DK‐520 is an acyl derivative of Niclosamide that exhibits a significant increase in both the plasma concentration and bioavailability. Like Niclosamide, DK‐520 effectively inhibits the early stages of RANKL‐induced osteoclastogenesis and may be a promising drug candidate for treatment of osteoclast‐related diseases.

Keywords: differentiation, DK‐520, Niclosamide, osteoclast, RANKL
Abstract
Niclosamide is a potent inhibitor of osteoclastogenesis and bone remodeling. DK‐520 is an acyl derivative of Niclosamide and significantly increased both the plasma concentration and the duration of exposure of Niclosamide when dosed orally. However, at present the effect of DK‐520 on osteoclastogenesis has not been reported. Here, we investigated whether DK‐520 can regulate receptor activator of nuclear factor‐κB ligand (RANKL)‐induced osteoclastogenesis of bone marrow macrophages (BMMs) in vitro. Following induction of BMMs with RANKL for three days, we detected differentiated osteoclasts with typical morphology and high levels of tartrate‐resistant acid phosphatase (TRAP), RANKL, and cathepsin K (CTSK) expression. Treatment with either Niclosamide or DK‐520 did not affect the viability of osteoclast precursors (OCPs), but significantly inhibited RANKL‐induced transdifferentiation of macrophages into OCPs, particularly in the early stage of osteoclastogenesis. Both Niclosamide and DK‐520 significantly decreased the relative levels of transcription factor PU.1 mRNA transcripts and dendritic cell‐specific transmembrane protein (DC‐STAMP), but not v‐ATPasev0d2 protein expression in OCPs. In addition, the inhibitory effect of DK‐520 on osteoclastogenesis is realized through impairment of the NF‐kB (nuclear factor‐κB) and MAPK (mitogen‐activated protein kinase) signaling pathways. These results demonstrate that DK‐520, like Niclosamide, effectively inhibits the early stage of osteoclastogenesis. The findings presented here, together with its increased oral plasma concentrations and bioavailability, suggest that DK‐520 may be a promising drug candidate for treatment of osteoclast‐related diseases.
Abbreviations
- BMMs
bone marrow macrophages
- c‐fms
colony‐stimulating factor 1 receptor
- CTSK
cathepsin K
- DC‐STAMP
dendritic cell‐specific transmembrane proteins
- ERK
extracellular signal‐regulated kinase
- FBS
fetal bovine serum
- IκB
inhibitor of NF‐κB
- JNK
Janus N‐terminal kinase
- MAPK
mitogen‐activated protein kinase
- M‐CSF
macrophage colony‐stimulating factor
- NFATc1
nuclear factor of activated T cells c1
- NF‐κB
nuclear factor‐κB
- OCPs
osteoclast precursors
- RANK
receptor activator of nuclear factor‐κβ
- RANKL
receptor activator of nuclear factor‐κβ ligand
- SD
standard deviation
- SERMs
selective estrogen receptor modulators
- TRAP
tartrate‐resistant acid phosphatase
- α‐MEM
alpha‐minimum essential medium
Osteoporosis is a common disease that affects many millions of peoples, particularly older men and postmenopausal women [1]. Pathologically, osteoporosis is characterized by reduced bone mass and altered bone microstructure, as well as increased risk for bone fractures. Osteoporotic fractures and their complications can cause disability and death in older patients. In addition, medical care for osteoporosis and osteoporotic fractures requires numerous resources and financial costs, which can be a socioeconomic burden for many families globally [2]. Osteoporosis is attributed to metabolic imbalance between bone formation by osteoblasts and bone resorption by osteoclasts [3].
Osteoclasts are multinucleated giant cells that are mainly located around vascular channels in the surface of the bone and are derived from the fusion of multiple monocytes/macrophages [4]. During the process of osteoclast differentiation and maturation, receptor activator of nuclear factor κβ ligand (RANKL) on osteoblasts and stromal cells, and macrophage colony‐stimulating factor (M‐CSF) activate nuclear factor‐kB (NF‐kB) and MAPK (mitogen‐activated protein kinase) signaling to induce expression of NFATc1 (nuclear factor of activated T cells c1) and other factors, causing macrophage activation and differentiation into osteoclast precursor cells (OCPs). Subsequently, OCPs fuse and further maturate into osteoclasts [5]. Hence, inhibition of osteoclastogenesis is valuable for controlling osteoporosis. Bisphosphonates, estrogen, or selective estrogen receptor modulators (SERMs) and calcitonin, which inhibit the different stages of osteoclastogenesis, have been used to treat osteoporosis [3]. However, long‐term treatment with these drugs may cause severe side effects, such as osteonecrosis, thromboembolism, atypical femoral fractures, and increased risk of endometrial cancer, breast cancer, and cardiovascular disease [6, 7, 8, 9]. In addition, experimental studies have revealed that inhibitors of cathepsin K (CTSK), c‐Src, and αVβ3 integrin receptor can inhibit the differentiation and resorption function of osteoclasts [10, 11, 12], but these drugs have not been used in clinical practice. Thus, discovery of new and safe drugs that inhibit osteoclastogenesis will be of significance for managing patients with osteoporosis and osteoporotic fractures.
Niclosamide, an anthelmintic drug, has been used to treat intestinal tapeworm infection since 1982 [13]. DK‐520 is an acyl derivative of Niclosamide and has the unique property that it increased both the plasma concentration and the duration of exposure of Niclosamide in vivo with no observable adverse effects [14, 15, 16]. Previous studies have shown that Niclosamide can suppress osteoclastogenesis and bone remodeling, although its precise mechanisms have not been clarified [17, 18]. Furthermore, it is unclear whether and how treatment with DK‐520, like Niclosamide, can inhibit the differentiation of mouse bone marrow macrophages (BMMs) into osteoclasts.
In the present study, we utilized a mouse BMM model to investigate the mechanisms by which DK‐520 and Niclosamide regulate osteoclastogenesis. Our data indicate that DK‐520, like Niclosamide, inhibits early differentiation of BMMs into osteoclasts in vitro. Therefore, DK‐520 may be a promising anti‐osteoporosis drug.
Materials and Methods
Special chemicals and reagents
Niclosamide and its derivative DK‐520 were provided by Chen in the Department of Medicine, Duke University Medical Center, Durham, USA; alpha‐minimum essential medium (α‐MEM) and fetal bovine serum (FBS) were from Gibco (Thermo Fisher Scientific, Waltham, MA, USA); soluble recombinant M‐CSF and RANKL were from PeproTech (London, UK); and antibodies against DC‐STAMP and ATPasev0d2 were from Thermo Fisher Scientific and Abcam (Cambridge, UK). The following primary antibodies and secondary antibodies were purchased from Cell Signaling Technology, Inc.: primary antibodies against NFATc1 and c‐Fos, and secondary antibodies against IκBα, phosphor‐IκBα, p44/42ERK, phospho‐44/42ERK, JNK, phospho‐JNK, p38, and phospho‐p38.
Animals
Female and male C57BL/6 mice (4–6 weeks of age) were obtained and maintained in the specific pathogen‐free animal facility of Shanxi Medical University (Taiyuan, China). The experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals and approved by the specific committee of Shanxi Medical University.
Induction of osteoclasts
BMMs were isolated from the hind limb long bones of mice. BMMs (5 × 104 cells/well) were treated with or without M‐CSF (44 ng·mL−1) and RANKL (100 ng·mL−1) in 10% FBS α‐MEM in 48‐well plates in the presence or absence of Niclosamide or DK‐520 at 37℃ and 5% CO2 for 3–4 days. The cells were exposed to fresh medium every 2 days. The differentiated cells were characterized for tartrate‐resistant acid phosphatase (TRAP) activity using a TRAP enzymatic staining kit (Sigma‐Aldrich, St. Louis, MO, USA). TRAP+ cells with multiple nuclei were counted as multinucleated osteoclasts in a blinded manner. In addition, BMMs were treated in triplicate with M‐CSF and RANKL for 3–4 days and the cells were treated with vehicle DMSO or each compound at 0.75 or 1.5 µm daily. TRAP expression was enzymatically stained, and the relative levels of PU.1 mRNA transcripts and DC‐STAMP and v‐ATPase0d2 protein expression were determined daily using quantitative Real‐time‐PCR (qRT‐PCR) and western blot, respectively, for the first 2 days.
CCK‐8 cell viability assay
BMMs (7 × 103 cells/well) were treated in triplicate with vehicle DMSO or varying concentrations of Niclosamide or DK‐520 (0.75, 1.0, 1.25, 1.5, 1.75, 2.0, and 2.25 μm) in the presence of M‐CSF (44 ng·mL−1) in 96‐well plates for 24 h to determine OCP viability. BMMs (8 × 103 cells/well) were then cultured with M‐CSF (44 ng·mL−1) and RANKL (100 ng·mL−1) for 3 days to induce osteoclast maturation. The cells were treated with DMSO or different concentrations of Niclosamide or DK‐520 in the presence of M‐CSF (44 ng·mL−1) and RANKL (100 ng·mL−1) for 24 h. Cell viability was measured using the CCK‐8 assay kit (Dojindo Molecular Technologies, Tokyo, Japan).
In vitro bone resorption assays
BMMs (1 × 105 cells/well) were seeded on bovine cortical bone slices plated in 24‐well tissue culture plates, and then cultured in the presence of M‐CSF (44 ng·mL−1) and RANKL (100 ng·mL−1) with or without DK‐520 for up to 5 days to promote osteoclastogenesis and bone resorption. Then, bone slices were harvested and cells were removed from bone slices with sodium hypochlorite solution, and bone slices were dyed with 0.5% Toluidine blue (Fisher Science Education). Images were obtained by microscope, and the data were quantified by measuring the number of resorbed pits in five random resorption areas of one slice.
Quantitative Real‐time‐PCR
Following osteoclast induction, we examined the impact of each compound on TRAP and CTSK mRNA transcripts using qRT‐PCR. Similarly, we detected the PU.1 mRNA transcript after treatment with each compound daily. Briefly, total RNA was extracted from osteoclasts using a miRNeasy Mini Kit (Qiagen, Germantown, MD, USA) and reverse‐transcribed into cDNA using the PrimeScript RT Master Mix Kit (Takara, Shiga, Japan), followed by qRT‐PCR with the SYBR Premix Ex Taq™ (Takara) and specific primers. The primer sequences were forward 5′AGCAGCCAAGGAGGACTACGTT3′ and reverse 5′TCGTTGATGTCGCACAGAGG3′ for TRAP; forward 5′CCTACGCACAAGGCGAAGATGC3′ and reverse 5′CTGAGACCACGATGATGTCGCC3′ for RANK; forward 5′CAGCAGAACGGAGGCATTGA3′ and reverse 5′CCTTTGCCGTGGCGTTATAC‐3′ for CtsK; forward 5′GGAGCTCAGCTGGATGTTACAGG3′ and reverse 5′GTAGTAGTCATGCATTGGACGTTGG3′ for PU.1; and forward 5′TTGTTACCAACTGGGACGACATGG3′ and reverse 5′GATCTTGATCTTCATGGTGCTAGG3′ for β‐actin. The data were analyzed using the 2−ΔΔCt method.
Western blot analysis
Following OCP induction on each day, we examined the impact of each compound on DC‐STAMP and ATPase0d2 expression daily using western blot analysis. Briefly, the cells were harvested and lysed. Their cytoplasmic and nuclear proteins were extracted using the special reagents (Thermo Fisher Scientific). Subsequently, we characterized these lysates using western blot analysis with anti‐DC‐STAMP and anti‐ATPasev0d2 primary antibodies and horseradish peroxidase‐conjugated secondary antibodies. In different time periods with or without impacting DK‐520, cells were washed twice with PBS and then lysed in the lysis buffer, supplemented with protease inhibitor (Sigma‐Aldrich) and phosphatase inhibitor cocktails 1 (Sigma‐Aldrich) and 2 (Sigma‐Aldrich). Cytoplasmic and nuclear extracts were prepared using the NE‐PER Nuclear and Cytoplasmic Extraction Reagents (Catalog Thermo Scientific). Lysates were then processed by western blot analysis as previously described [19]. Membranes were incubated with the primary antibodies and horseradish peroxidase‐conjugated secondary antibodies. An ECL detection assay was performed using a SuperSignal West Dura Kit from Pierce (Rockford, IL, USA). The data were analyzed using ImageJ software (National Institute of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as mean ± standard deviation (SD). We statistically analyzed the difference among groups using the Student's t‐test and the Statistical Package for the Social Sciences software (SPSS, version 21.0, Chicago, IL, USA). Statistical significance was accepted when the P‐value < 0.05.
Results
Characterization of induced osteoclasts
To induce osteoclasts, BBMs were isolated and stimulated with or without M‐CSF and RANKL for 3 days. The expression of TRAP was determined using enzymatic staining. As shown in Fig. 2A, there were many TRAP+ osteoclasts with several vacuoles and multiple nuclei, and the TRAP+ cells displayed a large cytoplasm and round, oval, or irregular body type with uniform red or purple particles. The qRT‐PCR results showed high levels of TRAP, RANK, and CTSK mRNA transcripts in these cells, but not in the control macrophages (Fig. 2B). Together, these data indicate that treatment with M‐CSF and RANKL induces transdifferentiation of BMMs into mature osteoclasts in vitro.
Fig. 2.

Characterization of differentiated osteoclasts, and both DK‐520 and Niclosamide inhibit osteoclast differentiation. BMMs were treated with M‐CSF and RANKL in the presence or absence of the indicated concentrations of Niclosamide or DK‐520 for 3–4 days. The cells were enzymatically assayed for TRAP activity, and the relative levels of TRAP, RANK, and CTSK mRNA transcripts were determined using qRT‐PCR. The TRAP+ cells with multiple nuclei were counted. Data are representative images (magnification × 40) or expressed as the mean ± SD of each group from three separate experiments. (A) Representative image of TRAP staining (magnification × 40). (B) Levels of TRAP, RANK, and CTSK mRNA transcripts. Data are representative images or expressed as the mean ± SD of each group from three separate experiments (n = 3). (C) Cell morphology and TRAP staining after 24 h of Niclosamide intervention with different concentrations under an inverted phase‐contrast microscope. (D) Cell morphology and TRAP staining after 24 h of DK‐520 intervention with different concentrations under an inverted phase‐contrast microscope. (E) TRAP staining of cells in the culture plate. (F) Cell number after 24 h of Niclosamide intervention with different concentrations under an inverted phase‐contrast microscope. Data are expressed as the mean ± SEM of each group from three separate experiments (n = 4) (G) Cell number after 24h of DK‐520 intervention with different concentrations under an inverted phase‐contrast microscope. Scale bar: 1000 μm. ** P < 0.001, # P < 0.01. Data are expressed as the mean ± SEM of each group from three separate experiments (n = 3). The data were statistically analyzed by Student’s t‐test.
The safe concentrations of Niclosamide and DK‐520
First, we investigated the impact of both Niclosamide and DK‐520 on the viability of OCPs and osteoclasts in vitro. BMMs were treated with vehicle or different concentrations of either Niclosamide or DK‐520 in the presence of M‐CFS for 24 h, and their viability was determined using the CCK‐8 assay. Treatment with Niclosamide at < 1.75 μm or DK‐520 at < 2.0 μm did not affect the viability of OCPs in our experimental conditions (Fig. 1). Furthermore, following induction of osteoclast maturation, treatment with Niclosamide at 2.25 μm or DK‐520 at dose did not alter the viability of osteoclasts. These data provided a safe dose of each compound for further experiments.
Fig. 1.

The effects of DK‐520 and Niclosamide on osteoclast viability. BMMs were treated in triplicate with vehicle DMSO or the indicated concentrations of Niclosamide or DK‐520 in the presence of M‐CSF (44 ng·mL−1) for 24 h to assay OCP viability. Following induction of osteoclast differentiation for 3 days, the differentiated cells were tested. Cell viability was determined using the CCK‐8 assay. (A) Effects of Niclosamide on OCP viability and mature osteoclasts. (B) The effects of DK‐520 on OCP viability and mature osteoclasts. Data are expressed as the mean ± SD of each group from three separate experiments (n = 3). The data were statistically analyzed by Student’s t‐test. * P < 0.05, ** P < 0.001, # P < 0.01.
Both Niclosamide and DK‐520 inhibit RANKL‐induced osteoclastogenesis and bone resorption in vitro
Next, we explored whether Niclosamide and DK‐520 affected the formation and function of mature osteoclasts. Following induction of osteoclast maturation for 3 days, we found that both Niclosamide and DK‐520 at 1.5 μm, but not a lower dose, significantly reduced the number of osteoclasts, particularly following treatment with DK‐520 (Fig. 2F,G). Morphological analysis revealed that treatment with either Niclosamide or DK‐520 decreased the cell size and number of nuclei in the induced osteoclasts (Fig. 2C–E). These data suggest that both Niclosamide and DK‐520 can inhibit RANKL‐induced transdifferentiation of macrophages into mature osteoclasts in vitro. To determine the impact of each compound on RANKL activation, we observed osteoclast formation using histology (Fig. 3F). We found that treatment with either Niclosamide or DK‐520 at the tested doses reduced the cell body size, especially in the early stage of osteoclastogenesis (Fig. 3A–E), and decreased the numbers of TRAP+ cells compared with the DMSO group (Fig. 3G,H). The inhibitory effects of different doses of each compound tended to be dose‐dependent. To determine the role of DK‐520 to the function of mature osteoclasts, we generated osteoclasts in the dishes with cow bone slices, preincubated with RANKL and M‐CSF 2 days and added DK‐520 with various doses, cells were maintained in growth medium for 3 days and then removed cells which were on slices. As exhibited in Fig. 4A–D, the bone resorption pits were markedly reduced in DK‐520 groups, showing that it suppressed the function of mature osteoclasts and in a dose‐dependent manner. These data indicate that both Niclosamide and DK‐520 treatment can inhibit RANKL‐induced osteoclastogenesis and bone resorption in vitro.
Fig. 3.

Both DK‐520 and Niclosamide inhibit osteoclast differentiation at the early stage of osteoclastogenesis. Following induction of osteoclast differentiation, cells were treated with vehicle or each compound at the indicated dose daily for 4 days. The morphology and TRAP expression in OCPs were assayed using enzymatic staining and counting the number of TRAP+ cells. (A–E) Both Niclosamide and DK‐520 altered the morphology and reduced TRAP expression in OCPs beginning on day 1. (F) Schematic illustration of treatments. (G,H) Both Niclosamide and DK‐520 decreased the number of TRAP+ cells. Data are representative images (magnification ×40) or expressed as the mean ± SD of each group from three separate experiments (n = 3). The data were statistically analyzed by Student’s t‐test. Scale bar: 1000 μm. * P < 0.05, ** P < 0.001, # P < 0.01
Fig. 4.

DK‐520 inhibits bone resorption in vitro with dose‐dependent manner. BMMs were seeded on bone slices and treated with M‐CSF (44 ng·mL−1) and RANKL (100 ng·mL−1), plus DMSO, 0.75 μm or 1.5 μm DK‐520 for 7 days. Cell culture medium was newly changed every 2 days. (A–C) Bone slices were stained with Toluidine blue, and resorbed pits were counted. (D) Data are expressed as the mean ± SD of each group from three separate experiments (n = 3). The data were statistically analyzed by Student’s t‐test, # P < 0.01.
Niclosamide and DK‐520 inhibit PU.1 mRNA expression in the early stage of osteoclast differentiation
Because PU.1 is a critical transcription factor in the early stage of osteoclastogenesis, we further measured the relative levels of PU.1 mRNA transcripts daily after treatment with each compound using qRT‐PCR. Following treatment with either compound on day 1 or 2, the relative levels of PU.1 mRNA transcripts were dramatically reduced in the compound‐treated cells compared to the vehicle‐treated control cells (Fig. 5A,B). There was no significant difference in the inhibitory effects between these doses of compounds. The reduction in PU.1 mRNA transcripts by either compound demonstrates that both Niclosamide and DK‐520 can inhibit RANKL‐induced early transdifferentiation of macrophages into osteoclasts in vitro.
Fig. 5.

Both DK‐520 and Niclosamide inhibit the transcription factor PU.1 mRNA in OCPs. Following induction of osteoclast differentiation, the cells were treated with vehicle DMSO, DK‐520, or Niclosamide at the indicated doses daily for 2 consecutive days. The relative levels of PU.1 mRNA transcripts were measured using qRT‐PCR. Data are expressed as the mean ± SD of each group from three separate experiments. (A,B) Both DK‐520 and Niclosamide inhibited PU.1 mRNA transcript level in OCPs. Data are expressed as the mean ± SD of each group from three separate experiments (n = 3). The data were statistically analyzed by Student’s t‐test, ** P < 0.001.
Both Niclosamide and DK‐520 inhibit DC‐STAMP expression in differentiating osteoclasts
Because DC‐STAMP and ATPaseV0d2 are crucial for the early process of osteoclastogenesis, we tested whether either Niclosamide or DK‐520 treatment modulated the expression of DC‐STAMP and ATPaseV0d2 in the RANKL‐treated cells using western blot analysis. The results indicated that treatment with either compound significantly decreased the relative levels of DC‐STAMP (Figs 6A,B and 7A,B), but not v‐ATPaseV0d2 (Figs 6A,C and 7A,C), expression in the differentiating cells compared to the control cells. Therefore, these data further support that both Niclosamide and DK‐520 can inhibit the early process of osteoclastogenesis in vitro.
Fig. 6.

Niclosamide inhibits DC‐STAMP protein expression in OCPs. Following induction of osteoclast differentiation, cells were treated with vehicle, DMSO, or Niclosamide at the indicated doses daily for 2 consecutive days. The relative levels of DC‐STAMP and v‐ATPAse0d2 protein expression in each group of cells were measured using western blot analysis. Data are representative images or expressed as the mean ± SD of each group from three separate experiments. (A–C): Western blot analysis indicated that Niclosamide inhibited DC‐STAMP, but not v‐ATPAse0d2, expression in OCPs. Data are expressed as the mean ± SD of each group from three separate experiments (n = 3). The data were statistically analyzed by Student’s t‐test, * P < 0.05, ** P < 0.001.
Fig. 7.
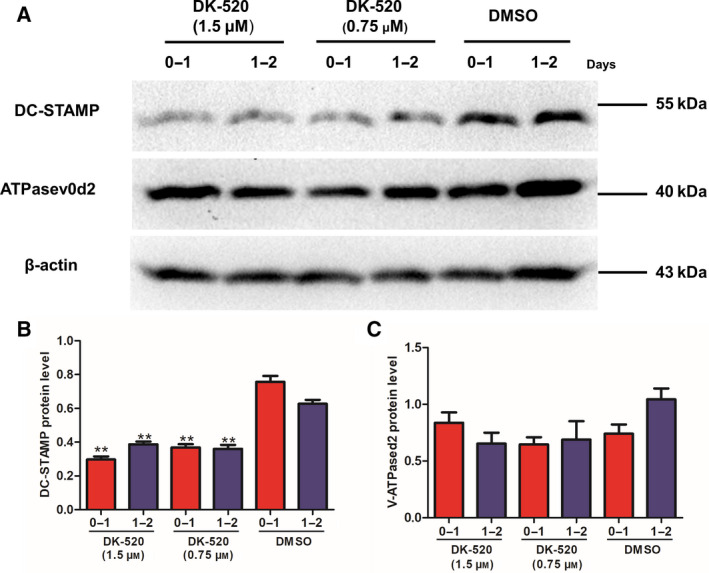
DK‐520 inhibits DC‐STAMP protein expression in OCPs. Following induction of osteoclast differentiation, cells were treated with vehicle, DMSO, or DK5‐520 at the indicated doses daily for 2 consecutive days. The relative levels of DC‐STAMP and v‐ATPAse0d2 protein expression in each group of cells were measured using western blot analysis. Data are representative images or expressed as the mean ± SD of each group from three separate experiments. (A–C): Western blot analysis indicated that DK‐520 inhibited DC‐STAMP, but not v‐ATPAse0d2, expression in OCPs. Data are expressed as the mean ± SD of each group from three separate experiments (n = 3). The data were statistically analyzed by Student’s t‐test, * P < 0.05, ** P < 0.001.
DK‐520 inhibits RANKL‐induced NFATc1 and C‐FOS expression
We next explored the effect of DK‐520 on the expression of RANKL‐induced NFATc1 and C‐FOS in osteoclasts. Western blot revealed that DK‐520 inhibited NFATc1 and C‐FOS expression level in 24h, 48h, and 72h (Fig. 8A–C).
Fig. 8.

DK‐520 inhibits RANKL‐induced NFATc1 and C‐FOS expression. BMMs were seeded in 6‐well plate to culture with M‐CSF (44 ng·mL−1) and RANKL (100ng·mL−1) for 3 days to promote osteoclastogenesis. 1.5 μm DK‐520 was added at meanwhile, and after 24 h, 48 h, and 72 h, osteoclasts were lysed separately for western blot analysis. β‐actin was used as a loading control. Data are representative images or expressed as the mean ± SD of each group from three separate experiments. (A–C): Western blot analysis indicated that DK‐520 inhibited RANKL‐induced NFATc1 and C‐FOS expression. Data are expressed as the mean ± SD of each group from three separate experiments (n = 3). The data were statistically analyzed by Student’s t‐test, ** P < 0.001.
DK‐520 inhibits osteoclastogenesis by affecting NF‐κB and MAPK signaling pathways
RANKL activates the NF‐κB and MAPK pathways. We then further assessed the effect of DK‐520 on the phosphorylation of ERK, JNK, P38, and IκB using western analysis. Our data showed that DK‐520 inhibited the RANKL‐mediated p38 and the phosphorylation of ERK and IκB activation (Fig. 9A–E).
Fig. 9.

DK‐520 inhibits osteoclastogenesis by affecting NF‐κB and MAPK signaling pathways. BMMs were seeded in 6‐well plate to culture with M‐CSF (10%) for 2 days and changed medium without fetal bovine serum for 6h; then, 1.5 μm DK‐520 was added for 1 h, and cells were harvested without adding RANKL (0 min) or with adding 100 ng·mL−1 RANKL (5, 10, 15 min) separately for western blot analysis. The activation of NF‐κB and MAPK signaling pathways was assessed as phosphorylation of ERK, JNK, P38, and IκB using western blot analysis. BMMs cultured with adding DMSO instead of DK‐520 were used as control. Data are representative images or expressed as the mean ± SD of each group from three separate experiments. A: Western blot analysis indicated that DK‐520 inhibited the RANKL‐mediated p38 and the phosphorylation of ERK and IκB activation. (B–D) Histograms, respectively, show the protein levels of P‐ERK/ERK, P‐p38/p38, P‐JNK/JNK, and P‐IκB/IκB. Data are expressed as the mean ± SD of each group from three separate experiments (n = 3). The data were statistically analyzed by Student’s t‐test, * P < 0.05, ** P < 0.001.
Discussion
In the current study, we assessed the effects and potential mechanism of Niclosamide and its derivative DK‐520 on RANKL‐induced osteoclast differentiation in vitro. We found that DK‐520, like Niclosamide, inhibited the early stage of osteoclast differentiation in vitro, supporting the findings that Niclosamide inhibits osteoclast differentiation and bone remodeling [17, 18]. More importantly, we found that DK‐520, like Niclosamide, significantly decreased both DC‐STAMP and PU.1, but not v‐ATPaseV0d2, expression in OCPs. Given that PU.1 and DC‐STAMP are crucial for early osteoclast differentiation, the significantly decreased expression of both molecules indicates that both Niclosamide and DK‐520 are able to inhibit the early process of M‐CSF‐ and RANKL‐dependent OCP fusion and differentiation in vitro [5, 20, 21]. Moreover, our results indicated that DK‐520 inhibits the RANKL‐induced osteoclastogenesis by attenuating the RANKL‐mediated p38 and the phosphorylation of ERK and IκB activation and suppressing the expression of c‐Fos and NFATc1 transcription factors. Our findings provide new insights into the pharmacological potential of DK‐520, as well as Niclosamide, for inhibiting osteoclast formation and subsequent bone resorption. Therefore, DK‐520 may be a promising new therapeutic for treating osteoclast‐related diseases [5].
PU.1 is a transcription factor in the ETS family that is crucial for the differentiation of macrophages, osteoclasts, and B cells [22, 23]. DC‐STAMP is a direct target of c‐Fos and NFATc1 and a master regulator of cell–cell fusion, and DC‐STAMP‐Tg mice display decreased bone mass and increased osteoclastogenesis, leading to the development of osteoporosis [24, 25]. We found that RANKL significantly increased PU.1 expression in OCPs, consistent with a previous report [26], and treatment with either DK‐520 or Niclosamide dramatically minimized PU.1 mRNA transcription and DC‐STAMP expression in OCPs. Given that PU.1 can induce NFATc1 expression, which promotes the expression of osteoclast‐specific genes and TRAP during osteoclastogenesis [27], and inhibition of OCP fusion reduces osteoclast activity and bone resorption and increases bone mass [24, 28], the decreased PU.1 and DC‐STAMP expression by either compound suggests that both compounds affect early OCP fusion during osteoclastogenesis. Since PU.1 deficiency or DC‐STAMP silencing completely abrogates cell–cell fusion and osteoclast formation during osteoclastogenesis [23, 29, 30, 31, 32, 33, 34, 35], PU.1 and DC‐STAMP may be new targets for inhibiting early osteoclastogenesis.
Interestingly, V‐ATPaseV0d2 is a component of the ATPase proton pump, which regulates osteoclast fusion and bone formation [36]. However, we observed that both DK‐520 and Niclosamide treatment failed to alter ATPaseV0d2 protein expression in OCPs. This suggests that neither compound affects ATPaseV0d2 expression and protein stability during OCP fusion. In future studies, we will investigate the pharmacological action of DK‐520 and Niclosamide in inhibiting osteoclastogenesis in vivo.
Osteoclasts are terminally differentiated myeloid cells. The formation of osteoclasts is a multistep process that is regulated by the NF‐κB and MAPK pathways leading to the induction and RANKL‐induced expression of c‐Fos and NFATc1 [37]. c‐Fos, a member of the AP family of transcription factors, is a critical switch involved in osteoclastogenesis, bone formation, and induction of downstream genes related to osteoclast differentiation [38]. RANKL‐RANK signaling leads to the robust induction of NFATc1 [37, 39]. A series of gene disruption studies has well‐documented the essential role of c‐Fos and NFTAC1 in bone homeostasis [39, 40]. Abrogation of these critical factors alleviates osteoclast formation and bone resorption [41, 42]. Early study reported that NFATc1−/−embryonic stem cells do not differentiate into osteoclasts [39]. Loss of c‐Fos shuttles redirects myeloid precursors toward macrophage commitment. Mice deficient in c‐Fos develop complete deficiency of osteoclasts with severe osteopetrosis [43]. Here, our results implied that DK‐520 drastically decreased the RANKL‐induced expression of c‐Fos and NFATc1 protein in osteoclast progenitors.
RANKL‐induced activation of c‐Fos and NFATC1 is downstream of the activation of the NF‐κB and MAPK pathways [44]. NF‐κB is a transcription factor and an inducible dimeric protein consisting of p65 and p50 subunits, which plays a central role in inflammation, autoimmune responses, differentiation, apoptosis, and cell proliferation [45, 46, 47]. Multiple previous studies have shown that NF‐κB plays a key role in osteoclastogenesis and its suppression affected NFATc1 expression [48, 49]. Genetic studies have shown that knockout of NF‐κB gene in mice results in severe osteopetrotic phenotype and failure of osteoclast formation [50]. Moreover, NF‐κB exists as inactive complex with inhibitory factor IκB which prevents nuclear translocation [48, 49]. Various inducers, including RANKL, can dissociate this complex and promote IκB degradation, presumably which liberates NF‐κB leading to its nuclear translocation and induces the gene transcription involved in osteoclast differentiation [51, 52]. In addition, the MAPK family includes JNK, ERK, and p38 which are relevant to osteoclast formation and play a crucial role in the modulation of osteoclast differentiation [53]. Abrogation of MAPK pathway severely alleviates osteoclast formation and bone resorption [53]. Inhibition of JNK abrogates RANKL‐stimulated osteoclastogenesis. Dominant‐negative ERK affects both osteoclast formation and survival, while inhibition of p38 activation severely blunts osteoclast differentiation but not osteoclast function [19, 54]. Therefore, NF‐κB and MAPK signaling pathways are a prerequisite for osteoclast differentiation and are critical to osteoclast function. In current study, we demonstrated that DK‐‐520 perturbed the RANKL‐induced phosphorylation and activation of p38, ERK, and IκB, whereas the expression of JNK was unaffected.
In summary, our results indicate that DK‐520, like Niclosamide, inhibits OCP fusion and osteoclast differentiation in vitro by significantly reducing PU.1 and DC‐STAMP expression. In addition, the inhibitory effect of DK‐520 on osteoclastogenesis is realized through impairment of the NF‐kB and MAPK signaling pathways. In addition, the inhibitory effect of DK‐520 on osteoclastogenesis is realized through impairment of the NF‐kB and MAPK signaling pathways. These findings provide new insights into the pharmacological action of DK‐520 and Niclosamide in inhibiting osteoclastogenesis and suggest that PU.1 and DC‐STAMP may be therapeutic targets. Our study also indicates that DK‐520, like Niclosamide, may be a promising new therapy for treating osteoclast‐related diseases.
Conflict of interest
The authors declare no conflict of interest.
Author contribution
YZ and XF conceived and designed the experiments. YJ and CC performed the experiments and prepared the manuscript. XH, ZS, JC, and QL analyzed the data.
Acknowledgements
We would like to thank Dr. Chen at Duke University Medical Center for kindly providing DK‐520. This work was supported by a grant from the Research Project Supported by Shanxi Scholarship Council of China (Number. 2017‐121).
Yurui Jiao, Chenglong Chen and Xijian Hu contributed equally to this work.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Sotorník I (2016) Osteoporosis – epidemiology and pathogenesis. Vnitr Lek 62 (Suppl 6), 84–87. [PubMed] [Google Scholar]
- 2. Cummings SR and Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet (London, England) 359, 1761–1767. [DOI] [PubMed] [Google Scholar]
- 3. Suen PK and Qin L (2016) Sclerostin, an emerging therapeutic target for treating osteoporosis and osteoporotic fracture: a general review. J Orthopaedic Trans 4, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teitelbaum SL (2006) Osteoclasts; culprits in inflammatory osteolysis. Arthritis Res Ther 8, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyce BF (2013) Advances in osteoclast biology reveal potential new drug targets and new roles for osteoclasts. J Bone Min Res 28, 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S and Lindsay R (2015) Erratum to: Clinician's guide to prevention and treatment of osteoporosis. Osteoporosis Int 26, 2045–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding Y, Zeng JC, Yin F, Zhang CL, Zhang Y, Li SX, Liu X, Zhang C, Xue QY, Lin H and et al (2017) Multicenter study on observation of acute‐phase responses after infusion of zoledronic acid 5 mg in Chinese women with postmenopausal osteoporosis. Orthopaedic Surg 9, 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O'Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S et al (2015) Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Min Res 30, 3–23. [DOI] [PubMed] [Google Scholar]
- 9. Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW et al (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Min Res 29, 1–23. [DOI] [PubMed] [Google Scholar]
- 10. Hannon RA, Clack G, Rimmer M, Swaisland A, Lockton JA, Finkelman RD and Eastell R (2010) Effects of the Src kinase inhibitor saracatinib (AZD0530) on bone turnover in healthy men: a randomized, double‐blind, placebo‐controlled, multiple‐ascending‐dose phase I trial. J Bone Min Res 25, 463–471. [DOI] [PubMed] [Google Scholar]
- 11. Murphy MG, Cerchio K, Stoch SA, Gottesdiener K, Wu M and Recker R (2005) Effect of L‐000845704, an alphaVbeta3 integrin antagonist, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab 90, 2022–2028. [DOI] [PubMed] [Google Scholar]
- 12. Ferrari S (2014) Future directions for new medical entities in osteoporosis. Best Pract Res Clin Endocrinol Metab 28, 859–870. [DOI] [PubMed] [Google Scholar]
- 13. Frayha GJ, Smyth JD, Gobert JG and Savel J (1997) The mechanisms of action of antiprotozoal and anthelmintic drugs in man. Gen Pharmacol 28, 273–299. [DOI] [PubMed] [Google Scholar]
- 14. Mook RA Jr, Wang J, Ren XR, Chen M, Spasojevic I, Barak LS, Lyerly HK and Chen W (2015) Structure‐activity studies of Wnt/β‐catenin inhibition in the Niclosamide chemotype: Identification of derivatives with improved drug exposure. Bioorg Med Chem 23, 5829–5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinbach EC and Garbus J (1969) Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature 221, 1016–1018. [DOI] [PubMed] [Google Scholar]
- 16. Osada T, Chen M, Yang XY, Spasojevic I, Vandeusen JB, Hsu D, Clary BM, Clay TM, Chen W, Morse MA and et al (2011) Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Can Res 71, 4172–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheon YH, Kim JY, Baek JM, Ahn SJ, So HS and Oh J (2016) Niclosamide suppresses RANKL‐induced osteoclastogenesis and prevents LPS‐induced bone loss. Biochem Biophys Res Comm 470, 343–349. [DOI] [PubMed] [Google Scholar]
- 18. Liu FL, Chen CL, Lee CC, Wu CC, Hsu TH, Tsai CY, Huang HS and Chang DM (2017) The simultaneous inhibitory effect of niclosamide on RANKL‐induced osteoclast formation and osteoblast differentiation. Int J Med Sci 14, 840–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu M, Chen X, Lv C, Yi X, Zhang Y, Xue M, He S, Zhu G, Wang HJB and Communications BR (2014) Curcumol suppresses RANKL‐induced osteoclast formation by attenuating the JNK signaling pathway. Biochem Biophys Res Commun 447, 364–370. [DOI] [PubMed] [Google Scholar]
- 20. So H, Rho J, Jeong D, Park R, Fisher DE, Ostrowski MC, Choi Y and Kim N (2003) Microphthalmia transcription factor and PU.1 synergistically induce the leukocyte receptor osteoclast‐associated receptor gene expression. J Biol Chem 278, 24209–24216. [DOI] [PubMed] [Google Scholar]
- 21. Crockett JC, Mellis DJ, Scott DI and Helfrich MH (2011) New knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: focus on the RANK/RANKL axis. Osteoporosis Int 22, 1–20. [DOI] [PubMed] [Google Scholar]
- 22. Scott EW, Fisher RC, Olson MC, Kehrli EW, Simon MC and Singh H (1997) PU.1 functions in a cell‐autonomous manner to control the differentiation of multipotential lymphoid‐myeloid progenitors. Immunity 6, 437–447. [DOI] [PubMed] [Google Scholar]
- 23. Tondravi MM, McKercher SR, Anderson K, Erdmann JM, Quiroz M, Maki R and Teitelbaum SL (1997) Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 386, 81–84. [DOI] [PubMed] [Google Scholar]
- 24. Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K et al (2005) DC‐STAMP is essential for cell‐cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iwasaki R, Ninomiya K, Miyamoto K, Suzuki T, Sato Y, Kawana H, Nakagawa T, Suda T, Miyamoto TJB and Communications BR (2008) Cell fusion in osteoclasts plays a critical role in controlling bone mass and osteoblastic activity. Biochem Biophys Res Communicat 377, 899–904. [DOI] [PubMed] [Google Scholar]
- 26. Chen W, Zhu G, Hao L, Wu M, Ci H and Li YP (2013) C/EBPα regulates osteoclast lineage commitment. Proc Natl Acad Sci USA 110, 7294–7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ishiyama K, Yashiro T, Nakano N, Kasakura K, Miura R, Hara M, Kawai F, Maeda K, Tamura N, Okumura K et al (2015) Involvement of PU.1 in NFATc1 promoter function in osteoclast development. Allergology Int 64, 241–247. [DOI] [PubMed] [Google Scholar]
- 28. Yagi M, Miyamoto T, Toyama Y and Suda T (2006) Role of DC‐STAMP in cellular fusion of osteoclasts and macrophage giant cells. J Bone Miner Metab 24, 355–358. [DOI] [PubMed] [Google Scholar]
- 29. Courtial N, Smink JJ, Kuvardina ON, Leutz A, Göthert JR and Lausen J (2012) Tal1 regulates osteoclast differentiation through suppression of the master regulator of cell fusion DC‐STAMP. FASEB J 26, 523–532. [DOI] [PubMed] [Google Scholar]
- 30. Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK et al (2006) v‐ATPase V0 subunit d2‐deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med 12, 1403–1409. [DOI] [PubMed] [Google Scholar]
- 31. Saginario C, Sterling H, Beckers C, Kobayashi R, Solimena M, Ullu E and Vignery A (1998) MFR, a putative receptor mediating the fusion of macrophages. Mol Cell Biol 18, 6213–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang C, Dou CE, Xu J and Dong S (2014) DC‐STAMP, the key fusion‐mediating molecule in osteoclastogenesis. J Cell Physiol 229, 1330–1335. [DOI] [PubMed] [Google Scholar]
- 33. Saginario C, Qian HY and Vignery A(1995) Identification of an inducible surface molecule specific to fusing macrophages. Proc Natl Acad Sci USA 92, 12210–12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartgers FC, Vissers JLM, Looman MWG, Zoelen CV, Huffine C, Figdor CG and Adema GJ(2000) DC‐STAMP, a novel multimembrane‐spanning molecule preferentially expressed by dendritic cells. Eur J Immunol 30, 3585–3590. [DOI] [PubMed] [Google Scholar]
- 35. Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H et al (2004) RANKL‐induced DC‐STAMP Is Essential for Osteoclastogenesis. J Exp Med 200, 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T and Lee SKJNM (2006) v‐ATPase V0 subunit d2–deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med 12, 1403–1409. [DOI] [PubMed] [Google Scholar]
- 37. Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A and Bravo R (1997) Osteopetrosis in mice lacking NF‐kappaB1 and NF‐kappaB2. Nat Med 3, 1285–1289. [DOI] [PubMed] [Google Scholar]
- 38. Teitelbaum SLJS (2000) Bone resorption by osteoclasts. Science 289, 1504–1508. [DOI] [PubMed] [Google Scholar]
- 39. Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T and Inoue JIJDC (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3, 889–901. [DOI] [PubMed] [Google Scholar]
- 40. Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ and Wagner EFJNG (2000) Fosl1 is a transcriptional target of c‐Fos during osteoclast differentiation. Nat Genet 24, 184–187. [DOI] [PubMed] [Google Scholar]
- 41. David J‐P, Sabapathy K, Hoffmann O, Idarraga MH and Wagner EF(2002) JNK1 modulates osteoclastogenesis through both c‐Jun phosphorylation‐dependent and ‐independent mechanisms. J Cell Sci 115, 4317–4325. [DOI] [PubMed] [Google Scholar]
- 42. Matsumoto M, Sudo T, Saito T, Osada H and Tsujimoto M (2000) Involvement of p38 mitogen‐activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF‐kappa B ligand (RANKL). J Biol Chem 275, 31155–31161. [DOI] [PubMed] [Google Scholar]
- 43. Johnson RS, Spiegelman BM and Papaioannou VJC (1992) Pleiotropic effects of a null mutation in the c‐fos proto‐oncogene. Cell 71, 577–586. [DOI] [PubMed] [Google Scholar]
- 44. Wada T, Nakashima T, Hiroshi N and Penninger JMJT(2006) RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 12, 17–25. [DOI] [PubMed] [Google Scholar]
- 45. Hinoi E, Iezaki T, Ozaki K, and Yoneda Y (2014) Nuclear factor‐κB is a common upstream signal for growth differentiation factor‐5 expression in brown adipocytes exposed to pro‐inflammatory cytokines and palmitate. Biochem Biophys Res Commun 452, 974–979. [DOI] [PubMed] [Google Scholar]
- 46. Perkins Neil D (2007) Integrating cell‐signalling pathways with NF‐κB and IKK function. Nat Rev Mol Cell Biol 8, 49–62. [DOI] [PubMed] [Google Scholar]
- 47. Baud V and Karin M(2009) Is NF‐kappaB a good target for cancer therapy? Hopes Pitfalls 8, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boyce BF, Xing L, Franzoso G and Siebenlist U (1999) Required and nonessential functions of nuclear factor‐kappa B in bone cells. Bone 25, 137–139. [DOI] [PubMed] [Google Scholar]
- 49. Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF and Siebenlist UJG (1997) Requirement for NF‐kappa B in osteoclast and B‐cell development. Genes Dev 11, 3482–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thanos D and Maniatis TJC (1995) NF‐kappa B: a lesson in family values. Cell 80, 529–532. [DOI] [PubMed] [Google Scholar]
- 51. Karin M and Ben‐Neriah YJAR(2000) Phosphorylation meets ubiquitination: the control of NF‐κB activity. Annu Rev Immunol 18, 621–663. [DOI] [PubMed] [Google Scholar]
- 52. Siebenlist U, Franzoso G and Brown KJAR(1994) Structure, regulation and function of NF‐kappaB. Annu Rev Cell Biol 10, 405–455. [DOI] [PubMed] [Google Scholar]
- 53. Kyunghee L, Ho CY, Heejin A, Hyunsoo K, Jaerang R and Daewon JJIJ(2016) Selective regulation of MAPK signaling mediates RANKL‐dependent osteoclast. Differentiation 12, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hotokezaka H, Sakai E, Kanaoka K, Saito K, Matsuo KI, Kitaura H, Yoshida N and Nakayama KJJ(2002) U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast‐like cells. J Biol Chem 277, 47366–47372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


