Abstract
Purpose
To assess the pharmacokinetic (PK)/pharmacodynamic (PD) relationship following intracameral Bimatoprost sustained-release (SR) implants (8, 15, 30, and 60 µg) in dogs to determine the optimal investigative dose in humans.
Methods
Forty-four male normotensive beagle dogs were assigned to 1 of 8 groups receiving 8-, 15-, 30-, and 60-µg implants (PD assessment [n = 8/group, 4 groups]; PK assessment [n = 3/group, 4 groups]). Intraocular pressure (IOP) in PD animals and aqueous humor/blood concentrations of bimatoprost and its acid in PK animals were assessed. PK/PD correlation analysis was performed using steady-state data. Residual implants were recovered to assess polymer degradation.
Results
Dose-dependent IOP lowering was observed for all dose groups for at least 3 months postdose. Blood concentrations of bimatoprost and bimatoprost acid were below the limit of quantification (<0.25 ng/mL), whereas dose-dependent concentration-time profiles were observed in the aqueous humor. At steady state, observed and predicted correlation between aqueous humor drug concentration and IOP lowering was similar and translatable to findings in humans following topical bimatoprost eyedrop administration. Implants at all doses were well tolerated and polymer degradation was apparent.
Conclusions
Dose-dependent IOP lowering with Bimatoprost SR was maintained for at least 3 months in dogs, and the implants were well tolerated. The established PK/PD relationship appears to translate to humans. Doses between 8 and 15 µg appear to provide the best benefit/risk profile for clinical development of the implants.
Translational Relevance
The close PK/PD relationship between dog and human helps inform which bimatoprost dose should be investigated in clinical studies.
Keywords: pharmacokinetics, pharmacodynamics, bimatoprost sustained-release, intracameral, nonclinical
Introduction
One of the most significant challenges to the successful management of glaucoma is poor patient adherence to topical ocular hypotensive medications that are prescribed to control intraocular pressure (IOP).1 Topical medications such as prostaglandin analogs (e.g., bimatoprost) are the most common initial intervention and, if the disease progresses, they can be used in combination with other classes of glaucoma medications and in conjunction with laser and/or surgical procedures. Reasons for poor adherence include the need for daily dosing, side effects, forgetfulness, and difficulty in instilling eye drops, especially in elderly patients.2–4
Efforts to ameliorate some of these barriers to compliance include the development of sustained-release implantable drug-delivery devices. Such devices may permit optimal dosing and targeted delivery of drug to ocular tissues for prolonged periods. This approach obviates the need for daily topical administration and minimizes the potential for adverse events associated with topical medications.
Bimatoprost sustained-release implant (Bimatoprost SR) is a rod-shaped implant containing bimatoprost within the biodegradable NOVADUR platform for drug delivery (Allergan plc, Dublin, Ireland).5 The implant was designed to slowly release bimatoprost with nonpulsatile, zero-order kinetics.6 Implantation in the intracameral space permits direct delivery of bimatoprost to key sites of action, the iris-ciliary body and trabecular meshwork, and may reduce adverse events associated with topical prostaglandin analogs by limiting distribution to the conjunctiva and periocular tissues. In a previous preclinical investigation in normotensive beagle dogs, Bimatoprost SR enhanced drug delivery to the iris-ciliary body, whereas the distribution to tissues associated with side effects of topical prostaglandin analogs (e.g., bulbar conjunctiva, eyelid margins, and periorbital fat) was limited compared with topical bimatoprost.7
A dose-ranging, phase 1/2 study of Bimatoprost SR containing 6-, 10-, 15-, or 20-µg doses showed effective IOP lowering in patients with open-angle glaucoma at all doses.6 A dose-dependent IOP-lowering effect was evident up to 12 weeks postimplantation, and through 16 weeks, the mean overall IOP reductions from baseline were 7.2, 7.4, 8.1, and 9.5 mm Hg with the 6-, 10-, 15-, and 20-µg doses, respectively.6 The ocular pharmacokinetic (PK) profile of the implant in humans is as yet unknown.
Beagle dogs are among the most common species used for nonclinical ocular assessments since they share similar ophthalmological anatomy and physiology with humans, and their eyes are relatively large, which facilitates the implantation of drug-delivery devices and ocular examination.8 The purpose of this study was to assess the PK and pharmacodynamic (PD) profiles of bimatoprost after intracameral injection of Bimatoprost SR (8, 15, 30, and 60 µg) in beagle dogs to support dose selection for testing in humans.
Methods
Animals and Ethics
The study was conducted by Covance Laboratories (Madison, WI, USA). Sixty male normotensive beagle dogs (Covance Research Products, Cumberland, VA, USA) were acclimated for approximately 11 weeks, and before dosing, weighed 9.1 to 13.4 kg and were approximately 7 to 8 months of age. Throughout the study, animals received a certified canine diet and fresh water daily ad libitum, were weighed regularly, and trained to tolerate study procedures without sedation or anesthesia.
All procedures were conducted in compliance with Animal Welfare Act Regulations and in accordance with the study protocol and amendments. The study adhered to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research and was approved by Allergan's Animal Care and Use Committee.
Bimatoprost SR and Study Design
Four treatments with varying implant size and bimatoprost doses were evaluated: implant length/dose of 1 mm/8 µg, 1.5 mm/15 µg, 1 mm/30 µg, and 2 mm/60 µg. Implants were administered using a 25-gauge applicator device. Eight study groups were investigated: PD values were assessed in 4 Bimatoprost SR-treated groups (groups 1–4, each group n = 8) in which the right eye of animals received a single administration of implant at doses of 8 µg, 15 µg, 30 µg, and 60 µg; the untreated left eye was used to evaluate within-animal differences between treated and fellow eyes. PK values were evaluated in 4 smaller Bimatoprost SR-treated groups (groups 5–8, each group n = 3) in which both eyes of animals received a single administration of the implants (8, 15, 30, or 60 µg). Aqueous humor and blood samples were collected from these animals. Ophthalmic safety assessment was carried out in all animals.
Animal Assignment and Injection Procedure
Forty-four animals were assigned to 1 of the 8 treatment groups. Both eyes were prepared for intracameral implant administration by applying topical ophthalmic anesthetic (1 or 2 drops of 0.5% proparacaine) approximately 5 minutes before dose administration. Eyes were rinsed with a diluted iodine solution for 2 to 3 minutes and the periorbital region cleaned with cotton-tipped applicators. The eyes were then irrigated with saline, and topical ophthalmic anesthetic instilled to each eye. The implant was injected into the anterior chamber using the sterile, preloaded applicator, and a broad-spectrum antibiotic was topically applied to the eye.
Assessments
Animals were observed twice daily for mortality and signs of pain/distress, and daily cage-side observations were conducted for general health and appearance. Throughout the study, assessments included IOP measurements, pachymetry, gonioscopy, noncontact specular microscopy, and slit-lamp ophthalmic examinations.
IOP was measured 3 times weekly for all animals in groups 1−4 in the treated and untreated eye. Measurements were taken once weekly by Covance in-life staff to maintain animal acclimation to the procedure, and twice weekly by a veterinary ophthalmologist for the IOP analysis. Each measurement consisted of 3 independent readings per eye per time point; the average of the 2 closest IOP readings (or if the 3 readings were equally spaced or equal, the average of the 3 readings) was used for analysis. To account for diurnal variation in IOP, mean IOP change between the treated right eye (OD) and the untreated left eye (OS) was calculated using the following equation:
Ophthalmic examinations were conducted throughout the study in all animals using a slit-lamp biomicroscope. The Standardization of Uveitis Nomenclature scoring system9 was used to evaluate anterior chamber cells (0 = cells in field <1 to +4 = cells in field >50), anterior chamber flare (0 = none to +4 = intense [fibrin and plastic aqueous]), and conjunctival hyperemia (0 = normal to +3 = severe). Three corneal thickness (pachymetry) measurements in each eye were taken using AccuPach V (Accutome, Malvern, PA, USA).
Gonioscopy was conducted using a slit-lamp biomicroscope and Oksma-Kaufman gonio lens (Ocular Instruments, Bellevue, WA, USA). Noncontact specular microscopy (TOPCON SP-300; Topcon Corporation, Tokyo, Japan) was conducted in both eyes.
Sample Collection
Blood (approximately 1 mL) and aqueous humor samples (at least 50 µL) were collected from all animals in groups 5−8 during weeks 2, 3, 5, 7, 9, 11, and 14. Blood samples were mixed with anticoagulant and stored at approximately -20°C. Aqueous humor samples were collected from anesthetized and sterilized eyes using a 25- to 30-gauge needle affixed to a zero dead volume tuberculin syringe that was inserted through the cornea near the limbus. Topical ophthalmic antibiotic was applied following aqueous humor collection. Samples were stored at approximately −20°C.
After 14 weeks, all animals were sacrificed in groups 5–8 for aqueous humor collection and implant recovery, and 2 animals per group were sacrificed in groups 1–4 at weeks 19 and 26 for the recovery of implants. Aqueous humor and residual implant (all groups) were collected, rinsed with saline, and weighed.
Determination of Drug Levels
Concentrations of bimatoprost and bimatoprost acid in the aqueous humor were determined using a liquid chromatography-tandem mass spectrometry method by Prevalere Life Sciences (Whitesboro, NY, USA). Concentrations of bimatoprost and bimatoprost acid in blood samples were determined using a liquid chromatography-tandem mass spectrometry method by Allergan (Irvine, CA, USA).
Remnant Implant Analysis
Remnant implants were retrieved from the eyes at necropsy when feasible and shipped back to Allergan for analysis. The implants were freeze-dried using a LyoStar II lyophilizer (FTS Systems, Inc., Stone Ridge, NY, USA) and weighed using a Mettler Toledo MT5 microbalance (Columbus, OH, USA). Degradation of the polymers in the implants was examined using a Gel Permeation Chromatography (GPC) system. The implant samples were dissolved in 100 µL tetrahydrofuran and analyzed using the GPC system. The average molecular weight of the polymers in the implants was determined based on the peak retention time in the GPC chromatograms. A Waters Alliance 2690D HPLC system (Waters Corporation, Milford, MA, USA) equipped with a W2487 detector was used for the quantitation of the residual bimatoprost. Scanning electron microscopy images of the implants were acquired using a Zeiss EVO40 electron microscope (Jena, Germany). Implant weight loss was calculated by subtracting the weight of bimatoprost released and polymer weight loss from the original implant.
PK/PD Data Analysis
The bimatoprost and bimatoprost acid metabolite concentration-time profile in aqueous humor suggested reasonable correlation with the in vitro implant drug release profile (Allergan, data on file). Corresponding to the in vitro data, the period between 5 and 11 weeks in vivo was considered to be “steady state.” Mean aqueous humor concentration of bimatoprost and bimatoprost acid (combined as both are pharmacologically active10,11) at steady state from all animals in groups 5−8 and IOP data from all animals in groups 1−4 across all 4 dose levels were used to establish the PK/PD relationship. Mean aqueous humor drug concentration (bimatoprost, bimatoprost acid, or combined concentration of both) versus the percentage decrease in IOP from baseline were fitted using Emax PD models (Phoenix WinNonlin version 64; Certara, Inc, Princeton, NJ, USA) assuming zero IOP lowering at zero aqueous humor drug concentration. Because aqueous humor samples and IOP data were collected from animals in different groups on different schedules, with IOP measurements taken much more frequently, IOP data collected on the days closest to the aqueous humor sample collection were used for the PK/PD correlation analysis, assuming comparable drug release and aqueous humor concentration profiles in animals receiving the same doses in different treatment groups.
Results
IOP Measurements
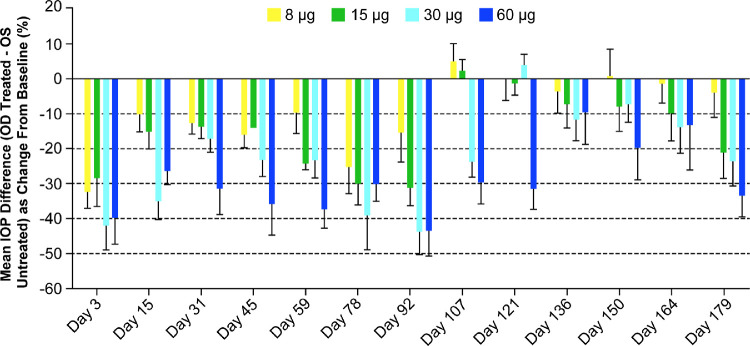
The mean baseline IOP values (combined mean IOP values on study days -5 and -7) for the right and left eyes, respectively, were 14.8 and 14.3 mm Hg (8-µg group), 14.0 and 13.6 mm Hg (15-µg group), 14.9 and 14.6 mm Hg (30-µg group), and 13.0 and 12.3 mm Hg (60-µg group), demonstrating comparable IOP values at baseline between the two eyes. A dose-dependent IOP-lowering effect in terms of mean IOP change in the treated right eyes from baseline (corrected for untreated left eye) was evident following Bimatoprost SR administration, with all dose strengths reducing IOP through at least 90 days post-dose (Fig. 1). A clinically significant IOP-lowering effect (>15%) was still evident after 6 months (day 179) with the 15-, 30-, and 60-µg doses.
Figure 1.
Mean difference in intraocular pressure (IOP) between treated (right eye [OD]) and untreated (left eye [OS]) eyes as percentage change from baseline. Error bars indicate the standard error of the mean.
Average IOP lowering as a function of the dose level during the 3 months after administration of Bimatoprost SR was evaluated, taking into account all IOP measurements. Analysis of the mean percentage change in IOP from baseline again demonstrated a clear dose response throughout the range of doses tested (Fig. 2).
Figure 2.
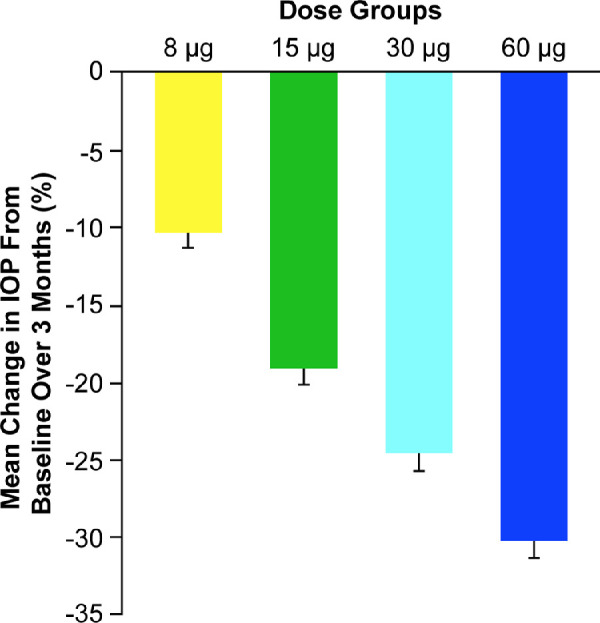
Mean percentage change in intraocular pressure (IOP) from baseline in beagle dogs in the treated eye (right eye) over 3 months after intracameral administration of Bimatoprost SR. Error bars indicate the standard error of the mean.
Concentrations of Bimatoprost and Bimatoprost Acid in Blood and Aqueous Humor
The concentrations of bimatoprost and bimatoprost acid in the blood were below the limit of quantification (<0.25 ng/mL) at all time points. A dose-dependent concentration-time profile of bimatoprost (Fig. 3A) and bimatoprost acid (Fig. 3B) was observed in the aqueous humor. Mean concentrations of bimatoprost in the aqueous humor were approximately 4 times higher than those for bimatoprost acid at 14 weeks postdose.
Figure 3.
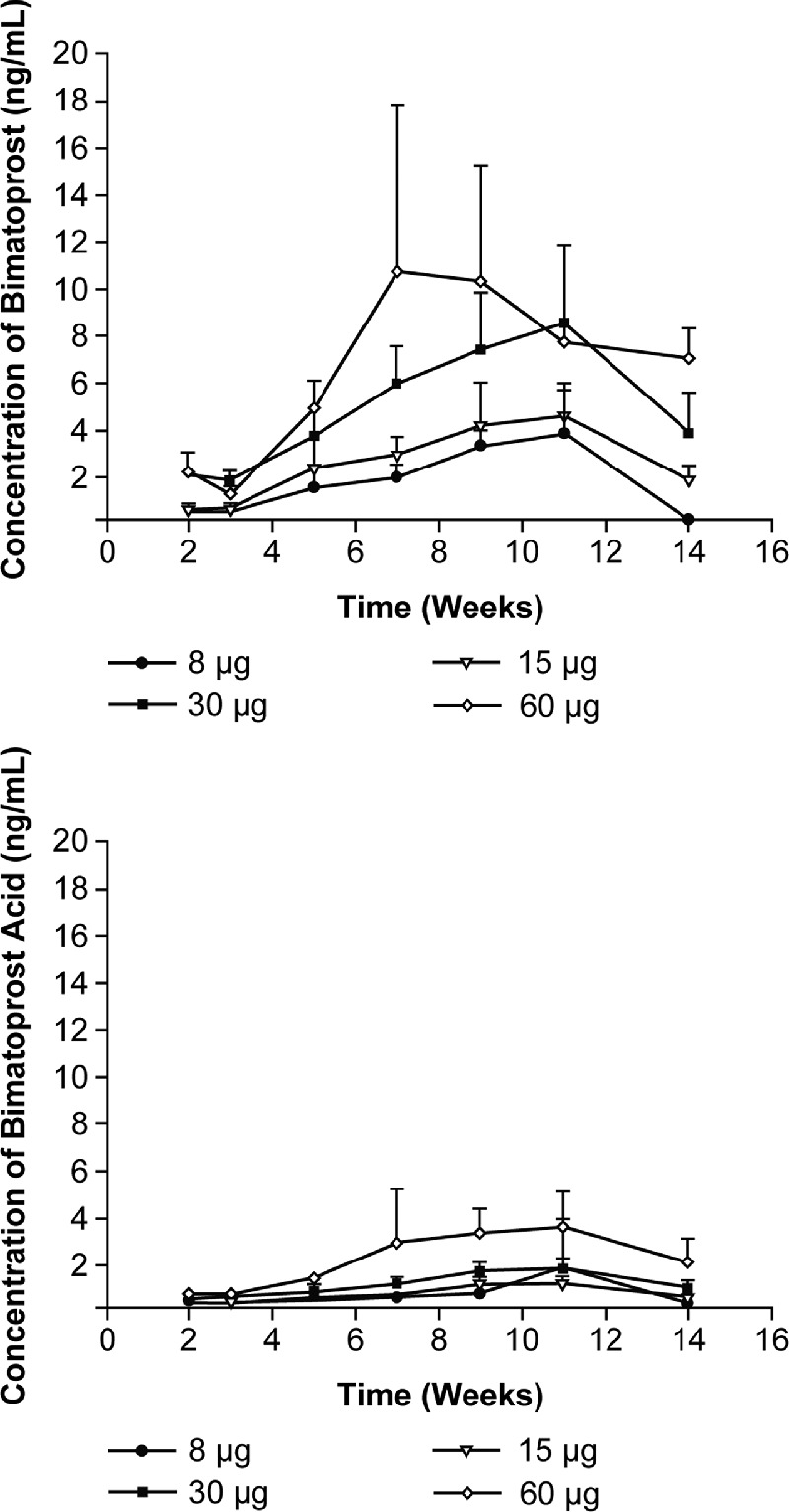
Mean (standard deviation) concentrations of (top) bimatoprost and (bottom) bimatoprost acid in aqueous humor collected from dogs after administration of Bimatoprost SR in both eyes.
PK/PD Analysis
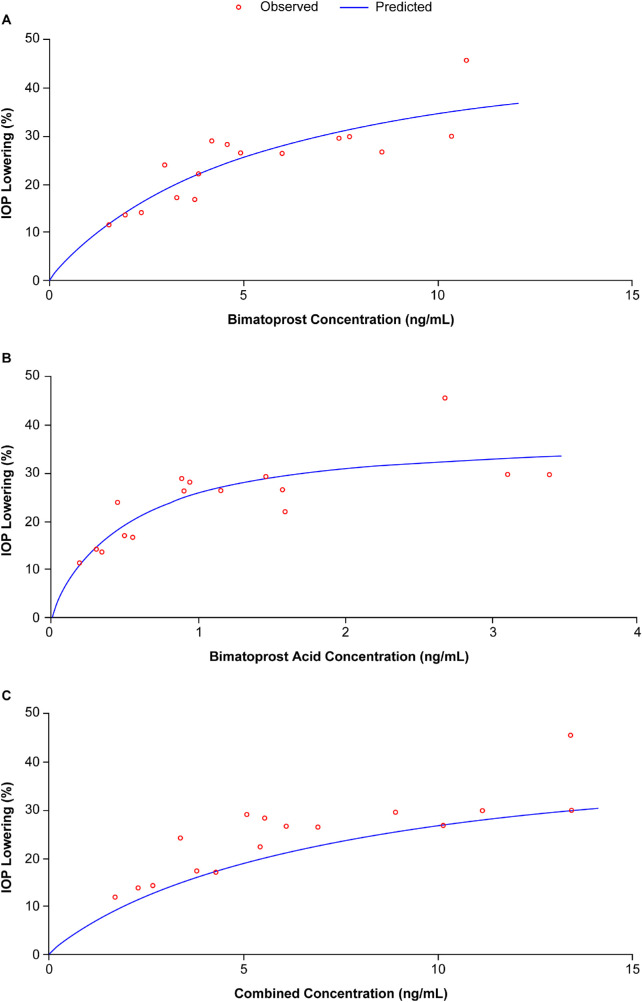
Because both bimatoprost and bimatoprost acid are pharmacologically active,10,11 PK/PD analysis was performed using concentrations of bimatoprost, bimatoprost acid, and the combined values against IOP-lowering data. Final parameter estimates and corresponding percentage coefficient of variation are listed for each scenario in Table 1. The predicted correlation between aqueous humor drug concentration and IOP lowering appeared to fit the observed data well in all 3 scenarios (Fig. 4).
Table 1.
PK/PD Parameter Estimates Obtained Based on the Best Fit Model (Simple Emax)
| Bimatoprost | Bimatoprost Acid | Combined (Bimatoprost and Acid) | ||||
|---|---|---|---|---|---|---|
| Parameters | Value | CV% | Value | CV% | Value | CV% |
| Emax | ||||||
| (% IOP lowering) | 53.4 | 19 | 39.0 | 11 | 45.8 | 33 |
| EC50 (ng/mL) | 5.42 | 38 | 0.503 | 34 | 7.22 | 66 |
CV%, percentage coefficient of variation; EC50, drug concentration required to achieve half maximal effect; Emax, maximum pharmacologic effect; IOP, intraocular pressure; PK/PD, pharmacokinetic/pharmacodynamic.
Figure 4.
Observed versus predicted correlation between aqueous humor drug concentration (A, bimatoprost; B, bimatoprost acid; C, combined) versus percentage intraocular pressure (IOP) lowering from baseline in dogs following intracameral administration of Bimatoprost SR (8 to 60 µg).
Based on reported peak human aqueous humor concentrations of bimatoprost and bimatoprost acid following topical administration of bimatoprost ophthalmic solution 0.03%,12,13 the PK/PD relationship established during steady state in the current study is consistent with the relationship in humans following topical bimatoprost dosing. Cantor et al. measured aqueous humor concentration of bimatoprost and bimatoprost acid at 1, 3, 6, and 12 hours after a single 30-µL drop of bimatoprost 0.03% solution.12 A maximum combined mean concentration for both analytes of 4.67 ng/mL (6.6 nM for bimatoprost and 5.0 nM for bimatoprost acid) was observed at 1 hour postdose (n = 8). Camras et al. measured aqueous humor concentration of bimatoprost and bimatoprost acid at 2 and 12 hours after 7 days of once-daily topical treatment of bimatoprost 0.03%.13 They reported a combined mean concentration for both analytes of 10.9 ng/mL (5.7 nM for bimatoprost and 22.0 nM for bimatoprost acid) observed at 2 hours postdose (n = 12), which was 3.4 times higher than the mean concentration observed at 12 hours postdose (n = 8). Considering topical bimatoprost 0.03% has been well established to elicit the maximum ∼30% IOP reduction clinically,14 the PK/PD relationship in humans, based on PK data established by Cantor and Camras, as discussed above, fit well with the PK/PD relationship established in dogs using data generated in this study, as shown in Figure 4. This supports the translatability of the dog PK/PD model to humans.
Dose-Response Prediction for Bimatoprost SR in Humans
To predict the clinical IOP dose response for the 4 dose strengths tested in this study, a few assumptions were made. Because bimatoprost is known to have minimal ocular metabolism beyond hydrolysis to form the acid (Allergan, data on file), aqueous humor turnover is expected to be the predominant route for drug clearance from the anterior chamber. Assuming the same in vivo Bimatoprost SR implant drug release rate in dog and human eyes, the steady-state aqueous humor drug concentration ratio between the 2 species would be inverse of the aqueous humor outflow rate ratio. In normal human subjects, aqueous humor outflow while awake has been reported to be 2 to 3 µL/min.15,16 With an anterior chamber approximately 4 times larger than that in humans, reported measurements of aqueous humor outflow in normal beagle dogs range from 4 to 6 µL/min.16–18 Assuming the same aqueous outflow rate in glaucoma patients with IOP controlled by Bimatoprost SR as normal human subjects, the steady-state drug concentration would be ∼2× higher in human eyes than in dog eyes dosed with the same Bimatoprost SR implants. Applying the estimated human aqueous humor drug concentration to the established PK/PD model as shown in Figure 4, the predicted IOP lowering in humans by the 4 different doses of Bimatoprost SR is presented in Table 2.
Table 2.
Predicted Steady-State Human IOP Response to 4 Doses of Intracameral Bimatoprost SR Implants Based on PK/PD Relationship Established in Dogs
| Implant Dose Strength (µg) | |||||
|---|---|---|---|---|---|
| 8 | 15 | 30 | 60 | ||
| Dog | Cav* (ng/mL) | 3.32 | 4.18 | 7.54 | 11.0 |
| % IOP lowering (observed) | 10 | 19 | 24 | 30 | |
| Human | Cav† (ng/mL) | 6.64 | 8.36 | 15.08 | 22.0 |
| % IOP lowering (predicted) | 29 | 31 | 33 | 33 | |
Bimatoprost SR, bimatoprost sustained-release; Cav, average concentration; IOP, intraocular pressure; PK/PD, pharmacokinetic/pharmacodynamic.
Average aqueous humor bimatoprost and bimatoprost acid concentration (combined) calculated based on PK data at steady state.
Average aqueous humor bimatoprost and bimatoprost acid concentration (combined) in humans estimated based on a 2:1 ratio of drug clearance rate assuming the same in vivo drug release rate in dogs versus humans.
Remnant Implant Analysis
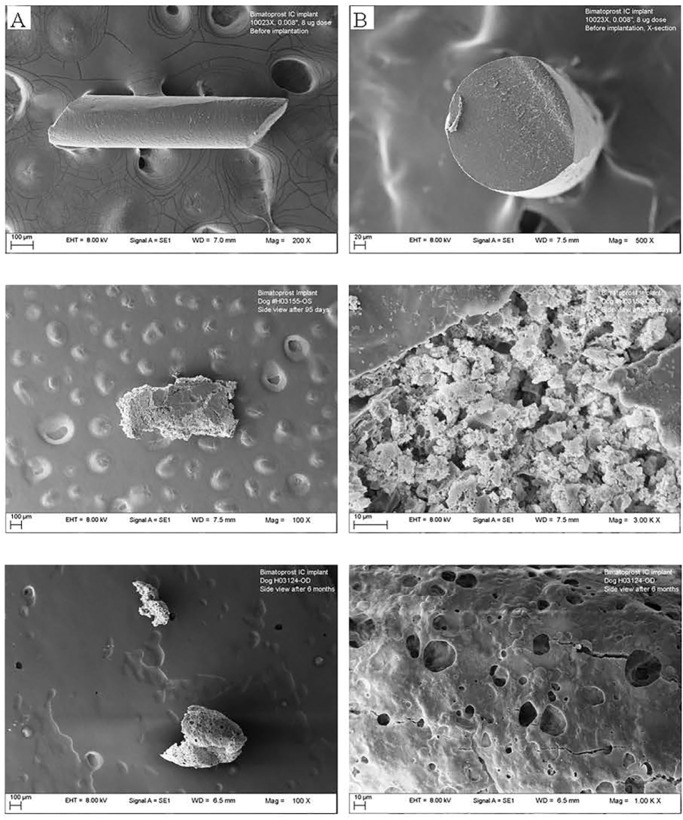
At 3 months postdose, the mean amount of bimatoprost in the recovered implants was 0 µg (8-µg dose), 0.4 µg (15-µg dose), 2.1 µg (30-µg dose), and 5.2 µg (60-µg dose), which translates to 100%, 97%, 93%, and 91% of drug released at that time point, respectively. There was no bimatoprost detected in implants retrieved at 6 months, indicating complete drug release. The total implant weight loss in the Bimatoprost SR implants ranged from 24% to 35% after 3 months, and from 46% to 66% after 6 months (Fig. 5). A highly porous and fragile structure was evident in all implants after 3 and 6 months, as illustrated in the representative scanning electron microscopy images of the 8-µg dose implant (Fig. 6).
Figure 5.
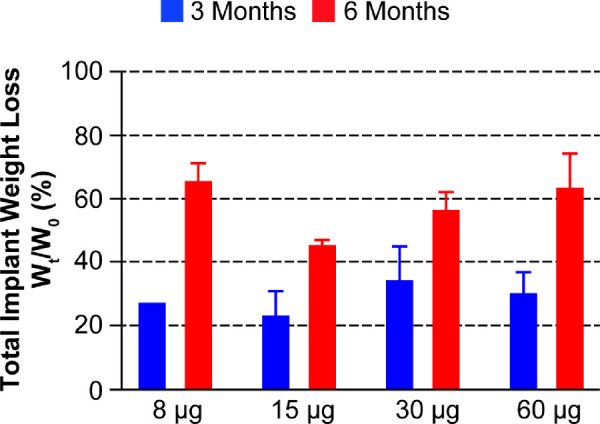
Percentage changes in total implant weight loss at 3 and 6 months (Wt) relative to the original implant weights (W0). The total implant weight loss was calculated based on the nominal weights of the implants (8-µg dose = 40 µg; 15-µg dose = 75 µg; 30-µg dose = 150 µg; 60-µg dose = 300 µg); error bars indicate the standard deviation.
Figure 6.
Representative scanning electron microscopy images of the implant (8-µg dose): (top) before implantation ([A] side view and [B] cross section); (middle) retrieved from the anterior chamber after 3 months (left side view, 100× magnification, right side view, 1000× magnification); (bottom) retrieved from the anterior chamber after 6 months (left side view, 100× magnification, right side view, 1000× magnification).
Ophthalmology Safety Assessments
Intracameral injection of bimatoprost implant at all doses was well tolerated. Generally, injection-site findings were minimal and resolved by day 8 or 9 postdose. Mild to moderate conjunctival hyperemia (+1 to +2) was evident in Bimatoprost SR-treated eyes with a dose-dependent trend (Fig. 7). Aqueous flare was sporadically and infrequently seen in all groups during the first week and resolved in all groups by day 8 or 9. There were no clear and consistent differences, or clinically meaningful abnormalities, in corneal thickness measurements between left and right eyes in groups 1 through 4. Iridocorneal angles appeared gonioscopically normal in all eyes with 1 exception, in which a small amount of blood in the trabecular meshwork was suspected at week 9 in an eye treated with Bimatoprost SR 60 µg; this resolved without sequelae. Pupil diameter measurements indicated a dose-associated miosis in the Bimatoprost SR-treated eye that diminished over the course of the study.
Figure 7.
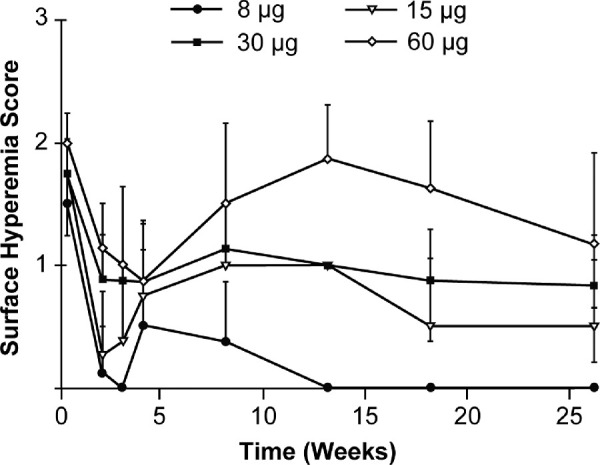
Mean (standard deviation) right eye surface hyperemia scores in male dogs administered bimatoprost via the intracameral drug delivery system. Note: values are the mean of 4 animals per group with the exception of week 26 (2 animals per group).
Bimatoprost SR had no effect on macroscopic or microscopic findings assessed at 4 or 6 months following implantation, other than minimal attenuation of the corneal endothelium in the Bimatoprost SR 60-µg-treated eye in 1 animal at 4 and another at 6 months, which was considered to be related to the implant rather than to bimatoprost.
Discussion
This nonclinical study evaluated PD values for up to 6 months and PK values for up to 3 months, following a single intracameral Bimatoprost SR implant in beagle dogs at dose strengths of 8, 15, 30, and 60 µg. A clear dose-dependent IOP-lowering effect was demonstrated through 3 months after Bimatoprost SR administration for all doses, with some persistent effects observed at 6 months.
This sustained benefit in IOP lowering with a single administration of Bimatoprost SR is consistent with a previous preclinical study in beagle dogs.19 In the 24-month analysis of the phase 1/2 study, a sustained decrease in IOP persisted in many patients after complete degradation of the implant and negligible drug exposure (Craven R, et al. Presentation at the American Academy of Ophthalmology 2017 Congress, Nov. 11–14, 2017, New Orleans, LA, USA). This is likely from bimatoprost-induced tissue remodeling in the ciliary body, whereby anatomic or physiological changes enhance aqueous humor outflow and IOP lowering. This remodeling has been reported in cynomolgus monkeys treated with topical bimatoprost20 and is thought to be secondary to prostaglandin analog-stimulated upregulation and release of matrix metalloproteases by ciliary muscle cells.21 The sustained IOP lowering at 6 months was evident despite the absence of bimatoprost in the remnant implants, which supports the concept of sustained bimatoprost-induced tissue remodeling in the ciliary body.
There was no detectable systemic exposure to bimatoprost or bimatoprost acid at any of the dose strengths evaluated. The plasma concentration-time profile is consistent with zero order kinetics, with no evidence of “drug dumping” in the eye postdose. Aqueous humor concentrations of both parent drug and metabolite generally increased with increasing Bimatoprost SR dose strength.
The PK/PD relationship established in the dog in the current study appears to match well with PK and PD data in humans for the bimatoprost 0.03% eye drops, affording us confidence to predict the dose-dependent IOP-lowering response to Bimatoprost SR implants in humans to assist dose selection for the ensuing clinical program. This analysis suggested that, with a smaller anterior chamber and slower aqueous humor turnover rate, close to maximum (∼30%) IOP lowering can already be achieved in humans between the 8- and 15-µg doses of Bimatoprost SR. It is worth considering potential differences in the PK and PD of bimatoprost in glaucomatous eyes compared with the healthy normotensive eyes assessed in this study. Although aqueous humor production is unlikely to be affected significantly, the restricted drainage of aqueous humor will reduce clearance of bimatoprost from the anterior chamber. This further supports the use of the lower 8- and 15-µg doses since impaired drug clearance in the glaucomatous eye will favor optimal exposure to bimatoprost in the target tissues.
The dose-dependent ocular findings 3 months postimplantation in the Bimatoprost SR-treated groups were mild or moderate conjunctival hyperemia and miosis. Conjunctival hyperemia following topical bimatoprost administration in dogs has been attributed to endothelial-derived nitric oxide-mediated vasodilation of ocular surface vessels and is not associated with ocular surface inflammation.22 Furthermore, in a previous study investigating the dose-response profiles of Bimatoprost SR in beagle dogs, vasodilation associated with ocular hyperemia was limited to the deeper aqueous outflow vessels and did not involve the more superficial conjunctival vessels that are commonly associated with topical bimatoprost administration.23 The conjunctival hyperemia observed in all groups during the first week was attributed to the ocular surface preparation and/or intracameral injection. Aqueous flare was infrequent and resolved after 1 week in all groups, suggesting that IOP reduction observed in this study was not attributable to ocular inflammation. The intensity of miosis tended to diminish over time and is an expected outcome attributed to the pharmacologic effect of bimatoprost in dogs.
There were no notable changes from baseline in other ophthalmological assessments across the range of Bimatoprost SR dose strength. Corneal thickness measurements in this study were consistent with those previously reported for this species.24 The single observation of bleeding in the trabecular meshwork upon gonioscopic examination may have been the result of a transient episode of hypotony in which IOP dropped below episcleral venous pressure and a small amount of blood refluxed into the trabecular meshwork. Implant-related pathological findings included minimal attenuation of the corneal endothelium with the largest implant size (60-µg dose strength). In general, Bimatoprost SR at dose levels of ≤30 µg was well tolerated in the beagle dog. Remnant implant analysis indicates significant polymer erosion with the formation of a highly porous structure in all implants at 3 months, as well as 6 months postdose administration.
In summary, the IOP-lowering effect following Bimatoprost SR administration is maintained for at least 3 months in dogs, and the implants are well tolerated except for the minimal attenuation of the corneal endothelium at the 60-µg dose. A PK/PD relationship was established and considered translatable to humans. Based on the predicted dose-dependent IOP-lowering in humans and taking into account safety findings in this study, doses between 8 and 15 µg are most likely to provide the best benefit/risk profile for clinical development of the implants.
Acknowledgments
The authors thank Tim Lam at Covance Laboratories who contributed to the IOP data analysis, Ruiwen Shi and Patrick Hughes for the implant analysis, and Paul Miller at Comparative Ophthalmic Research Laboratory, who performed the safety assessment in the study.
This study was sponsored by Allergan plc, Dublin, Ireland. Medical writing and editorial assistance were provided to the authors by Stuart Murray, MSc, of Evidence Scientific Solutions, Inc, Philadelphia, PA, USA, and funded by Allergan plc, Irvine, CA, USA. All authors met the ICMJE authorship criteria.
Disclosure: J. Shen, Allergan (E); M.R. Robinson, Allergan (E); C. Struble, Covance Laboratories (E); M. Attar, Allergan (E)
References
- 1. Schmidl D, Schmetterer L, Garhöfer G, Popa-Cherecheanu A. Pharmacotherapy of glaucoma. J Ocul Pharmacol Ther. 2015; 31: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dreer LE, Girkin CA, Campbell L, Wood A, Gao L, Owsley C. Glaucoma medication adherence among African Americans. Optom Vis Sci. 2013; 90: 883–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mansouri K, Iliev ME, Rohrer K, Shaarawy T. Compliance and knowledge about glaucoma in patients at tertiary glaucoma units. Int Ophthalmol. 2011; 31: 369–376. [DOI] [PubMed] [Google Scholar]
- 4. Newman-Casey PA, Robin AL, Blachley T, et al.. The most common barriers to glaucoma medication adherence. Ophthalmology. 2015; 122: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee SS, Hughes P, Ross AD, Robinson MR. Biodegradable implants for sustained drug release in the eye. Pharm Res. 2010; 27: 2043–2053. [DOI] [PubMed] [Google Scholar]
- 6. Lewis RA, Christie WC, Day DG, et al.. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a phase I/II clinical trial. Am J Ophthalmol. 2017; 175: 137–147. [DOI] [PubMed] [Google Scholar]
- 7. Seal JR, Robinson MR, Burke J, Bejanian M, Coote M, Attar M. Intracameral sustained-release bimatoprost implant delivers bimatoprost to target tissues with reduced drug exposure to off-target tissues. J Ocul Pharmacol Ther. 2018; 35: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrell Ramos M, Attar M, Stern ME, et al.. Safety evaluation of ocular drugs. In: Faqi AS. (ed), A Comprehensive Guide to Toxicology in Nonclinical Drug Development. London, UK: Academic Press, Elsevier; 2017: 757–811. [Google Scholar]
- 9. Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005; 140: 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharif NA, Kelly CR, Crider JY, Williams GW, Xu SX. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther. 2003; 19: 501–515. [DOI] [PubMed] [Google Scholar]
- 11. Woodward DF. Pharmacological characterization of a novel antiglaucoma agent, bimatoprost (AGN 192024). J Pharmacol Exp Ther. 2003; 305: 772–785. [DOI] [PubMed] [Google Scholar]
- 12. Cantor LB, Hoop J, Wudunn D, et al.. Levels of bimatoprost acid in the aqueous humour after bimatoprost treatment of patients with cataract. Br J Ophthalmol. 2006; 91: 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Camras CB, Toris CB, Sjoquist B, et al.. Detection of the free acid of bimatoprost in aqueous humor samples from human eyes treated with bimatoprost before cataract surgery. Ophthalmology. 2004; 111: 2193–2198. [DOI] [PubMed] [Google Scholar]
- 14. Laibovitz RA. Comparison of the ocular hypotensive lipid AGN 192024 with timolol: dosing, efficacy, and safety evaluation of a novel compound for glaucoma management. Arch Ophthalmol. 2001; 119: 994. [DOI] [PubMed] [Google Scholar]
- 15. McLaren JW. Measurement of aqueous humor flow. Exp Eye Res. 2009; 88: 641–647. [DOI] [PubMed] [Google Scholar]
- 16. Attar M, Brassard JA, Kim AS, Matsumoto S, Ramos M, Vangyi C. Safety evaluation of ocular drugs. In: Faqi AS. (Ed), A Comprehensive Guide to Toxicology in Preclinical Drug Development. London, UK: Academic Press, Elsevier; 2013: 567–617. [Google Scholar]
- 17. Ward DA, Cawrse MA, Hendrix DV. Fluorophotometric determination of aqueous humor flow rate in clinically normal dogs. Am J Vet Res. 2001; 62: 853–858. [DOI] [PubMed] [Google Scholar]
- 18. Cawrse MA, Ward DA, Hendrix DV. Effects of topical application of a 2% solution of dorzolamide on intraocular pressure and aqueous humor flow rate in clinically normal dogs. Am J Vet Res. 2001; 62: 859–863. [DOI] [PubMed] [Google Scholar]
- 19. Lee SS, Burke J, Shen J, et al.. Bimatoprost sustained-release intracameral implant reduces episcleral venous pressure in dogs. Vet Ophthalmol. 2018; 21: 376–381. [DOI] [PubMed] [Google Scholar]
- 20. Richter M, Krauss AH, Woodward DF, Lutjen-Drecoll E. Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest Ophthalmol Vis Sci. 2003; 44: 4419–4426. [DOI] [PubMed] [Google Scholar]
- 21. Lindsey JD, Kashiwagi K, Kashiwagi F, Weinreb RN. Prostaglandin action on ciliary smooth muscle extracellular matrix metabolism: implications for uveoscleral outflow. Surv Ophthalmol. 1997; 41: S53–S59. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Dinh T, Woodward DF, et al.. Bimatoprost: mechanism of ocular surface hyperemia associated with topical therapy. Cardiovasc Drug Rev. 2006; 23: 231–246. [DOI] [PubMed] [Google Scholar]
- 23. Lee SS, Dibas M, Almazan A, Robinson MR. Dose-response of intracameral bimatoprost sustained-release implant and topical bimatoprost in lowering intraocular pressure. J Ocul Pharmacol Ther. 2019; 35: 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bentley E, Campbell S, Woo HM, Murphy CJ. The effect of chronic corneal epithelial debridement on epithelial and stromal morphology in dogs. Invest Opthalmol Vis Sci. 2002; 43: 2136–2142. [PubMed] [Google Scholar]