Abstract
The human choroidal vasculature is subject to age-related structural and gene expression changes implicated in age-related macular degeneration (AMD). In this study, we performed both bulk and single-cell RNA sequencing on infant (n = 4 for bulk experiments, n = 2 for single-cell experiments) and adult (n = 13 for bulk experiments, n = 6 for single-cell experiments) human donors to characterize how choroidal gene expression changes with age. Differential expression analysis revealed that aged choroidal samples were enriched in genes encoding pro-inflammatory transcription factors and leukocyte transendothelial cell migration adhesion proteins. Such genes were observed to be differentially expressed specifically within choroidal endothelial cells at the single-cell level. Immunohistochemistry experiments support transcriptional findings that CD34 is elevated in infant choriocapillaris endothelial cells while ICAM-1 is enriched in adults. These results suggest several potential drivers of the pro-inflammatory vascular phenotype observed with advancing age.
Keywords: single-cell, choroid, choriocapillaris, pericytes, infant, age-related macular degeneration
Graphical Abstract
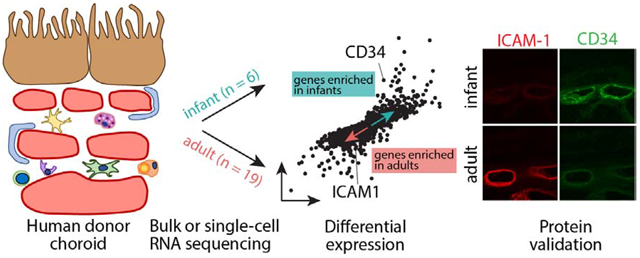
Introduction:
Age-related macular degeneration (AMD) is among the most common causes of irreversible vision loss in the western world. In AMD, photoreceptor cells in the central portion of the retina (the macula) degenerate, leading to a loss of central visual acuity required for sharp, detailed vision. Beneath the retina, a heterogenous vascular tissue known as the choroid extensively supports retinal physiology. Like other connective tissues, the choroid is comprised of diverse cell types including fibroblasts, melanocytes, resident leukocytes, and pericytes/smooth muscle cells. Yet most prominently, the choroid contains a rich vascular system that supplies approximately 85% of blood to the outer retina [1], with the majority of blood flowing through the superficial capillary network known as the choriocapillaris. Histological and imaging data suggest that choroidal vascular degeneration, particularly degeneration of the choriocapillaris, is an initiating event in AMD pathogenesis [2–4]. Such vascular loss often precedes the breakdown of the overlying retinal pigment epithelium (RPE) and photoreceptor cells, suggesting that microvascular disease contributes to AMD onset and progression.
Age is the most important risk factor for AMD, thus understanding age-related changes in the choroid can offer insight into its pathogenesis. Although only one out of every 250–500 individuals in their 5th decade of life is afflicted with AMD, disease prevalence rises to one out of every 6–8 individuals over 80 years old [5]. In aging, the choroid undergoes numerous molecular changes related to AMD pathogenesis. For example, the membrane attack complex (MAC) (a lytic, multiprotein component of the innate immune system) is absent from the choriocapillaris at birth but gradually accumulates throughout life [6,7], especially in individuals with AMD [8]. Such MAC accumulation may progressively damage choriocapillaris endothelial cells, interrupting proper metabolic support to the overlying photoreceptor cells and leading to retinal degeneration [9]. In addition to complement buildup, the surface expression of inflammatory mediators changes on the choroidal vasculature over time. CD34, a highly glycosylated sialomucin, inhibits leukocyte extravasation through the choroidal vasculature and is decreased in the choriocapillaris with advancing age [10]. This evidence, coupled with the observation that the aging choroid thins each year in adulthood [11,12], suggests that the choroid is subject to increased inflammatory and degenerative pressures with advancing age.
To identify AMD-initiating mechanisms, several research groups have profiled gene expression in the human RPE and choroid using serial analysis of gene expression [13], microarrays [14–18], and RNA sequencing [19]. These studies have catalogued choroidal genes enriched in AMD samples [20] and characterized regional gene expression patterns within the choroid [21]. In mice, a microarray study of young and old RPE/choroid identified an age-related increase in pro-inflammatory gene expression [22]. Such investigations have informed models of AMD pathogenesis; however, microarray and bulk RNA sequencing methodologies pool mRNA from both RPE and choroid, blurring the diverse cellular responses to age and AMD.
Recent advances in single-cell RNA sequencing circumvent this limitation, and the transcriptome of individual RPE and choroidal cells have been identified with this technology [23–26]. In this study, we set out to identify age-related gene expression changes in human choroid, with a particular focus on the choroidal endothelial cells. In two experiments comprising a total of 24 patients, we performed either bulk or single-cell RNA sequencing on infant (n = 6) versus adult (n = 19) human donors. We found differentially regulated gene expression patterns in young versus aged human donor choroid. These patterns suggest underlying age-related inflammatory changes in the choroidal vasculature.
Methods:
Human donor eyes:
The adult and infant human donor eyes utilized in this study were acquired from Iowa Lions Eye Bank in accordance with the Declaration of Helsinki and following full consent from the donor’s next of kin. For infant donations, families were only approached if they first expressed interest in tissue donation. RPE-choroidal tissue was used in two transcriptomic experiments as described below.
Experiment #1: Bulk RNA sequencing:
Donor information for bulk RNA sequencing experiments is presented in Table 1. Tissue was collected from central RPE/choroid (4 mm infant and bisected 8 mm adult) with trephine punch biopsies and flash-frozen in liquid nitrogen. Total RNA was extracted from frozen RPE/choroidal tissue (Qiagen RNeasy kit). Poly-A selected RNA transcripts underwent 150 base-pair paired-end sequencing with an Illumina HiSeq 2000. Reads were mapped to the human genome hg19 with STAR (version = 020201) [27] and were quantified with the featureCounts function from the Rsubread package (version = 1.28.1) [28]. Differential expression analysis was performed with edgeR (version = 3.26.8) [29]. Overrepresentation analysis was performed on differentially expressed genes between infant and adult samples (p-value < 0.00001, FDR < 0.001, cpm >2, logFC > 1) with WebGestalt [30], using the Kegg pathway functional database and the weighted set cover as the redundancy reduction technique.
Table 1:
Bulk RNA sequencing donor information.
| Donor | Age | Sex | Time postmortem | Eye | Cause of death | Ophthalmologic history |
|---|---|---|---|---|---|---|
| Donor 1 | 4 Days | Male | 04:40 | OD | Metabolic Acidosis | None |
| Donor 2 | 1 Day | Male | 12:00 | OD | Perinatal Asphyxia | None |
| Donor 3 | 5 Weeks | Female | 07:27 | OD | Congenital Heart Defect | None |
| Donor 4 | 9 Months | Female | 07:05 | OD | Cardiac Failure/CHARGE Syndrome | None |
| Donor 5 | 82 | Male | 05:23 | OD | Heart Failure | Control |
| Donor 6 | 85 | Female | 04:52 | OS | Bladder Cancer with Mets | Control, Pseudophakia, PVD, Ocular Migraines |
| Donor 7 | 73 | Male | 06:10 | OD | Interstitial Lung Disease | Control, PVD, IOL |
| Donor 8 | 93 | Female | 05:20 | OD | CVA/Stroke | Control, Cataract Surgery, YAG |
| Donor 9 | 77 | Male | 05:36 | OS | Sepsis | Control, IOL, Cataracts |
| Donor 10 | 66 | Female | 05:13 | OD | G.I. Bleed | Control |
| Donor 11 | 84 | Male | 05:57 | OS | Prostate Cancer with Mets | Control |
| Donor 12 | 75 | Female | 04:56 | OS | Metastatic Renal Cell Carcinoma | Control, IOL, Cataract, Acular, Zymar |
| Donor 13 | 86 | Male | 03:33 | OS | Aortic Stenosis | Control, Cataract Surgery |
| Donor 14 | 76 | Female | 05:37 | OS | Cardiac Arrest | Control |
| Donor 15 | 76 | Female | 05:07 | OS | Sepsis | Control, IOL, Cataract, Glaucoma Suspect |
| Donor 16 | 88 | Male | 05:59 | OS | Renal Failure | Control, Cataract |
| Donor 17 | 77 | Male | 05:43 | OS | Cardiac Arrest/Liver Cirrhosis | Control |
Experiment #2: Single-cell RNA sequencing:
Donor information for single-cell RNA sequencing experiments is presented in Table 2. Briefly, 12 mm central punches of RPE/choroid were acquired with trephine punch biopsies. Tissue was gently diced into ~1 mm squares with a razor blade before dissociation in 450 units/mL of collagenase II (Gibco, Carlsbad CA) reconstituted in Hank’s Balanced Salt Solution supplemented with magnesium and calcium (Life Technologies, Carlsbad CA) [31]. Cells were incubated on a shaker at 37 °C for one hour before cryopreservation in DMSO-based Recovery Cell Cryopreservation Media (Life Technologies, Carlsbad CA). Cells were placed in a CryoSafe cooler (CryoSafe, Summerville SC) in a −80°C freezer to cool 1°C/minute. After 3–8 hours, cells were placed in liquid nitrogen for storage. Cryopreserved cells were subsequently thawed prior to enrichment for endothelial cells. Cells were incubated for 15 minutes with anti-CD31 microbeads (Miltenyi Biotech, Bergisch Gladbach Germany) and separated with the autoMACs magnetic separation system (Miltenyi Biotech, Bergisch Gladbach Germany) into CD31+ and CD31− fractions, according to the manufacturer’s instructions. Both CD31+ and CD31− fractions from donors 8–12 were immediately barcoded with the Chromium v3 single-cell reagent kit (10x Genomics, Pleasanton CA), and resulting libraries were pooled and sequenced with an Illumina NovaSeq 6000. Of note, CD31+ fractions from donors 18–21 in this study were described in a previous investigation (GSE135922) [23] and serve as adult controls that were processed independently.
Table 2:
Single-cell RNA sequencing donor information. Note donors 18–21 served as adult controls and were described in a previous study [23].
| Donor | Age | Sex | Time postmortem | Eye | Cause of death | Ophthalmologic history |
|---|---|---|---|---|---|---|
| Donor 18 | 92 | F | 5:04 | OD | Respiratory distress | Neovascular AMD |
| Donor 19 | 80 | M | 5:50 | OS | Cardiac arrest secondary to respiratory failure | Normal macula contralateral eye |
| Donor 20 | 75 | F | 6:44 | OS | COPD | Minor focal basal deposits contralateral eye |
| Donor 21 | 77 | M | 4:48 | OD | Lung Cancer | Normal macula contralateral eye |
| Donor 22 | 61 | M | 3:21 | OS | Tumor lysis syndrome | Normal macula contralateral eye |
| Donor 23 | 63 | M | 4:44 | OD | Respiratory failure | Myopia (−6.25), pigment dispersion syndrome. Thin choroid in contralateral macula, otherwise unremarkable |
| Donor 24 | 7 months | M | 10:01 | OS | Cardiac complications secondary to trisomy 21 | None |
| Donor 25 | 1 month | M | 8:59 | OD | Encephalopathy | None |
FASTQ files were generated from basecall files with bcl2fastq software (Illumina, San Diego CA) by the University of Iowa Institute of Human Genetics. FASTQ files were mapped to the pre-built hg19 genome with CellRanger (v3.0.1, 10x Genomics, Pleasanton CA). Cells with fewer than 800 and greater than 7500 unique genes per cell were filtered, as were cells with more than 60% of genes mapping to mitochondrial genes (to remove cellular debris) [32]. Filtered libraries from single-cell donors were log-normalized using a scale factor of 10,000 before aggregation with canonical clustering analysis (Seurat v3.1.1) [33]. Variable features were identified with the variance stabilizing transformation (vst) selection method before dimensionality reduction and clustering. Differential expression analysis was performed with the non-parametric Wilcoxon Rank Sum test to identify genes enriched in each cluster as well as genes enriched in cells from infant and adult libraries. Most single-cell experiments, including the investigation described in this manuscript, contain limited biologic replicates. Differential expression within Seurat treats each cell as an independent observation, which inflates p-values comparing cells originating from different biologic conditions (in this case, infant-versus-adult comparisons). Consequently, we do not report p-values for these comparisons from Seurat. Instead, in each cluster we sum the raw counts of all reads for each independent library (eg biological replicate), which is used as input for differential expression with edgeR. This pseudo-bulk comparison of counts in each cluster more accurately reflects the number of infant and adult replicates. For each cell type, p-values and log2FCs from the infant-versus-adult pseudo-bulk RNA count analysis are reported alongside the summary statistics output from Seurat, similar to previous methods [34].
Immunohistochemistry:
Immunohistochemistry experiments were performed on frozen human tissue sections fixed in 4% paraformaldehyde [35]. Infant (n=3) and aged adult (n=5) maculas were evaluated. Sections were blocked with 1 mg/mL of bovine serum albumin for fifteen minutes and were subsequently incubated with 50 μL of 1X TrueBlack autofluorescence quencher (Biotinium, Hayward CA) for 60 seconds followed by three five-minute washes, according to the manufacturer’s instructions. This treatment quenches the very abundant autofluorescence present in adult RPE cells. Sections were incubated with anti-CD34 (1:100, Abcam, EP373Y) and anti-ICAM1 (1:100, Developmental Studies Hybridoma Bank, Iowa City, IA, P2A4) primary antibodies for one hour. Negative controls were additionally obtained by omitting each primary antibody. After washing, Alexa-546-conjugated anti-mouse IgG (1:200, Invitrogen) and Alexa-488-conjugated anti-rabbit IgG (1:200, Invitrogen) secondary antibodies resuspended in PBS supplemented with 100 μg/mL diamidino-phenyl-indole (DAPI, Sigma) were added to each section for 30 minutes. Sections were washed and coverslipped, and photographs were acquired with a confocal microscope (Leica DM 2500 SPE). In all steps, from immunofluorescent labeling to finalization of figures, all infant and adult samples were treated identically.
Results:
Bulk RNA sequencing was performed on central RPE/choroid lysates from 13 adult (ages 66 – 93) and 4 liveborn infant (ages 1 day – 9 months) human donors (Table 1). Multidimensional scaling of the mapped reads clearly separates transcriptomic profiles from infants versus adults (Figure 1A), suggesting that the infant and adult RPE/choroid possess distinct transcriptomes. In order to identify specific genes enriched in infant and adult populations, differential expression analysis was performed (SI Table 1). Overrepresentation analysis of differentially expressed genes identified functional pathways enriched in both infant and adult RPE/choroid (Figure 1B). Similar to studies in aging mouse RPE-choroid [22], inflammation-related genes were upregulated in aging, and the most enriched pathway observed in adult human RPE/choroid was leukocyte transendothelial migration. Other inflammation-related pathways, such as hematopoietic cell lineage, antigen processing and presentation, and cytokine-cytokine receptor interaction pathways, were also upregulated in adult choroid. In contrast, infant RPE/choroid was most enriched in genes involved in protein digestion and absorption (Figure 1B).
Figure 1 [non-color]: Bulk RNA sequencing reveals differences between the transcriptomes of infant and adult RPE/choroid.
![Figure 1 [non-color]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/05c8/7396301/62d0dd150300/nihms-1607570-f0002.jpg)
A. Multidimensional scaling dimensionality reduction separates infant (grey) and adult (black) RPE/choroidal samples. B. Pathway analysis of genes enriched in adult (black) and infant (grey) samples was performed with WebGestalt. The enrichment ratio is the number of observed genes in each category divided by the number of expected genes in each category, given the size of the input gene list.
In order to characterize how age influences gene expression in each choroidal cell population, we performed single-cell RNA sequencing on RPE/choroid samples from 6 adult and 2 infant human donors (Table 2). Four of the adult samples (donors 18–21) were described in a previous report (GSE135922) [23]. As choroidal endothelial cells degenerate early in AMD and comprise only a modest fraction of all choroidal cell types [23], we enriched for CD31-expressing endothelial cells before single-cell barcoding and sequencing. After mapping and filtering of the data, a total of 37,070 cells were recovered (Figure 2A), including 17,229 cells from infant samples (Figure 2B) (SI Table 2). The uniform manifold approximation and projection (UMAP) algorithm was applied for dimensionality reduction, and the resulting clusters were subsequently classified by identifying enriched cell type specific genes (Figure 2C).
Figure 2 [color]: Single-cell RNA sequencing of infant and adult RPE-choroid samples from 8 human donors.
![Figure 2 [color]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/05c8/7396301/497b5a3976a7/nihms-1607570-f0003.jpg)
A. After single-cell RNA sequencing, UMAP dimensionality reduction was performed to visualize clusters of choroidal cell types. A total of 37,070 cells were recovered after filtering, and all major choroidal cell types were identified. B. Cells comprising the endothelial cell clusters (dashed box in A) were well represented from both adult (left) and infant (right) libraries. C. In each cell type, we identified the 100 most enriched genes, averaged gene expression across cells, and performed hierarchical clustering analysis on these values (left). Violin plots (right) indicate relative expression levels of previously reported cell-specific genes within all cells of each cluster.
Clusters corresponding to all major choroidal cell populations were identified. Interestingly, two distinct clusters of RGS5-expressing contractile mural cell types were observed (Figure 3A). Differential expression analysis was performed to identify enriched genes in these two clusters (SI Table 3, Figure 3B). One cluster of cells was classified as vascular smooth muscle cells (SMC), which demonstrated high and specific expression of the smooth muscle myosin heavy chain gene (MYH11) characteristic of contractile cells surrounding large caliber vessels [36] (Figure 3C). The other mural cell cluster was classified as capillary-surrounding pericytes, and cells in this cluster exhibited high expression of the pericyte-specific genes GGT5 and ABCC9 [36] (Figure 3C). Interestingly, the gene encoding complement factor H (CFH) was the fourth-most enriched gene in pericytes compared to smooth muscle cells (logFC = 1.33, absolute fold change = 3.72) (Figure 3B).
Figure 3 [color]: Pericyte versus smooth muscle cell gene expression.
![Figure 3 [color]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/05c8/7396301/41fe81b3c7e0/nihms-1607570-f0004.jpg)
A. Expression of RGS5, which is expressed in both pericytes and smooth muscle cells, is high in both contractile mural cell clusters. B. Differential expression analysis between cells originating from pericyte and smooth muscle cell clusters. The y-axis depicts log fold-change difference of each gene between infants and adults, with positive values equating to higher expression in pericytes. The x-axis depicts the percentage of cells that express each gene in pericyte populations minus SMC populations (delta percent). For example, if 50% of pericytes express a given gene while only 10% of SMCs express the gene, the delta percent is 0.4 (40%). C. Violin plots depicting expression of RGS5 (common pericyte/smooth muscle cell marker), MHY11 and ACTG2 (enriched in smooth muscle cells), and GGT5 and ABCC9 (enriched in pericytes). D. Cartoon of pericyte and smooth muscle cell localization. SMC = smooth muscle cell.
Within each population, differential expression analysis was conducted between cells originating from infant versus adult libraries (Figure 4, SI Table 4) and functional pathway enrichment of differentially expressed genes was performed (SI Table 5). All clusters of adult vascular cells (choriocapillaris, arteries, and veins) were significantly enriched in genes belonging to the hematopoietic cell lineage (SI Figure 1). In addition, adult cells originating from vascular clusters were enriched for the expression of genes involved in complement and coagulation cascades, including complement inhibitors CD55 and CD59 (SI Figure 1).
Figure 4 [non-color]: Transcriptome of the choroidal vasculature in infants versus adults.
![Figure 4 [non-color]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/05c8/7396301/9dd42193d64f/nihms-1607570-f0005.jpg)
Differential expression analysis was performed between cells originating from infant versus adult libraries in choriocapillaris (A), arterial (B), and venous (C) choroidal endothelial cells. The y-axis depicts log fold-change difference of each gene between infants and adults, with positive values equating to higher expression in infants. The x-axis depicts the percentage of cells that express each gene in infant populations minus adult populations (delta percent). For example, if 50% of choriocapillaris endothelial cells in infants express a given gene while only 10% of adult choriocapillaris endothelial cells express the gene, the delta percent is 0.4 (40%). Genes with an absolute log fold enrichment of greater than 1.15 and an absolute delta percent greater than 0.15 are labeled in each cell type.
In contrast, infant choriocapillaris, arteries, and veins were each enriched for expression of genes broadly involved in the MAP kinase signaling pathway (SI Figure 1). For example, NR4A1 (which encodes the orphan nuclear receptor NUR77), was enriched in infant choriocapillaris (logFC = 0.79, absolute fold-change = 2.20). Previous studies have shown NR4A1 (NUR77) overexpression reduced endothelial activation and decreased expression of the adhesion molecules VCAM-1 and ICAM-1 in vitro [37]. Similarly, the orphan nuclear receptor NR2F2 (COUP-TFII), which decreases pro-inflammatory adhesion molecules [38], was enriched in infant compared to adult veins (logFC = 0.58, absolute fold-change = 1.79). Infant endothelial cells were also enriched in components of the AP-1 transcription factor (FOS and JUND), which increases endothelial migration and proliferation [39] and protects against age-related endothelial dysfunction in mice [40].
The infant choriocapillaris was also enriched in transcriptional regulators of angiogenesis. For example, infant choriocapillaris cells had increased expression of ID3 (logFC = 1.31, absolute fold-change = 3.71), which induces a molecular stem cell-like phenotype in the microvasculature [41] (Figure 4A). Likewise, infant choriocapillaris was enriched in the transcription factor KLF2 (logFC = 1.33, absolute fold-change = 3.78), which inhibits the expression of inflammatory surface adhesion molecules such as VCAM-1 and E-selectin [42]. Infant arterial endothelial cells demonstrated increased expression of HES5 (logFC = 1.67, absolute fold-change = 5.31), a transcriptional repressor that has been shown to drive arterial specification in the central nervous system vasculature of mice (Figure 4B) [43]. Infant venous endothelial cells demonstrated increased expression of RHOB (logFC = 1.18, absolute fold-change = 3.25), which has been implicated in driving capillary sprouting during angiogenesis [44] (Figure 4C). Collectively, these results suggest that the infant choriocapillaris has a unique transcriptional composition that promotes angiogenesis and limits the expression of inflammatory adhesion molecules.
The transcriptome of contractile mural cell populations was also observed to change with age (SI Table 4). Infant pericytes and smooth muscle cells were enriched for the connective tissue growth factor (CTGF) gene, which has been shown to promote differentiation of choriocapillaris-like endothelial cells from iPSCs [45]. Infant pericytes exhibited increased expression of numerous genes involved in construction of the extracellular matrix, including COL1A1, COL1A2, COL3A1, and ELN. In contrast, adult smooth muscle cells and pericytes were enriched in metallothionein genes (MT1A, MT1M, and MT1X), which have been suggested to become dysregulated in AMD and lead to increased oxidative damage [46]. Likewise, adult smooth muscle cells and pericytes demonstrated increased expression of the nuclear receptor NR4A3 (NOR-1), which has been shown to increase expression of the vascular adhesion molecules ICAM-1 and VCAM-1 [47].
A subset of genes observed to be differentially expressed between infant and adult choriocapillaris were molecularly validated with immunohistochemistry. CD34, which was enriched in infant samples at the bulk RNA level and in infant choriocapillaris at the single-cell level, was co-labeled with ICAM-1, which demonstrated increased expression in adult samples at the bulk level and in adult choriocapillaris endothelial cells (Figure 5AB). Corroborating the transcriptional data, the infant choriocapillaris exhibited minimal ICAM-1 labeling but abundant CD34 expression (Figure 5C–E). The opposite pattern was observed in adult choriocapillaris, with high ICAM-1 labeling while CD34 expression was frequently nearly absent (Figure 5F–H).
Figure 5 [color]: Transcriptome of the choroidal vasculature in infants versus adults.
![Figure 5 [color]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/05c8/7396301/2ddc0a12b6e3/nihms-1607570-f0006.jpg)
(A) Bulk RNA sequencing and (B) single-cell RNA sequencing (choriocapillaris cluster) log2 fold-changes for ICAM1 and CD34 between infant and adult samples. Infant (age = 4 days) (C–E) and adult (age = 81 years) (F–H) choroidal tissue sections were dual-labeled with antibodies directed against ICAM-1 (red, panels C & F) and CD34 (green, panels D & G). Infant samples demonstrated minimal ICAM-1 labeling (C) but strong CD34 expression (D), while adult sections exhibited robust ICAM-1 staining (F) but relatively low CD34 expression (G). Of note, RPE lipofuscion autofluorescence was quenched prior to incubation with primary antibodies (see Methods). All treatments, microscope settings and post-processing were identical for all panels. RPE = retinal pigment epithelium, CC = choriocapillaris, CHO = deeper choroid (Sattler’s layer). Scalebar (H) is 10 microns.
Discussion:
Single-cell transcriptomic studies have advanced our understanding of genetic regulation in the human retina [48–50], RPE [24], and choroid [23,25,26]. In addition to cataloguing expression patterns within complex tissues, single-cell experiments can compare how different biological conditions, such as age, influence gene expression across many cell populations simultaneously [51]. As advancing age is the single greatest risk factor for AMD development, we set out to identify how gene expression in the human choroid changes between eyes from very young versus older individuals.
Degeneration of choriocapillaris endothelial cells is one of the earliest detectable events in AMD pathogenesis [2–4], and we were particularly interested in identifying age-related expression differences in this specialized population of endothelial cells. The choriocapillaris is a structurally and molecularly unique capillary bed, consisting of a dense network of fenestrated endothelial cells organized in lobular units [52]. Unlike most other vascular beds, the choriocapillaris endothelium develops locally by hemovasculogenesis from a distinct precursor (the hemangioblast) rather than by angiogenesis of the deeper choroidal vasculature [52]. This developmental origin, as well as the fact that the choriocapillaris forms under an already pigmented RPE, likely confers the choriocapillaris with its unique structural and molecular properties, including that the transcriptome of choriocapillaris endothelial cells is distinct from deeper choroidal arteries and veins at the single-cell level [23] (Figure 2).
Choriocapillaris endothelial cells, as well as choroidal arteries and veins, increase pro-inflammatory gene expression with advancing age. Such expression of surface adhesion molecules promotes leukocyte binding and extravasation into aged tissues. Previous reports have identified increased recruitment of macrophages from the vasculature into the choroidal stroma in AMD [53], which may initiate local tissue destruction and further increase vascular permeability [54]. ICAM-1 is one such adhesion molecule that is constitutively expressed on the choriocapillaris [55], and choriocapillaris ICAM1 expression is higher within the macula compared to the periphery [23,56]. In this study, we determined that ICAM1 expression is increased in adult choroidal samples at the RNA level (both in bulk samples and within the choriocapillaris cluster) and at the protein level in the choriocapillaris (Figure 5). CD34, a transmembrane glycoprotein, is also expressed by the choriocapillaris [10]. In accordance with previous reports, we identify increased CD34 expression within infant choriocapillaris endothelial cells at both the RNA and protein levels (Figure 5). The negatively charged sialic acid residues on CD34 inhibit binding of leukocytes to endothelial cells by acting as “molecular Teflon” [57], such that loss of this inhibitor with age may increase leukocyte adhesion to the choroidal endothelium.
Numerous transcription factors were differentially enriched in either infant or adult choroidal populations. Several of the identified transcription factors belonged to the family of nuclear receptors, which regulate a myriad of angiogenic and signaling pathways and have been associated with AMD pathophysiology [58,59]. Infant choriocapillaris cells were enriched with the nuclear receptors NR4A1 and NR2F2, which have both been shown to suppress endothelial expression of adhesion proteins such as ICAM-1 [37,38]. Infant endothelial cells were enriched in additional non-nuclear transcription factors that regulate a pro-inflammatory phenotype in the vasculature. This group includes ID3 (which increases CD34 expression [41]) and KLF2 (which decreases ICAM-1 and VCAM-1 expression [42]). Collectively, these transcription factors represent promising targets that may regulate the diverging molecular composition of infant and adult choroidal endothelial cells.
There are several limitations to the study. First, demographic characteristics of incoming eye donations cannot be controlled or predicted. As such, the sample size of infant donor groups was smaller than that of the adults, and the postmortem interval of infant eyes was slightly longer. Differing postmortem intervals have been shown to change gene expression in primate RPE/choroid [60], and may be responsible for the increased detection of heat-shock proteins enriched in infant cell populations.
However, expression of inflammatory pathway genes does not appreciably change in primate RPE/choroid with prolonged death-to-preservation [60]. This suggests that inflammatory genes enriched in adult choroidal endothelial cells are reflective of underlying biological differences in age and not differing postmortem intervals. In addition, in order to have adequate numbers of endothelial cells to characterize different subpopulations, we pre-enriched for CD31-expressing endothelial cells prior to single-cell RNA sequencing. While endothelial cells are of high interest in AMD pathophysiology, this enrichment precluded other important cell types such as RPE cells from being recovered in large quantities. Likewise, many high-throughput single-cell barcoding strategies limit sequencing to the 3’ region of each gene, precluding isoform or allele-specific expression analyses. However, our bulk RNA sequencing data allows for assessment of alternative splicing between infants and adults. Finally, while we identified several transcription factors implicated in regulating the pro-inflammatory vascular phenotype with advancing age, further functional studies are required to validate these findings.
The experiments included in this study provide a rich resource for comparing infant and aged gene expression between all major cell populations within the human choroid. All raw and processed data have been deposited in the Gene Expression Omnibus (GEO) database (GSE149009 for bulk studies and GSE149100 for single-cell studies), and differential expression results are reported in supplementary tables in their entirety. Together, these data demonstrate that choroidal endothelial cells adopt a pro-inflammatory molecular profile with advancing age and suggest several potential transcriptional drivers of this phenotype.
Supplementary Material
Highlights:
Bulk or single-cell RNA sequencing were performed on 6 infant and 19 adult human donor choroids
Differentially expressed genes were identified between infant and adult cells in all choroidal cell populations
Distinct clusters of pericytes and vascular smooth muscle cells were characterized
Aged human choroid demonstrated increased pro-inflammatory gene expression, particularly in endothelial cells
Acknowledgments:
We thank the donors, their families, and Iowa Lions Eye Bank for their generous and essential role in this research. The ICAM-1 (P2A4) monoclonal antibody was developed by the Fred Hutchison Cancer Research Center and was obtained by the Developmental Studies Hybridoma Bank, created by the National Institute of Child Health and Human Development of the NIH and maintained at the Department of Biology, The University of Iowa (Iowa City, IA). The data presented herein were obtained at the Flow Cytometry Facility, which is a Carver College of Medicine/Holden Comprehensive Cancer Center Core Research Facility at the University of Iowa. The facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center.
Funding:
These studies were funded by NIH grants T32 GM007337, EY024605, and P30 EY025580 with additional support from the Research to Prevent Blindness, the Elmer and Sylvia Sramek Charitable Foundation, and the Martin Carver Chair in Ocular Cell Biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nork TM; Kim CB; Shanmuganayagam D; Van Lysel MS; Ver Hoeve JN; Folts JD Measurement of regional choroidal blood flow in rabbits and monkeys using fluorescent microspheres. Archives of ophthalmology (Chicago, Ill. : 1960) 2006, 124, 860–868, doi: 10.1001/archopht.124.6.860. [DOI] [PubMed] [Google Scholar]
- 2.Biesemeier A; Taubitz T; Julien S; Yoeruek E; Schraermeyer U Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiology of aging 2014, 35, 2562–2573, doi: 10.1016/j.neurobiolaging.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Arya M; Sabrosa AS; Duker JS; Waheed NK Choriocapillaris changes in dry age-related macular degeneration and geographic atrophy: a review. Eye and vision (London, England) 2018, 5, 22, doi: 10.1186/s40662-018-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohn EH; Flamme-Wiese MJ; Whitmore SS; Workalemahu G; Marneros AG; Boese EA; Kwon YH; Wang K; Abramoff MD; Tucker BA, et al. Choriocapillaris Degeneration in Geographic Atrophy. The American journal of pathology 2019, 189, 1473–1480, doi: 10.1016/j.ajpath.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman DS; O’Colmain BJ; Munoz B; Tomany SC; McCarty C; de Jong PT; Nemesure B; Mitchell P; Kempen J Prevalence of age-related macular degeneration in the United States. Archives of ophthalmology (Chicago, Ill. : 1960) 2004, 122, 564–572, doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 6.Chirco KR; Tucker BA; Stone EM; Mullins RF Selective accumulation of the complement membrane attack complex in aging choriocapillaris. Experimental eye research 2016, 146, 393–397, doi: 10.1016/j.exer.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seth A; Cui J; To E; Kwee M; Matsubara J Complement-associated deposits in the human retina. Investigative ophthalmology & visual science 2008, 49, 743–750, doi: 10.1167/iovs.07-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirco KR; Flamme-Wiese MJ; Wiley JS; Potempa LA; Stone EM; Tucker BA; Mullins RF Evaluation of serum and ocular levels of membrane attack complex and C-reactive protein in CFH-genotyped human donors. Eye (London, England) 2018, 32, 1740–1742, doi: 10.1038/s41433-018-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitmore SS; Sohn EH; Chirco KR; Drack AV; Stone EM; Tucker BA; Mullins RF Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Progress in retinal and eye research 2015, 45, 1–29, doi: 10.1016/j.preteyeres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohn EH; Flamme-Wiese MJ; Whitmore SS; Wang K; Tucker BA; Mullins RF Loss of CD34 expression in aging human choriocapillaris endothelial cells. PloS one 2014, 9, e86538, doi: 10.1371/journal.pone.0086538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakatsuki Y; Shinojima A; Kawamura A; Yuzawa M Correlation of Aging and Segmental Choroidal Thickness Measurement using Swept Source Optical Coherence Tomography in Healthy Eyes. PloS one 2015, 10, e0144156, doi: 10.1371/journal.pone.0144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohn EH; Khanna A; Tucker BA; Abramoff MD; Stone EM; Mullins RF Structural and biochemical analyses of choroidal thickness in human donor eyes. Investigative ophthalmology & visual science 2014, 55, 1352–1360, doi: 10.1167/iovs.13-13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharon D; Blackshaw S; Cepko CL; Dryja TP Profile of the genes expressed in the human peripheral retina, macula, and retinal pigment epithelium determined through serial analysis of gene expression (SAGE). Proceedings of the National Academy of Sciences of the United States of America 2002, 99, 315–320, doi: 10.1073/pnas.012582799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radeke MJ; Peterson KE; Johnson LV; Anderson DH Disease susceptibility of the human macula: differential gene transcription in the retinal pigmented epithelium/choroid. Experimental eye research 2007, 85, 366–380, doi: 10.1016/j.exer.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Booij JC; van Soest S; Swagemakers SM; Essing AH; Verkerk AJ; van der Spek PJ; Gorgels TG; Bergen AA Functional annotation of the human retinal pigment epithelium transcriptome. BMC Genomics 2009, 10, 164, doi: 10.1186/1471-2164-10-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booij JC; ten Brink JB; Swagemakers SM; Verkerk AJ; Essing AH; van der Spek PJ; Bergen AA A new strategy to identify and annotate human RPE-specific gene expression. PloS one 2010, 5, e9341, doi: 10.1371/journal.pone.0009341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strunnikova NV; Maminishkis A; Barb JJ; Wang F; Zhi C; Sergeev Y; Chen W; Edwards AO; Stambolian D; Abecasis G, et al. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Human molecular genetics 2010, 19, 2468–2486, doi: 10.1093/hmg/ddq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Soest SS; de Wit GM; Essing AH; ten Brink JB; Kamphuis W; de Jong PT; Bergen AA Comparison of human retinal pigment epithelium gene expression in macula and periphery highlights potential topographic differences in Bruch’s membrane. Molecular vision 2007, 13, 1608–1617. [PubMed] [Google Scholar]
- 19.Li M; Jia C; Kazmierkiewicz KL; Bowman AS; Tian L; Liu Y; Gupta NA; Gudiseva HV; Yee SS; Kim M, et al. Comprehensive analysis of gene expression in human retina and supporting tissues. Human molecular genetics 2014, 23, 4001–4014, doi: 10.1093/hmg/ddu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman AM; Gallo NB; Hancox LS; Miller NJ; Radeke CM; Maloney MA; Cooper JB; Hageman GS; Anderson DH; Johnson LV, et al. Systems-level analysis of age-related macular degeneration reveals global biomarkers and phenotype-specific functional networks. Genome Med 2012, 4, 16, doi: 10.1186/gm315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitmore SS; Wagner AH; DeLuca AP; Drack AV; Stone EM; Tucker BA; Zeng S; Braun TA; Mullins RF; Scheetz TE Transcriptomic analysis across nasal, temporal, and macular regions of human neural retina and RPE/choroid by RNA-Seq. Experimental eye research 2014, 129, 93–106, doi: 10.1016/j.exer.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H; Liu B; Lukas TJ; Neufeld AH The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration. PloS one 2008, 3, e2339, doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voigt AP; Mulfaul K; Mullin NK; Flamme-Wiese MJ; Giacalone JC; Stone EM; Tucker BA; Scheetz TE; Mullins RF Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proceedings of the National Academy of Sciences of the United States of America 2019, 116, 24100–24107, doi: 10.1073/pnas.1914143116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y; Wang X; Hu B; Mao Y; Chen Y; Yan L; Yong J; Dong J; Wei Y; Wang W, et al. Dissecting the transcriptome landscape of the human fetal neural retina and retinal pigment epithelium by single-cell RNA-seq analysis. PLoS Biol 2019, 17, e3000365, doi: 10.1371/journal.pbio.3000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehmann GL; Hanke-Gogokhia C; Hu Y; Bareja R; Salfati Z; Ginsberg M; Nolan DJ; Mendez-Huergo SP; Dalotto-Moreno T; Wojcinski A, et al. Single-cell profiling reveals an endothelium-mediated immunomodulatory pathway in the eye choroid. J Exp Med 2020, 217, doi: 10.1084/jem.20190730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohlenova K; Goveia J; García-Caballero M; Subramanian A; Kalucka J; Treps L; Falkenberg KD; de Rooij L; Zheng Y; Lin L, et al. Single-Cell RNA Sequencing Maps Endothelial Metabolic Plasticity in Pathological Angiogenesis. Cell Metab 2020, 31, 862–877.e814, doi: 10.1016/j.cmet.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Dobin A; Davis CA; Schlesinger F; Drenkow J; Zaleski C; Jha S; Batut P; Chaisson M; Gingeras TR STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21, doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Y; Smyth GK; Shi W The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res 2019, 47, e47, doi: 10.1093/nar/gkz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson MD; McCarthy DJ; Smyth GK edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140, doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Y; Wang J; Jaehnig EJ; Shi Z; Zhang B WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res 2019, 47, W199–w205, doi: 10.1093/nar/gkz401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giacalone JC; Miller MJ; Workalemahu G; Reutzel AJ; Ochoa D; Whitmore SS; Stone EM; Tucker BA; Mullins RF Generation of an immortalized human choroid endothelial cell line (iChEC-1) using an endothelial cell specific promoter. Microvasc Res 2019, 123, 50–57, doi: 10.1016/j.mvr.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luecken MD; Theis FJ Current best practices in single-cell RNA-seq analysis: a tutorial. Mol Syst Biol 2019, 15, e8746, doi: 10.15252/msb.20188746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler A; Hoffman P; Smibert P; Papalexi E; Satija R Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nature Biotechnology 2018, 36, 411, doi: 10.1038/nbt.4096 https://www.nature.com/articles/nbt.4096#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lun ATL; Marioni JC Overcoming confounding plate effects in differential expression analyses of single-cell RNA-seq data. Biostatistics 2017, 18, 451–464, doi: 10.1093/biostatistics/kxw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barthel LK; Raymond PA Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem 1990, 38, 1383–1388, doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- 36.Chasseigneaux S; Moraca Y; Cochois-Guégan V; Boulay AC; Gilbert A; Le Crom S; Blugeon C; Firmo C; Cisternino S; Laplanche JL, et al. Isolation and differential transcriptome of vascular smooth muscle cells and mid-capillary pericytes from the rat brain. Sci Rep 2018, 8, 12272, doi: 10.1038/s41598-018-30739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You B; Jiang YY; Chen S; Yan G; Sun J The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circ Res 2009, 104, 742–749, doi: 10.1161/circresaha.108.192286. [DOI] [PubMed] [Google Scholar]
- 38.Cui X; Lu YW; Lee V; Kim D; Dorsey T; Wang Q; Lee Y; Vincent P; Schwarz J; Dai G Venous Endothelial Marker COUP-TFII Regulates the Distinct Pathologic Potentials of Adult Arteries and Veins. Sci Rep 2015, 5, 16193, doi: 10.1038/srep16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia J; Ye T; Cui P; Hua Q; Zeng H; Zhao D AP-1 transcription factor mediates VEGF-induced endothelial cell migration and proliferation. Microvasc Res 2016, 105, 103–108, doi: 10.1016/j.mvr.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paneni F; Osto E; Costantino S; Mateescu B; Briand S; Coppolino G; Perna E; Mocharla P; Akhmedov A; Kubant R, et al. Deletion of the activated protein-1 transcription factor JunD induces oxidative stress and accelerates age-related endothelial dysfunction. Circulation 2013, 127, 1229–1240, e1221–1221, doi: 10.1161/circulationaha.112.000826. [DOI] [PubMed] [Google Scholar]
- 41.Das JK; Voelkel NF; Felty Q ID3 contributes to the acquisition of molecular stem cell-like signature in microvascular endothelial cells: its implication for understanding microvascular diseases. Microvasc Res 2015, 98, 126–138, doi: 10.1016/j.mvr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.SenBanerjee S; Lin Z; Atkins GB; Greif DM; Rao RM; Kumar A; Feinberg MW; Chen Z; Simon DI; Luscinskas FW, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 2004, 199, 1305–1315, doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitagawa M; Hojo M; Imayoshi I; Goto M; Ando M; Ohtsuka T; Kageyama R; Miyamoto S Hes1 and Hes5 regulate vascular remodeling and arterial specification of endothelial cells in brain vascular development. Mech Dev 2013, 130, 458–466, doi: 10.1016/j.mod.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Adini I; Rabinovitz I; Sun JF; Prendergast GC; Benjamin LE RhoB controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes Dev 2003, 17, 2721–2732, doi: 10.1101/gad.1134603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Songstad AE; Worthington KS; Chirco KR; Giacalone JC; Whitmore SS; Anfinson KR; Ochoa D; Cranston CM; Riker MJ; Neiman M, et al. Connective Tissue Growth Factor Promotes Efficient Generation of Human Induced Pluripotent Stem Cell-Derived Choroidal Endothelium. Stem cells translational medicine 2017, 6, 1533–1546, doi: 10.1002/sctm.16-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito Y; Tanaka H; Hara H The potential roles of metallothionein as a therapeutic target for cerebral ischemia and retinal diseases. Curr Pharm Biotechnol 2013, 14, 400–407, doi: 10.2174/1389201011314040003. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y; Howatt DA; Gizard F; Nomiyama T; Findeisen HM; Heywood EB; Jones KL; Conneely OM; Daugherty A; Bruemmer D Deficiency of the NR4A orphan nuclear receptor NOR1 decreases monocyte adhesion and atherosclerosis. Circ Res 2010, 107, 501–511, doi: 10.1161/circresaha.110.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menon M; Mohammadi S; Davila-Velderrain J; Goods BA; Cadwell TD; Xing Y; Stemmer-Rachamimov A; Shalek AK; Love JC; Kellis M, et al. Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration. Nat Commun 2019, 10, 4902, doi: 10.1038/s41467-019-12780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukowski SW; Lo CY; Sharov AA; Nguyen Q; Fang L; Hung SS; Zhu L; Zhang T; Grünert U; Nguyen T, et al. A single-cell transcriptome atlas of the adult human retina. Embo j 2019, 38, e100811, doi: 10.15252/embj.2018100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voigt AP; Whitmore SS; Flamme-Wiese MJ; Riker MJ; Wiley LA; Tucker BA; Stone EM; Mullins RF; Scheetz TE Molecular characterization of foveal versus peripheral human retina by single-cell RNA sequencing. Experimental eye research 2019, 184, 234–242, doi: 10.1016/j.exer.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He X; Memczak S; Qu J; Belmonte JCI; Liu G-H Single-cell omics in ageing: a young and growing field. Nature Metabolism 2020, 2, 293–302, doi: 10.1038/s42255-020-0196-7. [DOI] [PubMed] [Google Scholar]
- 52.Lutty GA; Hasegawa T; Baba T; Grebe R; Bhutto I; McLeod DS Development of the human choriocapillaris. Eye (London, England) 2010, 24, 408–415, doi: 10.1038/eye.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLeod DS; Bhutto I; Edwards MM; Silver RE; Seddon JM; Lutty GA Distribution and Quantification of Choroidal Macrophages in Human Eyes With Age-Related Macular Degeneration. Investigative ophthalmology & visual science 2016, 57, 5843–5855, doi: 10.1167/iovs.16-20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kauppinen A; Paterno JJ; Blasiak J; Salminen A; Kaarniranta K Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci 2016, 73, 1765–1786, doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLeod DS; Lefer DJ; Merges C; Lutty GA Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. The American journal of pathology 1995, 147, 642–653. [PMC free article] [PubMed] [Google Scholar]
- 56.Mullins RF; Skeie JM; Malone EA; Kuehn MH Macular and peripheral distribution of ICAM-1 in the human choriocapillaris and retina. Molecular vision 2006, 12, 224–235. [PubMed] [Google Scholar]
- 57.Steen R; Egeland T CD34 molecule epitope distribution on cells of haematopoietic origin. Leuk Lymphoma 1998, 30, 23–30, doi: 10.3109/10428199809050926. [DOI] [PubMed] [Google Scholar]
- 58.Malek G; Lad EM Emerging roles for nuclear receptors in the pathogenesis of age-related macular degeneration. Cell Mol Life Sci 2014, 71, 4617–4636, doi: 10.1007/s00018-014-1709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choudhary M; Malek G Rethinking Nuclear Receptors as Potential Therapeutic Targets for Retinal Diseases. J Biomol Screen 2016, 21, 1007–1018, doi: 10.1177/1087057116659856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kallestad L; Blackshaw S; Khalil AM; Palczewski K Tissue- and Species-Specific Patterns of RNA metabolism in Post-Mortem Mammalian Retina and Retinal Pigment Epithelium. Sci Rep 2019, 9, 14821, doi: 10.1038/s41598-019-51379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


