Abstract
The vasculature is a key component of the tissue microenvironment. Traditionally known for its role to provide nutrients and oxygen to surrounding cells, the vasculature is now also acknowledged to provide signaling cues that influence biological outcomes in regeneration and disease. These cues come from the cells that comprise vasculature, as well as the dynamic biophysical and biochemical properties of the surrounding extracellular matrix that accompany vascular development and remodeling. In this review, we illustrate the larger role of the vasculature in the context of regenerative biology and cancer progression. We describe cellular, biophysical, biochemical, and metabolic components of these vascularized microenvironments. Moreover, we provide an overview of multidimensional angiogenic biomaterials that have been developed to promote therapeutic vascularization and regeneration, as well as to mimic elements of vascularized microenvironments as a means to uncover mechanisms by which vasculature influences cancer progression and therapy.
Keywords: angiogenesis, vascularization, biomaterials, hydrogels, microenvironment, angiocrine, regeneration, stem cell, cancer
1. Introduction
Tissue microenvironments contain distinct biophysical, biochemical, and metabolic cues that may directly influence cell behavior or indirectly influence signaling between diverse cell populations as a means to direct a wide range of phenotypic outcomes [1–6]. The vascular niche, which is the microenvironment immediately surrounding vasculature, has been implicated in supporting various biological processes such as tissue regeneration, stem cell maintenance, and cancer progression [3, 7–10]. While the vasculature serves an important role in supplying tissue with nutrients and oxygen [11], signaling between vascular and non-vascular cells residing in the niche environment, termed angiocrine signaling, is another essential mechanism by which the vasculature instructs biological activity [12]. Furthermore, knowledge that the mechanical properties and composition of the extracellular matrix (ECM) instruct cell phenotype implies that the deposition and remodeling of the perivascular ECM can also act as an instructional cue within the vascular niche [13–16].
Existing knowledge regarding the role of the vascular niche in regenerative and stem cell processes has encouraged the integration of niche components with biomaterial platforms [7]. Such systems seek to stimulate vascularization either in vitro or in vivo to enhance regenerative potential or stem cell expansion. Additionally, due to the complexity of the native vascular niche, biomaterials that recapitulate elements of the vascular niche have recently become a primary instrument to examine mechanisms by which the vascular niche impacts phenotypic outcomes. Knowledge gleaned from these models can then be applied to better engineer biomaterial systems for regeneration, stem cell processing, and even treatments for cancer.
In this review, we first discuss the cellular, matrix, and biomolecular components of the niche environment. We then provide an overview of literature supporting the role of the vascular niche in modulating regeneration, stem cell behavior, and cancer progression (Figure 1). Subsequently, we describe various efforts that have been undertaken to develop angiogenic biomaterial platforms that incorporate elements of the vascular niche or facilitate its development in vivo. We particularly emphasize the development of tumor angiogenesis models for mechanistic studies and drug screening. Finally, we provide a perspective on opportunities that remain in the development and usage of angiogenic biomaterial models of the vascular niche.
Figure 1.
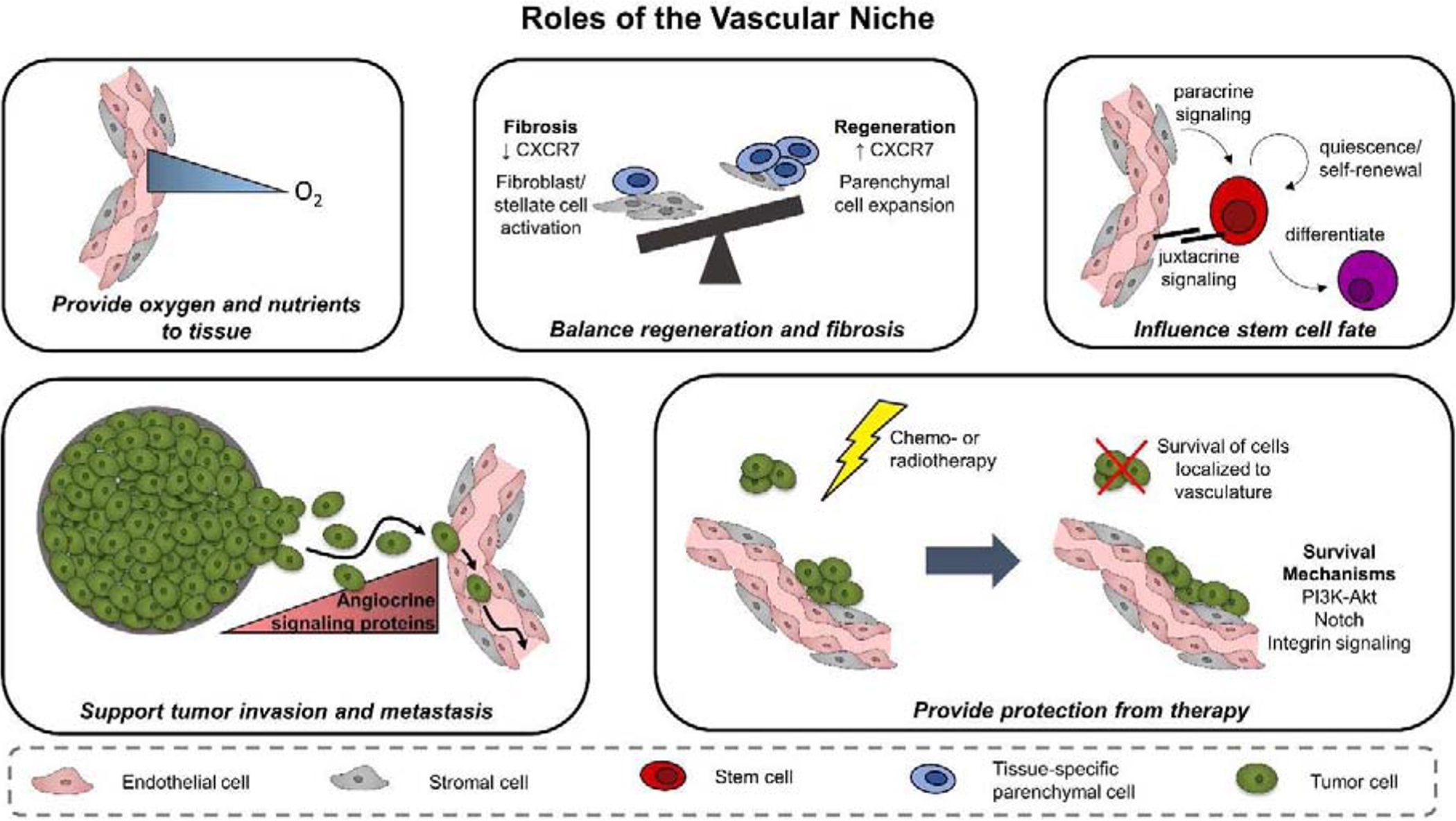
The vascular niche has various roles that influence regenerative outcome, stem cell fate, and cancer progression. In addition to providing oxygen and nutrients to the surrounding tissue, the vascular niche provides vascular-derived, or angiocrine, signals that influence parenchymal cell behavior. Angiocrine cues can direct a tissue towards a regenerative or fibrotic state, control stem cell maintenance versus differentiation, and facilitate tumor progression and therapeutic response.
2. Components of the Vascular Niche
Emergent behavior within the vascular niche results from the integration of microenvironmental cues that direct niche development and function. These cues include a wide range of cell, ECM, and biochemical signaling pathways that give rise to vascular microenvironments that vary between tissues and species [17–26] (Table 1). Considering the multiple classes of signals present within the vascular niche will inspire new tissue engineering approaches to replicate elements of these niche signals for therapeutic advantage.
Table 1.
Characteristics of vasculature in tissues
| Location | Species | Vessel Characteristics | References | |
|---|---|---|---|---|
| Brain | Human | |||
| Cerebral cortex capillaries | Average branch length | 0.060 mm | [17] | |
| Total network length | 461 mm/mm3 | |||
| Total vessels | 8400/mm3 | |||
| Brain | Mouse | |||
| Cerebral cortex capillaries | Average branch length | 0.045 mm | ||
| Total network length | 673 mm/mm3 | |||
| Total vessels | 17000/mm3 | |||
| Whole brain | Total network length | 400 – 1000 mm/mm3 | [18] | |
| Motor cortex capillaries | Average branch length | 0.020 – 0.14 mm | [19] | |
| Average diameter | 0.005 – 0.060 mm | |||
| Whole brain capillaries | Total network length | 465 mm/mm3 | ||
| Cortex | Total network length | 2090 mm/mm3 | [20] | |
| Average diameter | 0.00213 mm | |||
| Hippocampus | Total network length | 1690 mm/mm3 | ||
| Average diameter | 0.00171 mm | |||
| Thalamus | Total network length | 1770 mm/mm3 | ||
| Average diameter | 0.003090 mm | |||
| Bone | Murine | |||
| Metaphysis | Total vessels | 279/mm2 | [21] | |
| Average diameter | 0.027 mm | |||
| Diaphysis | Total vessels | 258/mm2 | ||
| Average diameter | 0.022 mm | |||
| Cortical bone | Average branch length | 0.1 mm | [22] | |
| Average diameter | 0.012 mm | |||
| Pancreas | Rat | |||
| Exocrine | Total vessels | 1050/mm2 | [23] | |
| Average diameter | 0.0054 mm | |||
| Endocrine | Total vessels | 1430/mm2 | ||
| Average diameter | 0.0064 mm | |||
| Lung | Human | |||
| Pulmonary artery, left lung | Average branch length | 0.2 – 10 mm | [24] | |
| Average diameter | 0.02 – 15 mm | |||
| Pulmonary vein, right lung | Average branch length | 0.12 – 36 mm | ||
| Average diameter | 0.018 – 13 mm | |||
| Pulmonary artery, whole lung | Average branch length | 0.13 – 90.5 mm | [25] | |
| Average diameter | 0.015 – 30 mm | |||
| Pulmonary vein, whole lung | Average branch length | 0.13 – 36.7 mm | ||
| Average diameter | 0.013 – 13.9 mm | |||
| Heart | Human | |||
| Coronary capillaries | Total vessels | 2249/mm2 | [26] | |
2.1. Cellular constituents
Vascular structures are comprised of endothelial cells that form hollow lumens and are supported by perivascular stromal or mural cells such as vascular smooth muscle cells and pericytes (Figure 2). Vascular structures can form via processes of vasculogenesis or angiogenesis. Vasculogenesis is the formation of de novo vasculature during embryonic development [27]. Here, mesodermal cells form blood islands, which contain endothelial precursors called angioblasts surrounding a core of hematopoietic precursors. Over time, these blood islands coalesce to form a primitive vascular network [28]. Comparatively, angiogenesis is the formation of new vessels from a pre-existing vascular network. Angiogenesis proceeds through coordinated action between endothelial and mural cells. Here, mural cells facilitate vessel sprouting, lumen formation, stabilize vasculature, and modulate vascular permeability [29–32]. They contribute to the induction of angiogenesis by secreting pro-angiogenic factors such as VEGF or FGF, and by regulating proteolytic activity [32–35]. As a result, endothelial cells begin to sprout through regions of degraded basement membrane. Endothelial cells assume tip- and stalk-cell phenotypes to form capillary structures, after which lumen development forms through intracellular vacuole fusion [36,37]. Finally, endothelial cells secrete growth factors such as PDGFB to recruit mural cells to line newly-formed sprouts [38]. Together, these cells secrete and become embedded in basement membrane proteins, which form a unique ECM environment in the proximity of vasculature [39].
Figure 2.

Several parameters in the vascular niche microenvironment influence the development of vasculature itself as well as the phenotype of other resident cells. Vasculature is comprised of endothelial cells and perivascular stromal cells embedded in a basement membrane. The composition and biophysical properties of the surrounding matrix environment affect the ability of the vasculature to remodel and is continuously altered by secretion of extracellular matrix proteins and degradative enzymes such as MMPs. Growth factors such as VEGF and bFGF can be bound by the extracellular matrix and basement membrane, or released upon degradation of the matrix. Flow (Q) within the vasculature imparts a shear stress on the vessel walls, which can impact the barrier function of the vessel as well as the behavior of the endothelial and perivascular stromal cells. Finally, oxygen availability can vary spatially and temporally throughout the niche and has been shown to impact both vascular development and resident cell behavior. Signaling within the niche can be categorized as paracrine, juxtacrine, or ECM-mediated. Signaling cues are bi-directional: the vasculature receives cues that influence angiogenic and vasculogenic processes, while simultaneously sending angiocrine cues that act on surrounding parenchymal cells.
Within the brain, vascular networks are characterized by a blood-brain barrier (BBB) which displays robust barrier function that is tighter than vasculature in other tissue [40]. In vitro measurements of transepithelial electrical resistance (TEER) and permeability reveal that brain microvascular endothelial cells have enhanced barrier function compared to model human umbilical vein endothelial cells [41]. To accomplish tight barrier function, the BBB is comprised not only of endothelial and mural cells, but also astrocytes. Vasculature in the brain also receives signals from proximal neurons and microglia [42]. Crosstalk between these various cell types results in the tight barrier function characteristic of the BBB. For instance, astrocytes secrete proteins such as bFGF and TGFB that enhance tight junction formation in endothelial cells [43]. Thus, BBB barrier function arises not only from inherent tissue-specific differences in endothelial cell phenotype, but also as a product of cell-cell signaling between the unique cellular population of the brain vascular niche.
2.2. Biochemical Signaling Pathways
While numerous signaling pathways influence cell behavior within the vascular environment, we choose to highlight a subset of these pathways based on their involvement in vascular development or their prevalence in mediating angiocrine interactions in regeneration, stem cell fate, and cancer progression (Table 2).
Table 2.
Common pathways involved in angiocrine signaling
| Pathway | Ligands | Receptors | Roles | References |
|---|---|---|---|---|
| VEGF | VEGFA - VEGFD, PIGF | VEGFR1 - VEGFR3 | Regulates endothelial proliferation, migration, survival, permeability; endothelial - mural crosstalk; liver regeneration; osteoblast differentiation; macrophage recruitment | [34, 44, 45, 49, 50] |
| Notch | DLL1, DLL3, DLL4, Jag1, Jag2 | NOTCH1 - NOTCH4 | Regulates angiogenic sprouting and vascular sprouting; endothelial adhesion and permeability; lung fibrosis; stem cell maintenance; cancer cell intravasation | [53–55, 57–62] |
| Ang/Tie | ANG1, ANG2, ANG4 | TIE1 and TIE2 | Regulates angiogenic sprouting; endothelial survival, adhesion, migration, permeability; vascular stability; cancer cell intravasation | [65–71,74,75] |
| CAMs | Integrins, immunoglobulin superfamily, cadherins, selectins | Regulates endothelial adhesion, proliferation, migration, survival, permeability; vascular stability; hematopoietic stem cell proliferation; chemoresistance | [79,81,84–86,150] | |
| CXC | CXCL1 - CXCL12, CXCL4L1, CXCL14 | CXCR1 - CXCR4, CXCR7 | Regulates endothelial proliferation, migration, survival; cancer stem cell growth, proliferation, migration; chemotaxis towards vasculature; hematopoeitic stem cell maintenance; lung and liver regeneration and fibrosis | [59,88–94] |
| Wnt | WNT1 - WNT11, WNT16 | Frizzled1 - Frizzled10 | Liver regeneration; regulates Notch signaling | [49,59] |
| Nitric oxide | Nitric oxide | Guanylate cyclase | Glioblastoma stem cell maintenance; endothelial tube formation | [156,177] |
| Other common ligands | PEDF, HGF, bFGF, IGFBP2 | [49,94,149,154] | ||
CAM: cell adhesion molecule
2.2.1. VEGF/VEGFR
VEGF signaling is central to formation and maintenance of vascular networks. In mammals, VEGF signaling is driven by five ligands (VEGFA, VEGFB, VEGFC, VEGFD, PIGF) and three VEGF receptors (VEGFR1, VEGFR2, VEGFR3) [44,45]. The ligands are either presented in soluble form or are sequestered by the ECM, which controls their availability and activity. While VEGFR1 and VEGFR2 are both involved in angiogenesis, VEGFR3 signaling is more predominant in lymphangiogenesis [45]. VEGF-VEGFR2 signaling is a predominant driver of endothelial cell activity and influences proliferation, migration, survival, and permeability through pathways such as MAPK/ERK, PKB/Akt, and eNOS [44,45]. VEGFR1 may regulate VEGF-VEGFR2 signaling by competitively binding VEGF, and is expressed by pericytes and may therefore be involved in mural stabilization and influence over vascularization [34,46,47].
VEGF signaling is further influenced by crosstalk with the Notch pathway and integrins, both of which play key roles in vascularization and are discussed below [36,48]. The VEGF pathway is a key mediator of vascular-parenchymal crosstalk; for instance, VEGF is produced by various cell types to promote vascularization. VEGF-VEGFR2 signaling also contributes to the angiocrine output of sinusoidal endothelial cells in the liver and promotes hepatocyte proliferation after acute injury [49]. It is worth mentioning that VEGF does not act exclusively on endothelial cells; for example, it is shown to promote osteoblast differentiation as well as macrophage recruitment [50]. In this manner, the effects of VEGF signaling extend beyond angiogenesis and vascularization.
2.2.2. Notch
The Notch pathway is ubiquitous across animal species and orchestrates signaling through juxtracrine or direct cell-cell contact. Essential components of the pathway include four receptors (NOTCH1, NOTCH2, NOTCH3, and NOTCH4), canonical ligands (DLL1, DLL3, DLL4, JAG1, JAG2), and a CSL family DNA-binding transcription factor (CBF1/RBPJκ) [51]. Notch receptors and ligands are commonly transmembrane proteins. When binding occurs between the receptor and the ligand in adjacent cells, an ADAM metalloendopeptidase cleaves the extracellular portion of the receptor, and further processing by γ-secretase releases the Notch intracellular domain (NICD) [52]. NICD translocates to the nucleus and associates with CBF1/RBFJκ to control transcription [51].
Notch signaling is heavily involved in angiogenesis and vascular patterning; in particular, signaling between DLL4 and NOTCH1 is implicated in defining the balance between tip and stalk cells in sprouting angiogenesis, influencing endothelial cell migration, and suppressing excessive tip formation to control vascular outgrowth [53–55]. DLL4/NOTCH1 signaling acts in coordination with VEGF/VEGFR signaling, in which tip cells responding to a VEGF gradient will activate DLL4 to signal via NOTCH1 to an adjacent cell [36]. Activated Notch signaling in the adjacent cell suppresses VEGFR2 and activates VEGFR1, which reduces its ability to respond to the VEGF gradient and prevents it from become a tip cell [56]. The ligand JAG1 competes with DLL4 and contributes to the balance of vascular branching [57]. While much emphasis has been placed on signaling induced by cleaving NICD, recent work by Polacheck and Kutys et al. discovers that the transmembrane domain that is left behind after cleavage plays a role in regulating endothelial cell adhesion and barrier function [58]. The ramifications of Notch signaling extend beyond vascular development, as it contributes to angiocrine signaling between vasculature and surrounding parenchymal cells and is involved in a range of other processes, including fibrosis, expansion of cancer and non-cancer stem cells, and transendothelial cancer cell migration [59–62].
2.2.3. ANG/TIE
The angiopoietin-tie pathway is involved in vessel stabilization and remodeling. There are two receptor tyrosine kinases (TIE1 and TIE2), three ligands (ANG1, ANG2, and ANG4), and a receptor phosphatase (VE-PTP) that serves a regulatory role by dephosphorylating TIE2 [63]. Most studies focus on signaling through TIE2, ANG1, and ANG2. TIE1 is considered an orphan receptor, but can be phosphorylated by the angiopoietins, interacts with TIE2, contributes to tip-stalk phenotype, and may regulate Notch signaling to promote endothelial sprouting [64–66] [67]. ANG1 - TIE2 signaling increases vessel stabilization and decreases permeability by localizing TIE2 to cell-cell junctions, supports endothelial survival through PI3K-Akt signaling, and binding of TIE2 to matrix-bound ANG1 supports adhesion and migration [68–70]. Meanwhile, the role of ANG2 is context-dependent: expression of ANG2 in the absence of VEGF leads to vessel regression, while the presence of both ANG2 and VEGF promotes sprouting [71]. Examples of the dual role of ANG2 can be seen in glioblastoma and in the ovaries, where there are cycles of vessel regression and growth [71,72]. Both angiopoietin ligands and Tie receptors interact with integrins. For example, ANG1 activation of a TIE2/integrin α5β1 complex improves endothelial cell survival and motility [73]. ANG2 binding by integrins α5β1, αVβ3, and αVβ5 facilitates sprouting angiogenesis by destabilizing existing vasculature and inducing endothelial cell migration [74]. Beyond endothelial cell biology, TIE2 is expressed in a subfraction of macrophages, which secrete VEGF to induce vessel permeability and allows for tumor cell intravasation during metastasis [75]. Imbalances in angiopoietin-tie signaling leads to vascular dysfunction in inflammatory and pathological disease, and thus this pathway regulates the stability of the vascular niche environment.
2.2.4. Cell adhesion molecules
There are four classes of cell adhesion molecules: integrins, cadherins, the immunoglobulin superfamily, and selectins [76]. Integrins are heterodimer proteins formed from α and β subunits. Integrins are predominantly known for binding to ECM proteins such as fibronectin, vitronectin, laminin, and collagen, but can also bind to members of the immunoglobulin superfamily [77]. Upon ligand binding, integrins cluster and form focal adhesion complexes that link the ECM and the cytoskeleton [78]. Signaling induced by integrin binding regulates endothelial cell survival, migration, and proliferation; specific subunits implicated in regulating angiogenesis include αv, β1, and β3 among others [79]. Integrin signaling often synergizes with signaling induced by growth factors such as VEGF, which can further regulate ECM binding by integrins or modulate the signaling output that arises from growth factor binding [80]. A wide range of cadherins contribute to cell-cell adhesion relevant to the vascular niche. VE-cadherin is expressed by endothelial cells and controls vessel permeability and stability [81]. VE-cadherin can regulate VEGFR2 signaling, and its own expression is mediated by signaling pathways activated by growth factors binding to tyrosine kinase receptors as well as Notch [70,82]. N-cadherin has a potential role in mediating contact between endothelial and mural cells to further promote vessel stability [81]. The immunoglobulin superfamily includes ICAM-1, VCAM-1, and PECAM-1 [83]. Broadly, these proteins regulate endothelial adhesion and migration. For example, soluble VCAM-1 promotes chemotactic migration of endothelial cells [84]. Furthermore, integrin α4β1 can bind to VCAM-1, and this interaction has been shown to confer chemoresistance to breast tumor cells [85]. Amongst the selectins, E-selectin is expressed by mature and progenitor endothelial cells and is active in soluble and bound forms [84,86]. In an ischemic limb model, E-selectin promotes homing and integration of endothelial progenitor cells (EPCs) into vasculature at ischemic sites by increasing ICAM-1 expression in endothelial cells and chemotaxis and tube formation by EPCs [86]. Overall, various forms of cell adhesion molecules are involved in regulating vascular niche development and angiocrine signaling, and crosstalk with other signaling pathways to exert their effect on cell behavior.
2.2.5. CXCL/CXCR
The family of CXC chemokines and receptors are instrumental to paracrine signaling. The chemokines are classified based on the presence of an ELR amino acid motif: ELR+ chemokines include CXCL1 – CXCL3 and CXCL5-CXCL8, and ELR− chemokines include CXCL4, CXCL4L1, CXCL9 – CXCL12, and CXCL14. The ELR+ chemokines bind to CXCR1 and CXCR2, while the ELR− chemokines predominantly bind to CXCR3 and CXCR7. CXCL12 additionally binds to CXCR4 [87]. The ELR+ chemokines as well as CXCL12 are angiogenic, while the remaining cytokines inhibit angiogenesis; signaling through the CXC receptors modulates endothelial cell chemotaxis, survival, and proliferation [88]. The anti-angiogenic chemokines may exert their influence by inhibiting chemotaxis by the ELR+ chemokines and other angiogenic growth factors such as VEGF. Literature demonstrates that the CXC family has a significant role in angiocrine signaling. In glioblastoma, for example, CXCL8/CXCR2 mediates cancer stem cell growth, proliferation, and migration in the perivascular niche [89]. CXCL12/CXCR4 promotes migration of neural progenitor cells and several cancer types towards vasculature, and CXCL12 is a critical regulator of HSC maintenance in the perivascular niche [90–93]. Angiocrine signaling through CXCR4 and CXCR7 also modulates regeneration and fibrosis in the lung and liver [59,94]. Taken together, these examples indicate a central role for CXC signaling in vascular niches.
2.3. ECM
A primary element of the ECM within the vascular niche is the basement membrane found immediately adjacent to the vessel. Cells interact with the basement membrane and surrounding ECM through integrin receptors, and such interactions lead to changes in gene expression and observed behaviors such as cell proliferation and motility [95–97]. In this manner, the vascular niche contributes to signaling within the microenvironment through presentation of basement membrane and other secreted matrix proteins. The basement membrane is comprised of several proteins including collagen type IV, laminin, heparin sulfate proteoglycan, fibronectin, and entactin [98,99]. Various other proteins have also been detected in a vessel-dependent manner, such as osteopontin, thrombospondin, tenascin C, and vitronectin. Outside of the basement membrane, collagens and elastins can additionally be found in proximity to vasculature. By showing that the extent of vessel formation varies in materials with differing ratios of collagen and fibrin, Rao et al. demonstrate the importance of matrix composition in vessel formation [100]. Specifically, total network length increased with increasing fibrin and decreasing collagen content, potentially because increasing fibrin content decreases the stiffness of the material. Cells not only deposit ECM proteins but also secrete proteins such as matrix metalloproteinases (MMPs) to remodel the microenvironment. Matrix remodeling is important in the context of the vascular niche to regulate capillary sprouting during vasculogenesis and angiogenesis. By cleaving the matrix, MMPS enable cell migration to initiate capillary sprouting, and they can release matrix-bound growth factors or expose binding sites that direct tube formation and vascular patterning [101]. Matrix degradation may also have anti-angiogenic effects, since excessive matrix breakdown can destabilize the matrix and induce vessel regression [102]. Trappmann et al. investigate angiogenic sprouting into a series of dextran hydrogels with varying degrees of crosslinking and discover that while intermediate crosslinking favors multicellular sprouting, single-cell migration is more prevalent in materials with low and high crosslinking [103]. Lowering crosslink susceptibility to degradation in low-crosslinked materials, however, results in multicellular sprouting. This demonstrates that degradation of the matrix affects the ability of endothelial cells to migrate either as single entities or collectively in strands, which further emphasizes the regulatory role of matrix remodeling in capillary formation.
2.4. Biophysical Properties
Numerous studies demonstrate that cells use mechanotransduction pathways to sense and respond differentially to environments with varying stiffnesses [14,16,104,105]. In the vascular microenvironment, the mechanical properties of the basement membrane and ECM surrounding vasculature can differ from the mechanical properties of the surrounding bulk tissue. Candiello et al. measure the mechanical properties of basement membrane in both chick embryos and mouse retinas, and find that the apparent Young’s modulus for both systems is approximately 3 – 4 MPa [106]. Furthermore, a review by Burton provides estimates for the mechanical properties of collagenous and elastic fibers found in the tissue of larger vascular structures [107]. Properties for collagenous fibers are approximated from tendon studies, which suggest that the Young’s modulus for this material is ~ 100 MPa. The Young’s modulus for elastic fibers isolated from the aorta and human veins is approximated between 0.3 and 0.5 MPa.
In vitro studies additionally demonstrate that matrix stiffness and density impact the extent of vascularization that develops in the microenvironment as well as the ability of the vasculature to remodel. Increasing matrix density has been shown to reduce capillary formation in in vitro models, possibly due to decreased diffusion of angiogenic factors [100,108]. Capillary formation also depends on the ability of cells to exert traction on the surrounding matrix [109,110]. Techniques to modulate the stiffness of 3D in vitro platforms often requires changes in material concentration or cross-linking density, which confounds the effect of stiffness on capillary formation with the effects of matrix density or pore size. To decouple stiffness from matrix architecture, Mason et al. demonstrate the cross-linking of collagen gels via non-enzymatic glycation to obtain gels with varying stiffnesses but similar fiber organization [111]. For example, glycation using 0 – 250 mM ribose results in gels with compressive moduli ranging from 200 – 800 Pa. Increasing stiffness leads to increased endothelial cell spreading, and a spheroid assay with endothelial cells also shows that increasing stiffness increases the number and length of capillary outgrowths.
Another biophysical parameter that contributes to the development of a vascular microenvironment is the shear stress of fluid flowing through lumenized vessels. Studies show that shear stress impacts endothelial barrier integrity and permeability, which could alter the influx of soluble factors into the vascular microenvironment from the bloodstream [112–114]. In the BBB, shear stress increases expression of tight junctions, transporters, and ion channels, while downregulating proliferation and glycolysis in favor of Krebs cycle upregulation [115]. Furthermore, endothelial barrier function directly relates to the ability for cells to intravasate and extravasate across blood vessels, which is critical in processes such as cancer metastasis [116]. Shear stress may also alter gene expression and signaling cascades within endothelial cells, thereby impacting their interactions with surrounding cells [112,117].
2.5. Nutrient and Oxygen Gradients
Spatial and temporal availability of nutrients and oxygen within the vascular niche can also modulate cell behavior. Hypoxia, for example, regulates angiogenic processes through several mechanisms such as HIF-1 signaling and reactive oxygen species (ROS) production [118]. In a recent study by Blatchley et al., endothelial colony-forming cells cultured in hypoxic biomaterials form clusters due to ROS production, while cells cultured in non-hypoxic biomaterials show vascular sprouting. Under hypoxia, vascular networks form by sprouting from the endothelial clusters, as opposed to traditional sprouting through elongation and connection between single cells [119]. The dynamic availability of oxygen and nutrients also contributes to glioblastoma expansion into the brain parenchyma. Various studies demonstrate that glioblastoma tumor cells migrate along and associate closely with blood vessels [120,121]. However, co-opting of these blood vessels leads to vessel damage and regression, and hypoxic tumor cells trigger pro-angiogenic signaling to stimulate vascular sprouting at the tumor periphery [72,122–124]. Tumor cells will then migrate towards and co-opt newly-formed vasculature. Overall, these examples demonstrate that nutrient and oxygen availability affect cell behavior within the vascular niche, notably through hypoxic versus non-hypoxic signaling.
3. The Role of the Vascular Niche
3.1. Regeneration
Tissue structures need nutrients and oxygen to survive. Since the diffusion of oxygen is limited to a length scale of approximately 100 to 200 μm [125], vascular ingrowth into developing tissue is necessary for the nourishment and regeneration of the entire tissue construct. The impact of vascularization on regenerative outcomes has been demonstrated for multiple tissue systems. For example, the success of pancreatic islet transplantation for diabetes treatment is highly dependent on oxygenation of the islets post-transplantation [126], and graft failure is associated with a lack of vascularization at the transplant site [126,127]. Stimulating angiogenesis within the graft site via VEGF-dependent mechanisms can increase the number of functional β-cells and improve insulin release and glucose tolerance in diabetic mice [127]. Access to vasculature is also important for the healing of bone. In a study of patients with tibial fractures, patients with compromised arterial circulation have increased incidence of delayed or no healing compared to patients with intact circulation [128]. A central challenge for induced regeneration of critical-sized defects is the design of strategies to fully re-vascularize large defects [129]. Vessel instability and the speed of vessel infiltration into the engineered tissue hampers adequate vascularization and can contribute to implant failure [130]. Neural tissue regeneration also benefits from vascularization at the injury site. The delivery of a hyaluronic acid (HA) hydrogel encapsulated with nanoparticle-bound VEGF to the stroke cavity of a murine model increases angiogenesis within the stroke cavity, which leads to increased neuroblast and axonal infiltration and the development of functional neural tissue [131]. Additional studies have shown that the incorporation of VEGF into regeneration chambers for sciatic nerve defect repair increases the infiltration of blood vessels along with axons and Schwann cells into the defect [132]. Overall, these studies suggest a role for vascularization in improving regenerative capacity.
The need for vascularization in regenerative processes is further supported by the initiation of angiogenic events during wound healing or regeneration [133]. In particular, immune cells that infiltrate the defect site stimulate angiogenesis through the secretion of growth factors and proteases to initiate endothelial proliferation and subsequently assist endothelial cells in migrating through the ECM to form capillary tubes [133–136]. Models with depleted immune cell populations, particularly macrophages, demonstrate reduced wound healing along with limited vascularization [135]. The specific role that macrophages play in stimulating angiogenesis depends on its activation status. Macrophages are activated upon receiving cues from the surrounding microenvironment, such as cytokines from T cells. While macrophage phenotypes exist on a spectrum, one form of classification labels classically-activated, pro-inflammatory macrophages as M1, while alternatively-activated and anti-inflammatory macrophages are classified as M2 [137,138]. A study by Spiller et al. revealed that M1 macrophages secrete VEGF, while M2a macrophages secrete PDGF-BB and M2c macrophages secrete MMP9 [139]. Thus, while M1 macrophages may play a role in initiating angiogenic processes, M2 macrophages also contribute by secreting factors that promote perivascular stromal or mural cell recruitment to stabilize developing vasculature and enable endothelial migration via matrix breakdown. Macrophages are further stimulated to secrete angiogenic factors by hypoxic conditions, which are present in defects [136,140]. Parenchymal cells can additionally modulate angiogenesis in addition to immune cells. Coordinated angiogenic and osteogenic processes are a hallmark of bone development and repair [141]. Osteoblasts and osteoclasts secrete PEDF and VEGF to control the extent of vascular remodeling and infiltration during embryonic and postnatal bone development, the initial inflammatory response after bone injury, and the subsequent bone repair process [50,142]. Thus, non-vascular cells contribute to the development of a vascular niche environment to deliver nutrients and oxygen that promote regeneration.
In addition to providing nutrients and oxygen, the vascular niche also can modulate regenerative activities via signaling between vascular cells (i.e. endothelial and perivascular stromal or mural cells) and parenchymal cells. A primary route for these interactions is via secreted, paracrine-mediated signaling between the diverse cell types found in the vascular niche. However, it is critical to also consider a wider class of cell-cell interactions, such as direct cell-cell (juxtacrine) contact and matrix-mediated signaling, that together define angiocrine-mediated signaling processes (Figure 2). For example, signaling from sinusoidal endothelial cells in the liver promotes hepatocyte proliferation in acute injury [49,94]. Sinusoidal endothelial cells upregulate expression of Id1 after CXCR7 or VEGFR2 signaling and produce Wnt2 and HGF to stimulate hepatocyte proliferation. In chronic liver injuries, however, FGF2 activates CXCR4 and suppresses CXCR7 to initiate a fibrotic response. This leads to reduced hepatocyte proliferation and increased expression of smooth muscle actin and collagen, which are both characteristic of a fibrotic response. Stellate-like cells or fibroblasts are observed in close proximity with endothelial cells, which express TGFB, PDGFC, and BMP2 to promote a fibrotic response. CXCR7 also balances regenerative and fibrotic responses following lung injury by activating Notch signaling [59]. Endothelial-specific Notch signaling also plays a role in bone regeneration. A study by Ramasamy et al. shows that inactivating the Notch pathway in endothelium in mice results in compromised bone and vessel formation [143]. In response to Notch signaling, bone endothelial cells upregulate Noggin, which regulates osteoprogenitor differentiation and restores bone formation when exogenously administered to models with inactivated Notch signaling. In an in vitro model, endothelial cells have also been shown to promote the proliferation of osteoblasts and mesenchymal stem cells and reduce apoptosis [144]. Overall, these studies demonstrate how signaling between cells residing in the vascular niche modulates regenerative processes.
3.2. Stem Cell Behavior
Vascular niches play an essential role in stem cell behavior, particularly influencing processes linked to self-renewal, differentiation, and quiescence. For hematopoietic stem cells (HSCs), which are responsible for the production of the body’s full complement of blood and immune cells, these processes of stem cell self-renewal, differentiation, and quiescence take place within the bone marrow [145]. HSCs are also one of the first stem cell types used to define the concept of the microenvironmental niche as providing critical signals to regulate activity. HSC activity is tightly linked to the type of vascular environment (e.g. arteriolar vs. sinusoidal) within which the HSC resides [90,146,147]. Interestingly, these niches may have very different effects on HSC behavior. HSCs located in proximity to arterioles are quiescent and protected from therapy [146]. NG2+ perivascular cells in the arteriolar niche are believed to contribute to mechanisms promoting HSC quiescence, as depletion of these cells reduces the number of quiescent HSCs and increases their distance from arterioles. NG2+ arteriolar and LepR+ sinuosoidal perivascular cells may differentially regulate HSC maintenance via CXCL12 and SCF [90]. Inhibition of CXCL12 from NG2+ arteriolar perivascular cells reduces the number of HSCs and increases their distance from arterioles. However, inhibition of CXCL12 from LepR+ sinusoidal perivascular cells does not affect the number of HSCs in the bone marrow nor the distribution of quiescent versus proliferating cells. Conversely, SCF from LepR+ cells, but not NG2+ cells, is necessary for maintaining HSC numbers in the bone marrow. Endothelial cells within these niches can also modulate long term maintenance of HSC populations [61,148,149]. Deletion of SCF from endothelial cells in mice reduces the number of HSCs in the bone marrow and reduces the functionality of these cells, as measured by transplantation assays [148]. A study by Winkler et al. demonstrates that E-selectin, a cell adhesion molecule expressed on endothelial cells, promotes HSC proliferation instead of quiescence [150]. Yet, endothelial cells can also enable HSC maintenance, potentially through Notch and Akt [61,149]. Endothelial cells express various Notch ligands and enhance Notch signaling in HSCs. Inhibition of Notch signaling by knocking down Notch receptors on HSCs or by targeting endothelial function reduces HSC expansion. Activation of Akt in endothelial cells, but not MAPK, promotes expansion of Lin- HSCs in an in vitro co-culture. HSCs expanded with Akt-activated endothelial cells also display increased engraftment in competitive serial transplantation assays, which demonstrates their improved functionality.
Neural stem cells (NSCs) are another population hypothesized to be regulated by vascular niches. Imaging of the subventricular zone (SVZ) in the brain reveals the presence of label-retaining GFAP+ stem cells and transit-amplifying progenitor cells juxtaposed to vasculature [151]. An additional study demonstrates that transplanted neural progenitor cells migrate towards vasculature in an in vivo model, potentially due to interactions between SDF1/CXCL12 expressed by vasculature and CXCR4 expressed on progenitor cells [91,152]. In vitro assays also demonstrate a role for endothelial cells in promoting NSC self-renewal and their capacity to differentiate into neurons [153]. Inhibition of Notch1 in endothelial - NSC co-cultures leads to increased differentiation of NSCs, suggesting a role for Notch signaling in maintaining NSC self-renewal. PEDF is another vascular-derived factor that increases neurosphere formation of NSCs in an in vitro assay [154]. Moreover, PEDF may also influence differentiation, as NSCs exposed to PEDF have an increased potential to differentiate into neurons. Thus, this literature survey suggests that the vascular niche influences NSC migration, self-renewal, and differentiation profile.
Signaling between vascular cells and stem cells is not a one-way street, however. In parallels observed with regenerative biology, stem cells can also influence vascular development and remodeling of the niche environment. Takakura et al., for example, use an AML1-deficient mouse model to determine that ANG1 expression by HSCs is responsible for pro-angiogenic signaling and facilitates endothelial migration and network formation via TIE2 [155]. Furthermore, work by Li et al. shows that co-culturing NSCs and brain endothelial cells increases endothelial tube formation [156]. Conditioned media from cultures containing NSCs increases phosphorylation of VEGFR2 and TrkB in endothelial cells, as well as expression of VEGF and BDNF. NSCs also induce signaling changes in endothelial cells through production of nitric oxide (NO), which together with VEGF and BDNF regulate tube formation. These studies reveal that crosstalk (e.g., NO, VEGF, and BDNF) in the form of reciprocal signaling between NSCs and endothelial cells is a critical component of long-term niche development and remodeling.
Overall, these studies reveal key considerations for developing an angiocrine niche for stem cells. First, signaling between vascular and stem cells occurs via paracrine and juxtracine mechanisms. Crosstalk between stem and vascular cells simultaneously influences stem cell behavior and vascular development, and signaling pathways can continually feed into each other to initiate long-term communication. Finally, different subtypes of endothelial and perivascular cells can divergently influence stem cell fate. Thus, when designing artificial environments to control stem cell fate using angiocrine signals, it is necessary to consider cell source or cellular engineering methodologies to actively direct angiocrine signaling.
3.3. Cancer Progression
In 2000, Hanahan and Weinberg identified the induction of angiogenesis as one of the hallmarks of cancer [157]. Analogous to non-cancerous tissue, tumors need nutrients and oxygen delivered via vasculature in order to survive and expand [158]. However, the vascular niche is more than a source for nutrients and oxygen in the context of cancer progression; it also contributes to invasion and metastasis, cancer stem cell maintenance, dormancy, and therapeutic resistance.
In metastatic tumors, the development of secondary tumor metastases is a primary driver of patient mortality [159]. The vascular niche plays a direct role in the development of metastases: tumor cells migrate towards vasculature, intravasate into circulation, and extravasate through vasculature to populate the metastatic site [160,161]. Interactions between tumor cells, immune cells, and endothelial cells within the vascular niche facilitate tumor cell migration towards vasculature as well as transendothelial migration [162]. For example, Notch1 expression by endothelial cells is increased by the presence of tumor and myeloid cells and is associated with increased intravasation and development of metastatic sites by promoting tumor cell migration and compromising vessel integrity [62]. Crosstalk between macrophages and endothelial cells may also regulate endothelial permeability [75]. An additional mechanism that may regulate transendothelial migration involves MMP1 expression by tumor cells, which signals to endothelial cells via PAR1 [163]. Signaling between endothelial cells and tumor cells can also influence tumor cell migration, particularly through CXCR4-CXCL12 signaling [92,93]. Additionally, in glioblastoma, which is not a metastatic tumor but is lethal due to diffuse infiltration [164,165], tumor cells migrate into the surrounding brain parenchyma by crawling along vasculature [120,166]. The vascular niche also regulates the initiation of tumor growth at the metastatic site post-extravasation. Tumor cells may remain dormant for extended periods of time, and Ghajar et al. demonstrates that dormant disseminated breast cancer cells are adjacent to microvasculature in the lung, bone marrow, and brain [167]. More specifically, tumor cells are dormant when associated with stable vasculature, while tumor cells in proximity of neovasculature are proliferative. Thus, not only can the vascular niche regulate intravasation and extravasation processes, but it can also regulate the initiation of growth at the metastatic site.
In addition to promoting metastasis and invasion, the vascular niche is implicated as a home for cancer stem cells (CSCs). CSCs are a rare, self-renewing cell population capable of repopulating the entirety of the tumor population [168,169]. CSCs can also be therapeutically-resistant, which contributes to disease recurrence [170,171]. Imaging of human specimens identifies CSCs in proximity to blood vessels in brain tumors and head and neck squamous cell carcinomas [172,173]. In vitro co-cultures of endothelial cells and CSCs maintain the proliferation and self-renewal capacity of the CSCs [172,174]. Several studies identify the Notch pathway as a prominent signaling mechanism in the vascular niche that promotes the maintenance of the CSC population [174–176]. In B cell lymphoma, for example, bi-directional crosstalk between endothelial cells and tumor cells promotes expansion of an aggressive CD44+ IGF1R+ CSF1R+ lymphoma initiating cell (LIC) subpopulation [60]. Signaling between endothelial cells and LICs is regulated by NOTCH2 on LICs and JAG1 on endothelial cells, and LICs support endothelial JAG1 induction by producing FGF4 to activate FGFR1 signaling in endothelial cells. Besides JAG1, nitric oxide production by endothelial cells can initiate Notch signaling in glioblastoma cells to promote stem-like behavior by acting through the cGMP/PKG pathway [177].
The vascular niche also contributes to therapeutic resistance in a variety of cancers [60,175]. Inhibition and knock-down studies demonstrate that decreasing angiocrine signaling can benefit therapeutic response. For example, mice with deleted JAG1 in endothelial cells are more responsive to doxorubicin compared to those that express JAG1. Another relevant pathway is PI3K - Akt. In medulloblastoma, a form of pediatric brain tumor, tumor cells adjacent to vasculature showed increased capacity to overcome radiation therapy [178]. Akt activation in these cells increases after radiation and inhibiting Akt prior to radiation leads to increased apoptosis. Endothelial Akt activation may also contribute to therapeutic resistance in ovarian cancer [179]. Finally, a recent study by Carlson et al. demonstrates that vasculature protects disseminated tumor cells in the bone marrow from chemotherapy [85]. Using both in vitro and in vivo models, the authors propose integrin engagement by tumor cells as a mechanism for therapeutic resistance. Disruption of integrins αvβ3 and α4β1 binding to endothelial VWF and VCAM1 respectively increases tumor cell apoptosis in response to doxorubicin.
Overall, these studies demonstrate that angiocrine signaling is involved in multiple aspects of tumor progression and dissemination. Importantly, it is evident that signaling pathways used for angiocrine communication in cancer are also seen to modulate stem cell behavior and regenerative outcomes, suggesting a conserved set of direct and indirect cell-cell communication modes by which the vascular niche exerts its influence. For example, paracrine signaling often occurs via CXC ligand and receptor interactions, which the Notch pathway is heavily utilized for juxtacrine signaling.
4. Engineered Biomaterial Models of the Vascular Niche
4.1. General Vascularization Models
Given the known avenues by which the vascular niche regulates many fundamental processes associated with wound healing, stem cell biology, and cancer progression, a wide range of efforts have engineered biomaterial platforms that promote vascularization for therapeutic purposes or disease modeling. We first discuss general top-down and bottoms-up approaches for generating angiogenic biomaterials (Figure 3A). In a top-down approach, naturally-derived materials that already contain bioactive components are used to instruct the desired cell behavior. In a bottoms-up approach, materials without inherent biological functionality are outfitted with bioactive modalities (i.e. growth factors, binding and degradative protein sequences) that recapitulate aspects of the native tissue. We then demonstrate how these top-down and bottoms-up methodologies are used in specialized subfields, including the generation of porous scaffolds, microfluidic devices, and 3D-printed platforms. We finally highlight cell sources and methods for characterizing vasculature. As a reference, Table 3 provides a list of in vitro biomaterial-based vascularization models and their design parameters.
Figure 3.

A) Biomaterials that support vascularization include naturally-derived polymers with inherent cell binding and degradation motifs, as well as constructs designed by combining synthetic polymers with protein sequences for cell attachment and degradation. B) In order to develop models with perfused vasculature, vascular cells can be encapsulated in biomaterials embedded into microfluidic devices. Media ports in the devices enable application of interstitial and intraluminal flow through the biomaterial. C) Vascular cell types can be sourced from primary tissue or differentiated from pluripotent stem cells. Endothelial cells or endothelial progenitor cells are often derived from umbilical cords or cord blood, while stromal cells such as mesenchymal stem cells and fibroblasts can be derived from bone marrow, lung, or dermal tissue among other sources. Several groups have shown that vascular cells can also be derived from induced pluripotent stem cells or embryonic stem cells.
Table 3.
Summary of in vitro angiogenic biomaterials
| Macroscale Platforms | ||||
|---|---|---|---|---|
| Material | Cell Types | Media | Vessel Network Quantification |
Reference |
| Collagen/fibrin | 5:1 – 1:5 HUVEC:MSC 6 × 105 cells/mL |
-- | Total length: 0.75 – 7.5 mm/mm2 Branches: 30 – 240/mm2 | [100] |
| GelMA | 1:1 ECFC:MSC 2 × 106 cells/mL |
EGM2 | Total length: 0.5 – 4 mm/mm2 Branches: 10 – 30/mm2 | [187] |
| Collagen | 1:5:1 RBE4:AC:PC 5 × 105 RBE4s/mL |
Alpha MEM/ Ham’s F10 + 20% rat serum | Average branch length: 0.15 – 0.25 mm Branches: 100 – 400/construct | [182] |
| Fibrin | 5:1 HUVEC:NHLF 0.1 × 105 – 3 × 106 HUVECs/mL |
EGM2 | Total length: 4 – 12 mm/mm2 Branches: 1 – 1.5/vessel network | [188] |
| Fibrin | 5:1 – 1:2 EC:FB 1 × 106 HUVECs/mL |
EGM2 | Total length: 4 – 12 mm/mm2 Branches: 20 – 70/mm2 | [189] |
| PEGGGGPQGIWGQGK + RGD + VEGF | 4:1 HUVEC:10T1/2 30 × 106 cells/mL |
EGM2 | Total length: 0.8 mm | [191] |
| GelMA | 1:2 HUVEC:NHLF 1.5 × 106 – 6 × 106 cells/mL |
EGM2 | Total length: 25–50 mm/mm3 Branches: 500 – 7500/mm3 | [202] |
| PEG/PLL | 10:1 EC:NPC 1 × 106 HUVECs/implant | DMEM + 10% FBS | Branches: 2/area |
[277] |
| Collagen | 5.5 × 105 – 2.2 × 106 iPSC-EP | EGM2/SMGM2 | Total length: 0.4 – 10 mm/slice Branches: 1020/slice | [181] |
| PEG Heparin + GCGGPQGIWGQGGCG + RGD + VEGF + bFGF + SDF1a |
6 × 106 HUVECs/mL MSC/HDF/SMC/10T1/2 |
ECGM | Total length: 520 mm/mm2 Branches: 0–60/mm2 | [201] |
| PEG/PPLGgPEG GPQIWQG + RGD |
12 × 106 iPS-ECs/mL | VascuLife + iCell supplement | Total length: 560 mm/image volume | [193] |
| PEGKCGGPQGIWGQGCK + RGD | 2:1 ESC-EC:PC 1.7 × 106 HUVECs/mL |
E7V | Total area: 3040 mm2/mm2 Branch points: 0.1–0.15/mm2 | [251] |
| PEGGCRDVPMSMRGGDRCG + RGD | 1:1 HUVEC:NHDF 4 × 106 cells/mL |
VascuLife | Total length: 20–50 mm/mm2 | [194] |
| Hyaluronic acid RGD | 1:1 HUVEC:HFF-1 4 × 106 cells/mL |
EGM2 | Average branch length: 0.07 – 0.30 mm | [321] |
| PCL/collagen/hyaluronic acid VEGF + PDGFBB |
1:1, 1:3, 1:5 HUVEC:FB 5 × 104 – 5 × 105 HUVECs/mL |
EBM2 | Average branch length: 0.15 mm | [210] |
| PEG/collagen | 1:1 HUVEC:NHLF | EGM2 | Total length: 15 mm/ROI | [211] |
| Microfluidic Platforms | ||||
| Fibrin | 1:2 – 2:1 HUVEC:NHLF 1 × 106 – 4 × 106 HUVECs/mL |
EGM2-MV | Average branch length: 0.05 – 0.11 mm Branches: 90–250/mm2 | [224] |
| Fibrin | 2:1 HUVEC:MSC 12 × 106 HUVECs/mL |
EGM2-MV | Total length: 813 mm/ROI Branches: 90–150/mm2 | [225] |
| Fibrin | 3:1:1 iPS-EC:PC:AC 6 × 106 HUVECs/mL |
VascuLife + iCell supplement | Average branch length: 0.15 – 0.25 mm | [226] |
| Fibrin | 2:1 iPS-EC:NHLF 1 × 107 HUVECs/mL |
EGM2 | Total length: 6 mm/ROI | [243] |
EC: endothelial cell; FB: fibroblast; HUVEC: human umbilical vein endothelial cell; MSC: mesenchymal stem cell; ECFC: endothelial colony-forming cell; RBE: rat brain endothelial; AC: astrocyte; PC: pericyte; NHLF: normal human lung fibroblast; HFF-1: human foreskin fibroblast; NPC: neural progenitor cell; iPSC-EP: induced pluripotent stem cell - endothelial progenitor; HDF: human dermal fibroblast; SMC: smooth muscle cell; iPS-EC: induced pluripotent stem - endothelial cell; ESC-EC: embryonic stem cell - endothelial cell; NHDF: normal human dermal fibroblast; ROI: region of interest; GelMA: methacrylamide-functionalized gelatin; PEG: polyethylene glycol; PLL: poly-L-lysine; PPLG: poly (propargyl-L-glutamate); PCL: polycaprolactone
4.1.1. Top-Down Vascularization Approaches
Naturally-derived materials used for vascularization include fibrin and collagen. Collagen is ubiquitous across various tissue microenvironments and is therefore a prime candidate for recapitulating ECM structure and composition. Collagen supports general endothelial network formation and has also been used to develop tissue-specific vascularization models [180,181]. For example, Ahmad et al. co-culture rat brain endothelial cells with astrocytes and pericytes to develop a blood-brain barrier (BBB) model [182]. This platform recapitulates the tight junctions and adherens junctions seen in the native BBB. A potential drawback of using collagen, however, is the challenge of controlling gelation and microstructure via temperature and pH and limited material stiffness. An alternative approach is to use gelatin, which is the denatured form of collagen. While gelatin lacks the fibril microstructure of native collagen, it can be chemically modified to enable photopolymerization for facile and rapid gelation. The mechanical properties and microstructure of methacrylamide-functionalized gelatin (GelMA) hydrogels, for example, can be tuned not only by modulating the polymer content of the hydrogels, but also by the degree of substitution of methacrylamide groups and UV polymerization parameters [183–185]. This allows for fabrication of hydrogels with elastic moduli greater than 10 kPa [183]. Gelatin is also more soluble and less antigenic than collagen [186]. Chen et al. demonstrate successful vascularization of GelMA in vivo and in vitro by co-culturing endothelial colony-forming cells (ECFCs) with mesenchymal stem cells (MSCs) [187]. The extent of vascularization is dependent on the degree of functionalization of the material, which affect its stiffness and degradation rate.
Fibrin is formed by the polymerization of fibrinogen via thrombin and is involved in blood clot formation. The George lab has demonstrated that fibrin gels encapsulating human umbilical vein endothelial cells (HUVECs) and fibroblasts can be implanted in vivo to promote anastomosis with native vasculature [188]. Anastomosis is promoted by allowing vascularization to occur in the constructs in vitro before implantation. A subsequent study demonstrates that endothelial progenitor cell-derived endothelial cells promote anastomosis more effectively than HUVECs, and that higher densities of fibroblasts also facilitate anastomosis in vivo [189]. These studies highlight a key criteria in developing vascularized materials for clinical translation: the need to generate connected vascular networks that can form lumen, connect to host vasculature, and perfuse nutrients and oxygens throughout the entire injury or implant site. The examples discussed previously establish that both cell and material parameters must be considered to design vascularization. Cell-material interactions must also be considered, and one of the mechanisms by which cell-material interactions occur is through integrin binding of specific protein sequences. Fibrinogen and other natural materials contain a myriad of protein sequences that engage different integrins, which can lead to varying cell behaviors. In an effort to control vascular patterning in biomaterials, Li et al. create fibrin matrices that incorporated recombinant fragments of fibronectin that each bind specific integrins, α3/α5β1 or αvβ3 [190]. Their results demonstrate that αvβ3 leads to clumping of neighboring endothelial sprouts, while α3/α5β1 promotes the development of individual sprouts into branches. While fibrin strongly supports vascularization, it may be challenging to control its gelation and resulting microstructure. Collagen and fibrin are also limited by the fact that matrix density and cell-binding and cell-degrading ligand densities are interdependent, making it difficult to attribute cell behavior to any one of these variables. Additionally, these materials may suffer from batch-to-batch variability. However, because collagen and fibrin are essential components of the ECM and in vivo angiogenic processes, they are useful materials with which to begin to investigate vascularization and develop in vitro microenvironments for tissue engineering and disease modeling.
4.1.2. Bottoms-up Vascularization Approaches
Vascularization within synthetic biomaterials such as poly (ethylene glycol) (PEG) has been an area of focus, particularly for regenerative medicine strategies. While devoid of traditional ligands, approaches typically functionalize PEG with sequences containing binding motifs such as RGD and MMP degradable motifs to enable cell spreading and remodeling of the material, both essential for effective vascularization [191–194]. This allows for decoupling of matrix density from biochemical ligand density. Angiogenic growth factors such as VEGF can be incorporated into the synthetic matrices to promote vascularization. Growth factor presentation can occur through soluble supplementation, transient sequestration, or covalent tethering. While growth factors are traditionally supplemented in soluble form in cell culture media for in vitro studies, sequestration strategies or covalent tethering mimic the natural sequestration of growth factors by ECM in vivo, in which growth factor activity and the resulting effect on cell behavior is dependent on growth factor binding and release to and from the matrix [195–200]. For example, Chwalek et al. incorporate heparin into PEG hydrogels to sequester VEGF, bFGF, and SDF1α to stimulate angiogenesis [201]. From a practical standpoint, sequestration and covalent tethering enable extended release of growth factor over time, enabling the delivery of a single dose that can be as effective as continuous supplementation of soluble growth factor during the culture or regeneration period [200,202]. The combination of a material backbone, cell binding and degradation peptide sequences, and angiogenic growth factors facilitates vascular morphogenesis both in vitro and in vivo. While the platforms cited thus far need to be implanted, microgels formulated from PEG functionalized with RGD and VEGF and crosslinked with an MMP-degradable protein sequence can be injected into mice to promote vascularization and tissue infill after degradation of the material [203].
Glycosaminoglycans are another ubiquitous component of the microenvironment across various tissues. As such, there have been efforts to develop matrices from polysaccharides for in vitro and in vivo vascularization. A common material is hyaluronic acid (HA). HA does not contain integrin-binding domains or MMP sites, and thus the incorporation of these components can be decoupled from matrix density. HA is also incredibly amenable to chemical modification, enabling the development of materials with a vast range of mechanical properties and orthogonal biochemical presentation [204]. Using acrylated HA hydrogels, for example, Hanjaya-Putra et al. demonstrate that incorporation of RGD sites is necessary for lumen formation while MMP-degradable sites support vascular network expansion [205]. A potential drawback of HA is that it interacts with cells through CD44 and RHAMM receptors and is actively remodeled in the ECM via hyaluronic acid synthases (HAS) and hyaluronidases [206], and these interactions cannot be decoupled from other experimental variables. Alternative polysaccharides that have been utilized for angiogenic biomaterials include dextran and alginate [103,207–209]. These materials can also be modified with various chemical moieties but do not inherently present cell-interaction motifs, enabling full user control over the biochemical composition of the matrix. Compared to PEG, a potential advantage of using polysaccharide-based materials is that polysaccharides contain myriad sites for chemical modification, allowing for the synthesis of materials with a wide range of degree of substitution for optimizing ligand tethering and crosslinking.
The use of bottoms-up approaches is useful for methodical and controlled mechanistic studies interrogating the effect of individual matrix properties on angiogenesis. However, it may be difficult to recapitulate native cell behavior to the fullest extent because bottoms-up methods do not comprehensively capture the complexity of the native ECM. As such, it is important to consider that top-down and bottoms-up approaches are not mutually exclusive and can be combined to generate materials with optimal biochemical and biophysical properties for a given application. For example, Ekaputra et al. develop a scaffold containing electrospun PCL/collagen fibers electrosprayed with hyaluronic acid loaded with VEGF and PDGF-BB [210]. Overall, the fibers capture the fibrous nature of ECM while the hyaluronic acid enables cell infiltration and releases angiogenic factors over time. In another example, PEG-diacrylamide is conjugated to collagen and mixed with varying amounts of unconjugated PEG-diacrylamide to obtain different mechanical properties with the same biochemical ligand density [211]. Successful vascularization of these materials is dependent on MMP degradation. Thus, combining top-down and bottoms-up approaches enables the creation of hybrid materials that further expand the toolbox for angiogenesis.
4.1.3. Porous Scaffold Vascularization Approaches
The generation of porous scaffolds for tissue engineering is of great interest for implantable materials, because the porous structure enables proper cell infiltration, diffusion of nutrients and oxygen, and waste removal. The generation of a porous structure can be achieved through multiple processes, including freeze-drying, particulate leaching, and sintering. Porous scaffolds have been generated from both natural (e.g. collagen, alginate) and synthetic (e.g. PEG, PLGA, PCL) polymers. With these materials, the porous architecture becomes another parameter that impacts vascularization. Interest lies not only in the amount of vasculature that can infiltrate the scaffold, but also the depth to which the vasculature can penetrate. Computational models have been generated to investigate how variables such as pore size, porosity, and pore size distribution impact vascular infiltration [212, 213]. In idealized homogenous scaffolds, in which all pores have the same size and are equally spaced, porosity correlates with the interconnectivity between pores. Increasing pore size as well as interconnectivity increases vascular density and infiltration depth, but both vascular metrics are more dependent on interconnectivity and plateau with larger pore sizes. In heterogeneous scaffolds, pores are randomly distributed and differ in size, pore size, pore size distribution, and porosity, all of which dictate the extent of vascularization. Experimental results confirm the effect of increased pore size in improving scaffold vascularization. In porous PEG hydrogels, pore sizes greater than 50 μm support a significant increase in vascular infiltration [214]. Similarly, pore sizes greater than 140 μm reduce the time for vascular infiltration and increase vascular density in calcium phosphate particles, while imbuing electrospun PCL scaffolds with pore sizes greater than 160 μm enhanced vascular infiltration compared to controls without pores [215, 216]. Opportunities exist for tailoring additional variables such as pore size distribution and interconnectivity to further optimize vascular infiltration in wet-lab experiments.
In addition to scaffold architecture, biochemical modifications are another area of interest to improve the vascularization of porous scaffolds. Strategies include coating synthetic polymers with natural biopolymers such as heparin, Matrigel, or collagen to facilitate growth factor sequestration and prolonged release [217–220]. In one example, alginate is sulfated to mimic the binding behavior of heparin before being freeze-dried to form a scaffold, leading to increased retention of VEGF, PDGF-BB, and TGF-β1 [221]. Alternative strategies include directly immobilizing growth factors to the scaffold or incorporating microspheres to release the growth factors over time [222, 223].
4.1.4. Microfluidic Vascularization Approaches
Microfluidic models are an area of rapid growth, in part due to inherent advantages associated with the integration of flow with vasculature that allow for biological studies of the effects of luminal flow on vascular development (Figure 3B). These platforms can be used to study drug transport and perform high-throughput screening assays, assess variables that impact vascular permeability, and investigate chemotactic factors that regulate endothelial sprouting. The Kamm lab has developed various microfluidic devices which enable vascularization in collagen or fibrin gels [224–225]. Endothelial cells and stromal cells such as MSCs or fibroblasts are encapsulated in either the same channel or separate channels separated by media channels. When cells were cultured in separate channels, Whisler et al. show that branch formation and vessel diameter depend upon cell seeding density, fibrin density, and growth factor (i.e. VEGF and S1P) supplementation [224]. In a design where cells are co-cultured in a single channel, MSCs express mural cell markers such as αSMA and assume a perivascular location to the developing vascular network [225]. Supplementation of the culture medium with VEGF, TGFB1, and ANG1 also facilitates differentiation towards a pericyte-like phenotype. Addition of these growth factors modulates vessel branching, diameter, and average branching length. In order to obtain a perfusable network, endothelial cells can be seeded in lateral media channels to form monolayers to which the vascular network can anastomose. The Kamm lab has extended these design principles to develop microfluidic platforms for examining cancer metastasis (discussed later in this review), establishing fundamental transport principles for drug delivery, as well as creating tissue-specific vascular platforms [116,226–228]. For example, Campisi et al. establish a microfluidic blood-brain barrier model by co-culturing iPS-ECs with astrocytes and pericytes [226]. The resulting vascular network demonstrates tight junctions and decreased permeability in the presence of astrocytes and pericytes, as well as deposition of basement membrane proteins such as laminin.
An existing challenge in the development of microfluidic vascularized platforms is the use of biomaterials beyond collagen and fibrin. One proposed issue that hinders this advancement in the field is the fact that many biomaterials swell, and this swelling behavior prevents the biomaterial from remaining in confined regions and recapitulating the desired dimensions of the platform. Furthermore, restriction of swelling hydrogels alters the matrix density and ligand concentration sensed by encapsulated cells, leading to non-optimal vascularization. Potential solutions include tuning the hydrophobicity of the biomaterial, with Trappmann et al. demonstrating that increased hydrophobicity through increasing the methacrylation of a dextran backbone diminishes swelling [103]. Alternatively, hybrid materials may also be utilized to tune swelling behavior. Brown et al. demonstrate that combining PEG with increasing amounts PPLGgPEG, a rigid alpha-helical polymer, reduces swelling and rescues endothelial network formation in a microfluidic device [193]. The ability to incorporate materials beyond collagen and fibrin into microfluidic devices will increase batch-to-batch similarity and enable heightened control over material architecture and composition.
4.1.5. 3D - Printed Vascularization Approaches
While microfluidic platforms are useful for modeling tissue and optimizing design, there is an evident need to create larger, millimeter-scale biomaterials for clinical translation. Such constructs are valuable for regenerating critical-sized defects. Infiltration of host vasculature or self-assembly of implanted vascular cells may occur too slowly in these cases to nourish the artificial tissue as it develops, leading to necrosis and ultimately implant failure. 3D printing provides a solution to this issue by enabling user-defined fabrication of vascular structures that can span the entire biomaterial construct. Various strategies can be used to print conduits that physically resemble vasculature. For example, Cui et al. use thermal inkjet printing to deposit a bioink consisting of thrombin and endothelial cells on a fibrinogen substrate to create a grid of fibrin fibers in which cells proliferate and form channels with lumen [229]. A disadvantage of this approach is the limited ability to print in the z-direction; this challenge has been tackled by supporting the bioprint with secondary materials during the fabrication process and by developing bioinks with rheological properties that allow the structure to support itself before curing or polymerization. These bioinks are often blends of materials with different crosslinking mechanisms (i.e. photopolymerization, ionic, thermal), such that the bioink can flow during printing, retain its shape after deposition, and then undergo a final crosslinking after printing to reinforce the completed structure. For example, a bioink made of Pluronic F127 and GelMA is used to create conduits that are subsequently seeded with cells [230]. Pure Pluronic F127 is used as a support material in this case, and is removed post-print by liquifying the polymer at 4 °C. Alginate is another popular material found in bioinks; using a coaxial nozzle, an alginate shell can be printed around a core of calcium chloride solution that then diffuses into the alginate to crosslink the polymer and leaves behind a hollow lumen [231]. Cells can be suspended in the alginate shell to directly encapsulate them in the printed structure. However, alginate on its own does not contain cell-binding and degradative components to enable cell-matrix interactions, and therefore Jia et al. have created a bioink from alginate, GelMA, and PEGTA such that GelMA provides an ECM for cell interactions as well as a modality for secondary covalent crosslinking along with PEGTA [232] (Figure 4A). This bioink can be printed in a grid-like array of tubes with up to ten layers, which when encapsulated with HUVECs and MSCs results in a perfusable vascular network.
Figure 4.

3D printing approaches are increasingly being used to create large-scale vascular networks. A) One method of printing vasculature consists of encapsulating cells in bioink before depositing the bioink in a user-defined pattern. Here, cells are encapsulated in a bioink comprised of GelMA, alginate, and PEGTA. The bioink is loaded into the outer shell of a nozzle, while a solution containing calcium ions is in the core. Co-deposition of these solutions leads to calcium ions diffusing into the bioink to preliminarily crosslink the structure and leaves behinds an open lumen. Afterwards, UV light is used to crosslink the GelMA and PEGTA. B) Another strategy involves printing of vascular networks using sacrificial material, which is then removed after the networks are cast in extracellular matrix. Vascular networks can be lined with endothelial cells and perfused. In this example, Kolesky et al. use Pluronic F127 to print vascular networks while a combination of gelatin and fibrin acts as the surrounding extracellular matrix and is used to encapsulate cells. The entire construct can be placed inside a perfusion chamber to provide intravascular flow. C) Light-based patterning strategies allow for printing of fine and complex structures. Laser-mediated degradation is used to degrade portions of bulk hydrogel to leave behind hollow structures. Using this strategy, Heintz et al. pattern microfluidic networks within a PEGDA hydrogel that recapitulates a section of murine cerebral cortex vasculature. Images are reprinted with permission from [232], [236], and [241].
While the aforementioned examples highlight strategies to print vascular structures, they do not embed the constructs in ECM. This component is necessary to implant parenchymal cells alongside printed vasculature and provides structural support to the vascular network. In one approach, layer-by-layer photolithography is used to print a construct consisting of a grid of cell-encapsulated HA and GelMA channels surrounding by pure GelMA ECM [233]. In this case, lumen are not present initially, but form when enzymes such as hyaluronidase degrade the ECM in the channels. The delay in lumen development is a potential drawback, and thus multiple groups have developed methods of printing channels from sacrificial material that can be removed once the channels are embedded in ECM. For example, by using a layer-by-layer approach Lee et al. print a gelatin bioink with encapsulated HUVECs surrounded by a collagen matrix [234]. Culturing the construct at 37 °C polymerizes the collagen while liquifying the gelatin, which allows the HUVECs to settle and line the remaining hollow channel. The construct can then be placed into a flow chamber to perfuse the vessel, with the application of flow resulting in quiescent and stable vasculature. However, the presence of only a single vascular channel limits the scalability of the construct. Kolesky et al. demonstrate the ability to print large-scale intertwined networks of vasculature and parenchymal cells embedded in ECM [235,236] (Figure 4B). The vascular networks are printed using Pluronic F127, while GelMA or a combination of gelatin and fibrinogen are used to print non-vascular cells and the surrounding ECM. Shifting from GelMA to a gelatin/fibrinogen bioink eliminates the need to crosslink with UV light, which limits the size of the construct due to depth-dependent UV penetration. Pluronic F127 is evacuated after cooling to 4 °C, after which the vascular channels can be seeded with endothelial cells and perfused. An alternative strategy is to print a vascular network from carbohydrate glass, which can then be embedded in ECM containing cells [237]. Subsequent heating of the construct evacuates the carbohydrate glass to leave behind channels that can be lined with endothelial cells. This technique has been used to create implants for liver regeneration and relieving hind-limb ischemia [238,239].
A potential limitation of using nozzle-based printing techniques is that the size of the printed structures is a function of nozzle diameter. As such, it may be difficult to print dense networks of vasculature, or capillary-sized vasculature (diameter < 10 μm). Light-based approaches offer improved resolution and allow for the generation of complex and dense structures. Grigoryan et al. have incorporated food dyes into pre-polymer solutions to control gelation in a stereolithography set-up in order to print vascular channels that are faithful in size to the inputted design [240]. Such control enables printing of intertwined vascular networks, as well as a model of vascularized alveoli. In another approach, lasers are used to degrade bulk hydrogel material, leaving behind channels that can be subsequently perfused and lined with cells [241] (Figure 4C). Using this technology, it is possible to print channels that mimic the dense organization of cerebral cortex vasculature, or even separate networks within the same construct to model lymphangiogenesis. Taken together, there are multiple 3D printing strategies to fabricate vascularized biomaterials of different sizes, materials, and complexity.
4.1.6. Cell Sources for Angiogenic Biomaterials
Most studies utilize primary endothelial cells to vascularize biomaterials. HUVECs are used as a model endothelial cell source, while fibroblasts, MSCs, and pericytes are all used interchangeably as mural cells. There are increasing opportunities to generate vascular cell populations from stem cell sources (Figure 3C). Such technologies, particularly those leveraging induced pluripotent stem (iPS) cells, may eventually enable platform development from patient-derived cells and would be particularly valuable as disease models. Recent efforts have largely focused on deriving endothelial cells from more primitive stem cells or precursors. For example, the George lab has utilized endothelial cells derived from endothelial colony forming or progenitor cells (ECFC-ECs or EPC-ECs). The EPC-ECs have higher CD31 expression and form vessel networks with increased length and branching compared to HUVECs [189]. Belair et al. show that iPS-ECs form capillary networks in bulk Matrigel constructs as well as fibrin loaded into microfluidic devices [242]. In another study, Kurokawa et al. differentiate iPS cells transformed with an mCherry reporter for VE-cadherin into endothelial cells [243]. These cells form vascular networks within microfluidic devices when co-cultured with fibroblasts. Additionally, human pluripotent stem cells (hPSCs) can be induced into a differentiated population containing both endothelial and pericyte precursors [244]. Culturing differentiated hPSCs with VEGF and TGFB inhibition results in a CD105+CD146+ population that contains VEcad+ PDGFRβlo and VEcad+ PDGFRβhi subpopulations corresponding to endothelial cells and pericytes respectively. Culturing these CD105+CD146+ derived cells in collagen or HA hydrogels results in vascular network formation, complete with lumen formation and pericytes lining the endothelial tubes. Finally, induced pluripotent stem cells have been recently used to develop blood vessel organoids, in which organoids are three-dimensional cultures of cells that self-organize into structures that recapitulate tissue form and function [245, 246]. Here, pluripotent stem cells are induced towards mesoderm and then vascular lineage to generate a mixed population of endothelial cells and pericytes. During the differentiation process, cell aggregates are embedded in collagen and Matrigel and eventually give rise to miniature vascular networks with lumen-lined endothelial cords, perivascular pericytes, and basement membrane. When implanted, the vascular networks form vessels ranging from capillaries to arteries to recapitulate the vascular tree.
4.1.7. Characterization of Angiogenic Biomaterials
Various methodologies exist to characterize and benchmark the function and complexity of vascular morphogenesis within biomaterials. Vascular structures can be visualized in situ using fluorescent reports or dyes to label cells, or post-culture through immunohistochemical staining for endothelial-specific CD31, or VE-cadherin and ZO-1 to visualize adherens and tight junctions [226,243]. To functionally assess barrier function, fluorescent solutions can be perfused through vascular networks encapsulated in microfluidic devices, and fluorescent permeation into the surrounding extracellular space is monitored over time to quantify diffusion and permeability [226]. In vivo, fluorescent dyes or beads can be injected into the animal, and fluorescent perfusion into the implanted biomaterial confirms lumen formation and anastomosis with the host vasculature [238]. Micro computed tomography (micro-CT) can also be used to obtain high-resolution three-dimensional renderings of the developed vascular structure [247,248]. Here, the vascular network is cleared, fixed, and filled with a contrast agent before imaging [249]. In order to quantify the vascular network complexity, various pipelines exist to extract metrics such as network length, number of branches, and average branch length from two- and three-dimensional images [181,250,251]. Advances that enhance the accuracy and speed of these analysis tools will facilitate the characterization of increasingly complex vascular network morphologies.
4.2. Regeneration
The role of vascularization in improving regeneration has led to the development of various biomaterials with combined pro-angiogenic and regenerative design elements for recovering tissue-specific parenchymal function. These biomaterials have been developed for several tissue systems, including pancreatic islet, neural, and musculoskeletal applications.
4.2.1. Islet Transplantation
For islet transplantation for diabetic patients, the Garcia lab has developed a PEG-MAL hydrogel functionalized with RGD, VEGF, and a protease-degradable crosslinker for islet encapsulation [252,253] (Figure 5A). The hydrogel can be placed on the surgical site and crosslinked in situ. Compared to intrahepatic islet delivery, which is the current clinical standard, delivery using the hydrogel construct results in lower blood glucose levels and increased weight gain. In another design, a PDMS scaffold loaded with islets as well as a fibrin gel is combined with recombinant fibronectin fragments to bind VEGF and PDGF-BB and provide integrin-binding sites [254]. Upon implantation in the epididymal fat pat, mice that receive islets in the PDMS + fibrin construct experience faster diabetic reversal and return to normal blood glucose levels compared to mice that receive islets in PDMS scaffolds alone. Denser vascularization observed in the PDMS + fibrin construct further suggests that vascularization contributes to improved islet transplantation.
Figure 5.

Biomaterials that promote vascularization have been used to improve regenerative outcomes for numerous applications, including islet transplantation, post-stroke intervention, and musculoskeletal repair. A) PEG-MAL hydrogels decorated with RGD, VEGF, and crosslinked with an MMP-degradable sequence are used to deliver pancreatic islets. Inclusion of VEGF in the hydrogel construct leads to increased vascularization of implanted islets, seen by lectin perfusion (green) in the figure, and improved islet function. B) Hyaluronic acid hydrogels are optimized for neural progenitor cell encapsulation and transplantation into a stroke model. Implanted materials supported vascularization, and optimized concentrations of bioactive signals and growth factors promoted initial neural progenitor cell proliferation and survival in vivo. C) Endothelial cells and osteoblasts co-cultured in PCL/hydroxyapatite scaffolds form vasculature (arrows in upper image) lined with Flk-1+ endothelial cells (inset in upper image) and deposit osteoid (arrow in lower image) and type I collagen (green in lower image) when implanted in a rat bone defect. D) Decellularized bone scaffolds are loaded with IFNγ and IL4 to promote M1 and M2 polarization of infiltrating macrophages. When implanted in a murine model, scaffolds containing IFNγ display increased vascularization compared to those containing no cytokines or IL4 alone. Images are reprinted with permission from [253], [255], [257], and [259].
4.2.2. Neural Regeneration
Biomaterials for neural regeneration are a potential therapy for patients who have suffered a stroke or for patients with neurodegenerative diseases. The dense vascular network associated with physiological tissue motivates efforts that focus on improving vasculogenic processes to aid healing. In a study by Moshayedi et al., the authors develop a HA hydrogel with integrated binding sites for fibronectin (RGD) and laminin (YIGSR and IKVAV) and a MMP-degradable crosslinker that can be injected into the stroke cavity of mice to deliver induced pluripotent stem cell-derived neural progenitor cells (iPS-NPCs) [255] (Figure 5B). Variations of this hydrogel also include heparin to bind BDNF and BMP4. iPS-NPCs show increased survival when encapsulated in hydrogels before transplantation. Interestingly, hydrogels with only binding sites for fibronectin and laminin promote differentiation into neurons, while hydrogels that additionally include bound BDNF and BMP-4 promote astrocytic differentiation. While vessel in-growth is observed in the hydrogels, a correlation is not made between angiogenic activity and iPS-NPC behavior. In a subsequent study, the same HA starting material is modified with RGD and encapsulated with heparin nanoparticles that were left blank or contained adsorbed VEGF [131]. This material is injected into the stroke cavity without encapsulated cells. Hydrogel injection decreases inflammation and increases neuroblast and axonal infiltration into the stroke cavity. Vascularization of the stroke cavity correlates to new axon formation, thus demonstrating that angiogenic biomaterials are effective in promoting neural regeneration.
4.2.3. Musculoskeletal Regeneration
Angiogenic biomaterials have been a primary focus for regenerative repair of many musculoskeletal tissues. In this case, the scale of the defect (e.g. critical-sized bone defects; volumetric muscle loss; tendon, ligament, and insertion injuries) often requires significant vascular repair in order to support the cell-mediated remodeling necessary for mature tissue regeneration. In one example, a PLG scaffold loaded with VEGF and coated with a biomineral film is implanted into a rat critical-sized cranial defect model [256]. The addition of VEGF enhances vessel infiltration as well as mineralized tissue formation. In another study, a PCL - hydroxyapatite scaffold implanted into a rat femur defect improves bone regeneration when seeded with both endothelial cells and osteoblasts compared to osteoblasts alone (Figure 5C) [257]. In a study focused on muscle regeneration, alginate gels incorporating VEGF and IGF1 are injected into mice with hindlimb ischemia [258]. Delivery of hydrogels containing both growth factors leads to increased muscle mass post-injury compared mice receiving a blank hydrogel, along with increased vascularization and perfusion. This biomaterial system also supports regeneration of the neuromuscular junction, and increased muscle contraction force post-treatment confirms functionality of regenerated muscle with combined VEGF and IGF1 delivery.
Interestingly, several studies use biomaterials to demonstrate a role for immune cells in modulating heterotypic cell-cell crosstalk to simultaneously promote angiogenesis and musculoskeletal regeneration. Building upon previous work demonstrating that M1 macrophages secrete growth factors that promote angiogenesis while M2 macrophages secrete factors that promote vessel stability, Spiller et al. design decellularized bone scaffolds with an initial release of IFN-gamma to promote M1 phenotype followed by IL4 to promote phenotype switching to M2 [259] (Figure 5D). In vivo, vascularization occurs in scaffolds containing either growth factor separately or in combination, while scaffolds that do not contain growth factors do not have any vessel in-growth. In another study, a decellularized collagen scaffold containing intrafibrillar silica promotes angiogenesis and bone regeneration in a murine calvarial defect [260]. Scaffolds with silica contain increased bone in-fill and vascularization compared to those without silica after three months. An increased presence of CD14+TRAP+ monocytes in the silica-containing scaffolds leads to increased recruitment of bone marrow-derived mesenchymal stem cells and endothelial progenitor cells into the defect site, potentially via increased secretion of SDF1A, TGFB1, VEGFA and PDGF-BB.
In addition to being developed as regenerative therapies, biomaterials have been used as 3D culture systems to uncover new cell-cell signaling mechanisms governing regeneration. Latroche et al. encapsulate beads coated with endothelial cells in fibrin gels and seed myogenic progenitor cells (MPCs) either on top of the gels or within the gels [261]. The presence of MPCs increases capillary formation, while the presence of endothelial cells increases myotube formation. Microarray analysis of endothelial cells and MPCs after in vivo culture reveals expression of apelin, oncostatin M, and periostin by both cell types. Blocking these proteins in co-cultures within fibrin gels reduces capillary and myotube formation, suggesting that expression of these proteins by MPCs and endothelial cells serves as a signaling mechanism to simultaneously promote angiogenesis and myogenesis. Macrophages also promote angiogenesis and myogenesis via oncostatin M expression. Overall, these studies demonstrate that biomaterials can be designed to improve regeneration in vivo by promoting concurrent vascularization, and that biomaterials can also be used to develop in vitro model systems to form new insights regarding mechanisms that couple regeneration and vascularization.
4.3. Stem Cell Behavior
Stem cell transplantation holds significant potential to accelerate therapeutic regeneration. However, difficulties in maintaining cell viability post-transplantation, inadequate cell number expansion, and limited engraftment reduce transplant effectiveness [262–264]. Biomaterials inspired by the natural environments in which stem cells are found may provide an alternative perspective to generate platforms to both expand and deliver stem cell populations [265,266]. By incorporating cues that natively direct stem cell survival, self-renewal, and differentiation, the use of biomaterials to culture and deliver stem cells may provide solutions to the various issues facing standard transplantations via injection. As various types of stem cells have been shown to reside in vascular niches, biomaterials that incorporate vascular cell types or cues that mimic the extracellular microenvironment of the vascular niche may enable controlled regulation of stem cell behavior. Here, we provide examples of biomaterials developed for hematopoietic and neural stem cell culture and delivery.
4.3.1. Hematopoietic Stem Cell Culture
HSC transplantation is a potential treatment for several forms of cancer (e.g. leukemia, lymphoma), blood disorders, and auto-immune diseases [267]. One challenge associated with expanding the use of HSC transplants is the rarity of donor HSCs; currently ex vivo strategies to facilitate HSC expansion without the loss of quiescent long-term repopulating HSC fractions are poorly realized. The association of HSCs with vasculature in arteriolar and sinusoidal niches in the bone marrow suggest that approaches that combine HSCs with biomaterial-induced vascular networks may be advantageous [268]. However, biomaterials that co-culture vascular cells with HSCs have not been developed until recently. In one study, endothelial cells line a vascular network patterned into a collagen gel using soft lithography and injection molding, while bone marrow fibroblast cells can be encapsulated in the surrounding gel [269]. CD34+/CD45+ hematopoietic progenitor cells are perfused through the vascular network, and their adhesion and transendothelial migration are analyzed in the presence or absence of fibroblasts and monocytes. While the presence of fibroblasts alone does not affect hematopoietic cell adhesion to the vessel walls, perfusing monocytes through the vascular network prior to hematopoietic cell perfusion decreases hematopoietic cell adherence in systems containing encapsulated fibroblasts. Furthermore, the presence of fibroblasts increases the adhesion of leukemic cells migrating through the vascular network. However, perfusion of monocytes eliminates differences in leukemic cell adhesion arising from fibroblast encapsulation, demonstrating differential responses between healthy and leukemic hematopoietic cells in response to stromal cues.
A series of other studies have examined HSC interactions with elements of the marrow vascular niche, notably via ECM signals (e.g., Matrigel) or in co-culture with combinations of undifferentiated mesenchymal stem cells (MSCs), differentiated MSCs (adipogenic or osteogenic), and endothelial progenitor cells (EPCs) [270]. Co-culturing HSCs with adipogenic- and osteogenic-differentiated MSCs and EPCs increases the colony-forming potential of the HSCs through seven days of culture compared to co-culture with MS-5 feeder cells and maintains the number of CFU-GEMM colonies through ten days of culture. Culturing these cells in Matrigel increases HSC numbers compared to alginate culture by facilitating cell-cell contact and providing a biochemical environment containing ECM components relevant to the vascular niche. The platform is also hypoxic; in vivo, HSCs reside in regions of low oxygen perfusion, and reduced oxygen tension regulates the balance between quiescence and proliferation as well as lineage commitment of progenitor cells [271–273]. Overall, these studies suggest that vascular niche cells can be used as a powerful regulator of HSC maintenance and expansion in vitro and may provide clues about migration processes via the vasculature that occur in vivo.
4.3.2. Neural Stem Cell Culture
Neural stem cell (NSC) transplantation is a potential therapy for stroke, traumatic brain injury, and neurodegenerative diseases [274–276]. We previously discussed work by Moshayedi et al. and Nih et al., which both describe HA-based biomaterials that promote angiogenesis and either increases the survival of implanted neural progenitor cells (NPCs) or supports neurogenesis by resident stem cells in vivo [131,255]. In a separate example, a macroporous PEG hydrogel is seeded with brain endothelial cells and NPCs to determine the effect of NPCs on angiogenesis [277]. The presence of macroscale pores enables tube formation by endothelial cells alone, whereas these cells cannot form tubes in hydrogels without the pores. NPCs associate with endothelial tubes, and co-culture of NPCs and BECs increases vascular density and uniform vascularization of the implants. While these studies demonstrate that engineered systems containing both vascular cells and NSCs hold promise for promoting neural regeneration and angiogenesis, opportunities remain to use biomaterials to better understand the angiocrine cues that govern NSC fate, which can subsequently inform design rules to control NSC expansion and differentiation in vivo. In many delivery systems for NSCs developed thus far, vascular infiltration occurs passively within the implant, but incorporating biomaterial cues to actively promote a more robust angiogenesis response can also bolster regeneration.
4.4. Cancer Progression
The essential nature of vascular growth and remodeling in tumor growth, invasion, metastasis, and drug response has spurred the development of in vitro culture systems that model interactions between tumor cells and vasculature. These platforms are increasingly used to uncover new angiocrine mechanisms or to accelerate drug screening studies.
4.4.1. General Angiogenic Models to Study Cancer Progression
Both synthetic and natural biomaterials have been used to develop co-culture models containing vasculature and tumor cells. One type of system features tumor cells encapsulated in the bulk material while endothelial cells are seeded on the surface of the material [180,278]. This approach enables analysis of paracrine signals between tumor and endothelial cells and the resulting shifts in migration or cell morphology. However, endothelial cells initially seeded as a monolayer do not mimic the native vasculature. Alternatively, endothelial cells can be encapsulated inside of materials while cancer cells are seeded on top (Figure 6A). This provides an opportunity for the endothelial cells to under capillary morphogenesis [279]. Tracking endothelial sprout formation from beads or spheroids provides an approach to rapidly assess potential mechanisms or therapeutic approaches regulating angiogenesis. While these platforms are useful for studying the effect of tumor-derived soluble signals on endothelial migration and proliferation, it is difficult to account for cell behavior that arises from contact between tumor cells and vasculature. Furthermore, the spatial organization of tissues observed in vivo is extraordinarily different than monolayer-based structures. Thus, various groups have developed platforms that co-encapsulate vascular (i.e. endothelial and mural cells) and tumor cells within the same biomaterial environment as single-cell suspensions or spheroids (Figures 6B and 6C). These efforts rely on endothelial and mural cell capacity to self-assemble into vascular structures that can physically interact with each other and surrounding tumor cells. For example, Bray et al. encapsulate endothelial cells and mesenchymal stromal cells along with various breast and prostate cell lines in PEG hydrogels functionalized with RGD and heparin to develop a tumor angiogenesis model [280]. The platform facilitates endothelial tube formation and interactions between resulting tube structures and tumor cells. This model also enables screening of drug combinations in the tumor angiogenesis environment, particularly with regards to combining anti-angiogenic agents or receptor tyrosine kinase inhibitors with traditional chemo- or radiotherapy. In another model using PEG hydrogels, Roudsari et al. create a bilayer model, in which one hydrogel layer contains lung adenocarcinoma cells while a second hydrogel layer contains endothelial cells and pericytes [281]. The tumor cells form clusters, while vascular networks form in the vascular layer. Not surprisingly, a complex signaling environment is formed between the bilayer culture, with tumor cells secreting angiogenic factors such as VEGF and PDGF-BB, while vascular networks secrete TGF-β1, implicated in epithelial-to-mesenchymal transition. The interface of the two hydrogel layers provides a defined zone to consider the consequences of these interactions; notably, tumor cell clusters are larger, more disorganized, and display physical interactions with vascular networks. The close proximity between vascular and tumor cells afforded by this platform is necessary to alter tumor cell cluster growth and disorganization and provides an opportunity to explore spatially-driven cancer cell responses.
Figure 6.

Tissue engineered models of tumor-vascular interactions in non-perfused biomaterials. A) i) Endothelial or tumor cells can be coated onto beads and embedded in the biomaterial, while the opposing cell type is seeded as a monolayer on top of the biomaterial. ii) When human umbilical vein endothelial cells (HUVECs) are coated onto beads and embedded in a fibrin gel overlaid with glioblastoma cells, endothelial cells organize into sprouts that extend away from the beads. B) i) Endothelial, stromal, and tumor cells can be encapsulated in biomaterials and allowed to self-assemble. ii) Bray et al. demonstrate that when HUVECs, mesenchymal stromal cells (MSCs) and either breast or prostate cancer cells are cultured in PEG-based hydrogels, the tumor cells form clusters that show some physical interactions with capillary structures formed by the HUVECs and MSCs. C) i) Spheroids of tumor cells and vascular cell types can also be encapsulated into biomaterials. ii) Correa de Sampiao et al. form spheroids of breast cancer cells, endothelial cells, and fibroblasts, and then encapsulate the spheroids within a collagen matrix. Endothelial cells (orange) form sprouts emanating away from the spheroid, and fibroblasts (green) align in perivascular positions around the endothelial sprouts. Images are reprinted with permission from [279], [280], and [283].
In addition to PEG, a wide range of naturally-derived materials have also been used to create co-cultures of tumor and vascular cells [282,283]. To model intravasation, Ehsan et al. embed spheroids containing endothelial and tumor cells in fibroblast-laden fibrin gels and show that SW620 epithelial colon cancer cells enter the lumens of the endothelial sprouts that develop [284]. Intravasation distance increases when cells are cultured in 5% O2 and is Slug-dependent. Slug is a regulator for EMT, and thus these results suggest that hypoxia regulates intravasation by promoting EMT progression. Our research group has adapted methacrylamide-functionalized gelatin (GelMA) hydrogels to model the perivascular niche environment for glioblastoma [202,285]. By co-culturing endothelial cells, fibroblasts, and glioblastoma cells, we demonstrate that glioblastoma cells will associate closely with the developed endothelial networks and induce their regression, similar to the co-option and subsequent regression of vasculature in native glioblastoma [202]. Furthermore, interactions between glioblastoma and perivascular cells produces a transcriptomic profile that supports angiogenesis, signaling, and regulation of motility, adhesion, and ECM remodeling, while cell replication processes are downregulated [285]. Co-culturing endothelial cells, fibroblasts, and glioblastoma cells results in a culture model that is less responsive to temozolomide compared to glioblastoma cells alone. Thus, these results show that the three-dimensional co-culture of GBM and vascular cells provides a platform to investigate how angiocrine signals impact GBM invasion and therapeutic response. For example, we recently established that secreted proteins from endothelial networks contribute to the temozolomide resistance observed in our co-culture model [286]. Vascularized tumor models have also been used to investigate mechanisms underlying dormancy, which is a potential cause of tumor recurrence and therapeutic resistance to drugs that target the cell cycle [287,288]. In one example, Ghajar et al. develop organotypic models of the perivascular niche by seeding endothelial and stromal cells in a laminin-rich ECM before adding breast tumor cells [167]. Breast tumor cells display inhibited growth, or quiescence, on matrices containing both endothelial and stromal cells compared to matrices containing only stromal cells. This approach allows the group to identify TSP1 as an endothelial-derived factor regulating tumor cell quiescence in regions with stable vasculature, while periostin and TGFB1 promote tumor cell proliferation in sprouting neovascular regions. These results reveal that even within the vascular niche environment, sub-regions of active angiogenesis versus stable vasculature can differentially regulate tumor behavior. In another study, Marlow et al. co-culture breast cancer cells with bone marrow-derived stromal cells, osteoblasts, and endothelial cells in a collagen matrix to mimic the bone marrow microenvironment [289]. Cancer cells cultured in this model do not proliferate, while cancer cells do proliferate when cultured with only stromal cells. Cancer cells can be prevented from entering quiescence upon co-culture with stromal, endothelial, and osteoblastic cells when receptor tyrosine kinase (RTK), p38, or Alk5 signaling are inhibited. Broadly, these examples reinforce the need to couple biomaterial design strategies with evaluation of next-generation drugs. Here, evidence that cell behavior and therapeutic efficacy can be strongly modified by the presence of soluble perivascular signals or vascular structures suggests the urgent need to re-design drug screening assays that currently rely on high-throughput 2D screening tools and do not consider vascular signals.
While several models rely on the self-assembly of various cell types into vascularized microenvironments, efforts have been made to develop platforms with controlled spatial organization of tumor cells and vasculature. For example, Agarwal et al. develop a platform that consists of tumor cells first encapsulated in collagen droplets with an alginate shell; afterwards, these tumor-cell droplets are suspended in a bulk collagen gel with endothelial cells and adipose-derived stem cells [290]. Media perfusion through the construct results in capillary network formation surrounding the tumor droplets. Implantation of the spatially-patterned construct in vivo results in larger tumors compared to constructs containing only tumor cells or without spatial patterning. The authors also perform drug studies with doxorubicin and show that the vascularized tumor model is more resistant to the drug compared to a non-vascularized model. This study highlights not only the role of vascularization in therapeutic response, but also suggests the importance of spatial organization in facilitating proper signaling between tumor and vascular cells. Such spatial control may not be possible in self-organized culture platforms. Thus, ongoing innovations in 3D printing and microfluidic-enabled material development are expected to enable additional spatial control over the construction of vascularized biomaterials.
4.4.2. Perfused Angiogenic Models to Study Cancer Progression
Given the known role of flow in vascular physiology, another area of significant innovation is the development of perfused vascularized tumor models. Modeling vascular perfusion in vitro is critical towards understanding the role of shear stress in modulating tumor progression, investigating metastatic intravasation and intravasation, and studying drug transport and diffusion across vasculature. One method of forming a perfused structure involves the formation of a hydrogel around a needle or other cylindrical molding agent, which can then be removed to leave behind a hollow tube in the hydrogel (Figure 7A). The tube is subsequently seeded with endothelial cells and perfused with media. This approach allows the hydrogel to encapsulate tumor cells, additional stromal cells, and/or additional biomolecular signals to create a complex co-culture [112,291,292]. To increase complexity, two parallel channels can be embedded into a gel [293]. In this approach, one channel can be lined with endothelial cells, while the other channel can be filled with tumor cells or cytokines. In one study, Wolf et al. embed two parallel channels in a HA hydrogel to study the biophysical aspects of glioblastoma migration towards vasculature [294]. One channel is seeded with tumor cells, while the other channel is passively filled with media to represent a blood vessel. Tumor cells exhibit collective migration through the HA matrix, but elongate and separate upon reaching the open channel to migrate along the channel wall. Furthermore, the hydrogel space between the two channels can be used to incorporate additional axes of complexity. For example, in another glioblastoma model, macrophages can be encapsulated in the gel to investigate the impact of immune-derived signaling on angiogenesis [293].
Figure 7.

Tissue engineered models of tumor-vascular interactions using perfusable biomaterials. A) i) Biomaterials can be cast around a needle, and after polymerization the needle can be removed to leave behind a hollow channel. Seeding the channel with endothelial cells results in a vessel mimic that can be perfused. ii) A collagen gel containing an endothelial-lined channel is encapsulated with either renal cell carcinoma cells or cells from normal tissue. Cells from normal tissue do not induce endothelial sprouting from the channel, while gels containing tumor cells do induce endothelial sprouting. B) i) Multi-compartment microfluidics have also been developed to model tumor-vascular interactions. In one configuration, tumor cells can be encapsulated in a hydrogel in one region, while an adjacent media channel is coated with endothelial cells to mimic a blood vessel. ii) A multi-channel microfluidic device that contains a central gel channel, two flanking media channels, and a side gel channel is used to model angiogenesis and lymphangiogenesis in tumors. When spheroids of tumor cells and fibroblasts are embedded in fibrin in the central gel channel and blood and lymphatic endothelial cells are seeded into the flanking media channels, blood and lymphatic vessel sprouts invade into the fibrin gel and interact with the tumor spheroids. C) i) Alternatively, vascular cell types can be encapsulated in a hydrogel to self-assemble into microvasculature in one channel of the hydrogel, and tumor cells can be perfused into the vasculature by injecting the cells into an adjacent media channel. ii) Sobrino et al. create a microfluidic device containing diamond-shaped tissue chambers flanked by media channels. Endothelial cells, fibroblasts, and tumor cells can be encapsulated in fibrin gels and introduced into the tissue chambers. The endothelial cells and fibroblasts self-assemble into a vascular network, while the tumor cells form clusters that a dispersed amongst the vascular network. D) Vascularized tumor models can be used to investigate mechanisms of tumor intravasation and extravasation. i) Bersini et al. introduce breast cancer cells into a endothelial-lined media channel and measure the extravasation rate of cancer cells into the adjacent collagen gel. Extravasation increases when the collagen gel is seeded with mesenchymal stromal cells differentiated in osteogenic media compared to an empty collagen gel. ii) Jeon et al. investigate extravasation of breast cancer cells from a self-assembled microvascular network. Extravasation into the surrounding extracellular matrix increases when osteo-differentiated mesenchymal stromal cells are included in the matrix compared to myoblasts and a negative control containing no stromal cells. Images are reprinted with permission from [116], [291], [295], [299], and [302].
Perfusion can also be achieved by using microfluidic devices (Figures 7B and 7C). In one simple design, a hydrogel channel is flanked by two media channels that can be lined or filled with cells or cytokines. The Kamm lab has pioneered the use of this design to study tumor metastasis (Figure 7D). In one configuration, an endothelial monolayer is established against one side of the hydrogel and cancer cells are encapsulated in the hydrogel or are injected into the side channel with the endothelial monolayer to model intravasation and extravasation respectively [227,295]. In another application, microfluidic devices where channels of endothelial and glioblastoma cells are separated by a gel can be used to determine how paracrine signals from endothelial cells influence glioblastoma cell migration [296–298]. Recently, we have used this parallel-channel microfluidic assay to identify TIMP1 as a potential chemokine promoting GBM chemotaxis [286]. Microvasculature can also be established by encapsulating vascular cells within the hydrogel [299,300]. Jeon et al. use this set-up to develop a model where breast cancer cells preferentially extravasate into bone-mimetic matrices compared to muscle-mimetic ones [116]. A recent study by Boussommier-Calleja et al. uses the same system to explore the effect of monocytes on extravasation [301]. Microvasculature is formed from fibroblasts and endothelial cells, and monocytes perfused through the microvasculature extravasate into the surrounding tissue microenvironment. When tumor cells and monocytes are simultaneously perfused through the microvasculature, the presence of monocytes decreases the frequency of tumor cell extravasation. However, if monocytes are perfused first and extravasate before the introduction of tumor cells, the effect on tumor cell extravasation is abolished. Thus, the use of a microfluidic platform reveals differential roles for monocytes in tumor cell extravasation. In another application, Sobrino et al. use a microfluidic device with perfused microvasculature for drug screening [299]. The microvasculature networks display close association with the embedded tumor cells, and the platform allows studies of tumor response to various chemotherapies and anti-angiogenic agents. The authors monitor the effect of chemotherapy or receptor tyrosine kinase inhibition on both tumor growth and vessel network stability and find that vessel network stability post-treatment is largely a function of the mechanism of action for a particular receptor tyrosine kinase. For example, anti-angiogenic treatments such as Sorafenib limit new capillary formation, whereas mitotic inhibitors such as Vincristine lead to overall vascular disintegration. The ability of this platform to recapitulate cell response correlated to mechanistic expectations suggests its usefulness in drug screening, particularly in evaluating novel drug combinations to holistically target the entire tumor microenvironment. Moreover, this platform enables monitoring post-treatment to establish potential therapeutic resistance outcomes such as tumor or vascular re-growth, which is highly relevant because the widespread clinical use of anti-angiogenic therapy has been hindered by tumor evasion mechanisms and subsequent relapse post-treatment.
An important advantage of microfluidic devices is that they are modular and easily customizable in design. Chung et al., for example, expand beyond the single hydrogel channel design with a microfluidic device with two gel channels flanked by media channels to control the spatial distribution of tumor, stromal, and vascular cell types [302]. Such a design allows for user-defined patterning of juxtacrine and paracrine cues; for example, tumor and fibroblast cells may be cultured separately to define the reciprocal effects of tumor-stromal signaling, or tumor and fibroblast cells can be cultured together and separated from endothelial cells to determine how juxtacrine tumor-stromal interactions affect endothelial sprouting. In another conformation, spheroids containing tumor cells and fibroblasts are embedded in fibrin in one gel channel, and the gel is subsequently lined by vascular and lymphatic endothelial cells. This configuration enables both vascular and lymphatic vessel formation, with sprouts extending into the fibrin gel and interacting with the tumor-fibroblast spheroids. Microfluidic devices can also be fabricated with channels mimicking the structures of natural vasculature by using images of in vivo vasculature to guide design [303]. In this manner, microfluidic devices can faithfully reproduce the flow profiles generated by the natural geometry of vasculature. Overall, microfluidic platforms are a powerful tool to design tumor models that are spatially ordered and incorporate physiologically-relevant flow, and have limitless applications in drug screening and fundamental biology.
5. Future Directions and Conclusions
Many biomaterial platforms have been developed thus far to promote vascularization in vivo as well as to investigate the signaling contributions of the vascular microenvironment in vitro. These platforms can be fabricated from both naturally-derived and synthetic polymers in top-down and bottoms-up approaches respectively. Furthermore, microfluidic platforms can be used to include luminal flow and shear stress and to provide spatial and temporal control. The approaches described in this review have provided valuable insights into the mechanisms governing crosstalk between vascular and non-vascular cells, have begun to recapitulate therapeutic responses observed in vivo, and have led to improved success in transplantation and restoration of function in regenerated tissue. Here, we offer our perspective on strategies to further the development and application of angiogenic biomaterials.
Precision vasculature.
Several opportunities exist to design vascularized biomaterials more precisely for both in vitro and in vivo applications. Controlling vascular architecture, for example, can be critical for proper regenerative outcomes and accurate recapitulation of the native microenvironment. Examples of tissues that contain vasculature organized in a hierarchal fashion include the brain and long bone [17,304–308]. Achieving control of vascular patterning using biomaterials can be facilitated by improved understanding of the biological cues governing vascular development. Once identified, these cues (e.g. matrix proteins, growth factors, structural components) can be incorporated into biomaterial design [190]. Cues may need to be presented in a spatially and temporally controlled manner. Spatial resolution can be achieved by using 3D printing or laser degradation techniques [241,309]. Recent advances have enabled printing of features with unparalleled resolution, and several groups have already demonstrated the ability to 3D print vascular networks [232,237,240]. However, printing has predominantly focused on either spatially organizing cells or matrix components individually and not simultaneously. Integrating both vascular and non-vascular components into printed designs will enable greater spatial control over the resulting microenvironment architecture [235,310]. Spatial as well as temporal control of protein incorporation can be achieved by using orthogonal chemistries to conjugate proteins to the biomaterial backbone combined with templating with photomasks or computer-aided design [311]. Proteins can be added or released selectively at different points in time. Utilizing these techniques to precisely tune biomaterial architecture can provide the capability to control the development of a vascularized microenvironment, which will increase the predictability of regenerative outcomes in vivo and the physiological accuracy of in vitro culture models.
Benchmarking physiological relevance.
Vasculature serves not only as a conduit for nutrients and oxygen, but also as an active regulator of biological activity. In vitro, the ability to develop vascularized microenvironments enables researchers to investigate how synergies between heterotypic cell-cell crosstalk, biophysical variables such as shear stress, and ECM organization and remodeling dictate processes such as regeneration, stem cell maintenance and expansion, and disease progression. However, care should be taken to validate the biomaterial platform against an in vivo model to provide greater confidence that mechanistic findings are clinically relevant. Biomaterials designed to recapitulate an in vivo microenvironment should faithfully mimic aspects of the microenvironment, such as mechanical properties, matrix composition, and cellular organization. In addition to engineering the ECM, it may also be necessary to engineer the vascular cells that are incorporated into the biomaterial. The transcriptomic and proteomic signature of a vascular cell is dependent upon its microenvironment, whether the tissue is in a healthy or disease state, and the vascular sub-niche in which it resides (e.g. stable vasculature vs. active angiogenesis, tip vs. stalk). A large portion of biomaterials research uses “model” vascular cell types such as HUVECs and MSCs, which may not inherently mimic the vascular cells of a given tissue, disease/injury state, or vascular environment/phenomena, even with instruction from an engineered ECM. It may be possible to isolate vascular cells from the desired tissue or pathophysiological model, but in vitro phenotype may still differ from in vivo behavior. Thus, it may be necessary to program vascular cells to recapitulate essential transcriptomic or proteomic characteristics of the cells as they exist in vivo to direct their interactions with the ECM and surrounding cells. This can be achieved using genetic engineering and synthetic biology techniques to introduce, amplify, or knock down genes of interest [312]. Being able to trigger transcriptomic or proteomic changes on demand through light- or chemically-induced constructs also provides the ability to temporally control cell behavior [313,314], which is relevant for modeling transitions or onsets to pathophysiological states. Taken together, a successful in vitro model can yield insights to design biomaterials for in vivo applications, in which infiltrating vasculature not only provides tissue nourishment but also active signaling guidance for regeneration.
Integrating “-omics” with biomaterial design.
Uncovering the mechanisms by which angiocrine signals impact biological outcome often involves co-culture of heterotypic cell types, i.e. vascular, parenchymal, and immune cells. Methodologies to uncover signaling networks between heterogeneous cell types also need to be incorporated into studies utilizing biomaterials. Here, single-cell RNA sequencing can be used to identify potential receptor-ligand interactions at the transcriptome level [315]. Cell-specific proteomic labeling will be vital for identifying reciprocal changes in secreted, deposited ECM, and intracellular protein levels [316–318]. These tools will need to be incorporated with strategies to degrade biomaterials post-culture to retrieve cells and proteomic samples without damage [319]. The use of next-generation sequencing and mass spectrometry-based proteomics analysis often results in a multidimensional dataset, in which simultaneous changes in multiple genes and proteins need to be correlated to cell phenotype in a useful manner. Here, statistical analysis techniques such as partial least squares regression can be used to generate models to extract information from big data, as seen in recent efforts to identify components of the MSC-generated secretome responsible for improving hematopoietic stem cell quiescence in in vitro culture [320]. Such tools can be leveraged through collaborations between the fields of biomaterials, chemical biology, and bioinformatics.
Overall, ongoing advances in engineering angiogenic biomaterials will improve the effectiveness of regenerative therapies and provide greater insight into the mechanisms governing regeneration, stem cell fate, and disease progression. These innovations will have significant impact on the delivery and design of health care solutions for a wide range of injuries as well as the identification of innovative new treatments for a large spectrum of diseases such as neurodegeneration and cancer.
Acknowledgements
This work was supported by the National Cancer Institute of the National Institutes of Health (R01 CA197488) and the National Science Foundation Graduate Research Fellowship (DGE 1144245 to MTN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors also acknowledge additional funding provided by the Department of Chemical and Biomolecular Engineering and the Carl R. Woese Institute for Genomic Biology at the University of Illinois at Urbana-Champaign.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Scadden DT, The stem-cell niche as an entity of action, Nature 441(7097) (2006) 1075–9. [DOI] [PubMed] [Google Scholar]
- [2].Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A, Oxygen in stem cell biology: a critical component of the stem cell niche, Cell Stem Cell 7(2) (2010) 150–61. [DOI] [PubMed] [Google Scholar]
- [3].Yin H, Price F, Rudnicki MA, Satellite cells and the muscle stem cell niche, Physiol Rev 93(1) (2013) 23–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jones DL, Wagers AJ, No place like home: anatomy and function of the stem cell niche, Nat Rev Mol Cell Biol 9(1) (2008) 11–21. [DOI] [PubMed] [Google Scholar]
- [5].Lane SW, Williams DA, Watt FM, Modulating the stem cell niche for tissue regeneration, Nat Biotechnol 32(8) (2014) 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schofield R, The relationship between the spleen colony-forming cell and the haemopoietic stem cell, Blood Cells 4(1–2) (1978) 7–25. [PubMed] [Google Scholar]
- [7].Putnam AJ, The Instructive Role of the Vasculature in Stem Cell Niches, Biomater Sci 2(11) (2014) 1562–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gilbertson RJ, Rich JN, Making a tumour’s bed: glioblastoma stem cells and the vascular niche, Nat Rev Cancer 7(10) (2007) 733–6. [DOI] [PubMed] [Google Scholar]
- [9].Yin T, Li L, The stem cell niches in bone, J Clin Invest 116(5) (2006) 1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Butler JM, Kobayashi H, Rafii S, Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors, Nat Rev Cancer 10(2) (2010) 138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moon JJ, West JL, Vascularization of engineered tissues: approaches to promote angiogenesis in biomaterials, Curr Top Med Chem 8(4) (2008) 300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rafii S, Butler JM, Ding BS, Angiocrine functions of organ-specific endothelial cells, Nature 529(7586) (2016) 316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gattazzo F, Urciuolo A, Bonaldo P, Extracellular matrix: a dynamic microenvironment for stem cell niche, Biochim Biophys Acta 1840(8) (2014) 2506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chaudhuri O, Koshy ST, Branco da Cunha C, Shin JW, Verbeke CS, Allison KH, Mooney DJ, Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium, Nat Mater 13(10) (2014) 970–8. [DOI] [PubMed] [Google Scholar]
- [15].Kleinman HK, Philp D, Hoffman MP, Role of the extracellular matrix in morphogenesis, Curr Opin Biotechnol 14(5) (2003) 526–32. [DOI] [PubMed] [Google Scholar]
- [16].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification, Cell 126(4) (2006) 677–89. [DOI] [PubMed] [Google Scholar]
- [17].Smith AF, Doyeux V, Berg M, Peyrounette M, Haft-Javaherian M, Larue AE, Slater JH, Lauwers F, Blinder P, Tsai P, Kleinfeld D, Schaffer CB, Nishimura N, Davit Y, Lorthois S, Brain Capillary Networks Across Species: A few Simple Organizational Requirements Are Sufficient to Reproduce Both Structure and Function, Front Physiol 10 (2019) 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu J, He Y, Yang Z, Guo C, Luo Q, Zhou W, Chen S, Li A, Xiong B, Jiang T, Gong H, 3D BrainCV: Simultaneous visualization and analysis of cells and capillaries in a whole mouse brain with one-micron voxel resolution, NeuroImage 87 (2014) 199–208. [DOI] [PubMed] [Google Scholar]
- [19].Di Giovanna AP, Tibo A, Silvestri L, Müllenbroich MC, Costantini I, Allegra Mascaro AL, Sacconi L, Frasconi P, Pavone FS, Whole-Brain Vasculature Reconstruction at the Single Capillary Level, Scientific Reports 8(1) (2018) 12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang X, Yin X, Zhang J, Li A, Gong H, Luo Q, Zhang H, Gao Z, Jiang H, High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer’s disease mice, National Science Review 6(6) (2019) 1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ellis SL, Grassinger J, Jones A, Borg J, Camenisch T, Haylock D, Bertoncello I, Nilsson SK, The relationship between bone, hemopoietic stem cells, and vasculature, Blood 118(6) (2011) 1516–1524. [DOI] [PubMed] [Google Scholar]
- [22].Schneider P, Krucker T, Meyer E, Ulmann-Schuler A, Weber B, Stampanoni M, Müller R, Simultaneous 3D visualization and quantification of murine bone and bone vasculature using micro-computed tomography and vascular replica, Microscopy Research and Technique 72(9) (2009) 690–701. [DOI] [PubMed] [Google Scholar]
- [23].Vetterlein F, Pethö A, Schmidt G, Morphometric investigation of the microvascular system of pancreatic exocrine and endocrine tissue in the rat, Microvascular Research 34(2) (1987) 231–238. [DOI] [PubMed] [Google Scholar]
- [24].Huang W, Yen RT, McLaurine M, Bledsoe G, Morphometry of the human pulmonary vasculature, Journal of Applied Physiology 81(5) (1996) 2123–2133. [DOI] [PubMed] [Google Scholar]
- [25].Townsley MI, Structure and Composition of Pulmonary Arteries, Capillaries, and Veins, Comprehensive Physiology (2012) 675–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rakusan K, Flanagan MF, Geva T, Southern J, Van Praagh R, Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy, Circulation 86(1) (1992) 38–46. [DOI] [PubMed] [Google Scholar]
- [27].Patan S, Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling, J Neurooncol 50(1–2) (2000) 1–15. [DOI] [PubMed] [Google Scholar]
- [28].Risau W, Flamme I, Vasculogenesis, Annu Rev Cell Dev Biol 11 (1995) 73–91. [DOI] [PubMed] [Google Scholar]
- [29].Jain RK, Molecular regulation of vessel maturation, Nat Med 9(6) (2003) 685–93. [DOI] [PubMed] [Google Scholar]
- [30].Armulik A, Abramsson A, Betsholtz C, Endothelial/pericyte interactions, Circ Res 97(6) (2005) 512–23. [DOI] [PubMed] [Google Scholar]
- [31].von Tell D, Armulik A, Betsholtz C, Pericytes and vascular stability, Exp Cell Res 312(5) (2006) 623–9. [DOI] [PubMed] [Google Scholar]
- [32].Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC, The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation, Mol Biol Cell 22(20) (2011) 3791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA, Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival, Dev Biol 264(1) (2003) 275–88. [DOI] [PubMed] [Google Scholar]
- [34].Eilken HM, Dieguez-Hurtado R, Schmidt I, Nakayama M, Jeong HW, Arf H, Adams S, Ferrara N, Adams RH, Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1, Nat Commun 8(1) (2017) 1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ghajar CM, Kachgal S, Kniazeva E, Mori H, Costes SV, George SC, Putnam AJ, Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms, Exp Cell Res 316(5) (2010) 813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Blanco R, Gerhardt H, VEGF and Notch in Tip and Stalk Cell Selection, Cold Spring Harb Perspect Med 3(1) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kamei M, Brian Saunders W, Bayless KJ, Dye L, Davis GE, Weinstein BM, Endothelial tubes assemble from intracellular vacuoles in vivo, Nature 442(7101) (2006) 453–456. [DOI] [PubMed] [Google Scholar]
- [38].Bergers G, Song S, The role of pericytes in blood-vessel formation and maintenance, Neuro Oncol 7(4) (2005) 452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE, Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation, Blood 114(24) (2009) 5091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ, Structure and function of the blood-brain barrier, Neurobiology of Disease 37(1) (2010) 13–25. [DOI] [PubMed] [Google Scholar]
- [41].Man S, Ubogu EE, Williams KA, Tucky B, Callahan MK, Ransohoff RM, Human brain microvascular endothelial cells and umbilical vein endothelial cells differentially facilitate leukocyte recruitment and utilize chemokines for T cell migration, Clin Dev Immunol 2008 (2008) 384982–384982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Abbott NJ, Ronnback L, Hansson E, Astrocyte-endothelial interactions at the blood-brain barrier, Nat Rev Neurosci 7(1) (2006) 41–53. [DOI] [PubMed] [Google Scholar]
- [43].Haseloff RF, Blasig IE, Bauer HC, Bauer H, In Search of the Astrocytic Factor(s) Modulating Blood-Brain Barrier Functions in Brain Capillary Endothelial Cells In Vitro, Cellular and Molecular Neurobiology 25(1) (2005) 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shibuya M, Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies, Genes & cancer 2(12) (2011) 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Koch S, Claesson-Welsh L, Signal transduction by vascular endothelial growth factor receptors, Cold Spring Harb Perspect Med 2(7) (2012) a006502–a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cao Y, Positive and Negative Modulation of Angiogenesis by VEGFR1 Ligands, Science Signaling 2(59) (2009) re1. [DOI] [PubMed] [Google Scholar]
- [47].Rahimi N, VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy, Front Biosci 11 (2006) 818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F, Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2, The EMBO Journal 18(4) (1999) 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, Sato TN, Rabbany SY, Rafii S, Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration, Nature 468(7321) (2010) 310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hu K, Olsen BR, Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair, J Clin Invest 126(2) (2016) 509–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kopan R, Ilagan MXG, The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism, Cell 137(2) (2009) 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Andersson ER, Sandberg R, Lendahl U, Notch signaling: simplicity in design, versatility in function, Development 138(17) (2011) 3593. [DOI] [PubMed] [Google Scholar]
- [53].Gridley T, Notch signaling in vascular development and physiology, Development 134(15) (2007) 2709. [DOI] [PubMed] [Google Scholar]
- [54].Arima S, Nishiyama K, Ko T, Arima Y, Hakozaki Y, Sugihara K, Koseki H, Uchijima Y, Kurihara Y, Kurihara H, Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement, Development 138(21) (2011) 4763–76. [DOI] [PubMed] [Google Scholar]
- [55].Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J, Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis, Development 134(5) (2007) 839. [DOI] [PubMed] [Google Scholar]
- [56].Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H, Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting, Nat Cell Biol 12(10) (2010) 943–53. [DOI] [PubMed] [Google Scholar]
- [57].Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH, The Notch Ligands Dll4 and Jagged1 Have Opposing Effects on Angiogenesis, Cell 137(6) (2009) 1124–1135. [DOI] [PubMed] [Google Scholar]
- [58].Polacheck WJ, Kutys ML, Yang J, Eyckmans J, Wu Y, Vasavada H, Hirschi KK, Chen CS, A non-canonical Notch complex regulates adherens junctions and vascular barrier function, Nature 552(7684) (2017) 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, Fong GH, Sakmar TP, Rafii S, Ding BS, Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis, Nat Med 22(2) (2016) 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cao Z, Ding BS, Guo P, Lee SB, Butler JM, Casey SC, Simons M, Tam W, Felsher DW, Shido K, Rafii A, Scandura JM, Rafii S, Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance, Cancer Cell 25(3) (2014) 350–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, Witte L, May C, Shawber C, Kimura Y, Kitajewski J, Rosenwaks Z, Bernstein ID, Rafii S, Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells, Cell Stem Cell 6(3) (2010) 251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wieland E, Rodriguez-Vita J, Liebler SS, Mogler C, Moll I, Herberich SE, Espinet E, Herpel E, Menuchin A, Chang-Claude J, Hoffmeister M, Gebhardt C, Brenner H, Trumpp A, Siebel CW, Hecker M, Utikal J, Sprinzak D, Fischer A, Endothelial Notch1 Activity Facilitates Metastasis, Cancer Cell 31(3) (2017) 355–367. [DOI] [PubMed] [Google Scholar]
- [63].Thurston G, Daly C, The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway, Cold Spring Harb Perspect Med 2(9) (2012) a006550–a006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Saharinen P, Kerkelä K, Ekman N, Marron M, Brindle N, Lee GM, Augustin H, Koh GY, Alitalo K, Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2, Journal of Cell Biology 169(2) (2005) 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Savant S, La Porta S, Budnik A, Busch K, Hu J, Tisch N, Korn C, Valls Aida F., Benest Andrew V., Terhardt D, Qu X, Adams Ralf H., Baldwin HS, Ruiz de Almodóvar C, Rodewald H-R, Augustin Hellmut G., The Orphan Receptor Tie1 Controls Angiogenesis and Vascular Remodeling by Differentially Regulating Tie2 in Tip and Stalk Cells, Cell Reports 12(11) (2015) 1761–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Eklund L, Kangas J, Saharinen P, Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems, Clin Sci (Lond) 131(1) (2017) 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].D’Amico G, Korhonen EA, Anisimov A, Zarkada G, Holopainen T, Hägerling R, Kiefer F, Eklund L, Sormunen R, Elamaa H, Brekken RA, Adams RH, Koh GY, Saharinen P, Alitalo K, Tie1 deletion inhibits tumor growth and improves angiopoietin antagonist therapy, The Journal of Clinical Investigation 124(2) (2014) 824–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K, Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts, Nature Cell Biology 10(5) (2008) 527–537. [DOI] [PubMed] [Google Scholar]
- [69].Kim I, Kim Hwan G, So J-N, Kim Joo H, Kwak Hee J, Koh Gou Y, Angiopoietin-1 Regulates Endothelial Cell Survival Through the Phosphatidylinositol 3′-Kinase/Akt Signal Transduction Pathway, Circulation Research 86(1) (2000) 24–29. [DOI] [PubMed] [Google Scholar]
- [70].Saharinen P, Eklund L, Alitalo K, Therapeutic targeting of the angiopoietin-TIE pathway, Nature Reviews Drug Discovery 16(9) (2017) 635–661. [DOI] [PubMed] [Google Scholar]
- [71].Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD, Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis, Science 277(5322) (1997) 55–60. [DOI] [PubMed] [Google Scholar]
- [72].Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ, Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF, Science 284(5422) (1999) 1994–8. [DOI] [PubMed] [Google Scholar]
- [73].Cascone I, Napione L, Maniero F, Serini G, Bussolino F, Stable interaction between α5β1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1, Journal of Cell Biology 170(6) (2005) 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S, Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG, Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling, The Journal of Clinical Investigation 122(6) (2012) 1991–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS, Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA, Cancer Discov 5(9) (2015) 932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Brooks PC, Cell adhesion molecules in angiogenesis, Cancer and Metastasis Reviews 15(2) (1996) 187–194. [DOI] [PubMed] [Google Scholar]
- [77].Francavilla C, Maddaluno L, Cavallaro U, The functional role of cell adhesion molecules in tumor angiogenesis, Seminars in Cancer Biology 19(5) (2009) 298–309. [DOI] [PubMed] [Google Scholar]
- [78].Hodivala-Dilke KM, Reynolds AR, Reynolds LE, Integrins in angiogenesis: multitalented molecules in a balancing act, Cell and Tissue Research 314(1) (2003) 131–144. [DOI] [PubMed] [Google Scholar]
- [79].Avraamides CJ, Garmy-Susini B, Varner JA, Integrins in angiogenesis and lymphangiogenesis, Nature Reviews Cancer 8(8) (2008) 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Eliceiri Brian P, Integrin and Growth Factor Receptor Crosstalk, Circulation Research 89(12) (2001) 1104–1110. [DOI] [PubMed] [Google Scholar]
- [81].Cavallaro U, Liebner S, Dejana E, Endothelial cadherins and tumor angiogenesis, Experimental Cell Research 312(5) (2006) 659–667. [DOI] [PubMed] [Google Scholar]
- [82].Bentley K, Franco CA, Philippides A, Blanco R, Dierkes M, Gebala V, Stanchi F, Jones M, Aspalter IM, Cagna G, Westrom S, Claesson-Welsh L, Vestweber D, Gerhardt H, The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis, Nat Cell Biol 16(4) (2014) 309–21. [DOI] [PubMed] [Google Scholar]
- [83].Wai Wong C, Dye DE, Coombe DR, The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis, Int J Cell Biol 2012 (2012) 340296–340296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ, Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1, Nature 376(6540) (1995) 517–9. [DOI] [PubMed] [Google Scholar]
- [85].Carlson P, Dasgupta A, Grzelak CA, Kim J, Barrett A, Coleman IM, Shor RE, Goddard ET, Dai J, Schweitzer EM, Lim AR, Crist SB, Cheresh DA, Nelson PS, Hansen KC, Ghajar CM, Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy, Nat Cell Biol 21(2) (2019) 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Oh I-Y, Yoon C-H, Hur J, Kim J-H, Kim T-Y, Lee C-S, Park K-W, Chae I-H, Oh B-H, Park Y-B, Kim H-S, Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle, Blood 110(12) (2007) 3891–3899. [DOI] [PubMed] [Google Scholar]
- [87].Keeley EC, Mehrad B, Strieter RM, CXC chemokines in cancer angiogenesis and metastases, Advances in cancer research 106 (2010) 91–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP, CXC chemokines in angiogenesis, Cytokine & Growth Factor Reviews 16(6) (2005) 593–609. [DOI] [PubMed] [Google Scholar]
- [89].Infanger DW, Cho Y, Lopez BS, Mohanan S, Liu SC, Gursel D, Boockvar JA, Fischbach C, Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche, Cancer Res 73(23) (2013) 7079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS, Differential cytokine contributions of perivascular haematopoietic stem cell niches, Nat Cell Biol 19(3) (2017) 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S, Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling, Cell Stem Cell 7(2) (2010) 163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rao S, Sengupta R, Choe EJ, Woerner BM, Jackson E, Sun T, Leonard J, Piwnica-Worms D, Rubin JB, CXCL12 mediates trophic interactions between endothelial and tumor cells in glioblastoma, PLoS One 7(3) (2012) e33005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gassmann P, Haier J, Schluter K, Domikowsky B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider SW, Homey B, Muller A, CXCR4 regulates the early extravasation of metastatic tumor cells in vivo, Neoplasia 11(7) (2009) 651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S, Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis, Nature 505(7481) (2014) 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hielscher A, Ellis K, Qiu C, Porterfield J, Gerecht S, Fibronectin Deposition Participates in Extracellular Matrix Assembly and Vascular Morphogenesis, PLoS One 11(1) (2016) e0147600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Choi JS, Harley BA, Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells, Sci Adv 3(1) (2017) e1600455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Paulsson M, Basement membrane proteins: structure, assembly, and cellular interactions, Crit Rev Biochem Mol Biol 27(1–2) (1992) 93–127. [DOI] [PubMed] [Google Scholar]
- [98].Xu J, Shi GP, Vascular wall extracellular matrix proteins and vascular diseases, Biochim Biophys Acta 1842(11) (2014) 2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Leblond CP, Inoue S, Structure, composition, and assembly of basement membrane, Am J Anat 185(4) (1989) 367–90. [DOI] [PubMed] [Google Scholar]
- [100].Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP, Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials, Angiogenesis 15(2) (2012) 253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ghajar CM, George SC, Putnam AJ, Matrix metalloproteinase control of capillary morphogenesis, Crit Rev Eukaryot Gene Expr 18(3) (2008) 251–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Saunders WB, Bayless KJ, Davis GE, MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices, J Cell Sci 118(Pt 10) (2005) 2325–40. [DOI] [PubMed] [Google Scholar]
- [103].Trappmann B, Baker BM, Polacheck WJ, Choi CK, Burdick JA, Chen CS, Matrix degradability controls multicellularity of 3D cell migration, Nat Commun 8(1) (2017) 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ulrich TA, de Juan Pardo EM, Kumar S, The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells, Cancer Res 69(10) (2009) 4167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wells RG, The role of matrix stiffness in regulating cell behavior, Hepatology 47(4) (2008) 1394–400. [DOI] [PubMed] [Google Scholar]
- [106].Candiello J, Balasubramani M, Schreiber EM, Cole GJ, Mayer U, Halfter W, Lin H, Biomechanical properties of native basement membranes, FEBS J 274(11) (2007) 2897–908. [DOI] [PubMed] [Google Scholar]
- [107].Burton AC, Relation of structure to function of the tissues of the wall of blood vessels, Physiol Rev 34(4) (1954) 619–42. [DOI] [PubMed] [Google Scholar]
- [108].Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CC, Jeon NL, Putnam AJ, George SC, The effect of matrix density on the regulation of 3-D capillary morphogenesis, Biophys J 94(5) (2008) 1930–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sieminski AL, Hebbel RP, Gooch KJ, The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro, Exp Cell Res 297(2) (2004) 574–84. [DOI] [PubMed] [Google Scholar]
- [110].Kniazeva E, Putnam AJ, Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D, Am J Physiol Cell Physiol 297(1) (2009) C179–87. [DOI] [PubMed] [Google Scholar]
- [111].Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA, Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior, Acta Biomater 9(1) (2013) 4635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Buchanan CF, Verbridge SS, Vlachos PP, Rylander MN, Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model, Cell Adh Migr 8(5) (2014) 517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Phelps JE, DePaola N, Spatial variations in endothelial barrier function in disturbed flows in vitro, Am J Physiol Heart Circ Physiol 278(2) (2000) H469–76. [DOI] [PubMed] [Google Scholar]
- [114].Ogunrinade O, Kameya GT, Truskey GA, Effect of fluid shear stress on the permeability of the arterial endothelium, Ann Biomed Eng 30(4) (2002) 430–46. [DOI] [PubMed] [Google Scholar]
- [115].Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D, The role of shear stress in Blood-Brain Barrier endothelial physiology, BMC Neurosci 12 (2011) 40–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD, Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation, Proc Natl Acad Sci U S A 112(1) (2015) 214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ohura N, Yamamoto K, Ichioka S, Sokabe T, Nakatsuka H, Baba A, Shibata M, Nakatsuka T, Harii K, Wada Y, Kohro T, Kodama T, Ando J, Global analysis of shear stress-responsive genes in vascular endothelial cells, J Atheroscler Thromb 10(5) (2003) 304–13. [DOI] [PubMed] [Google Scholar]
- [118].Krock BL, Skuli N, Simon MC, Hypoxia-induced angiogenesis: good and evil, Genes Cancer 2(12) (2011) 1117–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Blatchley MR, Hall F, Wang S, Pruitt HC, Gerecht S, Hypoxia and matrix viscoelasticity sequentially regulate endothelial progenitor cluster-based vasculogenesis, Sci Adv 5(3) (2019) eaau7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Winkler F, Kienast Y, Fuhrmann M, Von Baumgarten L, Burgold S, Mitteregger G, Kretzschmar H, Herms J, Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis, Glia 57(12) (2009) 1306–15. [DOI] [PubMed] [Google Scholar]
- [121].Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H, Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells, Nat Commun 5 (2014) 4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zagzag D, Amirnovin R, Greco MA, Yee H, Holash J, Wiegand SJ, Zabski S, Yancopoulos GD, Grumet M, Vascular apoptosis and involution in gliomas precede neovascularization: a novel concept for glioma growth and angiogenesis, Lab Invest 80(6) (2000) 837–49. [DOI] [PubMed] [Google Scholar]
- [123].Rong Y, Durden DL, Van Meir EG, Brat DJ, ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis, J Neuropathol Exp Neurol 65(6) (2006) 529–39. [DOI] [PubMed] [Google Scholar]
- [124].Tate MC, Aghi MK, Biology of angiogenesis and invasion in glioma, Neurotherapeutics 6(3) (2009) 447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Carmeliet P, Jain RK, Angiogenesis in cancer and other diseases, Nature 407(6801) (2000) 249–57. [DOI] [PubMed] [Google Scholar]
- [126].Emamaullee JA, Shapiro AM, Factors influencing the loss of beta-cell mass in islet transplantation, Cell Transplant 16(1) (2007) 1–8. [DOI] [PubMed] [Google Scholar]
- [127].Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, Brandhorst D, Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets, Clin Exp Immunol 144(2) (2006) 179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Dickson K, Katzman S, Delgado E, Contreras D, Delayed unions and nonunions of open tibial fractures. Correlation with arteriography results, Clin Orthop Relat Res (302) (1994) 189–93. [PubMed] [Google Scholar]
- [129].Roux BM, Cheng M-H, Brey EM, Engineering clinically relevant volumes of vascularized bone, J Cell Mol Med 19(5) (2015) 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Santos MI, Reis RL, Vascularization in Bone Tissue Engineering: Physiology, Current Strategies, Major Hurdles and Future Challenges, Macromolecular Bioscience 10(1) (2010) 12–27. [DOI] [PubMed] [Google Scholar]
- [131].Nih LR, Gojgini S, Carmichael ST, Segura T, Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke, Nat Mater 17(7) (2018) 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Hobson MI, Green CJ, Terenghi G, VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy, J Anat 197 Pt 4 (2000) 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Li J, Zhang YP, Kirsner RS, Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix, Microsc Res Tech 60(1) (2003) 107–14. [DOI] [PubMed] [Google Scholar]
- [134].Li WW, Talcott KE, Zhai AW, Kruger EA, Li VW, The role of therapeutic angiogenesis in tissue repair and regeneration, Adv Skin Wound Care 18(9) (2005) 491–500; quiz 501–2. [DOI] [PubMed] [Google Scholar]
- [135].Nucera S, Biziato D, De Palma M, The interplay between macrophages and angiogenesis in development, tissue injury and regeneration, Int J Dev Biol 55(4–5) (2011) 495–503. [DOI] [PubMed] [Google Scholar]
- [136].Crowther M, Brown NJ, Bishop ET, Lewis CE, Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors, J Leukoc Biol 70(4) (2001) 478–90. [PubMed] [Google Scholar]
- [137].Mosser DM, Edwards JP, Exploring the full spectrum of macrophage activation, Nature Reviews Immunology 8(12) (2008) 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Liu Y-C, Zou X-B, Chai Y-F, Yao Y-M, Macrophage Polarization in Inflammatory Diseases, International Journal of Biological Sciences 10(5) (2014) 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G, The role of macrophage phenotype in vascularization of tissue engineering scaffolds, Biomaterials 35(15) (2014) 4477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ, Oxygen tension regulates the expression of angiogenesis factor by macrophages, Science 221(4617) (1983) 1283–5. [DOI] [PubMed] [Google Scholar]
- [141].Grosso A, Burger MG, Lunger A, Schaefer DJ, Banfi A, Di Maggio N, It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration, Frontiers in Bioengineering and Biotechnology 5 (2017) 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Tombran-Tink J, Barnstable CJ, Osteoblasts and osteoclasts express PEDF, VEGF-A isoforms, and VEGF receptors: possible mediators of angiogenesis and matrix remodeling in the bone, Biochem Biophys Res Commun 316(2) (2004) 573–9. [DOI] [PubMed] [Google Scholar]
- [143].Ramasamy SK, Kusumbe AP, Wang L, Adams RH, Endothelial Notch activity promotes angiogenesis and osteogenesis in bone, Nature 507(7492) (2014) 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Steiner D, Lampert F, Stark GB, Finkenzeller G, Effects of endothelial cells on proliferation and survival of human mesenchymal stem cells and primary osteoblasts, J Orthop Res 30(10) (2012) 1682–9. [DOI] [PubMed] [Google Scholar]
- [145].Pinho S, Frenette PS, Haematopoietic stem cell activity and interactions with the niche, Nature Reviews Molecular Cell Biology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS, Arteriolar niches maintain haematopoietic stem cell quiescence, Nature 502(7473) (2013) 637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ, SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells, Cell 121(7) (2005) 1109–21. [DOI] [PubMed] [Google Scholar]
- [148].Ding L, Saunders TL, Enikolopov G, Morrison SJ, Endothelial and perivascular cells maintain haematopoietic stem cells, Nature 481(7382) (2012) 457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, Rafii S, Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells, Nat Cell Biol 12(11) (2010) 1046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Levesque JP, Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance, Nat Med 18(11) (2012) 1651–7. [DOI] [PubMed] [Google Scholar]
- [151].Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F, A specialized vascular niche for adult neural stem cells, Cell Stem Cell 3(3) (2008) 279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Schmidt NO, Koeder D, Messing M, Mueller FJ, Aboody KS, Kim SU, Black PM, Carroll RS, Westphal M, Lamszus K, Vascular endothelial growth factor-stimulated cerebral microvascular endothelial cells mediate the recruitment of neural stem cells to the neurovascular niche, Brain Res 1268 (2009) 24–37. [DOI] [PubMed] [Google Scholar]
- [153].Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S, Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells, Science 304(5675) (2004) 1338–40. [DOI] [PubMed] [Google Scholar]
- [154].Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, Mira H, Escribano J, Farinas I, Pigment epithelium-derived factor is a niche signal for neural stem cell renewal, Nat Neurosci 9(3) (2006) 331–9. [DOI] [PubMed] [Google Scholar]
- [155].Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T, A role for hematopoietic stem cells in promoting angiogenesis, Cell 102(2) (2000) 199–209. [DOI] [PubMed] [Google Scholar]
- [156].Li Q, Ford MC, Lavik EB, Madri JA, Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study, J Neurosci Res 84(8) (2006) 1656–68. [DOI] [PubMed] [Google Scholar]
- [157].Hanahan D, Weinberg RA, The hallmarks of cancer, Cell 100(1) (2000) 57–70. [DOI] [PubMed] [Google Scholar]
- [158].Folkman J, Role of angiogenesis in tumor growth and metastasis, Semin Oncol 29(6 Suppl 16) (2002) 15–8. [DOI] [PubMed] [Google Scholar]
- [159].Chaffer CL, Weinberg RA, A perspective on cancer cell metastasis, Science 331(6024) (2011) 1559–64. [DOI] [PubMed] [Google Scholar]
- [160].Jahroudi N, Greenberger JS, The role of endothelial cells in tumor invasion and metastasis, J Neurooncol 23(2) (1995) 99–108. [DOI] [PubMed] [Google Scholar]
- [161].Gupta GP, Massague J, Cancer metastasis: building a framework, Cell 127(4) (2006) 679–95. [DOI] [PubMed] [Google Scholar]
- [162].Joyce JA, Pollard JW, Microenvironmental regulation of metastasis, Nat Rev Cancer 9(4) (2009) 239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Juncker-Jensen A, Deryugina EI, Rimann I, Zajac E, Kupriyanova TA, Engelholm LH, Quigley JP, Tumor MMP-1 activates endothelial PAR1 to facilitate vascular intravasation and metastatic dissemination, Cancer Res 73(14) (2013) 4196–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Mourad PD, Farrell L, Stamps LD, Chicoine MR, Silbergeld DL, Why are systemic glioblastoma metastases rare? Systemic and cerebral growth of mouse glioblastoma, Surg Neurol 63(6) (2005) 511–9; discussion 519. [DOI] [PubMed] [Google Scholar]
- [165].Bernstein JJ, Woodard CA, Glioblastoma cells do not intravasate into blood vessels, Neurosurgery 36(1) (1995) 124–32; discussion 132. [DOI] [PubMed] [Google Scholar]
- [166].Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P, Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis, Glia 53(8) (2006) 799–808. [DOI] [PubMed] [Google Scholar]
- [167].Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, Bissell MJ, The perivascular niche regulates breast tumour dormancy, Nat Cell Biol 15(7) (2013) 807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Clarke MF, Hass AT, Cancer Stem Cells, Reviews in Cell Biology and Molecular Medicine. [Google Scholar]
- [169].Jordan CT, Guzman ML, Noble M, Cancer stem cells N Engl J Med 355(12) (2006) 1253–61. [DOI] [PubMed] [Google Scholar]
- [170].Dean M, Fojo T, Bates S, Tumour stem cells and drug resistance, Nat Rev Cancer 5(4) (2005) 275–84. [DOI] [PubMed] [Google Scholar]
- [171].Rich JN, Cancer stem cells in radiation resistance, Cancer Res 67(19) (2007) 8980–4. [DOI] [PubMed] [Google Scholar]
- [172].Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ, A perivascular niche for brain tumor stem cells, Cancer Cell 11(1) (2007) 69–82. [DOI] [PubMed] [Google Scholar]
- [173].Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nor JE, Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells, Cancer Res 70(23) (2010) 9969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, He X, Hervey-Jumper SL, Heth JA, Muraszko KM, DiMeco F, Vescovi AL, Fan X, Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells, Cancer Res 71(18) (2011) 6061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, Tozzi F, Sceusi E, Zhou Y, Tachibana I, Maru DM, Hawke DH, Rak J, Mani SA, Zweidler-McKay P, Ellis LM, Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1, Cancer Cell 23(2) (2013) 171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Ghiabi P, Jiang J, Pasquier J, Maleki M, Abu-Kaoud N, Rafii S, Rafii A, Endothelial cells provide a notch-dependent pro-tumoral niche for enhancing breast cancer survival, stemness and pro-metastatic properties, PLoS One 9(11) (2014) e112424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC, Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells, Cell Stem Cell 6(2) (2010) 141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC, PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo, Genes Dev 22(4) (2008) 436–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Guerrouahen BS, Pasquier J, Kaoud NA, Maleki M, Beauchamp MC, Yasmeen A, Ghiabi P, Lis R, Vidal F, Saleh A, Gotlieb WH, Rafii S, Rafii A, Akt-activated endothelium constitutes the niche for residual disease and resistance to bevacizumab in ovarian cancer, Mol Cancer Ther 13(12) (2014) 3123–36. [DOI] [PubMed] [Google Scholar]
- [180].Cross VL, Zheng Y, Won Choi N, Verbridge SS, Sutermaster BA, Bonassar LJ, Fischbach C, Stroock AD, Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro, Biomaterials 31(33) (2010) 8596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Crosby CO, Valliappan D, Shu D, Kumar S, Tu C, Deng W, Parekh SH, Zoldan J, Quantifying the Vasculogenic Potential of Induced Pluripotent Stem Cell-Derived Endothelial Progenitors in Collagen Hydrogels, Tissue Engineering Part A 25(9–10) (2019) 746–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO, Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult, J Cereb Blood Flow Metab 31(2) (2011) 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A, Cell-laden microengineered gelatin methacrylate hydrogels, Biomaterials 31(21) (2010) 5536–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Chen JE, Pedron S, Harley BAC, The Combined Influence of Hydrogel Stiffness and Matrix-Bound Hyaluronic Acid Content on Glioblastoma Invasion, Macromol Biosci 17(8) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Gilchrist AE, Lee S, Hu Y, Harley BAC, Soluble Signals and Remodeling in a Synthetic Gelatin-Based Hematopoietic Stem Cell Niche, Advanced Healthcare Materials 8(20) (2019) 1900751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A, Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels, Biomaterials 73 (2015) 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Chen YC, Lin RZ, Qi H, Yang Y, Bae H, Melero-Martin JM, Khademhosseini A, Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels, Adv Funct Mater 22(10) (2012) 2027–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CC, George SC, Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature, Tissue Eng Part A 15(6) (2009) 1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Chen X, Aledia AS, Popson SA, Him L, Hughes CC, George SC, Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts, Tissue Eng Part A 16(2) (2010) 585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Li S, Nih LR, Bachman H, Fei P, Li Y, Nam E, Dimatteo R, Carmichael ST, Barker TH, Segura T, Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability, Nat Mater 16(9) (2017) 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Moon JJ, Saik JE, Poche RA, Leslie-Barbick JE, Lee SH, Smith AA, Dickinson ME, West JL, Biomimetic hydrogels with pro-angiogenic properties, Biomaterials 31(14) (2010) 3840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ, Bioartificial matrices for therapeutic vascularization, Proc Natl Acad Sci U S A 107(8) (2010) 3323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Brown A, He H, Trumper E, Valdez J, Hammond P, Griffith LG, Engineering PEG-based hydrogels to foster efficient endothelial network formation in free-swelling and confined microenvironments, Biomaterials 243 (2020) 119921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Juliar BA, Beamish JA, Busch ME, Cleveland DS, Nimmagadda L, Putnam AJ, Cell-mediated matrix stiffening accompanies capillary morphogenesis in ultra-soft amorphous hydrogels, Biomaterials 230 (2020) 119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Park JE, Keller GA, Ferrara N, The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF, Mol Biol Cell 4(12) (1993) 1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Belair DG, Le NN, Murphy WL, Design of growth factor sequestering biomaterials, Chem Commun (Camb) 50(99) (2014) 15651–15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Flaumenhaft R, Rifkin DB, Extracellular matrix regulation of growth factor and protease activity, Current Opinion in Cell Biology 3(5) (1991) 817–823. [DOI] [PubMed] [Google Scholar]
- [198].Anderson SM, Chen TT, Iruela-Arispe ML, Segura T, The phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) by engineered surfaces with electrostatically or covalently immobilized VEGF, Biomaterials 30(27) (2009) 4618–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML, Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors, The Journal of cell biology 169(4) (2005) 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA, Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth, FASEB J 17(15) (2003) 2260–2. [DOI] [PubMed] [Google Scholar]
- [201].Chwalek K, Tsurkan MV, Freudenberg U, Werner C, Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models, Scientific Reports 4(1) (2014) 4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Ngo MT, Harley BA, The Influence of Hyaluronic Acid and Glioblastoma Cell Coculture on the Formation of Endothelial Cell Networks in Gelatin Hydrogels, Adv Healthc Mater 6(22) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Foster GA, Headen DM, Gonzalez-Garcia C, Salmeron-Sanchez M, Shirwan H, Garcia AJ, Protease-degradable microgels for protein delivery for vascularization, Biomaterials 113 (2017) 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Burdick JA, Prestwich GD, Hyaluronic Acid Hydrogels for Biomedical Applications, Advanced Materials 23(12) (2011) H41–H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Hanjaya-Putra D, Bose V, Shen Y-I, Yee J, Khetan S, Fox-Talbot K, Steenbergen C, Burdick JA, Gerecht S, Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix, Blood 118(3) (2011) 804–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Wolf KJ, Kumar S, Hyaluronic Acid: Incorporating the Bio into the Material, ACS Biomaterials Science & Engineering 5(8) (2019) 3753–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Sun G, Shen Y-I, Kusuma S, Fox-Talbot K, Steenbergen CJ, Gerecht S, Functional neovascularization of biodegradable dextran hydrogels with multiple angiogenic growth factors, Biomaterials 32(1) (2011) 95–106. [DOI] [PubMed] [Google Scholar]
- [208].Ruvinov E, Leor J, Cohen S, The effects of controlled HGF delivery from an affinity-binding alginate biomaterial on angiogenesis and blood perfusion in a hindlimb ischemia model, Biomaterials 31(16) (2010) 4573–4582. [DOI] [PubMed] [Google Scholar]
- [209].Wang W, Guo L, Yu Y, Chen Z, Zhou R, Yuan Z, Peptide REDV-modified polysaccharide hydrogel with endothelial cell selectivity for the promotion of angiogenesis, Journal of Biomedical Materials Research Part A 103(5) (2015) 1703–1712. [DOI] [PubMed] [Google Scholar]
- [210].Ekaputra AK, Prestwich GD, Cool SM, Hutmacher DW, The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (ɛ-caprolactone)/collagen fibers and hyaluronic acid hydrogel, Biomaterials 32(32) (2011) 8108–8117. [DOI] [PubMed] [Google Scholar]
- [211].Singh RK, Seliktar D, Putnam AJ, Capillary morphogenesis in PEG-collagen hydrogels, Biomaterials 34(37) (2013) 9331–9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Wang MO, Vorwald CE, Dreher ML, Mott EJ, Cheng M-H, Cinar A, Mehdizadeh H, Somo S, Dean D, Brey EM, Fisher JP, Evaluating 3D-Printed Biomaterials as Scaffolds for Vascularized Bone Tissue Engineering, Advanced Materials 27(1) (2015) 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Mehdizadeh H, Sumo S, Bayrak ES, Brey EM, Cinar A, Three-dimensional modeling of angiogenesis in porous biomaterial scaffolds, Biomaterials 34(12) (2013) 2875–2887. [DOI] [PubMed] [Google Scholar]
- [214].Chiu Y-C, Cheng M-H, Engel H, Kao S-W, Larson JC, Gupta S, Brey EM, The role of pore size on vascularization and tissue remodeling in PEG hydrogels, Biomaterials 32(26) (2011) 6045–6051. [DOI] [PubMed] [Google Scholar]
- [215].Klenke FM, Liu Y, Yuan H, Hunziker EB, Siebenrock KA, Hofstetter W, Impact of pore size on the vascularization and osseointegration of ceramic bone substitutes in vivo, Journal of Biomedical Materials Research Part A 85A(3) (2008) 777–786. [DOI] [PubMed] [Google Scholar]
- [216].Joshi VS, Lei NY, Walthers CM, Wu B, Dunn JCY, Macroporosity enhances vascularization of electrospun scaffolds, Journal of Surgical Research 183(1) (2013) 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Bezuidenhout D, Davies N, Black M, Schmidt C, Oosthuysen A, Zilla P, Covalent Surface Heparinization Potentiates Porous Polyurethane Scaffold Vascularization, Journal of Biomaterials Applications 24(5) (2008) 401–418. [DOI] [PubMed] [Google Scholar]
- [218].Singh S, Wu BM, Dunn JCY, The enhancement of VEGF-mediated angiogenesis by polycaprolactone scaffolds with surface cross-linked heparin, Biomaterials 32(8) (2011) 2059–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Laschke MW, Rücker M, Jensen G, Carvalho C, Mülhaupt R, Gellrich NC, Menger MD, Incorporation of growth factor containing Matrigel promotes vascularization of porous PLGA scaffolds, Journal of Biomedical Materials Research Part A 85A(2) (2008) 397–407. [DOI] [PubMed] [Google Scholar]
- [220].Singh S, Wu BM, Dunn JCY, Delivery of VEGF using collagen-coated polycaprolactone scaffolds stimulates angiogenesis, Journal of Biomedical Materials Research Part A 100A(3) (2012) 720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Freeman I, Cohen S, The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization, Biomaterials 30(11) (2009) 2122–2131. [DOI] [PubMed] [Google Scholar]
- [222].Chiu LLY, Radisic M, Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues, Biomaterials 31(2) (2010) 226–241. [DOI] [PubMed] [Google Scholar]
- [223].Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S, Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres, Journal of Biomedical Materials Research Part A 65A(4) (2003) 489–497. [DOI] [PubMed] [Google Scholar]
- [224].Whisler JA, Chen MB, Kamm RD, Control of perfusable microvascular network morphology using a multiculture microfluidic system, Tissue Eng Part C Methods 20(7) (2014) 543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, Moretti M, Kamm RD, Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems, Integr Biol (Camb) 6(5) (2014) 555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD, 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes, Biomaterials 180 (2018) 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD, Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function, Proc Natl Acad Sci U S A 109(34) (2012) 13515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Offeddu GS, Possenti L, Loessberg-Zahl JT, Zunino P, Roberts J, Han X, Hickman D, Knutson CG, Kamm RD, Application of Transmural Flow Across In Vitro Microvasculature Enables Direct Sampling of Interstitial Therapeutic Molecule Distribution, Small 15(46) (2019) 1902393. [DOI] [PubMed] [Google Scholar]
- [229].Cui X, Boland T, Human microvasculature fabrication using thermal inkjet printing technology, Biomaterials 30(31) (2009) 6221–6227. [DOI] [PubMed] [Google Scholar]
- [230].Suntornnond R, Tan EYS, An J, Chua CK, A highly printable and biocompatible hydrogel composite for direct printing of soft and perfusable vasculature-like structures, Scientific Reports 7(1) (2017) 16902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Zhang Y, Yu Y, Akkouch A, Dababneh A, Dolati F, Ozbolat IT, In Vitro Study of Directly Bioprinted Perfusable Vasculature Conduits, Biomater Sci 3(1) (2015) 134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A, Direct 3D bioprinting of perfusable vascular constructs using a blend bioink, Biomaterials 106 (2016) 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J, Wang P, Lai CSE, Gou M, Xu Y, Zhang K, Chen S, Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture, Biomaterials 124 (2017) 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Lee VK, Kim DY, Ngo H, Lee Y, Seo L, Yoo S-S, Vincent PA, Dai G, Creating perfused functional vascular channels using 3D bio-printing technology, Biomaterials 35(28) (2014) 8092–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA, 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs, Adv Mater 26(19) (2014) 3124–30. [DOI] [PubMed] [Google Scholar]
- [236].Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA, Three-dimensional bioprinting of thick vascularized tissues, Proceedings of the National Academy of Sciences 113(12) (2016) 3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS, Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues, Nat Mater 11(9) (2012) 768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Mirabella T, MacArthur JW, Cheng D, Ozaki CK, Woo YJ, Yang M, Chen CS, 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia, Nature biomedical engineering 1 (2017) 0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Stevens KR, Scull MA, Ramanan V, Fortin CL, Chaturvedi RR, Knouse KA, Xiao JW, Fung C, Mirabella T, Chen AX, McCue MG, Yang MT, Fleming HE, Chung K, de Jong YP, Chen CS, Rice CM, Bhatia SN, In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease, Science Translational Medicine 9(399) (2017) eaah5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, Johansson F, Randles A, Rosenkrantz JE, Louis-Rosenberg JD, Galie PA, Stevens KR, Miller JS, Multivascular networks and functional intravascular topologies within biocompatible hydrogels, Science 364(6439) (2019) 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Heintz KA, Bregenzer ME, Mantle JL, Lee KH, West JL, Slater JH, Fabrication of 3D Biomimetic Microfluidic Networks in Hydrogels, Adv Healthc Mater 5(17) (2016) 2153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Belair DG, Whisler JA, Valdez J, Velazquez J, Molenda JA, Vickerman V, Lewis R, Daigh C, Hansen TD, Mann DA, Thomson JA, Griffith LG, Kamm RD, Schwartz MP, Murphy WL, Human vascular tissue models formed from human induced pluripotent stem cell derived endothelial cells, Stem Cell Rev 11(3) (2015) 511–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Kurokawa YK, Yin RT, Shang MR, Shirure VS, Moya ML, George SC, Human Induced Pluripotent Stem Cell-Derived Endothelial Cells for Three-Dimensional Microphysiological Systems, Tissue Eng Part C Methods 23(8) (2017) 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Kusuma S, Shen YI, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S, Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix, Proc Natl Acad Sci U S A 110(31) (2013) 12601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Wimmer RA, Leopoldi A, Aichinger M, Wick N, Hantusch B, Novatchkova M, Taubenschmid J, Hämmerle M, Esk C, Bagley JA, Lindenhofer D, Chen G, Boehm M, Agu CA, Yang F, Fu B, Zuber J, Knoblich JA, Kerjaschki D, Penninger JM, Human blood vessel organoids as a model of diabetic vasculopathy, Nature 565(7740) (2019) 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Wimmer RA, Leopoldi A, Aichinger M, Kerjaschki D, Penninger JM, Generation of blood vessel organoids from human pluripotent stem cells, Nature Protocols 14(11) (2019) 3082–3100. [DOI] [PubMed] [Google Scholar]
- [247].Zagorchev L, Oses P, Zhuang ZW, Moodie K, Mulligan-Kehoe MJ, Simons M, Couffinhal T, Micro computed tomography for vascular exploration, J Angiogenes Res 2 (2010) 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Schmidt C, Bezuidenhout D, Beck M, Van der Merwe E, Zilla P, Davies N, Rapid three-dimensional quantification of VEGF-induced scaffold neovascularisation by microcomputed tomography, Biomaterials 30(30) (2009) 5959–5968. [DOI] [PubMed] [Google Scholar]
- [249].McFadden TM, Duffy GP, Allen AB, Stevens HY, Schwarzmaier SM, Plesnila N, Murphy JM, Barry FP, Guldberg RE, O’Brien FJ, The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen-glycosaminoglycan scaffold in vivo, Acta Biomaterialia 9(12) (2013) 9303–9316. [DOI] [PubMed] [Google Scholar]
- [250].Zudaire E, Gambardella L, Kurcz C, Vermeren S, A Computational Tool for Quantitative Analysis of Vascular Networks, PLOS ONE 6(11) (2011) e27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Kaushik G, Gil DA, Torr E, Berge ES, Soref C, Uhl P, Fontana G, Antosiewicz-Bourget J, Edington C, Schwartz MP, Griffith LG, Thomson JA, Skala MC, Daly WT, Murphy WL, Quantitative Label-Free Imaging of 3D Vascular Networks Self-Assembled in Synthetic Hydrogels, Advanced Healthcare Materials 8(2) (2019) 1801186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Phelps EA, Headen DM, Taylor WR, Thule PM, Garcia AJ, Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes, Biomaterials 34(19) (2013) 4602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Weaver JD, Headen DM, Aquart J, Johnson CT, Shea LD, Shirwan H, Garcia AJ, Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites, Sci Adv 3(6) (2017) e1700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Brady AC, Martino MM, Pedraza E, Sukert S, Pileggi A, Ricordi C, Hubbell JA, Stabler CL, Proangiogenic hydrogels within macroporous scaffolds enhance islet engraftment in an extrahepatic site, Tissue Eng Part A 19(23–24) (2013) 2544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Moshayedi P, Nih LR, Llorente IL, Berg AR, Cinkornpumin J, Lowry WE, Segura T, Carmichael ST, Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain, Biomaterials 105 (2016) 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Murphy WL, Simmons CA, Kaigler D, Mooney DJ, Bone regeneration via a mineral substrate and induced angiogenesis, J Dent Res 83(3) (2004) 204–10. [DOI] [PubMed] [Google Scholar]
- [257].Yu H, VandeVord PJ, Mao L, Matthew HW, Wooley PH, Yang SY, Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization, Biomaterials 30(4) (2009) 508–17. [DOI] [PubMed] [Google Scholar]
- [258].Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ, Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors, Proc Natl Acad Sci U S A 107(8) (2010) 3287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G, Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds, Biomaterials 37 (2015) 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Sun JL, Jiao K, Niu LN, Jiao Y, Song Q, Shen LJ, Tay FR, Chen JH, Intrafibrillar silicified collagen scaffold modulates monocyte to promote cell homing, angiogenesis and bone regeneration, Biomaterials 113 (2017) 203–216. [DOI] [PubMed] [Google Scholar]
- [261].Latroche C, Weiss-Gayet M, Muller L, Gitiaux C, Leblanc P, Liot S, Ben-Larbi S, Abou-Khalil R, Verger N, Bardot P, Magnan M, Chretien F, Mounier R, Germain S, Chazaud B, Coupling between Myogenesis and Angiogenesis during Skeletal Muscle Regeneration Is Stimulated by Restorative Macrophages, Stem Cell Reports 9(6) (2017) 2018–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Bongso A, Fong CY, Gauthaman K, Taking stem cells to the clinic: Major challenges, J Cell Biochem 105(6) (2008) 1352–60. [DOI] [PubMed] [Google Scholar]
- [263].Mattsson J, Ringden O, Storb R, Graft failure after allogeneic hematopoietic cell transplantation, Biol Blood Marrow Transplant 14(1 Suppl 1) (2008) 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Baldari S, Di Rocco G, Piccoli M, Pozzobon M, Muraca M, Toietta G, Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies, Int J Mol Sci 18(10) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Cha C, Liechty WB, Khademhosseini A, Peppas NA, Designing biomaterials to direct stem cell fate, ACS Nano 6(11) (2012) 9353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Dawson E, Mapili G, Erickson K, Taqvi S, Roy K, Biomaterials for stem cell differentiation, Adv Drug Deliv Rev 60(2) (2008) 215–28. [DOI] [PubMed] [Google Scholar]
- [267].Copelan EA, Hematopoietic stem-cell transplantation N Engl J Med 354(17) (2006) 1813–26. [DOI] [PubMed] [Google Scholar]
- [268].Morrison SJ, Scadden DT, The bone marrow niche for haematopoietic stem cells, Nature 505(7483) (2014) 327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Kotha SS, Hayes BJ, Phong KT, Redd MA, Bomsztyk K, Ramakrishnan A, Torok-Storb B, Zheng Y, Engineering a multicellular vascular niche to model hematopoietic cell trafficking, Stem Cell Res Ther 9(1) (2018) 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Braham MVJ, Li Yim ASP, Garcia Mateos J, Minnema MC, Dhert WJA, Oner FC, Robin C, Alblas J, A Human Hematopoietic Niche Model Supporting Hematopoietic Stem and Progenitor Cells In Vitro, Adv Healthc Mater 8(10) (2019) e1801444. [DOI] [PubMed] [Google Scholar]
- [271].Parmar K, Mauch P, Vergilio J-A, Sackstein R, Down JD, Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia, Proceedings of the National Academy of Sciences 104(13) (2007) 5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Eliasson P, Rehn M, Hammar P, Larsson P, Sirenko O, Flippin LA, Cammenga J, Jönsson J-I, Hypoxia mediates low cell-cycle activity and increases the proportion of long-term-reconstituting hematopoietic stem cells during in vitro culture, Experimental Hematology 38(4) (2010) 301–310.e2. [DOI] [PubMed] [Google Scholar]
- [273].Roy S, Tripathy M, Mathur N, Jain A, Mukhopadhyay A, Hypoxia improves expansion potential of human cord blood-derived hematopoietic stem cells and marrow repopulation efficiency, European Journal of Haematology 88(5) (2012) 396–405. [DOI] [PubMed] [Google Scholar]
- [274].Lindvall O, Kokaia Z, Martinez-Serrano A, Stem cell therapy for human neurodegenerative disorders-how to make it work, Nat Med 10 Suppl (2004) S42–50. [DOI] [PubMed] [Google Scholar]
- [275].Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK, Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage, Stroke 34(9) (2003) 2258–63. [DOI] [PubMed] [Google Scholar]
- [276].Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, Raghupathi R, Lenzlinger PM, Lifshitz J, Boockvar J, Neugebauer E, Snyder EY, McIntosh TK, Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury, Neurosurgery 51(4) (2002) 1043–52; discussion 1052–4. [DOI] [PubMed] [Google Scholar]
- [277].Rauch MF, Michaud M, Xu H, Madri JA, Lavik EB, Co-culture of primary neural progenitor and endothelial cells in a macroporous gel promotes stable vascular networks in vivo, J Biomater Sci Polym Ed 19(11) (2008) 1469–85. [DOI] [PubMed] [Google Scholar]
- [278].Furlan A, Vercamer C, Heliot L, Wernert N, Desbiens X, Pourtier A, Ets-1 drives breast cancer cell angiogenic potential and interactions between breast cancer and endothelial cells, Int J Oncol 54(1) (2019) 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Chen Z, Htay A, Dos Santos W, Gillies GT, Fillmore HL, Sholley MM, Broaddus WC, In vitro angiogenesis by human umbilical vein endothelial cells (HUVEC) induced by three-dimensional co-culture with glioblastoma cells, J Neurooncol 92(2) (2009) 121–8. [DOI] [PubMed] [Google Scholar]
- [280].Bray LJ, Binner M, Holzheu A, Friedrichs J, Freudenberg U, Hutmacher DW, Werner C, Multi-parametric hydrogels support 3D in vitro bioengineered microenvironment models of tumour angiogenesis, Biomaterials 53 (2015) 609–20. [DOI] [PubMed] [Google Scholar]
- [281].Roudsari LC, Jeffs SE, Witt AS, Gill BJ, West JL, A 3D Poly(ethylene glycol)-based Tumor Angiogenesis Model to Study the Influence of Vascular Cells on Lung Tumor Cell Behavior, Sci Rep 6 (2016) 32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Del Bufalo F, Manzo T, Hoyos V, Yagyu S, Caruana I, Jacot J, Benavides O, Rosen D, Brenner MK, 3D modeling of human cancer: A PEG-fibrin hydrogel system to study the role of tumor microenvironment and recapitulate the in vivo effect of oncolytic adenovirus, Biomaterials 84 (2016) 76–85. [DOI] [PubMed] [Google Scholar]
- [283].Correa de Sampaio P, Auslaender D, Krubasik D, Failla AV, Skepper JN, Murphy G, English WR, A heterogeneous in vitro three dimensional model of tumour-stroma interactions regulating sprouting angiogenesis, PLoS One 7(2) (2012) e30753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Ehsan SM, Welch-Reardon KM, Waterman ML, Hughes CC, George SC, A three-dimensional in vitro model of tumor cell intravasation, Integr Biol (Camb) 6(6) (2014) 603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Ngo MT, Harley BAC, Perivascular signals alter global gene expression profile of glioblastoma and response to temozolomide in a gelatin hydrogel, Biomaterials 198 (2019) 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Ngo MT, Karvelis E, Harley BAC, Multidimensional hydrogel models reveal endothelial network angiocrine signals increase glioblastoma cell number, invasion, and temozolomide resistance, bioRxiv (2020) 2020.01.18.911396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Almog N, Molecular mechanisms underlying tumor dormancy, Cancer Lett 294(2) (2010) 139–46. [DOI] [PubMed] [Google Scholar]
- [288].Hedley BD, Chambers AF, Tumor dormancy and metastasis, Adv Cancer Res 102 (2009) 67–101. [DOI] [PubMed] [Google Scholar]
- [289].Marlow R, Honeth G, Lombardi S, Cariati M, Hessey S, Pipili A, Mariotti V, Buchupalli B, Foster K, Bonnet D, Grigoriadis A, Rameshwar P, Purushotham A, Tutt A, Dontu G, A novel model of dormancy for bone metastatic breast cancer cells, Cancer Res 73(23) (2013) 6886–99. [DOI] [PubMed] [Google Scholar]
- [290].Agarwal P, Wang H, Sun M, Xu J, Zhao S, Liu Z, Gooch KJ, Zhao Y, Lu X, He X, Microfluidics Enabled Bottom-Up Engineering of 3D Vascularized Tumor for Drug Discovery, ACS Nano 11(7) (2017) 6691–6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Miller CP, Tsuchida C, Zheng Y, Himmelfarb J, Akilesh S, A 3D Human Renal Cell Carcinoma-on-a-Chip for the Study of Tumor Angiogenesis, Neoplasia 20(6) (2018) 610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Mannino RG, Santiago-Miranda AN, Pradhan P, Qiu Y, Mejias JC, Neelapu SS, Roy K, Lam WA, 3D microvascular model recapitulates the diffuse large B-cell lymphoma tumor microenvironment in vitro, Lab Chip 17(3) (2017) 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Cui X, Morales RT, Qian W, Wang H, Gagner JP, Dolgalev I, Placantonakis D, Zagzag D, Cimmino L, Snuderl M, Lam RHW, Chen W, Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis, Biomaterials 161 (2018) 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Wolf KJ, Lee S, Kumar S, A 3D topographical model of parenchymal infiltration and perivascular invasion in glioblastoma, APL Bioeng 2(3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, Moretti M, Kamm RD, A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone, Biomaterials 35(8) (2014) 2454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Chonan Y, Taki S, Sampetrean O, Saya H, Sudo R, Endothelium-induced three-dimensional invasion of heterogeneous glioma initiating cells in a microfluidic coculture platform, Integr Biol (Camb) 9(9) (2017) 762–773. [DOI] [PubMed] [Google Scholar]
- [297].Truong D, Fiorelli R, Barrientos ES, Melendez EL, Sanai N, Mehta S, Nikkhah M, A three-dimensional (3D) organotypic microfluidic model for glioma stem cells - Vascular interactions, Biomaterials 198 (2019) 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD, Cell migration into scaffolds under co-culture conditions in a microfluidic platform, Lab Chip 9(2) (2009) 269–75. [DOI] [PubMed] [Google Scholar]
- [299].Sobrino A, Phan DT, Datta R, Wang X, Hachey SJ, Romero-Lopez M, Gratton E, Lee AP, George SC, Hughes CC, 3D microtumors in vitro supported by perfused vascular networks, Sci Rep 6 (2016) 31589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Xiao Y, Kim D, Dura B, Zhang K, Yan R, Li H, Han E, Ip J, Zou P, Liu J, Chen AT, Vortmeyer AO, Zhou J, Fan R, Ex vivo Dynamics of Human Glioblastoma Cells in a Microvasculature-on-a-Chip System Correlates with Tumor Heterogeneity and Subtypes, Advanced Science 6(8) (2019) 1801531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Boussommier-Calleja A, Atiyas Y, Haase K, Headley M, Lewis C, Kamm RD, The effects of monocytes on tumor cell extravasation in a 3D vascularized microfluidic model, Biomaterials 198 (2019) 180–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Chung M, Ahn J, Son K, Kim S, Jeon NL, Biomimetic Model of Tumor Microenvironment on Microfluidic Platform, Advanced Healthcare Materials 6(15) (2017) 1700196. [DOI] [PubMed] [Google Scholar]
- [303].Pradhan S, Smith AM, Garson CJ, Hassani I, Seeto WJ, Pant K, Arnold RD, Prabhakarpandian B, Lipke EA, A Microvascularized Tumor-mimetic Platform for Assessing Anti-cancer Drug Efficacy, Scientific Reports 8(1) (2018) 3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Filipowska J, Tomaszewski KA, Niedzwiedzki L, Walocha JA, Niedzwiedzki T, The role of vasculature in bone development, regeneration and proper systemic functioning, Angiogenesis 20(3) (2017) 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Hankenson KD, Dishowitz M, Gray C, Schenker M, Angiogenesis in bone regeneration, Injury 42(6) (2011) 556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Ramasamy SK, Kusumbe AP, Schiller M, Zeuschner D, Bixel MG, Milia C, Gamrekelashvili J, Limbourg A, Medvinsky A, Santoro MM, Limbourg FP, Adams RH, Blood flow controls bone vascular function and osteogenesis, Nat Commun 7 (2016) 13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Shih AY, Ruhlmann C, Blinder P, Devor A, Drew PJ, Friedman B, Knutsen PM, Lyden PD, Mateo C, Mellander L, Nishimura N, Schaffer CB, Tsai PS, Kleinfeld D, Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture, Microcirculation 22(3) (2015) 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Sivaraj KK, Adams RH, Blood vessel formation and function in bone, Development 143(15) (2016) 2706–15. [DOI] [PubMed] [Google Scholar]
- [309].Paulsen SJ, Miller JS, Tissue vascularization through 3D printing: Will technology bring us flow?, Dev Dyn 244(5) (2015) 629–40. [DOI] [PubMed] [Google Scholar]
- [310].Grolman JM, Zhang D, Smith AM, Moore JS, Kilian KA, Rapid 3D Extrusion of Synthetic Tumor Microenvironments, Adv Mater 27(37) (2015) 5512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Shadish JA, Benuska GM, DeForest CA, Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials, Nat Mater (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Bacchus W, Aubel D, Fussenegger M, Biomedically relevant circuit-design strategies in mammalian synthetic biology, Molecular Systems Biology 9(1) (2013) 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Polstein LR, Gersbach CA, Light-Inducible Spatiotemporal Control of Gene Activation by Customizable Zinc Finger Transcription Factors, Journal of the American Chemical Society 134(40) (2012) 16480–16483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Liu L, Huang W, Huang J-D, Synthetic circuits that process multiple light and chemical signal inputs, BMC Systems Biology 11(1) (2017) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Kumar MP, Du J, Lagoudas G, Jiao Y, Sawyer A, Drummond DC, Lauffenburger DA, Raue A, Analysis of Single-Cell RNA-Seq Identifies Cell-Cell Communication Associated with Tumor Characteristics, Cell Rep 25(6) (2018) 1458–1468 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Stone SE, Glenn WS, Hamblin GD, Tirrell DA, Cell-selective proteomics for biological discovery, Curr Opin Chem Biol 36 (2017) 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Tape CJ, Ling S, Dimitriadi M, McMahon KM, Worboys JD, Leong HS, Norrie IC, Miller CJ, Poulogiannis G, Lauffenburger DA, Jorgensen C, Oncogenic KRAS Regulates Tumor Cell Signaling via Stromal Reciprocation, Cell 165(7) (2016) 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Loebel C, Mauck RL, Burdick JA, Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels, Nat Mater 18(8) (2019) 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Valdez J, Cook CD, Ahrens CC, Wang AJ, Brown A, Kumar M, Stockdale L, Rothenberg D, Renggli K, Gordon E, Lauffenburger D, White F, Griffith L, On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks, Biomaterials 130 (2017) 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Gilchrist AE, Harley BAC, Connecting secretome to hematopoietic stem cell phenotype shifts in an engineered bone marrow niche, bioRxiv (2020) 2020.01.19.911800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Wang L-S, Lee F, Lim J, Du C, Wan ACA, Lee SS, Kurisawa M, Enzymatic conjugation of a bioactive peptide into an injectable hyaluronic acid-tyramine hydrogel system to promote the formation of functional vasculature, Acta Biomaterialia 10(6) (2014) 2539–2550. [DOI] [PubMed] [Google Scholar]


