Abstract
Inhalation of particulate matter (PM) radioactivity is an important pathway of ionizing radiation exposure. We investigated the associations between short-term exposures to PM gamma radioactivity with oxidative stress in COPD patients. Urinary concentrations of 8-hydroxy-2’-deoxyguanosine (8-OHdG) and malondialdehyde (MDA) of 81 COPD patients from Eastern Massachusetts were measured 1-4 times during 2012-2014. Daily ambient and indoor PM gamma activities (gamma-3 through gamma-9) were calculated based on EPA RadNet data and indoor-outdoor infiltration ratios. Linear mixed effects models were used to examine the associations between biomarkers with PM gamma activities for moving averages from urine collection day to 7 days before. Our results indicate that ambient and indoor PM gamma activities were positively associated with 8-OHdG, with stronger effects for exposure windows closer to urine collection day. For per IQR increase in indoor PM gamma activities averaged over urine collection day and 1 day before, 8-OHdG increased from 3.41% (95%CI: −0.88, 7.88) to 8.87% (95%CI: 2.98, 15.1), adjusted for indoor black carbon. For MDA, the timing of greatest effects across the exposure week varied but was nearly all positive. These findings provide insight into the toxigenic properties associated with PM radioactivity and suggest that these exposures promote systemic oxidative stress.
Keywords: exposure, radiation, particle gamma radioactivity, oxidative stress, chronic obstructive pulmonary disease (COPD)
Introduction
Chronic obstructive pulmonary disease (COPD) is an irreversible chronic inflammatory lung disease and is one of the leading causes of morbidity and mortality worldwide.1 In 2015, 174 million people had COPD2 and 3.2 million people died from COPD3, while in the U.S., COPD was the 4th most common cause of death in 20164. Oxidative stress may promote more severe disease when free radicals react with biomolecules (e.g., DNA and lipids).5,6 Oxidative stress biomarkers increase during COPD exacerbations, which also contribute to COPD-related morbidity and mortality.7-9
Exposure to particulate matter (PM) and its components has been linked to increases in oxidative stress biomarkers in the general population, including COPD patients, who have a higher vulnerability to air pollution exposure.10-16 As COPD patients spend most of their time indoors, exposures to indoor pollutants may be especially important for their health. Indoor pollution exposure has been shown to be responsible for over one-third of premature COPD deaths in adults in low and middle-income countries.17 We have previously demonstrated that greater exposure to indoor black carbon (BC) in PM was associated with an increase of oxidative stress biomarkers in COPD patients.16
One hypothesized mechanism that contributes to the adverse effects of PM involves natural radionuclides that attach to airborne particles. Environmental radioactive nuclei from background sources, i.e., natural terrestrial and extra-terrestrial, can attach to respirable particulate matter (PM).18 PM radioactivity can be carried into the human body by inhalation.18,19 Previous studies have found associations between PM radioactivity with biomarkers of increased systemic inflammation in a general population cohort20 and increased blood pressure and reduced pulmonary function in a cohort of elderly men.21-22 As COPD patients are more vulnerable to air pollution exposure,15,23 it is possible that PM associated radioactivity, even with short-term ambient and indoor exposures, is associated with increases in oxidative stress in COPD patients. However, no study has linked these exposures to oxidative stress in COPD patients.
The aim of this study is to investigate the association between exposures to PM radioactivity and biomarkers of oxidative stress in COPD patients. We investigated the associations between short-term (up to one week) exposures to PM gamma activities (surrogates of PM radioactivity), including ambient and indoor exposures, with urinary oxidative stress biomarkers, 8-hydroxy-2’-deoxyguanosine (8-OHdG) and malondialdehyde (MDA), in 81 COPD patients from Eastern Massachusetts, USA. Previously in this cohort we reported associations between exposures to gamma PM radioactivity with increases in systemic inflammation and decreases in pulmonary function assessed up to one week after exposures.18,24
Methods
Study population
Participants were enrolled in the COPD and Air Pollution Study.16,25 We recruited participants at the VA Boston Healthcare System between November 2012 and December 2014. We identified potential participants by reviewing medical records of VA Boston pulmonary, primary care, and pulmonary function clinic encounters, with ICD-9 codes 490–493 and 496. We included participants if they were at least 40 years old, former smokers with a smoking history of at least 10 pack years, had physician-diagnosed COPD, and had a ratio of forced expiratory volume in one second (FEV1) over forced vital capacity (FVC) on post-bronchodilator spirometry less than 0.70 or emphysema on a clinical CT scan report. We excluded individuals if they had a history of any malignancies other than stable skin cancer or prostate cancer; were smokers or lived with smokers; had important indoor sources such as wood stove, fireplace or candles at the time of study entry. Each participant attended clinic visits 1-4 times per year (roughly three months apart) for urine collection and completed questionnaires about demographics, lifestyle factors, health history and home characteristics.
Our study protocol was approved by Institutional Review Boards at VA Boston and Harvard Medical School. We obtained informed consent from all participants prior to study procedures.
Urinary oxidative stress biomarkers
Urine samples were analysed for 8-OHdG, which represents the oxidative damage of DNA; and MDA, which represents the oxidative degradation of lipids. There are two forms of MDA: total and free MDA. Free MDA is unbound to proteins, while total MDA includes both the unbound and bound forms. As free MDA is likely explained by the binding of MDA to protein molecules in the urine, total urinary MDA may be a better indicator of lipid peroxidation.16,26 Therefore, we used total MDA in the urine to represent lipid peroxidation in the current study.
Urinary concentration of 8-OHdG was measured using a HPLC-ESI-MS system.27 MDA was measured using a HPLC system with fluorescent detection, with the exception of an added alkaline hydrolysis step prior to sample extraction procedures.28 Details regarding analyses have been published elsewhere.16
Assessment of PM gamma activities
Hourly ambient PM gamma activities of different energy ranges (101-2200 keV) were obtained in counts per minute (CPM) from the US Environmental Protection Agency (EPA) RadNet website.29,30 The energy ranges are represented as separate gamma channels as shown in Table S1 in the Supplementary Information. Each EPA air radiation monitoring station is equipped with a Total Suspended Particle (TSP) high volume air sampler. The gamma radiation detector is a NaI(Tl) scintillation crystal, sampling at 60m3/hr and positioned above the TSP filter and connected to a 1,024-channel multi-channel analyzer and a local processing unit. The hourly PM gamma activity data (from 0:00 to 23:00) were averaged into daily measurements for each channel. We did not include gamma2 in our analysis as over 25% of the data were missing.
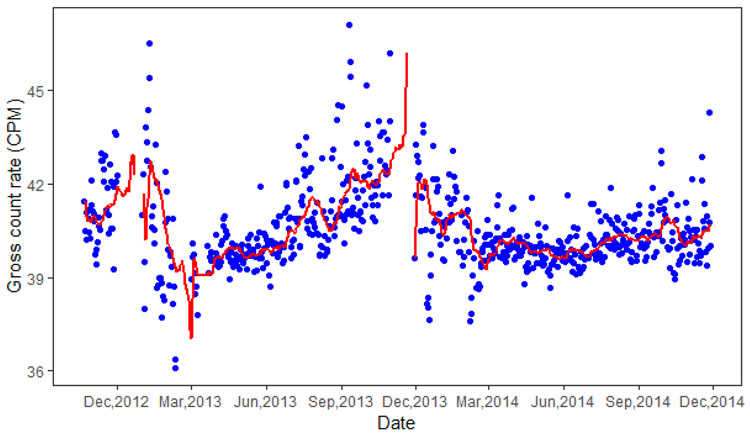
The gamma spectrometer from RadNet stationary air monitors are not well-shielded, therefore, measurement of gamma activities may be influenced by background terrestrial and cosmic radiation, which exhibit a small seasonal variability.31 In order to estimate the PM associated gamma activities (PM gamma activities), we subtracted from the RadNet daily gamma activities an average baseline signal that represents background radiation. We assessed different averaging periods that included 7, 21, 28 and 90 days before measurement day for the background levels. Finally, we selected the average of the previous 21 days to represent the background level, as corrected ambient PM gamma activities using this averaging period had the best correlation between ambient PM2.5 mass exposures (p<0.05).24 Fig. 1 shows the variability of the RadNet and corrected PM gamma-9 activity radiation. Similar plots for the other PM gamma activities (gamma-3 to gamma-8) are shown in Fig. S1 in Supplementary Information.
Fig. 1.
Daily measured gamma-9 activity (blue dots) from RadNet and 21-day moving average (red line) representing background radiation during study period.
Footnote: The red line is not continuous because of the missing of gamma activities for some dates from RadNet.
Assessment of ambient and indoor exposures to other pollutants
Prior to each clinic visit, we collected weekly indoor PM samples in the main activity room of each home, using the Harvard T.H. Chan School of Public Health (HSPH) Micro-environmental Automated Particle Sampler (APS).32 Ambient PM2.5 samples were collected daily at the Harvard Supersite located on the roof of the Harvard Medical School Library (Boston, MA). Filters were analysed using an electronic microbalance (Micro-Gravimetric M5, Mettler Instruments Corp, Hightstown, NJ) for indoor and ambient PM2.5 mass concentrations. Subsequently, a smoke stain reflectometer (model EEL m43d, Diffusion System Ltd., United Kingdom) for indoor BC concentrations and an aethalometer (Magee Scientific Company, model AE-16, Berkeley, CA) for ambient BC concentrations.33,34 Indoor nitrogen dioxide (NO2) was collected using Ogawa passive sampling badges clipped to the HSPH Micro-environmental APS, and then extracted from the filters and analyzed using ion chromatography.35 Ambient NO2 and O3 concentrations were obtained from US EPA regulatory Boston-area monitoring stations and the adjusted mean values calculated.36 We calculated the weekly ambient exposures concurrent with the indoor weekly exposures by averaging daily ambient concentrations. We used the indoor-to-outdoor sulfur (S) ratio measured in indoor and outdoor PM2.5 filter samples37 as proxy for the PM2.5 infiltration ratio. Previous studies have shown that the greatest activity fraction of ambient radioactive aerosols is in the accumulation mode, which includes particle sizes between 0.1 and 2.5 μm.38,39 Therefore, in the absence of major indoor combustion sources, indoor PM gamma activities can be estimated by multiplying the ambient PM gamma measurements by indoor-outdoor sulfur ratio for each home. For the indoor analysis we excluded observations with sulfur ratios greater than 1.1, as this was an indicator of indoor sources.32 To examine patterns of effects over the week before the clinic visit, we constructed different exposure windows averaged from the day of urine collection (day 0) up to seven days before urine collection (day7) for ambient and indoor PM-gamma activities
Covariates
We a priori included variables known to be associated with oxidative stress or PM gamma activities in our model as potential confounders or precision variables. These variables included demographics, such as age and race; health information, such as heart disease, diabetes, and body mass index (BMI); and meteorological parameters, such as temperature, humidity, season and time of the day for urine collection. We also adjusted for urine dilution using urinary creatinine concentration.
Information on demographics and health conditions were obtained from questionnaires. Age was calculated by subtracting date of birth from date of visit, and BMI was calculated using height and weight measurements taken at the visit. Daily ambient temperature at each home was estimated using an exposure model based on satellite remote sensing, land use, and ground level temperature data.40 Daily ambient relative humidity was obtained from measurements at the Boston Logan International Airport weather station.34 Season was categorized into winter (December, January, February), spring (March, April, May), summer (June, July, August), or fall (September, October, November) based on the month of the clinic visit. Urinary creatinine was measured by a colorimetric method.28 As we previously found positive associations between indoor BC and oxidative stress biomarkers in the same cohort,16 we adjusted for weekly ambient BC and indoor BC exposures for the ambient and indoor gamma models, respectively. In order to consider the possible confounding effects of other pollutants, we also performed sensitivity analyses by adjusting the indoor and ambient PM gamma radioactivity models for indoor weekly and corresponding ambient exposures to other pollutants. Specifically, we adjusted for indoor PM2.5 and NO2 in the regression models that included indoor PM gamma radioactivity and adjusted for ambient PM2.5, NO2, and O3 in the regression models that included ambient PM gamma radioactivity. We also assessed models unadjusted for any other pollutants for comparison.
Statistical methods
We fit linear mixed-effects models (using the “lme4” package in R) with random intercepts for each participant to account for the correlation among repeated observation within individuals41 to examine the associations between exposures to each of the ambient and indoor PM gamma activities (gamma-3 through gamma-9) in different exposure windows with oxidative stress biomarkers. Each biomarker was natural log-transformed to meet the linear model assumption, i.e., an approximate normal distribution for residuals. The results are presented as percent changes of each biomarker per interquartile range (IQR) increase in exposure to each of the PM gamma activities.
We examined that we had enough sample size for our linear mixed-effects models based on power-calculation for standard design (using package “sjstats” in R). The validity of model assumptions was confirmed by examination of model residuals. We consider results with p < 0.05 as statistically significant. All statistical analyses were performed using R software.
Results and Discussion
Characteristics of participants and exposures
A flow chart of participants in our cohort is shown in Fig. S2 in Supplementary Information. We included data from 81 participants with 231 clinic visits, including 28 participants followed for 4 clinic visits, 22 with 3 clinic visits, 22 with 2 clinic visits, and 9 with 1 clinic visit. Clinic visits were conducted evenly throughout the seasons. Characteristics of the 81 study participants as well as levels of biomarkers of oxidative stress are shown in Table 1. All participants were male, and 89% were white, with an average age of 72.7 years old. Furthermore, 45.2% of the subjects were obese (BMI ≥ 30 kg/m2), 24.4% had healthcare provider-diagnosed diabetes, and 50% had heart disease requiring treatment in the past 10 years.
Table 1.
Descriptive information for the 81 participants, and urinary oxidative stress biomarkers collected during up to 4 clinic visits (n=231)
| (a) Descriptive information of 81 participants | |||
| Variables | Mean (SD) | Range | |
| Age (yrs) | 72.7 (8.4) | 46.7 – 90.7 | |
| BMI (kg/m2) | 29.8 (5.8) | 18.8 – 50.8 | |
| N (%) | |||
| Race | White | 72 (88.9%) | |
| Non-White | 9 (11.1%) | ||
| Diabetes | Yes | 20 (24.7%) | |
| No | 61 (75.3%) | ||
| Heart disease | Yes | 41 (50.6%) | |
| No | 40 (59.4%) | ||
| (b) Seasons for clinic visits | |||
| N (%) | |||
| Season | Winter | 41 (17.7%) | |
| Spring | 52 (22.5%) | ||
| Summer | 72 (31.2%) | ||
| Fall | (28.6%) | ||
| (c) Biomarkers for 231 observations | |||
| Biomarker | Median (25 – 75 percentile) | Range | |
| 8-OHdG (mg/mL) | 4.4 (2.6 – 7.5) | 0.5 – 28.1 | |
| MDA (μM) | 12.6 (8.9 – 19.7) | 1.2 – 275.6 | |
| Creatinine (mg/dL) | 114.0 (71.9 – 158.1) | 10.1 – 626.8 | |
Table 2 presents the distributions of the ambient and indoor daily PM-gamma activities, ambient temperature and relative humidity on the urine collection day, and ambient and indoor weekly BC concentrations before urine collection. The estimated PM gamma activities have both negative and positive values, as they are relative estimates of daily fluctuations from average background exposures. The distributions of ambient and indoor PM gamma activities for each of the different moving averages are shown in Tables S2 and S3.
Table 2.
Ambient and indoor PM gamma activities, temperature and relative humidity on the day of urine collection (day 0), weekly BC and season of assessment in 81 COPD patients (n=231 for ambient and n=222 for indoor).
| Variables | Median (25-75th percentile) |
Range |
|---|---|---|
| Daily temperature (°C) | 13.1 (4.4 – 20.0) | −12.2 – 28.0 |
| Daily relative humidity (%) | 63.8 (52.3 – 77.6) | 30.7 – 97.0 |
| Ambient weekly BC concentration (μg/m3) | 0.54 (0.41 – 0.78) | 0.27 – 1.20 |
| Ambient weekly PM concentration (μg/m3) | 6.03 (5.02 – 7.97) | 3.64 –13.4 |
| Ambient weekly NO2 concentration (ppb) | 24.8 (21.2 – 29.7) | 14.5 – 41.4 |
| Ambient weekly O concentration (ppb) | 53.1 (44.2 – 62.6) | 26.2 – 75.6 |
| Ambient daily PM-gamma3 activity (CPM) | 4.3 (−25.6-51.1) | −195.6 - 265.5 |
| Ambient daily PM-gamma4 activity (CPM) | −0.01 (−6.1-10.0) | −53.9 - 43.4 |
| Ambient daily PM-gamma5 activity (CPM) | 1.0 (−4.5-6.6) | −37.2 - 31.4 |
| Ambient daily PM-gamma6 activity (CPM) | 0.23 (−1.6-2.7) | −25.3 - 14.3 |
| Ambient daily PM-gamma7 activity (CPM) | −0.04 (−2.4-3.7) | −26.3 - 18.6 |
| Ambient daily PM-gamma8 activity (CPM) | 0.60 (−2.9-4.3) | −26.7 - 43.4 |
| Ambient daily PM-gamma9 activity (CPM) | 0.08 (−0.43-0.44) | −3.2 - 3.2 |
| Indoor weekly BC concentration (μg/m3) | 0.18 (0.09 – 0.31) | −0.42 – 0.95 |
| Indoor weekly PM2.5 concentration (μg/m3) | 6.90 (4.78 – 8.93) | 0.26 – 42.8 |
| Indoor weekly NO2 concentration (ppb) | 12.3 (8.45 – 19.5) | 1.09 – 112 |
| Indoor weekly O concentration (ppb) | 19.6 (26.4 – 47.6) | 0.02 – 463 |
| Indoor daily PM-gamma3 activity (CPM) | −0.03 (−22.2-32.5) | −151.1 - 205.2 |
| Indoor daily PM-gamma4 activity (CPM) | −0.26 (−4.3-5.5) | −38.4 - 38.8 |
| Indoor daily PM-gamma5 activity (CPM) | 0.00 (−3.3-3.9) | −24.4 - 29.1 |
| Indoor daily PM-gamma6 activity (CPM) | 0.01 (−1.1-1.6) | −15.1 - 15.7 |
| Indoor daily PM-gamma7 activity (CPM) | −0.19 (−2.1-2.2) | −16.1 - 17.3 |
| Indoor daily PM-gamma8 activity (CPM) | 0.37 (−1.9-3.4) | −22.0 - 29.8 |
| Indoor daily PM-gamma9 activity (CPM) | 0.00 (−0.31-0.30) | −2.0 - 3.0 |
The number of observations included in each analysis varied depending on the availability of the exposures, with a maximum number of 231 for ambient models and up to 222 for indoor models (Supplemental Tables S1 and S2).
Associations between PM gamma activities exposure and biomarkers of oxidative stress
We assessed the associations between ambient and indoor PM gamma activities starting with the urine collection day through 7 days before. Figs. 2 and 3 show the associations between ambient and indoor PM gamma activities with 8-OHdG and MDA, expressed in percent increases in each biomarker per IQR of exposure, adjusted for black carbon exposure. The values of percent increase with per IQR PM gamma activities across all channels, and the p-values are summarized in Table S4 and S5 in Supplementary Information.
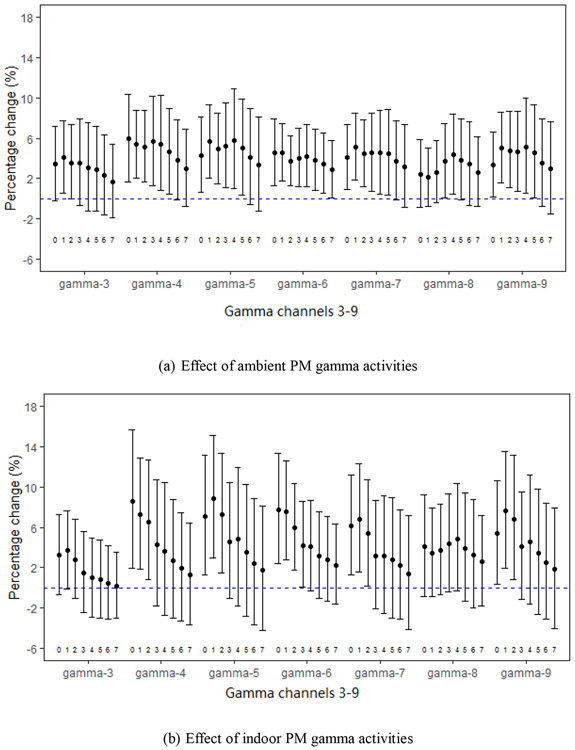
Fig. 2.
Percent changes in 8-OHdG per IQR increases in PM-gamma activities (gamma-3 through gamma-9) for daily moving averages (2a for ambient and 2b for indoor) starting with the day of urine collection (day 0) through 7 days before urine collection (day 7), adjusted for weekly black carbon.
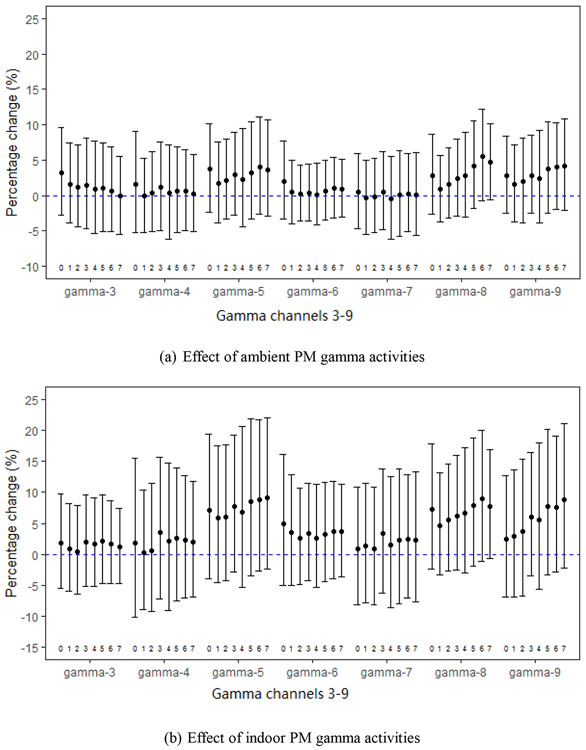
Fig. 3.
Percent changes in MDA per IQR increases in PM-gamma activities (gamma-3 through gamma-9) for daily moving averages (3a for ambient and 3b for indoor) starting with the day of urine collection (day 0) through 7 days before urine collection (day 7), adjusted for weekly black carbon.
We observed positive associations between 8-OHdG with ambient and indoor PM gamma activities (Fig. 2a and 2b) with stronger associations for shorter exposure windows. The increases in 8-OHdG with per IQR indoor PM gamma activities were slightly higher than those with ambient exposure, with the strongest associations and greatest effects for the moving average starting 1 or 2 days before urine collection across all gamma channels. For example, for the moving average starting from 1 day before urine collection: percent increases with per IQR increase of PM gamma activities ranged from 2.09% (95%CI: −0.79, 5.05) to 5.65% (95%CI: 2.08, 9.34) for the ambient, and from 3.41% (95CI: −0.88, 7.88) to 8.87% (95%CI: 2.98, 15.1) for the indoor.
The associations between MDA with ambient and indoor PM gamma activities were not as consistent as 8-OHdG. However, the associations across each gamma channel were all generally positive (Fig. 3a and 3b). For moving averages starting from 1 day before urine collection, the percent changes in MDA ranged from −0.37% (95%CI: −5.47, 5.00) to 1.68% (95%CI: −3.88, 7.57) per IQR increases of ambient PM activities. For moving averages of indoor PM gamma activities starting from 1 day before urine collection percent increases in MDA ranged from 0.23% (95%CI: −8.97, 10.4) to 5.89% (95%CI: −4.64, 17.6) with per IQR across gamma channels 3 through 9.
We also explored the effects of other pollutants, including ambient PM2.5, NO2 and O3, and indoor PM2.5 and NO2 as potential confounders in the models for ambient and indoor gamma activities, respectively. The results of these models (Fig. S3-S8 in the Supplementary Information) were similar to the results of models adjusted for ambient and indoor BC (Fig. 2 and 3). The results of models unadjusted for other pollutants (Fig. S9-S10 in the Supplementary Information) were similar to the adjusted models.
Effects of BC, PM2.5, NO2 and O3
For models including ambient PM gamma activities, the effect of ambient BC on 8-OHdG ranged from 5.86% (95CI%: −1.20, 13.4) to 8.16% (95CI%: 1.02, 15.8) increase per IQR (Fig. S11(a), Supplementary Information). For models including indoor PM gamma activity, the effects of indoor BC on 8-OHdG ranged from 1.38% (95CI%: −3.78, 6.81) to 3.18% (95CI%: −2.01, 8.65) increase per IQR, depending on the specific gamma channel (Fig. S11(b), Supplementary Information). For MDA, the effect of ambient BC ranged from 5.27% (95%CI: −6.05, 17.9) to 6.64% (95%CI: −4.70, 19.3) increase per IQR and 3.10% (95%CI: −3,64 10.3) to 3.90% (95%CI: −2.82, 11.1) for indoor BC exposures (Fig. S12, Supplementary Information). These results are similar to our previous findings regarding indoor BC.16
The associations between ambient PM2.5, NO2 and O3, and indoor PM2.5 and NO2 with 8-OHdG and MDA are shown in Fig. S13-S18. Although not statistically significant, we found positive associations between ambient PM2.5 (Fig. S13(a)) and ambient NO2 (Fig. S15(a)) with 8-OHdG, but not with indoor PM2.5 (Fig. S13(b)), indoor NO2 (Fig. S15(b)) or ambient O3 (Fig. S17). There were also positive associations between indoor PM2.5 (Fig. S14(b)) and ambient NO2 (Fig. S16(a)) with MDA, but not with ambient PM2.5 (Fig. S14(a)) indoor NO2 (Fig. S16(b)), nor ambient O3 (Fig. S18). The reasons for the differences in PM2.5, NO2 or O3 on MDA and 8-OHdG are uncertain.
Discussion
In this study, we observed positive associations between PM gamma activities and biomarkers of oxidative stress. Specifically, we found positive associations between both ambient and indoor PM gamma activities with 8-OHdG (a biomarker of DNA oxidation), with slightly higher effects for indoor exposures; and more consistent positive associations between MDA (biomarker of lipid oxidation) with indoor PM gamma activities than the associations with ambient PM gamma activities. The more consistent associations with 8-OHdG than those with MDA suggest that exposure to PM radioactivity maybe more likely to cause oxidative damage to DNA than to lipids. The increases in biomarkers with the increase indoor PM gamma activities suggest that there are effects of ambient exposures infiltrating the homes of COPD patients. As these COPD patients spend most of their time indoors, our results suggest that staying indoors are not protective of the effects of ambient PM gamma activities. We adjusted for ambient and indoor weekly BC, and found positive effects of BC, consistent with the effects of BC in our previous study. We also adjusted for PM2.5, NO2 and O3 respectively, and found the results of PM gamma activities were similar to models adjusted for BC and unadjusted models.
To the best of our knowledge, this is the first study investigating the short-term effects of exposures to PM radioactivity on oxidative stress biomarkers in COPD patients. Exposure to ionizing radiation has been found to be associated with an increase in oxidative stress.42,43 In the only other study to date of the impacts of PM radioactivity on oxidative stress, there was no association between PM gross beta radioactivity with plasma 8-epi-prostaglandin F2α or myeloperoxidase (MPO).20 This may be due to differences in the populations of these two studies; our study was composed of males with COPD who were receiving treatment at VA Boston, while the other analysis was conducted among male and female volunteers from the Framingham Heart Study who may have been less susceptible to adverse effects. In addition, other studies also suggested systemic effects of exposures to PM radioactivity. These studies include report of a positive relationship between PM radiation and diastolic and systolic blood pressure in elderly men,21 as well as the association between exposure to PM radiation and decrease in lung function22. The above studies20-22 used PM gross beta activity as the surrogate of PM radioactivity. However, as gamma radiation is mostly emitted after the emission of alpha or beta radiation, it is possible that PM gamma activities are better surrogates for the sum of environmental PM alpha and beta activities.44,24 Huang et al.18 and Vieira et al.24 used PM gamma activities as surrogates of PM radioactivity and found that ambient and indoor PM gamma activities were associated with increases in plasma inflammation biomarkers and decreases in pulmonary function (FEV1 and FVC) in the same COPD cohort as in this report. All these previous studies indicate potential adverse health effects of PM associated radioactivity and support our findings.
There are some limitations in this study. One limitation is that we did not measure PM gamma activities directly in the indoor environment. As we estimated indoor PM gamma activities using infiltration ratio (sulfur ratio) without considering the contribution of indoor radiation sources (such as radon) we may underestimate the full burden of indoor exposures. Second, our study participants were all white males living in the Northeastern US, which may restrict the generalizability of the study if the mechanisms or exposure levels are different in individuals of other ages, races, or in females. Third, our study only included participants who were able to travel to VA Boston for a study visit and were able to receive and ship samplers back to the field team. Thus, or results may not be generalizable to individuals with very severe COPD who may have greater systemic response to PM gamma activities.
Our study also has several strengths. To the best of our knowledge, we ae the first to describe the effects of previously unrecognized exposures to PM radioactivity on oxidative stress biomarkers in COPD patients. Additionally, our study considers exposures to PM gamma activities in indoor environment where COPD patients spend most of their time. Finally, all participants in our study have well documented COPD.
Conclusion
Our results indicated positive associations between ambient and indoor PM gamma activities (surrogates of PM radioactivity) with urinary oxidative stress biomarkers. Our findings suggest that PM with attached radionuclides may promote the oxidative degradation of DNA and lipids in COPD patients and contributes to understanding effects of low-level ambient radiation.
Supplementary Material
Footnotes
Conflict of Interest Disclosures:
The authors declare no conflict of interests, including relevant financial interests, activities, relationships, and affiliations.
References
- [1].Senior RM, Anthonisen NR. 1998. Chronic obstructive pulmonary disease (COPD). Am J Resp Critcare 1998; 157: S139–S147. [DOI] [PubMed] [Google Scholar]
- [2].GBD 2015 DALYs and HALE Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hurst JR, Elborn JS, De Soyza A. COPD-bronchiectasis overlap syndrome. Eur. Respir. J. 2015; 45: 310–313. [DOI] [PubMed] [Google Scholar]
- [4].Kochanek KD, Murphy SL, Xu J, Arias E. Mortality in the united states, 2016. NCHS Data Brief 293. Centers for Disease Control and Prevention, National Center for Health Statistics, 2017.
- [5].Therond P Oxidative stress and damages to biomolecules (lipids, proteins, DNA). Ann Pharm Fr 2006; 64(6):383–389. [DOI] [PubMed] [Google Scholar]
- [6].Van Eeden SF, Sin DD. Oxidative stress in chronic obstructive pulmonary disease: A lung and systemic process. Can Respir J 2013; 20: 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cheng WE, Shih CM, Hang LW, Wu KY, Yang HL, Hsu WH, et al. Urinary biomarker of oxidative stress correlating with outcome in critically septic patients. Intensive Care Med 2007; 33: 1468–1472. [DOI] [PubMed] [Google Scholar]
- [8].Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 2003; 60: 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tramuto F, Cusimano R, Cerame G, Vultaggio M, Calamusa G, Maida CM, et al. Urban air pollution and emergency room admissions for respiratory symptoms: a case-crossover study in Palermo, Italy. Environ Health 2001; 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barregard L, Sallsten G, Andersson L, Almstrand AC, Gustafson P, Andersson M, Olin AC. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med 2008; 65: 319–324. [DOI] [PubMed] [Google Scholar]
- [11].Ceylan E, Kocyigit A, Gencer M, Aksoy N, Selek S. Increased DNA damage in patients with chronic obstructive pulmonary disease who had once smoked or been exposed to biomass. Respir Med 2006; 100: 1270–1276. [DOI] [PubMed] [Google Scholar]
- [12].Neophytou AM, Hart JE, Chang Y, Zhang JJ, Smith TJ, Garshick E, Laden F. Short-term traffic related exposures and biomarkers of nitro-PAH exposure and oxidative DNA damage. Toxics 2014; 2: 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Romieu I, Barraza-Villarreal A, Escamilla-Nunez C, Almstrand AC, Diaz-Sanchez D, Sly PD, Olin AC. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. J Allergy Clin Immunol 2008; doi: 10.1016/j.jaci.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [14].Zhang J, Zhu T, Kipen H, Wang G, Huang W, Rich D, Zhu P, Wang Y, Lu SE, Ohman-Strickland P, Diehl S, Hu M, Tong J, Gong J, Thomas D. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res Rep Health Eff Inst 2013; 174:5–174. [PMC free article] [PubMed] [Google Scholar]
- [15].Heinrich J, Schikowski T. COPD patients as vulnerable subpopulation for exposure to ambient air pollution. Curr Environ Health Rep 2018; 5:70–76. [DOI] [PubMed] [Google Scholar]
- [16].Grady ST, Koutrakis P, Hart JE, Coull BA, Schwartz J, Laden F, Zhang JF, Gong JC, Moy ML, Garshick E. Indoor Black Carbon Exposure and Oxidative Stress Biomarkers in COPD Patients. Environment International. 2018; 115: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bruce N, Perez-Padilla R, Albalak R. Indoor air pollution in developing countries: a major environmental and public health challenge. B World Health Organ 2000; 78:1078–92. [PMC free article] [PubMed] [Google Scholar]
- [18].Huang S, Garshick E, Vieira CLZ, Grady ST, Schwartz JD, Coull BA, Hart JE, Laden F, Koutrakis P. Short-term exposures to particulate matter gamma radiation activities and biomarkers of systemic inflammation and endothelial activation in COPD patients. Environ Res. 2019; doi: 10.1016/j.envres.2019.108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marsh JW, Bailey MR. 2013. A review of lung-to-blood absorption rates for radon progeny. Radiat Prot Dosimetry 2013; 157: 499–514. [DOI] [PubMed] [Google Scholar]
- [20].Li W, Nyhan MM, Wiker EH, Vieira CLZ, Lin HH, Schwartz JD, et al. Recent exposure to particle radioactivity and biomarkers of oxidative stress and inflammation: The Framingham Heart Study. Environ Int 2018; 121: 1210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nyhan MM, Coull BA, Blomberg AJ, Vieira CLZ, Garshick E, Aba A, et al. Associations Between Ambient Particle Radioactivity and Blood Pressure: The NAS (Normative Aging Study). J Am Heart Assoc. 2018; doi: 10.1161/JAHA.117.008245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nyhan MM, Rice M, Blomberg A, Coull BA, Garshick E, Vokonas P, et al. Associations between ambient particle radioactivity and lung function. Environ Int 2019; 130: 104795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Garshick E Effects of short- and long-term exposures to ambient air pollution on COPD. Eur Respir J 2014; 44: 558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vieira CLZ, Koutrakis P, Huang S, Grady S, Hart JE, Coull BA, Laden F, et al. Short-term effects of particle gamma radiation activities on pulmonary function in COPD patients. Environ Res 2019; 175: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garshick E, Grady ST, Hart JE, Coull BA, Schwartz J, Laden F, et al. Indoor Black Carbon and Biomarkers of Systemic Inflammation and Endothelial Activation in COPD Patients. Environ Res 2018; 165: 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cui X, Gong J, Han H, He L, Teng Y, Tetley T, Sinharay R, Chung KF, Islam T, Gilliland F, Grady S, Garshick E, Li Z, Zhang JJ. Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J Thorac Dis 2018. 10: 3088–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Commodore AA, Zhang JJ, Chang Y, Hartinger SM, Lanata CF, Mausezahl D, et al. Concentrations of urinary 8-hydroxy-2′-deoxyguanosine and 8-isoprostane in women exposed to woodsmoke in a cookstove intervention study in San Marcos, Peru. Environ Int 2013; 60, 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gong J, Zhu T, Kipen H, Wang G, Hu M, Ohman-Strickland P, et al. Malondialdehyde in exhaled breath condensate and urine as a biomarker of air pollution induced oxidative stress. J. Expo. Sci. Environ Epidemiol 2013; 23, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Environmental Protection Agency (EPA). Expansion and Upgrade of the RadNet Air Monitoring Network. U.S. EPA. 2005.
- [30].Environmental Protection Agency (EPA). Radiological Laboratory Sample Analysis Guide for Incidents of National Significance - Radionuclides in Air. U.S. EPA. 2009.
- [31].Ramadhan RA, Abdullah KMS. Background reduction by Cu/Pb shielding and efficiency study of NaI (TI) detector. Nucl Eng Technol 2018; 50: 462–469. [Google Scholar]
- [32].Tang CH, Garshick E, Grady S, Coull B, Schwartz J, Koutrakis P. Development of a modeling approach to estimate indoor to-outdoor sulfur ratios and predict indoor PM2.5 and black carbon concentrations for Eastern Massachusetts households. J Expo Sci Env Epid 2018; 28: 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huang SD, Lawrence J, Kang CM, Li J, Martins M, Vokonas P, et al. Road proximity influences indoor exposures to ambient fine particle mass and components. Environ Pollut 2018; 243: 978–987. [DOI] [PubMed] [Google Scholar]
- [34].Kang CM, Koutrakis P, Suh HH. Hourly measurements of fine particulate sulfate and carbon aerosols at the Harvard-U.S. Environmental Protection Agency Supersite in Boston. J Air Waste Manag Assoc 2010; 60: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ruiz PA, Toro C, Cáceres J, López G, Oyola P, Koutrakis P. Effect of Gas and Kerosene Space Heaters on Indoor Air Quality: A Study in Homes of Santiago, Chile. J Air Waste Manage 2000; 60: 98–108. [DOI] [PubMed] [Google Scholar]
- [36].Schwartz J The distributed lag between air pollution and daily deaths. Epidemiology. 2000; 11: 320–6. [DOI] [PubMed] [Google Scholar]
- [37].Sarnat J, Long CM, Koutrakis P. Using sulfur as a tracer of outdoor fine particulate matter. Environ Sci Technol 2002; 36: 5305–5314. [DOI] [PubMed] [Google Scholar]
- [38].Porstendörfer J Physical parameters and dose factors of the radon and thoron decay products. Radiat Prot Dosimetry 2001; 94(4): 365–973. [DOI] [PubMed] [Google Scholar]
- [39].Porstendörfer J, Gründel M. Radon decay products in outdoor air. Radioactivity in the Environment 2005; 7: 56–65. [Google Scholar]
- [40].Kloog I, Nordio F, Coull BA, Schwartz J. Predicting spatiotemporal mean air temperature using MODIS satellite surface temperature measurements across the NorthEastern USA. Remote Sens. Environ 2014; 150, 132–139. [Google Scholar]
- [41].Reinhold K, Wei P, Dambacher M, Yan M, Zhou XL. Experimental effects and individual differences in linear mixed models: estimating the relationship between spatial, object, and attraction effects in visual attention. Front. Psychol. 2011; 1: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 2012; 327: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Szumiel I Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: the pivotal role of mitochondria. Int J Radiat Biol 2015; 91:1–12. [DOI] [PubMed] [Google Scholar]
- [44].Stacey WM. Introductory nuclear physics, 2nd, completely revised and enlarged edition. Wiley-VCH, Verlag GmbH & Co. KgaA, Weinheim. 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.