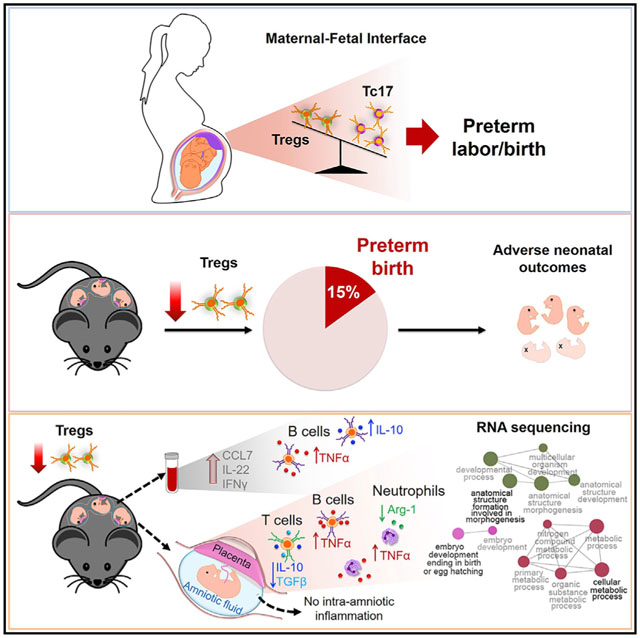
SUMMARY
Regulatory T cells (Tregs) have been exhaustively investigated during early pregnancy; however, their role later in gestation is poorly understood. Herein, we report that functional Tregs are reduced at the maternal-fetal interface in a subset of women with idiopathic preterm labor/birth, which is accompanied by a concomitant increase in Tc17 cells. In mice, depletion of functional Tregs during late gestation induces preterm birth and adverse neonatal outcomes, which are rescued by the adoptive transfer of such cells. Treg depletion does not alter obstetrical parameters in the mother, yet it increases susceptibility to endotoxin-induced preterm birth. The mechanisms whereby depletion of Tregs induces adverse perinatal outcomes involve tissue-specific immune responses and mild systemic maternal inflammation, together with dysregulation of developmental and cellular processes in the placenta, in the absence of intra-amniotic inflammation. These findings provide mechanistic evidence supporting a role for Tregs in the pathophysiology of idiopathic preterm labor/birth and adverse neonatal outcomes.
Graphical Abstract
In Brief
Understanding of the role of regulatory T cells in late gestation has been limited. Gomez-Lopez et al. provide evidence that Tregs modulate immune responses in the third period of pregnancy. Treg deficiency contributes to a subset of formerly idiopathic preterm births and adverse perinatal outcomes.
INTRODUCTION
Preterm birth is a leading cause of perinatal morbidity and mortality worldwide (Blencowe et al., 2012; Liu et al., 2015). Preterm neonates are at high risk for multiple short- and long-term complications, accounting for more than two million neonatal deaths in 2010 and representing an enormous burden for society and the health care system (Howson et al., 2013). Preterm birth is preceded by spontaneous preterm labor (PTL), a syndrome of multiple putative etiologies (Romero et al., 2014a). Among these, only acute pathological inflammation (i.e., intra-amniotic infection/inflammation and clinical chorioamnionitis) has been well characterized and causally linked to preterm labor (Romero et al., 1988; Gravett et al., 1994; Combs et al., 2014; Oh et al., 2017; Deng et al., 2019). The remaining etiologies are poorly understood; therefore, most cases of preterm birth (approximately 60%) are characterized as idiopathic (Goldenberg et al., 2008; Barros et al., 2015). A breakdown of maternal-fetal tolerance has been suggested as a mechanism of disease for idiopathic preterm labor and birth (Romero et al., 2014a; Gomez-Lopez et al., 2014). However, to date, no causal evidence has been provided connecting impaired maternal-fetal tolerance with preterm labor/birth and its adverse perinatal outcomes.
Maternal-fetal tolerance is established locally (e.g., the maternal-fetal interface) and systemically by the mother toward the allogeneic conceptus (Chaouat et al., 1979; Bonney and Onyekwuluje, 2003; Aluvihare et al., 2004; Zenclussen et al., 2005; Robertson et al., 2009; Kahn and Baltimore, 2010; Shima et al., 2010, 2015; Samstein et al., 2012; Rowe et al., 2012). At the maternal-fetal interface, this tolerance is sustained by an immune repertoire composed of regulatory T cells (Tregs) (Aluvihare et al., 2004; Sasaki et al., 2004; Heikkinen et al., 2004; Tsuda et al., 2018; Salvany-Celades et al., 2019), as well as homeostatic innate immune cells such as macrophages (Hunt et al., 1984; Gustafsson et al., 2008; Houser et al., 2011; Svensson et al., 2011; Svensson-Arvelund et al., 2015; Xu et al., 2016), natural killer (NK) cells (Kieckbusch et al., 2015; Li et al., 2017), and innate lymphoid cells (Vacca et al., 2015; Doisne et al., 2015; Xu et al., 2018; Miller et al., 2018). To date, most research has focused on investigating the processes of maternal-fetal tolerance during early pregnancy (Zenclussen et al., 2005; Kahn and Baltimore, 2010; Shima et al., 2010; Samstein et al., 2012; Rowe et al., 2012; Chen et al., 2013), given that this is the period in which the growing conceptus educates the maternal immune system to sustain pregnancy and promote its survival (Arck and Hecher, 2013; Robertson et al., 2018; Tsuda et al., 2019). However, the role of Tregs later in gestation (third trimester in humans and third week in mice) has not been mechanistically investigated.
Clinical studies have shown a negative association between the numbers and/or function of peripheral Tregs and the diagnosis of PTL leading to preterm birth (Xiong et al., 2010; Schober et al., 2012; Steinborn et al., 2012; Gomez-Lopez and Laresgoiti-Servitje, 2012). However, whether Tregs are reduced in number and/or function at the maternal-fetal interface (i.e., decidua) in women with PTL is unknown. Herein, we undertook an extensive investigation that included both human decidual samples from different subsets of PTL and animal models, which allowed us to provide translational and mechanistic evidence of a role for Tregs in the pathophysiology of idiopathic preterm labor/birth and adverse neonatal outcomes.
RESULTS
Functional Tregs Are Reduced at the Maternal-Fetal Interface in a Subset of Women with Idiopathic PTL and birth
The maternal-fetal interface represents the site of immune interactions between the mother and the conceptus (Chaouat et al., 1983; Petroff, 2005; Erlebacher, 2013; PrabhuDas et al., 2015; Bonney, 2016). The human maternal-fetal interface includes (1) the decidua parietalis, the area of contact between the endometrium and the chorioamniotic membranes, and (2) the decidua basalis, the interface between the endometrium and the placenta (Sindram-Trujillo et al., 2003; Gomez-Lopez et al., 2013). Different subsets of Tregs have been localized in both the decidua basalis (Sindram-Trujillo et al., 2004; Sasaki et al., 2004; Tilburgs et al., 2006, 2008; Inada et al., 2013; Tsuda et al., 2018; Salvany-Celades et al., 2019) and the decidua parietalis (Sindram-Trujillo et al., 2004; Heikkinen et al., 2004; Tilburgs et al., 2006, 2008; Salvany-Celades et al., 2019); however, their suppressive activity has yet to be established. We first tested whether third-trimester-derived decidual Tregs had suppressive activity. Decidual Tregs were sorted from preterm and term decidual tissues (Table S1) and co-cultured with matched isolated effector T cells (Teffs) (Figure 1A), and the percentage of suppression was calculated (see STAR Methods). Decidual Tregs exhibited suppressive activity at a 1:1 Treg:Teff ratio, yet such a function seemed to be lower in preterm pregnancies compared to term pregnancies (Figure 1B). These data show that third-trimester decidual Tregs display suppressive activity toward Teffs at the human maternal-fetal interface.
Figure 1. Functional Tregs Are Reduced at the Human Maternal-Fetal Interface in a Subset of Idiopathic PTL and birth.
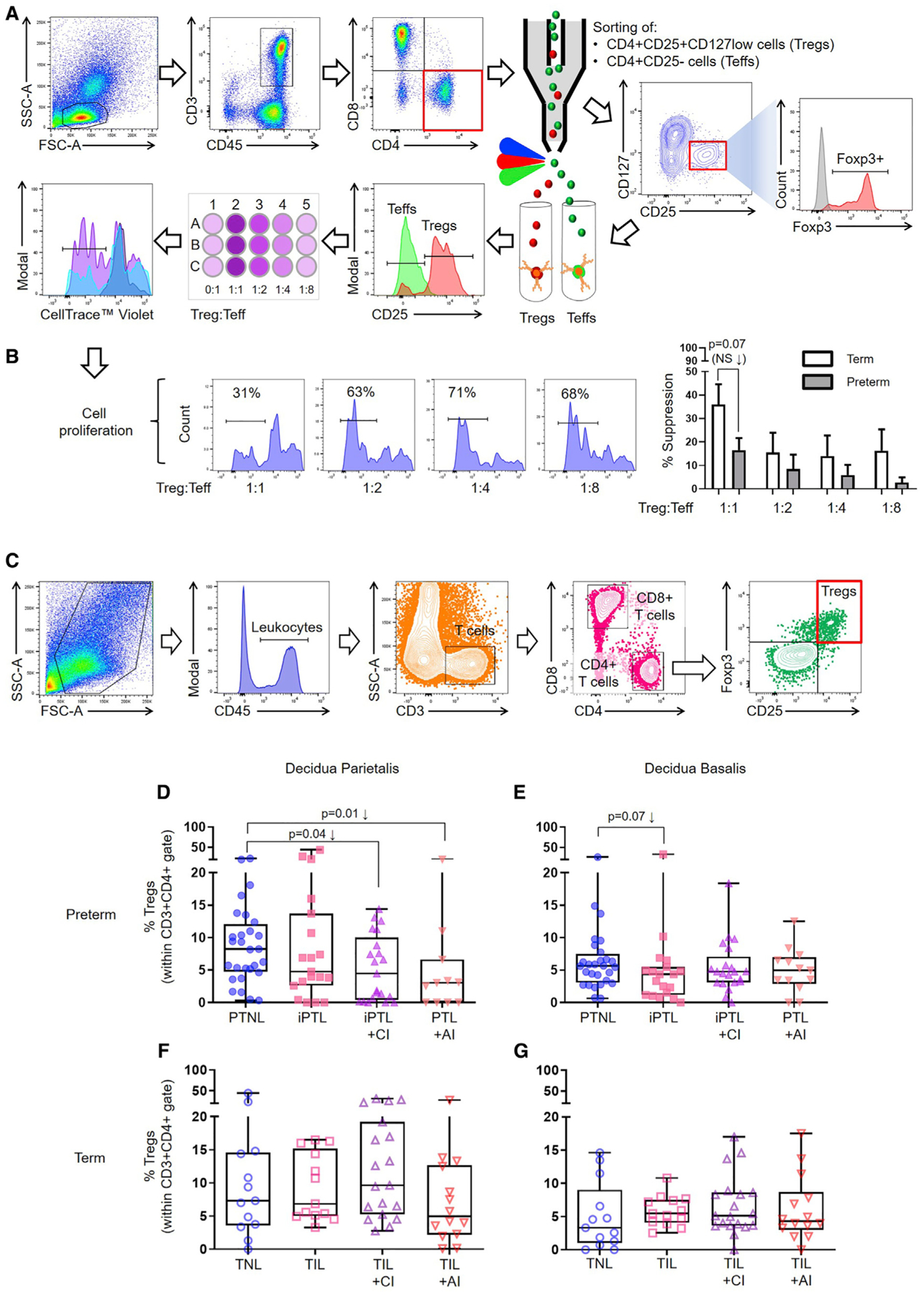
(A) Representative gating strategy used to sort Tregs and Teffs from the decidua. Tregs were co-cultured with Teffs, and Teff proliferation was measured by flow cytometry using CellTrace violet.
(B) Representative plots showing the proliferation of Teffs, with the percentage of decidual Treg suppression of Teffs from preterm and term pregnancies. Suppression data are shown as means ± SEM. n = 6–8 per group.
(C) Representative gating strategy used to identify Tregs in the decidua parietalis and decidua basalis.
(D and E) Frequency of Tregs in the (D) decidua parietalis (n = 11–28 per group) or (E) decidua basalis (n = 13–28 per group) of women with PTNL, iPTL, iPTL+CI, or PTL+AI.
(F and G) Frequency of Tregs in the (F) decidua parietalis (n = 13–19 per group) or (G) decidua basalis (n = 13–19 per group) of women with TNL, TIL, TIL+CI, or TIL+AI.
Data are represented as medians with interquartile and minimum/maximum ranges. Statistical analysis was performed using the Mann-Whitney U-test. Demographic and clinical characteristics of the study population are shown in Tables S1 and S2.
Next, we investigated whether Tregs were altered at the maternal-fetal interface of women who underwent the pathological (preterm) or physiological (term) process of labor. To address this research question, we collected decidual tissues from women with PTL and birth or term labor/birth (TIL), as well as gestational age-matched non-labor controls (preterm without labor [PTNL] or term without labor [TNL]) (Table S2). Given that the process of labor can occur in the setting of acute inflammation of the placenta (Kim et al., 2015a), resulting from intra-amniotic inflammation/infection, the well-known etiology of preterm birth (Romero et al., 1989), we subdivided the preterm and term labor groups according to the presence or absence of acute chorioamnionitis (AI) (Table S2). Idiopathic PTL (iPTL) was subdivided by the presence (iPTL+CI) or absence (iPTL) of chronic chorioamnionitis (CI) (Kim et al., 2015b), a placental lesion associated with maternal anti-fetal rejection (Lee et al., 2011). Neither of the iPTL groups included the presence of acute inflammation of the placental tissues. Leukocytes were isolated from the decidua basalis and decidua parietalis based on our previously established method (Xu et al., 2015), and flow cytometry was used to determine the frequencies of Tregs (CD45+CD3+CD4+CD25+Foxp3+ cells) (Figure 1C). In the decidua parietalis, Tregs were significantly reduced in women with iPTL+CI compared to non-labor controls (Figure 1D). A slight non-significant reduction in Tregs was also observed in women with iPTL compared to non-labor controls (Figure 1D). Moreover, in the decidua basalis, Tregs tended to be reduced in women with iPTL (Figure 1E). Decidual Tregs were also reduced in women whose placentas displayed acute inflammatory lesions (Figure 1D), as previously shown in the uterine tissues of mice injected with an endotoxin (animal model of acute systemic inflammation-induced preterm birth) (Arenas-Hernandez et al., 2016). No differences were observed among the term groups (Figures 1F and 1G). These findings show that Tregs are reduced at the maternal-fetal interface in a subset of women who underwent the pathological process of idiopathic PTL and birth, but not in those who underwent the physiological process of labor at term.
Tc17 Cells Are Increased at the Maternal-Fetal Interface in Women with Idiopathic PTL and birth
It has been proposed that a balance exists during normal pregnancy between homeostatic Tregs and pro-inflammatory Th17 cells, and a breakdown of this balance leads to obstetrical disease (Saito et al., 2010; Figueiredo and Schumacher, 2016). Therefore, we investigated whether the reduction in decidual Tregs in women with PTL and birth was accompanied by a concomitant increase in decidual Th17 cells. Flow cytometry was performed to determine the frequencies of Th17 cells (CD45+CD3+CD4+IL-17A+ cells) (Figure 2A) in the decidua (Table S2). Neither the decidua parietalis nor the decidua basalis displayed significant changes in the frequencies of Th17 cells among the preterm and term study groups (Figures 2B–2E). Given that interleukin (IL)-17A is also produced by CD8+ T cells, Tc17 cells (CD45+CD3+CD8+IL-17A+ cells), and that such cells display a pro-inflammatory phenotype (Hamada et al., 2009; Huber et al., 2009), these cells were identified in the decidua (Figure 2A). Consistently, both in the decidua parietalis and decidua basalis, the frequencies of Tc17 cells were greater in women with iPTL compared to non-labor controls (Figures 2F and 2G). Again, no differences were observed in the frequencies of Tc17 cells among the term study groups (Figures 2H and 2I). These findings show that an imbalance between functional Tregs and Tc17 cells occurs at the maternal-fetal interface in women with idiopathic PTL and birth.
Figure 2. Tc17 Cells Are Increased at the Human Maternal-Fetal Interface in Idiopathic PTL and birth.
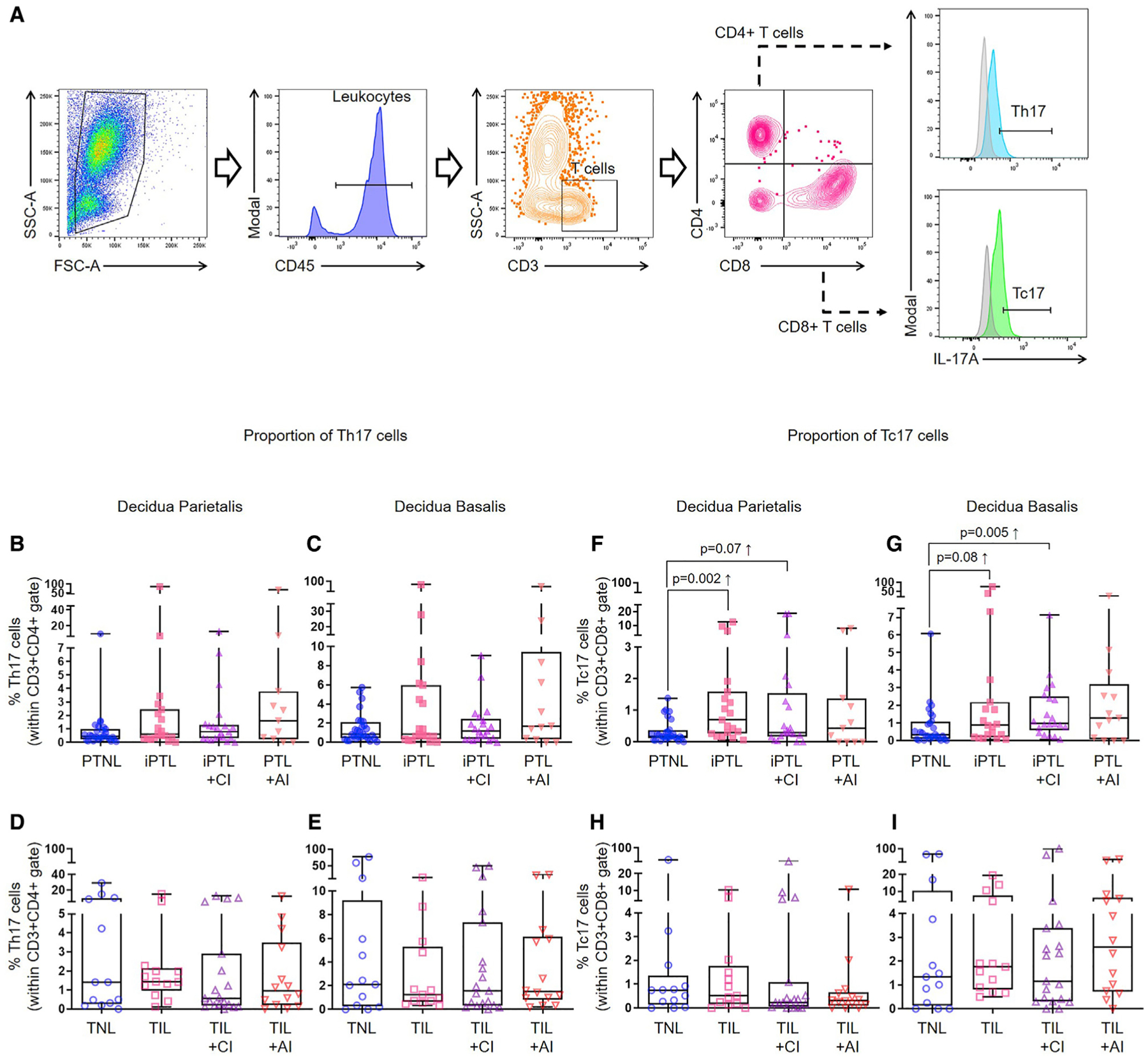
(A) Representative gating strategy used to identify Th17 cells and Tc17 cells in the decidua parietalis and decidua basalis.
(B and C) Frequency of Th17 cells in the (B) decidua parietalis (n = 11–28 per group) or (C) decidua basalis (n = 13–28 per group) of women with PTNL, iPTL, iPTL+CI, or PTL+AI.
(D and E) Frequency of Th17 cells in the (D) decidua parietalis (n = 13–19 per group) or (E) decidua basalis (n = 13–19 per group) of women with TNL, TIL, TIL+CI, or TIL+AI.
(F and G) Frequency of Tc17 cells in the (F) decidua parietalis (n = 11–28 per group) or (G) decidua basalis (n = 13–28 per group) of women with PTNL, iPTL, iPTL+CI, or PTL+AI.
(H and I) Frequency of Tc17 cells in the (H) decidua parietalis (n = 13–19 per group) or (I) decidua basalis (n = 13–19 per group) of women with TNL, TIL, TIL+CI, or TIL+AI.
Data are represented as medians with interquartile and minimum/maximum ranges. Statistical analysis was performed using the Mann-Whitney U-test. Demographic and clinical characteristics of the study population are shown in Table S2.
Establishment of a Model to Study the Role of Systemic Tregs in Late Pregnancy
Together with clinical studies (Xiong et al., 2010; Schober et al., 2012; Steinborn et al., 2012; Gomez-Lopez and Laresgoiti-Servitje, 2012), our first set of data (Figure 1) indicates that women with PTL and birth have reduced Tregs both in the peripheral circulation and at the maternal-fetal interface. Next, we undertook in vivo experimentation to demonstrate a causal link between systemic Tregs and preterm birth.
Previous studies showed that the systemic depletion of Tregs before implantation or in early gestation results in failure to implant or pregnancy loss (Zenclussen et al., 2005; Darrasse-Jèze et al., 2006; Kahn and Baltimore, 2010; Shima et al., 2010; Rowe et al., 2011, 2012; Samstein et al., 2012; Chen et al., 2013). However, to date, no studies have established a role for systemic Tregs in the third week of murine pregnancy (i.e., third trimester in humans). An earlier study depleted allogeneic CD25+ T cells on 10.5 and 13.5 days postcoitum (dpc), and no adverse pregnancy outcomes were recorded (Shima et al., 2010). A possible explanation as to why the depletion of CD25+ T cells did not cause disease in the previously mentioned study is that the depletion of CD25+ T cells is incomplete and not specific for Foxp3+ Tregs (Kohm et al., 2006; Couper et al., 2007; Fan et al., 2018). Alternatively, it could be that Tregs were not functional during the third week of murine pregnancy, as they were in earlier pregnancy (Polanczyk et al., 2005). Therefore, we first determined whether murine Tregs displayed suppressive activity in the second and third weeks of pregnancy (Figure S1A). As expected, second-week-derived Tregs suppressed Teff proliferation (Figure S1B). Third-week-derived Tregs also suppressed Teff proliferation (Figure S1C). Indeed, at a 1:8 Treg:Teff ratio, third-week-derived Tregs were more suppressive than second-week-derived Tregs (Figure S1D). Thus, Tregs are functional during the second and third weeks of murine pregnancy.
We then proceeded to establish a murine model in which the partial or total depletion of Tregs can be achieved in the third week of pregnancy. We reasoned that the partial depletion of Tregs will resemble the clinical condition in which women have reduced Tregs (e.g., PTL) (Figure 1), whereas the total depletion of Tregs will allow us to definitively establish a role for such cells in pregnancy. We used Foxp3DTR mice (Kim et al., 2007), in which the administration of diphtheria toxin (DT) allows for depletion of all Foxp3+ Tregs. The administration of 25 μg/kg (Rowe et al., 2011, 2012) or 50 μg/kg (Kim et al., 2007; Samstein et al., 2012) of DT to naive non-pregnant Foxp3DTR mice induced the partial or total depletion of Tregs, respectively, as indicated by the reduction of these cells in the uterine-draining lymph nodes (ULNs), spleen, and thymus (Figure S2). Next, we mated Foxp3DTR females with BALB/c males to obtain an allogeneic pregnancy (Figure 3A; Figure S3A). To induce the partial or total depletion of Tregs, Foxp3DTR dams were injected with 25 or 50 μg/kg of DT on 14.5 dpc, followed by daily administration of 5 or 50 μg/kg of DT, respectively, until delivery (Figure 3A; Figure S3A). The partial or total depletion of Tregs was confirmed in the decidua, myometrium, peripheral blood, and placenta (Figure 3B), as well as the ULNs, inguinal lymph nodes, mesenteric lymph nodes, spleen, and thymus (Figures S3B–S3F). These experiments established the murine models to study the role of Tregs in the third week of pregnancy (i.e., third trimester in humans, when the onset of preterm labor occurs).
Figure 3. Depletion of Tregs Induces a Fraction of Preterm Births and Adverse Neonatal Outcomes.
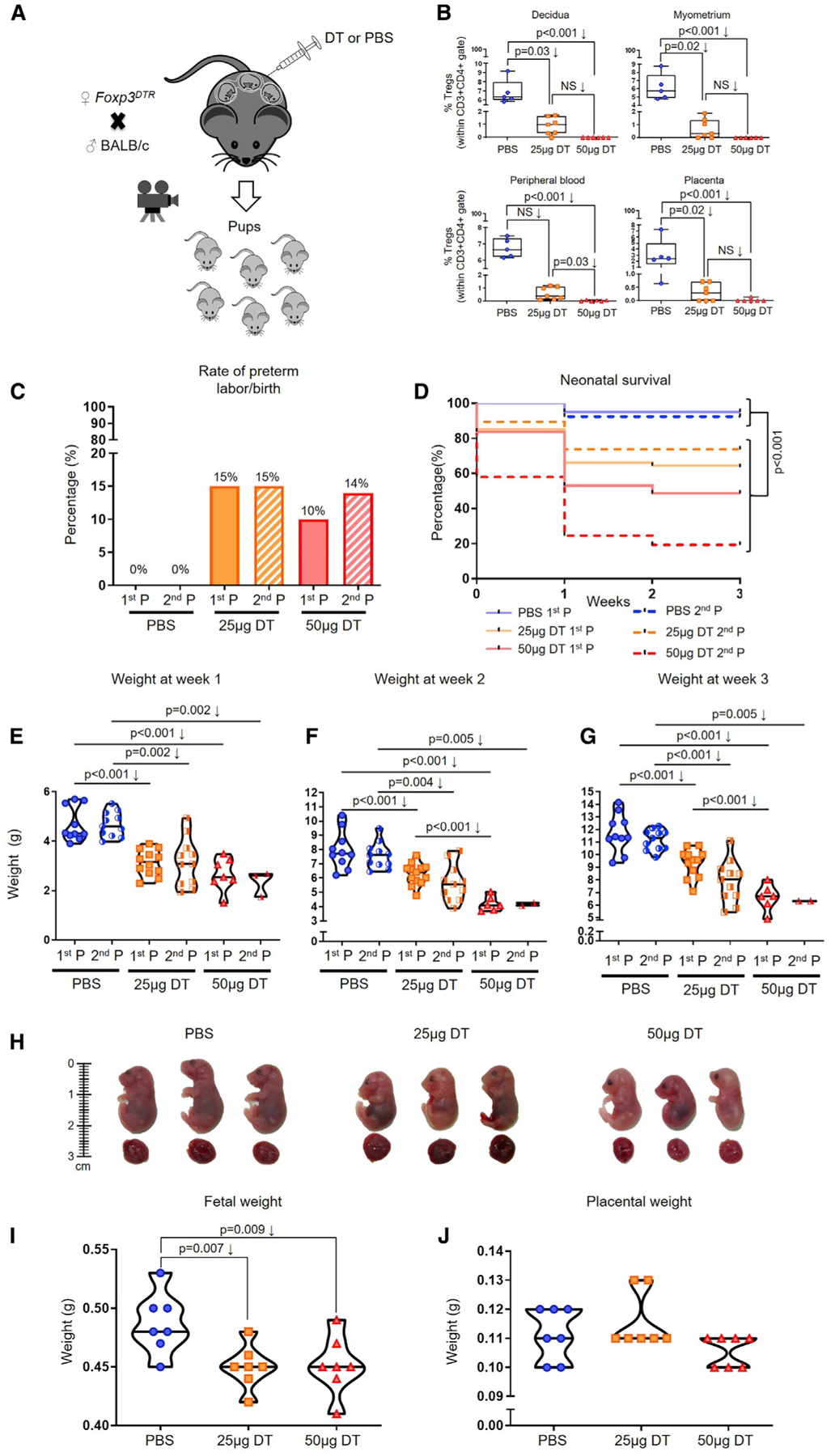
(A) Foxp3DTR dams underwent partial or total Treg depletion. Controls were injected with sterile 13 PBS. After the first pregnancy (P), a subset of Foxp3DTR dams underwent a second P and were again partially or totally Treg-depleted or were injected with sterile 13 PBS.
(B) Frequencies of Tregs in the decidua, myometrium, peripheral blood, and placenta of partially or totally Treg-depleted Foxp3DTR dams (n = 5–7 per group). Data are represented as medians with interquartile and minimum/maximum ranges.
(C) Preterm birth rates of non-Treg-depleted-, partially Treg-depleted-, and totally Treg-depleted-Foxp3DTR dams (1st or 2nd P, n = 9–20 per group). Data are represented as means of percentages.
(D) Percentage of survival from birth until 3 weeks postpartum for neonates born to non-Treg-depleted-, partially Treg-depleted-, and totally Treg-depleted-Foxp3DTR dams (1st or 2nd P, n = 7–18 per group).
(E–G) Weights of neonates born to non-Treg-depleted-, partially Treg-depleted-, and totally Treg-depleted-Foxp3DTR dams at weeks (E) 1, (F) 2, and (G) 3 postpartum (1st or 2nd P, n = 2–12 litters per group). Data are represented as violin plots with medians and minimum/maximum ranges.
(H) Representative images of fetuses (and their placentas) from non-Treg-depleted-, partially Treg-depleted-, and totally Treg-depleted-Foxp3DTR dams (n = 8–9 per group).
(I and J) Weights of (I) fetuses and (J) their placentas from non-Treg-depleted-, partially Treg-depleted-, and totally Treg-depleted-Foxp3DTR dams (n = 7 litters per group).
Statistical analysis was performed using the Mantel-Cox test for survival curves, and Kruskal-Wallis or ANOVA tests with correction for multiple comparisons. See also Figures S1–S4.
Systemic Depletion of Tregs Induces a Fraction of Preterm Births and Causes Adverse Neonatal Outcomes in the First Pregnancy
During the first pregnancy, the partial or total depletion of Tregs in the third week induced a 15% or 10% rate of preterm birth, respectively (Figure 3C). In addition, neonates born to partially or totally Treg-depleted Foxp3DTR dams displayed increased mortality from birth to three weeks of age (Figure 3D). However, no differences were observed between those born to partially and those born to totally Treg-depleted Foxp3DTR dams (Figure 3D). Moreover, surviving neonates born to partially or totally Treg-depleted Foxp3DTR dams were leaner than those from controls at one, two, and three weeks postpartum (Figures 3E–3G), but this effect was more drastic in neonates born to totally Treg-depleted Foxp3DTR dams (Figures 3F–3G). Given the adverse effects of the depletion of Tregs on neonatal outcomes, we also investigated whether the partial or total depletion of Tregs affected fetal growth in utero. Representative images of the fetuses and placentas from control (PBS), partially Treg-depleted, or totally Treg-depleted Foxp3DTR dams are shown in Figure 3H. Fetuses from partially and totally Treg-depleted Foxp3DTR dams were smaller and leaner than those from controls (Figures 3H and 3I), indicating that the depletion of Tregs affects fetal growth. However, placental weights were similar between controls and Treg-depleted mice (Figure 3J). These results show that the partial or total depletion of Tregs in the third week of the first pregnancy induces a fraction of preterm birth and, importantly, adverse fetal and neonatal outcomes.
Systemic Depletion of Tregs Induces a Fraction of Preterm Births and Causes Adverse Neonatal Outcomes in Repeat Pregnancies
A prior study has shown that Tregs undergo an accelerated expansion during the second pregnancy, which confers resilience against pregnancy loss (Rowe et al., 2012). Therefore, we reasoned that the total depletion of Tregs during the first and second pregnancy will worsen the rate of preterm birth and/or adverse neonatal outcomes observed during the first pregnancy. To examine this research question, Tregs were partially or totally depleted during the first and second pregnancy. The repeated partial or total depletion of Tregs induced comparable rates of preterm birth to the first pregnancy (15% and 14%, respectively) (Figure 3C). As observed in the first pregnancy, neonates born to partially and totally Treg-depleted Foxp3DTR dams during repeat pregnancies had higher rates of mortality compared to controls (Figure 3D). The repeated total depletion of Tregs in the first and second pregnancy induced a greater rate of neonatal mortality than the partial depletion of Tregs, a phenomenon that was not observed during the first pregnancy (Figure 3D). Like the first pregnancy, surviving neonates from partially or totally Treg-depleted Foxp3DTR dams during repeat pregnancies were leaner than their control counterparts at weeks one, two, and three postpartum (Figures 3E–3G). These results show that the repeated partial or total depletion of Tregs in the first and second pregnancy results in rates of preterm birth that are comparable to those of the first pregnancy. Nonetheless, the total depletion of Tregs in the first and second pregnancy had more deleterious effects on neonatal survival than the depletion of such cells in the first pregnancy alone.
The Adoptive Transfer of Tregs Prevents Preterm Birth and Adverse Neonatal Outcomes
Next, we evaluated whether the adoptive transfer of Tregs could prevent the adverse pregnancy outcomes induced by the depletion of such cells. Tregs were isolated from allogeneic pregnancies and adoptively transferred to partially Treg-depleted Foxp3DTR dams on 14.5 and 16.5 dpc, and pregnancy outcomes were recorded (Figure 4A). The rate of preterm birth in partially Treg-depleted Foxp3DTR dams was rescued by the adoptive transfer of Tregs (15% versus 0%) (Figure 4B). Moreover, neonatal survival at one, two, and three weeks of age was significantly improved after the adoptive transfer of Tregs (Figure 4C). The proportion of adoptively transferred Tregs was also determined using EGFP-expressing mice (see STAR Methods and Key Resources Table) (Figure 4D). Adoptively transferred Tregs were observed in the peripheral blood, ULNs, spleen, decidua, myometrium, and placenta (Figure 4E). These results provide a mechanistic demonstration that Tregs play a role in the timing of parturition and neonatal survival.
Figure 4. The Adoptive Transfer of Tregs Prevents Preterm Birth and Adverse Neonatal Outcomes.
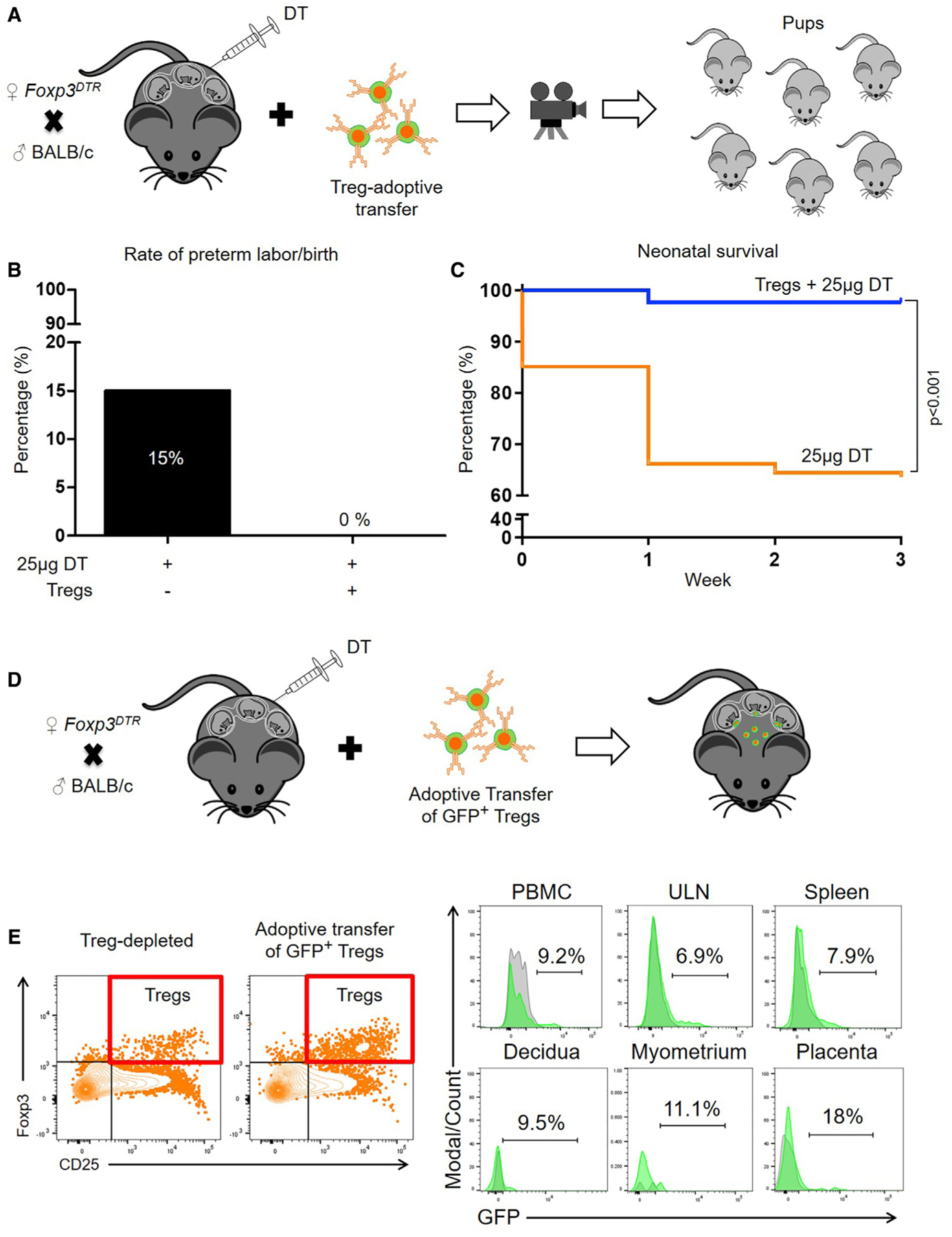
(A) Foxp3DTR dams underwent partial Treg depletion. On 14.5 and 16.5 dpc, Foxp3DTR dams received an adoptive transfer of Tregs from wild-type mice.
(B) Preterm birth rates of partially Treg-depleted Foxp3DTR dams without or with the adoptive transfer of Tregs (n = 6–20 per group). Data are represented as means of percentages.
(C) Percentage of survival from birth until 3 weeks postpartum for neonates born to partially Treg-depleted Foxp3DTR dams without or with the adoptive transfer of Tregs (n = 6–20 per group).
(D) Foxp3DTR dams underwent partial Treg depletion. On 14.5 and 16.5 dpc, Foxp3DTR dams received an adoptive transfer of Tregs from EGFP mice.
(E) Representative gating and histograms showing adoptively transferred GFP+ Tregs in the decidua, myometrium, placenta, and peripheral tissues (peripheral blood mononuclear cells [PBMCs], uterine-draining lymph nodes [ULNs], and spleen) of recipient Treg-depleted dams. Statistical analysis was performed using the Mantel-Cox test for survival curves.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD45-AlexaFluor700; Clone HI30 | BD Biosciences | Cat# 560566; RRID:AB_1645452 |
| CD3-APC-Cy7; Clone SK7 | BD Biosciences | Cat# 557832; RRID:AB_396890 |
| CD4-PE-CF594; Clone RPA-T4 | BD Biosciences | Cat# 562316; RRID:AB_11154394 |
| CD8-PE; Clone RPA-T8 | BD Biosciences | Cat# 561949; RRID:AB_10897146 |
| CD25-PE-Cy7; Clone M-A251 | BD Biosciences | Cat# 557741; RRID:AB_396847 |
| FoxP3-V450; Clone 259D/C7 | BD Biosciences | Cat# 560459; RRID:AB_1645591 |
| L-17A-AlexaFluor488; Clone N49–653 | BD Biosciences | Cat# 560489; RRID:AB_1645355 |
| gG1, κ Isotype Control-V450; Clone MOPC-21 | BD Biosciences | Cat# 560373; RRID:AB_1645606 |
| gG2b, κ Isotype Control-AlexaFluor 488; Clone 27–35 | BD Biosciences | Cat# 558716; RRID:AB_1645613 |
| CD3-APC-Cy7; Clone 145–2C11 | BD Biosciences | Cat# 557596; RRID:AB_396759 |
| CD4-APC; Clone RM4–5 | BD Biosciences | Cat# 553051; RRID:AB_398528 |
| CD8-PE-CF594; Clone 53–6.7 | BD Biosciences | Cat# 562283; RRID:AB_11152075 |
| CD25-PE; Clone 7D4 | Miltenyi Biotec | Cat# 130-118-550; RRID:AB_2784088 |
| Foxp3-V450; Clone MF23 | BD Biosciences | Cat# 561293; RRID:AB_10611728 |
| CD45-AF700; Clone 30-F11 | BD Biosciences | Cat# 560510; RRID:AB_1645208 |
| CD11b-BV737; Clone M1/70 | BD Biosciences | Cat# 564443; RRID:AB_2738811 |
| F4/80-APC-eFluor780; Clone BM8 | eBioscience | Cat# 12-4801-82; RRID:AB_465923 |
| Ly6G-BV395; Clone 1A8 | BD Biosciences | Cat# 563978; RRID:AB_2716852 |
| CD11c-BV711; Clone HL3 | BD Biosciences | Cat# 563048; RRID:AB_2734778 |
| CD49b-PE-CF594; Clone DX5 | BD Biosciences | Cat# 562453; RRID:AB_11153857 |
| CD19-BV421; Clone 1D3 | BD Biosciences | Cat# 562701; RRID:AB_2737731 |
| CD205-PerCP eFluor710; Clone 205yekta | eBioscience | Cat# 46-2051-82; RRID:AB_1834423 |
| NOS-PE; Clone CXNFT | eBioscience | Cat # 12-5920-82; RRID:AB_2572642 |
| Arg1-FITC; Polyclonal | R&D Systems | Cat # IC5868F; RRID:AB_10718118 |
| IFNγ-BV786; Clone XMG1.2 | BD Biosciences | Cat# 563773; RRID:AB_2738419 |
| IL10-BV605; Clone JES5–16E3 | BD Biosciences | Cat# 564082; RRID:AB_2738582 |
| TNFα-PECy7; Clone MP6-XT22 | BD Biosciences | Cat# 557644; RRID:AB_396761 |
| IL6-APC; Clone MP5–20F3 | BD Biosciences | Cat# 561367; RRID:AB_10679354 |
| CD3-BV650; Clone 145–2C11 | BD Biosciences | Cat# 564378; RRID:AB_2738779 |
| CD4-PECy5; Clone RM4–5 | BD Biosciences | Cat# 553050; RRID:AB_394586 |
| CD8-APC-Cy7; Clone 53–6.7 | BD Biosciences | Cat# 557654; RRID:AB_396769 |
| CD25-BV711; Clone PC61 | BD Biosciences | Cat# 740714; RRID:AB_2740396 |
| IL2-AF700; Clone JES6–5H4 | BD Biosciences | Cat# 561287; RRID:AB_10679118 |
| IL4-BV421; Clone 11B11 | BD Biosciences | Cat# 562915; RRID:AB_2737889 |
| Foxp3-AF488; Clone MF23 | BD Biosciences | Cat# 560403; RRID:AB_1645192 |
| IL17A-BV786; Clone TC11–18H10 | BD Biosciences | Cat# 564171; RRID:AB_2738642 |
| IgG2a, κ Isotype Control-PE; Clone eBR2a | eBioscience | Cat# 12-4321-80; RRID:AB_1834380 |
| IgG Control-FITC; Polyclonal | R&D Systems | Cat# IC016F; RRID:AB_1267476 |
| IgG1, κ Isotype Control-BV786; Clone R3–34 | BD Biosciences | Cat# 563847; RRID:N/A |
| IgG2b, κ Isotype Control-BV605; Clone R35–38 | BD Biosciences | Cat# 563145; RRID:N/A |
| IgG1, κ Isotype Control-PE-Cy7; Clone R3–34 | BD Biosciences | Cat# 557645; RRID:AB_396762 |
| IgG1, κ Isotype Control-APC; Clone R3–34 | BD Biosciences | Cat# 554686; RRID:AB_39857 |
| IgG1, κ Isotype Control-BV421; Clone R3–34 | BD Biosciences | Cat# 562868; RRID:AB_2734711 |
| IgG2b, κ Isotype Control AF488; Clone A95–1 | BD Biosciences | Cat# 557726; RRID:AB_396834 |
| CD45-APC-Cy7; Clone 2D1 | BD Biosciences | Cat# 557833, RRID: AB_396891 |
| CD3-PerCP-Cy5.5; Clone SK7 | BD Biosciences | Cat# 340949, RRID: AB_400190 |
| CD4-BUV737; Clone SK3 | BD Biosciences | Cat# 564305, RRID: AB_2713927 |
| CD8-BUV395; Clone RPA-T8 | BD Biosciences | Cat# 563795, RRID: AB_2722501 |
| CD25-PE-Cy7; Clone M-A251 | BD Biosciences | Cat# 557741, RRID: AB_396847 |
| IL-17A-BV650; Clone N49–653 | BD Biosciences | Cat# 563746, RRID: AB_2738402 |
| IgG1, κ Isotype Control-BV650; Clone X40 | BD Biosciences | Cat# 563231, RRID: N/A |
| FoxP3-Alexa Fluor 647; Clone 295DD/C7 | BD Biosciences | Cat# 560045, RRID: AB_1645411 |
| IgG1, κ Isotype Control-Alexa Fluor 647; Clone MOPC-21 | BD Biosciences | Cat# 557714, RRID: AB_396823 |
| FoxP3-Alexa Fluor 488; Clone 295DD/C7 | BD Biosciences | Cat# 560047, RRID: AB_1645349 |
| IgG1, κ Isotype Control-Alexa Fluor 488; Clone MOPC-21 | BD Biosciences | Cat# 557702, RRID: AB_396811 |
| Biological Samples | ||
| Human placental basal plate (decidua basalis) and chorioamniotic membrane (decidua parietalis) samples | Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver NICHD, NIH, DHHS, Wayne State University (Detroit, MI, USA), and the Detroit Medical Center (Detroit, MI, USA) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Diphtheria toxin (Corynebacterium diptheriae) | Sigma-Aldrich | Cat# D0564 |
| Diphtheria toxin (Corynebacterium diptheriae) | Calbiochem, EMD Millipore Corp | Cat# 322326-1MG |
| Diphtheria toxin (Corynebacterium diptheriae) | Enzo Life Sciences | Cat# BML-G135 |
| Lipopolysaccharides from Escherichia coli O55:B5 | Sigma-Aldrich | Cat# L6259-1MG |
| BD Horizon Fixable Viability Stain 510 | BD Biosciences | Cat# 564406 |
| BD Horizon Fixable Viability Stain 575V | BD Biosciences | Cat# 565694 |
| LIVE/DEAD Fixable Green Dead Cell Stain Kit | Invitrogen, Thermo Fisher Scientific | Cat# L23101 |
| CellTrace Violet Cell Proliferation Kit | Molecular Probes, Thermo Fisher Scientific | Cat# C34557 |
| IL2 Recombinant Human Protein | GIBCO, Thermo Fisher Scientific | Cat# PHC0026 |
| 2-Mercaptoethanol | GIBCO, Thermo Fisher Scientific | Cat# 21985-023 |
| Mouse IL-2 | Miltenyi Biotec | Cat# 130-094-054 |
| Animal Free Recombinant Mouse IFNγ | PeproTech | Cat# AF-315-05 |
| Recombinant Mouse CCL7 | R&D Systems | Cat# 456-MC-010/CF |
| Recombinant Mouse IL-22 | Biolegend | Cat# 576202 |
| Deposited Data | ||
| RNA-Seq data of murine placental tissues | This manuscript | GEO: GSE145357 |
| Experimental Models: Organisms/Strains | ||
| B6.129(Cg)-Foxp3tm3(DTR/GFP)Ayr (Foxp3DTR) | The Jackson Laboratory | Stock# 016958 |
| C57BL/6-Tg (CAG-EGFP)131Osb/LeySopJ (EGFP) | The Jackson Laboratory | Stock# 006567 |
| C57BL/6 | The Jackson Laboratory | Stock# 000664 |
| BALB/cBy | The Jackson Laboratory | Stock# 001026 |
| Other | ||
| Stain buffer | BD Biosciences | Cat# 554656 |
| Foxp3/Transcription Factor Fixation/Permeabilization solution | eBioscience | Cat# 00-5523-00 |
| CD4+ CD25+ Regulatory T Cell Isolation Kit, mouse | Miltenyi Biotec | Cat# 130-091-041 |
| Treg Expansion Kit, mouse (CD3/CD28 MACSiBead particles) | Miltenyi Biotec | Cat# 130-095-925 |
| Dynabeads Human T-Activator CD3/CD28 for T Cell Expansion and Activation | GIBCO, Thermo Fisher Scientific | Cat# 11161D |
| ProcartaPlex Mouse Cytokine & Chemokine Panel 1A 36-plex | ThermoFisher | Cat# EPX360-26092-901 |
Systemic Depletion of Tregs Increases the Susceptibility to Endotoxin-Induced Preterm Birth
The depletion of Tregs did not always induce preterm birth (Figure 3C). Therefore, we reasoned that the loss of such cells, in some cases, could increase the susceptibility to lipopolysaccharide (LPS or endotoxin)-induced preterm birth. Partially Treg-depleted Foxp3DTR dams received a mild dose of LPS (that does not cause preterm birth in wild-type mice) on 16.5 dpc and were monitored until delivery (Figure S4A). The injection of LPS in Treg-depleted Foxp3DTR dams induced a 33% rate of preterm birth, whereas all non-Treg-depleted Foxp3DTR dams delivered at term (Figure S4B). Moreover, the injection of LPS caused a higher rate of neonatal mortality in Treg-depleted Foxp3DTR dams than in non-Treg-depleted Foxp3DTR dams (Figure S4C). Foxp3DTR dams injected with DT alone (on 14.5 and 15.5 dpc) did not deliver preterm, and their neonates thrived for up to 3 weeks, as controls did (data not shown). These findings indicate that the depletion of Tregs also increases the susceptibility of the mother to deliver preterm upon encountering inflammatory agents (e.g., bacterial infection).
Systemic Depletion of Tregs Does Not Negatively Affect Maternal Obstetrical Parameters
Previous studies have shown that the long-term depletion of Tregs induces systemic disease (Kim et al., 2007; Gousopoulos et al., 2016), including severe acute inflammation (Kim et al., 2007). This finding raised the question as to whether the total or partial depletion of Tregs used in the current study could cause systemic acute disease that, in turn, may be the cause of mice delivering preterm. We argue that this could not be the case because the Treg depletion used herein was solely performed during the third week of pregnancy. Nonetheless, we conducted a series of obstetrical determinations that are commonly performed in pregnant women to evaluate maternal and fetal well-being (Sotiriadis et al., 2019; Poon et al., 2019). Partially and totally Treg-depleted Foxp3DTR dams, as well as control dams, underwent body temperature monitoring, blood pressure determination, and high-resolution ultrasound imaging (Figure 5A) (see STAR Methods). Systemic acute inflammation induces hypothermia in mice (Gomez-Lopez et al., 2018); however, the depletion of Tregs did not cause hypothermia. Thus, partially and totally Treg-depleted Foxp3DTR dams displayed body temperature similar to that of control dams (Figure 5B). In addition, partially and totally Treg-depleted Foxp3DTR dams had blood pressure similar to that of control dams (Figure 5C). Uterine artery Doppler allowed the determination of the maternal heart rate and uterine artery pulsatility index (Figure 5D). Partially and totally Treg-depleted Foxp3DTR dams had heart rates similar to those of control dams (Figure 5E). Totally Treg-depleted Foxp3DTR dams had reduced uterine artery pulsatility indices compared to control dams (Figure 5F). However, only an increased uterine artery Doppler is associated with hypertensive disorders (e.g., preeclampsia) and adverse perinatal outcomes (Poon et al., 2019). Umbilical artery Doppler allowed the determination of the fetal heart rate and umbilical artery pulsatility index (Figure 5G). Fetuses of totally, but not partially, Treg-depleted Foxp3DTR dams were bradycardic (reduced heart rates compared to controls) (Figure 5H), which is consistent with our earlier observations showing that Treg depletion causes fetal compromise (Figures 3H and 3I). However, fetuses of partially and totally Treg-depleted Foxp3DTR dams displayed umbilical artery pulsatility indices similar to those of control fetuses (Figure 5I). The latter finding is relevant, because an abnormal umbilical artery pulsatility index is associated with a cytokine storm in the maternal circulation (Gomez-Lopez et al., 2016a), indicating that Treg depletion is not associated with severe acute inflammation in the maternal circulation.
Figure 5. Maternal-Fetal Obstetrical Parameters upon Partial or Total Treg Depletion.
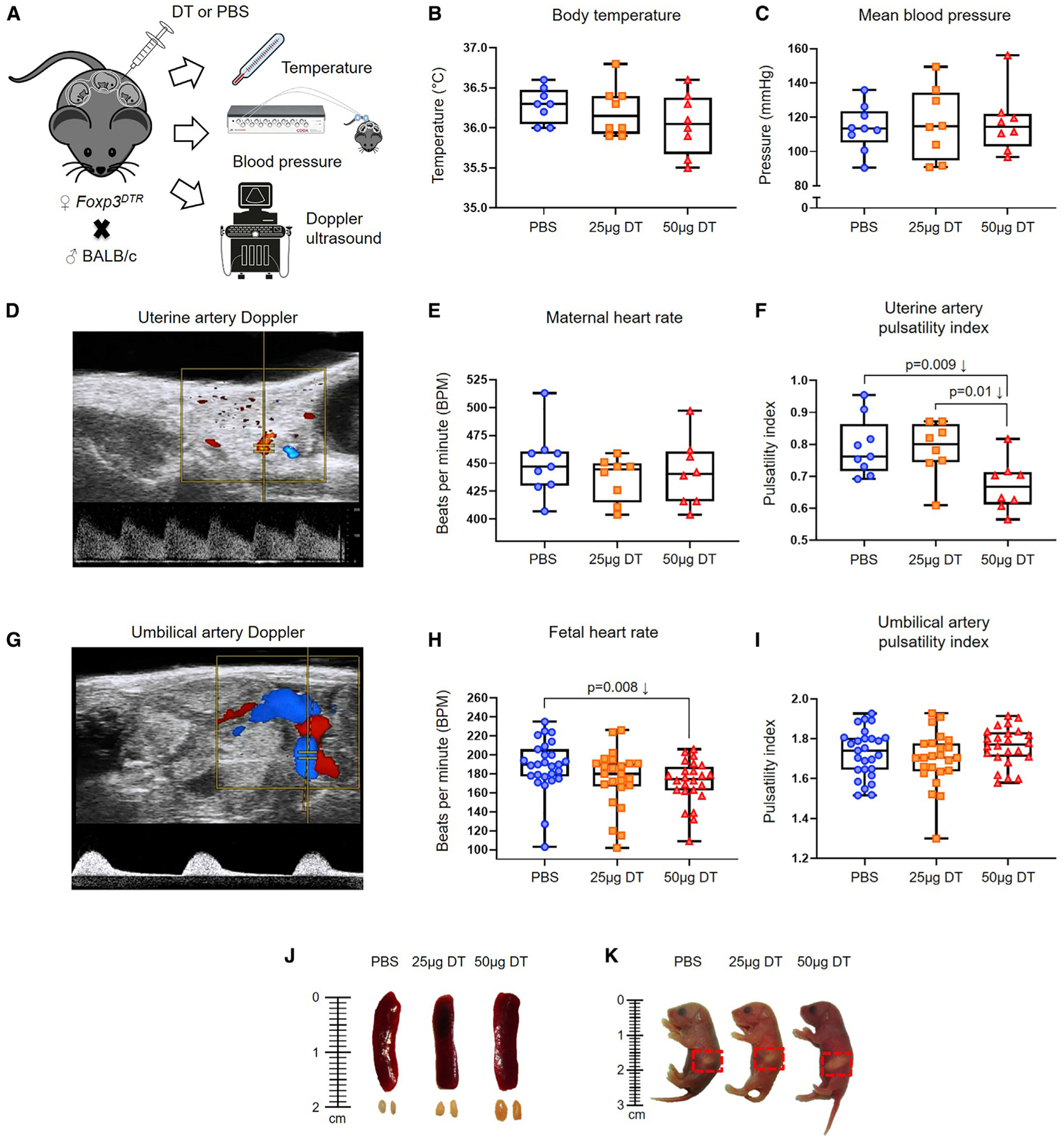
(A) Foxp3DTR dams underwent partial or total Treg depletion until 17.5 dpc on which body temperature, blood pressure, and Doppler determinations were performed (n = 8–9 per group).
(B and C) Body temperature (B) and mean blood pressure (C) of non-Treg-depleted-, partially Treg-depleted-, and totally Treg-depleted-Foxp3DTR dams (n = 8–9 per group).
(D–F) Representative Doppler image of the uterine artery (D), which was used to determine (E) maternal heart rate, and (F) uterine artery pulsatility index of non-Treg-depleted-, partially Treg-depleted-, and totally Treg-depleted-Foxp3DTR dams (n = 8–9 per group).
(G–I) Representative Doppler image of the umbilical artery (G), which was used to determine (H) fetal heart rate, and (I) umbilical artery pulsatility index in fetuses of non-Treg-depleted-, partially Treg-depleted-, and totally Treg-depleted-Foxp3DTR dams (n = 24–27 per group).
(J) Representative images of the spleens and ULNs from non-Treg-depleted-, partially Treg-depleted-, and totally Treg-depleted-Foxp3DTR dams (n = 3 per group).
(K) Representative images of neonates born to non-Treg-depleted-, partially Treg-depleted-, and totally Treg-depleted-Foxp3DTR dams on the day of birth (n = 3 per group). Red dotted squares indicate the presence of the milk band. Statistical analysis was performed using the Kruskal-Wallis or ANOVA tests with correction for multiple comparisons.
The long-term depletion of Tregs causes splenomegaly (Kim et al., 2007); therefore, we also evaluated the size of the spleens and ULNs. Partially and totally Treg-depleted Foxp3DTR dams had a similar-sized spleen compared to control dams, but ULNs of totally Treg-depleted dams were slightly enlarged (Figure 5J). However, neonates of partially or totally Treg-depleted Foxp3DTR dams that survived were breastfed by the dams (Figure 5K; see milk band indicating that newborns were breastfed).
These physiological determinations show that the depletion of Tregs during the third week of pregnancy neither negatively affects maternal obstetrical parameters nor induces splenomegaly or alters breastfeeding, yet it induces fetal compromise.
Systemic Depletion of Tregs Induces a Mild Inflammatory Response in the Maternal Circulation that Can Induce Early-Term Delivery
Next, we investigated whether the depletion of Tregs alters cytokine responses in the maternal circulation. This question arose from some cases of preterm labor being characterized by diverse inflammatory responses in the maternal circulation (Gervasi et al., 2001; Sorokin et al., 2010; Cruciani et al., 2010; Cobo et al., 2013; Park et al., 2018). However, these systemic immune responses vary between subsets of preterm labor; therefore, the determination of specific cytokines in the maternal circulation is not useful to predict preterm birth. Regardless, we reasoned that by using targeted approaches (e.g., Treg depletion), we may be able to observe stereotypical immune responses in the maternal circulation that may other-wise be difficult to identify in humans. Foxp3DTR dams underwent partial or total depletion of Tregs, and in preterm gestations, the maternal plasma was collected for determination of 36 cytokines using a multiplex system (see STAR Methods and Key Resources Table). Consistent with our hypothesis, the depletion of Tregs did not increase the conventional acute pro-inflammatory cytokines IL-6, CCL2, IL-1β, and tumor necrosis factor alpha (TNF-α) in the maternal circulation (Figures 6A–6D). As expected (Garcia-Flores et al., 2018), these and other acute pro-inflammatory cytokines were increased in a model of endotoxin-induced preterm birth, but not in the partially or totally Treg-depleted Foxp3DTR dams (Figure S5). Interestingly, totally Treg-depleted Foxp3DTR dams displayed higher plasma concentrations of interferon gamma (IFNγ), CCL7, and IL-22 compared to control dams (Figures 6E–6G). The plasma concentrations of IL-10 (a cytokine produced by Tregs) (Kemper et al., 2003) tended to be reduced in Treg-depleted Foxp3DTR dams; however, this reduction did not reach statistical significance (Figure 6H). To complement these observations, we tested whether systemic administration of IFNγ, CCL7, and IL-22 at pathological concentrations (concentrations found in the maternal circulation upon Treg depletion) could induce preterm birth and neonatal mortality (see STAR Methods and Key Resources Table), as Treg depletion does. Systemic administration of CCL7 and IL-22, but not IFNγ, shortened the gestational length but was insufficient to induce preterm birth (i.e., delivery before 18.5 dpc) (Figure 6I). Thus, a fraction of dams injected with CCL7 and/or IL-22 underwent early-term delivery (Figure 6J). However, pups born to dams injected with CCL7 and/or IL-22 did not show significantly higher mortality rates at birth compared to controls (Figure 6K). The combined administration of CCL7 and IL-22 resulted in similar outcomes to those observed with the single administration of each cytokine (data not shown). Collectively, these results indicate that the mild inflammatory response observed in Treg-depleted dams partially participates in the timing of parturition but alone is not sufficient to induce preterm birth or neonatal mortality.
Figure 6. Depletion of Tregs Induces a Mild Systemic Inflammatory Response in the Absence of Intra-Amniotic Inflammation.
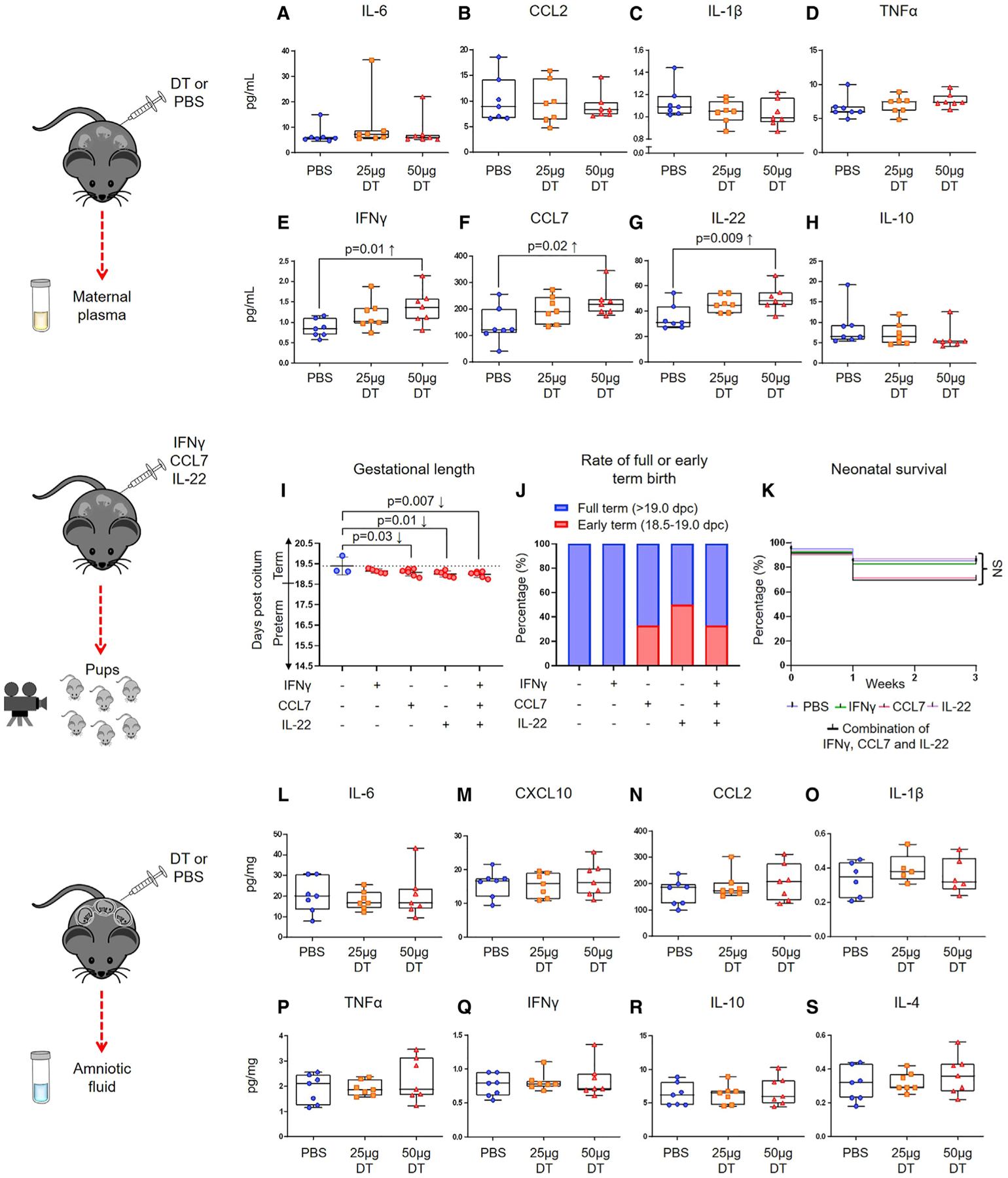
(A–H) Foxp3DTR dams underwent partial or total Treg depletion. Controls were injected with sterile 13 PBS. Mice were euthanized approximately 4 h after the second DT or PBS injection, and maternal plasma samples and amniotic fluid were collected. Concentrations of (A) IL-6, (B) CCL2, (C) IL-1β, (D) TNF-α, (E) IFNγ, (F) CCL7, (G) IL-22, and (H) IL-10 in the maternal plasma (n = 7 per group). Data are shown as medians with interquartile ranges and minimum/maximum ranges. C57BL/6 dams were intravenously injected with recombinant mouse IFNγ (1.4 pg/100 μL), CCL7 (218 pg/100 μL), IL-22 (48 pg/100 μL), or a combination of all three. Controls were injected with 100 μL of sterile 13 PBS alone.
(I) Gestational length of cytokine-injected dams. Data are shown as means with standard deviations (n = 3–6 per group).
(J) Rate of early-term or full-term delivery of cytokine-injected dams (n = 3–6 per group).
(K) Percentage of survival from birth until 3 weeks postpartum for neonates born to cytokine-injected dams (n = 3–6 litters per group).
(L–S) Concentrations of (L) IL-6, (M) CXCL10, (N) CCL2, (O) IL-1β, (P) TNF-α, (Q) IFNγ, (R) IL-10, and (S) IL-4 in the amniotic fluid (n = 5–7 per group). Data are shown as medians with interquartile ranges and minimum/maximum ranges.
Statistical analysis was performed using the Mantel-Cox test for survival curves, and Kruskal-Wallis or ANOVA tests with correction for multiple comparisons. See also Figure S5.
Systemic Depletion of Tregs Does Not Cause Intra-amniotic Inflammation
Until this point, we have shown that the depletion of Tregs causes fetal compromise and adverse neonatal outcomes in the absence of a severe acute inflammatory response in the maternal circulation. This scenario resembles the subclinical presentation of PTL (Barros et al., 2015). However, some of these cases occur in the presence of localized sterile intra-amniotic inflammation (Romero et al., 2015). Therefore, we next explored whether the partial or total depletion of Tregs affects the cytokine network in the amniotic cavity. Foxp3DTR dams underwent partial or total depletion of Tregs, and in preterm gestations, amniotic fluid was collected to determine 36 cytokines by using a multiplex system (see STAR Methods and Key Resources Table). The depletion of Tregs did not induce an intra-amniotic inflammatory response (Figures 6L–6S). Indeed, pro-inflammatory cytokines—IL-6 (Yoon et al., 2001; Gervasi et al., 2012; Combs et al., 2014; Romero et al., 2014b, 2014c), CXCL10 (Kim et al., 2010; Romero et al., 2017), CCL2 (Esplin et al., 2005), and others (Romero et al., 1992, 2015; Cox et al., 1997; Dudley et al., 1997)—that have been reported to be elevated in some cases of intra-amniotic inflammation/infection-associated preterm labor were unchanged in Treg-depleted dams compared to controls (Figures 6L–6Q). Stereotypical anti-inflammatory cytokines, namely, IL-10 (D’Andrea et al., 1993; de Waal Malefyt et al., 1993; Wei et al., 2017) and IL-4 (Hart et al., 1989; Fenton et al., 1992), were also unchanged upon the depletion of such cells (Figures 6R and 6S). These results show that the reduction of Tregs can lead to adverse perinatal outcomes in the absence of an intra-amniotic inflammatory response, resembling idiopathic PTL.
Systemic Depletion of Tregs Induces Specific Cellular Immune Responses at the Maternal-Fetal Interface, in the Placenta, and in the Maternal Circulation
To further explore the mechanisms whereby the loss of Tregs induces adverse perinatal outcomes, we explored the cellular repertoire at the maternal-fetal interface, in the placenta, and in the maternal circulation. This research question was based on previous studies showing that PTL involves stereotypical cellular responses at the maternal-fetal interface (Arenas-Hernandez et al., 2016, 2019; St Louis et al., 2016; Xu et al., 2016; Gomez-Lopez et al., 2017a; Rinaldi et al., 2017; Garcia-Flores et al., 2018; Xu et al., 2018; Leng et al., 2019; Slutsky et al., 2019), in the placenta (Salafia et al., 1991; Redline and Patterson, 1994; Kim et al., 2009, 2015b; Gill et al., 2019), and in the maternal circulation (Gervasi et al., 2001; Pique-Regi et al., 2019). Foxp3DTR dams underwent partial or total depletion of Tregs, and the decidua, myometrium, placenta, and peripheral blood were collected in preterm gestations. Immunophenotyping was performed, and data were represented in heatmaps (see STAR Methods). Overall, the partial or total depletion of Tregs in Foxp3DTR dams induced specific immune alterations in the cellular repertoire among compartments (Figure 7A; Figure S6). Specifically, the partial depletion of Tregs in Foxp3DTR dams reduced Arg1+ B cells and increased total macrophages in the decidual tissues (Figure 7A). In the myometrium, the total depletion of Tregs consistently reduced transforming growth factor β (TGF-β)+ and IL-10+ CD4+ T cells (Figure S6), as well as Arg1+ neutrophils (Figure 7A). In the maternal circulation, the depletion of Tregs caused the reduction of inducible nitric oxide synthase (iNOS)+ dendritic cells and an increase in TNF-α+ B cells (Figure 7A). Similar to the maternal circulation, Treg depletion induced cellular responses in the placental tissues; however, these changes did not hold statistical significance after adjustment for multiple comparisons (Figure 7A). Altogether, these data show that Tregs modulate the expression of specific mediators by immune cells present at the maternal-fetal interface, in the placenta, and in the maternal circulation.
Figure 7. Depletion of Tregs Is Associated with Altered Systemic and Local Cellular Immune Responses and Dysregulation of Developmental and Cellular Processes in the Placenta.
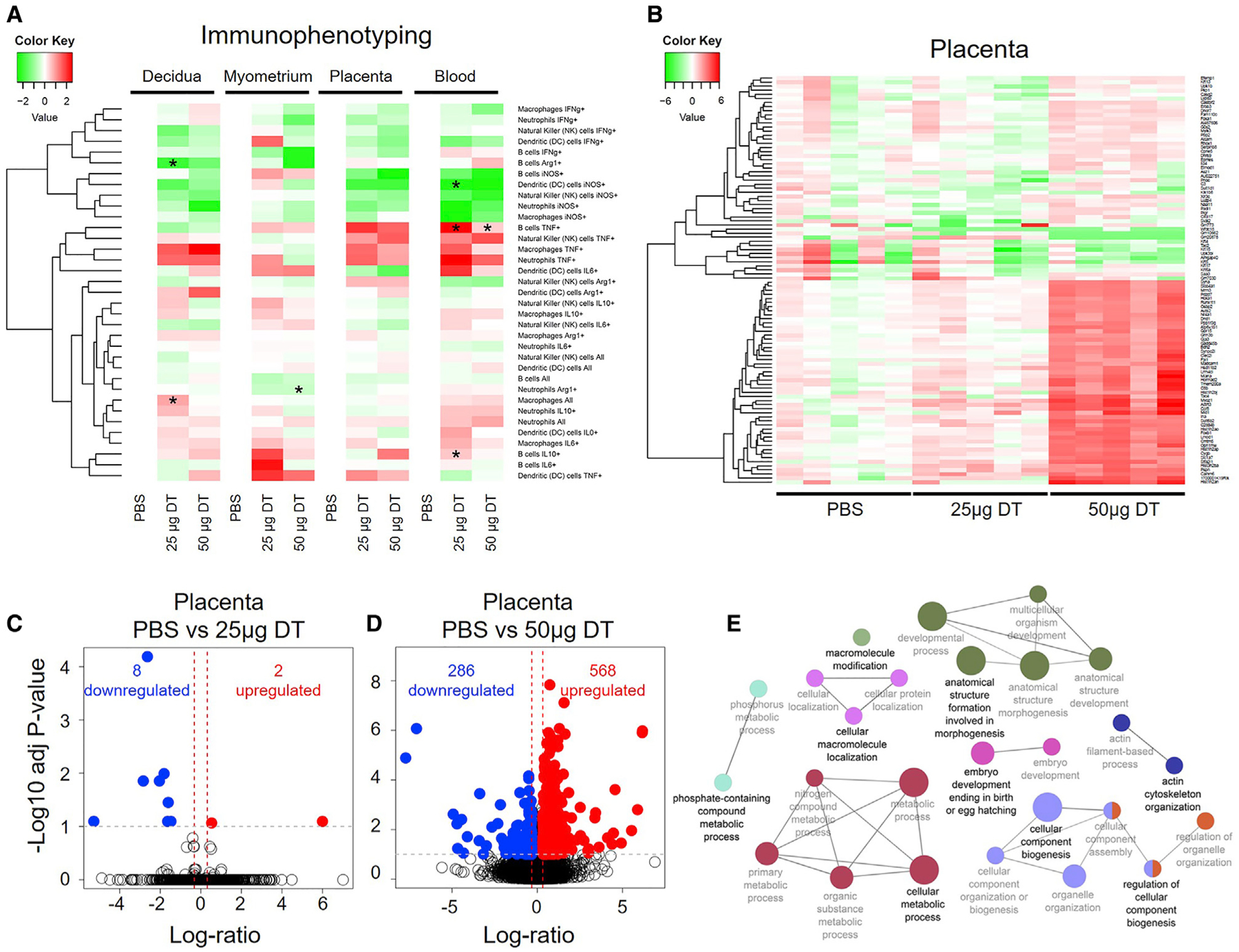
Foxp3DTR dams underwent partial or total Treg depletion. Controls were injected with sterile 13 PBS. Mice were euthanized approximately 4 h after the second injection and the decidua, myometrium, placenta, and peripheral blood were collected for flow cytometry (all tissues, n = 5–7 per group) or for RNA-seq analysis (placenta only, n = 5 per group).
(A) Heatmap visualization of changes in the log2-transformed frequencies of immune cell subsets in the decidua, myometrium, placenta, and peripheral blood of partially and totally Treg-depleted Foxp3DTR dams relative to controls. Red and green indicate increased and reduced abundance, respectively, relative to PBS controls.
(B) Heatmap visualization of changes in gene expression in the placentas of partially and totally Treg-depleted Foxp3DTR dams and controls. Red indicates gene upregulation and green indicates gene downregulation relative to the average value in the PBS group.
(C) Volcano plot showing genes differentially expressed between placentas from partially Treg-depleted Foxp3DTR dams and those from control dams.
(D) Volcano plot showing genes differentially expressed between placental tissues from totally Treg-depleted Foxp3DTR dams and those from control dams.
(E) Network of biological processes dysregulated in the placentas of totally Treg-depleted Foxp3DTR dams.
Statistical analysis was performed using t-tests with false discovery rate adjustment. Asterisks indicate significant differences compared to controls after adjustment. See also Figure S6 and Tables S3, S4, S5, S6, S7, and S8.
RNA-Seq Analysis Reveals that Tregs Are Central for Regulation of Developmental and Cellular Processes in the Placenta
The loss of Tregs induces fetal growth restriction (Figures 3H and 3I), whose pathophysiology involves placental disease (Burton and Jauniaux, 2018). Therefore, we next used RNA sequencing (RNA-seq) to survey the molecular processes dysregulated in the placenta upon Treg depletion (see STAR Methods). The partial depletion of Tregs caused mild dysregulation of the placental transcriptome (Figures 7B and 7C; Tables S3 and S4). However, the total depletion of Tregs caused significant dysregulation of several physiological processes in the placenta (Figures 7B and 7D). Specifically, total depletion of Tregs induced the upregulation of 568 genes and the downregulation of 286 genes (Figure 7D; Tables S5 and S6). The dysregulated pathways included embryo development ending in birth, anatomical structure formation involved in morphogenesis, cellular macromolecule localization, cellular metabolic process, cellular component biogenesis, and actin cytoskeleton organization (Figure 7E; Tables S7 and S8). This last set of data demonstrates that Tregs play a role in fetal life and provides a possible explanation as to why the offspring of Treg-depleted dams display impaired growth and, more importantly, adverse neonatal outcomes.
DISCUSSION
This study provides descriptive and mechanistic evidence showing a role for Tregs in the pathophysiology of a subset of idiopathic preterm labors/births and adverse neonatal outcomes. First, we showed that functional Tregs are reduced at the human maternal-fetal interface in a subset of women with idiopathic PTL and birth. This finding is consistent with previous clinical reports showing that women with PTL display a reduction in the suppressive activity of peripheral Tregs (Schober et al., 2012; Gomez-Lopez and Laresgoiti-Servitje, 2012). Therefore, both peripheral and local Tregs are reduced in women who undergo spontaneous PTL and birth. However, the reduction in Tregs at the maternal-fetal interface does not occur in the physiological process of labor at term. This latter finding is in line with the hypothesis that some cases of preterm labor are mediated through a mechanism different from term parturition (Holt et al., 2011; Gomez-Lopez et al., 2017b; Yellon, 2017; Willcockson et al., 2018; Paquette et al., 2018; Pereyra et al., 2019). The most marked reduction of Tregs occurred at the area of contact between the endometrium and the fetal chorioamniotic membranes (i.e., decidua parietalis) in the presence of chronic inflammation of the placenta, which is a pathological process mediated by effector activated T cells (Kim et al., 2015b; Arenas-Hernandez et al., 2019). These data allowed us to hypothesize that the decline in Tregs in the decidua parietalis is accompanied by augmented T-cell responses at the maternal-fetal interface in a subset of women who underwent idiopathic PTL and birth.
Effector memory CD4+ and CD8+ T cells are present at the human maternal-fetal interface in term and preterm gestations (van der Zwan et al., 2018; Arenas-Hernandez et al., 2019; Slutsky et al., 2019). These T cells are increased in women with preterm labor, leading to preterm birth (Arenas-Hernandez et al., 2019). In the current study, we provide further data indicating that decidual CD8+ T cells expressing IL-17A are implicated in the pathophysiology of preterm parturition in the absence of acute inflammation in the placenta (i.e., idiopathic preterm birth). In addition to the inflammatory cytokine IL-17A (Yao et al., 1995; Fossiez et al., 1996; Onishi and Gaffen, 2010; McGeachy et al., 2019), most Tc17 cells secrete pro-inflammatory mediators such as TNF-α and IL-2 but have reduced cytotoxic activity (Hamada et al., 2009; Huber et al., 2009); therefore, we suggest that the imbalance between Tregs and Tc17 cells at the human maternal-fetal interface creates a hostile inflammatory milieu that leads to preterm parturition, even in the absence of acute inflammation in the placenta or amniotic cavity. This study demonstrates that Tc17 cells participate in the pathophysiology of preterm birth, yet further research is required to reveal their specific functions during pregnancy.
Consistent with the human findings, the depletion of Tregs during the third week of pregnancy induced preterm birth that was restored upon the adoptive transfer of such cells. The rates of preterm birth resulting from the depletion of Tregs were modest compared to animal models of systemic and local acute inflammation-induced preterm birth (St Louis et al., 2016; Gomez-Lopez et al., 2016a, 2016b, 2018, 2019; Garcia-Flores et al., 2018). However, this is consistent with the current belief that different subsets of preterm labor and birth are mediated through different mechanisms (Romero et al., 2014a). Thus, in line with this concept, the depletion of Tregs should be responsible only for a subset of idiopathic preterm labors and births. The partial and total depletion of Tregs induced similar rates of preterm birth, which suggests that only a fraction of Tregs need to be depleted to cause preterm birth, regardless of parity. This is relevant to the clinical scenario, in which both nulliparous and multiparous women can present with PTL associated with a reduction of Tregs.
Importantly, the depletion of Tregs induces a fraction of preterm births in the absence of severe systemic acute inflammation, alterations in obstetrical parameters, and intra-amniotic inflammation. These features resemble the clinical scenario of idiopathic PTL (Barros et al., 2015), which is not observed in other established animal models of preterm birth, such as systemic inflammation-induced (Arenas-Hernandez et al., 2019), acute intra-amniotic inflammation-induced (Garcia-Flores et al., 2018; Gomez-Lopez et al., 2018), and progesterone antagonist-induced (Arenas-Hernandez et al., 2019) preterm birth, as well as in another acute inflammatory model (St Louis et al., 2016). Therefore, we surmise that the loss of Tregs is directly implicated in the pathophysiology of this subset of preterm labor/birth. However, the underlying etiologies leading to reduced Tregs, such as dysregulation of the vaginal microbiome (Elovitz et al., 2019; Fettweis et al., 2019) or intestinal microbiome (Shiozaki et al., 2014), require further investigation.
In searching for the mechanisms implicated in this model of idiopathic preterm labor/birth, we found that the depletion of Tregs induced elevated concentrations of CCL7, IL-22, and IFNγ in the maternal circulation. However, only systemic administration of CCL7 and IL-22 at pathological concentrations shortened the length of gestation, inducing early-term delivery. Previous clinical reports have shown that CCL7 (Laudanski et al., 2014) and IL-22 (Bersani et al., 2015) are slightly increased in women with preterm labor and preeclampsia, respectively. Furthermore, the administration of IL-22 reduces Tregs and increases alloreactive Teffs (Zhao et al., 2014). Hence, we propose that the depletion of Tregs shortens the length of gestation by increasing these inflammatory cytokines in the maternal circulation, even though neither the individual nor the combined systemic effects of these cytokines can entirely explain the pathophysiology of preterm birth resulting from the loss of Tregs.
Further investigation revealed that the depletion of Tregs altered specific cellular responses in the maternal circulation, at the maternal-fetal interface, and in the placenta. Overall, Treg depletion increased the abundance of pro-inflammatory immune cells such as B cells (Menard et al., 2007) and neutrophils (Bazzoni et al., 1991) expressing TNF-α and reduced homeostatic immune cells such as arginase 1-expressing neutrophils (Munder et al., 2006). As expected, IL-10- and TGF-β-expressing CD4+ T cells (i.e., Tregs) were also reduced. Therefore, it is tempting to suggest that Tregs play a central role during late pregnancy in modulating systemic and local cellular responses and that their absence can cause a pro-inflammatory environment, leading to preterm birth.
Importantly, fetuses of Treg-depleted dams exhibited signs of fetal growth restriction, which allowed us to hypothesize that the loss of maternal Tregs causes alterations in placental development. In line with this hypothesis, we found that several key biological processes primarily related to embryo development and cellular metabolic processes were dysregulated in the placental tissues upon Treg depletion. These findings are consistent with previous reports showing that Tregs participate in the development and function of the placenta (Kahn and Baltimore, 2010; Samstein et al., 2012; Loewendorf et al., 2015; Nguyen et al., 2017, 2018; Care et al., 2018). Recently, it was shown that the adoptive transfer of uterine-like NK cells can serve as a treatment for growth-restricted fetuses (Fu et al., 2017), suggesting that Tregs and NK cells may contribute to the development of offspring.
We also showed that the deleterious effects of Treg depletion on the placenta were carried over to neonatal life, given that a subset of neonates born to Treg-depleted dams died before weaning age. This finding is consistent with clinical studies showing that newborns with growth restriction have fewer cord blood Tregs (Steinborn et al., 2010; Mukhopadhyay et al., 2014). Herein, we also found that adverse neonatal outcomes were more prevalent when Tregs were depleted in repeat pregnancies, supporting the concept that pregnancy imprints protective regulatory memory (Rowe et al., 2012) and that the resulting enhanced Treg expansion in a second pregnancy is involved not only in maternal-fetal tolerance but also in neonatal well-being. Lastly, we provided a causal link between neonatal mortality and Treg depletion since the adoptive transfer of these cells mitigated adverse perinatal outcomes.
In summary, we have shown that a subset of women with idiopathic PTL and birth, formerly considered to have an unknown cause, is associated with a reduction of functional Tregs at the maternal-fetal interface. This finding is in tandem with clinical reports showing that women who underwent preterm labor and birth have reduced numbers and function of Tregs in the maternal circulation (Xiong et al., 2010; Schober et al., 2012). Consistently, the systemic deficiency of Tregs results in a fraction of preterm births in first and repeat pregnancies and, more importantly, induces adverse outcomes in offspring. The mechanisms whereby the loss of Tregs induces adverse perinatal outcomes involve alterations in cellular and soluble immune responses in the mother and at the maternal-fetal interface, as well as dysregulation of developmental and cellular processes in the placenta. This study provides insight into the mechanisms of disease for idiopathic PTL and birth, the leading cause of neonatal morbidity and mortality worldwide.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Nardhy Gomez-Lopez (nardhy.gomez-lopez@wayne.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The accession number for the RNA-seq data reported in this paper is GEO: GSE145357.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human subjects, clinical specimens, and definitions
Human placental basal plate (decidua basalis) and chorioamniotic membrane (decidua parietalis) samples were obtained at the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, U.S. Department of Health and Human Services, Wayne State University (Detroit, MI, USA), and the Detroit Medical Center (Detroit, MI, USA). The collection and use of human materials for research purposes were approved by the Institutional Review Boards of NICHD and Wayne State University. All participating women provided written informed consent prior to sample collection. The study groups included women who delivered at term with labor/birth (TIL), at term without labor (TNL), preterm with labor (PTL), or preterm without labor (PTNL). Patients with PTL were further categorized into those who underwent idiopathic preterm labor/birth without inflammation (iPTL), those with idiopathic preterm labor/birth with chronic inflammatory lesions of the placenta (iPTL+CI), and those with preterm labor/birth with acute inflammatory lesions of the placenta (PTL+AI). Patients with TIL were further categorized into those who underwent term labor without inflammation (TIL), those with chronic inflammatory lesions of the placenta (TIL+CI), and those with acute inflammatory lesions of the placenta (TIL+AI). The demographic and clinical characteristics of the study groups are shown in Tables S1 and S2. Labor was defined by the presence of regular uterine contractions at a frequency of at least two contractions every 10 min with cervical changes resulting in delivery. Preterm delivery was defined as delivery < 37 weeks of gestation. Patients with multiple births or neonates that had congenital or chromosomal abnormalities were excluded from this study.
Placental histopathological examination
Placentas were examined histologically by a perinatal pathologist blinded to clinical diagnoses and obstetrical outcomes according to standardized Perinatology Research Branch protocols. Briefly, three to nine sections of the placenta were examined, and at least one full-thickness section was taken from the center of the placenta; others were taken randomly from the placental disc. Chronic and acute inflammatory lesions of the placenta were diagnosed following established Perinatology Research Branch protocols (Redline et al., 2003, Redline, 2006, Kim et al., 2015a, 2015b).
Mice
B6.129(Cg)-Foxp3tm3(DTR/GFP)Ayr (Foxp3DTR), BALB/cBy (BALB/c), C57BL/6-Tg (CAG-EGFP)131Osb/LeySopJ (EGFP), and C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were bred in the animal care facility at the C.S. Mott Center for Human Growth and Development (Wayne State University, Detroit, MI, USA) and housed under a circadian cycle (light:-dark = 12:12 h). Eight- to twelve-week-old Foxp3DTR, EGFP, or C57BL/6 females were mated with BALB/c or C57BL/6 males of proven fertility. Female mice were examined daily between 8:00 and 9:00 a.m. for the presence of a vaginal plug, which indicated 0.5 days post coitum (dpc). After observation of vaginal plugs, female mice were removed from the mating cages and housed separately. A weight gain ≥2 g confirmed pregnancy at 12.5 dpc. All animal experiments were approved by the Institutional Animal Care and Use Committee at Wayne State University (Protocol No: A-09-08-12, A-07-03-15 and 18-03-0584).
METHOD DETAILS
Decidual leukocyte isolation from human samples
Leukocytes were isolated from human decidual tissue (Table S2) as previously described (Xu et al., 2015). Briefly, the decidua basalis was collected from the basal plate of the placenta, and the decidua parietalis was separated from the chorioamniotic membranes. Decidual tissue was homogenized using a gentleMACS Dissociator (Miltenyi Biotec, San Diego, CA, USA) in StemPro Cell Dissociation Reagent (Life Technologies, Grand Island, NY, USA). Homogenized tissues were incubated for 45 min at 37°C with gentle agitation. After incubation, tissues were washed with 1X PBS (Life Technologies) and filtered through a 100 μm cell strainer. Cell suspensions were collected and centrifuged at 300 × g for 10 min at 4°C, and the cell pellet was suspended in stain buffer (BD Biosciences, San Jose, CA, USA). Mononuclear cells were purified using a density gradient (Ficoll-Paque Plus; GE Healthcare Bio-Sciences, Up-psala, Sweden), following the manufacturer’s instructions. Then, mononuclear cell suspensions were washed using stain buffer prior to immunophenotyping.
Immunophenotyping of human decidual leukocytes
Mononuclear cell suspensions were washed with stain buffer and centrifuged. Cell pellets were incubated for 10 min at 4°C with 20 μL of human FcR Blocking Reagent (Miltenyi Biotec) in 80 μL of stain buffer. Next, mononuclear cell suspensions were incubated with extracellular fluorochrome-conjugated anti-human mAbs (Key Resources Table) for 30 min at 4°C in the dark. After extracellular staining, the cells were fixed. For intracellular staining, the cells were fixed and permeabilized using the Foxp3/Transcription Factor Fixation/Permeabilization solution (eBioscience) prior to incubation with intracellular anti-human mAbs (Key Resources Table). Finally, mononuclear cell suspensions were washed and resuspended in 0.5 mL of stain buffer and acquired using the BD LSR Fortessa flow cytometer and FACSDiva 6.0 software. Flow cytometry analysis was performed using the FlowJo software v10 (FlowJo, Ashland, OR, USA).
Human Treg suppression assays
Decidual leukocytes were isolated from the decidual basalis from women who delivered preterm or at term (Table S1), as described above. Leukocytes were incubated with BD Horizon Fixable Viability Stain 510 for 30 min at 4°C, then washed with 1X PBS. The cells were resuspended in stain buffer and incubated with fluorochrome conjugated anti-human mAbs (Key Resources Table) for 30 min at 4°C in the dark. The cells were washed with 1X PBS to remove excess antibody, resuspended in 0.5 mL of presort buffer (BD Bio-sciences), and effector T cells (Teff cells; CD45+CD3+CD4+CD25cells) and regulatory T cells (Tregs; CD45+CD3+CD4+CD25+CD127low cells) were sorted using the BD FACSMelody cell sorter (BD Biosciences) and BD FACSChorus version 1.3 software (BD Biosciences). The expression of Foxp3 by sorted Tregs was confirmed by flow cytometry using the BD LSR Fortessa flow cytometer and FACSDiva 6.0 software.
Sorted Tregs were resuspended in RPMI media (Thermo Fisher Scientific, Grand Island, NY, USA) supplemented with 10% FBS and 1% penicillin/streptomycin (Thermo Fisher Scientific) at 4×104 cells/mL. Purified CD4+CD25-Teff cells were labeled with the CellTrace Violet Cell Proliferation Kit (Molecular Probes, Eugene, OR, USA), following the manufacturer’s instructions. Labeled Teff cells were adjusted to 4×104 cells/mL in supplemented RPMI medium. The Tregs and Teff cells were co-cultured in a round-bottom 96-well plate at a ratio of 1:1, 1:2, 1:4, and 1:8, respectively. Dynabeads Human T-Activator CD3/CD28 were added to the co-culture to obtain a bead:cell ratio of 1:1. The culture plate was then incubated at 37°C with 5% CO2 for 96 h. Human recombinant IL-2 (30 U/mL; Thermo Fisher Scientific) was used to stimulate T cell expansion, and 2-mercaptoethanol (55 μM; Thermo Fisher Scientific) was added to maintain cell viability. The suppressive capacity of Treg cells was determined using flow cytometry to analyze the proliferation of Teff cells under different co-culture conditions, according to the CellTrace Violet fluorescence intensity of the Teff cells. The percentage of Treg suppression of Teff cell expansion was calculated using the following formula: percentage of suppression = [(Number of proliferated Teff cells cultured alone – Number of proliferated Teff cells co-cultured with Tregs)/Number proliferated Teff cells cultured alone] × 100.
Treg suppression assays in the second and third week of murine pregnancy
C57BL/6 female mice were mated with BALB/c males as described above. Dams were sacrificed either in the 2nd week (10.5–13.5 dpc) or 3rd week (16.5 dpc) of pregnancy and the spleens were harvested. Splenocytes were isolated from the spleen as previously described (Arenas-Hernandez et al., 2016), filtered through a 30 μm cell strainer (Miltenyi Biotec), and washed with sterile 1X PBS. Splenocytes were counted using an automatic cell counter (Cellometer Auto 2000; Nexcelom, Lawrence, MA, USA) to obtain a preliminary cell number for Treg isolation. Approximately 1×108 splenocytes were taken for Treg isolation using the mouse CD4+CD25+ Regulatory T cell Isolation Kit (Miltenyi Biotec), following the manufacturer’s instructions. The counts of isolated Tregs (CD3+CD4+CD25+Foxp3+ cells) and Teff (CD3+CD4+CD25-cells) cells were determined by flow cytometry, using CountBright Absolute Counting Beads (Molecular Probes).
Purified Tregs were adjusted to 5×105 cells/mL in supplemented RPMI. Purified Teff cells were labeled with the CellTrace Violet Cell Proliferation Kit, following the manufacturer’s instructions. Labeled Teff cells were adjusted to 5×105 cells/mL in supplemented RPMI medium. The Tregs and Teff cells were co-cultured in a round-bottom 96-well plate at a ratio of 1:1, 1:2, 1:4, and 1:8, respectively, and cultured with CD3/CD28-loaded MACSiBeads (5×105 beads/mL), mouse recombinant IL-2 (200 U/mL; Miltenyi Biotec), and 2-mercaptoethanol (55mM). The culture plate was then incubated at 37°C with 5% CO2 for 96 h. The suppressive capacity of Tregs was determined using flow cytometry to analyze the proliferation of Teff cells under different co-culture conditions, according to the CellTrace Violet fluorescence intensity of the Teff cells. Dead cells were excluded using the Live/Dead Fixable Green Dead Cell Stain Kit (Molecular Probes). The percentage of Treg suppression of Teff cell expansion was calculated using the following formula: percentage of suppression = [(Number of proliferated Teff cells cultured alone – Number of proliferated Teff cells co-cultured with Tregs)/Number proliferated Teff cells cultured alone] × 100.
Depletion of Tregs in non-pregnant mice and leukocyte isolation from lymphatic tissues
Naive non-pregnant Foxp3DTR mice were injected intraperitoneally (i.p.) with 25 μg/kg (partial depletion of Tregs) or 50 μg/kg (total depletion of Tregs) of diphtheria toxin from Corynebacterium diphtheria (DT; Sigma-Aldrich, St. Louis, MO, USA; Calbiochem, EMD Millipore Corp, Billerica, MA, USA; or Enzo Life Sciences, Inc., Farmingdale, NY, USA) in 200 μL of sterile 1X PBS (Fisher Scientific, Fair Lawn, NY, USA) or with 200 μL sterile 1X PBS using a 26-gauge needle. Twenty-four hours post-injection, mice received a second injection of 25 or 50 μg/kg of DT, respectively, in 200 μL of sterile 1X PBS, or with 200 μL sterile 1X PBS. Four hours later, the uterine-draining lymph nodes (ULN), spleen, and thymus were collected and leukocyte suspensions were prepared as previously reported (Arenas-Hernandez et al., 2016) to determine the proportion of Tregs (CD3+CD4+CD25+Foxp3+ cells) by flow cytometry.
Depletion of Tregs in pregnant mice
Foxp3DTR dams were injected i.p. with 25 μg/kg of DT (Rowe et al., 2011, 2012) (partial depletion of Tregs) or 50 μg/kg of DT (Kim et al., 2007, Samstein et al., 2012) (total depletion of Tregs) dissolved in 200 μL of sterile 1X PBS using a 26-gauge needle on 14.5 dpc. Controls were injected with 200 μL of sterile 1X PBS alone. After 24 h, dams were injected with 5 μg/kg of DT or 50 μg/kg of DT, respectively, in 200 μL of sterile 1X PBS, or with 200 μL of sterile 1X PBS alone. Four hours later, dams were euthanized and the decidual, myometrial, placental, and lymphatic tissues were collected, as well as the peripheral blood, to determine the proportion of Tregs by flow cytometry.
Leukocyte isolation from murine decidua, myometrium, placenta, lymphatic tissues, and peripheral blood to verify Treg depletion
Isolation of leukocytes from decidual, myometrial, and placental tissues was performed as previously described (Arenas-Hernandez et al., 2015). Briefly, tissues were minced into small pieces using fine scissors and enzymatically digested with StemPro Cell Dissociation Reagent for 35 minutes at 37°C. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient using Ficoll-Paque Plus and washed with 1X PBS. The uterine-draining lymph nodes (ULN), inguinal lymph nodes (ILN), mesenteric lymph nodes (MLN), spleen, and thymus were also collected and leukocyte suspensions were prepared, as previously reported (Arenas-Hernandez et al., 2016). Leukocytes were filtered using a 100 μm cell strainer and washed with FACS buffer [0.1% BSA (Sigma-Aldrich) and 0.05% sodium azide (Fisher Scientific Chemicals) in 1X PBS)] before immunophenotyping.
Leukocyte suspensions prepared from the decidua, myometrium, placenta, lymphatic tissues, and peripheral blood were centrifuged at 1,250 × g for 10 min at 4°C in the dark. Cells were stained with the Live/Dead Fixable Green Dead Cell Stain Kit for 10 min at 4°C in the dark, followed by washing with 1X PBS. Cell pellets were then incubated with the CD16/CD32 mAb (FcγIII/II receptor; BD Biosciences) for 10 min and subsequently incubated with specific fluorochrome-conjugated anti-mouse mAbs (Key Resources Table) for 30 min at 4°C in the dark. Leukocyte suspensions were fixed/permeabilized with the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) prior to staining with intranuclear anti-mouse mAbs. Cells were acquired for determination of Tregs (CD3+CD4+CD25+Foxp3+ cells) using the BD LSR Fortessa flow cytometer and FACSDiva 6.0 software. Data were analyzed using FlowJo software v10.
Animal models of Treg depletion and preterm birth in the first and second pregnancy
Partial depletion (25 μg/kg) or total depletion (50 μg/kg) of Tregs was performed in Foxp3DTR dams as described above, and secondary injections (5 μg/kg or 50 μg/kg, respectively) were repeated every 24 h until delivery. Control dams were injected with 200 μL of sterile 1X PBS. Following injection, dams were monitored by infrared camera (Sony, Tokyo, Japan) until delivery. Gestational length was calculated from the presence of the vaginal plug until the appearance of the first pup in the cage bedding. Preterm birth was defined as delivery of all pups < 18.5 dpc. Neonatal survival and weights were recorded at birth and 1, 2, and 3 weeks postpartum. Photographs of neonates were also taken on the day of birth to verify breastfeeding. After 3 weeks of observation, dams were returned to mating cages to undergo a second pregnancy, underwent partial or total Treg depletion (or PBS injection) as described above, and were again monitored to determine pregnancy and neonatal outcomes until 3 weeks postpartum.
Fetal and placental weights from Treg-depleted dams
Foxp3DTR dams were injected with 25 or 50 μg/kg of DT dissolved in 200 μL of sterile 1X PBS, or 200 μL of sterile 1X PBS alone, on 14.5 dpc. Twenty-four hours after injection, dams were injected with 5 or 50 μg/kg of DT dissolved in 200 μL of sterile 1X PBS, or 200 μL of sterile 1X PBS alone, respectively. Four hours later, dams were euthanized and the fetal and placental weights were measured using a scale (DIA-20; American Weights Scales, Norcross, GA, USA). Photographs of the fetuses and placentas were also taken.
Cell sorting and adoptive transfer of Tregs
Splenic Tregs were isolated from C57BL/6 dams at 10.5–13.5 dpc. Tregs were isolated using the mouse CD4+CD25+ Regulatory T Cell Isolation Kit, according to the manufacturer’s instructions. The isolated Tregs were characterized (CD3+CD4+CD25+Foxp3+ cells) and quantified by flow cytometry prior to injection. Foxp3DTR dams were injected intravenously with 1×105 – 1×106 Tregs/100 μL of sterile 1X PBS on 14.5 and 16.5 dpc. Two hours after the initial adoptive transfer, dams were i.p injected with 25 μg/kg of DT diluted in 200 μL of sterile 1X PBS. After 24 h, dams were injected with 5 μg/kg of DT diluted in 200 μL of sterile 1X PBS, which was repeated every 24 h until delivery. Following the second adoptive transfer, dams were monitored by infrared camera until delivery to record the rates of preterm birth and neonatal survival at birth. Neonatal survival was recorded at birth and 1, 2, and 3 weeks postpartum.
To determine the proportion of adoptively transferred Tregs present in peripheral and reproductive tissues, splenic Tregs were isolated from EGFP dams at 10.5–13.5 dpc. Tregs were isolated using the mouse CD4+CD25+ Regulatory T Cell Isolation Kit, according to the manufacturer’s instructions. The isolated Tregs were characterized (CD3+CD4+CD25+Foxp3+ cells) and quantified by flow cytometry prior to injection. Foxp3DTR dams were injected intravenously with 1×105 – 1×106 Tregs/100 μL of sterile 1X PBS on 14.5 and 16.5 dpc. Two hours after the initial adoptive transfer, dams were i.p injected with 25 μg/kg of DT diluted in 200 μL of sterile 1X PBS. After 24 h, dams were injected with 5 μg/kg of DT diluted in 200 μL of sterile 1X PBS, which was repeated until 17.5 dpc. On 18.5 dpc, dams were euthanized and the decidual, myometrial, and placental tissues were collected, as well as the peripheral blood and lymphatic tissues. Leukocyte isolation and immunophenotyping of Tregs was performed as described above to determine the proportion of adoptively transferred Tregs present in each tissue.
Measurement of maternal-fetal obstetrical parameters
Partial depletion (25 μg/kg) or total depletion (50 μg/kg) of Tregs was performed in Foxp3DTR dams as described above, and secondary injections (5 μg/kg or 50 μg/kg, respectively) were repeated every 24 h until 17.5 dpc. Control dams were injected with 200 μL of sterile 1X PBS. On 17.5 dpc, three hours after injection, body temperature was evaluated using a rectal probe (TMH-150; VisualSonics Inc., Toronto, ON, Canada). Next, dams were placed in a restrainer and the arterial blood pressure was measured in the tail using a non-invasive method, according to manufacturer’s instructions (Noninvasive Blood Pressure System, CODA High Throughput System; Kent Scientific Corporation, Torrington, CT, USA). The systolic, diastolic, and mean pressures were registered. Afterward, dams were anesthetized by inhalation of 2%–3% isoflurane (Aerrane; Baxter Healthcare Corporation, Deerfield, IL, USA) and 1–2 L/min of oxygen in an induction chamber. Anesthesia was maintained with a mixture of 1.75%–2% isoflurane and 2.0 L/min of oxygen during the ultrasound procedure, which was performed using the Vevo 2100 Imaging System (VisualSonics Inc.) as previously described (St Louis et al., 2016, Gomez-Lopez et al., 2016a, Arenas-Hernandez et al., 2019). Three fetuses from each dam were evaluated, including the first fetus in each uterine horn. The fetal and maternal heart rates and the pulsatility indices (PI) were examined in the right and left uterine arteries as well as in the umbilical artery using the 55-MHz linear ultrasound probe (VisualSonics Inc.). The PI was calculated using the following formula: [PI = (peak systolic velocity – end diastolic velocity)/mean velocity]. Ultrasound signals were processed, displayed, and stored using the Vevo Imaging Station (VisualSonics Inc.). Following the ultrasound procedure, dams were euthanized and photographs of the maternal spleen and ULN were taken.
Model of susceptibility to endotoxin-induced preterm birth after Treg depletion
Foxp3DTR dams were injected i.p. with an initial dose of 25 μg/kg of DT diluted in 200 μL of sterile 1X PBS on 14.5dpc. Twenty-four hours post-injection, dams received a second injection of 25 μg/kg of DT diluted in 200 μL of sterile 1X PBS. On 16.5 dpc, Foxp3DTR dams were injected with 2 μg/200 μL of lipopolysaccharide (LPS) from Escherichia coli (O55:B5; Sigma-Aldrich). Non-Treg-depleted control Foxp3DTR dams were injected with sterile 1X PBS on 14.5 and 15.5 dpc and 2 μg/200 μL of LPS on 16.5 dpc. Controls also included Foxp3DTR dams injected with DT only on 14.5 and 15.5 dpc. Following injection, dams were monitored by infrared camera until delivery to record the rates of preterm birth. Neonatal survival was recorded at birth and 1, 2, and 3 weeks postpartum.
Determination of cytokine concentrations in the maternal circulation and amniotic fluid
Foxp3DTR dams were injected with 25 or 50 μg/kg of DT dissolved in 200 μL of sterile 1X PBS, or 200 μL of sterile 1X PBS alone, on 14.5dpc. Twenty-four hours after injection, dams were injected with 5 or 50 μg/kg of DT, respectively, dissolved in 200 μL of sterile 1X PBS, or 200 μL of sterile 1X PBS alone. Four hours later, dams were euthanized and peripheral blood was collected by cardiac puncture for plasma separation. Amniotic fluid was also obtained from each amniotic sac with a 26-gauge needle. To determine the cytokine storm in endotoxin-induced preterm birth, C57BL/6 dams were i.p. injected with 10 μg of LPS dissolved in 200 μL of sterile 1X PBS on 16.5 dpc. Dams were euthanized on 17.5 dpc and peripheral blood was collected by cardiac puncture for plasma separation. Maternal plasma and amniotic fluid samples were centrifuged at 800 or 1300 × g, respectively, for 10 min at 4°C and the supernatants were separated and stored at −20°C until analysis. The ProcartaPlex Mouse Cytokine & Chemokine Panel 1A 36-plex (Invitrogen by Thermo Fisher Scientific, Vienna, Austria) was used to measure the concentrations of IFNγ, IFNα, IL-12p70, IL-1β, TNFα, GM-CSF, IL-18, IL-17A, IL-22, IL-23, IL-27, IL-9, IL-15/IL-15R, IL-13, IL-2, IL-4, IL-5, IL-6, IL-10, CCL11, IL-28, IL-3, LIF, IL-1α, IL-31, CXCL1, CCL3, CXCL10, CCL2, CCL7, CCL4, CXCL2, CCL5, G-CSF, M-CSF, and CXCL5 in maternal plasma and amniotic fluid samples, according to the manufacturer’s instructions. Plates were read using the FLEXMAP 3D (Luminex Corporation, Austin, TX, USA), and analyte concentrations were calculated with ProcartaPlex Analyst 1.0 Software from Affymetrix, San Diego, CA, USA. The sensitivities of the assays were: 0.09 pg/mL (IFNγ), 3.03 pg/mL (IFNα), 0.21 pg/mL (IL-12p70), 0.14 pg/mL (IL-1β), 0.39 pg/mL (TNFα), 0.19 pg/mL (GM-CSF), 9.95 pg/mL (IL-18), 0.08 pg/mL (IL-17A), 0.24 pg/mL (IL-22), 2.21 pg/mL (IL-23), 0.34 pg/mL (IL-27), 0.28 pg/mL (IL-9), 0.42 pg/mL (IL-15/IL-15R), 0.16 pg/mL (IL-13), 0.10 pg/mL (IL-2), 0.03 pg/mL (IL-4), 0.32 pg/mL (IL-5), 0.21 pg/mL (IL-6), 0.69 pg/ mL (IL-10), 0.01 pg/mL (CCL11), 20.31 pg/mL (IL-28), 0.11 pg/mL (IL-3), 0.28 pg/mL (LIF), 0.32 pg/mL (IL-1α), 0.45 pg/mL (IL-31), 0.05 pg/mL (CXCL1), 0.13 pg/mL (CCL3), 0.26 pg/mL (CXCL10), 3.43 pg/mL (CCL2), 0.15 pg/mL (CCL7), 1.16 pg/mL (CCL4), 0.37 pg/mL (CXCL2), 0.35 pg/mL (CCL5), 0.19 pg/mL (G-CSF), 0.02 pg/mL (M-CSF), and 5.67 pg/mL (CXCL5). Inter-assay and intra-assay co-efficients of variation were less than 10%. Amniotic fluid cytokine concentrations were adjusted by protein concentration, which were determined using the Pierce BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA), following the manufacturer’s instructions.
Systemic injection of cytokines/chemokines in pregnant mice
C57BL/6 dams were intravenously injected with 1.4 pg of recombinant mouse IFNγ (Peprotech, Rocky Hill, NJ, USA), 218 pg of recombinant mouse CCL7 (R&D Systems, Minneapolis, MN, USA), or 48 pg of recombinant mouse IL-22 (Biolegend, San Diego, CA, USA), or a combination of these three cytokines, dissolved in 100 μL of sterile 1X PBS using a 26-gauge needle on 15.5, 16.5, and 17.5 dpc. Controls were injected with 100μL of sterile 1X PBS alone. Following injection, dams were monitored by infrared camera until delivery to record the rates of full or early term birth and neonatal mortality at birth, and neonates were monitored for up to 3 weeks after delivery.
Immunophenotyping of leukocytes from the decidual, myometrial, and placental tissues and peripheral blood
Foxp3DTR dams were injected with 25 or 50 μg/kg of DT dissolved in 200 μL of sterile 1X PBS, or 200 μL of sterile 1X PBS alone, on 14.5dpc. Twenty-four hours after injection, dams were injected with 5 or 50 μg/kg of DT, respectively, dissolved in 200 μL of sterile 1X PBS, or 200 μL of sterile 1X PBS alone. Four hours later, dams were euthanized and the decidual, myometrial, and placental tissues, as well as the peripheral blood, were collected. The PBMCs were isolated from peripheral blood by density gradient using Ficoll-Paque Plus and washed with 1X PBS. Leukocyte suspensions were prepared from decidual, myometrial, and placental tissues as previously described (Arenas-Hernandez et al., 2015), and were stained using the BD Fixable Viability Stain 510 prior to incubation with anti-mouse mAbs (Key Resources Table). Leukocyte suspensions were centrifuged at 1,250 × g for 10 min at 4°C in the dark. Cells were then incubated with the CD16/CD32 mAb (FcγIII/II receptor) for 10 min and subsequently incubated with specific fluorochrome conjugated anti-mouse mAbs (Key Resources Table) for 30 min at 4°C in the dark. Leukocyte suspensions were fixed/permeabilized with the BD Cytofix/Cytoperm Fixation/Permeabilization Kit or Foxp3/Transcription Factor Staining Buffer Set prior to straining with intracellular or intranuclear anti-mouse mAbs. Cells were acquired using the BD LSR Fortessa flow cytometer and FACSDiva 6.0 software. Immunophenotyping included the identification of macrophages (CD45+CD11b+F4/80+Ly6G-cells), neutrophils (CD45+CD11b+F4/80-Ly6G+ cells), dendritic cells (DCs; CD45+CD11b+F4/80-CD11c+CD205+ cells), NK cells (CD45+CD11b-CD49b+ cells), T cells (CD3+ cells), CD4+ T cells (CD3+CD4+ cells), CD8+ T cells (CD3+CD8+ cells), and B cells (CD45+CD11b-CD19+ cells) in the decidual, myometrial, and placental tissues as well as in the maternal circulation. The activation status of these cells was determined by cytokine expression (Key Resources Table). Data were analyzed using FlowJo software v10. Log2 fold changes in cell abundance (frequencies) were determined and represented in heatmaps.
RNA-seq analysis of placental tissues
RNA isolation, library preparation, and sequencing:
Partial depletion (25 μg/kg) or total depletion (50 μg/kg) of Tregs was performed in Foxp3DTR dams on 14.5 dpc as described above, and secondary injections (5 μg/kg or 50 μg/kg, respectively) were performed 24 h after (15.5 dpc). Four hours later, dams were euthanized and the placental tissues were collected from implantation sites and placed in RNAlater Stabilization Solution (Life Technologies). Total RNA was isolated from tissues using the RNeasy mini kit (QIAGEN, Hilden, Germany), following the manufacturer’s instructions. RNA concentrations and purity were assessed with the NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific), and RNA integrity was evaluated with the 2100 Bioanalyzer system (Agilent Technologies, Wilmington, DE) using the Agilent RNA 6000 Nano Kit (Agilent Technologies). The RNA-Seq library was prepared by the Beijing Genomics Institute (BGI; Wuhan, China) using the MGIEasy RNA Library Prep kit (MGI Americas Inc., San Jose, CA, USA). Paired-end reads of 150 bp length were sequenced on the BGISEQ-500RS sequencer (BGI), and raw data were provided by BGI.
Pre-processing of RNA-Seq data:
Paired-end RNA-Seq sequence data in fastq format were processed using Salmon aligner v0.12.0 (Patro et al., 2017) in quasi-mapping mode using a transcriptome index prepared from Mus musculus genome assembly GRCm38 (mm10). Transcripts per million (TPM) values from Salmon output were scaled up to library size and imported into R (version 3.5.2) using the tximport package of Bioconductor (Gentleman et al., 2004, Soneson et al., 2015).
Assessment of differences between treatment groups:
Count data were analyzed using negative binomial models implemented in the DESeq2 package (Anders et al., 2013). Only genes that had counts > 5 in at least five samples were retained for further analysis. Genes were considered to be differentially expressed if the fold change between groups was > 1.25 and the false discovery rate (Benjamini and Hochberg, 1995) adjusted p value was < 0.1.
RNA-Seq data visualization:
The RNA-Seq data (log2 counts) for the top 50 upregulated and top 50 downregulated genes were visualized using heatmaps with hierarchical clustering of genes.
Biological process determination:
Over-representation of biological processes among significantly up- and down- regulated genes was determined separately by performing hypergeometric tests using the stringApp v1.5.0 (Doncheva et al., 2019) in Cytoscape v3.7.2 (Otasek et al., 2019). The stringApp was also used to visualize known interactions between proteins coded by the differentially expressed genes in selected biological processes. Over-representation of biological processes among all differentially expressed genes, regardless of their direction of change, was also tested with ClueGO v2.5.5 (Mlecnik et al., 2019) and the significantly disrupted biological processes were grouped based on their functional annotation and visualized as a network of gene ontologies. A q-value cutoff of < 0.05 was used to determine significance of enrichment analysis.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical Analysis
Statistical analyses were performed using SPSS v19.0 (IBM, Armonk, NY, USA) or the R package (version 3.5.2). For human demographic data, the group comparisons were performed using the Fisher’s test with Monte-Carlo simulation for proportions and Kruskal-Wallis tests for non-normally distributed continuous variables. The Mann-Whitney U-test was used to compare differences between the groups for human flow cytometry data. The Fisher’s exact test was used to compare the rates of preterm birth. The Kaplan-Meier survival curves were used to plot and compare the neonatal survival data (Mantel-Cox test). The Kruskal-Wallis and ANOVA tests were utilized for comparisons among groups for murine observational and obstetrical data as well as for maternal plasma and amniotic fluid cytokine concentrations. A p value % 0.05 was considered statistically significant. For flow cytometry heatmaps, the statistical significance of differences between study groups were assessed using t tests followed by the false discovery rate adjustment of p values, with a q < 0.1 being considered significant (represented as asterisks in the heatmap). RNA-Seq analysis was performed as described above.
Supplementary Material
Highlights.
Tregs are reduced at the maternal-fetal interface in idiopathic preterm labor/birth
The loss of Tregs induces a fraction of preterm births
The loss of Tregs induces adverse fetal and neonatal outcomes
Tregs modulate systemic and local immune responses in the third period of pregnancy
ACKNOWLEDGMENTS
We thank the physicians, nurses, and research assistants from the Center for Advanced Obstetrical Care and Research, Intrapartum Unit, Perinatology Research Branch Clinical Laboratory, and Perinatology Research Branch Perinatal Translational Science Laboratory for help with collecting and processing samples. We also thank Tatjana Milovic, Amapola Balancio, Hong Meng, Gaurav Bhatti, and Rebecca Slutsky for help with carrying out some experiments in mice, data analysis, and/or helpful discussion of the findings. This research was supported in part by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), and in part with federal funds from NICHD/NIH/DHHS under contract HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. R.R. contributed to this work as part of his official duties as an employee of the U.S. federal government.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.107874.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Aluvihare VR, Kallikourdis M, and Betz AG (2004). Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol 5, 266–271. [DOI] [PubMed] [Google Scholar]
- Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, and Robinson MD (2013). Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat. Protoc 8, 1765–1786. [DOI] [PubMed] [Google Scholar]
- Arck PC, and Hecher K (2013). Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat. Med 19, 548–556. [DOI] [PubMed] [Google Scholar]
- Arenas-Hernandez M, Sanchez-Rodriguez EN, Mial TN, Robertson SA, and Gomez-Lopez N (2015). Isolation of Leukocytes from the Murine Tissues at the Maternal-Fetal Interface. J. Vis. Exp 99, e52866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, and Gomez-Lopez N (2016). An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell. Mol. Immunol 13, 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Hernandez M, Romero R, Xu Y, Panaitescu B, Garcia-Flores V, Miller D, Ahn H, Done B, Hassan SS, Hsu CD, et al. (2019). Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone. J. Immunol 202, 2585–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros FC, Papageorghiou AT, Victora CG, Noble JA, Pang R, Iams J, Cheikh Ismail L, Goldenberg RL, Lambert A, Kramer MS, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (2015). The distribution of clinical phenotypes of preterm birth syndrome: implications for prevention. JAMA Pediatr. 169, 220–229. [DOI] [PubMed] [Google Scholar]
- Bazzoni F, Cassatella MA, Laudanna C, and Rossi F (1991). Phagocytosis of opsonized yeast induces tumor necrosis factor-alpha mRNA accumulation and protein release by human polymorphonuclear leukocytes. J. Leukoc. Biol 50, 223–228. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, and Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300. [Google Scholar]
- Bersani I, De Carolis MP, Foell D, Weinhage T, Rossi ED, De Carolis S, Rubortone SA, Romagnoli C, and Speer CP (2015). Interleukin-22: biomarker of maternal and fetal inflammation? Immunol. Res 61, 4–10. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, and Lawn JE (2012). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172. [DOI] [PubMed] [Google Scholar]
- Bonney EA (2016). Immune Regulation in Pregnancy: A Matter of Perspective? Obstet. Gynecol. Clin. North Am 43, 679–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney EA, and Onyekwuluje J (2003). The H-Y response in mid-gestation and long after delivery in mice primed before pregnancy. Immunol. Invest 32, 71–81. [DOI] [PubMed] [Google Scholar]
- Burton GJ, and Jauniaux E (2018). Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol 218 (2S), S745–S761. [DOI] [PubMed] [Google Scholar]
- Care AS, Bourque SL, Morton JS, Hjartarson EP, Robertson SA, and Davidge ST (2018). Reduction in Regulatory T Cells in Early Pregnancy Causes Uterine Artery Dysfunction in Mice. Hypertension 72, 177–187. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Voisin GA, Escalier D, and Robert P (1979). Facilitation reaction (enhancing antibodies and suppressor cells) and rejection reaction (sensitized cells) from the mother to the paternal antigens of the conceptus. Clin. Exp. Immunol 35, 13–24. [PMC free article] [PubMed] [Google Scholar]
- Chaouat G, Kolb JP, and Wegmann TG (1983). The murine placenta as an immunological barrier between the mother and the fetus. Immunol. Rev 75, 31–60. [DOI] [PubMed] [Google Scholar]
- Chen T, Darrasse-Jèze G, Bergot AS, Courau T, Churlaud G, Valdivia K, Strominger JL, Ruocco MG, Chaouat G, and Klatzmann D (2013). Self-specific memory regulatory T cells protect embryos at implantation in mice. J. Immunol 191, 2273–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo T, Tsiartas P, Kacerovsky M, Holst RM, Hougaard DM, Skogstrand K, Wennerholm UB, Hagberg H, and Jacobsson B (2013). Maternal inflammatory response to microbial invasion of the amniotic cavity: analyses of multiple proteins in the maternal serum. Acta Obstet. Gynecol. Scand 92, 61–68. [DOI] [PubMed] [Google Scholar]
- Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, Rael J, Grove T, Morgan TK, Clewell W, et al. ; ProteoGenix/Obstetrix Collaborative Research Network (2014). Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am. J. Obstet. Gynecol 210, 125.e1–125.e15. [DOI] [PubMed] [Google Scholar]
- Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, and Riley EM (2007). Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J. Immunol 178, 4136–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SM, Casey ML, and MacDonald PC (1997). Accumulation of interleukin-1beta and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Hum. Reprod. Update 3, 517–527. [DOI] [PubMed] [Google Scholar]
- Cruciani L, Romero R, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Mazaki-Tovi S, Dong Z, Kim SK, Ogge G, Yeo L, et al. (2010). Pentraxin 3 in maternal circulation: an association with preterm labor and preterm PROM, but not with intra-amniotic infection/inflammation. J. Matern. Fetal Neonatal Med 23, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, and Trinchieri G (1993). Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med 178, 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrasse-Jèze G, Klatzmann D, Charlotte F, Salomon BL, and Cohen JL (2006). CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol. Lett 102, 106–109. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R, Yssel H, and de Vries JE (1993). Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J. Immunol 150, 4754–4765. [PubMed] [Google Scholar]
- Deng W, Yuan J, Cha J, Sun X, Bartos A, Yagita H, Hirota Y, and Dey SK (2019). Endothelial Cells in the Decidual Bed Are Potential Therapeutic Targets for Preterm Birth Prevention. Cell Rep. 27, 1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doisne JM, Balmas E, Boulenouar S, Gaynor LM, Kieckbusch J, Gardner L, Hawkes DA, Barbara CF, Sharkey AM, Brady HJ, et al. (2015). Composition, Development, and Function of Uterine Innate Lymphoid Cells. J. Immunol 195, 3937–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncheva NT, Morris JH, Gorodkin J, and Jensen LJ (2019). Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res 18, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DJ, Hunter C, Mitchell MD, and Varner MW (1997). Amniotic fluid interleukin-10 (IL-10) concentrations during pregnancy and with labor. J. Reprod. Immunol 33, 147–156. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, and Ravel J (2019). Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat. Commun 10, 1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A (2013). Immunology of the maternal-fetal interface. Annu. Rev. Immunol 31, 387–411. [DOI] [PubMed] [Google Scholar]
- Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, and Adashi EY (2005). Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J. Matern. Fetal Neonatal Med 17, 365–373. [DOI] [PubMed] [Google Scholar]
- Fan MY, Low JS, Tanimine N, Finn KK, Priyadharshini B, Germana SK, Kaech SM, and Turka LA (2018). Differential Roles of IL-2 Signaling in Developing versus Mature Tregs. Cell Rep. 25, 1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton MJ, Buras JA, and Donnelly RP (1992). IL-4 reciprocally regulates IL-1 and IL-1 receptor antagonist expression in human monocytes. J. Immunol 149, 1283–1288. [PubMed] [Google Scholar]
- Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, Huang B, Arodz TJ, Edupuganti L, Glascock AL, et al. (2019). The vaginal microbiome and preterm birth. Nat. Med 25, 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo AS, and Schumacher A (2016). The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 148, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, et al. (1996). T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med 183, 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B, Zhou Y, Ni X, Tong X, Xu X, Dong Z, Sun R, Tian Z, and Wei H (2017). Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity 47, 1100–1113. [DOI] [PubMed] [Google Scholar]
- Garcia-Flores V, Romero R, Miller D, Xu Y, Done B, Veerapaneni C, Leng Y, Arenas-Hernandez M, Khan N, Panaitescu B, et al. (2018). Inflammation-Induced Adverse Pregnancy and Neonatal Outcomes Can Be Improved by the Immunomodulatory Peptide Exendin-4. Front. Immunol 9, 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. (2004). Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, and Romero R (2001). Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am. J. Obstet. Gynecol 185, 1124–1129. [DOI] [PubMed] [Google Scholar]
- Gervasi MT, Romero R, Bracalente G, Erez O, Dong Z, Hassan SS, Yeo L, Yoon BH, and Chaiworapongsa T (2012). Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J. Perinat. Med 40, 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill N, Leng Y, Romero R, Xu Y, Panaitescu B, Miller D, Arif A, Mumuni S, Qureshi F, Hsu CD, et al. (2019). The immunophenotype of decidual macrophages in acute atherosis. Am. J. Reprod. Immunol 81, e13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, and Romero R (2008). Epidemiology and causes of preterm birth. Lancet 371, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, and Laresgoiti-Servitje E (2012). T regulatory cells: regulating both term and preterm labor? Immunol. Cell Biol 90, 919–920. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, and Vadillo-Ortega F (2013). Evidence for a role for the adaptive immune response in human term parturition. Am. J. Reprod. Immunol 69, 212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, and Arenas-Hernandez M (2014). Immune cells in term and preterm labor. Cell. Mol. Immunol 11, 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Arenas-Hernandez M, Ahn H, Panaitescu B, Vadillo-Ortega F, Sanchez-Torres C, Salisbury KS, and Hassan SS (2016a). In vivo T-cell activation by a monoclonal aCD3ε antibody induces preterm labor and birth. Am. J. Reprod. Immunol 76, 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, and Hassan SS (2016b). Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am. J. Reprod. Immunol 75, 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Arenas-Hernandez M, Schwenkel G, St Louis D, Hassan SS, and Mial TN (2017a). In vivo activation of invariant natural killer T cells induces systemic and local alterations in T-cell subsets prior to preterm birth. Clin. Exp. Immunol 189, 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Plazyo O, Schwenkel G, Garcia-Flores V, Unkel R, Xu Y, Leng Y, Hassan SS, Panaitescu B, et al. (2017b). Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am. J. Obstet. Gynecol 217, 592.e1–592.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Arenas-Hernandez M, Panaitescu B, Garcia-Flores V, Mial TN, Sahi A, and Hassan SS (2018). Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J. Matern. Fetal Neonatal Med 31, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, Hsu CD, and Panaitescu B (2019). Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes. Biol. Reprod 100, 1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousopoulos E, Proulx ST, Bachmann SB, Scholl J, Dionyssiou D, Demiri E, Halin C, Dieterich LC, and Detmar M (2016). Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function. JCI Insight 1, e89081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, and Novy MJ (1994). An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am. J. Obstet. Gynecol 171, 1660–1667. [DOI] [PubMed] [Google Scholar]
- Gustafsson C, Mjösberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, and Ernerudh J (2008). Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS ONE 3, e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Garcia-Hernandez Mde.L., Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, and Dutton RW (2009). Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol 182, 3469–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PH, Vitti GF, Burgess DR, Whitty GA, Piccoli DS, and Hamilton JA (1989). Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc. Natl. Acad. Sci. USA 86, 3803–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen J, Möttönen M, Alanen A, and Lassila O (2004). Phenotypic characterization of regulatory T cells in the human decidua. Clin. Exp. Immunol 136, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R, Timmons BC, Akgul Y, Akins ML, and Mahendroo M (2011). The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology 152, 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser BL, Tilburgs T, Hill J, Nicotra ML, and Strominger JL (2011). Two unique human decidual macrophage populations. J. Immunol 186, 2633–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howson CP, Kinney MV, McDougall L, and Lawn JE; Born Too Soon Preterm Birth Action Group (2013). Born too soon: preterm birth matters. Reprod. Health 10 (Suppl 1), S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, Hünig T, Mittrücker HW, Brüstle A, Kamradt T, and Lohoff M (2009). A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol 39, 1716–1725. [DOI] [PubMed] [Google Scholar]
- Hunt JS, Manning LS, and Wood GW (1984). Macrophages in murine uterus are immunosuppressive. Cell. Immunol 85, 499–510. [DOI] [PubMed] [Google Scholar]
- Inada K, Shima T, Nakashima A, Aoki K, Ito M, and Saito S (2013). Characterization of regulatory T cells in decidua of miscarriage cases with abnormal or normal fetal chromosomal content. J. Reprod. Immunol 97, 104–111. [DOI] [PubMed] [Google Scholar]
- Kahn DA, and Baltimore D (2010). Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc. Natl. Acad. Sci. USA 107, 9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, and Atkinson JP (2003). Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 421, 388–392. [DOI] [PubMed] [Google Scholar]
- Kieckbusch J, Balmas E, Hawkes DA, and Colucci F (2015). Disrupted PI3K p110d Signaling Dysregulates Maternal Immune Cells and Increases Fetal Mortality In Mice. Cell Rep. 13, 2817–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, and Rudensky AY (2007). Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol 8, 191–197. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, et al. (2009). Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J. Immunol 182, 3919–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, and Kim JS (2010). The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod. Pathol 23, 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, and Kim YM (2015a). Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol 213 (Suppl 4), S29–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Romero R, Chaemsaithong P, and Kim JS (2015b). Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol 213 (Suppl 4), S53–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, and Miller SD (2006). Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol 176, 3301–3305. [DOI] [PubMed] [Google Scholar]
- Laudanski P, Lemancewicz A, Kuc P, Charkiewicz K, Ramotowska B, Kretowska M, Jasinska E, Raba G, Karwasik-Kajszczarek K, Kraczkowski J, and Laudanski T (2014). Chemokines profiling of patients with preterm birth. Mediators Inflamm. 2014, 185758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, Kusanovic JP, Chaiworapongsa T, Hassan SS, Yoon BH, and Kim CJ (2011). A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS ONE 6, e16806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Romero R, Xu Y, Galaz J, Slutsky R, Arenas-Hernandez M, Garcia-Flores V, Motomura K, Hassan SS, Reboldi A, and Gomez-Lopez N (2019). Are B cells altered in the decidua of women with preterm or term labor? Am. J. Reprod. Immunol 81, e13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang J, Zhang D, Hong X, Tao Y, Wang S, Xu Y, Piao H, Yin W, Yu M, et al. (2017). Tim-3 signaling in peripheral NK cells promotes maternal-fetal immune tolerance and alleviates pregnancy loss. Sci. Signal 10, eaah4323. [DOI] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, and Black RE (2015). Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385, 430–440. [DOI] [PubMed] [Google Scholar]
- Loewendorf AI, Nguyen TA, Yesayan MN, and Kahn DA (2015). Pre-eclampsia is Characterized by Fetal NK Cell Activation and a Reduction in Regulatory T Cells. Am. J. Reprod. Immunol 74, 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ, and Gaffen SL (2019). The IL-17 Family of Cytokines in Health and Disease. Immunity 50, 892–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard LC, Minns LA, Darche S, Mielcarz DW, Foureau DM, Roos D, Dzierszinski F, Kasper LH, and Buzoni-Gatel D (2007). B cells amplify IFN-gamma production by T cells via a TNF-alpha-mediated mechanism. J. Immunol 179, 4857–4866. [DOI] [PubMed] [Google Scholar]
- Miller D, Motomura K, Garcia-Flores V, Romero R, and Gomez-Lopez N (2018). Innate Lymphoid Cells in the Maternal and Fetal Compartments. Front. Immunol 9, 2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlecnik B, Galon J, and Bindea G (2019). Automated exploration of gene ontology term and pathway networks with ClueGO-REST. Bioinformatics 35, 3864–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D, Weaver L, Tobin R, Henderson S, Beeram M, Newell-Rogers MK, and Perger L (2014). Intrauterine growth restriction and prematurity influence regulatory T cell development in newborns. J. Pediatr. Surg 49, 727–732. [DOI] [PubMed] [Google Scholar]
- Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, Kropf P, Mueller I, Kolb A, Modolell M, and Ho AD (2006). Suppression of T-cell functions by human granulocyte arginase. Blood 108, 1627–1634. [DOI] [PubMed] [Google Scholar]
- Nguyen TA, Kahn DA, and Loewendorf AI (2017). Maternal-Fetal rejection reactions are unconstrained in preeclamptic women. PLoS ONE 12, e0188250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TA, Kahn DA, and Loewendorf AI (2018). Placental implantation over prior cesarean scar causes activation of fetal regulatory T cells. Immun. Inflamm. Dis 6, 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KJ, Kim SM, Hong JS, Maymon E, Erez O, Panaitescu B, Gomez-Lopez N, Romero R, and Yoon BH (2017). Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am. J. Obstet. Gynecol 216, 604.e1–604.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi RM, and Gaffen SL (2010). Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otasek D, Morris JH, Bouças J, Pico AR, and Demchak B (2019). Cytoscape Automation: empowering workflow-based network analysis. Genome Biol. 20, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, Brockway HM, Price ND, and Muglia LJ (2018). Comparative transcriptomic analysis of human placentae at term and preterm delivery. Biol. Reprod 98, 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Park KH, Kim YM, Kook SY, Jeon SJ, and Yoo HN (2018). Plasma inflammatory and immune proteins as predictors of intra-amniotic infection and spontaneous preterm delivery in women with preterm labor: a retrospective study. BMC Pregnancy Childbirth 18, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, and Kingsford C (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra S, Sosa C, Bertoni B, and Sapiro R (2019). Transcriptomic analysis of fetal membranes reveals pathways involved in preterm birth. BMC Med. Genomics 12, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff MG (2005). Immune interactions at the maternal-fetal interface. J. Reprod. Immunol 68, 1–13. [DOI] [PubMed] [Google Scholar]
- Pique-Regi R, Romero R, Tarca AL, Sendler ED, Xu Y, Garcia-Flores V, Leng Y, Luca F, Hassan SS, and Gomez-Lopez N (2019). Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 8, e52004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, and Offner H (2005). Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J. Neuroimmunol 170, 85–92. [DOI] [PubMed] [Google Scholar]
- Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAu-liffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, et al. (2019). The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet 145 (Suppl 1), 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, et al. (2015). Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat. Immunol 16, 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline RW (2006). Inflammatory responses in the placenta and umbilical cord. Semin. Fetal Neonatal Med 11, 296–301. [DOI] [PubMed] [Google Scholar]
- Redline RW, and Patterson P (1994). Patterns of placental injury. Correlations with gestational age, placental weight, and clinical diagnoses. Arch. Pathol. Lab. Med 118, 698–701. [PubMed] [Google Scholar]
- Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, and Vogler C; Society for Pediatric Pathology, Perinatal Section, Amniotic Fluid Infection Nosology Committee (2003). Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr. Dev. Pathol 6, 435–448. [DOI] [PubMed] [Google Scholar]
- Rinaldi SF, Makieva S, Saunders PT, Rossi AG, and Norman JE (2017). Immune cell and transcriptomic analysis of the human decidua in term and preterm parturition. Mol. Hum. Reprod 23, 708–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Guerin LR, Moldenhauer LM, and Hayball JD (2009). Activating T regulatory cells for tolerance in early pregnancy—the contribution of seminal fluid. J. Reprod. Immunol 83, 109–116. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Care AS, and Moldenhauer LM (2018). Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Invest 128, 4224–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, and Hobbins JC (1988). Infection in the pathogenesis of preterm labor. Semin. Perinatol 12, 262–279. [PubMed] [Google Scholar]
- Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, and Hobbins JC (1989). Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am. J. Obstet. Gynecol 161, 817–824. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, and Williams J (1992). Tumor necrosis factor in preterm and term labor. Am. J. Obstet. Gynecol 166, 1576–1587. [DOI] [PubMed] [Google Scholar]
- Romero R, Dey SK, and Fisher SJ (2014a). Preterm labor: one syndrome, many causes. Science 345, 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, et al. (2014b). A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am. J. Reprod. Immunol 71, 330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, et al. (2014c). Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod. Immunol 72, 458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, and Margolis L (2015). Evidence of perturbations of the cytokine network in preterm labor. Am. J. Obstet. Gynecol 213, 836.e1–836.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaemsaithong P, Chaiyasit N, Docheva N, Dong Z, Kim CJ, Kim YM, Kim JS, Qureshi F, Jacques SM, et al. (2017). CXCL10 and IL-6: Markers of two different forms of intra-amniotic inflammation in preterm labor. Am. J. Reprod. Immunol 78, e12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, and Way SS (2011). Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe 10, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, and Way SS (2012). Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Nakashima A, Shima T, and Ito M (2010). Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol 63, 601–610. [DOI] [PubMed] [Google Scholar]
- Salafia CM, Vogel CA, Vintzileos AM, Bantham KF, Pezzullo J, and Silberman L (1991). Placental pathologic findings in preterm birth. Am. J. Obstet. Gynecol 165, 934–938. [DOI] [PubMed] [Google Scholar]
- Salvany-Celades M, van der Zwan A, Benner M, Setrajcic-Dragos V, Bougleux Gomes HA, Iyer V, Norwitz ER, Strominger JL, and Tilburgs T (2019). Three Types of Functional Regulatory T Cells Control T Cell Responses at the Human Maternal-Fetal Interface. Cell Rep. 27, 2537–2547. [DOI] [PubMed] [Google Scholar]
- Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, and Rudensky AY (2012). Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, and Saito S (2004). Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod 10, 347–353. [DOI] [PubMed] [Google Scholar]
- Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, and Steinborn A (2012). Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol. Cell Biol 90, 935–944. [DOI] [PubMed] [Google Scholar]
- Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, and Saito S (2010). Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol 85, 121–129. [DOI] [PubMed] [Google Scholar]
- Shima T, Inada K, Nakashima A, Ushijima A, Ito M, Yoshino O, and Saito S (2015). Paternal antigen-specific proliferating regulatory T cells are increased in uterine-draining lymph nodes just before implantation and in pregnant uterus just after implantation by seminal plasma-priming in allogeneic mouse pregnancy. J. Reprod. Immunol 108, 72–82. [DOI] [PubMed] [Google Scholar]
- Shiozaki A, Yoneda S, Yoneda N, Yonezawa R, Matsubayashi T, Seo G, and Saito S (2014). Intestinal microbiota is different in women with preterm birth: results from terminal restriction fragment length polymorphism analysis. PLoS ONE 9, e111374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindram-Trujillo A, Scherjon S, Kanhai H, Roelen D, and Claas F (2003). Increased T-cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am. J. Reprod. Immunol 49, 261–268. [DOI] [PubMed] [Google Scholar]
- Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, and Claas FH (2004). Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J. Reprod. Immunol 62, 125–137. [DOI] [PubMed] [Google Scholar]
- Slutsky R, Romero R, Xu Y, Galaz J, Miller D, Done B, Tarca AL, Gregor S, Hassan SS, Leng Y, and Gomez-Lopez N (2019). Exhausted and Senescent T Cells at the Maternal-Fetal Interface in Preterm and Term Labor. J. Immunol. Res 2019, 3128010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneson C, Love MI, and Robinson MD (2015). Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 4, 1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin Y, Romero R, Mele L, Wapner RJ, Iams JD, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Harper M, et al. (2010). Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth <32 weeks and adverse neonatal outcomes. Am. J. Perinatol 27, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriadis A, Hernandez-Andrade E, da Silva Costa F, Ghi T, Glanc P, Khalil A, Martins WP, Odibo AO, Papageorghiou AT, Salomon LJ, and Thilaganathan B; ISUOG CSC Pre-eclampsia Task Force (2019). ISUOG Practice Guidelines: role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound Obstet. Gynecol 53, 7–22. [DOI] [PubMed] [Google Scholar]
- St Louis D, Romero R, Plazyo O, Arenas-Hernandez M, Panaitescu B, Xu Y, Milovic T, Xu Z, Bhatti G, Mi QS, et al. (2016). Invariant NKT Cell Activation Induces Late Preterm Birth That Is Attenuated by Rosiglitazone. J. Immunol 196, 1044–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinborn A, Engst M, Haensch GM, Mahnke K, Schmitt E, Meuer S, and Sohn C (2010). Small for gestational age (SGA) neonates show reduced suppressive activity of their regulatory T cells. Clin. Immunol 134, 188–197. [DOI] [PubMed] [Google Scholar]
- Steinborn A, Schmitt E, Kisielewicz A, Rechenberg S, Seissler N, Mahnke K, Schaier M, Zeier M, and Sohn C (2012). Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin. Exp. Immunol 167, 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, and Ernerudh J (2011). Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol 187, 3671–3682. [DOI] [PubMed] [Google Scholar]
- Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G, Lash GE, Jenmalm MC, and Ernerudh J (2015). The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J. Immunol 194, 1534–1544. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, Kanhai HHH, Claas FHJ, and Scherjon SA (2006). Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancy. Placenta 27 (Suppl A), S47–S53. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, and Claas FH (2008). Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J. Immunol 180, 5737–5745. [DOI] [PubMed] [Google Scholar]
- Tsuda S, Zhang X, Hamana H, Shima T, Ushijima A, Tsuda K, Muraguchi A, Kishi H, and Saito S (2018). Clonally Expanded Decidual Effector Regulatory T Cells Increase in Late Gestation of Normal Pregnancy, but Not in Preeclampsia, in Humans. Front. Immunol 9, 1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda S, Nakashima A, Shima T, and Saito S (2019). New Paradigm in the Role of Regulatory T Cells During Pregnancy. Front. Immunol 10, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca P, Montaldo E, Croxatto D, Loiacono F, Canegallo F, Venturini PL, Moretta L, and Mingari MC (2015). Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol. 8, 254–264. [DOI] [PubMed] [Google Scholar]
- van der Zwan A, Bi K, Norwitz ER, Crespo AC, Claas FHJ, Strominger JL, and Tilburgs T (2018). Mixed signature of activation and dysfunction allows human decidual CD8+ T cells to provide both tolerance and immunity. Proc. Natl. Acad. Sci. USA 115, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Zhang J, Gu Q, Huang M, Zhang W, Guo J, and Zhou X (2017). Reciprocal Expression of IL-35 and IL-10 Defines Two Distinct Effector Treg Subsets that Are Required for Maintenance of Immune Tolerance. Cell Rep. 21, 1853–1869. [DOI] [PubMed] [Google Scholar]
- Willcockson AR, Nandu T, Liu CL, Nallasamy S, Kraus WL, and Mahendroo M (2018). Transcriptome signature identifies distinct cervical pathways induced in lipopolysaccharide-mediated preterm birth. Biol. Reprod 98, 408–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Zhou C, and Qi G (2010). Proportional changes of CD4+CD25+Foxp3+ regulatory T cells in maternal peripheral blood during pregnancy and labor at term and preterm. Clin. Invest. Med 33, E422. [DOI] [PubMed] [Google Scholar]
- Xu Y, Plazyo O, Romero R, Hassan SS, and Gomez-Lopez N (2015). Isolation of Leukocytes from the Human Maternal-fetal Interface. J. Vis. Exp 99, e52863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, Garcia-Flores V, Hassan SS, Xu Z, Tarca AL, et al. (2016). An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J. Immunol 196, 2476–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Romero R, Miller D, Silva P, Panaitescu B, Theis KR, Arif A, Hassan SS, and Gomez-Lopez N (2018). Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am. J. Reprod. Immunol 79, e12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, and Armitage RJ (1995). Human IL-17: a novel cytokine derived from T cells. J. Immunol 155, 5483–5486. [PubMed] [Google Scholar]
- Yellon SM (2017). Contributions to the dynamics of cervix remodeling prior to term and preterm birth. Biol. Reprod 96, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, and Jun JK (2001). Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol 185, 1130–1136. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, and Volk HD (2005). Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am. J. Pathol 166, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Zhao D, Huang D, Yin L, Chen C, Pan B, Wu Q, Li Z, Yao Y, Shen E, et al. (2014). Interleukin-22 aggravates murine acute graft-versus-host disease by expanding effector T cell and reducing regulatory T cell. J. Interferon Cytokine Res 34, 707–715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the RNA-seq data reported in this paper is GEO: GSE145357.


