Abstract
Tumor research has largely relied on xenograft models created by the engraftment of cultured cell lines derived from tumor tissues into immunodeficient mice for in vivo studies. Like in vitro models, such models retain the ability of tumor cells to continuously proliferate, so they have been used to predict the clinical relevance of studies on proliferating cells. However, these models are composed of a limited population of tumor cells, which include only those tumor cells that are able to adapt to culture conditions, and thus they do not reflect the diversity and heterogeneity of tumors. This, at least in part, explains the poor predictivity of non-clinical data in the research and development of molecularly targeted drugs. Recently, research focus has been directed towards patient-derived xenograft (PDX) models created by directly engrafting tumor tissues, which have not been cultured in vitro, into immunodeficient mice. PDX models reflect the diversity and heterogeneity of tumors, and the evidence they provide can be verified in the patient tissues from which they were derived originally. PDX models are anticipated to efficiently bridge non-clinical and clinical data in translational research. Based on the evidence obtained from our research experience, this review describes the characteristics of PDX models for acting as tumor models, and elucidates the points to consider when attempting to establish these models.
Keywords: patient derived xenograft (PDX) model, engrafting tumor tissue, immunodeficient mouse, NOG mouse, EBV-related lymphoproliferative disorder
Tumor research has largely relied on xenograft models created by the engraftment of cultured cell lines derived from tumor tissues into immunodeficient mice for in vivo studies. Like in vitro models, such models retain the ability of tumor cells to continuously proliferate, so they have been used to predict the clinical relevance of studies on proliferating cells or of agents that exert anti-tumor effects by damaging and killing tumor cells, including DNA-damaging agents and agents that target driver mutations. But these models are composed of a limited population of tumor cells, only those able to adapt to culture conditions, and so do not reflect the diversity and heterogeneity of tumors. This, at least in part, explains the low predictivity of non-clinical data in the research and development of molecularly targeted drugs1, 2, 3.
Recently, as a possible solution for this, there has been a focus on patient derived xenograft (PDX) models created by directly engrafting tumor tissues which were not cultured in vitro into immunodeficient mice4, 5. PDX models reflect the diversity and heterogeneity of tumors6, 7, 8, and the evidence they provide can be verified in the patient tissues from which they were originally derived. Thus, the models are anticipated to efficiently bridge non-clinical and clinical data in translational research.
The development of immunodeficient mice has been a major contributor to the use of PDX models in medical research. The history of immunodeficient mice dates back to the 1960s when the athymic nude mouse was first discovered. In the following decades, efforts were made to improve the efficiency of establishing xenograft models. After the discovery and development of scid and NOD-scid mice, the NOD.Cg-Prkdcscid Il2rgtm1Sug/Jic mice (NOG mice) and the NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice (NSG mice) were developed as attempts to further improve the efficiency of xenotransplantation9-12. NOG and NSG mice lack mature T, B, and NK cells, and have multiple defects in dendritic cell and macrophage function. They are currently considered to be the animals best suited for engraftment of human tissues4, 11.
Using evidence gathered from our research experience, in this review, we will describe the characteristics of PDX tumor models using NOG mice, and the points to consider when establishing such models.
Characteristics of PDX Models
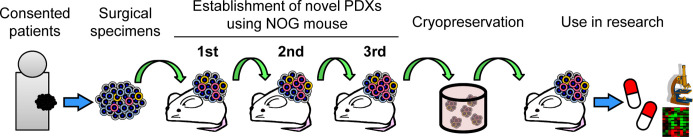
Our group has successfully established PDX models using NOG mice at PharmaLogicals Research Pte. Ltd., which is a joint venture founded by Chugai Pharmaceutical Co., Ltd., Mitsui & Co., Ltd., and the Central Institute for Experimental Animals. The establishment process of PDX models is outlined in Fig. 1. Surgically resected tumor tissues were engrafted subcutaneously into NOG mice, followed by three generations of serial transplantations and cryopreservation after the third generation. The cryopreserved tissues were thawed and transplanted into NOG mice for use as PDX models in various studies, including drug-response analyses and tumor biology research.
Fig. 1.
Scheme for establishment process of patient-derived xenograft (PDX) models.
By engrafting several types of tumors from a variety of organs, we were able to establish epithelial and non-epithelial PDX models13. These models included epithelial tumors, such as, gastric, colorectal, and mammary cancers, as well as, non-epithelial tumors, such as, rhabdomyosarcoma, gastrointestinal stromal tumor (GIST), and astrocytoma. In addition to tumors from the original site, models were established from lymph nodes and other sites of metastasis. Similarly, successful engraftment of epithelial tumors including lung, mammary, and ovarian cancers, has been reported for the NSG mouse models14, 15.
The established PDX models retained the same tissue structure, cell morphology, and differentiation found in PDX models of the original surgically excised tissues13. For example, in the three cases of colorectal adenocarcinoma with varying differentiation, the engrafted tissues that were serially transplanted for three generations successfully retained the pathological characteristics of the original tissues (Fig. 2). With well differentiated colorectal adenocarcinoma, ductal structures consisting of tall columnar tumor cells with slight atypia and clear polarization were retained. With moderately differentiated colorectal adenocarcinoma, ductal structures or fused cribriform structures consisting of atypical cells were retained. With poorly differentiated colorectal adenocarcinoma, there was no duct formation, and a growth pattern showing sheet or cord-like or single cell invasion of tumor cells into tumor stroma was well preserved.
Fig. 2.
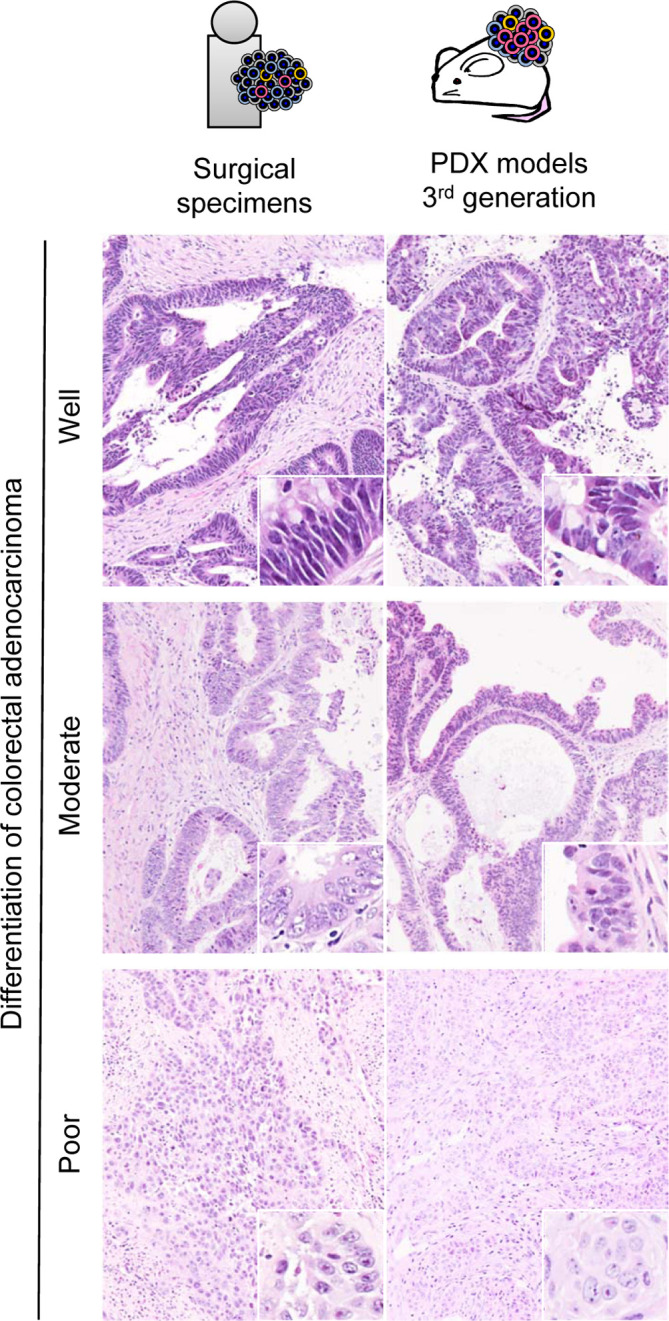
Comparison of surgical specimens and transplanted tissues of colorectal cancer. Ductal structures are maintained with tall columnar cells in the well differentiated case and shorter columnar cells in the moderately differentiated case. Small nests of epithelial cells are formed in the poorly differentiated case.
Besides colorectal cancer, PDX models of other tumor types also successfully retained morphological characteristics. For example, with lung squamous cell carcinoma tissues, there was a tendency for differentiation from the basal side to the center of tumor nests, and a cornified layer or so-called cancer pearls were observed in the center of tumor nests (Fig. 3A). In renal clear cell carcinoma tissues, large clear cells formed sheet-like growths with a fine capillary network (Fig. 3A). With rhabdomyosarcoma, tumor cells with round nuclei with low cell adhesion proliferated in a diffuse manner, and some of these cells were racket-shaped, which is a noted feature of rhabdomyosarcoma (Fig. 3B). Engrafted GIST tissues consisted of tightly packed spindle cells (Fig. 3B).
Fig. 3.
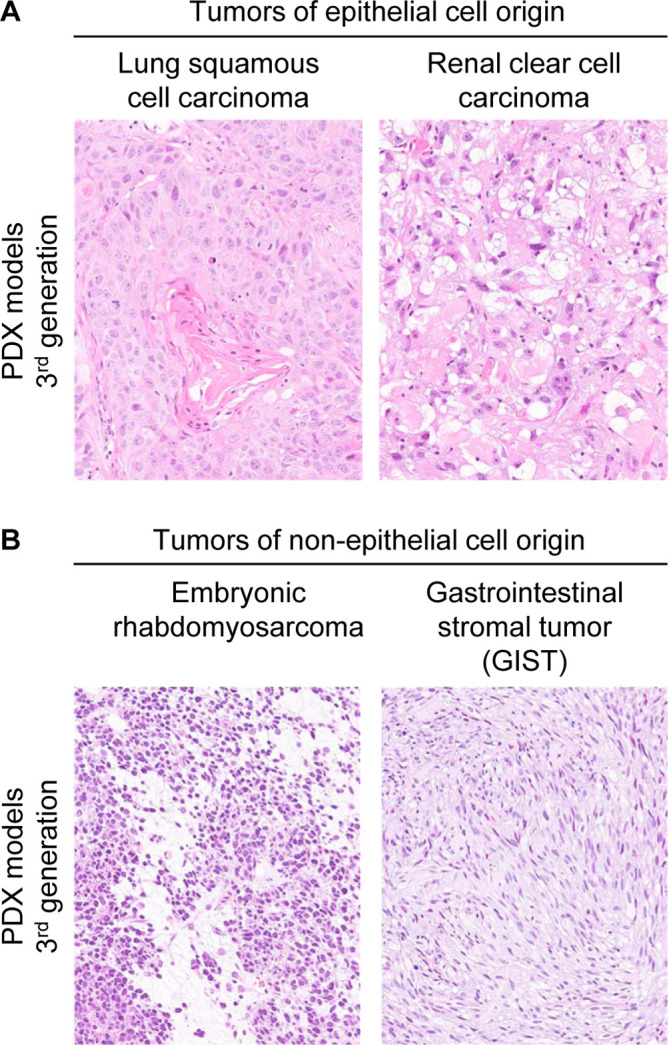
Characteristics of representative epithelial and non-epithelial patient-derived xenograft (PDX) models established with the NOD.Cg-Prkdcscid Il2rgtm1Sug/Jic mouse (NOG mouse). A: Tumors of epithelial cell origin. 3rd generation tissues from a case of lung squamous cell carcinoma with characteristic cancer pearls, and renal clear cell carcinoma with sarcomatous features. B: Tumors of non-epithelial cell origin. 3rd generation tissues from a case of embryonic rhabdomyosarcoma with poorly adhering, round-shaped cells and occasional racquet shaped or myofibroblast-like features, and gastrointestinal stromal tumor (GIST) with an interlacing growth pattern of spindle cells.
In addition to the preservation of tissue structure and cell morphology, PDX models are known to retain the molecular biological and genetic features of the original tumor4, 6, 7, 16, 17, 18, 19, 20.Results of global gene expression analyses show that PDX models are designated to the same clusters as the original tumor, and that these PDX models retain key genetic and pathway activation4, 20. DNA copy numbers, genetic mutations, chromosome abnormalities and gene fusions are also retained in these models19.
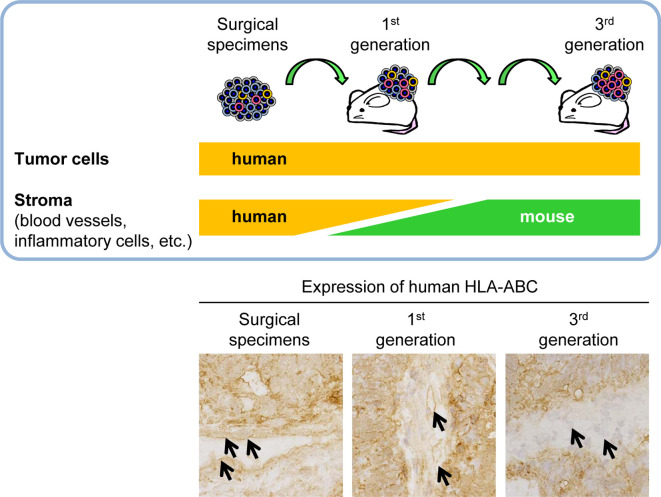
Next, we will take a look at interstitial cells, such as fibroblasts and endothelial cells. In primary engrafted tissues that were surgically removed from patients, cells originating from humans and mice were mixed together in the interstitial tissue13, 21 (Fig. 4). By the third generation, there were no longer any human interstitial cells and the interstitial tissue constituted only of mouse cells13. Along with tumor cells, human cells were also maintained, whereas the interstitial cells were replaced by mouse cells during engraftment and passaging (Fig. 4). This is a defining characteristic of PDX models.
Fig. 4.
Changes in the species origin of stromal cells during the establishment process in patient-derived xenograft (PDX) models. Expression of human HLA-ABC in surgical specimens and engrafted tissues of a case of colorectal cancer. In the primary engraftment tissue, there was a mixture of human and mouse stromal cells in the xenograft tissue. After 3 generations of passage, the stroma consisted of only mouse cells. Arrows show endothelium of the tumor interstitium.
In PDX models, unlike in vitro cell-engrafted models, the tissue structure and cell morphology, along with their molecular biological and genetic features, are well preserved, and the models are anticipated to be applicable to various oncology studies. Multiple myeloma cells, which are difficult to maintain by passage in vivo, can be engrafted into NOG mice, and the NOG mouse models are known to preserve the characteristics of clinical tumors22. Thus, using NOG mice as hosts might enable the establishment of PDX models with tumor types that were difficult to establish in other immunodeficient mice.
Points to Consider in Establishing PDX Models
PDX models are promising for oncology research, but there are also a number of obstacles for their successful establishment. Not all patient tissues can be successfully established as PDX models and PDX models of some tumor types are difficult to establish1, 4. Additionally, establishment of these models requires a lot of resources in terms of cost, time, and labor1, 4. Thus, in order to better utilize PDX models in oncology research, we should be able to establish them more efficiently for a wider variety of tumors.
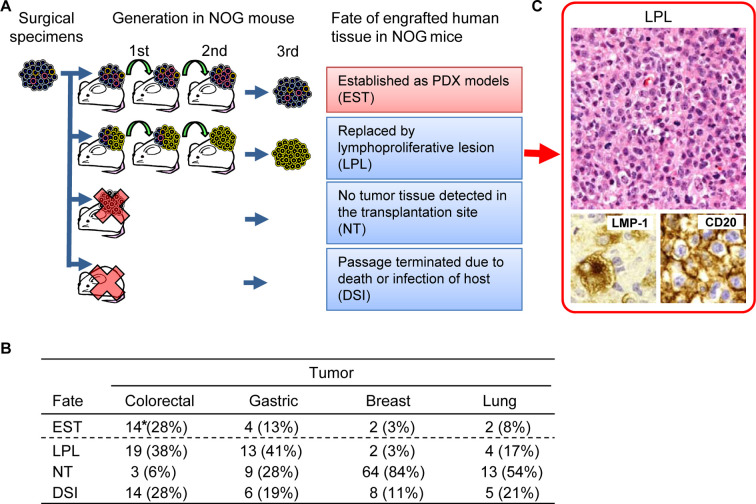
In our previous study, the rate of establishment was 54/436 cases (16.6%), which was much lower than we had expected13. For various tumor types, the rates of establishment were approximately 30% for colorectal cancer, but less than 5% for breast cancer models, and there were no successful cases established for testicular and prostatic tumors. To address this, we analyzed all the causes of failure and categorized them into three types, namely, replacement of engrafted tissues by lymphoproliferative lesions (LPL), no tumor growth (NT), and attrition due to unscheduled death or host infections (DSI) (Fig. 5A). The causes differed with the tumor type, but the major causes for all types were determined to be LPL and NT23 (Fig. 5B). With gastrointestinal tumors, LPL was observed in 40% of the engrafted cases, making it the leading cause of attrition.
Fig. 5.
Fate of engrafted human tissues in NOD.Cg-Prkdcscid Il2rgtm1Sug/Jic mice (NOG mice). A: Analysis of the outcome of engrafted human tumor tissues. The tissues were passaged through 3 generations for establishment. In some cases, the palpable mass formed after engraftment consisted of a lymphoproliferative lesion (LPL) that was thought to replace the original tumor cells. In other cases, establishment was unsuccessful because no palpable mass was formed after engraftment or due to an unscheduled death of the mouse. B: Incidence of the fate of engrafted human tumor tissues. * number of cases. C: Analysis of LPL. LPL was characterized by examining leukocyte marker (CD20) and Epstein-Barr virus (EBV)-related antigen (LMP-1).
In cases of LPL, Epstein-Barr virus (EBV)-infected B cells proliferate, resulting in the replacement of engrafted tissues with proliferating lymphocytes23 (Fig. 5C). This change is thought to arise in EBV-infected B lymphocytes that are engrafted with the tumor tissue into the severely immunodeficient NOG mice, resulting in a state resembling lymphoproliferative disorders in human24, 25, 26, 27. There are similar reports in NOD-scid and NSG mice engrafted with tumor tissues and 16–80% of the cases that were originally judged to be successfully established were in fact replaced by LPLs28, 29. LPL occurs often when tumor tissues are engrafted into immunodeficient mice, and hence it should be taken into consideration when attempting to establish a PDX model.
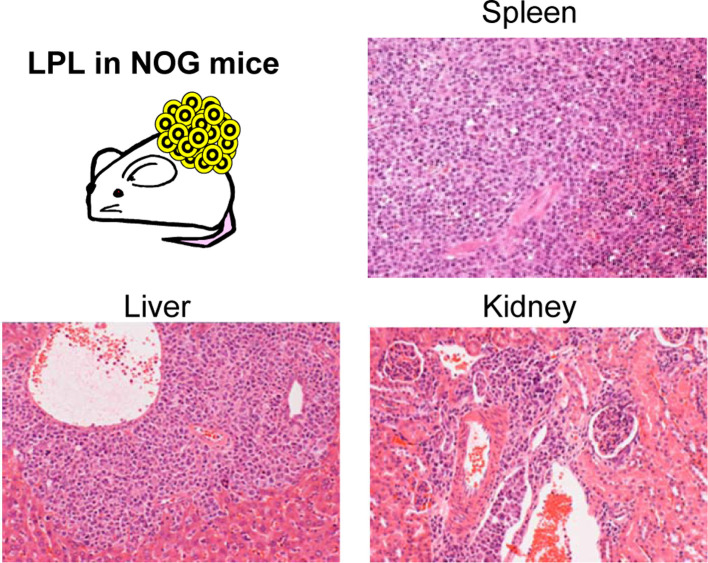
The infection rate of EBV in human is high—about 90% of the world population is infected with EBV30—so it is not feasible to eliminate EBV-infected cases for engraftment. Thus, we might improve the efficiency of establishing these models through early detection and attrition of affected cases during the establishment process. The most sensitive method for detecting LPL is to perform a thorough histopatholgical examination. However, this takes time, making it difficult to make a timely decision for all cases. Therefore, we recommend a scheme for deciding at necropsy. LPL is distributed in various organs of NOG mice (Fig. 6), and is frequently accompanied by splenomegaly (Fig. 7A). Based on this, we deemed that early detection and attrition could be achieved by monitoring gross findings at the time of passage24 (Fig, 7B). Additionally, we have experimentally explored the possibility of prevention by applying an anti-CD20 antibody, rituximab, to eliminate EBV-infected lymphocytes23.
Fig. 6.
Distribution of lymphoproliferative lesions (LPL) in NOD.Cg-Prkdcscid Il2rgtm1Sug/Jic mice (NOG mice). LPL is observed in the spleen, liver, and kidney.
Fig. 7.
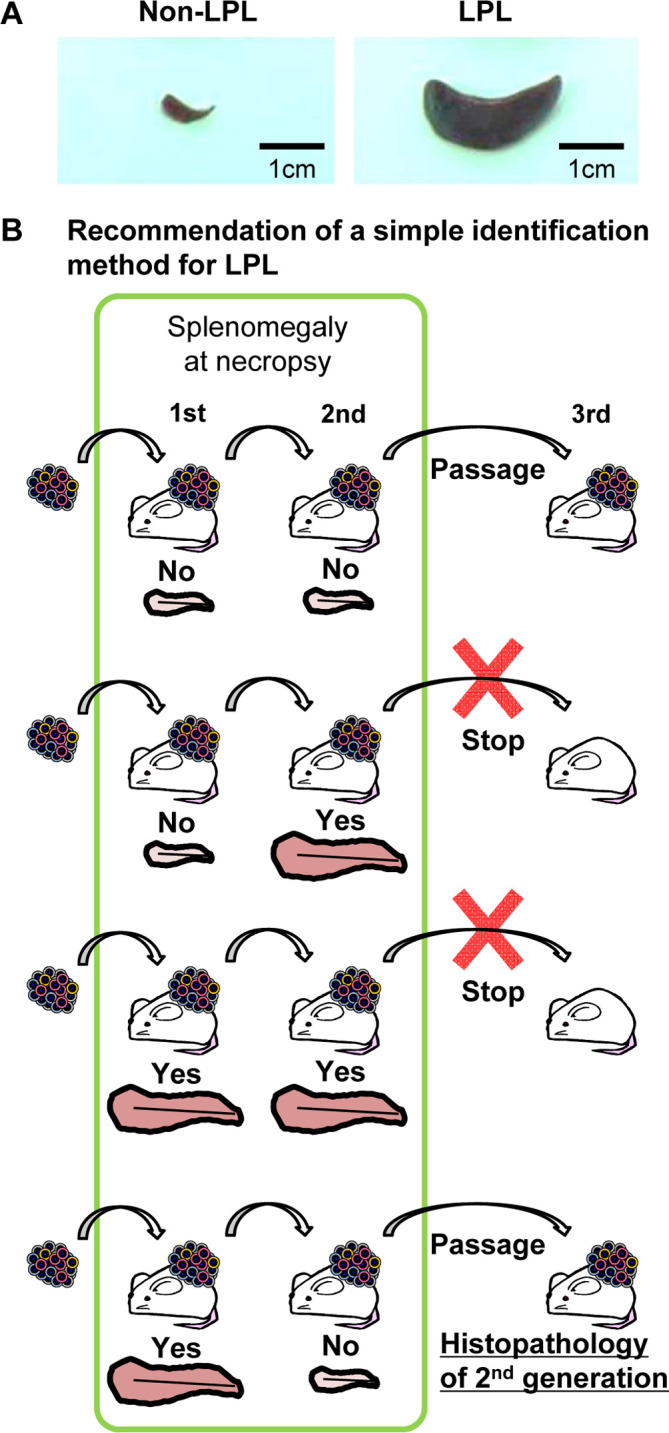
Proposal of a method for early detection and early termination of lymphoproliferative lesions (LPL) cases at necropsy. A: Splenomegaly is observed in LPL-NOD.Cg-Prkdcscid Il2rgtm1Sug/Jic mice (NOG mice). B: The following decisions can be made at necropsy by gross examination of the mouse spleen: If there is no splenomegaly in the 1st and 2nd generations, passage should proceed, but if there is splenomegaly in the 2nd generation regardless of the results of the 1st generation, further passage should be terminated. If there is splenomegaly in the 1st generation but not in the 2nd generation, the tissue should be passaged, and the 2nd generation spleen should be examined histopathologically.
With regards to the other major leading cause, NT, we analyzed various cases of colorectal cancer. We found that tumor-infiltrating lymphocytes (TIL) from the primary generation (first generation) mice reflect the immune contexture of excised patient tissue, and can suppress the progression and growth of tumor cells31, 32, 33, 34. Thus, methods to manipulate the engrafted TIL, such as, elimination of lymphocytes and engrafting only tumor cells, or suppressing TIL by administering immunosuppressive agents to NOG mice might be effective in increasing the efficiency of engraftment.
With tumors that are affected by sex hormones, such as, breast or prostate cancer, the engraftment rate was notably low. There are reports of successful establishment of a breast cancer PDX panel that reflects the heterogeneity of the disease, by implantation into the mammary fat pad and subcutaneous administration of estrogen pellets 4, 20. With prostate cancer, administration of testosterone is reported to improve the efficiency of engraftment. Hence, for hormone-dependent tumors, treatment with hormones is thought to be effective for improving the rate of engraftment. We have only studied the establishment process by sub-cutaneous engraftment, but orthotopic or sub-capsular engraftment into the renal capsule has been shown to be more efficient compared to sub-cutaneous engraftment, so considering the site of engraftment might also improve the rates of model establishment.
Future Perspectives of PDX Models
PDX models reflect the heterogeneity and diversity of clinical tumors, so they are utilized for studies in drug response or drug resistance mechanisms5, 11, 35. Especially for non-clinical research of molecular targeted drugs, the genetic characteristics of PDX models are verified, and PDX models with several specific types of genetic characteristics are selected to analyze drug responses20. Additionally, the models have been proven to be applicable for studying predictive biomarkers for drug responses and drug resistance. Because of these features, there is now a concept called co-clinical trials, which are studies conducted alongside clinical trials, and in such studies, PDX models are utilized to obtain data to support clinical trials5, 20. Furthermore, our research group has previously used PDX models for cancer stem cell research and we found that cancer stem cells transition between a proliferative and non-proliferative state according to the presence of drugs or other environmental factors36. In that study, we revealed a possibility that transition between states is one of the major features of cancer stem cells that contributes to resistance against cytotoxic agents and recurrence36.
Thus, PDX models can be used to obtain novel evidence that could not be gained with in vitro cultured tumor cell models. While the types of tumors that can be established as PDX models are currently limited, the accumulation of further evidence towards stable and efficient establishment of PDX models might lead to expanding their use in oncology research.
Furthermore, there is active research being undertaken concerning regulation of tumor immunity, such as with immune check point molecules. For these studies, models are constructed to reconstitute the human immune system through the engraftment of hematopoietic stem cells into severely immunodeficient animals, including NOG mice37, 38.The selection of appropriate hosts that mimic the human immune system, in combination with PDX models that reflect the heterogeneity and diversity of clinical tumors, might broaden the scope of novel research fields, such as tumor immunity.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Williams SA, Anderson WC, Santaguida MT, and Dylla SJ. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21st century. Lab Invest. 93: 970–982. 2013. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri BA, Camp F, and Miknyoczki S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol. 87: 150–161. 2014. [DOI] [PubMed] [Google Scholar]
- 3.Wilding JL, and Bodmer WF. Cancer cell lines for drug discovery and development. Cancer Res. 74: 2377–2384. 2014. [DOI] [PubMed] [Google Scholar]
- 4.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, Arcaroli JJ, Messersmith WA, and Eckhardt SG. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 9: 338–350. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, Clarke RB, Clevers H, Coukos G, Dangles-Marie V, Eckhardt SG, Gonzalez-Suarez E, Hermans E, Hidalgo M, Jarzabek MA, de Jong S, Jonkers J, Kemper K, Lanfrancone L, Mælandsmo GM, Marangoni E, Marine JC, Medico E, Norum JH, Palmer HG, Peeper DS, Pelicci PG, Piris-Gimenez A, Roman-Roman S, Rueda OM, Seoane J, Serra V, Soucek L, Vanhecke D, Villanueva A, Vinolo E, Bertotti A, and Trusolino L. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 17: 254–268. 2017. [DOI] [PubMed] [Google Scholar]
- 6.Dong X, Guan J, English JC, Flint J, Yee J, Evans K, Murray N, Macaulay C, Ng RT, Gout PW, Lam WL, Laskin J, Ling V, Lam S, and Wang Y. Patient-derived first generation xenografts of non-small cell lung cancers: promising tools for predicting drug responses for personalized chemotherapy. Clin Cancer Res. 16: 1442–1451. 2010. [DOI] [PubMed] [Google Scholar]
- 7.Burgenske DM, Monsma DJ, Dylewski D, Scott SB, Sayfie AD, Kim DG, Luchtefeld M, Martin KR, Stephenson P, Hostetter G, Dujovny N, and MacKeigan JP. Establishment of genetically diverse patient-derived xenografts of colorectal cancer. Am J Cancer Res. 4: 824–837. 2014. [PMC free article] [PubMed] [Google Scholar]
- 8.Malaney P, Nicosia SV, and Davé V. One mouse, one patient paradigm: New avatars of personalized cancer therapy. Cancer Lett. 344: 1–12. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, and Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 100: 3175–3182. 2002. [DOI] [PubMed] [Google Scholar]
- 10.Ito M, Kobayashi K, and Nakahata T. NOD/Shi-scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models. Curr Top Microbiol Immunol. 324: 53–76. 2008. [DOI] [PubMed] [Google Scholar]
- 11.Shultz LD, Ishikawa F, and Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 7: 118–130. 2007. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Facciponte J, Jin M, Shen Q, and Lin Q. Humanized NOD-SCID IL2rg–/– mice as a preclinical model for cancer research and its potential use for individualized cancer therapies. Cancer Lett. 344: 13–19. 2014. [DOI] [PubMed] [Google Scholar]
- 13.Fujii E, Suzuki M, Matsubara K, Watanabe M, Chen YJ, Adachi K, Ohnishi Y, Tanigawa M, Tsuchiya M, and Tamaoki N. Establishment and characterization of in vivo human tumor models in the NOD/SCID/gamma(c)(null) mouse. Pathol Int. 58: 559–567. 2008. [DOI] [PubMed] [Google Scholar]
- 14.Bankert RB, Balu-Iyer SV, Odunsi K, Shultz LD, Kelleher RJ, Jr , Barnas JL, Simpson-Abelson M, Parsons R, and Yokota SJ. Humanized mouse model of ovarian cancer recapitulates patient solid tumor progression, ascites formation, and metastasis. PLoS One. 6: e24420 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Claerhout S, Prat A, Dobrolecki LE, Petrovic I, Lai Q, Landis MD, Wiechmann L, Schiff R, Giuliano M, Wong H, Fuqua SW, Contreras A, Gutierrez C, Huang J, Mao S, Pavlick AC, Froehlich AM, Wu MF, Tsimelzon A, Hilsenbeck SG, Chen ES, Zuloaga P, Shaw CA, Rimawi MF, Perou CM, Mills GB, Chang JC, and Lewis MT. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 73: 4885–4897. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, Neumayer L, Randall RL, Stijleman IJ, Welm BE, and Welm AL. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 17: 1514–1520. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monsma DJ, Monks NR, Cherba DM, Dylewski D, Eugster E, Jahn H, Srikanth S, Scott SB, Richardson PJ, Everts RE, Ishkin A, Nikolsky Y, Resau JH, Sigler R, Nickoloff BJ, and Webb CP. Genomic characterization of explant tumorgraft models derived from fresh patient tumor tissue. J Transl Med. 10: 125 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin D, Wyatt AW, Xue H, Wang Y, Dong X, Haegert A, Wu R, Brahmbhatt S, Mo F, Jong L, Bell RH, Anderson S, Hurtado-Coll A, Fazli L, Sharma M, Beltran H, Rubin M, Cox M, Gout PW, Morris J, Goldenberg L, Volik SV, Gleave ME, Collins CC, and Wang Y. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 74: 1272–1283. 2014. [DOI] [PubMed] [Google Scholar]
- 19.Sia D, Moeini A, Labgaa I, and Villanueva A. The future of patient-derived tumor xenografts in cancer treatment. Pharmacogenomics. 16: 1671–1683. 2015. [DOI] [PubMed] [Google Scholar]
- 20.Cho SY, Kang W, Han JY, Min S, Kang J, Lee A, Kwon JY, Lee C, and Park H. An integrative approach to precision cancer medicine using patient-derived xenografts. Mol Cells. 39: 77–86. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii E, Kato A, Chen YJ, Matsubara K, Ohnishi Y, and Suzuki M. Histopathological characteristics of human non-tumor thyroid tissues in a long-term model of adenomatous goiter xenografts in the NOD/Shi-scid, IL-2Rγ(null) mouse. Exp Toxicol Pathol. 66: 203–209. 2014. [DOI] [PubMed] [Google Scholar]
- 22.Ninomiya M, Abe A, Yokozawa T, Ozeki K, Yamamoto K, Ito M, Ito M, Kiyoi H, Emi N, and Naoe T. Establishment of a myeloid leukemia cell line, TRL-01, with MLL-ENL fusion gene. Cancer Genet Cytogenet. 169: 1–11. 2006. [DOI] [PubMed] [Google Scholar]
- 23.Fujii E, Kato A, Chen YJ, Matsubara K, Ohnishi Y, and Suzuki M. Characterization of EBV-related lymphoproliferative lesions arising in donor lymphocytes of transplanted human tumor tissues in the NOG mouse. Exp Anim. 63: 289–296. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe T, Tada M, Shinohara N, Okada F, Itoh T, Hamada J, Harabayashi T, Chen Q, Moriuchi T, and Nonomura K. Establishment and characterization of human urothelial cancer xenografts in severe combined immunodeficient mice. Int J Urol. 13: 47–57. 2006. [DOI] [PubMed] [Google Scholar]
- 25.Tse E, and Kwong YL. Epstein Barr virus-associated lymphoproliferative diseases: the virus as a therapeutic target. Exp Mol Med. 47: e136 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heslop HE. How I treat EBV lymphoproliferation. Blood. 114: 4002–4008. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morscio J, Dierickx D, and Tousseyn T. Molecular pathogenesis of B-cell posttransplant lymphoproliferative disorder: what do we know so far? Clin Dev Immunol. 2013: 150835 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Ahmed S, Adeyi O, Dick JE, and Ghanekar A. Human solid tumor xenografts in immunodeficient mice are vulnerable to lymphomagenesis associated with Epstein-Barr virus. PLoS One. 7: e39294 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.John T, Yanagawa N, Kohler D, Craddock KJ, Bandarchi-Chamkhaleh B, Pintilie M, Sykes J, To C, Li M, Panchal D, Chen W, Shepherd FA, and Tsao MS. Characterization of lymphomas developing in immunodeficient mice implanted with primary human non-small cell lung cancer. J Thorac Oncol. 7: 1101–1108. 2012. [DOI] [PubMed] [Google Scholar]
- 30.Niller HH, Wolf H, and Minarovits J. Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity. 41: 298–328. 2008. [DOI] [PubMed] [Google Scholar]
- 31.Camus M, Tosolini M, Mlecnik B, Pagès F, Kirilovsky A, Berger A, Costes A, Bindea G, Charoentong P, Bruneval P, Trajanoski Z, Fridman WH, and Galon J. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 69: 2685–2693. 2009. [DOI] [PubMed] [Google Scholar]
- 32.Fridman WH, Dieu-Nosjean MC, Pagès F, Cremer I, Damotte D, Sautès-Fridman C, and Galon J. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron. 6: 117–122. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fridman WH, Pagès F, Sautès-Fridman C, and Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 12: 298–306. 2012. [DOI] [PubMed] [Google Scholar]
- 34.Fujii E, Kato A, Chen YJ, Matsubara K, Ohnishi Y, and Suzuki M. The status of donor cancer tissues affects the fate of patient-derived colorectal cancer xenografts in NOG mice. Exp Anim. 64: 181–190. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosfjord E, Lucas J, Li G, and Gerber HP. Advances in patient-derived tumor xenografts: from target identification to predicting clinical response rates in oncology. Biochem Pharmacol. 91: 135–143. 2014. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi S, Yamada-Okabe H, Suzuki M, Natori O, Kato A, Matsubara K, Jau Chen Y, Yamazaki M, Funahashi S, Yoshida K, Hashimoto E, Watanabe Y, Mutoh H, Ashihara M, Kato C, Watanabe T, Yoshikubo T, Tamaoki N, Ochiya T, Kuroda M, Levine AJ, and Yamazaki T. LGR5-positive colon cancer stem cells interconvert with drug-resistant LGR5-negative cells and are capable of tumor reconstitution. Stem Cells. 30: 2631–2644. 2012. [DOI] [PubMed] [Google Scholar]
- 37.Manz MG, and Di Santo JP. Renaissance for mouse models of human hematopoiesis and immunobiology. Nat Immunol. 10: 1039–1042. 2009. [DOI] [PubMed] [Google Scholar]
- 38.Katano I, Ito R, Kamisako T, Eto T, Ogura T, Kawai K, Suemizu H, Takahashi T, Kawakami Y, and Ito M. NOD-Rag2null IL-2Rγnull mice: an alternative to NOG mice for generation of humanized mice. Exp Anim. 63: 321–330. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]