donor pig-specific (DS) IgG was significantly lower in NHPs transplanted with GT-KO porcine corneas under anti-CD20 Ab-based immunosuppressive regimen than in NHPs transplanted WT porcine corneas at postoperative 4 weeks (Kruskal-Wallis test followed by an uncorrected Dunn's test). (I) Anti-αGal IgG level was not different among the groups at postoperative 4 weeks. Abbreviations: Ab: antibody, aCD20: anti-CD20 Ab, αGal: Galα1-3Galβ1-4GlcNAc-R, GT-KO: α1,3-galactosyltransferase gene-knockout, Ig: Immunoglobulin, MFI: mean fluorescence intensity, ns.: not significant, Preop: preoperative, W: weeks, WT: wild-type. Notes: All NHPs received methylprednisolone intramuscularly at an initial dose of 2 mg/kg/d and tapered over 5 weeks. Steroid combined with tacrolimus group (S + T): Tacrolimus (Prograf®; Astellas Pharma US, Deerfield, IL, USA) was intramuscularly administered twice daily at a dose of 0.035 mg/kg. Anti-CD20 Ab group (aCD20): Anti-CD20 Ab (Rituximab; MabThera®, Hoffmann-La Roche, Basel, Switzerland) was intravenously administered at a dose of 20 mg/kg on postoperative days 0 and 7, and every 2 months. Tacrolimus (Prograf®; Astellas Pharma US, Deerfield, IL, USA) was intramuscularly administered twice daily at a dose of 0.05 mg/kg. WT (S + T, n = 3), WT (aCD20, n = 7), GT-KO (S + T, n = 5), GT-KO (aCD20, n = 4). All data are described as mean ± standard error; *P < 0.05.
6.3. Endothelial keratoplasty
EK shows better visual outcomes and less rejection than PKP (Hos et al., 2019). EK does not have to consider thickness matching, and then does not need to choose a young donor for size-matching. Moreover, EK may need a weaker immunosuppressive regimen and shows less risk of xenozoonosis than PKP since there are fewer porcine cells in the posterior lamellar graft. Porcine corneas, unlike humans, have strong Descemet membrane-stroma adhesion (Liu et al., 2018). Therefore, a donor graft preparation for Descemet membrane endothelial keratoplasty (DMEK) is challenging. Hence, pig-to-NHP DMEK has not been reported yet. Recently, a study on how to produce DMEK graft using porcine cornea has been published (Liu et al., 2018). Regarding Descemet stripping automated endothelial keratoplasty (DSAEK), there was one study reporting long-term survivals of the porcine grafts in NHPs with CS (Liu et al., 2019). However, the graft size was too small (6.0 mm) to apply in human (Liu et al., 2019). EK with porcine corneal graft may be beneficial to overcome xenogeneic rejection. Therefore, further studies of EK are required using a large size of porcine graft to verify preclinical efficacy in NHPs.
In summary, pig-to-NHP full-thickness corneal transplantation shows preclinical efficacy with a clinically applicable graft size under co-stimulation blockade agents or anti-CD20 Abs/calcineurin inhibitor (CNI) combined regimen, while preclinical efficacy of pig-to-NHP ALK or DSAEK has not been proved sufficient so far.
7. Development of decellularized cornea and efficacy data
7.1. Concept of decellularization and various methods
7.1.1. Concept of decellularized porcine cornea (DPC)
Decellularization is a process to remove cells from tissues that will be used in cell-free scaffolds. Since cellular components of the porcine cornea are the main source of the xenoantigens (Lee et al., 2014c), decellularized cornea has the advantage of reducing the immune response after transplantation (Hargrave et al., 2003). Although DPC is regarded as a medical device (FDA, 2016b; Kim et al., 2013b), whether xenotransplantation includes DPC grafting is still debated in the international xenotransplantation community. Its efficacy and possible feasibility have also received attention and have been compared with native xenografting.
Corneal endothelial cells are a crucial barrier to prevent aqueous humor from entering the corneal stroma. Indeed, dysfunction of the endothelial cells result in corneal swelling and reduction of transparency (Brunette et al., 2017). Since DPC does not contain corneal endothelial cells, therapeutic indications for DPC are limited to only be used as anterior lamellar grafts for the cornea with healthy endothelial cells. DPC seeding of cultivated human corneal endothelial cells has been reported, but there have been no in vivo transplantation study using those grafts (Ju et al., 2012; Yoeruek et al., 2012a).
DPCs as anterior lamellar graft show long-term graft survival not only in NHP models but also in human studies (Choi et al., 2011b; Zhang et al., 2015; Zheng et al., 2019). Therefore, herein, efficacy and legal status of DPC products are updated to clarify the position of DPC as a medical device or a xenotransplant.
7.1.2. Various methods for decellularized porcine cornea
The decellularized methods for DPCs (used alone or in combination) are classified into three categories: chemical, physical, and biological (Fernandez-Perez and Ahearne, 2020). Chemical decellularization applies detergents including sodium dodecyl sulfate (SDS), sodium deoxycholate, Triton X-100, peracetic acid, formic acid, ammonium hydroxide, sodium chloride, and ethylenediaminetetraacetic acid (EDTA). Physical decellularization includes agitation, freeze-thawing, electrophoresis, high hydrostatic pressure, osmotic pressure, supercritical CO2, ultrasound, glycerol, and lyophilization (Li et al., 2017b). Biological methods are mostly enzymes that include trypsin, dispase, phospholipase A2, human serum, and nucleases.
The ideal decellularization aims to remove all cellular components while maintaining the transparency of the cornea and the structure of extracellular matrices (Isidan et al., 2019). The following methods have been reported to be sufficient for the decellularization process: (1) 1.5 M NaCl alone or 0.05% trypsin/0.02% EDTA application after 1.5 M NaCl (Lee et al., 2014c; Oh et al., 2009d), (2) 0.05% or 0.1% SDS (Du and Wu, 2011; Gonzalez-Andrades et al., 2015), (3) high-hydrostatic pressurization (Hashimoto et al., 2010), (4) freeze-thaw followed by treated with DNase, Rnase (Li et al., 2017b), (5) N2 with Triton X-100 (Lee et al., 2014c), (6) 200 U/ml phospholipase A2 with 0.5% sodium deoxycholate (Lee et al., 2014c), and (7) Glycerol followed by chemical crosslinking (Lin et al., 2017).
7.1.3. Characteristics of decellularized porcine cornea
DPCs have shown little immunogenicity with less binding of human immunoglobulin regardless of the decellularization methods when compared with native porcine corneas (NPCs) (Lee et al., 2014c). Lesser graft rejection and reduced infiltration of T and B cells were shown in DPC-grafted NHPs (Choi et al., 2011b). Although αGal was not completely removed, DPCs showed long-term survival in NHP studies compared to NPC grafts (Choi et al., 2011b; Lee et al., 2014c), suggesting that reduction of antigen load is beneficial. Since chemicals are used for decellularization making cytotoxicity an issue, thorough washout processes should be included in the decellularization procedures. Several studies show that extracts of DPCs have no significant cytotoxicity when mixed with human or rabbit corneal stromal cells (Du and Wu, 2011; Li et al., 2017b) and L929 cells (Zhou et al., 2011) or when injected intravenously in mice (Du and Wu, 2011).
Transparency should be kept after decellularization. The transparency of DPC may vary depending on the decellularization method, and DPCs through certain methods are not satisfactory for clinical use (Lee et al., 2014c; Oh et al., 2009d; Riau et al., 2020). Maintaining transparency have been reported in the following methods: N2, SDS, hyper/hypotonic solution, hydrostatic pressure/DNase, freezing, hypertonic NaCl, freeze-thaw followed by incubation with DNase/RNase, or distilled water/trypsin/freeze-thaw/NaOH/DNase/RNase (Hashimoto et al., 2010; Lee et al., 2014c; Li et al., 2017b; Lin et al., 2008; Luo et al., 2013; Oh et al., 2009d). Dehydration with glycerol is widely used to re-gain transparency after decellularization (Hashimoto et al., 2010; Riau et al., 2020). DPC may become opaque by swelling during storage or early after surgery. The swelling reduces days or weeks after transplantation and collagen fibers have redistributed, therefore restoring DPC transparency (Hashimoto et al., 2015; Li et al., 2011). DPC showed similar mechanical properties compared to NPCs in tensile strengths, elasticity and light transmittance (Du and Wu, 2011; Du et al., 2011; Hashimoto et al., 2010; Luo et al., 2013; Wu et al., 2009).
7.2. Efficacy, safety, and histological studies in keratoplasty using decellularized porcine cornea
The efficacy and histology of lamellar keratoplasties using DPCs are shown in Table 11 . Out of 22 studies that used rabbits or NHPs as recipients, 16 studies reported graft survival more than 6 months (24 weeks). Among these 16 studies, in 2 studies long-term survival of the DPC grafts was achieved even without the use of CSs (Oh et al., 2009d; Zhang et al., 2007). In NHP studies, longer than 6-month survivals was shown in 14 recipients out of 15 (Choi et al., 2011b; Li et al., 2011). Histological results showed that most DPC grafts had no or mild inflammatory cell infiltration depending on the decellularization method. Therefore, preclinical efficacy using DPC is proven in keratoplasty.
Table 11.
Preclinical efficacies of decellularized porcine corneal xenotransplantation in rabbit or non-human primate studiesa.
| References | Type | Decellularization method | Donor | Recipient | Graft size (mm)/Thickness (㎛) | Survival days of each recipient | Immunosuppression | Histology |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. (2007) | ALK | Dispase/Triton X-100/Trypsin/DNase/RNase/Freeze-thaw | Domestic WT | Rabbit | 4.0/NA | >224 (n = 20)b | None |
|
| Amano et al. (2008) | ALK | N2 | Domestic WT | Rabbit | 5.0/100-200 | >180 (n = 3) | None |
|
| Lin et al. (2008) | ALK | Distilled water/Trypsin/freeze-thaw/NaOH/DNase/RNase | Domestic WT | Rabbit | 6.5/200 | Transparency maintained in 83% (10/12) of grafts | None |
|
| Xu et al. (2008) | ALK | Dispase/Triton X-100/Trypsin/EDTA/DNase/RNase | Domestic WT | Rabbit | 4.0/NA | >28 (n = 15)b | None |
|
| Oh et al. (2009d) | ALK | Freezing | SNU miniature WT | Rabbit | 8.0/250 | 20, 20, 20, 24, 24, 28, 28 | None |
|
| Freeze–thaw | 43, 43, 47, 47, 60, 60, 60 | |||||||
| Hypertonic NaCl/Trypsin/EDTA | >30c, >60c, >180, >180, >180, >180, >180 | |||||||
| Trypsin/EDTA/Glycerol | 16, 18, 18, 18, 20, 20, 20 | |||||||
| Trypsin/Dispase/SDS | All 0 (n = 7) | |||||||
| NaOH/DNase/RNase | All 0 (n = 7) | |||||||
| Wu et al. (2009) | ALK | PLA2/sodium deoxycholate | Yorkshire WT | Rabbit | 6.0/100 | >10 (n = 2)c,> 50 (n = 2)c, >84 ± 11 (n = 10)c, >360 (n = 10) | Topical steroid |
|
| Sasaki et al. (2009) | IST | Hydrostatic pressure/DNase | Domestic WT | Rabbit | 2.0/160 | >56 (n = 11) | None |
|
| Hashimoto et al. (2010) | IST | Hydrostatic pressure/DNase | Domestic WT | Rabbit | 2.0/160 | >360 (n = 6) | None |
|
| Pang et al. (2010) | IST | SDS | Domestic WT | Rabbit | 6.0/150 | >14 (n = 2)c, >28 (n = 2)c, >84 (n = 2)c, >168 (n = 4) | Subconjunctival steroid |
|
| Lee et al. (2011) | ALK + substance-P |
DNase/RNase/Distilled water/Freeze-thaw/Glycerol/Lyophilization | Domestic WT | Rabbit | 4.0/300 | >56 (n = 5)b | None |
|
| Zhou et al. (2011) | IST | SDS | Yorkshire WT | Rabbit | 10.0/150 | >330 (n = 30)b | Subconjunctival steroid |
|
| Li et al. (2011) | ALK | Calcium chloride | WZS miniature WT | NHP | 7.0/AHL | >180 (n = 5), >180 (n = 5) | None/Subconjunctival steroid |
|
| Choi et al. (2011b) | DALK | Hypertonic NaCl/Trypsin/EDTA | SNU miniature WT | NHP | 7.5/312.5–375 | >391, >265, >208, >195, 28 | Topical, subconjunctival, and systemic steroid |
|
| Du and Wu (2011) | IST | SDS | Domestic WT | Rabbit | 5.0/150 | >14 (n = 4)c, >28 (n = 4)c, >56 (n = 4)c, >84 (n = 4)c, >168 (n = 4) | None |
|
| Yoeruek et al. (2012b) | IST | EDTA/SDS/DNase/RNase | Domestic WT | Rabbit | 3.0/100 | >180 (n = 10) | Topical steroid |
|
| Luo et al. (2013) | ALK | Ultrapure water/Hypertonic saline/Triton X-100/Glycerol | Domestic WT | Rabbit (alkali burn) | NA/300 | <15 (n = 6) | Topical steroid |
|
| Hashimoto et al. (2015) | DALK | Hydrostatic pressure/DNase | Domestic WT | Rabbit | 6.0/300 | >180 (n = 6) | Topical steroid |
|
| Shao et al. (2015) | IST, ALK | Human serum/electrophoresis | NA | Rabbit | 6.0/300 | >60 (n = 13)b | None |
|
| Hashimoto et al. (2016) | ALK | Hydrostatic pressure/DNase | Domestic WT | Rabbit | 4.0/160 | >180 (n = 6) | None |
|
| Li et al. (2017b) | ALK | Deionized water/Freeze–thaw/DNase/RNase/Ultrasound | Domestic WT | Rabbit | 6.5/450 | >84 (n = 3) | None |
|
| ALK (cross-linked) | Glycerol, crosslinked with NHS and EDC | Rabbit (ulcer) | 7.75/250 | 72.3% (8/11) >168 | Topical tacrolimus |
|
||
| Huang et al. (2017) | ALK | Ultrapure water/NaCl/SCCO2/Glycerol | Domestic WT | Rabbit | 6.0/NA | >60 (n = 3)c, >180 (n = 3) | Topical steroid |
|
| Zheng et al. (2018) | IST | SDS | Domestic WT | Rabbit | 7.0/126 | >168 (n = 8) | Topical steroid |
|
Abbreviations: AHL: anterior half lamella, ALK: anterior lamellar keratoplasty, CD: cluster of differentiation, DALK: deep anterior lamellar keratoplasty, EDC: 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, EDTA: ethylenediaminetetraacetic acid, IST: intrastromal transplantation, NA: not available, NHS: N-Hydroxysulfosuccinimide, SCCO2: supercritical carbon dioxide, SDS: sodium dodecyl sulfate, SNU: Seoul National University, WT: wild-type, WZS: Wuzhishan.
Studies using tissue engineered decellularized porcine cornea are not included in this table.
Some recipients were sacrificed at specific time points for histologic examinations.
Sacrificed for histology.
Graft survival seems to be affected not only by cytotoxicity and antigenicity after the decellularization, but also by the size and thickness of the graft. Residual donor cells or cellular debris after decellularization may have acted as inflammatory inducers (He et al., 2016). The larger the graft is, the more residual donor cells and debris can be transplanted into the recipient cornea. Moreover, the larger the graft size, the closer the graft-recipient junction is to the limbus, therefore increasing exposure to recipient immune cells in the limbus. Since the reported preclinical study designs are not the same, the most suitable method for DPC is unknown. When considering clinical application, NHP data are mostly pertinent. Thus, the methods that showed efficacy in the NHP studies (survival ≥ 6 months) such as calcium chloride or the hypertonic NaCl combined with trypsin/EDTA are presumed to be clinically suitable methods (Choi et al., 2011b; Li et al., 2011). When considering the effect of graft size, the method with a larger graft size (7–10 mm) that shows good outcome (survival ≥ 6 months) may be feasible method. The methods that showed efficacy (survival ≥ 6 months) with a graft size of 7.0 mm or more in rabbit or NHP studies are as follows; 1) Hypertonic NaCl (Trypsin/EDTA), 2) sodium dodecyl sulfate, 3) calcium chloride, and 4) glycerol crosslinked with N-Hydroxysulfosuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) (Choi et al., 2011b; Li et al., 2011, 2017b; Oh et al., 2009d; Zheng et al., 2018). Taken together, hypertonic NaCl combined with Trypsin/EDTA method or calcium chloride may be clinically feasible. However, further comparative studies should be investigated.
7.3. Clinical trials in keratoplasty using decellularized porcine cornea
To date, DPC was approved to be used for LKP only by the Chinese National Institutes for Food and Drug Control in 2015 (Shi and Xie, 2016). Three clinical trials and one case report using DPC in China have been published; all the studies were performed using the same commercially available DPC products (Li et al., 2019, 2020; Shi et al., 2017; Zhang et al., 2015; Zheng et al., 2019). The DPC was decellularized by hypertonic NaCl-ultrapure water alternating treatments, preserved in glycerol, and sterilized by 60Co irradiation (Zheng et al., 2019). The first clinical trial reported a visual acuity improvement and gradual restoration of graft transparency in 34 out of 47 eyes with fungal keratitis (Zhang et al., 2015). In the second clinical trial, improvement of visual acuity was also reported in 9 out of 13 eyes with herpes keratitis but graft melting was found in 3 eyes (Zheng et al., 2019). In the third clinical trial, 39 patients with progressive infectious keratitis underwent ALK using DPC for a 12 month-follow up. Twelve patients (30.8%) showed graft failure associated with herpetic keratitis and a graft size larger than 8.0 mm (Li et al., 2019, 2020). In a case report, DPC tissue was transplanted in a patient with a corneal ulcer and histology showed minimal infiltration of the inflammatory cells and migration of recipient's stromal cells. This suggests that DPC may be biocompatible scaffold (Shi et al., 2017).
Although not registered as a clinical trial, two DPC clinical studies have also been reported in Ukraine (Drozhzhyna et al., 2017; Turchyn et al., 2019). The authors used the same lyophilized cornea manufactured in Ukraine. In one study, DPC was transplanted in 32 eyes with necrotizing keratitis but all the grafts were rejected (Drozhzhyna et al., 2017). In the other study, DPC was transplanted in 49 eyes with complicated corneal ulcers (Turchyn et al., 2019). They reported that the ulcers were healed in all patients although the xenografts were completely absorbed between 2 and 3 months (Turchyn et al., 2019). Outcomes of the clinical studies from Ukraine were not as good as the ones from China. This may be due to different decellularization methods, the diameter of the graft or the different diagnosis of the recipients. In Zhang's study, hypertonic treated DPC were used in fungal keratitis with variable size, and no patient experienced perforation (Zhang et al., 2015). Conversely, Drozhzhyna et al. included perforating, autoimmune or rosacea cornea, and used cryolyophilized DPC with different graft sizes, and thereby reported opaque grafts, of which diameter is 7–10 mm (Drozhzhyna et al., 2017). Larger size of DPC or combined autoimmune disase may lead to a poorer outcome.
The melting of DPC was more frequent in clinical studies than in the preclinical rabbit or NHP studies. It is presumed by that 1) different hierarchical discordance may affect different host immune responses (Wen et al., 2014), 2) human corneal endothelial cells, unlike rabbits, have limited mitotic capacity in vivo (Joyce, 2003; Van Horn et al., 1977), 3) residual donor cells or necrotic debris due to insufficient or toxic decellularization may have acted as inflammatory inducers (He et al., 2016), and 4) unlike preclinical animal model with normal cornea, the patients had diseases such as infectious keratitis, corneal perforation, or autoimmune disease. Uncontrolled infections also cause melting of the grafts by releasing matrix metalloproteinase from the recipient corneal cells. Taken together, whether DPC would be biocompatible as a lamellar graft for diseased corneas is still controversial. Hence, further clinical study data related to DPC transplantation is required.
7.4. The legal position of decellularized porcine cornea
DPC is regarded as a medical device by Food and Drug Administration (FDA) and IXA (and not as a xenoproduct) only when the complete removal of the cells is verified (Kim et al., 2013b). FDA does not address DPC in the regulation of xenotransplantation products, but instead requires DPC to follow guidelines under the Medical Devices Containing Materials Derived from Animal Sources (FDA, 2016b, c). Furthermore, FDA requires to document the sourcing and handling of animal tissues and empathizes sufficient sterilization of xenozoonotic pathogens as well as validation of the sterilization methods (FDA, 2016b).
To date, more than 1000 DPCs have been clinically transplanted in China (Fernandez-Perez and Ahearne, 2020). Given that long term follow-up results have not yet been reported (Fernandez-Perez and Ahearne, 2020), monitoring of xenozoonosis transmission may be necessary during clinical trials using DPCs. Because current decellularization procedures usually assure sufficient removal of the cells, some porcine cells or PERV virus can remain in DPC. Although hematoxylin and eosin (H&E) staining did not identify the cells, detection of nuclear debris or residual porcine deoxyribonucleic acid (DNA) was reported in DPC (Gonzalez-Andrades et al., 2011; Huang et al., 2017; Lin et al., 2017; Wu et al., 2009; Zheng et al., 2005). Therefore, unless complete removal of the cells is ensured, the legal definition of DPC lies somewhere between medical device and xenotransplant. Currently, there is no consensus on how to completely remove the cells and to verify it in DPC (Isidan et al., 2019). To confirm the absence of nucleus, variable methods are suggested that include H&E or 4′, 6-diamidino-2-phenylindole (DAPI) staining, quantifying dsDNA, and identifying the maximum length of DNA remnants (Fernandez-Perez and Ahearne, 2020).
Evidence emerged that LKP using DPC is effective. For clinical application in humans, not only standard manufacturing procedures of DPC but also standard procedures to verify complete decellularization should be developed in order to be appropriately regulated by the relevant laws as a medical device.
8. Safety and xenozoonosis
After xenotransplantation, infectious disease may be caused by microorganisms from the donors and prior colonization or latent infection in the recipients. The overall risk of infection is determined by 1) the epidemiologic exposures of the recipient and the donor to specific microorganisms and 2) the net state of immunosuppression of the recipient, which includes type and intensity of immunosuppression, metabolic derangement, neutropenia, lymphopenia, hypogammaglobulinemia, and mucocutaneous barrier integrity (Fishman, 2017, 2019). Potential common pathogens that may cause infection in xenotransplant recipients are shown in Table 12 .
Table 12.
Potential common pathogens which cause infection in xenotransplant recipients.
| Origin | Pathogens | Risk | Recipient testing assays | |
|---|---|---|---|---|
| Donor | Virus | PERV | Unknown | QNAT, Ab-based tests* |
| PCMV | Known (NHP) | NAT, Ab-based tests* | ||
| PLHV | Unknown | QNAT | ||
| HEV | Known (human) | NAT | ||
| PCV | Unknown | NAT | ||
| Recipient | Virus | CMV | Known | QNAT, histopathology |
| VZV | NAT, DFA | |||
| HSV | Clinical, NAT, culture, Tzanck smear, histopathology | |||
| EBV | QNAT, histopathology | |||
| BKV | QNAT, histopathology | |||
| HPV | Histopathology, speculum examination, cervical pap test with NAT | |||
| Fungus | Candida species | Culture, histopathology | ||
| Aspergillus species | BAL galactomannan, BAL NAT, chest CT, culture | |||
| Cryptococcus species | Lateral flow assay over latex agglutination assay, culture, imaging of lung and CNS | |||
| Pneumocystis jiroveci | Chest radiograph, serum lactic dehydrogenase, serum (1 → 3) β-d-glucan assay, sputum examination, direct immunofluorescent staining, histopathology | |||
| Parasite | Strongyloides stercoralis | Parasitological or molecular tests of stool, lower respiratory or affected body fluids/tissues samples | ||
Abbreviations: Ab: antibody, BAL: bronchoalveolar lavage, BKV: BK polyomavirus, CMV: Cytomegalovirus, CNS: central nervous system, CT: computed tomography, DFA: direct fluorescent-antibody, EBV: Epstein-Barr virus, HEV: hepatitis E virus, HPV: human papilloma virus, HSV: herpes simplex virus, NAT: nucleic acid testing, NHP: non-human primate, PCMV: porcine cytomegalovirus, PCV: porcine circovirus, PERV: porcine endogenous retrovirus, PLHV: porcine lymphotropic herpes virus, QNAT: quantitative nucleic acid testing, VZV: varicella-zoster virus, *Antibody-based tests include serology, ELIZA, and western blot.
8.1. Donor-related infections
8.1.1. Xenozoonosis
The term ‘xenosis’, ‘direct zoonosis’, and ‘xenozoonosis’ is used to reflect the epidemiology of infection transmitted from xenogeneic grafts (Fishman, 2018, 2019; Fishman et al., 2012). Corneal xenotransplantation procedure does not exempt the risk of transmission of pathogens from animals to the recipients and the public. The challenges are the paucity of data on the microbiology of pigs, which can cause xenozoonosis in humans, and the absence of standardized screening and monitoring modalities for potential pathogens.
8.1.2. Porcine endogenous retrovirus (PERV)
Transmission of bacteria, fungi, and parasites can be largely prevented by controlling donor pig breeding and animal husbandry practices. Whereas viral infections can still occur in host because of immunosuppression. In particular, PERVs are a key concern since PERVs are capable of infecting human cells in vitro (Patience et al., 1997) and PERV-A receptors have been identified in various human tissues (Ericsson et al., 2003). The major concern about retroviral transmission is associated with altered gene regulation, oncogenesis, or DNA recombination in the recipient by unapparent infection (Fishman et al., 2012).
PERV is present in the genome of all swine. PERV-A and –B can infect human and porcine cells, while PERV-C only infects porcine cells (Scobie and Takeuchi, 2009). The presence of PERV-A/C recombinants is of special concern because they demonstrate high replication efficacy and can be transmitted in human cells (Denner, 2008). PERV expression is different depending on the pig strain, the individual pig of a given strain, and different organs (Bittmann et al., 2012; Tacke et al., 2003). To date, PERV is found in all tested porcine organs (Nellore, 2018) and the cornea (Choi et al., 2017; Li et al., 2017a).
8.1.2.1. PERV infection in pig-to-NHP studies
Experiences with pig-to-NHP transplantation provide insights into the potential risk of xenozoonosis. There is no evidence for PERV transmission to NHP recipients from various transplanted porcine cells, tissues, and organs (Garkavenko et al., 2008; Issa et al., 2008; Loss et al., 2001; Martin et al., 1998; Switzer et al., 2001). Regarding the cornea, transmission of PERV was not evident in both in vitro (Choi et al., 2017) and in vivo corneal transplantation studies (Choi et al., 2017; Li et al., 2017a). However, PERV was not detectable up to 3.2 years post-PKP (Table 13 ) (Choi et al., 2017). Since the main receptor for PERV entry, PERV-A receptor 1, is dysfunctional in NHPs (Denner et al., 2018) and NHP cells are not permissive to replication by PERV (Ritzhaupt et al., 2002), human studies are essential to verify the actual potential of transmission of PERV.
Table 13.
No evidence of porcine endogenous retrovirus transmission after xenotransplantation in non-human primate and human recipients.
| Recipient | Xenotransplantation type | Detection method | Longest FU (Y)a | references |
|---|---|---|---|---|
| NHP | Aortic endothelial cells | PCR | 2 | (Martin et al., 1998) |
| Kidney | PCR, RT-PCR | 0.64 | (Loss et al., 2001) | |
| Heart, skin, and encapsulated isles | PCR, RT-PCR, Ab assay | 2.58 | (Switzer et al., 2001) | |
| Extracorporeal liver perfusion | PCR | 1 | (Nishitai et al., 2005) | |
| Encapsulated islets | PCR, RT-PCR, Ab assay | 0.5 | (Garkavenko et al., 2008) | |
| Thymocyte infusion, vascularized thymic lobe, vascularized thymic lobe and kidney, kidney, thymokidney, heart | PCR, RT-PCR | 0.5 | (Issa et al., 2008) | |
| LKP using freshly preserved and decellularized cornea, DALK, PKP | PCR, RT-PCR | 3.22 | (Choi et al., 2017) | |
| LKP using freshly preserved and dehydrated cornea | PCR, RT-PCR | 2 | (Lee et al., 2017a) | |
| Human | Fetal islets | PCR, RT-PCR for viral RNA and RT, Ab assay | 7 | (Heneine et al., 1998) |
| Extracorporeal connection to kidney | Nested PCR, Ab assay | 3 | (Patience et al., 1998) | |
| Extracorporeal splenic, liver, kidney perfusion, extracorporeal bioartificial liver perfusion, skin, islet cells | PCR, RT-PCR, Ab assay | 8.45 | (Paradis et al., 1999) | |
| Extracorporeal bioartificial liver perfusion | PCR | 5 | (Pitkin and Mullon, 1999) | |
| Porcine fetal neuronal cells | PCR | 2 | (Dinsmore et al., 2000) | |
| Encapsulated porcine islets | PCR, RT-PCR | 1.58 | (Elliott et al., 2000) | |
| Extracorporeal porcine liver perfusion | PCR | 0.82 | (Levy et al., 2000) | |
| Extracorporeal bioartificial liver perfusion | Ab assay | 5 | (Irgang et al., 2003) | |
| Extracorporeal bioartificial liver perfusion | PCR, RT-PCR | 8.7 | (Di Nicuolo et al., 2010) | |
| Porcine skin | PCR, RT-PCR, Ab assay | 34 | (Scobie et al., 2013) |
Abbreviations: Ab: antibody, DALK: deep anterior lamellar keratoplasty, FU: follow-up, LKP: lamellar keratoplasty, NHP: non-human primate, PCR: polymerase chain reaction, PKP: penetrating keratoplasty, RNA: ribonucleic acid, RT: reverse transcriptase, RT-PCR: reverse transcription-polymerase chain reaction, Y: years.
This table summarizes the results of studies which reported evidence of porcine endogenous retrovirus transmission in long-term (≥6 months) follow-up samples from the recipients.
8.1.2.2. PERV infection in clinical studies
To date, all clinical studies have failed to demonstrate the transmission of PERV to human recipients (Table 13). Extracorporeal spleen/liver/kidney perfusion (Levy et al., 2000; Paradis et al., 1999; Patience et al., 1998), as well as ex vivo bioartificial liver support system (Di Nicuolo et al., 2010; Irgang et al., 2003; Pitkin and Mullon, 1999), have not shown PERV transmission for up to 8.7 years (Di Nicuolo et al., 2010). A study in burn patients treated with porcine skin grafts showed a negative result for up to 34 years (Scobie et al., 2013). Early clinical studies using fetal neuronal cells (Dinsmore et al., 2000), islet cells (Heneine et al., 1998; Paradis et al., 1999), or encapsulated islet cells (Elliott et al., 2000) also showed no evidence of PERV infection for up to 7 years (Heneine et al., 1998).
A recent clinical trial with twenty-three diabetic patients using porcine islets showed that neither PERV DNA nor ribonucleic acid (RNA) was detectable in blood samples for up to 8 years (Valdes-Gonzalez et al., 2010). The clinical trial of encapsulated porcine islet cell transplantation in New Zealand showed no transmission of PERV for up to a year in 14 patients (Wynyard et al., 2014). In a subsequent clinical trial of encapsulated porcine islet cell transplantation in Argentina, all 8 recipients also showed no Abs against PERVs in the sera for up to 52 weeks and no PERV DNA/RNA in blood samples for up to 113 weeks (Morozov et al., 2017).
8.1.3. Other viral infection
Porcine cytomegalovirus (PCMV) can be excluded from pig colonies but is easily reintroduced into herds (Clark et al., 2003). Once established, PCMV infection is restricted to xenotransplant causing consumptive coagulopathy and early renal graft loss in pig-to-NHP transplantation (Gollackner et al., 2003; Yamada et al., 2014), however, it appears to be resistant to ganciclovir (Mueller et al., 2003). Porcine lymphotropic herpes virus (PLHV)-1 has a homology to EBV. Most of the porcine herds are positive for PLHV and is difficult to be eliminated (Mueller et al., 2005). Neither PLHV reactivation nor PTLD-like disease has been reported in pig-to-NHP xenotransplantation with potent immunosuppression (Nellore, 2018). Hepatitis E virus (HEV) is highly prevalent in pigs. HEV genotypes 3 and 4 infect both pig and human causing neurological disorders or hepatitis (Nellore, 2018). Porcine circovirus (PCV) type 2 is considered an important pathogen of the immunosuppressed pig but not for NHP and human recipients (Richmond et al., 2015). Regarding coronaviruses (CoV), pigs can carry Severe Acute Respiratory Syndrome (SARS)-CoV developing antibodies and amplify Middle East Respiratory Syndrome-CoV. In contrast, there is no evidence that pigs can become infected with SARS-CoV-2 or are capable of amplifying the virus. Although the risk of transmission of porcine CoV to human seems to be low, a proper surveillance in the pig herd would be needed (Opriessnig and Huang, 2020).
8.2. Recipient-related infections after transplantation
The actual risk of recipient-related infection in clinical corneal xenotransplantation is unknown. Given that potent immunosuppression is mandatory in corneal xenotransplantation, perioperative potential pathogens and timeline for the occurrence of infections can be presumed in recipients with xenotransplants based on experiences with solid organ allotransplantations (Fishman, 2017; Personett and Laub, 2017).
Actually, an outbreak of simian varicella virus (SVV) infection has been reported in pig-to-rhesus corneal transplantation studies. Furthermore, a non-significant trend of a higher death rate was observed among immunosuppressed SVV-infected hosts receiving rituximab, tacrolimus, and basiliximab (Choi et al., 2018), which emphasizes the importance of antiviral prophylaxis. In addition, two cases of shigelloses and a case of pneumonia have been reported in rhesus recipients receiving rituximab, tacrolimus, and basiliximab (Choi et al., 2018; Kim et al., 2018a). Meanwhile, CSs-, costimulatory blockade agents-, and rituximab/tacrolimus/basiliximab-based immunosuppressive regimens do not reportedly induce clinically significant rhesus cytomegalovirus (CMV) reactivation in pig-to-rhesus corneal transplantation studies (Choi et al., 2018; Kim et al., 2017, 2018a). CMV infection is one of the most common opportunistic infections after human solid organ allotransplantations (Razonable and Humar, 2019).
8.3. Surveillance and monitoring of safety
The development of surveillance and safety programs for clinical trials in xenotransplantation is guided by a “Precautionary Principle” (Fishman et al., 2012). Specifically, appropriate screening procedures and assays for source animals and xenotransplant recipients should be deployed even in the absence of data suggesting infectious risks.
8.3.1. Screening of source animal for xenotransplantation
A designated pathogen-free (DPF) environment means the absence of pathogens that affect the health status of the herd and exclusion of microorganisms, which have zoonotic potential in human recipients (Fishman, 2018; Fishman et al., 2012). DPF pigs should be isolated from other animals, and optimally derived by hysterectomy with rapid weaning to limit transmission of microorganisms by breast milk. Standard veterinary practice includes routine vaccination, microbe-restricted and mammalian protein-free diets, filtered water, a biosecure facility, and restriction of routine antimicrobial use. To evaluate the presence of pathogens on a detailed designated exclusion list (Fishman, 2018, 2019; Fishman et al., 2012), source pigs should be routinely screened using microbe-specific assays. However, the exclusion list may vary depending on the xenotransplant, common human pathogens and specific porcine pathogens with the potential of xenozoonosis should be included. These microbiological standards are subject to updates based on experimental and clinical experiences, geography, and the evolution of testing strategies. Blood and/or tissue samples should be archived.
8.3.2. Screening and monitoring of recipients
8.3.2.1. Preoperative screening
Candidates should be evaluated for the risk of infection with a comprehensive medical history. All xenotransplant recipients should be screened for active or latent infections as for allograft recipients. Routine screening includes human immunodeficiency virus (HIV)-1/2, Hepatitis B virus, Hepatitis C virus, CMV, EBV, Toxoplasma gondii, Treponema pallidum, and Mycobacterium tuberculosis. According to donor and recipient screening results and regional disease patterns, prophylactic antimicrobials can be administered (Fishman, 2018; Malinis et al., 2019). Screening of varicella-zoster virus (VZV), measles, mumps, and rubella of the recipient is also important, with vaccination of the seronegative recipient should be performed (Malinis et al., 2019).
8.3.2.2. Perioperative surveillance and monitoring
All recipients should be closely monitored according to a schedule for the detection of donor- and recipient-related infections. There is no need to monitor for pig-derived microorganisms absent in the donor pig (Denner et al., 2016). Since PERV infection is a main concern its monitoring requires a stepwise approach: detection of PERV RNA in serum by reverse transcription-polymerase chain reaction (RT-PCR) and PERV DNA in peripheral blood mononuclear cells (PBMCs) by PCR, differentiation between infection and microchimerism, detection of serum Abs to PERV proteins via Western blot, ELISA, or immunoperoxidase assay and in vitro co-culture assays of PBMCs or other cells from the recipients and human HEK-293 cells (Hering et al., 2009; Kim et al., 2014). If recipients develop signs of infection, intensive monitoring is mandatory to reveal the exact cause and empiric anti-microbial therapy can be initiated.
8.3.2.3. Bio-archiving of specimens
For future epidemiologic studies, the storage of frozen PBMCs, sera, and/or tissue biopsies are required preoperatively and at standard time points post-transplantation.
8.4. Future directions in xenozoonosis
Recently, public concerns about zoonosis have increased. The latest pandemic of coronavirus disease 2019 (COVID-19) (Sohrabi et al., 2020; Zhu et al., 2020), which has caused hundreds of thousands of deaths worldwide and brought the world to an economic standstill, is a good example of such concern. This might justify public refusal of xenotransplantation. Therefore, investigators should make every effort to minimize xenozoonosis and assure a safe clinical application of xenotransplantation. Outcomes of any clinical xenotransplantation should be reported in the scientific literature and to regulatory authorities. Standardized surveillance programs for known and unknown pathogens should be developed and validation of microbiological assays are necessary.
In particular, to prevent PERV infection, the following strategies are suggested: 1) careful screening of the source pig herd, 2) selection of pigs that exhibit low-level expressions of PERV-A and PERV-B, 3) use of pigs that do not contain PERV-C in their germline, to prevent recombination of PERV-A/C, 4) application of vaccines or anti-viral therapy, and 5) regular screening of recipients for PERV transmission using assays that are sufficiently sensitive (Fishman, 2018, 2019; Hering et al., 2009; Kimsa et al., 2014; McGregor et al., 2018). The recent success of genome-wide inactivation of PERVs using CRISPR-Cas9 technique may reduce the potential of PERV infection (Niu et al., 2017; Yang et al., 2015). Taken together, we can infer that the actual risk of PERV transmission may be low in future clinical trials.
9. Regulatory framework and ethical issues in xenotransplantation
9.1. Regulatory framework in xenotransplantation
9.1.1. Special regulatory issues in clinical trials of xenotransplantation
Special regulatory issues in clinical trials of xenotransplantation are shown in Table 14 . (1) As any xenotransplant procedures possess the possibility of transmission of porcine pathogens to human recipients, proper regulatory oversight is necessary for safe clinical trials. Since infectious risk may extend to close contacts, medical caregivers, and even general population; nationwide policies and intimate global networks should be established based on the active involvement of the public. (2) Participants of xenotransplantation clinical trials might be asked long-term (even life-long and after death) postoperative surveillance and waiver of autonomic withdrawal and their close contacts also might share their obligations. (3) Adds-on informed consent forms should contain critical issues mentioned above and be obtained from close contacts as a separate procedure.
Table 14.
Special regulatory issues in clinical trials of xenotransplantation.
| Uncertainty of risks to the public |
|---|
| Porcine endogenous retrovirus and other new infection outbreak |
| Regulatory oversight |
| Long-term follow-up |
| Even life-long surveillance and after death |
| Feasibility of the follow-up system |
| Add-on consent from the close contacts of the subject |
| Risks of zoonotic infection to the close contacts |
| Share the obligations of the subject with closer contacts (usually family members) |
9.1.2. Global consensus and guidelines
To conduct xenotransplantation safely in clinical settings, the establishment of a regulatory framework has been of special concern worldwide. In 1997, the US FDA placed ongoing trials using porcine xenoproducts on hold until the development of appropriate PERV detection methods and implementation of detection methods in the participant monitoring (Bloom, 2001, 2003). Hence the “World Health Organization (WHO) Guidance on Xenogeneic Infection/disease Surveillance and Response: A Strategy for International Cooperation and Coordination” was published in 2001 (WHO, 2001).
In resolution 57.18, WHO urged Member States to allow xenogeneic transplantation only when effective national regulatory control is in place and emphasized on collaboration (WHO, 2004). Subsequently, the IXA Ethics Committee provided an overview of the major regulatory issues and stated the position for clinical application of xenotransplantation as follows (Sykes et al., 2004): 1) there must be adequate preclinical data to justify a clinical trial, 2) the trial must be conducted with regulatory oversight from a national authority that ensures qualities of source animals, 3) the clinical trial must be conducted with the approval and oversight from an institutional review panel.
The WHO regulation was followed by a statement from the xenotransplantation advisory consultation (WHO, 2005), resulting in the First WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials (The Changsha Communiqué) in 2008 (WHO, 2009). During that time, porcine islet cell xenotransplantation clinical trials in China were put on hold until the establishment of a proper regulatory oversight. Ten principles of The Changsha Communiqué reflected the contents of IXA's position and resolution by the WHO described above. It was emphasized that the regulation should be a legal basis with powers to ban unregulated procedures and enforce compliance with regulatory requirements. The regulatory system should be transparent including scientific and ethical assessments. Patient selection should be based on informed consent and participants and close contacts should be effectively educated. Additionally, the long-term storage of samples and records, the possibility of life-long surveillance of participants and/or close contacts were postulated. In 2011, the Second WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials (Geneva Consultation) focused on reviewing the first regulated xenotransplantation clinical trial using encapsulated porcine islets in New Zealand and the Changsha principles (WHO, 2011). In particular, the consultation stressed the need for “Principle of Transparency” in the conduct of any xenotransplantation trial, including the design of the trials and the development of national policies and procedures to regulate them. Transparency includes full publication of data, independent experts' advice, and timely independent data review (Noel, 2012). Recently, the Third WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials (The, 2018 Changsha Communiqué) was held, in which the original ten principles were modified. The definition of xenotransplantation was amended based on that of the US FDA. Moreover, monitoring post-market entry after regulatory approval of a xenoproduct was emphasized and succession should be assured for surveillance of the patients, samples, and records.
In line with global consensus mentioned above, required conditions for undertaking xenotransplantation clinical trials were suggested for the porcine heart (Cooper et al., 2000), islet (Hering et al., 2009, 2016), and cornea (Kim et al., 2013b, 2014) products.
9.1.3. Current regulation status
Nowadays, many countries recognize the importance of a regulatory framework for the safe implementation of xenotransplantation and have implemented regulations or guidelines for the clinical use of xenotransplantation or follow the FDA guidelines.
9.1.3.1. The United States (US)
The development of US policy on xenotransplantation began around 25 years ago. Based on the public comments received and the advances in xenotransplantation fields, the US Department of Health and Human Services (DHHS) published a “Public Health Service Guideline on Infectious Disease Issues in Xenotransplantation” to address the infectious disease concerns raised by xenotransplantation in 2001 (DHHS, 2001). Secretary's Advisory Committee on Xenotransplantation was established in DHHS and provided the draft of informed consent for participants (DHHS, 2004).
All aspects of xenoproduct development and clinical trials conducted within US are subject to regulation by the FDA. The US FDA considers four target items in regulatory aspects: 1) the source herd, 2) the source animal, 3) the processing and manufacture of the product, and 4) the monitoring of the participants. This is further specified for the three types of products: 1) whole organs, 2) cells and tissues, and 3) combination products (cells and device). In 2003, the FDA Center for Biologics Evaluation and Research has published a guidance document titled “Guidance for Industry: Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans”, which was revised in 2016 (FDA, 2016c). Regulatory requirements for GE xenoproducts outlined in “Draft Guidance for Industry: Regulation of Intentionally Altered Genomic DNA in Animals” are currently being finalized by the FDA Center for Veterinary Medicine (FDA, 2017b). These guidelines focus on reducing the potential risks in using xenoproducts. To design and conduct early-phase clinical trials of xenogeneic cellular products, the regulatory principles are referred to as a guidance document titled “Guidance for Industry: Considerations for the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products” published by the FDA in 2017 (FDA, 2017a).
Recently, the 21st Century Cures Act, signed into law on December 13, 2016, was designed to help accelerate medical product development and bring new innovations to patients who need them faster (FDA, 2016a). The law builds on FDA's ongoing work to incorporate the perspectives of patients into the development of drugs, biological products, and devices in FDA's decision-making process. In particular, it describes harmonization of differences between the HHS Human Subject Regulations and FDA Human Subject Regulations to reduce regulatory duplication and unnecessary delays.
9.1.3.2. European Union (EU)
In Europe, the main regulatory actions focused on a xenogeneic cell therapy product considered as Advanced Therapy Medicinal Product (ATMP). ATMPs are defined as medicinal products for human use that are based on gene therapy, somatic-cell therapy, tissue-engineered products, and combination products. The regulatory framework on ATMPs is centered around the Regulation 1394/2007 (The European Parliament and of the Council, 2007). The Committee for Advanced Therapies (CAT) at the European Medicines Agency (EMA) has been established to meet the scientific and regulatory challenges with advanced therapies. The CAT plays a central role in the regulatory process. In 2009, EMA issued a “Guideline on Xenogeneic Cell-based Medicinal Products”, which is a key document in the clinical development of xenogeneic cell-based therapy (EMA, 2009). The quality, safety and efficacy requirements of GE cells are defined in a guideline titled “Guideline on quality, non-clinical and clinical aspects of 4 medicinal products containing genetically modified cells”, of which public consultation has been finalized (EMA, 2018).
Since 2004, any clinical trial of medicinal products must be approved in Europe, which has increased both safety for the subjects and validity of the data. In 2014, the European Parliament and Council adopted the new Clinical Trial Regulation (EU) No 536/2014, with the hope of increasing the number of clinical trials (Abou-El-Enein and Schneider, 2016; The European Parliament and of the Council, 2014). In 2020, the Regulation is most likely to replace the current European Clinical Trial Directive 2001/20/EC. The assessment of a clinical trial application will proceed through twofold procedures: The Reporting Member State should evaluate the scientific features of the trial (part 1), and each Concerned Member State should conduct a separate assessment covering national features (part 2) (Hawthorne et al., 2019).
9.1.3.3. South Korea
The establishment of a regulatory framework in South Korea has been initiated to meet global standards. Over the last 10 years, there have been debates on whether xenogeneic cells, tissues, and organs can be included in the category of medicinal products and who has the authority to regulate xenoproducts. Recently, the Ministry of Food and Drug Safety (MFDS) decided to regulate porcine islets and cornea under the category of cell therapy products. The Advanced Regenerative Medicine and Biopharmaceutical Act (ARMBA), which will guide a clinical trial of xenotransplantation, was enacted on August 27, 2019 and will be enforced from August 28, 2020. The purpose of the ARMBA is to ensure the safety and efficacy of Advanced Regenerative Medicines (ARMs) and support the commercialization of the products. ARMs include cell, genetic, tissue-engineered, and combination therapy, which could be used to regenerate the human structures and functions with allo- or xeno-geneic materials.
The overall process of upcoming xenotransplantation clinical trials will adhere to ARMBA (Fig. 8 ). The institutions, which procure, investigate, and apply ARMs for clinical researches must be equipped with the necessary facilities, devices, and professionals and obtain the permission of Cell Treatment Facility from the director of MFDS. ARMs applying institutions must prepare clinical research protocols, conduct clinical trials with ARMs, make and keep records, and report adverse events to ARMs Safety Agency. ARMs Review Committee, which is appointed by the Ministry of Health and Welfare (MOHW) will review and approve research protocols with ARMs and decide whether life-long surveillance is necessary. ARMs Safety Agency assigned by the MOHW will oversee ARMs Applying Institutions, establish surveillance systems, execute life-long surveillance, take reports of, and investigate adverse events after application of ARMs. Life-long surveillance seems to be feasible in South Korea due to compulsory National Health Insurance Service, that is, all Koreans must enroll in the National Health Insurance Service and get regular health examinations.
Fig. 8.
Regulatory process of upcoming xenotransplantation clinical trials based on Advanced Regenerative Medicine and Biopharmaceutical Act (ARMBA) in South Korea. Abbreviations: ARMs: Advanced Regenerative Medicines, IRB: institutional review board, NHI: national health insurance.
Several national regulatory authorities are also involved in safe clinical researches with xenogeneic ARMs. MOHW controls all health-related issues and its agency, the Center for Disease Control (CDC), oversees disease prevention. That is, the CDC takes charge of the control of epidemic zoonotic infections according to the Infectious Disease Control and Prevention Act. The Ministry of Agriculture, Food and Rural Affairs controls laboratory animal care and veterinarian quarantine. Providing protection and care system for laboratory animals should abide by the Laboratory Animal Act. National Bioethics Committee reviews any bioethical issues and is responsible for public engagement and establishment of consensus, based on the Bioethics and Safety Act, which regulates all human subject researches demanding ethical principles.
9.1.3.4. Japan
In Japan, the Regenerative Medicine Promotion Act was approved and came into effect in 2013 to promote the comprehensive policies regarding regenerative medicine. The Act on the Safety of Regenerative Medicine, effective since 2014, covers xenogeneic cell transplantation as Class I (high-risk), including islet xenotransplantation, but not xenogeneic organ transplantation. A certified special committee for regenerative medicine reviews a submitted application by a medical institution and the result is submitted to the Ministry of Health, Labor and Welfare (MHLW), which can order changes to the plan based on the opinions of the health science council members. According to the Revised Pharmaceutical Affairs Act effective from 2014, a provisional approval of xenogeneic cells will grant the product a conditional term of 7 years, during which additional efficacy and safety data should be collected. To prevent infections and the spread of emerging infectious diseases caused by xenotransplantation, the Research and Development Division, Health Policy Bureau, MHLW updated and published the “Public Health Guidelines on Infectious Disease Issues in Xenotransplantation” in 2016 (Shimoda and Matsumoto, 2019).
9.1.3.5. Australia
In Australia, the National Health & Medical Research Council (NHMRC) implemented the Australian xenotransplantation moratorium (ban on all forms of xenotransplantation clinical trials) after two rounds of national consultation from 2005 to 2009. The major public concerns were the fear of xenozoonosis, welfare of animals and doubt regarding significant benefits to research participants. Then, the NHMRC issued research guidelines for scientists and ethics committees and recommended that clinical trials involving animal to human transplantation could begin as soon as the Therapeutic Goods Administration (TGA) could implement the appropriate regulatory and surveillance frameworks (Cheng, 2015). Currently, goods that comprise or contain live animal cells, tissues or organs are regulated as biologicals (Class 4, high-risk). Therapeutic Goods (Manufacturing Principles) Determination 2018 and Therapeutic Goods Order No. 87 (General requirements for labelling of biologicals) are two standards applicable to these xenogeneic products (TGA, 2011, 2017). The TGA at Department of Health has adopted the “Guideline on Xenogeneic Cell-based Medicinal Products” issued by EMA for quality, safety and efficacy aspects for live animal cells, tissues and organs.
9.2. Ethical dilemma and moral framework
As with all research on the frontiers of biomedicine, xenotransplantation raises its own ethical quandaries. Although xenotransplantation may solve ethical issues related to allotransplantation (Smetanka and Cooper, 2005), xenotransplatation has its own ethical concerns regarding self-identity, xenozoonosis, and animal sacrifice for human benefit.
9.2.1. Self-identity and psychological issues
Xenotransplantation is unique because it involves the creation of a chimera of live cells from two different biological species. Psychosocial aspects related to xenotransplantation might include a distorted self-image about acquiring animal features as reported among allotransplantation patients who fantasize about receiving physical characteristics from the donor (Appel et al., 2000). Debates on self-identity was stimulated by the ‘Baby Fae’ case in which a baboon heart was transplanted into a neonate (Bailey et al., 1985).
Nevertheless, self-identity is not generally compromised by xenotransplantation as a current view of allograft considered as purely a functioning organ without affecting the recipient's identity. Actually, it has been reported that although some participants for porcine islet cell transplantation had erroneous notions about the implantation of a non-human source into their bodies, such notions were abandoned after the procedure (Teran-Escandon et al., 2005). The aforementioned issues seem to be affected by the individual's cultural background and public attitudes toward xenotransplantation in their communities.
9.2.2. Breaches of autonomy and confidentiality
Precedents ensure that participation in the studies is voluntary and that leaving the study is the autonomous decision of each participant (Barker and Polcrack, 2001). However, requiring that volunteers for xenotransplantation comply with long-term surveillance seems reasonable to protect the public health from the threat of xenozoonosis. The participant could potentially be isolated based on the evidence of unexplained infections among their contacts. Once exposed to a porcine xenotransplant, the recipient will remain at risk of a porcine infection, even if the xenotransplant is removed. This means that the recipient is asked to waive the right to withdraw from the surveillance, once the transplant has taken place (Spillman and Sade, 2007). The dilemma is that it is contrary to the fundamental human right of autonomy as articulated by the Belmont Report and the Declaration of Helsinki for research on human subjects (Anderson, 2006; George, 2006; Smetanka and Cooper, 2005; Sykes et al., 2004).
For the same reason mentioned above, the xenotransplant recipient would be advised to inform close contacts about its potential risk of xenozoonosis. Moreover, long-term access by the appropriate public health agencies to the recipient's medical records is ensured if necessary. Notification to close contacts and agencies about the potential infectious risk surrounding a participant could violate the principle of confidentiality (Sykes et al., 2004).
9.3. Public awareness and acceptance
9.3.1. Public attitudes to xenotransplantation
There has been a lot of quantitative public opinion surveys conducted regarding xenotransplantation. Most of these public opinions were obtained from the mid-1990s to the mid-2000s and the results of 35 sources in 23 countries are well summarized (Hagelin, 2004). Briefly, there was no overwhelming support for xenotransplantation, average 40% morally acceptable, and a proportion of 54% the application potentially useful. The proportion of opposition seems to be lower as time went on. The supporters of xenotransplantation tend to be male and have higher formal education (Hagelin, 2004).
The results of attitude surveys on xenotransplantation from different specific cohort groups are shown in Supplementary Table 1. The cohorts encompass university students, medical caregivers, patients on the transplant waiting list or already transplanted, and various ethnic groups. The acceptance rate ranges from 10% to 97% according to the specific cohort and the type of xenotransplantation. Generally, medical caregivers, university students, and patients on the transplant waiting list or already transplanted were more favorable than public ethnic groups. The acceptance rate of xenogeneic cells or tissues was higher than organs. Interestingly, a positive response was related to a favorable attitude toward organ donation, a family member's favorable attitude toward transplantation, and previous experience of organ donation or transplantation. Notably, there were significantly more positive responses from clinical staff members in a hospital where a clinical trial of encapsulated islet cell xenotransplantation was performed previously compared to that in a hospital where no such xenotransplantation has been conducted (Abalovich et al., 2017). Therefore, attitudes toward xenotransplantation seem to be influenced by individuals' experience and their social activities.
Currently, there has been only one survey on corneal xenotransplantation. In the report, 42% of patients on the corneal transplant waiting list or already transplanted showed a favorable attitude toward corneal xenotransplantation. Among patients expressing favorable views, the willingness to participate in clinical trials was 63% after being informed that both they and their spouse must get periodic medical check-ups for a long time. About 29% of subjects expressed their concerns about self-identity (Lee et al., 2014a).
Given public concerns about pandemic COVID-19 this year, public attitude towards xenotransplantation may have changed. Therefore, further studies should be mandatory to investigate public acceptance on xenotransplantation.
9.3.2. Public engagement
To settle down safe xenotransplantation, it is mandatory to involve the public in a decision-making procedure regarding to the possible risks (Sobbrio and Jorqui, 2014). Public engagement is a two-way process with the sharing of the activity and benefits of higher education and research with the public. During the process, the involvement of specialists is necessary to listen to, develop their understanding of, and interact with non-specialists. People can participate in the process in various ways including surveys, consensus conferences, and public hearings on the issue of xenotransplantation. A greater understanding of the risks and benefits of xenotransplantation can increase public acceptance. As seen in public surveys mentioned above, rigorous preclinical data on the efficacy and safety of xenotransplantation is mandatory to move the public's opinion.
10. Current status on clinical trials in xenotransplantation
10.1. Clinical trials in organ or cellular xenotransplantation
10.1.1. Current worldwide status of clinical trials in xenotransplantation
Case reports of xenotransplantation had been anecdotally published using NHP organs (Bailey et al., 1985; Lambrigts et al., 1998; Michel et al., 2015; Starzl et al., 1993). Given the major drawbacks in using NHP donor organs (see chapter 2–1), NHP-to-human xenotransplantation is no longer conducted. Cellular xenotransplantation using porcine or rabbit islet cells has been performed in Russia, Ukraine, China, Mexico, New Zealand, and Sweden. However, in most of these studies, no proper regulatory oversight was ensured (Benikova et al., 1987; Danilova et al., 1989; Elliott et al., 2007; Groth et al., 1994; Karabun et al., 1994; Matsumoto et al., 2016; Sgroi et al., 2010; Shalimov et al., 1990; Valdes-Gonzalez et al., 2005; Wang, 2007; Wang et al., 2011). Among the studies, some clinical trials have been conducted with an institutional review board (IRB) approval (Groth et al., 1994; van der Windt et al., 2012).
The IXA has stated guidelines for undertaking clinical trials of heart, lung, islet cell and cornea xenotransplantation since the year 2000 (Cooper et al., 2000; Hering et al., 2009, 2016; Kim et al., 2014). Since then, the clinical trial of organ xenotransplantation has not been performed to date; only clinical trials using porcine islet cell have been conducted. The first clinical xenotransplantation trial under the appropriate regulatory framework was conducted in New Zealand in 2009 (Matsumoto et al., 2014). The study was a Phase 1/2a clinical trial in which encapsulated porcine islets were transplanted into the peritoneal cavity of 14 diabetic patients. A second clinical trial using encapsulated porcine islets was also performed with the proper regulatory oversight in Argentina (Cooper et al., 2016). Between 2013 and 2017, pig islets were transplanted in 10 diabetic patients in China (Wang et al., 2019). However, details about the study design, regulatory framework, and the results have not been published yet.
By using the term ‘xenotransplantation’ and ‘xeno’ in clinicaltrials.gov (accessed May 20, 2020) we have found two registered studies of cellular xenotransplantation. One study was completed using encapsulated porcine choroid plexus in patients with Parkinson's disease in New Zealand (ClinicalTrials.gov Identifier NCT02683629); while the other study is ongoing and using porcine islets xenotransplantation combined with Tregs in diabetic patients in China (ClinicalTrials.gov Identifier NCT03162237). The detailed design and the results of those studies have not been published yet.
10.1.2. Requirements for investigational new drug (IND) submission for xenotransplant product
General items required for IND submission of xenotransplant products are summarized in Fig. 9 . The US FDA regards four target items as being regulated: 1) source herd, 2) source animal, 3) the manufacturing process of the xenotransplant product, and 4) monitoring of the patient (FDA, 2016c).
Fig. 9.
Items to be established for IND submission of corneal xenotransplant product.
Abbreviation: cGMP: current Good Manufacturing Practice.
Regarding the source herd, the following items are required: (i) appropriated breeding programs and closed herd system; (ii) maintenance of animal health with Standard Operating Procedures (SOPs) of veterinary care, caretakers, feeding, water, and bedding, and monitoring of the health screenings, etc. Source pig herds should be screened regularly for designated pathogens and the results should be documented; and (iii) maintenance of the animal facility for all physical attributes of the animal environment. Regarding source animal, the following items are required; (i) animal qualification to minimize infectious diseases. Source animals should be derived only from closed herds with documented health screening programs and animal history. All individual animals should be screened for the presence of the same infectious agents used for herd qualification. Source animals should be quarantined for at least 3 weeks before harvesting xenotransplantation products. SOPs for animal and personnel traffic through the source animal facility should be documented; (ii) anatomic and physiologic feasibility of whether the organ is of the appropriate size and functions adequately across species barriers; and (iii) sample archiving at −70 °C for a certain period of time is recommended before use by the Public Health Service if needed.
The following items are required for xenotransplant products stored or processed: (i) current Good Manufacturing Practice (cGMP); procession qualification that includes appropriate procedures, reagents, and test methods; appropriate controls for tracking, labeling, and cross-contamination; and appropriate conditions for processing, storage, and shipping; (ii) product characterization of identity, purity, and potency; (iii) safety testing; products should be tested for bacterial and fungal sterility, mycoplasma, viral agents and endotoxin level by validated technologies; and (iv) lot release criteria should be established based on product characterization and safety testing, and the final product must meet these criteria. Post-release assessment must include confirmation of sterility in the final product.
Items to monitor the patient include (i) preoperative screening; (ii) perioperative surveillance; and (iii) and bio-archiving of samples, which are discussed in detail in chapter 8.
10.2. Process of clinical trial in corneal xenotransplantation
Since preclinical efficacy data in NHP studies is sufficient to justify initiating a clinical trial of corneal xenotransplantation (Kim et al., 2014, 2018a), preparations for anterior lamellar or full thickness porcine corneal xenotransplantation clinical trial is under way in Korea. The clinical trial is designed as a non-randomized, open-label, single-center, and investigator-initiated phase I clinical trial to investigate safety and efficacy of corneal xenotransplantation. The participant should be bilaterally legally blind with the BCVA of operating eye ≤20/1000. The primary disease is a corneal opacity. Although, the clinical trial has not yet started in Korea, it will be conducted after the IND approval by the MFDS (Choi et al., 2019b; Kim et al., 2018a).
10.2.1. Quality control of source herds and pigs
According to FDA guidelines, to minimize the possibility of xenozoonosis (FDA, 2016c) mentioned above, appropriate source herd and pig qualifications should be developed. Source pigs should be derived only from closed herds and certified for at least two generations to minimize the risk of pathogen introduction (Kim et al., 2014). Pigs obtained from slaughterhouses or raised under free-ranging conditions should not be used as a source (FDA, 2016c). Pigs should be housed in a well-controlled DPF environment with high standards of animal welfare (Hering et al., 2009; Kim et al., 2013b), with SOPs for entry and exit of pigs, and regular tests for pathogens (FDA, 2016c).
In Korea, Seoul National University (SNU) Biomedical Center for Animal Resource Development has been operating as the first pig facility for xenotransplantation since 2004. This facility has maintained DPF colony of SNU miniature pigs. Recently, SNU and National Institute of Animal Science established qualified DPF pig facilities additionally that can accommodate 100–150 minipigs (Park et al., 2019).
10.2.2. Quality control of the corneal xenotransplant products
The quality control provisions for corneal xenotransplantation products are based on that of human corneal allotransplantation. The preparation of corneal xenoproducts should follow the standard guidelines of the European Eye Bank Association or Eye Bank Association of America (EBAA) or EU Legislation concerning standards for human tissues, and the Guide to the quality and safety of tissues and cells for human application that has been published by the European Directorate for the Quality of Medicines & HealthCare (EDQM) of the Council of Europe to ensure adequate procurement, processing, preservation, and storage (EBAA, 2019; EDQM, 2019; Jones et al., 2009; Kim et al., 2014; The European Parliament and of the Council, 2006). The SOPs, including corneal procurement, preservation, and transportation from the site of procurement to the site of the clinical trial must be established. All procedures must be recorded, including enucleation-to-preservation time, gross examination of the cornea, findings of the slit-lamp examination, endothelial evaluation by a specular microscope, and culture report (Choi et al., 2019b).
Porcine eyeball is harvested in the animal surgery room that maintains positive laminar flow with regular temperature monitoring (20-26 °C), humidity (30–70%), and air quality (settle plates (90 mm diameter) < 30 CFU/30 min)). The corneoscleral button is excised from the eyeball and preserved in the biosafety cabinet (Class II, Type A2) that is in the GMP certified cleanroom (Grade B).
The biological property of porcine corneal xenotransplant is well maintained using the same preservation protocol of human allograft (Kim et al., 2016). Based on CED changes depending on age, the age of WT SNU miniature pig is limited to less than 72 month-old for a suitable product (Kim et al., 2016). The remaining ocular tissues after harvest of the cornea must be cultured to exclude contamination with mycoplasma, bacteria, and fungi, and should be stored for a certain period of time (Kim et al., 2014). The test outcome is retrospectively available; therefore, action must be pre-planned in case the microbiologic tests come back positive.
10.2.3. Indications for the patient selection
Participants must be enrolled after full assessment of risks and benefits of corneal xenotransplantation based on the international consensus statement (Kim et al., 2014). The clinical protocol for corneal xenotransplantation received the approval from the IRB in Korea (Choi et al., 2019b) and was modified by the IXA and the Transplantation Society ethics committee to ensure its safety and transparency after the field inspection (Park et al., 2019). The latest revised inclusion and exclusion criteria of subjects for a clinical trial in corneal xenotransplantation are shown in Table 15 .
Table 15.
Inclusion and exclusion criteria of subjects for a clinical trial in corneal xenotransplantation.
| Inclusion criteria |
|---|
| • ≥ 19 years old |
| • Corneal opacity |
| • Legal blindness (best corrected visual acuity ≤ 20/200 in the better eye)a |
| • Best corrected vision of the operating eye ≤ 20/1000. |
| • Corneal impairment that cannot be cured with any other method except corneal transplantation. |
| Exclusion criteria |
| • A keratoconus |
| • Emergency surgical case such as impending perforation or perforation |
| • Endothelial dysfunction without corneal opacity (e.g. Fuchs' Dystrophy) |
| • Pregnant women, wish to fall pregnant in the future, breast-feeding a baby |
| • A mentally ill incapacitated or a non-compliance |
| • Chronic alcoholism, Cardiac disease, CVA, Liver disease, History of malignancy, COPD |
| • Hypersensitivity to medication which will be used after transplantation |
Abbreviations: COPD: chronic obstructive pulmonary disease, CVA: cerebrovascular accident.
Legal blindness is defined by American Medical Association and the United States Congress.
10.2.4. Standardization of the protocols for subject monitoring in a clinical trial
All processes of xenotransplantation clinical trials are standardized according to global guidelines to ensure harmonized corneal xenotransplantation practices and levels of safety. Corneal xenotransplantation must be regulated by a responsible governmental authority in collaboration with international xenotransplantation society and WHO (Hawthorne et al., 2019; WHO, 2009, 2011).
All the process will be conducted under the surveillance of the multidisciplinary consulting boards to ensure the safety of the participants (Fig. 10 ). Given the proof-of-concept NHP study that included anti-CD20 Ab, tacrolimus, and CS (Choi et al., 2019a), similar immunosuppressant regimen will be adopted in a clinical trial (Choi et al., 2019b). The protocol was developed based on the advice obtained from multidisciplinary consulting boards as follows. (1) The board members of the executive council of Korea Cornea Society have been involved in determining inclusion and exclusion criteria and have advised detailed ophthalmic screening tools and frequency of postoperative ocular examinations. (2) An infectious disease physician, a transplantation specialist, and a clinical pharmacologist have provided advice on systemic screening tests, infection prophylaxis, immunosuppressive drug regimen, drug level monitoring, and postoperative surveillance tests. (3) A neuropsychiatrist has designed comprehensive programs for preoperative counseling and perioperative mental support, and ethicists have thoroughly reviewed the informed consent. Moreover, (4) a microbiologist will monitor postoperative humoral responses and PERV transmission in the participants.
Fig. 10.
Multidisciplinary approaches for protocol inspection and subject monitoring system in clinical trials of corneal xenotransplantation.
MFDS will inspect the IRB-approved protocol and the qualities of xenotransplant products. Data and Safety Monitoring Board (DSMB) is independently organized, and will evaluate the safety, monitor the overall process of the trial and advise to continue/discontinue or modify the trial. The principal investigator should respond promptly to any suggestions of the DSMB (Choi et al., 2019a, 2019b). During clinical trials, the results will be periodically submitted to the IRB and DSMB. The CDC is responsible for the prevention of xenozoonosis. If xenozoonosis is suspected, the event should be reported to the CDC.
Detailed activities and surveillance programs in the protocol are as follows. Each participant and his/her family members will be informed using IRB-approved consent forms. There will be two separate deliberation time between signing two informed consent documents (before and after screening tests). If necessary, other neutral advocate ophthalmologists will explain the study in detail again (Table 16 ) (Choi et al., 2019b).
Table 16.
Activities of corresponding persons, participants and family members at each timeline during the screening period.
| Timelinea | Activities | Subjects |
|---|---|---|
| 12 W | First explanation of the study protocol | Ophthalmologists, screening team and neuropsychiatrist |
| First sign on the informed consent form | Participants and family members | |
| 12 W - 6 W | First deliberation time with free question and answer | Participants and family members |
| 6 W | Second explanation of the study protocol | Ophthalmologists, screening team, neuropsychiatrist Neutral advocate ophthalmologists, if necessary |
| 6 W - 20 days | Second deliberation time with free question and answer | Participants and family members |
| 20 days | Second sign on the informed consent form Notarization, if necessary |
Participants and family members |
| Day of surgery | Final confirmation of the informed consent form | Ophthalmologists |
Abbreviation: W: weeks.
Before surgery.
Regarding infection prophylaxis, the patients will receive prophylactic medication for opportunistic infections based on blood test outcomes (Table 17 ). Adverse reactions and opportunistic infections will be closely monitored by regular blood tests and physical examination (Table 18 ). The participant will be evaluated by a neuropsychiatrist for mental competence of understanding informed consent. The first interview will evaluate (i) cognitive ability, (ii) emotional competency, and (iii) psychopathological state at 12 weeks prior to surgery. The second interview will evaluate (i) the understanding of xenotransplantation, and (ii) the understanding of the side effects of xenotransplantation 6 weeks prior to the surgery (Table 19 ). If the score remains below a designated level, the subjects will be excluded from the clinical trial.
Table 17.
Prophylactic medications after corneal xenotransplantation.
| Prophylaxis | Medication |
|---|---|
| Routine antibiotics | IV: cefazolin |
| Topical: moxifloxacin, voriconazole, anti-viral eye drop | |
| CMV, herpes virus | If CMV IgG (−): valacyclovir for 3Mo (herpes prophylaxis) |
| If CMV IgG (+): valganciclovir for 3Mo | |
| PCP | Trimethoprim/sulfamthoxazole for 6Mo |
| Tuberculosis | If Interferon gamma release assay (+), Isoniazid + pyridoxine (9Mo) |
| Oral candidiasis | Nystatin oral gargle for 6Mo |
Abbreviations: CMV: cytomegalovirus, Ig: immunoglobulin, IV: intravenous, Mo: months, PCP: pneumocystis pneumonia.
Table 18.
Regular Blood tests before and after corneal xenotransplantation.
| Test | Time |
|---|---|
| Screening testa | Before surgery |
| PERV | 12 W, 24 W, 52 W, 1Y6M, 2Y |
| CMV antigenemia | 2 W, 4 W, 8 W, 12 W, 24 W, 52 W, 2Y |
| EBV RT-PCR | 24 W |
| HBs Ag | 24 W, 2Y |
| Anti-HCV, HIV, RPR | 52 W, 2Y |
| AFP, PSA (only for man) | 2Y |
Abbreviations: Ab: antibody, AFP: alpha-fetoprotein, Anti-HCV: hepatitis C Antibody, CMV: cytomegalovirus, EBV: Epstein–Barr virus, Ig: immunoglobulin, HBs Ag: hepatitis B surface antigen, HIV: human immunodeficiency virus, PERV: porcine endogenous retrovirus, PSA: prostate specific antigen, RPR: rapid plasma reagin test, RT-PCR: reverse transcription-polymerase chain reaction, W: weeks, Y: years.
Screening test: Hepatitis A virus IgG, Hepatitis B e antibody, Hepatitis B e antigen, Varicella zoster virus IgG, Epstein–Barr nuclear antigen IgG, Toxoplasma IgG Ab, Mumps IgG, Measles IgG, Rubella IgG, Tuberculosis specific antigen induced interferon gamma, Herpes simplex virus IgG, CMV IgG, EBV viral-capsid antigen IgG, CMV antigenemia, PSA (only for man), AFP, EBV RT-PCR, sputum acid-fast bacillus culture, Panel reactive antibody (class I, II), HBs Ag, Hepatitis B surface antibody, Hepatitis B core antibody (IgM, IgG), Anti-HCV, HIV, RPR, Blood/urine/sputum culture, sputum fungus culture, PERV.
Table 19.
Neuropsychiatric assessment for psychosocial status of the subject.
| Assessment | Timeline | Examination |
|---|---|---|
| Suitability for TPL | 12 W before TPL | Cognitive assessment: K-WAIS-IV short form Psychiatric disorder & Psychopathology: MINI |
| Pre-TPL | 6 W before TPL | Semi-structured interview |
| Just before TPL | D −1 | Psychiatric consultation during admission (20 min) |
| Just after TPL | D0-D3 | |
| Post-TPL | D7, W4 ± D4, W8 ± D4, W12 ± D4, as required thereafter | |
Abbreviations: D: days, K-WAIS-IV: Korean Wechsler Adult Intelligence Scale-IV, MINI: Mini International Neuropsychiatric Interview, TPL: transplantation, W: weeks.
An IND submission for a corneal xenotransplant product and investigator-initiated corneal xenotransplantation clinical trial are being prepared in Korea. Pre-IND meetings are currently in progress with the Biopharmaceutical Policy Division, Cell, and Gene Therapy Products Division, Biologics Division, and Clinical Trials Management Division of MFDS (Fig. 10). Through the pre-IND meetings, lot release criteria of corneal xenotransplant product will be established that include (1) tests for purity, potency, and identity, (2) sterility test, (3) tests for mycoplasma, and (4) endotoxin level tests.
Since it will be the world's first corneal xenotransplantation clinical trial, it should be conducted under the proper supervision of the national regulatory authority. This process is expected to be a standardized model for future xenotransplantation studies.
11. Discussion and future perspectives
Since GT-KO pigs were generated in the early 2000s, serious progress has been made in xenotransplantation research as an alternative for allografts. However, immune rejection and the requirement of heavy immunosuppression remains the main barrier. For the clinically acceptable immunosuppressive regimen in a clinical trial, production of less antigenic GE pigs is required. Understanding how xenogeneic rejections are elicited can help to choose GE pigs appropriate for corneal xenotransplantation. The recently announced gene-editing technology using CRISPR-Cas9 can manipulate multiple genes in a short period of time and may give us the least immunogenic and PERV-free GE pigs. These scientific advances, along with the launch of huge commercial funding from industries, will reinvigorate research on xenotransplantation for market-entry (Editors, 2016; Perkel, 2016).
In xenotransplantation, safety is a critical concern for both individual participants and the public. The actual risk for xenozoonosis is still unknown therefore, standardized surveillance should be developed for potential pathogens through oversight of the source herd and animals, xenotransplant processing, and participant monitoring. The selection of PERV-C free pig or genomic inactivation of PERV may reduce the potential of PERV transmission. However, as with the regulatory framework for safe implementation of xenotransplantation, public engagement is essential with education for potential benefits and risks of xenotransplantation, and the participation of the decision-making procedures.
With accumulating evidence and recent achievements for preclinical efficacy, corneal xenotransplants may be offered for a clinical trial. Efficacy data is obvious with prolonged survival of WT grafts under the administration of co-stimulation blockade agents or anti-CD20 Abs/CNI combined regimens. As for corneal grafts from GE pig, further experiments should be conducted to verify their efficacies. Moreover, a well-controlled clinical trial of corneal xenotransplantation should be set under the proper supervision of regulatory authorities. Immunosuppression should be standardized before conducting a clinical trial. Furthermore, close communication with the xenotransplantation research community will lead a global consensus to bring corneal xenotransplantation closer to reality.
In South Korea, investigator-initiated phase I clinical trial is designed to investigate the safety and efficacy of corneal xenotransplantation. The participants should be bilaterally legally blind with a corneal opacity. During the consent process, alternatives such as allotransplant and keratoprosthesis should be introduced to the participant. If the outcome of the clinical trial is proven to be safe and effective, then xenotransplant may be used to bridge the operation in the bilateral corneal blind patients for whom the allograft is not timely available or for the emergent perforation in a clinical practice.
However, it is still controversial whether corneal xenotransplantation can be a clinically relevant option to replace allotransplantation. The COVID-19 pandemic hugely impacts both public and personal lives and brings us to a worldwide economic crisis (Adam, 2020). Given that COVID-19 is also suspected as a zoonotic infection with presumably bat origin (Lau et al., 2020; Zhou et al., 2020), the possibility of a xenozoonosis is considered as a major limiting factor in the clinical use of corneal xenotransplantation. The requirement of heavy immunosuppression with xenotransplantation may also be a barrier in regards with possible systemic adverse reaction and the expensive cost of the immunosuppressants. Current high cost to maintain DPF pig or to produce GE pigs as well as the costly process to provide corneal xenoproduct may be regarded as a practical concern to distribute corneal xenoproduct in developing countries where xenotransplantation may actually be required. Finally, many countries still do not have a regulatory framework for corneal xenotransplantation that should be set before a clinical implementation. These issues should be addressed and attempted to be solved in collaboration with the ophthalmologic and xenotransplantation society.
Although the preclinical efficacy using DPC is proven in keratoplasty, the legal definition of DPC lies somewhere between a medical device and a xenotransplant unless complete removal of the cells is ensured. Standard procedures to verify complete decellularization should be established before going into clinics. There are no long-term safety data using DPC; therefore, safety should be additionally evaluated in further clinical trial studies.
This review sheds light on how to perceive the status of xenotransplantation, the scientific advancement, and legal positioning in corneal xenotransplantation. The study provides the perspectives for the development of a new therapeutic strategy applicable to corneal disease.
Funding
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea [grant number: HI13C0954].
CRediT authorship contribution statement
Chang Ho Yoon: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Project administration, Visualization, Writing - original draft. Hyuk Jin Choi: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing - original draft. Mee Kum Kim: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing - review & editing.
Declarations of competing interest
None by Chang Ho Yoon, Hyuk Jin Choi, and Mee Kum Kim.
Glossary
- Ab
Antibody
- ACAID
Anterior chamber-associated immune deviation
- aCD20
anti-CD20 Ab
- ACR
Acute cellular rejection
- AFP
Alpha-fetoprotein
- αGal
Galα1-3Galβ1-4GlcNAc-R
- AHL
Anterior half lamella
- AL
Anterior lamella
- ALK
Anterior lamellar keratoplasty
- AMR
Antibody-mediated rejection
- α-MSH
Alpha melanocyte stimulating hormone
- Anti-HCV
Hepatitis C Antibody
- AP-1
Activating protein-1
- APC
Antigen presenting cells
- ARMBA
Advanced Regenerative Medicine and Biopharmaceutical Act
- ARMs
Advanced Regenerative Medicines
- ATG
Anti-thymocyte globulin
- ATMP
Advanced Therapy Medicinal Product
- BAL
Bronchoalveolar lavage
- BMT
Bone marrow transplantation
- C3a
Complement component 3a
- C3c
Complement component 3c
- CD
Cluster of differentiation
- CDC
Center for Disease Control
- CDR
Complementarity determining region
- CED
Corneal endothelial cell density
- cGMP
Current Good Manufacturing Practice
- CIITA-DN
MHC II transactivator dominant negative
- CMAH
Cytidine monophospho-N-acetylneuraminic acid hydroxylase
- COVID-19
Coronavirus disease 2019
- CMV
Cytomegalovirus
- CNI
Calcineurin inhibitor
- CNS
Central nervous system
- COPD
Chronic obstructive pulmonary disease
- CRISPR
Clustered Regularly Interspaced Short Palindromic Repeats
- CS
Corticosteroid
- CsA
Cyclosprine A
- CT
Computed tomography
- CTLA
Cytotoxic T lymphocyte antigen
- CVA
Cerebrovascular accident
- CVF
Cobra venom factor
- D
Days
- DALK
Deep anterior lamellar keratoplasty
- DFA
Direct fluorescent-antibody
- DHHS
Department of Health and Human Services
- DMEK
Descemet membrane endothelial keratoplasty
- DNA
Deoxyribonucleic acid
- DPC
Decellularized porcine cornea
- DPF
Designated pathogen-free
- DS
Donor pig-specific
- DSAEK
Descemet stripping automated endothelial keratoplasty
- DSMB
Data and Safety Monitoring Board
- DXR
Delayed xenograft rejection
- EBAA
Eye Bank Association of America
- EBV
Epstein–Barr virus
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- EDQM
European Directorate for the Quality of Medicines & HealthCare
- EDTA
Ethylenediaminetetraacetic acid
- EK
Endothelial keratoplasty
- EMA
European Medicines Agency
- ES
Embryonic stem cells
- EU
European Union
- FDA
Food and Drug Administration
- FKBP
FK506-binding protein
- FT
Fucosyltransferase
- FU
Follow-up
- G1
Cell cycle gap phase 1
- G2
Cell cycle gap phase 2
- GE
Genetically-engineered
- GT-KO
α1,3-galactosyltransferase gene-knockout
- H&E
Hematoxylin and eosin
- HAR
Hyperacute rejection
- HBs Ag
Hepatitis B surface antigen
- hCTLA4-Ig
Human cytotoxic T-lymphocyte associated antigen4-immunoglobulin
- HCV
Hepatitis C virus
- HEV
Hepatitis E virus
- HIV
Human immunodeficiency virus
- HLA
Human leukocyte antigen
- HSV
Herpes simplex virus
- IFN
Interferon
- Ig
Immunoglobulin
- iGb3s
Isoglobotrihexosylceramide 3 synthase
- IL
Interleukin
- IND
Investigational New Drug
- iPS
Induced pluripotent stem cells
- IRB
Institutional review board
- IST
Intrastromal transplantation
- IV
Intravenous
- IVIG
Intravenous immunoglobulin
- IXA
International Xenotransplantation Association
- K-WAIS-IV
Korean Wechsler Adult Intelligence Scale-IV
- LFA
Leukocyte function-associated antigen
- LKP
Lamellar keratoplasty
- LSCD
Limbal stem cell deficiency
- M
Cell cycle mitosis phase
- mAb
Monoclonal antibody
- MFDS
Ministry of Food and Drug Safety
- MFI
Mean fluorescence intensity
- MHC
Major histocompatibility complex
- MINI
Mini International Neuropsychiatric Interview
- MMF
Mycophenolate mofetil
- Mo
Months
- MOHW
Ministry of Health and Welfare
- MPA
Mycophenolic acid
- MSC
Mesenchymal stem cells
- MST
Median survival time
- mTOR
Mammalian target of rapamycin
- NA
Not available
- NAT
Nucleic acid testing
- NeuGc
N-glycolylneuraminic acid
- NFAT
Nuclear factor of activated T cells
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NHI
National health insurance
- NHP
Non-human primate
- NHS
N-Hydroxysulfosuccinimide
- NK
Natural killer
- NOG
NOD/SCID/γcnull
- NPC
Native porcine corneas
- ns
Not significant
- PCMV
Porcine cytomegalovirus
- PCP
Pneumocystis pneumonia
- PCR
Polymerase chain reaction
- PCV
Porcine circovirus
- PEDF
Pigment epithelium-derived factor
- PERV
Porcine endogenous retrovirus
- PKP
Penetrating keratoplasty
- PLHV
Porcine lymphotropic herpes virus
- PLK
Posterior lamellar keratoplasty
- PMN
Polymorphonuclear
- Preop
Preoperative
- PTLD
Posttransplant lymphoproliferative disorder
- PSA
Prostate specific antigen
- QNAT
Quantitative nucleic acid testing
- RNA
Ribonucleic acid
- RPR
Rapid plasma reagin test
- RT
Reverse transcriptase
- RT-PCR
Reverse transcription-polymerase chain reaction
- S
Cell cycle synthesis phase
- SCCO2
Supercritical carbon dioxide
- SCID
Severe combined immunodeficiency
- SDS
Sodium dodecyl sulfate
- sFlt-1
Soluble fms-like tyrosine kinase 1
- SIRPα
Signal regulatory protein alpha
- SNU
Seoul National University
- SOPs
Standard operating procedures
- TCR
T cell receptor
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
- Tregs
Regulatory T cells
- TSP
Thrombospondin
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
- VIP
Vasoactive intestinal peptide
- VZV
Varicella-zoster virus
- W
Weeks
- WHO
World Health Organization
- WT
Wild-type
- WZS
Wuzhishan
- Y
Years
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.preteyeres.2020.100876.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abalovich A., Matsumoto S., Wechsler C.J., Carulla M.E., Siciliano M.E., Sznaider D., Denner J., Elliott R.B. Level of acceptance of islet cell and kidney xenotransplants by personnel of hospitals with and without experience in clinical xenotransplantation. Xenotransplantation. 2017;24 doi: 10.1111/xen.12315. [DOI] [PubMed] [Google Scholar]
- Abou-El-Enein M., Schneider C.K. Deciphering the EU clinical trials regulation. Nat. Biotechnol. 2016;34:231–233. doi: 10.1038/nbt.3492. [DOI] [PubMed] [Google Scholar]
- Abud T.B., Di Zazzo A., Kheirkhah A., Dana R. Systemic immunomodulatory strategies in high-risk corneal transplantation. J. Ophthalmic Vis. Res. 2017;12:81–92. doi: 10.4103/2008-322X.200156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam D. Special report: the simulations driving the world's response to COVID-19. Nature. 2020;580:316–318. doi: 10.1038/d41586-020-01003-6. [DOI] [PubMed] [Google Scholar]
- Ahearne M., Yang Y., Then K.Y., Liu K.K. An indentation technique to characterize the mechanical and viscoelastic properties of human and porcine corneas. Ann. Biomed. Eng. 2007;35:1608–1616. doi: 10.1007/s10439-007-9323-9. [DOI] [PubMed] [Google Scholar]
- Almeida H.G., Kara-Jose N., Hida R.Y., Kara-Junior N. A 15-year review of corneal transplant in Brazil. Eye Contact Lens. 2018;44(Suppl. 2):S376–S381. doi: 10.1097/ICL.0000000000000554. [DOI] [PubMed] [Google Scholar]
- Amano S., Shimomura N., Kaji Y., Ishii K., Yamagami S., Araie M. Antigenicity of porcine cornea as xenograft. Curr. Eye Res. 2003;26:313–318. doi: 10.1076/ceyr.26.5.313.15440. [DOI] [PubMed] [Google Scholar]
- Amano S., Shimomura N., Yokoo S., Araki-Sasaki K., Yamagami S. Decellularizing corneal stroma using N2 gas. Mol. Vis. 2008;14:878–882. [PMC free article] [PubMed] [Google Scholar]
- Anderson M. Xenotransplantation: a bioethical evaluation. J. Med. Ethics. 2006;32:205–208. doi: 10.1136/jme.2005.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel J.Z., 3rd, Alwayn I.P., Cooper D.K. Xenotransplantation: the challenge to current psychosocial attitudes. Prog. Transplant. 2000;10:217–225. doi: 10.1177/152692480001000405. [DOI] [PubMed] [Google Scholar]
- Ayalew M., Tilahun Y., Holsclaw D., Indaram M., Stoller N.E., Keenan J.D., Rose-Nussbaumer J. Penetrating keratoplasty at a tertiary referral center in Ethiopia: indications and outcomes. Cornea. 2017;36:665–668. doi: 10.1097/ICO.0000000000001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babel J., Bourquin J.B. Experimental research with corneal heterografts. Br. J. Ophthalmol. 1952;36:529–536. doi: 10.1136/bjo.36.10.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey L.L., Nehlsen-Cannarella S.L., Concepcion W., Jolley W.B. Baboon-to-human cardiac xenotransplantation in a neonate. J. Am. Med. Assoc. 1985;254:3321–3329. [PubMed] [Google Scholar]
- Barker J.H., Polcrack L. Respect for persons, informed consent and the assessment of infectious disease risks in xenotransplantation. Med. Health Care Philos. 2001;4:53–70. doi: 10.1023/a:1009972928996. [DOI] [PubMed] [Google Scholar]
- Belin M.W., Bouchard C.S., Frantz S., Chmielinska J. Topical cyclosporine in high-risk corneal transplants. Ophthalmology. 1989;96:1144–1150. doi: 10.1016/s0161-6420(89)32756-4. [DOI] [PubMed] [Google Scholar]
- Benikova E.A., Turchin I.S., Beliakova L.S., Bol'shova E.V., Lysenko A.G. Experience with the treatment of children with diabetes mellitus using allo- and xenografts of cultures of pancreatic islet cells. Probl. Endokrinol. 1987;33:19–22. [PubMed] [Google Scholar]
- Bigan G., Puyraveau M., Saleh M., Gain P., Martinache I., Delbosc B., Gauthier A.S. Corneal transplantation trends in France from 2004 to 2015: a 12-year review. Eur. J. Ophthalmol. 2018;28:535–540. doi: 10.1177/1120672118762224. [DOI] [PubMed] [Google Scholar]
- Birnbaum F., Reis A., Bohringer D., Sokolowska Y., Mayer K., Voiculescu A., Oellerich M., Sundmacher R., Reinhard T. An open prospective pilot study on the use of rapamycin after penetrating high-risk keratoplasty. Transplantation. 2006;81:767–772. doi: 10.1097/01.tp.0000191291.71003.1b. [DOI] [PubMed] [Google Scholar]
- Bittmann I., Mihica D., Plesker R., Denner J. Expression of porcine endogenous retroviruses (PERV) in different organs of a pig. Virology. 2012;433:329–336. doi: 10.1016/j.virol.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Bloom E.T. Xenotransplantation: regulatory challenges. Curr. Opin. Biotechnol. 2001;12:312–316. doi: 10.1016/s0958-1669(00)00218-4. [DOI] [PubMed] [Google Scholar]
- Bloom E.T. Xenotransplantation–federal regulatory considerations. Curr. Top. Microbiol. Immunol. 2003;278:239–251. [PubMed] [Google Scholar]
- Bobba S., Di Girolamo N., Munsie M., Chen F., Pebay A., Harkin D., Hewitt A.W., O'Connor M., McLenachan S., Shadforth A.M.A., Watson S.L. The current state of stem cell therapy for ocular disease. Exp. Eye Res. 2018;177:65–75. doi: 10.1016/j.exer.2018.07.019. [DOI] [PubMed] [Google Scholar]
- Bock F., Maruyama K., Regenfuss B., Hos D., Steven P., Heindl L.M., Cursiefen C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog. Retin. Eye Res. 2013;34:89–124. doi: 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Boisgerault F., Liu Y., Anosova N., Ehrlich E., Dana M.R., Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J. Immunol. 2001;167:1891–1899. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- Bora N.S., Gobleman C.L., Atkinson J.P., Pepose J.S., Kaplan H.J. Differential expression of the complement regulatory proteins in the human eye. Invest. Ophthalmol. Vis. Sci. 1993;34:3579–3584. [PubMed] [Google Scholar]
- Bourne R.R.A., Flaxman S.R., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., Leasher J., Limburg H., Naidoo K., Pesudovs K., Resnikoff S., Silvester A., Stevens G.A., Tahhan N., Wong T.Y., Taylor H.R. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob. Health. 2017;5:e888–e897. doi: 10.1016/S2214-109X(17)30293-0. Vision Loss Expert, G. [DOI] [PubMed] [Google Scholar]
- Brunette I., Roberts C.J., Vidal F., Harissi-Dagher M., Lachaine J., Sheardown H., Durr G.M., Proulx S., Griffith M. Alternatives to eye bank native tissue for corneal stromal replacement. Prog. Retin. Eye Res. 2017;59:97–130. doi: 10.1016/j.preteyeres.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Byrne G.W. Does human leukocyte antigens sensitization matter for xenotransplantation? Xenotransplantation. 2018;25 doi: 10.1111/xen.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S., Chandraker A. Cell therapy in solid organ transplantation. Curr. Gene Ther. 2019;19:71–80. doi: 10.2174/1566523219666190603103840. [DOI] [PubMed] [Google Scholar]
- Cardona K., Korbutt G.S., Milas Z., Lyon J., Cano J., Jiang W., Bello-Laborn H., Hacquoil B., Strobert E., Gangappa S., Weber C.J., Pearson T.C., Rajotte R.V., Larsen C.P. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat. Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- Carlsson D.J., Li F., Shimmura S., Griffith M. Bioengineered corneas: how close are we? Curr. Opin. Ophthalmol. 2003;14:192–197. doi: 10.1097/00055735-200308000-00004. [DOI] [PubMed] [Google Scholar]
- Chakrabarty K., Shetty R., Ghosh A. Corneal cell therapy: with iPSCs, it is no more a far-sight. Stem Cell Res. Ther. 2018;9:287. doi: 10.1186/s13287-018-1036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.L., Mohiuddin M.M. Heart xenotransplantation. Curr. Opin. Organ Transplant. 2017;22:549–554. doi: 10.1097/MOT.0000000000000461. [DOI] [PubMed] [Google Scholar]
- Chandler J.W., Leder R., Kaufman H.E., Caldwell J.R. Quantitative determinations of complement components and immunoglobulins in tears and aqueous humor. Invest. Ophthalmol. 1974;13:151–153. [PubMed] [Google Scholar]
- Chang D.H., Kittleson M.M., Kobashigawa J.A. Immunosuppression following heart transplantation: prospects and challenges. Immunotherapy. 2014;6:181–194. doi: 10.2217/imt.13.163. [DOI] [PubMed] [Google Scholar]
- Chatel M.A., Larkin D.F. Sirolimus and mycophenolate as combination prophylaxis in corneal transplant recipients at high rejection risk. Am. J. Ophthalmol. 2010;150:179–184. doi: 10.1016/j.ajo.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Chauhan S.K., Saban D.R., Lee H.K., Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 2009;182:148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. Islet Xeno/transplantation and the risk of contagion: local responses from Canada and Australia to an emerging global technoscience. Life Sci. Soc. Policy. 2015;11:12. doi: 10.1186/s40504-015-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.J., Hyon J.Y., Lee H.K., Song J.S., Chung T.Y., Mo H., Kim J., Kim J.E., Yoo H., Lee S.H., Kwon I., Kim M.K. Standardization of the proceedings for preparing clinical trials of corneal xenotransplantation in South Korea. Xenotransplantation. 2019;26 doi: 10.1111/xen.12448. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Kim J., Kim J.Y., Lee H.J., Wee W.R., Kim M.K., Hwang E.S. Long-term safety from transmission of porcine endogenous retrovirus after pig-to-non-human primate corneal transplantation. Xenotransplantation. 2017;24 doi: 10.1111/xen.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.J., Kim M.K., Lee H.J., Jeong S.H., Kang H.J., Park C.S., Park C.G., Joon Kim S., Wee W.R. Effect of alphaGal on corneal xenotransplantation in a mouse model. Xenotransplantation. 2011;18:176–182. doi: 10.1111/j.1399-3089.2011.00641.x. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Kim M.K., Lee H.J., Ko J.H., Jeong S.H., Lee J.I., Oh B.C., Kang H.J., Wee W.R. Efficacy of pig-to-rhesus lamellar corneal xenotransplantation. Invest. Ophthalmol. Vis. Sci. 2011;52:6643–6650. doi: 10.1167/iovs.11-7273. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Lee J.J., Kim D.H., Kim M.K., Lee H.J., Ko A.Y., Kang H.J., Park C., Wee W.R. Blockade of CD40-CD154 costimulatory pathway promotes long-term survival of full-thickness porcine corneal grafts in nonhuman primates: clinically applicable xenocorneal transplantation. Am. J. Transplant. 2015;15:628–641. doi: 10.1111/ajt.13057. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Yoon C.H., Hyon J.Y., Lee H.K., Song J.S., Chung T.Y., Mo H., Kim J., Kim J.E., Hahm B.J., Yang J., Park W.B., Kim M.K. Protocol for the first clinical trial to investigate safety and efficacy of corneal xenotransplantation in patients with corneal opacity, corneal perforation, or impending corneal perforation. Xenotransplantation. 2019;26 doi: 10.1111/xen.12446. [DOI] [PubMed] [Google Scholar]
- Choi S.H., Yoon C.H., Lee H.J., Kim H.P., Kim J.M., Che J., Roh K.M., Choi H.J., Kim J., Hwang E.S., Park C., Kim M.K. Long‐term safety outcome of systemic immunosuppression in pig‐to‐nonhuman primate corneal xenotransplantation. Xenotransplantation. 2018;25 doi: 10.1111/xen.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claerhout I., Kestelyn P., Debacker V., Beele H., Leclercq G. Role of natural killer cells in the rejection process of corneal allografts in rats. Transplantation. 2004;77:676–682. doi: 10.1097/01.tp.0000114964.07637.b4. [DOI] [PubMed] [Google Scholar]
- Clark D.A., Fryer J.F., Tucker A.W., McArdle P.D., Hughes A.E., Emery V.C., Griffiths P.D. Porcine cytomegalovirus in pigs being bred for xenograft organs: progress towards control. Xenotransplantation. 2003;10:142–148. doi: 10.1034/j.1399-3089.2003.01128.x. [DOI] [PubMed] [Google Scholar]
- Cohen D., Miyagawa Y., Mehra R., Lee W., Isse K., Long C., Ayares D.L., Cooper D.K., Hara H. Distribution of non-gal antigens in pig cornea: relevance to corneal xenotransplantation. Cornea. 2014;33:390–397. doi: 10.1097/ICO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- Cooper D.K. Clinical xenotransplantion–how close are we? Lancet. 2003;362:557–559. doi: 10.1016/S0140-6736(03)14118-9. [DOI] [PubMed] [Google Scholar]
- Cooper D.K., Gollackner B., Sachs D.H. Will the pig solve the transplantation backlog? Annu. Rev. Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- Cooper D.K., Keogh A.M., Brink J., Corris P.A., Klepetko W., Pierson R.N., Schmoeckel M., Shirakura R., Warner Stevenson L. Report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. J. Heart Lung Transplant. 2000;19:1125–1165. doi: 10.1016/s1053-2498(00)00224-2. Xenotransplantation Advisory Committee of the International Society for, H., Lung, T. [DOI] [PubMed] [Google Scholar]
- Cooper D.K., Matsumoto S., Abalovich A., Itoh T., Mourad N.I., Gianello P.R., Wolf E., Cozzi E. Progress in clinical encapsulated islet xenotransplantation. Transplantation. 2016;100:2301–2308. doi: 10.1097/TP.0000000000001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D.K., Satyananda V., Ekser B., van der Windt D.J., Hara H., Ezzelarab M.B., Schuurman H.J. Progress in pig-to-non-human primate transplantation models (1998-2013): a comprehensive review of the literature. Xenotransplantation. 2014;21:397–419. doi: 10.1111/xen.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D.K.C., Gaston R., Eckhoff D., Ladowski J., Yamamoto T., Wang L., Iwase H., Hara H., Tector M., Tector A.J. Xenotransplantation-the current status and prospects. Br. Med. Bull. 2018;125:5–14. doi: 10.1093/bmb/ldx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J., Benvenuto L.J., Sonett J.R. Long-term outcomes and management of lung transplant recipients. Best Pract. Res. Clin. Anaesthesiol. 2017;31:285–297. doi: 10.1016/j.bpa.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Dai Y., Vaught T.D., Boone J., Chen S.H., Phelps C.J., Ball S., Monahan J.A., Jobst P.M., McCreath K.J., Lamborn A.E., Cowell-Lucero J.L., Wells K.D., Colman A., Polejaeva I.A., Ayares D.L. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat. Biotechnol. 2002;20:251–255. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- Danilova A.I., Zubkova S.T., Efimov A.S., Komissarenko I.V., Turchin I.S. Effect of the transplantation of cultured pancreatic islet cell on the status of diabetic microangiopathy. Probl. Endokrinol. 1989;35:9–14. [PubMed] [Google Scholar]
- Denis J. Extrait d’une Lettre de M.Denis, professeur de philosophie et de mathématique à M… touchant la transfusion du sang. Journal des Sçavants. 1667;69:72. [Google Scholar]
- Denner J. Recombinant porcine endogenous retroviruses (PERV-A/C): a new risk for xenotransplantation? Arch. Virol. 2008;153:1421–1426. doi: 10.1007/s00705-008-0141-7. [DOI] [PubMed] [Google Scholar]
- Denner J., Scobie L., Schuurman H.J. Is it currently possible to evaluate the risk posed by PERVs for clinical xenotransplantation? Xenotransplantation. 2018;25 doi: 10.1111/xen.12403. [DOI] [PubMed] [Google Scholar]
- Denner J., Tonjes R.R., Takeuchi Y., Fishman J., Scobie L. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes–Chapter 5: recipient monitoring and response plan for preventing disease transmission. Xenotransplantation. 2016;23:53–59. doi: 10.1111/xen.12227. [DOI] [PubMed] [Google Scholar]
- Dhaliwal J.S., Mason B.F., Kaufman S.C. Long-term use of topical tacrolimus (FK506) in high-risk penetrating keratoplasty. Cornea. 2008;27:488–493. doi: 10.1097/ICO.0b013e3181606086. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran R. Management of immunosuppression in liver transplantation. Clin. Liver Dis. 2017;21:337–353. doi: 10.1016/j.cld.2016.12.007. [DOI] [PubMed] [Google Scholar]
- DHHS . 2001. PHS Guideline on Infectious Disease Issues in Xenotransplantation.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/phs-guideline-infectious-disease-issues-xenotransplantation accessed 20 May 2020. [Google Scholar]
- DHHS . 2004. Informed Consent in Clinical Research Involving Xenotransplantation.https://www.tts.org/images/stories/ixa/regulatory_documents/8_SACX_Informed_consent_US_DHHS_June_2004.pdf accessed 20 May 2020. [Google Scholar]
- Di Nicuolo G., D'Alessandro A., Andria B., Scuderi V., Scognamiglio M., Tammaro A., Mancini A., Cozzolino S., Di Florio E., Bracco A., Calise F., Chamuleau R.A. Long-term absence of porcine endogenous retrovirus infection in chronically immunosuppressed patients after treatment with the porcine cell-based Academic Medical Center bioartificial liver. Xenotransplantation. 2010;17:431–439. doi: 10.1111/j.1399-3089.2010.00617.x. [DOI] [PubMed] [Google Scholar]
- Di Zazzo A., Kheirkhah A., Abud T.B., Goyal S., Dana R. Management of high-risk corneal transplantation. Surv. Ophthalmol. 2017;62:816–827. doi: 10.1016/j.survophthal.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsmore J.H., Manhart C., Raineri R., Jacoby D.B., Moore A. No evidence for infection of human cells with porcine endogenous retrovirus (PERV) after exposure to porcine fetal neuronal cells. Transplantation. 2000;70:1382–1389. doi: 10.1097/00007890-200011150-00020. [DOI] [PubMed] [Google Scholar]
- Dong X., Hara H., Wang Y., Wang L., Zhang Y., Cooper D.K., Dai Y., Pan Z. Initial study of alpha1,3-galactosyltransferase gene-knockout/CD46 pig full-thickness corneal xenografts in rhesus monkeys. Xenotransplantation. 2017;24:202–210. doi: 10.1111/xen.12282. [DOI] [PubMed] [Google Scholar]
- Drozhzhyna G.I., Gaidamaka T.B., Cursiefen C., Bachmann B.O., Ivanovska O.V., Ostashevsky V.L., Kogan B.M., Usov V.J., Pasyechnikova N.V. Emergency keratoplasty with porcine xenografts in necrotizing keratitis. Klin. Monbl. Augenheilkd. 2017;234:1387–1395. doi: 10.1055/s-0043-109694. [DOI] [PubMed] [Google Scholar]
- Du L., Wu X. Development and characterization of a full-thickness acellular porcine cornea matrix for tissue engineering. Artif. Organs. 2011;35:691–705. doi: 10.1111/j.1525-1594.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- Du L., Wu X., Pang K., Yang Y. Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold. Br. J. Ophthalmol. 2011;95:410–414. doi: 10.1136/bjo.2008.142539. [DOI] [PubMed] [Google Scholar]
- EBAA . Eye Bank Association of America (EBAA); Washington, DC: 2019. Medical Standards of the. [Google Scholar]
- Editors Xenotransplantation 2.0. Nat. Biotechnol. 2016;34:1. doi: 10.1038/nbt.3466. [DOI] [PubMed] [Google Scholar]
- EDQM . 2019. Guide to the Quality and Safety of Tissues and Cells for Human Application.https://register.edqm.eu/freepub accessed 20 May 2020. [Google Scholar]
- Ekser B., Ezzelarab M., Hara H., van der Windt D.J., Wijkstrom M., Bottino R., Trucco M., Cooper D.K. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- Ekser B., Li P., Cooper D.K.C. Xenotransplantation: past, present, and future. Curr. Opin. Organ Transplant. 2017;22:513–521. doi: 10.1097/MOT.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R.B., Escobar L., Garkavenko O., Croxson M.C., Schroeder B.A., McGregor M., Ferguson G., Beckman N., Ferguson S. No evidence of infection with porcine endogenous retrovirus in recipients of encapsulated porcine islet xenografts. Cell Transplant. 2000;9:895–901. doi: 10.1177/096368970000900616. [DOI] [PubMed] [Google Scholar]
- Elliott R.B., Escobar L., Tan P.L., Muzina M., Zwain S., Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157–161. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- Elsheikh A., Alhasso D., Rama P. Biomechanical properties of human and porcine corneas. Exp. Eye Res. 2008;86:783–790. doi: 10.1016/j.exer.2008.02.006. [DOI] [PubMed] [Google Scholar]
- EMA . 2009. Guideline on Xenogeneic Cell-Based Medicinal Products.http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/12/WC500016936.pdf accessed 20 May 2020. [Google Scholar]
- EMA . 2018. Guideline on Quality, Non-clinical and Clinical Aspects of 4 Medicinal Products Containing Genetically Modified Cells.https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-non-clinical-clinical-aspects-medicinal-products-containing-genetically_en.pdf accessed 20 May 2020. [Google Scholar]
- Ericsson T.A., Takeuchi Y., Templin C., Quinn G., Farhadian S.F., Wood J.C., Oldmixon B.A., Suling K.M., Ishii J.K., Kitagawa Y., Miyazawa T., Salomon D.R., Weiss R.A., Patience C. Identification of receptors for pig endogenous retrovirus. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6759–6764. doi: 10.1073/pnas.1138025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber C., Wang M., Scherfig E., Sorensen K.E., Prause J.U., Ehlers N., Nissen M.H. Orthotopic porcine corneal xenotransplantation using a human graft. Acta Ophthalmol. 2009;87:917–919. doi: 10.1111/j.1755-3768.2008.01489.x. [DOI] [PubMed] [Google Scholar]
- Fasciani R., Mosca L., Giannico M.I., Ambrogio S.A., Balestrazzi E. Subconjunctival and/or intrastromal bevacizumab injections as preconditioning therapy to promote corneal graft survival. Int. Ophthalmol. 2015;35:221–227. doi: 10.1007/s10792-014-9938-4. [DOI] [PubMed] [Google Scholar]
- FDA . 2016. 21st Century Cures Act.https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act accessed 20 May 2020. [Google Scholar]
- FDA . 2016. Medical Devices Containing Materials Derived from Animal Sources (Except for in Vitro Diagnostic Devices); Guidance for Industry and Food and Drug Administration Staff.https://www.fda.gov/media/87251/download accessed 20 May 2020. [Google Scholar]
- FDA . 2016. Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans; Guidance for Industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/source-animal-product-preclinical-and-clinical-issues-concerning-use-xenotransplantation-products accessed 20 May 2020. [Google Scholar]
- FDA . 2017. Considerations for the Design of Early-phase Clinical Trials of Cellular and Gene Therapy Products; Guidance for Industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-design-early-phase-clinical-trials-cellular-and-gene-therapy-products accessed 20 May 2020. [Google Scholar]
- FDA . 2017. CVM GFI #187 Regulation of Intentionally Altered Genomic DNA in Animals.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-187-regulation-intentionally-altered-genomic-dna-animals accessed 20 May 2020. [Google Scholar]
- Fernandez-Perez J., Ahearne M. Decellularization and recellularization of cornea: progress towards a donor alternative. Methods. 2020;171:86–96. doi: 10.1016/j.ymeth.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Fishman J.A. Infection in organ transplantation. Am. J. Transplant. 2017;17:856–879. doi: 10.1111/ajt.14208. [DOI] [PubMed] [Google Scholar]
- Fishman J.A. Infectious disease risks in xenotransplantation. Am. J. Transplant. 2018;18:1857–1864. doi: 10.1111/ajt.14725. [DOI] [PubMed] [Google Scholar]
- Fishman J.A. Infection in xenotransplantation: opportunities and challenges. Curr. Opin. Organ Transplant. 2019;24:527–534. doi: 10.1097/MOT.0000000000000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J.A., Scobie L., Takeuchi Y. Xenotransplantation-associated infectious risk: a WHO consultation. Xenotransplantation. 2012;19:72–81. doi: 10.1111/j.1399-3089.2012.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., Leasher J., Limburg H., Naidoo K., Pesudovs K., Silvester A., Stevens G.A., Tahhan N., Wong T.Y., Taylor H.R. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob. Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. Vision Loss Expert Group of the Global Burden of Disease, S. [DOI] [PubMed] [Google Scholar]
- Flockerzi E., Maier P., Bohringer D., Reinshagen H., Kruse F., Cursiefen C., Reinhard T., Geerling G., Torun N., Seitz B. Trends in corneal transplantation from 2001 to 2016 in Germany: a report of the DOG-section cornea and its keratoplasty registry. Am. J. Ophthalmol. 2018;188:91–98. doi: 10.1016/j.ajo.2018.01.018. all German Keratoplasty Registry, C. [DOI] [PubMed] [Google Scholar]
- Forrester J.V., Steptoe R.J., Klaska I.P., Martin-Granados C., Dua H.S., Degli-Esposti M.A., Wikstrom M.E. Cell-based therapies for ocular inflammation. Prog. Retin. Eye Res. 2013;35:82–101. doi: 10.1016/j.preteyeres.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Fujita M., Mehra R., Lee S.E., Roh D.S., Long C., Funderburgh J.L., Ayares D.L., Cooper D.K., Hara H. Comparison of proliferative capacity of genetically-engineered pig and human corneal endothelial cells. Ophthalmic Res. 2013;49:127–138. doi: 10.1159/000342978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gain P., Jullienne R., He Z., Aldossary M., Acquart S., Cognasse F., Thuret G. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167–173. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- Garkavenko O., Dieckhoff B., Wynyard S., Denner J., Elliott R.B., Tan P.L., Croxson M.C. Absence of transmission of potentially xenotic viruses in a prospective pig to primate islet xenotransplantation study. J. Med. Virol. 2008;80:2046–2052. doi: 10.1002/jmv.21272. [DOI] [PubMed] [Google Scholar]
- George J.F. Xenotransplantation: an ethical dilemma. Curr. Opin. Cardiol. 2006;21:138–141. doi: 10.1097/01.hco.0000203183.81534.f9. [DOI] [PubMed] [Google Scholar]
- Gollackner B., Mueller N.J., Houser S., Qawi I., Soizic D., Knosalla C., Buhler L., Dor F.J., Awwad M., Sachs D.H., Cooper D.K., Robson S.C., Fishman J.A. Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation. 2003;75:1841–1847. doi: 10.1097/01.TP.0000065806.90840.C1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Andrades M., Carriel V., Rivera-Izquierdo M., Garzon I., Gonzalez-Andrades E., Medialdea S., Alaminos M., Campos A. Effects of detergent-based protocols on decellularization of corneas with sclerocorneal limbus. Evaluation of regional differences. Transl. Vis. Sci. Technol. 2015;4:13. doi: 10.1167/tvst.4.2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Andrades M., de la Cruz Cardona J., Ionescu A.M., Campos A., Del Mar Perez M., Alaminos M. Generation of bioengineered corneas with decellularized xenografts and human keratocytes. Invest. Ophthalmol. Vis. Sci. 2011;52:215–222. doi: 10.1167/iovs.09-4773. [DOI] [PubMed] [Google Scholar]
- Griffith M., Alarcon E.I., Brunette I. Regenerative approaches for the cornea. J. Intern. Med. 2016;280:276–286. doi: 10.1111/joim.12502. [DOI] [PubMed] [Google Scholar]
- Groth C.G., Korsgren O., Tibell A., Tollemar J., Moller E., Bolinder J., Ostman J., Reinholt F.P., Hellerstrom C., Andersson A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Hagelin J. Public opinion surveys about xenotransplantation. Xenotransplantation. 2004;11:551–558. doi: 10.1111/j.1399-3089.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- Hamrah P., Haskova Z., Taylor A.W., Zhang Q., Ksander B.R., Dana M.R. Local treatment with alpha-melanocyte stimulating hormone reduces corneal allorejection. Transplantation. 2009;88:180–187. doi: 10.1097/TP.0b013e3181ac11ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Aglyamov S.R., Li J., Singh M., Wang S., Vantipalli S., Wu C., Liu C.H., Twa M.D., Larin K.V. Quantitative assessment of corneal viscoelasticity using optical coherence elastography and a modified Rayleigh-Lamb equation. J. Biomed. Optic. 2015;20:20501. doi: 10.1117/1.JBO.20.2.020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq M. Fish cornea for grafting. Br. Med. J. 1972;2:712–713. doi: 10.1136/bmj.2.5815.712-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Cooper D.K. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation. 2010;17:338–349. doi: 10.1111/j.1399-3089.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- Hara H., Cooper D.K. Xenotransplantation–the future of corneal transplantation? Cornea. 2011;30:371–378. doi: 10.1097/ICO.0b013e3181f237ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Koike N., Long C., Piluek J., Roh D.S., SundarRaj N., Funderburgh J.L., Mizuguchi Y., Isse K., Phelps C.J., Ball S.F., Ayares D.L., Cooper D.K. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Invest. Ophthalmol. Vis. Sci. 2011;52:5278–5286. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Witt W., Crossley T., Long C., Isse K., Fan L., Phelps C.J., Ayares D., Cooper D.K., Dai Y., Starzl T.E. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology. 2013;140:39–46. doi: 10.1111/imm.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J.D., Kurrus F.D., Chavez C.M., Neely W.A., Eraslan S., Turner M.D., Fabian L.W., Labecki T.D. Heart transplantation in man. Developmental studies and report of a case. J. Am. Med. Assoc. 1964;188:1132–1140. [PubMed] [Google Scholar]
- Hargrave S.L., Mayhew E., Hegde S., Niederkorn J. Are corneal cells susceptible to antibody-mediated killing in corneal allograft rejection? Transpl. Immunol. 2003;11:79–89. doi: 10.1016/S0966-3274(02)00082-5. [DOI] [PubMed] [Google Scholar]
- Hargrave S.L., Taherzadeh S., Hegde S., Niederkorn J. High-risk corneal allografts are capable of stimulating complement dependent cytolytic antibodies. Cornea. 2000;19:521–525. doi: 10.1097/00003226-200007000-00024. [DOI] [PubMed] [Google Scholar]
- Harland R.C., Klintmalm G., Jensik S., Yang H., Bromberg J., Holman J., Kumar M.S.A., Santos V., Larson T.J., Wang X. Efficacy and safety of bleselumab in kidney transplant recipients: a phase 2, randomized, open-label, noninferiority study. Am. J. Transplant. 2020;20:159–171. doi: 10.1111/ajt.15591. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Funamoto S., Sasaki S., Honda T., Hattori S., Nam K., Kimura T., Mochizuki M., Fujisato T., Kobayashi H., Kishida A. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials. 2010;31:3941–3948. doi: 10.1016/j.biomaterials.2010.01.122. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Funamoto S., Sasaki S., Negishi J., Honda T., Hattori S., Nam K., Kimura T., Mochizuki M., Kobayashi H., Kishida A. Corneal regeneration by deep anterior lamellar keratoplasty (DALK) using decellularized corneal matrix. PloS One. 2015;10 doi: 10.1371/journal.pone.0131989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Hattori S., Sasaki S., Honda T., Kimura T., Funamoto S., Kobayashi H., Kishida A. Ultrastructural analysis of the decellularized cornea after interlamellar keratoplasty and microkeratome-assisted anterior lamellar keratoplasty in a rabbit model. Sci. Rep. 2016;6:27734. doi: 10.1038/srep27734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne W.J., Cowan P.J., Buhler L.H., Yi S., Bottino R., Pierson R.N., 3rd, Ahn C., Azimzadeh A., Cozzi E., Gianello P., Lakey J.R.T., Luo M., Miyagawa S., Mohiuddin M.M., Park C.G., Schuurman H.J., Scobie L., Sykes M., Tector J., Tonjes R.R., Wolf E., Nunez J.R., Wang W. Third WHO global consultation on regulatory requirements for xenotransplantation clinical trials, Changsha, hunan, China december 12-14, 2018: "the 2018 Changsha communique" the 10-year anniversary of the international consultation on xenotransplantation. Xenotransplantation. 2019;26 doi: 10.1111/xen.12513. [DOI] [PubMed] [Google Scholar]
- He Y.G., Ross J., Niederkorn J.Y. Promotion of murine orthotopic corneal allograft survival by systemic administration of anti-CD4 monoclonal antibody. Invest. Ophthalmol. Vis. Sci. 1991;32:2723–2728. [PubMed] [Google Scholar]
- He Z., Forest F., Bernard A., Gauthier A.S., Montard R., Peoc'h M., Jumelle C., Courrier E., Perrache C., Gain P., Thuret G. Cutting and decellularization of multiple corneal stromal lamellae for the bioengineering of endothelial grafts. Invest. Ophthalmol. Vis. Sci. 2016;57:6639–6651. doi: 10.1167/iovs.16-20256. [DOI] [PubMed] [Google Scholar]
- Hecht G., Eventov-Friedman S., Rosen C., Shezen E., Tchorsh D., Aronovich A., Freud E., Golan H., El-Hasid R., Katchman H., Hering B.J., Zung A., Kra-Oz Z., Shaked-Mishan P., Yusim A., Shtabsky A., Idelevitch P., Tobar A., Harmelin A., Bachar-Lustig E., Reisner Y. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8659–8664. doi: 10.1073/pnas.0812253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneine W., Tibell A., Switzer W.M., Sandstrom P., Rosales G.V., Mathews A., Korsgren O., Chapman L.E., Folks T.M., Groth C.G. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet. 1998;352:695–699. doi: 10.1016/S0140-6736(98)07145-1. [DOI] [PubMed] [Google Scholar]
- Henry M.L., Rajab A. The use of basiliximab in solid organ transplantation. Expet Opin. Pharmacother. 2002;3:1657–1663. doi: 10.1517/14656566.3.11.1657. [DOI] [PubMed] [Google Scholar]
- Hering B.J., Cooper D.K., Cozzi E., Schuurman H.J., Korbutt G.S., Denner J., O’Connell P.J., Vanderpool H.Y., Pierson R.N., 3rd The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes– executive summary. Xenotransplantation. 2009;16:196–202. doi: 10.1111/j.1399-3089.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- Hering B.J., Cozzi E., Spizzo T., Cowan P.J., Rayat G.R., Cooper D.K., Denner J. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes–Executive summary. Xenotransplantation. 2016;23:3–13. doi: 10.1111/xen.12231. [DOI] [PubMed] [Google Scholar]
- Higginbotham L., Ford M.L., Newell K.A., Adams A.B. Preventing T cell rejection of pig xenografts. Int. J. Surg. 2015;23:285–290. doi: 10.1016/j.ijsu.2015.07.722. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Streilein J.W. CD8+ T cell-mediated delayed rejection of orthotopic Guinea pig cornea grafts in mice deficient in CD4+ T cells. Invest. Ophthalmol. Vis. Sci. 2003;44:175–182. doi: 10.1167/iovs.02-0050. [DOI] [PubMed] [Google Scholar]
- Hill J.C. Systemic cyclosporine in high-risk keratoplasty. Short- versus long-term therapy. Ophthalmology. 1994;101:128–133. doi: 10.1016/s0161-6420(13)31253-6. [DOI] [PubMed] [Google Scholar]
- Hjortdal J. In: Biomechanics of the Eye. Roberts C.J., Dupps W.J., Downs J.C., editors. Kugler publications; Amsterdam: 2018. Basics of biomechanics; pp. 3–13. [Google Scholar]
- Holan V., Vitova A., Krulova M., Zajicova A., Neuwirth A., Filipec M., Forrester J.V. Susceptibility of corneal allografts and xenografts to antibody-mediated rejection. Immunol. Lett. 2005;100:211–213. doi: 10.1016/j.imlet.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Hori J., Yamaguchi T., Keino H., Hamrah P., Maruyama K. Immune privilege in corneal transplantation. Prog. Retin. Eye Res. 2019;72:100758. doi: 10.1016/j.preteyeres.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Hos D., Matthaei M., Bock F., Maruyama K., Notara M., Clahsen T., Hou Y., Le V.N.H., Salabarria A.C., Horstmann J., Bachmann B.O., Cursiefen C. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog. Retin. Eye Res. 2019;73:100768. doi: 10.1016/j.preteyeres.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Huang Y.H., Tseng F.W., Chang W.H., Peng I.C., Hsieh D.J., Wu S.W., Yeh M.L. Preparation of acellular scaffold for corneal tissue engineering by supercritical carbon dioxide extraction technology. Acta Biomater. 2017;58:238–243. doi: 10.1016/j.actbio.2017.05.060. [DOI] [PubMed] [Google Scholar]
- Inoue K., Amano S., Kimura C., Sato T., Fujita N., Kagaya F., Kaji Y., Oshika T., Tsuru T., Araie M. Long-term effects of topical cyclosporine A treatment after penetrating keratoplasty. Jpn. J. Ophthalmol. 2000;44:302–305. doi: 10.1016/s0021-5155(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US) National Academy Press; Washington DC: 1996. Committee on Xenograft Transplantation: Ethical Issues and Public Policy. Xenotransplantation: Science, Ethics, and Public Policy. Xenotransplantation: Science, Ethics, and Public Policy. [PubMed] [Google Scholar]
- Irgang M., Sauer I.M., Karlas A., Zeilinger K., Gerlach J.C., Kurth R., Neuhaus P., Denner J. Porcine endogenous retroviruses: no infection in patients treated with a bioreactor based on porcine liver cells. J. Clin. Virol. 2003;28:141–154. doi: 10.1016/s1386-6532(02)00275-5. [DOI] [PubMed] [Google Scholar]
- Isidan A., Liu S., Li P., Lashmet M., Smith L.J., Hara H., Cooper D.K.C., Ekser B. Decellularization methods for developing porcine corneal xenografts and future perspectives. Xenotransplantation. 2019;26 doi: 10.1111/xen.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa N.C., Wilkinson R.A., Griesemer A., Cooper D.K., Yamada K., Sachs D.H., Fishman J.A. Absence of replication of porcine endogenous retrovirus and porcine lymphotropic herpesvirus type 1 with prolonged pig cell microchimerism after pig-to-baboon xenotransplantation. J. Virol. 2008;82:12441–12448. doi: 10.1128/JVI.01278-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaboulay M. Greffe de reins au pli du coude par soudures arterielles et veineuses. Lyon Med. 1906;107:575. [Google Scholar]
- Jie Y., Liu L., Pan Z., Wang L. Survival of pig-to-rhesus corneal xenografts prolonged by prior donor bone marrow transplantation. Mol. Med. Rep. 2013;7:869–874. doi: 10.3892/mmr.2013.1294. [DOI] [PubMed] [Google Scholar]
- Jones G.L.A., Ponzin D., Pels E., Maas H., Tullo A.B., Claerhout I. European eye bank association. Dev. Ophthalmol. 2009;43:15–21. doi: 10.1159/000223835. [DOI] [PubMed] [Google Scholar]
- Jordan S.C., Toyoda M., Kahwaji J., Vo A.A. Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am. J. Transplant. 2011;11:196–202. doi: 10.1111/j.1600-6143.2010.03400.x. [DOI] [PubMed] [Google Scholar]
- Joseph A., Raj D., Shanmuganathan V., Powell R.J., Dua H.S. Tacrolimus immunosuppression in high-risk corneal grafts. Br. J. Ophthalmol. 2007;91:51–55. doi: 10.1136/bjo.2006.097428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce N.C. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003;22:359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Ju C., Gao L., Wu X., Pang K. A human corneal endothelium equivalent constructed with acellular porcine corneal matrix. Indian J. Med. Res. 2012;135:887–894. [PMC free article] [PubMed] [Google Scholar]
- Karabun P.M., Malik A., Skrobons'ka N.A., Stepura N.M. The immunological indices of diabetic patients following the transplantation of a pancreatic islet cell culture. Lik. Sprava. 1994;77:80. [PubMed] [Google Scholar]
- Kawai T., Andrews D., Colvin R.B., Sachs D.H., Cosimi A.B. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat. Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Kim J., Jeong H.J., Lee H.J., Kim M.K., Wee W.R. Biophysico-functional compatibility of Seoul National University (SNU) miniature pig cornea as xenocorneal graft for the use of human clinical trial. Xenotransplantation. 2016;23:202–210. doi: 10.1111/xen.12234. [DOI] [PubMed] [Google Scholar]
- Kim H.K., Choi J.A., Uehara H., Zhang X., Ambati B.K., Cho Y.K. Presurgical corticosteroid treatment improves corneal transplant survival in mice. Cornea. 2013;32:1591–1598. doi: 10.1097/ICO.0b013e31829ebb0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Choi S.H., Lee H.J., Kim H.P., Kang H.J., Kim J.M., Hwang E.S., Park C.G., Kim M.K. Comparative efficacy of anti-CD40 antibody-mediated costimulation blockade on long-term survival of full-thickness porcine corneal grafts in nonhuman primates. Am. J. Transplant. 2018;18:2330–2341. doi: 10.1111/ajt.14913. [DOI] [PubMed] [Google Scholar]
- Kim J., Kim D.H., Choi H.J., Lee H.J., Kang H.J., Park C.G., Hwang E.S., Kim M.K., Wee W.R. Anti-CD40 antibody-mediated costimulation blockade promotes long-term survival of deep-lamellar porcine corneal grafts in non-human primates. Xenotransplantation. 2017;24 doi: 10.1111/xen.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Kim J., Choi S.H., Shin J.S., Min B.H., Jeong W.Y., Lee G.E., Kim M.S., Kwon S., Kim M.K., Park C.G. Tacrolimus-induced asymptomatic thrombotic microangiopathy diagnosed by laboratory tests in pig-to-rhesus corneal xenotransplantation: a case report. Xenotransplantation. 2018;25 doi: 10.1111/xen.12404. [DOI] [PubMed] [Google Scholar]
- Kim M.K. Commentary: current status and future of corneal xenotransplantation. Transplantation Technologies & Research. 2016;6:3. [Google Scholar]
- Kim M.K. In: Xenotransplantation - New Insights. Miyagawa S., editor. IntechOpen; London: 2017. Current progress in corneal xenotransplantation; pp. 25–46.https://www.intechopen.com/books/xenotransplantation-new-insights/current-progress-in-corneal-xenotransplantation [Google Scholar]
- Kim M.K., Choi H.J., Kwon I., Pierson R.N., 3rd, Cooper D.K., Soulillou J.P., O'Connell P.J., Vabres B., Maeda N., Hara H., Scobie L., Gianello P., Takeuchi Y., Yamada K., Hwang E.S., Kim S.J., Park C.G., International Xenotransplantation A. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of xenocorneal transplantation. Xenotransplantation. 2014;21:420–430. doi: 10.1111/xen.12129. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Hara H. Current status of corneal xenotransplantation. Int. J. Surg. 2015;23:255–260. doi: 10.1016/j.ijsu.2015.07.685. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Lee J.J., Choi H.J., Kwon I., Lee H., Song J.S., Kim M.J., Chung E.S., Wee W.R., Park C.G., Kim S.J., Xenotransplatation Research C., Clinical Research I. Ethical and regulatory guidelines in clinical trials of xenocorneal transplantation in Korea; the Korean xenocorneal transplantation consensus statement. Xenotransplantation. 2013;20:209–218. doi: 10.1111/xen.12036. Korean External Eye Disease, S. [DOI] [PubMed] [Google Scholar]
- Kim Y.G., Oh J.Y., Gil G.C., Kim M.K., Ko J.H., Lee S., Lee H.J., Wee W.R., Kim B.G. Identification of alpha-Gal and non-Gal epitopes in pig corneal endothelial cells and keratocytes by using mass spectrometry. Curr. Eye Res. 2009;34:877–895. doi: 10.3109/02713680903184243. [DOI] [PubMed] [Google Scholar]
- Kimelman M., Brandacher G. Trends in immunosuppression after pancreas transplantation: what is in the pipeline? Curr. Opin. Organ Transplant. 2013;18:76–82. doi: 10.1097/MOT.0b013e32835c6eda. [DOI] [PubMed] [Google Scholar]
- Kimsa M.C., Strzalka-Mrozik B., Kimsa M.W., Gola J., Nicholson P., Lopata K., Mazurek U. Porcine endogenous retroviruses in xenotransplantation–molecular aspects. Viruses. 2014;6:2062–2083. doi: 10.3390/v6052062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk A.D., Blair P.J., Tadaki D.K., Xu H., Harlan D.M. The role of CD154 in organ transplant rejection and acceptance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:691–702. doi: 10.1098/rstb.2001.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissam R. Ceratoplastice in man. N. Y. J. Med. Collat. Sci. 1844;2:281. [Google Scholar]
- Koyama I., Kawai T., Andrews D., Boskovic S., Nadazdin O., Wee S.L., Sogawa H., Wu D.L., Smith R.N., Colvin R.B., Sachs D.H., Cosimi A.B. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77:460–462. doi: 10.1097/01.TP.0000110291.29370.C0. [DOI] [PubMed] [Google Scholar]
- Lai L., Kolber-Simonds D., Park K.W., Cheong H.T., Greenstein J.L., Im G.S., Samuel M., Bonk A., Rieke A., Day B.N., Murphy C.N., Carter D.B., Hawley R.J., Prather R.S. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- Lambrigts D., Sachs D.H., Cooper D.K. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998;66:547–561. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- Lamm V., Hara H., Mammen A., Dhaliwal D., Cooper D.K. Corneal blindness and xenotransplantation. Xenotransplantation. 2014;21:99–114. doi: 10.1111/xen.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langin M., Mayr T., Reichart B., Michel S., Buchholz S., Guethoff S., Dashkevich A., Baehr A., Egerer S., Bauer A., Mihalj M., Panelli A., Issl L., Ying J., Fresch A.K., Buttgereit I., Mokelke M., Radan J., Werner F., Lutzmann I., Steen S., Sjoberg T., Paskevicius A., Qiuming L., Sfriso R., Rieben R., Dahlhoff M., Kessler B., Kemter E., Kurome M., Zakhartchenko V., Klett K., Hinkel R., Kupatt C., Falkenau A., Reu S., Ellgass R., Herzog R., Binder U., Wich G., Skerra A., Ayares D., Kind A., Schonmann U., Kaup F.J., Hagl C., Wolf E., Klymiuk N., Brenner P., Abicht J.M. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564:430–433. doi: 10.1038/s41586-018-0765-z. [DOI] [PubMed] [Google Scholar]
- Larkin D.F., Williams K.A. The host response in experimental corneal xenotransplantation. Eye. 1995;9:254–260. doi: 10.1038/eye.1995.49. [DOI] [PubMed] [Google Scholar]
- Lau S.K.P., Luk H.K.H., Wong A.C.P., Li K.S.M., Zhu L., He Z., Fung J., Chan T.T.Y., Fung K.S.C., Woo P.C.Y. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Ni M.Y., Luk A.C., Lau J.K., Lam K.S., Li T.K., Wong C.S., Wong V.W. Trends and determinants of familial consent for corneal donation in Chinese. Cornea. 2017;36:295–299. doi: 10.1097/ICO.0000000000001091. [DOI] [PubMed] [Google Scholar]
- Lee H.I., Kim M.K., Oh J.Y., Ko J.H., Lee H.J., Wee W.R., Lee J.H. Gal alpha(1-3)Gal expression of the cornea in vitro, in vivo and in xenotransplantation. Xenotransplantation. 2007;14:612–618. doi: 10.1111/j.1399-3089.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- Lee H.I., Kim M.K., Oh J.Y., Ko J.H., Lee H.J., Wee W.R., Lee J.H. The role of cyclosporine and mycophenolate in an orthotopic porcine-to-rat corneal xenotransplantation. J. Kor. Med. Sci. 2008;23:492–501. doi: 10.3346/jkms.2008.23.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.J., Kim D.H., Jang Y.E., Choi H.J., Kim M.K., Wee W.R. The attitude toward xenocorneal transplantation in wait-listed subjects for corneal transplantation in Korea. Xenotransplantation. 2014;21:25–34. doi: 10.1111/xen.12069. [DOI] [PubMed] [Google Scholar]
- Lee J.K., Lee S.H., Kim J.C. In: Biomaterials Science and Engineering. Pignatello R., editor. IntechOpen; London: 2011. Xenotransplantation using lyophilized acellular porcine cornea with cells grown in vivo and stimulated with substance-p; pp. 387–403.http://www.intechopen.com/books/biomaterials-science-and-engineering/xenotransplantation-using-lyophilized-acellular-porcine-cornea-with-cells-grown-in-vivo-and-stimulat [Google Scholar]
- Lee J.K., Ryu Y.H., Ahn J.I., Kim M.K., Lee T.S., Kim J.C. The effect of lyophilization on graft acceptance in experimental xenotransplantation using porcine cornea. Artif. Organs. 2010;34:37–45. doi: 10.1111/j.1525-1594.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- Lee S.E., Mehra R., Fujita M., Roh D.S., Long C., Lee W., Funderburgh J.L., Ayares D.L., Cooper D.K., Hara H. Characterization of porcine corneal endothelium for xenotransplantation. Semin. Ophthalmol. 2014;29:127–135. doi: 10.3109/08820538.2013.787104. [DOI] [PubMed] [Google Scholar]
- Lee W., Mammen A., Dhaliwal D.K., Long C., Miyagawa Y., Ayares D., Cooper D.K., Hara H. Development of retrocorneal membrane following pig-to-monkey penetrating keratoplasty. Xenotransplantation. 2017;24 doi: 10.1111/xen.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Miyagawa Y., Long C., Cooper D.K., Hara H. A comparison of three methods of decellularization of pig corneas to reduce immunogenicity. Int. J. Ophthalmol. 2014;7:587–593. doi: 10.3980/j.issn.2222-3959.2014.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Miyagawa Y., Long C., Ekser B., Walters E., Ramsoondar J., Ayares D., Tector A.J., Cooper D.K., Hara H. Expression of NeuGc on pig corneas and its potential significance in pig corneal xenotransplantation. Cornea. 2016;35:105–113. doi: 10.1097/ICO.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M.F., Crippin J., Sutton S., Netto G., McCormack J., Curiel T., Goldstein R.M., Newman J.T., Gonwa T.A., Banchereau J., Diamond L.E., Byrne G., Logan J., Klintmalm G.B. Liver allotransplantation after extracorporeal hepatic support with transgenic (hCD55/hCD59) porcine livers: clinical results and lack of pig-to-human transmission of the porcine endogenous retrovirus. Transplantation. 2000;69:272–280. doi: 10.1097/00007890-200001270-00013. [DOI] [PubMed] [Google Scholar]
- Li A., Pan Z., Jie Y., Sun Y., Luo F., Wang L. Comparison of immunogenicity and porcine-to-rhesus lamellar corneal xenografts survival between fresh preserved and dehydrated porcine corneas. Xenotransplantation. 2011;18:46–55. doi: 10.1111/j.1399-3089.2011.00626.x. [DOI] [PubMed] [Google Scholar]
- Li A., Zhang Y., Liu Y., Pan Z. Corneal xenotransplantation from pig to rhesus monkey: No signs of transmission of endogenous porcine retroviruses. Transplant. Proc. 2017;49:2209–2214. doi: 10.1016/j.transproceed.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Li Q., Wang H., Dai Z., Cao Y., Jin C. Preparation and biomechanical properties of an acellular porcine corneal stroma. Cornea. 2017;36:1343–1351. doi: 10.1097/ICO.0000000000001319. [DOI] [PubMed] [Google Scholar]
- Li S., Deng Y., Tian B., Huang H., Zhang H., Yang R., Zhong J., Wang B., Peng L., Yuan J. Healing characteristics of acellular porcine corneal stroma following therapeutic keratoplasty. Xenotransplantation. 2020;27 doi: 10.1111/xen.12566. [DOI] [PubMed] [Google Scholar]
- Li S., Li M., Gu L., Peng L., Deng Y., Zhong J., Wang B., Wang Q., Xiao Y., Yuan J. Risk factors influencing survival of acellular porcine corneal stroma in infectious keratitis: a prospective clinical study. J. Transl. Med. 2019;17:434. doi: 10.1186/s12967-019-02192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Waer M., Billiau A.D. Xenotransplantation: role of natural immunity. Transpl. Immunol. 2009;21:70–74. doi: 10.1016/j.trim.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Lim M.A., Kohli J., Bloom R.D. Immunosuppression for kidney transplantation: where are we now and where are we going? Transplant. Rev. 2017;31:10–17. doi: 10.1016/j.trre.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Lin X.C., Hui Y.N., Wang Y.S., Meng H., Zhang Y.J., Jin Y. Lamellar keratoplasty with a graft of lyophilized acellular porcine corneal stroma in the rabbit. Vet. Ophthalmol. 2008;11:61–66. doi: 10.1111/j.1463-5224.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- Lin Y., Zheng Q., Hua S., Meng Y., Chen W., Wang Y. Cross-linked decellularized porcine corneal graft for treating fungal keratitis. Sci. Rep. 2017;7:9955. doi: 10.1038/s41598-017-08207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang J., Zhang Y., Yin M., Miao S., Liang Q., Pan Z. The feasibility and efficacy of preparing porcine Descemet's membrane endothelial keratoplasty (DMEK) grafts by two techniques: an ex-vivo investigation for future xeno-DMEK. Xenotransplantation. 2018;25 doi: 10.1111/xen.12407. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang Y., Liang Q., Yan C., Wang L., Zhang J., Pan Z. Porcine endothelial grafts could survive for a long term without using systemic immunosuppressors: an investigation of feasibility and efficacy of xeno-Descemet's stripping automated endothelial keratoplasty from WZS-pig to rhesus monkey. Xenotransplantation. 2019;26 doi: 10.1111/xen.12433. [DOI] [PubMed] [Google Scholar]
- Liu Z., Hu W., He T., Dai Y., Hara H., Bottino R., Cooper D.K.C., Cai Z., Mou L. Pig-to-Primate islet xenotransplantation: past, present, and future. Cell Transplant. 2017;26:925–947. doi: 10.3727/096368917X694859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss M., Arends H., Winkler M., Przemeck M., Steinhoff G., Rensing S., Kaup F.J., Hedrich H.J., Winkler M.E., Martin U. Analysis of potential porcine endogenous retrovirus (PERV) transmission in a whole-organ xenotransplantation model without interfering microchimerism. Transpl. Int. 2001;14:31–37. doi: 10.1111/j.1432-2277.2001.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Luo H., Lu Y., Wu T., Zhang M., Zhang Y., Jin Y. Construction of tissue-engineered cornea composed of amniotic epithelial cells and acellular porcine cornea for treating corneal alkali burn. Biomaterials. 2013;34:6748–6759. doi: 10.1016/j.biomaterials.2013.05.045. [DOI] [PubMed] [Google Scholar]
- Ma D.H., Chen J.K., Zhang F., Lin K.Y., Yao J.Y., Yu J.S. Regulation of corneal angiogenesis in limbal stem cell deficiency. Prog. Retin. Eye Res. 2006;25:563–590. doi: 10.1016/j.preteyeres.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Magalhaes O.A., Marinho D.R., Kwitko S. Topical 0.03% tacrolimus preventing rejection in high-risk corneal transplantation: a cohort study. Br. J. Ophthalmol. 2013;97:1395–1398. doi: 10.1136/bjophthalmol-2013-303639. [DOI] [PubMed] [Google Scholar]
- Malinis M., Boucher H.W. Screening of donor and candidate prior to solid organ transplantation-guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019;33 doi: 10.1111/ctr.13548. Practice, A.S.T.I.D.C.o. [DOI] [PubMed] [Google Scholar]
- Martin D.E., Van Assche K., Dominguez-Gil B., Lopez-Fraga M., Garcia Gallont R., Muller E., Rondeau E., Capron A.M. A new edition of the Declaration of Istanbul: updated guidance to combat organ trafficking and transplant tourism worldwide. Kidney Int. 2019;95:757–759. doi: 10.1016/j.kint.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Martin U., Steinhoff G., Kiessig V., Chikobava M., Anssar M., Morschheuser T., Lapin B., Haverich A. Porcine endogenous retrovirus (PERV) was not transmitted from transplanted porcine endothelial cells to baboons in vivo. Transpl. Int. 1998;11:247–251. doi: 10.1007/s001470050136. [DOI] [PubMed] [Google Scholar]
- Mathews P.M., Lindsley K., Aldave A.J., Akpek E.K. Etiology of global corneal blindness and current practices of corneal transplantation: a focused review. Cornea. 2018;37:1198–1203. doi: 10.1097/ICO.0000000000001666. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Tan P., Baker J., Durbin K., Tomiya M., Azuma K., Doi M., Elliott R.B. Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant. Proc. 2014;46:1992–1995. doi: 10.1016/j.transproceed.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Tomiya M., Sawamoto O. Current status and future of clinical islet xenotransplantation. J. Diabetes. 2016;8:483–493. doi: 10.1111/1753-0407.12395. [DOI] [PubMed] [Google Scholar]
- Matthaei M., Sandhaeger H., Hermel M., Adler W., Jun A.S., Cursiefen C., Heindl L.M. Changing indications in penetrating keratoplasty: a systematic review of 34 Years of global reporting. Transplantation. 2017;101:1387–1399. doi: 10.1097/TP.0000000000001281. [DOI] [PubMed] [Google Scholar]
- Matthyssen S., Van den Bogerd B., Dhubhghaill S.N., Koppen C., Zakaria N. Corneal regeneration: a review of stromal replacements. Acta Biomater. 2018;69:31–41. doi: 10.1016/j.actbio.2018.01.023. [DOI] [PubMed] [Google Scholar]
- McGregor C.G.A., Takeuchi Y., Scobie L., Byrne G. PERVading strategies and infectious risk for clinical xenotransplantation. Xenotransplantation. 2018;25 doi: 10.1111/xen.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar P.B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- Mehta J.S., Kocaba V., Soh Y.Q. The future of keratoplasty: cell-based therapy, regenerative medicine, bioengineering keratoplasty, gene therapy. Curr. Opin. Ophthalmol. 2019;30:286–291. doi: 10.1097/ICU.0000000000000573. [DOI] [PubMed] [Google Scholar]
- Michel S.G., Madariaga M.L.L., Villani V., Shanmugarajah K. Current progress in xenotransplantation and organ bioengineering. Int. J. Surg. 2015;13:239–244. doi: 10.1016/j.ijsu.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Mondino B.J., Ratajczak H.V., Goldberg D.B., Schanzlin D.J., Brown S.I. Alternate and classical pathway components of complement in the normal cornea. Arch. Ophthalmol. 1980;98:346–349. doi: 10.1001/archopht.1980.01020030342023. [DOI] [PubMed] [Google Scholar]
- Morozov V.A., Wynyard S., Matsumoto S., Abalovich A., Denner J., Elliott R. No PERV transmission during a clinical trial of pig islet cell transplantation. Virus Res. 2017;227:34–40. doi: 10.1016/j.virusres.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Mueller N.J., Kuwaki K., Knosalla C., Dor F.J., Gollackner B., Wilkinson R.A., Arn S., Sachs D.H., Cooper D.K., Fishman J.A. Early weaning of piglets fails to exclude porcine lymphotropic herpesvirus. Xenotransplantation. 2005;12:59–62. doi: 10.1111/j.1399-3089.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- Mueller N.J., Sulling K., Gollackner B., Yamamoto S., Knosalla C., Wilkinson R.A., Kaur A., Sachs D.H., Yamada K., Cooper D.K., Patience C., Fishman J.A. Reduced efficacy of ganciclovir against porcine and baboon cytomegalovirus in pig-to-baboon xenotransplantation. Am. J. Transplant. 2003;3:1057–1064. doi: 10.1034/j.1600-6143.2003.00192.x. [DOI] [PubMed] [Google Scholar]
- Mulder A., Kardol M.J., Arn J.S., Eijsink C., Franke M.E., Schreuder G.M., Haasnoot G.W., Doxiadis, Sachs D.H., Smith D.M., Claas F.H. Human monoclonal HLA antibodies reveal interspecies crossreactive swine MHC class I epitopes relevant for xenotransplantation. Mol. Immunol. 2010;47:809–815. doi: 10.1016/j.molimm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Murray A.G., Khodadoust M.M., Pober J.S., Bothwell A.L. Porcine aortic endothelial cells activate human T cells: direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity. 1994;1:57–63. doi: 10.1016/1074-7613(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Inatomi T., Sotozono C., Koizumi N., Kinoshita S. Ocular surface reconstruction using stem cell and tissue engineering. Prog. Retin. Eye Res. 2016;51:187–207. doi: 10.1016/j.preteyeres.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Nellore A. Infections after xenotransplantation. Curr. Opin. Organ Transplant. 2018;23:628–632. doi: 10.1097/MOT.0000000000000580. [DOI] [PubMed] [Google Scholar]
- Niederkorn J.Y. Immune mechanisms of corneal allograft rejection. Curr. Eye Res. 2007;32:1005–1016. doi: 10.1080/02713680701767884. [DOI] [PubMed] [Google Scholar]
- Niederkorn J.Y., Larkin D.F. Immune privilege of corneal allografts. Ocul. Immunol. Inflamm. 2010;18:162–171. doi: 10.3109/09273948.2010.486100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn J.Y., Mellon J. Anterior chamber-associated immune deviation promotes corneal allograft survival. Invest. Ophthalmol. Vis. Sci. 1996;37:2700–2707. [PubMed] [Google Scholar]
- Niederkorn J.Y., Stevens C., Mellon J., Mayhew E. CD4+ T-cell-independent rejection of corneal allografts. Transplantation. 2006;81:1171–1178. doi: 10.1097/01.tp.0000203140.70742.cb. [DOI] [PubMed] [Google Scholar]
- Niederkorn J.Y., Stevens C., Mellon J., Mayhew E. Differential roles of CD8+ and CD8- T lymphocytes in corneal allograft rejection in 'high-risk' hosts. Am. J. Transplant. 2006;6:705–713. doi: 10.1111/j.1600-6143.2006.01237.x. [DOI] [PubMed] [Google Scholar]
- Nishida K. Tissue engineering of the cornea. Cornea. 2003;22:S28–S34. doi: 10.1097/00003226-200310001-00005. [DOI] [PubMed] [Google Scholar]
- Nishitai R., Ikai I., Shiotani T., Katsura N., Matsushita T., Yamanokuchi S., Matsuo K., Sugimoto S., Yamaoka Y. Absence of PERV infection in baboons after transgenic porcine liver perfusion. J. Surg. Res. 2005;124:45–51. doi: 10.1016/j.jss.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Niu D., Wei H.J., Lin L., George H., Wang T., Lee I.H., Zhao H.Y., Wang Y., Kan Y., Shrock E., Lesha E., Wang G., Luo Y., Qing Y., Jiao D., Zhao H., Zhou X., Wang S., Wei H., Guell M., Church G.M., Yang L. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357:1303–1307. doi: 10.1126/science.aan4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel L. Global regulatory requirements for xenotransplantation clinical trials. Xenotransplantation. 2012;19:71. doi: 10.1111/j.1399-3089.2012.00699.x. [DOI] [PubMed] [Google Scholar]
- Ochando J., Ordikhani F., Jordan S., Boros P., Thomson A.W. Tolerogenic dendritic cells in organ transplantation. Transpl. Int. 2020;33:113–127. doi: 10.1111/tri.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.Y., Kim M.K., Ko J.H., Lee H.J., Kim Y., Park C.S., Park C.G., Kim S.J., Wee W.R., Lee J.H. Acute cell-mediated rejection in orthotopic pig-to-mouse corneal xenotransplantation. Xenotransplantation. 2009;16:74–82. doi: 10.1111/j.1399-3089.2009.00514.x. [DOI] [PubMed] [Google Scholar]
- Oh J.Y., Kim M.K., Ko J.H., Lee H.J., Lee J.H., Wee W.R. Rat allogeneic mesenchymal stem cells did not prolong the survival of corneal xenograft in a pig-to-rat model. Vet. Ophthalmol. 2009;12(Suppl. 1):35–40. doi: 10.1111/j.1463-5224.2009.00724.x. [DOI] [PubMed] [Google Scholar]
- Oh J.Y., Kim M.K., Ko J.H., Lee H.J., Park C.G., Kim S.J., Wee W.R., Lee J.H. Histological differences in full-thickness vs. lamellar corneal pig-to-rabbit xenotransplantation. Vet. Ophthalmol. 2009;12:78–82. doi: 10.1111/j.1463-5224.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- Oh J.Y., Kim M.K., Lee H.J., Ko J.H., Kim Y., Park C.S., Kang H.J., Park C.G., Kim S.J., Lee J.H., Wee W.R. Complement depletion with cobra venom factor delays acute cell-mediated rejection in pig-to-mouse corneal xenotransplantation. Xenotransplantation. 2010;17:140–146. doi: 10.1111/j.1399-3089.2010.00574.x. [DOI] [PubMed] [Google Scholar]
- Oh J.Y., Kim M.K., Lee H.J., Ko J.H., Wee W.R., Lee J.H. Processing porcine cornea for biomedical applications. Tissue Eng. C Methods. 2009;15:635–645. doi: 10.1089/ten.TEC.2009.0022. [DOI] [PubMed] [Google Scholar]
- Oh J.Y., Kim M.K., Wee W.R. Lamellar corneal pig-to-rabbit xenotransplantation. Xenotransplantation. 2008;15:198–199. doi: 10.1111/j.1399-3089.2008.00469.x. [DOI] [PubMed] [Google Scholar]
- Olsen T., Sperling S. The swelling pressure of the human corneal stroma as determined by a new method. Exp. Eye Res. 1987;44:481–490. doi: 10.1016/s0014-4835(87)80159-8. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Huang Y.W. Coronavirus disease 2019 (COVID-19) outbreak: could pigs be vectors for human infections? Xenotransplantation. 2020;27 doi: 10.1111/xen.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oura T., Cosimi A.B., Kawai T. Chimerism-based tolerance in organ transplantation: preclinical and clinical studies. Clin. Exp. Immunol. 2017;189:190–196. doi: 10.1111/cei.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang K., Du L., Wu X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials. 2010;31:7257–7265. doi: 10.1016/j.biomaterials.2010.05.066. [DOI] [PubMed] [Google Scholar]
- Paradis K., Langford G., Long Z., Heneine W., Sandstrom P., Switzer W.M., Chapman L.E., Lockey C., Onions D., Otto E. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- Park C.G., Shin J.S., Min B.H., Kim H., Yeom S.C., Ahn C. Current status of xenotransplantation in South Korea. Xenotransplantation. 2019;26 doi: 10.1111/xen.12488. [DOI] [PubMed] [Google Scholar]
- Patience C., Patton G.S., Takeuchi Y., Weiss R.A., McClure M.O., Rydberg L., Breimer M.E. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet. 1998;352:699–701. doi: 10.1016/S0140-6736(98)04369-4. [DOI] [PubMed] [Google Scholar]
- Patience C., Takeuchi Y., Weiss R.A. Infection of human cells by an endogenous retrovirus of pigs. Nat. Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- Paunicka K.J., Mellon J., Robertson D., Petroll M., Brown J.R., Niederkorn J.Y. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am. J. Transplant. 2015;15:1490–1501. doi: 10.1111/ajt.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez B.C., Morris H.J., Hart R.T., Liu J. Finite element modeling of the viscoelastic responses of the eye during microvolumetric changes. J. Biomed. Sci. Eng. 2013;6:29–37. doi: 10.4236/jbise.2013.612A005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel J.M. Xenotransplantation makes a comeback. Nat. Biotechnol. 2016;34:3–4. doi: 10.1038/nbt0116-3. [DOI] [PubMed] [Google Scholar]
- Personett H.A., Laub M.R. Review of infectious disease prophylaxis in solid organ transplantation. Crit. Care Nurs. Q. 2017;40:383–398. doi: 10.1097/CNQ.0000000000000175. [DOI] [PubMed] [Google Scholar]
- Pindjakova J., Vitova A., Krulova M., Zajicova A., Filipec M., Holan V. Corneal rat-to-mouse xenotransplantation and the effects of anti-CD4 or anti-CD8 treatment on cytokine and nitric oxide production. Transpl. Int. 2005;18:854–862. doi: 10.1111/j.1432-2277.2005.00112.x. [DOI] [PubMed] [Google Scholar]
- Pitkin Z., Mullon C. Evidence of absence of porcine endogenous retrovirus (PERV) infection in patients treated with a bioartificial liver support system. Artif. Organs. 1999;23:829–833. doi: 10.1046/j.1525-1594.1999.06444.x. [DOI] [PubMed] [Google Scholar]
- Porth J.M., Deiotte E., Dunn M., Bashshur R. A review of the literature on the global epidemiology of corneal blindness. Cornea. 2019;38:1602–1609. doi: 10.1097/ICO.0000000000002122. [DOI] [PubMed] [Google Scholar]
- Princeteau M. Greffe renale. J Med Bordeaux. 1905;26:549. [Google Scholar]
- Qian Y., Dana M.R. Molecular mechanisms of immunity in corneal allotransplantation and xenotransplantation. Expet Rev. Mol. Med. 2001;3:1–21. doi: 10.1017/S1462399401003246. [DOI] [PubMed] [Google Scholar]
- Razonable R.R., Humar A. Cytomegalovirus in solid organ transplant recipients-guidelines of the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019;33 doi: 10.1111/ctr.13512. [DOI] [PubMed] [Google Scholar]
- Reemtsma K., McCracken B.H., Schlegel J.U., Pearl M.A., Pearce C.W., Dewitt C.W., Smith P.E., Hewitt R.L., Flinner R.L., Creech O., Jr. Renal heterotransplantation in man. Ann. Surg. 1964;160:384–410. doi: 10.1097/00000658-196409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders M.E.J., van Kooten C., Rabelink T.J., de Fijter J.W. Mesenchymal stromal cell therapy for solid organ transplantation. Transplantation. 2018;102:35–43. doi: 10.1097/TP.0000000000001879. [DOI] [PubMed] [Google Scholar]
- Reisinger F. Die keratoplastik, ein versuch zur enweiterund der augenheilkunde. Bayerische Annalem. 1824;1:207–215. [Google Scholar]
- Riau A.K., Liu Y.C., Yam G.H.F., Mehta J.S. Stromal keratophakia: corneal inlay implantation. Prog. Retin. Eye Res. 2020;75:100780. doi: 10.1016/j.preteyeres.2019.100780. [DOI] [PubMed] [Google Scholar]
- Richmond O., Cecere T.E., Erdogan E., Meng X.J., Pineyro P., Subramaniam S., Todd S.M., LeRoith T. PD-L1 expression is increased in monocyte derived dendritic cells in response to porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus infections. Vet. Immunol. Immunopathol. 2015;168:24–29. doi: 10.1016/j.vetimm.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Ristov J., Espie P., Ulrich P., Sickert D., Flandre T., Dimitrova M., Muller-Ristig D., Weider D., Robert G., Schmutz P., Greutmann B., Cordoba-Castro F., Schneider M.A., Warncke M., Kolbinger F., Cote S., Heusser C., Bruns C., Rush J.S. Characterization of the in vitro and in vivo properties of CFZ533, a blocking and non-depleting anti-CD40 monoclonal antibody. Am. J. Transplant. 2018;18:2895–2904. doi: 10.1111/ajt.14872. [DOI] [PubMed] [Google Scholar]
- Ritzhaupt A., Van Der Laan L.J., Salomon D.R., Wilson C.A. Porcine endogenous retrovirus infects but does not replicate in nonhuman primate primary cells and cell lines. J. Virol. 2002;76:11312–11320. doi: 10.1128/JVI.76.22.11312-11320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues E.B., Farah M.E., Maia M., Penha F.M., Regatieri C., Melo G.B., Pinheiro M.M., Zanetti C.R. Therapeutic monoclonal antibodies in ophthalmology. Prog. Retin. Eye Res. 2009;28:117–144. doi: 10.1016/j.preteyeres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Romano M., Fanelli G., Albany C.J., Giganti G., Lombardi G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front. Immunol. 2019;10:43. doi: 10.3389/fimmu.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J.R., Howell D.N., Sanfilippo F.P. Characteristics of corneal xenograft rejection in a discordant species combination. Invest. Ophthalmol. Vis. Sci. 1993;34:2469–2476. [PubMed] [Google Scholar]
- Ross J.R., Sanfilippo F.P., Howell D.N. Histopathologic features of rejecting orthotopic corneal xenografts. Curr. Eye Res. 1994;13:725–730. doi: 10.3109/02713689409047006. [DOI] [PubMed] [Google Scholar]
- Sachs D.H. Transplantation tolerance through mixed chimerism: from allo to xeno. Xenotransplantation. 2018;25 doi: 10.1111/xen.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S., Funamoto S., Hashimoto Y., Kimura T., Honda T., Hattori S., Kobayashi H., Kishida A., Mochizuki M. In vivo evaluation of a novel scaffold for artificial corneas prepared by using ultrahigh hydrostatic pressure to decellularize porcine corneas. Mol. Vis. 2009;15:2022–2028. [PMC free article] [PubMed] [Google Scholar]
- Schroder P.M., Fitch Z.W., Schmitz R., Choi A.Y., Kwun J., Knechtle S.J. The past, present, and future of costimulation blockade in organ transplantation. Curr. Opin. Organ Transplant. 2019;24:391–401. doi: 10.1097/MOT.0000000000000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkopff J., Schlereth S.L., Berger M., Bredow L., Birnbaum F., Bohringer D., Reinhard T. NK cell depletion delays corneal allograft rejection in baby rats. Mol. Vis. 2010;16:1928–1935. [PMC free article] [PubMed] [Google Scholar]
- Scobie L., Padler-Karavani V., Le Bas-Bernardet S., Crossan C., Blaha J., Matouskova M., Hector R.D., Cozzi E., Vanhove B., Charreau B., Blancho G., Bourdais L., Tallacchini M., Ribes J.M., Yu H., Chen X., Kracikova J., Broz L., Hejnar J., Vesely P., Takeuchi Y., Varki A., Soulillou J.P. Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. J. Immunol. 2013;191:2907–2915. doi: 10.4049/jimmunol.1301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie L., Takeuchi Y. Porcine endogenous retrovirus and other viruses in xenotransplantation. Curr. Opin. Organ Transplant. 2009;14:175–179. doi: 10.1097/mot.0b013e328327984d. [DOI] [PubMed] [Google Scholar]
- Sedlakova K., Muckersie E., Robertson M., Filipec M., Forrester J.V. FTY720 in corneal concordant xenotransplantation. Transplantation. 2005;79:297–303. doi: 10.1097/01.tp.0000151005.37985.de. [DOI] [PubMed] [Google Scholar]
- Seitz B., Langenbucher A., Kuchle M., Naumann G.O. Impact of graft diameter on corneal power and the regularity of postkeratoplasty astigmatism before and after suture removal. Ophthalmology. 2003;110:2162–2167. doi: 10.1016/S0161-6420(03)00659-6. [DOI] [PubMed] [Google Scholar]
- Sgroi A., Buhler L.H., Morel P., Sykes M., Noel L. International human xenotransplantation inventory. Transplantation. 2010;90:597–603. doi: 10.1097/TP.0b013e3181eb2e8c. [DOI] [PubMed] [Google Scholar]
- Shalimov A.A., Turchin I.S., Lifshits Iu Z., Tishchenko A.V., Kozhara S.P., Usenko A. Experience in xenografts of pancreatic islet cell cultures after surgical treatment of chronic pancreatitis. Klin. Khir. (Kiev) 1990;1–2 [PubMed] [Google Scholar]
- Shao Y., Tang J., Zhou Y., Qu Y., He H., Liu Q., Tan G., Li W., Liu Z. A novel method in preparation of acellularporcine corneal stroma tissue for lamellar keratoplasty. Am. J. Transl. Res. 2015;7:2612–2629. [PMC free article] [PubMed] [Google Scholar]
- Sharifi R., Yang Y., Adibnia Y., Dohlman C.H., Chodosh J., Gonzalez-Andrades M. Finding an optimal corneal xenograft using comparative analysis of corneal matrix proteins across species. Sci. Rep. 2019;9:1876. doi: 10.1038/s41598-018-38342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W.Y., Xie L.X. Focus on the clinical application of the first artificial bioengineered cornea in China. Zhonghua Yan Ke Za Zhi. 2016;52:161–163. doi: 10.3760/cma.j.issn.0412-4081.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Shi Y., Bikkuzin T., Song Z., Jin X., Jin H., Li X., Zhang H. Comprehensive evaluation of decellularized porcine corneal after clinical transplantation. Xenotransplantation. 2017;24 doi: 10.1111/xen.12338. [DOI] [PubMed] [Google Scholar]
- Shimazaki J., Den S., Omoto M., Satake Y., Shimmura S., Tsubota K. Prospective, randomized study of the efficacy of systemic cyclosporine in high-risk corneal transplantation. Am. J. Ophthalmol. 2011;152:33–39. doi: 10.1016/j.ajo.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Shimoda M., Matsumoto S. Update regarding xenotransplantation in Japan. Xenotransplantation. 2019;26 doi: 10.1111/xen.12491. [DOI] [PubMed] [Google Scholar]
- Smetanka C., Cooper D.K. The ethics debate in relation to xenotransplantation. Rev. Sci. Tech. 2005;24:335–342. [PubMed] [Google Scholar]
- Snellingen T., Rao G.N., Shrestha J.K., Huq F., Cheng H. Quantitative and morphological characteristics of the human corneal endothelium in relation to age, gender, and ethnicity in cataract populations of South Asia. Cornea. 2001;20:55–58. doi: 10.1097/00003226-200101000-00011. [DOI] [PubMed] [Google Scholar]
- Sobbrio P., Jorqui M. An overview of the role of society and risk in xenotransplantation. Xenotransplantation. 2014;21:523–532. doi: 10.1111/xen.12120. [DOI] [PubMed] [Google Scholar]
- Sohn J.H., Kaplan H.J., Suk H.J., Bora P.S., Bora N.S. Chronic low level complement activation within the eye is controlled by intraocular complement regulatory proteins. Invest. Ophthalmol. Vis. Sci. 2000;41:3492–3502. [PMC free article] [PubMed] [Google Scholar]
- Sohn J.H., Kaplan H.J., Suk H.J., Bora P.S., Bora N.S. Complement regulatory activity of normal human intraocular fluid is mediated by MCP, DAF, and CD59. Invest. Ophthalmol. Vis. Sci. 2000;41:4195–4202. [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondergaard A.P., Ivarsen A., Hjortdal J. Corneal resistance to shear force after UVA-riboflavin cross-linking. Invest. Ophthalmol. Vis. Sci. 2013;54:5059–5069. doi: 10.1167/iovs.12-10710. [DOI] [PubMed] [Google Scholar]
- Sondergaard A.P., Ivarsen A., Hjortdal J. Reduction of stromal swelling pressure after UVA-riboflavin cross-linking. Invest. Ophthalmol. Vis. Sci. 2013;54:1625–1634. doi: 10.1167/iovs.12-10346. [DOI] [PubMed] [Google Scholar]
- Soomsawasdi B., Romayanonda N., Bhadrakom S. Heterokeratoplasty using gibbon donor cornea. Trans. Asia Pacific Acad. Ophthalmol. 1964;2:251–261. [Google Scholar]
- Spillman M.A., Sade R.M. Clinical trials of xenotransplantation: waiver of the right to withdraw from a clinical trial should be required. J. Law Med. Ethics. 2007;35:265–272. doi: 10.1111/j.1748-720X.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- Sprangers B., Waer M., Billiau A.D. Xenotransplantation: where are we in 2008? Kidney Int. 2008;74:14–21. doi: 10.1038/ki.2008.135. [DOI] [PubMed] [Google Scholar]
- Starzl T.E., Fung J., Tzakis A., Todo S., Demetris A.J., Marino I.R., Doyle H., Zeevi A., Warty V., Michaels M., et al. Baboon-to-human liver transplantation. Lancet. 1993;341:65–71. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steering Committee of the Istanbul S. Organ trafficking and transplant tourism and commercialism: the Declaration of Istanbul. Lancet. 2008;372:5–6. doi: 10.1016/S0140-6736(08)60967-8. [DOI] [PubMed] [Google Scholar]
- Stern J.H., Tian Y., Funderburgh J., Pellegrini G., Zhang K., Goldberg J.L., Ali R.R., Young M., Xie Y., Temple S. Regenerating eye tissues to preserve and restore vision. Cell Stem Cell. 2018;22:834–849. doi: 10.1016/j.stem.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J.W. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Streilein J.W., Bradley D., Sano Y., Sonoda Y. Immunosuppressive properties of tissues obtained from eyes with experimentally manipulated corneas. Invest. Ophthalmol. Vis. Sci. 1996;37:413–424. [PubMed] [Google Scholar]
- Streilein J.W., Okamoto S., Sano Y., Taylor A.W. Neural control of ocular immune privilege. Ann. N. Y. Acad. Sci. 2000;917:297–306. doi: 10.1111/j.1749-6632.2000.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Switzer W.M., Michler R.E., Shanmugam V., Matthews A., Hussain A.I., Wright A., Sandstrom P., Chapman L.E., Weber C., Safley S., Denny R.R., Navarro A., Evans V., Norin A.J., Kwiatkowski P., Heneine W. Lack of cross-species transmission of porcine endogenous retrovirus infection to nonhuman primate recipients of porcine cells, tissues, or organs. Transplantation. 2001;71:959–965. doi: 10.1097/00007890-200104150-00022. [DOI] [PubMed] [Google Scholar]
- Sykes M., d'Apice A., Sandrin M., Committee I.X.A.E. Position paper of the ethics committee of the international xenotransplantation association. Transplantation. 2004;78:1101–1107. doi: 10.1097/01.tp.0000142886.27906.3e. [DOI] [PubMed] [Google Scholar]
- Tacke S.J., Specke V., Denner J. Differences in release and determination of subtype of porcine endogenous retroviruses produced by stimulated normal pig blood cells. Intervirology. 2003;46:17–24. doi: 10.1159/000068120. [DOI] [PubMed] [Google Scholar]
- Tan D.T., Dart J.K., Holland E.J., Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sonoda K., Streilein J.W. Acute rejection of orthotopic corneal xenografts in mice depends on CD4(+) T cells and self-antigen-presenting cells. Invest. Ophthalmol. Vis. Sci. 2001;42:2878–2884. [PubMed] [Google Scholar]
- Tanaka K., Yamada J., Streilein J.W. Xenoreactive CD4+ T cells and acute rejection of orthotopic Guinea pig corneas in mice. Invest. Ophthalmol. Vis. Sci. 2000;41:1827–1832. [PubMed] [Google Scholar]
- Teran-Escandon D., Teran-Ortiz L., Ormsby-Jenkins C., Evia-Viscarra M.L., White D.J., Valdes-Gonzalez-Salas R. Psychosocial aspects of xenotransplantation: survey in adolescent recipients of porcine islet cells. Transplant. Proc. 2005;37:521–524. doi: 10.1016/j.transproceed.2005.01.001. [DOI] [PubMed] [Google Scholar]
- TGA D.o.H. 2011. Therapeutic Goods Order No. 87.https://www.legislation.gov.au/Details/F2011L01493 Available at: accessed 20 May 2020. [Google Scholar]
- TGA D.o.H. 2017. Therapeutic Goods (Manufacturing Principles) Determination 2018.https://www.legislation.gov.au/Details/F2017L01574 Available at: accessed 20 May 2020. [Google Scholar]
- The European Parliament and of the Council . 2006. Commission Directive 2006/17/EC of 8 February 2006 Implementing Directive 2004/23/EC.http://www.legislation.gov.uk/eudr/2006/17/introduction/adopted accessed 20 May 2020. [Google Scholar]
- The European Parliament and of the Council . 2007. REGULATION (EC) No 1394/2007.https://www.legislation.gov.uk/eur/2007/1394/contents accessed 20 May 2020. [Google Scholar]
- The European Parliament and of the Council . 2014. Clinical Trial Regulation EU No 536/2014.https://ec.europa.eu/health/human-use/clinical-trials/regulation_en accessed 20 May 2020. [Google Scholar]
- The Global Alliance of Eye Bank Associations Inc The Barcelona principles: an agreement on the use of human donated tissue for ocular transplantation, research, and future technologies(c) Cornea. 2018;37:1213–1217. doi: 10.1097/ICO.0000000000001675. [DOI] [PubMed] [Google Scholar]
- Thompson P., Badell I.R., Lowe M., Cano J., Song M., Leopardi F., Avila J., Ruhil R., Strobert E., Korbutt G., Rayat G., Rajotte R., Iwakoshi N., Larsen C.P., Kirk A.D. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am. J. Transplant. 2011;11:2593–2602. doi: 10.1111/j.1600-6143.2011.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P., Badell I.R., Lowe M., Turner A., Cano J., Avila J., Azimzadeh A., Cheng X., Pierson R.N., 3rd, Johnson B., Robertson J., Song M., Leopardi F., Strobert E., Korbutt G., Rayat G., Rajotte R., Larsen C.P., Kirk A.D. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. Am. J. Transplant. 2012;12:1765–1775. doi: 10.1111/j.1600-6143.2012.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure T. The Falun Gong, organ transplantation, the holocaust and ourselves. J. R. Soc. Med. 2007;100:119–121. doi: 10.1258/jrsm.100.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchyn M., Marushchak M., Krynytska I., Klishch I. Clinical efficacy of therapeutic keratoplasty using corneal xenografts in patients with corneal ulcers. Rom. J. Ophthalmol. 2019;63:257–263. [PMC free article] [PubMed] [Google Scholar]
- Vabres B., Le Bas-Bernardet S., Riochet D., Cherel Y., Minault D., Hervouet J., Ducournau Y., Moreau A., Daguin V., Coulon F., Pallier A., Brouard S., Robson S.C., Nottle M.B., Cowan P.J., Venturi E., Mermillod P., Brachet P., Galli C., Lagutina I., Duchi R., Bach J.M., Blancho G., Soulillou J.P., Vanhove B. hCTLA4-Ig transgene expression in keratocytes modulates rejection of corneal xenografts in a pig to non-human primate anterior lamellar keratoplasty model. Xenotransplantation. 2014;21:431–443. doi: 10.1111/xen.12107. [DOI] [PubMed] [Google Scholar]
- Valdes-Gonzalez R., Dorantes L.M., Bracho-Blanchet E., Rodriguez-Ventura A., White D.J. No evidence of porcine endogenous retrovirus in patients with type 1 diabetes after long-term porcine islet xenotransplantation. J. Med. Virol. 2010;82:331–334. doi: 10.1002/jmv.21655. [DOI] [PubMed] [Google Scholar]
- Valdes-Gonzalez R.A., Dorantes L.M., Garibay G.N., Bracho-Blanchet E., Mendez A.J., Davila-Perez R., Elliott R.B., Teran L., White D.J. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur. J. Endocrinol. 2005;153:419–427. doi: 10.1530/eje.1.01982. [DOI] [PubMed] [Google Scholar]
- van der Windt D.J., Bottino R., Kumar G., Wijkstrom M., Hara H., Ezzelarab M., Ekser B., Phelps C., Murase N., Casu A., Ayares D., Lakkis F.G., Trucco M., Cooper D.K. Clinical islet xenotransplantation: how close are we? Diabetes. 2012;61:3046–3055. doi: 10.2337/db12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Essen T.H., Roelen D.L., Williams K.A., Jager M.J. Matching for Human Leukocyte Antigens (HLA) in corneal transplantation - to do or not to do. Prog. Retin. Eye Res. 2015;46:84–110. doi: 10.1016/j.preteyeres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Van Horn D.L., Sendele D.D., Seideman S., Buco P.J. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest. Ophthalmol. Vis. Sci. 1977;16:597–613. [PubMed] [Google Scholar]
- Vassileva P.I., Hergeldzhieva T.G. Avastin use in high risk corneal transplantation. Graefes Arch. Clin. Exp. Ophthalmol. 2009;247:1701–1706. doi: 10.1007/s00417-009-1170-y. [DOI] [PubMed] [Google Scholar]
- Vellara H.R., Patel D.V. Biomechanical properties of the keratoconic cornea: a review. Clin. Exp. Optom. 2015;98:31–38. doi: 10.1111/cxo.12211. [DOI] [PubMed] [Google Scholar]
- Vincenti F., Larsen C., Durrbach A., Wekerle T., Nashan B., Blancho G., Lang P., Grinyo J., Halloran P.F., Solez K., Hagerty D., Levy E., Zhou W., Natarajan K., Charpentier B., Belatacept Study G. Costimulation blockade with belatacept in renal transplantation. N. Engl. J. Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- Visvanathan S., Daniluk S., Ptaszynski R., Muller-Ladner U., Ramanujam M., Rosenstock B., Eleftheraki A.G., Vinisko R., Petrikova A., Kellner H., Dokoupilova E., Kwiatkowska B., Alten R., Schwabe C., Baum P., Joseph D., Fine J.S., Padula S.J., Steffgen J. Effects of BI 655064, an antagonistic anti-CD40 antibody, on clinical and biomarker variables in patients with active rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase IIa study. Ann. Rheum. Dis. 2019;78:754–760. doi: 10.1136/annrheumdis-2018-214729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. A pilot trial with pig-to-man islet transplantation at the 3rd Xiang-Ya Hospital of the Central South University in Changsha. Xenotransplantation. 2007;14 358-358. [Google Scholar]
- Wang W., Mo Z., Ye B., Hu P., Liu S., Yi S. A clinical trial of xenotransplantation of neonatal pig islets for diabetic patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:1134–1140. doi: 10.3969/j.issn.1672-7347.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lei T., Wei L., Du S., Girani L., Deng S. Xenotransplantation in China: present status. Xenotransplantation. 2019;26 doi: 10.1111/xen.12490. [DOI] [PubMed] [Google Scholar]
- Wen J., Chan R.H., Yau S.C., He R.L., Yau S.S. K-mer natural vector and its application to the phylogenetic analysis of genetic sequences. Gene. 2014;546:25–34. doi: 10.1016/j.gene.2014.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2001. WHO Guidance on Xenogeneic Infection/disease Surveillance and Response: a Strategy for International Cooperation and Coordination.http://whqlibdoc.who.int/hq/2001/WHO_CDS_CSR_EPH_2001.2.pdf No. WHO/CDS/CSR/EPH/2001.2. accessed 20 May 2020. [Google Scholar]
- WHO . 2004. Fifty-seventh World Health Assembly.http://www.who.int/gb/ebwha/pdf_files/WHA57/A57_R18-en.pdf Agenda Item 12.14, May 22, 2004. Resolution 57.18. accessed 20 May 2020. [PubMed] [Google Scholar]
- WHO . 2005. Statement from the Xenotransplantation Advisory Consultation.http://www.who.int/transplantation/XenoEnglish.pdf Geneva, 18-20 April 2005. accessed 20 May 2020. [Google Scholar]
- WHO First WHO global consultation on regulatory requirements for xenotransplantation clinical trials: Changsha, China, 19-21 november 2008. The Changsha Communique. Xenotransplantation. 2009;16:61–63. doi: 10.1111/j.1399-3089.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- WHO . 2011. Second WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials.http://www.who.int/transplantation/xeno/report2nd_global_consultation_xtx.pdf?ua=1 accessed 20 May 2020. [Google Scholar]
- Wijkstrom M., Iwase H., Paris W., Hara H., Ezzelarab M., Cooper D.K. Renal xenotransplantation: experimental progress and clinical prospects. Kidney Int. 2017;91:790–796. doi: 10.1016/j.kint.2016.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.H., Kam K.W., Chen L.J., Young A.L. Corneal blindness and current major treatment concern-graft scarcity. Int. J. Ophthalmol. 2017;10:1154–1162. doi: 10.18240/ijo.2017.07.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Zhou Y., Li N., Huang M., Duan H., Ge J., Xiang P., Wang Z. The use of phospholipase A(2) to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials. 2009;30:3513–3522. doi: 10.1016/j.biomaterials.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Wynyard S., Nathu D., Garkavenko O., Denner J., Elliott R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation. 2014;21:309–323. doi: 10.1111/xen.12102. [DOI] [PubMed] [Google Scholar]
- Xu H., Zhang X., Mannon R.B., Kirk A.D. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. J. Clin. Invest. 2006;116:769–774. doi: 10.1172/JCI27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.G., Xu Y.S., Huang C., Feng Y., Li Y., Wang W. Development of a rabbit corneal equivalent using an acellular corneal matrix of a porcine substrate. Mol. Vis. 2008;14:2180–2189. [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Tasaki M., Sekijima M., Wilkinson R.A., Villani V., Moran S.G., Cormack T.A., Hanekamp I.M., Hawley R.J., Arn J.S., Fishman J.A., Shimizu A., Sachs D.H. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig to baboon xenotransplantation model. Transplantation. 2014;98:411–418. doi: 10.1097/TP.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Yazawa K., Shimizu A., Iwanaga T., Hisashi Y., Nuhn M., O'Malley P., Nobori S., Vagefi P.A., Patience C., Fishman J., Cooper D.K., Hawley R.J., Greenstein J., Schuurman H.J., Awwad M., Sykes M., Sachs D.H. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat. Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- Yamagami S., Isobe M., Yamagami H., Hori J., Tsuru T. Mechanism of concordant corneal xenograft rejection in mice: synergistic effects of anti-leukocyte function-associated antigen-1 monoclonal antibody and FK506. Transplantation. 1997;64:42–48. doi: 10.1097/00007890-199707150-00009. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Hamrah P., Shimazaki J. Bilateral alterations in corneal nerves, dendritic cells, and tear cytokine levels in ocular surface disease. Cornea. 2016;35(Suppl. 1):S65–S70. doi: 10.1097/ICO.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Guell M., Niu D., George H., Lesha E., Grishin D., Aach J., Shrock E., Xu W., Poci J., Cortazio R., Wilkinson R.A., Fishman J.A., Church G. Genome-wide inactivation of porcine endogenous retroviruses (PERVs) Science. 2015;350:1101–1104. doi: 10.1126/science.aad1191. [DOI] [PubMed] [Google Scholar]
- Yoeruek E., Bayyoud T., Maurus C., Hofmann J., Spitzer M.S., Bartz-Schmidt K.U., Szurman P. Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts. Acta Ophthalmol. 2012;90:e125–131. doi: 10.1111/j.1755-3768.2011.02261.x. [DOI] [PubMed] [Google Scholar]
- Yoeruek E., Bayyoud T., Maurus C., Hofmann J., Spitzer M.S., Bartz-Schmidt K.U., Szurman P. Reconstruction of corneal stroma with decellularized porcine xenografts in a rabbit model. Acta Ophthalmol. 2012;90:e206–210. doi: 10.1111/j.1755-3768.2011.02300.x. [DOI] [PubMed] [Google Scholar]
- Yoon C.H., Choi S.H., Choi H.J., Lee H.J., Kang H.J., Kim J.M., Park C.G., Choi K., Kim H., Ahn C., Kim M.K. Long-term survival of full-thickness corneal xenografts from alpha1,3-galactosyltransferase gene-knockout miniature pigs in non-human primates. Xenotransplantation. 2020;27 doi: 10.1111/xen.12559. [DOI] [PubMed] [Google Scholar]
- Yoon C.H., Choi S.H., Lee H.J., Kang H.J., Kim M.K. Predictive biomarkers for graft rejection in pig-to-non-human primate corneal xenotransplantation. Xenotransplantation. 2019;26 doi: 10.1111/xen.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York N.J., Tinley C. Corneal donations in South Africa: a 15-year review. S. Afr. Med. J. 2017;107:697–701. doi: 10.7196/SAMJ.2017.v107i8.12482. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Yang J., Huang K., Lee Z., Lee X. A comparison of biomechanical properties between human and porcine cornea. J. Biomech. 2001;34:533–537. doi: 10.1016/s0021-9290(00)00219-0. [DOI] [PubMed] [Google Scholar]
- Zhang C., Nie X., Hu D., Liu Y., Deng Z., Dong R., Zhang Y., Jin Y. Survival and integration of tissue-engineered corneal stroma in a model of corneal ulcer. Cell Tissue Res. 2007;329:249–257. doi: 10.1007/s00441-007-0419-1. [DOI] [PubMed] [Google Scholar]
- Zhang M.C., Liu X., Jin Y., Jiang D.L., Wei X.S., Xie H.T. Lamellar keratoplasty treatment of fungal corneal ulcers with acellular porcine corneal stroma. Am. J. Transplant. 2015;15:1068–1075. doi: 10.1111/ajt.13096. [DOI] [PubMed] [Google Scholar]
- Zheng J., Huang X., Zhang Y., Wang Y., Qin Q., Lin L., Jin X., Lam C., Zhang J. Short-term results of acellular porcine corneal stroma keratoplasty for herpes simplex keratitis. Xenotransplantation. 2019;26 doi: 10.1111/xen.12509. [DOI] [PubMed] [Google Scholar]
- Zheng M.H., Chen J., Kirilak Y., Willers C., Xu J., Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2005;73:61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- Zheng X., Zhang D., Li S., Zhang J., Zheng J., Du L., Gao J. An experimental study of femto-laser in assisting xenograft acellular cornea matrix lens transplantation. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2018;24:5208–5215. doi: 10.12659/MSM.909294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiqiang P., Cun S., Ying J., Ningli W., Li W. WZS-pig is a potential donor alternative in corneal xenotransplantation. Xenotransplantation. 2007;14:603–611. doi: 10.1111/j.1399-3089.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- Zhou H., Hara H., Cooper D.K.C. The complex functioning of the complement system in xenotransplantation. Xenotransplantation. 2019;26 doi: 10.1111/xen.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wu Z., Ge J., Wan P., Li N., Xiang P., Gao Q., Wang Z. Development and characterization of acellular porcine corneal matrix using sodium dodecylsulfate. Cornea. 2011;30:73–82. doi: 10.1097/ICO.0b013e3181dc8184. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus I., Research T. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirm E.K. Eine erfolgreiche totale Keratoplastik (A successful total keratoplasty) Refract. Corneal Surg. 1989;5:258–261. 1906. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.