Abstract
The Mycobacterium fortuitum complex comprises several closely related species, causing pulmonary and extra-pulmonary infections. However, there is very limited knowledge about the disease pathogenesis involved in M. fortuitum infections, particularly due to the lack of suitable animal models. Using the zebrafish model, we show that embryos are susceptible to M. fortuitum infection in a dose-dependent manner. Furthermore, zebrafish embryos form granulomas from as early as 2 days post-infection, recapitulating critical aspects of mycobacterial pathogenesis observed in other pathogenic species. The formation of extracellular cords in infected embryos highlights a previously unknown pathogenic feature of M. fortuitum. The formation of large corded structures occurs also during in vitro growth, suggesting that this is not a host-adapted stress mechanism deployed during infection. Moreover, transient macrophage depletion led to rapid embryo death with increased extracellular cords, indicating that macrophages are essential determinants of M. fortuitum infection control. Importantly, morpholino depletion of the cystic fibrosis transmembrane conductance regulator (cftr) significantly increased embryo death, bacterial burden, bacterial cords and abscesses. There was a noticeable decrease in the number of cftr-deficient infected embryos with granulomas as compared to infected controls, suggesting that loss of CFTR leads to impaired host immune responses and confers hypersusceptiblity to M. fortuitum infection. Overall, these findings highlight the application of the zebrafish embryo to study M. fortuitum and emphasizes previously unexplored aspects of disease pathogenesis of this significant mycobacterial species.
Keywords: Mycobacterium fortuitum, granuloma, infection, cording, cystic fibrosis, CFTR, pathogenesis, zebrafish
Introduction
The incidence of non-tuberculous mycobacterial (NTM) infections is increasing globally, surpassing the infection rate of tuberculosis in many developed countries (Johansen et al., 2020). NTM are increasingly acknowledged pathogens capable of infecting a vast array of tissues and inducing a wide spectrum of clinical symptoms in humans, with pulmonary infections being the most common clinical manifestations (Ratnatunga et al., 2020). NTM infections are particularly prevalent in immunocompromised individuals and those with underlying genetic and structural defects. They have emerged in recent years as prevalent pathogens in cystic fibrosis (CF) patients, with several global CF centers reporting the isolation of NTM in the respiratory tract of CF patients (Roux et al., 2009; Skolnik et al., 2016). NTM prevalence in CF patients varies between 5 and 20%, with the Mycobacterium avium complex and the Mycobacterium abscessus complex being the most significant species (Richards and Olivier, 2019). However, less frequently isolated species in CF patients include other NTM, such as Mycobacterium gordonae, Mycobacterium kansasii or M. fortuitum (Sermet-Gaudelus et al., 2003; Cândido et al., 2014; Martiniano et al., 2016; Richards and Olivier, 2019).
The M. fortuitum complex includes M. fortuitum, Mycobacterium peregrinum, Mycobacterium porcinum, Mycobacterium septicum, Mycobacterium conceptionense, Mycobacterium boenickei, Mycobacterium houstonense, Mycobacterium neworleansense, Mycobacterium brisbanense, Mycobacterium farcinogenes, Mycobacterium senegalense, and Mycobacterium setense (Brown-Elliott and Wallace, 2002; Brown-Elliott and Philley, 2017; Tortoli et al., 2017). M. fortuitum is a rapid-growing and frequently identified NTM species, causing localized skin and soft tissue infections (Wallace et al., 1983). Several clinical cases have also reported catheter infections, post-surgical infections, peritonitis, eye infections, and pulmonary infections (Brown-Elliott et al., 2012; Brown-Elliott and Philley, 2017). Unfortunately, our understanding surrounding the pathogenicity of M. fortuitum has been hampered by the lack of genetic tools and by the restricted panel of cellular and animal models available. Among the few animal models reported, a murine infection model was characterized by a sustained persistent infection of M. fortuitum in the kidneys (Parti et al., 2005, 2008). However, while the bacilli proliferated freely inside murine macrophages they did not invade a murine kidney cell line (Parti et al., 2005). A murine neutropenic model, in which neutropenia is induced by intraperitoneal doses of cyclophosphamide, has also been exploited to demonstrate the in vivo efficacy of drugs against M. fortuitum and M. abscessus (Das et al., 2019). In addition, a recent study highlighted the suitability of the Galleria mellonella moth larvae as a cheap, efficient and rapid in vivo model for the screening of antibiotic combinations and novel treatments against NTM, including M. fortuitum (Entwistle and Coote, 2018; García-Coca et al., 2019).
Infection of mice with M. fortuitum are also accompanied by visible symptoms, such as spinning disease (Saito and Tasaka, 1969; Parti et al., 2005), often associated with neurological disorders. Interestingly, spinning disease due to invasive abscess formation within the central nervous system has been described both in mice as well as in zebrafish embryos infected with M. abscessus (Saito and Tasaka, 1969; Bernut et al., 2014a), suggesting that both M. abscessus and M. fortuitum display common pathogenic traits in these animals. Interestingly, M. fortuitum, formerly Mycobacterium ranae, was originally recovered from frogs in 1905 and considered a pathogen for animals and humans since its first isolation from a human abscess in 1938. Together with Mycobacterium marinum, it is commonly associated with fish tuberculosis, a systemic and chronic disease characterized by the presence of granulomatous reactions. Supporting the view that M. fortuitum infects amphibians and fish, inoculation in adult goldfish led to the development of a characteristic chronic granulomatous response similar to that associated with natural mycobacterial infection (Talaat et al., 1999). Overall, these observations suggest that zebrafish may represent a valuable model to study pathogenesis of this understudied mycobacterial species.
Zebrafish have recently gained favor as a useful and amenable model to study host-bacterial interactions (Davis et al., 2002; van der Sar et al., 2004; Clay et al., 2007; Prajsnar et al., 2008, 2013; Vergunst et al., 2010; Alibaud et al., 2011; Cambier et al., 2014; Gomes and Mostowy, 2020). Due to genetic tractability and optical transparency, zebrafish embryos represent an exquisite model to study important aspects of infectious diseases. Whilst the adult zebrafish possess a complex immune system similar to that of humans, comprising both the innate and adaptive arms of immunity, the early embryonic stages solely harbor innate immunity (Davis et al., 2002). Infection of embryos, thus allows a more in-depth focus on the role of innate immunity during the very early stages of infection (Davis et al., 2002; Torraca and Mostowy, 2018). Zebrafish infection with the fish pathogen M. marinum has led to remarkable insights into the understanding of human tuberculosis (Davis et al., 2002; Berg and Ramakrishnan, 2012), the role of macrophages in pathogen dissemination (Davis and Ramakrishnan, 2009), infection-induced antibiotic tolerance (Adams et al., 2011), the contribution of ESX secretion system in granuloma formation (Volkman et al., 2004) and induced granuloma-associated angiogenesis which promotes mycobacterial growth and facilitates the spread of infection to distant tissue sites (Oehlers et al., 2015). However, a noticeably important breakthrough came from the M. abscessus model of infection in zebrafish, which unraveled the importance of cording as mechanism of immune evasion (Bernut et al., 2014a) and the role of TNF signaling in controlling infection (Bernut et al., 2016a). Because many conserved virulence mechanisms and host susceptibility determinants identified during zebrafish infection have been validated in humans (Tobin et al., 2010; Bernut et al., 2019), we reasoned that zebrafish may also represent a useful experimental model to decipher the virulence and immunopathology of M. fortuitum infections.
Herein, we have exploited the zebrafish embryo as an amenable model for the study of systemic M. fortuitum infections. We describe the aggressive and lethal infections caused by M. fortuitum, which develops in the absence of either functional innate immunity or CFTR.
Materials and Methods
Mycobacterial Strains and Culture Conditions
Mycobacterium abscessus CIP104536T, M. fortuitum subsp. fortuitum (ATCC 6841) and Mycobacterium smegmatis mc2155 were routinely grown and maintained at 37°C in Middlebrook 7H9 broth (BD Difco) supplemented with 10% oleic acid, albumin, dextrose, catalase (OADC; BD Difco) and 0.025% Tyloxapol (Sigma-Aldrich) (7H9OADC/T) or on Middlebrook 7H10 supplemented with 10% OADC enrichment (7H10OADC) and in the presence of antibiotics if required. To observe in vitro liquid growth phenotypes, bacteria were grown in Cation-adjusted Mueller-Hinton Broth (CaMHB; Sigma-Aldrich).
Creation of Fluorescent M. fortuitum
Fluorescent M. fortuitum was generated using the pVV16-eGFP replicative vector (Vilchèze et al., 2014). Electro-competent M. fortuitum were generated as previously described (Viljoen et al., 2018). M. fortuitum was placed on ice for 2 h prior to pelleting by centrifugation (3,000 × g at 4°C for 15 min). Following centrifugation, bacteria were resuspended in decreasing volumes of wash buffer (10% glycerol (v/v) and 0.025% Tyloxapol in distilled water) and pelleted by centrifugation for a total of 4 washes. For electroporation transformation, 1 μg of plasmid DNA was added to 200 μL of electrocompetent bacteria and transferred to a chilled 0.2 cm electrode gap GenePulser electroporation cuvette (Bio-rad) and transformed using a GenePulser Cxell electroporator (Bio-rad) (2.5 kV, 1, 000 Ω and 25 μF). Bacteria were recovered in 800 μL of 7H9OADC/T and placed at 37°C overnight. For selection of green fluorescent colonies, M. fortuitum was plated on 7H10OADC supplemented 50 μg/mL kanamycin (Euromedex). Positive colonies were selected based on fluorescence and maintained in 7H9OADC/T supplemented with 50 μg/mL kanamycin. Fluorescent M. abscessus has been previously described (Bernut et al., 2014a).
Preparation of Single Cell M. fortuitum
Single cell preparations of fluorescent M. fortuitum were created, as previously described (Bernut et al., 2015). Exponential-phase M. fortuitum were pelleted at 3000 × g for 10 min at room temperature and resuspended in 1 mL of 7H9OADC/T. Bacteria were passed through a 26 gauge needle 15 times, followed by two rounds of 10 s sonication, made up to 50 mL with 7H9OADC/T and centrifuged at 3000 × g for 10 min at room temperature. Pelleted bacteria were resuspended in up to 1 mL of 7H9OADC/T and 10 μL aliquots of single cell M. fortuitum prepared and placed at −80°C until required.
Zebrafish Maintenance
Zebrafish experiments were completed in accordance with the Comité d'Ethique pour l'Expérimentation Animale de la Région Languedoc Roussillon under the reference CEEALR36-1145. All experiments in the current study were performed using the golden mutant and macrophage reporter Tg(mpeg1:mCherry) lines as previously described (Bernut et al., 2014a, 2015). Zebrafish embryos were obtained and maintained as previously described (Bernut et al., 2015).
Morpholino Injection and cftr Knockdown
Morpholinos were designed and purchased from GeneTools. A splice-blocking morpholino specifically targeting zebrafish gene cftr (ZEBRAFISHIN, ZDB-GENE-050517-20) (5′-GACACATTTTGGACACTCACACCAA-3′) was injected into zebrafish embryos at the 1–4 cell stage (1 mM, 2 nL). Furthermore, details of the morpholino efficacy and specificity and cftr knockdown was previously validated as described (Bernut et al., 2019). Briefly, this splice blocking morpholino was designed against the exon 3-intron 3 junction within the cftr gene. Sequencing analysis indicated that the cftr morpholino blocks normal splicing, resulting in a 54 bp deletion in exon 3, and leading to the knockdown of CFTR expression (Bernut et al., 2019).
Zebrafish Microinjection and Infection
At 24 h post-fertilization, embryos were dechorionated using Pronase (10 mg/mL; Sigma-Aldrich) for up to 5 min at room temperature, followed by extensive washing in zebrafish water. Following dechorionation, embryos were injected with either liposomal clodronate or PBS-filled liposomes (Liposoma) (2 nL) via caudal vein injection, as previously described (Bernut et al., 2014a, 2015). At 30 h post-fertilization, embryos were anesthetized in 0.02% tricaine solution and infected with fluorescent M. fortuitum via caudal vein injection (3 nL containing ≈100 bacteria/nL). Bacterial inoculum was checked a posteriori by injection of 3 nL into sterile PBS and plating onto 7H10OADC. Following infection, embryos were transferred to 24-well plates (2 embryos/well) and incubated at 28.5°C for the duration of the experiment. Embryo age is expressed as days post-infection (dpi).
Zebrafish Monitoring and Live Imaging
Embryo survival was monitored daily based on the presence or absence of a heartbeat. Survival curves were determined by counting dead larvae for up to 12 days, or until uninfected embryos begin to die. At designated key time points post-infection, embryos were anesthetized in 0.02% tricaine solution and mounted on 3% (w/v) methylcellulose solution for live imaging. Images were taken using a Zeiss Axio Zoom.V16 coupled with an Axiocam 503 monochrome camera (Zeiss). Fluorescent Pixel Count (FPC) measurements were determined using the “Analyse particles” function in ImageJ. Granulomas were identified based on the co-localization of fluorescent macrophages and fluorescent M. fortuitum. All experiments were completed at least two times independently.
Statistical Analysis
Survival curve analysis was completed using the log-rank (Mantel-Cox) statistical test. Abscess, cord, granuloma and bacterial burden (FPC) analyses were completed using unpaired Student′s t-test. All statistical tests were completed using Graphpad Prism (Version 8.0.1).
Results
M. fortuitum Forms Large Corded Aggregates in vitro
Zebrafish naturally require lower ambient temperatures of ~28°C for normal growth and development. In establishing a new model to study the pathogenesis of a disease-causing agent, it is critical that the replication of the pathogen is not affected by these temperatures. As has been reported for other NTM such as M. abscessus (Bernut et al., 2014a) and M. kansasii (Johansen and Kremer, 2020), these species are able to replicate at lower ambient temperatures, albeit at a slower rate, highlighting their adaptability to the zebrafish platform. We firstly wanted to determine whether M. fortuitum is able to grow at lower temperatures compared to traditional 37°C incubation. When grown in 7H9OADC/T at 30°C, we observed that M. fortuitum was able to reach early stationary phase around Day six, which was comparable to other rapid growers that are known to thrive at lower temperatures, such as M. abscessus and M. smegmatis (Figure 1A). Comparatively, all species grew at a much faster rate and reached stationary phase earlier when grown at 37°C, which was to be expected and has been reported for other NTM species (Johansen and Kremer, 2020) (Figure 1B).
Figure 1.
Mycobacterium fortuitum forms large corded bacterial aggregates in vitro. (A) M. abscessus CIP104536T smooth (S) and rough (R) variants, M. fortuitum subsp. fortuitum (ATCC 6841) and M. smegmatis mc2155 were inoculated in 7H9OADC/T at an optical density of 0.05 (OD620) and incubated at 30°C and (B) 37°C under shaking at 100 rpm. Growth measurements were taken daily over a 7-day period until cultures reached stationary phase. Data shown is the merge of two independent experiments. Error bars represent standard deviation. (C) In vitro liquid growth properties of the different NTM species in CaMHB medium following 3–4 days static culture at 30°C in a 96-well plate. The boxed area depicts the zoomed section of liquid growth in the bottom left hand corner of each well. Scale bars represent 2 mm. (D) In vitro solid agar growth properties of M. fortuitum and M. abscessus R variant following 3 days growth at 30°C. Scale bars represent 1 mm.
It has been well-described that specific mycobacteria are able to grow in large cord-like aggregates; a trait that has been often regarded for the most pathogenic mycobacteria such as Mycobacterium tuberculosis, M. marinum, M. abscessus and recently M. kansasii (Glickman et al., 2000; Staropoli and Branda, 2008; Bernut et al., 2014a, 2016b; Johansen and Kremer, 2020). As such, we wanted to determine whether M. fortuitum also possessed similar traits, which may explain why it is regarded as an opportunistic pathogen and frequently isolated in pulmonary and extra-pulmonary sites (Wallace et al., 1983; Brown-Elliott and Philley, 2017). Remarkably, when grown at both 30 and 37°C in CaMHB, a liquid medium commonly used for testing of antimicrobial susceptibility, we observed the formation of large cord-like aggregates for M. fortuitum (Figure 1C). These cords were different in size and appearance when compared to rough M. abscessus; an NTM species well-known for its large extracellular cord formation both in vitro and in vivo (Bernut et al., 2014a, 2016a,b). When we observed bacterial growth on 7H10OADC solid agar, we also identified the presence of large cord-like structures for M. fortuitum, which were noticeably different in appearance to rough M. abscessus (Figure 1D).
M. fortuitum Is Pathogenic in a Zebrafish Model in a Dose-Dependent Manner
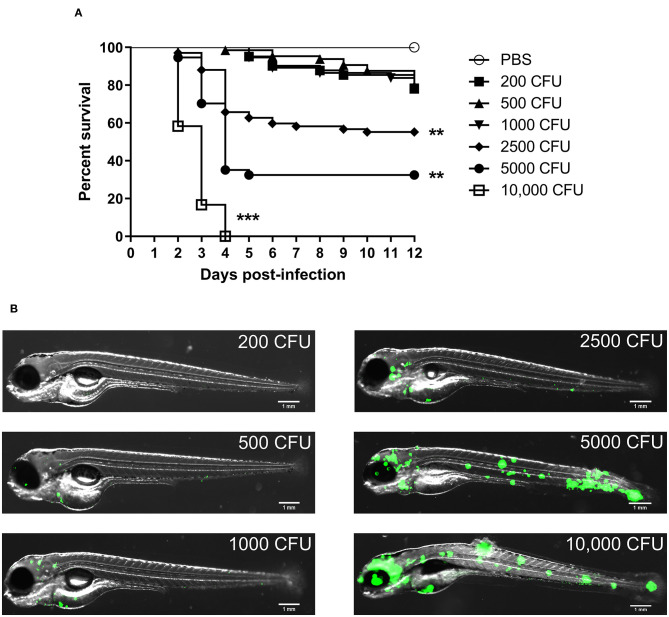
We firstly wanted to determine whether M. fortuitum was able to promote embryonic death in a dose-dependent manner. At 30 h post-fertilization, bacterial suspensions of M. fortuitum were injected in the caudal vein of zebrafish embryos. We observed that embryos infected with 200, 500 or 1,000 CFU of M. fortuitum induced ~20% embryonic death in a 12-day period (Figure 2A). This is similar to what has been observed for other NTM, such as the smooth variant of M. abscessus (Bernut et al., 2014a) or M. kansasii (Johansen and Kremer, 2020). Moreover, infection with higher doses, corresponding to 2,500 or 5,000 CFU, led to 40 and 70% of embryo death, respectively, within a 12-day period. Interestingly, larval killing occurred rapidly (within 4 days post-infection) after which the percentage of survival remained stable. Embryos infected with 10,000 CFU all succumbed within a 4-day period. Whole embryo imaging demonstrates that larval killing was directly correlated to the bacterial burden and that bacteria were disseminated throughout the entire embryo, suggestive of a systemic infection (Figure 2B). Collectively, these findings demonstrate the feasibility of the zebrafish model to study acute infections with M. fortuitum.
Figure 2.
Zebrafish embryos are susceptible to Mycobacterium fortuitum infection in a dose-dependent manner. (A) Zebrafish embryos were injected with varying dosages of GFP-expressing M. fortuitum at 30 h post-fertilization via caudal vein injection. Embryo survival was monitored over a 12 day period. N = 20–25 embryos/group. Statistical analysis was completed using the log-rank (Mantel-Cox) statistical test for survival curves. Data shown is the merge of two independent experiments. (B) Representative images of zebrafish embryos infected with varying dosages of GFP-expressing M. fortuitum at 2 days post-infection. Green represents M. fortuitum. Scale bars represent 1 mm. **P ≤ 0.01, ***P ≤ 0.001.
The Zebrafish/M. fortuitum Model Recapitulates Important Mycobacterial Pathophysiological Features
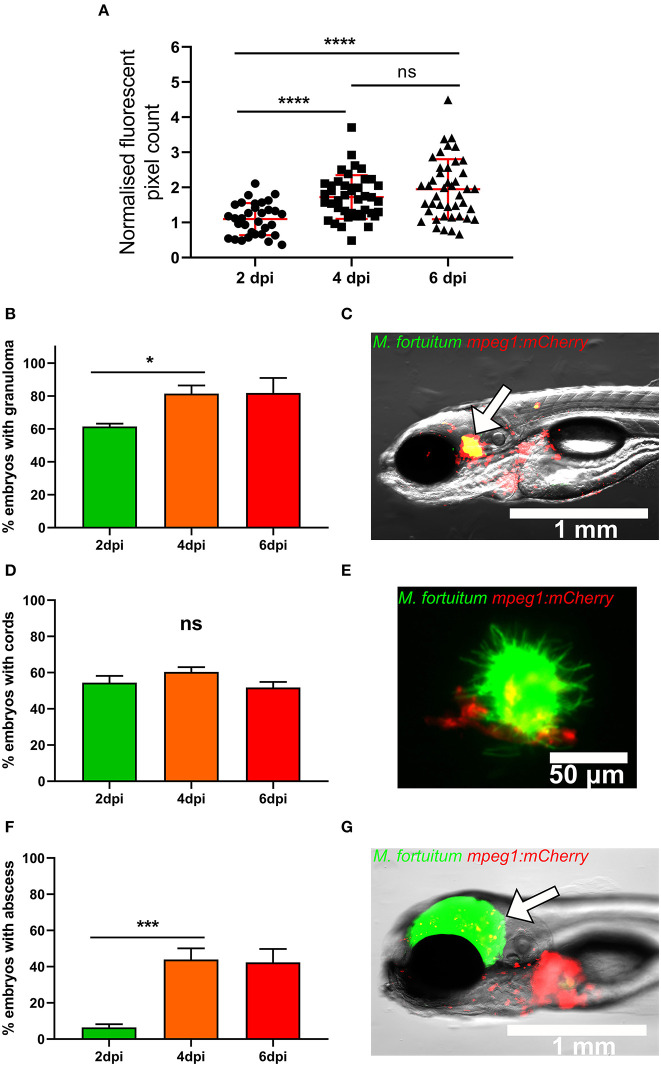
We next monitored the kinetic of the bacterial burden upon intravenous infection of embryos with 300 CFU of M. fortuitum expressing GFP by determination of fluorescent pixel counts (FPC). There was a significant increase in the bacterial loads between 2 and 4 dpi, after which bacterial loads remained stable between 4 and 6 dpi, suggesting that the bacterial expansion is controlled at later time points (Figure 3A).
Figure 3.
Zebrafish infected with Mycobacterium fortuitum recapitulate the hallmark features of other pathogenic mycobacteria. (A) Following infection mpeg1::mCherry zebrafish embryo at 30 h post-fertilization via caudal vein injection with 300 CFU of GFP-expressing M. fortuitum, bacterial burden was quantified using fluorescent pixel count (FPC determination using ImageJ software) at 2, 4, and 6 days post-infection. For each experiment, all groups were normalized against the 2 days post-infection group. Each datapoint represents an individual embryo. Error bars represent standard deviation. Statistical significance was determined by Student′s t-test. Plots represent a pool of 2 independent experiments containing approximately 20 embryos per group. (B) The granuloma formation kinetic in embryos following infection with M. fortuitum. Error bars represent standard deviation. Data shown is the merge of two independent experiments. Statistical significance was determined by Student′s t-test. (C) Representative image of a granuloma at 4 days post-infection. The white arrow highlights the granuloma. Scale bar represents 1 mm. (D) The kinetic of bacterial cord formation in zebrafish embryos following M. fortuitum infection. Error bars represent standard deviation. Data shown is the merge of two independent experiments. Statistical significance was determined by Student′s t-test. (E) Representative image of a bacterial corded M. fortuitum aggregate surrounded by recruited macrophages at 4 days post-infection. Note the sheer size of the bacterial aggregate in comparison to the smaller macrophages. Green represents M. fortuitum, while red represents macrophages. Scale bar represents 50 μm. (F) The kinetic of abscess formation in zebrafish embryos infected with M. fortuitum. Error bars represent standard deviation. Data shown is the merge of two independent experiments. Statistical significance was determined by Student′s t-test. (G) Representative image of an M. fortuitum abscess at 4 days post-infection. The white arrow highlights the abscess. Green represents M. fortuitum, while red represents macrophages. Scale bar represents 1 mm. *P ≤ 0.05, ***P ≤ 0.001, ****P ≤ 0.0001.
A major feature of mycobacterial infection is the formation of the granuloma (Pagán and Ramakrishnan, 2018). Importantly, zebrafish embryos do not possess adaptive immunity and so granuloma identification is strictly based on the presence of macrophages at the infection foci (Davis et al., 2002). This host immune aggregate acts to restrict pathogen spread, however mycobacteria have developed unique mechanisms allowing their prolonged survival within these host structures (Volkman et al., 2004; Davis and Ramakrishnan, 2009; Bernut et al., 2014a; Johansen and Kremer, 2020). Comparatively, extracellular cord formation within the zebrafish embryo is often representative of acute infection and generally is indicative of mycobacterial escape from the phagosome and macrophage destruction (Bernut et al., 2014a, 2015, 2016a). Abscess formation often represents loss of infection control and typically occurs following extracellular cord formation and expansion (Bernut et al., 2014a, 2015, 2016a). Abscesses represent a marker of disease severity and are often associated with cellular debris, tissue destruction, and acute infection in zebrafish (Bernut et al., 2014a). As such, we wanted to determine whether zebrafish embryos infected with M. fortuitum are able to recapitulate critical determinants of mycobacterial infection. To visualize and monitor the presence of granulomas, infections with M. fortuitum expressing GFP were carried out in the caudal vein of the mpeg1:mCherry zebrafish reporter line harboring red fluorescent macrophages. We observed the presence of granulomas in approximately 60% of infected embryos at 2 dpi, which increased to 80% of embryos by 4 dpi and then remained constant between 4 and 6 dpi (Figures 3B,C). This coincides with the arrest of bacterial proliferation between 4 and 6 dpi as seen in Figure 3A. When we counted the number of embryos with cords, ~50% of embryos displayed these extracellular bacterial structures, which was consistent throughout the experiment until 6 dpi (Figures 3D,E). We observed a significant increase in the number of embryos with abscesses from 5 to 40% between 2 and 4 dpi (Figures 3F,G). These findings demonstrate that the zebrafish model effectively recapitulates critical aspects of mycobacterial pathophysiology following M. fortuitum infection and highlights the applicability of this model for the study of M. fortuitum pathogenesis. These results also suggest that acute infection is typified by rapid bacterial expansion and that cord formation occurs very rapidly within 2 dpi, while the bacterial growth is controlled once granulomas are fully formed at 4 dpi.
Macrophages Are Essential Host Determinants of M. fortuitum Infection Control
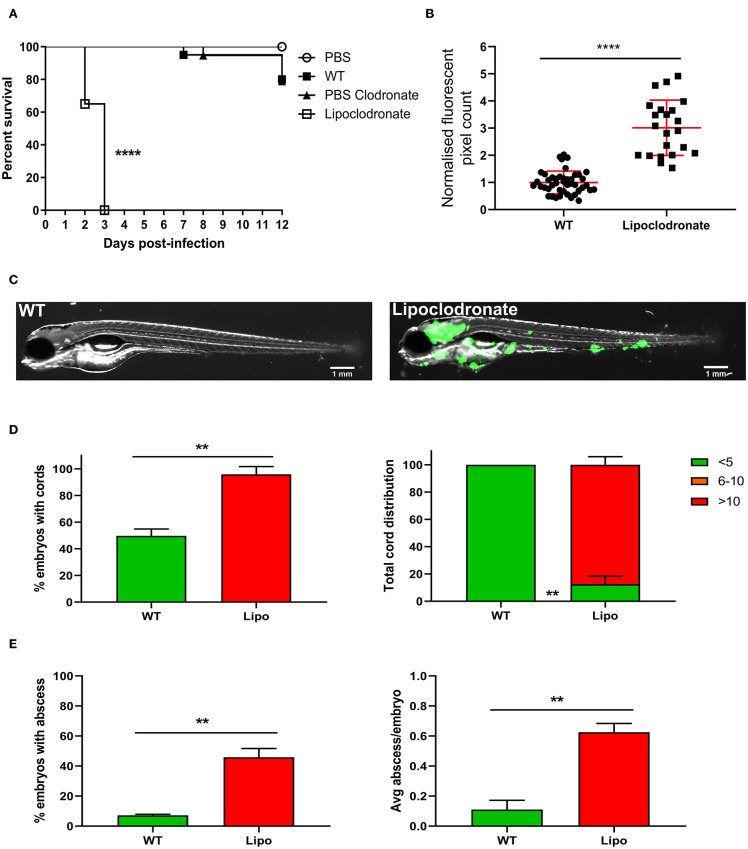
Macrophages are the first responders to infection and one of the major cell populations within the mycobacterial granuloma (Volkman et al., 2004; Bernut et al., 2016a; Pagán and Ramakrishnan, 2018). It is well-known that macrophage depletion has a detrimental outcome for mycobacterial infection in zebrafish embryos, leading to premature larval death within several days post-infection (Clay et al., 2007; Bernut et al., 2014a). Currently, it is not known whether macrophages play such a crucial role in M. fortuitum infection as compared to other mycobacterial species. As such, we aimed to elucidate whether macrophages are essential determinants of M. fortuitum infection control. Liposomal clodronate is highly effective in depleting macrophages within the zebrafish embryo for up to 6 days as previously reported (Bernut et al., 2014a, 2015). Using Lipoclodronate macrophage depletion, 100% killing was achieved in animals infected with ≈300 CFU at 3 dpi (Figure 4A). This was associated with a massive increase in bacterial loads in macrophage-depleted embryos as compared to wild-type fish receiving PBS clodronate at 2 dpi, thus highlighting the critical role of macrophages in controlling M. fortuitum infection (Figure 4B). Further, embryo imaging clearly highlighted the very high bacterial loads in the macrophage-depleted embryos as compared to the wild-type fish (Figure 4C), consistent with bacterial burden quantification. Importantly, we observed a greater proportion of embryos with cords and an increase in the number of cords per embryo in Lipoclodronate-treated embryos as compared to PBS-injected animals, emphasizing the need of macrophages to restrict intracellular growth and preventing extracellular cord formation (Figure 4D). There was also a greater proportion of Lipoclodronate treated embryos with abscesses and a higher number of abscesses per embryo when compared to PBS groups at 2 dpi (Figure 4E). Together, these results demonstrate that the hypersusceptibility of Lipoclodronate-treated embryos to M. fortuitum stems from their lack of macrophages.
Figure 4.
Lipoclodronate macrophage depletion results in lethal M. fortuitum infection. (A) At 24 h post-fertilization, embryos were treated with liposomal clodronate via caudal vein injection to transiently deplete macrophages. At 30 h post-fertilization, embryos were injected intravenously with 300 CFU of GFP-expressing M. fortuitum with embryo survival monitored over a 12 day period. n = 20–25 embryos/group. Statistical analysis was completed using the log-rank (Mantel-Cox) statistical test for survival curves. Data shown is the merge of two independent experiments. (B) Bacterial burden at 2 days post-infection was calculated using fluorescent pixel count (FPC) determination with ImageJ software. Lipoclodronate-treated embryos were normalized against corresponding controls in each experiment. Each datapoint represents an individual embryo. Error bars represent standard deviation. Statistical significance was determined by Student′s t-test. Plots represent a pool of 2 independent experiments containing approximately 20 embryos per group. (C) Representative images of wild-type (WT) and Lipoclodronate-treated embryos infected with 300 CFU of GFP-expressing M. fortuitum at 2 days post-infection. Scale bars represent 1 mm. (D) The proportion of embryos with bacterial cords and the total distribution of cords categorized as low (<5 cords/embryo), moderate (6–10 cords/embryo) and high (>10 cords/embryo) in M. fortuitum-infected embryo at 2 days post-infection. Error bars represent standard deviation. Data shown is the merge of two independent experiments. Statistical significance was determined by Student′s t-test. (E) The proportion of embryos with abscesses and the average number of abscesses per infected embryo at 2 days post-infection. Error bars represent standard deviation. Data shown is the merge of two independent experiments. Statistical significance was determined by Student′s t-test. **P ≤ 0.01, ****P ≤ 0.0001.
CFTR Depletion Leads to Increased Susceptibility to M. fortuitum Infection
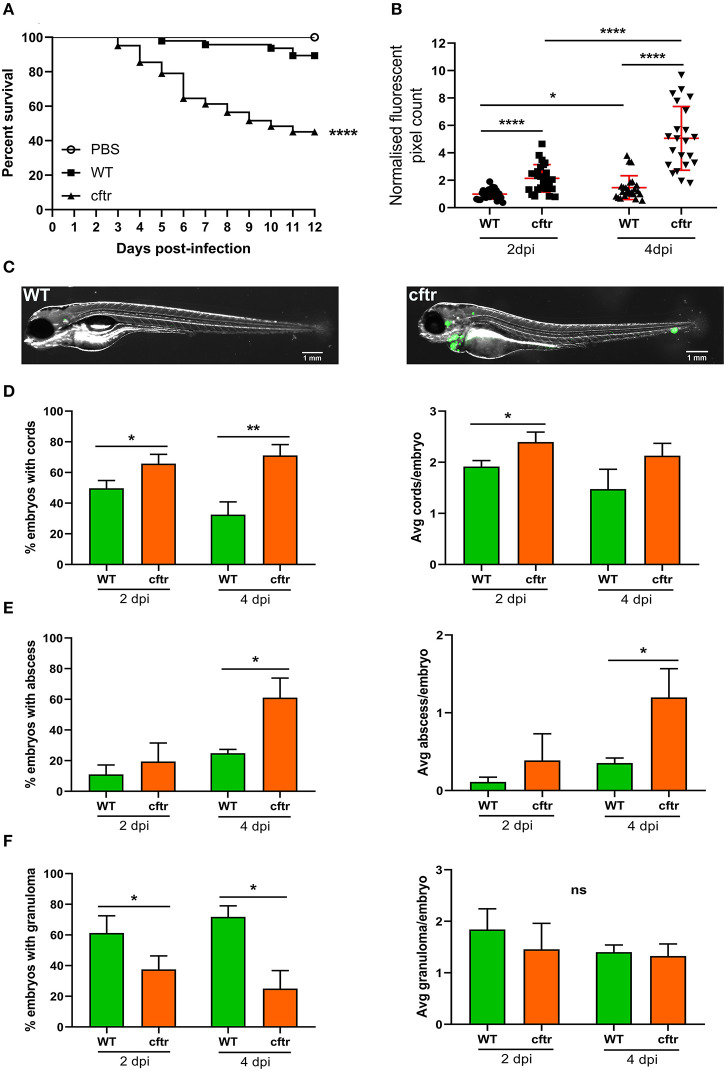
It is estimated that 5–20% of CF patients will develop NTM infection (Richards and Olivier, 2019), indicating that these patients are at increased risk of infection due to genetic susceptibility. Although less frequently encountered than M. avium or M. abscessus, several studies have reported the presence of M. fortuitum in CF patients (Sermet-Gaudelus et al., 2003; Cândido et al., 2014; Martiniano et al., 2016; Richards and Olivier, 2019). Recent work has shown that CFTR is important for fine-tuning host oxidative stress and restricting intracellular growth of M. abscessus in a zebrafish model (Bernut et al., 2019). These embryos also possessed fewer granulomas and granulomas displayed a disorganized structure, suggesting that CFTR is also an important determinant of infection containment within the granuloma microenvironment. We thus inquired whether CFTR depletion also conferred increased susceptibility to M. fortuitum infection. Using a morpholino knockdown strategy previously validated to target the zebrafish cftr gene (Bernut et al., 2019), we observed a massive increase in embryo mortality (almost 60% mortality at 12 dpi) following M. fortuitum infection in cftr morphants when compared to wild-type infected embryos (Figure 5A). In addition, mortality started earlier in cftr morphants than in wild-type fish. When we further examined bacterial burden, we observed an increase in CFTR-depleted embryos at 2 dpi when compared to wild-type infection, which was greatly increased by 4 dpi (Figures 5B,C). Strikingly, in CFTR-depleted embryos, there was an enhanced proportion of embryos with cords at both 2 and 4 dpi when compared to wild-type infection. Moreover, there was an increase in the number of cords per embryo in CFTR-depleted embryos at 2 dpi when compared to wild-type (Figure 5D). There was also a significantly greater number of embryos with abscesses and a greater number of abscesses per embryo at 4dpi in the CFTR-depleted group as compared to wild-type infections (Figure 5E). In contrast, infected cftr morphants produced less granulomas when compared to wild-type infections, however the number of granulomas per embryo remained constant over time (Figure 5F), similarly to what was previous observed in M. abscessus infected cftr morphants (Bernut et al., 2019). In contrast to wild-type embryos where bacterial proliferation is constrained when granulomas are formed (Figure 3), the opposite scenario occurs in cftr morphants whereby bacterial proliferation progresses whilst the number of granulomas remains unchanged. These findings suggest that depletion of CFTR impairs host immune responses resulting in granuloma formation.
Figure 5.
CFTR ablation leads to rapid larval death and uncontrolled bacterial expansion. (A) Cftr mrophants at 30 h post-fertilization were infected with 300 CFU of GFP-expressing M. fortuitum via caudal vein injection and embryo survival was monitored over a 12 day period. N = 20–25 embryos/group. Statistical analysis was completed using the log-rank (Mantel-Cox) statistical test for survival curves. Data shown is the merge of three independent experiments. (B) Bacterial burden was quantified by fluorescent pixel count (FPC) determination using ImageJ software, with each data point representing an individual embryo. Each group was normalized against the wild-type cohort at 2 days post-infection. Error bars represent standard deviation. Statistical significance was determined by Student′s t-test. Plots represent a pool of three independent experiments containing ~20–25 embryos per group. (C) Representative images of wild-type (WT) and CFTR depleted (cftr) embryos infected with 300 CFU of GFP-expressing M. fortuitum at 2 days post-infection. Scale bars represent 1 mm. (D) The proportion of embryos with bacterial cords and the average number of cords per infected embryo at 2 and 4-days post-infection. Error bars represent standard deviation. Data shown is the merge of three independent experiments. Statistical significance was determined by Student′s t-test. (E) The proportion of embryos with abscesses and the average number of abscesses per infected embryo at 2 and 4-days post-infection. Error bars represent standard deviation. Data shown is the merge of three independent experiments. Statistical significance was determined by Student′s t-test. (F) The proportion of embryos with granulomas and the average number of granulomas per infected embryo at 2 and 4-days post-infection. Error bars represent standard deviation. Data shown is the merge of three independent experiments. Statistical significance was determined by Student′s t-test. *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001.
Overall, these findings emphasize the importance of CFTR as a host factor in controlling M. fortuitum pathogenesis through granuloma formation and preventing extracellular cord formation, indicating that CFTR dysfunction results in hypersusceptibility to M. fortuitum infection.
Discussion
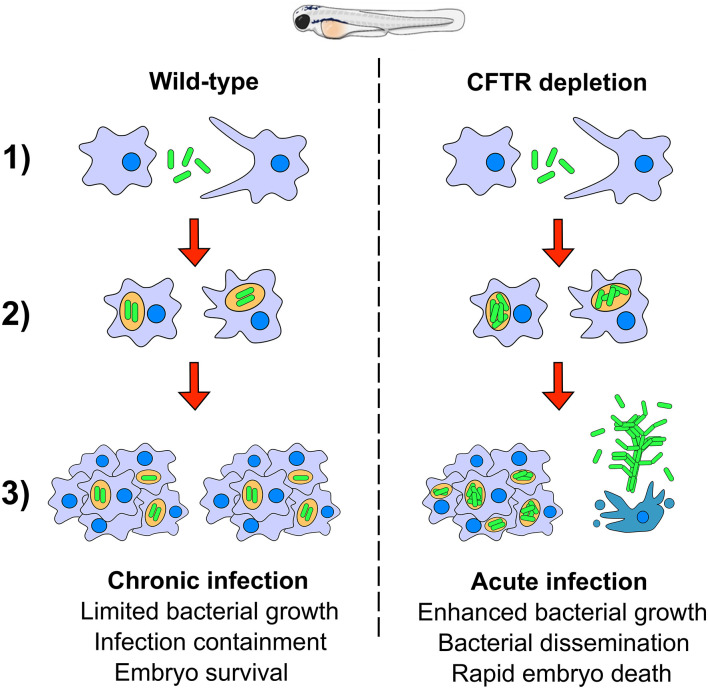
Mycobacterium fortuitum is increasingly recognized as an opportunistic nosocomial pathogen responsible for a wide panel of clinical infections, including lung diseases. However, our understanding of the pathogenic mechanisms of this important NTM species remains obscured by the lack of relevant animal models. Herein, we exploited the zebrafish model to directly and non-invasively dissect critical steps in the pathogenesis of M. fortuitum. Genetic manipulation of the host, combined with a fluorescent reporter bacterial strain were used to decipher the interplay between M. fortuitum and host macrophages. We provide evidence that zebrafish embryos, displaying only innate immunity, represent a suitable and permissive host that is susceptible to high doses of M. fortuitum. Chemical depletion of macrophages led to a rapid expansion of M. fortuitum, establishing a systemic and acute infection leading to rapid larval killing. Most importantly, morpholino depletion of CFTR led to hypersusceptibility of zebrafish embryos to M. fortuitum infection, providing the first insight into the significance of CFTR in M. fortuitum pathogenesis. The pathophysiological events of M. fortuitum infection are reminiscent to those reported earlier for M. abscessus, a well-acknowledged CF pathogen and can be summarized in a sequential manner (Figure 6): (1) Following intravenous injection, M. fortuitum is rapidly phagocytosed by macrophages; (2) Once inside the phagosome, M. fortuitum is initially able to replicate until the recruitment of additional macrophages to the site of infection; (3) Infection is contained with the formation of the mycobacterial granuloma and leads to a chronic infection. However, in a subset of wild-type embryos and to a much greater extent in CFTR embryos, it is very likely that the escape or release of M. fortuitum from infected macrophages is favoring extracellular bacterial multiplication in the form or serpentine cords (highly stimulated in macrophage-depleted embryos). Bacterial cords can withstand phagocytosis by macrophages which further exacerbates uncontrolled bacterial multiplication and ultimately results in abscess formation, tissue damage and larval death. This is supported by recent findings indicating that caspase-mediated apoptosis of catfish macrophages occurs rapidly after infection with M. fortuitum (Datta et al., 2018). Thus, one can envisage a similar scenario where apoptosis of zebrafish macrophages releases M. fortuitum into the extracellular milieu, promoting the production of extracellular cords.
Figure 6.
A hypothetical schematic summarizing the pathogenesis of Mycobacterium fortuitum in both wild-type and CFTR backgrounds. (1) Following intravenous injection of M. fortuitum into zebrafish embryos, macrophages are rapidly recruited to the site of infection and phagocytose bacilli; (2) Once inside the phagosome, M. fortuitum is able to initially replicate, however in a wild-type scenario, host defenses are able to slow the bacterial expansion until further macrophages are recruited. Comparatively, following CFTR depletion, bacteria are able to rapidly expand within the phagosome, presumably due to defective host oxidative defense mechanisms. (3) Additional macrophages are recruited to the infection foci, forming the granuloma which acts to contain infection dissemination in wild-type embryos. In a proportion of wild-type embryos, we propose that macrophage apoptosis triggers the escape of bacilli to the extracellular space which facilitates the growth of bacilli into large corded aggregates, promoting zebrafish embryo death. In CFTR-depleted embryos, there are fewer granulomas and a greater proportion of cords which leads to more rapid embryonic death and a greater proportion of cords and abscesses.
Multiple hypotheses may explain why high doses of M. fortuitum are required to induce efficient and rapid killing of zebrafish larvae, as compared to M. marinum (Volkman et al., 2004; Davis and Ramakrishnan, 2009). One plausible explanation could be attributed to the fact that M. fortuitum remains phagolysosomal, presumably because of the lack of ESAT-6 secretion (Houben et al., 2012), although pan-genomic analyses seem to indicate that the M. fortuitum genome possesses an ESX-1 cluster (Dumas et al., 2016). Indeed, a clear link between translocation and mycobacterial virulence has been proposed, relying on a functional ESX-1 system and ESAT-6 secretion, which facilitates translocation of members of the M. tuberculosis complex and M. marinum from the phagolysosome to the cytosol (Houben et al., 2012). However, M. abscessus which escapes the phagosome lacks ESX-1, but it has been postulated that communication with the cytosolic compartment is favored by the presence of a functional ESX-4 secretion system (Roux et al., 2016; Laencina et al., 2018; Johansen et al., 2020). Future work is therefore required to investigate the contribution of the different ESX systems in the pathogenicity of M. fortuitum.
Moreover, we have harnessed the CFTR zebrafish model of infection as an innovative vertebrate recapitulating aspects of CF immuno-pathogenesis (Bernut et al., 2019). M. fortuitum-infected CFTR-depleted zebrafish rapidly succumbed to infection, reflecting a hypersusceptibility phenotype to this mycobacterial species in CF and providing a first glance into the vulnerability of CF patients to M. fortuitum infection. Previous work indicated CFTR participates in neutrophil chemotaxis to the infected sites and the adjustment of oxidative host defenses, conditioning efficient phagocyte-mediated bacterial killing, together generating a protective granulomatous response (Bernut et al., 2019). However, the role of neutrophils in the defense against M. fortuitum remains to be investigated in future studies. CFTR depletion is also associated with a deficiency in radical oxygen species production altering phagocyte-mediated killing. It is, therefore, very likely that this low oxidative response in cftr morphants results in increased intracellular loads of M. fortuitum and premature cell death. Thus, the reduced number of protective granulomas together with the uncontrolled extracellular mycobacterial spread leads to acute infection and larval death in cftr morphants. Our study indicates that CFTR is a regulator of host immunity to M. fortuitum, as suggested previously for M. abscessus. Importantly, CFTR depletion has been shown to have no impact on the control of host immunity to the non-pathogenic M. smegmatis nor to the pathogenic M. marinum (Bernut et al., 2019). These findings imply that species-specific restriction mechanisms occur for various NTM, an interesting observation that will require further investigation. Overall, this new animal model allows us to propose that cftr represents a gene of susceptibility to M. fortuitum infection.
This study also paves the way to new translational possibilities in the fight against M. fortuitum infections. The zebrafish model described here, particularly conducive to spatiotemporal imaging of M. fortuitum infections, may also be exploited to test the in vivo efficacy of known antibiotics or new drug treatments. It may represent a unique biological system allowing non-invasive observations to evaluate, in real time, the efficacy of compounds in a living vertebrate, as shown previously for M. marinum (Takaki et al., 2012) and M. abscessus (Bernut et al., 2014b; Dubée et al., 2015; Dupont et al., 2017; Raynaud et al., 2019) and applied to high-throughput in vivo testing of drug efficacy (Carvalho et al., 2011).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The animal study was reviewed and approved the Comité d'Ethique pour l'Expérimentation Animale de la Région Languedoc Roussillon under the reference CEEALR36-1145.
Author Contributions
MJ conducted experiments, analyzed the data, and wrote the paper. LK conceived the idea of the project, analyzed the data, and wrote the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. MJ received a post-doctoral fellowship granted by Labex EpiGenMed, an Investissements d'avenir program (ANR-10-LABX-12-01).
References
- Adams K. N., Takaki K., Connolly L. E., Wiedenhoft H., Winglee K., Humbert O., et al. (2011). Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145, 39–53. 10.1016/j.cell.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibaud L., Rombouts Y., Trivelli X., Burguière A., Cirillo S. L. G., Cirillo J. D., et al. (2011). A Mycobacterium marinum TesA mutant defective for major cell wall-associated lipids is highly attenuated in Dictyostelium discoideum and zebrafish embryos. Mol. Microbiol. 80, 919–934. 10.1111/j.1365-2958.2011.07618.x [DOI] [PubMed] [Google Scholar]
- Berg R. D., Ramakrishnan L. (2012). Insights into tuberculosis from the zebrafish model. Trends Mol. Med. 18, 689–690. 10.1016/j.molmed.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Bernut A., Dupont C., Ogryzko N. V., Neyret A., Herrmann J.-L., Floto R. A., et al. (2019). CFTR protects against Mycobacterium abscessus infection by fine-tuning host oxidative defenses. Cell Rep. 26, 1828–1840. 10.1016/j.celrep.2019.01.071 [DOI] [PubMed] [Google Scholar]
- Bernut A., Dupont C., Sahuquet A., Herrmann J.-L., Lutfalla G., Kremer L. (2015). Deciphering and imaging pathogenesis and cording of Mycobacterium abscessus in zebrafish embryos. J. Vis. Exp. 103:e53130. 10.3791/53130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernut A., Herrmann J.-L., Kissa K., Dubremetz J.-F., Gaillard J.-L., Lutfalla G., et al. (2014a). Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. U.S.A. 111, E943–E952. 10.1073/pnas.1321390111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernut A., Le Moigne V., Lesne T., Lutfalla G., Herrmann J.-L., Kremer L. (2014b). In vivo assessment of drug efficacy against Mycobacterium abscessus using the embryonic zebrafish test system. Antimicrob. Agents Chemother. 58, 4054–4063. 10.1128/AAC.00142-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernut A., Nguyen-Chi M., Halloum I., Herrmann J.-L., Lutfalla G., Kremer L. (2016a). Mycobacterium abscessus-induced granuloma formation is strictly dependent on TNF signaling and neutrophil trafficking. PLoS Pathog. 12:e1005986. 10.1371/journal.ppat.1005986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernut A., Viljoen A., Dupont C., Sapriel G., Blaise M., Bouchier C., et al. (2016b). Insights into the smooth-to-rough transitioning in Mycobacterium bolletii unravels a functional Tyr residue conserved in all mycobacterial MmpL family members. Mol. Microbiol. 99, 866–883. 10.1111/mmi.13283 [DOI] [PubMed] [Google Scholar]
- Brown-Elliott B. A., Mann L. B., Hail D., Whitney C., Wallace R. J. (2012). Antimicrobial susceptibility of nontuberculous mycobacteria from eye infections. Cornea 31, 900–906. 10.1097/ICO.0b013e31823f8bb9 [DOI] [PubMed] [Google Scholar]
- Brown-Elliott B. A., Philley J. V. (2017). Rapidly growing mycobacteria. Microbiol. Spectr. 5, 1–19. 10.1128/microbiolspec.TNMI7-0027-2016 [DOI] [PubMed] [Google Scholar]
- Brown-Elliott B. A., Wallace R. J. (2002). Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15, 716–746. 10.1128/CMR.15.4.716-746.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cândido P. H. C., Nunes Lde S., Marques E. A., Folescu T. W., Coelho F. S., de Moura V. C. N., et al. (2014). Multidrug-resistant nontuberculous mycobacteria isolated from cystic fibrosis patients. J. Clin. Microbiol. 52, 2990–2997. 10.1128/JCM.00549-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier C. J., Takaki K. K., Larson R. P., Hernandez R. E., Tobin D. M., Urdahl K. B., et al. (2014). Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 505, 218–222. 10.1038/nature12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho R., de Sonneville J., Stockhammer O. W., Savage N. D. L., Veneman W. J., Ottenhoff T. H. M., et al. (2011). A high-throughput screen for tuberculosis progression. PLoS ONE 6:e16779. 10.1371/journal.pone.0016779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H., Davis J. M., Beery D., Huttenlocher A., Lyons S. E., Ramakrishnan L. (2007). Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe 2, 29–39. 10.1016/j.chom.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Garg T., Chopra S., Dasgupta A. (2019). Repurposing disulfiram to target infections caused by non-tuberculous mycobacteria. J. Antimicrob. Chemother. 74, 1317–1322. 10.1093/jac/dkz018 [DOI] [PubMed] [Google Scholar]
- Datta D., Khatri P., Singh A., Saha D. R., Verma G., Raman R., et al. (2018). Mycobacterium fortuitum-induced ER-mitochondrial calcium dynamics promotes calpain/caspase-12/caspase-9 mediated apoptosis in fish macrophages. Cell Death Discov. 4:30. 10.1038/s41420-018-0034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Clay H., Lewis J. L., Ghori N., Herbomel P., Ramakrishnan L. (2002). Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 17, 693–702. 10.1016/S1074-7613(02)00475-2 [DOI] [PubMed] [Google Scholar]
- Davis J. M., Ramakrishnan L. (2009). The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49. 10.1016/j.cell.2008.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubée V., Bernut A., Cortes M., Lesne T., Dorchene D., Lefebvre A.-L., et al. (2015). β-Lactamase inhibition by avibactam in Mycobacterium abscessus. J. Antimicrob. Chemother. 70, 1051–1058. 10.1093/jac/dku510 [DOI] [PubMed] [Google Scholar]
- Dumas E., Christina Boritsch E., Vandenbogaert M., Rodríguez de la Vega R. C., Thiberge J.-M., Caro V., et al. (2016). Mycobacterial pan-genome analysis suggests important role of plasmids in the radiation of type VII secretion systems. Genome Biol. Evol. 8, 387–402. 10.1093/gbe/evw001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont C., Viljoen A., Thomas S., Roquet-Banères F., Herrmann J.-L., Pethe K., et al. (2017). Bedaquiline inhibits the ATP synthase in Mycobacterium abscessus and is effective in infected zebrafish. Antimicrob. Agents Chemother. 61, e01225–17. 10.1128/AAC.01225-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entwistle F. M., Coote P. J. (2018). Evaluation of greater wax moth larvae, Galleria mellonella, as a novel in vivo model for non-tuberculosis mycobacteria infections and antibiotic treatments. J. Med. Microbiol. 67, 585–597. 10.1099/jmm.0.000696 [DOI] [PubMed] [Google Scholar]
- García-Coca M., Aguilera-Correa J. J., Ibáñez-Apesteguía A., Rodríguez-Sevilla G., Romera-García D., Mahíllo-Fernández I., et al. (2019). Non-pigmented rapidly growing mycobacteria smooth and rough colony phenotypes pathogenicity evaluated using in vitro and experimental models. Pathog. Dis. 77:ftz051. 10.1093/femspd/ftz051 [DOI] [PubMed] [Google Scholar]
- Glickman M. S., Cox J. S., Jacobs W. R. (2000). A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5, 717–727. 10.1016/S1097-2765(00)80250-6 [DOI] [PubMed] [Google Scholar]
- Gomes M. C., Mostowy S. (2020). The case for modeling human infection in zebrafish. Trends Microbiol. 28, 10–18. 10.1016/j.tim.2019.08.005 [DOI] [PubMed] [Google Scholar]
- Houben D., Demangel C., van Ingen J., Perez J., Baldeón L., Abdallah A. M., et al. (2012). ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell. Microbiol. 14, 1287–1298. 10.1111/j.1462-5822.2012.01799.x [DOI] [PubMed] [Google Scholar]
- Johansen M. D., Herrmann J.-L., Kremer L. (2020). Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 18, 392–407. 10.1038/s41579-020-0331-1 [DOI] [PubMed] [Google Scholar]
- Johansen M. D., Kremer L. (2020). A zebrafish model of Mycobacterium kansasii infection reveals large extracellular cord formation. J. Infect. Dis. 10.1093/infdis/jiaa187. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Laencina L., Dubois V., Le Moigne V., Viljoen A., Majlessi L., Pritchard J., et al. (2018). Identification of genes required for Mycobacterium abscessus growth in vivo with a prominent role of the ESX-4 locus. Proc. Natl. Acad. Sci. U.S.A. 115, E1002–E1011. 10.1073/pnas.1713195115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiniano S. L., Nick J. A., Daley C. L. (2016). Nontuberculous mycobacterial infections in cystic fibrosis. Clin. Chest Med. 37, 83–96. 10.1016/j.ccm.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Oehlers S. H., Cronan M. R., Scott N. R., Thomas M. I., Okuda K. S., Walton E. M., et al. (2015). Interception of host angiogenic signalling limits mycobacterial growth. Nature 517, 612–615. 10.1038/nature13967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán A. J., Ramakrishnan L. (2018). The formation and function of granulomas. Annu. Rev. Immunol. 36, 639–665. 10.1146/annurev-immunol-032712-100022 [DOI] [PubMed] [Google Scholar]
- Parti R. P. S., Shrivastava R., Srivastava S., Subramanian A. R., Roy R., Srivastava B. S., et al. (2008). A transposon insertion mutant of Mycobacterium fortuitum attenuated in virulence and persistence in a murine infection model that is complemented by Rv3291c of Mycobacterium tuberculosis. Microb. Pathog. 45, 370–376. 10.1016/j.micpath.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Parti R. P. S., Srivastava S., Gachhui R., Srivastava K. K., Srivastava R. (2005). Murine infection model for Mycobacterium fortuitum. Microbes Infect. 7, 349–355. 10.1016/j.micinf.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Prajsnar T. K., Cunliffe V. T., Foster S. J., Renshaw S. A. (2008). A novel vertebrate model of Staphylococcus aureus infection reveals phagocyte-dependent resistance of zebrafish to non-host specialized pathogens. Cell. Microbiol. 10, 2312–2325. 10.1111/j.1462-5822.2008.01213.x [DOI] [PubMed] [Google Scholar]
- Prajsnar T. K., Renshaw S. A., Ogryzko N. V., Foster S. J., Serror P., Mesnage S. (2013). Zebrafish as a novel vertebrate model to dissect enterococcal pathogenesis. Infect. Immun. 81, 4271–4279. 10.1128/IAI.00976-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnatunga C. N., Lutzky V. P., Kupz A., Doolan D. L., Reid D. W., Field M., et al. (2020). The rise of non-tuberculosis mycobacterial lung disease. Front. Immunol. 11:303. 10.3389/fimmu.2020.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud C., Daher W., Johansen M., Roquet-Baneres F., Blaise M., Onajole O. K., et al. (2019). Active benzimidazole derivatives targeting the MmpL3 transporter in Mycobacterium abscessus. ACS Infect. Dis. 6, 324–337. 10.1021/acsinfecdis.9b00389 [DOI] [PubMed] [Google Scholar]
- Richards C. J., Olivier K. N. (2019). Nontuberculous mycobacteria in cystic fibrosis. Semin. Respir. Crit. Care Med. 40, 737–750. 10.1055/s-0039-1693706 [DOI] [PubMed] [Google Scholar]
- Roux A.-L., Catherinot E., Ripoll F., Soismier N., Macheras E., Ravilly S., et al. (2009). Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in france. J. Clin. Microbiol. 47, 4124–4128. 10.1128/JCM.01257-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A.-L., Viljoen A., Bah A., Simeone R., Bernut A., Laencina L., et al. (2016). The distinct fate of smooth and rough Mycobacterium abscessus variants inside macrophages. Open Biol. 6:160185. 10.1098/rsob.160185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tasaka H. (1969). Comparison of the pathogenicity for mice of Mycobacterium fortuitum and Mycobacterium abscessus. J. Bacteriol. 99, 851–855. 10.1128/JB.99.3.851-855.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermet-Gaudelus I., Le Bourgeois M., Pierre-Audigier C., Offredo C., Guillemot D., Halley S., et al. (2003). Mycobacterium abscessus and children with cystic fibrosis. Emerging Infect. Dis. 9, 1587–1591. 10.3201/eid0912.020774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik K., Kirkpatrick G., Quon B. S. (2016). Nontuberculous mycobacteria in cystic fibrosis. Curr. Treat Options Infect. Dis. 8, 259–274. 10.1007/s40506-016-0092-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staropoli J. F., Branda J. A. (2008). Cord formation in a clinical isolate of Mycobacterium marinum. J. Clin. Microbiol. 46, 2814–2816. 10.1128/JCM.00197-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki K., Cosma C. L., Troll M. A., Ramakrishnan L. (2012). An in vivo platform for rapid high-throughput antitubercular drug discovery. Cell Rep. 2, 175–184. 10.1016/j.celrep.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat A. M., Trucksis M., Kane A. S., Reimschuessel R. (1999). Pathogenicity of Mycobacterium fortuitum and Mycobacterium smegmatis to goldfish, Carassius auratus. Vet. Microbiol. 66, 151–164. 10.1016/S0378-1135(99)00002-4 [DOI] [PubMed] [Google Scholar]
- Tobin D. M., Vary J. C., Ray J. P., Walsh G. S., Dunstan S. J., Bang N. D., et al. (2010). The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140, 717–730. 10.1016/j.cell.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torraca V., Mostowy S. (2018). Zebrafish infection: from pathogenesis to cell biology. Trends Cell Biol. 28, 143–156. 10.1016/j.tcb.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortoli E., Fedrizzi T., Meehan C. J., Trovato A., Grottola A., Giacobazzi E., et al. (2017). The new phylogeny of the genus Mycobacterium: the old and the news. Infect. Genet. Evol. 56, 19–25. 10.1016/j.meegid.2017.10.013 [DOI] [PubMed] [Google Scholar]
- van der Sar A. M., Appelmelk B. J., Vandenbroucke-Grauls C. M. J. E., Bitter W. (2004). A star with stripes: zebrafish as an infection model. Trends Microbiol. 12, 451–457. 10.1016/j.tim.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Vergunst A. C., Meijer A. H., Renshaw S. A., O'Callaghan D. (2010). Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection. Infect. Immun. 78, 1495–1508. 10.1128/IAI.00743-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchèze C., Molle V., Carrère-Kremer S., Leiba J., Mourey L., Shenai S., et al. (2014). Phosphorylation of KasB regulates virulence and acid-fastness in Mycobacterium tuberculosis. PLoS Pathog. 10:e1004115. 10.1371/journal.ppat.1004115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen A., Gutiérrez Ana Victoria Dupont C., Ghigo E., Kremer L. (2018). A simple and rapid gene disruption strategy in Mycobacterium abscessus: on the design and application of glycopeptidolipid mutants. Front. Cell. Infect. Microbial. 8:69. 10.3389/fcimb.2018.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman H. E., Clay H., Beery D., Chang J. C. W., Sherman D. R., Ramakrishnan L. (2004). Tuberculous granuloma formation is enhanced by a mycobacterium virulence determinant. PLoS Biol. 2:e367. 10.1371/journal.pbio.0020367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Swenson J. M., Silcox V. A., Good R. C., Tschen J. A., Stone M. S. (1983). Spectrum of disease due to rapidly growing mycobacteria. Rev. Infect. Dis. 5, 657–679. 10.1093/clinids/5.4.657 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.