Abstract
Background & Aims
Granulocyte colony-stimulating factor (G-CSF) treatment has been proposed as a therapeutic option for patients with severe alcoholic hepatitis (AH). The aim of this study was to synthesise available evidence on the efficacy of G-CSF in AH.
Methods
This is a meta-analysis of randomised controlled trials evaluating the risk of death at 90 days and the risk of infection.
Results
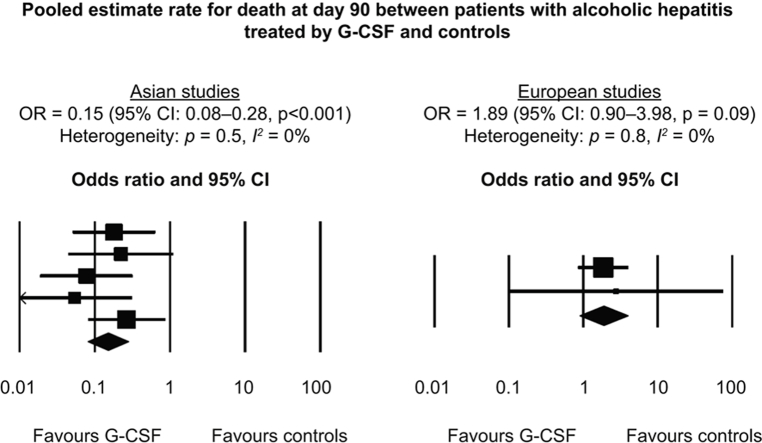
Seven studies were included. Of a total of 396 patients, 336 had AH, 197 patients were treated with G-CSF, and 199 received placebo or pentoxifylline. In overall meta-analysis, G-CSF therapy was associated with a reduced risk of death at 90 days (odds ratio [OR] 0.28; 95% CI 0.09–0.88; p = 0.03). There was high heterogeneity between studies (p <0.001; I2 = 80%). Five studies were performed in Asia and 2 in Europe. In the subgroup analysis of studies performed in Asia, G-CSF was associated with a reduced risk of death (OR 0.15; 95% CI 0.08–0.28; p <0.001; heterogeneity: p = 0.5, I2 = 0%). In European studies, G-CSF tended to increase mortality compared with controls, although the difference was not significant (OR 1.89; 95% CI 0.90–3.98; p = 0.09; heterogeneity: p = 0.8, I2 = 0%). In Asian studies, occurrence of infection was less frequent in G-CSF patients than in controls (OR 0.12; 95% CI 0.06–0.23; p <0.001; heterogeneity: p = 0.7, I2 = 0%), whilst in European studies, this occurrence was not statistically different (OR 0.92; 95% CI 0.50–1.68; p = 0.78; heterogeneity: p = 0.5, I2 = 0%). In sensitivity analyses, excluding studies that included patients with acute-on-chronic liver failure (ACLF) other than AH, patients with less severe AH, or patients with non-response to corticosteroids, results were similar to those of overall analyses, both for mortality and occurrence of infection.
Conclusions
Granulocyte colony-stimulating factor therapy may improve the prognosis of patients with severe AH. However, owing to the high heterogeneity observed in the overall analysis caused by conflicting results between the Asian and European studies, G-CSF cannot currently be recommended for AH, particularly in Europe. Whether these differences can be explained by ethnic differences or disparities in patient selection and disease severity remains unclear.
Lay summary
The main finding of this meta-analysis is that the use of granulocyte colony-stimulating factor (G-CSF) is associated with a mortality reduction of more than 70% at 3 months amongst patients with alcoholic hepatitis (AH) compared with controls who did not receive this therapy. However, owing to the high heterogeneity observed in the overall analysis caused by conflicting results between the Asian and European studies, G-CSF cannot currently be recommended for patients with AH, particularly in Europe. Whether these differences can be explained by ethnic differences or disparities in patient selection and disease severity remains unclear.
Keywords: Alcoholic hepatitis, Infection, Liver regeneration
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ACG, American College of Gastroenterology; AH, alcoholic hepatitis; EASL, European Association for the Study of the Liver; G-CSF, granulocyte colony-stimulating factor; MELD, model for end-stage liver disease; NA, not available; NIAAA, National Institute on Alcohol Abuse and Alcoholism; OR, odds ratio
Graphical abstract
Highlights
-
•
This meta-analysis reports pooled data from 7 studies on the use of G-CSF in patients with alcoholic hepatitis.
-
•
The favourable effect of G-CSF was only encountered in Asian not European studies.
-
•
Additional data are needed to clarify the usefulness of G-CSF in severe alcoholic hepatitis, particularly in Europe.
Introduction
Alcoholic hepatitis (AH) is a severe disease characterised by recent onset of jaundice in individuals with chronic alcohol abuse.1,2 Severe AH is commonly defined by a Maddrey's discriminant function (MDF) of 32 or higher,3,4 and is the form of alcoholic liver disease that carries the poorest prognosis. In this case, mortality rates as high as 50% have been reported at 3 months without treatment.2 Corticosteroids (prednisolone 40 mg/day given orally) are the most widely recommended treatment for severe AH.1,5,6 Although meta-analyses and recent trials have shown that corticosteroids reduced the risk of death at 1 month, the magnitude of this effect is limited. In addition, corticosteroids do not improve survival beyond 1 month.[7], [8], [9] Moreover, the applicability of corticosteroid therapy is restricted by concerns about the risk of sepsis.10
Aside from liver transplantation in highly selected patients, no other therapeutic options are currently available in non-responders to medical therapy.1,5,11 Granulocyte colony-stimulating factor (G-CSF) has been proposed as a treatment in this setting.12,13 G-CSF is a glycoprotein that stimulates the bone marrow to produce and release neutrophils and stem cells (CD34+) into the bloodstream. In animal models, administration of G-CSF has been shown to mobilise the haematopoietic stem cells, induce liver regeneration, and improve survival.14 In humans, recent studies have reported encouraging results concerning the use of G-CSF in patients with acute-on-chronic liver failure (ACLF),[15], [16], [17], [18], [19], [20], [21] with chronic liver disease at different stages of severity[22], [23], [24] and with AH.9,12,17,25,26 However, other studies have failed to demonstrate a survival benefit associated with G-CSF treatment in both advanced liver disease and in AH.13,[27], [28], [29]
Meta-analysis is a quantitative technique that enables pooling data from trials to decrease random errors. It also allows for assessment of a particular factor's magnitude of impact. In this study, we performed a meta-analysis of randomised controlled trials (RCTs) evaluating the usefulness of G-CSF in AH. Our primary objective was to assess the 90-day risk of death in AH patients treated with G-CSF compared with controls not treated with G-CSF. Our secondary objective was to assess the occurrence of infections.
Materials and methods
Literature search
Medline (PubMed), Embase, Cochrane Library, and manual searches were combined and last performed on January 6, 2020. Key search terms were ‘G-CSF’ or ‘GCSF’ or ‘Granulocyte Colony-Stimulating Factor’ and ‘alcoholic hepatitis’ and ‘acute-on-chronic liver failure’. Terms were combined within each database (see supplementary appendix 1). General reviews and references from published trials were also used. Two observers (AM and AKS) also screened all abstracts presented between 2015 and 2019 at the Liver Meeting of the American Association for the Study of Liver Diseases (AASLD) and the International Liver Congress of the European Association for the Study of the Liver (EASL).
Criteria for inclusion and exclusion of studies
All RCTs were included. To reduce the risk of bias, strict inclusion and exclusion criteria were defined before the literature search. To be considered, a study had to:
-
•
include patients with AH defined clinically or proven by liver biopsy1,5,6;
-
•
compare G-CSF to placebo or pentoxifylline (as this therapy has no impact on survival in AH8);
-
•
be prospective and randomised;
-
•
provide data on survival or on the occurrence of infections.
When several publications were found that covered the same study population, only the most recent was taken into account.
Endpoints and criteria for combinability
Endpoints were defined before the beginning of the meta-analysis. The main endpoint was 90-day mortality. The secondary endpoint was the occurrence of infections.
Data extraction
Data extraction was performed independently by 2 investigators (AM and AKS) using standardised data collection forms. Discrepancies in data interpretation were resolved by discussion, re-review of the studies, and consultation with one other author (PD) when necessary.
Quality score
The methodological quality of the studies was assessed using the Jadad score.30,31 Studies were scored according to the presence of 3 key methodological features of clinical trials, including randomisation, masking, accountability of all patients, and withdrawals.
Statistical analysis
We used a random effects model to obtain a summary estimate of 90-day mortality amongst patients treated with G-CSF and controls. The random model was chosen because it takes into account the possibility of heterogeneity between studies.32 Data on all patients were extracted to allow intention-to-treat analyses. The overall treatment effect was expressed as event rates, a measure of how often a particular statistical event occurs within a group included in an experiment, with 95% CI.
As a first step, an overall meta-analysis was performed. This analysis included studies performed in Asia and others performed in Europe. In a second step, subgroup analyses with the Asian studies and the European studies were performed.
Heterogeneity was assessed by Cochran's Q test33 and the I2. More specifically, the I2 statistic was used to estimate inconsistency in meta-analyses, representing the percentage of the between-study variability caused by heterogeneity rather than chance.34 A significant Cochran's Q-statistic (below 0.10) was chosen as a threshold for significant heterogeneity across studies. The following cut-offs were used to quantify heterogeneity with the I2 statistic: 0–25% (low), 25–50% (moderate), and >50% (high) heterogeneity.34 In cases of moderate or high heterogeneity, the methodological section of each study was re-reviewed to determine whether any discrepancy could be identified, and sensitivity analyses, excluding the discrepant study, were performed. To assess the extent of publication bias, the Egger test and the Begg and Mazumdar test were used.33,35 A p value <0.05 was considered statistically significant. All statistical analyses were performed using Comprehensive Meta-Analysis (Biostat, Englewood, NJ, USA).
Results
Study population
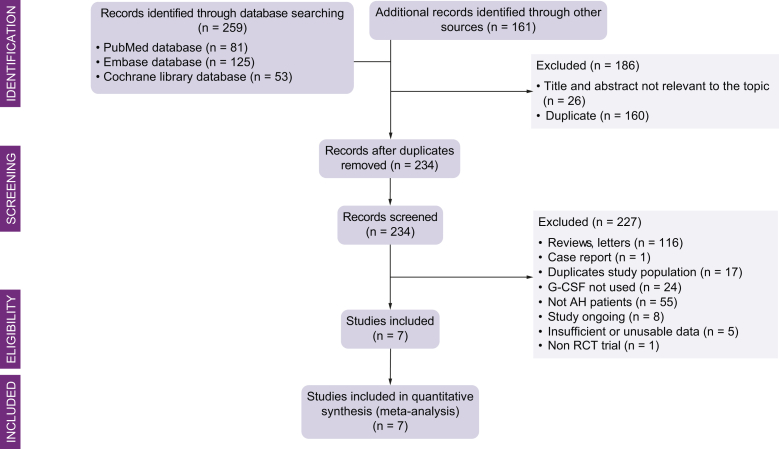
Figure 1 is a flow chart of the selection of studies for inclusion in the meta-analysis. We screened 420 references; 234 were selected for full-text retrieval. Of these, 7 were included in the analysis.12,13,17,25,26,28,36 Two studies also included patients with ACLF other than AH.17,28 Five studies were performed in Asia12,17,25,26,36 and 2 in Europe.13,28
Fig. 1.
Flow chart of the selection of studies for inclusion in the meta-analysis.
AH, alcoholic hepatitis; G-CSF, granulocyte colony-stimulating factor; RCT, randomised controlled trial.
Table 1 is a summary of the main characteristics of the studies included in the meta-analysis. Overall, 336 patients had AH (85%) and 60 patients had ACLF other than AH. A total of 197 patients were treated with G-CSF, and 199 received placebo or pentoxifylline and served as controls. In 6 studies,12,17,25,26,28,36 AH was diagnosed clinically in accordance with the EASL1, AASLD5, or American College of Gastroenterology6 guidelines. In 2 studies, AH was also confirmed by liver biopsy.13,25
Table 1.
Characteristics of the 7 included studies.
| Authors | Location of the study | Aetiology | Group of patients/study design | No. of patients | G-CSF doses | Age (years, range) | Sex ratio (No. of males, %) | MELD score at baseline∗ | Maddrey's discriminant function at baseline∗ |
|---|---|---|---|---|---|---|---|---|---|
| Spahr et al. (2008)13 | Geneva, Switzerland, Europe | AH (alcoholic steatohepatitis) | Patients randomly assigned to receive standard care + G-CSFc (5 patients received steroids) | 13 | 10 μg/kg/day (5 days) | 53 (34–69)∗ | 11 (85%) | 15 (13–22) | 34 (25–60) |
| Patients randomly assigned to receive standard care + placeboc (7 patients received steroids) | 11 | 54 (42–61)∗ | 6 (54%) | 16 (11–20) | 38.7 (21–59) | ||||
| Garg et al. (2012)17 | New Delhi, India, Asia | ACLFad | Patients randomly assigned to receive G-CSF (no patient received steroids)c | 23 | 5 μg/kg/day (5 days) and every 3 days (1 month) | 40 (30–65)∗ | 20 (87%) | 29 (21–40) | NA |
| Patients randomly assigned to receive placebo or pentoxifylline (no patient received steroids)c | 24 | 40 (19–55)∗ | 21 (87%) | 31.5 (20–40) | NA | ||||
| Singh et al. (2014)36 | Chandigarh, India, Asia | Severe AH | Patients randomly assigned to receive G-CSF + pentoxifylline (no patient received steroids) | 23 | 10 μg/kg/day (5 days) | 41.7 ± 7.5∗∗ | 23 (100%) | 27 | 85.5 |
| Patients randomly assigned to receive pentoxifylline (no patient received steroids) | 23 | 44.3 ± 13∗∗ | 23 (100%) | 30 | 79.2 | ||||
| Sharma et al. (2017)26 | Jaipur, India, Asia | Severe AH | Patients randomly assigned to receive G-CSF (no patient received steroids) | 25 | 5 μg/kg/day (5 days) | 49.4 ± 11.5∗∗ | 25 (100%) | 25.40 ± 9.07∗∗ | 84.54 ± 59.2∗∗ |
| Patients randomly assigned to receive placebo (no patient received steroids) | 25 | 48.6 ± 14.4∗∗ | 25 (100%) | 30.25 ± 10.42∗∗ | 124.78 ± 90.7∗∗ | ||||
| Singh et al. (2018)12 | Chandigarh, India, Asia | Severe AH | Patients randomly assigned to receive G-CSF + pentoxifylline (no patient received steroids) | 18 | 10 μg/kg/day (5 days) | 41.6 ± 8.1∗∗ | 18 (100%) | 26 (19–37) | 84 (56–185) |
| Patients randomly assigned to receive pentoxifylline (no patient received steroids) | 20 | 44.7 ± 9.4∗∗ | 20 (100%) | 27.5 (19–41) | 77.4 (37–235) | ||||
| Shasthry et al. (2019)25 | New Delhi, India, Asia | Severe AH with no responsiveness to steroids | Patients randomly assigned to receive G-CSF (all patients were non-responders to steroids) | 14 | 5 μg/kg/day (5 days) and every 3 days (1 month) | 39.6 ± 9.0∗∗ | 27 (96%) | 24.6 ± 3.9∗∗ | 74.8 ± 22.8∗∗ |
| Patients randomly assigned to receive placebo (all patients were non-responders to steroids) | 14 | 40.7 ± 11.7∗∗ | 27.6 ± 4.4∗∗ | 87.5 ± 28.7∗∗ | |||||
| Engelmann et al. (2019)28 | Multicentric, Europe | ACLFbd | Patients randomly assigned to receive G-CSF | 81e | 5 μg/kg/day (5 days) and every 3 days (1 month) | 54.2 ± 10.1∗∗ | 46 (57%) | 24.5 ± 6∗∗ | NA |
| Patients randomly assigned to receive placebo | 82e | 56.9 ± 9.6∗∗ | 56 (68%) | 23.9 ± 5.6∗∗ | NA |
ACLF, acute-on-chronic liver failure; AH, alcoholic hepatitis; EASL-CLIF, European Association for the Study of the Liver-chronic liver failure; G-CSF, granulocyte colony-stimulating factor; NA, not available; RCT, randomised controlled trial.
Expressed as median.
Expressed as mean ± SD.
ACLF was defined according to the Asian Pacific Association for the Study of the Liver criteria, as an acute hepatic insult manifesting as jaundice (serum bilirubin level ≥5 mg/dl) and coagulopathy (international normalised ratio ≥1.5), complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease.
ACLF according to the EASL-CLIF criteria.
65% of the patients had ACLF caused by AH in the G-CSF group and 50% of the patients had ACLF caused by AH in the control group. Patients with reactivation of hepatitis B and AH were treated with tenofovir and pentoxifylline, respectively.
69.2% of the patients had ACLF caused by AH in the G-CSF group and 69.4% of the patients had ACLF caused by AH in the control group.
Eighty-one patients were randomised in the G-CSF group, but 60 patients ended the study, and 82 patients were randomised in the control group, but 52 patients ended the study (interim analysis).
Study quality
The quality of the included studies is detailed in Table S1.
Methodological assessment of studies
Five studies have been published12,13,17,25,36 and 2 were available only in abstract form,26,28 one of which is an interim report of an ongoing trial.28 All trials were prospective and randomised (Table 1). Aside from geographic origin, the methodological analysis of each study identified several potential discrepancies amongst the included studies:
- •
-
•
1 study included patients with less severe AH.13
-
•
1 study included patients with non-response to corticosteroids.25
Hence, 3 sensitivity analyses were performed in case of moderate or high heterogeneity.
Outcomes
Primary outcome: 90-day mortality
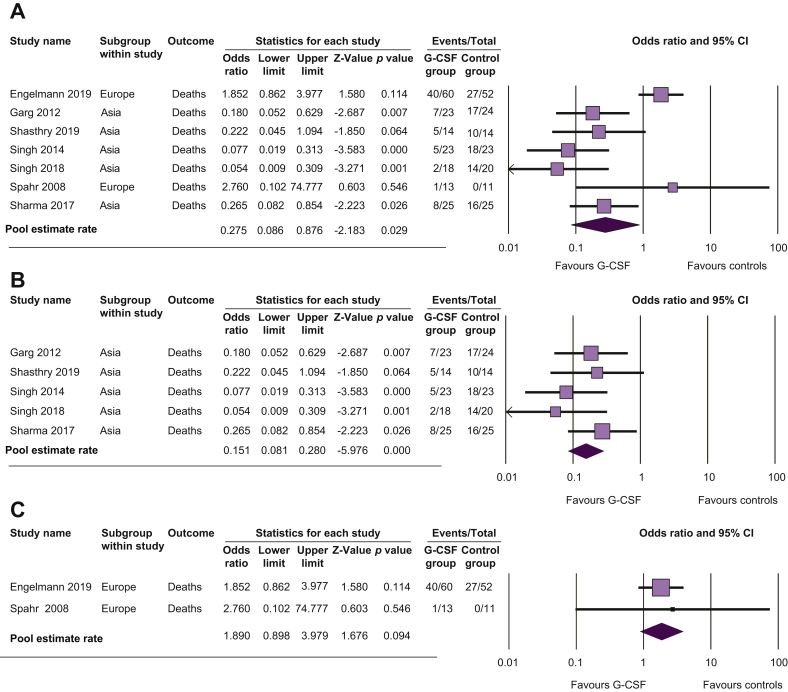
In overall meta-analysis, G-CSF therapy was associated with a reduced risk of death at 90 days compared with controls (odds ratio [OR] 0.28; 95% CI 0.09–0.88; p = 0.03; Fig. 2A and Table 2). There was high heterogeneity between studies (p <0.001; I2 = 80%). In the subgroup analysis of studies performed in Asia, G-CSF was associated with a reduced risk of death compared with controls (OR 0.15; 95% CI 0.08–0.28; p <0.001; Fig. 2B) with no heterogeneity between the studies (p = 0.5; I2 = 0%). In European studies, G-CSF tended to increase mortality compared with controls, although the difference was not significant (OR 1.89; 95% CI 0.90–3.98; p = 0.09; Fig. 2C), with no heterogeneity between studies (p = 0.8; I2 = 0%).
Fig. 2.
Pooled estimate rate for death at Day 90 between patients with alcoholic hepatitis.
Treated by (A) G-CSF and controls, (B) G-CSF and controls in Asian studies, and (C) G-CSF and controls in European studies. G-CSF, granulocyte colony-stimulating factor.
Table 2.
Outcomes in the 7 included studies.
| Authors | Group of patients/study design | 90-day mortality | Number of infections |
|---|---|---|---|
| Spahr et al. (2008)13 | Patients randomly assigned to receive standard care + G-CSF | 1/13 (7.7) | 3/13 (23.1) |
| Patients randomly assigned to receive standard care + placebo | 0/11 (0) | 4/11 (36.4) | |
| Garg et al. (2012)17 | Patients randomly assigned to receive G-CSF | 7/23 (30.4)a | 3/23 (13.0) |
| Patients randomly assigned to receive placebo | 17/24 (70.8)a | 10/24 (41.6) | |
| Singh et al. (2014)36 | Patients randomly assigned to receive G-CSF + pentoxifylline | 5/23 (21.7) | 5/23 (21.7) |
| Patients randomly assigned to receive pentoxifylline | 18/23 (78.3) | 18/23 (78.3) | |
| Sharma et al. (2017)26 | Patients randomly assigned to receive G-CSF | 8/25 (32.0) | 6/25 (24.0) |
| Patients randomly assigned to receive placebo | 16/25 (64.0) | 17/25 (68) | |
| Singh et al. (2018)12 | Patients randomly assigned to receive G-CSF + pentoxifylline | 2/18 (11.1) | 2/18 (11.1) |
| Patients randomly assigned to receive pentoxifylline | 14/20 (70.0) | 14/20 (70.0) | |
| Shasthry et al. (2019)25 | Patients randomly assigned to receive G-CSF | 5/14 (35.7) | 4/14 (28.6) |
| Patients randomly assigned to receive placebo | 10/14 (71.4) | 10/14 (71.4) | |
| Engelmann et al. (2019)28 | Patients randomly assigned to receive G-CSF | 40/60 (66.7)b | 32/74 (43.2) |
| Patients randomly assigned to receive placebo | 27/52 (51.9)b | 34/78 (43.6) |
Data are presented as n/N (%).
G-CSF, granulocyte colony-stimulating factor.
Data for mortality at 60 days (data at 90 days not available).
Data for death or transplantation.
No publication bias was detected by the Egger test (p = 0.2) or by the Begg and Mazumdar test (p = 0.7).
Results of sensitivity analyses, excluding studies that included patients with ACLF other than AH,17,28 patients with less severe AH,13 or patients with non-response to corticosteroids,25 are reported in the supplementary material. Results of sensitivity analyses were similar to those of overall analyses.
Secondary outcome: occurrence of infections
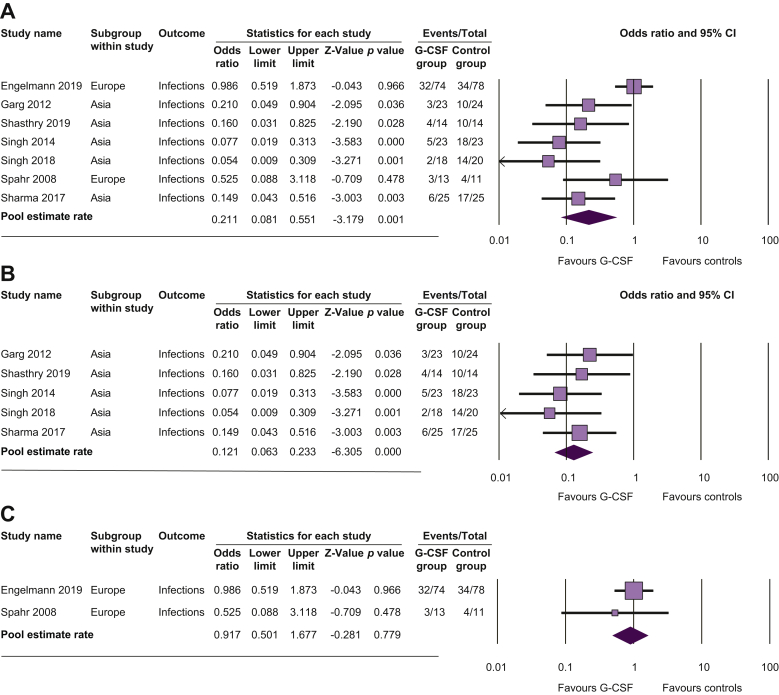
In overall meta-analysis, G-CSF therapy was associated with a reduced risk of developing infections compared with controls (OR 0.21; 95% CI 0.08–0.55; p = 0.001; Fig. 3A and Table 2). There was high heterogeneity between studies (p = 0.01; I2 = 73%). In Asian studies, occurrence of infection was less frequent in G-CSF patients than in controls (OR 0.12; 95% CI 0.06–0.23; p <0.001; Fig. 3B) with no heterogeneity between studies (p = 0.7; I2 = 0%), whilst in European studies, this occurrence was not statistically different (OR 0.92; 95% CI 0.50–1.68; p = 0.78; Fig. 3C) with no heterogeneity between studies (p = 0.5; I2 = 0%).
Fig. 3.
Pooled estimate rate for infection between patients with alcoholic hepatitis.
Treated by (A) G-CSF and controls, (B) G-CSF and controls in Asian studies, and (C) G-CSF and controls in European studies. G-CSF, granulocyte colony-stimulating factor.
No publication bias was detected by the Begg and Mazumdar test (p = 0.9), but a publication bias was detected by the Egger test (p = 0.02).
Results of sensitivity analyses, excluding studies that included patients with ACLF other than AH,17,28 patients with less severe AH,13 or patients with non-response to corticosteroids,25 are reported in the supplementary material. Results of sensitivity analyses were similar to those of overall analyses.
Discussion
In its severe form, AH is characterised by mortality rates as high as 50% at 3 months without treatment. A number of reports have provided encouraging results for patients treated with G-CSF. These individual publications address only a restricted number of patients. As enough data have accumulated in the literature for an initial meta-analytic assessment of the effects of G-CSF in AH, a meta-analysis was required to synthesise available data on the efficacy of G-CSF in this setting. The main finding of this meta-analysis is that the use of G-CSF is associated with a mortality reduction of more than 70% at 3 months amongst patients with AH compared with controls who did not receive this therapy. However, there was high heterogeneity between studies caused by conflicting results between the Asian and European studies. The favourable effect of G-CSF was only encountered in Asian studies and not in European studies. In fact, no significant increase in infection rates was observed in patients treated in Europe compared with a lower risk of infections in G-CSF-treated patients in Asia. Although the difference was not significant, in the European studies, survival of patients treated with G-CSF tended to be worse than that of untreated patients. Hence, the usefulness of G-CSF in AH remains a subject of debate, and currently, G-CSF cannot be recommended in Europe.
The precise mechanism of action by which G-CSF may promote clinical benefit remains unclear. Granulocyte colony-stimulating factor could have dual mechanisms of action. First, it has been shown that G-CSF stimulates liver regeneration. Both anti-inflammatory and liver regeneration mechanisms participate in the repair of liver damage in AH. Although a decrease in proinflammatory cytokines (the main mechanism of action of corticosteroids in AH) is likely to be helpful, hepatocyte regeneration also plays a crucial role in restoring liver function.37 After liver injury, bone-marrow-derived circulating pluripotent cells contribute to hepatocyte regeneration by providing cytokines and growth factors that promote liver repair.38 It has been shown that G-CSF stimulates the bone marrow to release stem cells (CD34+) and their differentiation into mature hepatocytes, as well as the proliferation of hepatocyte progenitor cells in cholangiocytes.13,39 These effects are associated with increased markers of hepatic regeneration and neutrophilia along with increased neutrophil density on liver tissue. This translates into improved liver function, decreased risk of complications of liver disease, reduced risk of infections, and improved survival. The effect of G-CSF on liver regeneration may explain, at least in part, why a survival benefit was observed in Asian studies and not in European studies. Indeed, the Asian population seemed to have more severe disease with MELD scores of 25–31 and/or MDF scores of 32–235,12,17,25,26,36 which makes liver regeneration even more important. By comparison, the Western population had lower MELD scores at 15–24 and/or MDF scores of 21–60.13,28 However, differences in the severity of liver disease reflected by the MELD score do not seem to explain all of the differences in treatment effects observed between Asian and European patients, as the median MELD score of the Engelmann et al. study28 was close to that of the Asian studies.12,17,25,26,36 As a consequence, other parameters that could influence study results may also differ across studies. The second mechanism of action of G-CSF is related to improved antibacterial pathways related to stimulation of neutrophil function and restoration of immune function.38,39 This additional benefit of G-CSF may be particularly important in patients with AH given the high risk of infection. In this meta-analysis, the occurrence of infections differed between Asian and European studies, which made definite conclusions difficult to drawn. Nevertheless, the absence of evidence of increased risk of infection with the use of G-CSF in AH may represent an advantage over corticosteroids, the use of which is often restricted by concerns about the risk of sepsis.
Granulocyte colony-stimulating factor has an excellent safety profile with most studies reporting minor adverse effects, mainly associated with bone pain, rash, and increase in spleen size.9,12,13,17,25 In this meta-analysis, no major safety issues and no deaths were attributable to treatment. This favourable safety profile is in accordance with that observed in patients with cancer in whom G-CSF has been used for a long time.40 One other consideration, however, is the fact that G-CSF is an off-label drug in patients with severe AH and might be expensive if used in daily clinical practice.
Despite the overall positive effect on short-term survival observed in this meta-analysis, we cannot yet recommend using G-CSF in patients with severe AH. As Engelmann et al.28 reported a trend towards poorer survival amongst G-CSF-treated patients, the benefit of G-CSF in AH is currently unsettled amongst European patients. Therefore, a firm conclusion on the effectiveness of G-CSF in AH cannot be made without additional data, particularly in Europe, where G-CSF therapy is not currently recommended. Hence, further larger, prospective, multicentre, well-designed studies are needed to precisely determine the therapeutic role of G-CSF in patients with AH, particularly in the severe form of this disease, where therapeutic options are urgently needed. Also, trials performed outside India are needed to assess the usefulness of G-CSF in patients with severe AH. This should include determinations regarding schedules of administration, dosing of G-CSF, and the selection of patients. In this context, the results of an ongoing clinical trial from the National Institute on Alcohol Abuse and Alcoholism (ClinicalTrials.gov NCT04072822), evaluating the efficacy of G-CSF in patients with severe AH, as well as the results of the GraCiAH trial evaluating the efficacy of G-CSF in patients with severe alcoholic hepatitis with partial or null respons to steroids (ClinicalTrials.gov NCT02442180)41 would be of interest.
We acknowledge that this meta-analysis has several limitations. First, a liver biopsy was not systematically performed. However, according to current guidelines, diagnosis of AH is probable without a liver biopsy in cases of typical clinical and biological presentation and no other confounding variables.42 Another set of limitations is related to the fact that there is an imbalance between the regions of the included studies, as the majority of them came from India1.2,17,25,26,36 Regarding the European studies, the Swiss study published more than 10 years ago was not designed for assessing clinical outcomes,13 and only an interim analysis was available for the multicentre European study presented in abstract form at the past AASLD meeting.28 However, this study is the largest randomised study on 160 patients with ACLF, the majority of whom had AH as the aetiology. Another study was also only available in abstract form.26 Pooling of abstracts and full papers enables analysis of the most recent ongoing studies, or those that have been terminated but not yet published, as already done in several systematic reviews.[43], [44], [45], [46], [47], [48] This strategy may reduce publication bias that is caused by the probability of less frequently reporting negative studies as full papers. Lastly, owing to a lack of individual data, we were not able to evaluate the pooled evolution of liver function and Child-Pugh score or MELD score during therapy.
In summary, this meta-analysis is the first to report pooled data from 7 studies on the use of G-CSF in patients with AH. Even though a survival benefit was observed in G-CSF-treated patients, definite conclusions regarding the usefulness of G-CSF in AH cannot be made, as high heterogeneity was observed in the overall analysis caused by conflicting results between the Asian and European studies. Whether these differences can be explained by ethnic differences or disparities in patient selection and disease severity remains unclear. Additional data are needed to clarify the usefulness of G-CSF in severe AH, particularly in Europe. In this regard, the results of 2 ongoing clinical trials are awaited. These studies may help to identify patients with severe AH who would benefit from this exciting therapy.
Financial support
The authors did not receive financial support for this study.
Authors' contributions
AM: data acquisition, analysis, and interpretation; drafting and critical revision of the article for important intellectual content. AKS: data acquisition; drafting and critical revision of the article for important intellectual content. CM: critical revision of the article for important intellectual content. PD: study design; data acquisition, analysis, and interpretation; statistical analysis; study supervision; drafting and critical revision of the article for important intellectual content. All authors approved the final version of the article.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors acknowledge the contribution of Sandy Field, Ph.D., for her assistance concerning English-language editing.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100139.
Supplementary data
References
- 1.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69:154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Lucey M.R., Mathurin P., Morgan T.R. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 3.Carithers R.L., Jr., Herlong H.F., Diehl A.M., Shaw E.W., Combes B., Fallon H.J. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989;110:685–690. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 4.Maddrey W.C., Boitnott J.K., Bedine M.S., Weber F.L., Jr., Mezey E., White R.I., Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 5.Crabb D.W., Im G.Y., Szabo G., Mellinger J.L., Lucey M.R. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–333. doi: 10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- 6.Singal A.K., Bataller R., Ahn J., Kamath P.S., Shah V.H. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113:175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathurin P., O'Grady J., Carithers R.L., Phillips M., Louvet A., Mendenhall C.L. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–260. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 8.Thursz M.R., Richardson P., Allison M., Austin A., Bowers M., Day C.P. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–1628. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 9.Singh S., Murad M.H., Chandar A.K., Bongiorno C.M., Singal A.K., Atkinson S.R. Comparative effectiveness of pharmacological interventions for severe alcoholic hepatitis: a systematic review and network meta-analysis. Gastroenterology. 2015;149:958–970.e912. doi: 10.1053/j.gastro.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Vergis N., Atkinson S.R., Knapp S., Maurice J., Allison M., Austin A. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology. 2017;152:1068–1077.e1064. doi: 10.1053/j.gastro.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marot A., Dubois M., Trepo E., Moreno C., Deltenre P. Liver transplantation for alcoholic hepatitis: a systematic review with meta-analysis. PLoS One. 2018;13:e0190823. doi: 10.1371/journal.pone.0190823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh V., Keisham A., Bhalla A., Sharma N., Agarwal R., Sharma R. Efficacy of granulocyte colony-stimulating factor and N-acetylcysteine therapies in patients with severe alcoholic hepatitis. Clin Gastroenterol Hepatol. 2018;16:1650–1656.e2. doi: 10.1016/j.cgh.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Spahr L., Lambert J.F., Rubbia-Brandt L., Chalandon Y., Frossard J.L., Giostra E. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221–229. doi: 10.1002/hep.22317. [DOI] [PubMed] [Google Scholar]
- 14.Mark A.L., Sun Z., Warren D.S., Lonze B.E., Knabel M.K., Melville Williams G.M. Stem cell mobilization is life saving in an animal model of acute liver failure. Ann Surg. 2010;252:591–596. doi: 10.1097/SLA.0b013e3181f4e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavez-Tapia N.C., Mendiola-Pastrana I., Ornelas-Arroyo V.J., Norena-Herrera C., Vidana-Perez D., Delgado-Sanchez G. Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Ann Hepatol. 2015;14:631–641. [PubMed] [Google Scholar]
- 16.Duan X.Z., Liu F.F., Tong J.J., Yang H.Z., Chen J., Liu X.Y. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol. 2013;19:1104–1110. doi: 10.3748/wjg.v19.i7.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg V., Garg H., Khan A., Trehanpati N., Kumar A., Sharma B.C. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505–512.e501. doi: 10.1053/j.gastro.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Saha B.K., Mahtab M.A., Akbar S.M.F., Noor E.A.S.M., Mamun A.A., Hossain S.M.S. Therapeutic implications of granulocyte colony stimulating factor in patients with acute-on-chronic liver failure: increased survival and containment of liver damage. Hepatol Int. 2017;11:540–546. doi: 10.1007/s12072-017-9814-1. [DOI] [PubMed] [Google Scholar]
- 19.Di Campli C., Zocco M.A., Saulnier N., Grieco A., Rapaccini G., Addolorato G. Safety and efficacy profile of G-CSF therapy in patients with acute on chronic liver failure. Dig Liver Dis. 2007;39:1071–1076. doi: 10.1016/j.dld.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Khanam A., Trehanpati N., Garg V., Kumar C., Garg H., Sharma B.C. Altered frequencies of dendritic cells and IFN-gamma-secreting T cells with granulocyte colony-stimulating factor (G-CSF) therapy in acute-on- chronic liver failure. Liver Int. 2014;34:505–513. doi: 10.1111/liv.12415. [DOI] [PubMed] [Google Scholar]
- 21.Yang Q., Yang Y., Shi Y., Lv F., He J., Chen Z. Effects of granulocyte colony-stimulating factor on patients with liver failure: a meta-analysis. J Clin Transl Hepatol. 2016;4:90–96. doi: 10.14218/JCTH.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kedarisetty C.K., Anand L., Bhardwaj A., Bhadoria A.S., Kumar G., Vyas A.K. Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology. 2015;148:1362–1370.e7. doi: 10.1053/j.gastro.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 23.Verma N., Kaur A., Sharma R., Bhalla A., Sharma N., De A. Outcomes after multiple courses of granulocyte colony-stimulating factor and growth hormone in decompensated cirrhosis: a randomized trial. Hepatology. 2018;68:1559–1573. doi: 10.1002/hep.29763. [DOI] [PubMed] [Google Scholar]
- 24.Gaia S., Smedile A., Omede P., Olivero A., Sanavio F., Balzola F. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45:13–19. doi: 10.1016/j.jhep.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Shasthry S.M., Sharma M.K., Shasthry V., Pande A., Sarin S.K. Efficacy of granulocyte colony-stimulating factor in the management of steroid-nonresponsive severe alcoholic hepatitis: a double-blind randomized controlled trial. Hepatology. 2019;70:802–811. doi: 10.1002/hep.30516. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A., Setia A., Rai R.R. Effect of granulocyte colony-stimulating factor (G-CSF) on mortality and complications viz. sepsis, encephalopathy, hepatorenal syndrome, and gastrointestinal bleed in severe alcoholic hepatitis: a randomized controlled study. United Eur Gastroenterol J. 2017;5:A17. [Google Scholar]
- 27.Newsome P.N., Fox R., King A.L., Barton D., Than N.N., Moore J. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:25–36. doi: 10.1016/S2468-1253(17)30326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelmann C.H.A., Bruns T., Schiefke I., Zipprich A., Schiedeknecht A., Zeuzem S. G-CST to treat ACLF (GRAFT TRIAL): interim analysis of the first randomised European multicentral trial. AASLD. 2019 abstract 17. [Google Scholar]
- 29.Philips C.A., Augustine P., Rajesh S., Ahamed R., George T., Padsalgi G. Granulocyte colony-stimulating factor use in decompensated cirrhosis: lack of survival benefit and probable predisposition to hepatocellular carcinoma. J Clin Exp Hepatol. 2020;10:124–134. doi: 10.1016/j.jceh.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 31.Moher D., Jadad A.R., Tugwell P. Assessing the quality of randomized controlled trials. Current issues and future directions. Int J Technol Assess Health Care. 1996;12:195–208. doi: 10.1017/s0266462300009570. [DOI] [PubMed] [Google Scholar]
- 32.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Deeks J.J., Altman D.G., Bradburn M.J. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M., Davey Smith G., Altman D.G., editors. Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Books; London: 2005. [Google Scholar]
- 34.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 36.Singh V., Sharma A.K., Narasimhan R.L., Bhalla A., Sharma N., Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109:1417–1423. doi: 10.1038/ajg.2014.154. [DOI] [PubMed] [Google Scholar]
- 37.Dubuquoy L., Louvet A., Lassailly G., Truant S., Boleslawski E., Artru F. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015;64:1949–1960. doi: 10.1136/gutjnl-2014-308410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustot T. Beneficial role of G-CSF in acute-on-chronic liver failure: effects on liver regeneration, inflammation/immunoparalysis or both? Liver Int. 2014;34:484–486. doi: 10.1111/liv.12356. [DOI] [PubMed] [Google Scholar]
- 39.Moreau R., Rautou P.E. G-CSF therapy for severe alcoholic hepatitis: targeting liver regeneration or neutrophil function? Am J Gastroenterol. 2014;109:1424–1426. doi: 10.1038/ajg.2014.250. [DOI] [PubMed] [Google Scholar]
- 40.Dale D.C., Crawford J., Klippel Z., Reiner M., Osslund T., Fan E. A systematic literature review of the efficacy, effectiveness, and safety of filgrastim. Support Care Cancer. 2018;26:7–20. doi: 10.1007/s00520-017-3854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho Y., Park Y.S., Kim H.Y., Kim W., Lee H.J., Kim D.J. Efficacy of granulocyte colony stimulating factor in patients with severe alcoholic hepatitis with partial or null response to steroid (GRACIAH trial): study protocol for a randomized controlled trial. Trials. 2018;19:696. doi: 10.1186/s13063-018-3092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thursz M., Kamath P.S., Mathurin P., Szabo G., Shah V.H. Alcohol-related liver disease: areas of consensus, unmet needs and opportunities for further study. J Hepatol. 2019;70:521–530. doi: 10.1016/j.jhep.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 43.Deltenre P., Henrion J., Canva V., Dharancy S., Texier F., Louvet A. Evaluation of amantadine in chronic hepatitis C: a meta-analysis. J Hepatol. 2004;41:462–473. doi: 10.1016/j.jhep.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Marot A., Trepo E., Doerig C., Moreno C., Moradpour D., Deltenre P. Systematic review with meta-analysis: self-expanding metal stents in patients with cirrhosis and severe or refractory oesophageal variceal bleeding. Aliment Pharmacol Ther. 2015;42:1250–1260. doi: 10.1111/apt.13424. [DOI] [PubMed] [Google Scholar]
- 45.Mathurin P., Rixe O., Carbonell N., Bernard B., Cluzel P., Bellin M.F. Review article: overview of medical treatments in unresectable hepatocellular carcinoma—an impossible meta-analysis? Aliment Pharmacol Ther. 1998;12:111–126. doi: 10.1046/j.1365-2036.1998.00286.x. [DOI] [PubMed] [Google Scholar]
- 46.Best L.M., Mughal M., Gurusamy K.S. Laparoscopic versus open gastrectomy for gastric cancer. Cochrane Database Syst Rev. 2016;3:CD011389. doi: 10.1002/14651858.CD011389.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grace A.G., Mittal A., Jain S., Tripathy J.P., Satyanarayana S., Tharyan P. Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis. Cochrane Database Syst Rev. 2019;12:CD012918. doi: 10.1002/14651858.CD012918.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreiber J., Moreno C., Garcia B.G., Louvet A., Trepo E., Henrion J. Meta-analysis: the impact of IL28B polymorphisms on rapid and sustained virological response in HCV-2 and -3 patients. Aliment Pharmacol Ther. 2012;36:353–362. doi: 10.1111/j.1365-2036.2012.05197.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.