Abstract
Human embryonic stem cells (hESCs) possess the potential of long-term self-renewal and three primary germ layers differentiation, and thus hESCs are expected to have broad applications in cell therapy, drug screening and basic research on human early embryonic development. Many efforts have been put to dissect the regulation of pluripotency and direct differentiation of hESCs. TGFβ/Activin/Nodal signal pathway critically regulates pluripotency maintenance and cell differentiation through the main signal transducer SMAD2/3 in hESCs, but the action manners of SMAD2/3 in hESCs are sophisticated and not documented yet. Here we review and discuss the roles of SMAD2/3 in hESC pluripotency maintenance and differentiation initiation separately. We summarize that SMAD2/3 regulates pluripotency and differentiation mainly through four aspects, (1) controlling divergent transcriptional networks of pluripotency and differentiation; (2) interacting with chromatin modifiers to make the chromatin accessible or recruiting METTL3-METTL14-WTAP complex and depositing m6A to the mRNA of pluripotency genes; (3) acting as a transcription factor to activate endoderm-specific genes to thus initiate definitive endoderm differentiation, which happens as cyclin D/CDK4/6 downstream target in later G1 phase as well; (4) interacting with endoderm specific lncRNAs to promote differentiation.
Keywords: SMAD2/3, human embryonic stem cell, pluripotency, cell cycle, differentiation, lncRNAs, epigenetic modification
Introduction
The TGF-β superfamily comprises TGF-βs, activins, nodal, growth and differentiation factors (GDFs) and bone morphogenetic proteins (BMPs). More than 60 TGF-β family members have been identified in multicellular organisms, with at least a half of proteins are encoded in human genome (Feng and Derynck, 2005). TGF-β signal originates from the binding of ligand dimers and heteromeric complex of type I (ALK1-7) and type II transmembrane serine/threonine kinase receptors. Activated type II receptors phosphorylate the type I receptors kinase domain, and then phosphorylate the intracellular SMAD proteins (Wu and Hill, 2009). There are three functional classes containing eight SMAD proteins: receptor-regulated SMADs (R-SMAD) including SMAD1/2/3/5/8, Co-mediator SMAD4 (Co-SMAD), and the inhibitory SMAD6 and 7 (I-SMAD). All SMAD proteins share Mad Homology domains MH1 and MH2: the MH1 domain contains a β-hairpin structure that mediates DNA binding, and the MH2 domain mediates SMAD oligomerization and establishes signal specificity by mediating proper type I receptor interaction with specific DNA binding partners (Weiss and Attisano, 2013). TGF-β/Activin/Nodal signaling occurs through ALKs 4, 5, and 7 and SMAD2/3 (Massague et al., 2005). It is reported that inhibition of Activin/Nodal signaling causes hESCs differentiation (James et al., 2005; Vallier et al., 2005; Xiao et al., 2006). On the other hand, Activin/Nodal signaling, through the TGF-β receptors and its effector SMAD2/3, initiates definitive endoderm differentiation in human and mouse ESC (Tremblay et al., 2000; Kubo et al., 2004; D’Amour et al., 2005). Accumulated reports show that TGF-β/Activin/Nodal signaling and its effectors SMAD2/3 are not only necessary for hESC self-renewal but also required for germ layer differentiation. However, the mechanism that SMAD2/3 coordinates different partners to determine different cell fate decisions is not systematically elaborated. Herein, this review is focusing on the roles and mechanisms of SMAD2/3 in regulating hESC pluripotency maintenance and germ layer differentiation.
SMAD2/3 Controls Divergent Transcriptional Networks of Pluripotency and Endoderm Differentiation
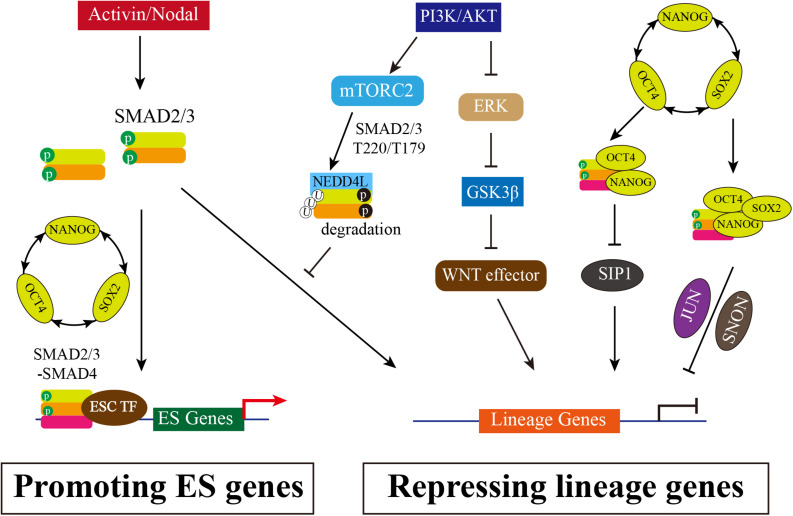
Human ESCs are derived from the blastocyst with the potential of long-term self-renewal and three primary germ layers differentiation (Thomson et al., 1998) and cultured in medium with basic fibroblast growth factor (bFGF) and Activin or TGF-β. TGF-β/Activin/Nodal signaling is essential for the maintenance of hESC pluripotency and self-renewal, while FGF2 serving as a competence factor (James et al., 2005; Xiao et al., 2006). Inhibition of Activin/Nodal signaling by Follistatin or by Activin receptor inhibitor SB431542 causes hESC differentiation (James et al., 2005; Vallier et al., 2005; Xiao et al., 2006). Pluripotency is maintained by the OCT4-SOX2-NANOG transcriptional network, which forms a positive feedback loop that activates pluripotency genes and inhibits the expression of differentiation associated genes (Boyer et al., 2005; He et al., 2009). Activin A is sufficient for the maintenance of self-renewal and pluripotency and induces the expression of OCT4, NANOG and SOX2 (Xiao et al., 2006; Xu et al., 2008), suggesting that pluripotent transcription factors might be the targets of TGF-β/Activin/Nodal signaling in hESCs. Inhibition of Activin/Nodal results in a more decrease of NANOG expression than OCT4 and SOX2 in hESCs, and thus induces differentiation toward neuroectoderm. Constitutive expression of NANOG is sufficient to maintain the pluripotency of hESC in the absence of Activin/Nodal signaling (Vallier et al., 2009a, b). In addition, transfection of small interfering RNAs targeting NANOG in hESCs causes differentiation to extraembryonic endoderm and trophectoderm lineages (Hyslop et al., 2005). Therefore, NANOG is considered as a direct target of TGF-β/Activin/Nodal signaling, which is further demonstrated by the study that SMAD2/3 can directly bind with the NANOG proximal promoter to activate NANOG expression and repress autocrine BMP signaling (Xu et al., 2008; Vallier et al., 2009a; Brown et al., 2011; Sakaki-Yumoto et al., 2013). Meanwhile, NANOG and SMAD2/3 can bind to regulatory elements of endoderm genes with other transcription repressors to inhibit differentiation (Xu et al., 2008; Brown et al., 2011). For instance, NANOG and SMAD2/3 inhibits Smad-interacting protein 1 (SIP1) expression to block neuroectoderm differentiation (Chng et al., 2010). Like SIP1, SNON (also named as SKIL), a potent SMAD2/3 corepressor, is expressed in hESCs but rapidly down-regulated upon differentiation. In pluripotent state, SNON is transcriptionally activated by OCT4/SOX2/NANOG complex and is selectively recruited by SMAD2, and then SMAD2/SNON together binds to mesendodermal genes to suppress the expression (Tsuneyoshi et al., 2012). A recent report shows that Jun N-terminal kinase (JNK)-JUN family genes JUN co-occupies ESC-specific enhancers with OCT4, NANOG, SMAD2/3, and specifically inhibits the exit from the pluripotent state by impeding the decommissioning of ESC enhancers and inhibiting the reconfiguration of SMAD2/3 chromatin binding from ESC-specific to endoderm-specific enhancers (Li et al., 2019). Overall, SMAD2/3 is necessary for self-renewal and pluripotency maintenance: SMAD2/3 not only targets NANOG to positively regulate OCT4-SOX2-NANOG transcriptional network, but also interacts with repressors such as SIP1, SNON and JUN to inhibit lineage genes associated with primary germ layer differentiation (Figure 1).
FIGURE 1.
The Multiple Roles of SMAD2/3 in Maintenance of hESCs. Activated TGF-β/Activin/Nodal signaling causes SMAD2/3 phosphorylation in distal C-terminal SXS motif (in green), which thus interacts with SMAD4 to form heterooligomeric complex and enters nucleus. Accumulated SMAD2/3/4 complex in nucleus can directly bind to the promotor regions of pluripotency marker genes, such as NANOG and OCT4, promoting the positive feedback loop of OCT4-SOX2-NANOG. In addition, SMAD2/3 can inhibit SIP1 expression to block neuroectodermal differentiation. SMAD2/3 also interacts with NANOG to repress differential genes expression, or recruits SNON activated by OCT4/SOX2/NANOG, or interacts with JUN, and then together with OCT4/SOX2/NANOG complex to suppress lineage genes expression. Meanwhile, PI3K/AKT is highly activated in hESCs. PI3K/AKT on one hand inhibits ERK and WNT signaling, allowing SMAD2/3 to activate pluripotency associated target genes rather than lineage genes; on the other hand, the downstream of PI3K/AKT mTORC2 can phosphorylate SMAD2/3 resulting the degradation mediated by NEDD4Land thus blocking the direct activation of lineage genes.
However, highly activated Activin/Nodal signal results in definitive endoderm differentiation. High concentrations of Activin A induces efficient differentiation of hESCs towards definitive endoderm: 50–100 ng/ml Activin A drives endoderm differentiation (D’Amour et al., 2005), whereas 5 ng/ml Activin A is supportive to maintain pluripotency of hESCs (Xiao et al., 2006; Tsai et al., 2010; Tomizawa et al., 2013). SMAD2/3 directly binds to endodermal lineage specifiers such as SOX17, FOXA2, GATA6, and GSC to induce endoderm differentiation (Brown et al., 2011; Kim et al., 2011). In addition, the pluripotent factors OCT4, SOX2, and NANOG control EOMES expression at onset of endoderm specification, and SMAD2/3 further interacts with EOMES to initiate endodermal transcription network expression (Teo et al., 2011). Activin/Nodal signaling also interacts with other signal pathways, mainly including BMP and WNT to synergistically promote endoderm differentiation (D’Amour et al., 2005; Loh et al., 2014). Higher activity of WNT/β-catenin stimulated by high dosage of WNT3A or glycogen synthase kinase-3 (GSK-3) inhibitors is required for maximally inducing definitive endoderm differentiation together with Activin A (Gadue et al., 2006; Teo et al., 2014). PI3K/AKT signaling, stimulated by fibroblast growth factor (FGF), directly modulates the downstream of WNT pathway to maintain undifferentiated hESCs (Ding et al., 2010). PI3K/AKT can also inhibit SMAD3 phosphorylation causing blocking of nuclear translocation (Remy et al., 2004). In addition, the inhibition of PI3K activity causes decrease of the mRNA and protein levels of Nanog (Storm et al., 2007), and activation of PI3K/AKT signaling is sufficient to maintain pluripotency of primate ESCs (Watanabe et al., 2006). Furthermore, Activin/Nodal specifies definitive endoderm from hESCs only when PI3K signaling is low (McLean et al., 2007). It seems that PI3K/AKT signaling is the important switcher for Activin/Nodal signaling in pluripotency or differentiation. Supporting with this notion, a later study suggests that PI3K governs Activin A/SMAD2/3 to promote pluripotency or differentiation cell fate in hESCs when PI3K activity is in high or low level, respectively (Singh et al., 2012). Singh and colleagues found that PI3K/AKT inhibits RAF/MEK/ERK and canonical WNT signaling, allowing SMAD2/3 to activate pluripotency associated target genes, such as NANOG, at high level of PI3K in undifferentiated state. When PI3K/AKT signaling is absent, the ERK activates canonical WNT signaling pathways and then the WNT effectors such as β-catenin and SNAIL can permit SMAD2/3 to activate differentiation associated genes (Singh et al., 2012). However, there is a controversial: Na and colleagues observed the inhibition of MEK/ERK prevents differentiation and promotes hESCs self-renewal (Na et al., 2010) while others showed that MEK/ERK signaling positively contributes to maintain self-renewal of hESCs (Armstrong et al., 2006; Li et al., 2007). Yu and colleagues provide more details in molecular mechanism. PI3K antagonizes the Activin/Nodal signaling in definitive endoderm differentiation by activated rapamycin complex 2 (mTORC2). Activation mTORC2 induces SMAD2/3 phosphorylation at T220/T179 residues which is not dependent on AKT, CDK, or ERK activity. The phosphorylated SMAD2/3 recruits E3 ubiquitin ligase NEDD4L, which causes SMAD2/3 degradation and blocks endoderm differentiation (Yu et al., 2015).
Taken together, Activin/Nodal signaling via SMAD2/3 on one hand is involved in controlling divergent transcriptional networks by interacting with different partners to regulating pluripotency or differentiation in hESCs. On the other hand, Activin/Nodal signaling antagonizes with PI3K/AKT pathway in pluripotency and differentiation. High PI3K and low Activin activity in hESCs promotes SMAD2/3 binding and activating gene expression of pluripotent transcription factors (Figure 1).
SMAD2/3 Interacts With Epigenetic Modifiers
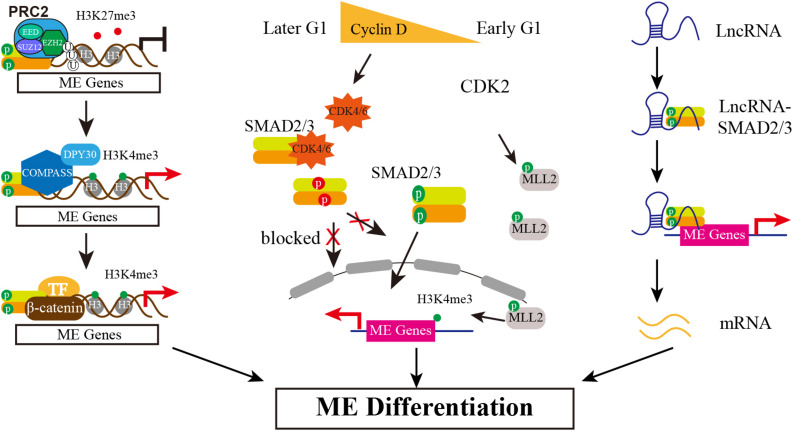
The pluripotency and differentiation of ESC is regulated not only by the core transcriptional network but also epigenetic modifiers, as epigenetic modification supports a permissive or repressive chromatin environment for transcription. In mouse ESCs, SMAD2/3 recruits the histone demethylase JMJD3 to target genes Nodal and Brachyury, thereby counteracting repression of Polycomb (Dahle et al., 2010). A follow-up study reveals that SMAD2/3 can also counteract Polycomb repression to regulate Oct4 expression during initiation of ESC differentiation (Dahle and Kuehn, 2013). Similarly, helix-loop-helix (HLH) proteins HEB also interacts with SMAD2/3 at distal enhancer elements and associates with PRC2 at promotors of mesendodermal genes (Yoon et al., 2015). In addition, it is reported that TGF-β/Nodal signals trigger differentiation in mouse ESC by influencing H3K9me3 modification, the hallmark of heterochromatin. The activation of Nodal signal induces the formation of TRIM33-SMAD2/3 complex. The PHD and Bromo cassette of TRIM33, respectively, recognizes K9me3 and binds an adjacent K18ac, making the chromatin accessible, which in turns allows SMAD2/3-SMAD4 to bind to the promotor of Gsc and Mixl1 to finally promote stem cell differentiation (Xi et al., 2011). Whether there is similar mechanism that SMAD2/3 interacts with Polycomb complex to respond to pluripotency maintenance or differentiation initiation in hESCs is rarely reported until recent years. Wang and colleagues show that in hESCs Activin signal impairs PRC2 activity by SMAD2-mediate reduction of EZH2 protein level, which is the catalytic subunit of PRC2 (Wang et al., 2017). Further study documents that the global reduction of H3K27me3 by Activin signal causes the forkhead protein FOXH1 to recruit into open chromatin regions, which together with SMAD2 and β-catenin activates mesendodermal genes expression, such as HAS2 and ALDH3A2 (Xu et al., 2018). Except for inhibitory epigenetic modification, it is also reported that Activin/Nodal signaling is relevant with the H3K4me3 maintenance on the master regulators of both pluripotency and germ layer specification in hESCs (Bertero et al., 2015). SMAD2/3 interacts with H3K4 methyltransferases complex COMPASS and its cofactor DPY30 to maintain H3K4me3 marks on pluripotency and mesendoderm genes. Inhibiting Activin/Nodal signaling causes specifically impaired H3K4me3 level on promotor and enhancer regions. Knockdown DPY30 in hESCs reduces expression of pluripotency and endoderm markers but increases neuroectoderm genes. Further mechanistic studies show that SMAD2/3 cooperates with NANOG and DPY30 to regulate H3K4me3 deposition on pluripotency and cell fate decision associated genes (Bertero et al., 2015). Recently the same group shows SMAD2/3 interacts with the METTL3-METTL14-WTAP complex identified by genome-wide analysis of SMAD2/3 interactome, which deposits N6-methyladenosine (m6A) on RNA. The interaction between SMAD2/3 and METTL3-METTL14-WTAP complex promotes m6A deposition on specific regulators of pluripotency such as NANOG, which resulting the degradation of NANOG mRNA and thus facilitating pluripotency exit and differentiation initiation (Bertero et al., 2018). Taken together, SMAD2/3 can interact with multiple epigenetic modifiers to function in different levels, together controlling pluripotency and mesendoderm differentiation initiation (Figure 2).
FIGURE 2.
The Multiple Mechanisms of SMAD2/3 Regulating Mesendoderm Differentiation of hESCs. Epigenetically, SMAD2/3 recruits JMJD3 to its target genes, erasing H3K27me3 marker. Then, SMAD2/3 interacts with COMPASS and its cofactor DPY30 to maintain H3K4me3 marks on these genes. In addition, differentially expressed Cyclin D in early and later G1 phase determines the biased lineage differentiation propensity. Low expression of Cyclin D in early G1 causes low level of CDK4/6, blocking the phosphorylation of SMAD2/3 in the linker region (in red) and thus allowing SMAD2/3 enter nucleus to activate mesendodermal genes expression. Meanwhile, CDK2 may phosphorylate MLL2 during G1 and causes H3K4me3 transiently increased at developmental genes, thereby opening a “window of opportunity” for lineage specification. The third action model is mediated by lncRNAs. Certain highly expressed lncRNAs interact with SMAD2/3 and recruit SMAD2/3 to and activate the expression of endodermal transcription factors such as FOXA2/GSC.
SMAD2/3 Mediates hESC Differentiation Propensity in G1 Phase
Human ESCs in different cell cycle phases exhibit biased differentiation propensity, which involves in CDK4/6-mediated SMAD2/3 phosphorylation. Primate ESCs exhibit unusual cell cycle features which are different from mouse ESCs, including dramatically shortened G1 phase (Becker et al., 2006; Fluckiger et al., 2006). Subsequent studies show that shortened G1 phase of cell cycle in hESCs is a cause, rather than a consequence of pluripotency. A short G1 limits the “window of opportunity” for which a cell can be responsive to differentiation cues (Neganova et al., 2009; Lange and Calegari, 2010), and lengthening G1 phase of pluripotent cell promotes differentiation in mouse and human (Filipczyk et al., 2007; Koledova et al., 2010; Lange and Calegari, 2010; Sela et al., 2012). Human ESCs express all G1-specific Cyclins (D1, D2, D3, and E) and cyclin-dependent kinases (CDK) (CDK2, CDK4, and CDK6) at variable levels (Neganova et al., 2009; Lange and Calegari, 2010). Knockdown of CDK2 in hESCs results in arrest at G1 phase and differentiation to extraembryonic (Neganova et al., 2009), suggesting that CDK2 is very important for cell cycle regulation and pluripotency maintenance in hESCs. Recent reports have provided some explanations for how to determine cell fate propensity in G1 phase. Using FUCCI reporter system, which is a sensor of cell cycle, Pauklin and Vallier observed hESCs in early G1 phase can only initiate endoderm differentiation but neuroectoderm differentiation is limited in later G1 phase. Further mechanistic analysis shows that the activity of Activin/Nodal signaling is controlled by Cyclin D, which activates CDK4/6 to phosphorylate SMAD2/3 in the linker region and thus makes the phosphorylated fail to enter nucleus. Since the Cyclin D expression is low in early G1 and high in later G1, thus Cyclin D/CDK4/6 inhibits the transcriptional activity of Activin/Nodal signaling to determine cell fate propensity by controlling the cellular localization of SMAD2/3 (Pauklin and Vallier, 2013). Consistently, SMAD3 is proved to be the substrate of CDK4 in MEF, and the phosphorylated SMAD3 by CDK4 inhibits its transcriptional activity (Matsuura et al., 2004).
Singh and colleagues also reported the heterogeneity correlated with cell cycle, but their results indicate that WNT/ERK signal promotes heterogeneity in late G1 cells, including developmental regulators expression such as GATA6, SOX17, FOXA2 (Singh et al., 2013). A later study by the same group further proved that the bivalent state is not stable in hESCs either, especially in G1 phase. H3K4me3 is transient increased at developmental genes by CDK2-dependent phosphorylation of the MLL2 histone methyl-transferase during G1, thereby opening a “window of opportunity” for lineage specification (Singh et al., 2015). In addition, a recent single-cell level study shows that hESCs exhibit high single-cell variation in absolute G1 length which is controlled by WNT/β-catenin pathway. A longer and wider distribution of G1 phase is regulated by WNT inhibition, allowing global reduction of 5-hydroxymethylcytosine (5hmC) on lineage-specific genes and thus causing biased differentiation toward neuroectoderm lineages but not affecting pluripotent genes expression. Transient decrease of G1 length by transgenic manipulation shows predominantly contributing to mesendoderm (Jang et al., 2019). Different with the previous report (Pauklin and Vallier, 2013), the single-cell analysis results show that the length of G1 has no effect on SMAD2/3 activity (Jang et al., 2019). Taken together, it is consistent that G1 phase creating a “window of opportunity” for mesendodermal genes expression, which endows hESC differentiation propensity to mesendoderm in early G1 phase (Figure 2). However, how to initiate lineage specification and whether SMAD2/3 is controlled in G1 phase are still controversial and need to be further clarified.
SMAD2/3 Interacts With lncRNA Promoting Differentiation
Long non-coding RNAs (lncRNAs) are transcribed with longer than 200 nucleotides and have been found wildly expressed from mammal genome, and play an important role in diverse biological processes, including regulation of stem cell (Fatica and Bozzoni, 2014). During hESCs differentiates into endoderm, there are reported that SMAD2/3 serve as RNA-interacting protein to actively participate in endoderm differentiation by different action mechanism (Jiang et al., 2015; Daneshvar et al., 2016; Chen et al., 2020). DEANR1 is the first reported lncRNA that regulates human definitive endoderm differentiation. DEANR1 is highly expressed in endoderm and contributes to endoderm differentiation by positively regulating endoderm factor FOXA2. Mechanistically, DEANR1 facilitates FOXA2 activation by interacting with SMAD2/3 and recruiting to the FOXA2 promotor (Jiang et al., 2015). DIGIT, an endoderm-expressing lncRNA, is regulated by a SMAD3-occupied enhancer proximal to DIGIT. DIGIT regulates the transcription of GSC in trans, and deletion of the SMAD3-occupied enhancer inhibits DIGIT and GSC expression and definitive endoderm differentiation (Daneshvar et al., 2016). Very recently, lncRNA LINC00458 is reported up-regulated in hESCs upon cultured with soft substrate. Gain- and loss-of-function experiments confirm that LINC00458 is functionally required for endodermal lineage specification from hESCs induced by soft substrates. Importantly, the endoderm-promoting function of LINC00458 depends on the interaction with SMAD2/3 (Chen et al., 2020). These reports together suggest a new model that lncRNA may function as a new co-factor of SMAD2/3 to mediate lineage differentiation (Figure 2).
Summary and Prospect
SMAD2/3 is the major effector of TGFβ/Activin/Nodal signal, which plays different roles in undifferentiated hESCs and committed cells. Herein, we summarize the multiple action manners of SMAD2/3 in pluripotency maintenance and differentiation initiation in terms of transcription regulation, epigenetic modification, cell cycle related differentiation bias and interaction with lncRNAs. SMAD2/3 protects pluripotent state by directly targeting OCT4-SOX2-NANOG network to maintain pluripotency and interacts with other factors such as SIP1, SNON, and JUN to inhibit differentiation at high PI3K/AKT activity. When in differentiation context, SMAD2/3 is highly activated by higher Activin/Nodal signal while low PI3K/AKT activity. Accessible chromatin due to the increase of 5hmC and H3K4me3 allows SMAD2/3 binding to developmental regulators to perform cell-fate decision function. It is very likely that Activin/Nodal and its antagonistic PI3K/AKT signal together determine SMAD2/3 functional switch in pluripotency and differentiation. In addition, WNT pathway synergies with Activin/Nodal to permit SMAD2/3 to activate differentiation associated genes. Meanwhile, the co-regulators of SMAD2/3 also are decisive for SMAD2/3 function in different contexts. However, there are controversies about the role of SMAD2/3 in cell cycle dependent differentiation initiation. In addition, how SMAD2/3 chooses different co-regulators during the pluripotency maintenance and differentiation transition is still unclear but very important to understand the underlying mechanism of cell fate determination.
Furthermore, the SMAD2/3 interactome analysis suggests SMAD2/3 interacts with many other functional complexes except for classic TGF-β signaling associated pathways, including E3 ubiquitin ligase, mRNA processing and degradation, DNA repair and apoptosis (Bertero et al., 2018). Whether or which of these interactions with SMAD2/3 are important for ESCs pluripotency maintenance or differentiation is not uncovered yet. In addition, SMAD2/3 also participates in lipid metabolism in mouse hepatocytes (Yang et al., 2013) and epithelial-mesenchymal transition and metastasis induction in breast cancer (Rios Garcia et al., 2017). Of note, there is significant metabolic difference between pluripotent state and differentiation in stem cells (Mathieu and Ruohola-Baker, 2017). Does SMAD2/3 also directly regulate metabolism related protein or signal pathways to influence pluripotency or differentiation in hESCs? In addition to the roles of SMAD2/3 reviewed in this article, there are still many unknown roles of SMAD2/3 in hESCs that need to be clarified.
Author Contributions
JY conceived the project and drafted the manuscript. WJ and JY prepared the figures and finalized the manuscript. Both authors contributed to and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Donghui Zhang for figure preparation, and members of Jiang laboratory for helpful discussion on the topic. We apologize to those scientists whose outstanding works are not cited here due to limited space.
Footnotes
Funding. This work was supported by the National Key Research and Development Program of China (No. 2016YFA0503100 to WJ); the National Natural Science Foundation of China (Nos. 31970608 and 91740102); and the Fundamental Research Funds for the Central Universities and the Medical Science Advancement Program of Wuhan University (Nos. TFZZ2018053 and TFJC2018005).
References
- Armstrong L., Hughes O., Yung S., Hyslop L., Stewart R., Wappler I., et al. (2006). The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum. Mol. Genet. 15 1894–1913. 10.1093/hmg/ddl112 [DOI] [PubMed] [Google Scholar]
- Becker K. A., Ghule P. N., Therrien J. A., Lian J. B., Stein J. L., van Wijnen A. J., et al. (2006). Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J. Cell Physiol. 209 883–893. 10.1002/jcp.20776 [DOI] [PubMed] [Google Scholar]
- Bertero A., Brown S., Madrigal P., Osnato A., Ortmann D., Yiangou L., et al. (2018). The SMAD2/3 interactome reveals that TGFbeta controls m(6)A mRNA methylation in pluripotency. Nature 555 256–259. 10.1038/nature25784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero A., Madrigal P., Galli A., Hubner N. C., Moreno I., Burks D., et al. (2015). Activin/nodal signaling and NANOG orchestrate human embryonic stem cell fate decisions by controlling the H3K4me3 chromatin mark. Genes Dev. 29 702–717. 10.1101/gad.255984.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer L. A., Lee T. I., Cole M. F., Johnstone S. E., Levine S. S., Zucker J. P., et al. (2005). Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122 947–956. 10.1016/j.cell.2005.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Teo A., Pauklin S., Hannan N., Cho C. H., Lim B., et al. (2011). Activin/Nodal signaling controls divergent transcriptional networks in human embryonic stem cells and in endoderm progenitors. Stem Cells 29 1176–1185. 10.1002/stem.666 [DOI] [PubMed] [Google Scholar]
- Chen Y. F., Li Y. J., Chou C. H., Chiew M. Y., Huang H. D., Ho J. H., et al. (2020). Control of matrix stiffness promotes endodermal lineage specification by regulating SMAD2/3 via lncRNA LINC00458. Sci. Adv. 6:eaay0264. 10.1126/sciadv.aay0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chng Z., Teo A., Pedersen R. A., Vallier L. (2010). SIP1 mediates cell-fate decisions between neuroectoderm and mesendoderm in human pluripotent stem cells. Cell Stem Cell 6 59–70. 10.1016/j.stem.2009.11.015 [DOI] [PubMed] [Google Scholar]
- Dahle O., Kuehn M. R. (2013). Polycomb determines responses to smad2/3 signaling in embryonic stem cell differentiation and in reprogramming. Stem Cells 31 1488–1497. 10.1002/stem.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahle O., Kumar A., Kuehn M. R. (2010). Nodal signaling recruits the histone demethylase Jmjd3 to counteract polycomb-mediated repression at target genes. Sci. Signal. 3:ra48. 10.1126/scisignal.2000841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour K. A., Agulnick A. D., Eliazer S., Kelly O. G., Kroon E., Baetge E. E. (2005). Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23 1534–1541. 10.1038/nbt1163 [DOI] [PubMed] [Google Scholar]
- Daneshvar K., Pondick J. V., Kim B. M., Zhou C., York S. R., Macklin J. A., et al. (2016). DIGIT is a conserved long noncoding RNA that regulates GSC expression to control definitive endoderm differentiation of embryonic stem cells. Cell Rep. 17 353–365. 10.1016/j.celrep.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding V. M., Ling L., Natarajan S., Yap M. G., Cool S. M., Choo A. B. (2010). FGF-2 modulates Wnt signaling in undifferentiated hESC and iPS cells through activated PI3-K/GSK3beta signaling. J. Cell Physiol. 225 417–428. 10.1002/jcp.22214 [DOI] [PubMed] [Google Scholar]
- Fatica A., Bozzoni I. (2014). Long non-coding RNAs: new players in cell differentiation and development. Nat. Rev. Genet. 15 7–21. 10.1038/nrg3606 [DOI] [PubMed] [Google Scholar]
- Feng X. H., Derynck R. (2005). Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 21 659–693. 10.1146/annurev.cellbio.21.022404.142018 [DOI] [PubMed] [Google Scholar]
- Filipczyk A. A., Laslett A. L., Mummery C., Pera M. F. (2007). Differentiation is coupled to changes in the cell cycle regulatory apparatus of human embryonic stem cells. Stem Cell Res. 1 45–60. 10.1016/j.scr.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Fluckiger A. C., Marcy G., Marchand M., Negre D., Cosset F. L., Mitalipov S., et al. (2006). Cell cycle features of primate embryonic stem cells. Stem Cells 24 547–556. 10.1634/stemcells.2005-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P., Huber T. L., Paddison P. J., Keller G. M. (2006). Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 103 16806–16811. 10.1073/pnas.0603916103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Nakada D., Morrison S. J. (2009). Mechanisms of stem cell self-renewal. Annu. Rev. Cell Dev. Biol. 25 377–406. 10.1146/annurev.cellbio.042308.113248 [DOI] [PubMed] [Google Scholar]
- Hyslop L., Stojkovic M., Armstrong L., Walter T., Stojkovic P., Przyborski S., et al. (2005). Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cell 23 1035–1043. 10.1634/stemcells.2005-0080 [DOI] [PubMed] [Google Scholar]
- James D., Levine A. J., Besser D., Hemmati-Brivanlou A. (2005). TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132 1273–1282. 10.1242/dev.01706 [DOI] [PubMed] [Google Scholar]
- Jang J., Han D., Golkaram M., Audouard M., Liu G., Bridges D., et al. (2019). Control over single-cell distribution of G1 lengths by WNT governs pluripotency. PLoS Biol. 17:e3000453. 10.1371/journal.pbio.3000453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Liu Y., Liu R., Zhang K., Zhang Y. (2015). The lncRNA DEANR1 facilitates human endoderm differentiation by activating FOXA2 expression. Cell Rep. 11 137–148. 10.1016/j.celrep.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. W., Yoon S. J., Chuong E., Oyolu C., Wills A. E., Gupta R., et al. (2011). Chromatin and transcriptional signatures for Nodal signaling during endoderm formation in hESCs. Dev. Biol. 357 492–504. 10.1016/j.ydbio.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Koledova Z., Kafkova L. R., Calabkova L., Krystof V., Dolezel P., Divoky V. (2010). Cdk2 inhibition prolongs G1 phase progression in mouse embryonic stem cells. Stem Cells Dev. 19 181–194. 10.1089/scd.2009.0065 [DOI] [PubMed] [Google Scholar]
- Kubo A., Shinozaki K., Shannon J. M., Kouskoff V., Kennedy M., Woo S., et al. (2004). Development of definitive endoderm from embryonic stem cells in culture. Development 131 1651–1662. 10.1242/dev.01044 [DOI] [PubMed] [Google Scholar]
- Lange C., Calegari F. (2010). Cdks and cyclins link G1 length and differentiation of embryonic, neural and hematopoietic stem cells. Cell Cycle 9 1893–1900. 10.4161/cc.9.10.11598 [DOI] [PubMed] [Google Scholar]
- Li J., Wang G., Wang C., Zhao Y., Zhang H., Tan Z., et al. (2007). MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation 75 299–307. 10.1111/j.1432-0436.2006.00143.x [DOI] [PubMed] [Google Scholar]
- Li Q. V., Dixon G., Verma N., Rosen B. P., Gordillo M., Luo R., et al. (2019). Genome-scale screens identify JNK-JUN signaling as a barrier for pluripotency exit and endoderm differentiation. Nat. Genet. 51 999–1010. 10.1038/s41588-019-0408-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K. M., Ang L. T., Zhang J., Kumar V., Ang J., Auyeong J. Q., et al. (2014). Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell 14 237–252. 10.1016/j.stem.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J., Seoane J., Wotton D. (2005). Smad transcription factors. Genes Dev. 19 2783–2810. 10.1101/gad.1350705 [DOI] [PubMed] [Google Scholar]
- Mathieu J., Ruohola-Baker H. (2017). Metabolic remodeling during the loss and acquisition of pluripotency. Development 144 541–551. 10.1242/dev.128389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura I., Denissova N. G., Wang G., He D., Long J., Liu F. (2004). Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 430 226–231. 10.1038/nature02650 [DOI] [PubMed] [Google Scholar]
- McLean A. B., D’Amour K. A., Jones K. L., Krishnamoorthy M., Kulik M. J., Reynolds D. M., et al. (2007). Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 25 29–38. 10.1634/stemcells.2006-0219 [DOI] [PubMed] [Google Scholar]
- Na J., Furue M. K., Andrews P. W. (2010). Inhibition of ERK1/2 prevents neural and mesendodermal differentiation and promotes human embryonic stem cell self-renewal. Stem Cell Res. 5 157–169. 10.1016/j.scr.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Neganova I., Zhang X., Atkinson S., Lako M. (2009). Expression and functional analysis of G1 to S regulatory components reveals an important role for CDK2 in cell cycle regulation in human embryonic stem cells. Oncogene 28 20–30. 10.1038/onc.2008.358 [DOI] [PubMed] [Google Scholar]
- Pauklin S., Vallier L. (2013). The cell-cycle state of stem cells determines cell fate propensity. Cell 155 135–147. 10.1016/j.cell.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy I., Montmarquette A., Michnick S. W. (2004). PKB/Akt modulates TGF-beta signalling through a direct interaction with Smad3. Nat. Cell Biol. 6 358–365. 10.1038/ncb1113 [DOI] [PubMed] [Google Scholar]
- Rios Garcia M., Steinbauer B., Srivastava K., Singhal M., Mattijssen F., Maida A., et al. (2017). Acetyl-CoA carboxylase 1-dependent protein acetylation controls breast cancer metastasis and recurrence. Cell Metab. 26 842.e5–855.e5. 10.1016/j.cmet.2017.09.018 [DOI] [PubMed] [Google Scholar]
- Sakaki-Yumoto M., Liu J., Ramalho-Santos M., Yoshida N., Derynck R. (2013). Smad2 is essential for maintenance of the human and mouse primed pluripotent stem cell state. J. Biol. Chem. 288 18546–18560. 10.1074/jbc.M112.446591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela Y., Molotski N., Golan S., Itskovitz-Eldor J., Soen Y. (2012). Human embryonic stem cells exhibit increased propensity to differentiate during the G1 phase prior to phosphorylation of retinoblastoma protein. Stem Cells 30 1097–1108. 10.1002/stem.1078 [DOI] [PubMed] [Google Scholar]
- Singh A. M., Chappell J., Trost R., Lin L., Wang T., Tang J., et al. (2013). Cell-cycle control of developmentally regulated transcription factors accounts for heterogeneity in human pluripotent cells. Stem Cell Rep. 1 532–544. 10.1016/j.stemcr.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. M., Reynolds D., Cliff T., Ohtsuka S., Mattheyses A. L., Sun Y., et al. (2012). Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell 10 312–326. 10.1016/j.stem.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. M., Sun Y., Li L., Zhang W., Wu T., Zhao S., et al. (2015). Cell-cycle control of bivalent epigenetic domains regulates the exit from pluripotency. Stem Cell Rep. 5 323–336. 10.1016/j.stemcr.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm M. P., Bone H. K., Beck C. G., Bourillot P. Y., Schreiber V., Damiano T., et al. (2007). Regulation of nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J. Biol. Chem. 282 6265–6273. 10.1074/jbc.M610906200 [DOI] [PubMed] [Google Scholar]
- Teo A. K., Arnold S. J., Trotter M. W., Brown S., Ang L. T., Chng Z., et al. (2011). Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 25 238–250. 10.1101/gad.607311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo A. K., Valdez I. A., Dirice E., Kulkarni R. N. (2014). Comparable generation of activin-induced definitive endoderm via additive Wnt or BMP signaling in absence of serum. Stem Cell Rep. 3 5–14. 10.1016/j.stemcr.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282 1145–1147. 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Tomizawa M., Shinozaki F., Sugiyama T., Yamamoto S., Sueishi M., Yoshida T. (2013). Activin A is essential for Feeder-free culture of human induced pluripotent stem cells. J. Cell Biochem. 114 584–588. 10.1002/jcb.24395 [DOI] [PubMed] [Google Scholar]
- Tremblay K. D., Hoodless P. A., Bikoff E. K., Robertson E. J. (2000). Formation of the definitive endoderm in mouse is a Smad2-dependent process. Development 127 3079–3090. [DOI] [PubMed] [Google Scholar]
- Tsai Z. Y., Singh S., Yu S. L., Kao L. P., Chen B. Z., Ho B. C., et al. (2010). Identification of microRNAs regulated by activin A in human embryonic stem cells. J. Cell Biochem. 109 93–102. 10.1002/jcb.22385 [DOI] [PubMed] [Google Scholar]
- Tsuneyoshi N., Tan E. K., Sadasivam A., Poobalan Y., Sumi T., Nakatsuji N., et al. (2012). The SMAD2/3 corepressor SNON maintains pluripotency through selective repression of mesendodermal genes in human ES cells. Genes Dev. 26 2471–2476. 10.1101/gad.201772.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L., Alexander M., Pedersen R. A. (2005). Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 118(Pt 19), 4495–4509. 10.1242/jcs.02553 [DOI] [PubMed] [Google Scholar]
- Vallier L., Mendjan S., Brown S., Chng Z., Teo A., Smithers L. E., et al. (2009a). Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development 136 1339–1349. 10.1242/dev.033951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L., Touboul T., Brown S., Cho C., Bilican B., Alexander M., et al. (2009b). Signaling pathways controlling pluripotency and early cell fate decisions of human induced pluripotent stem cells. Stem Cell 27 2655–2666. 10.1002/stem.199 [DOI] [PubMed] [Google Scholar]
- Wang L., Xu X., Cao Y., Li Z., Cheng H., Zhu G., et al. (2017). Activin/Smad2-induced Histone H3 Lys-27 Trimethylation (H3K27me3) reduction is crucial to initiate mesendoderm differentiation of human embryonic stem cells. J. Biol. Chem. 292 1339–1350. 10.1074/jbc.M116.766949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Umehara H., Murayama K., Okabe M., Kimura T., Nakano T. (2006). Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 25 2697–2707. 10.1038/sj.onc.1209307 [DOI] [PubMed] [Google Scholar]
- Weiss A., Attisano L. (2013). The TGFbeta superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2 47–63. 10.1002/wdev.86 [DOI] [PubMed] [Google Scholar]
- Wu M. Y., Hill C. S. (2009). Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell 16 329–343. 10.1016/j.devcel.2009.02.012 [DOI] [PubMed] [Google Scholar]
- Xi Q., Wang Z., Zaromytidou A. I., Zhang X. H., Chow-Tsang L. F., Liu J. X., et al. (2011). A poised chromatin platform for TGF-beta access to master regulators. Cell 147 1511–1524. 10.1016/j.cell.2011.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Yuan X., Sharkis S. J. (2006). Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cell 24 1476–1486. 10.1634/stemcells.2005-0299 [DOI] [PubMed] [Google Scholar]
- Xu R. H., Sampsell-Barron T. L., Gu F., Root S., Peck R. M., Pan G., et al. (2008). NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell 3 196–206. 10.1016/j.stem.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wang L., Liu B., Xie W., Chen Y. G. (2018). Activin/Smad2 and Wnt/beta-catenin up-regulate HAS2 and ALDH3A2 to facilitate mesendoderm differentiation of human embryonic stem cells. J. Biol. Chem. 293 18444–18453. 10.1074/jbc.RA118.003688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Inokuchi S., Roh Y. S., Song J., Loomba R., Park E. J., et al. (2013). Transforming growth factor-beta signaling in hepatocytes promotes hepatic fibrosis and carcinogenesis in mice with hepatocyte-specific deletion of TAK1. Gastroenterology 144 1042.e4–1054.e4. 10.1053/j.gastro.2013.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. J., Foley J. W., Baker J. C. (2015). HEB associates with PRC2 and SMAD2/3 to regulate developmental fates. Nat. Commun. 6:6546. 10.1038/ncomms7546 [DOI] [PubMed] [Google Scholar]
- Yu J. S., Ramasamy T. S., Murphy N., Holt M. K., Czapiewski R., Wei S. K., et al. (2015). PI3K/mTORC2 regulates TGF-beta/Activin signalling by modulating Smad2/3 activity via linker phosphorylation. Nat. Commun. 6:7212. 10.1038/ncomms8212 [DOI] [PMC free article] [PubMed] [Google Scholar]