Abstract
Background:
For pancreatic adenocarcinoma (PDAC), no studies have established any association between earlier treatment initiation and long-term outcomes. In addition, an optimal type of initial treatment for the localized disease remains ill-defined.
Methods:
Patients in the National Cancer Database (2004-2015) with clinical stage I (CS-I) and II (CS-II) PDAC who underwent curative-intent resection were included. Optimal time from diagnosis-to-treatment including neoadjuvant chemotherapy, neoadjuvant chemoradiation, or upfront surgery was assessed. An optimal type of treatment was evaluated. The primary outcome was overall survival (OS).
Results:
Among 29 167 patients, starting any treatment within 0 to 6 weeks was associated with improved median OS compared with 7 to 12 weeks (21.0 vs 20.1 months; P = .004). This persisted when accounting for sex, race, and Charlson-Deyo score (hazard ratio [HR], 0.94; P = 0.02) and on subset analysis for CS-I (23.5 vs 21.8 months; P = .04) and CS-II (19.4 vs 18.3 months; P = .03). Neoadjuvant chemotherapy was associated with improved OS compared with neoadjuvant chemoradiation (25.6 vs 22.7 months; P <.0001) or US (25.6 vs 20.1 months; P <.0001) even when accounting for sex, race, and Charlson-Deyo score (neoadjuvant chemoradiation: HR, 0.86; P < .001; US: HR, 0.79; P < .001). This improvement persisted in subset analysis with NC compared with neoadjuvant chemoradiation (CS-I: 28.6 vs 25.0 months; CS-II: 25.0 vs 22.9 months; both P < .0001) and to US (CS-I: 28.6 vs 22.9 months; CS-II: 24.7 vs 18.4 months; both P < .0001). On multivariable analysis for each CS-I/CS-II, NC remained associated with 20% improved survival compared with neoadjuvant chemoradiation or upfront surgery.
Conclusions:
For PDAC, initiation of therapy within 6 weeks from diagnosis is associated with improved survival, with neoadjuvant chemotherapy associated with the best survival compared with neoadjuvant chemoradiation or upfront surgery.
Keywords: neoadjuvant therapy, optimal treatments, pancreas cancer, time-to-treatment, treatment delay
1 |. INTRODUCTION
Pancreatic adenocarcinoma (PDAC) comprises 3% of all cancer diagnoses each year in the United States and yet accounts for nearly 8% of all cancer-related deaths due to its aggressive biology, late symptom presentation, and propensity for early metastasis.1,2 Over the last two decades, though the 5-year survival rates for other gastrointestinal cancers have steadily improved, the 5-year survival for PDAC has remained dismally low at 5% to 20%.3,4 As such, strategies to standardize treatment guidelines and better define the optimal treatment sequence are urgently needed for systematic improvement of long-term outcomes in this disease.
Given this aggressive tumor biology, it is conceivable that timely treatment initiation may result in an improvement in survival. Increasing the diagnosis-to-treatment interval beyond a critical time point could allow for the progression of disease to either locally advanced and/or metastatic and preclude surgical resection. Indeed, a 2017 retrospective study found that even short delays in treatment by 1-week intervals were associated with increased mortality by 0.5% to 3.2% for breast, prostate, colon, lung, and kidney cancer.5 Importantly, based on these data and a series of other retrospective studies, three time-dependent measures are currently endorsed by the National Quality Forum, Commission of Cancer, American Society of Clinical Oncology, and the American College of Surgeons’ National Accreditation Program for Breast Centers for the treatment of breast cancer.2,6–11 The effect of the time interval between diagnosis and start of neoadjuvant therapy or upfront surgery on the prognosis of patients with PDAC has not been well studied and presents an important metric that can be integrated into clinical practice guidelines to improve long-term outcomes.
To date, there is also no consensus regarding the optimal initial treatment for patients with early-stage PDAC. The treatment armamentarium currently includes combined modality treatment approaches with neoadjuvant chemotherapy or neoadjuvant chemoradiation or upfront surgery. As there are no randomized controlled trials to demonstrate the superiority of one treatment strategy over another, clinical practices vary widely with some advocating upfront surgery in those with resectable disease and others advocating the routine use of neoadjuvant therapy. The latter approach has been shown to facilitate a margin negative resection and allow improved patient selection by identifying those who would not benefit from resection due to occult, micrometastatic disease that may become evident during therapy.12–15
Given these important gaps in treatment standardization for PDAC, this study aimed to evaluate if delay from diagnosis to initial treatment impacts survival and to better define the optimal initial treatment. The results of this study will help establish time-to-treatment benchmarks and to develop future quality improvement initiatives to address barriers to achieving timely treatment.
2 |. METHODS
2.1 |. Data source
The National Cancer Database (NCDB) is a hospital-based registry, a joint program of the American College of Surgeons Committee on Cancer and the American Cancer Society, with data sources from more than 1500 Commission on Cancer-accredited hospitals.16 The NCDB was queried for patients with clinical stage I or II PDAC according to the American Joint Committee on Cancer (AJCC) sixth and seventh edition staging manual who underwent curative-intent resection from 2004 to 2015. The diagnosis was elicited according to ICD-3 codes (Table S1). The analysis excluded patients with locally advanced or unresectable disease due to vascular involvement, patients who underwent palliative resection, those with missing data regarding treatment sequence, and those with missing vital status. Patient demographics included age, race, sex, Charlson-Deyo score, insurance status (private, government, and uninsured), educational attainment as estimated by the percentage of adults in the patient’s reported zip code that did not obtain a high school diploma (<6.3%, 6.3%-10.8%, 10.9%-17.5%, and ≥17.6%), and survival time in months. Clinicopathologic variables included tumor size, tumor grade, AJCC clinical and pathologic T and N stage, and lymphovascular invasion. Treatment groups included neoadjuvant chemotherapy, neoadjuvant chemoradiation, or upfront surgery as defined by treatment-surgery sequence codes within the NCDB.
2.2 |. Statistical analysis
Statistical analysis was conducted using SAS, version 9.4 (SAS Institute), and SAS macros or software developed at the Biostatistics and Bioinformatics at Winship Cancer Institute.17 Time from diagnosis to first treatment including neoadjuvant chemotherapy, neoadjuvant chemoradiation, or upfront surgery was elicited based on time intervals (in days) provided by the NCDB. Optimal time from diagnosis to treatment was assessed by sequential survival analysis at varying cut-points to create two groups with a statistically significant difference in survival. Statistical significance was predefined as two-tailed P < .05. Descriptive statistics for each variable were reported. The χ2 test was used for comparison of discrete variables, and the analysis of variance test was used for comparison of continuous variables between the two-time interval cohorts and between the three treatment groups. Overall survival (OS) was calculated as the time from initial treatment after diagnosis to the date of death or date of last available follow-up. The median follow-up time was calculated based on the reversed Kaplan-Meier method. Univariate Cox regression was used to identify clinicopathologic factors associated with OS. A multivariable Cox regression was produced using variables statistically significant for OS on univariate regression. Univariate binary logistic regression analysis was used to determine the association of demographic factors with delayed time to treatment. Multivariable logistic regression was produced by backward elimination steps with an alpha level of 0.02 as removal criteria. Results were reported as hazard ratios (HRs) with a 95% confidence interval (95% CI).
3 |. RESULTS
3.1 |. Demographic and clinicopathologic characteristics
Among 29 167 patients, the mean age was 67 (±10) years, 51% were male (n = 14 758) and the median follow-up was 20.9 months. The clinical-stage of the presentation included 45% stage I (n = 13 160) and 55% stage II (n = 16 007). Patient and tumor characteristics of these groups are listed in Table 1.
TABLE 1.
Demographic and clinicopathologic characteristics for clinical stage I and clinical stage II
| All patients n = 29 167 |
Clinical stage I n = 13 160 |
Clinical stage II n = 16 007 |
|
|---|---|---|---|
| Age at diagnosis (mean ± std) | 66 ± 10 | 67 ± 10 | 66 ± 10 |
| Sex | |||
| Male | 14 409 (49) | 6424 (49) | 8334 (52) |
| Female | 14 758 (51) | 6736 (51) | 7673 (48) |
| Race | |||
| White | 25 167 (86) | 11 304 (86) | 13 863 (87) |
| Black | 2745 (10) | 1264 (10) | 1481 (9) |
| Other | 1255 (4) | 592 (4) | 663 (4) |
| Insurance status | |||
| Not insured | 616 (2) | 237 (2) | 379 (2) |
| Government insurance | 17 448 (61) | 8196 (63) | 9252 (59) |
| Private insurance | 10 780 (37) | 4621 (35) | 6159 (39) |
| Missing | 323 | 106 | 217 |
| Educational attainmenta | |||
| ≥17.6% | 5011 (17) | 2206 (17) | 2805 (18) |
| 10.9%-17.5% | 7213 (25) | 3257 (25) | 3956 (25) |
| 6.3%-10.8% | 8157 (28) | 3644 (28) | 4513 (28) |
| <6.3% | 7853 (27) | 3625 (28) | 4228 (26) |
| Facility location | |||
| Northeast | 6381 (22) | 2801 (21) | 3580 (23) |
| South | 10 723 (37) | 4671 (36) | 6052 (38) |
| Midwest | 7939 (27) | 3801 (29) | 4138 (26) |
| West | 3898 (14) | 1803 (14) | 2095 (13) |
| Missing | 226 | 84 | 142 |
| Charlson-Deyo score | |||
| 0 | 18 758 (64) | 8255 (63) | 10 503 (66) |
| 1 | 7951 (27) | 3680 (28) | 4271 (27) |
| ≥2 | 2458 (8) | 1225 (9) | 1233 (7) |
| Tumor differentiation | |||
| Well | 2540 (10) | 1334 (11) | 1206 (8) |
| Moderate | 13 969 (53) | 6428 (54) | 7541 (53) |
| Poor/undifferentiated | 9652 (37) | 4110 (35) | 5542 (39) |
| Missing | 3006 | 1288 | 1718 |
| Tumor size, cm | |||
| <1 | 454 (2) | 331 (3) | 123 (1) |
| 1-2 | 2786 (10) | 1610 (12) | 1176 (8) |
| 2-4 | 16 621 (58) | 7612 (59) | 9009 (58) |
| >4 | 8733 (31) | 3426 (26) | 5318 (34) |
| Missing | 562 | 181 | 381 |
| Time to treatment (mean ± std), d | 22 ±27 | 22 ± 27 | 22 ±27 |
| Time to treatment, wk | |||
| 0-6 | 26 310 (92) | 11 846 (92) | 14 464 (92) |
| 7-12 | 2250 (8) | 1042 (8) | 1209 (8) |
| Missing | 607 | 273 | 334 |
| Treatment approach | |||
| Upfront surgery | 24 884 (86) | 11 889 (90) | 12 995 (81) |
| Neoadjuvant chemotherapy | 3293 (11) | 942 (7) | 2351 (15) |
| Neoadjuvant chemoradiation | 973 (3) | 322 (3) | 651 (4) |
| Missing | 17 | 7 | 10 |
| Surgery type | |||
| Local excision | 70 (0.2) | 27 (0.2) | 43 (0.3) |
| Partial pancreatectomy | 4364 (15) | 2416 (18) | 1948 (12) |
| Pacreatoduodenectomy | 18 641 (64) | 8184 (62) | 10 457 (65) |
| Total pancreatectomy | 3766 (13) | 1642 (13) | 2124 (13) |
| Extended pancreatoduodenectomy | 1736 (6) | 671 (5) | 1065 (7) |
| Not otherwise specified | 590 (2) | 220 (2) | 370 (2) |
| Surgical approach | |||
| Open | 14 512 (85) | 6835 (84) | 7677 (85) |
| Minimally invasive | 2671 (16) | 1319 (16) | 1352 (15) |
| Missing | 11 984 | 5006 | 6978 |
| Margin status | |||
| R0 | 22 499 (78) | 10 526 (81) | 11 973 (76) |
| R1 | 3753 (13) | 1578 (12) | 2175 (14) |
| R2 | 211 (1) | 76 (0.6) | 135 (1) |
| Indeterminate | 2308 (8) | 837 (6) | 1471 (9) |
| Missing | 396 | 143 | 253 |
| Adjuvant therapy | 16 726 (57) | 7646 (58) | 9080 (57) |
| Median follow-up (95% CI), mo | 20.9 (20.6-21.3) | 23.4 (22.8-24) | 19.3 (19-19.6) |
Note: Percentages in parentheses exclude missing data.
Abbreviation: CI, confidence interval.
% in zip code without a high-school diploma.
3.2 |. Survival analysis: Time-to-treatment
For all patients treated during the study period, the mean interval to any treatment was 22 (±27) days. Sequential survival analysis at varying cut-points demonstrated improved survival if patients started any treatment (neoadjuvant chemotherapy, neoadjuvant chemoradiation, or upfront surgery) before 7 weeks and no significant difference in survival was found with shorter time intervals. Treatment intervals were then divided into 0 to 6 weeks (n = 26 310) and 7 to 12 weeks (n = 2250). Compared with patients who initiated treatment 7 to 12 weeks after diagnosis, those who started treatment before 7 weeks were younger, more likely to have private insurance, have a lower Charlson-Deyo score, and more likely to undergo upfront surgery instead of neoadjuvant therapy, regardless of the clinical stage. Importantly, the rate of upstaging from clinical stage I to pathologic stage II was 73% for patients in a 0- to 6-week time interval and 72% for patients in the 7- to 12-week time interval (P = .36; Table 2).
TABLE 2.
Comparison of demographic and clinicopathologic characteristics between time intervals for clinical stage I and clinical stage II
| 0-6 wk | 7-12 wk | P value | |
|---|---|---|---|
| Clinical stage I | n = 11 846 | n = 1041 | |
| Age at diagnosis (mean) | 67 ± 10 | 70 ± 10 | <.001 |
| Sex | |||
| Male | 5787 (50) | 496 (48) | .46 |
| Female | 5958 (50) | 545 (52) | |
| Race | |||
| White | 10 211 (86) | 870 (84) | <.001 |
| Black | 1088 (9) | 139 (13) | |
| Other | 547 (5) | 32 (3) | |
| Insurance status | |||
| Not insured | 213 (2) | 17 (2) | <.001 |
| Government insurance | 7285 (62) | 730 (71) | |
| Private insurance | 4254 (36) | 285 (28) | |
| Educational attainmenta | |||
| ≥17.6% | 2023 (17) | 183 (18) | <.001 |
| 10.9%-17.5% | 2950 (25) | 307 (30) | |
| 6.3%-10.8% | 3332 (28) | 312 (30) | |
| <6.3% | 3398 (29) | 227 (22) | |
| Facility location | |||
| Northeast | 2497 (21) | 235 (23) | .21 |
| South | 4216 (36) | 358 (35) | |
| Midwest | 3452 (29) | 285 (27) | |
| West | 1601 (14) | 159 (15) | |
| Charlson-Deyo score | |||
| 0 | 7517 (64) | 572 (55) | <.001 |
| 1 | 3277 (28) | 332 (32) | |
| ≥2 | 1052 (9) | 137 (13) | |
| Tumor differentiation | |||
| Well | 1195 (11) | 107 (12) | .49 |
| Moderate | 5811 (54) | 500 (55) | |
| Poor/undifferentiated | 3739 (35) | 298 (33) | |
| Tumor size, cm | |||
| <1 | 283 (2) | 32 (3) | .54 |
| 1-2 | 1455 (12) | 127 (12) | |
| 2-4 | 6862 (59) | 602 (59) | |
| >4 | 3092 (26) | 262 (26) | |
| AJCC pathologic stage (sixth or seventh edition) | |||
| Stage I | 2972 (25) | 266 (26) | .36 |
| Stage II | 8618 (73) | 747 (72) | |
| Stage III | 167 (1) | 15 (1) | |
| Treatment approach | |||
| Upfront surgery | 10 768 (91) | 882 (85) | <.001 |
| Neoadjuvant chemotherapy | 817 (7) | 106 (10) | |
| Neoadjuvant chemoradiation | 254 (2) | 53 (5) | |
| Surgery type | |||
| Local excision | 25 (0.2) | 0(0) | .14 |
| Partial pancreatectomy | 2149 (18) | 210 (20) | |
| Pacreatoduodenectomy | 7383 (62) | 655 (63) | |
| Total pancreatectomy | 1483 (13) | 118 (11) | |
| Extended pancreatoduodenectomy | 612 (5) | 41 (4) | |
| Not otherwise specified | 194 (2) | 17 (2) | |
| Surgical approach | |||
| Open | 6135 (84) | 562 (84) | .75 |
| Minimally invasive | 1187 (16) | 105 (16) | |
| Margin status | |||
| R0 | 9481 (81) | 826 (80) | .66 |
| R1 | 1415 (12) | 132 (13) | |
| R2 | 69 (1) | 6(1) | |
| Indeterminate | 756 (6) | 63 (6) | |
| Clinical stage II | n = 14 464 | n = 1209 | |
| Age at diagnosis (mean) | 66 ± 10 | 68 ± 10 | <.001 |
| Sex | |||
| Male | 7522 (52) | 643 (53) | .43 |
| Female | 6942 (48) | 566 (47) | |
| Race | |||
| White | 12 538 (87) | 1033 (85) | .157 |
| Black | 1322 (9) | 130 (11) | |
| Other | 604 (4) | 46 (4) | |
| Insurance status | |||
| Not insured | 341 (2) | 27 (2) | <.001 |
| Government insurance | 8261 (58) | 798 (67) | |
| Private insurance | 5666 (40) | 369 (31) | |
| Educational attainmenta | |||
| ≥17.6% | 2581 (18) | 224 (19) | .003 |
| 10.9%-17.5% | 3622 (25) | 334 (28) | |
| 6.3%-10.8% | 4150 (29) | 363 (30) | |
| <6.3% | 3957 (28) | 271 (23) | |
| Facility location | |||
| Northeast | 3193 (22) | 283 (24) | .635 |
| South | 5498 (38) | 449 (37) | |
| Midwest | 3760 (26) | 319 (27) | |
| West | |||
| Charlson-Deyo score | |||
| 0 | 9545 (66) | 748 (62) | .001 |
| 1 | 3833 (27) | 339 (28) | |
| ≥2 | 1086 (7) | 122 (10) | |
| Tumor differentiation | |||
| Well | 1069 (8) | 106 (10) | .04 |
| Moderate | 6864 (53) | 548 (53) | |
| Poor/undifferentiated | 5069 (39) | 376 (37) | |
| Tumor size, cm | |||
| <1 | 109 (1) | 10 (1) | .87 |
| 1-2 | 1069 (8) | 86 (7) | |
| 2-4 | 8149 (58) | 669 (57) | |
| >4 | 4797 (34) | 413 (35) | |
| AJCC pathologic stage (sixth or seventh edition) | |||
| Stage I | 628 (4) | 69 (6) | .17 |
| Stage II | 13 548 (94) | 1114 (92) | |
| Stage III | 244 (2) | 22 (2) | |
| Treatment approach | |||
| Upfront surgery | 11 860 (82) | 879 (73) | <.001 |
| Neoadjuvant chemotherapy | 2083 (14) | 228 (19) | |
| Neoadjuvant chemoradiation | 515 (4) | 99 (8) | |
| Surgery type | |||
| Local excision | 39 (0.3) | 4 (0.3) | .42 |
| Partial pancreatectomy | 1748 (12) | 169 (14) | |
| Pacreatoduodenectomy | 9484 (66) | 761 (63) | |
| Total pancreatectomy | 1911 (13) | 162 (13) | |
| Extended pancreatoduodenectomy | 950 (7) | 85 (7) | |
| Not otherwise specified | 332 (2) | 28 (2) | |
| Surgical approach | |||
| Open | 6937 (85) | 573 (86) | .58 |
| Minimally invasive | 1226 (15) | 95 (14) | |
| Margin status | |||
| R0 | 10 810 (76) | 915 (77) | .57 |
| R1 | 1978 (14) | 155 (13) | |
| R2 | 127 (1) | 6(1) | |
| Indeterminate | 1329 (9) | 106 (9) | |
Note: Percentages in parentheses exclude missing data. Bold indicates statistical significance at P < .05.
Abbreviation: AJCC, American Joint Committee on Cancer.
% in zip code without a high-school diploma.
For both clinical stage I and II, starting any treatment (neoadjuvant chemotherapy, neoadjuvant chemoradiation, or upfront surgery) within 0 to 6 weeks from diagnosis was associated with improved median OS compared with 7 to 12 weeks (21.0 vs 20.1 months; P = .004; Figure 1A). On subset analysis, this association of earlier time-to-treatment with improved median OS was consistent for clinical stage I (0-6 weeks: 23.5 months vs 7-12 weeks: 21.8 months; P = .04; Figure 1B) and clinical stage II disease (0-6 weeks: 19.4 months vs 7-12 weeks: 18.3 months; P = .03; Figure 1C). This again persisted on univariate Cox regression for OS for both clinical stage I (HR, 0.93; 95% CI, 0.86-1.00; P = .049; Table 3) and clinical stage II (HR, 0.93; 95% CI, 0.87-0.99; P = .025; Table 3). On multivariable Cox regression when accounting for other prognostic factors including sex, race, and Charlson-Deyo score, treatment between 0 and 6 weeks remained associated with the improved OS for patients with clinical stage II disease (HR, 0.93; 95% CI, 0.87-1.00; P = .045; Table 3 multivariable Cox regression A). On binary logistic regression, factors associated with delayed treatment included age older than 67 years (odds ratio [OR], 1.33; 95% CI, 1.14-1.55; P < .001), Black race (OR, 1.48; 95% CI, 1.21-1.80; P < .001), Charlson-Deyo score 1 (OR, 1.26; 95% CI, 1.09-1.45; P = .002) or Charlson-Deyo score > 2 (OR, 1.56; 95% CI, 1.27-1.91; P < .001), uninsured status (OR, 1.10; 95% CI, 0.66-1.83; P = .72), and lack of high school degree attainment (6.3%-10.8%: OR, 1.38; 95% CI, 1.16-1.65; P < .001; 10.9%-17.5%: OR, 1.48; 95% CI, 1.24-1.78; P < .001; >17.6%: OR, 1.26; 95% CI, 1.02-1.55; P = .033).
FIGURE 1.
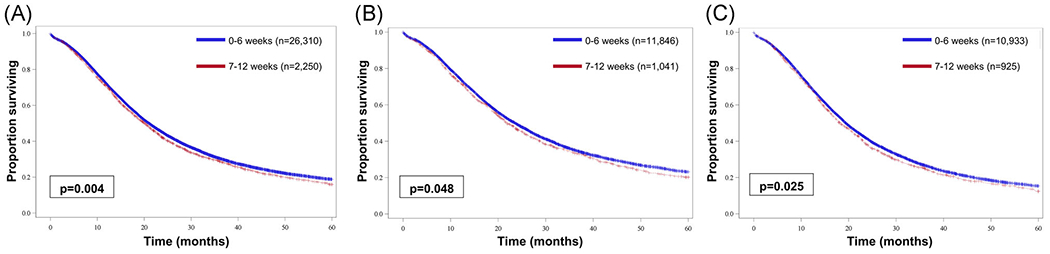
Kaplan-Meier curves comparing overall survival between patients who initiated any treatment at 0 to 6 weeks from diagnosis or 7 to 12 weeks from diagnosis among (A) all patients, (B) patients with clinical stage I disease, and (C) patients with clinical stage II disease [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 3.
Clinicopathologic factors associated with overall survival for all patients and stratified by clinical stage I and clinical stage II
| Univariable Cox regression |
Multivariable Cox regression (A) |
Multivariable Cox regression (B) |
Multivariable Cox regression (C) |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| All patients | ||||||||
| Age at diagnosis | 1.01 (1.01-1.01) | <.001 | ||||||
| Sex | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 0.96 (0.93-0.98) | .001 | 0.96 (0.93-0.99) | .004 | 0.96 (0.94-0.99) | .004 | 0.96 (0.94-0.99) | .004 |
| Race | ||||||||
| White | Reference | Reference | ||||||
| Black | 1.00 (0.95-1.04) | .898 | 0.99 (0.94-1.04) | .642 | 0.99 (0.94-1.04) | .619 | 0.99 (0.94-1.04) | 619 |
| Other | 0.86 (0.80-0.93) | <.001 | 0.87 (0.81-0.93) | <.001 | 0.86 (0.80-0.92) | <.001 | 0.86 (0.80-0.92) | <.001 |
| Insurance status | ||||||||
| Not insured | Reference | |||||||
| Government insurance | 1.07 (0.97-1.18) | .156 | ||||||
| Private insurance | 0.85 (0.77-0.93) | <.001 | ||||||
| Charlson-Deyo score | ||||||||
| 0 | Reference | Reference | ||||||
| 1 | 1.10 (1.07-1.14) | <.001 | 1.10 (1.07-1.13) | <.001 | 1.10 (1.07-1.13) | <.001 | 1.10 (1.07-1.13) | <.001 |
| ≥2 | 1.28 (1.21-1.34) | <.001 | 1.27 (1.21-1.34) | <.001 | 1.27 (1.21-1.33) | <.001 | 1.27 (1.21-1.33) | <.001 |
| Tumor differentiation | ||||||||
| Well | Reference | |||||||
| Moderate | 1.49 (1.41-1.57) | <.001 | ||||||
| Poor/undifferentiated | 1.97 (1.86-2.08) | <.001 | ||||||
| Tumor size | ||||||||
| Time to treatment, wk | ||||||||
| 0-6 | 0.93 (0.88-0.98) | .004 | 0.94 (0.89-0.99) | .017 | ||||
| 7-12 | Reference | Reference | ||||||
| Treatment approach | ||||||||
| Upfront surgery | Reference | Reference | 1.10 (1.02-1.18) | .015 | ||||
| Neoadjuvant chemotherapy | 0.79 (0.75-0.82) | <.001 | 0.79 (0.75-0.82) | <.001 | 0.86 (0.79-0.94) | <.001 | ||
| Neoadjuvant chemoradiation | 0.92 (0.85-0.99) | .023 | 0.91 (0.85-0.98) | .015 | Reference | |||
| Margin status | ||||||||
| R0 | Reference | |||||||
| R1 | 1.62 (1.56-1.68) | <.001 | ||||||
| R2 | 2.01 (1.74-2.33) | <.001 | ||||||
| Clinical stage I | ||||||||
| Age at diagnosis | 1.02 (1.01-1.02) | <.001 | ||||||
| Sex | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 0.93 (0.89-0.97) | .001 | 0.94 (0.90-0.98) | .002 | 0.94 (0.90-0.98) | .002 | 0.94 (0.90-0.98) | .002 |
| Race | ||||||||
| White | Reference | Reference | Reference | Reference | ||||
| Black | 1.01 (0.94-1.08) | .834 | 0.99 (0.92-1.07) | .859 | 1.00 (0.93-1.07) | .963 | 1.00 (0.93-1.07) | .963 |
| Other | 0.84 (0.76-0.94) | .002 | 0.84 (0.75-0.93) | .001 | 0.84 (0.76-0.94) | .002 | 0.84 (0.76-0.94) | .002 |
| Insurance status | ||||||||
| Not insured | Reference | |||||||
| Government insurance | 1.10 (0.93-1.28) | .264 | ||||||
| Private insurance | 0.83 (0.71-0.98) | .025 | ||||||
| Charlson-Deyo score | ||||||||
| 0 | Reference | Reference | Reference | Reference | ||||
| 1 | 1.14 (1.09-1.19) | <.001 | 1.14 (1.08-1.19) | <.001 | 1.14 (1.08-1.19) | <.001 | 1.14 (1.08-1.19) | <.001 |
| ≥2 | 1.31 (1.22-1.40) | <.001 | 1.31 (1.22-1.41) | <.001 | 1.30 (1.21-1.40) | <.001 | 1.30 (1.21-1.40) | <.001 |
| Tumor differentiation | ||||||||
| Well | Reference | |||||||
| Moderate | 1.60 (1.48-1.74) | <.001 | ||||||
| Poor/undifferentiated | 2.18 (2.00-2.37) | <.001 | ||||||
| Tumor size | 1.03 (1.03-1.04) | <.001 | ||||||
| Time to treatment, wk | ||||||||
| 0-6 | 0.93 (0.86-1.00) | .049 | 0.94 (0.87-1.02) | .132 | ||||
| 7-12 | Reference | Reference | ||||||
| Treatment approach | ||||||||
| Upfront surgery | Reference | Reference | 0.97 (0.85-1.10) | .636 | ||||
| Neoadjuvant chemotherapy | 0.81 (0.74-0.89) | <.001 | 0.81 (0.74-0.89) | <.001 | 0.79 (0.68-0.92) | .002 | ||
| Neoadjuvant chemoradiation | 1.04 (0.92-1.18) | .528 | 1.03 (0.91-1.17) | .636 | Reference | |||
| Margin status | ||||||||
| R0 | Reference | |||||||
| R1 | 1.76 (1.66-1.87) | <.001 | ||||||
| R2 | 2.55 (2.02-3.23) | <.001 | ||||||
| Clinical stage II | ||||||||
| Age at diagnosis | 1.01 (1.01-1.01) | <.001 | ||||||
| Sex | ||||||||
| Male | Reference | Reference | ||||||
| Female | 0.99 (0.95-1.03) | .552 | 1.00 (0.96-1.03) | .793 | 1.00 (0.97-1.04) | .982 | 1.00 (0.97-1.04) | .982 |
| Race | ||||||||
| White | Reference | Reference | ||||||
| Black | 0.99 (0.93-1.06) | .823 | 0.99 (0.93-1.05) | .682 | 0.98 (0.92-1.05) | .572 | 0.98 (0.92-1.05) | 572 |
| Other | 0.89 (0.81-0.98) | .018 | 0.90 (0.82-0.99) | .038 | 0.88 (0.80-0.97) | .011 | 0.88 (0.80-0.97) | .011 |
| Insurance status | ||||||||
| Not insured | Reference | |||||||
| Government insurance | 1.09 (0.97-1.23) | .143 | ||||||
| Private insurance | 0.87 (0.77-0.98) | .026 | ||||||
| Charlson-Deyo score | ||||||||
| 0 | Reference | Reference | ||||||
| 1 | 1.08 (1.04-1.13) | <.001 | 1.08 (1.04-1.13) | <.001 | 1.09 (1.04-1.13) | <.001 | 1.09 (1.04-1.13) | <.001 |
| ≥2 | 1.29 (1.21-1.38) | <.001 | 1.28 (1.20-1.37) | <.001 | 1.29 (1.21-1.38) | <.001 | 1.29 (1.21-1.38) | <.001 |
| Tumor differentiation | ||||||||
| Well | Reference | |||||||
| Moderate | 1.35 (1.25-1.45) | <.001 | ||||||
| Poor/undifferentiated | 1.74 (1.61-1.88) | <.001 | ||||||
| Tumor size | 1.01 (1.01-1.01) | <.001 | ||||||
| Time to treatment, wk | ||||||||
| 0-6 | 0.93 (0.87-0.99) | .025 | 0.93 (0.87-1.00) | .045 | ||||
| 7-12 | Reference | Reference | ||||||
| Treatment approach | ||||||||
| Upfront surgery | Reference | Reference | 1.24 (1.13-1.36) | <.001 | ||||
| Neoadjuvant chemotherapy | 0.72 (0.68-0.76) | <.001 | 0.72 (0.68-0.76) | <.001 | 0.89 (0.81-0.99) | .029 | ||
| Neoadjuvant chemoradiation | 0.81 (0.74-0.89) | <.001 | 0.81 (0.74-0.88) | <.001 | Reference | |||
| Margin status | ||||||||
| R0 | Reference | |||||||
| R1 | 1.50 (1.42-1.57) | <.001 | ||||||
| R2 | 1.70 (1.41-2.05) | <.001 | ||||||
Note: Bold values indicate HRs statistically significant at P < .05.
Abbreviations: CI, confidence interval; HR, hazard ratio.
3.3 |. Survival analysis: Optimal treatment strategy
The majority of patients (86%) underwent upfront surgery (n = 24 884), with only 11% undergoing neoadjuvant chemotherapy (n = 3293) and 3% undergoing neoadjuvant chemoradiation (n = 973). Importantly, the rate of adjuvant therapy was only 57% for the entire cohort and 61% (n = 15 112) among the patients who underwent upfront surgery. For clinical stage I, 90% of patients underwent upfront surgery (n = 11 889) with only 7% (n = 942) and 3% (n = 322) undergoing noeadjuvant chemotherapy and neoadjuvant chemoradiation, respectively. For clinical stage II, 81% of patients underwent upfront surgery (n = 12 995), 15% neoadjuvant chemotherapy (n = 2351), and 4% neoadjuvant chemoradiation (n = 651).
When analyzing the optimal type of initial treatment regardless of stage, neoadjuvant chemotherapy was associated with improved median OS compared with either neoadjuvant chemoradiation (25.6 vs 22.7 months; P < .0001; Figure 2A) or upfront surgery (25.6 vs 20.1 months; P < .0001; Figure 2A). Neoadjuvant chemotherapy proved to be the optimal treatment strategy compared with upfront surgery (HR, 0.79; 95% CI, 0.75-0.92; P < .001; Table 3 multivariable Cox regression B) and neoadjuvant chemoradiation (HR, 0.86; 95% CI, 0.79-0.94; P < .001; Table 3 multivariable Cox regression C), even when accounting for sex, race, and Charlson-Deyo score.
FIGURE 2.
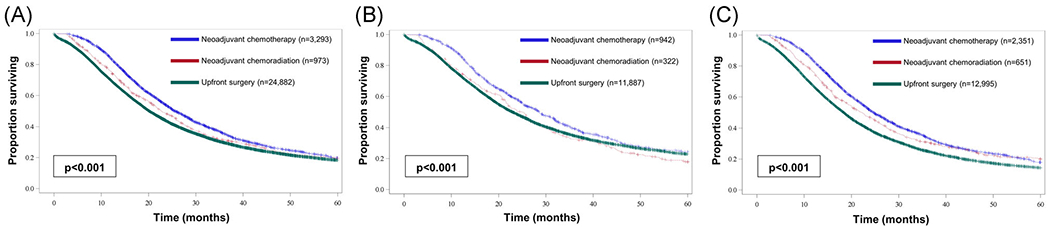
Kaplan-Meier curves comparing overall survival between patients treated with neoadjuvant chemotherapy, neoadjuvant chemoradiation, or upfront surgery among (A) all patients, (B) patients with clinical stage I disease, and (C) patients with clinical stage II disease [Color figure can be viewed at wileyonlinelibrary.com]
Neoadjuvant chemotherapy was also associated with improved median OS on subset analysis when compared to neoadjuvant chemoradiation (clinical stage I: 28.6 vs 25.0 months; Figure 2B; clinical stage II: 25.0 vs 22.9 months; Figure 2C; both P < .0001) and when compared with upfront surgery (clinical stage I: 28.6 vs 22.9 months; Figure 2B; clinical stage II: 24.7 vs 18.4 months; Figure 2C; both P < .0001). On multivariable Cox regression subset analysis for each clinical-stage, neoadjuvant chemotherapy was still associated with improved OS when compared with upfront surgery (clinical stage I: HR, 0.81; 95% CI, 0.74-0.89; P < .001; Table 3 multivariable Cox regression B; clinical stage II: HR, 0.72; 95% CI, 0.68-0.76; P <.001; Table 3 multivariable Cox regression B) or neoadjuvant chemoradiation (clinical stage I: HR, 0.79; 95% CI, 0.68-0.92; P = .002; Table 3 multivariable Cox regression C; clinical stage II: HR, 89; 95% CI, 0.81-0.99; P = .029; Table 3 multivariable Cox regression C). Similar results were obtained when these analyses were performed excluding patients who died within 30 days of curative resection.
4 |. DISCUSSION
Pancreatic cancer is an aggressive malignancy with a high recurrence rate even after pancreaticoduodenectomy. For those with resectable disease, surgery remains the mainstay of treatment. However, resection alone is often not sufficient as studies of recurrence patterns demonstrate that approximately 50% to 60% of patients will recur distantly, 20% to 25% locally, and 15% to 20% both locally and distantly.18–20 As such, multimodal therapies including neoadjuvant chemotherapy or chemoradiation are often employed to improve long-term outcomes, though no trial has been able to establish the superiority of either neoadjuvant treatment approach in resectable disease. As some studies have evaluated the impact of defining the optimal timing between completion of neoadjuvant therapy and the start of curative surgery for pancreatic cancer, the National Comprehensive Cancer Network guidelines currently recommend that definitive surgery is performed within 4 to 8 weeks of completion of radiation to balance optimal tumor downstaging and resultant radiation-induced fibrosis which may make surgery more challenging.21,22 However, no studies have established the impact of earlier initiation of neoadjuvant therapy or upfront surgery on long-term survival in a stage-stratified analysis.23–25 The results of the current analyses demonstrate that initiating any treatment including upfront surgery, neoadjuvant chemotherapy, or neoadjuvant chemoradiation within 6 weeks of diagnosis is associated with improved median survival for both clinical stage I (0-6 weeks: 23.5 months vs 7-12 weeks: 21.8 months; P = .04; Figure 1B) and clinical stage II (0-6 weeks: 19.4 months vs 7-12 weeks: 18.3 months; P = .03; Figure 1C). Although the absolute effect of 1 to 2 months is not large, this difference is comparable with the addition of some standard cancer therapies while not having the adverse effects or costs found with other interventions. When evaluating the optimal initial treatment, our results show that neoadjuvant chemotherapy was associated with improved median OS when compared with neoadjuvant chemoradiation (clinical stage I: 28.6 vs 25.0 months; clinical stage II: 25.0 vs 22.9 months; both P < .0001) and when compared with upfront surgery (clinical stage I: 28.6 vs 22.9 months; clinical stage II: 24.7 vs 18.4 months; both P < .0001).
The concept of time to treatment has been most robustly studied for breast cancer, and these findings have led to the establishment of three time-dependent measures including time between diagnosis and start of chemotherapy less than 120 days for women under 70 with AJCC T1c, stage II/III hormone receptor-negative breast cancer; an interval between diagnosis and start of radiation less than 365 days for women under 70 having breast conservation surgery; and time between diagnosis and start of endocrine therapy less than 365 days for women with AJCC T1c, stage II or III, hormone receptor-positive breast cancer.2,6–11 Despite major strides to answer this important question in breast cancer, there is a knowledge gap regarding this time-to-treatment metric for pancreatic cancer.
Defining a stage-specific time interval from diagnosis to initial treatment can subsequently lead to the development of quality improvement efforts in the medical community to address barriers to timely care to ensure that this metric is consistently met. In addition, the establishment of an optimal time interval on the basis of weeks from an initial cancer diagnosis can allay both clinician and patient concerns that initiating cancer treatment is an urgency. Indeed, on sequential survival analysis, our study failed to identify any time interval shorter than 6 weeks that would result in improved survival. Although there is little doubt that prompt treatment initiation just days after diagnosis may reduce patient anxiety, a 6-week time interval allows adequate preoperative diagnostic and multidisciplinary evaluation and ensures that patients and their families have sufficient time to incorporate anticipated treatment plans into their lifestyles. Given that the majority of patients (92%) underwent treatment before 7 weeks in our study, this metric seems attainable nationwide at all cancer centers. Our results also demonstrate that factors associated with delayed treatment include older age, Black race, Charlson-Deyo score ≥ 2, government insurance, and less educational attainment. Intuitively, it is likely that older patients and those with more comorbidities require a more extensive preoperative workup that may delay cancer treatment. However, clinicians should be aware of these factors to ensure that the evaluation of these patients is expedited when possible.
The initial treatment approach for early-stage pancreatic cancer remains highly varied with some clinicians opting for neoadjuvant therapy regardless of clinical stage and some preferring upfront surgery in those with resectable disease. Proponents of neoadjuvant therapy prefer this approach as it ensures delivery of therapy, may result in downstaging, facilitates a margin negative resection, decreases the risk of positive lymph nodes, and most importantly, allows for improved identification of patients that would not benefit from resection due to occult, micrometastatic disease that may become evident during therapy.12–15 However, there is still no clear consensus regarding the optimal treatment strategy. Data to support the use of neoadjuvant chemotherapy are based on findings from a randomized phase II trial that demonstrated that more patients receiving gemcitabine-based preoperative chemotherapy were able to undergo resection.26 In terms of chemoradiation, the Dutch PREOPANC-1 phase III trial randomized 246 patients with resectable or borderline resectable PDAC to either neoadjuvant chemoradiotherapy followed by surgery and adjuvant gemcitabine or upfront surgery followed by adjuvant gemcitabine.27 Results demonstrated that neoadjuvant chemoradiotherapy achieved a median OS of 16.0 months compared with 14.3 months with upfront surgery and adjuvant chemotherapy in the intention-to-treat analysis (HR, 78; P = .096). On subgroup analysis of patients who were able to undergo resection, the median OS in the neoadjuvant therapy arm was 35.2 months compared with 19.8 months for those who had upfront surgery, likely due to improved selection of surgical candidates. In addition, 89% of patients were able to complete neoadjuvant treatment and achieve a complete resection rate of 72% compared with only 40% in those who underwent upfront surgery.28 There is currently no level I evidence to guide the use of neoadjuvant chemotherapy vs chemoradiation. The Alliance for Clinical Trials in Oncology Trial A02150 sought to answer this question by comparing the efficacy of neoadjuvant mFOLFIRINOX alone and neoadjuvant mFOLFIRINOX combined with hypofractionated SBRT in patients with borderline resectable PDAC, but unfortunately, accrual was suspended in 2018 secondary to reaching the futility boundary of margin positive resections.29
It is important to recognize that though upfront surgery may be considered a viable and reasonable treatment strategy for clinical stage I disease, only 58% of these patients (Table 1) were able to undergo adjuvant therapy in our study, a clinical reality that is also evident in randomized control trials of adjuvant therapy for pancreatic cancer.3,30 This is largely due to delayed recovery from surgery secondary to postoperative complications or inability to tolerate the chemotherapeutic regimen. In addition, nearly 75% of patients with clinical stage I disease (lymph node-negative) were upstaged to pathologic stage II disease (lymph node-positive) postoperatively thus highlighting that clinical staging frequently underestimates disease burden. The inability to preoperatively predict patient tolerance of adjuvant therapy combined with the fact that a majority of patients already have lymph node-positive disease at diagnosis further highlights the fact that most patients will benefit from neoadjuvant systemic therapy without risk for “overtreatment.” In the absence of a clinical trial to guide the optimal approach to multimodal therapy, our results suggest that preoperative chemotherapy may be superior to preoperative chemoradiation or upfront surgery for clinical stage I or II disease.
The results of this study should be interpreted with several limitations predominantly arising from its retrospective design. In addition, there is some selection bias in the treatment cohorts as the neoadjuvant therapy groups only include the select group of patients who fail to demonstrate the progression of disease while on therapy. There are also several limitations inherent to the NCDB including lack of recurrence data or disease-specific survival, and lack of chemotherapy agents and dosage. For pancreatic cancer, however, OS is an optimal surrogate endpoint given the aggressive natural history of this disease.
5 |. CONCLUSION
For pancreatic adenocarcinoma, initiation of therapy within 6 weeks from diagnosis is associated with improved survival, with neoadjuvant chemotherapy being associated with the best survival compared with neoadjuvant chemoradiation or upfront surgery. Future clinical trials that assess different treatment strategies for pancreas cancer should stratify for the time interval between diagnosis and treatment.
Supplementary Material
ACKNOWLEDGMENTS
The research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. It was also supported in parts by the Katz Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
The Abraham J. and Phyllis Katz Foundation
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the National Cancer Database (NCDB). Restrictions apply to the availability of these data, which were used under license for this study. Data are available authors with the permission of the NCDB.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Pancreatic Cancer. 2019. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013; 310(14):1473–1481. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073): 1011–1024. [DOI] [PubMed] [Google Scholar]
- 5.Khorana AA, Tullio K, Elson P, et al. Increase in time to initiating cancer therapy and association with worsened survival in curative settings: a U.S. analysis of common solid tumors. J Clin Oncol. 2017; 35(15_suppl):6557. [Google Scholar]
- 6.Wagner JL, Warneke CL, Mittendorf EA, et al. Delays in primary surgical treatment are not associated with significant tumor size progression in breast cancer patients. Ann Surg. 2011;254(1):119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazda A, Estroff J, Euhus D, et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol. 2010;17(suppl 3): 291–296. [DOI] [PubMed] [Google Scholar]
- 8.Polverini AC, Nelson RA, Marcinkowski E, et al. Time to treatment: measuring quality breast cancer care. Ann Surg Oncol. 2016;23(10): 3392–3402. [DOI] [PubMed] [Google Scholar]
- 9.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012;30(36):4493–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Surgeons. National Accreditation Program For Breast Centers Standards Manual. 2018 ed.Chicago, IL: American College of Surgeons; 2018. [Google Scholar]
- 12.Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8(2):123–132. [DOI] [PubMed] [Google Scholar]
- 13.Tsai S, Erickson B, Dua K, Ritch P, Tolat P, Evans D. Evolution of the management of resectable pancreatic cancer. J Oncol Pract. 2016;12:772–778. [DOI] [PubMed] [Google Scholar]
- 14.Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997; 15(3):928–937. [DOI] [PubMed] [Google Scholar]
- 15.Yeung RS, Weese JL, Hoffman JP, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A phase II study. Cancer. 1993; 72(7):2124–2133. [DOI] [PubMed] [Google Scholar]
- 16.American College of Surgeons. National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb. Accessed October 17, 2018.
- 17.Liu Y, N D, Chao Z, Switchenko JM, Kowalski J. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS® macros. F1000Res. 2019;7:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267(5):936–945. [DOI] [PubMed] [Google Scholar]
- 19.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997; 21(2):195–200. [DOI] [PubMed] [Google Scholar]
- 20.Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35(6):600–604. [DOI] [PubMed] [Google Scholar]
- 21.Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250(4):582–589. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 2.2019). https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed May 8, 2019.
- 23.Chen KT, Devarajan K, Milestone BN, et al. Neoadjuvant chemoradiation and duration of chemotherapy before surgical resection for pancreatic cancer: does time interval between radiotherapy and surgery matter? Ann Surg Oncol. 2014;21(2):662–669. [DOI] [PubMed] [Google Scholar]
- 24.Du D, Su Z, Wang D, Liu W, Wei Z. Optimal interval to surgery after neoadjuvant chemoradiotherapy in rectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer. 2018;17(1):13–24. [DOI] [PubMed] [Google Scholar]
- 25.Kwak YK, Kim K, Lee JH, et al. Timely tumor response analysis after preoperative chemoradiotherapy and curative surgery in locally advanced rectal cancer: a multi-institutional study for optimal surgical timing in rectal cancer. Radiother Oncol. 2016;119(3): 512–518. [DOI] [PubMed] [Google Scholar]
- 26.Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14(7):2088–2096. [DOI] [PubMed] [Google Scholar]
- 27.Versteijne E, van Eijck CHJ, Punt CJA, et al. Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials. 2016;17(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020:JCO1902274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz MHG, Ou FS, Herman JM, et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer. 2017;17(1):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer. Japanese study group of adjuvant therapy for pancreatic cancer. Br J Cancer. 2009;101(6):908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


