Abstract
Cardiogenic shock remains a major cause of morbidity and mortality for patients with acute myocardial infarction and advanced heart failure. Intra-aortic balloon pump has been the most widely used short-term mechanical circulatory support device to rapidly stabilize hemodynamics. However, it provides modest support, current evidence does not show a decrease in mortality, and the latest guidelines no longer recommend its routine use. Several percutaneous mechanical circulatory support devices have been introduced into clinical practice (Impella, extracorporeal membrane oxygen, TandemHeart), providing a greater level of hemodynamic support. These resource-intensive devices demand a careful selection of patients that stand to benefit the most. Premature initiation of mechanical circulatory support exposes the patient to unnecessary risk, whereas delaying therapy leads to irreversible end-organ injury, rendering any intervention medically futile. Cannulation methods, pump designs, and circuit configurations differ between devices, as do the adverse effects and physiological impact on the myocardium, which needs to be factored into consideration before deployment on the patient in cardiogenic shock. This article will review the commonly used percutaneous mechanical circulatory support devices in the setting of cardiogenic shock, compare their advantages and disadvantages, evaluate key clinical trials, and discuss a practical approach to guide clinicians’ decision and management.
Keywords: Mechanical circulatory support (MCS), intra-aortic balloon pump (IABP), Impella, extracorporeal membrane oxygenation (ECMO), TandemHeart (TH)
Introduction
Cardiogenic shock (CS) is a clinical syndrome characterized by low cardiac output and its inability to meet the body’s systemic demand, leading to multiple end-organ hypoperfusion. Diagnosis is made clinically according to the following signs and symptoms: systolic blood pressure (SBP) <90 mmHg or requirement of vasopressor support to maintain an SBP of 90 mmHg, pulmonary congestion, absence of hypovolemia, and signs of organ hypoperfusion (cool extremities, altered mental status, oliguria, and elevated serum lactate). Other advanced hemodynamic parameters used in clinical studies include cardiac index (CI) <1.8 L/min/m2 without support or <2.2 L/min/m2 with support, pulmonary capillary wedge pressure >15 mmHg, and elevated left ventricular end-diastolic pressure (LVEDP) >18 mmHg. The vast majority of cases are secondary to acute myocardial infarction (AMI). Current management involves early recognition, optimizing fluid balance, and stabilizing hemodynamics. Vasopressors and inotropes augment cardiac output and organ perfusion but at the expense of increasing myocardial oxygen consumption, compromising tissue microcirculation, and inducing arrhythmias (1,2). Intra-aortic balloon pump (IABP) modestly improves cardiac output; however, the latest guidelines do not recommend its routine use in CS after AMI (3). Early revascularization remains the only evidence-based therapy that improves survival in cases caused by acute coronary events (4); nevertheless, mortality remains at about 50%.
The implementation of mechanical circulatory support (MCS) devices aims to overcome these limitations, breaking the downward spiral seen in CS and allowing the heart to rest and recover (5). In the past, these devices were given preference to those who were eligible for eventual heart transplantation (bridge-to-transplantation) or left ventricular assist device (LVAD) implantation (bridge-to-bridge). Technological advancement and design improvements have made these devices [Impella (Abiomed, Danvers, MA, USA), venoarterial extracorporeal membrane oxygenation (VA ECMO), TandemHeart (TandemLife, LivaNova, London, UK) (TH)] more effective, reliable, and easily deployable without the need for sternotomy. As a result, there has been an increasing trend and an expanding role of short-term MCS device use over the last decade, including facilitating high-risk procedures, buying time for definitive therapy to take effect (bridge-to-recovery), and initial hemodynamic stabilization to allow delayed evaluation of treatment goals (bridge-to-decision) (6). However, it remains unclear which patients stand to benefit the most, when best to initiate MCS, and how to decide which device to use.This article reviews the features of short-term MCS devices by describing their hemodynamic effects, contra-indications, complications, and highlighting key publications in this rapidly expanding therapeutic field.
Short-term MCS
IABP
For the past 5 decades, IABP has been the most widely used device to improve hemodynamics in CS, with more than 50,000 devices inserted in the United States alone (6). It is most commonly utilized in AMI-CS, improving end-organ and coronary perfusion with diastolic augmentation while reducing left ventricular (LV) workload and myocardial oxygen consumption by reducing afterload. Despite its popularity and beneficial impact, supporting evidence for its use is limited to registry data. The IABP-SHOCK II was the first randomized multicenter trial to examine whether IABP improved mortality in AMI-CS. With 600 patients randomized to IABP or medical treatment, no differences in mortality were identified at 30 days, 1 year, and 6 years (7-9). IABP usage has been decreasing since this landmark trial. The latest European Society of Cardiology guidelines no longer recommend routine placement of IABP in the management of AMI-CS (class III) (3). However, its role in other causes of CS (e.g., acute decompensated heart failure, post craniotomy shock) and high-risk percutaneous coronary intervention (PCI) remains unclear. Nevertheless, its simplicity, ease of bedside insertion, staff familiarity, and low complication rate make IABP a popular choice amongst cardiologists.
Device features and hemodynamic impact
The device consists of a 7–8 Fr dual lumen vascular catheter with a cylindrical polyurethane balloon at the distal tip. The catheter is inserted percutaneously via the common femoral artery (CFA) into the descending aorta, with the balloon tip positioned 2 cm distal to the left subclavian artery (Figure 1). A central lumen allows for guidewire insertion and aortic pressure transduction, while the other lumen is connected to an external console that regulates balloon inflation/deflation synchronized to the cardiac cycle. Balloon inflation occurs immediately at the onset of diastole, while deflation takes place just before the onset of systole, shifting approximately 40 mL of blood volume in each cardiac cycle. Depending on a number of factors (balloon size and positioning, vascular compliance), IABP can provide up to 1 L/min of cardiac output. This counterpulsation mechanism augments diastolic blood flow to the coronaries and peripheral organs and improves stroke volume by lowering LV afterload, LV wall stress, and myocardial oxygen demand.
Figure 1.
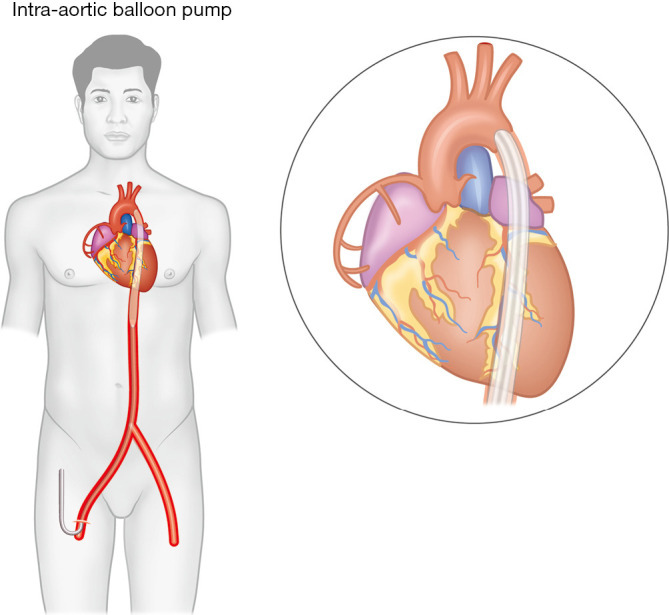
Intra-aortic balloon pump inserted via the femoral artery. Balloon tip is positioned just distal to the origin of the left subclavian artery.
Contraindications and complications
Balloon inflation/deflation is timed according to the cardiac cycle, triggered by either electrocardiogram (ECG) or pressure waveform. Poor quality ECG and cardiac arrhythmias may lead to inaccurately timed balloon pumping, not only negating the hemodynamic benefits but also increasing LV workload as the myocardium contracts against an inflated balloon. Balloon inflation in the presence of aortic regurgitation increases LV end-diastolic volume and pressure, inducing further ischemia. Screening for aortic insufficiency is necessary prior to deployment of IABP. Tortuous aortas, aortic aneurysms, and severe peripheral vascular disease preclude its usage. Subclavian artery access can be considered in severe vasculopathy or when maintaining ambulatory status is of high priority. Interestingly, no thromboembolic events occurred with upper body cannulation in one prospective study (10).
Major complications associated with IABP insertion are relatively rare, with an overall reported incidence of 2.6% (11). Most are vascular-related, including limb ischemia, bleeding at puncture sites, and vessel injury requiring surgical repair. Proper device positioning is important; insertion distal to the CFA obstructs blood flow to the lower limb, necessitating device removal and placement in the contralateral limb; a balloon too long or positioned too low compromises spinal cord and visceral perfusion, while a balloon too high up can obstruct flow in the left subclavian and left common carotid arteries. Thrombocytopenia is commonly observed, presumably due to mechanical destruction by the balloon, and is usually not clinically significant. In patients anticoagulated with heparin, heparin-induced-thrombocytopenia must be considered. Data on other anticoagulants use, such as bivalirudin, in patients receiving IABP are limited to case reports (12). Some centers do not routinely use anticoagulants and have seen a reduced incidence in bleeding without worsening of ischemic events (13). Balloon rupture is rare but may cause air embolism resulting in significant neurological injury.
Impella
Impella provides hemodynamic support similarly to ventricular assist devices (VAD) but with the advantages of being minimally invasive and miniaturized. Since its approval by the Food and Drug Administration in 2008, the use of this device has been increasing. In 2016, among patients undergoing PCI, Impella accounted for approximately 32% of all MCS devices (14).
Device features and hemodynamic impact
Four versions of Impella are currently available: Impella 2.5 (12 Fr pump motor, maximum flow rate 2.5 L/min), Impella CP (14 Fr pump motor, maximum flow rate 4.3 L/min), Impella 5.0 (21 Fr pump motor, maximum flow rate 5.0 L/min), and Impella 5.5 (19 Fr pump motor, maximum flow rate 6.2 L/min). In each of these devices, 9 Fr flexible-tip pigtail catheters mounted with microaxial pumps are inserted into the CFA and positioned across the aortic valve (Figure 2). LV blood is aspirated into the catheter via the inlet area and expelled by the pump motor through the outlet area into the proximal aorta using the Archimedes screw principle. Devices are either inserted percutaneously (Impella 2.5 and Impella CP) into the CFA, or by surgical cutdown (Impella 5.0 and Impella 5.5) into the CFA or axillary/subclavian artery. Motor pump size determines the maximum achievable flow rate; however, it is also dependent on the LV preload and afterload. An external console is attached to the catheter and allows adjustment of the pump speed to achieve the desired blood flow. With higher pump speeds, more blood is actively drawn into the aorta to provide hemodynamic support. As a result, LV unloading reduces LVEDP, LV wall stress, and myocardial oxygen demand. Blood flow delivered by Impella improves CI, mean arterial pressure (MAP), coronary flow, and end-organ perfusion. Currently, it is the only MCS device that provides forward flow to unload the LV.
Figure 2.
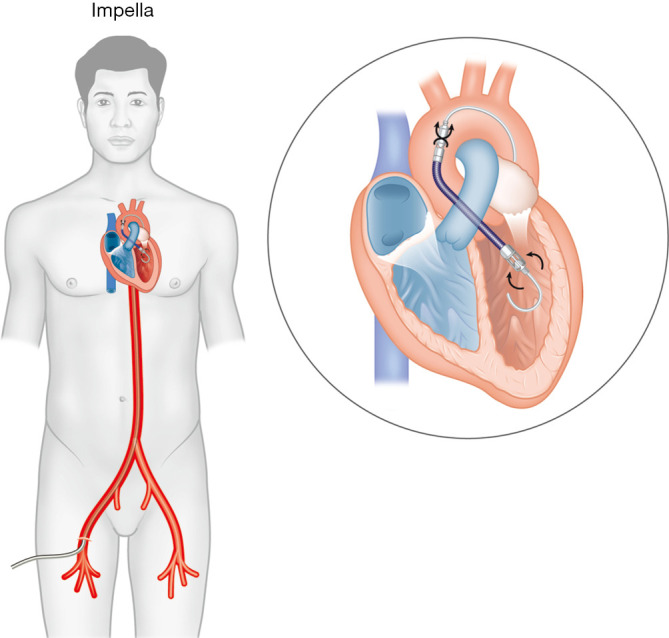
Impella inserted via the femoral artery. A pigtail catheter is positioned across the aortic valve, where left ventricular blood is aspirated into the catheter via the inlet area and expelled by the microaxial motor pump through the outlet area into the proximal aorta.
Contraindications and complications
The presence of a metallic aortic valve precludes the use of this device. Aortic stenosis has traditionally been considered a relative contra-indication for the use of Impella. However, there has been a growing number of centers reporting its successful use in aortic stenosis requiring PCI and in transcatheter aortic valve replacement (15-17). Impella should be avoided in patients with conditions intolerant to anticoagulation. Severe peripheral vascular disease is contraindicated, as femoral cannulation in diseased vessels increases the risk of limb ischemia. Use of this device in pre-existing ventricular septal defects creates a massive right-to-left shunt. Unlike IABP, Impella can continue to provide circulatory support during arrhythmias as long as there is adequate LV preload. Therefore, satisfactory right ventricular (RV) function is necessary for Impella to remain effective.
Potential complications from the use of Impella include hemolysis, ventricular arrhythmia, and cardiac valve injury (18). Significant bleeding and vascular complications are a genuine concern with the larger-sized catheters. Removal of the device with embolectomy should be considered in acute limb ischemia. Manzo-Silberman et al. reported a 6% hemolytic anemia and 26% puncture site bleeding risk requiring transfusion in their single-registry study (19). Major adverse events in one observational study included hemolysis (10.3%), infection (12.9%), limb ischemia (3.9%), bleeding requiring transfusion (17.5%), bleeding requiring surgery (2.6%), and vascular complication with surgical repair (9.7%), all more commonly observed than in IABP registries (20). A meta-analysis comparing Impella to IABP or control showed increased risk of short-term major bleeding [relative risk (RR) =3.11] and peripheral ischemic complications (RR =2.58) with the novel device (21).
VA ECMO
ECMO technology originated from cardiopulmonary bypassing (CPB) in 1950 and was mainly used in the pediatric population for the treatment of cardiorespiratory failure. The use of ECMO in adults was infrequent until the publication of CESAR trial, which coincided with the H1N1 epidemic in 2009 (22). The promising study results and rising incidence of fulminant acute respiratory failure led physicians to consider ECMO as a rescue strategy. Increasing research and improved circuit components further reduced complication rates, encouraging new centers to initiate their own ECMO program. As of 2019, there are more than 400 ECMO centers, with more than 10,000 instances of ECMO use per year over the last 3 years, compared to 150 prior to the epidemic (23).
Device specifics and hemodynamic impact
ECMO can be configured in 2 different ways: venovenous and VA. Both provide respiratory support, but only the latter provides circulatory support for CS. In VA ECMO, a multi-staged 21–25 Fr access cannula is inserted into the right atrium (RA), where venous blood is drained into the circuit, undergoes gas exchange in a polymethylpentene hollow-fiber membrane oxygenator, and is returned to the systemic circulation via a shorter 15–19 Fr return catheter (Figure 3). The femoral vessels are common sites for cannulation due to their size and accessibility. Unlike other MCS devices that require fluoroscopic guidance, ECMO cannulation can be done at the bedside percutaneously using ultrasound. This is particularly advantageous in hemodynamically unstable patients who are too ill for transfer or when ECMO needs to be established rapidly when extracorporeal cardiopulmonary resuscitation (ECPR) is required. In patients requiring prolonged support or awaiting organ transplant, surgical cannulation of upper body vessels (e.g., right internal jugular vein drainage and outflow to axillary, subclavian, or innominate arteries) are preferable for rehabilitation and maintaining transplant candidacy (24). When peripheral cannulation is not possible, central ECMO can be established with direct cannulation into the RA and ascending aorta via a median sternotomy. However, this is usually reserved for patients unable to come off CPB post-cardiac surgery by connecting the ECMO circuit to the existing central cannulas.
Figure 3.
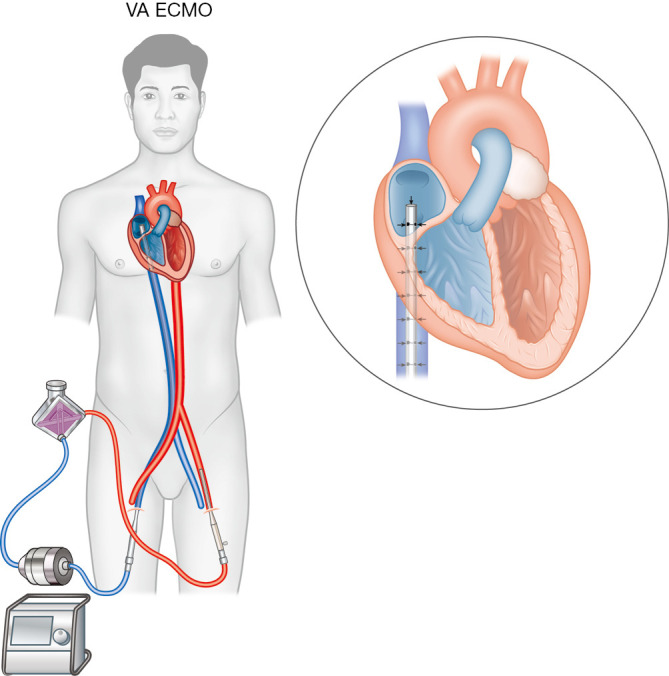
Venoarterial extracorporeal membrane oxygenation (femoral-femoral configuration). A multi-staged access cannula is inserted via the femoral vein into the right atrium, where venous blood is drained into the circuit, undergoes gas exchange in the membrane oxygenator, and returned to the systemic circulation via a single-staged return cannula in the femoral artery.
Among all temporary MCS devices, VA ECMO is unique in that it can provide both heart and lung support. As venous blood is diverted away from the failing heart, RA pressure, and pulmonary artery (PA) pressure are reduced. Initially, this reduces LV preload, prevents LV distention, lowers myocardial wall stress, reduces myocardial oxygen consumption, and improves coronary perfusion. Retrograde aortic blood flow provides oxygenated blood to end-organs, improves MAP, and increases LV afterload.
Contraindications and complications
The use of VA ECMO in the presence of aortic regurgitation is contraindicated as LV distention and wall stress worsens. Traditionally, anticoagulation is needed to prevent circuit thrombosis. However, with improving biocompatibility of circuit components, the absence of routine systemic anticoagulation has not resulted in higher mortality or thrombotic events and has led to lower incidence of bleeding complications (25). Furthermore, heparin-free ECMO has been frequently reported in trauma settings with successful outcomes (26). Peripheral cannulation should be avoided in severe peripheral arterial disease, with central ECMO considered as an alternative for hemodynamic support. When performing ECPR, unwitnessed cardiac arrest or prolonged downtime are contra-indications for VA ECMO.
As with most MCS devices, complications related to VA ECMO include access site bleeding, thromboembolic events, and pump-induced hemolysis. Incidence of intracranial hemorrhage varies from 1% to 20%, with mortality rates as high as 57% (27). Distal limb ischemia occurs due to disruption of blood flow from femoral cannulation, with a reported incidence of 10–16% (28). Inserting a reperfusion catheter to the superficial femoral artery is a common preventive strategy, given the significant impact amputation can have on patients’ quality of life. Limiting return cannula size to 15 Fr does not jeopardize overall outcomes and may further reduce vascular complications (29).
Elevated LV afterload due to retrograde aortic blood flow may not relieve LV wall stress and may increase myocardial oxygen requirements. Progressively, this hinders LV recovery and worsens survival outcomes (30). LV venting can be performed using IABP, balloon atrial septostomy, or open surgical vents, with the former being the most commonly used alternative (31-33). Active LV unloading with Impella is theoretically more efficient at mitigating the afterload effects from VA ECMO. Its prophylactic usage with VA ECMO is being investigated in an ongoing randomized trial to further improve overall outcomes (34).
TH
TH is a percutaneous ventricular assisting device designed to temporarily support patients undergoing high-risk cardiac procedures or in CS. Like other novel devices, it provides superior hemodynamic support compared to IABP. However, evidence supporting its use in these high-risk groups is scarce and limited to case studies and observational data. The need for trans-septal cannulation makes it the most invasive of the reviewed devices. Furthermore, trained interventional cardiologists who regularly performing trans-septal punctures are required, which limits its clinical application only to specialized centers.
Device specifics and hemodynamic impact
Similar to ECMO, TH consists of drainage and return cannulas, a centrifugal pump, and a console regulating the pump, but without a membrane oxygenator. A 21-Fr drainage catheter is inserted into the left atrium (LA) using a trans-septal approach from the femoral vein, wherein LA blood is aspirated by the centrifugal pump and propelled into the arterial circulation via a 15–17-Fr return catheter (Figure 4). Placement of the drainage catheter is performed by an experienced interventional cardiologist trained in trans-septal puncture with a 2-stage dilator (14 Fr then 21 Fr). Final positioning is confirmed with fluoroscopy to ensure the catheter tip and 14 side holes are within the LA. Screening angiogram of the femoral arteries is performed to determine return catheter size. Depending on catheter size and pump rotary speed, maximum circulatory support can reach 5 L/min. Similarly to Impella but unlike IABP, TH can continue to provide circulatory support in spite of transient arrhythmias or extreme tachycardia as long as RV function is satisfactory. Adequate systemic anticoagulation with unfractionated heparin is necessary to prevent thromboembolism.
Figure 4.
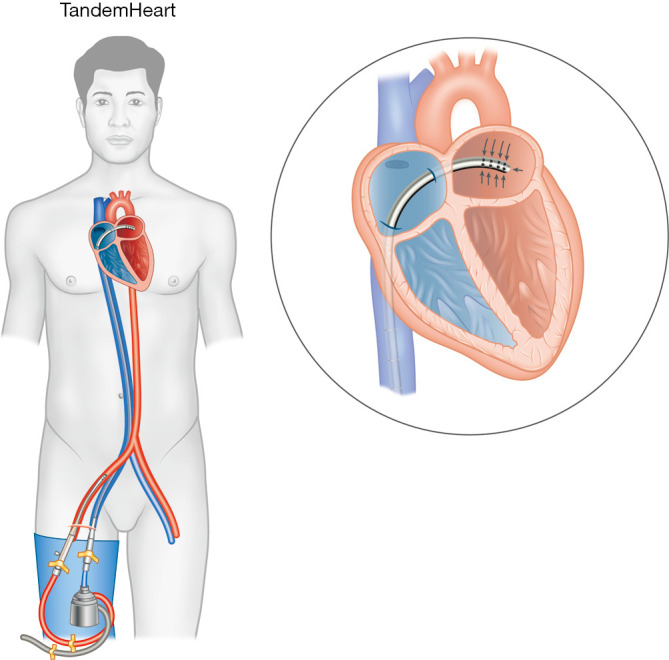
TandemHeart. A multi-staged access cannula is inserted via the femoral vein, placed in the left atrium via a trans-septal puncture, where left atrial blood is drained into the circuit, and returned to the systemic circulation via a single-staged return cannula in the femoral artery.
During TH support, blood flow is redirected from the LA to the aorta. As a result, LV preload, filling pressures, workload, and myocardial oxygen demand are reduced. Mechanical circulatory flow improves MAP and systemic circulation, working in tandem with the native cardiac output to provide adequate end-organ perfusion. However, like in VA ECMO, retrograde aortic blood flow increases afterload, potentially negating the myocardial protective benefits from offloading the LV. This is in contrast to Impella devices, where direct LV unloading may theoretically provide superior myocardial protection.
Contraindications and complications
As with other MCS devices, femoral artery cannulation may compromise distal limb circulation and should not be performed in patients with severe peripheral vascular disease. Anticoagulation is necessary to prevent thromboembolism; conditions that preclude the use of unfractionated heparin are contraindications. TH support in the presence of ventricular septal defect can theoretically cause a right-to-left shunt; satisfactory RV function is necessary for maintaining adequate LA volume. Concurrent RV failure, pulseless ventricular arrhythmias, and asystole are poorly tolerated by the system. The addition of right VAD (RVAD) or conversion to VA ECMO may be necessary. Other contraindications include the presence of intra-atrial thrombus and aortic insufficiency. Unlike when using Impella, LV thrombus and aortic stenosis are not contraindications.
Complications with large vessel cannulations are similar to those of other devices, including vascular injury, bleeding requiring transfusion, and distal limb ischemia. In selected patients, deploying a reperfusion catheter, as in VA ECMO, may mitigate these risks. Alternatively, the use of 2 smaller return cannulas (12 Fr) in both femoral arteries has been reported (35), but at the expense of reduced maximum achievable circulatory support. Cardiac tamponade may occur during puncture and dilatation of the intra-atrial septum. Drainage catheter migration of side holes into the RA will lead to massive right-to-left shunt, resulting in severe hypoxia. Catheters should be secured in place to prevent dislodgment, particularly during patient transport and cardiopulmonary resuscitation (CPR). Stroke and intracranial hemorrhage may occur with longer device use. Hemolysis is rare but should be considered when an unexplained drop in hemoglobin occurs.
RVAD
With improved LV supporting devices, RV failure has become clinically more relevant and is associated with poor outcomes. Currently, 2 percutaneous RVAD (pRVAD) are available: Impella RP (Abiomed, Danvers, MA, USA) and Protek Duo (TandemLife; LivaNova, London, UK). Impella RP, similar to other Impella devices, consists of a 22-Fr microaxial pump mounted on a 11-Fr pigtail catheter. The device is inserted in an anterograde fashion via the femoral vein, crossing the tricuspid and pulmonary valves. The inlet area drains venous blood from the inferior vena cava and expels it through the outlet area into the PA, bypassing the RV. Protek Duo is the RV version of TH. Unlike TH, Protek Duo is a dual-lumen cannula, draining RA and superior vena cava blood via proximal vents into an extracorporeal centrifugal pump, which is then delivered back directly to the PA. Unlike Impella RP, Protek Duo can be inserted via the right internal jugular vein, allowing for greater mobility and lower infection risk. Like a PA catheter, Protek Duo traverses the tricuspid and pulmonary valve, with its end hole in the PA. An oxygenator membrane can be spliced into the Protek Duo circuit to provide systemic oxygenation. Both devices reduce RA and RV preload and increase PA pressure and LV preload. Fluoroscopy is necessary to guide placement and positioning confirmation. VA ECMO provides RV support by diverting blood away from the RV, but at the expense of decreasing native cardiac output.
As with other short-term MCS devices, bleeding from large cannula insertions are commonly encountered. Presence of mechanical right heart valves, severe valvular stenosis or regurgitation of the tricuspid or pulmonary valve, RV thrombus, and conditions that preclude the usage of anticoagulants are contra-indications for pRVAD devices.
Device comparison
Although all novel MCS devices provide superior hemodynamic support compared to IABP, they differ in their physiological effects, pump and circuit designs, cannulation method, anticoagulation needs, and adverse effects profile (Tables 1-3). However, randomized clinical trials (RCTs) comparing short-term MCS to IABP are limited and do not favor the use of one device over another in the setting of AMI-CS. Ouweneel et al. reported a meta-analysis of 3 RCTs that compared Impella to IABP (n=95) and found no difference in 30-day mortality (36). Similarly, when RCTs that compared Impella and TH to IABP were analyzed (n=148), 30-day mortality was similar in both groups (RR 1.01, P=0.98), despite the improvement in MAP and serum lactate levels seen with novel devices (37). It should be noted, however, that many patients enrolled into these RCTs were in profound CS; in the IMPRESS trial, the only study powered for survival analysis, 92% of patients had resuscitated cardiac arrest prior to randomization, making this a sample where any intervention may be medically futile (38). Furthermore, the majority of patients had PCI performed prior to device implantation (38-40). Registry studies have observed significantly improved survival rates when Impella is initiated prior to PCI, emphasizing the importance of early hemodynamic stabilization (door-to-support time) over door-to-needle time (20,41). Nevertheless, meta-analyses that included observational data and available RCTs also found no difference in short-term mortality rates (21,42). Significantly higher bleeding events with use of novel MCS devices was a consistent finding in all meta-analyses, whereas reports on limb ischemia rates were variable.
Table 1. Characteristics of mechanical circulatory support devices.
| Characteristics | IABP | Impella | VA ECMO | TandemHeart | Protek Duo | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2.5 | CP | 5.0 | 5.5 | RP | |||||
| Hemodynamic support | LV | LV | LV | LV | LV | RV | RV + LV | LV | RV |
| Maximum blood flow (L/min) | 1.0 | 2.5 | 4.3 | 5.0 | 6.2 | 4.0 | 7.0 | 5.0 | 5.0 |
| Duration of use | Weeks | 4 days | 4 days | 14 days | 14 days | 14 days | 14–28 days | 14 days | 14 days |
| Sheath size (Fr) | 7–8 | 12 | 14 | 21 | 19 | 22 | Venous: 21–25; arterial: 15–19 | Venous: 21; arterial: 15–17 | 29–31 |
| Percutaneous insertion | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Pulmonary Support | No | No | No | No | No | No | Yes | Yes (oxygenator add on) | Yes (oxygenator add on) |
| Cardiac Synchronization | Yes | No | No | No | No | No | No | No | No |
| Anticoagulation | Debatable | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
IABP, intra-aortic balloon pump; LV, left ventricle; LVAD, left ventricular assist device; RV, right ventricle; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Table 2. Comparison of hemodynamic impact between mechanical circulator support devices.
| Hemodynamics | IABP | Impella | VA ECMO | TandemHeart |
|---|---|---|---|---|
| Mechanism | Counterpulsation | LV → Ao | RA → CFA | LA → CFA |
| Pump | Pneumatic | Axial | Centrifugal | Centrifugal |
| CVP | – | – | ↓ | – |
| PAP | – | – | ↓ | – |
| LVEDP | ↓ | ↓↓ | – | ↓↓ |
| MAP | ↑ | ↑↑ | ↑↑ | ↑↑ |
| LV afterload | ↓ | – | ↑ | ↑ |
| CI | ↑ | ↑↑ | ↑↑ | ↑↑ |
| Native CO | ↑ | ↓ | ↓ | ↓ |
Ao, aorta; CI, cardiac index; CFA, common femoral artery; CO, cardiac output; CVP, central venous pressure, IABP, intra-aortic balloon pump; LA, left atrium; LV, left ventricle; LVEDP, left ventricular end-diastolic pressure; MAP, mean arterial pressure; PAP, pulmonary artery pressure; RA, right atrium; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Table 3. Summary of advantages, disadvantages, contraindications, and complications of short-term mechanical circulatory support devices.
| Characteristics | IABP | Impella | VA ECMO | TandemHeart |
|---|---|---|---|---|
| Advantages | Readily available, bedside insertion, small size cannula single vascular access, less vascular injury, low cost, can tolerate without anticoagulation | Better hemodynamic support than IABP, small size cannula, bedside position adjustment, efficacy independent of rhythm, direct LV unloading | Better hemodynamic support than IABP, bedside insertion, can provide full cardiopulmonary support, efficacy independent of cardiac rhythm | Better hemodynamic support than IABP, efficacy independent of cardiac rhythm, respiratory support possible with addition of oxygenator |
| Disadvantages | Modest support, reduced efficacy during arrhythmias, no RV support | Risk of vascular injury, surgical cutdown for larger cannulas, no RV support, fluoroscopy needed for insertion | Risk of vascular injury, large sized cannula, may need LV unloading | Risk of vascular injury, large sized cannula, fluoroscopy needed for insertion, trans-septal puncture, no RV support |
| Contraindications | PVD, aortic insufficiency, aortic aneurysm, aortic dissection | Mechanical AV, LV thrombus, VSD, intolerant to anticoagulation, PVD, severe aortic stenosis, recent CVA | LV thrombus, intolerant to anticoagulation, PVD, aortic insufficiency, aortic dissection | LA thrombus, intolerant to anticoagulation, PVD, aortic insufficiency, aortic dissection, VSD |
| Complications | Limb ischemia, bleeding, thrombocytopenia, hemolysis, ischemic bowel, aortic rupture, balloon rupture | Limb ischemia, bleeding, hemolysis, AV injury, ventricular arrhythmia, LV perforation | Limb ischemia, bleeding, hemolysis, differential hypoxia, thromboembolism, LV dilatation, pulmonary edema, air embolism | Limb ischemia, bleeding, hemolysis, right-to-left shunt, cardiac tamponade, thromboembolism |
AV, aortic valve; CVA, cerebral vascular accident; IABP, intra-aortic balloon pump; LV, left ventricle; PVD, peripheral vascular disease; RV, right ventricle; VA ECMO, venoarterial extracorporeal membrane oxygenation; VSD, ventricular septal defect.
Comparative data on VA ECMO are limited to observational studies. The 30-day mortality in CS favored VA ECMO over IABP in a meta-analysis of 4 studies (n=95, risk difference 33%, P=0.0008, number needed to treat =3), but no significant difference was observed when compared to Impella and TH (n=140, risk difference −3%, P=0.70, number needed to harm =33) (43). Recently, a retrospective two-center study comparing VA ECMO and Impella 5.0 also did not find any significant difference in 30-day mortality (49% vs. 53%, P=0.30) (44). Although these results contradict the Impella/TH versus IABP study findings of a lack of survival benefit between these devices, they also highlight the need for better case selection and timely initiation of MCS support in CS. The ongoing DanGer Shock, a prospective multicenter clinical study of AMI-CS patients randomized to early Impella or guideline-driven therapy, excluding out-of-hospital cardiac arrest, aims to address these issues (45).
Practical considerations
Which patients to consider for MCS?
Although existing RCTs are not in favor of MCS devices, it is clear that when patients are unresponsive to conventional intensive care management, the use of MCS devices remains the only option for survival. However, its resource-intensive nature demands a careful selection of patients that stand to benefit the most. The goal of MCS deployment should be well documented before its initiation. Patients with conditions that are reversible (e.g., acute myocarditis, postpartum cardiomyopathy, Takotsubo cardiomyopathy) generally have better outcomes and would be ideal candidates for bridge-to-recovery (46). On the other hand, deploying MCS in patients with end-stage diseases will not lead to improvements unless they are candidates for organ transplant (bridge-to-transplant) or for implementation of more durable devices (bridge-to-device). Patients occasionally present with unstable hemodynamics before the diagnosis and prognosis can be determined. In these circumstances, MCS can be used as a bridge-to-decision. Underlying comorbidities and physiological age are important factors to consider during the triage process. With improving medical treatments and prognosis in diseases which used to be deemed terminal, the indication for MCS will likely further expand with time. Clinicians will also need to take these factors into consideration.
After initiating MCS, it should be noted that these bridging destinations are fluid depending on how the patient responds to the therapy. For example, a patient with heart failure on MCS initially listed for organ transplantation may be de-listed because of complications, de-conditioning, or personal choice. A patient with acute decompensated heart failure and multi-organ failure put on MCS trial for bridging-to-recovery may become transplant-eligible should all other organs recover. ECPR performed on a patient in cardiac arrest with the intention of bridging-to-recovery may ultimately require palliative care due to severe neurological injury from prolonged downtime. Regular reassessment of patient condition is paramount in determining the eventual exit strategy. Early involvement of a multidisciplinary team, including palliative care, would help establish feasible goals and provide the patient and family with realistic expectations (47).
When to consider MCS?
Early use of MCS devices in CS is associated with better outcomes, as it mitigates the negative effects of systemic hypoperfusion on end-organs and of inotropes/vasopressors on myocardial oxygen consumption and microcirculation. This is evident in retrospective studies with multiple devices. In one retrospective analysis of 46 patients with AMI-CS who required VA ECMO support, survival outcomes were compared between initiating circulatory support before or after PCI. Despite a delay in door-to-balloon time (145 vs. 115 min, P=0.469), early ECMO was associated with a significantly better 6-month survival (58.3% vs. 14.7%, P=0.006) (48). The timing of Impella use in AMI-CS was studied by Basir et al. In their cohort, 52% of patients experienced cardiac arrest, and 9% had ongoing CPR during implantation. Overall survival was 44%, with better survival rates when Impella was implanted <1.25 hours from shock onset (66%). The later Impella was implanted after shock onset, the worse the survival outcome (1.25–4.25 hours, 37%; >4.25 hours, 26%). Deployment of Impella prior to PCI was also associated with survival benefit (46% vs. 35%, P<0.01), further suggesting the importance of establishing systemic circulation over coronary intervention in the setting of AMI-CS (41). Delaying MCS initiation by increasing inotropic/vasopressor support may be detrimental, as prolonged microcirculatory dysfunction leads to irreversible end-organ injury, rendering any intervention medically futile. Although evidence supports the early use of MCS, clinicians should be wary of premature initiation, as it exposes patients to unnecessary risks and complications. Identifying the therapeutic window remains a challenging task for clinicians. The SAVE score, developed from 3,846 Extracorporeal Life Support Organization registry patients, predicts VA ECMO survival using clinical parameters and may be helpful in addition to clinical judgement (49). The results of the ongoing ECMO-CS study, a multicenter RCT of AMI-CS patients to early ECMO or standard therapy, will likely influence and change our approach on how to support these patients (50).
Which MCS device should be chosen?
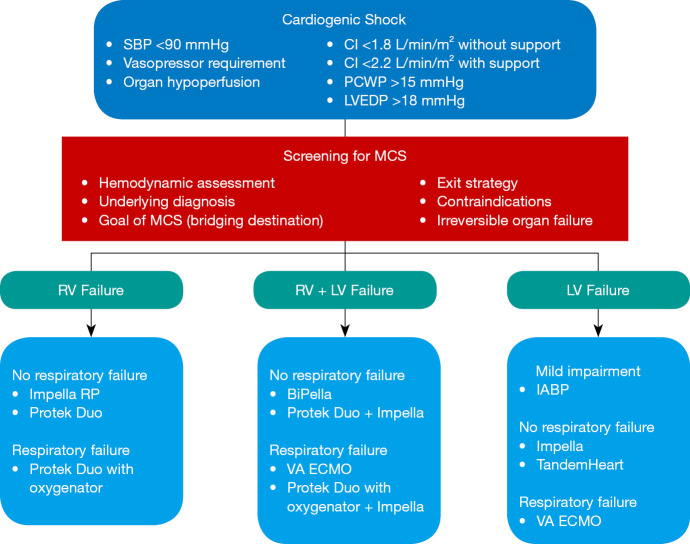
Multiple factors need to be considered when determining the MCS device to be used: the device hemodynamic impact, disease severity, situation urgency, available access sites, coexisting mechanical supports, structural abnormalities, and institutional factors. In mild hemodynamic instability or emergent situations, IABP is often considered in view of its low cost, ease of insertion, and operator familiarity. Concomitant pharmacological support is often necessary and may be adequate for temporary support. Centers with experience may prefer the similarly low-profile Impella 2.5, which is inserted just as quickly and provides superior hemodynamic support compared to IABP. Escalating requirement of vasopressors suggests the need for more powerful devices, although the definition of this threshold is currently unclear in the existing guidelines. In cardiac arrest or peri-arrest when detailed assessment of underlying pathophysiology is not possible, VA ECMO can be rapidly deployed at the bedside and provide both circulatory and respiratory support. Similarly, VA ECMO may be preferable in the setting of concurrent RV dysfunction. Although combination of LV and RV supporting devices have been described (Bi-Pella, Impella + Protek Duo), concurrent multiple consoles increase the complexity of patient management (51). Otherwise, if the hemodynamic profile can be thoroughly assessed, choosing a device that is best suited to meet the specific needs of the physiological derangement and underlying diagnosis is recommended (Figure 5).
Figure 5.
Approach to percutaneous mechanical circulatory support device in cardiogenic shock. CI, cardiac index; IABP, intra-aortic balloon pump; LV, left ventricle; LVEDP, left ventricle end-diastolic pressure; MCS, mechanical circulatory support; PCWP, pulmonary capillary wedge pressure; RV, right ventricle; SBP, systolic blood pressure; VA ECMO, venoarterial extracorporeal membrane oxygenation.
The anticipated duration of support, anatomical variations, and exit strategy are also factors that need consideration. Femoral vessel cannulation is less invasive, easily accessible, and preferable in patients who require peri-procedural short-term hemodynamic support. In patients who require prolonged support or scheduled for organ transplantation, devices permitting cannulation of upper body vessels are more suitable to facilitate mobility, undergo rehabilitation, and maintain transplant candidacy. Central cannulation is more invasive but may be the only feasible option in patients with severe peripheral vascular disease. Existing post-cardiac surgery central cannulas in patients who are unable to wean off CPB can simply be reused and connected to a TH or ECMO circuit. Mechanical aortic valves preclude the use of transaortic devices, whereas devices that generate retrograde aortic blood flow (IABP, TH, VA ECMO) increase LV wall stress in aortic insufficiency. The need for anticoagulation precludes the use of novel devices, but this is likely to change in the future as manufactures strive to reduce bleeding risk by producing more blood biocompatible circuit components.
Conclusions
CS remains a significant cause for morbidly and mortality. Increasing demand, advances in technology, and innovative designs in MCS devices have led to an unprecedented growth in this field. Their superior hemodynamic profile over IABP is unquestioned; however, this has not led to improved outcomes. Future prospective clinical trials on defining MCS candidacy, timing of implementation, and comparing device efficacy are needed to inform treatment guidelines.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kamen Valchanov) for the series “Perioperative Management of Patients with undergoing Mechanical Circulatory Support” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form. (available at http://dx.doi.org/10.21037/atm-20-2171). The series “Perioperative Management of Patients with undergoing Mechanical Circulatory Support” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779-89. 10.1056/NEJMoa0907118 [DOI] [PubMed] [Google Scholar]
- 2.Tarvasmäki T, Lassus J, Varpula M, et al. Current real-life use of vasopressors and inotropes in cardiogenic shock - adrenaline use is associated with excess organ injury and mortality. Crit Care 2016;20:208. 10.1186/s13054-016-1387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 4.Hochman JS, Sleeper L, Webb J, et al. Early Revascularization and Long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA 2006;295:2511-5. 10.1001/jama.295.21.2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy B, Buzon J, Kimmoun A, et al. Inotropes and vasopressors use in cardiogenic shock: when, which and how? Curr Opin Crit Care 2019;25:384-90. 10.1097/MCC.0000000000000632 [DOI] [PubMed] [Google Scholar]
- 6.Stretch R, Sauer C, Yuh D, et al. National trends in utilization of short-term mechanical circulatory support. J Am Coll Cardiol 2014;64:1407-15. 10.1016/j.jacc.2014.07.958 [DOI] [PubMed] [Google Scholar]
- 7.Thiele H, Zeymer U, Neumann F, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 8.Thiele H, Zeymer U, Neumann F, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomized, open-label trial. Lancet 2013;382:1638-45. 10.1016/S0140-6736(13)61783-3 [DOI] [PubMed] [Google Scholar]
- 9.Thiele H, Zeymer U, Thelemann N, et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation 2018. [Epub ahead of print]. doi:. 10.1161/CIRCULATIONAHA.118.038201 [DOI] [PubMed] [Google Scholar]
- 10.Tanaka A, Tuladhar S, Onsager D, et al. The subclavian intraaortic balloon pump: a compelling bridge device for advanced heart failure. Ann Thorac Surg 2015;100:2151-7. 10.1016/j.athoracsur.2015.05.087 [DOI] [PubMed] [Google Scholar]
- 11.Ferguson JJ, 3rd, Cohen M, Freedman RJ, Jr, et al. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J Am Coll Cardiol 2001;38:1456-62. 10.1016/S0735-1097(01)01553-4 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Xiao H, Zhang J, et al. Impacts of anticoagulation with bivalirudin versus low molecular weight heparin on thrombopenia during IABP therapy. J Am Coll Cardiol 2015;66:C166. 10.1016/j.jacc.2015.06.635 [DOI] [Google Scholar]
- 13.Pucher PH, Cummings IG, Shipolini AR, et al. Is heparin needed for patients with an intra-aortic balloon pump? Interact Cardiovasc Thorac Surg 2012;15:136-9. 10.1093/icvts/ivs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin AP, Spertus J, Curtis J, et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation 2020;141:273-84. 10.1161/CIRCULATIONAHA.119.044007 [DOI] [PubMed] [Google Scholar]
- 15.Spiro J, Venugopal V, Raja Y, et al. Feasibility and efficacy of the 2.5 L and 3.8 L Impella percutaneous left ventricular support device during high-risk, percutaneous coronary intervention in patients with severe aortic stenosis. Catheter Cardiovasc Interv 2015;85:981-9. 10.1002/ccd.25355 [DOI] [PubMed] [Google Scholar]
- 16.Ludeman DJ, Schwartz B, Burstein S. Impella-assisted balloon aortic valvuloplasty. J Invasive Cardiol 2012:24:E19-20. [PubMed] [Google Scholar]
- 17.Singh V, Mendirichaga R, Inglessis-Azuaje I, et al. The role of Impella for hemodynamic support in patients with aortic stenosis. Curr Treat Options Cardiovasc Med 2018;20:44. 10.1007/s11936-018-0644-9 [DOI] [PubMed] [Google Scholar]
- 18.Dixon SR, Henriques J, Mauri L, et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial U.S. experience. JACC Cardiovasc Interv 2009;2:91-6. 10.1016/j.jcin.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 19.Manzo-Silberman S, Fichet J, Mathonnet A, et al. Percutaneous left ventricular assistance in post cardiac arrest shock: comparison of intra aortic blood pump and Impella Recover LP2.5. Resuscitation 2013;84:609-15. 10.1016/j.resuscitation.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 20.O’Neill WW, Schreiber T, Wohns W, et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella registry. J Interv Cardiol 2014;27:1-11. 10.1111/joic.12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wernly B, Seelmaier C, Leistner D, et al. Mechanical circulatory support with Impella versus intra-aortic balloon pump or medical treatment in cardiogenic shock - a critical appraisal of current data. Clin Res Cardiol 2019;108:1249-57. 10.1007/s00392-019-01458-2 [DOI] [PubMed] [Google Scholar]
- 22.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. 10.1016/S0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 23.Extracorporeal Life Support Organization. ECLS registry report, international summary 2020. [Internet]. 2020 Jan [cited 2020 Feb 26]; [1 p.]. Available online: https://www.elso.org/Registry/Statistics.aspx
- 24.Javidfar J, Brodie D, Costa J, et al. Subclavian artery cannulation for venoarterial extracorporeal membrane oxygenation. ASAIO J 2012;58:494-8. 10.1097/MAT.0b013e318268ea15 [DOI] [PubMed] [Google Scholar]
- 25.Wood K, Ayers B, Gosev I, et al. Venoarterial ECMO without routine systemic anticoagulation decreases adverse events. Ann Thorac Surg. [Internet]. 2019 Sept [cited 2020 Feb 26]; [26 p.]. Available online: https://doi.org/ 10.1016/j.athoracsur.2019.08.040 [DOI] [PubMed]
- 26.Della Torre V, Robba C, Pelosi P net al. Extra corporeal membrane oxygenation in the critical trauma patient. Curr Opin Anaesthesiol 2019;32:234-41. 10.1097/ACO.0000000000000698 [DOI] [PubMed] [Google Scholar]
- 27.Lorusso R, Barili F, Di Mauro M, et al. In-hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med 2016:44:e964-72. 10.1097/CCM.0000000000001865 [DOI] [PubMed] [Google Scholar]
- 28.Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc 2013;15:172-8. [PubMed] [Google Scholar]
- 29.Takayama H, Landes E, Truby L, et al. Feasibility of smaller arterial cannulas in venoarterial extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2015:149:1428-33. 10.1016/j.jtcvs.2015.01.042 [DOI] [PubMed] [Google Scholar]
- 30.Russo JJ, Aleksova N, Pitcher I, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol 2019;73: 654-62. 10.1016/j.jacc.2018.10.085 [DOI] [PubMed] [Google Scholar]
- 31.Nuding S, Werdan K. IABP plus ECMO - is one and one more than two? J Thorac Dis 2017;9:961-4. 10.21037/jtd.2017.03.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin YN, Chen YH, Wang HJ, et al. Atrial Septostomy for Left Atrial Decompression During Extracorporeal Membrane Oxygenation by Inoue Balloon Catheter. Circ J 2017;81:1419-23. 10.1253/circj.CJ-16-1308 [DOI] [PubMed] [Google Scholar]
- 33.Pappalardo F, Schulte Cm Pieri M, et al. Concomitant implantation of Impella on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19:404-12. 10.1002/ejhf.668 [DOI] [PubMed] [Google Scholar]
- 34.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03431467, Impella CP with VA ECMO for cardiogenic shock (REVERSE); 2018 Feb [cited 2020 Feb 26]; [about 4 screens]. Available online: https://clinicaltrials.gov/ct2/show/NCT03431467
- 35.Burkhoff D, O’Neill W, Brunckhorst C, et al. Feasibility study of the use of the TandemHeart percutaneous ventricular assist device for treatment of cardiogenic shock. Catheter Cardiovasc Interv 2006;68:211-7. 10.1002/ccd.20796 [DOI] [PubMed] [Google Scholar]
- 36.Ouweneel DM, Eriksen E, Seyfarth, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump for treating cardiogenic shock. J Am Coll Cardiol 2017;69:358-60. 10.1016/j.jacc.2016.10.026 [DOI] [PubMed] [Google Scholar]
- 37.Thiele H, Jobs A, Ouweneel D, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 2017;38:3523-31. 10.1093/eurheartj/ehx363 [DOI] [PubMed] [Google Scholar]
- 38.Ouweneel DM, Eriksen E, Sjauw K, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017;69:278-87. 10.1016/j.jacc.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 39.Burkhoff D, Cohen H, Brunckhorst C, et al. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J 2006;152:469.e1-8. 10.1016/j.ahj.2006.05.031 [DOI] [PubMed] [Google Scholar]
- 40.Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52:1584-8. 10.1016/j.jacc.2008.05.065 [DOI] [PubMed] [Google Scholar]
- 41.Basir M, Khandelwal A, Dixon S, et al. Mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: insights from the cVAD registry. J Am Coll Cardiol 2016;68:B49. 10.1016/j.jacc.2016.09.027 [DOI] [Google Scholar]
- 42.Romeo F, Acconcia M, Sergi D, et al. Percutaneous assist devices in acute myocardial infarction with cardiogenic shock: review, meta-analysis. World J Cardiol 2016;8:98-111. 10.4330/wjc.v8.i1.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouweneel DM, Schotborgh J, Limpens J, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systemic review and meta-analysis. Intensive Care Med 2016;42:1922-34. 10.1007/s00134-016-4536-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karami M, den Uil CA, Ouweneel DM, et al. Mechanical circulatory support in cardiogenic shock from acute myocardial infarction: Impella CP/5.0 versus ECMO. Eur Heart J Acute Cardiovasc Care 2020;9:164-72. 10.1177/2048872619865891 [DOI] [PubMed] [Google Scholar]
- 45.Udesen NJ, Møller J, Lindholm M, et al. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am Heart J 2019;214:60-8. 10.1016/j.ahj.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 46.Keebler ME, Haddad E, Choi C, et al. Venoarterial extracorporeal membrane oxygenation in cardiogenic shock. JACC Heart Fail 2018;6:503-16. 10.1016/j.jchf.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 47.Goldstein NE, May C, Meier D. Comprehensive care for mechanical circulatory support: a new frontier for synergy with palliative care. Circ Heart Fail 2011;4:519-27. 10.1161/CIRCHEARTFAILURE.110.957241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C, Liao P, Ke S, et al. Door-to-ECMO before door-to-balloon? Early implementation of ECMO might improve the survival of patients with STEMI complicated by refractory cardiogenic shock. J Am Coll Cardiol 2016;68:S89. 10.1016/j.jacc.2016.09.897 [DOI] [Google Scholar]
- 49.Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015;36:2246-56. 10.1093/eurheartj/ehv194 [DOI] [PubMed] [Google Scholar]
- 50.Ostadal P, Rokyta R, Kruger A, et al. Extra corporeal membrane oxygenation in the therapy of cardiogenic shock (ECMO-CS): rationale and design of the multicenter randomized trial. Eur J Heart Fail 2017;19:124-7. 10.1002/ejhf.857 [DOI] [PubMed] [Google Scholar]
- 51.Pappalardo F, Scandroglio A, Latib A. Full percutaneous biventricular support with two Impella pumps: the Bi-Pella approach. ESC Heart Fail 2018;5:368-71. 10.1002/ehf2.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as