Introduction
Clear cell carcinoma (CCC) of ovarian and uterine cancer is associated with unique clinical behavior. While other histologies show microarray gene expression patterns that are unique to their organ of origin, CCC shows a remarkably similar gene expression pattern in the endometrium, kidney, and ovary(1).
Clear cell endometrial carcinoma is a rare subtype, accounting for 1–6% of all endometrial cancers and this unique morphology is an independent predictor of poor prognosis (2). Similarly, ovarian CCC constitutes approximately 5% all ovarian malignancies and is characterized by a high degree of recurrence, frequent early metastases, and chemotherapy resistance resulting in poor prognosis(3). Given their rarity, optimal management strategies are extrapolated from large studies of more common endometrial and ovarian histologies and incorporates comprehensive surgical staging and often combination cytotoxic platinum-based chemotherapy and/or radiotherapy.
Additional therapeutic strategies are urgently needed to improve survival in this population. In clear cell carcinomas of the kidney, checkpoint inhibition has shown considerable promise. Checkpoint inhibitors including nivolumab, nivolumab plus ipilimumab, pembrolizumab plus axitinib, and atezolizumab plus bevacizumab have been shown to improve outcomes in phase III studies (4–6). We hypothesize that given homogeneity among clear cell phenotypes, uterine and ovarian clear cell cancers express PD-L1 and have high PD-1 expression within tumor lymphocytes. This expression pattern may correlate with tumor stage and survival and provide insights into plausibility of these as biomarkers and their role in choosing therapeutic targets.
Materials and Methods
Patient Selection
Patients were identified through the University of California, Irvine Tissue Biorepository from 1992–2017, as 1992 marked the beginning of storage of usable paraffin embedded formalin fixed tissue for our study. Inclusion criteria included females, age 18 and over, with a pure clear cell histology and adequate available tissue for staining as determined by the pathologists. Both histology and standard IHC expression, including absence of WT1, p53 and ER as well as presence of Napsin A, CK7, and HNF1B, were used to confirm clear cell phenotype. Only tumors with 100% clear cell histology were included and any mixed component or lack of complete medical records merited exclusion. Mixed tumors that had any other histology were excluded, as were patients with incomplete medical records. Two pathologists interpreted each result, with any discrepancies in scoring being deferred to the more senior of the two. A total of 46 patients met the above criteria.
A HIPAA-exempt IRB approval was obtained (UCI IRB HS#2015–2464). Available clinical information included age, ethnicity, tumor origin, clinical presence or absence of endometriosis, primary vs. secondary cytoreductive surgery as tissue source, FIGO tumor stage (1–4), date of diagnosis, and date of death or last documented encounter. This data was abstracted from the electronic medical record system as well as the University of California, Irvine Tumor Registry. Overall survival was calculated in months by subtracting date of death from the date of original tissue diagnosis. Censored survival time was used for living patients, utilizing the last documented follow up exam date to calculate survival in months.
Immunohistochemistry
After a standard optimization protocol for both PD-1 and PD-L1 using placental tissue known to express the target of interest as the positive control, we stained both ovarian and uterine clear cell paraffin embedded formalin fixed tissue with PD-L1 and PD-1 (both from Ventana Medical System) and evaluated for the presence of tumor infiltrating lymphocyte population (TILs). All immunohistochemistry was done using the automated Ventana Medical Systems-Ultra Roche BenchMark Ultra IHC Detection Kit. (Ventana Medical Systems, CA, USA). For PD-1 staining, Ventana NAT105 mouse monoclonal antibody was used to stain tumor and adjacent tissue. Expression was described in two ways - broadly, any positive PD-1 lymphocyte seen on the slide, to include those surrounding or infiltrating the tumor, was reported as 0 if no uptake was visualized, or greater than 0 if any uptake at all was visualized. The PD-1 positive tumor infiltrating lymphocytes alone were then reported as Negative/Rare if <1 TIL per high power fields, or Elevated if >1 TIL per high power fields. For PD-L1, Ventana SP263 rabbit monocloncal antibody was used to stain tumor and adjacent tissue. The PD-L1 expression within viable tumor cells was reported as 0% or >0% based on institutional standards as well as similar models in gynecologic and non-gynecologic studies(7, 8). PD-L1 positive lymphocytes and macrophages were also evaluated to facilitate the PD-L1 combined positive score (CPS). The CPS was calculated as the number of PD-L1 positive cells (to include tumor cells, lymphocytes and macrophages) divided by total number of viable tumor cells, multiplied by 100. The CPS results were reported as <1 or >= 1 (9).
Statistical Analysis
Key patient characteristics were summarized by tumor site, including means and standard deviations for quantitative variables and frequencies and percentages for categorical variables. Biomarker variables of PD-1, PD-L1, PD-1 positive lymphocytes and PD-L1 CPS score were categorized into dichotomous variables indicating elevated or not elevated level. The distributions of binary biomarker variables were also assessed in each tumor site. Fisher’s exact tests were used to test the association between tumor site and higher levels of biomarkers. Moreover, the association between stage and levels of biomarker was also tested using Fisher’s exact test for each tumor site.
All-cause survival time was calculated from date of diagnosis to date of death, or if censored, the date of last contact or visit. Survival time was considered to be right-censored if the patient was lost-to-follow-up or alive at the end of the study period. Survival curves were then estimated based on the Kaplan-Meier estimator. The log-rank statistic was used to test for equality of survival outcomes when comparing key sub-populations based on organ system and PD-L1 CPS of ≥1. Univariate and multivariable Cox proportional hazards model was used in the ovarian cancer cohort to estimate hazard ratios for positive versus negative PD-L1 CPS. Results are considered to be statistically significant if two-tailed P-value is <.05. All analyses were performed on SAS 9.4 (SAS Institute Inc. Cary, NC).
Results
Patient Characteristics
There were a total of 58 patients with pure clear cell carcinoma identified during the available time period. Of these, 12 patients were excluded due to mixed histology tumors, missing medical record data or insufficient paraffin embedded tissue. Thus, there were 46 evaluable cases in our study population, including a total of 35 ovarian cancers and 11 uterine cancers. Key patient characteristics are summarized by group in Table 1. The IHC expression was uninterpretable for 1 patient in the uterine cohort and there was insufficient tissue to allow restaining, thus this patient was excluded from IHC expression correlations resulting in 10 evaluable patients in the uterine cohort.
Table 1.
Key Patient Characteristics by Tumor Site
| Overall (n=46) | Ovary (n=35, 76.1%) | Uterine (n=11, 23.9%) | p value a | ||||
|---|---|---|---|---|---|---|---|
| Age at diagnosis (mean ± SD) | 58.4±13.8 | 57.7±12.4 | 60.9±18.4 | ||||
| n | % | n | % | n | % | ||
| Race/ethnicity | |||||||
| White | 25 | 54.3% | 20 | 57.1% | 5 | 45.5% | |
| Asian | 14 | 30.4% | 11 | 31.4% | 3 | 27.3% | |
| Others | 7 | 15.2% | 4 | 11.4% | 3 | 27.3% | |
| Tumor stage | |||||||
| I or II | 29 | 63.0% | 21 | 60.0% | 8 | 72.7% | |
| III | 10 | 21.7% | 9 | 25.7% | 1 | 9.1% | |
| IV | 7 | 15.2% | 5 | 14.3% | 2 | 18.2% | |
| Endometriosis | |||||||
| Yes | 5 | 10.9% | 5 | 14.3% | 0 | 0.0% | |
| No | 41 | 89.1% | 30 | 85.7% | 11 | 100.0% | |
| Surgery | |||||||
| Primary surgery | 41 | 91.1% | 32 | 91.4% | 9 | 90.0% | |
| Secondary surgery | 4 | 8.9% | 3 | 8.6% | 1 | 10.0% | |
| PD-1 TILs | |||||||
| Negative/rare | 23 | 53.5% | 20 | 60.6% | 3 | 30% | |
| Elevated | 20 | 46.5% | 13 | 39.4% | 7 | 70% | 0.148 |
| PD-L1 IHC | |||||||
| ’0% | 15 | 34.9% | 14 | 42.4% | 1 | 10% | |
| More than 0% | 28 | 65.1% | 19 | 57.6% | 9 | 90% | 0.127 |
| PD-1 positive lymphocytes | |||||||
| ’0 | 23 | 51.1% | 21 | 60.0% | 2 | 20.0% | |
| More than 0 | 22 | 48.9% | 14 | 40.0% | 8 | 80.0% | 0.0351 |
| PD-L1 CPS score | |||||||
| <1 | 27 | 60.0% | 23 | 65.7% | 4 | 40.0% | |
| ≥1 | 18 | 40.0% | 12 | 34.3% | 6 | 60.0% | 0.1656 |
Abbreviations: SD: standard deviation; CRS: cytoreductive surgery; CPS: combined positive score; IHC: Immunohistochemistry; TILS: tumor infiltrating lymphocytes
Endometriosis
Endometriosis was documented in either operative notes or clinical diagnoses in 5/35 (14.3%) cases of ovarian clear cell carcinoma and 0/11 of the uterine carcinomas. Given this rare incidence of endometriosis, well below the 30% incidence noted in other clear cell carcinoma literature, additional correlations with IHC staining patterns was not feasible (10, 11).
Tissue of origin and IHC staining patterns
In the ovarian cohort, PD-1 positive lymphocytes were present in 40% of tumors (14/35) and PD-1+ TILS in 39.4% (13/35). The ovarian tumors had any positive percentage of PD-L1 expression in 57.6% of tumors (19/35) and the PD-L1 CPS was positive in 34.3% of ovarian tumors (12/35). In the uterine cancer cohort, PD-1 positive lymphocytes were present in 80% (8/10) and PD-1+ TILs in 70% of tumors (7/10). The uterine tumors had any positive percentage of PD-L1 expression in 90% of tumors (9/10) and the PD-L1 score was positive in 60% of uterine tumors (6/10). (Table 1)
Tumor stage and IHC staining patterns
In univariate analysis of any positive PD-L1 CPS score, a significant correlation to stage in the ovarian cancer population was noted (Table 3). Clear cell ovarian cancer PD-L1 CPS was =>1 in 28.6% of stage I/II, 66.7% of stage III, and 0% of stage IV cancers (p = 0.03). Other IHC expression markers did not have statistically significant correlations with stage in the ovarian cohort. Within the uterine cohort, PD-L1 CPS was =>1 in 57.1% of stage I/II, 100% of stage III, and 50% of stage IV cancers. (Table 2).
Table 3.
Univariate analysis of IHC markers and correlation with patient and tumor factors in ovarian cohort
| Hazard Ratio | 95% CI | p value | |
|---|---|---|---|
| PD-1 TILs | |||
| Negative/rare | Referent | ||
| Elevated | 1.29 | 0.91 – 1.84 | 0.154 |
| PD-L1 IHC | |||
| ’0% | Referent | ||
| More than 0% | 1.26 | 0.91 – 1.75 | 0.168 |
| PD-1 positive lymphocytes | |||
| ’0 | Referent | ||
| More than 0 | 0.91 | 0.323 – 2.59 | 0.8662 |
| PD-L1 CPS | |||
| <1 | Referent | ||
| ≥1 | 0.89 | 0.303 – 2.59 | 0.8245 |
Abbreviations: TILS: tumor infiltrating lymphocytes; CPS: combined positive score; IHC: Immunohistochemistry
Table 2:
Association between biomarker level and tumor stage.
| Ovary (n=35) | Uterine (n=10) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage I or II | Stage III | Stage IV | Stage I or II | Stage III | Stage IV | ||||||||||
| n | % | n | % | n | % | p value a | n | % | n | % | n | % | p value a | ||
| PD-1 TILs | 0.2251 | 1 | |||||||||||||
| Negative/rare | 15 | 71.4% | 3 | 37.5% | 2 | 50.0% | 2 | 28.6% | 0 | 0.0% | 1 | 50.0% | |||
| Elevated | 6 | 28.6% | 5 | 62.5% | 2 | 50.0% | 5 | 71.4% | 1 | 100.0% | 1 | 50.0% | |||
| PD-L1 IHC | 0.578 | 1 | |||||||||||||
| ’0% | 10 | 47.6% | 2 | 25.0% | 2 | 50.0% | 1 | 14.3% | 0 | 0.0% | 0 | 0.0% | |||
| More than 0% | 11 | 52.4% | 6 | 75.0% | 2 | 50.0% | 6 | 85.7.% | 1 | 100.0% | 2 | 100.0% | |||
| PD-1 positive lymphocytes | 0.227 | 0.5333 | |||||||||||||
| ’0 | 14 | 66.7% | 3 | 33.3% | 4 | 80.0% | 1 | 14.3% | 0 | 0.0% | 1 | 50.0% | |||
| More than 0 | 7 | 33.3% | 6 | 66.7% | 1 | 20.0% | 6 | 85.7% | 1 | 100.0% | 1 | 50.0% | |||
| PD-L1 CPS score | 0.0331 | 1 | |||||||||||||
| <1 | 15 | 71.4% | 3 | 33.3% | 5 | 100.0% | 3 | 42.9% | 0 | 0.0% | 1 | 50.0% | |||
| ≥1 | 6 | 28.6% | 6 | 66.7% | 0 | 0.0% | 4 | 57.1% | 1 | 100.0% | 1 | 50.0% | |||
Abbreviations: TILS: tumor infiltrating lymphocytes; CPS: combined positive score; IHC: Immunohistochemistry
P value from Fisher’s exact test for association between biomarker level and tumor stage
P value from Kendall’s Tau-b correlation coefficient testing correlation between CPS score and stage
Survival Analysis
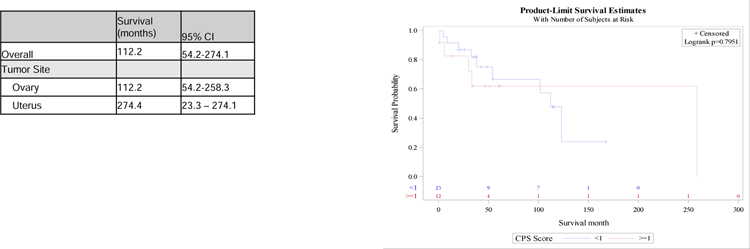
The median overall survival of all women with clear cell carcinoma in this sample was 9.35 years (95% CI 4.51–22.84). Median survival in the uterine cohort was 22.84 years (95% CI 1.94–22.84) and in the ovarian cancer cohort was 9.35 years (95% CI 4.51–21.52). The log rank between the PD-L1 positive and negative ovarian clear cell cohorts was p=0.795. (Figure 2). No IHC expression pattern was predictive of survival in univariate modeling, with a positive PD-L1 CPS HR of 0.89 (95%CI 0.3–2.59), p= 0.82, any positive PD-L1 percentage with HR 1.24 (95%CI 0.42–3.66), p=0.69, and PD-1 TILS with HR 1.29 (95%CI 0.91–1.84), p= 0.154). (Table 3) In the multivariate analysis, stage was correlated with survival independent of biomarker expression, however age and tumor site of origin were not. (Figure 2). Kaplan Meier for uterine CCC was not feasible due to small sample size.
Figure 2.
Kaplan Meier estimates of overall survival in ovarian cancer cohort based on PD-L1 CPS
Abbreviations: CCC: Clear cell carcinoma; CPS: combined positive score
a P value from logrank test
Discussion
A novel area of investigation is in manipulation of tumor induced immune suppression to achieve a more lasting response with biomarker driven approval for anti-PD-L1 therapy receiving recent FDA approval(12). Programmed cell death receptor (PD-1) and its ligand PD-L1 are expressed on antigen presenting cells and facilitate T cell suppression. A monoclonal antibody blocking either PD-1 or PD-L1 allows T cell activation and induction of tumor cell death. This therapeutic strategy has tolerable side effects and acceptable safety profiles, and has shown durable objective responses in metastatic melanoma, non-small cell lung cancer, as well as renal cell carcinoma(13–16). While PD-L1 IHC has not yet been established as a reliable biomarker, emerging research does imply that IHC expression may improve therapeutic targeting with anti-PD1 or anti-PD-L1 antibodies and correlate with improved survival(14, 16, 17). Given the remarkable similarity in histologic appearance, IHC staining patterns and microarray gene expression among clear cell histology regardless of organ of origin, it is reasonable to conclude that clear cell ovarian and endometrial carcinomas may benefit from targeted immune therapy. Clinical trials are upcoming or underway for recurrent ovarian cancers which include clear cell subtypes, utilizing combination regimens which include checkpoint inhibitors such as pembrolizumab and epicadostat (NCT03602586, NRG-GY016), nivolumab and ipilimumab (NCT03355976), tremelimumab and olaparib (NCT04034927, NRG-GY021), pegylated liposomal doxorubicin with atezolizumab and bevacizumab (NCT02839707, NRG-GY009), durvalumab (NCT03405454), and pembrolizumab with bevacizumab and cyclophosphamide (NCT02853318). In recurrent uterine clear cell cancers, there is a single study evaluating the addition of pembrolizumab to standard chemotherapy (NCT03914612, NRG-GY018) (18).
This descriptive study better characterizes expected IHC expression patterns for clear cell ovarian and uterine cancers. There is minimal existing literature evaluating IHC expression of PD-1 and PD-L1 and none within clear cell gynecologic cancers specifically in assessing their role as biomarkers. A 2015 study by Herzog et al showed that in mixed, non-pure clear cell endometrial cancer 69.2% (9/13) expressed PD-1 at 1+ or greater (19). Within their mixed, non-pure clear cell ovarian cancer population, 46.3% (19/41) of tumors expressed PD-1 at 1+ or greater. This study differs from ours in that it allowed mixed tumors, whereas ours is confined to pure clear cell tumors which may represent a different population. Our study suggests greater frequency of PD-L1 percent positivity in uterine cancers at 90%, and novel uterine cancer data including a positive PD-L1 CPS in 60%. Similarly, there was greater PD-L1 positivity in our ovarian cohort, at 57.6% as compared to 46% in the data by Hertzog et al, and novel ovarian cancer data including a positive PD-L1 CPS in 34.3%.
Interestingly, stage was significantly associated with positive PD-L1 CPS in ovarian tumors, with 67% of stage 3 tumors and 0% of stage 4 tumors showing expression. While this did not translate into prognostic implications, it is interesting to note as it has been established in lung cancers that tumors at more advanced stage become MHC-I negative and that this transition is characterized by immune escape. (20, 21) We hypothesize that this loss of PD-L1 expression may reflect a de-differentiation of more advanced and aggressive tumors with the development of more sophisticated immune-evasion mechanisms.
In our study, PD-L1 expression using CPS of =>1 was not correlated with survival in the ovarian cancer population. In a 2015 study of PD-1 and PD-L1 expression in patients with renal clear cell carcinoma, elevated PD-1 and PD-L1 expression are correlated with worse progression free survival and trended toward worse overall survival(22). The limited sample size in this study likely influenced our ability to detect any true correlation in survival outcome, such as that seen in the renal clear cell carcinoma cohort.
The primary limitation in this study was sample size. This was in part due to the stringent criteria that only pure clear cell carcinoma be included. These data were observational and the associations derived here do not represent causal inferences. Additionally, as this is a retrospective assessment of a single institution, it is subject to selection bias as well as inherent limitations of medical record review. Given the limitations of coding and documentation related to concurrent endometriosis, this was only found in a small subset of the ovarian cancer population and a larger cohort would need to be identified to draw any meaningful conclusions about the role of endometriosis in PD-1 and PD-L1 expression.
Conclusion:
Despite the limitations of the study, we are able to report baseline IHC expression frequencies within pure clear cell uterine and ovarian carcinoma populations. More advanced ovarian cancer was associated with a positive PD-L1 CPS score. There is no association between IHC expression and survival in our sample to suggest a role as a prognostic biomarker. Both TILs and PD-L1 play important roles in the anti-tumor immune response and, in contrast, tumor evasion of the host immune system. In the era of emerging use of targeted therapies and immunotherapy, these data are hypothesis generating and support further investigation into PD-L1 in the clear cell histologic subset as immune checkpoint inhibitor remain an intriguing therapeutic target for these rare gynecologic tumors.
Figure 1.
Clear cell carcinoma cells showing moderate positivity for PD-L1 in a predominantly membranous pattern.
A) H&E stain; B) PD-L1 stain
Table 4.
Multivariate analysis of IHC markers and correlation with patient and tumor factors
| PD-1 TILS | PD-L1 IHC | PD-1 positive lymphocytes | PD-L1 CPS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (by 10 years) | 1.29 | 0.91 – 1.83 | 0.154 | 1.26 | 0.91 – 1.75 | 0.168 | 1.21 | 0.857 – 1.70 | 0.2827 | 1.20 | 0.853 – 1.69 | 0.2964 |
| Tumor site | ||||||||||||
| Ovary | Referent | Referent | Referent | Referent | ||||||||
| Uterine | 1.52 | 0.32 – 7.25 | 0.603 | 2.01 | 0.43 – 9.35 | 0.3754 | 2.39 | 0.454 – 12.57 | 0.3041 | 2.48 | 0.484 – 12.70 | 0.2759 |
| Stage | ||||||||||||
| I or II | Referent | Referent | Referent | Referent | ||||||||
| III | 3.37 | 0.94 – 11.99 | 0.061 | 3.77 | 1.01 – 14.02 | 0.048 | 3.82 | 1.084 – 13.48 | 0.0371 | 3.99 | 1.096 – 14.53 | 0.0358 |
| IV | 7.21 | 1.68 – 31.03 | 0.008 | 7.11 | 1.64 – 30.86 | 0.009 | 6.27 | 1.456 – 27.03 | 0.0137 | 6.23 | 1.444 – 26.89 | 0.0142 |
Abbreviations: TILS: tumor infiltrating lymphocytes; CPS: combined positive score; IHC: Immunohistochemistry
Highlights – Clear cell IHC.
PD-L1 CPS ≥1 was found in 34.3% of ovarian and 60% of uterine CCC tumors
PD-1 associated lymphocytes were found in 40% of ovarian and 80% of uterine tumors
More advanced CC ovarian cancer is less likely to express PD-L1 using CPS
Uterine CCC is more likely to have PD-1 associated TILS than ovarian CCC
No difference in OS for PD-L1 CPS cohorts among the ovarian cancer population
Acknowledgments:
This study was supported by an institutional NIH T-32 training grant (Ruth L. Kirschstein NRSA Institutional Training Research Grant, 2 T32 CA06039611) as well as support through the Queen of Hearts Foundation.
Sources of financial support:
1. UCI Obstetrics and Gynecology Department
2. Institutional NIH T-32 training grant (Ruth L. Kirschstein NRSA Institutional Training Research Grant, 2 T32 CA06039611)
3. Queen of Hearts Foundation
Footnotes
Presentation information: 34th Annual Meeting of the Western Association of Gynecologic Oncologists, June 2017 – poster presentation
Conflicts of Interest: The authors report no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No authors have any conflicts of interest.
References
- 1.Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ, et al. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(18):6422–30. [DOI] [PubMed] [Google Scholar]
- 2.Olawaiye AB, Boruta DM 2nd. Management of women with clear cell endometrial cancer: a Society of Gynecologic Oncology (SGO) review. Gynecologic oncology. 2009;113(2):277–83. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida S, Furukawa N, Haruta S, Tanase Y, Kanayama S, Noguchi T, et al. Theoretical model of treatment strategies for clear cell carcinoma of the ovary: focus on perspectives. Cancer Treat Rev. 2009;35(7):608–15. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet (London, England). 2019;393(10189):2404–15. [DOI] [PubMed] [Google Scholar]
- 5.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2019;380(12):1116–27. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2018;378(14):1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips T, Simmons P, Inzunza HD, Cogswell J, Novotny J Jr., Taylor C, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Applied immunohistochemistry & molecular morphology : AIMM. 2015;23(8):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Annals of oncology : official journal of the European Society for Medical Oncology. 2015;26(2):259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karina Kulangara DAH, Stephanie Waldroup, Lindsay Peltz, Supriya Shah, Charlotte Roach, Jonathan Wes Juco, Kenneth Emancipator, and Dave Stanforth. Development of the combined positive score (CPS) for the evaluation of PD-L1 in solid tumors with the immunohistochemistry assay PD-L1 IHC 22C3 pharmDx. Journal of Clinical Oncology. 2017;35. [Google Scholar]
- 10.Kobayashi. Molecular pathogenesis of endometriosis-associated clear cell carcinoma of the ovary (Review). Oncology Reports. 2009. [PubMed]
- 11.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. The Lancet Oncology. 2012;13(4):385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. Journal for immunotherapy of cancer. 2018;6(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369(2):134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, et al. Survival, Durable Response, and Long-Term Safety in Patients With Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(18):2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grosso J Association of tumor PD-L1 expression and immune biomarkers and clinical activity in patients with advanced solid tumors treated with nivolumab. American Society of Clinical Oncology 2013.
- 18.NIH UNLoM. ClinicalTrials.gov Accessed 8.23.2019.
- 19.Thomas Herzog DA, Sandeep Reddy, Zoren Gatallica. PD-1 and PD-L1 expression in 1599 gynecologic malignancies - implications for immunotherapy.
- 20.Perea F, Sanchez-Palencia A, Gomez-Morales M, Bernal M, Concha A, Garcia MM, et al. HLA class I loss and PD-L1 expression in lung cancer: impact on T-cell infiltration and immune escape. Oncotarget. 2018;9(3):4120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Current opinion in immunology. 2016;39:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kammerer-Jacquet SF, Crouzet L, Brunot A, Dagher J, Pladys A, Edeline J, et al. Independent association of PD-L1 expression with noninactivated VHL clear cell renal cell carcinoma-A finding with therapeutic potential. International journal of cancer. 2017;140(1):142–8. [DOI] [PubMed] [Google Scholar]