Abstract
Sophora flavescens is used as a traditional herbal medicine to modulate inflammatory responses. However, little is known about the impact of (‐)‐maackiain, a compound derived from S. flavescens, on the activation of inflammasome/caspase‐1, a key factor in interleukin‐1β (IL‐1β) processing. Here, we report that (‐)‐maackiain potently amplified caspase‐1 cleavage in macrophages in response to nigericin (Nig). In macrophages primed with either lipopolysaccharide or monophosphoryl lipid A, Nig‐mediated caspase‐1 cleavage was also markedly promoted by (‐)‐maackiain. Notably, (‐)‐maackiain induced the production of vimentin, an essential mediator for the activation of the NOD‐, LRR‐, and pyrin domain‐containing protein 3 inflammasome, thereby contributing to promotion of the formation of the inflammasome complex to activate caspase‐1. Taken together, our data suggest that (‐)‐maackiain exerts an immunostimulatory effect by promoting IL‐1β production via activation of the inflammasome/caspase‐1 pathway. Thus, the potent inflammasome‐activating effect of (‐)‐maackiain may be clinically useful as an acute immune‐stimulating agent.
Keywords: (‐)‐maackiain, inflammasome, monophosphoryl lipid A, nigericin, Sophora flavescens
Proinflammatory cytokine interleukin‐1β (IL‐1β) plays critical roles in defending against infections. Sophora flavescens‐derived (‐)‐maackiain potently amplifies caspase‐1 cleavage in response to nigericin. Notably, (‐)‐maackiain promotes caspase‐1 cleavage and IL‐1β production even in monophosphoryl lipid A‐primed macrophages. The potent inflammasome‐activating effect of (‐)‐maackiain may be clinically useful as an acute immune‐stimulating agent.
Abbreviations
- alum
Aluminum salts
- GSDMD
gasdermin D
- IL‐1β
interleukin‐1β
- LPS
lipopolysaccharide
- MPL
monophosphoryl lipid A
- NF‐κB
nuclear factor kappa B
- NLRP3
NOD‐, LRR‐, and pyrin domain‐containing protein 3
- TLR
Toll‐like receptor
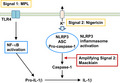
In diverse cell types, including monocytes and macrophages, the key proinflammatory cytokine interleukin‐1β (IL‐1β) is produced by a two‐step process: an initial priming signal and a second activating signal [1]. Production of the pro‐form of IL‐1β is driven by nuclear factor kappa B (NF‐κB), which is activated by Toll‐like receptor (TLR) signaling in response to a priming signal, for example, pathogen‐associated molecular patterns including lipopolysaccharide (LPS). In response to the activating signal [e.g., nigericin (Nig), a toxin derived from Streptomyces hygroscopicus [2]], pro‐IL‐1β is cleaved to its active form by the inflammasome, a multiprotein complex comprising apoptosis‐associated speck‐like protein containing a CARD (ASC), NOD‐like receptor, and pro‐caspase‐1. Once the inflammasome is activated, pro‐caspase‐1 is cleaved to yield its enzymatically active heterodimer, consisting of subunits p10 and p20. The active caspase cleaves not only pro‐IL‐1β, but also gasdermin D (GSDMD), a caspase‐1 substrate that forms membrane pores [2, 3]. The cleaved form of GSDMD then facilitates the release of biologically active IL‐1β.
Released IL‐1β stimulates acute inflammatory responses involved in defense against microbial infections, including influenza [4]. Consistent with this, intranasal delivery of IL‐1β or mucosal delivery of IL‐1β‐encoding adenoviral vectors along with influenza vaccine boosts immune responses against influenza infections, demonstrating that its immune‐stimulatory effect is physiologically important [5, 6]. Elderly mice infected with influenza produce less amounts of active IL‐1β due to reductions in the levels of inflammasome‐related proteins, although they produce normal levels of pro‐IL‐1β [7]. This suggests that these animals have normal NF‐κB activation, but are deficient in stimulation of the inflammasome. Notably in this regard, the impairment in inflammasome activity is rescued to some extent by Nig treatment, which increases caspase‐1‐augmented IL‐1β production during influenza infection [7]. This observation implies that it could be clinically useful to amplify the activation of inflammasome/caspase‐1.
In East Asian countries, the root of Sophora flavescens is used as a traditional herbal medicine for the treatment of infectious diseases [8], cancer [9], and inflammatory disorders [10]. Typically, it is well known as a traditional antipyretic medicine by reducing inflammatory responses, and the anti‐inflammatory activity was proven by inhibiting the release of proinflammatory cytokines, including TNF‐α, IL‐6, and MCP‐1, on LPS‐induced RAW264.7 cells [11]. Given that S. flavescens and its preparations, such as Fufang Kushen Lotion and Fufang Kushen Injection, have been clinically used to treat the diseases [12, 13], phytochemical studies have shown that it contains obvious flavonoids with pleiotropic activities including anti‐inflammatory [14, 15]. In addition, (‐)‐maackiain, which was originally isolated from Maackia amurensis var [16], is widely distributed as a flavonoid analog in different plant genus including Sophora [17, 18], and its antimicrobial effects have been reported [17].
Previously, we reported that treatment with total S. flavescens extracts decreases Pseudomonas aeruginosa‐mediated expression of IL‐1β [19]. Unlike the anti‐inflammatory effects shown by the extract, we found the stimulating effect of (‐)‐maackiain, one of the compounds derived from S. flavescens, on the activation of inflammasome in macrophages following treatment with Nig, a microbial toxin that activates the NOD‐, LRR‐, and pyrin domain‐containing protein 3 (NLRP3) inflammasome. In these experiments, macrophages were first primed with either LPS or monophosphoryl lipid A (MPL), both of which activate key innate immune pathways through TLR4. The activating effect of (‐)‐maackiain was proven by the increase in caspase‐1 cleavage once treated with Nig, and this can lead to enhanced production of IL‐1β in either LPS‐ or MPL‐primed cells. In light of the physiological importance of inflammasome/caspase‐1 activation, our results highlight the potential use of (‐)‐maackiain to boost acute immune‐stimulatory responses.
Materials and methods
Reagents
Dried extract of S. flavescens and its derivative compounds were purchased from KPEB (Korea Plant Extract Bank, Cheongju, Republic of Korea; http://extract.kribb.re.kr). P. aeruginosa‐derived LPS, Nig, and acetyl‐tyrosyl‐valyl‐alanyl‐aspartyl‐chloromethylketone (ac‐YVAD‐cmk) were purchased from Sigma‐Aldrich (St. Louis, MO, USA). MPL and alum were purchased from InvivoGen (San Diego, CA, USA).
Instruments
NMR spectra were recorded on a Bruker AM500 instrument (1H NMR at 500 MHz, 13C NMR at 125 MHz; Bruker, Karlsruhe, Germany), and tetramethylsilane was used as the internal standard. Chemical shifts are reported in p.p.m. (parts per million) and coupling constants (J) in Hz. Ultra‐performance liquid chromatography (UPLC) was performed using an ACQUITY UPLC™ system (Waters Corporation, Milford, MA, USA) equipped with a binary solvent delivery manager, a photodiode array (PDA). HRESIMS was performed on a Waters Q‐Tof Premier™ Mass Spectrometer equipped with an electrospray interface (Waters Corporation). Separations were conducted on an Armen Spot Prep II 250 medium‐pressure liquid chromatograph (MPLC) and on a PLC 2020 prep/high‐performance liquid chromatograph (prep‐HPLC; Gilson, Inc., Middleton, WI, USA) using an appropriately sized reversed‐phase silica gel column purchased from Waters. All solvents used for column chromatography were of analytical grade (SK Chemicals Co. Ltd., Seongnam, Korea), and all solvents used for HPLC were of HPLC grade (SK Chemicals Co., Ltd.). NMR solvents were purchased from Cambridge Isotope Laboratories Inc. (Andover, MA, USA).
Extraction and isolation
Sophora flavescens was collected from Yeongwol‐gun, Gangwon‐do, Korea, in 2016. The plant (50 g) dried in the shade and powdered was added to 1 L of methanol (MeOH; HPLC Grade) with an ultrasonicator (SDN‐900H; SD Ultrasonic Cleaner, Seoul, Korea) at room temperature for 3 days (15‐min ultrasonication followed by 120‐min standing per cycle; repeated 30 cycles). After filtration and drying under reduced pressure, S. flavescens extract (5.63 g, 11.2%) was obtained. Crude extract (about 950 mg) was submitted to MPLC (Armen Spot Prep II 250; Gilson, Inc) reversed‐phase silica gel (YMC ODS‐AQ, 10 µm, 220 g) using a stepwise MeOH‐H2O gradient (35–100% MeOH, 20 mL·min−1, 90 min) to give nine fractions. Each of SF Fr.4 (117.6 mg), SF Fr.7 (99.6 mg), and SF Fr.8 (156.0 mg) was subjected to preparative chromatography using PLC2020 prep‐HPLC eluted with H2O‐MeOH gradient (30–60% MeOH) to yield 4‐hydroxy‐3‐methoxy‐8,9‐methylenedioxy pterocarpan (7; 8.8 mg), formononetin (1; 11.3 mg), (‐)‐maackiain (5; 8.5 mg), and sophoraflavanone B (8; 15.7 mg) from SF Fr.4; noranhydroicaritin (6; 10.4 mg), kushenol F (4; 37.5 mg), and (2S)‐2'‐methoxykurarinone (2; 80.5 mg) from SF Fr.7; and kushenol E (3; 13.2 mg) from SF Fr.8, respectively. Purities (> 98%) were confirmed by UPLC‐PDA‐Q/TOF‐M. Purified compounds were identified by comparing 1H NMR, 13C NMR, MS, MS/MS, HRESIMS, and optical rotation data with literature values (Supporting information).
Bacterial culture and infection condition
Pseudomonas aeruginosa PAO1 wild‐type strain [20] was cultured in Luria (L) broth or on L agar plates at 37 °C. The cultured bacterial cells were harvested by centrifugation at 10 000 g for 20 min at 4 °C after overnight broth culture. The bacterial pellet was suspended in PBS for the preparation of live bacteria. Bacterial infection was performed according to the conditions previously described [19]. Briefly, cells were infected with P. aeruginosa strain PAO1 at a multiplicity of infection of 10 for 4 h.
Cell culture and treatment condition
RAW‐Blue cells (mouse macrophage reporter cells; InvivoGen) were maintained in Dulbecco’s modified Eagle’s medium (containing high glucose, l‐glutamine, and sodium pyruvate; HyClone, Logan, UT, USA). A549 (human alveolar epithelial) and THP‐1 (human monocyte) cells were cultured in RPMI‐1640 (HyClone). Media supplemented with 10% heat‐inactivated FBS (Access, Vista, CA, USA), penicillin (100 units per mL), and streptomycin (0.1 mg·mL−1) were used to cultivate cells. Cells were maintained at 37 °C in a humidified 5% CO2 air‐jacketed incubator. Differentiation of THP‐1 cells (dTHP‐1) was achieved by the treatment with PMA (phorbol‐12‐myristate‐13‐acetate; 50 ng·mL−1) for 48 h followed by resting for 24 h in the presence of FBS. Unless otherwise indicated, cells were exposed to the following condition as described. For the assay of alkaline phosphatase activity, cells were pretreated with either S. flavescens extract (Ext; 50 ng·mL−1) or the derivative compounds (1‐8; 50 ng·mL−1) for the indicated time. For the stimulation of inflammasome activity, cells were primed by treatment with 100 ng·mL−1 of either LPS or MPL for 3 h. Then, cells were sequentially treated with Nig (5 µm) for 1 h and either (‐)‐maackiain (MK; 100 ng·mL−1) or alum (300 ng·mL−1) for 1 h.
Alkaline phosphatase assay
RAW‐Blue cells are derived from RAW 264.7 macrophages with chromosomal integration of a secreted embryonic alkaline phosphatase (SEAP) reporter gene inducible by NF‐κB. Measurement of SEAP was performed according to the method previously described [19].
Determination of cell viability
The cell culture media from component‐treated cells were analyzed for lactate dehydrogenase (LDH) release using a commercially available kit (CytoTox 96 Non‐Radioactive Cytotoxicity Assay; Promega, Madison, WI, USA) following the manufacturer’s instructions.
Immunoblot analysis
Antibodies were used to analyze total cell lysates as per the manufacturers’ instructions. Antibodies of pro‐caspase‐1 (D7F10), caspase‐1 p20 (D57A2), pro‐IL‐1β (D3U3E), mature IL‐1β (D3U3E), cleaved GSDMD (L60), NLRP3 (D2P5E), NEK7 (C34C3), vimentin (D21H3), and β‐actin (D6A8) were purchased from Cell Signaling (Danvers, MA, USA). GSDMD (PA5‐30823) antibody was purchased from Thermo Scientific (Rockford, IL, USA). Immunoblot analysis was performed according to the method previously described [19].
Cell ELISA
The level of IL‐1β in culture supernatants was determined by microplate sandwich ELISA using human IL‐1β ELISA Kits (Thermo, Rockford, IL, USA) following the manufacturer's instructions.
Statistics
All experiments were carried out in triplicate. Results were expressed as mean ± SD. The Student t‐test was used to perform statistical analysis, with significance considered to be P < 0.05.
Results
Screening of S. flavescens‐derived compounds
Previously, we reported that treatment with total S. flavescens extracts to RAW‐Blue cells decreases P. aeruginosa‐mediated expression of IL‐1β by inhibiting NF‐κB activation [19]. To extend this observation, we obtained eight compounds derived from S. flavescens from KPEB (Supporting information) and examined the effect of each compound on NF‐κB activity in RAW‐Blue cells. The cells are mouse macrophage reporter cells, which are typically designed for the detection of NF‐κB activation through a SEAP reporter gene assay. As shown in Fig. 1A, six of the eight compounds (i.e., all except for 1 and 7) suppressed activation of NF‐κB induced by P. aeruginosa strain PAO1, similar to the effects of the total extract (Ext). Next, we analyzed cytotoxicity of A549 cells by measuring the release of LDH. Compounds 1 and 5 as well as total Ext significantly decreased the release of LDH relative to the other compounds (Fig. 1B), suggesting that two compounds have low cytotoxicity. Thus, these data suggested that compound 5‐mediated NF‐κB suppression is not caused by its cytotoxicity so that we select the compound 5 [(‐)‐maackiain; C16H12O5; MW 284.3] for further study.
Fig. 1.
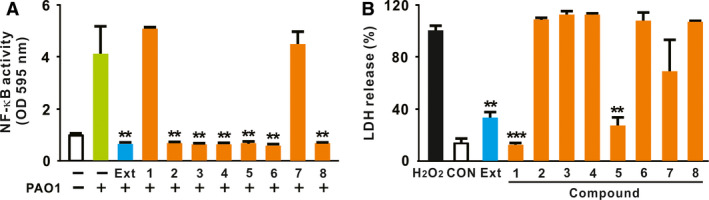
Screening of S. flavescens‐derived compounds. (A) RAW‐Blue cells were seeded in 12‐well plates at a concentration of 1 × 106 cell per mL, and the cells were pretreated with either S. flavescens total Ext or the derivative compounds (1‐8) for 1 h. Cells were then infected with P. aeruginosa strain PAO1. After treatment, bacteria‐induced NF‐κB activation was measured by alkaline phosphatase assay. (B) A549 cells were seeded in 12‐well plates at a concentration of 2 × 105 cell per mL, and the cells were treated with either total Ext or the derivative compounds (1‐8) for 24 h. After treatment, cytotoxicity was assessed by LDH release assay. Data are expressed as means ± SD (n = 3). Student’s t‐test: **P < 0.01, ***P < 0.001 vs. treatment with PAO1 alone (A) and H2O2 treatment (B). PBS was used as a control (CON). Compounds: 1, formononetin; 2, (2S)‐2’‐methoxykurarinone; 3, kushenol E; 4, kushenol F; 5, (‐)‐maackiain; 6, noranhydroicaritin; 7, 4‐hydroxy‐3‐methoxy‐8,9‐methylenedioxypterocarpan; 8, sophoraflavanone B.
(‐)‐Maackiain amplifies the activation of caspase‐1 induced by nigericin
Previously, it was reported that (‐)‐maackiain 3‐O‐glucoside as a (‐)‐maackiain analog inhibits LPS‐mediated expression of proinflammatory mediators, such as TNFα, IL‐6, and COX‐2, in macrophages probably through the inhibition of NF‐κB activation [10]. This led us to examine the effect of (‐)‐maackiain on the second activating signal, which is required for the maturation of IL‐1β through the activation of inflammasome/caspase‐1 [2]. To determine the effect, we first primed cells with LPS and then treated sequentially with nigericin and (‐)‐maackiain. Intriguingly, as shown in Fig. 2A, formation of the cleaved form (p20) of caspase‐1 was stimulated by treatment with nigericin and (‐)‐maackiain (Lane 7) to a much greater extent than treatment with nigericin alone (Lane 3). No cleavage was observed in response to treatment with (‐)‐maackiain alone (Lane 5). These imply that (‐)‐maackiain itself is not sufficient to initiate cleavage, but has an amplifying effect on the cleavage. Caspase‐1 cleavage was induced by the combined treatment with nigericin and (‐)‐maackiain (Lane 7) much more strongly than by treatment with two well‐known agonistic ligands, LPS and nigericin (Lane 4). However, treatment with LPS and (‐)‐maackiain (Lane 6) did not increase production of pro‐IL‐1β relative to treatment with LPS alone (Lane 2), suggesting that (‐)‐maackiain does not have an effect on the LPS‐mediated stimulation. This was further confirmed by measuring the release of mature IL‐1β, as shown in Fig. 2B. Taken together, these results demonstrate that (‐)‐maackiain plays a critical but discrete role in boosting the cleavage of caspase‐1 in response to nigericin.
Fig. 2.
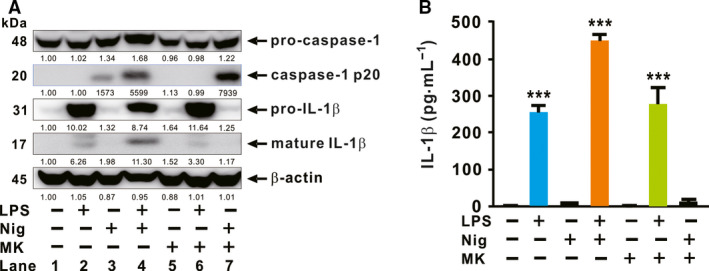
(‐)‐Maackiain amplifies the activation of caspase‐1 induced by nigericin. dTHP‐1 cells were seeded in 12‐well plates at a concentration of 7 × 105 cell per mL, and the cells were primed by treatment with LPS and then treated sequentially with Nig and (‐)‐maackiain (MK). (A) Cleavages of pro‐caspase‐1 and pro‐IL‐1β were assessed by immunoblot analysis. Densitometry was shown below each immunoblot for quantitative analysis. (B) IL‐1β level was measured by cell ELISA. Immunoblot data in A are representative of three separate experiments. Data in B are expressed as means ± SD (n = 3). Student’s t‐test: ***P < 0.001 vs. no treatment.
(‐)‐Maackiain‐activated caspase‐1 is biologically functional
To obtain insight into the biological function of cleaved caspase‐1, we measured the level of cleaved GSDMD in macrophages. Cleavage of GSDMD is facilitated by the action of enzymatically active caspase‐1, which is crucial for canonical inflammasome activation. Then, the cleaved form of GSDMD induces pore formation in the plasma membrane, resulting in a distinctive form of programmed cell death known as pyroptosis, which facilitates the release of biologically active IL‐1β [3]. As shown in Fig. 3A, treatment with nigericin and (‐)‐maackiain promoted cleavage of pro‐caspase‐1 and GSDMD, which are assessed by immunoblot analysis. However, this treatment did not markedly alter production of NLRP3, which is under the control of NF‐κB, further supporting the idea that (‐)‐maackiain mainly stimulates caspase‐1 activation. No cleavage was observed in response to treatment with (‐)‐maackiain alone. Next, given that caspase‐4/5/11 required for noncanonical inflammasome activation triggers the cleavage of GSDMD, we sought to determine whether the (‐)‐maackiain promoted cleavage depends on caspase‐1 by pretreating with ac‐YVAD‐cmk (YVAD), a specific chemical inhibitor of the caspase‐1. As shown in Fig. 3B, the pretreatment clearly blocked the cleavage of GSDMD, indicating that (‐)‐maackiain‐mediated enhancement of GSDMD cleavage is specific to caspase‐1. The efficacy of inhibitor was confirmed by immunoblotting for cleaved form of caspase‐1. Consistent with the elevated level of cleaved GSDMD, cytotoxicity also gradually increased whereas treatment with (‐)‐maackiain alone did not cause cytotoxicity (Fig. 3C). Furthermore, treatment of LPS‐primed macrophages with nigericin and (‐)‐maackiain amplified not only the cleavage of pro‐caspase‐1, but also the production of mature IL‐1β, in a (‐)‐maackiain dose‐dependent manner (Fig. 3D). Next, we looked for mechanistic insights to address how (‐)‐maackiain might work to enhance the activation of caspase‐1. To achieve this, we analyzed the expression level of regulator proteins, such as vimentin and NEK7, which are required for the activation of NLRP3 inflammasome [21, 22]. As shown in Fig. 3E, (‐)‐maackiain treatment markedly increased the production of vimentin in response to nigericin but not NEK7, implying that (‐)‐maackiain‐mediated caspase‐1 activation could be attributed to the action of vimentin.
Fig. 3.
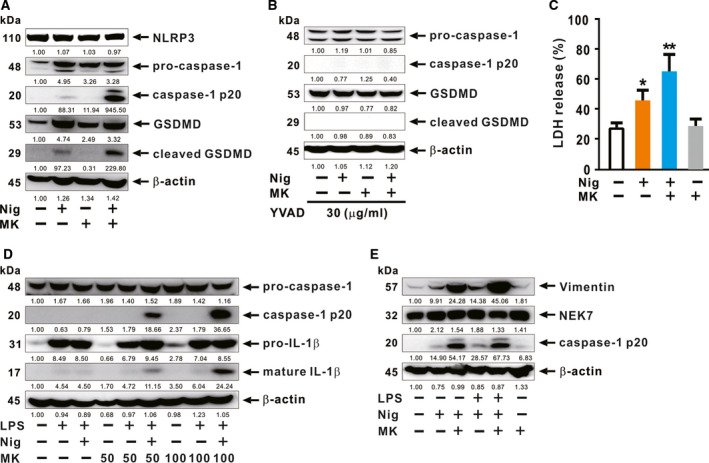
(‐)‐Maackiain‐activated caspase‐1 is biologically functional. dTHP‐1 cells were seeded in 12‐well plates at a concentration of 7 × 105 cell per mL. (A‐C) The cells were treated sequentially with nigericin (Nig) and (‐)‐maackiain (MK). Cells were pretreated with the 30 µg·mL−1 of caspase‐1 inhibitor (YVAD) for 1 h (B). Immunoblot analysis (A, B) and LDH release assay (C) were performed. (D, E) The cells were primed by treatment with LPS and then treated sequentially with Nig and (‐)‐maackiain (MK). Immunoblot analysis was performed. Densitometry was shown below each immune blot for quantitative analysis. Immunoblot data are representative of three separate experiments. Data in C are expressed as means ± SD (n = 3). Student’s t‐test: *P < 0.05, **P < 0.01 vs. no treatment.
(‐)‐Maackiain amplifies nigericin‐mediated caspase‐1 activation in MPL‐primed macrophages
In contrast to LPS, MPL cannot activate the NLRP3 inflammasome and therefore fails to induce maturation of IL‐1β [23]. To determine whether (‐)‐maackiain can rescue the impaired activation of inflammasome in response to MPL, we compared the (‐)‐maackiain‐mediated cleavage of caspase‐1 between LPS‐ and MPL‐primed macrophages. As shown in Fig. 4A, consistent with previous reports, LPS priming (Lane 3) increased caspase‐1 cleavage to a greater extent than MPL priming (Lane 8). Furthermore, caspase‐1 activation in MPL‐primed macrophages was increased to a greater extent by supplement with (‐)‐maackiain in addition to nigericin (Lane 9) than treatment with nigericin (Lane 8). The increase shown at Lane 9 in MPL‐primed macrophages was even greater than that induced by treatment with nigericin in LPS‐primed macrophages (Lane 3; a well‐known agonistic ligand combination), implying that (‐)‐maackiain rescues impaired inflammasome activation by MPL. Next, because nigericin may cause cytotoxicity by decreasing the intracellular levels of ATP, GTP, and ADP through inhibition of cellular respiration [24], we asked whether the stimulatory effect of (‐)‐maackiain would still be observed upon treatment with lower concentrations of nigericin (1 or 2 µm). As shown in Fig. 4B, a clear increase in IL‐1β production was still observed upon treatment with lower levels of nigericin, providing further proof of the compound’s ability to stimulate inflammasome activation.
Fig. 4.
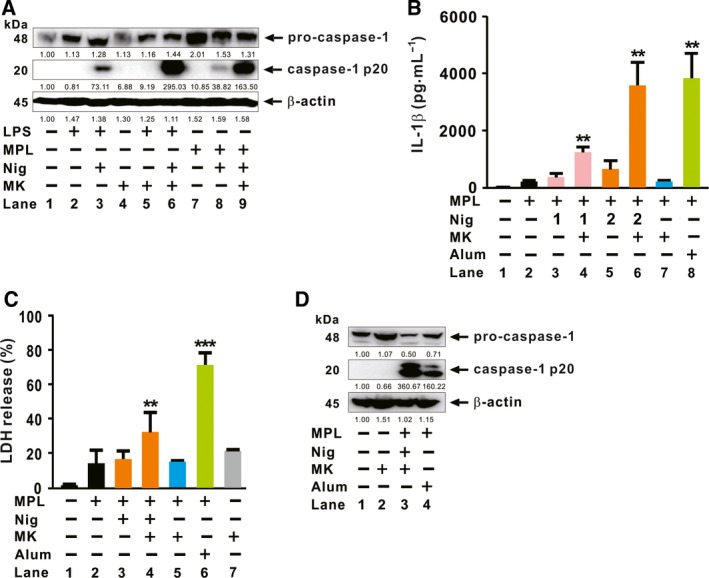
(‐)‐Maackiain amplifies nigericin‐mediated caspase‐1 activation in MPL‐primed macrophages. dTHP‐1 cells were seeded in 12‐well plates at a concentration of 7 × 105 cell per mL, and the cells were primed with either LPS or MPL. (A) The cells were treated sequentially with Nig and (‐)‐maackiain (MK). Activation of caspase‐1 was assessed by immunoblot analysis. (B‐D) The cells were treated sequentially with Nig (1 or 2 µm for B; 2 µm for C and D) and either (‐)‐maackiain (MK) or alum. ELISA (B), LDH release assay (C), and immunoblot analysis (D) were performed. Densitometry was shown below each immune blot for quantitative analysis. Immunoblot data in A and D are representative of three separate experiments. Data in B and C are expressed as means ± SD (n = 3). Student’s t‐test: **P < 0.01, ***P < 0.001 vs. no treatment.
To boost the adjuvant potential of MPL, GlaxoSmithKline Biologicals developed a combination adjuvant designated AS04, which contains alum [25, 26]. Given that MPL cannot activate NLRP3 inflammasome, the addition of alum complements the potential of MPL, resulting in activation of the NLRP3 inflammasome and in subsequent production of mature IL‐1β [27, 28]. We sought to determine whether (‐)‐maackiain‐mediated activation of the inflammasome is comparable to the effect mediated by alum in MPL‐primed macrophages. As shown in Fig. 4B, in MPL‐primed macrophages, IL‐1β release was stimulated by treatment with nigericin and (‐)‐maackiain (Lane 6), similar to the effect of treatment with alum (Lane 8), indicating that (‐)‐maackiain had a comparable stimulatory effect on the release of IL‐1β. Consistent with Fig. 4A (Lanes 8 and 9), treatment of MPL‐primed macrophages with (‐)‐maackiain in addition to nigericin (Lane 6 in Fig. 4B) stimulated release of IL‐1β to a greater extent than treatment with nigericin (Lane 5 in Fig. 4B). In addition, treatment of MPL‐primed macrophages with (‐)‐maackiain (Lane 7 in Fig. 4B) did not increase IL‐1β release relative to MPL priming alone (Lane 2 in Fig. 4B), again verifying the effect of (‐)‐maackiain on inflammasome activation, but not on the state created by the priming signal (i.e., MPL treatment). Then, we compared the cytotoxicity of (‐)‐maackiain and alum. As shown in Fig. 4C, cytotoxicity was lower in MPL‐primed macrophages treated with nigericin (2 µm) and (‐)‐maackiain (Lane 4) than in cells treated with alum (Lane 6), although cytotoxicity in Lane 4 was slightly higher than that induced by the treatment of MPL‐primed macrophages with either nigericin (Lane 3) or (‐)‐maackiain (Lane 5). Finally, we analyzed the cleavage of caspase‐1 to understand whether (‐)‐maackiain treatment stimulates more efficiently inflammasome activation than alum. As shown in Fig. 4D, treatment of MPL‐primed macrophages with nigericin and (‐)‐maackiain (Lane 3) stimulated the cleavage of caspase‐1 to a greater extent than treatment with alum (Lane 4). Taken together, these findings demonstrate that, in MPL‐primed macrophages, (‐)‐maackiain is less cytotoxic than alum but more efficiently stimulates activation of the inflammasome.
Discussion
In this study, we demonstrated that S. flavescens‐derived compound, (‐)‐maackiain, has an activating effect on inflammasome, which is proven by the increase in caspase‐1 cleavage once treated with nigericin, and this can lead to enhanced production of IL‐1β in either LPS‐ or MPL‐primed cells. (‐)‐Maackiain does not have an effect on stimulation by the first priming signal, LPS treatment (Lane 6 in Fig. 2A), but can intensify the state promoted by nigericin treatment (Lane 7 in Figs 2A and 3A), which is used as the second activating signal required for stimulation of inflammasome. (‐)‐Maackiain has inhibiting effects on the activation of NF‐κB based on a reporter assay with RAW‐Blue cells (Fig. 1A) so that we first primed cells with LPS or MPL and then treated sequentially with nigericin and (‐)‐maackiain to determine its role on the stimulation of inflammasome. In addition, we switched the target cell to THP‐1 because RAW‐Blue cells do not express ASC [29] and A549 cells do not produce caspase‐1 [30]. Nigericin treatment alone slightly promoted the cleavage of caspase‐1 (Figs 2A and 3A), suggesting that the nigericin‐mediated cleavage is to some extent independent of a priming signal such as LPS. This is in agreement with a previous report that ATP‐induced release of mature IL‐18 is independent of new protein synthesis in response to LPS [31]. Thus, (‐)‐maackiain plays a role in boosting the cleavage of caspase‐1 in response to nigericin. In addition, we clearly observed the increase of IL‐1β production in response to LPS alone as shown in Fig. 2B. Unlike classical inflammasome promoted by priming and activating signals, this could be mediated by alternative inflammasome, which promotes the secretion of IL‐1β in response to LPS alone [32]. These imply that (‐)‐maackiain‐mediated stimulation depends on the action of nigericin but not that of LPS. Notably, the upregulation of caspase‐1 cleavage depicted in Lane 7 in Fig. 2A was only observed in macrophages cultured in the presence of serum, suggesting that (‐)‐maackiain‐mediated activation of caspase‐1 is dependent on the action of additional factors present in serum.
Like ATP, nigericin stimulates the P2X7 receptor, thereby decreasing intracellular potassium levels and activating the NLRP3‐associated inflammasome via the classical pathway [33, 34]. Here, (‐)‐maackiain is actively involved in the activation of inflammasome by contributing to the action of nigericin on the classical inflammasome pathway after the priming step. In the course of study, we examined the level of regulator proteins, NEK7 and vimentin, which have shown their roles as essential mediators for the activation of NLRP3 inflammasome [21, 22], to understand mechanisms underlying the process of enhancement. As a result, we found that (‐)‐maackiain increased the production of intermediate filaments, vimentin (Fig. 3E), which acts as a scaffold for the assembly of NLRP3 inflammasome complex by promoting interaction with caspase‐1 [22]. However, the enhancement of vimentin expression was not observed by treatment with (‐)‐maackiain alone, further supporting the effect of (‐)‐maackiain in promoting the formation of inflammasome complex to activate caspase‐1 in response to nigericin. In addition, treatment with nigericin and (‐)‐maackiain more strongly increased the production of vimentin than with LPS and nigericin, leading to higher cleavage of caspase‐1.
Monophosphoryl lipid A, a detoxified component of LPS, has been used as an adjuvant to increase vaccine efficacy [35], and it activates key innate immune pathways through the action of TLR4 [36]. However, unlike LPS‐mediated induction of TLR4 activation through the TRIF and MyD88 pathways, MPL leads to activation only through TRIF, resulting in lower levels of inflammatory responses [37]. In addition, MPL cannot activate the NLRP3 inflammasome in contrast to LPS, and NLRP3 inflammasome plays a critical role in limiting lung damage resulting from influenza infections [38, 39]. The resultant production of IL‐1β stimulates acute inflammatory responses that play critical roles in defending against influenza infections [4]. Therefore, MPL fails to induce maturation of IL‐1β [23] and consequently fails to achieve efficient vaccination against influenza infection [38, 39, 40]. This implies that inflammasome signaling represents a promising target for improving MPL‐mediated vaccine efficacy. Especially, elderly mice infected with influenza produce less amounts of active IL‐1β even though they have normal levels of pro‐IL‐1β [7]. Thus, the potent inflammasome‐activating compound like (‐)‐maackiain could be useful clinically as an immune‐stimulating agent and supplemental influenza vaccine adjuvant to further increase caspase‐1‐augmented IL‐1β production.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
JWH and JHL planned the experiments. JWH, JHL, EJ, and HWR performed the experiments. HWR, SRO, and KSA analyzed the data. HSJ and UHH supervised the study. JWH and JHL wrote the original draft of the manuscript. UHH wrote, reviewed, and edited the manuscript.
Supporting information
Fig. S1‐1. MPLC fractionation of Sophora flavescens extract.
Fig. S1‐2. UPLC‐PDA‐QTof‐MS of fractions of Sophora flavescens extract.
Fig. S1‐3. UPLC‐PDA‐QTof‐MS of fractions of Sophora flavescens extract.
Fig. S2‐1. UV, MS/MS, and MS data of formononetin 1.
Fig. S2‐2. UV, MS/MS, and MS data of (2S)‐2'‐methoxy kurarinone 2.
Fig. S2‐3. UV, MS/MS, and MS data of kushenol E 3.
Fig. S2‐4. UV, MS/MS, and MS data of kushenol F 4.
Fig. S2‐5. UV, MS/MS, and MS data of (‐)‐maackiain 5.
Fig. S2‐6. UV, MS/MS, and MS data of noranhydroicaritin 6.
Fig. S2‐7. UV, MS/MS, and MS data of (‐)‐4‐hydroxy‐3‐methoxy‐8,9‐methylenedioxypterocarpan 7.
Fig. S2‐8. UV, MS/MS, and MS data of sophoraflavanone B 8.
Fig. S3‐1. 1H‐NMR spectrum of formononetin 1 (400 MHz, DMSO‐d6).
Fig. S3‐2. 13C‐NMR spectrum of formononetin 1 (100 MHz, DMSO‐d6).
Fig. S3‐3. 1H‐NMR spectrum of (2S)‐2'‐methoxy kurarinone 2 (400 MHz, DMSO‐d6).
Fig. S3‐4. 13C‐NMR spectrum of (2S)‐2'‐methoxy kurarinone 2 (100 MHz, DMSO‐d6).
Fig. S3‐5. 1H‐NMR spectrum of kushenol E 3 (400 MHz, DMSO‐d6).
Fig. S3‐6. 13C‐NMR spectrum of kushenol E 3 (100 MHz, DMSO‐d6).
Fig. S3‐7. 1H‐NMR spectrum of kushenol F 4 (400 MHz, DMSO‐d6).
Fig. S3‐8. 13C‐NMR spectrum of kushenol F 4 (100 MHz, DMSO‐d6).
Fig. S3‐9. 1H‐NMR spectrum of (‐)‐maackiain 5 (400 MHz, DMSO‐d6).
Fig. S3‐10. 13C‐NMR spectrum of (‐)‐maackiain 5 (100 MHz, DMSO‐d6).
Fig. S3‐11. 1H‐NMR spectrum of noranhydroicaritin 6 (400 MHz, DMSO‐d6).
Fig. S3‐12. 13C‐NMR spectrum of noranhydroicaritin 6 (100 MHz, DMSO‐d6).
Fig. S3‐13. 1H‐NMR spectrum of (‐)‐4‐hydroxy‐3‐methoxy‐8,9‐methylenedioxypterocarpan 7 (400 MHz, DMSO‐d6).
Fig. S3‐14. 13C‐NMR spectrum of (‐)‐4‐hydroxy‐3‐methoxy‐8,9‐methylenedioxypterocarpan 7 (100 MHz, DMSO‐d6).
Fig. S3‐15. 1H‐NMR spectrum of sophoraflavanone B 8 (400 MHz, DMSO‐d6).
Fig. S3‐16. 13C‐NMR spectrum of sophoraflavanone B 8 (100 MHz, DMSO‐d6).
Acknowledgements
This work was supported by a Korea University Grant (K1709641).
Jin‐Won Huh and Jung‐Hoon Lee contributed equally to this work
References
- 1. Dinarello CA (2009) Immunological and inflammatory functions of the interleukin‐1 family. Annu Rev Immunol 27, 519–550. [DOI] [PubMed] [Google Scholar]
- 2. Afonina IS, Muller C, Martin SJ and Beyaert R (2015) Proteolytic processing of interleukin‐1 family cytokines: variations on a common theme. Immunity 42, 991–1004. [DOI] [PubMed] [Google Scholar]
- 3. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F and Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. [DOI] [PubMed] [Google Scholar]
- 4. Schmitz N, Kurrer M, Bachmann MF and Kopf M (2005) Interleukin‐1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol 79, 6441–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kayamuro H, Yoshioka Y, Abe Y, Arita S, Katayama K, Nomura T, Yoshikawa T, Kubota‐Koketsu R, Ikuta K, Okamoto S et al (2010) Interleukin‐1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus. J Virol 84, 12703–12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lapuente D, Storcksdieck Genannt Bonsmann M, Maaske A, Stab V, Heinecke V, Watzstedt K, Hess R, Westendorf AM, Bayer W et al (2018) IL‐1beta as mucosal vaccine adjuvant: the specific induction of tissue‐resident memory T cells improves the heterosubtypic immunity against influenza A viruses. Mucos Immunol 11, 1265–1278. [DOI] [PubMed] [Google Scholar]
- 7. Stout‐Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ and Harrod KS (2012) Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with nigericin. J Immunol 188, 2815–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L, Cheng X, Shi W, Lu Q, Go VL, Heber D and Ma L (2005) Inhibition of growth of Streptococcus mutans, methicillin‐resistant Staphylococcus aureus, and vancomycin‐resistant enterococci by kurarinone, a bioactive flavonoid isolated from Sophora flavescens . J Clin Microbiol 43, 3574–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and Su C (2014) Anti‐tumor activities of matrine and oxymatrine: literature review. Tumour Biol 35, 5111–5119. [DOI] [PubMed] [Google Scholar]
- 10. Zhou H, Lutterodt H, Cheng Z and Yu LL (2009) Anti‐Inflammatory and antiproliferative activities of trifolirhizin, a flavonoid from Sophora flavescens roots. J Agric Food Chem 57, 4580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma H, Huang Q, Qu W, Li L, Wang M, Li S and Chu F (2018) In vivo and in vitro anti‐inflammatory effects of Sophora flavescens residues. J Ethnopharmacol 224, 497–503. [DOI] [PubMed] [Google Scholar]
- 12. He X, Fang J, Huang L, Wang J and Huang X (2015) Sophora flavescens Ait.: traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol 172, 10–29. [DOI] [PubMed] [Google Scholar]
- 13. Zhao Z, Fan H, Higgins T, Qi J, Haines D, Trivett A, Oppenheim JJ, Wei H, Li J, Lin H et al (2014) Fufang Kushen injection inhibits sarcoma growth and tumor‐induced hyperalgesia via TRPV1 signaling pathways. Cancer Lett 355, 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim DW, Chi YS, Son KH, Chang HW, Kim JS, Kang SS and Kim HP (2002) Effects of sophoraflavanone G, a prenylated flavonoid from Sophora flavescens, on cyclooxygenase‐2 and in vivo inflammatory response. Arch Pharm Res 25, 329–335. [DOI] [PubMed] [Google Scholar]
- 15. Lee SW, Lee HS, Nam JY, Kwon OE, Baek JA, Chang JS, Rho MC and Kim YK (2005) Kurarinone isolated from Sophora flavescens Ait inhibited MCP‐1‐induced chemotaxis. J Ethnopharmacol 97, 515–519. [DOI] [PubMed] [Google Scholar]
- 16. Suginome H (1962) Oxygen heterocycles. Maackiain, a new naturally occurring chromanocoumaran. Experientia 18, 161–163. [DOI] [PubMed] [Google Scholar]
- 17. Honda G and Tabata M (1982) Antidermatophytic substance from Sophora angustifolia . Planta Med 46, 122–123. [DOI] [PubMed] [Google Scholar]
- 18. Zhang GP, Xiao ZY, Rafique J, Arfan M, Smith PJ, Lategan CA and Hu LH (2009) Antiplasmodial isoflavanones from the roots of Sophora mollis . J Nat Product 72, 1265–1268. [DOI] [PubMed] [Google Scholar]
- 19. Lee JH, Shin H, Kim YJ, Paek SH, Jin S and Ha UH (2014) Pseudomonas aeruginosa‐induced IL‐1beta production is inhibited by Sophora flavescens via the NF‐kappaB/inflammasome pathways. J Microbiol 52, 1044–1049. [DOI] [PubMed] [Google Scholar]
- 20. Holloway BW (1955) Genetic recombination in Pseudomonas aeruginosa . J General Microbiol 13, 572–581. [DOI] [PubMed] [Google Scholar]
- 21. He Y, Zeng MY, Yang D, Motro B and Nunez G (2016) NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. dos Santos G, Rogel MR, Baker MA, Troken JR, Urich D, Morales‐Nebreda L, Sennello JA, Kutuzov MA, Sitikov A, Davis JM et al (2015) Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun 6, 6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Embry CA, Franchi L, Nunez G and Mitchell TC (2011) Mechanism of impaired NLRP3 inflammasome priming by monophosphoryl lipid A. Sci Signal 4, ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varnes ME, Glazier KG and Gray C (1989) pH‐dependent effects of the ionophore nigericin on response of mammalian cells to radiation and heat treatment. Radiat Res 117, 282–292. [PubMed] [Google Scholar]
- 25. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS and Flavell RA (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F et al (2009) AS04, an aluminum salt‐ and TLR4 agonist‐based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 183, 6186–6197. [DOI] [PubMed] [Google Scholar]
- 27. Li H, Willingham SB, Ting JP and Re F (2008) Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol 181, 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B and Nemazee D (2006) Adjuvant‐enhanced antibody responses in the absence of toll‐like receptor signaling. Science 314, 1936–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pelegrin P, Barroso‐Gutierrez C and Surprenant A (2008) P2X7 receptor differentially couples to distinct release pathways for IL‐1beta in mouse macrophage. J Immunol 180, 7147–7157. [DOI] [PubMed] [Google Scholar]
- 30. Motani K, Kushiyama H, Imamura R, Kinoshita T, Nishiuchi T and Suda T (2011) Caspase‐1 protein induces apoptosis‐associated speck‐like protein containing a caspase recruitment domain (ASC)‐mediated necrosis independently of its catalytic activity. J Biol Chem 286, 33963–33972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghonime MG, Shamaa OR, Das S, Eldomany RA, Fernandes‐Alnemri T, Alnemri ES, Gavrilin MA and Wewers MD (2014) Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J Immunol 192, 3881–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid‐Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T and Hornung V (2016) Human monocytes engage an alternative inflammasome pathway. Immunity 44, 833–846. [DOI] [PubMed] [Google Scholar]
- 33. Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose‐Girma M, Lee WP, Weinrauch Y, Monack DM and Dixit VM (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232. [DOI] [PubMed] [Google Scholar]
- 34. Gurcel L, Abrami L, Girardin S, Tschopp J and van der Goot FG (2006) Caspase‐1 activation of lipid metabolic pathways in response to bacterial pore‐forming toxins promotes cell survival. Cell 126, 1135–1145. [DOI] [PubMed] [Google Scholar]
- 35. Dubensky TW Jr and Reed SG (2010) Adjuvants for cancer vaccines. Semin Immunol 22, 155–161. [DOI] [PubMed] [Google Scholar]
- 36. Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D and Persing D (2004) Taking a Toll on human disease: Toll‐like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Exp Opin Biol Ther 4, 1129–1138. [DOI] [PubMed] [Google Scholar]
- 37. Mata‐Haro V, Cekic C, Martin M, Chilton PM, Casella CR and Mitchell TC (2007) The vaccine adjuvant monophosphoryl lipid A as a TRIF‐biased agonist of TLR4. Science 316, 1628–1632. [DOI] [PubMed] [Google Scholar]
- 38. Allen IC, Scull MA, Moore CB, Holl EK, McElvania‐TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ and Ting JP (2009) The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30, 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thomas PG, Dash P, Aldridge JR Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL et al (2009) The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase‐1. Immunity 30, 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ichinohe T, Lee HK, Ogura Y, Flavell R and Iwasaki A (2009) Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med 206, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1‐1. MPLC fractionation of Sophora flavescens extract.
Fig. S1‐2. UPLC‐PDA‐QTof‐MS of fractions of Sophora flavescens extract.
Fig. S1‐3. UPLC‐PDA‐QTof‐MS of fractions of Sophora flavescens extract.
Fig. S2‐1. UV, MS/MS, and MS data of formononetin 1.
Fig. S2‐2. UV, MS/MS, and MS data of (2S)‐2'‐methoxy kurarinone 2.
Fig. S2‐3. UV, MS/MS, and MS data of kushenol E 3.
Fig. S2‐4. UV, MS/MS, and MS data of kushenol F 4.
Fig. S2‐5. UV, MS/MS, and MS data of (‐)‐maackiain 5.
Fig. S2‐6. UV, MS/MS, and MS data of noranhydroicaritin 6.
Fig. S2‐7. UV, MS/MS, and MS data of (‐)‐4‐hydroxy‐3‐methoxy‐8,9‐methylenedioxypterocarpan 7.
Fig. S2‐8. UV, MS/MS, and MS data of sophoraflavanone B 8.
Fig. S3‐1. 1H‐NMR spectrum of formononetin 1 (400 MHz, DMSO‐d6).
Fig. S3‐2. 13C‐NMR spectrum of formononetin 1 (100 MHz, DMSO‐d6).
Fig. S3‐3. 1H‐NMR spectrum of (2S)‐2'‐methoxy kurarinone 2 (400 MHz, DMSO‐d6).
Fig. S3‐4. 13C‐NMR spectrum of (2S)‐2'‐methoxy kurarinone 2 (100 MHz, DMSO‐d6).
Fig. S3‐5. 1H‐NMR spectrum of kushenol E 3 (400 MHz, DMSO‐d6).
Fig. S3‐6. 13C‐NMR spectrum of kushenol E 3 (100 MHz, DMSO‐d6).
Fig. S3‐7. 1H‐NMR spectrum of kushenol F 4 (400 MHz, DMSO‐d6).
Fig. S3‐8. 13C‐NMR spectrum of kushenol F 4 (100 MHz, DMSO‐d6).
Fig. S3‐9. 1H‐NMR spectrum of (‐)‐maackiain 5 (400 MHz, DMSO‐d6).
Fig. S3‐10. 13C‐NMR spectrum of (‐)‐maackiain 5 (100 MHz, DMSO‐d6).
Fig. S3‐11. 1H‐NMR spectrum of noranhydroicaritin 6 (400 MHz, DMSO‐d6).
Fig. S3‐12. 13C‐NMR spectrum of noranhydroicaritin 6 (100 MHz, DMSO‐d6).
Fig. S3‐13. 1H‐NMR spectrum of (‐)‐4‐hydroxy‐3‐methoxy‐8,9‐methylenedioxypterocarpan 7 (400 MHz, DMSO‐d6).
Fig. S3‐14. 13C‐NMR spectrum of (‐)‐4‐hydroxy‐3‐methoxy‐8,9‐methylenedioxypterocarpan 7 (100 MHz, DMSO‐d6).
Fig. S3‐15. 1H‐NMR spectrum of sophoraflavanone B 8 (400 MHz, DMSO‐d6).
Fig. S3‐16. 13C‐NMR spectrum of sophoraflavanone B 8 (100 MHz, DMSO‐d6).


