Abstract
The Chronic Kidney Disease in Children (CKiD) cohort study is a North American (US and Canada) multicenter, prospective study of children with chronic kidney disease (CKD). The original aims of the study were (1) to identify novel risk factors for CKD progression, (2) to measure the impact of kidney function decline on growth, cognition, and behavior, and (3) to characterize the evolution of cardiovascular disease risk factors. CKiD has developed into a national and international resource for the investigation of a variety of factors related to CKD in children. This review highlights notable findings in the area of CKD progression and outlines ongoing opportunities to enhance understanding of CKD progression in children. CKiD’s contributions to the clinical care of children with CKD include updated and more accurate glomerular filtration rate estimating equations for children and young adults, and resources designed to help estimate the CKD progression timeline. In addition, results from CKiD have strengthened the evidence that treatment of hypertension and proteinuria should continue as a primary strategy for slowing the rate of disease progression in children.
Keywords: Children, CKD, Cohort, Hypertension, Proteinuria, Risk Factors
Introduction
The Chronic Kidney Disease in Children (CKiD) cohort study was designed to fill critical gaps in our knowledge of kidney disease in children. The primary aims of the study were (1) to identify novel risk factors for chronic kidney disease (CKD) progression, (2) to measure the impact of kidney function decline on growth, cognition, and behavior, and (3) to characterize the evolution of cardiovascular disease risk factors [1]. Since study recruitment began in 2005, more than 1,000 children have been enrolled in the study and followed longitudinally with annual study visits for data collection. Across the more than 100 published analyses generated by CKiD study investigators since 2006, not only have several known risk factors for adult CKD been confirmed in children enrolled in the study, but additional novel risk factors have also been identified. In addition, given the long-term and continuous nature of the study, characterization of the transition from CKD to end stage disease and the initiation of renal replacement therapy is now possible and has been a recent focus of the study.
CKiD Study Design
The CKiD study is a multicenter observational prospective cohort study of children with CKD recruited from 59 centers in North America. The initial CKiD study design and methods have been described previously [1]. The study population comprised participants recruited in three distinct periods; April 2005-August 2009 (Cohort I), February 2011-March 2015 (Cohort II), and October 2016-present (Cohort III). Inclusion criteria have evolved throughout the course of the study in an effort to enrich the cohort with glomerular diseases expected to have faster progression, and to capture and observe early CKD stages and characterize progression to renal replacement therapy (RRT) in younger children with higher estimated glomerular filtration rate (eGFR) at baseline. Cohort I (n=586) targeted children aged 1–16 years with mild-to-moderate CKD with GFR estimated using the original Schwartz formula between 30–75 ml/min/1.73m2 [2]. Cohort II (n=305) targeted the same 1–16 year old age group but changed the eGFR range for inclusion to 45–90 ml/min/1.73m2 using the updated CKiDSCr equation to confirm eligibility as will be discussed below, given that the original Schwartz formula was identified as overestimating kidney function in this population. Finally, cohort III inclusion criteria specify age 6 months to 17 years in children with non-glomerular disease who were within first 5 years of CKD onset, with no upper limit of eGFR and the presence of either urinary sediment abnormalities, anatomic abnormalities of the kidneys and urinary tract, or hypertension. This evolution of inclusion criteria over the course of the study has served to capture as much data as possible during early/mild CKD, as the window in which therapeutic interventions to delay or prevent GFR decline are more likely to be successful is earlier in the course of disease. Children enrolled in the CKiD cohort are followed through annual in-person study visits at which anthropometric, biochemical, and clinical data are collected, including medication use, quality of life, environmental exposures, and neurocognitive assessments [2]. Specific details of the data collection schedule have been previously published [1].
Demographic and Clinical Characteristics of CKiD Study Participants
As of April 2019, 1,043 children have been enrolled in the CKiD cohort. The cohort is 63% male and 23% African-American, with additional characteristics listed in Table 1. Twenty-six percent of the cohort had glomerular disease as the underlying cause of CKD, with the most common non-glomerular causes of CKD being obstructive uropathy, renal aplasia/hypoplasia/dysplasia, and reflux nephropathy. Since study initiation, 273 children have met an initial study endpoint of preemptive kidney transplant (n=110), initiation of dialysis (n=158), or death (n=7, with 2 deaths occurring after RRT initiation). Although participants were disenrolled from the main CKiD study once they reached the study endpoint of initiating renal replacement therapy (RRT), clinical information including serum creatinine and mode of renal replacement therapy are still collected either by phone, online, or at a routine nephrology clinic visit through the CKiD Phone or In-Person follow-up program (PIP) for consenting participants (n=238 of participants reaching RRT to date).
Table 1.
Baseline Demographic and Clinical Characteristics of All CKiD Study Subjects Enrolled by April 2019 (Median or %)
| OVERALL (N=1032) | NG (N=757) | G (N=275) | |
|---|---|---|---|
| MALE | 63% | 67% | 53% |
| AA | 23% | 20% | 31% |
| HISPANIC | 14% | 14% | 16% |
| AGE (YEARS) | 10 | 9 | 14 |
| INCOME ≤ $36,000 | 40% | 40% | 41% |
| YEARS SINCE CKD ONSET | 6.5 | 7.9 | 3.5 |
| SERUM CREATININE (MG/DL) | 1.0 | 1.0 | 1.1 |
| IOHEXOL GFR (ML/MIN/1.73M2) | 49 | 46 | 59 |
| URINE PROTEIN-CREATININE RATIO | 0.4 | 0.3 | 0.7 |
| SELF-REPORTED HYPERTENSION | 46% | 42% | 56% |
NG=non-glomerular; G=glomerular; AA=African American; GFR=glomerular filtration rate
CKiD Study Contributions to Measuring and Estimating Glomerular Filtration Rate
One of the key contributions of the CKiD study to the clinical care of children with progressive CKD has been the development of new GFR estimating equations. GFR has been directly measured in CKiD cohort participants by plasma disappearance of iohexol, a non-toxic low-osmolar contrast agent of molecular weight 821 Da. To date, the CKiD study has obtained more than 2,800 iohexol-based measured GFR studies. These studies were the foundation for the development of improved GFR estimating equations in children. In the early years of the study, 5 ml of iohexol was infused intravenously and GFR was subsequently determined from 4 blood samples obtained at 10, 30, 120, and 300 minutes after iohexol infusion; the protocol was further simplified to a 3-timepoint collection in June 2008, and to a 2-timepoint sample collection post-infusion at 120 and 300 minutes in November 2010 [3]. The initial four-blood sample protocol data allowed for the derivation of an equation for GFR measurement from plasma samples taken at time points after 120 minutes in children with CKD and adult men with normal kidney function [4]. This equation was recently validated in elderly minority men and women providing a basis for universal application [5]. In 2009, the CKiD study published both a simple bedside GFR estimating equation utilizing only height in centimeters, serum creatinine, and a proportionality constant 0.413, which we will refer to as CKiDSCr in this review, and a longer equation utilizing additional factors (CKiD2009) [3]. The simple equation was found to represent a good approximation of the results obtained from the more complex formula, and with improved feasibility for implementation in the context of routine clinical care. The cystatin C measurements utilized in the development of the CKiD2009 equation were measured by an immunoturbidimetric method in which the correlation coefficient of cystatin C and measured GFR (0.69) was less robust than expected [6]. Given this, 495 samples were later re-assayed for cystatin C using immunonephelometry, and improved correlation between cystatin C and measured GFR was observed. Subsequently in 2012, an updated CKiD estimating equation was published utilizing serum creatinine, cystatin C, blood urea nitrogen, height, and sex, which will be referred to as CKiDSCr-Cys [6]. These equations were developed and validated in CKiD subjects less than 18 years of age, and likely underestimate GFR when applied to children with GFR > 75 ml/min/1.73m2) [7]. (Table 2) Valid and reliable measures and estimates of GFR are critical for accurate assessments of CKD progression.
Table 2.
| ESTIMATING EQUATION | FORMULA |
|---|---|
| CKiDSCr | 0.413[height (cm)/serum creatinine (mg/dl) |
| CKiD2009 | 39.1[height (m)/serum creatinine (mg/dl)](0.516) × [1.8/cystatin C (mg/L)](0.294)[30/BUN (mg/dl)](0.169)[1.099](male)[height (m)/1.4](0.188) |
| CKiDScr-Cys | eGFR=39.8 × (ht(m)/Scr)0.456(1.8/cystatin C)0.418(30/BUN)0.0791.076male(ht(m)/1.4)0.179 |
Estimating GFR is also challenging as patients get older, as most GFR-estimating equations, including those derived in the CKiD study population, are specific to either children or adults. Previous work has shown that among young adults over the age of 18 years, adult formulas overestimated and pediatric formulas underestimated measured GFR [8]. To address this, Ng et al. evaluated commonly used, creatinine-only GFR estimating equations (CKiD and CKD-EPI equations) and measured iohexol GFRs in young-adult CKiD study participants 18–26 years of age [7]. This study found creatinine-based equations derived in adult or pediatric populations were both biased (substantially overestimated and underestimated, respectively) when applied to the young adult group. Ultimately, an unbiased creatinine-based GFR estimation was obtained by taking the simple mathematical average of the CKD-EPI and the CKiDSCr equations. Notably, the averaging estimate performed best when GFR was > 30 ml/min/1.73m2, and with an underestimation bias when applied to young adults with GFR < 30 ml/min/1.73m2 [7]. It should be also noted that cystatin-based and cystatin-creatinine-based equations offered unbiased consistent agreement between the CKD-EPI and CKiD equations. Averaging adult and pediatric creatinine-based GFR offers a simple, valid clinical solution for a young adult patient with a history of pediatric kidney disease.
Relationships between different GFR assessment methods (measured vs. estimated) and clinical indicators of CKD severity were examined by Ng et al in 2017 to investigate how methods affected epidemiologic inferences [9]. Specifically, markers of CKD severity included biomarkers of proteinuria (urine protein-creatinine ratio), hemoglobin, serum bicarbonate, potassium and phosphate, systolic and diastolic blood pressure ɀ scores, and height ɀ scores and were treated as outcomes in models including different GFR estimation methods as the independent variable. They also sought to assess whether the characterization of GFR decline over time by proteinuria level at baseline was different when using measured or estimated GFR. The study sample ultimately included 730 children who contributed 1,539 person-visits with both measured iohexol GFR and estimated GFR. They found that when compared to measured GFR, use of the CKiDSCr-Cys equation did not alter the observed relationship of CKD biomarkers to disease progression. In contrast, the simpler CKiDSCr estimating equation showed attenuated associations with biomarkers, and overestimated GFR decline compared to measured or CKiDSCr-Cys-estimated GFR. From this, the authors concluded that although the CKiDSCr is simple to use and is an excellent clinical tool that has been shown to be robust in diverse external populations [9], for research purposes when obtaining measured GFR is not feasible but a more accurate assessment of GFR is needed, CKiDSCr-Cys should be the estimating equation of choice. The inclusion of cystatin C and blood urea nitrogen levels in the CKiDSCr-Cys equation added to the predictive power of the equation as an indicator of severity, supporting its use in research studies of pediatric CKD when direct measures of GFR are not feasible.
Underlying cause of chronic kidney disease
Clinicians caring for children with CKD know well that the time course for the progression of CKD can be variable and is affected by a number of contributing factors that may be modifiable (e.g. blood pressure) or unmodifiable (e.g. underlying cause of kidney disease, race). One of the key goals of the CKiD study has been to identify specific risk factors for CKD progression toward the goal of developing therapeutic interventions to prevent or delay CKD progression, with the potential to be implemented early in the course of the disease. The convention in analyses performed in the CKiD cohort has been to classify the underlying cause of kidney disease into two broad categories of “glomerular” or “non-glomerular” disease, as disease characteristics and effects on change in kidney function over time can differ significantly between these groups. Table 3 displays the disease classification system utilized in the CKiD study. The higher prevalence and severity of proteinuria observed in children with glomerular vs. non-glomerular disease is likely a contributor to the increased progression risk noted in children with glomerular disease; the prevalence of nephrotic-range proteinuria at baseline is 25% in CKiD subjects with glomerular disease vs. 9% in those with non-glomerular disease.
Table 3.
Classification of CKD diagnoses in the CKiD Study, adapted from Warady et al., AJKD “Predictors of Rapid Progression of Glomerular and Non-glomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort”[13]
| Glomerular | Non-glomerular |
|---|---|
| Chronic glomerulonephritis | Aplastic/hypoplastic/dysplastic kidneys |
| Congenital nephrotic syndrome | Autosomal dominant polycystic kidney disease |
| Denys-Drash syndrome | Autosomal recessive polycystic kidney disease |
| Familial nephritis | Branchio-oto-renal syndrome |
| Focal segmental glomerulosclerosis | Cystinosis |
| Hemolytic uremic syndrome | Medullary cystic disease/juvenile nephronophthisis |
| Henoch Schönlein nephritis | Obstructive uropathy |
| Idiopathic crescentic glomerulonephritis | Oxalosis |
| IgA nephropathy | Pyelonephritis/Interstitial nephritis |
| Membranoproliferative glomerulonephritis types I & II | Reflux nephropathy |
| Sickle cell nephropathy | Renal infarct |
| Systemic immunological disease (including SLE) | Syndrome of agenesis of abdominal musculature |
| Wilms tumor |
Progression of CKD and associated risk factors
Although CKD is characterized by a progressive decline in kidney function, it remains challenging to accurately predict time to reach end stage kidney disease (ESKD), which is needed when planning for dialysis access and transplant evaluation. In 2012, the Kidney Disease: Improving Global Outcomes (KDIGO) guideline for CKD presented an international classification system which ranked a patient’s risk for CKD progression, but the guideline was developed with minimal pediatric data [10]. In response to this, CKiD investigators performed an analysis using eGFR and proteinuria at study entry to define progression-risk categories for children [11]. “Progression” was defined as a composite event of either the initiation of renal replacement therapy, 50% reduction in eGFR, or reaching an eGFR < 15 ml/min/1.73m2. To enrich the study population, data from CKiD subjects were combined with data from children enrolled in the European “Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients” (ESCAPE) trial [12]. Using eGFR and proteinuria at baseline along with underlying cause of disease dichotomized as glomerular or non-glomerular, they constructed six progression risk stages (A-F) and reported percentiles of median time-to-event (Figure 1) (Supplement 1). This progression risk model has been adapted into a clinical tool for predicting CKD progression risk for individual patients, and is available at https://form.jotform.com/81565256783164 [11].
Figure 1.
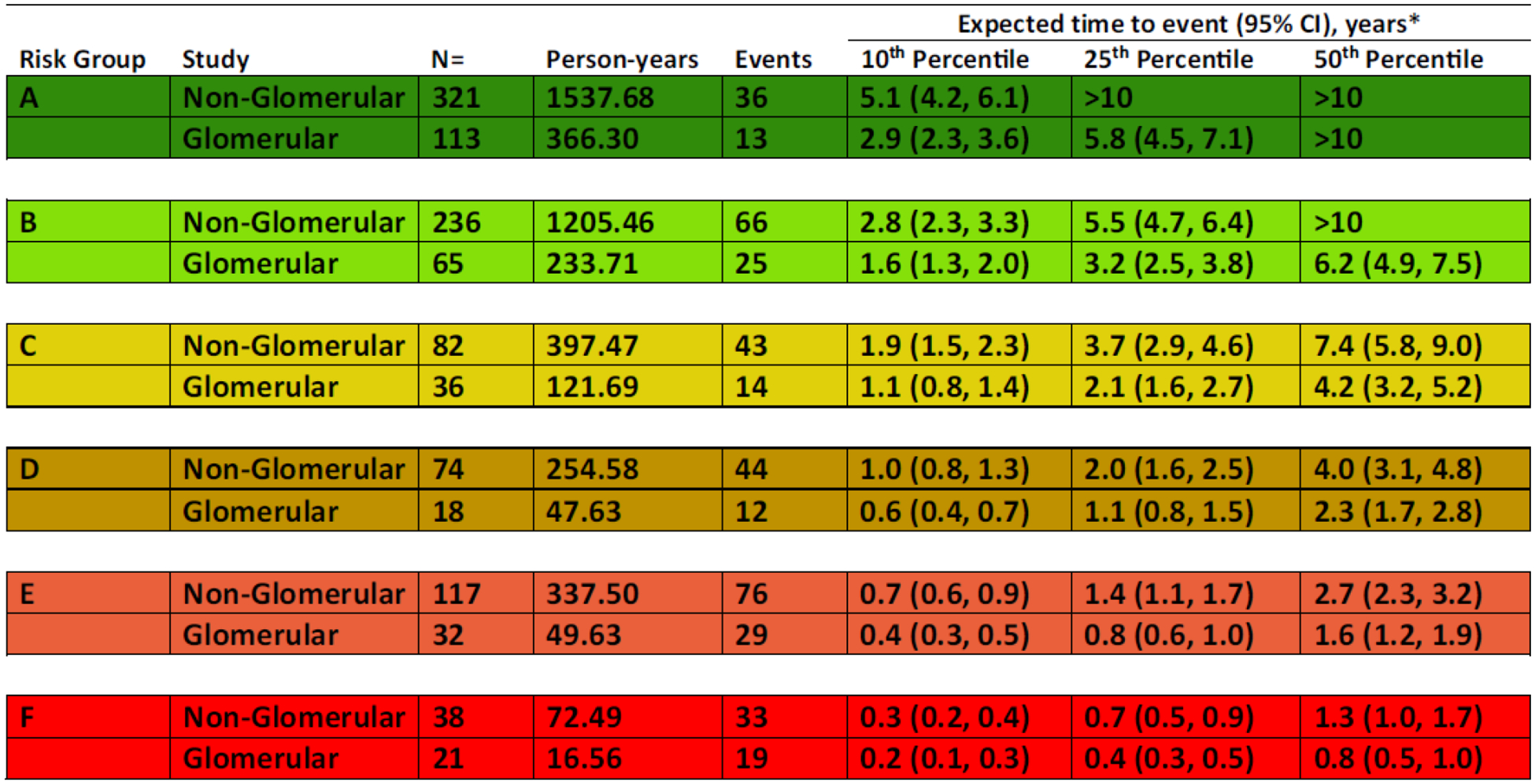
Expected times, 10th, 25th, and 50th (percentile), from glomerular filtration rate (GFR)/proteinuria risk characterization (study baseline) to clinical composite event (50% GFR decline, renal replacement therapy [RRT], or GFR < 15 mL/min/1.73 m2), for 6 GFR/proteinuria risk stages, by chronic kidney disease (CKD) diagnosis. Estimated event times are generated from an accelerated failure time model using a conventional generalized gamma distribution with 7 beta indicator parameters: 6 risk stages (A-F) and glomerular CKD. Time to event is from baseline (time at which risk level was defined). Event is defined as RRT, GFR < 15 mL/min/1.73 m2, or 50% GFR decline. (Reproduced from [11])
In 2015 Warady et al. examined factors associated with rapid CKD progression, with progression defined using the composite endpoint of initiation of RRT (dialysis or transplant) or a 50% reduction in GFR [13]. The analysis was stratified by underlying cause of CKD, based on known pathology and expected differences in disease progression. Participants with glomerular disease were more likely to be African American, female, and older with higher prevalence of significant and nephrotic-range proteinuria, hypoalbuminemia (serum albumin < 3.8 g/dL), anemia, and dyslipidemia. Those with glomerular disease (n=98) also demonstrated significantly faster progression compared to the 398 subjects with non-glomerular disease: 41% vs. 29% progressed to the composite endpoint after median follow-up times of 5.2 and 3.7 years, respectively [13].
All survival models used to characterize progression risk in this analysis were adjusted for baseline GFR (i.e., disease severity at study entry). Among participants with glomerular disease, factors associated with significantly shorter time to the composite end-point included urinary protein-creatinine ratio > 2 mg/mg, hypoalbuminemia, and elevated blood pressure (defined as systolic or diastolic blood pressure > 90th percentile for age, sex, and height based on the BP categories from the 2004 Fourth Report) [14]. Reduction in time to the composite end-point for each of these factors was 94%, 71%, and 67%, respectively. Among participants with non-glomerular disease the factors associated with a significant reduction in time to the end-point were urinary protein-creatinine ratio > 2 mg/mg (79% reduction), hypoalbuminemia (69% reduction), elevated blood pressure (38% reduction), dyslipidemia (40% reduction), male sex (38% reduction), and anemia (45% reduction). Notably, proteinuria, hypoalbuminemia, and elevated blood pressure were risk factors for progression to the end-point in both underlying disease groups, but the relative magnitude of the reduction in time to the composite endpoint was higher for the subjects with glomerular disease. This analysis confirmed proteinuria reduction and blood pressure control as potential therapeutic targets for reduction of progression risk. In addition, the models constructed and validated with internal cross-validation methods provide a means to stratify risk and estimate time to RRT or 50% reduction in GFR based on a particular clinical profile. The CKiD study plans to provide an online version of these models for clinical use in the future.
Disease-Specific CKD Progression
Since participants in the CKiD cohort are affected by a variety of underlying causes of kidney disease, many of which are relatively rare, analyses have been published describing and comparing disease progression among those with specific diagnoses. Work has been done exploring disease-specific progression risk in the CKiD cohort as well. Dell et al. examined GFR decline in a small (n=22) but well-characterized subset of subjects with autosomal recessive polycystic kidney disease (ARPKD) compared to matched participants with congenital kidney disease control groups (44 participants with aplastic/hypopastic/dysplastic disorders, and 44 with obstructive uropathy) [15]. They found that the median [IQR] annualized GFR decline in subjects with ARPKD was -1.4 [-3.5, -0.3] ml/min/1.73m2, with greater decline noted in subjects aged 10 years or older of -11.5 ml/min/1.73m2. Notably, the overall rates of GFR decline did not differ significantly between the ARPKD and the matched controls. In a separate analysis of children with non-glomerular causes of CKD overall, GFR declined 1.3 ml/min/1.73m2 on average [16].
Blood Pressure, Proteinuria/Albuminuria, and Progression
Work by Flynn et al. has demonstrated that elevated blood pressure is very common in children in the CKiD cohort. When hypertension was defined as either casual blood pressure > 95th percentile or as self-reported hypertension plus current treatment with hypertensive medications, 54% of subjects were hypertensive at study entry [17]. Nearly 36% of children prescribed anti-hypertensive therapy still demonstrated casual systolic or diastolic blood pressure values > 90th percentile, indicating uncontrolled hypertension [17]. Factors associated with blood pressure > 90th percentile included African American race, absence of anti-hypertensive use, and shorter duration of kidney disease, while factors associated with uncontrolled hypertension included male sex and absence of angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB) use. Thus, enhanced blood pressure control was identified as a potentially actionable target for decreasing CKD progression risk. Ten years later, Barletta et al. conducted a follow-up analysis to compare blood pressure control over two CKiD study time periods to explore whether publication of the initial CKiD blood pressure data in 2008 had translated into longitudinally improved blood pressure control in the cohort [18]. They examined both casual and ambulatory blood pressure data collected from 851 subjects, comparing time periods 2005–2008 and 2010–2013, and found no difference in either casual blood pressure status or uncontrolled hypertension between groups [18]. This confirmed that screening for and treatment of hypertension remain suboptimal in CKiD study participants, highlighting an opportunity for slowing CKD progression rates by intensifying therapy for this important CKD comorbidity.
As discussed above, analyses in the CKiD study have identified proteinuria as a significant risk factor for disease progression in all CKiD subjects, independent of underlying cause of disease. However, given that proteinuria is more strongly associated with glomerular disease and that subjects with glomerular disease demonstrate more rapid disease progression overall than subjects with non-glomerular disease, there is utility to examining risk factors within specific disease groups more deeply. Fathallah-Shaykh et al. examined the joint contribution of baseline blood pressure and proteinuria levels on GFR decline in a cohort of 522 subjects with non-glomerular CKD [16]. They found that a 2-fold higher baseline urine protein-creatinine ratio was associated with a significantly accelerated GFR decline of -0.3 ml/min/1.73m2 (95% CI 0.4 to 0.1) per year in addition to the overall average GFR decline of -1.3 ml/min/1.73m2 (95% CI 1.6 to 1.1) per year for the cohort overall. A 1-unit higher baseline systolic blood pressure z-score was associated with an additional GFR decline of -0.4 ml/min/1.73m2 (95% CI 0.7 to 0.1). They also found that among normotensive participants, higher baseline urine protein-creatinine ratio was associated with increased rates of GFR decline. However, among children with elevated blood pressure GFR declines were not significantly higher in those with higher levels of proteinuria [16]. Thus, while elevated blood pressure and proteinuria demonstrated additive effects on GFR decline in children with non-glomerular disease, the rate of decline among children with elevated blood pressure did not appear to be modified by the level of proteinuria in this sub-cohort.
The role of albuminuria in CKD progression was examined by Fuhrman et al. in a 2017 analysis using albumin-to-creatinine ratio and the composite endpoint of RRT or 50% reduction in GFR [19]. The association between elevated albumin-to-creatinine ratio ≥ 30 mg/g and disease progression was not significantly different than the association between urine protein-creatinine ratio ≥ 0.2 mg/mg and progression [19]. This suggests that in children with CKD, in whom disease secondary to diabetes is uncommon, urine protein-creatinine ratio is sufficient for monitoring proteinuria as a risk factor for CKD progression over time and measurement of albuminuria is unlikely to further contribute to categorizing progression risk.
Randomized controlled trial data in adults suggest that renin-angiotensin II-aldosterone system (RAAS) blockers using ACE-I or ARB treatment may slow CKD progression [20]–[23]. Abraham et al. examined the association of the use of RAAS blockers and time to renal replacement therapy (RRT) (initiation of dialysis or renal transplant) in the CKiD cohort [24]. The analysis included 851 children, and RAAS use was reported at annual study visits. For this study, Cox proportional hazards models with time-varying RAS exposure were used to evaluate the effect of RAAS use on time to RRT. The analyses were adjusted or weighted to control for age, sex, underlying cause of CKD, GFR, nephrotic range proteinuria, anemia, elevated blood pressure, acidosis, elevated phosphate, and elevated potassium levels using inverse probability weighting methods. In addition, given that ACE-I or ARB use may vary based on evolving clinical factors, RAAS blockade was treated as time-varying to account for switching and lagging exposure over time. At baseline, 55% of children reported RAAS use, and those prescribed RAAS were more likely to be older, to have glomerular disease, to have more proteinuria, and to take more medications, but were less likely to have elevated blood pressure compared to non-users. RAAS use was found to reduce the hazard for RRT by 21% to 37%, suggesting a benefit associated with maintaining children on ACE-I/ARB therapy compared to children never started on RAAS blockade [24]. They also examined characteristics of subjects whose RAAS blockade was discontinued, finding those who discontinued were more likely to have lower GFR (median 26 vs. 47 ml/min/1.73m2), higher urine protein-creatinine ratio, anemia, elevated blood pressure, and glomerular disease. Although the clinical manifestations of the reduction in GFR triggered by RAAS blockade including hyperkalemia and other electrolyte abnormalities may lead to discontinuation of the therapy in children with more advanced CKD, these results suggest that there may be benefit to continuing the therapy, even if medical management of side effects such as hyperkalemia is required. Of note, those who discontinued were not noted to have significantly higher prevalence of hyperkalemia or acidosis compared to those who continued. Finally, the benefit of RAAS blockade extended to normotensive subjects as well, suggesting that the benefits extend beyond blood pressure control and initiation of RAAS blockade should be considered for all CKD patients.
Fibroblast Growth Factor 23 (FGF23) and Uric Acid and Progression
Portale et al. examined the association between FGF23 and CKD progression in 419 study participants. In cross-sectional analysis, baseline FGF23 tertile was significantly associated with GFR; median GFR was highest in those with FGF23 levels in the lowest tertile (52 ml/min/1.73m2), and lowest in those with FGF23 levels in the highest tertile (35 ml/min/1.73m2) [25] In multivariable Cox proportional hazards regression adjusted for baseline GFR, proteinuria, underlying cause of CKD and other factors, risk for progression was 2.5 times higher in the highest vs. lowest FGF23 tertile [25]. Higher baseline FGF23 levels were independently associated with CKD progression in CKiD study subjects, with 40% shorter time to progression noted in those with FGF23 levels in the highest compared with the lowest FGF23 tertile [25]. FGF23 was also identified as an independent predictor of left ventricular hypertrophy in children with eGFR > 45 ml/min/1.73m2 [26]. Uric acid has also been found to be independently associated with progression risk in CKiD subjects. Children with baseline uric acid levels of 5.5–7.5 mg/dL or > 7.5 mg/dL had 17% and 38% shorter time to progression, respectively, compared to those with levels < 5.5 mg/dL [27]. Additionally, FGF23 was a strong predictor of current and future GFR and explained between 20% and 30% of longitudinal GFR variability [28]. Further investigation is required to explore whether urate-lowering therapy could slow disease progression.
Genetic Risk Factors for CKD Progression
Potential causes for the racial differences in rates of CKD incidence and progression observed in adults with CKD include genetic factors, in particular the high-risk APOL1 genotype which is prevalent in approximately 13% of the African American population and is associated with FSGS and an increased incidence of ESKD [29]–[32]. Within the CKiD cohort, African American race has not been independently associated with accelerated CKD progression, but analyses have largely been stratified by underlying cause of disease due to concern about confounding by race, since subjects with glomerular disease are more likely to be African American. All CKiD participants were offered the opportunity to consent to genotyping at study enrollment, with the goal of using genome-wide association studies to identify genetic variants associated with disease progression. In Ng et al.’s 2016 analysis of race and RRT, only 5 out of 84 (6%) African American children with a non-glomerular diagnosis and who consented for genetic testing harbored the high risk APOL1 alleles [28]. In a subsequent collaborative analysis, 104 African American children with glomerular disease by APOL1 genotype using participants enrolled from both CKiD and the Nephrotic Syndrome Study Network (NEPTUNE) studies [32]. They identified that 50% of African American CKiD subjects (and 46% of subjects in the combined cohort) with glomerular disease harbored the high risk APOL1 genotype. Compared to African American children with glomerular disease and free of the high risk APOL1 genotype, GFR at entry was lower and longitudinal GFR decline was significantly faster among those with the high risk genotype [32].
The international Pediatric Investigation for Genetic Factors Linked with Renal Progression (PediGFR) Consortium is composed of 3 pediatric CKD cohorts; CKiD, ESCAPE, and the Cardiovascular Comorbidity in Children with CKD (4C) studies [33]. Collaboration between these cohort studies offers the benefit of increasing sample size of children with CKD for genetic analyses, which is always a concern when conducting genome-wise association studies (GWAS) examining factors related to CKD progression. Data from 1136 children (262 from the CKiD study) were examined, and although some single nucleotide polymorphisms associated with GFR and proteinuria were identified, none were associated with genome-wide significance [33]. However, as CKiD study recruitment continues there will be more opportunities for exploration of genetic factors associated with CKD progression with increased power as the number of both participants and CKD progression outcomes increase.
Additional Risk Factors for CKD Progression
Adverse events in utero may increase the risk for chronic disease, and children with CKD are more likely to have been premature, low birth weight (LBW), or small for gestational age (SGA) [34]. This was explored in the CKiD cohort, and subjects with an abnormal birth history (defined as SGA, birthweight < 2500 grams, or gestational age < 36 weeks) were more likely to be female, of African American race or Hispanic ethnicity, and to have low household income (< $30,000/year). However the rate of change in GFR over time did not differ significantly between subjects with abnormal birth history and those without, suggesting that other, contemporary factor are more closely associated with GFR decline in children diagnosed with CKD [34].
Anemia, defined as hemoglobin level less that the 5th percentile using age- and sex-specific normative values, has also been identified as a risk factor for CKD progression in subjects with non-glomerular disease [13]. The mechanism for this is not clear, but anemia may serve as a marker for tissue hypoxia, potentially contributing to preexisting renal tissue damage. Anemia is also more common in African American CKiD study participants, and is an additional potentially modifiable risk factor for CKD progression [35].
In the general pediatric population, inadequate parental health literacy is associated with worse health outcomes [36]. In order to explore this association in children with CKD, Ricardo et al. assessed the parental health literacy of 367 children enrolled in the CKiD cohort using the Short Test of Functional Health Literacy (STOFHLA) which included 2 reading passages and 4 numeracy items [37]. The STOFHLA was completed by parents of CKiD participants between 2013 and 2015 in English or Spanish depending on their language of preference. Possible scores range from 0–100; 0–55 indicates inadequate health literacy, 56–66 marginal literacy, and 67–100 adequate literacy [38]. The association between STOFHLA scores and CKD progression, defined as initiation of RRT or 50% decline in eGFR, was assessed. The median STOFHLA score was higher for white parents vs. non-white parents, and for those with maternal education levels above high school. The median (IQR) STOFHLA score was 98 (93, 100), indicating overall excellent health literacy in parents of CKiD subjects. The analysis to determine relative time to CKD progression was adjusted for clinical center, baseline age, sex, race, baseline eGFR, proteinuria, glomerular disease, age at CKD onset, hypetension, BMI, history of being small for gestational age, serum albumin, dyslipidemia, and baseline maternal education, income, and health insurance. Even after adjustment for these factors and over a median follow-up time of 3.7 years, the relative time to CKD progression was 1.32 (95% CI 1.15 to 1.50) or 32% longer per 1 standard deviation (9.7) increase in STOFHLA score. This suggests that lower health literacy represents a potentially modifiable risk factor for CKD progression, even in a cohort of children engaged enough with their clinical center and care to participate in a long-term research study. Timely introduction of patient/family education targeted toward both the clinical progression risk profile of patients and the health literacy level of caregivers may be an effective adjunctive strategy for reducing progression risk.
Lower SES is also associated with poor health outcomes in multiple health domains, and this has also been explored in the CKiD cohort with regard to CKD progression. In a sample of 572 CKiD subjects, self-reported annual household income was used as a proxy for SES and categorized as high (≥ $75,000/year), middle ($30,000 to < $75,000/year) or low (< $30,000/year) income [39]. Low and middle household incomes compared to high income were associated with African American race, Hispanic ethnicity, lower maternal education, and having at least 1 comorbid clinical condition (e.g. hypertension, anemia). After adjustment for CKD severity at baseline and comorbid conditions, the average GFR decline per year did not differ significantly by income category at -1.9%, -2.7%, and -2.3%, respectively. Blood pressure control improved longitudinally in all income groups, but higher income was associated with more rapid improvement in blood pressure as indicated by casual blood pressure [39].
Other factors that have been examined in the CKiD cohort but have not thus far been correlated with increased risk for CKD progression are vitamin D deficiency [40], [41], obesity [42], medication non-adherence [43], blood lead level[44], and dietary sources of energy and nutrient intake including caloric, sodium, and protein intake [45]. With the additional follow-up time made possible by the continuation of the CKiD cohort study, understanding of the specific and potentially additive contributions of these factors to CKD progression will be enhanced.
Progression to Renal Replacement Therapy
Adult data have demonstrated that African Americans with CKD have higher rates of disease progression to ESKD, and that those who do progress to ESKD are more likely to be treated with dialysis than with transplant as their first RRT [46], [47]. In 2016 Ng et al. examined the whether there were racial differences in RRT initiation in CKiD subjects [28]. Because African American race is strongly related to glomerular disease due to the APOL1 genotype [32], [48], and because glomerular disease has been associated with more rapid progression in previous CKD analyses, they restricted the analysis to children with non-glomerular CKD who were free of the APOL1 genotype in order to assess the independent effect of race on initial RRT characteristics. A total of 603 were included, 110 of whom were African American. African American children demonstrated a shorter time to RRT with a median time to RRT of 3.2 years (95% CI -6.1 to -0.3 years) earlier than non-African American children. However, after adjusting for socioeconomic factors including household income, low birth weight or pre-term birth, food assistance, public insurance, and maternal education, the difference was diminished and no longer significant (1.6 years shorter time to RRT, 95% CI -4.6 to + 1.5 years). When RRT was stratified into either dialysis or transplant, the time to dialysis was 37.5% shorter for African American children, whereas the time to transplant for African Americans was 53.7% longer. The racial differences in time to RRT were almost fully accounted for by only a few socioeconomic factors, but as in adults, time to transplant was longer even after accounting SES, perhaps due to a lack of organ availability.
Although short stature has been identified as a risk factor for adverse outcomes in children with CKD, it has not been independently associated with risk for CKD progression in the CKiD study [13]. However, short stature defined as age-sex-specific height < 3rd percentile has been associated with poorer post-transplant kidney function in CKiD subjects. Of 138 subjects who progressed to renal transplant, 20% had short stature prior to transplant. The children with short stature had a 40% shorter time to decreased transplant kidney function (eGFR < 45 ml/min/1.73m2) after transplant compared to children with normal stature when both groups had the same distribution of observed pre-transplant characteristics including SES, measures of disease severity, and parental height [49]. This finding suggests that while short stature may not be a specific risk factor for CKD progression, it is associated with adverse effects of post-transplant disease progression and potentially modifiable risk factors for this should be explored further.
Generalizability of CKiD Study Data
The CKiD study is the largest prospective cohort study focused on risk factors for CKD in children ever conducted, and has served to confirm several risk factors for disease progression that could be targeted for therapeutic modulation. However, it must be acknowledged that the composition of the CKiD cohort consists of a subset of the pediatric CKD population at large, and may not be representative of all with CKD. Families who have enrolled their children in the CKiD study are more likely to have been offered participation, to receive their clinical care at a larger academic pediatric nephrology center, to have regular clinical follow up care and an established relationship with a primary pediatric nephrology provider and ancillary care team. They also have the flexibility and resources to schedule and attend regular follow-up visits, and to perceive participation in a research study as valuable. The effects of biochemical/disease pathologic risk factors for CKD progression such as hypertension and proteinuria may well be similar in CKiD participants and in the larger pediatric CKD population. However, the effects of other social or environmental risk factors like health literacy, race, SES and lead exposure may be attenuated in CKiD subjects, a group with excellent access to and engagement with longitudinal health care. Even if these factors have not been independently identified as risk factors in CKiD subjects, they may still contribute to CKD progression risk on the pediatric CKD population at large and are deserving of study in other contexts/patient populations.
Summary and Future Directions
The CKiD study is a novel and successful longitudinal cohort study in children with CKD that has benefited from excellent subject recruitment and retention. The CKiD study’s contributions to the clinical care of children with CKD include updated and more accurate GFR estimating equations for children and young adults, and resources designed to help estimate the CKD progression timeline. In addition, results from the CKiD cohort have strengthened the evidence that treatment of hypertension and proteinuria, including through RAAS blockade, should continue as a primary strategy for slowing the rate of disease progression in children. Moving forward, the ongoing CKiD study will not only accelerate the study of patients early in their course of CKD, looking for therapeutic targets which might modify CKD progression, but participants who meet a RRT study endpoint will be followed more closely for factors influencing post-transplant kidney function and a variety of comorbidities, particularly cardiovascular function. This will provide a new and exciting opportunity to explore factors related to outcomes after RRT and into adulthood, providing critical information to guide the care of patients during childhood and beyond.
Multiple Choice Questions
- The primary aim of the CKiD study is to:
- Identify CKD progression risk factors
- Measure the impact of kidney function on growth
- Describe cardiovascular risk factors in children with CKD
- All of the above
1. D
- Which study design describes CKiD?
- Cross-sectional study
- Prospective cohort study
- Case control study
- Clinical trial
2. B
- The most unbiased estimate of GFR in 18–26 year olds with CKD can be obtained using:
- The CKiDSCr-Cystatin equation
- The classic Schwartz formula
- The simple mathematical average of the CKiDSCr and CKD-EPI equations
- The CKD-EPI equation
3. C
- All of the following have been identified as risk factors for CKD progression in the CKiD cohort EXCEPT:
- Elevated blood pressure
- Income
- Proteinuria
- Glomerular disease
4. B
- Short stature in CKiD subjects has been identified with:
- Poorer renal function post-transplant
- Faster CKD progression
- Hypertension
- Glomerular disease
5. A
Supplementary Material
Key Summary Points.
The CKiD study has provided clinicians with updated and more accurate GFR estimating equations for children and young adults, and prognostic tools to estimate CKD progression
Both elevated blood pressure and proteinuria have been confirmed as critical risk factors for CKD progression, with RAAS blockade associated with decreased risk of progression to renal replacement therapy
While African-American race was not identified as an independent risk factor for CKD progression, after accounting for socioeconomic factors, African-American participants with glomerular disease and the high risk APOL1 genotype demonstrated significantly faster GFR decline compared to other African-American children with glomerular diseases
As the study moves forward, participants who experience renal replacement therapy will continue to be observed in the CKiD cohort, focusing on cardiovascular health and iohexol-measured GFR to characterize outcomes after renal replacement therapy
Funding information
The CKiD study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, U01-DK-082194, and UO1-DK66116).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- [1].Furth SL et al. , “Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study.,” Clin. J. Am. Soc. Nephrol, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schwartz GJ, Haycock GB, Edelmann CM, and Spitzer A, “A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine.,” Pediatrics, 1976. [PubMed] [Google Scholar]
- [3].Schwartz GJ et al. , “New Equations to Estimate GFR in Children with CKD,” J. Am. Soc. Nephrol, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ng DKS et al. , “Universal GFR determination based on two time points during plasma iohexol disappearance,” Kidney Int, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ng DK, Levey AS, Shlipak MG, Muñoz A, Inker LA, and Shafi T, “Validation of a simple equation for glomerular filtration rate measurement based on plasma iohexol disappearance,” Clin. Kidney J, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schwartz GJ et al. , “Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C,” Kidney Int, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ng DK, Schwartz GJ, Schneider MF, Furth SL, and Warady BA, “Combination of pediatric and adult formulas yield valid glomerular filtration rate estimates in young adults with a history of pediatric chronic kidney disease,” Kidney Int, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Selistre L et al. , “GFR Estimation in Adolescents and Young Adults,” J. Am. Soc. Nephrol, 2012. [DOI] [PubMed] [Google Scholar]
- [9].Ng DK, Schwartz GJ, Warady BA, Furth SL, and Muñoz A, “Relationships of Measured Iohexol GFR and Estimated GFR With CKD-Related Biomarkers in Children and Adolescents,” Am. J. Kidney Dis, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Levin A et al. , “Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease,” Kidney International Supplements. 2013. [Google Scholar]
- [11].Furth SL et al. , “Estimating Time to ESRD in Children With CKD,” Am. J. Kidney Dis, vol. 71, no. 6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].ESCAPE Trial Group et al. , “No Title,” N. Engl. J. Med, vol. 361, no. 17, pp. 1639–1650, 2009. [DOI] [PubMed] [Google Scholar]
- [13].Warady BA et al. , “Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort.,” Am. J. Kidney Dis, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, “The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents.,” Pediatrics, 2004. [Google Scholar]
- [15].Dell KM, Matheson M, Hartung EA, Warady BA, and Furth SL, “Kidney Disease Progression in Autosomal Recessive Polycystic Kidney Disease,” in Journal of Pediatrics, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fathallah-Shaykh SA et al. , “Progression of pediatric CKD of nonglomerular origin in the CKiD cohort,” Clin. J. Am. Soc. Nephrol, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Flynn JT et al. , “Blood pressure in children with chronic kidney disease: A report from the Chronic Kidney Disease in Children study,” Hypertension, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barletta GM et al. , “Is blood pressure improving in children with chronic kidney disease?: A period analysis,” Hypertension, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fuhrman DY et al. , “Albuminuria, proteinuria, and renal disease progression in children with CKD,” Clin. J. Am. Soc. Nephrol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lewis EJ, Hunsicker LG, Bain RP, and Rohde RD, “The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group.,” N. Engl. J. Med, 1993. [DOI] [PubMed] [Google Scholar]
- [21].Ruggenenti P, Perna A, Gherardi G, Benini R, and Remuzzi G, “Chronic proteinuric nephropathies: Outcomes and response to treatment in a prospective cohort of 352 patients with different patterns of renal injury,” Am. J. Kidney Dis, 2000. [DOI] [PubMed] [Google Scholar]
- [22].Ruggenenti P et al. , “Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria,” Lancet, 1999. [DOI] [PubMed] [Google Scholar]
- [23].Wright JT et al. , “Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial,” J. Am. Med. Assoc, 2002. [DOI] [PubMed] [Google Scholar]
- [24].Abraham AG et al. , “Renin–angiotensin II–aldosterone system blockers and time to renal replacement therapy in children with CKD,” Pediatr. Nephrol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Portale AA et al. , “Fibroblast growth factor 23 and risk of CKD progression in children,” Clin. J. Am. Soc. Nephrol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mitsnefes MM et al. , “FGF23 and left ventricular hypertrophy in children with CKD,” Clin. J. Am. Soc. Nephrol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rodenbach KE et al. , “Hyperuricemia and progression of CKD in children and adolescents: The Chronic Kidney Disease in Children (CKiD) cohort study,” Am. J. Kidney Dis, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ng DK, Moxey-Mims M, Warady BA, Furth SL, and Muñoz A, “Racial differences in renal replacement therapy initiation among children with a nonglomerular cause of chronic kidney disease,” Ann. Epidemiol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Genovese G et al. , “A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9,” Kidney Int, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Parsa A et al. , “APOL1 Risk Variants, Race, and Progression of Chronic Kidney Disease ,” N. Engl. J. Med, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Friedman DJ, Kozlitina J, Genovese G, Jog P, and Pollak MR, “Population-Based Risk Assessment of APOL1 on Renal Disease,” J. Am. Soc. Nephrol, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ng DK et al. , “APOL1-Associated glomerular disease among African-American children: A collaboration of the chronic kidney disease in children (CKiD) and nephrotic syndrome study network (NEPTUNE) cohorts,” Nephrol. Dial. Transplant, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wuttke M et al. , “Genetic loci associated with renal function measures and chronic kidney disease in children: The Pediatric Investigation for Genetic Factors Linked with Renal Progression Consortium,” Nephrol. Dial. Transplant, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Flynn JT et al. , “The effect of abnormal birth history on ambulatory blood pressure and disease progression in children with chronic kidney disease,” J. Pediatr, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Atkinson MA et al. , “Hemoglobin Differences by Race in Children With CKD,” Am. J. Kidney Dis, vol. 55, no. 6, pp. 1009–1017, June 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].DeWalt DA and Hink A, “Health Literacy and Child Health Outcomes: A Systematic Review of the Literature,” Pediatrics, 2009. [DOI] [PubMed] [Google Scholar]
- [37].Ricardo AC et al. , “Parental health literacy and progression of chronic kidney disease in children,” Pediatr. Nephrol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Baker DW, Williams MV, Parker RM, Gazmararian JA, and Nurss J, “Development of a brief test to measure functional health literacy,” in Patient Education and Counseling, 1999. [DOI] [PubMed] [Google Scholar]
- [39].Hidalgo G et al. , “Association of income level with kidney disease severity and progression among children and adolescents with CKD: A report from the chronic kidney disease in children (CKiD) study,” Am. J. Kidney Dis, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kumar J et al. , “Prevalence and correlates of 25-hydroxyvitamin D deficiency in the Chronic Kidney Disease in Children (CKiD) cohort,” Pediatr. Nephrol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Altemose KE et al. , “Vitamin D insufficiency, hemoglobin, and anemia in children with chronic kidney disease,” Pediatr. Nephrol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Patel HP et al. , “Waist Circumference and Body Mass Index in Children with Chronic Kidney Disease and Metabolic, Cardiovascular, and Renal Outcomes,” J. Pediatr, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Blydt-Hansen TD et al. , “Medication treatment complexity and adherence in children with CKD,” Clin. J. Am. Soc. Nephrol, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fadrowski JJ, Abraham AG, Navas-Acien A, Guallar E, Weaver VM, and Furth SL, “Blood lead level and measured glomerular filtration rate in children with chronic kidney disease,” Environ. Health Perspect, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen W et al. , “Dietary sources of energy and nutrient intake among children and adolescents with chronic kidney disease,” Pediatr. Nephrol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Saran R et al. , “US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States,” Am. J. Kidney Dis, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gore JL, Danovitch GM, Litwin MS, Pham PTT, and Singer JS, “Disparities in the utilization of live donor renal transplantation,” Am. J. Transplant, 2009. [DOI] [PubMed] [Google Scholar]
- [48].Anyaegbu EI, Shaw AS, Hruska KA, and Jain S, “Clinical phenotype of APOL1 nephropathy in young relatives of patients with end-stage renal disease,” Pediatr. Nephrol, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li Y, Greenbaum LA, Warady BA, Furth SL, and Ng DK, “Short stature in advanced pediatric CKD is associated with faster time to reduced kidney function after transplant,” Pediatr. Nephrol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


