Abstract
Carbon nanotubes (CNTs) are recently developed tubular nanomaterials, with diameters ranging from a few nanometers to tens of nanometers, and the length reaching up to several micrometers. They can be either single-walled carbon nanotubes (SWCNTs) or multi-walled carbon nanotubes (MWCNTs). Due to their nano-scaled structure, CNTs have a unique set of mechanical, electrical, and chemical properties that make them useful in information technologies, optoelectronics, energy technologies, material sciences, medical technologies, and other fields. However, with the wide application and increasing production of CNTs, their potential risks have led to concerns regarding their impact on environment and health. The shape of some types of CNTs is similar to asbestos fibers, which suggests that these CNTs may cause characteristic pleural diseases similar to those found in asbestos-exposed humans, such as pleural plaques and malignant mesothelioma. Experimental data indicate that CNTs can induce lung and pleural lesions, inflammation, pleural fibrosis, lung tumors, and malignant mesothelioma upon inhalation in the experimental animals. In this review, we focus on the potential of MWCNTs to induce diseases similar to those by asbestos, molecular and cellular mechanisms associated with these diseases, and we discuss a method for evaluating the pleural toxicity of MWCNTs.
Keywords: multi-walled carbon nanotubes, pleural translocation, pleural lesions, asbestos
Introduction
Carbon nanotubes (CNTs) are hollow carbon fibers with diameters ranging from a few nanometers to tens of nanometers and lengths of up to several micrometers. According to their structures, CNTs are divided into two classes: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs)1. There are two types of MWCNTs, the Russian Doll model and the Parchment model2. In the Russian Doll model, the MWCNTs are formed by multiple coaxially arranged graphene cylinders, in which smaller-diameter graphene tubes are encased by the larger-diameter tubes. In the Parchment model, the MWCNTs are formed by a single graphene sheet that is wrapped around itself, resulting in a CNT with more than one layer, resembling the rolled up scroll of paper. In contrast to MWCNTs, SWCNTs are composed of a single graphene cylinder. CNTs have many unique mechanical, electrical, and chemical properties, and have a broad range of applications in many fields, such as electronic devices, composite materials, and biomedicine3.
As compared to the wide application of CNTs, and the increasing scale of their production, their safety assessment is lagging behind, and there is an increasing concern regarding their harmful effects on the environment and human health. People can be exposed to carbon nanotubes through various routes in their work and daily life. Exposure to CNTs takes place mainly through the smoke generated by combustion of CNT-containing materials. In addition, workers can be exposed to particles released during the production of CNTs. In medical practice, CNTs may be used as drug carriers and for other purposes.
Nowadays, the global production of carbon nanomaterials each year is several hundred tons, increasing the risk of exposure to CNTs. Long-term exposure triggers a series of toxic reactions in animal models. Due to their small size and low density, CNTs can accumulate in the lungs of the workers, when inhaled through aerosols, at their occupational sites. Song et al.4 investigated the pathological changes in a group of patients in Chaoyang Hospital, Beijing, China, after 5 to 13 month-long exposure to polyacrylic acid paint in a poorly ventilated workplace, and found the nanoparticles in the lungs and pleural effusions of these patients. Although similar respiratory toxicity of CNTs in human beings has not been reported yet, it is likely that long-term exposure to CNTs could cause respiratory lesions. Many studies have shown that CNTs induce oxidative stress, significant inflammation, and apoptosis in a variety of cells, and eventually lead to genetic damage and mutations, resulting in lung cancer and mesothelioma5. Pleural plaques and malignant mesothelioma are characteristic lesions in asbestos-exposed humans6. The fibrous structure of asbestos impedes the clearance of these fibers after translocation from the lung into the pleural cavity. Some CNTs have structures similar to asbestos fibers, suggesting that these CNTs, like asbestos, can induce lesions in the pleura.
According to a classic pathogenesis paradigm for fibrous materials suggested by Standon et al.7, a high length to diameter ratio is an important parameter in assessment of the safety of the CNTs. Donaldson et al.8 have proposed that a fraction of fibers in the lung are routinely transported into the pleural cavity, and cannot be cleared effectively through pleural stomata, resulting in trapping and deposition of the fibers in the pleura, leading to subsequent pro-inflammatory, genotoxic, and mitogenic responses at the sites of deposition. Therefore, pleural pathogenicity of CNTs is length-dependent. It has been reported that intra-pleural injection of long CNT elicits a substantial inflammatory response in the pleural space and granuloma formation on the diaphragm and parietal mesothelium, although the length threshold has not been determined6.
In this review we focus on pleural toxicology of MWCNTs based on recent research, and discuss translocation of MWCNTs from the lung to the pleural cavity, the pleural lesions induced by respiratory exposure to MWCNTs, and mechanisms associated with these lesions.
Translocation of MWCNTs from the Lung to the Pleural Cavity
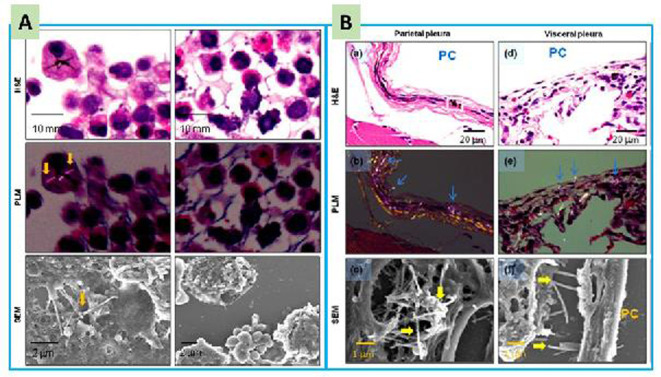
There are several reports9, 10, 11, 12 that investigated the distribution of MWCNTs in test animals, following respiratory exposure. It was found that the MWCNTs were mainly retained in the lung, but over time they were also transported to extra-pulmonary organs and tissues, including lymph nodes, liver, spleen, kidney, and bone marrow. To assess the extent of translocation of MWCNTs into the pleural cavity, we developed a simple and effective method using pleural cavity lavage (PCL)6, 11. After intratracheal spraying of MWCNTs into the lungs, they were found in cell pellets from PCL samples and were observed in the parietal and visceral pleura5, 13 (Fig. 1). Similar results were also reported by other researchers14. These results indicate that MWCNTs are able to translocate form the lung to the pleural cavity, although the proportion of translocated MWCNTs is small.
Fig. 1.
Evidence of multi-walled carbon nanotube (MWCNT) fibers in the cell pellets of pleural cavity lavage (PCL) (A) and in the pleura (B): H&E, hematoxylin and eosin staining; PLM, polarized light microscopy; SEM; scanning electronic microscopy; and PC, pleural cavity. Arrows indicate MWCNT fibers. Adapted from Xu, J. et al. Cancer Science 2014, 105, 763–769. MWCNT fibers were observed in the PCL and pleura by using PLM and SEM.
It is still not clear how MWCNTs from the lungs migrate to the pleura and pleural cavity. Several studies5, 13, 14, 15 have observed the penetration of MWCNTs from the lung into the visceral pleura, suggesting that needle-like MWCNTs directly pierce the lining, and when the pierced cells die, the fibers are released into the pleural cavity. Another possibility is that MWCNT-burdened macrophages carry the fibers into the pleural cavity during the process of inflammatory infiltration. The second scenario is supported by the observation that macrophages, some with phagocytosed MWCNTs, accumulated in the pleural side of visceral pleura16. It is unlikely that MWCNTs are transported into the pleural cavity through blood or lymphatic fluids.
Induction of Pleural Lesions in Animal Models
One of the physical characteristics of MWCNTs is a high aspect ratio and some MWCNTs have a needle-like shape, similar to that of asbestos. Thus, like asbestos, some MWCNTs are suspected to be able to cause lesions such as pleural plaque and mesothelioma14, 17. In the initial studies, injection of MWCNTs into the peritoneal cavity or scrotum, a surrogate method for observation of pleural toxicity especially for mesothelial lesions and malignant mesothelioma17, 18, 19, 20, as both these tissues are lined by mesothelium. Intraperitoneal exposure to different types of MWCNT at different doses resulted in the development of malignant mesothelioma in all cases21. These results suggest that MWCNTs have the potential to cause pleural lesions, if deposited in the pleura.
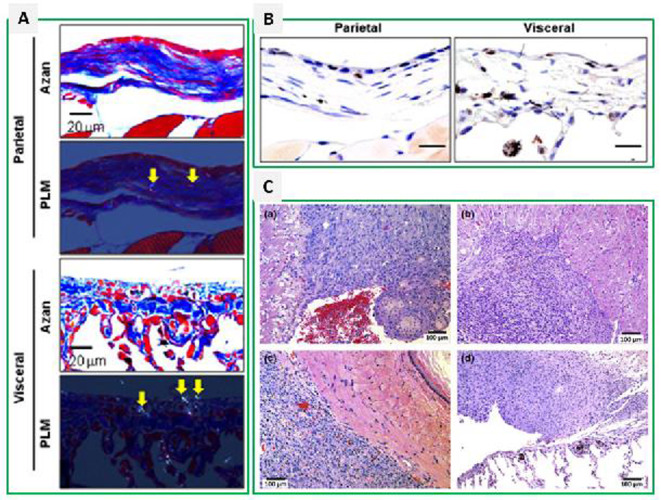
Short-term toxicity studies5, 13, 15 show that intratracheal spraying of MWCNTs causes acute inflammation in the pleural cavity, fibrosis, and mesothelial cell proliferation in rodents. Fibrosis is observed in both parietal and visceral pleura, and is often associated with deposition of fibers (Fig. 2A). Although neoplastic lesions were not found in these short-term studies, proliferative cell nuclear antigen (PCNA) staining showed increased mesothelial cell proliferation, especially in the inflammatory and fibrotic sites, suggesting that cell proliferation is involved in an acute phase responsive lesion (Fig. 2B). A 2-year long study22showed that, when the MWCNTs were intratracheally sprayed for 2 weeks at the beginning of the experiment, 15.8% of the Fisher rats developed malignant mesothelioma in the pleural cavity (Fig. 2C). Importantly, MWCNTs were also deposited in the pleura, suggesting that these depositions were the cause of the mesothelioma.
Fig. 2.
Pleural lesions induced by multi-walled carbon nanotubes (MWCNTs) in rats: A, pleural fibrosis by Azan-Mallory staining; B, mesothelial cell proliferation by PCNA staining; and C, malignant mesothelioma. PLM, polarized light microscopy. Arrows indicate MWCNT fibers. Adapted from Xu, J. et al. Cancer Science 2014, 105, 763–769, and from Suzui, M. et al. Cancer Science 2016, 107, 924-935.
It should be noted that pleural lesions induced by MWCNTs are dependent on their length and rigidity23. Short tangled MWCNTs do not cause pleural fibrosis and mesothelial proliferation13. Long and thick MWCNTs are more likely to cause DNA damage and inflammation than the short MWCNTs24. Also, tumorigenicity can occur at very low concentrations of MWCNT fibers (single peritoneal injection, 3 μg/mouse, median length of 2 μm)20, very similar to the asbestos-induced malignant mesothelioma in humans.
The Mechanisms of Pleural Toxicity
A major factor determining the pleural toxicity of engineered materials is whether they translocate and persist in the pleural cavity. According to the fiber pathogenicity paradigm described by Donaldson8, fibrous materials with diameters small enough to allow the deposition beyond the ciliated airways, and with a length and rigidity sufficient to impede clearing by macrophages, and are biopersistent, are potential lung carcinogens. If such fibers are translocated from the lungs into the pleural cavity and if they cannot be cleared through the parietal pleura stomata, they pose the risk of pleural diseases6. As these fibers cannot be effectively cleared, their deposition results in cellular and molecular changes that can eventually progress into pleural lesions at the deposition sites. In rats, intratracheal instillation of MWCNTs for 2 weeks leads to persistent pleural inflammation, fibrosis, and mesothelial cell proliferation, and the administered fibers can be detected in the pleura 3 months after instillation15.
The pathogenic mechanism is quite complex and involves cytotoxicity, generation of reactive oxygen species (ROS), genetic toxicity, and inflammatory responses. Many in vitro studies have demonstrated that MWCNTs are toxic to several cell types, including lung epithelial cells, airway epithelial cells, mesothelial cells, and macrophages11, 25, 26, 27, 28, 29, although lethal doses vary, depending on the type of the cell and the MWCNT used. One reason for the cytotoxicity of MWCNTs is high ROS generation. Normally, antioxidant systems and ROS production are in equilibrium in the cell. When the ROS generation exceeds the capacity of the antioxidant system to neutralize them, the cell faces oxidative stress30. Elevated levels of ROS cause oxidation of membrane lipids, cellular proteins, and DNA31, 32. Metal residues in MWCNTs, usually from the catalytic substances used in their production, also contribute to ROS production, probably by the Fenton reaction, in which transition elements like iron, catalyze the generation of oxygen free radicals, which enhance the levels of reduced coenzyme II and xanthine oxidase in macrophages and neutrophils33, 34. Exposure to MWCNTs can cause genetic alterations, like DNA damages in rat bone marrow cells, chromosome aberration, and micronucleus formation in alveolar type II epithelial cells35, 36.
The respiratory tract is a major exposure route for air-borne particles. Inhaled nanomaterials, including MWCNTs, can induce pulmonary inflammation. Their intrinsic cytotoxicity and induction of elevated ROS are responsible for triggering an inflammatory response in the lung37, 38. Excessive ROS causes NF-κB to transfer from the cytoplasm to the nucleus, activating the NF-κB signal transduction pathway, which in turn initiates transcription of a variety of pro-inflammatory cytokines such as TNF-α and IL-833. It is worth noting that MWCNT-mediated oxidative damage may have a synergistic effect on the inflammatory responses, associated with formation and activation of NLRP3 inflammasomes39. In vitro and animal experiments have shown that, in addition to phagocytosis by macrophages and mesothelial cells, MWCNTs can also penetrate and kill non-phagocytic cells34. Induction of cell damage and death by MWCNTs stimulate the release of inflammatory mediators (TNF-α, IL-6, IL-8, IL-33, etc.) and promote the formation of inflammation and fibrosis34, 40. Many MWCNTs are characterized by high surface reactivity that causes inflammation and damage at the sites of deposition41. In the pleural cavity and the pleural tissue, MWCNTs elicit similar inflammatory reactions at the deposit sites. Intratracheally instilled MWCNT are found to induce increased number of inflammatory cells and higher levels of cytokines in the PCL, promoting inflammation, fibrosis, and proliferation of mesothelial cells5, 13. If the translocated MWCNTs have a shape that impedes clearance from the pleural cavity, these reactions will be persistent15.
Macrophages play an important role in the MWCNT-induced lung toxicity and development of pleural lesions. Numerous studies have shown that macrophages are major infiltrating cells after MWCNTs enter the lung tissue. Alveolar macrophages, together with ciliary epithelial cells, are the first line of defense against the inhaled dust and invading microbes. Interaction of macrophages with MWCNTs triggers inflammation, fibrosis, and other reactions by releasing IL-33, CCL2/7, IL-12, and other chemokines. These chemokines recruit other immune cells to the site of inflammation42, 43, 44. Typical inflammatory lesions induced by MWCNTs are granulomas45. Production of PDGF-AA by MWCNT-burdened alveolar macrophages46 and interaction of macrophages with MWCNTs47 also promote fibrosis. Approximately 80% of the infiltrating cells in pleural cavity of MWCNT-administered test animals are CD68 positive cells5, indicating that macrophages are the principal infiltrating cell-type, and most MWCNT fibers in the pleural cavity of these test animals are within the macrophages5. Interaction of macrophages with MWCNTs also induces pleural inflammation, enhances fibrosis, and stimulates cytokine release by mesothelial cells47, promotes the production of a variety of cytokines5, 13, 15. Accumulation of macrophages loaded with phagocytosed MWCNTs are observed on the surface of the visceral pleura16. As the long MWCNTs cannot be completely engulfed by the macrophages, leading to frustrated phagocytosis, there is constant interaction of macrophages in the pleural cavity with these MWCNTs and this results in persistent inflammatory reactions, fibrosis, and stimulation of mesothelial cells. Thus, macrophages in the pleural cavity promote pleural lesions.
How to Evaluate the Chest Toxicity of MWCNTs
Current research data indicate that, like asbestos, MWCNTs have the potential to induce pleural diseases. However, there is no uniform standard method to evaluate the pleural toxicity of inhaled material. Observation of pleural lesions in patients with pleural effusion, usually involves imaging and cytological techniques, while in animal experiments, pleural histopathology is the most common method. Although these methods have their own advantages, they cannot evaluate the elimination and retention of nanomaterials in the thoracic cavity, the exudation of inflammatory cells, and the production of cytokines. Therefore, an effective method for evaluating toxicity in the chest and pleura is urgently needed.
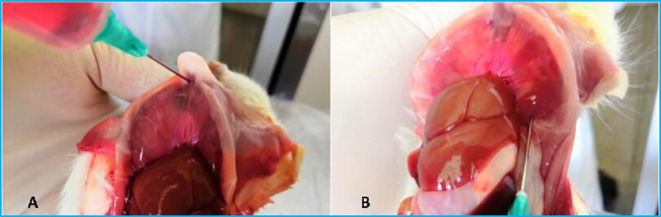
Previously we developed a new method using the PCL and collection of cells and materials from it. Collection of the PLC from rats is shown in Fig. 3. Histological examination of cell pellets obtained from the PLC allows the observation of the infiltration of inflammatory cells and MWCNT fiber retention in the pleural cavity5, 13. Examination of the PCL and the PCL cell pellet is a simple and effective method for evaluating pleural toxicity. This method has the following advantages: 1) it allows observation of the degree of infiltration of various inflammatory cells into the pleural cavity; 2) it is also possible to observe the nanomaterials in the cell pellets under polarized light microscope and electron microscope, which reflects the removal and retention of nanomaterials in the pleural cavity; 3) it allows the analysis of the types and levels of cytokines in the PCL supernatant by methods such as ELISA; 4) combined with pathological examination, it can effectively evaluate the presence and retention of nanomaterials in the pleural cavity and the relationship between inflammatory responses and pleural lesions. This method is economical and effective in the assessment of pleural toxicity.
Fig. 3.
Pleural cavity lavage in rats: A, injection of 10 ml RPMI 1640 medium into the pleural cavity at the diaphragm near the sternum; B, collection the injected medium from the left bottom of the diaphragm. Approximately 9 ml or more of the medium can be collected (not published data).
Conclusion
Studies in vitro and in animal models suggest that MWCNTs, like asbestos fibers, have the potential to induce pleural lesions, such as chronic inflammation, fibrosis, and malignant mesothelioma. Production and application of MWCNTs and other fibrous materials should be strictly controlled to prevent another asbestos disaster.
Disclosure of Potential Conflicts of Interest
The authors have no conflict of interest.
Acknowledgments
The work is supported by Health and Labor Sciences Research Grants of Japan (Research on Risk of Chemical Substance 21340601,grant numbers H19-kagaku-ippan-006, H22-kagaku-ippan-005, H25-kagaku-ippan-004, H24-kagaku-sitei-009),Foundation of Education Bureau of Anhui Province China (KJ2016SD29), and Foundation of Sciences and Technologies Bureau of Anhui Province China (201904a07020064).
References
- 1.Pondman KM, Salvador-Morales C, Paudyal B, Sim RB, and Kishore U. Interactions of the innate immune system with carbon nanotubes. Nanoscale Horiz. 2: 174–186. 2017. [DOI] [PubMed] [Google Scholar]
- 2.Eatemadi A, Daraee H, Karimkhanloo H, Kouhi M, Zarghami N, Akbarzadeh A, Abasi M, Hanifehpour Y, and Joo SW. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett. 9: 393 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shvedova AA, Kisin ER, Porter D, Schulte P, Kagan VE, Fadeel B, and Castranova V. Mechanisms of pulmonary toxicity and medical applications of carbon nanotubes: Two faces of Janus? Pharmacol Ther. 121: 192–204. 2009. [DOI] [PubMed] [Google Scholar]
- 4.Song Y, Li X, Wang L, Rojanasakul Y, Castranova V, Li H, and Ma J. Nanomaterials in humans: identification, characteristics, and potential damage. Toxicol Pathol. 39: 841–849. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Futakuchi M, Shimizu H, Alexander DB, Yanagihara K, Fukamachi K, Suzui M, Kanno J, Hirose A, Ogata A, Sakamoto Y, Nakae D, Omori T, and Tsuda H. Multi-walled carbon nanotubes translocate into the pleural cavity and induce visceral mesothelial proliferation in rats. Cancer Sci. 103: 2045–2050. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donaldson K, Poland CA, Murphy FA, MacFarlane M, Chernova T, and Schinwald A. Pulmonary toxicity of carbon nanotubes and asbestos - similarities and differences. Adv Drug Deliv Rev. 65: 2078–2086. 2013. [DOI] [PubMed] [Google Scholar]
- 7.Stanton MF, Layard M, Tegeris A, Miller E, May M, Morgan E, and Smith A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 67: 965–975. 1981. [PubMed] [Google Scholar]
- 8.Donaldson K, Murphy FA, Duffin R, and Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 7: 5 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czarny B, Georgin D, Berthon F, Plastow G, Pinault M, Patriarche G, Thuleau A, L’Hermite MM, Taran F, and Dive V. Carbon nanotube translocation to distant organs after pulmonary exposure: insights from in situ (14)C-radiolabeling and tissue radioimaging. ACS Nano. 8: 5715–5724. 2014. [DOI] [PubMed] [Google Scholar]
- 10.Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, McKinney W, Castranova V, and Porter DW. Extrapulmonary transport of MWCNT following inhalation exposure. Part Fibre Toxicol. 10: 38 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, Arras M, Fonseca A, Nagy JB, and Lison D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 207: 221–231. 2005. [DOI] [PubMed] [Google Scholar]
- 12.Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T, Matsumoto M, and Fukushima S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part Fibre Toxicol. 13: 53 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Alexander DB, Futakuchi M, Numano T, Fukamachi K, Suzui M, Omori T, Kanno J, Hirose A, and Tsuda H. Size- and shape-dependent pleural translocation, deposition, fibrogenesis, and mesothelial proliferation by multiwalled carbon nanotubes. Cancer Sci. 105: 763–769. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Schwegler-Berry D, Castranova V, and Porter DW. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol. 7: 28 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao D, Wang Q, He J, Alexander DB, Abdelgied M, El-Gazzar AM, Futakuchi M, Suzui M, Kanno J, Hirose A, Xu J, and Tsuda H. Persistent pleural lesions and inflammation by pulmonary exposure of multiwalled carbon nanotubes. Chem Res Toxicol. 31: 1025–1031. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, and Bonner JC. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 4: 747–751. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, Kitajima S, and Kanno J. Induction of mesothelioma in p53+/- mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 33: 105–116. 2008. [DOI] [PubMed] [Google Scholar]
- 18.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, and Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 3: 423–428. 2008. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto Y, Nakae D, Fukumori N, Tayama K, Maekawa A, Imai K, Hirose A, Nishimura T, Ohashi N, and Ogata A. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci. 34: 65–76. 2009. [DOI] [PubMed] [Google Scholar]
- 20.Takagi A, Hirose A, Futakuchi M, Tsuda H, and Kanno J. Dose-dependent mesothelioma induction by intraperitoneal administration of multi-wall carbon nanotubes in p53 heterozygous mice. Cancer Sci. 103: 1440–1444. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rittinghausen S, Hackbarth A, Creutzenberg O, Ernst H, Heinrich U, Leonhardt A, and Schaudien D. The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Part Fibre Toxicol. 11: 59 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzui M, Futakuchi M, Fukamachi K, Numano T, Abdelgied M, Takahashi S, Ohnishi M, Omori T, Tsuruoka S, Hirose A, Kanno J, Sakamoto Y, Alexander DB, Alexander WT, Jiegou X, and Tsuda H. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Sci. 107: 924–935. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, Yamashita K, Yoshikawa Y, Yasui H, Jiang L, Ohara H, Takahashi T, Ichihara G, Kostarelos K, Miyata Y, Shinohara H, and Toyokuni S. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci USA. 108: E1330–E1338. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita K, Yoshioka Y, Higashisaka K, Morishita Y, Yoshida T, Fujimura M, Kayamuro H, Nabeshi H, Yamashita T, Nagano K, Abe Y, Kamada H, Kawai Y, Mayumi T, Yoshikawa T, Itoh N, Tsunoda S, and Tsutsumi Y. Carbon nanotubes elicit DNA damage and inflammatory response relative to their size and shape. Inflammation. 33: 276–280. 2010. [DOI] [PubMed] [Google Scholar]
- 25.Visalli G, Bertuccio MP, Iannazzo D, Piperno A, Pistone A, and Di Pietro A. Toxicological assessment of multi-walled carbon nanotubes on A549 human lung epithelial cells. Toxicol In Vitro. 29: 352–362. 2015. [DOI] [PubMed] [Google Scholar]
- 26.Tabet L, Bussy C, Amara N, Setyan A, Grodet A, Rossi MJ, Pairon J-C, Boczkowski J, and Lanone S. Adverse effects of industrial multiwalled carbon nanotubes on human pulmonary cells. J Toxicol Environ Health A. 72: 60–73. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang W, Wang Q, Qu X, Wang L, Wei X, Zhu D, and Yang K. Effects of charge and surface defects of multi-walled carbon nanotubes on the disruption of model cell membranes. Sci Total Environ. 574: 771–780. 2017. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee N, Yang J, Kim S, Joo SW, and Choi J. Diameter size and aspect ratio as critical determinants of uptake, stress response, global metabolomics and epigenetic alterations in multi-wall carbon nanotubes. Carbon. 108: 529–540. 2016. [Google Scholar]
- 29.Vlaanderen J, Pronk A, Rothman N, Hildesheim A, Silverman D, Hosgood HD, Spaan S, Kuijpers E, Godderis L, Hoet P, Lan Q, and Vermeulen R. A cross-sectional study of changes in markers of immunological effects and lung health due to exposure to multi-walled carbon nanotubes. Nanotoxicology. 11: 395–404. 2017. [DOI] [PubMed] [Google Scholar]
- 30.Kruk J, Aboul-Enein HY, Kładna A, and Bowser JE. Oxidative stress in biological systems and its relation with pathophysiological functions: the effect of physical activity on cellular redox homeostasis. Free Radic Res. 53: 497–521. 2019. [DOI] [PubMed] [Google Scholar]
- 31.Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, and Capobianco JA. Upconverting nanoparticles: assessing the toxicity. Chem Soc Rev. 44: 1561–1584. 2015. [DOI] [PubMed] [Google Scholar]
- 32.Kermanizadeh A, Vranic S, Boland S, Moreau K, Baeza-Squiban A, Gaiser BK, Andrzejczuk LA, and Stone V. An in vitro assessment of panel of engineered nanomaterials using a human renal cell line: cytotoxicity, pro-inflammatory response, oxidative stress and genotoxicity. BMC Nephrol. 14: 96 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He X, Young S-H, Schwegler-Berry D, Chisholm WP, Fernback JE, and Ma Q. Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation. Chem Res Toxicol. 24: 2237–2248. 2011. [DOI] [PubMed] [Google Scholar]
- 34.Jacobsen NR, Møller P, Clausen PA, Saber AT, Micheletti C, Jensen KA, Wallin H, and Vogel U. Biodistribution of carbon nanotubes in animal models. Basic Clin Pharmacol Toxicol. 121(Suppl 3): 30–43. 2017. [DOI] [PubMed] [Google Scholar]
- 35.Muller J, Decordier I, Hoet PH, Lombaert N, Thomassen L, Huaux F, Lison D, and Kirsch-Volders M. Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells. Carcinogenesis. 29: 427–433. 2008. [DOI] [PubMed] [Google Scholar]
- 36.Patlolla AK, Hussain SM, Schlager JJ, Patlolla S, and Tchounwou PB. Comparative study of the clastogenicity of functionalized and nonfunctionalized multiwalled carbon nanotubes in bone marrow cells of Swiss-Webster mice. Environ Toxicol. 25: 608–621. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton RF, Jr , Wu Z, Mitra S, Shaw PK, and Holian A. Effect of MWCNT size, carboxylation, and purification on in vitro and in vivo toxicity, inflammation and lung pathology. Part Fibre Toxicol. 10: 57 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allegri M, Perivoliotis DK, Bianchi MG, Chiu M, Pagliaro A, Koklioti MA, Trompeta AA, Bergamaschi E, Bussolati O, and Charitidis CA. Toxicity determinants of multi-walled carbon nanotubes: The relationship between functionalization and agglomeration. Toxicol Rep. 3: 230–243. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palomäki J, Välimäki E, Sund J, Vippola M, Clausen PA, Jensen KA, Savolainen K, Matikainen S, and Alenius H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 5: 6861–6870. 2011. [DOI] [PubMed] [Google Scholar]
- 40.Duke KS, and Bonner JC. Mechanisms of carbon nanotube-induced pulmonary fibrosis: a physicochemical characteristic perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 10: e1498 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercer RR, Scabilloni JF, Hubbs AF, Battelli LA, McKinney W, Friend S, Wolfarth MG, Andrew M, Castranova V, and Porter DW. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 10: 33 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albini A, Pagani A, Pulze L, Bruno A, Principi E, Congiu T, Gini E, Grimaldi A, Bassani B, De Flora S, de Eguileor M, and Noonan DM. Environmental impact of multi-wall carbon nanotubes in a novel model of exposure: systemic distribution, macrophage accumulation, and amyloid deposition. Int J Nanomedicine. 10: 6133–6145. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rydman EM, Ilves M, Koivisto AJ, Kinaret PAS, Fortino V, Savinko TS, Lehto MT, Pulkkinen V, Vippola M, Hämeri KJ, Matikainen S, Wolff H, Savolainen KM, Greco D, and Alenius H. Inhalation of rod-like carbon nanotubes causes unconventional allergic airway inflammation. Part Fibre Toxicol. 11: 48 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morimoto Y, Hirohashi M, Ogami A, Oyabu T, Myojo T, Todoroki M, Yamamoto M, Hashiba M, Mizuguchi Y, Lee BW, Kuroda E, Shimada M, Wang W-N, Yamamoto K, Fujita K, Endoh S, Uchida K, Kobayashi N, Mizuno K, Inada M, Tao H, Nakazato T, Nakanishi J, and Tanaka I. Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology. 6: 587–599. 2012. [DOI] [PubMed] [Google Scholar]
- 45.Kim JE, Lim HT, Minai-Tehrani A, Kwon JT, Shin JY, Woo CG, Choi M, Baek J, Jeong DH, Ha YC, Chae CH, Song KS, Ahn KH, Lee JH, Sung HJ, Yu IJ, Beck GR, Jr , and Cho MH. Toxicity and clearance of intratracheally administered multiwalled carbon nanotubes from murine lung. J Toxicol Environ Health A. 73: 1530–1543. 2010. [DOI] [PubMed] [Google Scholar]
- 46.Poulsen SS, Saber AT, Williams A, Andersen O, Købler C, Atluri R, Pozzebon ME, Mucelli SP, Simion M, Rickerby D, Mortensen A, Jackson P, Kyjovska ZO, Mølhave K, Jacobsen NR, Jensen KA, Yauk CL, Wallin H, Halappanavar S, and Vogel U. MWCNTs of different physicochemical properties cause similar inflammatory responses, but differences in transcriptional and histological markers of fibrosis in mouse lungs. Toxicol Appl Pharmacol. 284: 16–32. 2015. [DOI] [PubMed] [Google Scholar]
- 47.Yuan X, Zhang X, Sun L, Wei Y, and Wei X. Cellular toxicity and immunological effects of carbon-based nanomaterials. Part Fibre Toxicol. 16: 18 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]