Abstract
Despite decades of messages warning about the dangers of tobacco use in pregnancy, 10% to 15% of pregnant women continue to smoke. Furthermore, an increased popularity of electronic nicotine delivery systems (ENDS) over the past decade in women of childbearing age raises parallel concerns regarding the effects of vaporized nicotine use in pregnancy. While research using animal models which mimic tobacco smoke and nicotine exposure in pregnancy have largely replicated findings in humans, few studies focus directly on the effects of these exposures on the placenta. Because the placenta is a fetal derived tissue, and nicotine and other components of tobacco smoke are either processed by or transported directly through the placenta, such studies help us understand the risks of these exposures on the developing fetus. In this review, we summarize research on the placenta and placental-derived cells examining either tobacco smoke or nicotine exposure, including both histologic and subcellular (ie, epigenetic and molecular) modifications. Collectively, these studies reveal that tobacco and nicotine exposure are accompanied by some common and several unique molecular and epigenomic placental modifications. Consideration of the nature and sequelae of these molecular mediators of risk may help to better inform the public and more effectively curtail modifiable behavior.
1. INTRODUCTION
Since the 1950s, the adverse impact and negative effects of maternal tobacco smoking on pregnancy outcomes and fetal development have been documented. The first of these observations signaled a role for the placenta as a conduit of maternal tobacco use, given the seminal observation that infants born to mothers who smoke had a lower birthweight than those born to nonsmoking mothers.1,2 Since these seminal studies, the numerous effects of smoking on maternal, fetal, and neonatal outcomes have been well documented across geographically and racially distinct populations and inclusive of both male and female offspring, including the surprising finding that smoking appears to be protective against preeclampsia in these women.3,4 The public health message advising pregnant women to quit smoking has been unequivocally directive. Nonetheless, smoking rates and prevalence during pregnancy continues to be a public health concern. Rates of smoking during pregnancy reported at a state-wide level varies between 6.8% (Utah) and as high as 25% (West Virginia).5 Interventions to help women quit smoking have kept up with technological advancements predictive of effective behavioral modifications in other spheres of medicine. Examples include digital tools such as phone apps to track smoking status and designate a specific quit date,6,7 as well as text messaging services specifically geared to the unique health concerns of pregnant women to encourage smoking cessation.8-10 However, despite such interventions and prompts, tobacco exposure persists as the leading modifiable behavior rendering maternal and neonatal risk of the most prevalent perinatal morbidities, namely preterm birth and fetal growth restriction.
Of the 4000+ chemicals that have been characterized from first and second-hand tobacco smoke, nicotine has been a major focus of research due to its highly addictive quality. For decades, studies have focused on nicotine addiction through smoking combustible tobacco cigarettes. However, the advent of electronic nicotine delivery systems (ENDS), which includes electronic cigarettes, vape pens, and Juul devices, has altered the field of addiction research, changing our understating of who is at risk for initiation, chronic use, dual ENDS & tobacco cigarette use, and use in pregnancy.
Electronic cigarettes, which are popular ENDS devices, have been on the market in the United States since 2007.11 Initially created as a tool for smoking cessation, and marketed as a healthier alternative to smoking, there is a concerning lack of data supporting these claims. Similarly, the claims of less harmful side effects from ENDS use appear to be untrue, instead, introducing new risks to these users compared with traditional combustible tobacco smoke.12,13 With regards to reproductive health and effects in pregnancy, the marketing of ENDS devices to adolescents through the use of flavors and colors that appeal to children has initiated public health policy initiatives including a ban on the addition of flavors in these devices.14 Only within the past 5 years have studies begun to focus on pregnant women in studying the use (or dual use) of ENDS devices, and these studies only aim to understand the prevalence of use in the prenatal period. Studies specifically focused on maternal and neonatal outcomes with ENDS use are lacking.15
Much of our understanding of nicotine exposure on fetal development comes from studies using animal models, including mouse, rat, and nonhuman primates. With such models, doses of nicotine are administered through use of implanted osmotic pumps or daily subcutaneous injections. This work has collectively shown that nicotine alone (eg, in the absence of the other components of cigarette smoke) alters fetal lung development16-18 and has metabolic and neurological consequences for the offspring.19-24 A thorough review on the developmental toxicity of nicotine describes its effects on pregnancy outcomes, fetal brain development, infant stress response, and outcomes in childhood and adolescence.25
However, while many reviews are available summarizing studies that highlight tobacco smoke and/ or nicotine exposures on the developing fetus, few focus on how these exposures affect placental development and function. In this review, we focus on studies that help us understand how exposures directly affect the organ at the maternal- fetal interface, the implications of such exposures for maternal and fetal health, and studies of Vitamin C supplementation that are currently being used in clinical trials in pregnant smokers to help offset the oxidative damage from smoking.
2. WHY STUDY THE EFFECT OF TOBACCO AND NICOTINE EXPOSURES ON THE PLACENTA?
Proper placental development and function is essential for maintaining pregnancy and fundamentally influences the health of the developing fetus; therefore, anything which disrupts placental development and function has the potential to disrupt fetal growth and development. The first cellular differentiation event in human development, which occurs as the blastocyst forms, is the differentiation of stem cells to trophoblasts, which give rise to all extraembryonic tissues, including the placenta. Classically, the placenta has been considered to be an effective maternal-fetal barrier. However, a more holistic view suggests that the placenta functions as an effective conduit and channel of communication between mother and fetus.26 Thus, the placenta is unique as it serves as both a metabolic and endocrine organ and as a conduit by which potential toxins and reactive compounds can be converted to less harmful intermediates prior to reaching the fetus. Abnormal placentation has been implicated in anembryonic early miscarriage, abruption or abnormal placentation, preeclampsia /HELLP, and fetal growth restriction27,28; all of these disorders occur with varying degrees of prevalence among tobacco exposed pregnancies.
A second, and highly practical reason the placenta is a valuable tool for understanding the effects of tobacco and nicotine on fetal development is the availability of tissue for research. The placenta is typically discarded after delivery, and there are few if any ethical concerns about its use in research. Primary trophoblast cells from the chorionic villi, or the “functional unit” of the placenta, can be isolated, cultured, and treated to help understand placental function.29 Tissue can be subject to multi-omics analysis including transcriptomics,30 epigenomics,31,32 metagenomics,33-35 proteomics, and unbiased metabolomics, allowing us to compare changes on an “omics-wide level with such exposures.”
Thirdly and importantly, we and others have found that the placenta retains a footprint of the in utero experience. Specific changes have been documented in the placenta with exposures during pregnancy. The placenta is a portal and reservoir for a number of congenital viruses which do not result in evident gross placental inflammation, such as we and others demonstrated with Zika virus.36-38 Maternal obesity,39,40 gestational diabetes,41 and alcohol consumption42,43 are associated with highly predictive changes in the placenta. Levels of polycyclic aromatic hydrocarbons, a carcinogenic and toxic component of tobacco smoke, have been measured from the placenta in a study of preterm birth.44 The biochemical and molecular activities of the placenta both respond to, and are modified by, environmental insults, making the placenta a “footprint” of the in utero exposures the fetus experiences. Of interest, we have previously shown that the placenta has a unique footprint associated with exposure to maternal smoking.45-48
2.1. Tobacco smoke and placental development
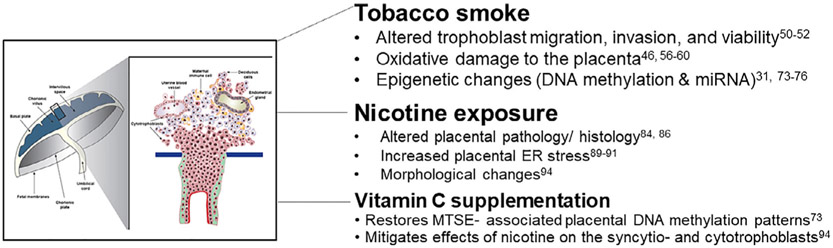
While the association between tobacco use and lower birthweight has been known for decades, how cigarette smoke exposure influences the growth restriction observed with these pregnancies remains unknown. Furthermore, not every neonate exposed in utero to maternal tobacco smoke exposure (MTSE) is growth restricted. Understanding the placental response to MTSE may help us to understand what occurs in the fetus and may lead to improved understanding of the physiology of MTSE. A summary of findings to-date can be seen in Figure 1.
FIGURE 1.
Alterations to the structure and function of the placenta with MTSE and nicotine exposure. Tobacco smoke and nicotine exposures have been implicated in changes in the structure and function of the placenta and trophoblast cells. Some of these effects can be mitigated by Vitamin C supplementation
To tackle this problem at the cellular level, in vitro culture techniques to model the placental response have been utilized. Immortalized cell lines representing first trimester and term placental cells are commercially available, as are choriocarcinoma cell lines. Utilization of these cell lines can yield insight into the molecular changes that occur in association with environmental exposures that could not otherwise be inferred through study of the more cellular heterogenous tissue en mass. Such studies have reported changes in trophoblast migration, viability, and invasion, as well as alterations of placental immune response.
Because cigarette smoke is known to have a plethora of chemicals, in order to study these chemicals in cell culture, cigarette smoke extract (CSE) is commonly used. This is created by bubbling the “mainstream” smoke, or the smoke drawn through the cigarette through puffing, through saline.49 This aqueous solution, or CSE, can be used to treat cells in vitro and contains representative chemicals from the cigarette smoke. in vitro cell culture models using placental cells treated with CSE have reported conflicting data concerning its effects; however, it should be noted that CSE does have an impact on the trophoblast cells. Using JEG3 choriocarcinoma cells as a model, CSE treatment was shown to decrease proliferation, migration, invasion, and viability.50 A second study using JEG3 cells confirmed the decreased viability and revealed this may be due to increased levels of trophoblast apoptosis, as observed by TUNEL staining.51 However, a third study which utilized an immortalized first trimester trophoblast line, HTR-8/SVneo cells, found increased viability with CSE, as well as increased migration, and increased levels of expression of placental growth factor (PlGF), VEGF, and adrenomeddulin, a potent vasodilator.52 It is possible that the discrepancy could be due to differences in cell type utilized in these studies.
It is also possible that MTSE influences changes in the immunological cellular profile at the maternal-fetal interface. Maintenance of this immunological profile is essential for fetal tolerance and protection against infection. A study of placental macrophages isolated from term deliveries found that CSE altered immune cell function. Specifically, CSE inhibited particle uptake in these macrophages, as well as inhibited their fusion into multicellular giant cells.53 Furthermore, production of cytokines from these cells, including IL-6 and IL-33, was altered with CSE treatment.
3. TOBACCO SMOKE AND OXIDATIVE DAMAGE TO THE FETO-PLACENTA UNIT
It has long been known that tobacco smoke causes oxidative damage in the lungs of smokers.54 Given the plethora of chemicals in tobacco smoke that create reactive oxygen species through activation of the CYP P450 pathway,55 it is not surprising that this is one of the many negative aspects of cigarette smoking. Because of the known deleterious effects of smoking on the lungs, studies of oxidative damage to the placenta have been performed in human tissue and in animal models, to determine if such damage could contribute to the known adverse effects on fetal growth associated with tobacco smoke exposure.
The footprint left by MTSE in the placenta can be observed under the microscope. Histopathology to detect markers of lipid peroxidation (4-hydroxynonenal or 4-HNE) and DNA oxidation (8-hydroxy-2′deoxyguanosine or 8-OHdG) revealed that placentae from mothers who smoke have increased levels of these oxidative markers.46 Furthermore, placentae from smoking mothers have higher levels of staining for the Phase I enzyme, CYP1A1.46 While another study that investigated 8-OHdG in placentae from smokers did not observe an increase with smoking, they did find that placental 8-OHdG positively correlated with maternal cotinine plasma levels56 revealing a correlation between smoking levels and oxidative damage in the placenta.
Cells naturally produce reactive oxygen species from enzymatic processes and are thus capable of dealing with oxidative stress. However, increased, chronic levels of oxidative stress can lead to damage of the cells, especially if there is not a balance between oxidative species and antioxidants.57 Studies using placenta tissue from mothers who smoke reveal an overall decreased total antioxidant capacity, and these tissues have a shift in the oxidative/ antioxidative balance towards the oxidative side.58 Smoking is associated with a higher oxidative stress index and a higher total oxidant status in the placenta. Others have found increased markers of lipid peroxidation measuring malondialdehyde and 4-hydroxyalkenal levels in placental homogenates59 as well as increased apoptosis in these placentas as visualized by TUNEL staining. Some of these markers are also reflected in the amniotic fluid (AF). AF from smokers also has lower total antioxidant capacity and increased levels of oxidative markers.60
Molecular characterization of the placenta is not the only way that markers of oxidative damage have been implicated in adverse pregnancy outcomes with maternal smoking. Whole genome transcriptomics of placenta tissue shows that pathways involved in the response to oxidative stress, xenobiotic metabolism, and inflammation are increased with smoking.61 A study comparing changes in transcriptomics and DNA methylation in the placenta further revealed an association between these changes and oxidative stress with smoking.62 In sum, oxidative damage of the placenta is a real concern with MTSE exposure. How this damage contributes to the observed growth restriction and other adverse neonatal outcomes remains to be studied.
4. VITAMIN C; AN ANTIOXIDANT TO COMBAT THE OXIDATIVE DAMAGE FROM SMOKING?
Because smoking is known to increase oxidative stress to exposed tissues, use of an antioxidant to help combat the oxidative damage has been utilized. Specifically, studies of Vitamin C supplementation reveal that it is able to prevent some of the adverse consequences to the fetus and neonate from tobacco smoke exposure. Vitamin C is a known potent antioxidant, and supplementation is safe in pregnancy.63 Pioneering work in this field has been done by Eliot Spindel and colleagues using animal models as well as clinical trials in pregnant smokers.
Using a Rhesus macaque model of nicotine exposure in pregnancy, where nicotine is administered through an implanted osmotic pump in the pregnant macaque from day 30 of gestation onward,64 the effects of nicotine on fetal brain and lung development65,66 have been delineated. In a subset of these animals, Vitamin C is administered daily in the form of a chewable vitamin supplement.64 With supplementation, the alterations to brainstem serotonin and cardiac norepinephrine levels observed in the fetuses exposed to maternal nicotine were offset64 as was the reduced pulmonary function observed in nicotine-exposed neonate.66
Vitamin C also appears to be beneficial for use in pregnant smokers. In a multicenter, randomized, double-blind, placebo-controlled trial, daily Vitamin C supplementation or placebo was given to pregnant women who smoked tobacco cigarettes.67 Vitamin C supplementation was found to be associated with improved pulmonary function in newborns within 72 hours of delivery as well as at 3 months of age, compared with those who received placebo.68,69
5. EPIGENETIC MODIFICATIONS IN THE PLACENTA ASSOCIATED WITH MATERNAL TOBACCO USE OR EXPOSURE
Decades of research into the Developmental Origins of Health and Disease (DOHaD) have revealed that adverse events experienced during fetal life can have lifelong consequences to the exposed individual.70,71 Because there is such an extensive timespan between insult and injury, the question arises: what molecular mechanisms are at play that “write” the memory of an in utero insult where the outcome only begins to emerge many decades later?
The contribution of epigenetic changes that occur during fetal development in response to the in utero environment are of interest as a potential “writer” of this memory. Correct establishment of epigenetic modifications during development is essential for the proper organization and maintenance of the genome. Furthermore, patterns of specific epigenetic modifications aid DNA-templated events such as DNA replication, DNA damage repair, and transcriptional activation and repression. By definition, epigenetic changes do not alter the underlying DNA sequence. Knowing that epigenetic changes in fetal life can cause long-term consequences, an understanding of changes, if any, to the epigenome that occur specifically by virtue of in utero insults such as MTSE is essential.
There are many published reviews which prove a far more comprehensive overview on which epigenetic modifications are observed in the placenta, cord blood, and even among exposed offspring (buccal cells) following maternal tobacco smoke in utero.48,55,72 A recent systematic review highlights 11 manuscripts reflecting changes in DNA methylation in the placenta with MTSE.31 Due to different methodologies to study changes to the methylome, the authors were unable to define a specific gene or region of interest with tobacco smoke exposure. Of note, Vitamin C supplementation was shown to “restore” placental DNA methylation levels of smokers to those of nonsmokers compared with placebo controlled.73 Suffice it to say, changes in DNA methylation are not the only epigenetic changes occurring in the placenta with MTSE. Specific noncoding micro-RNAs (miR-16, miR-21, and miR-146a) are altered in the placenta with MTSE.74 Furthermore, smoking is associated with a decrease in overall mitochondrial DNA (mtDNA) content and an increase in mtDNA methylation.75 In a study investigating associations between placental and fetal lung telomere length, smoking was associated with shortened relative fetal telomere length.76
5.1. Nicotine and placental development
5.1.1. Placental nAChRs
Nicotine is the addictive component of combustible tobacco cigarettes, and the primary exogenous additive of electronic nicotine delivery systems (ENDS). Nicotine crosses the placenta readily,77 and levels in fetal serum and AF have been reported to be higher than in corresponding maternal serum.78 Therefore, while the effects of ENDS devices like e-cigarettes are not fully understood,79-81 the effect of nicotine has been studied in animal models for decades.
Nicotine's effect in biological systems is due to binding of this compound with nicotinic acetyl choline receptors (nAChRs). nAChRs are stimulated by acetyl choline, which is released from cholinergic neurons as well as non-neuronal cells. While acetyl choline is the endogenous agonist, nicotine is able to bind to the alpha subunit of the complex and activate these receptors.82 nAChRs, their binding, activation, and signaling cascades have been well studied in the brain, but these receptors are also expressed in non-neuronal tissues (reviewed in Zoli et al83). nAChRs are found in skin, muscle, and bronchial epithelial cells as well as within the placenta. While the signaling cascades which occur upon activation of neuronal nAChRs have been well characterized, what cascades are activated in non-neuronal cells remain poorly understood.
nAChRs have been implicated in many cellular processes in non-neuronal cells including proliferation, adhesion, and migration, all of which are important processes in placental development. A characterization of the localization and distribution of nAChRs in human placenta revealed all mammalian nAChR α-subunits (α2e7 and α9e10) mRNAs were detected in human term placenta by RT-PCR.82 Immunofluorescence revealed the subunits to be localized to syncytiotrophoblasts and cytotrophoblasts, terminal villi, mesynchymal cells, and Hofbauer cells.82 Nicotine treatment in vivo and in vitro increased the alpha 4 subunit.84 In humans, maternal smoking is associated with altered levels of the nAChRs in term placenta.85
6. THE EFFECTS OF NICOTINE ON THE PLACENTA
Animal models have been instrumental in our understanding how nicotine affects placental development. Halloway et al published a comprehensive and elegant set of investigations on the effects of nicotine on the placenta in both an in vivo rat model as well as in vitro using cell culture.84 Nicotine was administered to pregnant rats through daily subcutaneous injections. At gestational day 15 of 21 days gestation, no change in fetal nor placental weight was observed. Pathological and histological analyses of the placenta showed decreased decidua and junctional zone along with decreased vascular branching of the placenta.84 Another study in a rat model of prenatal nicotine administration also found decreases in the labyrinth and junctional zone of the rodent placenta.86
While Holloway et al did not find changes in expression levels of genes involved in electron transport, changes in expression of trophoblast differentiation genes were observed.84 Histology revealed limited trophoblast migration in the placenta. Follow-up of these observations using a rat trophoblast cell culture model (RCHO-1 cell line) confirmed that differentiation was inhibited with nicotine treatment as was matrigel migration with the addition of nicotine. In vitro, nicotine treatment of HTR8-SVneo cells showed no change in cell viability, migration, culture growth, or proliferation.87 In 3A cells (trophoblasts), no change in cell viability with nicotine treatment was observed.88 In a rat model of nicotine exposure, placental levels of activated PERK were observed, which is an indicator of ER stress.89 Using BeWo cells, which is a choriocarcinoma cell line, treatment with nicotine for 72 hours leads to ER stress, as observed through an altered GRP78/BiP ratio and decreased amount of phosphorylated JNK.90 In a separate study using RCHO-1 cells, a rat trophoblast cell line, nicotine treatment increased phospho-PERK; this increase could be prevented with pretreatment with a nAChR antagonist. It was also found that nicotine treatment increased 11 beta HSD11b expression.91
Using the Rhesus macaque model of nicotine exposure in pregnancy,66,92,93 Vitamin C supplementation has been studied to determine if this potent antioxidant could counteract the oxidative damage from nicotine.94 The pregnant macaques underwent Dynamic Contrast Enhanced Magnetic Resonance Imaging (DCE-MRI) and ultrasound (US) to investigate placental blood flow in the presence or absence of nicotine and/or Vitamin C. Nicotine caused morphological changes to the placenta, specifically increased syncytiotrophoblasts sprouting and cytotrophoblast islands, both of which were mitigated with Vitamin C supplementation. Use of DCE-MRI showed no change in uterine artery volume blood flow. However, US revealed reduced placental blood flow. Of interest, levels of nicotine and cotinine were increased in AF in animals with Vitamin C supplementation compared with nicotine alone.
Soluble VEGF receptor-1 (sFlt-1) is a peptide secreted by the placenta and has been implicated in the etiology of preeclampsia.95 Therefore, an understanding of how nicotine may alter proper sFlt-1 expression is important for pregnancy health. Of interest, tobacco smoking during pregnancy has been repeatedly reported to protect against preeclampsia.4,96 Studies of nicotine treatment on the placenta using cell culture models show conflicting results. In one study using human cytotrophoblast cell line 3A, nicotine treatment reduced sFlt-1 secretion.88 Another study using BeWo cells found no change in sFlt-1 secretion with nicotine treatment unless the cells were cultured under hypoxic conditions, which then decreased expression.97 Using a primary culture of trophoblasts, nicotine decreases sFlt-1 release only in the presence of cotinine.98 Studies which investigate sFlt-1 levels in an in vivo model of pregnancy with nicotine exposure are necessary to determine how sFlt-1 levels are altered.
6.1. Summary of findings and future directions
The public health messages warning pregnant women not to smoke have been unequivocal: smoking during pregnancy harms both mother and the developing fetus. In the time since these warnings were issued, nicotine replacement products have been introduced. However, the public health message against nicotine use in pregnancy outside of smoking has not been as strong. With the popularity of ENDS devices continuing to grow, especially in adolescents and women of reproductive age, an evolving public health message will need to emerge; one that helps these young women understand the effects of ENDS use and nicotine exposure on fertility and pregnancy. Currently, studies of e-cigarette perception and use in pregnancy are available, and ENDS use in pregnancy is estimated to be between 1.2% and 5%.15,99 To-date, there are no published studies as to how ENDS use affects pregnancy outcome or fetal development. Such studies are lacking and yet sorely needed for physicians to understand the unique risks associated with ENDS use in pregnancy.
A second warning is thus now important to communicate: no amount of nicotine is known to be safe in pregnancy. None.
Epidemiological studies, research using animal models, and in vitro studies all support these messages. In association with maternal smoking, neonates are born at a lower birthweight and earlier gestational age, have a higher risk of developing lung problems, and are more prone to asthma and allergy in childhood. Furthermore, studies of the placenta and its component cells have allowed us to understand some of the cause and consequence of the adverse effects of tobacco and nicotine exposure in utero. It will be important, moving forward, to include studies of the placenta when interrogating the effects of ENDS use in pregnancy. These studies will be invaluable to inform the public health message about the inherent risk of ENDS use in pregnancy.
What is already known about this topic?
Tobacco and nicotine exposures have specific effects on fetal and placental development
No known amount of nicotine is safe in pregnancy
What does this study add?
This is the first review to our knowledge on the effects of nicotine and tobacco use on placenta function and development.
ACKNOWLEDGMENTS
The authors acknowledge funding from the NIH to support this work (HD075858 to M.A.S. and R01HD091731 and R01DK089201 to K.M.A.)
Funding information
NIH, Grant/Award Numbers: R01DK089201, R01HD091731, HD075858
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL STATEMENT
As this is a review of the published literature, ethical approval was not required.
REFERENCES
- 1.Simpson WJ. A preliminary report on cigarette smoking and the incidence of prematurity. Am J Obstet Gynecol. 1957;73(4):807–815. [PubMed] [Google Scholar]
- 2.Lowe CR. Effect of mothers' smoking habits on birth weight of their children. Br Med J. 1959;2(5153):673–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpoim PN, Godoi LC, Pinheiro MB, Freitas LG, Carvalho MDG, Dusse LM. The unexpected beneficial role of smoking in preeclampsia. Clin Chim Acta. 2016;459:105–108. [DOI] [PubMed] [Google Scholar]
- 4.Wei J, Liu CX, Gong TT, Wu QJ, Wu L. Cigarette smoking during pregnancy and preeclampsia risk: a systematic review and meta-analysis of prospective studies. Oncotarget. 2015;6(41):43667–43678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mo Dimes. March of Dimes: PeriStats Database [Available from: marchofdimes.org/peristats. 2019 [Google Scholar]
- 6.Heminger CL, Schindler-Ruwisch JM, Abroms LC. Smoking cessation support for pregnant women: role of mobile technology. Subst Abuse Rehabil. 2016;7:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths SE, Parsons J, Naughton F, Fulton EA, Tombor I, Brown KE. Are digital interventions for smoking cessation in pregnancy effective? A systematic review and meta-analysis. Health Psychol Rev. 2018;12(4):333–356. [DOI] [PubMed] [Google Scholar]
- 8.Sloan M, Hopewell S, Coleman T, Cooper S, Naughton F. Smoking cessation support by text message during pregnancy: a qualitative study of views and experiences of the MiQuit intervention. Nicotine Tob Res. 2017;19(5):572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naughton F, Cooper S, Foster K, et al. Large multi-centre pilot randomized controlled trial testing a low-cost, tailored, self-help smoking cessation text message intervention for pregnant smokers (MiQuit). Addiction. 2017;112(7):1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abroms LC, Johnson PR, Heminger CL, et al. Quit4baby: results from a pilot test of a mobile smoking cessation program for pregnant women. JMIR Mhealth Uhealth. 2015;3(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhalerao A, Sivandzade F, Archie SR, Cucullo L. Public health policies on E-cigarettes. Curr Cardiol Rep. 2019;21(10):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotts JE, Jordt SE, McConnell R, Tarran R. What are the respiratory effects of e-cigarettes? BMJ. 2019;366:l5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDonald A, Middlekauff HR. Electronic cigarettes and cardiovascular health: what do we know so far? Vasc Health Risk Manag. 2019;15:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statement from FDA Commissioner Scott Gottlieb, M.D., on advancing new policies aimed at preventing youth access to, and appeal of, flavored tobacco products, including e-cigarettes and cigars [press release]. March/13/2019 2019. [Google Scholar]
- 15.Cardenas VM, Fischbach LA, Chowdhury P. The use of electronic nicotine delivery systems during pregnancy and the reproductive outcomes: a systematic review of the literature. Tob Induc Dis. 2019;17:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehan VK, Wang Y, Sugano S, et al. In utero nicotine exposure alters fetal rat lung alveolar type II cell proliferation, differentiation, and metabolism. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L323–L333. [DOI] [PubMed] [Google Scholar]
- 17.Krebs M, Sakurai R, Torday JS, Rehan VK. Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp Lung Res. 2010;36(7):390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maritz GS. Perinatal exposure to nicotine and implications for subsequent obstructive lung disease. Paediatr Respir Rev. 2013;14(1):3–8. [DOI] [PubMed] [Google Scholar]
- 19.Gao YJ, Holloway AC, Su LY, Takemori K, Lu C, Lee RM. Effects of fetal and neonatal exposure to nicotine on blood pressure and perivascular adipose tissue function in adult life. Eur J Pharmacol. 2008;590(1–3):264–268. [DOI] [PubMed] [Google Scholar]
- 20.Gao YJ, Holloway AC, Zeng ZH, et al. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res. 2005;13(4):687–692. [DOI] [PubMed] [Google Scholar]
- 21.Holloway AC, Lim GE, Petrik JJ, Foster WG, Morrison KM, Gerstein HC. Fetal and neonatal exposure to nicotine in Wistar rats results in increased beta cell apoptosis at birth and postnatal endocrine and metabolic changes associated with type 2 diabetes. Diabetologia. 2005;48(12):2661–2666. [DOI] [PubMed] [Google Scholar]
- 22.Muneoka K, Ogawa T, Kamei K, Mimura Y, Kato H, Takigawa M. Nicotine exposure during pregnancy is a factor which influences serotonin transporter density in the rat brain. Eur J Pharmacol. 2001;411(3):279–282. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Rahman A, Dechkovskaia AM, Sutton JM, et al. Maternal exposure of rats to nicotine via infusion during gestation produces neuro-behavioral deficits and elevated expression of glial fibrillary acidic protein in the cerebellum and CA1 subfield in the offspring at puberty. Toxicology. 2005;209(3):245–261. [DOI] [PubMed] [Google Scholar]
- 24.Slotkin TA, Cho H, Whitmore WL. Effects of prenatal nicotine exposure on neuronal development: selective actions on central and peripheral catecholaminergic pathways. Brain Res Bull. 1987;18(5):601–611. [DOI] [PubMed] [Google Scholar]
- 25.England LJ, Aagaard K, Bloch M, et al. Developmental toxicity of nicotine: a transdisciplinary synthesis and implications for emerging tobacco products. Neurosci Biobehav Rev. 2017;72:176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lash GE. Molecular cross-talk at the feto-maternal Interface. Cold Spring Harb Perspect Med. 2015;5(12):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eastabrook G, Brown M, Sargent I. The origins and end-organ consequence of pre-eclampsia. Best Pract Res Clin Obstet Gynaecol. 2011; 25(4):435–447. [DOI] [PubMed] [Google Scholar]
- 28.Albu AR, Anca AF, Horhoianu VV, Horhoianu IA. Predictive factors for intrauterine growth restriction. J Med Life. 2014;7(2):165–171. [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas GC, King BF. Isolation of pure villous cytotrophoblast from term human placenta using immunomagnetic microspheres. J Immunol Methods. 1989;119(2):259–268. [DOI] [PubMed] [Google Scholar]
- 30.Pavlicev M, Wagner GP, Chavan AR, et al. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Res. 2017;27(3):349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palma-Gudiel H, Cirera F, Crispi F, Eixarch E, Fananas L. The impact of prenatal insults on the human placental epigenome: a systematic review. Neurotoxicol Teratol. 2018;66:80–93. [DOI] [PubMed] [Google Scholar]
- 32.Januar V, Desoye G, Novakovic B, Cvitic S, Saffery R. Epigenetic regulation of human placental function and pregnancy outcome: considerations for causal inference. Am J Obstet Gynecol. 2015;213(4 Suppl):S182–S196. [DOI] [PubMed] [Google Scholar]
- 33.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antony KM, Ma J, Mitchell KB, Racusin DA, Versalovic J, Aagaard K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obstet Gynecol. 2015;212(5):653 e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prince AL, Ma J, Kannan PS, Alvarez M, Gisslen T, Harris RA, et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol. 2016;214(5):627 e1– e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aagaard KM, Lahon A, Suter MA, et al. Primary human placental trophoblasts are permissive for Zika virus (ZIKV) replication. Sci Rep. 2017;7:41389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seferovic MD, Turley M, Valentine GC, et al. Clinical importance of placental testing among suspected cases of congenital Zika syndrome. Int J Mol Sci. 2019;20(3):712–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suter MA, Aagaard KM. Disease watch: Zika virus—placental passage and permissivity for infection. Nat Rev Endocrinol. 2016;12(8):437–438. [DOI] [PubMed] [Google Scholar]
- 39.Howell KR, Powell TL. Effects of maternal obesity on placental function and fetal development. Reproduction. 2017;153(3):R97–R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myatt L, Maloyan A. Obesity and placental function. Semin Reprod Med. 2016;34(1):42–49. [DOI] [PubMed] [Google Scholar]
- 41.Edu A, Teodorescu C, Dobjanschi CG, et al. Placenta changes in pregnancy with gestational diabetes. Rom J Morphol Embryol. 2016;57(2):507–512. [PubMed] [Google Scholar]
- 42.Davis-Anderson KL, Berger S, Lunde-Young ER, et al. Placental proteomics reveal insights into fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2017;41(9):1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tai M, Piskorski A, Kao JC, Hess LA, MdlM S, Gundogan F. Placental morphology in fetal alcohol spectrum disorders. Alcohol Alcohol. 2017;52(2):138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suter MA, Aagaard KM, Coarfa C, et al. Association between elevated placental polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts from superfund sites in Harris County, and increased risk of preterm birth (PTB). Biochem Biophys Res Commun. 2019;516(2):344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suter M, Abramovici A, Showalter L, et al. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism. 2010;59(10):1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sbrana E, Suter MA, Abramovici AR, Hawkins HK, Moss JE, Patterson L, et al. Maternal tobacco use is associated with increased markers of oxidative stress in the placenta. Am J Obstet Gynecol. 2011;205(3):246 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suter M, Ma J, Harris AS, et al. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics. 2011;6(11):1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suter MA, Aagaard K. What changes in DNA methylation take place in individuals exposed to maternal smoking in utero? Epigenomics. 2012;4(2):115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gellner CA, Reynaga DD, Leslie FM. Cigarette smoke extract: a pre-clinical model of tobacco dependence. Curr Protoc Neurosci. 2016;77:9 54 1–9 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim CW, Lee HM, Lee K, Kim B, Lee MY, Choi KC. Effects of cigarette smoke extracts on cell cycle, cell migration and endocrine activity in human placental cells. Reprod Toxicol. 2017;73:8–19. [DOI] [PubMed] [Google Scholar]
- 51.Lee HM, Choi KC. Cigarette smoke extract and isoprene resulted in the induction of apoptosis and autophagy in human placenta choriocarcinoma JEG-3 cells. Environ Toxicol. 2018;33(2):178–190. [DOI] [PubMed] [Google Scholar]
- 52.Beiswenger TR, Feng L, Brown HL, Heine RP, Murtha AP, Grotegut CA. The effect of cigarette smoke extract on trophoblast cell viability and migration: the role of adrenomedullin. Reprod Sci. 2012;19(5):526–533. [DOI] [PubMed] [Google Scholar]
- 53.Belhareth R, Mezouar S, Ben Amara A, et al. Cigarette smoke extract interferes with placenta macrophage functions: a new mechanism to compromise placenta functions? Reprod Toxicol. 2018;78:120–129. [DOI] [PubMed] [Google Scholar]
- 54.Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 2018;15(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suter MA, Anders AM, Aagaard KM. Maternal smoking as a model for environmental epigenetic changes affecting birthweight and fetal programming. Mol Hum Reprod. 2013;19(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossner P Jr, Milcova A, Libalova H, et al. Biomarkers of exposure to tobacco smoke and environmental pollutants in mothers and their transplacental transfer to the foetus. Part II. Oxidative Damage. Mutat Res 2009;669(1–2):20–26. [DOI] [PubMed] [Google Scholar]
- 57.Rahal A, Kumar A, Singh V, et al. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aycicek A, Varma M, Ahmet K, Abdurrahim K, Erel O. Maternal active or passive smoking causes oxidative stress in placental tissue. Eur J Pediatr. 2011;170(5):645–651. [DOI] [PubMed] [Google Scholar]
- 59.Garrabou G, Hernandez AS, Catalan Garcia M, et al. Molecular basis of reduced birth weight in smoking pregnant women: mitochondrial dysfunction and apoptosis. Addict Biol. 2016;21(1):159–170. [DOI] [PubMed] [Google Scholar]
- 60.Dittrich R, Schibel A, Hoffmann I, Mueller A, Beckmann MW, Cupisti S. Influence of maternal smoking during pregnancy on oxidant status in amniotic fluid. In Vivo. 2012;26(5):813–818. [PubMed] [Google Scholar]
- 61.Votavova H, Dostalova Merkerova M, Fejglova K, et al. Transcriptome alterations in maternal and fetal cells induced by tobacco smoke. Placenta. 2011;32(10):763–770. [DOI] [PubMed] [Google Scholar]
- 62.Suter M, Ma J, Harris A, et al. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics. 2011;6(11):1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rumbold A, Ota E, Nagata C, Shahrook S, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2015;9:CD004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slotkin TA, Seidler FJ, Qiao D, et al. Effects of prenatal nicotine exposure on primate brain development and attempted amelioration with supplemental choline or vitamin C: neurotransmitter receptors, cell signaling and cell development biomarkers in fetal brain regions of rhesus monkeys. Neuropsychopharmacology. 2005;30(1):129–144. [DOI] [PubMed] [Google Scholar]
- 65.Slotkin TA, Seidler FJ, Spindel ER. Prenatal nicotine exposure in rhesus monkeys compromises development of brainstem and cardiac monoamine pathways involved in perinatal adaptation and sudden infant death syndrome: amelioration by vitamin C. Neurotoxicol Teratol. 2011;33(3):431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Proskocil BJ, Sekhon HS, Clark JA, et al. Vitamin C prevents the effects of prenatal nicotine on pulmonary function in newborn monkeys. Am J Respir Crit Care Med. 2005;171(9):1032–1039. [DOI] [PubMed] [Google Scholar]
- 67.McEvoy CT, Milner KF, Scherman AJ, et al. Vitamin C to decrease the effects of smoking in pregnancy on infant lung function (VCSIP): rationale, design, and methods of a randomized, controlled trial of vitamin C supplementation in pregnancy for the primary prevention of effects of in utero tobacco smoke exposure on infant lung function and respiratory health. Contemp Clin Trials. 2017;58:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McEvoy CT, Shorey-Kendrick LE, Milner K, et al. Oral vitamin C (500 mg/d) to pregnant smokers improves infant airway function at 3 months (VCSIP). A randomized trial. Am J Respir Crit Care Med. 2019;199(9):1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McEvoy CT, Schilling D, Clay N, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311(20):2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suter MA, Aagaard-Tillery KM. Environmental influences on epigenetic profiles. Semin Reprod Med. 2009;27(5):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.El Hajj N, Schneider E, Lehnen H, Haaf T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction. 2014;148(6):R111–R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suter M, Abramovici A, Aagaard-Tillery K. Genetic and epigenetic influences associated with intrauterine growth restriction due to in utero tobacco exposure. Pediatr Endocrinol Rev. 2010;8(2):94–102. [PMC free article] [PubMed] [Google Scholar]
- 73.Shorey-Kendrick LE, McEvoy CT, Ferguson B, et al. Vitamin C prevents offspring DNA methylation changes associated with maternal smoking in pregnancy. Am J Respir Crit Care Med. 2017;196(6):745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF, Marsit CJ. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010;5(7):583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janssen BG, Gyselaers W, Byun HM, et al. Placental mitochondrial DNA and CYP1A1 gene methylation as molecular signatures for tobacco smoke exposure in pregnant women and the relevance for birth weight. J Transl Med. 2017;15(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirzakhani H, De Vivo I, Leeder JS, et al. Early pregnancy intrauterine fetal exposure to maternal smoking and impact on fetal telomere length. Eur J Obstet Gynecol Reprod Biol. 2017;218:27–32. [DOI] [PubMed] [Google Scholar]
- 77.Luck W, Nau H. Exposure of the fetus, neonate, and nursed infant to nicotine and cotinine from maternal smoking. N Engl J Med. 1984;311(10):672. [DOI] [PubMed] [Google Scholar]
- 78.Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8(6):384–395. [DOI] [PubMed] [Google Scholar]
- 79.Northrup TF, Klawans MR, Villarreal YR, et al. Family physicians' perceived prevalence, safety, and screening for cigarettes, marijuana, and electronic-nicotine delivery systems (ENDS) use during pregnancy. J Am Board Fam Med. 2017;30(6):743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kahr MK, Padgett S, Shope CD, et al. A qualitative assessment of the perceived risks of electronic cigarette and hookah use in pregnancy. BMC Public Health. 2015;15:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suter MA, Mastrobattista J, Sachs M, Aagaard K. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth Defects Res A Clin Mol Teratol. 2015;103(3):186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lips KS, Bruggmann D, Pfeil U, Vollerthun R, Grando SA, Kummer W. Nicotinic acetylcholine receptors in rat and human placenta. Placenta. 2005;26(10):735–746. [DOI] [PubMed] [Google Scholar]
- 83.Zoli M, Pucci S, Vilella A, Gotti C. Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr Neuropharmacol. 2018;16(4):338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holloway AC, Salomon A, Soares MJ, et al. Characterization of the adverse effects of nicotine on placental development: in vivo and in vitro studies. Am J Physiol Endocrinol Metab. 2014;306(4):E443–E456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Machaalani R, Ghazavi E, Hinton T, Waters KA, Hennessy A. Cigarette smoking during pregnancy regulates the expression of specific nicotinic acetylcholine receptor (nAChR) subunits in the human placenta. Toxicol Appl Pharmacol. 2014;276(3):204–212. [DOI] [PubMed] [Google Scholar]
- 86.Zhou J, Liu F, Yu L, et al. nAChRs-ERK1/2-Egr-1 signaling participates in the developmental toxicity of nicotine by epigenetically down-regulating placental 11beta-HSD2. Toxicol Appl Pharmacol. 2018;344:1–12. [DOI] [PubMed] [Google Scholar]
- 87.Correia-Branco A, Keating E, Martel F. Placentation-related processes in a human first-trimester extravillous trophoblast cell line (HTR-8/SVneo cells) are affected by several xenobiotics. Drug Chem Toxicol. Epub 2018 May 3. 2019. September; 42(5):541–545. 10.1080/01480545.2018.1463240 [DOI] [PubMed] [Google Scholar]
- 88.Kwon JY, Bai SW, Kwon YG, et al. The effect of nicotine on the production of soluble fms-like tyrosine kinase-1 and soluble endoglin in human umbilical vein endothelial cells and trophoblasts. Acta Obstet Gynecol Scand. 2010;89(4):565–571. [DOI] [PubMed] [Google Scholar]
- 89.Wong MK, Nicholson CJ, Holloway AC, Hardy DB. Maternal nicotine exposure leads to impaired disulfide bond formation and augmented endoplasmic reticulum stress in the rat placenta. PLoS One. 2015;10(3):e0122295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Repo JK, Pesonen M, Mannelli C, Vahakangas K, Loikkanen J. Exposure to ethanol and nicotine induces stress responses in human placental BeWo cells. Toxicol Lett. 2014;224(2):264–271. [DOI] [PubMed] [Google Scholar]
- 91.Wong MK, Holloway AC, Hardy DB. Nicotine directly induces endoplasmic reticulum stress response in rat placental Trophoblast Giant cells. Toxicol Sci. 2016;151(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sekhon HS, Jia Y, Raab R, et al. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest. 1999;103(5):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med. 2001;164(6):989–994. [DOI] [PubMed] [Google Scholar]
- 94.Lo JO, Schabel MC, Roberts VH, Morgan TK, Rasanen JP, Kroenke CD, et al. Vitamin C supplementation ameliorates the adverse effects of nicotine on placental hemodynamics and histology in nonhuman primates. Am J Obstet Gynecol. 2015;212(3):370 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao S, Gu Y, Fan R, Groome LJ, Cooper D, Wang Y. Proteases and sFlt-1 release in the human placenta. Placenta. 2010;31(6):512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giannakou K, Evangelou E, Papatheodorou SI. Genetic and non-genetic risk factors for pre-eclampsia: umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet Gynecol. 2018;51(6):720–730. [DOI] [PubMed] [Google Scholar]
- 97.Zhao H, Wu L, Wang Y, et al. Nicotine promotes vascular endothelial growth factor secretion by human trophoblast cells under hypoxic conditions and improves the proliferation and tube formation capacity of human umbilical endothelial cells. Reprod Biomed Online. 2017;34(4):406–413. [DOI] [PubMed] [Google Scholar]
- 98.Romani F, Lanzone A, Tropea A, Tiberi F, Catino S, Apa R. Nicotine and cotinine affect the release of vasoactive factors by trophoblast cells and human umbilical vein endothelial cells. Placenta. 2011;32(2):153–160. [DOI] [PubMed] [Google Scholar]
- 99.Hawkins SS, Wylie BJ, Hacker MR. Use of ENDS and cigarettes during pregnancy. Am J Prev Med. 2020;58(1):122–128. [DOI] [PubMed] [Google Scholar]