Abstract
Background
Pleomorphic carcinoma (PC) of the lung is a rare type of lung cancer with aggressive characteristics and a poor prognosis. Because it is rare, the molecular characteristics of PC remain unclear.
Methods
A gene mutation analysis was performed using next‐generation sequencing (NGS) in patients with PC of the lung who had undergone surgical resection.
Results
A total of nine patients were enrolled in the study. All the patients were male and eight had a history of smoking. Eight tumors contained spindle cells and three contained giant cells. Mutations considered significant were found in eight of the nine patients: in TP53 in five patients, in MET in two patients, and in ALK, ERBB2, PIK3CA, APC, NF1, and CDKN2A in one patient each. No EGFR mutation was detected in our analysis. Co‐mutations were detected in three patients: TP53 with MET and NF1, TP53 with ERBB2, and PIK3CA with CDKN2A.
Conclusions
TP53 mutations were detected most frequently in PC of the lung with NGS analysis. Different co‐mutations were seen in several specimens.
Key points
-
Significant findings of the study
This study demonstrates that mutations in the TP53 gene are frequently found and co‐mutations are sometimes found in pleomorphic carcinoma of the lung using genomic profiling analysis.
-
What this study adds
Our results will help to analogize the genetic characteristics and potential target of molecular‐targeted agents of pleomorphic carcinoma of the lung.
Keywords: Comutation, next‐generation sequencing, pleomorphic carcinoma, TP53 mutation
Introduction
Lung cancer is the leading cause of cancer death worldwide 1 and well‐known risk factors for lung cancer include smoking and some chemicals. 2 , 3 Gene mutations linked to carcinogenesis have been identified in the past decade, some of which strongly accelerate cancer growth and progression, and are called driver‐gene mutations. Research into non‐small cell lung cancer (NSCLC) has resulted in the development of several molecular therapeutic agents that target driver mutations, such as those in epidermal growth factor receptor (EGFR), 4 , 5 , 6 anaplastic lymphoma kinase (ALK), 7 , 8 ROS1, 9 , 10 and BRAF. 11 These have a greater tumor‐shrinking effect and lower toxicity and confer longer progression‐free survival (PFS) than conventional cytotoxic drugs. In contrast, lung cancers with other gene mutations, such as in TP53, have shown shorter overall survival (OS), regardless of their driver mutation status. 12 Therefore, genomic information on individual tumors is important to guide the selection of rational therapeutic strategies with which to improve the outcomes of patients with advanced NSCLC. Recently developed techniques to identify specific gene changes have accelerated the trend towards personalized therapies. PCR‐based testing, immunohistochemistry (IHC), and fluorescence in situ hybridization are routinely used to detect driver mutations, and next‐generation sequencing (NGS) has recently become a key technique for molecular screening. However, a personalized approach based on specific oncogenic variations is particularly useful in lung adenocarcinoma and several other pathological types of lung cancer.
Pleomorphic carcinoma (PC) of the lung accounts for 0.9%–1.6% of all primary lung cancers. 13 , 14 , 15 , 16 PC of the lung is a heterogeneous group of NSCLCs that contain at least 10% spindle and/or giant cells and carcinomas that consist of only spindle and giant cells. 17 PC of the lung is known to be highly progressive and less susceptible to cytotoxic anticancer agents. 18 , 19 , 20 The median PFS is only 1.5 months and the median OS is 7.2 months in patients with PC of the lung treated with platinum‐based chemotherapy. 20 Even in surgically‐resected patients, the median OS is about 14.7 months. 21 Therefore, the development of new treatment strategies is urgently required.
A few cases of pulmonary sarcomatoid carcinoma (PSC), which according to the pathological classification of the World Health Organization includes PC, that carry specific gene mutations have been reported. 22 , 23 However, the frequency of driver mutations in PC of the lung is still unknown. There may be several genetic factors that have a poor prognosis in patients with PC of the lung, and the detection of gene mutations carried by PC may clarify the cause of its rare pathological morphology, the mechanism of its treatment resistance, and targets for new treatment strategies. The aim of this study was to analyze the predictive factors for a poor outcome and potential targets of targeted therapeutic agents by determining genetic variation profiles of PC of the lung with NGS analyses.
Methods
Study population
We reviewed the records of 944 patients with primary lung cancer who had undergone surgical resection between 2007 and 2014 at Kanagawa Cancer Center, Yokohama, Japan, and extracted 21 specimens that had been diagnosed as PC. Nine of the 21 specimens, which had been stored as frozen tumor materials, were recruited for our study. All nine tumors were reviewed and diagnosed by two pathologists (Y.M. and T.Y.), according to the 2015 World Health Organization (WHO) classification of lung tumors. 17
All clinical information, such as patient age, sex, pathological stage, and smoking status, were obtained from the medical records. The study was approved by the Institutional Review Board of Kanagawa Cancer Center (approval No. Eki‐73‐2015), and written informed consent was obtained from each patient.
Design of original Ion AmpliSeq DNA panels for the detection of genetic changes
With reference to several studies that comprehensively examined genetic changes in tumors, we constructed an original gene cancer panel to detect genetic changes that have been reported at high frequencies in adenocarcinomas of the lung. 24 , 25 , 26 , 27 We then chose 41 genes that were frequently detected in these tumors and were considered to be biologically significant, to construct an original cancer gene panel, designated ‘KCC41’ (Table S1). 28
Genomic DNA and RNA extraction
To obtain tumor DNA and RNA samples, first, we prepared a thinly sliced section from each stored frozen specimen, stained with hematoxylin and eosin (HE), and confirmed morphologically that each tissue actually contained tumor cells. DNA and RNA were then extracted from the frozen tissues with the ZR‐Duet DNA/RNA Miniprep Kit (Zymo Research, Irvine, CA, USA), according to the manufacturer's instructions. DNA was also extracted from formalin‐fixed paraffin‐embedded (FFPE) tissues with NucleoSpin DNA FFPE (Macherey‐Nagel, Düren, Germany), according to the manufacturer's instructions.
Quantity and quality checks of DNA and RNA
DNA and RNA were quantified with Qubit 2 (Thermo Fisher Scientific, Waltham, MA, USA). The DNA and RNA quality was assessed with the optical density ratios A260/A280 and A260/A230, respectively, using a NanoPhotometer (Implen). Genomic DNA (10 ng) or total RNA (10 ng) was used to create panel libraries for each specimen. The degradation of the DNA extracts from the FFPE tissues was estimated with a TaqMan PCR assay using TaqMan RNase P Detection Reagents Kit (short assay: 87 bp) (Thermo Fisher Scientific) and TaqMan MGB gene expression detection kit for the FFPE DNA QC assay (long assay: 256 bp) (Thermo Fisher Scientific). The DNA samples for which the relative value of the long assay/short assay (Ct value) exceeded 0.2 were selected for the panel analysis.
Library preparation and sequencing with the original DNA panel, commercial DNA, and RNA panel
In this study, we used three types of panels: the KCC41 panel, the Cancer Hotspot Panel v2 (CHPv2) (Thermo Fisher Scientific), and the Ion AmpliSeq RNA Fusion Lung Cancer Research Panel (Thermo Fisher Scientific). Genomic DNA (10 ng) or total RNA (10 ng) was used to create the panel libraries for each specimen. The libraries were amplified with the Ion Torrent AmpliSeq technology, and sequenced with an Ion PGM System for Next‐Generation Sequencing (Thermo Fisher Scientific). Each pooled library was quantified with an Agilent High Sensitivity DNA Kit (Agilent Technologies, USA) with an Agilent 2100 Bioanalyzer (Agilent Technologies). Emulsion PCRs were performed with libraries diluted to 100 pM to create template‐positive Ion Sphere Particles (ISPs) using the OneTouch 2 (OT2) instrument (Thermo Fisher Scientific) and the Ion PGM Template OT2 200 Kit (Thermo Fisher Scientific). The template‐positive ISPs were enriched with Ion OneTouch ES (Thermo Fisher Scientific). The Ion PGM 200 Sequencing Kit was used for sequencing.
Variant call and validation
The Torrent Suite v4.0.2 and Ion Reporter version 4.4 software (Thermo Fisher Scientific) were used to process and analyze the Ion PGM sequence data. Quality control reports were obtained with the Torrent Suite. Single‐nucleotide variants (SNVs) and insertions/deletions (indels) with a coverage of ≥20 reads that encoded amino acid changes relative to the UCSC hg19 reference genome sequence were selected to identify somatic variants. Single‐nucleotide polymorphisms (SNPs) were then excluded by using the sequences as queries against the databases COSMIC (http://cancer.sanger.ac.uk/cosmic), dbSNPs (NCBI, NIH; https://www.ncbi.nlm.nih.gov/projects/SNP/), the 1000 Genomes Project (http://www.1000genomes.org), cBioPortal (https://www.cbioportal.org), and other publicly accessible databases. If the database analysis failed to clarify whether an SNV was an SNP or a somatic variant, Sanger sequencing of DNA from a non‐neoplastic counterpart of each specimen was performed to obtain a definitive result. Finally, sequence changes with an allele frequency of >2% were defined as somatic variants.
Detection of fusion transcripts and ALK allelic imbalance
Torrent Suite v4.0.2 and Ion Reporter version 4.4 were used to process and analyze the sequence data from the Ion AmpliSe RNA Fusion Lung Cancer Research Panel (Thermo Fisher Scientific). Quality control reports were obtained with the Torrent Suite server. The Ion AmpliSeq RNA Fusion Lung Cancer Research Panel is based on an amplicon target enrichment approach that allows the amplification and detection of 70 known fusion transcripts of the ALK, RET, ROS1, and NTRK1 genes, using a pair of primers specific for each fusion (Table S2). The 3′/5′ ratios are calculated for the four genes included in the panel. If no common fusion transcripts are detected, an imbalanced ratio may reflect the presence of fusion transcripts in the sample.
Immunohistochemistry for ALK protein expression
Thin‐sliced FFPE tissues were prepared on silane‐coated slides for immunohistochemistry (IHC). We used the highly sensitive N‐Histofine ALK Detection Kit (Nichirei Bioscience, Tokyo, Japan), which is based on the intercalated antibody‐enhanced polymer method and includes clone 5A4 as the anti‐ALK primary antibody. 29 , 30
A semiquantitative assessment was made by estimating the percentage of IHC‐positive tumor cells. ALK‐IHC scoring was as follows: 0 = no stained cells; 1 = 0%–50% stained tumor cells; 2 = 51%–80% stained tumor cells; 3 = >80% stained tumor cells. A score ≥ 1 was deemed to be positive.
Results
Patient characteristics
A total of nine patients pathologically diagnosed with PC, according to the WHO classification, were enrolled in the study. Table 1 provides the patients' characteristics, including age, sex, pathological subtype of the resected tissue, staging, and smoking status. The median age was 69 years (range, 44–78 years), all patients were male, and eight patients had a smoking history. Eight tumors contained spindle cells and three contained giant cells.
Table 1.
Clinical features of nine patients with pleomorphic carcinoma of the lung
| Pathological features | ||||||
|---|---|---|---|---|---|---|
| No. | Age | Sex | Epithelial component | Sarcomatoid component | p‐stage | Smoking status (Pack‐years) |
| 1 | 57 | M | Adeno | Spindle cell, Giant cell | IIB | 32 |
| 2 | 71 | M | Squamous | Spindle cell | IIIA | 72 |
| 3 | 67 | M | Squamous | Spindle cell | IB | 90 |
| 4 | 77 | M | Large | Spindle cell | IB | 52 |
| 5 | 78 | M | Adeno | Spindle cell | IIA | 64 |
| 6 | 73 | M | Adeno | Spindle cell, Giant cell | IIB | 104 |
| 7 | 69 | M | Adeno | Spindle cell | IIB | 51 |
| 8 | 54 | M | Large | Giant cell | IIB | 41 |
| 9 | 44 | M | Adeno | Spindle cell | IIIA | 0 |
M, male; p‐stage, pathological stage; Adeno, adenocarcinoma; Squamous, squamous cell carcinoma; Large, large cell carcinoma.
Gene variants identified with KCC41 and CHPv2 panel analyses
The DNA sequencing results from the KCC41 panel are shown in Figure 1. Because the tumor content of patient No. 5 was low (about 10%), we performed an additional panel analysis with the Cancer Hotspot Panel version 2. The confirmed somatic nucleotide sequence changes in nonsynonymous SNVs and indels, with or without frameshifts, were considered to be somatic variants in this study. Somatic variants were detected in all the patients with our panel analyses. A total of 58 somatic variants were identified in 23 of the 41 lung‐adenocarcinoma‐related genes in the KCC41 panel. The results are summarized in Figure 1 (detailed information is provided in Table S3). A total of 11 probable pathogenic variants in six genes were identified in the present KCC41 panel analysis. A known driver‐gene variant reported in the literature was detected in TP53 (p.R175H). 31 Although not well characterized as driver genes in the literature, somatic variants with high pathogenic scores predicted with Functional Analysis through Hidden Markov Models (FATHMM) (http://fathmm.biocompute.org.uk) were also identified in TP53 (p.H178D, p.R175H, p.H193Y, p.R249M, and p.C242S), PI3KCA (p.R357L), NF1 (p.R304*), APC (p.E1209*), MET (p.L211W, p.H1112Y), and ERBB2 (p.S1050L). Although a FATHMM score was not assigned to CDKN2A p.L120*, this causes the premature termination of a tumor suppressor protein and we consider CDKN2A (p.E120*) to be a pathogenic variant. The remaining two variants were considered to be variants of uncertain or unknown significance (VUSs). These variants were found in the COSMIC v90 database, with labels of “n/a” or “neutral” based on their FATHMM scores. The remaining 44 somatic mutations were not recorded in COSMIC.
Figure 1.
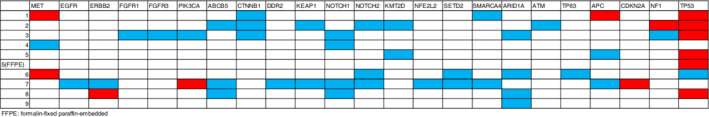
Mutational summary of nine patients with pleomorphic carcinoma. Genes in red were confirmed as pathogenic variants and genes in blue were considered variants of uncertain or unknown significance (VUSs).
In summary, eight of the nine patients showed more than one gene mutation considered to be pathogenic, and five patients showed a mutation in TP53. Combinations of gene mutations considered to be pathogenic were detected in three patients: two patients had two mutations (in PIK3CA and CDKN2A or in ERBB2 and TP53) and one patient had three mutations (in MET, APC, and TP53).
ALK‐IHC and fusion transcript analysis with the Ion AmpliSeq RNA fusion lung research panel
The results of the ALK mutation analysis are provided in Table 2. ALK‐IHC showed focal staining in patient No. 7 and diffuse staining in patient No. 9. In addition to IHC, we analyzed ALK fusion transcripts with the Ion AmpliSeq RNA Fusion Lung Research Panel, and demonstrated EML4 (E6)–ALK (E20) fusion transcripts in patient No. 9, but no common ALK fusion transcripts in patient No. 7. The allelic imbalance in the ALK gene was calculated and no ALK fusion was suggested in patient No. 7. Therefore, ALK mutation was only detected in patient No. 9, and patient No. 7 was negative for ALK fusion, although ALK‐IHC was positive in this patient.
Table 2.
Results of ALK immunohistochemistry (IHC), ALK fusion detection with sequencing, and an allelic imbalance analysis
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| ALK‐IHC (iScore*) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 |
| ALK‐fusion | − | − | − | − | − | − | − | − | EML4(E6)‐ALK(E20) |
| ALK allelic imbalance | − | − | − | − | − | − | − | − | + |
ALK, anaplastic lymphoma kinase; IHC, immunohistochemistry; EML4‐ALK, echinoderm microtubule associated protein like‐4‐anaplastic lymphoma kinase.
Discussion
PC is a rare pathological type of primary lung cancer, with an aggressive clinical course, that is resistant to cytotoxic chemotherapy. 18 , 32 Genetic profiling of PC should be helpful in the selection of treatment strategies, especially for advanced‐stage tumors. However, the pathological diagnosis of PC is generally difficult to substantiate with small biopsy samples. Therefore, we used a genomic profiling analysis of surgical samples from patients diagnosed with PC. There have been few reports of NGS analyses of PC of the lung. Here, we have shown that PC of the lung generally contains many somatic mutations and several driver‐gene mutations. Notably, multiple mutations were seen in some specimens.
Mutations were found in eight (88.9%) of the nine patients in this study, which is higher than expected for the common type of NSCLC. 33 Moreover, three (33.3%) of the nine patients had multiple mutations. A previous study of PSC demonstrated high gene‐mutation frequencies of 69%–100% and high multiple‐gene‐mutation frequencies of 39.5%–85.7%. 34 , 35 , 36 Conventionally, the driver‐gene mutation usually occurs in an early stage of tumor growth and tumor growth or relapse is only promoted by a single variant, 37 , 38 so driver‐gene mutations are considered to be mutually exclusive. PC of the lung includes several histological subtypes, so pathological heterogeneity may affect the co‐mutation profile. In this study, we did not evaluate the mutation types in tumor blocks from different pathological subtypes, but a previous study demonstrated the relative molecular homogeneity of the different histological component in PC of the lung. 36
Tumorigenesis is known to be related to the loss of suppressor genes, such as TP53. 39 TP53 is frequently mutated and the TP53 protein inactivated in many types of malignant tumor. 40 , 41 In the present study, TP53 gene mutations were observed in five (55.6%) of the nine patients. This result is similar to previous studies, which demonstrated that the TP53 gene is mutated in approximately 55% of PSCs. 34 , 35 TP53 is known to be the most frequently co‐mutated gene in all types of lung cancer. 42 A genome‐wide analysis of TP53 knockout rats demonstrated that the loss of TP53 is not the main driver of large‐scale structural variations of the genome, but that chromothripsis occurs primarily under TP53‐heterozygous conditions rather than under TP53‐null conditions. 43 This observation suggests that TP53 mutations increase genomic instability. TP53 mutations have also been associated with resistance to chemotherapy and radiation and with a poor prognosis. 44 PC of the lung generally has a poor prognosis and neither neoadjuvant nor chemotherapy improves patient survival, even in early stage disease. 18 , 19 , 21
The discovery of treatable driver mutations, including in EGFR and ALK, is associated with sensitivity to molecular targeted therapies and improved clinical outcomes in patients treated with those therapies. However, the frequency of treatable driver mutations in PC of the lung is controversial. The frequency of EGFR mutations was 50% in Asian patients with NSCLC. 45 Several previous studies reported that the frequency of EGFR mutations was 0%–23.8% in patients with PC of the lung. 15 , 46 , 47 No EGFR‐mutation‐positive PC of the lung was detected in our study. The frequency of EML4–ALK gene fusion is 2%–7% in NSCLC. 48 , 49 Several previous studies have reported that the frequency of the EML4–ALK gene fusion is 0–3.5% in patients with PC of the lung or PSC. 20 , 50 , 51 , 52 Because the frequency of ALK rearrangement in NSCLC is low, it is unclear whether it is lower in PC of the lung than in NSCLC. In the present study, ALK‐IHC detected two patients with suspected ALK protein fusion. However, patient No. 7 showed no ALK fusion in an RNA assay and was negative for ALK allelic imbalance, despite focal staining with ALK‐IHC, so we confirmed EML4–ALK gene fusion in only one patient. A comparative analysis of the ALK‐IHC and NGS panel results for fusion transcripts showed high agreement between the two test methods. 53 False‐positive ALK‐IHC results are possible in nonadenocarcinomas, although not in adenocarcinomas. 29 The fusion panel is theoretically the most reliable assay for the detection of mutant transcripts because it supplies direct evidence of translocation. However, in NSCLC there are many variants of the EML4–ALK fusion and many types of fusion partners, so this panel could miss ALK fusions that are not specifically included in the analysis. The efficacy of ALK‐TKI for PC with an ALK fusion gene is still unknown, but most reported cases suggest that ALK mutations in PC and PSC might be negative predictive factors of the efficacy of not only chemotherapy but also ALK‐TKI. 54 , 55
The MET exon‐14‐skipping mutation is considered one of the treatable driver mutations of NSCLC. The frequency of the MET exon‐14‐skipping mutation in pulmonary adenocarcinoma is about 3%. 56 Several previous studies have identified high frequencies of the MET exon‐14‐skipping mutation (approximately 20%) in both PSC 34 , 57 and PC of the lung, 58 which is higher than in adenocarcinoma. However, other studies have reported that the frequency of MET mutation is low. 36 , 59 In our study, two of the nine patients had a MET mutation, but not the exon‐14‐skipping mutation. In a case report, a MET inhibitor showed efficacy in the treatment of PSC with the MET exon‐14‐skipping mutation, 60 but there have been too few reports to estimate the clinical effect of MET inhibitors.
A programmed cell death 1 (PD‐1) blockade was recently reported to elicit good responses in some patients with PC of the lung. 61 PSC tumors have many somatic mutations, which is associated with the clinical efficacy of immune checkpoint inhibitors (ICIs). 62 NSCLC with SMARCA4 mutations displays a high mutation burden, so SMARCA4 mutations are associated with the sensitivity of ICIs and are expected to be one of the predictive biomarkers of ICIs. Some patients with SMARCA4‐deficient tumors reportedly show a dramatic response to PD‐1 blockade. 63 SMARCA4 mutations are found in approximately 10% of NSCLC. 64 , 65 However, the frequency of SMARCA4 mutations in PC of the lung is unknown. In the present study, we detected none of the SMARCA4 mutations reported in the COSMIC database. Therefore, the association between the presence of SMARCA4 mutations, a high tumor mutation burden, and the efficacy of ICIs for PC of the lung is still unclear.
This study had several limitations. First, we used an original gene panel containing 41 genes that are thought to be mutated frequently in lung cancer and to be biologically significant, which is fewer than can be detected with a commercial gene panel. Therefore, our panel may have missed some gene mutations related to the oncogenicity of PC of the lung. Second, the sample size was small, because this disease is rare and tissue availability consequently low. Consequently, the frequency of mutations may not have been accurately evaluated, and further extensive research is required. Finally, we did not evaluate the relationship between the detected gene mutations and the responsiveness of the tumors to candidate drugs or the clinical course of the disease. Therefore, the clinical value of the gene mutations detected in PC of the lung was not adequately evaluated.
In summary, we have demonstrated some driver‐gene mutations in PC of the lung. The driver‐gene mutation status of PC of the lung is still imprecise and controversial because it is a rare cancer. However, our data suggest that mutations in the TP53 gene are frequent and co‐mutations are sometimes present in PC of the lung, as in some previous studies. Further intensive genomic NGS analyses based on these findings should identify the genomic characteristics of and treatment options for PC patients.
Disclosure
All authors declare that they have no conflicts of interest.
Supporting information
Table S1 Original gene cancer panel, KCC41, including 41 genes thought to be associated with tumors of the lung.
Table S2 Fusion variants of the ALK, RET, ROS1, and NTRK1 genes from the Ion AmpliSeq RNA Fusion Lung Cancer Research Panel
Table S3 NGS results for nine patients with pleomorphic carcinoma
Acknowledgments
This work was supported, in part, by the Kanagawa Health Foundation and the Kanagawa Prefectural Hospitals Cancer Research Fund.
Contributor Information
Saki Manabe, Email: sakimana@yokohama-cu.ac.jp.
Shuji Murakami, Email: murakamis@kcch.jp.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 2. Wakai K, Inoue M, Mizoue T et al Tobacco smoking and lung cancer risk: An evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol 2006; 36: 309–24. [DOI] [PubMed] [Google Scholar]
- 3. t Mannetje A, Bencko V, Brennan P et al Occupational exposure to metal compounds and lung cancer. Results from a multi‐center case‐control study in central/Eastern Europe and UK. Cancer Causes Control 2011; 22: 1669–80. [DOI] [PubMed] [Google Scholar]
- 4. Mitsudomi T. Advances in target therapy for lung cancer. Jpn J Clin Oncol 2010; 40: 101–6. [DOI] [PubMed] [Google Scholar]
- 5. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 6. Mok TS, Wu YL, Thongprasert S et al Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 7. Shaw AT, Kim DW, Nakagawa K et al Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med 2013; 368: 2385–94. [DOI] [PubMed] [Google Scholar]
- 8. Shaw AT, Kim DW, Mehra R et al Ceritinib in ALK‐rearranged non‐small‐cell lung cancer. N Engl J Med 2014; 370: 1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shaw AT, Ou SH, Bang YJ et al Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med 2014; 371: 1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergethon K, Shaw AT, Ou SH et al ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012; 30: 863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Planchard D, Besse B, Groen HJM et al Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)‐mutant metastatic non‐small cell lung cancer: An open‐label, multicentre phase 2 trial. Lancet Oncol 2016; 17: 984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Labbe C, Cabanero M, Korpanty GJ et al Prognostic and predictive effects of TP53 co‐mutation in patients with EGFR‐mutated non‐small cell lung cancer (NSCLC). Lung Cancer 2017; 111: 23–9. [DOI] [PubMed] [Google Scholar]
- 13. Mochizuki T, Ishii G, Nagai K et al Pleomorphic carcinoma of the lung: Clinicopathologic characteristics of 70 cases. Am J Surg Pathol 2008; 32: 1727–35. [DOI] [PubMed] [Google Scholar]
- 14. Ito K, Oizumi S, Fukumoto S et al Clinical characteristics of pleomorphic carcinoma of the lung. Lung Cancer 2010; 68: 204–10. [DOI] [PubMed] [Google Scholar]
- 15. Kaira K, Horie Y, Ayabe E et al Pulmonary pleomorphic carcinoma: A clinicopathological study including EGFR mutation analysis. J Thorac Oncol 2010; 5: 460–5. [DOI] [PubMed] [Google Scholar]
- 16. Yuki T, Sakuma T, Ohbayashi C et al Pleomorphic carcinoma of the lung: A surgical outcome. J Thorac Cardiovasc Surg 2007; 134: 399–404. [DOI] [PubMed] [Google Scholar]
- 17. William D, Travis EB, Allen P, Burke AM, Nicholson AG. WHO Classifications of Tumours of the Lung, Pleura, Thymus and Heart, 4th edn IARC, Lyon: 2015. [DOI] [PubMed] [Google Scholar]
- 18. Bae HM, Min HS, Lee SH et al Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer 2007; 58: 112–5. [DOI] [PubMed] [Google Scholar]
- 19. Lin Y, Yang H, Cai Q et al Characteristics and prognostic analysis of 69 patients with pulmonary Sarcomatoid carcinoma. Am J Clin Oncol 2014; 39: 215–222. [DOI] [PubMed] [Google Scholar]
- 20. Tamura Y, Fujiwara Y, Yamamoto N et al Retrospective analysis of the efficacy of chemotherapy and molecular targeted therapy for advanced pulmonary pleomorphic carcinoma. BMC Res Notes 2015; 8: 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsubata Y, Sutani A, Okimoto T et al Comparative analysis of tumor angiogenesis and clinical features of 55 cases of pleomorphic carcinoma and adenocarcinoma of the lung. Anticancer Res 2015; 35: 389–94. [PubMed] [Google Scholar]
- 22. Pelosi G, Gasparini P, Cavazza A et al Multiparametric molecular characterization of pulmonary sarcomatoid carcinoma reveals a nonrandom amplification of anaplastic lymphoma kinase (ALK) gene. Lung Cancer 2012; 77: 507–14. [DOI] [PubMed] [Google Scholar]
- 23. Lee S, Kim Y, Sun JM et al Molecular profiles of EGFR, K‐ras, c‐met, and FGFR in pulmonary pleomorphic carcinoma, a rare lung malignancy. J Cancer Res Clin Oncol 2011; 137: 1203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Govindan R, Ding L, Griffith M et al Genomic landscape of non‐small cell lung cancer in smokers and never‐smokers. Cell 2012; 150: 1121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Imielinski M, Berger AH, Hammerman PS et al Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012; 150: 1107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hammerman PS, Lawrence MS, Voet D et al Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012; 489: 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Y, Shi JX, Pan XF, Feng J, Zhao H. Identification of candidate genes for lung cancer somatic mutation test kits. Genet Mol Biol 2013; 36: 455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oshita F, Kasajima R, Miyagi Y. Multiplex genomic test of mutation and fusion genes in small biopsy specimen of lung cancer. J Exp Ther Oncol 2016; 11: 189–94. [PubMed] [Google Scholar]
- 29. Takamochi K, Takeuchi K, Hayashi T, Oh S, Suzuki K. A rational diagnostic algorithm for the identification of ALK rearrangement in lung cancer: A comprehensive study of surgically treated Japanese patients. PLOS One 2013; 8: e69794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takeuchi K, Choi YL, Togashi Y et al KIF5B‐ALK, a novel fusion oncokinase identified by an immunohistochemistry‐based diagnostic system for ALK‐positive lung cancer. Clin Cancer Res 2009; 15: 3143–9. [DOI] [PubMed] [Google Scholar]
- 31. Sigal A, Rotter V. Oncogenic mutations of the p53 tumor suppressor: The demons of the guardian of the genome. Cancer Res 2000; 60: 6788–93. [PubMed] [Google Scholar]
- 32. Hong JY, Choi MK, Uhm JE et al The role of palliative chemotherapy for advanced pulmonary pleomorphic carcinoma. Med Oncol 2009; 26: 287–91. [DOI] [PubMed] [Google Scholar]
- 33. Wen S, Dai L, Wang L et al Genomic signature of driver genes identified by target next‐generation sequencing in Chinese non‐small cell lung cancer. Oncologist 2019; 24: e1070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li X, Wang D, Zhao Q et al Clinical significance and next‐generation sequencing of Chinese pulmonary Sarcomatoid carcinoma. Sci Rep 2017; 7: 3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lococo F, Gandolfi G, Rossi G et al Deep sequencing analysis reveals that KRAS mutation is a marker of poor prognosis in patients with pulmonary Sarcomatoid carcinoma. J Thorac Oncol 2016; 11: 1282–92. [DOI] [PubMed] [Google Scholar]
- 36. Fallet V, Saffroy R, Girard N et al High‐throughput somatic mutation profiling in pulmonary sarcomatoid carcinomas using the LungCarta panel: Exploring therapeutic targets. Ann Oncol 2015; 26: 1748–53. [DOI] [PubMed] [Google Scholar]
- 37. Pao W, Girard N. New driver mutations in non‐small‐cell lung cancer. Lancet Oncol 2011; 12: 175–80. [DOI] [PubMed] [Google Scholar]
- 38. Zhang J, Fujimoto J, Zhang J et al Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 2014; 346: 256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Surget S, Khoury MP, Bourdon JC. Uncovering the role of p53 splice variants in human malignancy: A clinical perspective. Onco Targets Ther 2013; 7: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang F, Syrjanen S, Tervahauta A, Syrjanen K. Tumourigenesis associated with the p53 tumour suppressor gene. Br J Cancer 1993; 68: 653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science 1991; 253: 49–53. [DOI] [PubMed] [Google Scholar]
- 42. Cancer Genome Atlas Research N . Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014; 511: 543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hermsen R, Toonen P, Kuijk E et al Lack of major genome instability in tumors of p53 null rats. PLOS One 2015; 10: e0122066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shetzer Y, Solomon H, Koifman G, Molchadsky A, Horesh S, Rotter V. The paradigm of mutant p53‐expressing cancer stem cells and drug resistance. Carcinogenesis 2014; 35: 1196–208. [DOI] [PubMed] [Google Scholar]
- 45. Shi Y, Au JS, Thongprasert S et al A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9: 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chang YL, Wu CT, Shih JY, Lee YC. EGFR and p53 status of pulmonary pleomorphic carcinoma: Implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann Surg Oncol 2011; 18: 2952–60. [DOI] [PubMed] [Google Scholar]
- 47. Italiano A, Cortot AB, Ilie M et al EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: Implications for anti‐EGFR treatment of a rare lung malignancy. Int J Cancer 2009; 125: 2479–82. [DOI] [PubMed] [Google Scholar]
- 48. Antoniu SA. Crizotinib for EML4‐ALK positive lung adenocarcinoma: A hope for the advanced disease? Evaluation of Kwak EL, Bang YJ, Camidge DR, et al. anaplastic lymphoma kinase inhibition in non‐small‐cell lung cancer. N Engl J med 2010;363(18):1693‐703. Expert Opin Ther Targets 2011; 15: 351–3. [DOI] [PubMed] [Google Scholar]
- 49. Shaw AT, Yeap BY, Mino‐Kenudson M et al Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol 2009; 27: 4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen X, Zhang Y, Lu J et al Pulmonary sarcomatoid carcinoma with ALK rearrangement: Frequency, clinical‐pathologic characteristics, and response to ALK inhibitor. Transl Oncol 2017; 10: 115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Terra SB, Jang JS, Bi L et al Molecular characterization of pulmonary sarcomatoid carcinoma: Analysis of 33 cases. Mod Pathol 2016; 29: 824–31. [DOI] [PubMed] [Google Scholar]
- 52. Forest F, Yvorel V, Karpathiou G et al Histomolecular profiling of pleomorphic, spindle cell, and giant cell carcinoma of the lung for targeted therapies. Hum Pathol 2016; 49: 99–106. [DOI] [PubMed] [Google Scholar]
- 53. Pekar‐Zlotin M, Hirsch FR, Soussan‐Gutman L et al Fluorescence in situ hybridization, immunohistochemistry, and next‐generation sequencing for detection of EML4‐ALK rearrangement in lung cancer. Oncologist 2015; 20: 316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Murakami Y, Saka H, Oki M. Response to Crizotinib and clinical outcome in ALK‐rearranged pulmonary pleomorphic carcinoma. J Thorac Oncol 2015; 10: e28–9. [DOI] [PubMed] [Google Scholar]
- 55. Kobayashi Y, Sakao Y, Ito S et al Transformation to sarcomatoid carcinoma in ALK‐rearranged adenocarcinoma, which developed acquired resistance to crizotinib and received subsequent chemotherapies. J Thorac Oncol 2013; 8: e75–8. [DOI] [PubMed] [Google Scholar]
- 56. Awad MM, Oxnard GR, Jackman DM et al MET exon 14 mutations in non‐small‐cell lung cancer are associated with advanced age and stage‐dependent MET genomic amplification and c‐met overexpression. J Clin Oncol 2016; 34: 721–30. [DOI] [PubMed] [Google Scholar]
- 57. Li Y, Gao L, Ma D et al Identification of MET exon14 skipping by targeted DNA‐ and RNA‐based next‐generation sequencing in pulmonary sarcomatoid carcinomas. Lung Cancer 2018; 122: 113–9. [DOI] [PubMed] [Google Scholar]
- 58. Kwon D, Koh J, Kim S et al MET exon 14 skipping mutation in triple‐negative pulmonary adenocarcinomas and pleomorphic carcinomas: An analysis of intratumoral MET status heterogeneity and clinicopathological characteristics. Lung Cancer 2017; 106: 131–7. [DOI] [PubMed] [Google Scholar]
- 59. Vieira T, Antoine M, Ruppert AM et al Blood vessel invasion is a major feature and a factor of poor prognosis in sarcomatoid carcinoma of the lung. Lung Cancer 2014; 85: 276–81. [DOI] [PubMed] [Google Scholar]
- 60. Liu X, Jia Y, Stoopler MB et al Next‐generation sequencing of pulmonary Sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol 2016; 34: 794–802. [DOI] [PubMed] [Google Scholar]
- 61. Tokuyasu H, Ishikawa S, Sakai H, Ikeuchi T, Miura H. Single pembrolizumab treatment causing profound durable response in a patient with pulmonary pleomorphic carcinoma. Respir Med Case Rep 2019; 28: 100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rizvi NA, Hellmann MD, Snyder A et al Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348: 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takada K, Sugita S, Murase K et al Exceptionally rapid response to pembrolizumab in a SMARCA4‐deficient thoracic sarcoma overexpressing PD‐L1: A case report. Thorac Cancer 2019; 10: 2312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dagogo‐Jack I, Schrock AB, Kem M et al Clinicopathologic characteristics of BRG1‐deficient non‐small cell lung cancer. J Thorac Oncol 2020; 15: 766–76. [DOI] [PubMed] [Google Scholar]
- 65. Consortium APG . AACR project GENIE: Powering precision medicine through an international consortium. Cancer Discov 2017; 7: 818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Original gene cancer panel, KCC41, including 41 genes thought to be associated with tumors of the lung.
Table S2 Fusion variants of the ALK, RET, ROS1, and NTRK1 genes from the Ion AmpliSeq RNA Fusion Lung Cancer Research Panel
Table S3 NGS results for nine patients with pleomorphic carcinoma


