Background:
The aim of our study was to explore the impact of elective-surgery deferment on the United States health-care system and subsequent recovery after COVID-19 containment. Using an orthopaedic elective surgery model, we aimed to answer the following: (1) What is the expected recovery time until the health-care system is back to nearly full capacity for performing elective surgery? (2) What will be the expected backlog of elective surgery over time? (3) How should health care change to address the backlog?
Methods:
A Monte Carlo stochastic simulation-based analysis was performed to forecast the post-pandemic volume of elective, inpatient total joint arthroplasty and spinal fusion surgical cases. The cumulative backlog was calculated and analyzed. We tested model assumptions with sensitivity analyses.
Results:
Assuming that elective orthopaedic surgery resumes in June 2020, it will take 7, 12, and 16 months—in optimistic, ambivalent, and pessimistic scenarios, respectively—until the health-care system can perform 90% of the expected pre-pandemic forecasted volume of surgery. In the optimistic scenario, there will be a cumulative backlog of >1 million surgical cases at 2 years after the end of elective-surgery deferment.
Conclusions:
The deferment of elective surgical cases during the SARS-CoV-2 pandemic will have a lasting impact on the United States health-care system. As part of disaster mitigation, it is critical to start planning for recovery now.
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in an ongoing pandemic. To reduce avoidable exposure of patients and health-care workers, and to prevent consumption of essential resources, in mid-March 2020 the American College of Surgeons recommended pausing all elective procedures until the spread of the virus is contained in the United States. The result has been a large-scale deferment of elective surgery.
Our goal was to explore the impact of this deferment on the United States health-care system and subsequent recovery after containment. Using an inpatient elective orthopaedic surgery model, we aimed to answer the following: (1) What is the expected recovery time until the health-care system is back to nearly full capacity for performing elective surgery? (2) What will be the expected backlog of elective surgery over time? (3) How should health care change to address the backlog?
Materials and Methods
Pre-Pandemic Surgical Volume
Trends in volume of elective orthopaedic surgery were identified using Agency for Healthcare Research and Quality (AHRQ) National Inpatient Sample (NIS) data1. The database was queried from 1993 to 2017 using a combination of procedural Clinical Classification Software (PRCCS), Medicare Severity Diagnosis Related Group (MS-DRG), and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) and ICD-10 codes to identify patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), or cervical or thoracolumbar spinal fusion (SF) (Table I). In order to isolate an elective population, surgical procedures performed for infections, tumors, fractures, and revision joint replacements were excluded using relevant codes (Table I). To our knowledge, the AHRQ has not released the 2018 to 2020 data, and thus the 2018 through May 2020 data were forecasted instead of using actual surgical numbers. A time series linear regression model was fit to each case category, and data for 2018 through 2022 were forecasted using regression (TKA time series regression r2 = 0.98, THA r2 = 0.97, and SF r2 = 0.98). The total elective inpatient orthopaedic surgical volume in a given month was defined as the sum of the TKA, THA, and SF surgical procedures performed in a given month. These forecasted data were used in the subsequent analysis as the pre-pandemic forecasted volume.
TABLE I.
Inclusion and Exclusion Criteria for Inpatient Elective Orthopaedic Surgery
| Code | |
| Inclusion criteria: procedures | |
| Total hip arthroplasty | MS-DRG: 469, 470; PRCCS: 153 |
| Total knee arthroplasty | MS-DRG: 469, 470; PRCCS: 152 |
| Cervical fusion | MS-DRG: 471, 472, 473; PRCCS: 158 |
| Thoracolumbar fusion | MS-DRG: 453, 454, 455, 459, 460; PRCCS: 158 |
| Exclusion criteria: diagnoses | |
| Revision joint replacement | MS-DRG: 466, 467, 468 |
| Fracture | MS-DRG: 533, 534, 535, 536, 562, 563 |
| Pathologic fracture | MS-DRG: 542, 543, 544 |
| Infection | MS-DRG: 853, 854, 855, 856, 857, 858, 862, 863 |
| Septic arthritis | MS-DRG: 548, 549, 550 |
| Osteomyelitis | MS-DRG: 539, 540, 541 |
| Spinal infection | MS-DRG: 094, 095, 096; ICD-10: M50.90, M46.44, M46.46, M46.20, T81.4 |
| Spinal tumor | MS-DRG: 456, 457, 458; ICD-10: M48.5, G95.2, D16.6, C41.2, C79.5 |
Post-Pandemic Surgical Volume Model
The post-pandemic elective orthopaedic surgical volume for February through April 2020 was forecasted with the assumption that 95% of the pre-pandemic forecasted volume was performed in February, 40% in March, and 3% in April 2020. These assumptions were based on the trends in volume of elective orthopaedic cases at the senior author’s institution (a major academic medical center) during this time period.
The post-pandemic volume from May 2020 through May 2022 was forecasted using a Monte Carlo stochastic simulation of a Gompertz function. Monte Carlo simulation is a technique used to model uncertainty in quantitative analysis using repeated random sampling. A Gompertz function is a sigmoid curve that has been used to model a variety of biological growth and ecological disaster recovery phenomena as well as to forecast the increase in demand for goods and services after industry disruption2. Three different growth velocities were arbitrarily chosen for the model: optimistic (growth velocity = 50% ± 5%), ambivalent (30% ± 3%), and pessimistic (20% ± 2%). The primary end point was the number of months after the pandemic required in each scenario for the health-care system to reach a steady-state surgical volume, defined as 90% of the expected pre-pandemic forecasted volume.
Cumulative Backlog Model
The cumulative backlog of surgical cases was defined as an aggregate of fixed backlog, which is created during the time when elective surgery is deferred (March to May 2020), plus the running sum of the variable backlog, which is created in the future while the health system is ramping up after the pandemic. The backlog for a given month was calculated by subtracting the volume of cases performed under the Gompertz function for that month from the pre-pandemic forecasted demand. We utilized the optimistic scenario to perform the cumulative backlog analysis.
Sensitivity Analyses
We performed a series of 1-way sensitivity analyses to investigate the impact of varying inputs on model outputs. In the first analysis, we varied the growth velocity in the Gompertz function from 10% to 90%. In the second analysis, we varied the volume of the fixed backlog (from March to May 2020) by ±50%. In the third analysis, instead of assuming that the post-pandemic demand for elective surgery would remain the same as the pre-pandemic demand, we simulated what would happen if post-pandemic surgical demand contracted by 10% to 50% in response to changes in patient factors and preferences. In addition, we simulated a backlog minimization scenario in which we assumed that growth velocity was 90% and the fixed backlog and elective surgery demand were 50% of expected.
Accelerated Growth Model
We then examined how the cumulative backlog would change if the health-care capacity grew in an accelerated growth scenario. In this model, we assumed that after ramping up to 90% of the volume forecasted before the pandemic, instead of the health system growth slowing down (as in the Gompertz model) the growth accelerated at a compound rate of 10% until the end of 2022.
Statistical Analysis
In the Monte Carlo analysis, 10,000 runs were performed for each scenario. The model input values were sampled randomly from a normal distribution for the corresponding model input. Statistical analysis was performed using Stata IC 15.0 (StataCorp). The numbers of cases have been rounded up to the nearest 10,000 in this article for ease of reading.
Results
Post-Pandemic Surgical Volume Recovery
Assuming that elective orthopaedic surgery resumes in June 2020, in an optimistic scenario it will take 7 months until the health-care system is able to revert to the steady-state volume—i.e., performance of 90% of the expected pre-pandemic forecasted volume (Fig. 1-A). In the ambivalent and pessimistic scenarios, it will take 12 and 16 months, respectively, to reach the steady-state volume.
Fig. 1.
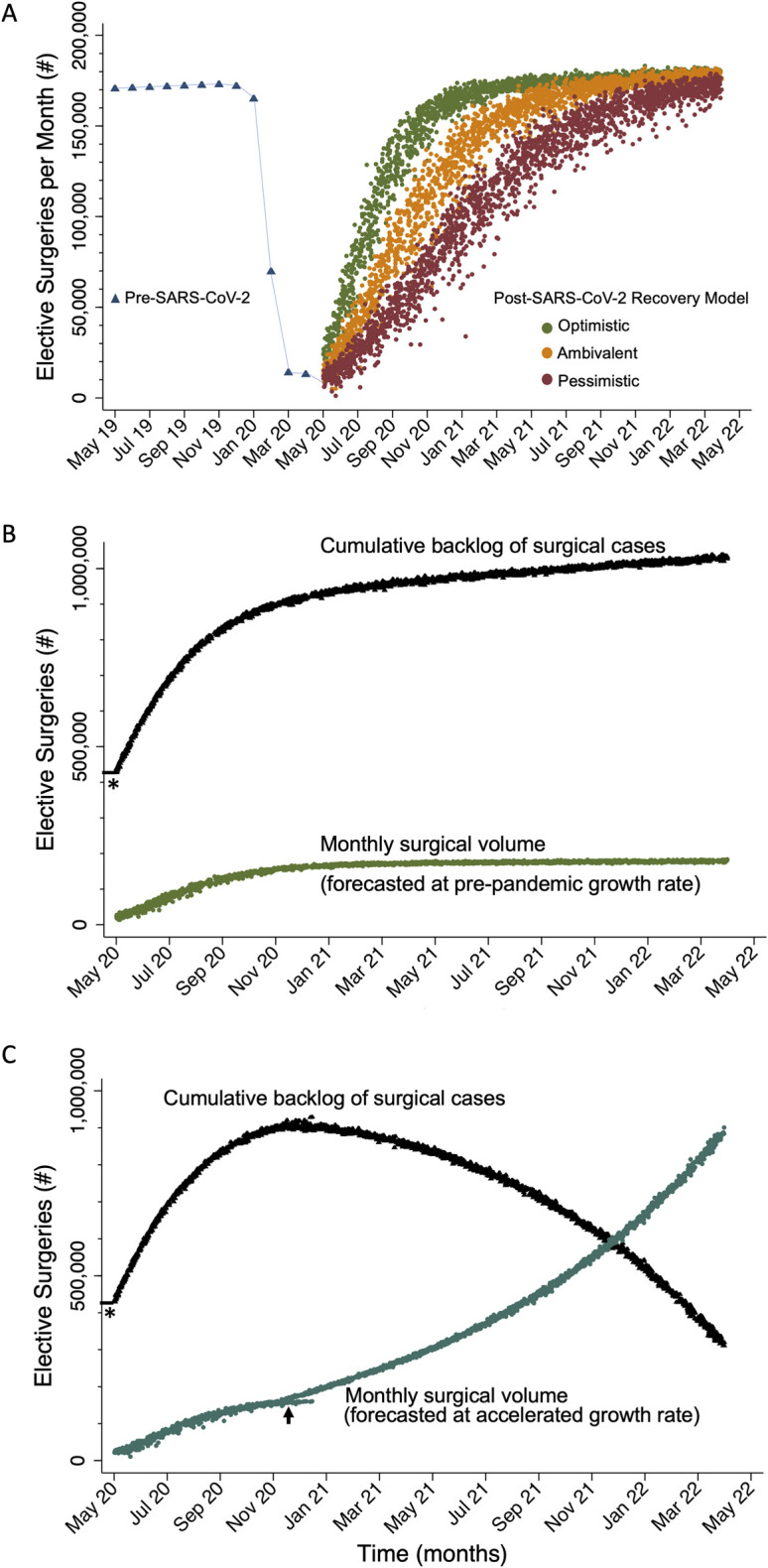
Figs. 1-A, 1-B and 1-C Monte Carlo simulation analyses of elective orthopaedic surgery in the United States after the SARS-CoV-2 pandemic. Fig. 1-A Recovery of elective surgical volume per month to the forecasted pre-pandemic volume under 3 different scenarios. Fig. 1-B The cumulative backlog of surgical cases is a summation of fixed backlog (asterisk) during the deferment period and the addition of new backlog created during the ramp-up period. Fig. 1-C Cumulative backlog decreases if an accelerated growth rate is assumed starting at 90% recovery of the forecasted pre-pandemic surgical volume (arrow).
Post-Pandemic Surgical Backlog
In the optimistic scenario, when the health-care system recovers to the pre-pandemic forecasted full capacity, there will be a cumulative backlog of >1 million surgical cases at 2 years after the end of deferment. Assuming a pre-pandemic growth rate, it appears to be impossible to close the gap on the accumulating backlog (Fig. 1-B).
Sensitivity Analysis Varying Growth Velocity
To investigate the impact of various growth velocities on the time to reach steady-state volume, we varied the growth velocities from 10% to 90%. The time to reach steady-state volume varied from 22 to 4 months, respectively. The expected backlog at 2 years after the pandemic was 3 million cases in the 10% growth scenario and 750,000 cases in the 90% growth scenario.
Sensitivity Analysis Varying Fixed Backlog
When we varied the fixed backlog (created during the deferment period) to be 50% less than expected, the cumulative backlog was >800,000 cases in the optimistic scenario. Varying the fixed backlog to 50% more than expected resulted in a cumulative backlog of 1,250,000 cases in the optimistic scenario.
Sensitivity Analysis Varying Surgical Demand
To test the impact of a decline in post-pandemic demand for elective surgery on the cumulative backlog, we conducted a sensitivity analysis simulating demand contraction by 10% to 50%. A 10% decline in surgical demand led to a backlog of 970,000 cases in the optimistic scenario. With a 50% decline in surgical demand, there still remained a backlog of 720,000 cases at 2 years after the end of deferment in the optimistic scenario.
When we simulated a backlog minimization scenario in which growth velocity was 90% during ramp-up, the fixed backlog was 50% less than expected, and the post-pandemic elective surgery demand was 50% less than the forecasted pre-pandemic demand, there was still a backlog of 380,000 cases at 2 years.
Backlog with Accelerated Growth
In an accelerated growth scenario in which health system growth accelerated instead of slowed down after reaching a steady-state volume, it would take >2 years to end the cumulative backlog (Fig. 1-C). In this model, the number of cases required to be performed at 2 years would be >5 times the forecasted pre-pandemic monthly volume of cases.
Discussion
The SARS-CoV-2 pandemic will have a lasting impact on health care in the United States. As this situation is unprecedented in modern medicine, there are no validated historical data to help predict how quickly and to what degree the health-care-system capacity will recover. We suspect that, in an optimistic scenario, it may take >6 months for the health-care system to return to 90% of its pre-pandemic forecasted level of production of elective inpatient orthopaedic surgery. In an ambivalent or pessimistic model, it would take even longer.
Once the elective-surgery deferment is lifted, it is extremely unlikely that we will be able to revert immediately to the fully functioning pre-pandemic production level. This is because the individual factors that affect the production function—i.e., capital and labor—will continue to remain affected3. Although health-care workers (labor) can probably, barring illnesses, return to nearly full capacity, the limiting factor would be capital. Fixed capital in terms of hospital beds and surgical equipment may be initially accessible. However, interruption of manufacturing and transportation resulting in disruption of the global supply chain may result in relative scarcity of consumables (circulating capital), such as those used in the perioperative setting, including personal protective equipment, surgical packs, implants, anesthesia supplies, and medications. As the cumulative backlog increases and the amount of net demand starts to exceed supply, it will create a relative shortage of fixed capital and labor as well. Furthermore, the existing process for presurgical care—with patients having to be evaluated, assessed for whether surgery is indicated, counseled, enabled to provide consent, and medically and financially cleared—will contribute to the ramp-up time once the deferment of effective surgery is lifted.
Our study has a number of limitations. First, the pre-pandemic surgical demand was forecasted using time series linear regression. This assumes that there will be no shifts in the population age and comorbidities during this time period and thus the baseline rate of growth in elective surgical volume will be constant. It is unlikely that there will be drastic changes in the patient population demographics in the 2-year post-pandemic horizon. Furthermore, we found it reassuring that our projected volumes were similar to those in a prior study that investigated the volume of primary total joint arthroplasty in the United States4.
Another limitation is that we chose 3 growth-velocity scenarios for recovery arbitrarily. Our sensitivity analysis revealed that, even with an extremely optimistic growth velocity of 90%, it would take the system 4 months to reach the pre-pandemic forecasted volume. Since there is no precedent for health-care disruption of this scale in modern medicine, it is impossible to predict the actual velocity at which recovery will occur. Thus, we wanted to provide likely scenarios in growth modeling. In the future, we can retrospectively review which of the scenarios was most accurate.
Another limitation of the study is that, in calculating backlog, we assumed that the demand for elective surgery will be similar to that forecasted before the pandemic. However, the demand for elective surgery may be influenced by patient factors such as insurance, employment, and post-pandemic financial and emotional stress, which could dampen patient enthusiasm for elective surgery in the future. In the sensitivity analysis, modeling a 50% decline in surgical demand showed that there would still be a backlog of 720,000 cases at 2 years after deferment of elective surgery ended. Furthermore, it is unlikely that demand will contract that drastically as prior literature has revealed that demand for elective surgery tends to be relatively inelastic5.
Despite the multiple limitations, the model and the sensitivity analyses were revealing. A backlog is inevitable even if production returns quickly to the forecasted pre-pandemic levels. Furthermore, it will be difficult to close the gap in the growing backlog without increasing production substantially. Thus, planning for post-pandemic recovery requires addressing the backlog reactively and proactively.
To deal with a backlog in computer servers and networks, our engineering colleagues have developed specific strategies that we can translate to health care; these strategies include scaling up of resources, queuing, buffering, and dropping6. In the health-care setting, scaling up implies increasing surgical throughput. While the number of surgeons will be a limited resource, a variety of other evidence-based strategies can be the focus of throughput enhancement. These strategies include greater utilization of telemedicine7, increased orthopaedic block times8, dedicated anesthesia and nursing teams for orthopaedic care9, a shift in care to ambulatory surgery centers10, and amplification of dedicated care coordination resources11,12 in the perioperative orthopaedic care pathways that aim to maximize efficiency and throughput while maintaining high-value care. Accomplishing this will require support from hospital leadership and investments from governmental agencies and private firms to provide dedicated capital to health-care systems and physician groups proactively.
Queuing and buffering would involve prioritizing some elective surgical patients on the basis of severity and urgency and scheduling other patients far out into the future. An alternate strategy is dropping, which would involve redefining surgical indications to render a subset of patients ineligible for surgical care. However, in health care, queuing, buffering, and dropping may propagate bias and potentially worsen preexisting disparities13, which is not in the best interest of patients. Ultimately, increasing throughput will likely be the most realistic and sustainable solution.
While based on multiple assumptions and having clear limitations, our analysis is timely in light of the unprecedented and rapidly evolving pandemic. To deal with the anticipated backlog in elective orthopaedic surgery, planning for post-pandemic recovery requires proactive action. Strategic investments focusing on capacity expansion are crucial. Staying ahead of the curve with innovations in efficiency and capacity enhancement will be critical to success.
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, Johns Hopkins University, Baltimore, Maryland
Disclosure: The authors indicated that no external funding was received for any aspect of this work. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/F885).
References
- 1.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. National Inpatient Sample (NIS) data 1993-2017.
- 2.Tjørve KMC, Tjørve E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: an addition to the Unified-Richards family. PLoS One. 2017. June 5;12(6):e0178691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobb CW, Douglas PH. A theory of production. Am Econ Rev. 1928. March;18(1):139-1-5. [Google Scholar]
- 4.Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018. September 5;100(17):1455-1-5. [DOI] [PubMed] [Google Scholar]
- 5.Riganti A, Siciliani L, Fiorio CV. The effect of waiting times on demand and supply for elective surgery: evidence from Italy. Health Econ. 2017. September;26(Suppl 2):92-1-5. [DOI] [PubMed] [Google Scholar]
- 6.Kafi MA, Djenouri D, Ben-Othman J, Badache N. Congestion control protocols in wireless sensor networks: a survey. IEEE Commun Surv. 2014;16(3):1369-1-5. [Google Scholar]
- 7.Buvik A, Bugge E, Knutsen G, Småbrekke A, Wilsgaard T. Patient reported outcomes with remote orthopaedic consultations by telemedicine: a randomised controlled trial. J Telemed Telecare. 2019. September;25(8):451-1-5 Epub 2018 Jul 4. [DOI] [PubMed] [Google Scholar]
- 8.Runner R, Moore T, Jr, Reisman W. Value of a dedicated Saturday orthopaedic trauma operating room. J Orthop Trauma. 2016. January;30(1):e24-1-5. [DOI] [PubMed] [Google Scholar]
- 9.Hiza EA, Gottschalk MB, Umpierrez E, Bush P, Reisman WM. Effect of a dedicated orthopaedic advanced practice provider in a level I trauma center: analysis of length of stay and cost. J Orthop Trauma. 2015. July;29(7):e225-1-5. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann JD, Kusnezov NA, Dunn JC, Zarkadis NJ, Goodman GP, Berger RA. The shift to same-day outpatient joint arthroplasty: a systematic review. J Arthroplasty. 2018. April;33(4):1265-1-5 Epub 2017 Nov 22. [DOI] [PubMed] [Google Scholar]
- 11.Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: an economic analysis. Clin Orthop Relat Res. 2016. January;474(1):222-1-5 Epub 2015 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfstadt JI, Wayment L, Koyle MA, Backstein DJ, Ward SE. The development of a standardized pathway for outpatient ambulatory fracture surgery: to admit or not to admit. J Bone Joint Surg Am. 2020. January 15;102(2):110-1-5. [DOI] [PubMed] [Google Scholar]
- 13.Dy CJ, Tipping AD, Nickel KB, Jiang W, O’Keefe RJ, Olsen MA. Variation in the delivery of inpatient orthopaedic care to Medicaid beneficiaries within a single metropolitan region. J Bone Joint Surg Am. 2019. August 21;101(16):1451-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


