Abstract
Background
Lung cancer is the leading cause of cancer‐related deaths. Although epidermal growth factor receptor tyrosine kinase inhibitors (EGFR‐TKIs) are effective for advanced non‐small cell lung cancer (NSCLC) harboring EGFR mutations, some patients experience little or no response. The Glasgow prognostic score (GPS) is an inflammation‐related score based on C‐reactive protein (CRP) and albumin concentrations, and has prognostic value in various cancer settings. This study aimed to evaluate whether GPS could predict response of NSCLC to EGFR‐TKIs.
Methods
This retrospective multicenter study evaluated patients with NSCLC harboring EGFR mutations who received EGFR‐TKI monotherapy from October 2006 to December 2016. GPS values were determined using CRP and albumin concentrations from before initiation of EGFR‐TKIs. The Kaplan‐Meier method and Cox proportional hazard models were used to evaluate progression‐free survival (PFS) and overall survival (OS).
Results
In 214 patients, 141, 43, and two patients had GPS values of 0, 1, and 2, respectively. The GPS independently predicted the efficacy of EGFR‐TKIs; good GPS (0–1) conferred significantly better PFS (hazard ratio [HR]: 0.59, 95% confidence interval [CI]: 0.38–0.96, P = 0.03) and OS (HR: 0.56, 95% CI: 0.33–0.96, P = 0.03). Multivariate analysis confirmed that a good GPS (0–1) independently predicted good PFS and OS among patients who had PS of 0–1. Good GPS (0–1) independently predicted good OS among patients receiving treatment in first‐line settings.
Conclusions
The GPS independently predicted the efficacy of EGFR‐TKIs for EGFR‐mutated NSCLC; however, further studies are needed to validate our findings.
Key points
Significant findings of the study
Glasgow prognostic score (GPS) independently predicted the efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR‐TKIs) treatment for EGFR‐mutated NSCLC.
What this study adds
The findings presented in this paper will help to identify patients who will be expected to experience limited or no response to EGFR‐TKI treatment by using GPS.
Keywords: Biomarker, EGFR‐TKI, Glasgow prognostic score, non‐small cell lung cancer
Introduction
Lung cancer is the global leading cause of cancer‐related mortality, and non‐small cell lung cancer (NSCLC) accounts for >80% of lung cancers. 1 , 2 Epidermal growth factor receptor (EGFR) mutations are key driver mutations in NSCLC, and patients with these mutations can be effectively treated using EGFR tyrosine kinase inhibitors (EGFR‐TKIs), such as gefitinib, erlotinib, afatinib, and osimertinib. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Clinical studies have indicated that EGFR‐TKI treatment provided an overall response rate (ORR) of 60%–80%, although approximately 10% of patients will have progressive disease. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 In addition, recent studies have demonstrated that immune checkpoint inhibitors had low efficacy in patients with EGFR mutations. 11 , 12 Therefore, it is important to identify patients who are expected to experience limited or no response to EGFR‐TKI treatment.
Most patients with NSCLC are diagnosed at an advanced or inoperable stage, with these cases often involving weight loss and a systemic inflammatory response, which reflects cancer cachexia. 13 , 14 The Glasgow prognostic score (GPS) is a systemic inflammation‐based scoring system that combines serum concentrations of C‐reactive protein (CRP) and albumin used as markers. 13 , 14 In this context, CRP is a nonspecific inflammation marker that can also be used to identify a poor nutritional status and a risk of poor overall survival, 15 , 16 , 17 while albumin is a nutritional marker that is inversely correlated with CRP. 18 The GPS is reportedly a better prognostic factor than performance status among patients with NSCLC, 19 and the GPS can be used to predict overall survival (OS) among patients receiving chemotherapy for NSCLC. 20 , 21 , 22 , 23 However, there is limited information regarding the association between the GPS and the response to EGFR‐TKI treatment. Therefore, this study evaluated whether the GPS could predict the response to EGFR‐TKI treatment in patients with EGFR‐mutated NSCLC.
Methods
Patients
This retrospective study protocol was approved by the institutional review board of Gunma University Hospital (no. 1528), and complied with the Declaration of Helsinki. The requirement for informed consent was waived based on the retrospective design. This study evaluated consecutive patients with histologically or cytologically diagnosed NSCLC, who harbored EGFR mutations and started EGFR‐TKI monotherapy between October 2006 and December 2016 at the Gunma University Hospital, Gunma Prefectural Cancer Center, and Hidaka Hospital.
Pretreatment values were used for the CRP and albumin concentrations, and the GPS values were defined as a GPS of 0 (CRP of <10 mg/L and albumin of >35 g/L), a GPS of 1 (CRP of ≥10 mg/L or albumin of <35 g/L), or a GPS of 2 (CRP of >10 mg/L and albumin of <35 g/L). For the present study, we defined gefitinib and erlotinib as first generation EGFR‐TKIs, and afatinib as a second generation EGFR‐TKI.
Adverse events were graded using the Common Terminology Criteria for Adverse Events (version 4.0), and the objective tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1). 24 The median follow‐up duration for censored cases was 30.2 months (range: 0.4–97.2 months).
Statistical analysis
Continuous variables were analyzed using the Student's t‐test and categorical variables were analyzed using the χ2 test. The relationships between different variables or matched pairs were examined using the nonparametric Spearman rank test or Wilcoxon signed‐rank test, as appropriate. The Kaplan‐Meier method and log‐rank test were used to compare the survival outcomes. The PFS interval was calculated from the start of EGFR‐TKI treatment to the first instance of disease relapse, death, or the last follow‐up visit (in cases with no evidence of relapse). The OS interval was calculated from the start of EGFR‐TKI treatment to death from any cause. Univariate and multivariate analyses of survival were also performed using Cox proportional hazard models. Differences were considered statistically significant at P‐values of <0.05, and all analyses were performed using JMP Pro software (version 12.0; SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
A total of 238 advanced NSCLC patients who had EGFR mutations were screened between October 2006 and December 2016. Among them, 10 patients had not received EGFR‐TKI monotherapy and pretreatment albumin, and CRP values were missing in seven patients each. Therefore, 214 patients were included for further analysis (Fig 1). The characteristics of the 214 patients (83 males and 131 females) are shown in Table 1. The median age at the start of EGFR‐TKI treatment was 69 years (range: 39–92 years) and 76 patients were smokers. The Eastern Cooperative Oncology Group performance status (ECOG PS) scores were 0–1 for 168 patients, two for 33 patients, three for eight patients, and four for five patients. The predominant histological type was adenocarcinoma (212 patients), although one patient had poorly differentiated carcinoma and one patient had the “not otherwise specified” histological type. The EGFR mutation subtypes were exon 19 deletions (107 patients), L858R (98 patients), and various other mutations (six patients had G719X, two patients had L861Q, and one patient had an exon 19 deletion plus L858R). Three patients had de novo T790M mutations and 17 patients developed T790M mutations during their treatment course. All patients received EGFR‐TKI monotherapy, which included gefitinib (144 patients), erlotinib (33 patients), and afatinib (37 patients). The GPS values at the start of EGFR‐TKI treatment were 0 (141 patients), one (43 patients), and two (30 patients). Box plots showing the CRP, albumin, and body mass index distributions in the three GPS groups are shown in Fig 2. The ECOG‐PS and GPS values were significantly correlated (P < 0.01).
Figure 1.
Flow chart of patient enrollment.
Table 1.
Patient characteristics
| All patients | GPS score | |||||
|---|---|---|---|---|---|---|
| n = 214 | 0 (n = 141) | 1 (n = 43) | 2 (n = 30) | P‐value | ||
| Age | <65 years/≥65 years | 62/152 | 44/97 | 10/33 | 8/22 | 0.57 |
| Sex | Male/Female | 83/131 | 51/90 | 18/25 | 14/16 | 0.50 |
| Stage | I/II/III/IV | 23/5/26/160 | 16/4/21/100 | 5/0/3/35 | 2/1/2/25 | 0.51 |
| Smoking status | Ever/never | 76/138 | 45/96 | 17/26 | 14/16 | 0.25 |
| ECOG‐PS | 0–1/2/3/4 | 168/33/8/5 | 126/12/2/1 | 30/10/2/1 | 12/11/4/3 | <0.01 |
| Histology | Ad/non‐Ad | 212/2 | 140/1 | 43/0 | 29/1 | 0.30 |
| EGFR mutation | Ex19del/L858R/other | 107/98/9 | 76/61/4 | 19/21/3 | 12/16/2 | 0.43 |
| T790M mutation | Positive/negative or unknown | 17/197 | 13/128 | 4/39 | 0/30 | 0.22 |
| EGFR‐TKI | Gefitinib/erlotinib/afatinib | 144/33/37 | 92/20/29 | 27/10/6 | 25/3/2 | 0.14 |
| Line of EGFR‐TKI | 1/2/≥3 | 163/41/10 | 109/26/6 | 31/8/4 | 23/7/0 | 0.42 |
Ad, adenocarcinoma; ECOG‐PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; GPS, Glasgow prognostic score; TKI, tyrosine kinase inhibitor.
Figure 2.
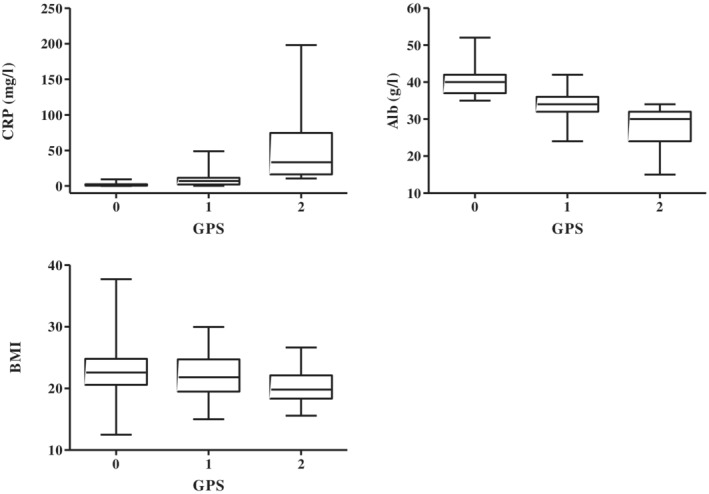
Box plot of the C‐reactive protein and albumin distributions in the Glasgow prognostic score groups.
Survival analysis
The overall median PFS was 11.3 months (95% confidence interval [CI]: 1.2–40.2 months) and 190 patients experienced disease progression. Table 2 shows the results of the univariate and multivariate analyses of PFS, which revealed significant associations with ECOG‐PS, type of EGFR‐TKI, and GPS. Multivariate analyses revealed that poor PFS was associated with a high ECOG‐PS (hazard ratio [HR]: 2.22, P < 0.01), first generation EGFR‐TKI treatment (HR: 1.55, P = 0.03), and a GPS of 2 (HR 1.66, P = 0.03). The Kaplan‐Meier curves for PFS are shown in Fig 3; a GPS of 0–1 was associated with significantly longer PFS than a GPS of 2 (P < 0.01).
Table 2.
Univariate and multivariate analyses of progression‐free and overall survival
| Characteristic | Subgroups | HR for PFS (95% CI) | HR for OS (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | P‐value | Multivariate | P‐value | Univariate | P‐value | Multivariate | P‐value | ||
| Age | <65 years/≥65 years | 1.16 (0.84–1.58) | 0.33 | 0.86 (0.58–1.23) | 0.41 | ||||
| Sex | Male/Female | 1.15 (0.85–1.54) | 0.33 | 1.42 (1.00–1.99) | 0.04 | 1.08 (0.71–1.64) | 0.68 | ||
| Smoking status | Ever/never | 1.12 (0.82–1.51) | 0.44 | 1.53 (1.08–2.15) | 0.01 | 1.59 (1.04–2.43) | 0.03 | ||
| ECOG‐PS | 2–4/0–1 | 2.82 (1.96–3.98) | <0.01 | 2.22 (1.48–3.28) | <0.01 | 4.12 (2.75–6.07) | <0.01 | 3.46 (2.15–5.47) | <0.01 |
| Line of EGFR‐TKI | 1/≥2 | 0.97 (0.70–1.36) | 0.87 | 0.94 (0.65–1.39) | 0.76 | ||||
| EGFR‐TKI | Gefitinib or erlotinib/afatinib | 1.75 (1.17–2.74) | <0.01 | 1.55 (1.03–2.43) | 0.03 | 1.75 (1.04–3.20) | 0.03 | 1.39 (0.81–2.57) | 0.23 |
| EGFR mutation | Ex19del or L858R/other | 0.55 (0.30–1.17) | 0.11 | 0.46 (0.23–1.11) | 0.08 | ||||
| T790M mutation | Yes/No or unknown | 0.86 (0.50–1.38) | 0.56 | 0.42 (0.19–0.81) | <0.01 | 0.54 (0.24–1.06) | 0.07 | ||
| GPS score | 2/0–1 | 2.44 (1.58–3.63) | <0.01 | 1.66 (1.03–2.61) | 0.03 | 3.59 (2.22–5.60) | <0.01 | 1.77 (1.03–2.97) | 0.03 |
CI, confidence interval; ECOG‐PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; GPS, Glasgow prognostic score; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival; TKI, tyrosine kinase inhibitor.
Figure 3.
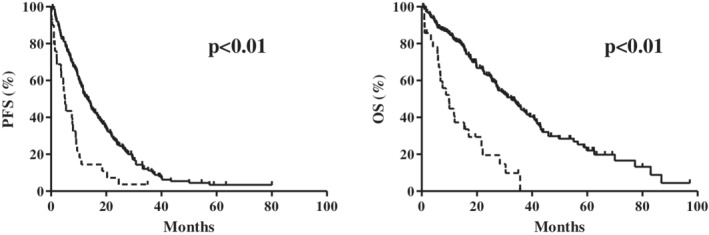
Kaplan‐Meier curves for (a) progression‐free survival (PFS) ( ) 0–1, (
) 0–1, ( ) 2 and (b) overall survival (OS) according to the Glasgow prognostic score (GPS) (
) 2 and (b) overall survival (OS) according to the Glasgow prognostic score (GPS) ( ) 0–1, (
) 0–1, ( ) 2. Solid lines indicate a post‐treatment GPS of 0–1 and dashed lines indicate a post‐treatment GPS of 2.
) 2. Solid lines indicate a post‐treatment GPS of 0–1 and dashed lines indicate a post‐treatment GPS of 2.
The median OS in all patients was 27.8 months (95% CI: 2.4–85.0 months) and 137 patients ultimately died. Table 2 shows the results of the univariate and multivariate analyses of OS, which revealed significant associations with sex, smoking status, ECOG‐PS, type of EGFR‐TKI, T790M status, and the GPS. Multivariate analyses revealed that poor OS was associated with past smoking (HR: 1.59, P = 0.03), a high ECOG‐PS (HR: 3.46, P < 0.01), and a GPS of 2 (HR: 1.77, P = 0.03) (Table 2). The Kaplan‐Meier curves for OS are shown in Fig 3; a GPS of 0–1 was associated with significantly longer OS than a GPS of 2 (P < 0.01).
Among patients with an ECOG‐PS of 0–1, multivariate analysis revealed that a poor PFS was associated with first generation EGFR‐TKIs (HR: 1.69, P = 0.01) and a GPS of 2 (HR: 2.51, P = 0.01) (Table 3). Univariate analyses revealed that OS was associated with smoking, T790M status, and GPS. Multivariate analysis revealed that poor OS was independently associated with past smoking (HR: 1.61, P = 0.02) and a GPS of 2 (HR: 3.00, P < 0.01) (Table 3). There were no independent predictors of PFS or OS among patients with an ECOG‐PS of ≥2 (Table S1).
Table 3.
Univariate and multivariate analyses of progression‐free and overall survival for performance status of 0–1
| Characteristic | Subgroups | HR for PFS (95% CI) | HR for OS (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | P‐value | Multivariate | P‐value | Univariate | P‐value | Multivariate | P‐value | ||
| Age | <65 years/≥65 years | 1.20 (0.83–1.70) | 0.30 | 0.84 (0.53–1.28) | 0.43 | ||||
| Sex | Male/Female | 1.02 (0.72–1.42) | 0.89 | 1.33 (0.88–2.00) | 0.16 | ||||
| Smoking status | Ever/never | 1.10 (0.78–1.54) | 0.56 | 1.70 (1.13–2.55) | 0.01 | 1.61 (1.07–2.42) | 0.02 | ||
| Line of EGFR‐TKI | 1/≥2 | 1.08 (0.73–1.63) | 0.69 | 1.11 (0.69–1.84) | 0.66 | ||||
| EGFR‐TKI | Gefitinib or erlotinib/afatinib | 1.59 (1.04–2.56) | 0.03 | 1.69 (1.10–2.73) | 0.01 | 1.45 (0.81–2.83) | 0.21 | ||
| EGFR mutation | Ex19del or L858R/other | 0.55 (0.27–1.31) | 0.16 | 0.46 (0.20–1.33) | 0.14 | ||||
| T790M mutation | Yes/no or unknown | 1.03 (0.59–1.69) | 0.88 | 0.47 (0.19–0.95) | 0.03 | 0.52 (0.21–1.05) | 0.07 | ||
| GPS score | 2/0–1 | 2.24 (1.13–3.98) | 0.02 | 2.51 (1.26–4.49) | 0.01 | 3.42 (1.58–6.58) | <0.01 | 3.00 (1.38–5.78) | <0.01 |
CI, confidence interval; EGFR, epidermal growth factor receptor; GPS, Glasgow prognostic score; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival; TKI, tyrosine kinase inhibitor.
Among patients who were treated in the first‐line setting, univariate analysis revealed that PFS was associated with ECOG‐PS, type of EGFR‐TKI, and GPS. Multivariate analysis revealed that poor PFS was independently associated with ECOG‐PS of ≥2 (HR: 2.58, P < 0.01), and first generation EGFR‐TKIs (HR: 1.65, P = 0.01) (Table 4). Univariate analysis revealed that OS was associated with ECOG‐PS, type of EGFR‐TKI, T790M status, and GPS. Multivariate analysis revealed that poor OS was independently associated with an ECOG‐PS of ≥2 (HR: 3.31, P < 0.01), and a GPS of 2 (HR: 2.24, P = 0.01) (Table 4). Among patients who were treated beyond the second‐line, only ECOG‐PS ≥2 was associated with poor PFS (HR: 0.43, P = 0.01) (Table S2). Univariate analysis revealed that OS was associated with sex, smoking, ECOG‐PS, and type of EGFR mutation. Multivariate analysis revealed that only ECOG‐PS ≥2 was associated with poor OS (HR: 3.98, P < 0.01) (Table S2).
Table 4.
Univariate and multivariate analyses of progression‐free and overall survival with first‐line setting
| Characteristic | Subgroups | HR for PFS (95% CI) | HR for OS (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | P‐value | Multivariate | P‐value | Univariate | P‐value | Multivariate | P‐value | ||
| Age | <65/≥65 years | 1.09 (0.74–1.56) | 0.64 | 0.82 (0.51–1.28) | 0.40 | ||||
| Sex | Male/Female | 1.02 (0.72–1.43) | 0.89 | 1.17 (0.77–1.75) | 0.44 | ||||
| Smoking status | Ever/never | 1.19 (0.83–1.68) | 0.33 | 1.40 (0.93–2.09) | 0.10 | ||||
| ECOG‐PS | 2–4/0–1 | 3.39 (2.12–5.23) | <0.01 | 2.58 (1.49–4.31) | <0.01 | 4.77 (2.87–7.68) | <0.01 | 3.31(1.83–5.79) | <0.01 |
| EGFR‐TKI | Gefitinib or erlotinib/afatinib | 1.82 (1.19–2.90) | <0.01 | 1.65 (1.08–2.65) | 0.01 | 1.87 (1.07–3.54) | 0.02 | 1.61 (0.92–3.07) | 0.09 |
| EGFR mutation | Ex19del or L858R/other | 067 (0.34–1.59) | 0.34 | 0.60 (0.26–1.71) | 0.30 | ||||
| T790M mutation | Yes/No or unknown | 0.86 (0.49–1.40) | 0.57 | 0.45 (0.19–0.92) | 0.02 | 0.55 (0.23–1.12) | 0.10 | ||
| GPS score | 2/0–1 | 2.42 (1.46–3.83) | <0.01 | 1.52 (0.84–2.64) | 0.15 | 4.15 (2.37–6.92) | <0.01 | 2.24 (1.17–4.14) | 0.01 |
CI, confidence interval; EGFR, epidermal growth factor receptor; GPS, Glasgow prognostic score; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival; TKI, tyrosine kinase inhibitor.
There were 132 of the 214 (61.7%) patients who exhibited an objective clinical response to EGFR‐TKIs. Only an ECOG‐PS of 0–1 was associated with treatment response (odds ratio: 3.28, P < 0.01) (Table S3).
Discussion
To the best of our knowledge, this is the first study to examine the relationship between the GPS and the efficacy of EGFR‐TKI treatment. Multivariate analyses revealed that the GPS was independently associated with PFS and OS; this suggests that the GPS may be used to predict the efficacy of EGFR‐TKI treatment for EGFR‐mutated NSCLC.
Previous studies have also identified factors that predicted the efficacy of EGFR‐TKI treatment; these included the neutrophil‐to‐lymphocyte ratio and the prognostic nutritional index. 25 , 26 , 27 , 28 GPS is a useful marker in clinical practice, as most centers are able to test serum CRP and albumin concentrations, and the GPS calculation was more objective and had greater prognostic value than the ECOG‐PS. 19 , 29 GPS is also associated with weight and muscle loss, drug metabolism, elevated cytokine and adipokine levels, and poor PS 14 , 30 , 31 , 32 , 33 , 34 , 35 , 36 ; these factors reflect the host's nutritional status. This may explain why the GPS could play a predictive role for EGFR‐TKIs. Therefore, it appears reasonable to use the GPS in clinical practice.
Although EGFR‐TKIs are key treatment agents for patients with EGFR‐mutated NSCLC, there are some populations who do not show clinical benefit with EGFR‐TKIs. In our analysis, among patients with ECOG‐PS of 0–1, the GPS was able to predict both, PFS and OS. This may suggest that the population with an ECOG‐PS of 0–1 is heterogeneous, and the GPS is useful for prognosis in this population. However, among patients treated in the first‐line setting, the GPS was able to predict OS, but not PFS. This may reflect the fact that patients with good GPS have more chances of receiving subsequent treatments after EGFR‐TKI.
The present study has several limitations. First, the study period precluded an analysis of third generation EGFR‐TKIs, such as osimertinib, which was only approved for use in Japan in 2016. Second, the study sample size was small, and may have introduced selection bias due to the retrospective nature of this study, although patients from three different institutions were included. Third, the study failed to consider other systemic inflammation‐based markers, such as the neutrophil‐to‐lymphocyte ratio. Further prospective studies are needed to overcome these limitations.
In conclusion, the GPS predicted the efficacy of EGFR‐TKI treatment and survival outcomes among NSCLC patients with EGFR mutations. Although further studies are needed to validate our findings, the GPS is an easily calculated biomarker, which might be ideal for routine clinical use in this setting.
Disclosure
The authors report no conflict of interest in this work.
Supporting information
Table S1 Univariate and multivariate analyses of progression‐free and overall survival for performance status of >2
Table S2 Univariate and multivariate analyses of progression‐free and overall survival beyond the second line
Table S3 Analysis of factors for association with response to EGFR‐TKI monotherapy.
Acknowledgments
This research was supported by the JSPS KAKENHI (19K16855).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Miller KD, Siegel RL, Lin CC et al Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016; 66: 271–89. [DOI] [PubMed] [Google Scholar]
- 3. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 4. Mitsudomi T, Morita S, Yatabe Y et al Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–8. [DOI] [PubMed] [Google Scholar]
- 5. Rosell R, Carcereny E, Gervais R et al Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 6. Zhou C, Wu YL, Chen G et al Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–42. [DOI] [PubMed] [Google Scholar]
- 7. Sequist LV, Yang JC, Yamamoto N et al Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–34. [DOI] [PubMed] [Google Scholar]
- 8. Wu YL, Zhou C, Hu CP et al Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–22. [DOI] [PubMed] [Google Scholar]
- 9. Mok TS, Wu Y‐L, Ahn M‐J et al Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017; 376: 629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soria JC, Ohe Y, Vansteenkiste J et al Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018; 378: 113–25. [DOI] [PubMed] [Google Scholar]
- 11. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fehrenbacher L, Spira A, Ballinger M et al Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–46. [DOI] [PubMed] [Google Scholar]
- 13. McMillan DC. An inflammation‐based prognostic score and its role in the nutrition‐based management of patients with cancer. Proc Nutr Soc 2008; 67: 257–62. [DOI] [PubMed] [Google Scholar]
- 14. Proctor MJ, Talwar D, Balmar SM et al The relationship between the presence and site of cancer, an inflammation‐based prognostic score and biochemical parameters. Initial results of the Glasgow inflammation outcome study. Br J Cancer 2010; 103: 870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahmoud FA, Rivera NI. The role of C‐reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep 2002; 4: 250–5. [DOI] [PubMed] [Google Scholar]
- 16. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009; 12: 223–6. [DOI] [PubMed] [Google Scholar]
- 17. Falconer JS, Fearon KC, Ross JA et al Acute‐phase protein response and survival duration of patients with pancreatic cancer. Cancer 1995; 75: 2077–82. [DOI] [PubMed] [Google Scholar]
- 18. McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer‐specific and non‐cancer survival in patients with cancer. Nutr Cancer 2001; 41: 64–9. [DOI] [PubMed] [Google Scholar]
- 19. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation‐based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum‐based chemotherapy for inoperable non‐small‐cell lung cancer. Br J Cancer 2004; 90: 1704–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gioulbasanis I, Pallis A, Vlachostergios PJ et al The Glasgow prognostic score (GPS) predicts toxicity and efficacy in platinum‐based treated patients with metastatic lung cancer. Lung Cancer 2012; 77: 383–8. [DOI] [PubMed] [Google Scholar]
- 21. Umihanic S, Umihanic S, Jamakosmanovic S et al Glasgow prognostic score in patients receiving chemotherapy for non‐small‐cell lung cancer in stages IIIb and IV. Med Arch 2014; 68: 83–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang AG, Chen HL, Lu HY. Comparison of Glasgow prognostic score and prognostic index in patients with advanced non‐small cell lung cancer. J Cancer Res Clin Oncol 2015; 141: 563–8. [DOI] [PubMed] [Google Scholar]
- 23. Jiang AG, Chen HL, Lu HY. Prognostic value of the Glasgow prognostic score in lung cancer: Evidence from 10 studies. Int J Biol Markers 2018; 33: 201–7. [DOI] [PubMed] [Google Scholar]
- 24. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 25. Sheng J, Yang YP, Ma YX et al Low prognostic nutritional index correlates with worse survival in patients with advanced NSCLC following EGFR‐TKIs. PLOS One 2016; 11: e0147226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park S, Park S, Lee SH et al Nutritional status in the era of target therapy: Poor nutrition is a prognostic factor in non‐small cell lung cancer with activating epidermal growth factor receptor mutations. Korean J Intern Med 2016; 31: 1140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minami S, Ogata Y, Ihara S, Yamamoto S, Komuta K. Neutrophil‐to‐lymphocyte ratio predicts overall survival of advanced non‐small cell lung cancer harboring mutant epidermal growth factor receptor. World J Oncol 2017; 8: 180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aguiar‐Bujanda D, Dueñas‐Comino A, Saura‐Grau S et al Neutrophil to lymphocyte ratio as a prognostic factor in European patients with epidermal growth factor receptor‐mutant non‐small cell lung cancer treated with tyrosine kinase inhibitors. Oncol Res Treat 2018; 41: 755–61. [DOI] [PubMed] [Google Scholar]
- 29. Dajczman E, Kasymjanova G, Kreisman H, Swinton N, Pepe C, Small D. Should patient‐rated performance status affect treatment decisions in advanced lung cancer? J Thorac Oncol 2008; 3: 1133–6. [DOI] [PubMed] [Google Scholar]
- 30. Brown DJ, Milroy R, Preston T et al The relationship between an inflammation‐based prognostic score (Glasgow prognostic score) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer. J Clin Pathol 2007; 60: 705–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim SJ, Ryu KJ, Hong M, Ko YH, Kim WS. The serum CXCL13 level is associated with the Glasgow prognostic score in extranodal NK/T‐cell lymphoma patients. J Hematol Oncol 2015; 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McMillan DC. The systemic inflammation‐based Glasgow prognostic score: A decade of experience in patients with cancer. Cancer Treat Rev 2013; 39: 534–40. [DOI] [PubMed] [Google Scholar]
- 33. Naito T, Tashiro M, Ishida T, Ohnishi K, Kawakami J. Cancer cachexia raises the plasma concentration of oxymorphone through the reduction of CYP3A but not CYP2D6 in oxycodone‐treated patients. J Clin Pharmacol 2013; 53: 812–8. [DOI] [PubMed] [Google Scholar]
- 34. Kerem M, Ferahkose Z, Yilmaz UT et al Adipokines and ghrelin in gastric cancer cachexia. World J Gastroenterol 2008; 14: 3633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giannousi Z, Gioulbasanis I, Pallis AG et al Nutritional status, acute phase response and depression in metastatic lung cancer patients: Correlations and association prognosis. Support Care Cancer 2012; 20: 1823–9. [DOI] [PubMed] [Google Scholar]
- 36. Naito T, Tashiro M, Yamamoto K, Ohnishi K, Kagawa Y, Kawakami J. Impact of cachexia on pharmacokinetic disposition of and clinical responses to oxycodone in cancer patients. Eur J Clin Pharmacol 2012; 68: 1411–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Univariate and multivariate analyses of progression‐free and overall survival for performance status of >2
Table S2 Univariate and multivariate analyses of progression‐free and overall survival beyond the second line
Table S3 Analysis of factors for association with response to EGFR‐TKI monotherapy.


