Abstract
Biomaterial systems have enabled the in vitro production of complex, emergent tissue behaviors that were not possible with conventional two-dimensional culture systems, allowing for analysis of both normal development and disease processes. We propose that the path towards developing the design parameters for biomaterial systems lies with identifying the molecular drivers of emergent behavior through leveraging technological advances in systems biology, including single cell omics, genetic engineering, and high content imaging. This growing research opportunity at the intersection of the fields of tissue engineering and systems biology – systems tissue engineering – can uniquely interrogate the mechanisms by which complex tissue behaviors emerge with the potential to capture the contribution of i) dynamic regulation of tissue development and dysregulation, ii) single cell heterogeneity and the function of rare cell types, and iii) the spatial distribution and structure of individual cells and cell types within a tissue. By leveraging advances in both biological and materials data science, systems tissue engineering can facilitate the identification of biomaterial design parameters that will accelerate basic science discovery and translation.
1. Introduction
Native tissues are composed of multiple cell types that reside within a complex, continuously changing three-dimensional microenvironment consisting of numerous inputs, including extracellular matrix (ECM), soluble factors, mechanical forces, and cell-cell contacts, all of which combine to drive collective tissue function [1, 2]. By mimicking and reproducing these input parameters, biomaterial and microsystems technologies [3, 4] can create diverse emergent tissue behaviors that would not otherwise be possible with conventional two-dimensional (2D) culture systems. By engineering input parameters across three categories i) physical (ECM stiffness and nonlinear elasticity, interstitial and microvascular flows, gradients), ii) chemical (soluble factors and ECM ligands), and iii) cellular (cell sources, cell types, and genetic manipulations) across 3D space and time, biomaterial microsystems can guide the emergence of tissues with diverse behaviors. These responses are exemplified by vasculogenic microcapillary networks [5–7], beating cardiomyocyte microtissues [8, 9], functional skeletal muscle [10, 11], and stem cell-derived tissue organoids [12, 13]. The tunable nature of biomaterial microsystems uniquely allows for the engineering of microenvironments to interrogate the contribution of specific inputs on the resulting cell and tissue behavior. In vitro biomaterial microsystems therefore have incredible potential as a tool for molecularly dissecting mechanisms behind tissue behaviors including tissue formation, disease initiation and progression, and the mechanism of action for therapeutic compounds [3, 14]. Biological systems have complex dynamic, multi-level (genetic, epigenetic, metabolic, protein interactions, intercellular signaling, etc.) regulatory networks making it challenging to identify any one molecular mechanism in isolation by which tissue behaviors emerge. Fully leveraging the potential of biomaterial microsystem platforms will require an analysis framework that can connect and explain the effects of design inputs on emergent cell and tissue behavior via the complex network of intracellular and intercellular molecular regulations.
The path towards developing improved microsystems and material platforms will likely involve the ability to apply tools from systems biology to the analysis of tissue dynamics and structure. Historically, the identification of biomaterial design parameters has ranged from reductionist approaches to high throughput screening systems, which have frequently involved a focused number of cell types or cellular responses. Materials data science approaches that integrate these experimental approaches with machine learning and artificial intelligence strategies[15–17] are emerging as a powerful tool in biomaterials development[18, 19]. Nevertheless, the approaches have often failed to fully capture the complex multivariate biology present in vivo [20], and more recent approaches have sought to capture increased complexity through co-culture to multi-culture systems, 2D and/or three dimensional (3D) culture across multi-well plates, and microphysiological systems that integrate multiple organ systems. Native in vivo tissues are increasingly analyzed with emerging molecular tools that provide measurements of thousands/millions of factors in a single experiment. These tools, including single cell RNA sequencing, have revealed the vast heterogeneity in molecular phenotype between cells of the same classical type even within the same tissue compartment [21, 22]. The large data sets generated have the potential to identify the interconnected responses inherent to spatially coordinated biological systems, the role of rare cells and behaviors within the complex systems, and the dynamic nature of the response networks. Descriptive single cell sequencing, no matter how detailed, cannot, in isolation, identify the dynamic cellular functions that drive tissue development and disease. In order to move from descriptive studies towards explanatory and predictive models, we need a better link between systems level molecular phenotypes and the underlying dynamic microenvironment-driven cell and tissue functions. A systems-level approach is necessary to identify nodes and times within the interconnected network of interactions that drive and control emergent tissue behaviors (Figure 1).
Figure 1. Systems tissue engineering for study of complex microenvironments.

A. The systems tissue engineering paradigm operates in a space spanning from the study of thousands of unique simplified microenvironments to just a few highly complex microenvironments. B. Systems tissue engineering measures complexity using tools from systems biology within high complexity microenvironments created using tissue engineering.
Here, we provide a perspective on utilizing recent technological advances in the areas of single cell systems biology, genetic engineering, and high content imaging to link dynamic single cell microenvironment-driven functions to their molecular drivers within engineered microenvironments. As the field of tissue engineering advances through improvements in biomaterial, cellular, and organoid microsystem technologies, it increasingly bridges the complexity gap between reductionist 2D in vitro cell culture and complex native in vivo tissues (Figure 1). This intermediate complexity allows for the production of realistic models and devices, while allowing for control and interrogation in ways that would not be possible in native tissues. We detail opportunities for application of tools from systems biology to the field of tissue engineering, an intersection we refer to as systems tissue engineering (Figure 1,2) for exploring sources of complexity within engineered microsystems including i) the dynamics of tissue development and dysregulation, ii) emergent heterogeneity and the function of rare cell types through single cell analysis, and iii) the spatial distribution of individual cells controlling structure and function within a tissue.
Figure 2:

Strategies for linking single cell functions to their molecular phenotype within engineered microenvironments.
2. Capturing dynamics within engineered microenvironments
Tissue behaviors emerge from many layers of dynamic cellular processes including cell cycling, migration, and matrix remodeling with time scales ranging from seconds to days, each regulated by subcellular molecular processes including protein-protein interactions and gene expression with timescales from microseconds to hours [23]. Epigenetic changes from these inputs may occur over days or longer guiding long-term cellular processes like cellular differentiation[24, 25]. The dynamic processes are ultimately integrated to regulate cell and tissue behavior. Recording the sequence of molecular regulatory events and the corresponding tissue properties would facilitate the identification of the key pathways that are driving tissue function across the stages of tissue development or disease progression. Biomaterial microsystems provide a means of creating systems that can be dynamically imaged to report on cell differentiation and maturation, and ultimately tissue function. Here we outline emerging methods and opportunities for dissecting dynamic tissue behaviors using biomaterial microsystems.
2.1. Dynamic microenvironments
Engineered 3D hydrogels and microsystems with the ability to analyze dynamics would facilitate the identification of the key drivers of tissue function (Figure 3)[26]. In transitioning cells from 2D tissue culture plastic to 3D culture, particularly within systems that can be remodeled, cells experience dynamic changes in geometry, structure, and composition of their environment. While the biochemical environment can be changed on demand without disrupting adherent cells by changing culture media, more recently, hydrogels have been designed whose biomechanical or biochemical properties can be switched on demand via an external signal, such as light [27, 28]. Cells across multiple material platforms respond with dramatic changes in behavior after altering the mechanical stiffness of the underlying substrate [29–32]. Similarly, material systems that allow for dynamic control of ECM ligands can guide diverse emergent behaviors[33–36]. These systems for modulating the signals on demand are enabling for analysis of dynamic cellular responses. More recent biomaterial designs are supporting fully reversible[37] mechanical or biochemical changes [38], allowing for in situ study of the dynamics of microenvironmental memory [39, 40] and its role on cell and tissue function. Furthermore, materials may sense and respond to their microenvironment, thereby providing biomimetic feedback loops to resident cells [41, 42]. These material systems provide critical tools for probing how dynamic microenvironmental changes control systems-level tissue function.
Figure 3. Monitoring dynamic transcriptional responses to engineered biomaterial.

Human foreskin fibroblasts bearing 50 transcription factor activity genetic reporters were cultured on polyethylene glycol (PEG) hydrogels with tunable stiffness and RGD ligand density. The fibroblasts exhibit distinct dynamic changes in transcription factor activity networks in response to material stiffness and material RGD ligand density. Adapted with permission from [26].
2.2. Dynamic cell and tissue monitoring with genetic reporters
The accessibility of biomaterial microsystem constructs to imaging is a key feature for analyzing dynamic responses. Optically clear biomaterial culture systems [43, 44] with homogenous microstructure (yet potentially with engineered nanostructures with length-scales less than half the wavelength of the light used for imaging) can transmit light much more efficiently than those containing high refractive index microscale fibers [45, 46] and micron-scale features which scatter light [47]. Light microscopy has long been employed for identifying cell and tissue structures and their dynamics, the spatial position of cells relative to each other, or intracellular distribution of organelles and proteins. Connecting cell and tissue structure and function to dynamic changes in molecular state is uniquely achieved by live cell imaging of genetic reporters.
With the use of live-cell reporters, fluorescence and luminescence can be employed to capture systems-level dynamics in molecular signaling, with emerging systems enabling large scale analysis. Analytical assays such as RNAseq and proteomics require destructive snapshot measurements, and while these techniques can be performed over a time-course experiment, they must be performed on replicate experiments of unique and distinct sets of cells and in themselves do not allow for direct correlation with tissue function. These limitations can be especially problematic when working with high complexity 3D biomaterial microsystems. Live cell imaging of genetically encoded reporters can uniquely monitor the dynamic signaling within living cells or tissues. High quality reporters have been generated for a variety of intracellular signaling processes, including transcription factors [26, 48–56], microRNA [57–60], protein kinase activity [61–65], metabolism, chromatin organization [66], and protein-protein interactions [67, 68] among others. Genetic reporters are also available for monitoring the dynamic physical state of the cell and extracellular signaling from the microenvironment including hypoxia[69], reactive oxygen species[70– 73], membrane potential/ion trafficking[74, 75], metabolism [76, 77], and neurotransmitters engagement[78, 79]. In additional to genetic reporters, chemical biology labeling strategies [80–82] can provide an additional toolset for imaging live-cell dynamics, and the commercialization of chemical probes for many applications including tracking hypoxia[83], reactive oxygen species[84], and organelles and structural features[85–87] within the cell has provided off-the-shelf tools for studying live cell dynamics.
Scaling dynamic genetic reporters to dozens of reporters in a single experiment has been achieved by pre-manufacturing libraries of ready to use reporter transduction agents, such as lentivirus, that can be used to transduce cells in a parallel format [26, 50, 54, 55, 60, 88, 89]. The transduced cells are then cultured and imaged dynamically in high-content array to investigate the dynamic molecular response networks triggered by micro-environmental stimuli. Using microwell plates with each well containing a distinct reporter, the signaling induced by RGD ligand or mechanical stiffness was investigated through identifying the transcriptional response networks of 50 transcription factors (Figure 3)[26]. These studies identified transcription factors that are specific to ligand density or mechanical stiffness, as well as transcription factors common to both stimuli [26]. Here, bioluminescence imaging of each well reported population level responses, yet was unable to capture cell specific responses.
Recent technological advances have furthered the potency and scalability of genetic reporters for monitoring dynamic cellular processes. Luminescent proteins have been attractive for detecting low levels of transcription factor activity, because the low background imparts a high signal to noise ratio [90, 91]. Advances in luminescent reporter proteins, including improvements in brightness [92] and in red-shifting for better tissue penetration and color multiplexing [93–96], allow them to outperform fluorescent proteins for reporting on whole-tissues or multicellular systems. Pairing the low read noise of Electron Multiplying Charge-Coupled Device (EMCCD) or scientific Complementary Metal-Oxide-Semiconductor (sCMOS) cameras with an appropriate objective and tube lens can enables bioluminescence microscopy [97, 98]. Nonetheless, fluorescent proteins with their much greater brightness still have advantages for single cell and 3D reporter imaging. Advances in commercial DNA synthesis [99, 100] allow for the straightforward and rapid synthesis of the genetic parts, with components assembled into multi-kilobase sequences with robust methods like Gibson Assembly [101]. Further, advances in genetic engineering including the advent of CRISPR cas9 technology [102, 103] and engineering of cas9 variants with improved specificity [104, 105] allows for scalable strategies for reporting on activity of endogenous promoters and enhancers [106–110], or for the insertion of large multi-kilobase [111] genetic cassettes capable of bearing several reporters at a genetic safe harbor site.
3. Capturing the role of single cell heterogeneity in emergent tissue behaviors
Heterogeneity of individual cells within a tissue is known to be of great clinical significance, as illustrated by intratumoral heterogeneity imparting resistance to cancer therapeutics [112]. On a more fundamental level, heterogeneity may facilitate basic tissue functions including fate plasticity and information coding [113]. Dynamic responses that appear continuous at the population scale can be heterogeneous and switch-like at the single cell level [114], indicating there is much to learn about how single cells make functional decision. However, linking systems scale single cell molecular information to the single cell functions within complex biomaterials microsystems requires experimental information linking molecular form to single cell function. New technologies including single cell RNA sequencing [115–117] provide molecular phenotype, and have revolutionized our understanding of the complexity and distribution of single cell phenotypes within in vivo tissues. Here, we describe approaches that aim to bridge single cell genomics technologies with high content imaging to capture the contribution of cellular heterogeneity or the function of rare cell populations on collective microtissue function (Figure 2).
3.1. Engineered biomaterial microsystems for single cell analysis
Biomaterial microsystem platforms provide a means by which to elicit and observe single cell functional responses to controlled environments (Figure 4)[46, 118–122]. For example, mature platforms for applying concentration gradients of soluble factors to cells in 3D culture allow observation of heterogeneous single cell chemotaxis responses of individual cells (Figure 4A)[123–126], with some rare cells displaying exceptional chemotaxis. Likewise, manipulation of the mechanical properties and structure of the ECM have allowed for dissection of individual cells exerting forces and migrating in response to the ECM (Figure 4B,D)[45, 46, 120, 127–131]. Single cell force generation can vary by orders of magnitude between cells and microenvironments [46, 132–134]. Using microsystems, the relative role of soluble and cell-cell contact factors in driving behavior has been explored in different cell types (Figure 4E–F)[121, 122] including single NK cell activation [135] and killing of cancer cells (Figure 4F)[122]. Single NK cell cytotoxicity experiments have demonstrated rare NK cells are responsible for the majority of cytotoxic activity against cancer cells[136–140]. Within organoid systems, engineered 3D biomaterial systems have been employed to guide stem cells to differentiate into organoids akin to diverse tissues including intestine [141], optic cup [142], and lung [143]. The stem cells within a colony under a complex process of self-organization and self-sorting [144] according to microenvironmental cues as they differentiate down the various lineages and spatially organize to form 3D organoids [145]. Engineered biomaterials can provide boundary conditions and gradients that break symmetry between individual cells and guide differentiation and organoid development [146]. Across these systems, a vast heterogeneity of single cell behaviors in both time and space has been observed, which motivates the application of single-cell analysis as well as the essential role of dynamic cell behaviors in the overall structure and function of tissues.
Figure 4: Recording dynamic single cell behaviors within engineered microenvironments.

A. Cancer cell chemotaxis up a linear concentration gradient within a 3D collagen matrix. Adapted with permission from[118] B. Cancer cell traction force generation and matrix alignment within a 3D collagen matrix. Adapted with permission from [46] C. Cancer cell stemness marker expression depends on proximity to edges and vertices in geometry. Adapted with permission from [119] D. 3D methacrylated dextran (DexMA) hydrogel crosslinking controls endothelial cell density and multicellularity in angiogenic sprouting. Adapted with permission from [120] E. Local collectivity of T cells within microwells promotes memory differentiation. Adapted with permission from [121] F. Microsystems with isolated or connected channels allow for study of soluble mediators driving single NK cell killing kinetics of cancer cells. Adapted with permission from [122].
3.2. Single cell pseudo-temporal omics analysis
Single cell transcriptomic and multi-omic technologies provide a means to capture a detailed molecular description of cell heterogeneity at any given time within engineered microenvironments. Single cell RNA sequencing technology [115–117] has scaled exponentially over the last decade [147] and now has a well-established ecosystem of analysis tools [148–150]. Further, multi-omic technologies provide a means to analyze non-transcriptionally controlled pathways by integrating single cell transcriptomic data with single cell proteomic and chromatin accessibility measurements [151]. CITE-seq [152] and REAP-seq [153] can simultaneously quantify mRNA and extracellular protein content in individual cells by using antibody cocktails barcoded with oligonucleotides which are sequenced along with endogenous mRNA. A complementary tool is the CRISPR loss of function screens, where a library of many guide RNA’s can be used to knock down or knock out genes in individual cells, allowing for detailed study of the role of proteins in gene regulatory networks[154–157]. Other sequencing modalities including single cell ATAC-seq [158, 159] and THS-seq [160] can be used to link single cell chromatin accessibility to the state of transcriptome of individual cells. Analysis methods for these genomics datasets provide descriptive clustering of cells into categories by their gene expression, but they cannot directly link gene expression to cell behavior without additional information. Information including single cell spatial location and orientation, single cell functional phenotype, and dynamic changes of individual cells over time are lost when the tissue is processed for sequencing.
Moving toward a predictive understanding of cellular heterogeneity and tissue function will require a means of linking and analyzing snapshot descriptions of single cell molecular phenotypes across time. Pseudo-temporal analysis of scRNAseq datasets[151], with methods such as pioneering work Monocle [149], achieve this goal by creating single cell trajectories across dynamic processes like differentiation [149, 150, 161–163] and the cell cycle[162]. Here, cells are harvested at several time points during a dynamic process such as cellular differentiation from replicate experiments, and scRNAseq or another omics method is conducted on the cells harvested at each timepoint. The transcriptome of each individual cell across replicate experiments harvested at distinct time points is then ordered on a trajectory of the biological process occurring over time, with this ordering referred to as psuedotime. The ordering and branchpoints of graph networks produced across pseudotime are then used to predict critical molecular regulators and decision points. While original algorithms allowed for only linear trajectories, newer methods support cycling and circular paths [151]. These methods are well suited for the study of dynamic heterogenous cellular responses in the microenvironment of biomaterial microsystems. While dynamic information can be captured by pseudo-temporal ordering, this ordering is obtained from the unique transcriptome of individual cells obtained from replicate experiments sequenced at multiple times; each individual cell is measured only once, and essential dynamic and spatial information is inherently lost in this process.
3.3. Connecting tissue function to molecular drivers with live single cell imaging
Directly linking dynamic single cell function to dynamic single cell molecular phenotypes requires observation of individual live cells over time. Modern high content live cell imaging assays can track morphology and biomarkers of many thousands of individual cells across time as they respond to stimuli from their microenvironment [164, 165]. In order to provide information on molecular phenotype, cell lines or progenitor cells [166–168] can be produced containing genetic reporter elements using fluorescent or luminescent proteins.
An automated live cell microscope equipped with an incubated stage can image individual cells across many wells of a microtiter plate over time, recording changes in single cell behavior simultaneously with changes in single cell reporter activity. Image analysis codes are then applied to link, correlate, and interrogate the order of changes in dynamic single cell functions with dynamic single cell molecular phenotype. For example, high content imaging of genetic reporters has been employed to screen the effects of thousands of drugs on cell function [169], and to elucidate the single cell toxicity pathways activated by these drugs [170–172] (Figure 5). Here, genetic reporters can be included in one or more cellular subsets of the microenvironment to facilitate imaging and record their specific contribution to the emergent behavior. These methodologies are directly applicable to the study of cells on planar 2D interfaces within biomaterials where multiple cell types or variation in the local microenvironment such as material or soluble gradients are present. We note that the dynamic physical movements and behaviors of individual cells residing within biomaterial microsystems are also routinely recorded to connect material microenvironment to cell function. Integration dynamic imaging of genetic reporters within such systems completes the linkage from microenvironment to function to molecular drivers within individual cells.
Figure 5. Case study: High-content live-cell imaging of drug-induced liver toxicity.

A. Human hepatoma HepG2 cell lines expressing various stress response pathway genetic reporters on bacterial artificial chromosomes were imaged in high-content live-cell format for 24 hours after addition of different drugs to culture. Adapted with permission from [171]. B. Automated image analysis was conducted to segment each cell nucleus and cytoplasm and record single-cell stress response from reporters. Reproduced with permission from [170]. C. Unsupervised hierarchical clustering was applied to infer the means and kinetics by which each drug compound and dose induced cellular stress. Reproduced with permission from [171].
For single cell studies, the process by which reporters are inserted must be considered to account for heterogeneity and bias in reporter function between individual cells. Viral and physical methods may result in integration of the reporter gene at different loci [173] or maintenance of the vector in the nucleus extrachromosomally [174] at variable copy number, which may necessitate production of a clonal cell line to improve consistency of reporter function between cells. Even so, bias may still exist in the reporter function of integrated clones from any adjacent genetic regulatory element. Insertion of reporters at so-called safe-harbor sites, such as the commonly used AAVS1 locus in humans, which are far from any other known genetic regulatory elements, is thought to reduce bias from adjacent regulatory elements [175, 176]. Considering these limitations, techniques such as those using CRISPR cas9[106–110] that can knock-in reporters in situ are especially powerful for capturing the full genetic regulatory context in single cells.
A staged discovery strategy may be necessary for incorporating single cell genomics and high content imaging of genetic reporters to efficiently link single cell functions to their molecular phenotypes. Here, an initial round of single cell genomics and pseudo-temporal analysis would be used to identify regulators stratifying the dataset. Then high content imaging on a more focused set of live cell genetic reporters can connect dynamic microenvironment driven functions to the larger systems-level molecular phenotypes. These methods can be computationally integrated with targets, with subsequent validation, such as through the use of high-throughput loss of function screening.
3.4. High content single cell imaging for 3D systems
Most cells live within a fully 3D rather than a 2D planar geometric context, and 3D cell culture is necessary for obtaining some complex phenotypic responses [177, 178]. However, 3D systems present unique challenges for high content imaging experiments including the requirement to acquire and process large 3D datasets, noise and bias from imaging through hydrogel or ECM material, and the requirements for highly specialized imaging systems for optimal performance. Conventional imaging systems including wide-field and confocal point scanning have substantial limitations for dynamic imaging of 3D microenvironments including phototoxicity and photobleaching as they illuminate through the whole sample [179]. Light sheet microscopy systems provide the unique ability to illuminate only the current imaging plane, greatly reducing phototoxicity in 3D imaging studies. Recent refinements including the advent of lattice light sheet microscopy [180] and the subsequent integration of aberration-correction adaptive optics [181] may further enhance performance. However, conventional light sheet microscopy requires complicated mounting schemes and small samples owing to the requirements of 2 orthogonal light paths. Recent work toward open top light sheet microscopy [182–184] and oblique plane microscopy [185] would allow for plate-based multi-well imaging enabling truly high content 3D imaging studies. For more on the current state of high-content cell imaging we refer the reader to an excellent review [164].
3.5. Tools and opportunities for machine learning in single cell image analysis
Accessible and accurate methods for automated image analysis tasks are needed to enable truly high-content experimental strategies. A mature array of proven tools and techniques for each step of the high-content 2D and 3D imaging workflows, from image acquisition, to segmentation, to downstream analysis, are available [164, 186–190]. Further, analysis methods new to cellular images that incorporate machine learning strategies can outperform classical methods, providing an opportunity to improve the power of high-content assays [191]. These strategies include both supervised, where annotated datasets are provided to train the algorithm, and unsupervised strategies[192]. Deep learning-based approaches to biological image analysis [193, 194], such as those that utilize artificial neural networks, show promise for both complex applications-specific tasks, such as detecting the location of early metastases in whole animal images [195] and common cell counting, detection, and morphology measurement tasks from noisy bright-field, phase-contrast, and fluorescence microscopy images [196]. For an assessment of the recent progress, challenges, and opportunities in applying deep learning to cell image analysis, an excellent review is available [197].
More generally, connecting the material design properties to emergent cell behaviors may be enabled through integration with materials data science. Materials data science involves techniques such as building databases of material properties, high throughput screening, meta-analysis, and molecular simulation[15–17] which is complementary [18, 19], to the detailed cellular and molecular analyses proposed herein for systems tissue engineering. This integration of data from multiple levels can provide a more comprehensive systems design approach that integrates the material properties, the mechanistic cellular and molecular responses, and ultimately function for the intended application.
4. Capturing spatial information driving tissue behavior
The spatial distribution of cells and features within and between niches in a tissue are integral for controlling collective tissue behavior and function [198–200]. Coordinated behaviors within tissues may at least in part ultimately emerge from changes in gene expression within subsets of spatially defined cells [201]. Cells communicate and provide spatial signals to neighbors through concentration gradients [202] and waves [203], cell-cell contacts, ECM remodeling, and mechanical forces [204, 205]. Spatial patterning produced by biomaterial microsystems can be used to recreate complex tissue architectures that would not be otherwise possible (Figure 6) [143, 206–209].
Figure 6. Spatial patterning of biomaterial microsystems.
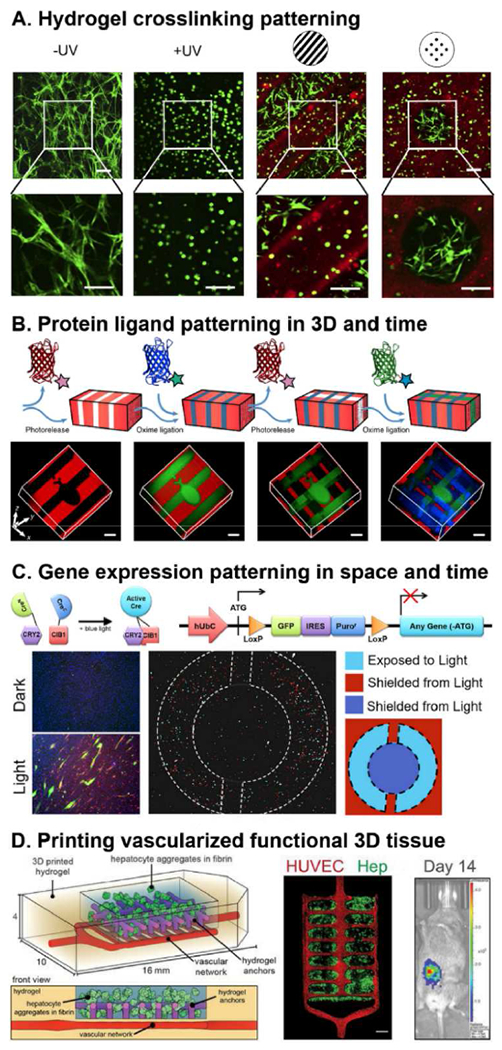
A. Application of photolithography techniques to a UV-reactive acrylated hyaluronic acid (AHA) hydrogels can create intricate spatial material patterns (red) that guide inhabitant cell behavior (green). Adapted with permission from [206]. B. Photopatterning of UV-reactive polyethylene glycol (PEG) hydrogel for reversible 3D patterning of several protein ligands within the same material. [207] C. Expressing optogenetic proteins paired with gene regulatory circuits in cells can be used to photopattern gene expression within tissue constructs [208]. D. Advances in 3D printing including stereolithography techniques can create intricate 3D tissue topologies. A vascularized 3D printed tissue construct containing hepatocyte aggregates exhibits functionality as measured by albumin promoter activity after implantation into mice with chronic liver injury. Adapted with permission from [209].
For example, geometric micropatterning of cell adhesion [210, 211] can drive a complex multi-cellular emergent behavior, such as where cancer cell stemness is concentrated at the geometric edges and vertices of colonies(Figure 4C)[119]. Patterning of gene delivery has been employed to control concentration profiles that spatially oriented neurite extension [212, 213]. Similarly, microfluidic generated concentration gradients [214–216] and material stiffness gradients [132, 217, 218] induce chemotaxis and durotaxis respectively in diverse cell types. Patterning of mechanical interfaces guide development of microtissues including the spontaneous polarized development of amnion-like tissue from stem cells [219]. We expect the use of biomaterial microsystems for control of spatial patterning of the tissue microenvironments paired with emerging spatially resolved sequencing [220–222] will further resolve the mechanisms and role of spatial signaling networks in tissue behaviors.
4.1. Spatially resolved omics within engineered microsystems
The most conceptually straightforward approach to link single cell microenvironment-driven function to a systems-level molecular phenotype is to isolate and index individual cells from culture after observing their behavior and function. Single cell isolation [223, 224] can be achieved by physically picking cells using a micromanipulation system, with microfluidic systems [225], or by culturing cells in isolation in microwells. However, current systems for cell picking by micromanipulation remain low throughput and labor intensive to operate [213, 223, 224]. This approach can however be effective for experiments with low cell numbers, as cells can be directly picked from complex multicellular microenvironments. Isolated culture approaches in contrast can be scaled to 10’s or 1,000’s of individual cells using microfluidic technology[225], but an inherent limitation is that cells will not receive the cell-cell contacts and soluble factors from other cells in their microenvironment. In one isolation culture approach, researchers used an ECM coated Fluidigm C1 chip to isolate individual cells into microwells then applied fluorescence microscopy to track NF-kB transcription factor reporter activity over time after lipopolysaccharide stimulation[226]. The chip was then used to create single cell cDNA libraries for single cell RNA sequencing providing both a history of dynamic NF-kB activity and transcriptome data for each individual cell. This capacity to link a history of dynamic functional behavior to the full transcriptome within the same individual cell is unique to the physical separation approach and allows for a direct link between microenvironment driven function and molecular phenotype.
Emerging methods to link spatial position and gene expression include MERFISH[220], seqFISH[221], and STARmap[222], which can detect from 100’s [220, 221] to up to 1000[222] genes per individual cell in situ within fixed tissue samples. This in situ approach would allow for dynamic monitoring of the sample before fixation to connect past behaviors of each cell to the molecular phenotype and spatial information in the sample at the time of fixation. However, current methods remain complex, time consuming, and low throughput, requiring dedicated facilities and expertise [227–229]. In STARmap, DNA nanoballs are produced locally at the site of each individual cell and entrapped within a 3D DNA hydrogel. Advances in nanoparticle and hydrogel technologies will be expected to facilitate improvements in the in situ sequencing technology. Even with all of the spatial information preserved, in situ spatial genomics remains a destructive snapshot technique and cannot give information about dynamic cell signaling networks.
4.2. Biomaterials and microsystems for spatial interrogation and control
Continuing advances in biomaterial microsystems uniquely allow for spatial control of cells to interrogate the role of spatial organization on tissue function. Synthetic hydrogel chemistries for 3D cell culture allow for high-resolution patterning of degradation [29], mechanical stiffness/crosslinking (Figure 6A)[206], and protein ligands(Figure 6B)[33, 207, 230] using photolithography methods. Just as materials can be patterned with light, optogenetic technology, where light and photosensitive proteins are used to control gene expression [231, 232], can be used to photo-pattern gene expression within inhabitant cells of a microtissue(Figure 6C)[208]. Through the application of 3D multi-phasic gel-in-gel material systems, cell can be patterned, and material characteristic can be controlled at both the microscale and the mesoscale (multi-microenvironment scale) to drive desired behaviors [233–235]. Application of computerized automated systems for 3D printing of these multi-phasic gels can allow for complex spatial patterning of several biological inks containing multiple cells and/or materials and/or voids in 3D space (Figure 6D)[209, 236, 237]. And patterning systems have also recently extended into the temporal domain with one system describing a method to simultaneously pattern 3 proteins, with spatial and temporal control, within a biomaterial (Figure 6B)[207]. An alternative approach with the ability to recapitulate true in vivo morphologies ex vivo is the decellularization of tissues into a vascularized ECM biomaterial that retains much of its native 3D spatial structure followed by subsequent recellularization by perfusion and ECM guided emergent behavior [238]. Integration of either intact decellularized ECM or decellularized ECM reconstituted into hydrogels is a promising strategy for studying tissue-specific behaviors within engineered microsystems [239, 240]. We anticipate that continuing advances [43, 241] in the spatiotemporal control of biomaterial microsystems will enable mechanistic studies of the spatial coordination and feedback loops driving tissue behaviors.
5. Conclusion
The integration of biomaterial technologies with genomics and high content imaging offers the opportunity to molecularly dissect normal and abnormal tissue responses, and ultimately to develop the design principles for biomaterial systems that direct cellular processes and tissue formation. This combination of techniques offers the opportunity to describe the complex spatio-temporal processes at the cellular scale, while also capturing the heterogeneity of cellular responses, with applications in basic science, drug development, and tissue engineering [143, 242, 243]. Given the complexity of emergent tissue behaviors, the path toward designing improved biomaterial microsystems will likely involve the application of systems-level experimental workflows that can elucidate the role of dynamics, spatial coordination, and rare cells in driving tissue functions (Figure 2). This overview of systems level analysis and the currently available tools highlights opportunities for innovation in the biomaterial platforms and their integration with computational algorithms that can process the data and identify the design parameters, which will advance the understanding of biomaterial function and potentially enhance the ultimate pace of translation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- [1].van Helvert S, Storm C, Friedl P, Mechanoreciprocity in cell migration, Nature Cell Biology 20(1) (2018) 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sasai Y, Cytosystems dynamics in self-organization of tissue architecture, Nature 493 (2013) 318. [DOI] [PubMed] [Google Scholar]
- [3].Ronaldson-Bouchard K, Vunjak-Novakovic G, Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development, Cell stem cell 22(3) (2018) 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang B, Korolj A, Lai BFL, Radisic M, Advances in organ-on-a-chip engineering, Nature Reviews Materials 3(8) (2018) 257–278. [Google Scholar]
- [5].Hsu YH, Moya ML, Hughes CC, George SC, Lee AP, A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays, Lab Chip 13(15) (2013) 2990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim S, Lee H, Chung M, Jeon NL, Engineering of functional, perfusable 3D microvascular networks on a chip, Lab on a Chip - Miniaturisation for Chemistry and Biology 13(8) (2013) 1489–1500. [DOI] [PubMed] [Google Scholar]
- [7].Morgan JP, Delnero PF, Zheng Y, Verbridge SS, Chen J, Craven M, Choi NW, Diaz-Santana A, Kermani P, Hempstead B, López JA, Corso TN, Fischbach C, Stroock AD, Formation of microvascular networks in vitro, Nat. Protocols 8(9) (2013) 1820–1836. [DOI] [PubMed] [Google Scholar]
- [8].Legant WR, Pathak A, Yang MT, Deshpande VS, McMeeking RM, Chen CS, Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues, Proceedings of the National Academy of Sciences of the United States of America 106(25) (2009) 10097–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS, A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues, Tissue engineering. Part A 18(9-10) (2012) 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Juhas M, Engelmayr GC, Fontanella AN, Palmer GM, Bursac N, Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo, Proceedings of the National Academy of Sciences 111(15) (2014) 5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rao L, Qian Y, Khodabukus A, Ribar T, Bursac N, Engineering human pluripotent stem cells into a functional skeletal muscle tissue, Nature Communications 9(1) (2018) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fatehullah A, Tan SH, Barker N, Organoids as an in vitro model of human development and disease, Nature Cell Biology 18 (2016) 246. [DOI] [PubMed] [Google Scholar]
- [13].Lancaster MA, Knoblich JA, Organogenesis in a dish: Modeling development and disease using organoid technologies, Science 345(6194) (2014) 1247125. [DOI] [PubMed] [Google Scholar]
- [14].Ranga A, Gjorevski N, Lutolf MP, Drug discovery through stem cell-based organoid models, Advanced Drug Delivery Reviews 69-70 (2014) 19–28. [DOI] [PubMed] [Google Scholar]
- [15].Agrawal A, Choudhary A, Perspective: Materials informatics and big data: Realization of the “fourth paradigm” of science in materials science, APL Materials 4(5) (2016) 053208. [Google Scholar]
- [16].Ramprasad R, Batra R, Pilania G, Mannodi-Kanakkithodi A, Kim C, Machine learning in materials informatics: recent applications and prospects, npj Computational Materials 3(1) (2017) 54. [Google Scholar]
- [17].Kalidindi SR, De Graef M, Materials Data Science: Current Status and Future Outlook, Annual Review of Materials Research, 2015, pp. 171–193. [Google Scholar]
- [18].Groen N, Guvendiren M, Rabitz H, Welsh WJ, Kohn J, de Boer J, Stepping into the omics era: Opportunities and challenges for biomaterials science and engineering, Acta Biomater 34 (2016) 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Darnell M, Mooney DJ, Leveraging advances in biology to design biomaterials, Nature Materials 16(12) (2017) 1178–1184. [DOI] [PubMed] [Google Scholar]
- [20].Seo J, Shin J-Y, Leijten J, Jeon O, Camci-Unal G, Dikina AD, Brinegar K, Ghaemmaghami AM, Alsberg E, Khademhosseini A, High-throughput approaches for screening and analysis of cell behaviors, Biomaterials (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Darnell M, O’Neil A, Mao A, Gu L, Rubin LL, Mooney DJ, Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells, Proceedings of the National Academy of Sciences 115(36) (2018) E8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Papalexi E, Satija R, Single-cell RNA sequencing to explore immune cell heterogeneity, Nature Reviews Immunology 18(1) (2018) 35. [DOI] [PubMed] [Google Scholar]
- [23].Lenstra TL, Rodriguez J, Chen H, Larson DR, Transcription Dynamics in Living Cells, Annual Review of Biophysics 45(1) (2016) 25–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kanherkar RR, Bhatia-Dey N, Csoka AB, Epigenetics across the human lifespan, Frontiers in cell and developmental biology 2 (2014) 49–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arney KL, Fisher AG, Epigenetic aspects of differentiation, J Cell Sci 117(Pt 19) (2004) 4355–63. [DOI] [PubMed] [Google Scholar]
- [26].Bernabé BP, Shin S, Rios PD, Broadbelt LJ, Shea LD, Seidlits SK, Dynamic transcription factor activity networks in response to independently altered mechanical and adhesive microenvironmental cues, Integr Biol (Camb) 8(8) (2016) 844–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Burdick JA, Murphy WL, Moving from static to dynamic complexity in hydrogel design, Nature Communications 3 (2012) 1269. [DOI] [PubMed] [Google Scholar]
- [28].DeForest CA, Anseth KS, Advances in bioactive hydrogels to probe and direct cell fate, Annual review of chemical and biomolecular engineering 3 (2012) 421–44. [DOI] [PubMed] [Google Scholar]
- [29].Kloxin AM, Kasko AM, Salinas CN, Anseth KS, Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties, Science 324(5923) (2009) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stowers RS, Allen SC, Suggs LJ, Dynamic phototuning of 3D hydrogel stiffness, Proceedings of the National Academy of Sciences 112(7) (2015) 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guvendiren M, Burdick JA, Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics, Nat Commun 3 (2012) 792. [DOI] [PubMed] [Google Scholar]
- [32].Ondeck MG, Kumar A, Placone JK, Plunkett CM, Matte BF, Wong KC, Fattet L, Yang J, Engler AJ, Dynamically stiffened matrix promotes malignant transformation of mammary epithelial cells via collective mechanical signaling, Proceedings of the National Academy of Sciences of the United States of America 116(9) (2019) 3502–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Farrukh A, Fan W, Zhao S, Salierno M, Paez JI, del Campo A, Photoactivatable Adhesive Ligands for Light-Guided Neuronal Growth, ChemBioChem 19(12) (2018) 1271–1279. [DOI] [PubMed] [Google Scholar]
- [34].Farrukh A, Paez JI, del Campo A, 4D Biomaterials for Light-Guided Angiogenesis, Advanced Functional Materials 29(6) (2019). [Google Scholar]
- [35].Petersen S, Alonso JM, Specht A, Duodu P, Goeldner M, Del Campo A, Phototriggering of cell adhesion by caged cyclic RGD peptides, Angewandte Chemie - International Edition 47(17) (2008) 3192–3195. [DOI] [PubMed] [Google Scholar]
- [36].Lee TT, García JR, Paez JI, Singh A, Phelps EA, Weis S, Shafiq Z, Shekaran A, Del Campo A, García AJ, Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials, Nature Materials 14(3) (2015) 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Boehler RM, Kuo R, Shin S, Goodman AG, Pilecki MA, Gower RM, Leonard JN, Shea LD, Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype, Biotechnol Bioeng 111(6) (2014) 1210–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rosales AM, Anseth KS, The design of reversible hydrogels to capture extracellular matrix dynamics, Nature Reviews Materials 1(2) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nasrollahi S, Walter C, Loza AJ, Schimizzi GV, Longmore GD, Pathak A, Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory, Biomaterials 146 (2017) 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang C, Tibbitt MW, Basta L, Anseth KS, Mechanical memory and dosing influence stem cell fate, Nature Materials 13 (2014) 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ooi HW, Hafeez S, Van Blitterswijk CA, Moroni L, Baker MB, Hydrogels that listen to cells: A review of cell-responsive strategies in biomaterial design for tissue regeneration, Materials Horizons 4(6) (2017)1020–1040. [Google Scholar]
- [42].Morris E, Chavez M, Tan C, Dynamic biomaterials: toward engineering autonomous feedback, Current Opinion in Biotechnology 39 (2016) 97–104. [DOI] [PubMed] [Google Scholar]
- [43].Caliari SR, Burdick JA, A practical guide to hydrogels for cell culture, Nature methods 13(5) (2016) 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Betz JF, Cheng Y, Tsao C-Y, Zargar A, Wu H-C, Luo X, Payne GF, Bentley WE, Rubloff GW, Optically clear alginate hydrogels for spatially controlled cell entrapment and culture at microfluidic electrode surfaces, Lab on a Chip 13(10) (2013) 1854–1858. [DOI] [PubMed] [Google Scholar]
- [45].Baker BM, Trappmann B, Wang WY, Sakar MS, Kim IL, Shenoy VB, Burdick JA, Chen CS, Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments, Nat Mater 14(12) (2015) 1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hall MS, Alisafaei F, Ban E, Feng X, Hui CY, Shenoy VB, Wu M, Fibrous nonlinear elasticity enables positive Mechanical feedback between cells and ECMs, Proceedings of the National Academy of Sciences of the United States of America 113(49) (2016) 14043–14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Arifler D, Pavlova I, Gillenwater A, Richards-Kortum R, Light scattering from collagen fiber networks: micro-optical properties of normal and neoplastic stroma, Biophysical journal 92(9) (2007) 3260–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Webb P, Lopez GN, Uht RM, Kushner PJ, Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens, Molecular endocrinology (Baltimore, Md.) 9(4) (1995) 443–56. [DOI] [PubMed] [Google Scholar]
- [49].Weiss MS, Penalver Bernabe B, Bellis AD, Broadbelt LJ, Jeruss JS, Shea LD, Dynamic, large-scale profiling of transcription factor activity from live cells in 3D culture, PLoS One 5(11) (2010) e14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bellis AD, Penalver-Bernabe B, Weiss MS, Yarrington ME, Barbolina MV, Pannier AK, Jeruss JS, Broadbelt LJ, Shea LD, Cellular arrays for large-scale analysis of transcription factor activity, Biotechnol Bioeng 108(2) (2011) 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Weiss MS, Bernabé BP, Shikanov A, Bluver DA, Mui MD, Shin S, Broadbelt LJ, Shea LD, The impact of adhesion peptides within hydrogels on the phenotype and signaling of normal and cancerous mammary epithelial cells, Biomaterials 33(13) (2012) 3548–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bellis AD, Bernabe BP, Weiss MS, Shin S, Weng S, Broadbelt LJ, Shea LD, Dynamic transcription factor activity profiling in 2D and 3D cell cultures, Biotechnol Bioeng 110(2) (2013) 563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Weiss MS, Bernabé B. Peñalver, Shin S, Asztalos S, Dubbury SJ, Mui MD, Bellis AD, Bluver D, Tonetti DA, Saez-Rodriguez J, Broadbelt LJ, Jeruss JS, Shea LD, Dynamic transcription factor activity and networks during ErbB2 breast oncogenesis and targeted therapy, Integr Biol (Camb) 6(12) (2014) 1170–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Decker JT, Hobson EC, Zhang Y, Shin S, Thomas AL, Jeruss JS, Arnold KB, Shea LD, Systems analysis of dynamic transcription factor activity identifies targets for treatment in olaparib resistant cancer cells, Biotechnol Bioeng (2017) n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Padmashali RM, Mistriotis P, Liang MS, Andreadis ST, Lentiviral arrays for live-cell dynamic monitoring of gene and pathway activity during stem cell differentiation, Molecular Therapy 22(11) (2014) 1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Moharil J, Lei P, Tian J, Gaile DP, Andreadis ST, Lentivirus live cell array for quantitative assessment of gene and pathway activation during myogenic differentiation of mesenchymal stem cells, PLoS ONE 10(10) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Doench JG, Sharp PA, Specificity of microRNA target selection in translational repression, Genes & Development 18(5) (2004) 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jin Y, Chen Z, Liu X, Zhou X, Evaluating the MicroRNA Targeting Sites by Luciferase Reporter Gene Assay, Methods in molecular biology (Clifton, N.J.) 936 (2013) 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lemus-Diaz N, Böker KO, Rodriguez-Polo I, Mitter M, Preis J, Arlt M, Gruber J, Dissecting miRNA gene repression on single cell level with an advanced fluorescent reporter system, Scientific Reports 7 (2017) 45197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Decker JT, Hall MS, Blaisdell RB, Schwark K, Jeruss JS, Shea LD, Dynamic microRNA activity identifies therapeutic targets in trastuzumab-resistant HER2(+) breast cancer, Biotechnol Bioeng (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhang J, Ma Y, Taylor SS, Tsien RY, Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering, Proc Natl Acad Sci U S A 98(26) (2001) 14997–15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Allen MD, DiPilato LM, Rahdar M, Ren YR, Chong C, Liu JO, Zhang J, Reading dynamic kinase activity in living cells for high-throughput screening, ACS Chem Biol 1(6) (2006) 371–6. [DOI] [PubMed] [Google Scholar]
- [63].Regot S, Hughey JJ, Bajar BT, Carrasco S, Covert MW, High-sensitivity measurements of multiple kinase activities in live single cells, Cell 157(7) (2014) 1724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kudo T, Jeknić S, Macklin DN, Akhter S, Hughey JJ, Regot S, Covert MW, Live-cell measurements of kinase activity in single cells using translocation reporters, Nature Protocols 13 (2017) 155. [DOI] [PubMed] [Google Scholar]
- [65].Gudernova I, Foldynova-Trantirkova S, Ghannamova BE, Fafilek B, Varecha M, Balek L, Hruba E, Jonatova L, Jelinkova I, Bosakova MK, Trantirek L, Mayer J, Krejci P, One reporter for in-cell activity profiling of majority of protein kinase oncogenes, eLife 6 (2017) e21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nozaki T, Imai R, Tanbo M, Nagashima R, Tamura S, Tani T, Joti Y, Tomita M, Hibino K, Kanemaki MT, Wendt KS, Okada Y, Nagai T, Maeshima K, Dynamic Organization of Chromatin Domains Revealed by Super-Resolution Live-Cell Imaging, Molecular Cell 67(2) (2017) 282–293.e7. [DOI] [PubMed] [Google Scholar]
- [67].Dixon AS, Schwinn MK, Hall MP, Zimmerman K, Otto P, Lubben TH, Butler BL, Binkowski BF, Machleidt T, Kirkland TA, Wood MG, Eggers CT, Encell LP, Wood KV, NanoLuc Complementation Reporter Optimized for Accurate Measurement of Protein Interactions in Cells, ACS Chemical Biology 11(2) (2016) 400–408. [DOI] [PubMed] [Google Scholar]
- [68].Truong K, Ikura M, The use of FRET imaging microscopy to detect protein-protein interactions and protein conformational changes in vivo, Current opinion in structural biology 11(5) (2001) 573–8. [DOI] [PubMed] [Google Scholar]
- [69].Erapaneedi R, Belousov VV, Schäfers M, Kiefer F, A novel family of fluorescent hypoxia sensors reveal strong heterogeneity in tumor hypoxia at the cellular level, The EMBO journal 35(1) (2016) 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ostergaard H, Henriksen A, Hansen FG, Winther JR, Shedding light on disulfide bond formation: engineering a redox switch in green fluorescent protein, Embo j 20(21) (2001) 5853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ, Investigating Mitochondrial Redox Potential with Redox-sensitive Green Fluorescent Protein Indicators, Journal of Biological Chemistry 279(13) (2004) 13044–13053. [DOI] [PubMed] [Google Scholar]
- [72].Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H, Superoxide flashes in single mitochondria, Cell 134(2) (2008) 279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S, Genetically encoded fluorescent indicator for intracellular hydrogen peroxide, Nat Methods 3(4) (2006) 281–6. [DOI] [PubMed] [Google Scholar]
- [74].Nakai J, Ohkura M, Imoto K, A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein, Nat Biotechnol 19(2) (2001) 137–41. [DOI] [PubMed] [Google Scholar]
- [75].Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL, Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators, Nature Methods 6(12) (2009) 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Berg J, Hung YP, Yellen G, A genetically encoded fluorescent reporter of ATP:ADP ratio, Nature Methods 6 (2009) 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hung YP, Albeck JG, Tantama M, Yellen G, Imaging cytosolic NADH-NAD(+) redox state with a genetically encoded fluorescent biosensor, Cell Metab 14(4) (2011) 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, Yong Z, Gao Y, Peng W, Wang L, Zhang S, Du J, Lin D, Xu M, Kreitzer AC, Cui G, Li Y, A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice, Cell 174(2) (2018) 481–496.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jing M, Zhang P, Wang G, Feng J, Mesik L, Zeng J, Jiang H, Wang S, Looby JC, Guagliardo NA, Langma LW, Lu J, Zuo Y, Talmage DA, Role LW, Barrett PQ, Zhang LI, Luo M, Song Y, Zhu JJ, Li Y, A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies, Nature Biotechnology 36(8) (2018) 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Marks KM, Nolan GP, Chemical labeling strategies for cell biology, Nature Methods 3(8) (2006) 591–596. [DOI] [PubMed] [Google Scholar]
- [81].Jung D, Min K, Jung J, Jang W, Kwon Y, Chemical biology-based approaches on fluorescent labeling of proteins in live cells, Mol Biosyst 9(5) (2013) 862–72. [DOI] [PubMed] [Google Scholar]
- [82].Li C, Tebo AG, Gautier A, Fluorogenic Labeling Strategies for Biological Imaging, Int J Mol Sci 18(7) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dmitriev RI, Papkovsky DB, Optical probes and techniques for O2 measurement in live cells and tissue, Cell Mol Life Sci 69(12) (2012) 2025–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Woolley JF, Stanicka J, Cotter TG, Recent advances in reactive oxygen species measurement in biological systems, Trends Biochem Sci 38(11) (2013) 556–65. [DOI] [PubMed] [Google Scholar]
- [85].Dolman NJ, Kilgore JA, Davidson MW, A review of reagents for fluorescence microscopy of cellular compartments and structures, part I: BacMam labeling and reagents for vesicular structures, Curr Protoc Cytom Chapter 12 (2013) Unit 12.30. [DOI] [PubMed] [Google Scholar]
- [86].Kilgore JA, Dolman NJ, Davidson MW, A review of reagents for fluorescence microscopy of cellular compartments and structures, Part II: reagents for non-vesicular organelles, Curr Protoc Cytom 66 (2013) 12.31.1–12.31.24. [DOI] [PubMed] [Google Scholar]
- [87].Kilgore JA, Dolman NJ, Davidson MW, A review of reagents for fluorescence microscopy of cellular compartments and structures, Part III: reagents for actin, tubulin, cellular membranes, and whole cell and cytoplasm, Curr Protoc Cytom 67 (2014) 12.32.1–12.32.17. [DOI] [PubMed] [Google Scholar]
- [88].Decker JT, Hall MS, Peñalver-Bernabé B, Blaisdell RB, Liebman LN, Jeruss JS, Shea LD, Design of Large-Scale Reporter Construct Arrays for Dynamic, Live Cell Systems Biology, ACS Synthetic Biology 7(9) (2018) 2063–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tian J, Alimperti S, Lei P, Andreadis ST, Lentiviral microarrays for real-time monitoring of gene expression dynamics, Lab on a Chip - Miniaturisation for Chemistry and Biology 10(15) (2010) 1967–1975. [DOI] [PubMed] [Google Scholar]
- [90].Tung JK, Berglund K, Gutekunst C-A, Hochgeschwender U, Gross RE, Bioluminescence imaging in live cells and animals, Neurophotonics 3(2) (2016) 025001–025001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Troy T, Jekic-McMullen D, Sambucetti L, Rice B, Quantitative comparison of the sensitivity of detection of fluorescent and bioluminescent reporters in animal models, Mol Imaging 3(1) (2004) 9–23. [DOI] [PubMed] [Google Scholar]
- [92].Hall MP, Unch J, Binkowski BF, Valley MP, Butler BL, Wood MG, Otto P, Zimmerman K, Vidugiris G, Machleidt T, Robers MB, Benink HA, Eggers CT, Slater MR, Meisenheimer PL, Klaubert DH, Fan F, Encell LP, Wood KV, Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate, ACS Chemical Biology 7(11) (2012) 1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Takai A, Nakano M, Saito K, Haruno R, Watanabe TM, Ohyanagi T, Jin T, Okada Y, Nagai T, Expanded palette of Nano-lanterns for real-time multicolor luminescence imaging, Proceedings of the National Academy of Sciences 112(14) (2015) 4352–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Suzuki K, Kimura T, Shinoda H, Bai G, Daniels MJ, Arai Y, Nakano M, Nagai T, Five colour variants of bright luminescent protein for real-time multicolour bioimaging, Nature Communications 7 (2016)13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chu J, Oh Y, Sens A, Ataie N, Dana H, Macklin JJ, Laviv T, Welf ES, Dean KM, Zhang F, Kim BB, Tang CT, Hu M, Baird MA, Davidson MW, Kay MA, Fiolka R, Yasuda R, Kim DS, Ng H-L, Lin MZ, A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo, Nat Biotech 34(7) (2016) 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yeh HW, Karmach O, Ji A, Carter D, Martins-Green MM, Ai HW, Red-shifted luciferase-luciferin pairs for enhanced bioluminescence imaging, Nat Methods 14(10) (2017) 971–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ogoh K, Akiyoshi R, May Maw T, Sugiyama T, Dosaka S, Hatta-Ohashi Y, Suzuki H, Bioluminescence microscopy using a short focal-length imaging lens, J. Microsc. 253(3) (2014) 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kim TJ, Tuerkcan S, Ceballos A, Pratx G, Modular platform for low-light microscopy, Biomed Opt Express 6(11) (2015) 4585–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kosuri S, Church GM, Large-scale de novo DNA synthesis: technologies and applications, Nature Methods 11 (2014) 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hughes RA, Ellington AD, Synthetic DNA Synthesis and Assembly: Putting the Synthetic in Synthetic Biology, Cold Spring Harbor perspectives in biology 9(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gibson DG, Young L, Chuang R-Y, Venter JC, Iii C.A. Hutchison, Smith HO, Enzymatic assembly of DNA molecules up to several hundred kilobases, Nature Methods 6 (2009) 343. [DOI] [PubMed] [Google Scholar]
- [102].Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E, A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity, Science 337(6096) (2012) 816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F, Multiplex genome engineering using CRISPR/Cas systems, Science 339(6121) (2013) 819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F, Rationally engineered Cas9 nucleases with improved specificity, Science 351(6268) (2016) 84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, Joung JK, High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects, Nature 529 (2016) 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lackner DH, Carré A, Guzzardo PM, Banning C, Mangena R, Henley T, Oberndorfer S, Gapp BV, Nijman SMB, Brummelkamp TR, Bürckstümmer T, A generic strategy for CRISPR-Cas9-mediated gene tagging, Nature Communications 6 (2015) 10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Schmid-Burgk JL, Honing K, Ebert TS, Hornung V, CRISPaint allows modular base-specific gene tagging using a ligase-4-dependent mechanism, Nat Commun 7 (2016) 12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kamiyama D, Sekine S, Barsi-Rhyne B, Hu J, Chen B, Gilbert LA, Ishikawa H, Leonetti MD, Marshall WF, Weissman JS, Huang B, Versatile protein tagging in cells with split fluorescent protein, Nature Communications 7 (2016) 11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Leonetti MD, Sekine S, Kamiyama D, Weissman JS, Huang B, A scalable strategy for high-throughput GFP tagging of endogenous human proteins, Proceedings of the National Academy of Sciences 113(25) (2016) E3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Chen B, Zou W, Xu H, Liang Y, Huang B, Efficient labeling and imaging of protein-coding genes in living cells using CRISPR-Tag, Nature Communications 9(1) (2018) 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].He X, Tan C, Wang F, Wang Y, Zhou R, Cui D, You W, Zhao H, Ren J, Feng B, Knock-in of large reporter genes in human cells via CRISPR/Cas9-induced homology-dependent and independent DNA repair, Nucleic Acids Research 44(9) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dagogo-Jack I, Shaw AT, Tumour heterogeneity and resistance to cancer therapies, Nature reviews. Clinical oncology 15(2) (2018) 81–94. [DOI] [PubMed] [Google Scholar]
- [113].Dueck H, Eberwine J, Kim J, Variation is function: Are single cell differences functionally important?: Testing the hypothesis that single cell variation is required for aggregate function, BioEssays : news and reviews in molecular, cellular and developmental biology 38(2) (2016) 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Tay S, Hughey JJ, Lee TK, Lipniacki T, Quake SR, Covert MW, Single-cell NF-[kgr]B dynamics reveal digital activation and analogue information processing, Nature 466(7303) (2010) 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Svensson V, Natarajan KN, Ly L-H, Miragaia RJ, Labalette C, Macaulay IC, Cvejic A, Teichmann SA, Power analysis of single-cell RNA-sequencing experiments, Nature Methods 14 (2017) 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ziegenhain C, Vieth B, Parekh S, Reinius B, Guillaumet-Adkins A, Smets M, Leonhardt H, Heyn H, Hellmann I, Enard W, Comparative Analysis of Single-Cell RNA Sequencing Methods, Molecular Cell 65(4) (2017) 631–643.e4. [DOI] [PubMed] [Google Scholar]
- [117].Hwang B, Lee JH, Bang D, Single-cell RNA sequencing technologies and bioinformatics pipelines, Experimental & Molecular Medicine 50(8) (2018) 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kim BJ, Hannanta-anan P, Chau M, Kim YS, Swartz MA, Wu M, Cooperative Roles of SDF-1? and EGF Gradients on Tumor Cell Migration Revealed by a Robust 3D Microfluidic Model, PLoS ONE 8(7) (2013) e68422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lee J, Abdeen AA, Wycislo KL, Fan TM, Kilian KA, Interfacial geometry dictates cancer cell tumorigenicity, Nat Mater 15(8) (2016) 856–862. [DOI] [PubMed] [Google Scholar]
- [120].Trappmann B, Baker BM, Polacheck WJ, Choi CK, Burdick JA, Chen CS, Matrix degradability controls multicellularity of 3D cell migration, Nature Communications 8(1) (2017) 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Polonsky M, Rimer J, Kern-Perets A, Zaretsky I, Miller S, Bornstein C, David E, Kopelman NM, Stelzer G, Porat Z, Chain B, Friedman N, Induction of CD4 T cell memory by local cellular collectivity, Science 360(6394) (2018). [DOI] [PubMed] [Google Scholar]
- [122].Xu Y, Zhou S, Lam YW, Pang SW, Dynamics of Natural Killer Cells Cytotoxicity in Microwell Arrays with Connecting Channels, Frontiers in immunology 8 (2017) 998–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Cheng S-Y, Heilman S, Wassweman M, Archer S, Shuler ML, Wu M, A hydrogel-based microfluidic device for the studies of directed cell migration, Lab-on-a-chip 7 (2007) 763–769. [DOI] [PubMed] [Google Scholar]
- [124].Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD, Cell migration into scaffolds under co-culture conditions in a microfluidic platform, Lab on a Chip 9(2) (2009) 269–75. [DOI] [PubMed] [Google Scholar]
- [125].Abhyankar VV, Lokuta MA, Huttenlocher A, Beebe DJ, Characterization of a membrane-based gradient generator for use in cell-signaling studies, Lab Chip 6(3) (2006) 389–93. [DOI] [PubMed] [Google Scholar]
- [126].Jeon NL, Dertinger SKW, Chiu DT, Choi IS, Stroock AD, Whitesides GM, Generation of solution and surface gradients using microfluidic systems, Langmuir 16(22) (2000) 8311–8316. [Google Scholar]
- [127].Pelham RJ Jr., Wang Y, Cell locomotion and focal adhesions are regulated by substrate flexibility, Proceedings of the National Academy of Sciences of the United States of America 94(25) (1997) 13661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Tse JR, Engler AJ, Preparation of hydrogel substrates with tunable mechanical properties, Curr Protoc Cell Biol Chapter 10 (2010) Unit 10.16. [DOI] [PubMed] [Google Scholar]
- [129].Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM, Tensional homeostasis and the malignant phenotype, Cancer Cell 8(3) (2005) 241–254. [DOI] [PubMed] [Google Scholar]
- [130].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification, Cell 126(4) (2006) 677–89. [DOI] [PubMed] [Google Scholar]
- [131].Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA, Nonlinear elasticity in biological gels, Nature 435(7039) (2005) 191–194. [DOI] [PubMed] [Google Scholar]
- [132].Lo CM, Wang HB, Dembo M, Wang YL, Cell movement is guided by the rigidity of the substrate, Biophysical journal 79(1) (2000) 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Califano JP, Reinhart-King CA, Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact, Cell Mol Bioeng 3(1) (2010) 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kraning-Rush CM, Reinhart-King CA, Controlling matrix stiffness and topography for the study of tumor cell migration, Cell Adhesion & Migration 6(3) (2012) 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kim M, Kim T-J, Kim HM, Doh J, Lee K-M, Multi-cellular natural killer (NK) cell clusters enhance NK cell activation through localizing IL-2 within the cluster, Scientific reports 7 (2017) 40623–40623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Bhat R, Watzl C, Serial Killing of Tumor Cells by Human Natural Killer Cells – Enhancement by Therapeutic Antibodies, PLOS ONE 2(3) (2007) e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Vanherberghen B, Olofsson PE, Forslund E, Sternberg-Simon M, Khorshidi MA, Pacouret S, Guldevall K, Enqvist M, Malmberg KJ, Mehr R, Onfelt B, Classification of human natural killer cells based on migration behavior and cytotoxic response, Blood 121(8) (2013) 1326–1334. [DOI] [PubMed] [Google Scholar]
- [138].Choi PJ, Mitchison TJ, Imaging burst kinetics and spatial coordination during serial killing by single natural killer cells, Proceedings of the National Academy of Sciences of the United States of America 110(16) (2013) 6488–6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Backes CS, Friedmann KS, Mang S, Knorck A, Hoth M, Kummerow C, Natural killer cells induce distinct modes of cancer cell death: Discrimination, quantification, and modulation of apoptosis, necrosis, and mixed forms, J Biol Chem 293(42) (2018) 16348–16363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Srpan K, Ambrose A, Karampatzakis A, Saeed M, Cartwright ANR, Guldevall K, De Matos GDSC, Onfelt B, Davis DM, Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells, The Journal of Cell Biology 217(9) (2018) 3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H, Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche, Nature 459(7244) (2009) 262–265. [DOI] [PubMed] [Google Scholar]
- [142].Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y, Self-organizing optic-cup morphogenesis in three-dimensional culture, Nature 472(7341) (2011) 51–56. [DOI] [PubMed] [Google Scholar]
- [143].Dye BR, Dedhia PH, Miller AJ, Nagy MS, White ES, Shea LD, Spence JR, A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids, eLife 5 (2016) e19732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Sasai Y, Eiraku M, Suga H, In vitro organogenesis in three dimensions: self-organising stem cells, Development 139(22) (2012) 4111. [DOI] [PubMed] [Google Scholar]
- [145].Brassard JA, Lutolf MP, Engineering Stem Cell Self-organization to Build Better Organoids, Cell Stem Cell 24(6) (2019) 860–876. [DOI] [PubMed] [Google Scholar]
- [146].Kratochvil MJ, Seymour AJ, Li TL, Paşca SP, Kuo CJ, Heilshorn SC, Engineered materials for organoid systems, Nature Reviews Materials 4(9) (2019) 606–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Svensson V, Vento-Tormo R, Teichmann SA, Exponential scaling of single-cell RNA-seq in the past decade, Nature Protocols 13 (2018) 599. [DOI] [PubMed] [Google Scholar]
- [148].Butler A, Hoffman P, Smibert P, Papalexi E, Satija R, Integrating single-cell transcriptomic data across different conditions, technologies, and species, Nature Biotechnology 36 (2018) 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL, The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells, Nature Biotechnology 32 (2014) 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C, Reversed graph embedding resolves complex single-cell trajectories, Nature Methods 14 (2017) 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Packer J, Trapnell C, Single-Cell Multi-omics: An Engine for New Quantitative Models of Gene Regulation, Trends in genetics : TIG 34(9) (2018) 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, Satija R, Smibert P, Simultaneous epitope and transcriptome measurement in single cells, Nat Methods 14(9) (2017) 865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Peterson VM, Zhang KX, Kumar N, Wong J, Li L, Wilson DC, Moore R, McClanahan TK, Sadekova S, Klappenbach JA, Multiplexed quantification of proteins and transcripts in single cells, Nature Biotechnology 35 (2017) 936. [DOI] [PubMed] [Google Scholar]
- [154].Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F, Genome-scale CRISPR-Cas9 knockout screening in human cells, Science 343(6166)(2014)84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, Qi LS, Kampmann M, Weissman JS, Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation, Cell 159(3) (2014) 647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Adamson B, Norman TM, Jost M, Cho MY, Nunez JK, Chen Y, Villalta JE, Gilbert LA, Horlbeck MA, Hein MY, Pak RA, Gray AN, Gross CA, Dixit A, Parnas O, Regev A, Weissman JS, A Multiplexed Single-Cell CRISPR Screening Platform Enables Systematic Dissection of the Unfolded Protein Response, Cell 167(7) (2016) 1867–1882.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Jaitin DA, Weiner A, Yofe I, Lara-Astiaso D, Keren-Shaul H, David E, Salame TM, Tanay A, van Oudenaarden A, Amit I, Dissecting Immune Circuits by Linking CRISPR-Pooled Screens with Single-Cell RNA-Seq, Cell 167(7) (2016) 1883–1896.e15. [DOI] [PubMed] [Google Scholar]
- [158].Cusanovich DA, Daza R, Adey A, Pliner HA, Christiansen L, Gunderson KL, Steemers FJ, Trapnell C, Shendure J, Multiplex single cell profiling of chromatin accessibility by combinatorial cellular indexing, Science 348(6237) (2015) 910–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, Chang HY, Greenleaf WJ, Single-cell chromatin accessibility reveals principles of regulatory variation, Nature 523 (2015) 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Lake BB, Chen S, Sos BC, Fan J, Kaeser GE, Yung YC, Duong TE, Gao D, Chun J, Kharchenko PV, Zhang K, Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain, Nature Biotechnology 36 (2017) 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Qiu X, Hill A, Packer J, Lin D, Ma Y-A, Trapnell C, Single-cell mRNA quantification and differential analysis with Census, Nature Methods 14 (2017) 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Sun N, Yu X, Li F, Liu D, Suo S, Chen W, Chen S, Song L, Green CD, McDermott J, Shen Q, Jing N, Han J-DJ, Inference of differentiation time for single cell transcriptomes using cell population reference data, Nature Communications 8(1) (2017) 1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Lummertz da Rocha E, Rowe RG, Lundin V, Malleshaiah M, Jha DK, Rambo CR, Li H, North TE, Collins JJ, Daley GQ, Reconstruction of complex single-cell trajectories using CellRouter, Nature Communications 9(1) (2018) 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Boutros M, Heigwer F, Laufer C, Microscopy-Based High-Content Screening, Cell 163(6) (2015) 1314–1325. [DOI] [PubMed] [Google Scholar]
- [165].Skylaki S, Hilsenbeck O, Schroeder T, Challenges in long-term imaging and quantification of single-cell dynamics, Nature Biotechnology 34 (2016) 1137. [DOI] [PubMed] [Google Scholar]
- [166].Zhang JZ, Guo H, Wu JC, Applications of genetically engineered human pluripotent stem cell reporters in cardiac stem cell biology, Curr Opin Biotechnol 52 (2018) 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Bak RO, Dever DP, Reinisch A, Cruz Hernandez D, Majeti R, Porteus MH, Multiplexed genetic engineering of human hematopoietic stem and progenitor cells using CRISPR/Cas9 and AAV6, Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Bressan RB, Dewari PS, Kalantzaki M, Gangoso E, Matjusaitis M, Garcia-Diaz C, Blin C, Grant V, Bulstrode H, Gogolok S, Skarnes WC, Pollard SM, Efficient CRISPR/Cas9-assisted gene targeting enables rapid and precise genetic manipulation of mammalian neural stem cells, Development 144(4) (2017) 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Kang J, Hsu C-H, Wu Q, Liu S, Coster AD, Posner BA, Altschuler SJ, Wu LF, Improving drug discovery with high-content phenotypic screens by systematic selection of reporter cell lines, Nature Biotechnology 34 (2015) 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Wink S, Hiemstra S, Huppelschoten S, Danen E, Niemeijer M, Hendriks G, Vrieling H, Herpers B, van de Water B, Quantitative high content imaging of cellular adaptive stress response pathways in toxicity for chemical safety assessment, Chem Res Toxicol 27(3) (2014) 338–55. [DOI] [PubMed] [Google Scholar]
- [171].Wink S, Hiemstra S, Herpers B, van de Water B, High-content imaging-based BAC-GFP toxicity pathway reporters to assess chemical adversity liabilities, Archives of Toxicology 91(3) (2017) 1367–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Wink S, Hiemstra SW, Huppelschoten S, Klip JE, van de Water B, Dynamic imaging of adaptive stress response pathway activation for prediction of drug induced liver injury, Archives of Toxicology 92(5) (2018) 1797–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Ciuffi A, Mechanisms governing lentivirus integration site selection, Curr Gene Ther 8(6) (2008) 419–29. [DOI] [PubMed] [Google Scholar]
- [174].Smith RH, Adeno-associated virus integration: virus versus vector, Gene Therapy 15(11) (2008) 817–822. [DOI] [PubMed] [Google Scholar]
- [175].Sadelain M, Papapetrou EP, Bushman FD, Safe harbours for the integration of new DNA in the human genome, Nature Reviews Cancer 12(1) (2012) 51–58. [DOI] [PubMed] [Google Scholar]
- [176].Papapetrou EP, Schambach A, Gene insertion into genomic safe harbors for human gene therapy, Molecular Therapy 24(4) (2016) 678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Griffith LG, Swartz MA, Capturing complex 3D tissue physiology in vitro, Nat Rev Mol Cell Biol 7(3) (2006) 211–24. [DOI] [PubMed] [Google Scholar]
- [178].Edmondson R, Broglie JJ, Adcock AF, Yang L, Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors, Assay Drug Dev Technol 12(4) (2014) 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Frigault MM, Lacoste J, Swift JL, Brown CM, Live-cell microscopy - tips and tools, Journal of Cell Science 122(6) (2009) 753. [DOI] [PubMed] [Google Scholar]
- [180].Chen B-C, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, Janetopoulos C, Wu XS, Hammer JA, Liu Z, English BP, Mimori-Kiyosue Y, Romero DP, Ritter AT, Lippincott-Schwartz J, Fritz-Laylin L, Mullins RD, Mitchell DM, Bembenek JN, Reymann A-C, Bohme R, Grill SW, Wang JT, Seydoux G, Tulu US, Kiehart DP, Betzig E, Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution, Science 346(6208) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Liu T-L, Upadhyayula S, Milkie DE, Singh V, Wang K, Swinburne IA, Mosaliganti KR, Collins ZM, Hiscock TW, Shea J, Kohrman AQ, Medwig TN, Dambournet D, Forster R, Cunniff B, Ruan Y, Yashiro H, Scholpp S, Meyerowitz EM, Hockemeyer D, Drubin DG, Martin BL, Matus DQ, Koyama M, Megason SG, Kirchhausen T, Betzig E, Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms, Science 360(6386) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].McGorty R, Liu H, Kamiyama D, Dong Z, Guo S, Huang B, Open-top selective plane illumination microscope for conventionally mounted specimens, Optics express 23(12) (2015) 16142–16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].McGorty R, Xie D, Huang B, High-NA open-top selective-plane illumination microscopy for biological imaging, Optics express 25(15) (2017) 17798–17810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Glaser AK, Reder NP, Chen Y, McCarty EF, Yin C, Wei L, Wang Y, True LD, Liu JTC, Light-sheet microscopy for slide-free non-destructive pathology of large clinical specimens, Nature Biomedical Engineering 1 (2017) 0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Dunsby C, Optically sectioned imaging by oblique plane microscopy, Optics express 16(25) (2008) 20306–16. [DOI] [PubMed] [Google Scholar]
- [186].Caicedo JC, Cooper S, Heigwer F, Warchal S, Qiu P, Molnar C, Vasilevich AS, Barry JD, Bansal HS, Kraus O, Wawer M, Paavolainen L, Herrmann MD, Rohban M, Hung J, Hennig H, Concannon J, Smith I, Clemons PA, Singh S, Rees P, Horvath P, Linington RG, Carpenter AE, Data-analysis strategies for image-based cell profiling, Nature Methods 14(9) (2017) 849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Smith K, Piccinini F, Balassa T, Koos K, Danka T, Azizpour H, Horvath P, Phenotypic Image Analysis Software Tools for Exploring and Understanding Big Image Data from Cell-Based Assays, Cell Systems 6(6) (2018) 636–653. [DOI] [PubMed] [Google Scholar]
- [188].McQuin C, Goodman A, Chernyshev V, Kamentsky L, Cimini BA, Karhohs KW, Doan M, Ding L, Rafelski SM, Thirstrup D, Wiegraebe W, Singh S, Becker T, Caicedo JC, Carpenter AE, CellProfiler 3.0: Next-generation image processing for biology, PLOS Biology 16(7) (2018) e2005970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Dao D, Fraser AN, Hung J, Ljosa V, Singh S, Carpenter AE, CellProfiler Analyst: interactive data exploration, analysis and classification of large biological image sets, Bioinformatics 32(20) (2016) 3210–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Mattiazzi Usaj M, Styles EB, Verster AJ, Friesen H, Boone C, Andrews BJ, High-Content Screening for Quantitative Cell Biology, Trends in Cell Biology 26(8) (2016) 598–611. [DOI] [PubMed] [Google Scholar]
- [191].Ulman V, Maška M, Magnusson KEG, Ronneberger O, Haubold C, Harder N, Matula P, Matula P, Svoboda D, Radojevic M, Smal I, Rohr K, Jaldén J, Blau HM, Dzyubachyk O, Lelieveldt B, Xiao P, Li Y, Cho S-Y, Dufour AC, Olivo-Marin J-C, Reyes-Aldasoro CC, Solis-Lemus JA, Bensch R, Brox T, Stegmaier J, Mikut R, Wolf S, Hamprecht FA, Esteves T, Quelhas P, Demirel Ö, Malmström L, Jug F, Tomancak P, Meijering E, Muñoz-Barrutia A, Kozubek M, Ortiz-de-Solorzano C, An objective comparison of cell-tracking algorithms, Nature methods 14(12) (2017) 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Grys BT, Lo DS, Sahin N, Kraus OZ, Morris Q, Boone C, Andrews BJ, Machine learning and computer vision approaches for phenotypic profiling, Journal of Cell Biology 216(1) (2017) 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Kraus OZ, Grys BT, Ba J, Chong Y, Frey BJ, Boone C, Andrews BJ, Automated analysis of high-content microscopy data with deep learning, Mol Syst Biol 13(4) (2017) 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Gupta A, Harrison PJ, Wieslander H, Pielawski N, Kartasalo K, Partel G, Solorzano L, Suveer A, Klemm AH, Spjuth O, Sintorn IM, Wahlby C, Deep Learning in Image Cytometry: A Review, Cytometry Part A 95(4) (2019) 366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Pan C, Schoppe O, Parra-Damas A, Cai R, Todorov MI, Gondi G, von Neubeck B, Bogurcu-Seidel N, Seidel S, Sleiman K, Veltkamp C, Forstera B, Mai H, Rong Z, Trompak O, Ghasemigharagoz A, Reimer MA, Cuesta AM, Coronel J, Jeremias I, Saur D, Acker-Palmer A, Acker T, Garvalov BK, Menze B, Zeidler R, Erturk A, Deep Learning Reveals Cancer Metastasis and Therapeutic Antibody Targeting in the Entire Body, Cell 179(7) (2019) 1661–1676.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Falk T, Mai D, Bensch R, Çiçek Ö, Abdulkadir A, Marrakchi Y, Böhm A, Deubner J, Jäckel Z, Seiwald K, Dovzhenko A, Tietz O, Dal Bosco C, Walsh S, Saltukoglu D, Tay TL, Prinz M, Palme K, Simons M, Diester I, Brox T, Ronneberger O, U-Net: deep learning for cell counting, detection, and morphometry, Nature Methods 16(1) (2019) 67–70. [DOI] [PubMed] [Google Scholar]
- [197].Moen E, Bannon D, Kudo T, Graf W, Covert M, Van Valen D, Deep learning for cellular image analysis, Nature Methods 16(12) (2019) 1233–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].O'Brien LE, Bilder D, Beyond the niche: tissue-level coordination of stem cell dynamics, Annu Rev Cell Dev Biol 29 (2013) 107–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Park S, Gonzalez DG, Guirao B, Boucher JD, Cockburn K, Marsh ED, Mesa KR, Brown S, Rompolas P, Haberman AM, BellaVche Y, Greco V, Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice, Nature cell biology 19(2) (2017) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Kim EJY, Korotkevich E, Hiiragi T, Coordination of Cell Polarity, Mechanics and Fate in Tissue Self-organization, Trends in Cell Biology 28(7) (2018) 541–550. [DOI] [PubMed] [Google Scholar]
- [201].Featherstone K, Hey K, Momiji H, McNamara AV, Patist AL, Woodburn J, Spiller DG, Christian HC, McNeilly AS, Mullins JJ, Finkenstadt BF, Rand DA, White MRH, Davis JRE, Spatially coordinated dynamic gene transcription in living pituitary tissue, eLife 5 (2016) e08494–e08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Carmona-Fontaine C, Deforet M, Akkari L, Thompson CB, Joyce JA, Xavier JB, Metabolic origins of spatial organization in the tumor microenvironment, Proc Natl Acad Sci U S A 114(11) (2017) 2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Deneke VE, Di Talia S, Chemical waves in cell and developmental biology, The Journal of Cell Biology 217(4) (2018) 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Reinhart-King CA, Dembo M, Hammer DA, Cell-Cell Mechanical Communication through Compliant Substrates, Biophysical Journal 95(12) (2008) 6044–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Winer JP, Oake S, Janmey PA, Non-Linear Elasticity of Extracellular Matrices Enables Contractile Cells to Communicate Local Position and Orientation, Plos One 4(7) (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Khetan S, Burdick JA, Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels, Biomaterials 31(32) (2010) 8228–8234. [DOI] [PubMed] [Google Scholar]
- [207].Shadish JA, Benuska GM, DeForest CA, Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials, Nature Materials 18(9) (2019) 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Polstein LR, Juhas M, Hanna G, Bursac N, Gersbach CA, An Engineered Optogenetic Switch for Spatiotemporal Control of Gene Expression, Cell Differentiation, and Tissue Morphogenesis, ACS Synthetic Biology 6(11) (2017) 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, Johansson F, Randles A, Rosenkrantz JE, Louis-Rosenberg JD, Galie PA, Stevens KR, Miller JS, Multivascular networks and functional intravascular topologies within biocompatible hydrogels, Science 364(6439) (2019) 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE, Geometric control of cell life and death, Science 276(5317) (1997) 1425–1428. [DOI] [PubMed] [Google Scholar]
- [211].Thery M, Micropatterning as a tool to decipher cell morphogenesis and functions, Journal of Cell Science 123(24) (2010) 4201. [DOI] [PubMed] [Google Scholar]
- [212].Houchin-Ray T, Whittlesey KJ, Shea LD, Spatially Patterned Gene Delivery for Localized Neuron Survival and Neurite Extension, Molecular Therapy 15(4) (2007) 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Houchin-Ray T, Swift LA, Jang J-H, Shea LD, Patterned PLG substrates for localized DNA delivery and directed neurite extension, Biomaterials 28(16) (2007) 2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Kim BJ, Wu M, Microfluidics for Mammalian Cell Chemotaxis, Annals of biomedical engineering 40(6) (2012) 1316–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Haessler U, Pisano M, Wu M, Swartz MA, Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19, Proc Natl Acad Sci U S A 108(14) (2011) 5614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Kim BJ, Hannanta-anan P, Chau M, Kim YS, Swartz MA, Wu M, Cooperative Roles of SDF-1α and EGF Gradients on Tumor Cell Migration Revealed by a Robust 3D Microfluidic Model, PLOS ONE 8(7) (2013) e68422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Isenberg BC, DiMilla PA, Walker M, Kim S, Wong JY, Vascular Smooth Muscle Cell Durotaxis Depends on Substrate Stiffness Gradient Strength, Biophysical Journal 97(5) (2009) 1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Raab M, Swift J, Dingal PCDP, Shah P, Shin J-W, Discher DE, Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain, The Journal of Cell Biology 199(4) (2012) 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Shao Y, Taniguchi K, Gurdziel K, Townshend RF, Xue X, Yong KMA, Sang J, Spence JR, Gumucio DL, Fu J, Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche, Nat Mater 16(4) (2017) 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Chen KH, Boettiger AN, Moffitt JR, Wang S, Zhuang X, RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells, Science 348(6233) (2015) aaa6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Shah S, Lubeck E, Zhou W, Cai L, In Situ Transcription Profiling of Single Cells Reveals Spatial Organization of Cells in the Mouse Hippocampus, Neuron 92(2) (2016) 342–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Wang X, Allen WE, Wright MA, Sylwestrak EL, Samusik N, Vesuna S, Evans K, Liu C, Ramakrishnan C, Liu J, Nolan GP, Bava F-A, Deisseroth K, Three-dimensional intact-tissue sequencing of single-cell transcriptional states, Science (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Gross A, Schoendube J, Zimmermann S, Steeb M, Zengerle R, Koltay P, Technologies for Single-Cell Isolation, Int J Mol Sci 16(8) (2015) 16897–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Hu P, Zhang W, Xin H, Deng G, Single Cell Isolation and Analysis, Frontiers in cell and developmental biology 4 (2016) 116–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Sun G, Lu H, Recent Advances in Microfluidic Techniques for Systems Biology, Anal Chem (2018). [DOI] [PubMed] [Google Scholar]
- [226].Lane K, Van Valen D, DeFelice MM, Macklin DN, Kudo T, Jaimovich A, Carr A, Meyer T, Pe’er D, Boutet SC, Covert MW, Measuring Signaling and RNA-Seq in the Same Cell Links Gene Expression to Dynamic Patterns of NF-kappaB Activation, Cell systems 4(4) (2017) 458–469.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Crosetto N, Bienko M, van Oudenaarden A, Spatially resolved transcriptomics and beyond, Nature Reviews Genetics 16 (2014) 57. [DOI] [PubMed] [Google Scholar]
- [228].Strell C, Hilscher MM, Laxman N, Svedlund J, Wu C, Yokota C, Nilsson M, Placing RNA in context and space – methods for spatially resolved transcriptomics, The FEBS journal 0(0) (2018). [DOI] [PubMed] [Google Scholar]
- [229].Lein E, Borm LE, Linnarsson S, The promise of spatial transcriptomics for neuroscience in the era of molecular cell typing, Science 358(6359) (2017) 64–69. [DOI] [PubMed] [Google Scholar]
- [230].DeForest CA, Polizzotti BD, Anseth KS, Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments, Nature Materials 8 (2009) 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Le C, Platt RJ, Scott DA, Church GM, Zhang F, Optical control of mammalian endogenous transcription and epigenetic states, Nature 500(7463) (2013) 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Polstein LR, Gersbach CA, A light-inducible CRISPR-Cas9 system for control of endogenous gene activation, Nature Chemical Biology 11(3) (2015) 198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Kühn S, Sievers J, Stoppa A, Träber N, Zimmermann R, Welzel PB, Werner C, Cell-Instructive Multiphasic Gel-in-Gel Materials, Advanced Functional Materials n/a(n/a) (2020) 1908857. [Google Scholar]
- [234].Husman D, Welzel PB, Vogler S, Bray LJ, Traber N, Friedrichs J, Korber V, Tsurkan MV, Freudenberg U, Thiele J, Werner C, Multiphasic microgel-in-gel materials to recapitulate cellular mesoenvironments in vitro, Biomater Sci 8(1) (2019) 101–108. [DOI] [PubMed] [Google Scholar]
- [235].Visser CW, Kamperman T, Karbaat LP, Lohse D, Karperien M, In-air microfluidics enables rapid fabrication of emulsions, suspensions, and 3D modular (bio)materials, Science Advances 4(1) (2018) eaao1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Mandrycky C, Wang Z, Kim K, Kim D-H, 3D bioprinting for engineering complex tissues, Biotechnology Advances 34(4) (2016) 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Murphy SV, Atala A, 3D bioprinting of tissues and organs, Nature Biotechnology 32 (2014) 773. [DOI] [PubMed] [Google Scholar]
- [238].Badylak SF, Taylor D, Uygun K, Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds, Annu Rev Biomed Eng 13 (2011) 27–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Hussey GS, Dziki JL, Badylak SF, Extracellular matrix-based materials for regenerative medicine, Nature Reviews Materials 3(7) (2018) 159–173. [Google Scholar]
- [240].Gilpin A, Yang Y, Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications, BioMed Research International 2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Leijten J, Seo J, Yue K, Santiago G.T.-d., Tamayol A, Ruiz-Esparza GU, Shin SR, Sharifi R, Noshadi I, Alvarez MM, Zhang YS, Khademhosseini A, Spatially and Temporally Controlled Hydrogels for Tissue Engineering, Mater Sci Eng R Rep 119 (2017) 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CCW, George SC, Prevascularization of a Fibrin-Based Tissue Construct Accelerates the Formation of Functional Anastomosis with Host Vasculature, Tissue Engineering Part A 15(6) (2008) 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Youngblood RL, Sampson JP, Lebioda KR, Shea LD, Microporous scaffolds support assembly and differentiation of pancreatic progenitors into β-cell clusters, Acta Biomaterialia (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


