Abstract
Background
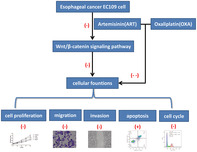
Esophageal cancer (EC) is a prevalent malignant cancer worldwide. Interestingly, the antimalaria compound artemisinin (ART) is also reported to have anticancer potential, although its underlying mechanism in EC is unclear. In this study, we explored the anticancer role of ART in EC109 and further explored the combination of ART and oxaliplatin (OXA) for their synergetic anticancer functions.
Methods
Human EC cell line EC109 was used. After ART or oxaliplatin (OXA) treatment, cell proliferation, migration, and invasion were measured by MTT, transwell, and scratch wound assays, respectively. Flow cytometry was performed to examine the cell cycle and apoptosis. The mRNA and protein levels were determined using qRT‐PCR and western blotting.
Results
The migration and invasion abilities of EC109 were suppressed by ART. This was due to the inhibitory effect of ART on the Wnt/β‐catenin signaling pathway. The levels of β‐catenin, c‐myc, and survivin were also downregulated by ART. ART inhibits the proliferation of EC109 cells by arresting the cells in the G1‐phase of cell cycle. By using LiCl, an activator of the Wnt/β‐catenin pathway, we further verified that the inhibition of the Wnt/β‐catenin pathway was indeed due to ART. Remarkably, ART enhanced the anticancer effects of OXA in EC109 cells. OXA combined with ART was found to be more efficient in decreasing tumor growth compared to the individual drugs.
Conclusions
ART could suppress tumor progression by inhibiting Wnt/β‐catenin signaling pathway, and it may also enhance the antitumor effect of OXA in EC. Thus, ART could be a novel anticancer drug for EC treatment.
Key points
Significant findings of the study
ART could be a novel anticancer drug for esophageal cancer (EC) treatment.
What this study adds
Combination treatment with artemisinin and oxaliplatin inhibits tumorigenesis in esophageal cancer EC109 cells through the Wnt/β‐catenin signaling pathway.
Keywords: Artemisinin, cell proliferation, esophageal cancer, Oxaliplatin, Wnt/β‐catenin
Artemisinin (ART) inhibited cell proliferation, migration, and invasion but promoted apoptosis in esophageal cancer EC109 cells. These effects were facilitated by the inhibition of the Wnt/β‐catenin signaling pathway. Furthermore, ART also enhanced the antitumor effect of oxaliplatin (OXA) in esophageal cancer cells.
Introduction
Esophageal cancer (EC) is one of the most common causes of cancer mortality in the world. 1 Although the treatment of EC has been greatly improved in recent years, the prognosis of EC remains unsatisfactory. 2 The present treatment for EC includes surgery, chemotherapy, and radiotherapy. 3 Chemotherapy is the key treatment for metastatic diseases which can improve quality of life. 4 As there are no easily distinguishable symptoms, the early prognosis of EC is difficult and at the time of diagnosis, many patients are already in the advanced stages. 5 Therefore, it is crucial to seek an effective treatment method that can improve the quality of life along with the survival time of EC patients.
A previous study suggests that the overall survival rate in cancer patients can be improved using broadly effective phytochemicals that inhibit metastasis and invasion with tolerable side effects. 6 Artemisinin (ART) is a sesquiterpene lactone isolated from the Chinese plant Artemisia annua (commonly known as qinghaosu or sweet wormwood) and has been used since 1970. 7 Presently, ART and its derivatives have been identified as the most effective drugs to treat chloroquine‐resistant malaria without the notable side effects. 8 , 9 In addition to the antimalarial properties, ART is also reported to exhibit an antitumor function. 10 , 11 , 12
Wnt/β‐catenin is a powerful signaling pathway that plays a crucial role in cell fate determination, survival, and proliferation in multiple tissues. 13 Like many other cancers, the occurrence and progress of EC is also closely related to the activation of oncogenic signaling pathways, and inactivation of tumor suppressor signaling pathways. 14 Specifically, the misregulation of the Wnt/β‐catenin signaling pathway mediated by the tumor suppressor or activating agents has been associated with EC. 15 , 16 Interestingly, several studies have suggested that ART imparts tumor attenuation through the Wnt/β‐catenin signaling pathway. 17 , 18 However, its exact role in regulating the Wnt/β‐catenin pathway in EC is unclear.
Oxaliplatin (OXA), a platinum‐based chemotherapeutic agent with a 1,2‐ diaminocyclohexane carrier ligand, has shown efficacy against many tumor cells, and possess no cross‐resistance with cisplatin and carboplatin. 19 , 20 OXA can also be used as an ideal chemotherapy drug for the treatment of esophageal related cancers but has limited effect in the single‐drug therapy. 21 Despite the initial efficiency, most anticancer drugs eventually develop chemoresistance in nearly all metastatic patients. This is the major reason for the failure of chemotherapy. 22 OXA is widely used in combination therapies with other anticancer drugs such as 5‐fluorouracil, leucovorin, irinotecan, and folinic acid. 23 , 24 However, the combined efficacy of ART and OXA in EC is unknown.
Therefore, in this study, we first tested whether ART interfered with EC tumor growth by blocking the unrestricted activation of the Wnt/β‐catenin signaling pathway. Further, we tested for the additive effects of OXA and ART against EC.
Methods
Cell cultures and material
The human EC cell line EC109 was obtained from Cell Bank of Chinese Academy of Sciences, Shanghai, China. The cells were cultured in Dulbecco's Modified Eagle Medium (DMEM, Gibco, USA) with 10% FBS and 1% streptomycin/penicillin at 37°C in a 5% CO2 incubator. Artemisinin (ART), oxaliplatin (OXA), and LiCl were purchased from Sigma‐Aldrich (Shanghai, China). ART and OXA were dissolved in dimethyl sulfoxide (DMSO; Sigma, USA) and added to 2 mg/mL phosphate‐buffered saline (PBS), used as a storage solution. The solution was then added into the cell culture medium at various concentrations. The final concentration of DMSO was <0.1% (v/v) in all experiments.
MTT assay
5‐Diphenyltetrazolium bromide (MTT) (Sigma‐Aldrich) assay was performed to measure cell proliferation. EC109 cells (2 × 104 cells/mL) were cultured in 96‐well plates with different doses of ART and OXA. After the drug treatment, 0.5 mg/mL MTT was added into each well at 24, 48, 72, and 96 hours and cells were further incubated for 4 hours at room temperature (RT). The supernatants were then discarded and colored formazan crystals were dissolved with 150 μL/well of DMSO. Further, cells were treated with ART and/or OXA (from 0–100 μM) to generate curves and calculate half‐maximal inhibitory concentration (IC50) values. A microplate reader (Bio‐Rad, USA) at 570 nm was used to analyze the OD values.
Scratch wound healing assay
Scratch wound healing assay was used to detect cell migration. EC109 cells were seeded into six‐well plates and treated with 0, 5, 10, or 20 μM ART and/or OXA. A wound was introduced to the cell layers using a 200 μL pipette tip and cells were cultured in 10% FBS‐supplemented DMEM medium. Cell migration was measured at 0 and 48 hours with an inverted microscope at 100× magnification.
Transwell assay
Transwell chambers (Corning, USA) were used to detect cell invasion. Briefly, 200 μL of EC109 cells (1 × 105 cells) treated with 0, 5, 10, or 20 μM ART and/or OXA were added in the upper chamber of a transwell apparatus coated with Matrigel (Corning, USA) and incubated in DMEM with 10% FBS for 48 hours. Cells that had migrated to the lower chamber were fixed for 20 minutes in 1% formaldehyde and stained for 20 minutes in crystal violet (0.1%). Stained cells were visualized with a microscope (Olympus) and five randomly selected fields were used to count the number of invaded cells.
Cell apoptosis analysis
The Annexin V‐FITC kit (Biosea Biotechnology Co., Beijing, China) was used to test cell apoptosis. EC109 cells (5.0 × 105 cells/mL) were treated with 0, 5, 10, or 20 μM ART and/or OXA and resuspended in PBS buffer. Subsequently, the cells were double‐stained using Annexin V‐Alexa Fluor 647 and propidium iodide (PI). Finally, the apoptotic rate was analyzed using a flow cytometer (BD Biosciences, San Diego, CA, USA).
Cell cycle analysis
EC109 cells (1 × 106 cells/mL) were treated with ART and/or OXA for up to 48 hours. For cell cycle analysis, cells were harvested, fixed with 70% cold ethanol, incubated with RNase, and stained with propidium iodide. The cell cycle was detected using flow cytometry (BD Biosciences) and data was analyzed using FlowJo v7.6 software (version 3.2, Verity Software House, USA).
Quantitative real‐time PCR (qRT‐PCR) assays
Total RNA from EC109 cells was extracted using TRIZOL reagent (Invitrogen). Sample concentrations were measured using a NanoDrop ND‐1000 spectrophotometer (NanoDrop, USA). Then, total RNA (500 ng) was reverse‐transcribed into cDNA using the PrimeScript RT reagent Kit (TaKaRa, Dalian, China) and analyzed by qRT‐PCR using a SYBR Green PCR kit (TaKaRa). The sequences of the used primers were as following: c‐myc: forward: 5′‐CCCAGCGAGGACATCTGGAAGAA‐3′, reverse: 5′‐GAGAAGCCGCTCCACATGCAGTC‐3′; survivin: forward: 5′‐AGCCCTTTCTCAAGGACCAC‐3′, reverse: 5′‐GCACTTTCTTCGCAGTTTCC‐3′; β‐actin forward primer: 5′‐ACACTGTGTGCCCATCTACGAGG‐3′, reverse primer: 5′‐AGGGGCCGGACTCGTCGTCATACT‐3′. The primers were obtained from GenePharma (GenePharma, Shanghai, China). The β‐actin was used to normalize the transcript levels of mRNA. Relative expression was calculated using the 2‐ΔΔCt method. 25
Western blot analysis
Total protein was extracted from EC109 cells using RIPA lysis buffer (Sigma, USA). The protein sample (50 μg per sample) was separated on a 10% SDS‐PAGE gel and transferred onto a polyvinylidene difluoride membrane. These membranes were blocked with 5% nonfat milk for 2 hours and incubated overnight with primary antibodies anti‐β‐actin (1:1000, ab179467, Abcam, UK), anti‐c‐myc (1:1000, ab32072, Abcam, UK), antisurvivin (1:1000, ab76424, Abcam, UK) at 4°C. After washing three times, the membranes were incubated with a peroxidase‐labeled secondary antibody (anti‐rabbit IgG, 1:2000, ab6721, Abcam, UK) for 2 hours. Enhanced chemiluminescence (ECL) (ThermoFisher, USA) was used to visualize the protein bands, and analysis was carried out using the Image Laboratory Software (Bio‐Rad, USA).
Statistical analysis
All data represent the mean ± SD from at least three independent experiments. A Student's t‐test was used to identify significant differences between two groups and a one‐way ANOVA with Tukey's post hoc test was used to compare the means of more than two groups. All statistical analyses were performed using SPSS 22.0 (Chicago, IL, USA) and GraphPad Prism 7.0 (GraphPad, San Diego, CA, USA). P < 0.05 was considered statistically significant.
Results
Artemisinin inhibits the malignant progression of EC cells
First, an MTT assay was conducted to investigate the effects of ART on the cell fate of EC109 cells (Fig 1a). Compared to the control group, artemisinin (ART, 0–20 μM) treatment significantly suppressed the cell viabilities (P < 0.001). Further, using the wound healing assay, migration in EC109 cells was evaluated. Here too, ART significantly inhibited the cell migration in a dose‐dependent manner, and 20 μM of ART was most effective in migration suppression compared with the control group (Fig 1b). Additionally, a transwell assay was used to explore the invasive ability of EC109 cells. Compared with the control group where abundant cells translocated to the underside well, a dose‐dependent decrease in cell invasion activities was noted upon treatment with 5, 10, and 20 μM of ART (Fig 1c). Furthermore, flow cytometry assay was conducted to measure the apoptosis and cell cycle of ART‐treated EC109 cells. As shown in Fig 1d, apoptosis in EC109 cells significantly increased with the increasing concentrations of ART compared to the control group (P < 0.001). Furthermore, an increase in concentrations of ART significantly accumulated the EC109 cells arrested in the G0/G1 phase. Especially, at 20 μM ART, a significant number of 58.89% ± 4.50% (P < 0.001) tumor cells were in the G0/G1 phase (Fig 1e). Therefore, these findings suggest that artemisinin increased apoptosis, but inhibited the proliferation, migration, and invasion of EC109 cells.
Figure 1.
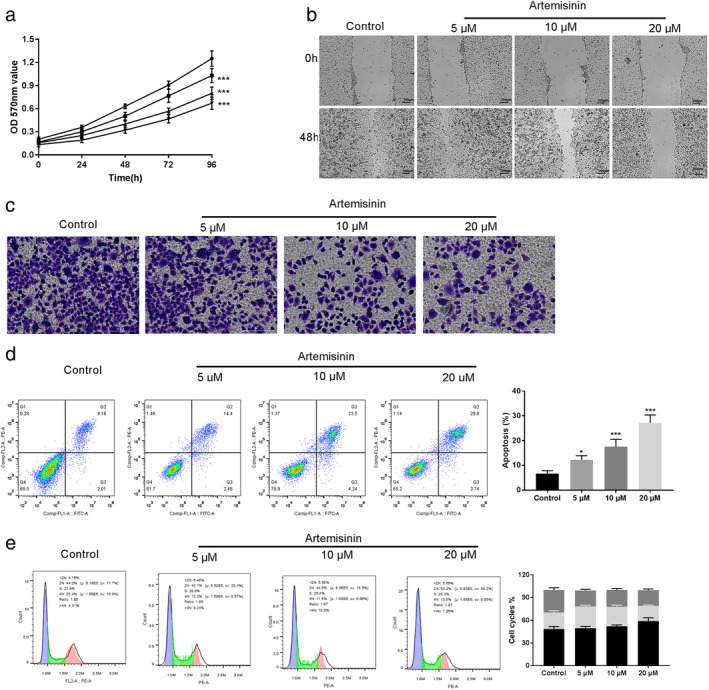
Artemisinin (ART) inhibits the malignant progression of EC109 cells. (a) Relative cell proliferation of EC109 treated with ART (0–20 μM) by MTT assay ( ) Control (
) Control ( ) 5 μM (
) 5 μM ( ) 10 μM (
) 10 μM ( ) 20 μM. (b) Representative photomicrographs of initial and final wounds at 0 and 48 hours are shown at 100× magnification by scratch wound healing assay. (c) Transwell assay shows the invasion of EC109 cells. (d) Effect of ART on EC109 cell apoptosis by flow cytometry. (e) Result of cell cycle of EC109 cells after treatment with ART by flow cytometry. Each group represented the mean ± standard deviation (SD) of at least three independent experiments. **P < 0.01, and ***P < 0.001 versus control group (
) 20 μM. (b) Representative photomicrographs of initial and final wounds at 0 and 48 hours are shown at 100× magnification by scratch wound healing assay. (c) Transwell assay shows the invasion of EC109 cells. (d) Effect of ART on EC109 cell apoptosis by flow cytometry. (e) Result of cell cycle of EC109 cells after treatment with ART by flow cytometry. Each group represented the mean ± standard deviation (SD) of at least three independent experiments. **P < 0.01, and ***P < 0.001 versus control group ( ) G2/M (
) G2/M ( ) S (
) S ( ) G0/G1.
) G0/G1.
Artemisinin inhibits Wnt/β‐catenin signaling pathway
To determine whether ART treatment affected the Wnt/β‐catenin signaling pathway in EC109 cells, the key proteins of the Wnt/β‐catenin pathway were analyzed by western blot. The results showed that the expression of β‐catenin had the greatest reduction with the increase of ART concentration (P < 0.001) (Fig 2a). Further, we tested if the ART effect on β‐catenin could inhibit the transcription of the target genes of Wnt/β‐catenin (c‐myc and survivin). The qRT‐PCR and western blot analysis showed a decrease in mRNA and protein levels of c‐myc and survivin. This effect was also apparent in a concentration‐dependent manner upon treating with different concentrations of ART (P < 0.001) (Fig 2b,c).
Figure 2.
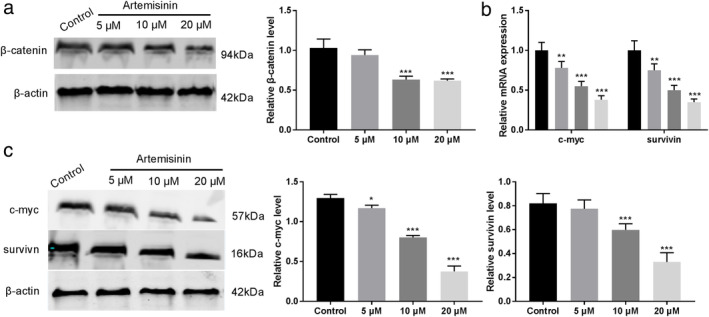
Artemisinin (ART) inhibits the Wnt/β‐catenin signaling pathway. (a) β‐catenin expression was downregulated in EC109 cells measured by western blot. (b) qRT‐PCR was used to measure the expression of c‐myc and survivin in EC109 cells treated with ART ( ) Control (
) Control ( ) 5 μM (
) 5 μM ( ) 10 μM (
) 10 μM ( ) 20 μM. (c) Western blot analysis was performed for c‐myc and survivin. Data shown are the means ± SD of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group.
) 20 μM. (c) Western blot analysis was performed for c‐myc and survivin. Data shown are the means ± SD of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group.
Artemisinin could suppress proliferation and metastasis of EC109 cells partially depending on the Wnt/β‐catenin pathway
To verify whether the aforementioned antitumor effects of ART were indeed associated with the Wnt/β‐catenin signaling pathway, ART (20 μM) treated cells were subjected to 20 mM LiCl, an activator of the Wnt/β‐catenin signaling pathway. Western blot assay demonstrated that ART mediated downregulation in the protein levels of β‐catenin, c‐myc, and survivin was attenuated upon treatment with LiCl (P < 0.001) (Fig 3a). Intriguingly, LiCl treatment also partially alleviated the inhibition of the cell proliferation caused by ART (P < 0.001) (Fig 3b). Moreover, ART mediated downregulation of cell migration and invasion was also recovered by LiCl treatment (Fig 3c,d). Also, cell flow cytometry assay elucidated that LiCl reduced the ART mediated apoptosis and decreased the cell arrest in the G0/G1 phase in EC109 cells (Fig 3e,f). Overall, these results strongly indicate the involvement of the Wnt/β‐catenin signaling pathway in ART‐mediated inhibition of EC progression.
Figure 3.
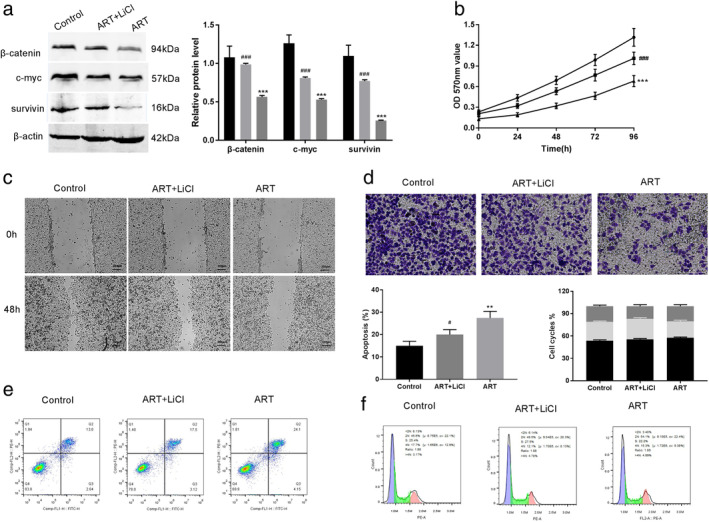
Artemisinin (ART) could suppress the proliferation and metastasis of EC109 cells depending on the Wnt/β‐catenin pathway. (a) Upon LiCl treatment, the increased protein level of β‐catenin, c‐myc, and survivin caused by ART was attenuated. (b) Cell proliferation impaired by the ART was drastically recovered ( ) Control (
) Control ( ) ART + LiCl (
) ART + LiCl ( ) ART. (c,d) The migration and invasive abilities of the ART‐treated cells were also recovered. (e) Apoptosis in the ART‐treated cells decreased (
) ART. (c,d) The migration and invasive abilities of the ART‐treated cells were also recovered. (e) Apoptosis in the ART‐treated cells decreased ( ) G2/M (
) G2/M ( ) S (
) S ( ) G0/G1. (f) The cell cycle arrest in the ART‐treated was also recovered. All results are presented as the mean ± SD of at least three independent experiments. ***P < 0.001 versus Control group, ###
P < 0.001 versus ART group.
) G0/G1. (f) The cell cycle arrest in the ART‐treated was also recovered. All results are presented as the mean ± SD of at least three independent experiments. ***P < 0.001 versus Control group, ###
P < 0.001 versus ART group.
Combination of artemisinin and oxaliplatin may reduce chemoresistance in EC109 cells
OXA, a chemotherapeutic drug is also used for the treatment of EC. We speculated that a combination of OXA and ART could improve the overall efficacy. Also, by reducing the amount of OXA in combination therapy compared to the single drug treatment might reduce the side effects of large doses. A MTT assay was conducted to investigate the effects of ART and OXA on the cell fate of EC109 cells. We found that after treating cells with ART, OXA, or ART+OXA (0–100 μM) for 48 hours, cell viabilities were significantly suppressed. Additionally, the efficiency of the cell inhibition was in the following order: ART+OXA > OXA > ART. The half‐maximal inhibitory concentration (IC50) of ART and OXA alone were 55.33 μM and 38.99 μM respectively. Interestingly, the IC50 of the combination (ART+OXA) was 18.31 μM (Fig 4).
Figure 4.
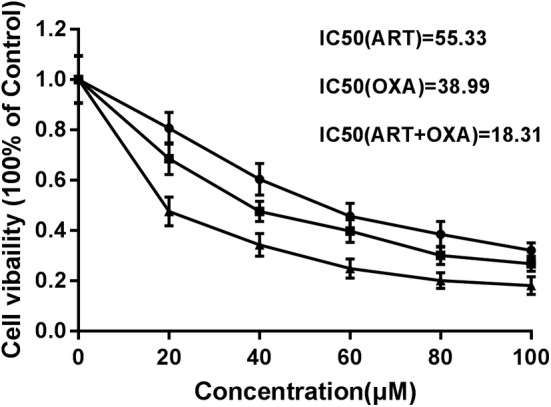
Artemisinin (ART) and oxaliplatin (OXA) reduces chemoresistance in EC109 cells. IC50 of ART, OXA, and the combination of ART and OXA were determined by treating parental EC109 cells in a dose‐dependent manner. The data are presented as the mean ± SD of at least three independent experiments ( ) ART (
) ART ( ) OXA (
) OXA ( ) ART+OXA.
) ART+OXA.
Artemisinin enhances the antitumor effects of oxaliplatin in EC109 cells
To further verify the combined effect of ART and OXA, we selected 20 μM ART and 18 μM OXA for the following experiments. MTT assay showed that cell proliferation against the ART+ OXA combination was significantly suppressed compared to the ART or OXA alone (P < 0.001) (Fig 5a). Similarly, in the wound healing assay, a combination of ART and OXA reduced cell migration more efficiently compared to either drug alone (Fig 5b). Also in the transwell assay, the effect of the combination of ART and OXA in reducing cell invasion was more pronounced compared to either ART or OXA (Fig 5c). Furthermore, when flow cytometry analysis was performed to determine the combined effect of ART and OXA on cell apoptosis, the apoptosis rate for the combination of ART + OXA was significantly higher compared to the individual drugs (P < 0.01) (Fig 5d). Similarly, greater accumulation of the cells arrested in the G0/G1 phase was observed for the combination than the ART or OXA treated cells (Fig 5e).
Figure 5.

Artemisinin (ART) enhances the antitumor effects of oxaliplatin (OXA) in EC109 cells. (a) An MTT assay showing the proliferation of EC cells treated with ART (20 μM) and/or OXA (18 μM) ( ) Control (
) Control ( ) ART (
) ART ( ) OXA (
) OXA ( ) ART+OXA. (b) Scratch wound healing assay showing the effect of ART and/or OXA on the migration of EC109 cells. (c) Transwell assay showing the effects of ART and/or OXA on the invasion of EC109 cells. (d) Effect of ART on EC109 cell apoptosis using flow cytometry. (e) Result of cell cycle of EC109 cells after treatment with ART using flow cytometry (
) ART+OXA. (b) Scratch wound healing assay showing the effect of ART and/or OXA on the migration of EC109 cells. (c) Transwell assay showing the effects of ART and/or OXA on the invasion of EC109 cells. (d) Effect of ART on EC109 cell apoptosis using flow cytometry. (e) Result of cell cycle of EC109 cells after treatment with ART using flow cytometry ( ) G2/M (
) G2/M ( ) S (
) S ( ) G0/G1. Each group represented the mean ± standard deviation (SD) of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control group. ##
P < 0.01, ###
P < 0.001 versus ART or OXA group.
) G0/G1. Each group represented the mean ± standard deviation (SD) of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control group. ##
P < 0.01, ###
P < 0.001 versus ART or OXA group.
Discussion
There is a high incidence of EC in China. 26 Despite recent advances in the treatment of EC, the prognosis for EC remains poor. 27 Several studies have shown the potential antitumor therapeutic effects of ART both in vitro and in vivo. 17 , 18 In this study, we assessed the efficacy of ART against EC cell line EC109. We found that ART suppressed the growth and promoted cell apoptosis in a dose‐dependent manner. Furthermore, wound healing and transwell assays also revealed the ART mediated dose‐dependent decrease in cell migration and invasion. More strikingly, cell cycle arrest of EC109 cells was also observed upon treatment with ART. In total, these results strongly suggest that ART could be a potential chemopreventive agent for EC.
The Wnt/β‐catenin pathway plays a critical role in every stage of cancer progression, including cell proliferation, development, and metastasis. 15 , 16 , 28 , 29 Furthermore, the Wnt/β‐catenin signaling pathway can be effectively inhibited by ART. 17 , 18 , 30 In this study, we also observed the inhibitory effect of ART on the hyperactive Wnt/β‐catenin signaling pathway. The Wnt/β‐catenin target genes, C‐myc and survivin, are reported to be upregulated in EC, 31 and a decrease in expression of these genes promote cell apoptosis and inhibits cell division. 32 Importantly, ART treatment significantly decreased the levels of β‐catenin, c‐myc, and survivin. Furthermore, the experiments were also carried out using the Wnt/β‐catenin signaling pathway activator LiCl 15 in combination with ART. The results from these experiments strongly suggested that inhibition of the Wnt/β‐catenin signaling pathway was indeed mediated by ART. Overall, these findings suggest that ART may impede the development of EC through the Wnt/β‐catenin signaling pathway.
Chemotherapy is a double‐edged sword, along with antitumor effectiveness; it can simultaneously lead to several side effects such as diarrhea, headache, and ototoxicity. 33 , 34 In the combination chemotherapy treating esophageal squamous carcinoma, OXA is more effective but less toxic than cisplatin. 4 However, its wider application is still limited by the side‐effects and drug resistance. Therefore, in this study, we explored the combinational antimetastatic effect of OXA with ART. Interestingly, in comparison to the individual drugs, the combination of ART and OXA was more efficient in inhibiting the cell proliferation, migration, and invasion in the EC109 cells. This strongly suggests that ART could also enhance the antitumor effects of OXA in EC.
In conclusion, our results showed that artemisinin inhibited cell proliferation, migration, and invasion but promoted apoptosis in EC109 cells. These effects were facilitated by the inhibition of the Wnt/β‐catenin signaling pathway. Furthermore, ART also enhanced the antitumor effect of OXA in EC. Although this study was limited to EC109 cells, in future more EC cell lines should be investigated to strengthen these findings. Nevertheless, these results significantly suggest that ART together with OXA can be used for the clinical treatment of EC.
Disclosure
The authors have no conflicts of interest to report.
Contributor Information
Tao Wang, Email: wangtao_med@163.com.
Xiao‐Mei Zhang, Email: zhangxm92_qhsu@163.com.
References
- 1. Fitzmaurice C, Dicker D, Pain A et al The global burden of cancer 2013. JAMA Oncol 2015; 1: 505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang Y, Liu G, Yuan D, Li C, Xue M, Chen L. Influence of exosome‐derived miR‐21 on chemotherapy resistance of esophageal cancer. Eur Rev Med Pharmacol Sci 2019; 23: 1513–9. [DOI] [PubMed] [Google Scholar]
- 3. Guo L, Zhang L, Zhao J. CT scan and magnetic resonance diffusion‐weighted imaging in the diagnosis and treatment of esophageal cancer. Oncol Lett 2018; 16: 7117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen Z, Xu L, Li J, Zhang N. Capilliposide C sensitizes esophageal squamous carcinoma cells to Oxaliplatin by inducing apoptosis through the PI3K/Akt/mTOR pathway. Med Sci Monit 2017; 23: 2096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi Q, Diao Y, Jin F, Ding Z. Anti‐metastatic effects of Aidi on human esophageal squamous cell carcinoma by inhibiting epithelial‐mesenchymal transition and angiogenesis. Mol Med Rep 2018; 18: 131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003; 3: 768–80. [DOI] [PubMed] [Google Scholar]
- 7. Tu Y. Artemisinin‐a gift from traditional Chinese medicine to the world (Nobel lecture). Angew Chem Int Ed Engl 2016; 55: 10210–26. [DOI] [PubMed] [Google Scholar]
- 8. Talisuna AO, Karema C, Ogutu B et al Mitigating the threat of artemisinin resistance in Africa: Improvement of drug‐resistance surveillance and response systems. Lancet Infect Dis 2012; 12: 888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Current perspectives on the mechanism of action of artemisinins. Int J Parasitol 2006; 36: 1427–41. [DOI] [PubMed] [Google Scholar]
- 10. Bhaw‐Luximon A, Jhurry D. Artemisinin and its derivatives in cancer therapy: Status of progress, mechanism of action, and future perspectives. Cancer Chemother Pharmacol 2017; 79: 451–66. [DOI] [PubMed] [Google Scholar]
- 11. Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol 2017; 46: 65–83. [DOI] [PubMed] [Google Scholar]
- 12. Slezakova S, Ruda‐Kucerova J. Anticancer activity of Artemisinin and its derivatives. Anticancer Res 2017; 37: 5995–6003. [DOI] [PubMed] [Google Scholar]
- 13. Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol 2004; 48: 477–87. [DOI] [PubMed] [Google Scholar]
- 14. Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and eastern countries. World J Gastroenterol 2015; 21: 7933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Q, Lv Q, Bian H et al A novel tumor suppressor SPINK5 targets Wnt/β‐catenin signaling pathway in esophageal cancer. Cancer Med 2019; 8: 2360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang LN, Zhao L, Yan XL, Huang YH. Loss of G3BP1 suppresses proliferation, migration, and invasion of esophageal cancer cells via Wnt/β‐catenin and PI3K/AKT signaling pathways. J Cell Physiol 2019; 234: 20469–84. [DOI] [PubMed] [Google Scholar]
- 17. Li LN, Zhang HD, Yuan SJ, Tian ZY, Wang L, Sun ZX. Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/beta‐catenin pathway. Int J Cancer 2007; 121: 1360–5. [DOI] [PubMed] [Google Scholar]
- 18. Tong Y, Liu Y, Zheng H et al Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β‐catenin signaling. Oncotarget 2016; 7: 31413–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang JW, Yeh HL, Hsu CP et al Phase II study of preoperative concurrent chemoradiotherapy with oxaliplatin for locally advanced esophageal cancer. J Chin Med Assoc 2017; 80: 401–7. [DOI] [PubMed] [Google Scholar]
- 20. Montagnani F, Turrisi G, Marinozzi C, Aliberti C, Fiorentini G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: A systematic review and meta‐analysis. Gastric Cancer 2011; 14: 50–5. [DOI] [PubMed] [Google Scholar]
- 21. Hecht JR, Bang YJ, Qin SK et al Lapatinib in combination with Capecitabine plus Oxaliplatin in human epidermal growth factor receptor 2‐positive advanced or metastatic gastric, esophageal, or Gastroesophageal adenocarcinoma: TRIO‐013/LOGiC–A randomized phase III trial. J Clin Oncol 2016; 34: 443–51. [DOI] [PubMed] [Google Scholar]
- 22. Hsu HH, Chen MC, Baskaran R et al Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J Cell Physiol 2018; 233: 5458–67. [DOI] [PubMed] [Google Scholar]
- 23. Cavanna L, Artioli F, Codignola C et al Oxaliplatin in combination with 5‐fluorouracil (5‐FU) and leucovorin (LV) in patients with metastatic gastric cancer (MGC). Am J Clin Oncol 2006; 29: 371–5. [DOI] [PubMed] [Google Scholar]
- 24. Peinert S, Grothe W, Stein AD et al Safety and efficacy of weekly 5‐fluorouracil/folinic acid/oxaliplatin/irinotecan in the first‐line treatment of gastrointestinal cancer. Ther Adv Med Oncol 2010; 2: 161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 26. Liu HB, Yang QC, Shen Y, Zhu Y, Zhang XJ, Chen H. A disintegrin and metalloproteinase 17 mRNA and protein expression in esophageal squamous cell carcinoma, as well as its clinicopathological factors and prognosis. Mol Med Rep 2015; 11: 961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu X, Wang Z, Zhang G et al Overexpression of asparaginyl endopeptidase is significant for esophageal carcinoma metastasis and predicts poor patient prognosis. Oncol Lett 2018; 15: 1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hubaux R, Thu KL, Lam WL. Re: The Wnt signaling pathway in non‐small cell lung cancer. J Natl Cancer Inst 2014; 106: dju188. [DOI] [PubMed] [Google Scholar]
- 29. Peng YY, He YH, Chen C et al NLRC5 regulates cell proliferation, migration and invasion in hepatocellular carcinoma by targeting the Wnt/β‐catenin signaling pathway. Cancer Lett 2016; 376: 10–21. [DOI] [PubMed] [Google Scholar]
- 30. Zhong G, Liang R, Yao J et al Artemisinin ameliorates osteoarthritis by inhibiting the Wnt/β‐catenin signaling pathway. Cell Physiol Biochem 2018; 51: 2575–90. [DOI] [PubMed] [Google Scholar]
- 31. Zhou SL, Yue WB, Fan ZM et al Autoantibody detection to tumor‐associated antigens of P53, IMP1, P16, cyclin B1, P62, C‐myc, Survivn, and Koc for the screening of high‐risk subjects and early detection of esophageal squamous cell carcinoma. Dis Esophagus 2014; 27: 790–7. [DOI] [PubMed] [Google Scholar]
- 32. Watson AJ. An overview of apoptosis and the prevention of colorectal cancer. Crit Rev Oncol Hematol 2006; 57: 107–21. [DOI] [PubMed] [Google Scholar]
- 33. Schramm A, De Gregorio N, Widschwendter P, Fink V, Huober J. Targeted therapies in HER2‐positive breast cancer ‐ a systematic review. Breast Care (Basel) 2015; 10: 173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kramer R, Bielawski J, Kistner‐Griffin E et al Neurotoxic 1‐deoxysphingolipids and paclitaxel‐induced peripheral neuropathy. FASEB J 2015; 29: 4461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


