Abstract
Background:
Esophageal adenocarcinoma (AC) and squamous cell carcinoma (SCC) have distinct outcomes, treatment strategies, and response profiles to therapy. Adenosquamous carcinoma (ASC) is thought to behave more aggressively than each of its counterparts. The aim of this study is to determine if ASC is best managed as AC or SCC.
Methods:
National Cancer Database (2004-2015) was queried for patients with nonmetastatic esophageal ASC. The analysis was stratified by clinical node-negative (cN0) or clinical node-positive (cN1-3). Treatment was categorized into chemoradiation alone, surgery alone, or preoperative chemoradiation followed by surgery. The primary outcome was 5-year overall survival (OS).
Results:
Among 352 patients, 43% were cN0 (n = 151), 57% were cN1-3 (n = 201) and 55% had chemoradiation alone (n = 194), 15% surgery alone (n = 53), and 30% preoperative chemoradiation (n = 105). Among patients who had preoperative chemoradiation, 20% had pathologic complete response (n = 17). For either cN0 or cN1-3, Charlson-Deyo Comorbidity Index did not differ among the treatment groups(all p > 0.05). On Kaplan-Meier analysis for cN0, treatment with surgery alone had comparable OS to preoperative chemoradiation (47% vs 34%; P = .5) and each had improved OS compared to chemoradiation alone (30%; P = .02; P = .06). On univariate analysis for cN0, clinical T category was not associated with OS. For cN1-3, however, preoperative chemoradiation was associated with improved OS when compared to chemoradiation alone or surgery alone (27% vs 19% vs 0%; P < .001). This persisted when accounting for age and clinical T category (hazard ratio: 0.45; P < .001).
Conclusion:
Esophageal ASC behaves more like AC in response to chemoradiation and survival based on treatment modality. A complete response to chemoradiation is only 20% unlike what has been shown for SCC, where chemoradiation is an acceptable definitive therapy. Esophageal ASC should be managed more like AC.
Keywords: adenocarcinoma, adenosquamous carcinoma, esophageal cancer, esophageal surgery, squamous cell carcinoma
1 |. INTRODUCTION
Esophageal cancer is an increasingly prevalent disease worldwide with approximately 572 000 new cases and 509 000 deaths in 2018.1 In the United States alone, there were an estimated 17 650 new cases in 2019, with a 5-year relative survival rate for all stages combined of 19%.2 The two predominant histological subtypes are adenocarcinoma (AC) and squamous cell carcinoma (SCC), the former with a rising incidence amongst Western countries.3,4 A less common histological subtype called adenosquamous carcinoma (ASC) has been described, which shares features of both AC and SCC.5 This rare tumor comprises approximately 1% of esophageal malignancies and has a higher risk of metastatic disease and worse overall survival (OS) than either of its counterparts.6,7 To date, only one large retrospective study has elucidated the natural history of this malignancy as compared to AC and SCC.6 Due to its rarity, there remains a lack of consensus regarding how this aggressive disease should be managed.
There is growing literature supporting different management strategies for esophageal cancer depending on its histology.8,9 Multiple clinical trials have sought to assess the optimal management of AC, including the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC), PreOperative therapy in Esophagogastric adenocarcinoma Trial (POET), Surgical Resection With or Without Preoperative Chemotherapy in Oesophageal Cancer, French Action Clinique Coordonnées en Cancérologie Digestive, and Chemoradiotherapy for Esophageal Cancer followed by Surgery Study (CROSS) trials.10–16 These have established the role for neoadjuvant therapy, resulting in decreased tumor burden and improved pathologic complete response (pCR) and OS.17,18 Long-term follow-up from the CROSS trial has shown a median OS of 43.2 vs 27.1 months for patients with AC who received neoadjuvant chemoradiotherapy with paclitaxel and carboplatin plus surgery vs surgery alone, respectively.16 As such, many practices now adopt this strategy, although the optimal regimen remains to be determined.
While the treatment of AC is augmented by neoadjuvant therapies, SCC management strategies are driven by its different pathogenesis, tumor biology, and prognosis. For instance, in the CROSS trial, the pCR in patients with SCC vs AC histology was 49% vs 23%, respectively, suggesting better responsiveness of SCC than AC to chemoradiation and potential nonoperative management in appropriately selected patients.15 In fact, some studies have shown little-to-no survival benefit with the addition of surgery for locally advanced SCC.9,19,20 As such, depending on the stage of the cancer, response to neoadjuvant therapy, and comorbidities and functional status of the patient, SCC can be potentially managed nonoperatively, unlike AC where neoadjuvant therapy and surgery are routinely incorporated into the treatment plan.
The different management strategies and outcomes for AC and SCC reflect the impact of histology on the cancer’s natural history and treatment efficacy. There is a clear need for additional data to assess this. Given the shared characteristics of ASC with both AC and SCC, it is difficult to decide whether its treatment should more closely reflect that of AC or SCC. The aim of this study is to determine whether ASC is better managed like AC, SCC, or as a unique entity.
2 |. METHODS
2.1 |. Data source
The National Cancer Database (NCDB) is a prospective data registry sponsored by the American Cancer Society and the American College of Surgeons that collects clinicopathological information from over 1500 Commission on Cancer-accredited facilities nationwide.21 The NCDB was queried for patients with nonmetastatic esophageal cancer from 2004 to 2015. The International Classification of Disease third revision code for adenosquamous histology 8560 was used to extract only patients with this confirmed histology. Patients were excluded if they had confirmed metastatic disease, underwent palliative management or no treatment, had missing clinical nodal status, and/or missing survival status. Demographics collected included age at diagnosis, sex, race, and Charlson-Deyo score. Clinical data collected included tumor grade, tumor size, specific tumor location, clinical and pathologic T and N categories as defined by the American Joint Committee on Cancer (AJCC) 8th Edition, radiation surgery sequence, systemic surgery sequence, last contact or death in terms of months from diagnosis, and vital status. Treatment groups were defined into three categories: (a) chemoradiation alone, (b) surgery alone, or (c) preoperative chemoradiation followed by surgery as defined by the treatment-surgery sequence codes within the NCDB. The primary outcome was OS as defined by months from diagnosis. pCR was defined for any patient who underwent preoperative chemoradiation with a clinical T (any) and/or clinical N (any) tumor that was converted to pathologic T0N0.
2.2 |. Statistical analysis
Data were analyzed using SAS, version 9.4 (SAS Institute), and SAS macros developed at the Biostatistics and Bioinformatics Shared Resource at Winship Cancer Institute.22 Demographic and clinicopathological variables were reported in descriptive statistics. The analysis was stratified by clinical node-negative (cN0) or clinical node-positive (cN1-3) status according to AJCC 8th edition given that lymph-node metastasis was the only independent prognostic factor on multivariable analysis in two reported series.23,24 Furthermore, the patients were separated by the three aforementioned treatment groups (chemoradiation alone, surgery alone, or preoperative chemoradiation followed by surgery). The χ2 test was used for comparison of discrete variables between treatment groups within each strata and the analysis of variance test was used for comparison of continuous variables. OS was calculated as the time from diagnosis to the date of death or date of last available follow-up. Survival was estimated using the Kaplan-Meier (KM) method, and the logrank test was used for comparison of survival between treatment groups within each strata. Univariate Cox regression was used to identify clinicopathologic factors associated with OS. A multivariable Cox regression was produced by controlling for age and clinical T category, if statistically significant on univariate analysis. Results were reported as hazard ratios (HR’s) with a 95% confidence interval (95% CI). Model assumptions were checked and verified.
3 |. RESULTS
3.1 |. Demographic and clinicopathologic characteristics
Initial query of the NCDB yielded a total of 1035 cases of esophageal ASC diagnosed in the United States between 2004 and 2015 (Figure 1) of which 53% (n = 352) had nonmetastatic disease and met inclusion criteria. The mean age was 66 years (±11), 80% were male (n = 281), and the median follow-up was 41 months (interquartile range, 19-73). The clinical stage of presentation included 18% stage I (n = 55), 34% stage II (n = 103), 37% stage III (n = 112), and 11% stage IVa (n = 35). Node status was cN0 in 43% (n = 151), and cN1-3 in 57% (n = 201). Concordance between cN0 and pathologic N0 was 76% while the rate of upstage from cN0 to pathologic N1-3 was 24%. Patient and tumor characteristics of these groups are listed in Table 1. Of all patients with cN0 disease, 49% underwent treatment with chemoradiation alone (n = 74), 28% underwent treatment with surgery alone (n = 43), and 23% underwent treatment with preoperative chemoradiation followed by surgery (n = 34). Of all patients with cN1-3 disease, 60% underwent treatment with chemoradiation alone (n = 120), 5% underwent treatment with surgery alone (n = 10), and 35% underwent treatment with preoperative chemoradiation followed by surgery (n = 71). Among all patients who had preoperative chemoradiation and had clinical and pathologic data available (n = 83), 20% had pCR (n = 17).
FIGURE 1.
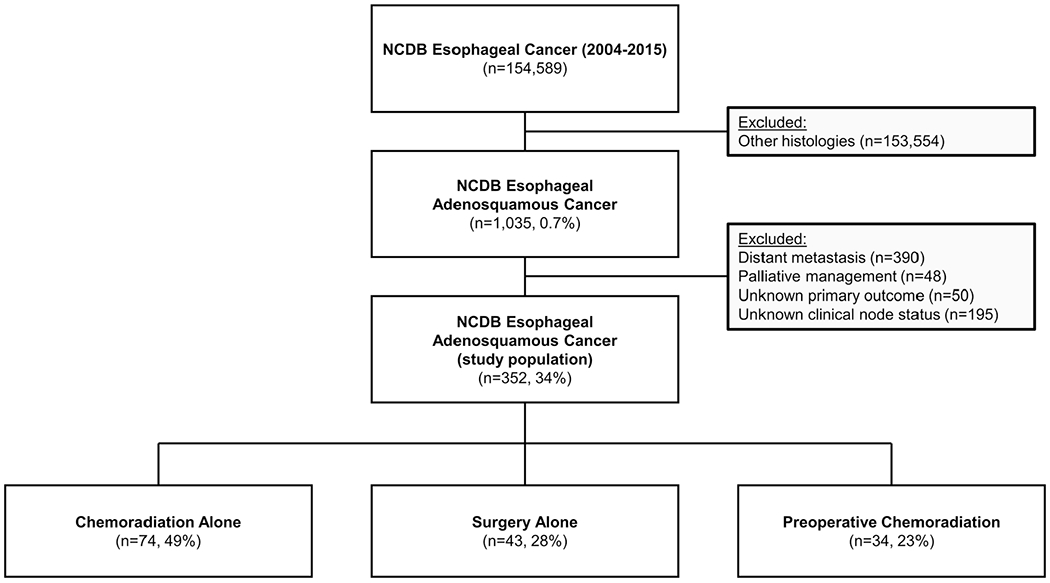
Inclusion/exclusion diagram of the study population. NCDB, National Cancer Database
TABLE 1.
Demographic and clinicopathologic factors of the entire cohort
| All patients n = 352 (%) | Clinical N0 n = 151 (%) | Clinical N1-3 n = 201 (%) | |
|---|---|---|---|
| Age at diagnosis (mean ± standard deviation) | 66 ± 11 | 67 ± 12 | 66 ± 10 |
| Sex | |||
| Male | 281 (80) | 111 (73) | 170 (85) |
| Female | 71 (20) | 40 (27) | 31 (15) |
| Race | |||
| White | 322 (92) | 136 (90) | 186 (93) |
| Black | 20 (6) | 11 (7) | 9 (5) |
| Other | 10 (3) | 4 (3) | 6 (3) |
| Charlson-Deyo Score | |||
| 0 | 232 (66) | 100 (66) | 132 (66) |
| 1 | 88 (25) | 36 (24) | 52 (26) |
| 2+ | 32 (9) | 15 (10) | 17 (9) |
| Tumor size (mean ± standard deviation) | 4.5 ± 2.8 | 3.7 ± 2.1 | 5.1 ± 3 |
| Tumor differentiation | |||
| Well/moderate | 61 (21) | 31 (25) | 30 (18) |
| Poor/undifferentiated | 230 (79) | 95 (75) | 135 (82) |
| Missing | 61 | 25 | 36 |
| Clinical T stage | |||
| T1 | 58 (19) | 44 (35) | 14 (8) |
| T2 | 70 (23) | 40 (32) | 30 (16) |
| T3 | 156 (50) | 39 (31) | 117 (64) |
| T4 | 26 (8) | 4 (3) | 22 (12) |
| Missing | 42 | 24 | 18 |
| Clinical stage | |||
| 0 | 2 (0.6) | 2 (2) | 0 (0) |
| I | 55 (18) | 43 (34) | 12 (7) |
| II | 103 (34) | 78 (61) | 25 (14) |
| III | 112 (37) | 0 (0) | 112 (62) |
| IVa | 35 (11) | 4 (3) | 31 (17) |
| Missing | 45 | 24 | 21 |
| Specific tumor location | |||
| Upper thoracic esophagus | 5 (2) | 3 (4) | 1 (2) |
| Middle thoracic esophagus | 41 (20) | 19 (23) | 2 (18) |
| Lower thoracic esophagus | 132 (64) | 47 (57) | 22 (68) |
| Unknown | 13 (6) | 6 (7) | 7 (6) |
| Missing | 144 | 69 | 75 |
| Lymphovascular invasion | |||
| Present | 25 (31) | 13 (32) | 28 (70) |
| Not present | 56 (69) | 28 (68) | 12 (30) |
| Missing | 271 | 110 | 161 |
| Treatment approach | |||
| Surgery alone | 53 (15) | 43 (29) | 10 (5) |
| Chemoradiation alone | 194 (55) | 74 (49) | 120 (60) |
| Preoperative chemoradiation | 105 (30) | 34 (23) | 71 (35) |
| Follow-up, mo (median, IQR) | 41 (19-73) | 50 (18-84) | 39 (25-55) |
Abbreviation: IQR, interquartile range.
3.2 |. Comparison of demographic and clinicopathologic characteristics by treatment groups
For patients with cN0 disease, those who underwent treatment with chemoradiation alone compared to surgery alone or preoperative chemoradiation were older (70 vs 67 vs 59 years; P < .01). Patients who underwent neoadjuvant chemoradiation had larger tumors (4.53 cm) compared to those who underwent surgery alone (2.95 cm) or chemoradiation alone (3.96 cm; P = 0.01). Notably, Charlson-Deyo Comorbidity Index did not differ among the treatment groups (all P > .05).
Patients with cN1-3 disease were well matched for most clinicopathologic characteristics including Charlson-Deyo Comorbidity Index aside from age (surgery alone: 69 years, chemoradiation alone: 67 years, and preoperative chemoradiation: 63 years; P = .002). A comparison of patient and tumor characteristics among treatment groups is listed in Table 2.
TABLE 2.
Univariate comparison of demographic and clinicopathologic factors by treatment approach
| Surgery alone | Chemoradiation alone | Preoperative chemoradiation | P value | |
|---|---|---|---|---|
| Clinical N0 | n = 43 (%) | n = 74 (%) | n = 34 (%) | |
| Variable | ||||
| Age at diagnosis (mean) | 67 | 70 | 59 | <.01 |
| Male | 33 (77) | 54 (73) | 24 (71) | .82 |
| Race | ||||
| White | 40 (93) | 66 (89) | 30 (88) | .39 |
| Black | 1 (2) | 6 (8) | 4 (12) | |
| Other | 2 (5) | 2 (3) | 0 (0) | |
| Charlson-Deyo score | ||||
| 0 | 24 (56) | 47 (64) | 29 (85) | .06 |
| 1 | 15 (35) | 18 (24) | 3 (9) | |
| 2+ | 4 (9) | 9 (12) | 2 (6) | |
| Tumor size (mean) | 2.95 | 3.96 | 4.53 | .01 |
| Tumor differentiation | ||||
| Well/moderate | 10 (25) | 14 (27) | 7 (21) | .79 |
| Poor/undifferentiated | 30 (75) | 38 (73) | 27 (79) | |
| Clinical T stage | ||||
| T1 | 18 (53) | 19 (31) | 7 (22) | .07 |
| T2 | 11 (32) | 19 (31) | 10 (31) | |
| T3 | 5 (15) | 20 (33) | 14 (44) | |
| T4 | 0 (0) | 3 (5) | 1 (3) | |
| Specific tumor location | ||||
| Upper thoracic esophagus | 0 (0) | 3 (8) | 0 (0) | .05 |
| Middle thoracic esophagus | 3 (14) | 13 (34) | 3 (14) | |
| Lower thoracic esophagus | 15 (68) | 14 (37) | 18 (82) | |
| Clinical N1-3 | n = 10 (%) | n = 120 (%) | n = 71 (%) | |
| Age at diagnosis (mean) | 69 | 67 | 63 | .002 |
| Male | 10 (100) | 99 (83) | 61 (86) | .31 |
| Race | ||||
| White | 10 (100) | 112 (93) | 64 (90) | .82 |
| Black | 0 (0) | 5 (4) | 4 (6) | |
| Other | 0 (0) | 3 (3) | 3 (4) | |
| Charlson-Deyo score | ||||
| 0 | 3 (30) | 81 (68) | 48 (68) | .07 |
| 1 | 4 (40) | 30 (3) | 18 (25) | |
| 2+ | 3 (30) | 9 (8) | 5 (7) | |
| Tumor size (mean) | 3.4 | 5.05 | 5.37 | .20 |
| Tumor differentiation | ||||
| Well/moderate | 1 (10) | 17 (18) | 12 (19) | .78 |
| Poor/undifferentiated | 9 (90) | 76 (82) | 50 (81) | |
| Clinical T stage | ||||
| T1 | 1 (10) | 9 (9) | 5 (6) | .24 |
| T2 | 3 (30) | 15 (14) | 12 (18) | |
| T3 | 6 (60) | 64 (60) | 47 (70) | |
| T4 | 0 (0) | 18 (17) | 4 (6) | |
| Specific tumor location | ||||
| Upper thoracic esophagus | 0 (0) | 1 (1) | 1 (2) | .98 |
| Middle thoracic esophagus | 0 (0) | 15 (20) | 7 (14) | |
| Lower thoracic esophagus | 2 (100) | 48 (64) | 35 (71) | |
Note: Bold value indicates statistical significance.
3.3 |. Survival analysis by treatment group
On KM analysis for all patients, treatment with preoperative chemoradiation followed by resection was associated with the improved 5-year OS when compared to chemoradiation alone (30% vs 18%; P < .001), but not when compared to surgery alone (38%; P = .29; Figure 2).
FIGURE 2.
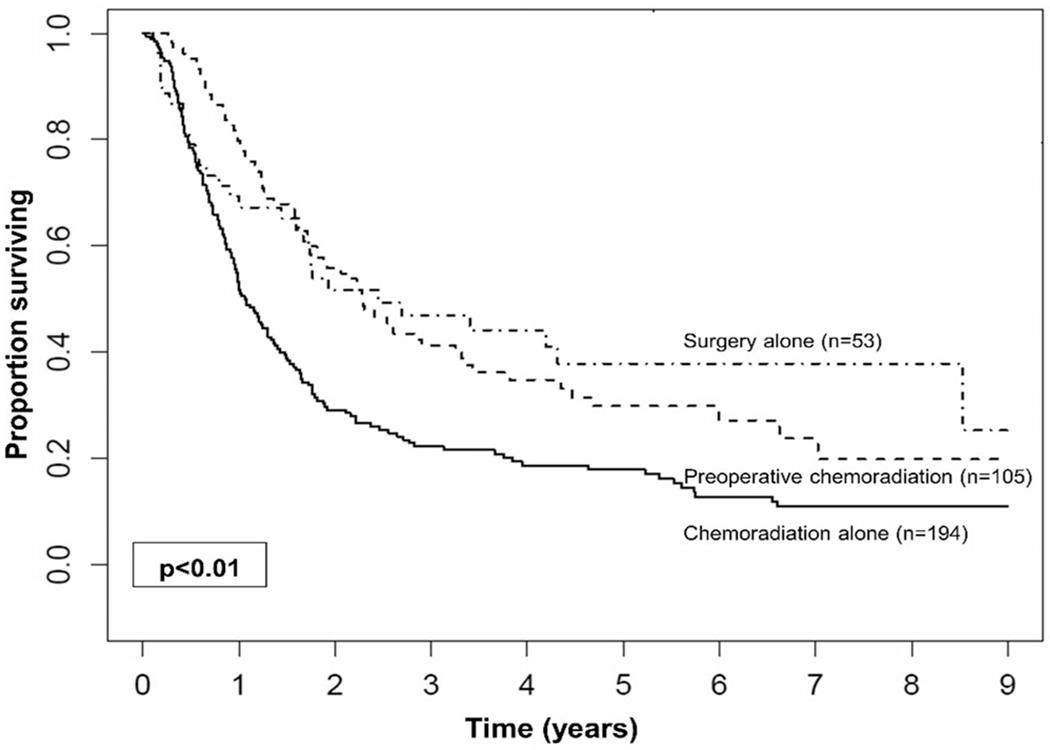
Kaplan-Meier curves for overall survival of all patients compared according to treatment approach modality
On KM analysis for patients with cN0 disease, treatment with surgery alone had a comparable 5-year OS to preoperative chemoradiation (47% vs 34%; P = .5; Figure 3A) and each had improved 5-year OS compared to chemoradiation alone (30%; P = .02; P = .06). On univariate analysis, increasing age was associated with worse OS (HR, 1.02; 95% CI, 1.00-1.04; P = .04) while treatment with surgery alone was associated with improved OS when compared to chemoradiation alone (HR, 0.57; 95% CI, 0.34-0.93; P = .02), and treatment with preoperative chemoradiation was comparable to chemoradiation alone (HR, 0.69; 95% CI, 0.42-1.13; P = .14). Notably, increasing clinical T category was not associated with worse OS. On multivariable analysis, while accounting for age, treatment with surgery alone remained associated with improved OS when compared to chemoradiation alone (HR, 0.59; 95% CI, 0.36-0.97; P = .04; Table 3).
FIGURE 3.
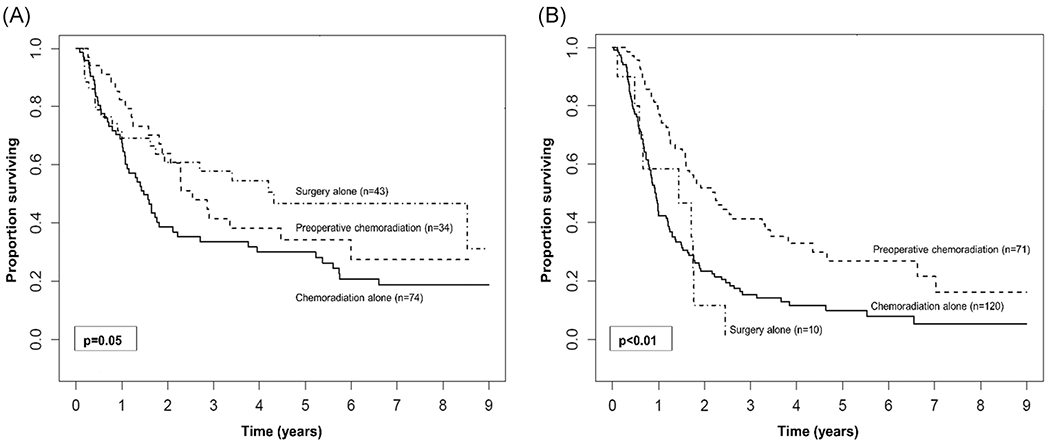
Kaplan-Meier curves for overall survival comparing treatment approach modality in patients with (A) clinically node-negative disease and (B) clinically node-positive disease
TABLE 3.
Cox regression for overall survival
| Univariate regression |
Multivariable regression |
|||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Clinical N0 | ||||
| Age at diagnosis | 1.02 (1.00-1.04) | .04 | 1.02 (1.00-1.04) | .08 |
| Male | 1.45 (0.90-2.33) | .12 | ||
| Race | ||||
| White | Reference | |||
| Black | 1.03 (0.50-2.12) | .94 | ||
| Other | 1.23 (0.39-3.91) | .73 | ||
| Charlson-Deyo score | ||||
| 0 | Reference | |||
| 1 | 0.79 (0.49-1.30) | .36 | ||
| 2+ | 1.49 (0.80-2.76) | .21 | ||
| Tumor size | 1.11 (1.00-1.24) | .05 | ||
| Tumor differentiation | ||||
| Well/moderate | Reference | |||
| Poor/undifferentiated | 1.35 (0.80-2.26) | .26 | ||
| Clinical T stage | ||||
| T1 | Reference | |||
| T2 | 1.09 (0.64-1.85) | .75 | ||
| T3 | 1.43 (0.83-2.47) | .20 | ||
| T4 | 1.13 (0.34-3.73) | .84 | ||
| Treatment group | ||||
| Chemoradiation alone | Reference | Reference | ||
| Surgery alone | 0.57 (0.34-0.93) | .02 | 0.59 (0.36-0.97) | .04 |
| Preoperative chemoradiation | 0.69 (0.42-1.13) | .14 | 0.83 (0.48-1.40) | .46 |
| Clinical N1-3 | ||||
| Age at diagnosis | 1.01 (0.99-1.02 | .33 | 1.00 (0.98-1.01) | .73 |
| Male | 1.36 (0.86-2.14) | .19 | ||
| Race | ||||
| White | Reference | |||
| Black | 0.61 (0.25-1.49) | .28 | ||
| Other | 1.23 (0.50-2.99) | .65 | ||
| Charlson-Deyo score | ||||
| 0 | Reference | |||
| 1 | 0.86 (0.60-1.24) | .42 | ||
| 2+ | 1.50 (0.87-2.59) | .14 | ||
| Tumor size | 1.11 (1.04-1.18) | <.01 | ||
| Tumor differentiation | ||||
| Well/moderate | Reference | |||
| Poor/undifferentiated | 1.53 (0.95-2.47) | .08 | ||
| Clinical T stage | ||||
| T1 | Reference | Reference | ||
| T2 | 1.20 (0.56-2.56) | .64 | 1.06 (0.49-2.29) | .88 |
| T3 | 1.54 (0.80-2.99) | .20 | 1.47 (0.75-2.91) | .27 |
| T4 | 2.17 (1.01-4.66) | .05 | 1.81 (0.83-3.93) | .14 |
| Treatment group | ||||
| Chemoradiation alone | Reference | Reference | ||
| Surgery alone | 1.15 (0.58-2.29) | .68 | 1.18 (0.58-2.41) | .66 |
| Preoperative chemoradiation | 0.45 (0.32-0.64) | <.01 | 0.45 (0.31-0.66) | <.01 |
Note: Bold value indicates statistical significance.
Abbreviations: CI, confidence interval; HR, hazard ratio.
For patients with cN1-3 disease, however, preoperative chemoradiation followed by resection was associated with improved 5-year OS when compared to chemoradiation alone or surgery alone (27% vs 19% vs 0%; P <.001; Figure 3B). On univariate analysis, clinical T4 category was associated with worse OS (HR, 2.17; 95% CI, 1.01-4.66; P = .05) while treatment with preoperative chemoradiation was associated with improved OS when compared with chemoradiation alone (HR, 0.45; 95% CI, 0.32-0.64; P < .01). Notably, treatment with surgery alone was comparable to treatment with chemoradiation alone for cN1-3 categories (HR, 1.15; 95% CI, 0.58-2.29; P = .68). The association between neoadjuvant chemoradiation and improved OS persisted on multivariable analysis even when accounting for age and clinical T category when compared to chemoradiation alone (HR, 0.45; 95% CI, 0.31-0.66; P < .001).
4 |. DISCUSSION
Adenosquamous carcinoma of the esophagus is a rare mixed histology with a propensity for aggressive clinical behavior for which the treatment paradigm remains to be robustly defined. At present, the treatment for this histology is similar to that for other types of esophageal carcinoma in which combined modality therapy has been shown to significantly increase survival. To the best of our knowledge, this is the largest analysis evaluating the impact of treatment modalities on long-term outcomes as data on this rare histology are largely limited to case reports or small case series.
In the current study, most of the patients were male with a mean age of 66 years. The primary lesions were most often found in the lower third of the thoracic esophagus and tended to be poorly differentiated or undifferentiated. Overall, 45% of the patients underwent formal resection, while 55% were treated with definitive chemoradiation. This resection rate is similar to that found in the two largest previously published series on ASC which have demonstrated resection rates up to 37%, a more comparable number to AC than SCC.6,25 As previous studies by Ni et al23 and Zhang et al24 have demonstrated lymph-node metastases to be the only independent prognostic factor for adenosquamous esophageal carcinoma, survival analysis was stratified by cN0 vs cN1-3. For the patients with clinically negative nodes, treatment with surgery alone had comparable 5-year OS to treatment with preoperative chemoradiation (47% vs 34%; P = .5) indicating a clear role for surgery even in low-risk lesions. Conversely, for patients with the cN1-3 disease, preoperative chemoradiation was associated with improved 5-year OS when compared to chemoradiation alone or surgery alone (27% vs 19% vs 0%; P < .001). These outcomes are concordant with previously published data on esophageal AC, where the effectiveness of preoperative chemoradiation and surgery has been clearly demonstrated.19 In addition, the pCR rate among patients who underwent preoperative chemoradiation was 20% which is similar to that of AC as shown in the CROSS trial (SCC 49% vs AC 23%; P = .008).16
As previously mentioned, patients with clinically negative nodes were shown to have a comparable 5-year OS of up to 47% when treated with surgery alone or preoperative chemoradiation suggesting that although preoperative chemoradiation may be pursued for this cohort, local control with esophagectomy may be the main driver of improved outcomes. In addition, due to the low pCR rate of 20%, our results suggest that consideration for upfront esophagectomy may be given to patients who may not be able to tolerate multimodality therapy. In this study, although the patients with cN0 disease did not differ in Charlson-Deyo Comorbidity Index, those who underwent preoperative chemoradiation were younger compared to those who underwent surgery alone (59 vs 67 years; P < .01), indicating that frailty considerations may have played a role in guiding the treatment plan. Given the survival benefit associated with preoperative chemoradiation in the CROSS trial, however, combined modality therapy may still be pursued for patients with cN0 disease. This decision should be made in the context of a multidisciplinary discussion with the goal of achieving adequate oncologic control while avoiding overtreatment.
In cN1-3 disease, however, our results demonstrate the value of multimodal therapy with preoperative chemoradiation and resection much like what has been shown for esophageal AC. In the CROSS trial, the largest trial to date comparing preoperative chemoradiation to surgery alone in patients with resectable esophageal cancers, median OS for AC was 43.2 vs 27.1 months, respectively (P = .038) after a median follow-up of 84.1 months.16 Conversely, the effectiveness of preoperative chemoradiation in patients with esophageal SCC remains controversial. A trial by Stahl et al26 randomized 172 patients with esophageal SCC to receive either induction chemotherapy followed by preoperative chemoradiation plus surgery or induction chemotherapy followed by chemoradiation alone. At a long-term follow-up of 10 years, results demonstrated no difference in 2-year OS between the two arms (39.9% vs 35.4%). The Chemoradiation Followed by Surgery Compared With Chemoradiation Alone in Squamous Cancer of the Esophagus (FFCD 9102) trial also showed that adding surgery to chemoradiation provides little benefit compared to treatment with chemoradiation alone in patients with locally advanced SCC.27 A meta-analysis of randomized controlled trials compared chemoradiation plus surgery with chemoradiation alone in patients with at least T3 and/or N+ thoracic esophageal squamous cancer and the authors19 concluded that the addition of surgery to chemoradiation in locally advanced esophageal SCC has little impact on OS, and may be associated with higher treatment-related mortality.
In contrast to what has been shown for SCC in which definitive therapy with chemoradiation alone may be considered, our findings indicate that single-modality treatment with chemoradiation for patients with esophageal ASC may not be sufficient. Indeed, this treatment group had the worst 5-year OS when compared to surgery alone or preoperative chemoradiation for both cN0 (chemoradiation alone: 30%, surgery alone: 47%, and preoperative chemoradiation: 34%; Figure 3A) and cN1-3 disease (chemoradiation: 0%, surgery alone: 27%, and preoperative chemoradiation: 34%; Figure 3B). Conversely, in a 2003 multi-institutional phase II study by Kato et al28 patients with stage I SCC underwent definitive chemoradiotherapy with a complete response rate of 96% and a 2-year survival rate of 93%. Furthermore, these results have also been shown for locally advanced disease in the previously mentioned trial by Stahl et al26 which demonstrated similar 2-year OS between patients treated with preoperative chemoradiation followed by surgery and those treated with chemoradiation alone. Lastly, for patients with T4 tumors and/or M1 lymph-node disease chemoradiotherapy with carboplatin and paclitaxel is considered standard treatment as survival outcomes following surgery have been historically poor in this select group of patients.29
The findings from this study should be interpreted with some limitations, primarily from its retrospective design. Due to lack of granularity regarding chemotherapy agents, our analysis could not further stratify within each treatment category. In addition, the NCDB lacks data on recurrence or disease-specific survival. However, OS is a commonly used endpoint for aggressive disease processes. Although our study is not propensity-matched due to a low incidence of this histology and resultant sample size, we attempted to create homogenous groups of patients by stratifying by clinical node status.
5 |. CONCLUSION
Esophageal adenosquamous carcinoma behaves more like adenocarcinoma both in response to chemoradiation and survival outcomes based on the treatment modality. The complete response rate to chemoradiation is only 20% unlike what has been shown for squamous cell carcinoma, where chemoradiation may be an acceptable definitive therapy. Esophageal adenosquamous carcinoma should be managed like adenocarcinoma and not squamous cell carcinoma.
ACKNOWLEDGMENTS
This study was supported by The Abraham J. & Phyllis Katz Foundation. The research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under Award Number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data used in the study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Funding information
The Abraham J. & Phyllis Katz Foundation
Footnotes
This manuscript was presented at the 2020 Gastrointestinal Cancer Symposium in San Francisco, CA, and the 2020 Society of Surgical Oncology Meeting.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the National Cancer Database. Restrictions apply to the availability of these data, which were used under license for this study. Data are available with authors permission on the National Cancer Database.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–2053. [PubMed] [Google Scholar]
- 4.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119(6):1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japan Esophageal S Japanese classification of esophageal cancer, 11th edition: part II and III. Esophagus. 2017;14(1):37–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans M, Liu Y, Chen C, et al. Adenosquamous carcinoma of the esophagus: an NCDB-based investigation on comparative features and overall survival in a rare tumor. Oncology. 2017;93(5):336–342. [DOI] [PubMed] [Google Scholar]
- 7.Yachida S, Nakanishi Y, Shimoda T, et al. Adenosquamous carcinoma of the esophagus. Oncology. 2004;66(3):218–225. [DOI] [PubMed] [Google Scholar]
- 8.Mariette C, Finzi L, Piessen G, Van Seuningen I, Triboulet JP. Esophageal carcinoma: prognostic differences between squamous cell carcinoma and adenocarcinoma. World J Surg. 2005;29(1):39–45. [DOI] [PubMed] [Google Scholar]
- 9.Bollschweiler E, Metzger R, Drebber U, et al. Histological type of esophageal cancer might affect response to neo-adjuvant radiochemotherapy and subsequent prognosis. Ann Oncol. 2009;20(2):231–238. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. [DOI] [PubMed] [Google Scholar]
- 11.Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27(6):851–856. [DOI] [PubMed] [Google Scholar]
- 12.Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183–190. [DOI] [PubMed] [Google Scholar]
- 13.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–5067. [DOI] [PubMed] [Google Scholar]
- 14.Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002; 359(9319):1727–1733. [DOI] [PubMed] [Google Scholar]
- 15.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomized controlled trial. Lancet Oncol. 2015;16(9):1090–1098. [DOI] [PubMed] [Google Scholar]
- 17.Donohoe CL, Reynolds JV. Neoadjuvant treatment of locally advanced esophageal and junctional cancer: the evidence-base, current key questions and clinical trials. J Thorac Dis. 2017;9:S697–S704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan KKW, Saluja R, Delos Santos K, et al. Neoadjuvant treatments for locally advanced, resectable esophageal cancer: a network meta-analysis. Int J Cancer. 2018;143(2):430–437. [DOI] [PubMed] [Google Scholar]
- 19.Vellayappan BA, Soon YY, Ku GY, Leong CN, Lu JJ, Tey JC. Chemoradiotherapy versus chemoradiotherapy plus surgery for esophageal cancer. Cochrane Database Syst Rev. 2017;8:Cd010511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310–2317. [DOI] [PubMed] [Google Scholar]
- 21.American College of Surgeons. National Cancer Database https://www.facs.org/quality-programs/cancer/ncdb. Accessed 17 October 2018.
- 22.Nickleach DC, Liu Y, Shrewsberry AB, Ogan K, Kim S-J. SAS macros to conduct common biostatistical analysis and generate reports. In Proc SouthEast SAS Users Group Conf (SESUG 2013), 2013. [Google Scholar]
- 23.Ni P-Z, Yang YS, Hu WP, Wang WP, Yuan Y, Chen LQ. Primary adenosquamous carcinoma of the esophagus: an analysis of 39 cases. J Thorac Dis. 2016;8(10):2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang HD, Chen CG, Gao YY, et al. Primary esophageal adenosquamous carcinoma: a retrospective analysis of 24 cases. Dis Esophagus. 2014;27(8):783–789. [DOI] [PubMed] [Google Scholar]
- 25.Yendamuri S, Malhotra U, Hennon M, et al. Clinical characteristics of adenosquamous esophageal carcinoma. J Gastrointest Oncol. 2017; 8(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310–2317. [DOI] [PubMed] [Google Scholar]
- 27.Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–1168. [DOI] [PubMed] [Google Scholar]
- 28.Kato H, Sato A, Fukuda H, et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol. 2009;39(10):638–643. [DOI] [PubMed] [Google Scholar]
- 29.Higuchi K, Koizumi W, Tanabe S, et al. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest Cancer Res. 2009;3(4):153–161. [PMC free article] [PubMed] [Google Scholar]


