Abstract
Background/purpose
Clinical outcomes in pediatric ulcerative colitis (UC) in the era of biologic agents are poorly defined. We aimed to describe risk factors for colectomy in pediatric UC in the era of infliximab therapy.
Methods
We reviewed 217 pediatric patients at Texas Children’s Hospital with newly diagnosed UC between 2003 and 2015; 117 had a minimum of 5 years of follow-up. Extent of disease at diagnosis, medication exposure, the presence of extraintestinal manifestations (EIMs), and need for surgery were noted.
Results
Average length of follow up was 5.02 ± 2.27 years. Forty-two percent presented with pancolitis. Infliximab was used in 39%, immunomodulators in 65%, and steroids in 89% of patients. EIMs occurred in 24.9% of patients. The cumulative rate of colectomy was 12.9% at 5 years. Children presenting as E2 (Paris Classification) and children prescribed oral steroid monotherapy at diagnosis progressed to surgery faster than any other group. Of the children who received infliximab, females and children less than 5 years old were less likely to respond to therapy.
Conclusions
The natural course of pediatric UC remains aggressive despite the addition of infliximab to the standard of care and suggests a need for early aggressive clinical intervention.
Level-of-evidence rating
Level IV.
Keywords: Natural history, Colectomy, Biologic therapies
The epidemiology of inflammatory bowel disease has been well studied in adults; however, clinical outcomes of pediatric ulcerative colitis (UC) are less understood. Most of the population-based studies have been conducted outside of the United States and prior to the introduction of biologic (anti-tumor necrosis factor α) agents in the standard treatment of pediatric UC [1–6]. Children with UC tend to present with more extensive disease and greater severity at diagnosis than adults [7,8]. Because of the early and aggressive onset of pediatric UC, these children may be at an increased risk of disease complications and the need for surgical intervention [4,9]. In addition, pediatric UC patients have significantly more complications after surgery, including pouchitis and even pouch failure, as compared to adults [8]. It is important that we understand the risk factors for these complications in order to better tailor therapeutic management of the disease.
In 2013, Malaty et al. conducted a retrospective review of pediatric UC patients presenting to Texas Children’s Hospital (TCH) from 1986 to 2003 [4]. They found that the cumulative colectomy rate was 4.1% at 1 year and 14% at 5 years. Twenty percent of the patients had extraintestinal manifestations (EIMs). Boys were twice as likely to undergo colectomy as girls. Age, time between onset of symptoms and diagnosis, and EIMs were not associated with increased risk of colectomy [4]. This analysis took place prior to the use of infliximab in the standard therapy for pediatric UC; therefore, it is unclear if we can apply its findings to our current patient population.
While initial optimism about the efficacy of biologics in IBD was high, it is unclear if outcomes have been significantly improved for these patients. Cannom et al. [10] found that between 1998 and 2005 overall rates of hospitalization for adult IBD patients increased significantly, while overall surgical rates remained unchanged, concluding that the increasing use of infliximab did not have an impact on surgical rates or hospitalizations. A more recent meta-analysis found that infliximab use decreased rates of hospitalization in adult IBD, but the impact on surgery was less clear [11]. Murthy et al. similarly demonstrated that introduction of infliximab has not led to significant declines in the rates of UC-related hospitalization rates or colectomies between 1995 and 2012 [12]. Finally, a pediatric IBD study found that from 2007 to 2012 hospital utilization decreased while surgical utilization increased [13]. Ultimately, the efficacy of biologic medications to decrease surgical rates remains unclear, with multiple studies showing conflicting results [14,15].
We aimed to describe the natural history of pediatric UC and to ascertain the risk factors associated with surgery in patients presenting to TCH in the era of infliximab therapy. We hypothesized that outcomes, including need for surgery, would be unchanged despite the availability and common use of biologics to treat pediatric UC.
1. Materials and methods
1.1. Patient population
Pediatric patients (≤17 years old) who presented with a new diagnosis of UC (using International Classification of Disease, Ninth Revision Clinical Modification codes 556.2, 556.5, 556.6, 556.8 and 556.9) between January 2003 and July 2015 were identified. The clinical (including available small bowel imaging) and histological diagnosis of UC was verified and patients who did not meet these criteria were excluded.
1.2. Data collection
All patient records through July 2015 were reviewed. A follow-up phone call was made to all patients who were lost to follow-up or transitioned to adult care within five years of diagnosis to assess clinical outcomes.
1.3. Outcome measures/variables descriptions
Disease diagnosis was based on clinical, endoscopy, radiology and pathology records. We specifically noted extent of disease at diagnosis, extension of disease at follow-up endoscopy, medication use, presence of EIMs, and need for surgery.
Extent of disease was categorized according to the Paris classification (the pediatric modification of the Montreal classification) [16,17]. Ulcerative proctitis (E1) was defined as involvement limited to the rectum. Left-sided UC (E2) was defined as involvement limited to a portion of the colorectum distal to the splenic flexure. Extensive UC (E3) was defined as involvement that extends proximal to the splenic flexure but distal to the hepatic flexure [16]. Pancolitis (E4) was defined as involvement proximal to the hepatic flexure [17]. Patients with incomplete colonoscopy (terminating in the transverse colon) demonstrating disease extension proximal to the splenic flexure were considered “E3”.
Medications included the following: oral 5-aminosalicylic acid (5-ASA); topical therapies (5-ASA enema or suppository, hydrocortisone foam or enema); corticosteroids (oral prednisone or budesonide, IV methylprednisolone); immunomodulators (azathioprine [AZA], 6-mercaptopurine [6-MP], methotrexate); and biologic therapy (infliximab). Patients were classified as being exposed to a medication if it was prescribed during the follow-up period.
EIMs were defined as arthritis, arthralgia, primary sclerosing cholangitis (PSC), autoimmune hepatitis (AIH), aphthous stomatitis, erythema nodosum and skin lesions. The treating physician determined diagnosis of EIMs.
1.4. Statistical analysis
Qualitative variables are reported numerically and as percentages; quantitative variables were calculated as averages with standard deviations. Data were analyzed using right-censored (to account for children lost to follow-up) Kaplan–Meier survival curves with subsequent log-rank analysis. The primary outcome was defined as colectomy. Predictive variables for colectomy included age, gender, race, extent of disease at diagnosis, EIMs, and medication exposure. Univariate Cox proportional hazards models were used to first identify risk factors for surgery to be included in multivariate Cox analysis — any risk factor with a p b 0.1 was included in the multivariate analysis to account for variable interaction and relative contribution. Hazard ratios (HR) were estimated with their 95% CI. All tests were two-tailed, and statistical significance was considered with p < 0.05. The “Overall” dataset (N = 217) was also used in the Cox proportional hazards analyses after accounting for children lost to follow up. The “Infliximab” dataset of children prescribed anti-TNFα therapy (N = 84) was used to evaluate the efficacy of infliximab therapy in various patient groups. All statistical analyses were performed with RStudio version 0.99.491 with the aid of the ‘survminer,”survival’, and ‘Rcmdr’ analysis packages (versions 0.4.0, 2.40–1, and 2.4–1 respectively).
1.5. Ethical considerations
This study received IRB approval (H-33527).
2. Results
2.1. Population characteristics
Between January 2003 and July 2015, 217 patients with newly diagnosed UC presented to Texas Children’s Hospital (Table 1). Mean age at diagnosis was 11.4 ± 3.9 years, with no significant difference between males and females (11.13 ± 4.2 and 11.72 ± 3.60 respectively, p = 0.27 one-way ANOVA). The average length of follow up was 5.02 ± 2.27 years (median 4.6 years, range 2–15 years). One hundred seventeen patients were followed for over 5 years or progressed to surgery within 5 years of diagnosis. There were 66 (30%) Caucasians, 37 (17%) Hispanics, 18 (8%) African Americans, 7 (3%) Asians, and the remaining 89 (41%) were unknown or chose not to report.
Table 1.
Descriptive statistics of pediatric ulcerative colitis cohort at diagnosis.
| Overall (N = 217) | Treated with Biologics (N = 84) | p-value | |
|---|---|---|---|
| Follow-Up (Years ± Std Dev) | 5.02 ± 2.27 | 4.69 ± 2.06 | 0.09 |
| Mean Age at Diagnosis (Years ± Std Dev) | 11.4 ± 3.9 | 11.5 ± 3.8 | 0.92 |
| Males/Females | 107/110 (49.3% / 50.7%) | 47/37 (55.9%/44.1%) | 0.3 |
| Race | 0.94 | ||
| Caucasian | 66 (30.4%) | 26 (33.3%) | |
| Hispanic | 37 (17.1%) | 13 (15.5%) | |
| African American | 18 (8.3%) | 9 (10.7%) | |
| Asian | 7 (3.2%) | 2 (2.4%) | |
| N/R | 89 (41.0%) | 32 (38.1%) | |
| Extent of Disease | 0.52 | ||
| E1 | 22 (11.9%) | 7 (8.3%) | |
| E2 | 19 (8.8%) | 13 (15.5%) | |
| E3 | 31 (14.4%) | 15 (17.9%) | |
| E4 (Pancolitis) | 91 (42.1%) | 31 (36.9%) | |
| Incomplete | 21 (9.7%) | 7 (8.3%) | |
| Unknown/Not Performed | 33 (15.2%) | 11 (13.1%) | |
| EIMs | 54 (24.9%) | 19 (22.6%) | 0.32 |
EIMS = Extraintestinal Manifestations.
2.2. Extent of disease
Of the 217 patients, 33 (15.2%) patients did not have endoscopic data available from the time of diagnosis. One hundred eighty-four (184, 84.8%) patients had initial endoscopic data available, of which 21 (9.7%) had an incomplete endoscopic evaluation. Twenty-two (22, 10.2%) patients presented at diagnosis with ulcerative proctitis (E1), 19 (8.8%) had left sided disease (E2), 31 (14.4%) had extensive UC (E3), and 91 (42.1%) had pancolitis (E4) (Table 1). Of note, 20 patients were included in the “E3” category who had incomplete colonoscopy, but with evidence of disease extension proximal to the splenic flexure.
2.3. Medication exposure
Initial therapy documentation was not available for 37 (17%) patients. Of the remaining 180 patients, 62.8% were treated with monotherapy at the time of diagnosis: 47.8% 5-ASA, 27.4% oral steroids, and 23% IV steroids. One patient was treated with biologic monotherapy, and one was treated with immunomodulator monotherapy at diagnosis. Thirty-seven percent (37%) of patients received a combination of medications at diagnosis.
Throughout the follow-up period, biologic therapy was used in 38.7% of patients, immunomodulators in 65.4%, oral 5-ASA in 95%, topical therapy (rectal 5-ASA and rectal steroids) in 43.3%, and 89.4% were exposed to systemic steroid therapy (Table 2). All patients who received biologics were treated with infliximab as their initial biologic therapy. Twelve patients (5.5%) also received adalimumab throughout the follow up period, and 5 patients (2.3%) were exposed to more than 2 biologics throughout the follow up period. Average time to infliximab exposure was 1.98 years from diagnosis, with some evidence that females were being prescribed infliximab earlier in their disease course than males (1.49 years versus 2.35 years respectively, p = 0.07, one-way ANOVA).
Table 2.
Medication exposure in pediatric ulcerative colitis patients.
| Overall cohort (N = 217) | Treated with biologics (N = 84) | p-value | |
|---|---|---|---|
| Topical Therapy | 94 (43.3%) | 45 (53.6%) | 0.9 |
| Hydrocortisone | 14 (14.9%) | 7 (15.6%) | |
| Mesalamine | 67 (71.3%) | 33 (73.3%) | |
| Both | 13 (13.8%) | 5 (11.1%) | |
| Steroids | 194 (89.4%) | 84 (100%) | 0.002 |
| 5-Aminosalycylic Acid | 205 (94.5%) | 79 (94.0%) | 0.89 |
| Immunomodulators | 142 (65.4%) | 62 (73.8%) | 0.37 |
| 6-mercaptopurine | 93 (65.5%) | 38 (61.3%) | |
| Azathioprine | 29 (20.4%) | 8 (12.9%) | |
| Biologics (Infliximab) | 84 (38.7%) | -- | -- |
2.4. Extraintestinal manifestations
EIMs occurred in 54 patients (24.9%). In respect to all patients, 4% had arthritis, 4% had arthralgia, 11% had primary sclerosing cholangitis, 4% had autoimmune hepatitis, and <1% had aphthous stomatitis. One patient (<1%) had psoriasis, but no patients were diagnosed with erythema nodosum or other skin lesions. Development of EIMs was not associated with age, gender, race, or extent of disease at diagnosis (data not shown).
2.5. Progression to colectomy
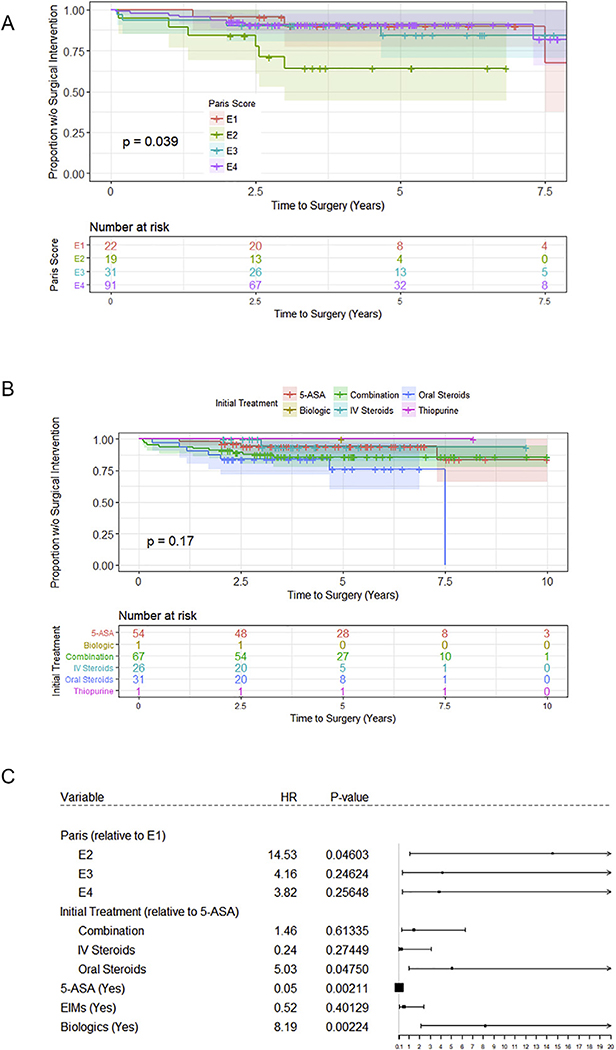
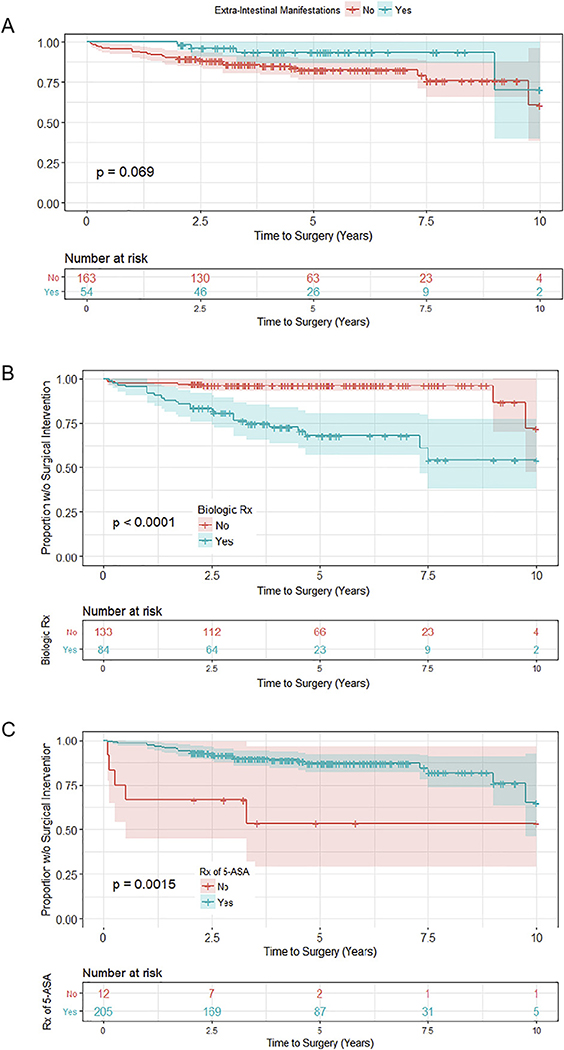
The cumulative rate of colectomy in our cohort was 4.6% by 1 year after diagnosis, 8.3% by 2 years, 10.6% by 3 years, and 12.9% within 5 years of diagnosis. All patients underwent colectomy for fulminant/severe, medically refractory colitis. There were no cases of toxic megacolon, and no cases were elective (data not shown). Additionally, all patients who required colectomy, received it prior to transition to adult care. Several factors were found to influence progression to surgery, as measured by univariate Cox regression (See Supplementary Fig. 1), in the 217 children included in the study. These included Paris Classification at diagnosis (Fig. 1A), initial treatment received (Fig. 1B), EIMS (Fig. 2A), infliximab exposure (Fig. 2B), and 5-ASA exposure (Fig. 2C). Following multivariate analysis, only Paris Classification, initial treatment at diagnosis, exposure to 5-ASA, and exposure to infliximab remained as significant predictors of colectomy within 5 years of diagnosis (Fig. 1C).
Fig. 1.
Risk factors for progression to colectomy in pediatric ulcerative colitis patients. (A) Paris classification at diagnosis and (B) initial treatment received significantly influenced progression to surgery. (C) Multivariate analysis demonstrated that children who presented with E2 disease (by Paris classification), those who received only oral steroids at diagnosis, those who received 5-ASA treatment throughout their course of disease, and those who received biologic medications progressed to colectomy the fastest.
Fig. 2.
Additional risk factors in progression to surgery. (A) Extraintestinal manifestations (EIMS), (B) exposure to biologic medications, and (C) 5-ASA exposure influenced progression to colectomy by univariate analysis.
Children who presented as E2 progressed to surgery 14.5 times faster than those who presented as E1 and approximately 3.5 times faster than children who presented as either E3 or E4 (p = 0.04, Fig. 1C). As for the effects of induction therapy, those patients initially treated with oral steroid monotherapy progressed to surgery faster than those prescribed 5-ASA, IV steroids, or combination therapy as first line medications (p < 0.05, Fig. 1C). Patients with any exposure to 5-ASA were less likely to progress to colectomy compared to those not exposed to this drug class (p < 0.01, Fig. 1C).
Patients prescribed infliximab at any time in the course of their disease progressed to colectomy 8.2 times faster than those not receiving biologics (p < 0.01, Fig. 1C). However, it is important to note that most children who received infliximab and ultimately underwent colectomy were prescribed infliximab therapy within 1 year of surgical intervention (data not shown). As demonstrated in Supplementary Fig. 2, patients who were prescribed anti-TNF biologic within 3 months of diagnosis had a lower incidence of surgery than those who were prescribed biologics later in their disease course (between 3 months and 2 years).
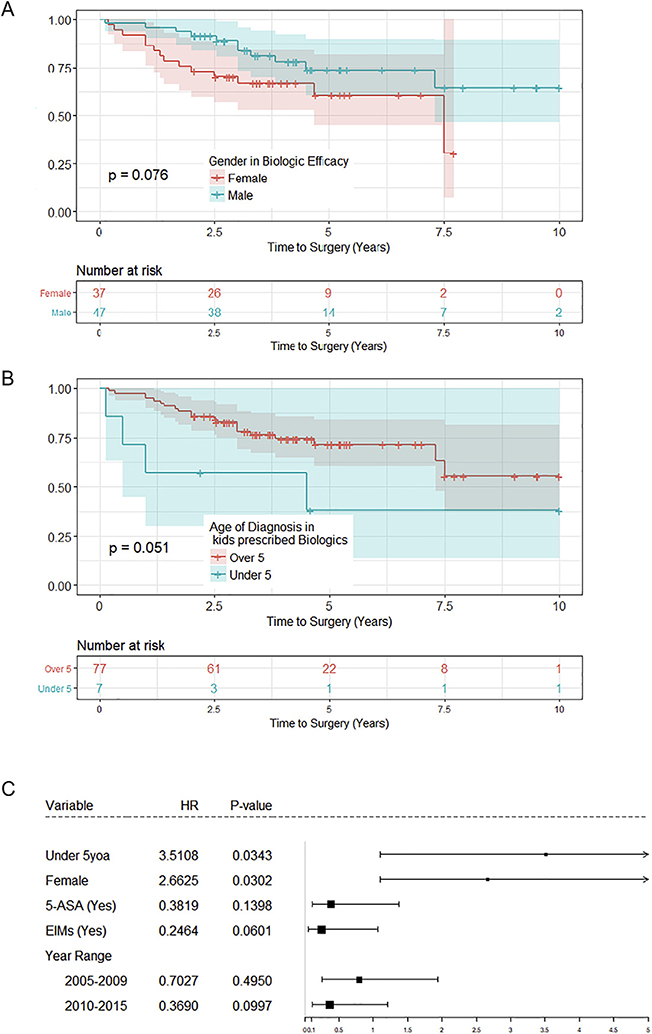
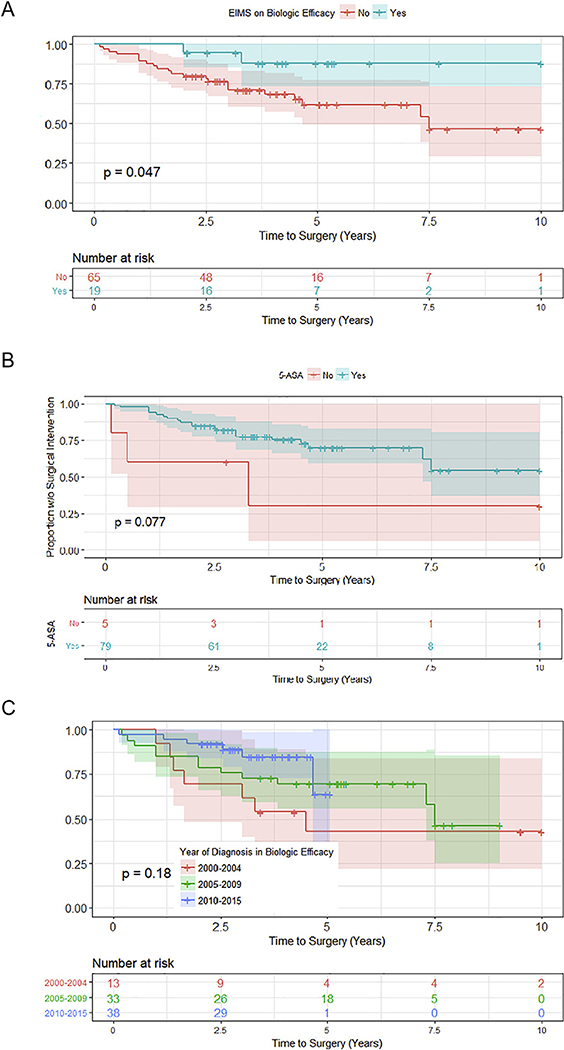
Several factors were found to influence progression to surgery in children who were prescribed infliximab, as measured by univariate Cox regression (See Supplementary Fig. 3). These included gender (Fig. 3A), age (Fig. 3B), development of EIMS (Fig. 4A) exposure to 5-ASA (Fig. 4B), and year of diagnosis (Fig. 4C). Following multivariate analysis, however, only age at diagnosis and gender remained significant (Fig. 3C). Of the children prescribed infliximab, those diagnosed with UC less than the age of 5 progressed to surgery 3.5 times faster than patients diagnosed at a later age (p = 0.03, Fig. 3C). Furthermore, females were less likely to respond to infliximab therapy and progressed to surgery 2.7 times faster than their male peers (p = 0.03, Fig. 3C).
Fig. 3.
Factors influencing progression to surgery in children receiving biologics (infliximab). (A) Gender and (B) age significantly influenced progression to surgery in children receiving infliximab therapy. (C) Multivariate analysis demonstrated that females and children less than the age of 5 years progressed to colectomy significantly faster than other children.
Fig. 4.
Additional factors influencing progression to surgery in children receiving biologics. (A) Extraintestinal manifestations (EIMS), (B) exposure to 5-ASA medications, and (C) year of diagnosis influenced progression to colectomy by univariate Cox regression in children receiving biologic medications.
3. Discussion
This is one of the largest cohorts of pediatric UC patients in the United States presenting in the infliximab era with 5 years of clinical follow up reported. The strengths of this study include its single center design, as well as the availability of detailed clinical records. Significant variation in the diagnostic accuracy and clinical care practices for IBD has been well described in the literature [18–20]. The largest prospective study in pediatric IBD to date also underscored significant intercenter practice variation [21]. A single center design with similar IBD care practices can limit multicenter bias [22]. Additionally, Texas Children’s Hospital (TCH) is one of the largest children’s hospitals in the nation with a diverse patient population, which has already allowed for unique demographic and clinical observations in pediatric IBD [23–25].
Our findings indicate that the natural course of pediatric UC remains highly morbid despite the addition of infliximab to the standard care for these patients. Infliximab use at TCH increased when it became approved for pediatric Crohn’s disease in 2006, but was available for use before that. From 2003 to 2015, it has been used for steroid-refractory and steroid-dependent UC, for both induction and maintenance therapy in these patients. Importantly, incidence of pediatric IBD has remained unchanged in the Houston Metro Area over recent years [25]. The colectomy rate for our patients was 4.6% by one year, and 12.9% by 5 years after diagnosis. These rates are in agreement with previously reported colectomy rates at our institution in the prebiologic era (4.1% at 1 year, 14% at 5 years) [4]. Notably, nationwide database surveys indicate a stable incidence of colectomy rates in pediatric UC patients over the prior decade, in spite of the use of biologic agents [26]. It is unclear if this is a reflection of more severe disease over time, or evidence that biologic therapy is not efficacious in significantly modulating the disease course in those pediatric UC patients who become refractory to available treatments within 5 years of diagnosis.
Our current study suggests that children who present with left-sided UC (E2) progress to surgery significantly faster than those with any other Paris Classification. Previous observations have shown that the majority of pediatric UC patients will ultimately progress to have extensive disease, regardless of initial presentation [4,9]. This emphasizes that extent of disease at diagnosis cannot be used alone to predict outcome in pediatric UC patients, and limited but intense colonic disease beyond the rectum may warrant as aggressive therapy as similarly active pancolitis. Gender and age at presentation were not associated with need for surgery in our cohort, which is in agreement with some previously reported studies [9,27], but not consistent with Malaty et al, who found that in the prebiologic era, males were twice as likely to require a colectomy as females [4].
A high percentage of our patients (42.1%) presented with pancolitis (E4) at diagnosis, with an additional 14.4% presenting with extensive (E3) disease. The percent of pediatric patients with extensive disease at diagnosis varies across different studies, with some reporting >80% [28,29] and others reporting 35%–43% [4,9,30]. One possible explanation for this discrepancy is that patients with less severe presenting symptoms are being referred to private care facilities, as opposed to tertiary care centers. However, this would not explain the discrepancy between disease presentation observed by Malaty, et al. (25% E1, 40% E2, 35% E3) [4] and our cohort (10.2% E1, 8.8% E2, 56.5% E3 or greater), as both findings are from the same tertiary care center, but during different time periods. Therefore, UC presentation may be shifting towards more extensive colonic disease in children of the Southern United States.
Along with location of disease, initial treatment at diagnosis was also associated with progression to colectomy. Children who received only oral steroids as their first line of treatment progressed to surgery 5 times faster than children who were prescribed 5-ASA or combination therapy (HR = 5.03, p < 0.05). Children who received oral steroid monotherapy were likely deemed more severe than those started on 5-ASA monotherapy, but not severe enough to require admission and IV steroid therapy or combination therapy with immunomodulators. It is possible that these patients would have benefitted from more aggressive initial therapy. These findings suggest that a more aggressive initial therapeutic intervention may improve long-term outcomes in children with UC. Importantly, children who received infliximab had a substantially greater risk (HR = 8.19, p < 0.01) of progressing to colectomy than those who did not receive infliximab, regardless of their location of disease at diagnosis. It is logical that children with more severe disease are both more likely to receive infliximab and more likely to require colectomy throughout the course of their disease. In fact, the majority of children were prescribed infliximab therapy within 1 year of colectomy, reflecting its use as a final therapeutic option in an effort to avoid surgery in patients with severe UC.
It is worth noting that patients who were prescribed biologics within 3 months of diagnosis had a lower incidence of surgery than those prescribed biologics between 1 and 2 years after diagnosis. This implies that more aggressive use of infliximab earlier in the disease course could improve outcomes in pediatric UC, especially for patients with severe disease who will likely need initiation of biologic therapy within 2 years of diagnosis. This is supported by a recent retrospective review which found that the use of infliximab was independently associated with a reduction in 2-year colectomy rates in pediatric ulcerative colitis [31]. A recent multicenter study identifying outcomes following standardized therapy after initial diagnosis of UC also supports this conclusion [32]. These and our findings suggest that patients presenting with more severe disease may warrant more aggressive therapeutic interventions early in the course of their disease.
This must be balanced by the recent finding that hospitalized patients with UC (adult and pediatric) who received accelerated infliximab dosing after initial standard infusion dosing (5 mg/kg) had a higher 30-day colectomy rate [33]. The authors proposed that these patients had a high inflammatory burden and altered infliximab pharmacokinetics leading to more rapid drug clearance, and may have benefited from high dose (10 mg/kg) initial treatment to decrease the risk of colectomy. This further emphasizes the potential importance of aggressive initial therapy in the setting of severe disease.
Interestingly, patients who did not initiate infliximab therapy for at least 2 years after diagnosis were also less likely to progress to surgery in our study, indicating that disease may be more responsive to therapy in patients who remain naive to anti-TNF therapy for at least 2 years after diagnosis. This is in agreement with a study by Mir et al. [34], which demonstrated that initiation of infliximab within 20 months of disease onset increased the likelihood of colectomy within one year, whereas those with longer disease duration at time of infliximab initiation were more likely to respond to therapy.
Additionally, we found that females treated with infliximab progressed to surgery significantly faster than males. This signifies that infliximab may be more effective in preventing surgery in males than in females. This finding has not been previously reported, and it will be important to investigate sex differences in the effectiveness of infliximab in future studies of pediatric UC. It is worth noting that a recent study investigating the effect of initial infliximab induction dose on 30-day colectomy rate found that females had a significantly increased odds of needing accelerated induction of infliximab compared to males [33]. This supports our finding that standard-dose infliximab is not as effective in females with UC. Also, in our cohort of patients, children younger than 5 years did not appear to benefit from infliximab therapy as much as older children. Clinically, such early-onset disease often has a genetic component and is substantially more aggressive, which likely contributed to this nonresponse to therapy [35] as has been further indicated in a recently published cohort [36].
Infliximab wasn’t approved by the Food and Drug Administration (FDA) for use in pediatric UC until 2011. Until recently, infliximab has been reserved for use in acute severe UC. However, with increasing reluctance to use immunomodulators (specifically thiopurines) in this population owing to concerns of malignancy induction [37,38], infliximab use is likely increasing, along with its use earlier on in the course of pediatric UC. Additionally, the way that infliximab is dosed in clinical practice has evolved over recent years, with a new emphasis on therapeutic drug monitoring [39,40] and mucosal healing as a target [41–43]. It is possible that these changes have led to overall higher and more aggressive infliximab dosing early on. It will be important for large, multicenter, placebo controlled trials to evaluate how these changes have affected colectomy rate in this patient population over time.
Although this is one of the largest pediatric UC cohorts in the United States with more than 5 years of clinical follow-up, we acknowledge the inherent limitations of a retrospective clinical study. No specific protocol was followed for the treatment of these patients, but the single center experience does provide advantages in this respect over multicenter retrospective explorations. Given the well documented intercenter practice variation that exists [21], it is likely that even without a specific protocol to follow, general practice within a single center will be more similar than between centers. Incomplete endoscopic data at the time of diagnosis restricted our sample size for certain analyses. It is not uncommon for physicians to limit the extent of a conoloscopic exam in the face of severe mucosal disease to decrease risks to the patient. Many of these patients had evidence of disease beyond the splenic flexure; however, because the colonoscopic examination did not extend to the ascending colon, we could not determine which of these patients presented with pancolitis by Paris Classification standards. Additionally, we were unable to stratify based on clinical disease severity, as PUCAI scores were not available for all patients. Similarly, race/ethnicity was not reported in 41% of our patients, and initial therapy was unknown in 17%. Finally, owing to the retrospective nature of this study, diagnosis of EIMs were based upon available records, and standard criteria for diagnosis could not be applied. Given these limitations, there is certainly risk for bias in the interpretation of our results.
3.1. Conclusion
Our study demonstrates that pediatric UC remains with unchanged 1- and 5-year colectomy rates, despite the addition of infliximab therapy to the standard of care for these patients. Based on our findings, early use of biologics (within 3 months of diagnosis) may decrease the risk of colectomy in pediatric UC. Our work also suggests that infliximab therapy may not be as effective in certain patient populations, including females and young children. Recent changes in infliximab dosing regimens based on therapeutic drug monitoring and mucosal healing as a target may have led to more intense and aggressive dosing of infliximab earlier on, which could affect colectomy rates in this patient population. Future studies with national level data may confirm and strengthen our findings. This work emphasizes the need for the development of novel preventative and therapeutic measures to combat pediatric UC.
Supplementary Material
Acknowledgments
Funding
This work was supported by philanthropic support from the Gutsy Kids Fund supported by the Karen and Brock Wagner family and by the Klaasmeyer family [to R.K.]; and the National Institutes of Health Training Grant [T32 DK007664-24S1 to F.D.I.]. These funding bodies played no role in the design of the study, the collection, analysis and interpretation of data, writing of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Declarations of interest
None.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpedsurg.2020.01.054.
References
- [1].da Silva BC, Lyra AC, Rocha R, et al. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol 2014;20:9458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].El Mouzan MI, Saadah O, Al-Saleem K, et al. Incidence of pediatric inflammatory bowel disease in Saudi Arabia: a multicenter national study. Inflamm Bowel Dis 2014;20:1085–90. [DOI] [PubMed] [Google Scholar]
- [3].Urlep D, Trop TK, Blagus R, et al. Incidence and phenotypic characteristics of pediatric IBD in northeastern Slovenia, 2002–2010. J Pediatr Gastroenterol Nutr 2014;58:325–32. [DOI] [PubMed] [Google Scholar]
- [4].Malaty HM, Abraham BP, Mehta S, et al. The natural history of ulcerative colitis in a pediatric population: a follow-up population-based cohort study. Clinical and experimental gastroenterology 2013;6:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schildkraut V, Alex G, Cameron DJ, et al. Sixty-year study of incidence of childhood ulcerative colitis finds eleven-fold increase beginning in 1990s. Inflamm Bowel Dis 2013;19:1–6. [DOI] [PubMed] [Google Scholar]
- [6].Jakobsen C, Paerregaard A, Munkholm P, et al. Pediatric inflammatory bowel disease: increasing incidence, decreasing surgery rate, and compromised nutritional status: a prospective population-based cohort study 2007–2009. Inflamm Bowel Dis 2011; 17:2541–50. [DOI] [PubMed] [Google Scholar]
- [7].Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric ibd consortium registry. J Pediatr 2005;146:35–40. [DOI] [PubMed] [Google Scholar]
- [8].Hirata A, Uchino M, Bando T, et al. Long-term outcomes and sex differences after restorative proctocolectomy in pediatric patients with ulcerative colitis. J Pediatr Surg 2016;51:454–60. [DOI] [PubMed] [Google Scholar]
- [9].Gower-Rousseau C, Dauchet L, Vernier-Massouille G, et al. The natural history of pediatric ulcerative colitis: a population-based cohort study. Am J Gastroenterol 2009; 104:2080–8. [DOI] [PubMed] [Google Scholar]
- [10].Cannom RR, Kaiser AM, Ault GT, et al. Inflammatory bowel disease in the United States from 1998 to 2005: has infliximab affected surgical rates? Am Surg 2009; 75:976–80. [PubMed] [Google Scholar]
- [11].Costa J, Magro F, Caldeira D, et al. Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2013;19:2098–110. [DOI] [PubMed] [Google Scholar]
- [12].Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: a population-based interrupted time series study. Gut; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park KT, Sin A, Wu M, et al. Utilization trends of anti-tnf agents and health outcomes in adults and children with inflammatory bowel diseases: a single-center experience. Inflamm Bowel Dis 2014;20:1242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sokol H, Seksik P, Cosnes J. Complications and surgery in the inflammatory bowel diseases biological era. Curr Opin Gastroenterol 2014;30:378–84. [DOI] [PubMed] [Google Scholar]
- [15].Olivera P, Spinelli A, Gower-Rousseau C, et al. Surgical rates in the era of biological therapy: up, down or unchanged? Curr Opin Gastroenterol 2017;33:246–53. [DOI] [PubMed] [Google Scholar]
- [16].Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- [17].Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- [18].Farmer M, Petras RE, Hunt LE, et al. The importance of diagnostic accuracy in colonic inflammatory bowel disease. Am J Gastroenterol 2000;95:3184–8. [DOI] [PubMed] [Google Scholar]
- [19].Ananthakrishnan AN, Kwon J, Raffals L, et al. Variation in treatment of patients with inflammatory bowel diseases at major referral centers in the United States. Clin Gastroenterol Hepatol 2015;13:1197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kronman MP, Gerber JS, Prasad PA, et al. Variation in antibiotic use for children hospitalized with inflammatory bowel disease exacerbation: a multicenter validation study. J Pediatric Infect Dis Soc 2012;1:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Krishnakumar C, Ballengee CR, Liu C, et al. Variation in care in the management of children with crohn’s disease: data from a multicenter inception cohort study. Inflamm Bowel Dis 2019;25:1208–17. [DOI] [PubMed] [Google Scholar]
- [22].Shiau H, Ihekweazu FD, Amin M, et al. Unique inflammatory bowel disease phenotype of pediatric primary sclerosing cholangitis: a single-center study. J Pediatr Gastroenterol Nutr 2017;65:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hattar LN, Abraham BP, Malaty HM, et al. Inflammatory bowel disease characteristics in hispanic children in Texas. Inflamm Bowel Dis 2012;18:546–54. [DOI] [PubMed] [Google Scholar]
- [24].Malaty HM, Fan X, Opekun AR, et al. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr 2010;50:27–31. [DOI] [PubMed] [Google Scholar]
- [25].Krishna M, Salako A, Fofanova T, et al. Parental education may differentially impact pediatric inflammatory bowel disease phenotype risk. Inflamm Bowel Dis; 2019. [DOI] [PubMed] [Google Scholar]
- [26].Debruyn JC Soon IS, Hubbard J, et al. Nationwide temporal trends in incidence of hospitalization and surgical intestinal resection in pediatric inflammatory bowel diseases in the United States from 1997 to 2009. Inflamm Bowel Dis 2013;19: 2423–32. [DOI] [PubMed] [Google Scholar]
- [27].Kelley-Quon LI, Jen HC, Ziring DA, et al. Predictors of proctocolectomy in children with ulcerative colitis. J Pediatr Gastroenterol Nutr 2012;55:534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kugathasan S, Judd RH, Hoffmann RG, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a state-wide population-based study. J Pediatr 2003;143:525–31. [DOI] [PubMed] [Google Scholar]
- [29].Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008; 135:1114–22. [DOI] [PubMed] [Google Scholar]
- [30].Hyams JS, Davis P, Grancher K, et al. Clinical outcome of ulcerative colitis in children. J Pediatr 1996;129:81–8. [DOI] [PubMed] [Google Scholar]
- [31].Bolia R, Rajanayagam J, Hardikar W, et al. Impact of changing treatment strategies on outcomes in pediatric ulcerative colitis. Inflamm Bowel Dis 2019;25:1838–44. [DOI] [PubMed] [Google Scholar]
- [32].Hyams JS, Davis S, Mack DR, et al. Factors associated with early outcomes following standardised therapy in children with ulcerative colitis (protect): a multicentre inception cohort study. Lancet Gastroenterol Hepatol 2017;2:855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shah SC, Naymagon S, Panchal HJ, et al. Accelerated infliximab dosing increases 30-day colectomy in hospitalized ulcerative colitis patients: a propensity score analysis. Inflamm Bowel Dis 2018;24:651–9. [DOI] [PubMed] [Google Scholar]
- [34].Mir SA, Nagy-Szakal D, Smith EO, et al. Duration of disease may predict response to infliximab in pediatric ulcerative colitis. J Clin Gastroenterol 2014;48:248–52. [DOI] [PubMed] [Google Scholar]
- [35].Kelsen JR, Baldassano RN. The role of monogenic disease in children with very early onset inflammatory bowel disease. Curr Opin Pediatr 2017;29:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kelsen JR, Conrad MA, Dawany N, et al. The unique disease course of children with very early onset-inflammatory bowel disease. Inflamm Bowel Dis; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol 2015;13:847–58 e844; quiz e848–850. [DOI] [PubMed] [Google Scholar]
- [38].Hyams JS, Dubinsky MC, Baldassano RN, et al. Infliximab is not associated with increased risk of malignancy or hemophagocytic lymphohistiocytosis in pediatric patients with inflammatory bowel disease. Gastroenterology 2017;152:1901–14 e1903. [DOI] [PubMed] [Google Scholar]
- [39].Singh N, Rosenthal CJ, Melmed GY, et al. Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2014;20:1708–13. [DOI] [PubMed] [Google Scholar]
- [40].Lega S, Phan BL, Rosenthal CJ, et al. Proactively optimized infliximab monotherapy is as effective as combination therapy in IBD. Inflamm Bowel Dis 2019;25:134–41. [DOI] [PubMed] [Google Scholar]
- [41].Santha SL, Shankar PR, Pan A, et al. Mucosal healing in clinical practice: a single-center pediatric IBD experience. Inflamm Bowel Dis 2017;23:1447–53. [DOI] [PubMed] [Google Scholar]
- [42].Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016;65:408–14. [DOI] [PubMed] [Google Scholar]
- [43].Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.