Abstract
Epithelial ovarian cancer (EOC) is a leading cause of cancer death in women. Standard of care treatment has remained platinum-containing cytotoxic chemotherapy for over three decades. Among the central challenges in treating ovarian CA are disease recurrence and the development of chemoresistance. Survival is uniformly poor for patients with chemoresistant recurrent disease and effective therapeutic options are limited. As such, delineating the mechanisms of chemoresistance and developing targeted therapies to prevent chemoresistance from occurring are of vital importance to improving survival for patients with EOC. Attempts to characterize mechanisms of chemoresistance have implicated numerous cellular pathways, but a rift remains between pre-clinical findings and translation to improving patient survival. More recently, the interplay among different cell types within the tumor microenvironment has become central to understanding how chemoresistance may develop and may be sustained. An improved understanding of how tumor cell-intrinsic and -extrinsic pathways converge during the development of chemoresistance may improve the likelihood of successful clinical translation. This review focuses on the roles of the EOC tumor microenvironment and tumor cell heterogeneity in the development of chemoresistance. We review recent studies into mechanisms of chemoresistance as they relate to tumor microenvironment and development of novel therapeutic approaches that exploit these mechanisms to prevent or reverse chemoresistance. This review attempts to cast these latest discoveries in a clinical context by summarizing trends in ongoing clinical trials for patients with EOC.
Keywords: Ovarian cancer, heterogeneity, microenvironment
Introduction
Epithelial ovarian cancer (EOC) is a leading cause of cancer death in women. The onset of EOC is typically insidious and patients usually present with advanced stage disease, consisting of metastases throughout the abdomen and pelvis (1). The cornerstones of treatment are combinations of surgery and cytotoxic chemotherapy, the order of which depends on disease distribution and patient factors (2-4). Despite ongoing work in refining current treatment strategies and the emergence of some targeted therapies, the standard of care for patients with advanced EOC has remained platinum-containing chemotherapy for over three decades. Among the central challenges in treating EOC are disease recurrence and the development of chemoresistance (5-7). Survival is uniformly poor for patients with recurrent chemoresistant disease and effective therapeutic options are extremely limited. As such, delineating the mechanisms of chemoresistance and developing targeted therapies to prevent chemoresistance from occurring are of vital importance to improving survival for patients with EOC.
There is an increasing appreciation of the role that the tumor microenvironment—consisting of tumor cells, surrounding stromal cells, and stromal elements—plays in promoting and sustaining EOC chemoresistance, recurrence, and metastasis (8-10). Traditionally, the aim of cytotoxic chemotherapy has been to act on EOC tumor cells per se to bring about their demise (11). However, attempts to characterize mechanisms of chemoresistance in EOC have implicated numerous signaling pathways occurring both within tumor cells themselves (tumor cell-intrinsic) as well as signaling between stromal cells and tumor cells (tumor cell-extrinsic) (6,12,13). This shift in focus has revealed new layers of complexity in EOC signaling, which may underlie the frustrating rift that has been encountered between promising pre-clinical findings and their failure to successfully translate into effective clinical therapeutics.
This review focuses on the EOC tumor microenvironment and recent insights into the signaling mechanisms that are believed to underlie tumor metastasis, recurrence, and chemoresistance. We review the various cell types that constitute the tumor microenvironment, emphasizing signaling pathways that have been implicated in tumor metastasis, recurrence, and chemoresistance. We cast these pathways in a clinical context by reviewing studies that have attempted to leverage these pathways as therapeutic targets to prevent or reverse chemoresistance, recurrence, and metastasis.
Tumor heterogeneity in EOC
Ovarian cancer can arise from epithelial, stromal, or germ cell tissues within the ovary. The vast majority of cases of ovarian cancer are EOC, and this sub-type of ovarian cancer is the subject of this review. Historically, EOC has been categorized based on histological appearance: high-grade serous, low-grade serous, endometrioid, clear cell, and other rarer histology. With the publication of The Cancer Genome Atlas (TCGA)—a program sponsored by the National Cancer Institute and the National Human Genome Research Institute that provided sequencing data on 33 different types of human tumors—there has been increasing emphasis on understanding the diverse molecular aberrations that underlie EOC. In 2011, sequencing data from 489 tumors from patients with high-grade serous ovarian (HGSOC) cancer (the most common and aggressive type of EOC) were published as the ovarian TCGA database (14). It provided insight into EOC pathobiology with the following major conclusions: (I) homologous recombination is impaired in approximately 50% of cases of EOC, and (II) there is considerable molecular heterogeneity in EOC, without a clear driver mutation (15). The TCGA analysis of EOC demonstrated significant heterogeneity both among different patient tumors as well as within a single patient’s tumor. This degree of heterogeneity became even further apparent with publication of integrated proteogenomic analyses that show the relationship between genomic aberrations and protein levels (16). There is also data to support genetic evolution of tumors as they metastasize. Using transcriptomic analysis on tumors taken from patients with advanced stage EOC (17), Hoogstraat and colleagues found a remarkable degree of genomic rearrangement among tumors that had metastasized to different regions within the abdominopelvic cavity (e.g., omentum vs peritoneum). Similar findings came from a study by Bashashati and colleagues (18) in which mutational profiling was performed on 31 HGSOC tumors from 6 patients. Similar to Hoogstraat and colleagues, they found genetic diversity in the tumors. Among patients, there was no common pattern of genetic evolution in tumors as they metastasized (18). Given most patients with EOC present in an advanced stage, often with multiple genetically and molecularly distinct metastases throughout various regions of the abdomen and pelvis, it is not surprising that single reagent chemotherapy is not always advantageous. Indeed, particularly in the recurrent setting, patients with metastases may show a clinical or radiographic response in some of their tumors but not in others (19).
The molecular heterogeneity of EOC poses a central challenge to its treatment. EOC displays heterogeneity at numerous levels: there are distinct histological subtypes, there is heterogeneity among the cell populations within a tumor, and there is molecular heterogeneity among different cell populations within the tumor microenvironment. Furthermore, chemotherapeutic treatments and other exposures can drive the evolution of the tumor, inducing further heterogeneity at various levels (7). Without a unique genomic aberration that can be specifically targeted, the classical therapeutic approach to treating EOC has involved non-specific targeting of rapidly dividing cells. Standard of care chemotherapy for front-line treatment of EOC includes combination therapy with platinum and taxane agents. Platinum agents damage DNA via adduct formation eventually leading to double-strand breaks in DNA. Taxane agents stabilize microtubules, preventing cell division. The rationale for using these agents is to target cancer cells that are rapidly dividing and rely on DNA synthesis and cytokinesis more than non-cancer cells (20). While effective in the majority of patients initially, use of these agents eventually leads to the development of chemoresistance, whereby they are no longer effective. This traditional approach to treating EOC can be characterized as tumor cell-intrinsic, in that the focus has been on disrupting processes in the cancer cells themselves to bring about their demise. However, as more data have emerged about the important role of the tumor stroma in influencing tumorigenesis, proliferation, metastasis, and chemoresistance, there is a growing appreciation that these additional cell types are critical targets as well. Thus, expanding the view beyond the cancer cells themselves to include a tumor cell-extrinsic approach to treating EOC, may allow for a therapeutic approach in which cells within the tumor microenvironment are exploited to prevent, or ideally, reverse chemoresistance. In this manner, understanding the molecular mechanisms by which these other cell populations within the EOC tumor microenvironment contribute to chemoresistance will hopefully identify key molecular targets that can be disrupted in a more precise manner to achieve greater efficacy and less toxicity.
Tumor microenvironment & the development of chemoresistance
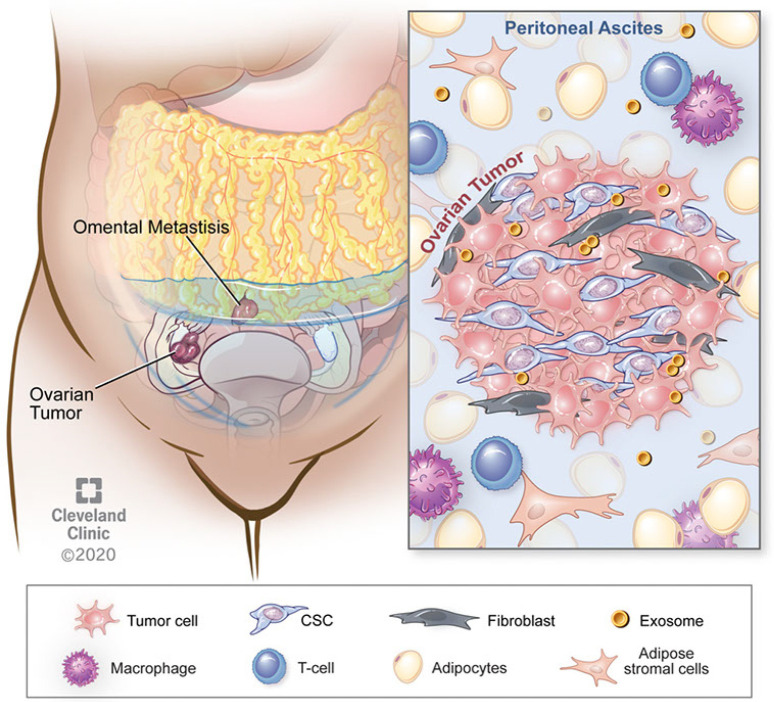
There is a growing body of evidence demonstrating that non-cancer cells that are present in the tumor microenvironment make significant contributions to tumorigenesis, metastasis, and, as we will review, chemoresistance. These effects are achieved through diverse signaling interactions, which may be via direct cell-to-cell contact or release of soluble factors (either on their own or compartmentalized, e.g., in exosomes). In contrast to certain other solid tumors, EOC also frequently has a non-solid component of its tumor microenvironment, namely the ascites fluid that is frequently present in patients with advanced cases of disease. Figure 1 provides an overview of some of these microenvironmental interactions as they pertain to chemoresistance. These tumor microenvironments are dynamic and different cell types exert effects on one another.
Figure 1.
Dynamics of tumor microenvironment interactions in EOC. Schematic demonstrates the key cell types that are within the microenvironment and can participate in EOC growth and responsiveness to chemotherapy. EOC, epithelial ovarian cancer.
Adipocytes
Epidemiological studies have demonstrated an association between obesity and multiple types of cancer. White adipose tissue contains numerous cell types—adipocytes, adipose stromal cells, immune cells—that appear to play complementary roles in promoting cancer proliferation and metastasis. Adipocytes also release adipokines that may participate in oncogenic signaling, angiogenesis, and immunomodulation—all serving to promote cancer cell survival and proliferation (21). In their review, Lengyel and colleagues make the interesting observation that therapy that is tailored to obese patients with cancer may actually be necessary to combat the ability of adipose tissue to promote chemoresistance (8). White adipose tissue is thought to play an important role in EOC in that the omentum—a large abdominal fat pad that drapes over the visceral organs of the abdomen and pelvis—is a frequent site of EOC metastasis. When EOC metastasizes to omentum, it establishes itself within an adipose-rich environment and adipocytes provide cancer cells with metabolic support in the form of fatty acids (22). Ladanyi and colleagues recently showed that CD36, a fatty acid transporter that is expressed on the surface of ovarian cancer cells to promote exogenous fatty acid uptake, is critical to establishing adipocyte-supported fatty acid metabolism in ovarian cancer cells. They co-cultured an ovarian cancer cell line with primary human omentum-derived adipocytes—which by themselves promote cancer cell proliferation—and showed that CD36 inhibition with small molecule inhibitors or siRNA leads to impairment of tumorigenic properties such as cell adhesion, invasion, migration, and proliferation. As altered metabolism appears to be a critical nexus for chemoresistance, targeting the adipose stromal-cancer cell interaction may be a way to prevent or reverse chemoresistance in EOC (23).
White adipose tissue also contains stromal cells, most notably adipose stromal cells (ASC; also referred to as adipose stem cells). Zhang and colleagues analyzed the role of ASCs in EOC cancer cell proliferation using a combination of gene expression, co-culture, and in vivo assays. They demonstrated that ASCs derived from the omental tissue of patients with EOC promoted resistance to paclitaxel and carboplatin when co-cultured with various EOC cell lines. Whether the mechanism by which ASC’s promote EOC chemoresistance is via metabolic alterations, adipokine signaling, and/or other mechanisms was not directly explored in their paper and remains an important area of investigation (24).
Fibroblasts
Cancer-associated fibroblasts (CAFs) have been implicated in tumor progression and immune regulation in pre-clinical animal models of cancer (10,25,26). Wang and colleagues (10) performed an elegant study in which they dissected the cellular and molecular mechanisms underlying the tumor microenvironment’s contribution to platinum-resistance in EOC. Through a series of detailed in vitro and EOC mouse model experiments, they showed that the presence of CAFs provides redox support for EOC cells in the form of glutathione (GSH), which leads to a decrease in the concentration of intra-nuclear cisplatin and a resultant blunting of cisplatin-mediated DNA damage and cytotoxicity to EOC cells (10). Beyond adding to the knowledge of the role of CAFs in chemoresistance, the authors also examined the contribution of effector T cells within the EOC microenvironment. They found that the production of interferon gamma (IFNg) by T cells reverses the CAF-mediated chemoresistance on EOC cells by modulating expression of genes that are involved in GSH metabolism—specifically, the up-regulation of GSH-degrading enzyme, GGT5 and the down-regulation of the GSH transporter component. Lastly, they correlated some of their cellular and molecular findings with human EOC outcomes, showing that higher expression of fibroblast markers in human EOC tumor correlates with poorer overall survival whereas higher expression of CD8+ T cells correlates with improved overall survival. Their findings not only strengthened the growing body of data showing an important role for immune cells of the tumor microenvironment in EOC, it also highlighted the potential utility of targeting the tumor microenvironment therapeutically to reverse or prevent the development of chemoresistance.
CAFs have also been shown to contribute to chemoresistance through interaction with microvascular endothelial cells (MECs) within the EOC tumor microenvironment. It is established that tumors can secrete factors that alter tumor vasculature affect resulting in tumor progression and chemoresistance. Agents that target vascular endothelial growth factor (VEGF) signaling are used in clinical practice for EOC, in both the front-line and recurrent settings; examples include bevacizumab, cediranib, and pazopanib. Leung and colleagues (9) asked how CAFs may be acting on MECs and performed a comparative transcriptomic analysis on MECs that were co-cultured with either human ovarian CAFs or normal ovarian fibroblasts. Among the most common MEC pathway alterations were cell motility, adhesion, and cytoskeletal effects. They found that the CAF-derived factor, MFAP5, increases expression of lipoma-preferred partner (LPP), in MECs, leading to alterations in endothelial cell permeability that result in compromised paclitaxel delivery. By combining LPP inhibition (via siRNA) with paclitaxel administration, they were able to demonstrate reversal of paclitaxel chemoresistance in a mouse model of EOC. They furthermore demonstrated that high expression of LPP in human EOC tumors correlates with poor overall survival, lending further relevance to the interaction of CAFs and MECs in human EOC. This work highlights the potential value in targeting EOC tumor microenvironment stromal cells as an adjunct to improve the efficacy of standard cytotoxic chemotherapy (9).
Cancer stem cells (CSCs)
A sub-population of neoplastic cells that is hypothesized to be a source of cancer recurrence and chemoresistance development based on their defining features of: (I) self-renewal, (II) tumorigenesis, (III) pluripotency (27). Based on single-cell RNA sequencing (scRNA-seq) studies, ovarian CSCs are thought to represent only 1% of cells within EOC tumors (28). Once thought to be a static entity, there are now data to support that CSCs exist in a dynamic state in which “stemness” can be induced by exogenous factors, importantly by the extracellular matrix (ECM) and stromal cells surrounding the CSC, in what is commonly referred to as the CSC “niche” (29). This induction of stemness is an epigenetic event referred to as the epithelial-mesenchymal transition (EMT). EMT, which can occur in both cancer and non-cancer settings (e.g., wound healing), is marked by the following epigenetic changes: (I) morphological changes, (II) decrease in cell-cell junctions, (III) loss of cell polarity, (IV) gain of cell motility, (V) degradation/reorganization of ECM, and (VI) expression of a coordinated program of genes (30). Since these changes are critical to metastasis, EMT is a critical factor in cancer metastasis. Therefore, rather than simply targeting CSCs per se, there is an increasing focus on targeting factors that contribute to the EMT and, in turn, the induction of stem-like phenotype that is associated with tumorigenesis, metastasis, and chemoresistance (30).
Studies have shown that pro-inflammatory cytokines can stimulate the CSC phenotype. For example, Xiang and colleagues (31) showed that IL-17, produced by various immune cells in the tumor microenvironment [CD4+ T cells, CD68+ macrophages, tumor-associated macrophages (TAMs)], acts on its receptor (IL-17R), which is expressed on CD133+ CSCs. This promotes a CSC phenotype, thereby contributing to chemoresistance insofar as CSCs are chemoresistant. Activation of IL-17R leads to tumorigenesis in an NFkB/p38/MAPK-dependent signaling pathway (31). There is evidence that CSCs can also induce an inflammatory cytokine profile in macrophages that creates a feed-forward process of CSC self-renewal (32). Additionally, treatment with standard chemotherapy enriches for CSCs. Studies have shown that exposure of an ovarian cancer cell line to cisplatin induces a CSC phenotype, as assessed using a functional assay in which a reporter gene is driven by the CSC-relevant NANOG promoter (12,33,34).
These findings provide insights on into the role of CSCs in EOC and development of chemotherapeutic resistance. CSCs directed therapies have the potential to overcome some of the limitations of current chemotherapeutic strategies by directly targeting an inherently chemoresistant tumor-associated cell type.
Targeting CSCs therapeutically has been a challenge since they bear many overlapping features with non-cancerous cells, making for a narrow therapeutic window. An additional challenge is that CSCs exist in a dynamic state between differentiated cells and CSCs. The plasticity and heterogeneity of CSCs—i.e., the extent to which they exist in a dynamic state, influenced by the tumor microenvironment—also pose a therapeutic challenge (27). In a recent review, Saygin and colleagues summarized clinical trials (mostly phase I–II studies) that have examined agents that target either CSC antigens (e.g., EpCAM, CD123, CD47) or CSC-relevant signaling pathways (Hedgehog, Notch, Wnt, TGFbeta signaling) in various cancer types. Targeting the stromal cells of the tumor microenvironment and/or the factors that they release to drive EMT may therefore be a more successful strategy, since they are known to influence stemness (27).
MicroRNAs & exosomes
MicroRNAs are non-coding RNA molecules that are about 22 nucleotides in length and regulate diverse cellular functions through post-transcriptional repression of various gene products. Due in part to their small size, they can act on many gene targets simultaneously, and thus post-transcriptionally regulate expression programs in a coordinated manner. They have been implicated in both cancer pathogenesis as well as the development of chemoresistance and have therefore been the focus of therapeutic development (35,36). In ovarian cancer, they have been implicated in chemoresistance insofar as they regulate expression of MDR transport proteins that function in efflux of chemotherapy drugs as well as non-transporter genes (37). In general, the various implicated miRNAs can be classified into those that regulate: ABCB1 (miR-27a, miR-451, let-7g, miR-186), tubulin gene (TUBB3; miR-200), EMT pathways (miR-20a), and ERCC2 (miR-770-5p) (37).
Exosomes are relatively stable structures that are found in various body fluids and contain miRNA, mRNA, and proteins. Exosomes, along with other extracellular vesicles, are detected in ascites both at the time of diagnosis and at recurrence in patients with advanced ovarian cancer. Although their physiology is still being delineated, evidence is accumulating that miRNA are contained in exosomes and trafficked in a paracrine fashion from tumor cells to other cells to effect metastasis, disease progression, and chemoresistance. MicroRNA contained within exosomes are the focus of efforts to use them as both predictive biomarkers (i.e., exo-miRNA signatures to predict response to therapy) and prognostic biomarkers (i.e., exo-miRNA signatures to predict development of chemoresistance) (37). Some studies have shown that these nanovesicles contain enzymes such as matrix metalloproteinases that can alter the extracellular matrix, facilitating metastasis and invasion (38-40). The proteins that are contained within exosomes in patients with ovarian CA can be categorized into: adhesion, angiogenesis, metabolism, and proliferation. Sinha and colleagues (41) performed a proteomic analysis on ascites fluid-derived exosomes and found enrichment in signaling pathways, secreted proteins, and biomarkers, whereas exosomes were deplete of nuclear, cytoplasmic, and intracellular proteins. These observations suggest utility of exosomes in biomarker discovery however, there is also evidence that exosomes act on immune cells within the tumor microenvironment (42).
Various exo-miRNAs have been linked to chemoresistance. Au Yeung and colleagues (43) showed that exo-miR21 derives from tumor stromal cells—CAFs and cancer-associated adipocytes—and delivers miR21 to nearby ovarian cancer cells, where it confers chemoresistance. Using a combination of cellular models (including mouse embryonic fibroblasts derived from knock-out mice that lack miR21 expression) and animal models, they showed that miR21 confers paclitaxel chemoresistance by targeting expression of APAF1, a component of the apoptosome (43). Weiner-Gorzel et al. and colleagues (44) showed that miR-433 confers chemoresistance to paclitaxel by altering metabolism to promote cellular senescence in a CDK6-dependent (44). They also showed that paclitaxel-sensitive cells became paclitaxel-resistant when they were exposed to exosomes containing miR-433, highlighting the ability of exo-miRNAs to communicate with and alter the chemosensitivity of other cells (44).
Feng and colleagues (45) showed exosomes secreted by the primary tumor can act on distant sites to condition the environment to be receptive to metastasis. Preparation of the pre-metastatic niche requires subsequent signaling among a variety of cell types—immune, vascular, epithelial cells—and via a variety of signaling pathways. Cross-talk within this network leads to remodeling of stroma to support metastasis. Part of this process involves creating an immune environment that is welcoming to tumor metastasis, namely an immunosuppressive environment. Exosomes isolated from ascites have been shown to suppress T-cell activation (46) via alteration of inflammatory cytokines among other mechanisms (47). Part of optimizing a pre-metastatic niche also involves rearrangement of the regional vascular supply so that it can support the subsequent tumor growth demand. Exosomes have been implicated in this process of altering angiogenesis by modulating VEGF signaling.
Just as pre-metastatic niche preparation involves altering the function of immune cells within the tumor microenvironment, it is also necessary to change the phenotype of other stromal cells, such as fibroblasts. Exosomes have been shown to convert fibroblasts to CAFs. This leads to signaling via TGF-b, S100A4, SDF-1a, matrix metalloproteinase, and fibronectin—that ultimately promotes extracellular matrix (ECM) remodeling to promote tumor metastasis. Similarly, exosomes can alter the phenotype of macrophages—converting them into tumor-associated macrophages (TAMs)—so that they are more welcoming of metastases by contributing factors that promote an immunosuppressive tumor environment (48). Many of these signaling pathways overlap with those induced by CAFs—TGF-b, S100A4, SDF-1, VEGF, STAT3 (48).
Chemoresistance—a moving target
Chemoresistance refers to a cell’s ability to survive the cytotoxic insults of chemotherapy. In clinical practice in EOC, chemoresistance is typically framed in terms of platinum resistance since platinum is the most effective known agent in EOC. Platinum resistance is defined as disease recurrence within 6 months of completion of front-line treatment (i.e., chemotherapy that is given at the time of initial diagnosis, rather than second-line chemotherapy that is given at the time of diagnosis of recurrence). Recurrent platinum-sensitive recurrent EOC is defined as disease that recurs 6 months or longer after completion of front-line treatment (11). As such, the definitions do not take into account any molecular changes that occur within cancer cells or the tumor microenvironment. As such, the clinical definition of chemoresistance is crude in that it does not take into account any molecular biology of the cancer cells or tumor microenvironment, however it is a useful prognostic definition in the clinical setting. Nonetheless, this bipartite categorization does effectively define prognosis in EOC, with survival directly correlating with time to recurrence (i.e., degree of platinum sensitivity). This is demonstrated in a recent study by Rose and colleagues (49), in which a survival nomogram was developed based on outcomes from multiple modern randomized clinical trials of EOC in which platinum agents were used. Patients with disease recurrence within 6 months had a median overall survival of 9.8 months, whereas patients with recurrence after 24 months had a median survival that ranged from 33 to 45 months (49). Given the difference in prognosis for platinum-resistant vs platinum-sensitive disease, this definition is frequently used to establish eligibility criteria for enrollment in clinical trials. The goal of defining platinum-resistance is to predict whether or not a patient would respond to repeat challenge with a platinum-containing regimen.
Laboratory-based studies define chemoresistance differently. Chemoresistance is defined by the demonstration of a cell’s decreasing susceptibility to the cytotoxic effects of drugs. In transformed cell lines, this is achieved with repeated treatment with a drug and selection for cells that are resistant (50). Therefore, there is an important distinction between chemoresistance as it is defined clinically and scientifically, and these definitions of chemoresistance must be kept in mind when extrapolating findings from the laboratory to the clinic. Although the term platinum-resistance is frequently applied, cells that acquire platinum-resistance are frequently chemoresistant to other agents as well, both in pre-clinical models and in practice (13,50).
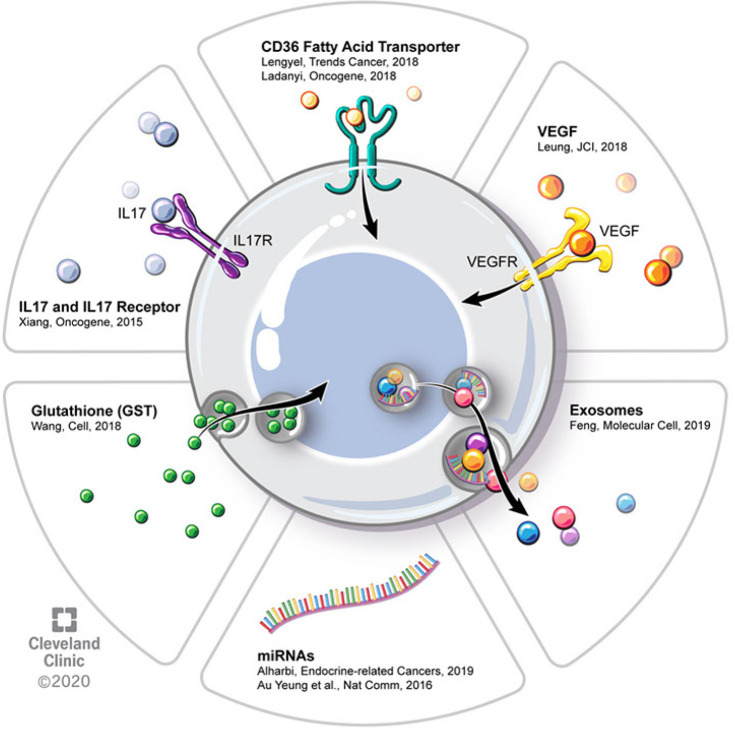
At the cellular level, platinum-resistance has been attributed to various cellular alterations that broadly achieve one or more of the following things: (I) increased efflux of platinum, (II) increased sequestering/conjugation/inactivation of platinum, (III) decreased uptake of platinum, (VI) increased repair of platinum-induced DNA damage, (V) increased anti-apoptotic/decreased pro-apoptotic signaling. Some resistance mechanisms are in fact agent-specific, such as mutations that develop in tubulin after exposure to taxanes that resist taxane binding (5,6). These review articles on chemoresistance frequently categorize mechanisms of chemoresistance according to whether they are innate (to the tumor cell) or acquired (after exposure to a cytotoxic agent). This classification focuses largely on tumor cell-specific intrinsic changes and does not fully capture the roles of cells in the tumor microenvironment that also contribute to chemoresistance. As outlined in this review, pertinent proposed mechanisms of chemoresistance are cited in Figure 2. These mechanisms of platinum resistance can be broadly classified as: (I) platinum buffering, (II) vascular alterations resulting in decreased platinum delivery, and (III) tissue hypoxia/acidification. Strategies to chemosensitize or prevent chemoresistance will involve overcoming both tumor cell-intrinsic and micro environmental (i.e., tumor cell-extrinsic) mechanisms of chemoresistance.
Figure 2.
Mechanisms of EOC chemoresistance. Schematic demonstrates potential interactions between extracellular signaling molecules and EOC cell. Potential signaling molecules in the microenvironment include CD36 fatty acid transporter, VEGF, exosomes, miRNAs, GST, IL17 and IL17 receptors. EOC, epithelial ovarian cancer; VEGF, vascular endothelial growth factor; GST, glutathione S-transferase.
Chemoresistance signatures
To address whether or not there is a common gene target in chemo-resistant tumors, Patch et al. and colleagues (15) investigated whole-genome sequencing and transcriptome analysis of patients with HGSOC. They analyzed 114 tumor samples from 92 patients, that included a mix of primary platinum-refractory (patients whose disease progressed on primary therapy or recurred within one month of completion of primary therapy), platinum-resistant, platinum-sensitive, and acquired platinum-resistant. Among the recurrent cases of platinum resistance, they did not detect any gene targets that were consistently altered. Up-regulation of multi-drug resistant protein 1 (MDR1), a drug efflux transporter that has been linked to chemoresistance—was only found in 8% of their acquired chemoresistance patients. Such a paucity of consistently altered genes makes a single targeted approach unlikely to lead to chemosensitization in the majority of recurrent EOC patients.
If chemoresistance/chemosensitivity can be predicted for each patient, then this would have multiple benefits. The therapeutic index of a drug could be maximized so that patients derive the most benefit relative to the adverse effects incurred. A chemoresistance signature could also be used as a basis for drug development to screen drugs that are more effective for a patient with a chemoresistant tumor. Some have applied transcriptome-based drug prediction models to ovarian cancer. For example, Wang et al. and colleagues (10) applied a drug prediction model to TCGA transcriptomic data. The identified five putative drugs that they predicted would have a more robust effect in chemoresistant tumors. And while a statistically significant difference was found in sensitivity, further validation beyond in vitro experiments is required. There has also been a move toward identifying molecular signatures for EOC that can be used to predict chemosensitivity and therefore prognosis. Tothill et al. (51) have outlined four molecular subtypes of HGSOC: C1/mesenchymal, C2/immune, C4/differentiated, C5/proliferative). Interestingly, these phenotypes are highly dependent on features of the tumor microenvironment, particularly the activity of the immune infiltrate in the tumor.
The difficulty that has been met in attempting to identify a universal signature of chemoresistance in EOC with biomarkers has been complicated by the great degree of heterogeneity in EOC. As mentioned previously, there is heterogeneity among different tumor histologies, among tumors of the same histology from different patients, and even among tumors from different anatomical regions of the same patient. This presents a challenge to finding a unified expression signature that can be utilized either for predicting sensitivity to certain drugs or for discovery of novel drug targets. Multiple studies have implicated multiple individual markers at the protein (52-54), RNA, or DNA (15,54) levels—see the review by Davidson et al. and colleagues (55) for examples of such markers. Very few of these individual markers have been validated in other studies. At present, these signatures may be able to be used prognostically (56,57), but fall short of informing rational chemotherapy selection.
It is also important to keep in mind that the transcriptomic profiling performed in the TCGA project was bulk RNA sequencing rather than single-cell sequencing. Therefore, the role of individual cell types within the tumor cannot be easily discerned. Expression of the same genes in one cell type versus another within the tumor microenvironment may have drastically different effects on tumor growth and patient outcomes. And while there is prognostic value in the TCGA data, the inability to understand transcriptomic derangements on the cell population level within the tumor microenvironment may underlie the current inability to translate this information to find drugs that have enhanced activity in chemoresistant EOC. Single-cell RNA sequencing studies in the EOC literature are limited and do more to define cell populations within the tumor microenvironment than to define a chemoresistance signature.
On the horizon—trends in clinical trials in EOC
The emergence of the tumor microenvironment as a critical component to EOC chemoresistance and recurrence has ushered in a new appreciation for the complexity of the disease and the approach that must be taken to effectively treat it. Trends in recent/current EOC clinical trials include: (I) combining standard front-line chemotherapy with targeted therapies (e.g., anti-angiogenic agents and/or PARP inhibitors), (II) use of maintenance therapy following front-line chemotherapy (e.g., anti-angiogenic agents or PARP inhibitors), and (III) inclusion of immune-targeting agents into multi-agent therapy (e.g., PD-L1 inhibitors). It is hoped that these approaches will prove successful in preventing or reversing chemoresistance and thereby improve patient survival in this devastating disease.
Although there have been some efforts to therapeutically target molecules (such as integrins) that underlie metastasis to certain organs (58). An approach that addresses more than just metastasis is essential for bringing about survival benefit in EOC.
Acknowledgments
The authors would like to acknowledge members of the Reizes laboratory for critical insights during preparation of the review. Dr. Reizes is the Laura J. Fogarty Endowed Chair in Uterine Cancer Research.
Funding: Research in the Reizes laboratory is funded by VeloSano Bike to Cure, Center of Research Excellence in Gynecologic Cancer, and the Department of Defense.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Khalid Sossey-Alaoui) for the series “Cancer Metastasis: Molecular signaling and therapeutic options” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-2019-cm-08). The series “Cancer Metastasis: Molecular signaling and therapeutic options” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Lheureux S, Gourley C, Vergote I, et al. Epithelial ovarian cancer. Lancet 2019;393:1240-53. 10.1016/S0140-6736(18)32552-2 [DOI] [PubMed] [Google Scholar]
- 2.Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi24-32. 10.1093/annonc/mdt333 [DOI] [PubMed] [Google Scholar]
- 3.Morgan RJ, Jr, Armstrong DK, Alvarez RD, et al. Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1134-63. 10.6004/jnccn.2016.0122 [DOI] [PubMed] [Google Scholar]
- 4.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943-53. 10.1056/NEJMoa0908806 [DOI] [PubMed] [Google Scholar]
- 5.Cornelison R, Llaneza DC, Landen CN. Emerging Therapeutics to Overcome Chemoresistance in Epithelial Ovarian Cancer: A Mini-Review. Int J Mol Sci 2017;18:2171. 10.3390/ijms18102171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freimund AE, Beach JA, Christie EL, et al. Mechanisms of Drug Resistance in High-Grade Serous Ovarian Cancer. Hematol Oncol Clin North Am 2018;32:983-96. 10.1016/j.hoc.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Han Y, Kim SI, et al. Tumor evolution and chemoresistance in ovarian cancer. NPJ Precis Oncol 2018;2:20. 10.1038/s41698-018-0063-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lengyel E, Makowski L, DiGiovanni J, et al. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer 2018;4:374-84. 10.1016/j.trecan.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung CS, Yeung TL, Yip KP, et al. Cancer-associated fibroblasts regulate endothelial adhesion protein LPP to promote ovarian cancer chemoresistance. J Clin Invest 2018;128:589-606. 10.1172/JCI95200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Kryczek I, Dostal L, et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 2016;165:1092-105. 10.1016/j.cell.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: A review. Gynecol Oncol 2019;152:416-25. 10.1016/j.ygyno.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 12.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 2008;100:672-9. 10.1093/jnci/djn123 [DOI] [PubMed] [Google Scholar]
- 13.Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 1991;9:389-93. 10.1200/JCO.1991.9.3.389 [DOI] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609-15. 10.1038/nature10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015;521:489-94. 10.1038/nature14410 [DOI] [PubMed] [Google Scholar]
- 16.Zhang AW, McPherson A, Milne K, et al. Interfaces of Malignant and Immunologic Clonal Dynamics in Ovarian Cancer. Cell 2018;173:1755-69.e22. 10.1016/j.cell.2018.03.073 [DOI] [PubMed] [Google Scholar]
- 17.Hoogstraat M, de Pagter MS, Cirkel GA, et al. Genomic and transcriptomic plasticity in treatment-naive ovarian cancer. Genome Res 2014;24:200-11. 10.1101/gr.161026.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashashati A, Ha G, Tone A, et al. Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling. J Pathol 2013;231:21-34. 10.1002/path.4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fields EC, McGuire WP, Lin L, et al. Radiation Treatment in Women with Ovarian Cancer: Past, Present, and Future. Front Oncol 2017;7:177. 10.3389/fonc.2017.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikula-Pietrasik J, Witucka A, Pakula M, et al. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell Mol Life Sci 2019;76:681-97. 10.1007/s00018-018-2954-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Zaman MM, Vlasakov I, et al. Adipocytes promote ovarian cancer chemoresistance. Sci Rep 2019;9:13316. 10.1038/s41598-019-49649-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cozzo AJ, Fuller AM, Makowski L. Contribution of Adipose Tissue to Development of Cancer. Compr Physiol 2017;8:237-82. 10.1002/cphy.c170008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladanyi A, Mukherjee A, Kenny HA, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 2018;37:2285-301. 10.1038/s41388-017-0093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Nowicka A, Solley TN, et al. Stromal Cells Derived from Visceral and Obese Adipose Tissue Promote Growth of Ovarian Cancers. PLoS One 2015;10:e0136361. 10.1371/journal.pone.0136361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719-34. 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010;330:827-30. 10.1126/science.1195300 [DOI] [PubMed] [Google Scholar]
- 27.Saygin C, Matei D, Majeti R, et al. Targeting Cancer Stemness in the Clinic: From Hype to Hope. Cell Stem Cell 2019;24:25-40. 10.1016/j.stem.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 28.Winterhoff B, Thomaier L, Mullany S, et al. Molecular characterization of endometrial cancer and therapeutic implications. Curr Opin Obstet Gynecol 2020;32:76-83. 10.1097/GCO.0000000000000602 [DOI] [PubMed] [Google Scholar]
- 29.Brown Y, Hua S, Tanwar PS. Extracellular matrix-mediated regulation of cancer stem cells and chemoresistance. Int J Biochem Cell Biol 2019;109:90-104. 10.1016/j.biocel.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 30.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 2017;14:611-29. 10.1038/nrclinonc.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang T, Long H, He L, et al. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene 2015;34:165-76. 10.1038/onc.2013.537 [DOI] [PubMed] [Google Scholar]
- 32.Deng X, Zhang P, Liang T, et al. Ovarian cancer stem cells induce the M2 polarization of macrophages through the PPARgamma and NF-kappaB pathways. Int J Mol Med 2015;36:449-54. 10.3892/ijmm.2015.2230 [DOI] [PubMed] [Google Scholar]
- 33.Kolev VN, Tam WF, Wright QG, et al. Inhibition of FAK kinase activity preferentially targets cancer stem cells. Oncotarget 2017;8:51733-47. 10.18632/oncotarget.18517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiechert A, Saygin C, Thiagarajan PS, et al. Cisplatin induces stemness in ovarian cancer. Oncotarget 2016;7:30511-22. 10.18632/oncotarget.8852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren L, Yu Y. The role of miRNAs in the diagnosis, chemoresistance, and prognosis of pancreatic ductal adenocarcinoma. Ther Clin Risk Manag 2018;14:179-87. 10.2147/TCRM.S154226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen SN, Chang R, Lin LT, et al. MicroRNA in Ovarian Cancer: Biology, Pathogenesis, and Therapeutic Opportunities. Int J Environ Res Public Health 2019;16:1510. 10.3390/ijerph16091510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alharbi M, Zuniga F, Elfeky O, et al. The potential role of miRNAs and exosomes in chemotherapy in ovarian cancer. Endocr Relat Cancer 2018;25:R663-85. 10.1530/ERC-18-0019 [DOI] [PubMed] [Google Scholar]
- 38.Kenny HA, Lengyel E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle 2009;8:683-8. 10.4161/cc.8.5.7703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiner AT, Tan S, Agreiter C, et al. EV-Associated MMP9 in High-Grade Serous Ovarian Cancer Is Preferentially Localized to Annexin V-Binding EVs. Dis Markers 2017;2017:9653194. 10.1155/2017/9653194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graves LE, Ariztia EV, Navari JR, et al. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res 2004;64:7045-9. 10.1158/0008-5472.CAN-04-1800 [DOI] [PubMed] [Google Scholar]
- 41.Sinha A, Ignatchenko V, Ignatchenko A, et al. In-depth proteomic analyses of ovarian cancer cell line exosomes reveals differential enrichment of functional categories compared to the NCI 60 proteome. Biochem Biophys Res Commun 2014;445:694-701. 10.1016/j.bbrc.2013.12.070 [DOI] [PubMed] [Google Scholar]
- 42.Filipazzi P, Burdek M, Villa A, et al. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol 2012;22:342-9. 10.1016/j.semcancer.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 43.Au Yeung CL, Co NN, Tsuruga T, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun 2016;7:11150. 10.1038/ncomms11150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner-Gorzel K, Dempsey E, Milewska M, et al. Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells. Cancer Med 2015;4:745-58. 10.1002/cam4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng H, Bao S, Rahman MA, et al. Modeling RNA-Binding Protein Specificity In Vivo by Precisely Registering Protein-RNA Crosslink Sites. Mol Cell 2019;74:1189-204.e6. 10.1016/j.molcel.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest 2016;126:1216-23. 10.1172/JCI81136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bretz NP, Ridinger J, Rupp AK, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem 2013;288:36691-702. 10.1074/jbc.M113.512806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen XW, Yu TJ, Zhang J, et al. CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene 2017;36:5045-57. 10.1038/onc.2017.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose PG, Java JJ, Salani R, et al. Nomogram for Predicting Individual Survival After Recurrence of Advanced-Stage, High-Grade Ovarian Carcinoma. Obstet Gynecol 2019;133:245-54. 10.1097/AOG.0000000000003086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malek A. In vivo and in vitro properties of ovarian cancer cells. Methods Mol Biol 2013;1049:315-21. 10.1007/978-1-62703-547-7_23 [DOI] [PubMed] [Google Scholar]
- 51.Tothill RW, Tinker AV, George J, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res 2008;14:5198-208. 10.1158/1078-0432.CCR-08-0196 [DOI] [PubMed] [Google Scholar]
- 52.Ahmed N, Greening D, Samardzija C, et al. Unique proteome signature of post-chemotherapy ovarian cancer ascites-derived tumor cells. Sci Rep 2016;6:30061. 10.1038/srep30061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonneau C, Rouzier R, Geyl C, et al. Predictive markers of chemoresistance in advanced stages epithelial ovarian carcinoma. Gynecol Oncol 2015;136:112-20. 10.1016/j.ygyno.2014.10.024 [DOI] [PubMed] [Google Scholar]
- 54.Ryner L, Guan Y, Firestein R, et al. Upregulation of Periostin and Reactive Stroma Is Associated with Primary Chemoresistance and Predicts Clinical Outcomes in Epithelial Ovarian Cancer. Clin Cancer Res 2015;21:2941-51. 10.1158/1078-0432.CCR-14-3111 [DOI] [PubMed] [Google Scholar]
- 55.Davidson B. Recently identified drug resistance biomarkers in ovarian cancer. Expert Rev Mol Diagn 2016;16:569-78. 10.1586/14737159.2016.1156532 [DOI] [PubMed] [Google Scholar]
- 56.Verhaak RG, Tamayo P, Yang JY, et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest 2013;123:517-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krzystyniak J, Ceppi L, Dizon DS, et al. Epithelial ovarian cancer: the molecular genetics of epithelial ovarian cancer. Ann Oncol 2016;27 Suppl 1:i4-10. 10.1093/annonc/mdw083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527:329-35. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]