Abstract
Advanced parental age is a well-replicated risk factor for autism spectrum disorder (ASD), a neurodevelopmental condition with a complex and not well-defined etiology. We sought to determine parental age associations with ASD-related outcomes in subjects at high familial risk for ASD. A total of 397 younger siblings of a child with ASD, drawn from existing prospective high familial risk cohorts, were included in these analyses. Overall, we did not observe significant associations of advanced parental age with clinical ASD diagnosis, Social Responsiveness Scale, or Vineland Adaptive Behavior Scales scores. Instead, increased odds of ASD were found with paternal age < 30 years (adjusted odds ratio [AOR] = 2.83 and 95% confidence intervals [CI] = 1.14–7.02). Likewise, younger age (<30 years) for both parents was associated with decreases in Mullen Scales of Early Learning early learning composite (MSEL-ELC) scores (adjusted β = −9.62, 95% CI = −17.1 to −2.15). We also found significant increases in cognitive functioning based on MSEL-ELC scores with increasing paternal age (adjusted β associated with a 10-year increase in paternal age = 5.51, 95% CI = 0.70–10.3). Results suggest the potential for a different relationship between parental age and ASD-related outcomes in families with elevated ASD risk than has been observed in general population samples.
Keywords: parental age, autism, autism-related traits, high familial risk
Lay Summary:
Previous work suggests that older parents have a greater likelihood of having a child with autism. We investigated this relationship in the younger siblings of families who already had a child with autism. In this setting, we found a higher likelihood of autism, as well as poorer cognitive scores, in the siblings with younger fathers, and higher cognitive scores in the siblings with older parents. These results suggest that parental age associations may differ based on children’s familial risk for autism.
Introduction
Advanced parental age is one of the most consistently identified perinatal risk factors for autism spectrum disorder (ASD), a complex neurodevelopmental condition with a multifactorial etiology [Lyall et al., 2017]. Though results are not uniform across studies [Wu et al., 2017], the literature on the whole supports increased risk of ASD with both older maternal and paternal ages independently [Shelton, Tancredi, & Hertz-Picciotto, 2010]. However, it is not known whether the association differs in the presence of increased familial risk. One prior study, conducted in families who already had one child with ASD from the Baby Siblings Research Consortium (BSRC), reported no association with maternal age but suggested an association between younger paternal age and ASD risk among siblings of children with ASD [Ozonoff et al., 2011]. This finding suggests that the parental age-ASD association could differ in families with increased recurrence risk for ASD, though existing evidence is limited.
A recent meta-analysis examining parental age and ASD [Wu et al., 2017], including nearly 30 studies, found increased risk of approximately 40% and 50% for the oldest maternal and paternal age categories, respectively, as well as decreased risk of 10% and 20% for the youngest maternal and paternal ages, as compared to mid-aged referent categories. Most studies observed increases in risk above maternal age of 35 [Sandin et al., 2012; Wu et al., 2017], and above paternal age of 40 [Hultman, Sandin, Levine, Lichtenstein, & Reichenberg, 2011]. Associations with younger parental age, or U-shaped relationships, have been reported with less consistency [Idring et al., 2014; Sandin et al., 2016]. There have also been suggestions of combined effects of maternal and paternal age [Sandin et al., 2016]. Mechanisms underlying these relationships are not well understood, though epigenetic modification or DNA damage due to aging, confounding or mediation by social determinants of reproductive age, and/or mediation by certain pregnancy complications are suspected [Lee & McGrath, 2015; Sandin et al., 2016]. Potential differences in associations with advanced parental age by families at low, general, or high familial risk may relate to these mechanisms.
Beyond the well-documented associations with chromosomal disorders like Down syndrome, parental age has also been associated with other conditions with relevance to ASD [de Kluiver, Buizer-Voskamp, Dolan, & Boomsma, 2017; McGrath et al., 2014], though patterns of associations may differ. For example, studies have reported a nonlinear association between paternal age and offspring verbal IQ [Gajos & Beaver, 2017], increased risk of child intellectual disability with younger mothers [McGrath et al., 2014], and increased risk of attention deficit hyper-activity disorder and Tourette’s/chronic tic disorder with younger parents [Janecka et al., 2019]. Prior work examining associations with quantitatively measured ASD-related traits is limited. However, consideration of such traits may reveal associations with subclinical deficits relevant to a larger proportion of the population than those for a single categorical diagnostic outcome.
In this study, we therefore examined the association between parental age and child ASD-related outcomes in an ASD high-familial risk setting, in order to shed light on whether associations differ in such families, and/or if associations extend across broader ASD-related outcomes assessed on quantitative scales. We used data available from prospective cohorts of younger siblings of a child with confirmed ASD in order to assess these relationships.
Methods
Study Population
Participants for this analysis are drawn from the ASD-enriched risk (ASD-ER) cohort, itself a “cohort of cohorts” including participants drawn from studies designed to examine early life factors associated with ASD and other neurodevelopmental outcomes in children at high familial-risk for autism. These studies enrolled the younger siblings, or pregnant mothers, of children already given an ASD diagnosis. This design capitalizes on the higher risk of ASD in younger siblings of a child with ASD [Newschaffer et al., 2012; Ozonoff et al., 2011] to efficiently enable prospective investigation of ASD. ASD-ER was formed for purposes of participation in the NIH-funded Environmental Influences on Child Health Outcomes (ECHO) program, which is a large collaborative effort to study environmental risks for a range of child outcomes [Blackwell, Wakschlag, Gershon, & Cella, 2018]. For analyses here, one younger sibling per family was included.
Participants included in ASD-ER were originally enrolled through the Early Autism Risk Longitudinal Investigation (EARLI) [Newschaffer et al., 2012], Markers of Autism Risk in Babies-Learning Early Signs (MARBLES) [Hertz-Picciotto et al., 2018], the Infant Brain Imaging Study (IBIS) [Hazlett et al., 2012; Wolff et al., 2012], and select sites participating in the Baby Siblings Research Consortium [McDonald et al., 2019; Messinger et al., 2013; Ozonoff et al., 2011, 2015] (BSRC; including UC Davis, University of Washington, Kennedy Krieger Institute, and University of Miami). Details of each of these studies have been published elsewhere. Selected enrollment features and sites included in ASD-ER are provided in Supporting Information Table 1. Briefly, EARLI and MARBLES enrolled pregnant mothers who already had given birth to a child with autism, and followed that mother through her subsequent pregnancy until the child was 3 years old. EARLI families were recruited at four sites. MARBLES families were recruited from UC Davis in the Sacramento, CA area. Both studies confirmed the proband/older sibling ASD diagnosis according to expert clinician evaluation. IBIS is a longitudinal brain imaging study network that includes four clinical data collection sites and two image processing sites. Infants were enrolled at 6 months and evaluated at 12 and 24 months. The BSRC is an international network of 26 individually funded research sites, which had procedures and measures similar enough to permit pooling, in order to study the development of ASD-high risk infants. Children were enrolled at <18 months of age and evaluated at 36 months of age or older (Note, the prior BSRC analysis suggesting younger paternal age in cases [Ozonoff et al., 2011] included eight of the same individuals here, representing 2% of our study population). Although recruitment methods varied somewhat by study (Supporting Information Table 1), all sites recruited through autism service, community, and advocacy organizations in their regions. Local institutional review board approvals were obtained at all study sites, and all participants provided informed consent.
IBIS and BSRC also enrolled some low-risk infants; we restricted analyses here to high-risk infants due to small numbers in the low-risk group. Furthermore, analyses here include only those participants with available data and enrollment into ECHO by January 30, 2019. Figure 1 presents further exclusions; individuals missing parental age or child outcome data (any of Social Responsiveness Scale [SRS], Mullen Scales of Early Learning [MSEL], Vineland Adaptive Behavior Scales [VABS] or diagnosis) were excluded from analyses.
Figure 1.
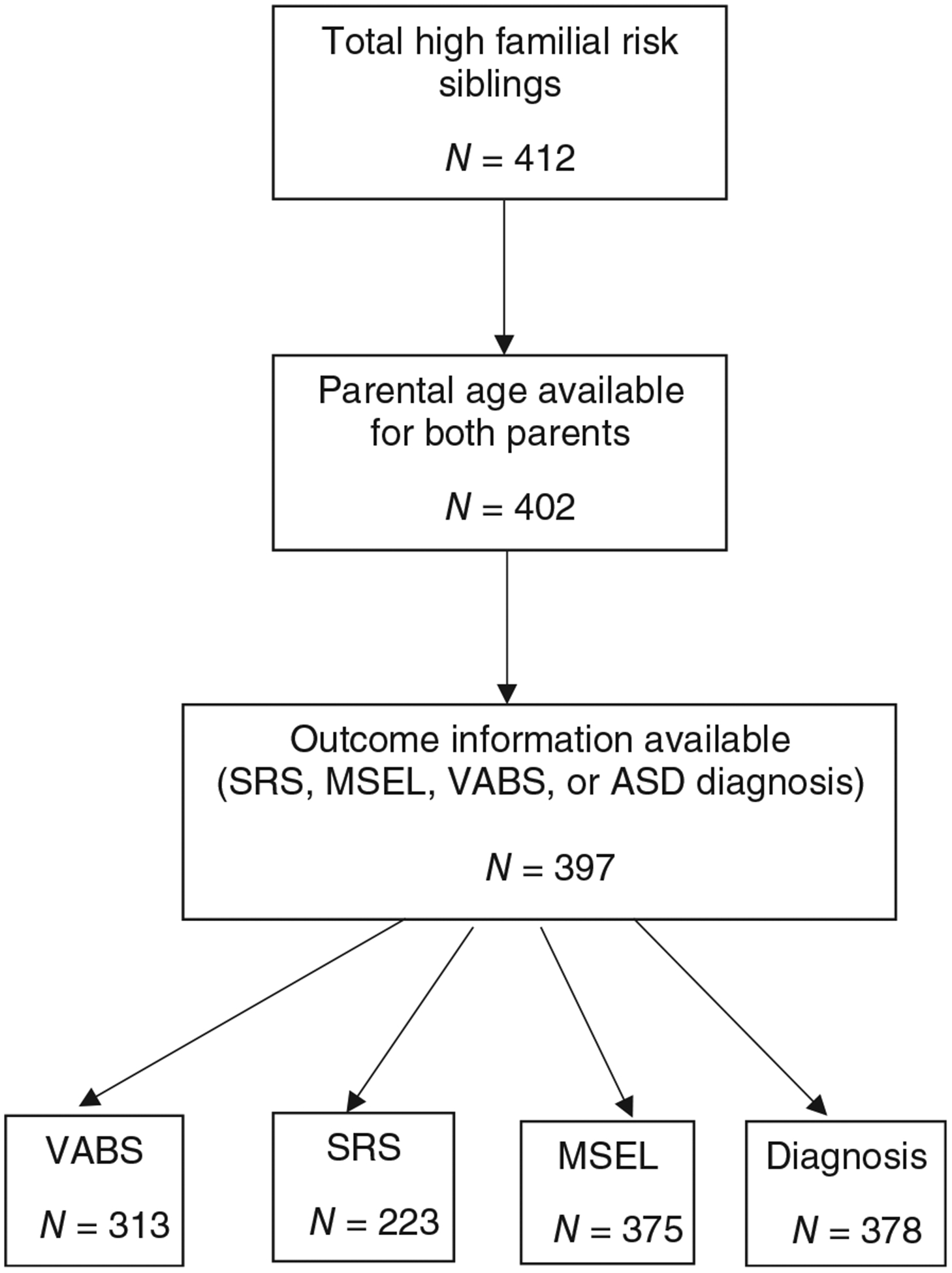
Study exclusions. ASD, autism spectrum disorder; MSEL, Mullen Scales of Early Learning; SRS, Social Responsiveness Scale; VABS, Vineland Adaptive Behavior Scales.
Parental Age and Covariate Information
Parental age was calculated based on self or maternally reported date of birth and child’s date of birth in questionnaires conducted at study enrollment. Information on parental race/ethnicity, education, child’s sex, and year of birth was also collected through questionnaires (administered to the mother). Additional variables, including preterm birth, parity, child gestational age, and maternal body mass index, were collected for prenatal cohorts (EARLI and MARBLES) only.
Outcome Assessments
All ASD-ER participants received extensive neurodevelopmental phenotyping over the course of follow-up by trained, research reliable clinicians at each site. For analyses here, we focus on outcome data collected at 36 months (or, if not available, at 24 months), since these are the time points that had the most uniform data collection across studies and sites and represent ages at which valid and reliable ASD-related outcomes can be obtained.
Clinical classification.
Across ASD-ER studies, clinical diagnoses of ASD were made by licensed, experienced psychologists according to the Diagnostic and Statistical Manual (DSM-IV) [American Psychiatric Association, 1994] or DSM-5 [American Psychiatric Association, 2013], depending on the site and study timing, based on clinical evaluations that included the Autism Diagnostic Observation Schedule (ADOS or ADOS-2) [Lord et al., 1989, 2012]. To be considered a case in these analyses, we used diagnoses according to these clinical assessments at 36 months, unless the child was missing an evaluation at that time (which occurred for some cases from the IBIS study only), in which case we used the outcome determined at 24 months (Ȉ10% of cases). Nontypical development (non-TD) was defined according to criteria defined in prior publications [Ozonoff et al., 2014]: failing to meet criteria for ASD but with cognitive development scores >1.5 standard deviations (SDs) below the mean for two or more subscales of the MSEL, or one or more subscales >2SDs below the mean, and/or ADOS scores ≤3 points below the ASD cutoff. TD here was defined as children not meeting the criteria for either ASD or non-TD. Children in the TD and non-TD groups were combined to form a general comparison group for analyses of ASD diagnosis described below (sensitivity analyses also examined ASD vs. TD only comparisons).
Quantitative trait outcomes.
The SRS is a 65-item informant-report questionnaire generating a raw score ranging from 0 to 195, with higher values indicating greater expression of the ASD-related phenotype [Constantino & Gruber, 2012]. SRS forms were administered to parents (primarily mothers) about their child at 36 month visits. SRS scores have been shown to be stable over time and, in most studies, not related to age [Constantino et al., 2003; Constantino & Gruber, 2005]. Raw scores are generated by summing individual item scores. Sex-normed T scores are obtained by converting raw scores according to publisher guidelines.
The MSEL assesses overall cognitive, language, and motor ability [Mullen, 1995]. The MSEL yields an overall standardized score (the Early Learning Composite [ELC]), which demonstrates high internal consistency, and convergent validity with other measures of IQ among children with and without ASD [Bishop, Guthrie, Coffing, & Lord, 2011]. MSEL data collected at 36 months were used in the present study.
Vineland Adaptive Behavior Scales 2nd Edition (VABS-II) is a standardized measure of adaptive functioning from 0 to 18 years that assesses adaptive behavior in each of five domains of functioning: communication, daily living skills, socialization, motor, and maladaptive behaviors [Sparrow, Balla, & Cicchetti, 1984]. Norms are available based on current U.S. census data. The VABS was administered at 36 months for most children, and at 24 months otherwise.
Statistical Analyses
Distributions of maternal and paternal age were examined prior to analyses. The correlation between maternal and paternal age was calculated using a Pearson correlation coefficient. To examine the association between parental age and quantitative outcomes, we used a series of crude and multivariable adjusted linear regression models. Across models, we examined maternal and paternal age separately, as well as in co-adjusted models. We modeled maternal and paternal age (separately) continuously in years; for comparison to prior studies, we conducted analyses using a unit of 10 years (to examine associations with 10 year increases in age). Because some reports have suggested U-shaped relationships or other nonlinear patterns, we also tested for nonlinearity in the parental age-ASD related outcomes associations using restricted cubic splines, using three knot points. Tests of nonlinearity in these models were performed in adjusted analyses based on a Wald test for the parental age term in the cubic spline model. In addition, we assessed potential incremental increases in risk by examining parental age in categories informed by prior work, as well as the distribution in our study (categories of <30, 30 to <35, and ≥35 for maternal age, and <30, 30–34, 35 to <40, and ≥40 for paternal age). Referent categories for these analyses were the 30–34 year-old age group. Finally, we also considered potential maternal–paternal age combined effects by examining categories of low (both parents < 30 years of age) and advanced (maternal age ≥ 35 and paternal age ≥ 40) parental age, relative to all other age combinations as the referent. Confounders included in adjusted models, selected on the basis of a priori associations and relationships in our study population, were parental education (categorized as high school or less, college, or graduate education), parental race/ethnicity (categorized as non-Hispanic white and other), and (in coadjusted models) the other parent’s age as a continuous variable. Child’s sex was also included in adjusted models given the known association with ASD diagnosis and raw SRS scores [Constantino & Gruber, 2012]. Missingness on any one of these primary covariates was generally low (<10%). For purposes of maintaining sample size across crude and adjusted models, missing values on these covariates were imputed following multiple imputation using the “aregImpute” function in the “Hmisc” package in R. We also examined adjustment for study region and individual cohorts included in ASD-ER; however, as these did not materially change results, these variables were not included in final models. Variables that may be on the pathway between parental age and ASD-related outcomes, including low birth weight and preterm birth, were examined in secondary analyses adjusting for these factors, using the subset of participants with this information available (n = 258 for birth weight, n = 355 for preterm birth). We also conducted secondary analyses adjusting for parity in the subset with this information (n = 217).
The above parameterizations of parental age were examined in association with each outcome: ASD clinical diagnosis (with the comparison group being children without an ASD diagnosis), SRS score, MSEL ELC score, and VABS composite score. Primary analyses of SRS scores used raw scores, as is suggested by the publisher for examination of quantitative traits across the population. However, to facilitate comparisons to diagnostic outcome, we also conducted analyses of SRS T scores. In secondary analyses, we also examined associations comparing individuals classified as non-TD to the TD group.
Sensitivity analyses.
In order to examine whether inclusion of non-TD individuals in the reference group influenced results, we conducted ASD diagnostic analyses excluding these individuals from the comparison group (n = 96). We also conducted sensitivity analyses excluding individuals whose outcomes were based on 24-month data (n = 31 with 24-month VABS analyses and n = 40 with 24-month ASD diagnostic analyses). To confirm missing covariate data did not influence results, we also conducted primary analyses in the subset with complete information on covariates used in final adjusted models (n = 352 for ASD diagnosis analyses, n = 214 for SRS scores, n = 350 for MSEL, and n = 310 for VABS).
Results
Characteristics of the study population overall and by advanced maternal and paternal age categories are presented in Table 1. The mean maternal and paternal ages in this study group were 34 years and 36 years, respectively. The correlation between maternal and paternal age was high (r = 0.67) and did not differ by case status. Advanced parental ages were associated with higher education, as has been observed in the US generally [Mathews & Hamilton, 2019; Mathews & Ventura, 1997]. Characteristics of the study group by ASD-ER cohorts are provided in Supporting Information Table 2. Child characteristics were similar across these cohorts, as were distributions of parental age and outcomes.
Table 1.
Basic Characteristics of the Full Study Group and by Advanced Maternal and Paternal Age Categories
| Maternal age | Paternal age <40 years (n = 306) | >40 years (n = 91) | |||
|---|---|---|---|---|---|
| Full study group (n = 397) | <35 years (n = 221) | >35 years (n = 176) | |||
| Child characteristics—n (%) unless noted | |||||
| Female | 148 (37.3%) | 95 (43.0%) | 53 (30.1%) | 117 (38.2%) | 31 (34.1%) |
| Male | 249 (62.7%) | 126 (57.0%) | 123 (69.9%) | 189 (61.8%) | 60 (65.9%) |
| ASD diagnosis | 76 (20.1%) | 40 (19.0) | 36 (21.4) | 55 (18.9) | 21 (24.1) |
| SRS raw score (mean, SD) | 38.6 (29.9) | 38.0 (30.4) | 39.4 (29.5) | 38.3 (30.2) | 39.9 (29.3) |
| SRS T score (mean, SD) | 50.3 (13.3) | 50.0 (13.4) | 50.6 (13.1) | 50.1 (13.2) | 50.7 (13.7) |
| MSEL ELC (mean, SD) | 98.5 (19.4) | 96.7 (19.8) | 100.8 (18.6) | 97.6 (19.9) | 101.9 (16.9) |
| VABS composite (mean, SD) | 95.7 (13.4) | 96.2 (14.2) | 95.2 (12.2) | 96.3 (13.8) | 93.9 (11.6) |
| Parent characteristics—n (%) unless noted | |||||
| Maternal race/ethnicity | |||||
| Non-Hispanic White | 207 (52%) | 110 (49.8%) | 97 (55.1%) | 155 (50.7%) | 52 (57.1%) |
| Hispanic | 60 (15.1%) | 36 (16.3%) | 24 (13.6%) | 48 (15.7%) | 12 (13.2%) |
| Other | 130 (32.7%) | 75 (33.9%) | 55 (31.2%) | 103 (33.7%) | 27 (29.7%) |
| Maternal education | |||||
| High school or less | 141 (38.0%) | 93 (44.9%) | 48 (29.3%) | 111 (39.4%) | 30 (33.7%) |
| College/college degree | 133 (35.8%) | 69 (33.3%) | 64 (39.0%) | 101 (35.8%) | 32 (36.0%) |
| Graduate degree | 97 (26.1%) | 45 (21.7%) | 52 (31.7%) | 70 (24.8%) | 27 (30.3%) |
| Missing | 26 (6.5%) | 14 (6.3%) | 12 (6.8%) | 24 (7.8%) | 2 (2.2%) |
| Paternal race/ethnicity | |||||
| Non-Hispanic White | 218 (54.9%) | 112 (50.7%) | 106 (60.2%) | 165 (53.9%) | 53 (58.2%) |
| Hispanics | 61 (15.4%) | 37 (16.7%) | 24 (13.6%) | 47 (15.4%) | 14 (15.4%) |
| Other | 118 (29.7%) | 72 (32.6%) | 46 (26.1%) | 94 (30.7%) | 24 (26.4%) |
| Paternal education | |||||
| High school or less | 156 (43.3%) | 96 (48.0%) | 60 (37.5%) | 118 (42.9%) | 38 (44.7%) |
| College/college degree | 105 (29.2%) | 57 (28.5%) | 48 (30.0%) | 83 (30.2%) | 22 (25.9%) |
| Graduate degree | 99 (27.5%) | 47 (23.5%) | 52 (32.5%) | 74 (26.9%) | 25 (29.4%) |
| Missing | 37 (9.3%) | 21 (9.5%) | 16 (9.1%) | 31 (10.1%) | 6 (6.6%) |
| Maternal age (mean, SD) | 34.2 (4.36) | ||||
| Paternal age (mean, SD) | 36.1 (5.38) | ||||
| Maternal age categories | |||||
| <30 | 61 (15.4%) | 61 (20%) | 0 | ||
| 30 to <35 | 160 (40.3%) | 145 (47%) | 15 (16%) | ||
| ≥35 | 176 (44.3%) | 100 (33%) | 76 (84%) | ||
| Paternal age categories | |||||
| <30 | 45 (11.3%) | 43 (19%) | 2 (1%) | ||
| 30 to <35 | 124 (31.2%) | 104 (47%) | 20 (11%) | ||
| 35 to <40 | 137 (34.5%) | 59 (27%) | 78 (44%) | ||
| ≥40 | 91 (22.9%) | 15(7%) | 76 (43%) | ||
Distributions of all continuous outcomes examined are provided in Supporting Information Fig. 1. Compared to general population samples, the distribution of SRS scores in ASD-ER is shifted slightly toward higher scores (indicating higher ASD-related traits/social communication deficits). This is consistent with many prior studies of familial quantitative traits [Constantino et al., 2006; Constantino, Zhang, Frasier, Abbacchi, & Law, 2010; Messinger et al., 2015; Ozonoff et al., 2014; Virkud, Todd, Abbacchi, Zhang, & Constantino, 2009], as well as with the increased recurrence risk of ASD diagnosis in these families of approximately 18–20% [Ozonoff et al., 2011]. The distribution of MSEL and VABS scores (for which higher scores indicate stronger cognitive ability and adaptive functioning) was shifted somewhat lower as compared to what is typically seen in general population samples, also consistent with the increased risk of other developmental delays in these populations [Messinger et al., 2013; Ozonoff et al., 2014].
Results of cubic spline analyses (Supporting Information Fig. S2) for associations with all outcomes did not reveal significant nonlinear associations, although the association between paternal age and ASD appeared weakly U-shaped, while MSEL scores demonstrated a more linear association and generally increased with increasing paternal age.
Results of associations with clinically defined ASD diagnosis are shown in Table 2. Nonsignificant increases in odds of ASD were observed for increasing continuously parametrized maternal, but not paternal, age (for a 10-year increase in maternal age, coadjusted model odds ratio [OR] = 1.65, 95% confidence intervals [CI] = 0.73–3.73; corresponding estimate for paternal age = 0.90, 95% CI = 0.47–1.72). In categorical models, comparing to the referent age group of 30–34, odds of ASD were also nonsignificantly increased with paternal age ≥ 35 (OR for 35–39 = 1.46, 95% CI = 0.73–2.96 and ≥40 OR = 1.87, 95% CI = 0.91–3.89, with slightly attenuated estimates in coadjusted models); a smaller increase was observed for maternal age of ≥35 (OR = 1.22, 95% CI = 0.69–2.19). A statistically significant increase in odds of ASD was observed in association with lower paternal age (<30 years OR = 2.57, 95% CI = 1.06–6.18), which persisted when also adjusting for maternal age (OR = 2.83, 95% CI = 1.14–7.02). Combined effects models did not suggest significant increases in risk when both parents were young (<30), or when both parents were older (≥35 for mothers and ≥40 for fathers). In secondary analyses comparing non-TD to TD, we did not find evidence for associations with parental age (Supporting Information Table 3).
Table 2.
Crude and Adjusted Associations (Odds Ratios and 95% Confidence Intervals) Between Parental Age and Offspring ASD Diagnosis According to Continuous and Categorical Parental Age Parameterizations
| N | Crude | Adjusteda | Coadjustedb | |
|---|---|---|---|---|
| Continuous models | ||||
| Maternal age (10 year increase) | 378 | 1.30 (0.73, 2.32) | 1.46 (0.79, 2.72) | 1.65 (0.73, 3.73) |
| Paternal age (10 year increase) | 378 | 1.06 (0.66, 1.70) | 1.10 (0.67, 1.77) | 0.90 (0.47, 1.72) |
| Categorical models | ||||
| Maternal age categories | ||||
| <30 | 57 (12) | 1.19 (0.54, 2.50) | 1.03 (0.46, 2.22) | 1.05 (0.45, 2.36) |
| 30–34 (referent) | 153 (12) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| ≥35 | 168 (36) | 1.22 (0.70, 2.13) | 1.22 (0.69, 2.19) | 1.20 (0.63, 2.30) |
| Paternal age categories | ||||
| <30 | 41 (13) | 2.84 (1.22, 6.54) | 2.57 (1.06, 6.18)* | 2.83 (1.14, 7.02)* |
| 30–34 (referent) | 121 (17) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| ≥35 to <40 | 129 (25) | 1.47 (0.76, 2.93) | 1.46 (0.73, 2.96) | 1.33 (0.65, 2.78) |
| ≥40 | 87 (21) | 1.95 (0.96, 4.00) | 1.87 (0.91, 3.89) | 1.50 (0.62, 3.63) |
| Combined effects | ||||
| Young parents | 25 (6) | 1.36 (0.48, 3.39) | 1.18 (0.40, 3.09) | |
| Referent | 281 (53) | 1.0 (referent) | 1.0 (referent) | |
| Old parents | 72 (17) | 1.33 (0.70, 2.44) | 1.29 (0.66, 2.41) | |
Note. Ns shown represent total n in analysis for continuous models, or n in that category for categorical models. Definitions as follows: young parents: both parents < 30 years; old parents: maternal age ≥ 35 and paternal age ≥ 40; referent combined effects category: all other combinations of parental age.
Adjusted for child’s sex, parental education, and parental race/ethnicity (maternal education and race/ethnicity used in maternal models; paternal education and race/ethnicity used in paternal age models).
Additionally adjusted for the other parent’s age. Combined effects models adjusted for maternal race and education, though estimates adjusting for paternal race and education instead were similar.
Statistically significant (P < 0.05) in adjusted model.
Results of parental age in association with child quantitative ASD-related traits as measured by the SRS (total raw scores) are shown in Table 3. No significant associations were observed with maternal age; scores were modestly increased (indicating greater social communication deficits) by approximately 2 points for a 10-year increase and for ≥35 years compared to 30–34 years. Both younger and older paternal age categories (relative to fathers 30–34 years) were also associated with nonsignificant increases in SRS scores (of approximately 11 and 5 points, respectively). Increased child SRS scores were suggested with younger parental age (both parents <30 years, β = 16.10, 95% CI = −1.84 to 34.10), though these associations were also nonsignificant. These general patterns were also observed when examining associations with SRS T scores rather than raw scores (Supporting Information Table 4).
Table 3.
Crude and Adjusted Associations (β and 95% Confidence Intervals) Between Parental Age and Offspring Social Communication Traits as Measured by Raw Total SRS Scores
| N | Crude | Adjusteda | Coadjustedb | |
|---|---|---|---|---|
| Continuous models | ||||
| Maternal age (10 years) | 223 | 1.17 (−8.40, 10.7) | 2.16 (−7.69, 12.0) | 2.70 (−9.60, 15.0) |
| Paternal age (10 years) | 223 | 0.42 (−6.78, 7.62) | 0.63 (−6.62, 7.88) | 0.08 (−8.82, 8.98) |
| Categorical models | ||||
| Maternal age categories | ||||
| <30 | 28 | 4.27 (−8.43, 17.0) | 1.58 (−10.9, 14.1) | 1.56 (−11.3, 14.4) |
| 30–34 (referent) | 97 | 0.0 (referent) | 0.0 (referent) | 0.0 (referent) |
| ≥35 | 98 | 2.40 (−6.07, 10.9) | 2.02 (−6.50, 10.5) | 2.04 (−7.45, 11.5) |
| Paternal age categories | ||||
| <30 | 26 | 17.0 (3.61, 30.5) | 10.7 (−3.00, 24.4) | 11.0 (−2.92, 24.9) |
| 30–34 (referent) | 70 | 0.0 (referent) | 0.0 (referent) | 0.0 (referent) |
| 35–39 | 79 | 7.89 (−1.70, 17.5) | 5.76 (−3.92, 13.2) | 5.47 (−4.51, 15.5) |
| ≥40 | 48 | 7.76 (−3.19, 18.7) | 5.15 (−5.84, 13.8) | 4.31 (−8.65, 17.3) |
| Combined effects | ||||
| Young parents | 11 | 18.8 (0.57, 37.1) | 16.1 (−1.84, 34.1) | |
| Referent | 172 | 0.0 (referent) | 0.0 (referent) | |
| Old parents | 40 | 1.27 (−9.03, 11.6) | 0.45 (−9.56, 10.5) | |
Note. Ns shown represent total n in analysis for continuous models, or n in that category for categorical models. Definitions as follows: young parents: both parents < 30 years; old parents: maternal age ≥ 35 and paternal age ≥ 40; referent combined effects category: all other combinations of parental age.
Adjusted for child’s sex, parental education, and parental race/ethnicity (maternal education and race/ethnicity used in maternal age and combined effects models; paternal education and race/ethnicity used in paternal age models).
Additionally adjusted for the other parent’s age. Combined effects models adjusted for maternal race and education, though estimates adjusting for paternal race and education instead were similar.
Associations with broader quantitative neurodevelopmental outcome scores are shown in Table 4. VABS composite scores decreased (indicating poorer adaptive functioning) with increasing maternal but not paternal age, particularly in coadjusted (e.g., including both parent’s ages) models, though this result did not reach statistical significance (β = −3.60, 95% CI = −8.20 to 1.00); a similar decrease in VABS scores was noted when both parents had advanced ages (β = −2.60, 95% CI = −6.17 to 0.98). In contrast, increases in MSEL ELC score (indicating higher cognitive functioning) were observed with increasing maternal and paternal age (of 5–6 points per 10-year increase, respectively; estimatesȈ—as for others summarized here—were from adjusted models including parental education). When adjusting for the other parent’s age, only the association with paternal age remained significant (β = 5.51, 95% CI = 0.70–10.30 for a 10-year increase). These associations were not observed with categorical parameterizations of maternal age where the referent group was parental age 30–34 and were attenuated for categorical paternal age. In combined effects models, having both parents under the age of 30 was associated with a significant decrease in offspring MSEL scores (β = −9.62, 95% CI = −17.10 to −2.15).
Table 4.
Crude and Adjusted Associations (β and 95% Confidence Intervals) Between Parental Age and Offspring Adaptive Behavior and Cognitive Functioning as Measured by VABS and MSEL Composite Scores
| N | Crude | Adjusteda | Coadjustedb | |
|---|---|---|---|---|
| VABS models | ||||
| Maternal age (10 years) | 313 | −1.29 (−4.7, 2.12) | −1.31 (−4.77, 2.14) | −3.60 (−8.20, 1.00) |
| Paternal age (10 years) | 313 | 0.63 (−2.05, 3.32) | 0.96 (−1.65, 3.57) | 2.26 (−1.22, 5.75) |
| Maternal age categories | ||||
| <30 | 48 | −1.42 (−5.88, 3.05) | −0.24 (−4.60, 4.11) | 0.53 (−4.08, 5.14) |
| 30–34 (referent) | 126 | 0.0 (referent) | 0.0 (referent) | 0.0 (referent) |
| ≥35 | 139 | −1.45 (−4.69, 1.80) | −0.64 (−3.81, 2.53) | −1.52 (−5.13, 2.09) |
| Paternal age categories | ||||
| <30 | 35 | −2.49 (−7.63, 2.65) | −2.04 (−7.03, 2.95) | −2.49 (−7.66, 2.68) |
| 30–34 (referent) | 100 | 0.0 (referent) | 0.0 (referent) | 0.0 (referent) |
| 35–39 | 104 | 2.47 (−1.20, 6.13) | 2.77 (−0.79, 6.33) | 3.17 (−0.58, 6.93) |
| ≥40 | 74 | −1.68 (−5.69, 2.33) | −1.09 (−4.95, 2.77) | −0.16 (−4.90, 4.58) |
| Combined effects | ||||
| Young parents | 21 | −1.62 (−7.61, 4.37) | −0.98 (−6.79, 4.84) | |
| Referent | 228 | 0.0 (referent) | 0.0 (referent) | |
| Old parents | 64 | −3.04 (−6.75, 0.68) | −2.60 (−6.17, 0.98) | |
| MSEL models | ||||
| Maternal age (10 years) | 375 | 6.12 (1.63, 10.6) | 4.87 (0.27, 9.46)* | −0.27 (−6.30, 5.77) |
| Paternal age (10 years) | 375 | 6.84 (3.17, 10.5) | 6.15 (2.53, 9.77)* | 5.51 (0.70, 10.3)* |
| Maternal age categories | ||||
| <30 | 56 | −7.33 (−13.2, −1.44) | −5.84 (−11.6, −0.06)* | −3.37 (−9.50, 2.76) |
| 30–34 (referent) | 152 | 0.0 (referent) | 0.0 (referent) | 0.0 (referent) |
| ≥35 | 167 | 2.08 (−2.14, 6.33) | 1.88 (−2.29, 6.05) | −0.49 (−5.11, 4.13) |
| Paternal age categories | ||||
| <30 | 43 | −7.81 (−14.5, −1.11) | −5.92 (−12.5, 0.71) | −5.20 (−12.1, 1.69) |
| 30–34 (referent) | 117 | 0.0 (referent) | 0.0 (referent) | 0.0 (referent) |
| 35–39 | 132 | 2.42 (−2.35, 7.19) | 2.02 (−2.66, 6.70) | 1.38 (−3.58, 6.34) |
| ≥40 | 83 | 4.23 (−1.16, 9.62) | 4.57 (−0.68, 9.82) | 3.21 (−3.07, 9.50) |
| Combined effects | ||||
| Young parents | 27 | −11.1 (−18.7, −3.54) | −9.62 (−17.1, −2.15)* | |
| Referent | 279 | 0.0 (referent) | 0.0 (referent) | |
| Old parents | 69 | 1.83 (−3.24, 6.89) | 1.75 (−3.20, 6.70) | |
Note. Ns shown represent total n in analysis for continuous models, or n in that category for categorical models. Definitions as follows: young parents: both parents < 30 years; old parents: maternal age ≥ 35 and paternal age ≥ 40; referent combined effects category: all other combinations of parental age.
Adjusted for child’s sex, parental education, and parental race/ethnicity (maternal education and race/ethnicity used in maternal age models; paternal education and race/ethnicity used in paternal age models).
Additionally adjusted for the other parent’s age. Combined effects models adjusted for maternal race and education, though estimates adjusting for paternal race and education instead were similar.
Statistical significance in adjusted model.
Secondary analyses examining additional adjustment for preterm birth, low birth weight, or parity in the sub-sets of individuals with information on these factors yielded similar findings, and estimates were similar not adjusted for child’s sex (Supporting Information Tables S5 and S6). Sensitivity analyses (Supporting Information - Table S7) excluding the non-TD group from the reference group also suggested very similar associations with ASD diagnosis as our primary results. Additional sensitivity analyses excluding those with 24-month rather than 36-month outcome data, or those with missing covariate information, also did not materially alter findings. Results across primary analyses are broadly summarized in Supporting Information Table S8.
Discussion
In this study, which examined outcomes in younger siblings of a child with autism, the strongest parental age associations observed were somewhat counter to expectation based on previous general population findings. In these high familial risk children, we observed significant increased odds of ASD diagnosis with younger paternal age, as well as increases in cognitive functioning with increasing paternal age, and decreases in these cognitive scores when both parents were younger. Potential explanations for our findings, which may relate to specific mechanisms operating in this high familial-risk setting, are discussed below.
As noted, the overwhelming majority of prior research suggests increased risk of ASD with advanced maternal and paternal age [Idring et al., 2014; Sandin et al., 2012; Wu et al., 2017]. We did not observe significant associations with increasing ASD-related outcomes and advanced parental age here. However, our point estimates for associations with ASD are broadly consistent with prior estimates suggesting increases in risk of ASD of 40–60% with advanced maternal and paternal age categories (typically defined as ages >35 and >40) [Idring et al., 2014; Sandin et al., 2012; Wu et al., 2017]. We may have lacked sufficient power to detect smaller increases in risk, as more modest associations have also been reported in larger samples [Grether, Anderson, Croen, Smith, & Windham, 2009]. Birth order may have also played a role in potentially attenuating associations with advanced parental age here, as our participants were by design younger siblings of children with ASD. A prior study that examined combined effects of parental age and birth order reported attenuated risk with higher birth order, although estimates with advanced parental age in that general population study were higher than observed here and statistically significant, even for later-born children [Durkin et al., 2008]. Differences in the trait distribution and associated features noted in these high familial risk cohorts [Constantino et al., 2006, 2010; McDonald et al., 2019; Virkud et al., 2009] may have also influenced findings, for example, if more of the “unaffected” comparison group had broader phenotype symptoms—although our analyses excluding the non-TD group yielded similar findings.
Instead, we observed stronger associations, which reached statistical significance in analyses of ASD diagnosis, with younger paternal age. We also observed some suggestion of a slight U-shaped relationship with paternal (but not maternal) age. These results are broadly consistent with some prior work. Two studies conducted within populations of twins have reported U-shaped relationships with paternal age and ASD or social-emotional development measured by the Strengths and Difficulties Questionnaire [Janecka, Haworth, et al., 2017; Lundstrom et al., 2010]. As noted, a prior BSRC study [Ozonoff et al., 2011] (including only 2% of the same children here) did not see significant associations with parental age, but did observe younger paternal age in ASD cases. Though not observed here, a few studies have also suggested U-shaped or increased risks of ASD with younger maternal age, as well [Idring et al., 2014; McGrath et al 2014; Sandin et al., 2016]. Potential mechanisms underlying an association with younger paternal age may include increases in risk of perinatal complications (supported independently for young paternal [Chen et al., 2008] as well as maternal age), or higher risk-taking behaviors (influencing either exposures to substances like alcohol linked with epigenetic modifications, or behaviors linked with younger age at first birth) potentially related to increased liability to psychiatric conditions [Lundstrom et al., 2010] (although the latter has primarily been suggested for paternal ages <20 years).
Prior work has not examined parental age associations with the specific quantitative trait measures used here. However, a recent study suggested that advanced paternal age was associated with a combination of traits (defined by the authors as the “geek index”) according to higher verbal IQ, higher social aloofness as measured by the Childhood Autism Spectrum Test, and a greater degree of restrictive repetitive behaviors [Janecka, Rijsdijk, Rai, Modabbernia, & Reichenberg, 2017]. In another study also conducted within Twin Early Development Study, both young and advanced paternal ages were associated with altered social development as measured by the Strengths and Difficulties Questionnaire [Janecka, Haworth, et al., 2017]. No associations with parental age were observed with scores on the Social and Communication Disorders Checklist in the Avon Longitudinal Study of Parents and Children [Robinson, Munir, McCormick, Koenen, & Santangelo, 2011]. A recent small study suggested an association between advanced paternal, but not maternal age, and autism severity in male children according to parent report of symptoms [Rieske & Matson, 2019]. Examination of these quantitative and broader traits is of interest not only for comparability to associations with ASD diagnosis, but also to understand how risk factors influence ASD-related traits across the population and to consider potential endophenotypes. We observed only modest and nonsignificant associations with adaptive behavior traits for both parents. Overall, associations with maternal and paternal ages were consistent across ASD diagnosis and quantitatively assessed ASD traits (e.g., higher SRS scores and increased odds of ASD with younger paternal age; modest and nonsignificant increases in SRS scores and odds of ASD with older maternal age).
Our observed associations between advanced parental age and improved cognitive scores as measured by the MSEL run somewhat counter to existing literature demonstrating negative impacts of advanced parental age on offspring psychiatric and neurodevelopmental outcomes [McGrath et al., 2014]. However, some recent studies have also suggested certain advantageous outcomes with older parental age. The previously mentioned study examining paternal age in association with the “geek index” captured higher nonverbal IQ and could be consistent with our MSEL findings [Janecka, Haworth, et al., 2017; Janecka, Rijsdijk, et al., 2017]. There has also been suggestion of improved cognitive ability (with evidence for time trends) [Goisis, Schneider, & Myrskyla, 2017], and social and emotional development, in children of older mothers [Trillingsgaard & Sommer, 2018]. Higher income, linked with improved parental educational attainment, is also associated with older parents [Goisis et al., 2017; Leung, Groes, & Santaeulalia-Llopis, 2016]. We also observed significantly decreased MSEL scores when both parents were younger (under the age of 30). As noted, a number of studies have suggested that young parental age may also increase risk of adverse neurodevelopmental outcomes [Janecka et al., 2019; Lundstrom et al., 2010; Merikangas et al., 2017]. Liabilities associated with younger age at childbearing [Chen et al., 2008; Lundstrom et al., 2010] (see also below) may also influence risk of adverse cognitive development. It is also possible that our results with cognitive scores may represent residual confounding or mediation by education or socio-economic status (SES)-related factors representing increased resources to the child that are often correlated with older parental age. In this setting of families who already have a child diagnosed with ASD, access to resources and social supports, which may be more limited and compounded in families with younger parents relative to those with older parents, may play a particularly influential role in subsequent child outcomes.
In addition to factors noted above, another potential explanation for differing results here from most general population studies may be that the mechanisms hypothesized to underlie the parental age association, or their relative contributions, differ in high-risk versus general population families. Differences in de novo mutation rates, copy number variants, and burden of these [Leppa et al., 2016], have been found between simplex and multiplex families, with some support for the theory of greater shared inherited genetic risk in multiplex families and higher de novo genetic risks in simplex families [Leppa et al., 2016; Sebat et al., 2007; Virkud et al., 2009]. A prevailing hypothesis is that the increased rate of de novo mutations with advanced paternal age [Girard et al., 2016] leads to the observed increased risk of ASD. Thus, a lack of association with advanced paternal age here may be consistent with a lesser role of de novo mutations in these high-risk families. However, it has also been suggested that de novo mutations may not fully explain advanced parental age associations with psychiatric disorders [Gratten et al., 2016], and that shared inherited risks play a role. This theory of broader autism phenotype in the parents resulting in delayed childbearing would not explain our findings here, and may be more relevant for first born children (as has been suggested elsewhere) [Petersen, Mortensen, & Pedersen, 2011]. Other potential mechanisms that could explain parental age associations may also differ in as yet unknown ways in the high familial risk setting. For example, mediation by pregnancy complications is a hypothesized mechanism for associations with maternal age, which may also have lesser influence in the context of shared genetics. As noted in prior work [Grether et al., 2009; Lee & McGrath, 2015], mechanisms are likely to differ for maternal and paternal age associations, and are suspected to be complex. Our results suggest that mechanisms for parental age associations may also differ according to familial risk for ASD. We were not able to examine mechanisms here, but future work should seek to do so, considering both high-familial risk and general population settings.
Strengths of this work include consideration of associations with both clinically ascertained ASD diagnosis as well as quantitative traits according to multiple measures, allowing for broader phenotypic comparisons. We also used data from established cohorts with rigorous methods and contribute novel information within a high familial-risk setting. However, a number of limitations should also be noted. The sample size was relatively small, limiting ability to detect modest associations with a high degree of accuracy, or to explore potential differences in subgroups, or other combinations of parental age categories. Only participants from these studies enrolled into ASD-ER were included here, and we cannot rule out the potential for selection bias (or variants of it in the high-familial risk setting, e.g., collider stratification bias) [Weisskopf, Sparrow, Hu, & Power, 2015], or differential loss to follow-up. However, the previously noted BSRC study included 664 individuals and also reported younger paternal age in children with ASD and nearly identical mean parental ages as in our sample [Ozonoff et al., 2011]. Due to the high familial risk design, which shifts the distribution of ASD quantitative traits higher [Constantino et al., 2006, 2010; Messinger et al., 2015; Ozonoff et al., 2014; Virkud et al., 2009], and also influences the parental age distribution, results should be considered within the context of these factors. Specifically, by nature of the study design, following ASD families and second or later-born children, parental ages are higher (e.g., 44% of mothers in this study fell in the highest category of age [≥35], compared to 10.9% in a general-population based sample including both first and later born children in the US Centers for Disease Control and Prevention’s Autism and Developmental Disabilities Monitoring [ADDM] Network) [Durkin et al., 2008]. While we cannot rule out potential rater biases in parent-informed measures (SRS, VABS), we observed overall consistency in the direction of associations between parental age and child outcome measured by quantitative ASD traits, adaptive behavior, and ASD diagnosis, suggesting this did not play a major role in our results. We did not have the ability to adjust for SES or specifically income, though it has been suggested that doing so in parental age analyses may result in inappropriate adjustment for a mediator [McGrath et al., 2014; Sandin et al., 2016] (We did adjust for parental education in our models, however, given its potential influence on outcome responses and associations with some outcomes). Finally, we conducted many comparisons, increasing the potential for chance findings. We did not perform correction for multiple testing as our focus was consideration of patterns, and consistency and precision of effect estimates across analyses, rather than statistical significance levels.
Comparing our results to the existing literature, our findings suggest that patterns of association between ASD-related outcomes and parental age, a well-established risk factor for ASD, may differ somewhat in the high familial-risk setting. Additional work is needed to understand whether and how the ASD risk-factor architecture differs in these high-familial risk populations versus those drawn from the general population. Given preliminary findings noted here, we believe continued work focused on such contrasts, considering risk not just of ASD diagnosis but also broader related traits, may shed light on etiologic and social mechanisms influencing these outcomes.
Supplementary Material
Acknowledgments
This work was supported by Environmental Influences on Child Health Outcomes (ECHO) grant number UH3OD023342 (Newschaffer). The EARLI Study was funded by the National Institute of Environmental Health Sciences, the National Institute of Mental Health, the National Institute of Child Health and Human Development, and the National Institute of Neurologic Disease and Stroke (R01 ES016443), with additional funding from Autism Speaks (AS 5938). The MARBLES Study has been supported by NIH grants: P01-ES011269, 1R01-ES020392, R01-ES025574, P30-ES023513, and R01-ES028089 from the National Institute of Environmental Health Sciences; U54-HD079125 from the Eunice Kennedy Shriver Intellectual and Developmental Disabilities Research Centers Network; NIH-UL1-TR000002 for the UC Davis Clinical and Translational Sciences Center and 2K12-HD051958; by the following U.S. EPA (U.S. Environmental Protection Agency) grants #R-829388 and R833292; by DOD #AR110194; and by the UC Davis MIND Institute. The BSRC sites included in this work were funded by grants 2R01 MH068398 (Ozonoff) from the National Institute of Mental Health, R01HD057284 (Messinger and Stone) from the National Institute of Child Health and Human Development (NICHD), and grant R01MH059630 (Landa) from the National Institute of Mental Health. The Infant Brain Imaging Study (IBIS) Network is an NIH funded Autism Centers of Excellence project and consists of a consortium of eight universities in the United States and Canada. IBIS is funded by R01-HD055741 and R01-HD055741-S1 (Piven) from National Institute of Child Health and Human Development (NICHD).
Footnotes
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Appendix S1. Supplemental Data.
Contributor Information
Kristen Lyall, AJ Drexel Autism Institute, Drexel University, Philadelphia, Pennsylvania;.
Lanxin Song, Department of Mental Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland;.
Kelly Botteron, Department of Psychiatry, Washington University, St Louis, Missouri;.
Lisa A. Croen, Kaiser Permanente Division of Research, Oakland, California;.
Stephen R. Dager, Department of Radiology, University of Washington, Seattle, Washington;
M. Daniele Fallin, Department of Mental Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland;.
Heather C. Hazlett, Department of Psychiatry, University of North Carolina, Chapel Hill, North Carolina;
Elizabeth Kauffman, AJ Drexel Autism Institute, Drexel University, Philadelphia, Pennsylvania;.
Rebecca Landa, Department of Psychiatry and Behavioral Sciences, Center for Autism and Related Disorders, Kennedy Krieger Institute, Johns Hopkins University, Baltimore, Maryland;.
Christine Ladd-Acosta, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland;.
Daniel S. Messinger, Department of Psychology, University of Miami, Miami, Florida;
Sally Ozonoff, MIND Institute, Department of Psychiatry and Behavioral Sciences, University of California Davis, Sacramento, California;.
Juhi Pandey, Department of Psychiatry, University of North Carolina, Chapel Hill, North Carolina;.
Joseph Piven, Center for Autism Research, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania;.
Rebecca J. Schmidt, Department of Public Health, University of California Davis, Davis, California;.
Rebecca J. Schmidt, MIND Institute, University of California Davis, Sacramento, California
Robert T. Schultz, Center for Autism Research, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania
Wendy L. Stone, Department of Psychology, University of Washington, Seattle, Washington
Craig J. Newschaffer, College of Health and Human Development, Pennsylvania State University, State College, Pennsylvania
Heather E. Volk, Department of Mental Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland;.
References
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed). Washington, DC: Author. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed). Washington, DC: Author. [Google Scholar]
- Bishop SL, Guthrie W, Coffing M, & Lord C (2011). Convergent validity of the Mullen Scales of Early Learning and the differential ability scales in children with autism spectrum disorders. American Journal on Intellectual and Developmental Disabilities, 116(5), 331–343. 10.1352/1944-7558-116.5.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell CK, Wakschlag LS, Gershon RC, & Cella D (2018). Measurement framework for the Environmental influences on Child Health Outcomes research program. Current Opinion in Pediatrics, 30(2), 276–284. 10.1097/mop.0000000000000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XK, Wen SW, Krewski D, Fleming N, Yang Q, & Walker MC (2008). Paternal age and adverse birth outcomes: Teenager or 40+, who is at risk? Human Reproduction, 23(6), 1290–1296. 10.1093/humrep/dem403 [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, … Reich W (2003). Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders, 33(4), 427–433. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Gruber C (2005). The social responsiveness scale (SRS). Los Angeles, CA: Western Pyschological Services. [Google Scholar]
- Constantino JN, & Gruber C (2012). Social responsiveness scale (2nd ed). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, … Todd RD (2006). Autistic social impairment in the siblings of children with prevasive developmental disorders. The American Journal of Psychiatry, 163(2), 294–296. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frasier T, Abbacchi AM, & Law P (2010). Sibling recurrence and the genetic epidemiology of autism. The American Journal of Psychiatry, 167(111), 1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kluiver H, Buizer-Voskamp JE, Dolan CV, & Boomsma DI (2017). Paternal age and psychiatric disorders: A review. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 174(3), 202–213. 10.1002/ajmg.b.32508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Newschaffer CJ, Lee LC, Cunniff CM, Daniels JL, … Schieve LA (2008). Advanced parental age and the risk of autism spectrum disorder. American Journal of Epidemiology, 168(11), 1268–1276. 10.1093/aje/kwn250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajos JM, & Beaver KM (2017). The role of paternal age in the prediction of offspring intelligence. The Journal of Genetic Psychology, 178(6), 319–333. 10.1080/00221325.2017.1377678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard SL, Bourassa CV, Lemieux Perreault LP, Legault MA, Barhdadi A, Ambalavanan A, … Rouleau GA (2016). Paternal age explains a major portion of de novo germline mutation rate variability in healthy individuals. PLoS One, 11(10), e0164212 10.1371/journal.pone.0164212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goisis A, Schneider DC, & Myrskyla M (2017). The reversing association between advanced maternal age and child cognitive ability: Evidence from three UK birth cohorts. International Journal of Epidemiology, 46(3), 850–859. 10.1093/ije/dyw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratten J, Wray NR, Peyrot WJ, McGrath JJ, Visscher PM, & Goddard ME (2016). Risk of psychiatric illness from advanced paternal age is not predominantly from de novo mutations. Nature Genetics, 48(7), 718–724. 10.1038/ng.3577 [DOI] [PubMed] [Google Scholar]
- Grether JK, Anderson MC, Croen LA, Smith D, & Windham GC (2009). Risk of autism and increasing maternal and paternal age in a large north American population. American Journal of Epidemiology, 170(9), 1118–1126. 10.1093/aje/kwp247 [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, McKinstry RC, Shaw DW, Botteron KN, Dager SR, … Piven J (2012). Brain volume findings in 6-month-old infants at high familial risk for autism. The American Journal of Psychiatry, 169(6), 601–608. 10.1176/appi.ajp.2012.11091425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Schmidt RJ, Walker CK, Bennett DH, Oliver M, Shedd-Wise KM, … Ozonoff S (2018). A prospective study of environmental exposures and early bio-markers in autism spectrum disorder: Design, protocols, and preliminary data from the MARBLES study. Environmental Health Perspectives, 126(11), 117004 10.1289/ehp535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman CM, Sandin S, Levine SZ, Lichtenstein P, & Reichenberg A (2011). Advancing paternal age and risk of autism: New evidence from a population-based study and a meta-analysis of epidemiological studies. Molecular Psychiatry, 16(12), 1203–1212. 10.1038/mp.2010.121 [DOI] [PubMed] [Google Scholar]
- Idring S, Magnusson C, Lundberg M, Ek M, Rai D, Svensson AC, … Lee BK (2014). Parental age and the risk of autism spectrum disorders: Findings from a Swedish population-based cohort. International Journal of Epidemiology, 43(1), 107–115. 10.1093/ije/dyt262 [DOI] [PubMed] [Google Scholar]
- Janecka M, Hansen SN, Modabbernia A, Browne HA, Buxbaum JD, Schendel DE, … Grice DE (2019). Parental age and differential estimates of risk for neuropsychiatric disorders: Findings from the Danish Birth Cohort. Journal of the American Academy of Child and Adolescent Psychiatry, 58 (6), 618–627. 10.1016/j.jaac.2018.09.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecka M, Haworth CMA, Ronald A, Krapohl E, Happe F, Mill J, … Rijsdijk F (2017). Paternal age alters social development in offspring. Journal of the American Academy of Child and Adolescent Psychiatry, 56(5), 383–390. 10.1016/j.jaac.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecka M, Rijsdijk F, Rai D, Modabbernia A, & Reichenberg A (2017). Advantageous developmental outcomes of advancing paternal age. Translational Psychiatry, 7(6), e1156 10.1038/tp.2017.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, & McGrath JJ (2015). Advancing parental age and autism: Multifactorial pathways. Trends in Molecular Medicine, 21(2), 118–125. 10.1016/j.molmed.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Leppa VM, Kravitz SN, Martin CL, Andrieux J, Le Caignec C, Martin-Coignard D, … Geschwind DH (2016). Rare inherited and de novo CNVs reveal complex contributions to ASD risk in multiplex families. American Journal of Human Genetics, 99(3), 540–554. 10.1016/j.ajhg.2016.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MY, Groes F, & Santaeulalia-Llopis R (2016). The relationship between age at first birth and mother’s lifetime earnings: Evidence from Danish data. PLoS One, 11(1), e0146989 10.1371/journal.pone.0146989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, & Schopler E (1989). Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders, 19(2), 185–212. [DOI] [PubMed] [Google Scholar]
- Lundstrom S, Haworth CM, Carlstrom E, Gillberg C, Mill J, Rastam M, … Reichenberg A (2010). Trajectories leading to autism spectrum disorders are affected by paternal age: Findings from two nationally representative twin studies. Journal of Child Psychology and Psychiatry, 51(7), 850–856. 10.1111/j.1469-7610.2010.02223.x [DOI] [PubMed] [Google Scholar]
- Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, … Newschaffer C (2017). The changing epidemiology of autism spectrum disorders. Annual Review of Public Health, 38, 81–102. 10.1146/annurev-publhealth-031816-044318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TJ, & Hamilton BE (2019). Educational attainment of mothers aged 25 and over: United States, 2017. [PubMed] [Google Scholar]
- Mathews TJ, & Ventura SJ (1997). Birth and fertility rates by educational attainment: United States. [Google Scholar]
- McDonald NM, Senturk D, Scheffler A, Brian JA, Carver LJ, Charman T, … Jeste SS (2019). Developmental trajectories of infants with multiplex family risk for autism: A Baby Siblings Research Consortium study. JAMA Neurology. 10.1001/jamaneurol.2019.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, & Pedersen CB (2014). A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry, 71(3), 301–309. 10.1001/jamapsychiatry.2013.4081 [DOI] [PubMed] [Google Scholar]
- Merikangas AK, Calkins ME, Bilker WB, Moore TM, Gur RC, & Gur RE (2017). Parental age and offspring psychopathology in the philadelphia neurodevelopmental cohort. Journal of the American Academy of Child and Adolescent Psychiatry, 56(5), 391–400. 10.1016/j.jaac.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, … Sigman M (2013). Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child and Adolescent Psychiatry, 52(3), 300–308. 10.1016/j.jaac.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger DS, Young GS, Webb SJ, Ozonoff S, Bryson SE, Carter A, … Zwaigenbaum L (2015). Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Molecular Autism, 6, 32 10.1186/s13229-015-0027-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E (1995). Mullen scales of early learning. Circle Pines, MN: American Guidance Services. [Google Scholar]
- Newschaffer CJ, Croen LA, Fallin MD, Hertz-Picciotto I, Nguyen DV, Lee NL, … Shedd-Wise KM (2012). Infant siblings and the investigation of autism risk factors. Journal of Neurodevelopmental Disorders, 4(1), 7 10.1186/1866-1955-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, … Iosif AM (2014). The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child and Adolescent Psychiatry, 53(4), 398–407. 10.1016/j.jaac.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL (2011). Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics, 128(3), e488–e495. 10.1542/peds.2010-2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Landa RJ, Brian J, Bryson S, Charman T, … Iosif AM (2015). Diagnostic stability in young children at risk for autism spectrum disorder: A Baby Siblings Research Consortium study. Journal of Child Psychology and Psychiatry, 56(9), 988–998. 10.1111/jcpp.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L, Mortensen PB, & Pedersen CB (2011). Paternal age at birth of first child and risk of schizophrenia. The American Journal of Psychiatry, 168(1), 82–88. 10.1176/appi.ajp.2010.10020252 [DOI] [PubMed] [Google Scholar]
- Rieske RD, & Matson JL (2019). Parental age at conception and the relationship with severity of autism symptoms. Developmental Neurorehabilitation, 1–6. 10.1080/17518423.2019.1645222 [DOI] [PubMed] [Google Scholar]
- Robinson EB, Munir K, McCormick MC, Koenen KC, & Santangelo SL (2011). Brief report: No association between parental age and extreme social-communicative autistic traits in the general population. Journal of Autism and Developmental Disorders, 41(12), 1733–1737. 10.1007/s10803-011-1202-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Hultman CM, Kolevzon A, Gross R, MacCabe JH, & Reichenberg A (2012). Advancing maternal age is associated with increasing risk for autism: A review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 51(5), 477–486. 10.1016/j.jaac.2012.02.018 [DOI] [PubMed] [Google Scholar]
- Sandin S, Schendel D, Magnusson P, Hultman C, Suren P, Susser E, … Reichenberg A (2016). Autism risk associated with parental age and with increasing difference in age between the parents. Molecular Psychiatry, 21, 693–700. 10.1038/mp.2015.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, … Wigler M (2007). Strong association of de novo copy number mutations with autism. Science, 316(5823), 445–449. 10.1126/science.1138659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Tancredi DJ, & Hertz-Picciotto I (2010). Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Research, 3(1), 30–39. 10.1002/aur.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, & Cicchetti DV (1984). Vineland adaptive behavior scales. Bloomington, MN: Pearson Assessments. [Google Scholar]
- Trillingsgaard T, & Sommer D (2018). Associations between older maternal age, use of sanctions, and children’s socioemotional development through 7, 11, and 15 years. European Journal of Developmental Psychology, 15(2), 141–155. [Google Scholar]
- Virkud YV, Todd RD, Abbacchi AM, Zhang Y, & Constantino JN (2009). Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. American Journal of Medical Genetics, 150B(3), 328–334. 10.1002/ajmg.b.30810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Sparrow D, Hu H, & Power MC (2015). Biased exposure-health effect estimates from selection in cohort studies: Are environmental studies at particular risk? Environmental Health Perspectives, 123(11), 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, … Piven J (2012). Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. The American Journal of Psychiatry, 169(6), 589–600. 10.1176/appi.ajp.2011.11091447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Wu F, Ding Y, Hou J, Bi J, & Zhang Z (2017). Advanced parental age and autism risk in children: A systematic review and meta-analysis. Acta Psychiatrica Scandinavica, 135(1), 29–41. 10.1111/acps.12666 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


