Abstract
Objectives
Coronavirus disease-19 (COVID-19) has spread worldwide and poses various challenges to healthcare services. The limited supply of medical and personal-protective equipment has affected the ability of many countries to respond to the crisis. Three-dimensional printing (3DP) is well suited to addressing these shortages. We assessed the medical role of 3DP during the COVID-19 outbreak in hospitals in France.
Design
Retrospective survey.
Setting and intervention
We included and questioned all French level-1 and -2 COVID-certified centers.
Participants
One hundred and thirty-eight COVID-certified centers were contacted across France: 38 (27.5 %) level 1 and 100 (72.5 %) level 2 centers. The analysis focused on 133 centers (96.37 %), among which 98 (73.68 %) used 3DP.
Main outcome measures
The primary endpoint was the number of pieces printed in 3D. The secondary endpoints were the mode, type, and benefits of 3DP.
Results
The total number of pieces printed in 3D nationwide was 84,886: 76,000 pieces of individual protective equipment (IPE) (89.53 %), 6335 pieces of biomedical equipment (7.47 %), and 2551 prototypes (3.01 %). In 91 cases (92.85 %), 3DP was performed using external printers. The pieces 3D-printed by the various centers helped around 6109 patients and protected around 41,091 caregivers.
Conclusions
3DP produced more than 84,000 pieces at 98 centers, helped more than 6000 patients, and protected more than 41,000 caregivers. Therefore, 3DP played a major role in medical aid during the COVID-19 outbreak in France.
Keywords: COVID-19, SARS COV-2, 3D printing, Additive manufacturing, Survey, France
1. Introduction
The coronavirus disease-19 (COVID-19) pandemic is thought to have originated in Wuhan, China, and has spread to 192 countries and administrative regions. As of 31 March 2020, it had infected nearly 800,000 individuals of all ages [1].
In France, up to June 9, 2020, the epidemic had affected 154,591 people, with 102,863 hospitalizations, and was responsible for 29,296 deaths, including 18,912 in hospital [2].
The worldwide spread of COVID-19 poses challenges to healthcare resources in every affected region [3]. Basic measures such as hand washing and social distancing are crucial. However, according to World Health Organization guidelines [1], protective materials are essential for all healthcare providers. The limited supply of N95 respirator masks, face shields, ventilator valves, testing kits and other personal protective equipment (PPE) [4], [5] has affected the ability of numerous countries to respond to the crisis.
Three-dimensional printing (3DP), a novel and innovative technology used to manufacture complex objects, is well suited to addressing these shortages [4], [6], [7], [8], [9]. 3DP is an essential technique in all specialties of medicine [10], [11], as well as for the manufacture of PPE [12], [13], [14], [15]. Tino described the potential of 3DP in the current outbreak [9], but no article that enables assessment of the impact, need, and solutions to increase access to 3DP based on precise nationwide data has been published. We assessed the medical role of 3DP during the COVID-19 outbreak in French hospitals.
2. Materials and methods
We analyzed all French level-1 and -2 COVID-certified centers, a list of which was provided by the French Directorate General for Health. These centers were contacted by telephone or email with a questionnaire regarding the use of 3DP in the center.
The primary endpoint was the number of 3DP pieces printed for managing COVID-19 in the level-1 and -2 COVID-certified centers. The secondary endpoints were:
-
•
In centers that used 3DP: number of printers, position of the individual responsible for introducing 3DP, and number of departments benefiting from 3DP.
-
•
Number of centers that did not use 3DP, and why.
-
•
Uses of 3DP, evaluated by the type of printed pieces: individual protective equipment (IPE), biomedical, and prototypes.
-
•
Ownership of the 3DP (external, purchase, the center).
-
•
The mode of introduction of 3DP (investment or donation).
-
•
The date of introduction of 3DP.
-
•
The benefits of 3DP, evaluated by estimating the number of patients and caregivers for whom 3DP was used.
-
•
The subjective opinion of users of the role of 3DP in this epidemic (major, moderate, or minor) and their intention to reuse 3DP if the situation were to recur.
-
•
Media coverage of use of 3DP, evaluated by the number of press articles.
We excluded centers that did not respond and those that did not care for COVID-19 patients. Statistical analysis was performed using Microsoft Excel software.
3. Results
3.1. Primary endpoint
The total number of 3DP pieces used in the management of COVID-19 was 84,886.
3.2. Centers that used 3DP
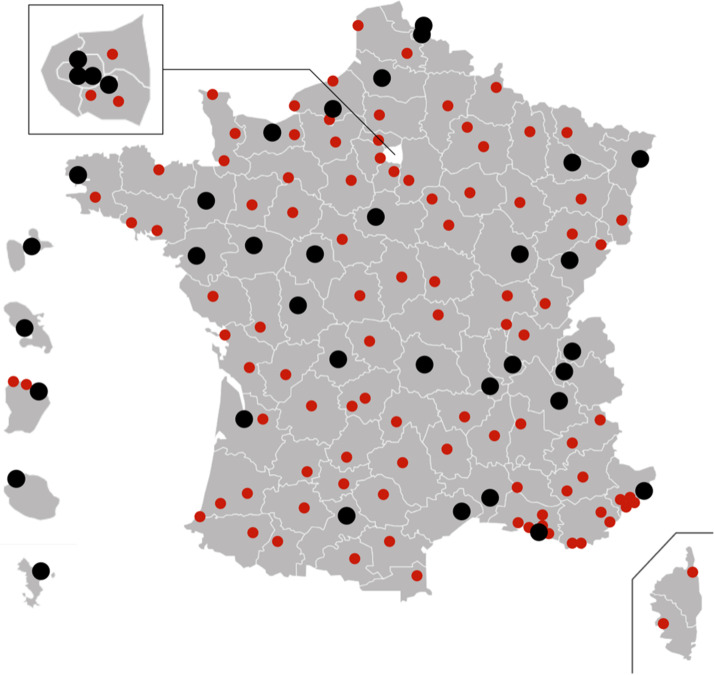
One hundred and thirty-eight COVID-certified centers across France were contacted: 38 (27.5 %) level-1 centers and 100 (72.5 %) level-2 centers (Fig. 1 ). Four centers (2.9 %) did not respond to the survey and were excluded. One center that did not support COVID patients was also excluded. The final analysis therefore focused on 133 centers (96.37 %). Of these 133 centers, 98 (73.68 %) used 3DP to help manage COVID-19, including 29 level-1 centers (76.3 %) and 69 level-2 centers (69 %).
Fig. 1.
Map of France, Corsica, and French Overseas Departments with the locations of level-1 (black dots) and level-2 (red dots) COVID-certified centers.
The proposal to use 3DP frequently originated outside the center (n = 24, 24.49 %). The individuals responsible for introducing 3DP were physicians (n = 21, 21.42 %), Center Directors (n = 10, 10.20 %), and hygiene/pharmacy departments (n = 8, 8.16 %) (Table 1 ). In each center, 3DP was used in an average of 14.2 departments (1–400; 44.96).
Table 1.
Origins and individuals responsible for the introduction of 3DP.
| n (%) | |
|---|---|
| External proposal | 24 (24.49 %) |
| Doctor or resident | 21(21.42 %) |
| Center director | 10 (10.20 %) |
| Hygiene and pharmacy departments | 8 (8.16 %) |
| Medical commission president | 6 (6.12 %) |
| Head of department | 6 (6.12 %) |
| Nurse | 6 (6.12 %) |
| Logistic/research & development departments | 5 (5.10 %) |
| Crisis staff | 3 (3.06 %) |
| Unknown | 5 (5.10 %) |
| Local elected people | 3 (3.06 %) |
| Informatics department | 1 (1.02 %) |
| Total | 98 (100 %) |
3.3. Centers that did not use 3DP
Thirty-five centers (26.31 %) did not use 3DP during the COVID-19 pandemic. Thirteen (37.14 %) of the centers did not need 3DP (no lack of equipment, small number of patients), 9 (25.71 %) were not offered 3DP (but were aware of it), 7 (20 %) refused to use non-certified material, 4 (11.42 %) were unaware of 3DP, and 2 (5.71 %) did not have access to a ‘maker’, i.e. a 3D printing manufacturer.
3.4. Type, mode, and date of introduction of 3DP
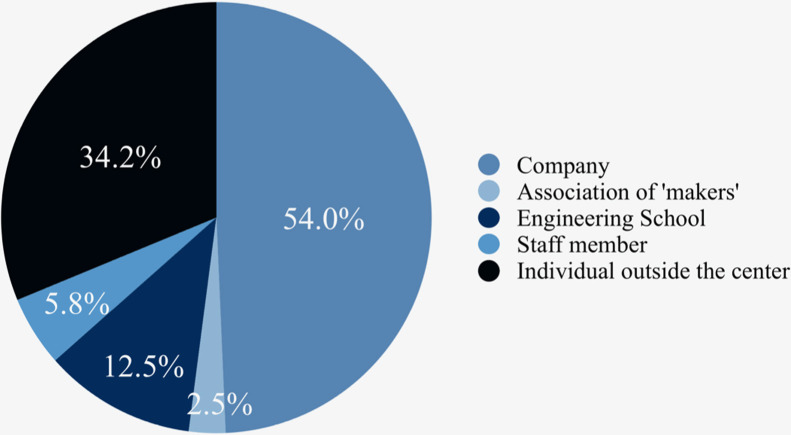
External printers were used in 91 centers (92.85 %) (Fig. 2 ). These external printers belonged in 54 % of cases to a company, in 34.2 % of cases to an individual outside the center, in 12.5 % of cases to an Engineering School, in 5.8 % of cases to a staff member, and in 2.5 % of cases to an association of makers.
Fig. 2.
Origins of 3D printers outside the centers.
Seven centers (7.15 %), owned 3D printers. Of them, five centers (5.1 %) owned them before the COVID-19 crisis, and two (2.04 %) acquired them to manage COVID-19, using donations.
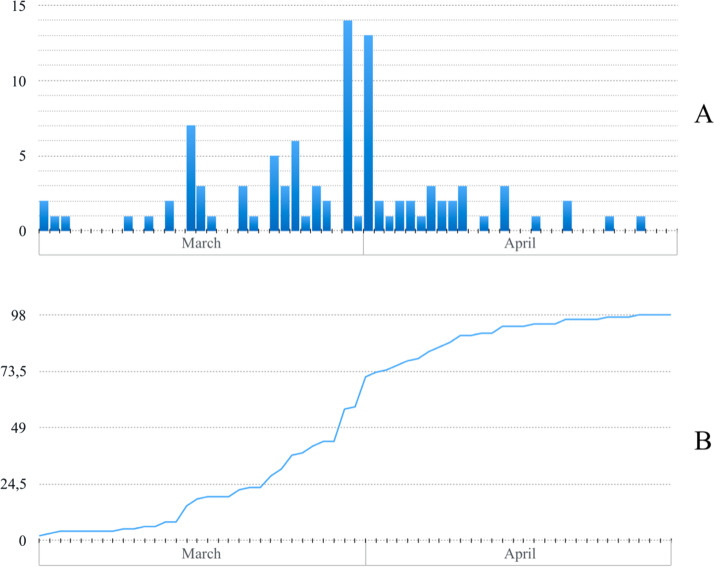
All the centers that used 3DP introduced it between March and April 2020. Fig. 3 shows the precise date of the decision to introduce 3DP (A), as well as the number of centers in which it was introduced (B). On March 23, March 30, April 03, and April 27, 25 %, 50 %, 75 % and 100 % respectively of the centers had introduced 3DP.
Fig. 3.
Numbers of centers introducing 3DP in March and April 2020 (A), and a running total (B).
3.5. Mode of use
Each center that used 3DP printed an average of 2.3 (1–47; 4.80) different types of pieces. Among the 84,886 pieces printed in 3D, there were 76,000 pieces of IPE (89.53 %), 6335 pieces of biomedical equipment (7.47 %), and 2551 prototypes (3.01 %) (Table 2 ).
Table 2.
Types and numbers of 3D-printed pieces.
| Type of piece | n (%) |
|---|---|
| Individual protective equipment | 76,000 (89.53 %) |
| Visors / supports | 67,485 (79.50 %) |
| Door handles | 5015 (5.91 %) |
| Glasses | 3500 (4.12 %) |
| Biomedical | 6335 (7.46 %) |
| EasyBreath® mask adapters | 3516 (4.14 %) |
| Suction cannula adapters | 2135 (2.51 %) |
| Maintenance objects | 500 (0.59 %) |
| Swabs | 100 (0.12 %) |
| Breathing tubes holders | 42 (0.05 %) |
| Bed frames | 20 (0.02 %) |
| Others | 22 (0.02 %) |
| Prototypes | 2551 (3.01 %) |
| Total | 84,886 (100 %) |
The pieces of IPE printed in 3D were visors (n = 67,485; 79.50 %); the main 3D-printed biomedical pieces were adapters for EasyBreath® masks (n = 3516; 4.14 %) and suction cannula adapters (n = 2135; 2.51 %).
3.6. Benefits and user reviews
According to our survey estimates, pieces printed in 3D by the various centers helped around 6109 patients and protected around 41,091 caregivers across France during the COVID-19 crisis.
Of the 98 centers that used 3DP, it played a major role in management of COVID-19 in 50 (51.02 %), a moderate role in 40 (40.81 %), and a minor role in 8 (8.17 %). Almost all (n = 97; 98.97 %) of the centers that used 3DP said they would use it again if the situation recurs.
3.7. Mediatization
The use of 3DP by French centers was the subject of 1223 press articles. Among them were 432 (35.32 %) articles in local print media, 395 (32.30 %) in regional print media, 62 (5.07 %) in national print media, and 334 (27.31 %) on the Internet.
4. Discussion
We reviewed the use of 3DP in France during the COVID-19 crisis. More than 96 % of the solicited COVID-certified centers (138 centers) responded to the survey. This implies the enthusiasm of medical staff for spreading 3DP utilization. Other hospitals and long-term care centers for the elderly also used 3DP, but we focused on COVID level-1 and -2 certified centers. We did not consider the 3DP materials used by other public bodies, such as police departments or schools.
Furthermore, the list of printed materials may have been incomplete, because other medical units might have used 3DP for COVID patients. Therefore, we may have underestimated the use of 3DP in France in early 2020.
Some of the materials were made from a single printed mold, making 3DP a step in the production process, and preventing us from quantifying its influence. Some 3D-printed objects were imported (Germany or China), and others were ordered by individual caregivers outside the hospital administrative channel or donated. Those were not considered.
The survey was limited by the fact that in some centers, pieces were printed in 3D but were not used as much as possible because of a lack of certification (see below) or effectiveness.
3DP is useful in two types of applications. First is the mass production of simple objects in a period of supply shortage and critical financial difficulty. This concerns IPE, which accounted for 89.53 % of the pieces in the survey. Most 3D-printed pieces used for COVID-19 were quick-fix solutions, often based on open-source prototypes or meeting basic requirements. Unfortunately, the emergency context prevented teams from evaluating more complex designs. However, 2551 prototypes were designed, sometimes without cooperation between centers, demonstrating the ease of use of 3DP, as well as the willingness of caregivers to innovate. This rendered healthcare facilities independent of the supply chain and provided unlimited pieces at low cost.
The second application is improvement or modification of an extant device using 3D-printed accessories, for repurposing or compatibility with a reprocessed object. Such pieces are typically not commercially available because they are developed locally for a specific purpose. An example is the use of 3D-printed connectors to adapt Easybreath® masks to artificial respirators (3516, 4.14 %).
The integration of 3DP devices in medical centers could also allow rapid development of innovative healthcare materials. Most internal requests for 3DP objects were from medical staff (21.42 %); therefore, caregivers are interested in owning a 3DP without having to approach corporations or negotiate a budget.
3DP has shown caregivers a new way of working. That is, not by means of an industry-caregiver relationship (typically one-sided), but an engineer-caregiver relationship (necessarily bilateral). 3DP bridges these two professions, bypassing intermediaries; this enables an idea to progress to a physical design, and modifications can be made immediately if necessary.
Only 9.62 % of French COVID centers did not express a need for 3DP during the crisis, but 16.69 % did not have access to 3DP, although they would have benefitted from it. More than half of the 3DP equipment used in France was provided by corporations; only 12 % of the printed items were produced by a 3D printer owned by the center. Thus, in this period of crisis, the safety of caregivers and their medical efficiency has relied on corporations and non-medical individuals, an unsatisfactory situation despite the solidarity shown by corporations small and large. More than 98 % of the centers were prepared to use 3DP again, and 3DP played major and moderate roles in COVID crisis management in 51.02 % and 40.81 % of the centers, respectively. Equipping medical facilities with 3DP technology would drive innovation in medicine.
Because the 3D-printed objects were produced rapidly, most had no European Conformity (EC) certification and were not tested or evaluated before being used by caregivers. One of the centers could not obtain administrative approval because of the lack of certification. In the other centers, this requirement was set aside for reasons of equipment shortages and the emergency situation. However, in non-crisis situations, in which funding is inadequate and supply insufficient, the need for medical equipment could be met by 3DP, if some administrative adjustments were made. Healthcare facilities could be reinforced by engineering teams under the authority of a national 3DP department working in conjunction with the French scientific committee. A dialogue could be opened with local medical departments each time 3DP is needed or 3DP products need to be tested or validated. A simplified validation process could be implemented in certain situations, although a thorough evaluation of the potential risks will typically be required.
The development of 3DP has been accelerated by crowdsourcing; e.g., the numerous open-source models in the COVID literature [9], [12], [13], [14]. 3DP is used in medicine in situations in which time, money, precision and reliability matter. It renders medical teams independent of the supply chain and is useful for research as well as patient care. Its main limitation lies in the validation of effectiveness and safety. The French healthcare system follows strict recommendations on medical material, submitted to EC certification. EC certification is manufacturers’ responsibility, but it requires time, effort, and specific knowledge that medical teams may well lack. This may be why 3DP has not yet been introduced in many healthcare facilities. In May 2020, the French National agency for drug safety exceptionally approved the use of non-validated products during the COVID-19 crisis, if the need was justified and supply was unavailable. This shows our ability to address critical situations with adapted measures. However, simplified procedures could have accelerated the availability of standardized and thoroughly tested equipment pieces and promoted their efficient distribution.
5. Conclusions
With more than 84,000 pieces printed by 98 centers, 3D printing helped more than 6000 patients and protected more than 41,000 caregivers. Almost all (98.9 %) of the centers that used it said they are ready to use it again if the situation should reoccur.
3DP played a major medical role during the COVID-19 outbreak in France. The COVID-19 crisis and the resulting supply shortages have facilitated innovation to find short-term solutions for caregiver protection and patient care. A reliable and safe means of introducing 3DP in medical facilities needs to be identified.
Transparency declaration
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Grant/funding
The authors declare no funding source.
Declaration of Competing Interest
All authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
The authors thank all those who responded to the survey and who contributed to the study.
References
- 1.World Health Organization . 2020. When and how to use masks. Coronavirus Dis. COVID-19 Advice Public Use Masks.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks (Accessed 26 April 2020) [Google Scholar]
- 2.SPF Santé Publique France. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/articles/infection-au-nouveau-coronavirus-sars-cov-2-covid-19-france-et-monde (Accessed 10 June 2020)
- 3.Shereen M.A., Khan S., Kazmi A., et al. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishack S., Lipner S.R. Applications of 3D printing technology to address COVID-19 related supply shortages. Am J Med. 2020;0 doi: 10.1016/j.amjmed.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages - the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020 doi: 10.1056/NEJMp2006141. Published Online First: 25 March 2020. [DOI] [PubMed] [Google Scholar]
- 6.Belhouideg S. Impact of 3D printed medical equipment on the management of the Covid19 pandemic. Int J Health Plann Manage. 2020 doi: 10.1002/hpm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox J.L., Koepsell S.A. 3D-printing to address COVID-19 testing supply shortages. Lab Med. 2020 doi: 10.1093/labmed/lmaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maracaja L., Blitz D., Maracaja D.L.V., et al. How 3D printing can prevent spread of COVID-19 among healthcare professionals during times of critical shortage of protective personal equipment. J Cardiothorac Vasc Anesth. 2020 doi: 10.1053/j.jvca.2020.04.004. Published Online First: 13 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tino R., Moore R., Antoline S., et al. COVID-19 and the role of 3D printing in medicine. 3D Print Med. 2020;6:11. doi: 10.1186/s41205-020-00064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aimar A., Palermo A., Innocenti B. The role of 3D printing in medical applications: a state of the art. J Healthc Eng. 2019 doi: 10.1155/2019/5340616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durfee W.K., Iaizzo P.A. In: Engineering in medicine. Iaizzo P.A., editor. Academic Press; 2019. Chapter 21 - medical applications of 3D printing.http://www.sciencedirect.com/science/article/pii/B978012813068100021X 527–. [Google Scholar]
- 12.Erickson M.M., Richardson E.S., Hernandez N.M., et al. Helmet modification to PPE with 3D printing during the COVID-19 pandemic at duke university medical center: a novel technique. J Arthroplasty. 2020 doi: 10.1016/j.arth.2020.04.035. Published Online First: 18 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swennen G.R.J., Pottel L., Haers P.E. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 doi: 10.1016/j.ijom.2020.03.015. Published Online First: 2 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessop Z.M., Dobbs T.D., Ali S.R., et al. Personal protective equipment (PPE) for surgeons during COVID-19 pandemic: a systematic review of availability, usage, and rationing: CUTTING EDGE REVIEW : british journal of surgery. Br J Surg. 2020 doi: 10.1002/bjs.11750. Published Online First: 12 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vordos N., Gkika D.A., Maliaris G., et al. How 3D printing and social media tackles the PPE shortage during Covid – 19 pandemic. Saf Sci. 2020;130 doi: 10.1016/j.ssci.2020.104870. [DOI] [PMC free article] [PubMed] [Google Scholar]