Abstract
BNIP3 is a proapoptotic protein that mediates apoptosis, necrosis and autophagy. However, the involvement of BNIP3 in cisplatin‐induced apoptosis in ovarian cancer is not clear. In this study, we examined the role of BNIP3 in ovarian cancer during cisplatin treatment and its correlation with clinical outcomes. We first measured cisplatin cytotoxicity and BNIP3 levels before and after cisplatin exposure for ovarian cancer cell lines A2780, SKOV3, OVCAR4, OV2008, ES2 and HO8910. BNIP3 was observed to be differentially expressed in these cell lines, and cisplatin induced a significant increase in BNIP3 levels in A2780 and OVCAR4. BNIP3 knockdown with siRNA in A2780 and OVCAR4 significantly reduced cisplatin cytotoxicity in these two cell lines and alleviated cisplatin‐induced apoptosis. We searched the online databases Gene Expression Omnibus and The Cancer Genome Atlas to analyze the correlation between BNIP3 level and overall survival and progression‐free survival in patients with ovarian cancer. Pooled analyses showed that higher BNIP3 level was correlated with poorer overall survival (95% confidence intervals; hazard ratio = 1.18, 1.04–1.34; P = 0.013) and progression‐free survival (95% confidence intervals; hazard ratio = 1.26, 1.10–1.43; P = 0.00049). However, the results of individual datasets and stratification analyses of histology, FIGO (Federation Internationale de Gynecolgie et d’Obstetrique) stage, chemotherapy regimen and P53 mutation status varied. These findings indicate that cisplatin‐induced apoptosis is dependent on BNIP3 level in ovarian cancer cell lines. Targeting BNIP3 may therefore be a potential way of restoring cisplatin sensitivity.
Keywords: apoptosis, BNIP3, cisplatin, cisplatin sensitization, ovarian cancer
BNIP3 is up‐regulated by cisplatin in ovarian cancer cells and contributes to cisplatin‐induced cellular apoptosis in a caspase‐dependent manner.
Abbreviations
- FIGO
Federation Internationale de Gynecolgie et d'Obstetrique
- HR
hazard ratio
- MTT
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐tetrazolium bromide
- NC
cell lines treated with saline
- OS
overall survival
- PFS
progression‐free survival
- polyAb
polyclonal antibody
- qRT‐PCR
quantitative RT‐PCR
- SD
standard deviation
- si‐BNIP3
cell lines transfected with BNIP3 siRNA
- si‐NC
cell lines transfected with NC siRNA
- TCGA
The Cancer Genome Atlas
Ovarian cancer is the most lethal gynecological malignancy. The asymptomatic early‐stage disease usually advances to FIGO (Federation Internationale de Gynecolgie et d’Obstetrique) stage III‐IV drastically almost unnoticed, leading to a prevalence of late diagnosis and poor outcome [1, 2]. The standard treatment for ovarian cancer with FIGO stage II‐IV is tumor‐debulking surgery followed by platinum‐based chemotherapy [3]. Around 70% of patients gradually develop platinum resistance, subsequent chemotherapy failure and recurrence [4]. The 5‐year survival rate of patients with ovarian cancer is only <30% [5]. Improving the overall survival (OS) of patients with ovarian cancer remains the most challenging task.
As the two most commonly used anticancer chemotherapeutic agents in treatment of multiple solid malignancies, including ovarian cancer, cisplatin and carboplatin share a similar cytotoxic mechanism [6]. The binding of platinum complex to DNA leads to DNA abducts formation and DNA configuration alteration, which causes cell‐cycle arrest and the release of both proapoptotic and prosurvival signals. The ultimate fate of the assaulted cells depends on the duration and intensity of the activated signals, the interaction of both proapoptotic and prosurvival pathways, and the extent of DNA damage [7]. The apoptosis of ovarian cancer cells triggered by cisplatin could be either caspase dependent or independent [8]. Bax/Bcl2 and Fas/FasL contribute to cisplatin‐induced apoptosis through caspase‐9/caspase‐3 and caspase‐8/caspase‐3 pathways, respectively [9], whereas the AKT/ERK‐FOXO3a pathway has a negative effect on caspase‐independent apoptosis induced by cisplatin [10].
BNIP3 is a BH3‐only protein involved in cell death through mediating apoptosis, necrosis and autophagy [11]. As a member of the Bcl‐2 family, it acts as a proapoptotic protein that interacts with Bcl‐2 and activates the downstream Bax/Bak caspase‐dependent apoptotic pathway [12]. In cardiomyocytes, BNIP3 mediates necrosis caused by ischemia–reperfusion through the Ripk3–JNK–Bnip3–mitochondria cascade [13]. In malignant glioma cells, BNIP3 causes mitochondria depolarization and autophagic cell death in reaction to ceramide [14]. As a key mediator protein in autophagy, BNIP3 is responsive to hypoxia via the carboxyl‐terminal transmembrane domain. Subsequent interaction of BNIP3 with LC3 through LC3‐interacting regions promotes hypoxia‐induced autophagy [15].
The aforementioned diverse effects of BNIP3 in cell death led to intricate roles of BNIP3 in cancer. The basic level of BNIP3 in tumors was tissue specific. BNIP3 was highly expressed in breast cancer, lung cancer, glioma and cervical cancer. BNIP3 was epigenetically silenced in pancreatic cancer, colorectal cancer, gastric cancer, leukemia and lymphoma [16]. Induction of BNIP3 by HIP‐1 under hypoxia found in various solid tumors was regarded as one of the factors promoting tumor aggressiveness, increasing resistance to therapy and worsening overall patient survival [17]. In ovarian cancer, BNIP3 was reported to influence the proliferation and migration of ovarian cancer cells [18]. In addition, the truncated form of BNIP3 lacking the transmembrane domain abolished the mitochondria‐dependent cellular apoptosis induced by BNIP3 in ovarian cancer cells [19]. Evidence suggested that BNIP3 also reacted to chemotherapy agents, such as paclitaxel [20]. However, the effect of BNIP3 regarding cell death in reaction to cisplatin in ovarian cancer is not clear.
Therefore, in this study, we aim to evaluate the function of BNIP3 in ovarian cancer during cisplatin treatment and its correlation with clinical outcomes.
Materials and methods
Cell culture
Human ovarian cancer cell lines A2780, SKOV3, OV2008, OVCAR4, HO8910 and ES2 were purchased from the China Center for Type Culture Collection (CCTCC, Wuhan, China). A2780 and ES2 were cultured with 1640 medium supplemented with 10% FBS, whereas SKOV3, OV2008, OVCAR4 and HO8910 were cultured with Dulbecco’s modified Eagle’s medium/F12 medium supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All media were added to 1% penicillin and streptomycin. All cells were maintained at 37 °C with 5% CO2 in a humidified incubator.
3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐tetrazolium bromide assay
The cytotoxic effect of cisplatin to ovarian cancer cell lines A2780, SKOV3, OV2008, OVCAR4, HO8910 and ES2 and siRNA‐transfected cell lines A2780siBNIP3/A2780siNC and OVCAR4siBNIP3/OVCAR4siNC was assessed using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐tetrazolium bromide (MTT) assay. Cells were seeded in 96‐well plates (Corning Life Sciences, Corning, NY, USA) at a density of 5000 cells per well. After attachment, the cells were exposed to different concentrations of cisplatin for 48 h (0, 2.5, 5, 10, 20, 40, 60, 80 and 100 μm) and followed by 4 h‐incubation at 37 °C in 5 mg·mL−1 medium diluted with MTT reagent (Sigma‐Aldrich, Merck KGaA, Darmstadt, Germany) [21, 22]. The medium was removed, and 100 μL pure dimethyl sulfoxide was added to each well overnight for consecutive absorbance (A) measurement at 490 nm wavelength using a plate reader (iMark‐10601; Bio‐Rad Laboratories, Inc., Hercules, CA, USA). Cell survival curves were generated while reads from wells with no cells served as blank controls and reads from wells with pure medium served as negative controls. All experiments were performed in triplicate.
Western blot analysis
Total cellular proteins were extracted using a protein extraction kit (Beyotime Institute of Biotechnology, Haimen, China). The cellular lysates were subjected to protein quantification by bicinchoninic acid assay (Beyotime Institute of Biotechnology) according to the manufacturer’s protocol. Fifty micrograms of each sample was loaded for the blotting process and then transferred to poly(vinylidene difluoride) membranes. The membranes were blocked with 5% nonfat milk at room temperature for 1 h followed by incubation of the primary antibodies at 4° C overnight. The primary antibodies used were Anti‐BNIP3 Rabbit serum, Anti‐PMS2 Rabbit serum (Abcam, Cambridge, MA, USA), anti‐(cleaved caspase‐3) rabbit serum, anti‐[Poly ADP‐ribose polymerase (PARP) cleavage rabbit] serum, Anti‐LC3B Rabbit serum (Cell Signaling Technology, Inc., Danvers, MA, USA) and Anti‐β‐actin Mouse serum (Epitomics Inc., Burlingame, CA, USA). Then the membranes were washed three times using Tris‐Buffered Saline Tween‐20 buffer before incubation in the secondary antibodies at room temperature for 1 h. The secondary antibodies used were horseradish peroxidase‐conjugated goat anti‐(rabbit Ig) and goat anti‐(mouse Ig) (both from Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Before visualization, the membranes were soaked in an enhanced chemiluminescence system (Beyotime Institute of Biotechnology) and exposed with Molecular Imager Chemi‐Doc XRS+ system (Bio‐Rad Laboratories, Inc.). The bands were quantified using quantity one v4.62 software (Bio‐Rad Laboratories, Inc.). All experiments were performed in triplicate.
Quantitative RT‐PCR
Quantitative RT‐PCR (qRT‐PCR) assays were carried out as previously described. In brief, total RNAs were extracted from the harvested cells using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Reverse transcription was performed using a Reverse Transcription Kit (Toyobo, Osaka, Japan). PCRs were performed using an Applied Biosystem StepOne Plus PCR system (ABI, Foster City, CA, USA) with SYBR Green Real‐time PCR Master Mix (TaKaRa, Otsu, Japan). The primers were designed based on National Center for Biotechnology Information (NCBI) reference sequences and synthesized by Invitrogen (Suzhou, China). The primers used were as follows: BNIP3, forward 5′‐GCCCACCTCGCTCGCAGACAC‐3′, reverse 5′‐CAATCCGATGGCCAGCAAATGAGA‐3′; and β‐actin, forward 5′‐CAGAGCCTCGCCTTTGCC‐3′, reverse 5′‐GTCGCCCACATAGGAATC‐3′. All primers were assessed by a standard curve, ensuring the amplification efficiencies to be approximately 100%. BNIP3 expression was normalized to β‐actin and calculated using the method. All experiments were performed in triplicate.
Cell transfection
Small interfering BNIP3‐specific siRNA and a scrambled control siRNA were synthesized by Univ‐bio (Shanghai, China). The sequence for siBNIP3 was 5′‐AAGGAACACGAGCGUCAUGAAdTdT‐3′. The siRNA transient transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. The transfected cells were exposed to puromycin selection for 3 days prior to further experiments.
Clonogenic assay
Cells were seeded to six‐well plates at a concentration of 500 cells per well. When cells were attached, they were exposed to different concentrations of cisplatin for 48 h; then media were changed with fresh media, and culturing continued for another 10–14 days until visible colonies were seen. The colonies were fixed using 4% formaldehyde for 30 min and stained with crystal violet for at least 1 h. The plates were dried and photographed for colony counting. All experiments were performed in triplicate.
Flow cytometry for apoptosis
A2780siBINP3/A2780siNC and OVCAR4siBNIP3/OVCAR4siNC cells were seeded in six‐well plates at a concentration of 1 × 105 cells per well. When grown to 90% of confluence, the cells were exposed to cisplatin (6 μm for A2780 and 8 μm for OVCAR4) for 48 h. The cells were harvested by trypsin without EDTA, washed twice with icy PBS and went on for apoptosis detection using a FITC Annexin V Apoptosis Detection kit (catalog no. 556547; BD Biosciences, San Jose, CA, USA) according to the manufacturer’s protocol. The cells were analyzed on a flow cytometer (FACSCalibur; BD Biosciences). The data were analyzed by cellquest™ analysis program software, version 5.1 (BD Biosciences). All experiments were performed in triplicate.
Bioinformatics analysis
RNA sequencing data of 10 datasets [The Cancer Genome Atlas (TCGA), GSE9891, GSE26712, GSE26193, GSE63885, GSE65986, GSE18520, GSE30161, GSE3149, GSE14764] were downloaded from the TCGA (https://tcga‐data.nci.nih.gov/tcga/) and Gene Expression Omnibus websites (http://www.ncbi.nlm.nih.gov/geo/). An open free online survival analysis website for the Kaplan–Meier plotter (http://kmplot.com/analysis/) was used to analyze the prognosis significance of BNIP3 in patients with ovarian cancer. The transcript for BNIP3 measured in each dataset was 201849_at with the best probe set. Median expression of BNIP3 within each dataset was selected as the cutoff point dividing BNIP3 expression as high or low.
Statistical analysis
Statistical analysis was performed using spss 17.0 statistics software (IBM, New York, NY, USA) and graphpad prism 5.0 software (GraphPad Software, San Diego, CA, USA). The statistical significance of differences between two groups was analyzed using two‐tailed Student’s t‐test. The statistical significance of differences among three or more groups was analyzed using one‐way ANOVA. Kaplan–Meier and log rank tests were used to evaluate OS and progression‐free survival (PFS). All data are expressed as the mean ± standard deviation (SD) (n ≥ 3).
Results
BNIP3 level in ovarian cancer cell lines
First, the cytotoxicity of cisplatin to ovarian cancer cell lines A2780, SKOV3, OVCAR4, OV2008, ES2 and HO8910 was determined using the MTT test. The general platinum cytotoxicity was estimated by the average the half maximal inhibitory concentration (IC50) of all cell lines included. The cell lines with an IC50 below the average level were considered more sensitive. As shown in Fig. 1A, A2780 (IC50, 3.103 μm), OVCAR4 (IC50, 12.63 μm), ES2 (IC50, 6.403 μm) and HO8910 (IC50, 12.57 μm) cells were relatively more sensitive to cisplatin treatment than the rest of the cell lines. Next, we evaluated the BNIP3 level in all of the earlier cell lines before and after cisplatin treatment. We found that BNIP3 is generally expressed in all six cell lines with lower basal level in A2780 and OVCAR4 and higher level in HO8910 (Fig. 1B). With cisplatin treatment, the protein level of BNIP3 in A2780 and OVCAR4 increased, respectively, with corresponding mRNA increase (Fig. 1B–D).
Fig. 1.
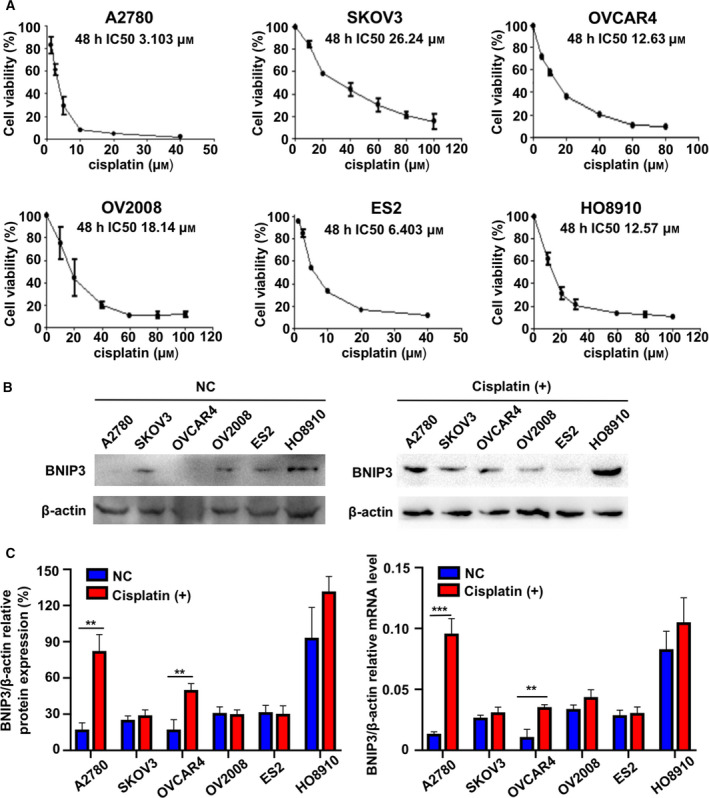
BNIP3 level in ovarian cancer cell lines. (A) The cell viability of ovarian cancer cell lines measured by MTT 48 h after exposure to indicated concentrations of cisplatin. The IC50 of the ovarian cells were A2780 3.103 μm, OV2008 18.14 μm, SKOV3 26.24 μm, OVCAR4 12.63 μm, ES2 6.403 μm and HO8910 12.57 μm. (B–D) BNIP3 level before and after cisplatin exposure measured by western blotting (B) and by qRT‐PCR (C, D) in A2780, SKOV3, OVCAR4, OV2008, ES2 and HO8910 cells. Data were presented as mean ± SD, N = 3. **P < 0.01, ***P < 0.001, Student’s t‐test.
BNIP3 and cisplatin cytotoxicity in ovarian cancer cell lines
To investigate whether BNIP3 level is correlated with cellular cisplatin cytotoxicity, we knocked down BNIP3 expression in relatively platinum‐sensitive ovarian cancer cell lines to determine whether any concurrent cisplatin sensitivity variation occurs. Because A2780 and OVCAR4 were of better cisplatin sensitivity, more significant BNIP3 increase in reaction to cisplatin treatment and better transfection efficiency, we proceeded the subsequent experiments using these two cell lines. The protein and mRNA levels of BNIP3 of A2780 and OVCAR4 are down‐regulated in A2780si‐BNIP3 and OVCAR4si‐BNIP3 (Fig. 2A,B). The decrease in BNIP3 leads to a declined cellular response to cisplatin measured by MTT assay and clonogenic assay (Fig. 2C,D).
Fig. 2.
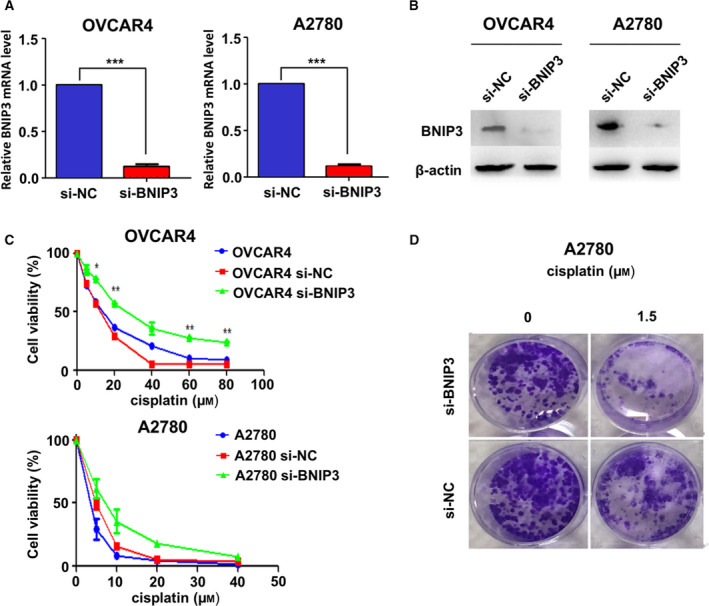
BNIP3 level is correlated with cisplatin cytotoxicity in ovarian cancer cell lines. (A, B) Relative BNIP3 mRNA level measured by qRT‐PCR (A) and BNIP3 protein level by western blotting (B) in A2780 and OVCAR4 cells after BNIP3 knockdown. (C, D) Cell viability was measured by MTT (C) and clonogenic ability was measured by clonogenic assay (D) in A2780 and OVCAR4 after BNIP3 knockdown. Data were presented as mean ± SD, N = 3. *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t‐test.
Cisplatin‐induced cellular apoptosis was dependent on BNIP3
Because BNIP3 correlated with cisplatin cytotoxicity in ovarian cancer cells, we hypothesized that cisplatin‐induced cellular apoptosis was to some extent dependent on BNIP3 level. To determine the cellular status after cisplatin exposure, we measured the markers of apoptosis, including cleaved PARP and cleaved caspase‐3, as well as the markers of autophagy, including LC3I and LC3II in A2780 and OVCAR4 cells. BNIP3 knockdown led to a significant decrease in platinum‐induced PARP/caspase‐3 cleavage and slight reduction of LC3I/LC3II level (Fig. 3A,B). According to flow cytometry results, cisplatin treatment caused apoptosis in both cell lines transfected with NC siRNA (si‐NC) and cell lines transfected with BNIP3 siRNA (si‐BNIP3) cells of A2780 and OVCAR4. BNIP3 down‐regulation did not bring about much change in apoptotic rate in saline‐treated cells, but significantly alleviated cisplatin‐induced apoptosis in both A2780 and OVCAR4 (Fig. 3C–F and Table 1). Based on the earlier results, we assumed that cisplatin‐induced cellular apoptosis was dependent on BNIP3.
Fig. 3.
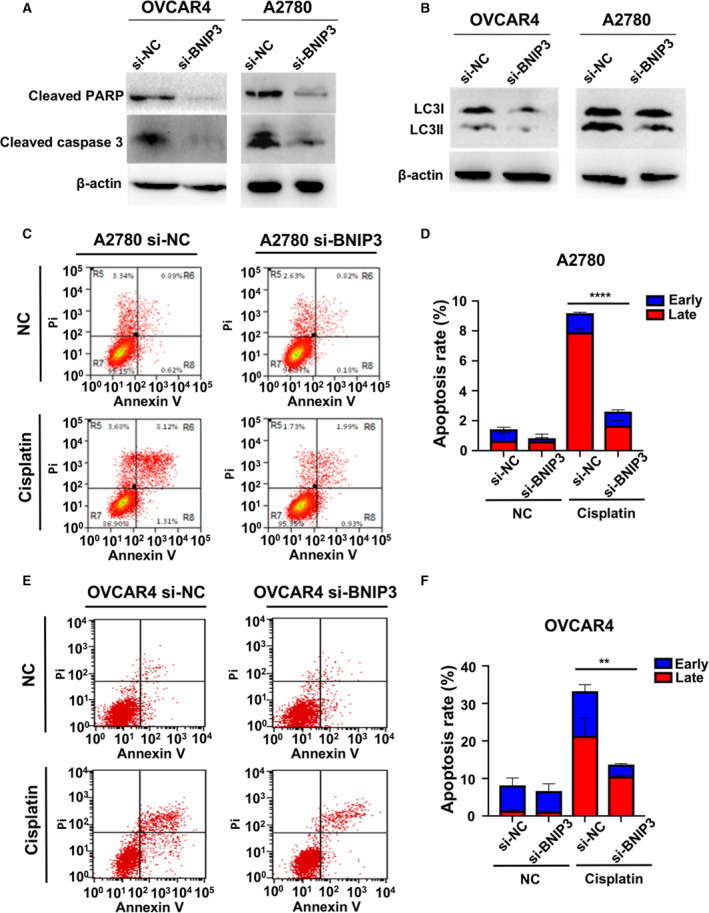
Cisplatin‐induced cellular apoptosis was dependent on BNIP3. (A, B) Cleaved PARP and caspase‐3 (A) and LC3I/LC3II (B) were measured by western blotting in A2780 and OVCAR4 cells when treated with 6 and 8 μm CDDP for 48 h after BNIP3 knockdown. (C, E) Apoptosis and necrosis in A2780 si‐NC/A2780 si‐BNIP3 (C) and OVCAR4 si‐NC/OVCAR4 si‐BNIP3 (E) cells assessed by flow cytometry following 48 h of 6 and 8 μm cisplatin treatment. (D, F) The apoptotic percentage in A2780 si‐NC/A2780 si‐BNIP3 (D) and OVCAR4 si‐NC/OVCAR4 si‐BNIP3 (F) cells after treatment of cisplatin. Data were presented as mean ± SD, N = 3. **P < 0.01, ****P < 0.0001, Student’s t‐test.
Table 1.
Apoptosis and necrosis of ovarian cancer cell lines A2780 and OVCAR4 in reaction to cisplatin after BNIP3 regulation. Cisplatin, cell lines treated with cisplatin. P‐value, unpaired student t‐test. Data are presented as mean ± SD.
| Apoptosis/Necrosis | A2780 | OVCAR4 | ||||
|---|---|---|---|---|---|---|
| NC | Cisplatin | P | NC | Cisplatin | P | |
| si‐NC | ||||||
| Early apoptosis | 0.77 ± 011 | 1.28 ± 0.05 | 0.0040 | 6.87 ± 1.63 | 11.85 ± 0.23 | 0.0276 |
| Late apoptosis | 0.67 ± 0.42 | 7.91 ± 0.18 | <0.0001 | 1.46 ± 0.17 | 21.39 ± 3.93 | 0.0020 |
| Apoptosis | 1.43 ± 0.36 | 9.18 ± 0.18 | <0.0001 | 8.15 ± 1.47 | 33.24 ± 5.32 | 0.0030 |
| Necrosis | 2.98 ± 0.26 | 3.10 ± 0.50 | 0.7909 | 0.40 ± 0.29 | 1.42 ± 0.14 | 0.0101 |
| si‐BNIP3 | ||||||
| Early apoptosis | 0.21 ± 0.03 | 0.93 ± 0.11 | 0.0008 | 5.49 ± 1.56 | 3.13 ± 0.23 | 0.1021 |
| Late apoptosis | 0.63 ± 0.08 | 1.67 ± 0.27 | 0.0368 | 1.17 ± 0.09 | 10.58 ± 0.18 | <0.0001 |
| Apoptosis | 0.83 ± 0.12 | 2.60 ± 0.23 | 0.0060 | 6.66 ± 1.47 | 13.71 ± 0.36 | 0.0028 |
| Necrosis | 3.00 ± 0.28 | 2.20 ± 0.35 | 0.0640 | 0.64 ± 0.28 | 0.99 ± 0.07 | 0.1576 |
| A2780 | OVCAR4 | |||||
|---|---|---|---|---|---|---|
| si‐NC | si‐BNIP3 | P | si‐NC | si‐BNIP3 | P | |
| NC | ||||||
| Early apoptosis | 0.77 ± 011 | 0.21 ± 0.03 | 0.0022 | 6.87 ± 1.63 | 5.49 ± 1.56 | 0.4951 |
| Late apoptosis | 0.67 ± 0.42 | 0.63 ± 0.08 | 0.9268 | 1.46 ± 0.17 | 1.17 ± 0.09 | 0.0995 |
| Apoptosis | 1.43 ± 0.36 | 0.83 ± 0.12 | 0.1946 | 8.15 ± 1.47 | 6.66 ± 1.47 | 0.3700 |
| Necrosis | 2.98 ± 0.26 | 3.00 ± 0.28 | 0.9437 | 0.40 ± 0.29 | 0.64 ± 0.28 | 0.4365 |
| Cisplatin | ||||||
| Early apoptosis | 1.28 ± 0.05 | 0.93 ± 0.11 | 0.0147 | 11.85 ± 0.23 | 3.13 ± 0.23 | 0.0010 |
| Late apoptosis | 7.91 ± 0.18 | 1.67 ± 0.27 | <0.0001 | 21.39 ± 3.93 | 10.58 ± 0.18 | 0.0177 |
| Apoptosis | 9.18 ± 0.18 | 2.60 ± 0.23 | <0.0001 | 33.24 ± 5.32 | 13.71 ± 0.36 | 0.0066 |
| Necrosis | 3.10 ± 0.50 | 2.20 ± 0.35 | 0.1085 | 1.42 ± 0.14 | 0.99 ± 0.07 | 0.0171 |
Values in bold represent values with statistical significance.
BNIP3 level and clinical outcome of patients with ovarian cancer
Because BNIP3 level was significantly related to cisplatin‐induced apoptosis, we wondered whether BNIP3 could act as a biomarker for clinical outcomes in patients with ovarian cancer. We performed the analyses to examine the correlation between BNIP3 level and clinical outcomes such as OS and PFS in patients with ovarian cancer using online datasets. Pooled analyses showed that higher BNIP3 level was correlated with poorer OS [95% confidence intervals (CI); hazard ratio (HR) = 1.18, 1.04–1.34; P = 0.013] and PFS (95% CI; HR = 1.26, 1.10–1.43; P = 0.00049; Table 2). However, this correlation might vary on account of histology, FIGO stage, chemotherapy regimen and P53 mutation status according to stratification analyses. High BNIP3 level remained correlated with poor OS in patients with serous (OS: 95% CI; HR = 1.23, 1.05–1.43; P = 0.0085) and advanced stage (OS: 95% CI; HR = 1.23, 1.06–1.43; P = 0.0058) ovarian cancer. Although in patients who received paclitaxel (OS: 95% CI; HR = 0.57, 0.36–0.90; P = 0.014; PFS: 95% CI; HR = 0.54, 0.35–0.84; P = 0.0053) or topotecan (OS: 95% CI; HR = 0.58, 0.37–0.90; P = 0.013) and patients with wild‐type p53 (PFS: 95% CI; HR = 0.47, 0.24–0.93; P = 0.027), high BNIP3 level was related with better OS or PFS (Table 2). Then an independent dataset was analyzed separately. GSE9891 (OS: 95% CI; HR = 1.46, 1–2.13; P = 0.046) and GSE65986 (PFS: 95% CI; HR = 3.53, 0.99–12.57; P = 0.038) reported increased risk for patients with higher BNIP3, whereas GSE3149 (OS: 95% CI; HR = 0.53, 0.31–0.91; P = 0.019), TCGA (PFS: 95% CI; HR = 0.76, 0.59–0.99; P = 0.038) and GSE63885 (PFS: 95% CI; HR = 0.51, 0.30–0.88; P = 0.015) reported the opposite results. The rest indicated no statistical relevance (Tables 3 and 4).
Table 2.
Correlation of BNIP3 level to OS and PFS in patients with ovarian cancer with varied clinical characteristics. CI, confidence interval.
| Clinical characteristics | OS | PFS | ||||
|---|---|---|---|---|---|---|
| N | HR (95% CI) | P | N | HR (95% CI) | P | |
| All | 1656 | 1.18 (1.04–1.34) | 0.013 | 1435 | 1.26 (1.1–1.43) | 0.000 |
| Histology | ||||||
| Serous | 1104 | 1.23 (1.05–1.43) | 0.0085 | 1104 | 0.89 (0.75–1.05) | 0.160 |
| FIGO stage | ||||||
| I + II | 135 | 0.68 (0.31–1.49) | 0.33 | 163 | 1.62 (0.84–3.13) | 0.140 |
| III + IV | 1220 | 1.23 (1.06–1.43) | 0.0058 | 1081 | 0.89 (0.75–1.06) | 0.180 |
| Chemotherapy | ||||||
| T + P | 776 | 1.16 (0.96–1.40) | 0.13 | 698 | 0.90 (0.74–1.10) | 0.300 |
| Paclitaxel | 220 | 0.57 (0.36–0.90) | 0.014 | 229 | 0.54 (0.35–0.84) | 0.0053 |
| Gemcitabine | 135 | 0.68 (0.45–1.02) | 0.062 | 131 | 1.29 (0.86–1.92) | 0.22 |
| Topotecan | 119 | 0.58 (0.37–0.9) | 0.013 | 118 | 0.72 (0.46–1.13) | 0.15 |
| P53 mutation | ||||||
| Mutated | 506 | 0.88 (0.70–1.11) | 0.28 | 483 | 1.19 (0.95–1.49) | 0.13 |
| Wild | 94 | 0.71 (0.37–1.34) | 0.28 | 84 | 0.47 (0.24–0.93) | 0.027 |
Values in bold represent values with statistical significance.
Table 3.
Separate analysis of correlation between BNIP3 level and OS in patients with ovarian cancer. The transcript for BNIP3 measured in each dataset was 201849_at. All studies were split by median expression of BNIP3 within each dataset. CI, confidence interval.
| Dataset | No. of patients | HR (95% CI) | P | ||
|---|---|---|---|---|---|
| Total | BNIP3 high | BNIP3 low | |||
| TCGA | 557 | 415 | 142 | 0.81 (0.62–1.07) | 0.130 |
| GSE9891 | 285 | 129 | 156 | 1.46 (1.00–2.13) | 0.046 |
| GSE26712 | 184 | 66 | 118 | 1.19 (0.83–1.71) | 0.340 |
| GSE26193 | 107 | 52 | 55 | 1.21 (0.77–1.89) | 0.420 |
| GSE63885 | 75 | 41 | 34 | 0.70 (0.43–1.14) | 0.150 |
| GSE18520 | 53 | 14 | 39 | 1.45 (0.74–2.85) | 0.280 |
| GSE3149 | 116 | 44 | 72 | 0.53 (0.31–0.91) | 0.019 |
| GSE30161 | 58 | 41 | 17 | 1.79 (0.79–4.05) | 0.160 |
| GSE14764 | 80 | 49 | 31 | 0.53 (0.22–1.26) | 0.150 |
Values in bold represent values with statistical significance.
Table 4.
Separate analysis of correlation between BNIP3 level and PFS in patients with ovarian cancer. The transcript for BNIP3 measured in each dataset was 201849_at. All studies were split by median expression of BNIP3 within each dataset. CI, confidence interval.
| Dataset | No. of patients | HR (95% CI) | P | ||
|---|---|---|---|---|---|
| Total | BNIP3 high | BNIP3 low | |||
| TCGA | 522 | 169 | 353 | 0.76 (0.59–0.99) | 0.038 |
| GSE9891 | 285 | 95 | 190 | 1.17 (0.86–1.58) | 0.31 |
| GSE26712 | 184 | 133 | 51 | 1.34 (0.93–1.93) | 0.11 |
| GSE26193 | 107 | 57 | 50 | 1.28 (0.82–1.99) | 0.27 |
| GSE65986 | 55 | 31 | 24 | 3.53 (0.99–12.57) | 0.038 |
| GSE63885 | 75 | 56 | 19 | 0.51 (0.30–0.88) | 0.015 |
| GSE30161 | 54 | 39 | 15 | 1.95 (0.95–3.99) | 0.063 |
| GSE14764 | 80 | 42 | 38 | 0.73 (0.43–1.22) | 0.23 |
Values in bold represent values with statistical significance.
Discussion
To evaluate the effect of BNIP3 in ovarian cancer during cisplatin treatment and whether it could work as a prognostic factor, we measured the level of BNIP3 before and after exposure to cisplatin in ovarian cancer cells, evaluated the relation of BNIP3 level to cisplatin cytotoxicity and cellular apoptosis, and examined the correlation between BNIP3 and clinical outcomes. According to our results, cisplatin‐induced apoptosis is dependent on BNIP3 level, and the depletion of BNIP3 remarkably alleviates the cytotoxic effect of cisplatin in ovarian cancer cells. These findings provide evidence of cisplatin sensitization by targeting BNIP3.
When evaluating the basic level of BNIP3 in ovarian cancer cells, we identified BNIP3 expression in all selected ovarian cancer cell lines. This finding was consistent with the former finding that BNIP3 expression was universally higher in tumor tissue compared with matched normal tissue because of hypoxia and metabolic change [23]. We also recognized big variation of basic BNIP3 level among the selected cell lines. The variation was possibly due to varied molecular background, metabolism and aggressiveness of each cell line because the basic level of BNIP3 was influenced by several factors: HIF1 level, BNIP3 promoter hypermethylation status, miRNA inhibition and hypoxia [24, 25, 26]. Cisplatin‐induced BNIP3 up‐regulation in both protein and mRNA levels was observed in ovarian cancer lines. Previous studies also reported cisplatin‐induced BNIP3 up‐regulation in lung cancer cell line A549 [27, 28]. The increase in BNIP3 could also be triggered by other drug treatments, such as rapamycin, LBH589, verticillin A and acidosis [27, 29].
In this study, BNIP3 silencing in A2780 and OVCAR4 cells led to a significant decrease in sensitivity to cisplatin, which suggested that cisplatin cytotoxicity was dependent on BNIP3 level in ovarian cancer cells. Similar results were reported in other cancer cell lines. Cisplatin cytotoxicity increased with ascending BNIP3 level in several clonal variants of human colon carcinoma cell line HT29, and up‐regulation of BNIP3 in HT29 significantly increased its cisplatin sensitivity [30]. In cisplatin‐resistant breast cancer cell line MCF‐7/R, BNIP3 expression was down‐regulated by miR‐944, and inhibition of miR‐944 restored cisplatin sensitivity of MCF7/R [26]. In lung cancer cell line A549, cisplatin‐based combination treatment triggered cell death by induction of BNIP3 [27].
BNIP3 was reported to contribute to both spontaneous and drug‐induced cell death through the apoptotic pathway. Previous studies reported that BNIP3 knockdown in the esophageal squamous cell carcinoma cell line down‐regulated spontaneous cellular apoptosis [31]. Up‐regulation of BNIP3 in colon carcinoma increased both spontaneous apoptosis and cisplatin‐induced apoptosis [30]. In this study, we observed similar results that BNIP3 silencing caused only slight reduction of spontaneous apoptosis in A2780 and a massive decrease in cisplatin‐induced apoptosis in both A2780 and OVCAR4. We found that BNIP3 silencing led to a significant decrease in cleaved PARP and cleaved caspase‐3 induced by cisplatin, which suggested that cisplatin‐induced BNIP3‐dependent apoptosis was caspase dependent. Although the underlying mechanism was not entirely clear, this result provided possible interpretation to some of the previous clinical findings. A comparative analysis and a patient‐matched analysis of 112 primary ovarian carcinoma and 63 metastatic samples reported by Nymoen et al. [32] showed that BNIP3 levels were significantly lower in metastatic sites than in primary ovarian cancer lesions. Also, these patients with metastasis did not respond well to cisplatin treatment, resulting in a poorer prognosis. According to our result, it was highly possible that a decrease in BNIP3 in metastatic sites alleviated cytotoxicity of cisplatin, which led to the overall failure of cisplatin‐based chemotherapy.
To unveil the mechanism of action of BNIP3 in cell death, other facets such as necrosis and autophagy should not be neglected. Although a previous study reported that BNIP3 induces programmed necrosis by an intrinsic caspase‐independent mitochondrial pathway [29], our results did not show a significant change in necrosis of ovarian cancer cells after BNIP3 modulation. One important concept that is often underappreciated is that autophagy is not necessarily responsible for promoting cell death in all cases. According to Zhu et al. [33], phosphorylation of BNIP3 at serines 17 and 24 initiated the binding of BNIP3 to LC3II and triggered autophagy. BNIP3‐induced autophagy counteracted apoptosis via reduction of cellular cytochrome c release capacity. It was demonstrated in breast cancer, pancreatic cancer, cervical cancer and other various cancers that autophagy protects against apoptosis in response to many different stimuli [34, 35, 36, 37]. In this study, we observed a significant decrease in LC3II after BNIP3 knockdown, suggesting the possible involvement of BNIP3 in autophagy. We did not explore the role of BNIP3 in autophagy further because the main consequence of BNIP3 silencing lay in reduction of cisplatin‐triggered apoptosis.
BNIP3 worked as a good prognostic indicator in pancreatic cancer [38], indicated poor prognosis in cervical cancer [34] and had inconclusive value of clinical outcome prediction in breast cancer [35]. In this study, the pooled analysis showed a correlation between high BNIP3 level with poor OS and PFS. However, considering the results of individual study and stratification analysis, the predictive value of BNIP3 in ovarian cancer should be interpreted delicately. On one hand, several individual analyses, such as the TCGA, the GSE63885 and the GSE3149 datasets, showed completely opposite results to the pooled analysis. On the other hand, the result of stratification analysis of histology, FIGO stage, chemotherapy regimen and P53 mutation status varied greatly. We assumed that such an intricate result of BNIP3 was linked to the highly inducible characteristic of BNIP3 itself and the multiple cell death pathways it was involved in.
Taken together, our results indicate that cisplatin‐induced apoptosis was dependent on BNIP3 level in ovarian cancer cell lines. Targeting BNIP3 is a promising way to restore cisplatin sensitivity.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
XH and XY supervised the project. JJ and XH designed the present study. QZ performed the experiments. JJ and QZ collected data. XY and XH analyzed and interpreted the data. SS drafted the manuscript. SS, FY and EC prepared data and the manuscript for revision. All authors contributed to producing the manuscript and approved the final manuscript.
Acknowledgements
We thank Prof Jing Cai for help with the study design. This work was supported by National Natural Science Foundation of China (grant 81302265 to JJ); Natural Science Foundation of Hubei Province (grant 2017CFB764 to XY); Health, Family Planning Commission of Wuhan Municipality (grant WX17C10 to XY); Natural Science Foundation of Hubei Province (grant 2019CFB702 to XH); and Union Hospital Scientific Research Fund (grant 2018‐229 to SS).
Jinghui Jia and Xiaoxin Yang contributed equally to this article
Data accessibility
All data generated or analyzed during this study are included in this article.
References
- 1. Siegel RL, Miller KD and Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL (2018) Ovarian cancer statistics, 2018. CA Cancer J Clin 68, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Covens A, Carey M, Bryson P, Verma S, Fung Kee Fung M and Johnston M(2002) Systematic review of first‐line chemotherapy for newly diagnosed postoperative patients with stage II, III, or IV epithelial ovarian cancer. Gynecol Oncol 85, 71–80. [DOI] [PubMed] [Google Scholar]
- 4. Agarwal R and Kaye SB (2003) Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer 3, 502–516. [DOI] [PubMed] [Google Scholar]
- 5. Tummala MK and McGuire WP (2005) Recurrent ovarian cancer. Clin Adv Hematol Oncol 3, 723–736. [PubMed] [Google Scholar]
- 6. Olszewski U and Hamilton G (2010) A better platinum‐based anticancer drug yet to come? Anticancer Agents Med Chem 10, 293–301. [DOI] [PubMed] [Google Scholar]
- 7. Descoteaux C, Leblanc V, Belanger G, Parent S, Asselin E and Berube G (2008) Improved synthesis of unique estradiol‐linked platinum(II) complexes showing potent cytocidal activity and affinity for the estrogen receptor alpha and beta. Steroids 73, 1077–1089. [DOI] [PubMed] [Google Scholar]
- 8. Henkels KM and Turchi JJ (1999) Cisplatin‐induced apoptosis proceeds by caspase‐3‐dependent and ‐independent pathways in cisplatin‐resistant and ‐sensitive human ovarian cancer cell lines. Cancer Res 59, 3077–3083. [PubMed] [Google Scholar]
- 9. Del Bello B, Valentini MA, Zunino F, Comporti M and Maellaro E (2001) Cleavage of Bcl‐2 in oxidant‐ and cisplatin‐induced apoptosis of human melanoma cells. Oncogene 20, 4591–4595. [DOI] [PubMed] [Google Scholar]
- 10. Yamaguchi H, Hsu JL, Chen CT, Wang YN, Hsu MC, Chang SS, Du Y, Ko HW, Herbst R and Hung MC (2013) Caspase‐independent cell death is involved in the negative effect of EGF receptor inhibitors on cisplatin in non‐small cell lung cancer cells. Clin Cancer Res 19, 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang J and Ney PA (2009) Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ 16, 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kubli DA, Ycaza JE and Gustafsson AB (2007) Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J 405, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song X and Li T (2019) Ripk3 mediates cardiomyocyte necrosis through targeting mitochondria and the JNK‐Bnip3 pathway under hypoxia‐reoxygenation injury. J Recept Signal Transduct Res 39, 331–340. [DOI] [PubMed] [Google Scholar]
- 14. Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y and Kondo S (2004) Pivotal role of the cell death factor BNIP3 in ceramide‐induced autophagic cell death in malignant glioma cells. Cancer Res 64, 4286–4293. [DOI] [PubMed] [Google Scholar]
- 15. Drake LE, Springer MZ, Poole LP, Kim CJ and Macleod KF (2017) Expanding perspectives on the significance of mitophagy in cancer. Semin Cancer Biol 47, 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee H and Paik SG (2006) Regulation of BNIP3 in normal and cancer cells. Mol Cells 21, 1–6. [PubMed] [Google Scholar]
- 17. Mellor HR and Harris AL (2007) The role of the hypoxia‐inducible BH3‐only proteins BNIP3 and BNIP3L in cancer. Cancer Metastasis Rev 26, 553–566. [DOI] [PubMed] [Google Scholar]
- 18. Jia XN, Yin SD, Wei Y and Chen L (2019) MiR‐182‐5p inhibited proliferation and migration of ovarian cancer cells by targeting BNIP3. Eur Rev Med Pharmacol Sci 23, 3270–3276. [DOI] [PubMed] [Google Scholar]
- 19. Bristow N, Burton TR, Henson ES, Ong‐Justiniano C, Brown M and Gibson SB (2011) Truncated forms of BNIP3 act as dominant negatives inhibiting hypoxia‐induced cell death. Biochim Biophys Acta 1812, 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bani MR, Nicoletti MI, Alkharouf NW, Ghilardi C, Petersen D, Erba E, Sausville EA, Liu ET and Giavazzi R (2004) Gene expression correlating with response to paclitaxel in ovarian carcinoma xenografts. Mol Cancer Ther 3, 111–121. [PubMed] [Google Scholar]
- 21. Hu S, Yu L, Li Z, Shen Y, Wang J, Cai J, Xiao L and Wang Z (2010) Overexpression of EZH2 contributes to acquired cisplatin resistance in ovarian cancer cells in vitro and in vivo . Cancer Biol Ther 10, 788–795. [DOI] [PubMed] [Google Scholar]
- 22. Cai J, Yang C, Yang Q, Ding H, Jia J, Guo J, Wang J and Wang Z (2013) Deregulation of let‐7e in epithelial ovarian cancer promotes the development of resistance to cisplatin. Oncogenesis 2, e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uberall I, Kolek V, Klein J, Krejci V, Stastna J, Radova L, Skarda J and Fridman E (2010) The immunohistochemical expression of BNIP3 protein in non‐small‐cell lung cancer: a tissue microarray study. APMIS 118, 565–570. [DOI] [PubMed] [Google Scholar]
- 24. Guo K, Searfoss G, Krolikowski D, Pagnoni M, Franks C, Clark K, Yu KT, Jaye M and Ivashchenko Y (2001) Hypoxia induces the expression of the pro‐apoptotic gene BNIP3. Cell Death Differ 8, 367–376. [DOI] [PubMed] [Google Scholar]
- 25. Chinnadurai G, Vijayalingam S and Gibson SB (2008) BNIP3 subfamily BH3‐only proteins: mitochondrial stress sensors in normal and pathological functions. Oncogene 27 (Suppl 1), S114–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He H, Tian W, Chen H and Jiang K (2016) MiR‐944 functions as a novel oncogene and regulates the chemoresistance in breast cancer. Tumour Biol 37, 1599–1607. [DOI] [PubMed] [Google Scholar]
- 27. Chung LY, Tang SJ, Wu YC, Yang KC, Huang HJ, Sun GH and Sun KH (2020) Platinum‐based combination chemotherapy triggers cancer cell death through induction of BNIP3 and ROS, but not autophagy. J Cell Mol Med 24, 1993–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu HM, Jiang ZF, Ding PS, Shao LJ and Liu RY (2015) Hypoxia‐induced autophagy mediates cisplatin resistance in lung cancer cells. Sci Rep 5, 12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Graham RM, Thompson JW and Webster KA (2014) Inhibition of the vacuolar ATPase induces Bnip3‐dependent death of cancer cells and a reduction in tumor burden and metastasis. Oncotarget 5, 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma Z, Chen C, Tang P, Zhang H, Yue J and Yu Z (2017) BNIP3 induces apoptosis and protective autophagy under hypoxia in esophageal squamous cell carcinoma cell lines: BNIP3 regulates cell death. Dis Esophagus 30, 1–8. [DOI] [PubMed] [Google Scholar]
- 31. Chen BC, Weng YJ, Shibu MA, Han CK, Chen YS, Shen CY, Lin YM, Viswanadha VP, Liang HY and Huang CY (2018) Estrogen and/or estrogen receptor alpha inhibits BNIP3‐induced apoptosis and autophagy in H9c2 cardiomyoblast cells. Int J Mol Sci 19, 1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nymoen DA, Hetland Falkenthal TE, Holth A, Ow GS, Ivshina AV, Trope CG, Kuznetsov VA, Staff AC and Davidson B (2015) Expression and clinical role of chemoresponse‐associated genes in ovarian serous carcinoma. Gynecol Oncol 139, 30–39. [DOI] [PubMed] [Google Scholar]
- 33. Zhu Y, Massen S, Terenzio M, Lang V, Chen‐Lindner S, Eils R, Novak I, Dikic I, Hamacher‐Brady A and Brady NR (2013) Modulation of serines 17 and 24 in the LC3‐interacting region of Bnip3 determines pro‐survival mitophagy versus apoptosis. J Biol Chem 288, 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leo C, Horn LC and Hockel M (2006) Hypoxia and expression of the proapoptotic regulator BNIP3 in cervical cancer. Int J Gynecol Cancer 16, 1314–1320. [DOI] [PubMed] [Google Scholar]
- 35. Tan EY, Campo L, Han C, Turley H, Pezzella F, Gatter KC, Harris AL and Fox SB (2007) BNIP3 as a progression marker in primary human breast cancer; opposing functions in in situ versus invasive cancer. Clin Cancer Res 13, 467–474. [DOI] [PubMed] [Google Scholar]
- 36. Giatromanolaki A, Koukourakis MI, Sowter HM, Sivridis E, Gibson S, Gatter KC and Harris AL (2004) BNIP3 expression is linked with hypoxia‐regulated protein expression and with poor prognosis in non‐small cell lung cancer. Clin Cancer Res 10, 5566–5571. [DOI] [PubMed] [Google Scholar]
- 37. Sington J, Giatromanolaki A, Campo L, Turley H, Pezzella F and Gatter KC (2007) BNIP3 expression in follicular lymphoma. Histopathology 50, 555–560. [DOI] [PubMed] [Google Scholar]
- 38. Erkan M, Kleeff J, Esposito I, Giese T, Ketterer K, Buchler MW, Giese NA and Friess H (2005) Loss of BNIP3 expression is a late event in pancreatic cancer contributing to chemoresistance and worsened prognosis. Oncogene 24, 4421–4432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


