Abstract
Anomalous marginal veins of the trunk or extremities are congenitally incompetent entities found in association with phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA)-related overgrowth syndromes, such as Klippel-Trénaunay syndrome and congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and skeletal deformities (CLOVES) syndrome. When present, they can be a major source of venous hypertension-related morbidity and potentially lethal thromboembolic events. Herein, we describe a rare case of an upper extremity marginal vein in a patient with CLOVES syndrome. Through a multimodal therapeutic approach, we identified a somatic PIK3CA mutation in the excised anomalous vein. This finding questions the validity of commonly employed terminology, such as persistent embryonic vein, in reference to these anomalous entities.
Keywords: CLOVES syndrome, Marginal veins, PIK3CA, Coil embolization
Anomalous marginal veins of the trunk and extremities—found in association with Klippel-Trénaunay syndrome (KTS) and congenital lipomatous overgrowth, vascular malformations, epidermal nevus, and skeletal deformities (CLOVES) syndrome—are a major source of venous hypertension-related morbidity and potentially lethal thromboembolic events.1,2 These congenitally incompetent anomalous veins have been referred to as persistent embryonic veins, suggesting that they are noninvoluting embryonic remnants.3,4 Our staged, multimodal therapeutic approach incorporating paired exome sequencing to identify a somatic mutation provides further insight into the pathophysiologic mechanism of these anomalous entities and questions the validity of traditionally applied nomenclature.
Case report
Clinical presentation
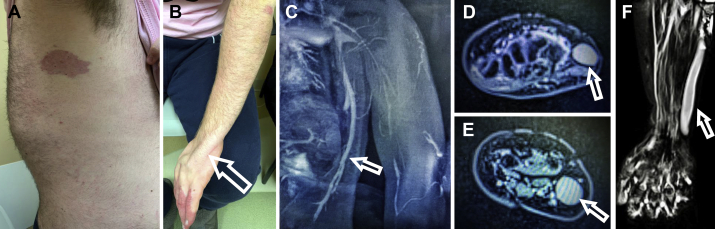
A 17-year-old boy presented with chronic pain and swelling of the left upper extremity in the setting of spinal scoliosis, lipomatous overgrowth of the left posterolateral upper trunk, and multifocal port-wine stains of the left hand and trunk (Fig 1, A and B). Magnetic resonance and duplex ultrasound imaging revealed thoracic phlebectasia draining the anomalous veins of the left lateral chest wall into the left axillary vein (Fig 1, C) as well as an enlarged incompetent anomalous marginal vein extending from the dorsum of the left hand to the axilla, draining into an intact deep system (Fig 1, D-F). A clinical diagnosis of CLOVES syndrome was made. Treatment of these incompetent, anomalous venous structures was warranted to address symptoms, to prevent hematologic derangements, and to minimize risk of thromboembolic events.1 The patient consented to publication of this report.
Fig 1.
A, Congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and skeletal deformities (CLOVES) syndrome with its characteristic port-wine stain. B, Subdermal, ulnar location of an incompetent anomalous vein in the left upper extremity (arrow). C, Magnetic resonance venography of thoracic phlebectasia of CLOVES syndrome (arrow). D-F, Magnetic resonance venography of the incompetent anomalous vein at the level of (D) wrist and (E) forearm, (F) extending from the dorsum of the left hand along the ulnar aspect (arrow).
Surgical technique
Under general endotracheal anesthesia, we gained ultrasound-guided distal micropuncture access of the left chest wall thoracic phlebectasia and performed ascending phlebography, which confirmed the lateral marginal position of the incompetent truncal vein with multiple venous tributaries, draining into the axillosubclavian venous system (Fig 2, A-C). We verified the presence of an intact and competent deep venous system by duplex ultrasound and tourniquet-assisted venography. Through a coaxial, microcatheter-based platform, we embolized the major venous outflow channels draining the lateral marginal vein using platinum-based, mechanically detachable Ruby POD, standard, and soft coils (Penumbra Inc, Alameda, Calif; Fig 2, D). Once the outflow tract was controlled, mechanochemical ablation (MOCA) of the remaining venous trunk was performed with a 45-cm ClariVein system (Merit Medical, South Jordan, Utah) using a total of 3.5 mL of 1.5% sodium tetradecyl sulfate (STS). Completion venography and duplex ultrasound showed no residual flow through the lateral marginal vein and the embolized venous tributaries (Fig 2, E).
Fig 2.
A-C, Ascending venography demonstrating the thoracic phlebectasia. Note the significant reflux into chest varicosities. D and E, Coil embolization of the venous outflow into the ipsilateral axillary vein was performed before mechanochemical ablation (MOCA) of the incompetent anomalous vein. F, Ascending venography showing an enlarged, incompetent anomalous vein draining the dorsum of the hand, crossing medially at the antecubital fossa, and draining into the distal left axillary vein. G, Coil embolization of the outflow portion of the anomalous vein before MOCA was performed.
Next and in a similar fashion, we accessed the left upper extremity marginal vein distally at the dorsum of the hand. Ascending phlebography confirmed presence of an enlarged, incompetent anomalous vein draining the dorsum of the hand, coursing marginally along the ulnar aspect of the forearm, crossing medially at the antecubital fossa, and draining into the distal left axillary vein (Fig 2, F). An intact and competent deep system was verified by tourniquet-based techniques. The venous outflow portion of the marginal vein into the axillary vein was embolized (Fig 2, G) with a combination of Ruby standard and soft coils. Next, MOCA of the remaining venous trunk was performed using a 45-cm ClariVein with 10 mL of 1.5% STS. Completion venography and duplex ultrasound showed obliteration of flow through the main venous trunk. A multilayer compression wrap was applied to the left upper extremity extending from the hand to the axilla. The patient tolerated the procedure well without complications and was discharged home in stable condition on the same day.
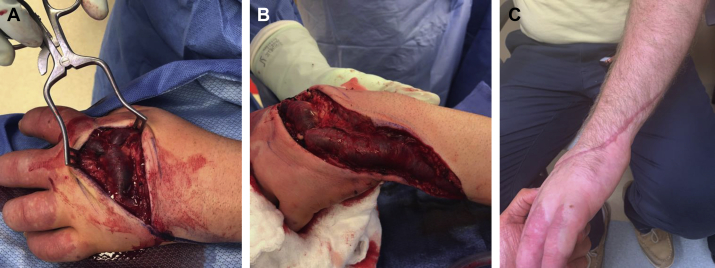
At the 2-week follow-up appointment, duplex ultrasound examination verified durable closure of the treated veins. However, size and length of the thrombosed upper extremity vein had caused symptomatic thrombophlebitis refractory to conservative, nonoperative measures including compression therapy and anti-inflammatory medications (ketorolac and acetaminophen used at weight-based standard doses). Therefore, 4 weeks after the index procedure, we proceeded with surgical excision of the thrombosed anomalous vein under general endotracheal anesthesia. The thrombosed anomalous vein was excised from the dorsum of the hand up to the mid forearm, and all tributary and draining veins were ligated (Fig 3, A and B). The patient was discharged home on postoperative day 1.
Fig 3.
A and B, Surgical resection of main trunk of the anomalous embryonic vein and ligation of dorsal hand and ulnar wrist tributaries after mechanochemical ablation (MOCA). C, The site of surgical resection had healed at follow-up.
Postoperative follow-up
The patient had an uneventful postoperative course without complications and with resolution of all presenting symptoms (Fig 3, C). Duplex ultrasound revealed successful obliteration of all treated veins with no recurrence at 6-month follow-up. The patient was maintained on customized compression therapy (30-40 mm Hg) of the left upper extremity from the time of the initial visit until the most recent follow-up.
Exome sequencing analysis
Lesional genomic DNA was prepared using DNeasy (Qiagen, Valencia, Calif) per the manufacturer's protocol. Paired exome sequencing was performed (Yale Center for Genome Analysis) using the patient's blood to identify mutations unique to the lesion. After alignment of the exome data to the human reference genome (by the Burrows-Wheeler MEM Aligner),5 polymerase chain reaction duplicates or mismapped sequences were trimmed using Picard (Broad Institute, Cambridge, Mass). All genetic mutations present in the lesion and blood were identified using GATK Best Practices and Haplotype Caller, and those mutations occurring only within the vascular tissue were enriched and annotated through Mutect2 and ANNOVAR, respectively.6, 7, 8 Reference control data sets, including 1000 Genomes (release 05/2011), the National Heart, Lung, and Blood Institute, and the ExAC control set (version 0.3), were employed to crosscheck all mutations to enrich for pathogenic mutations. Finally, all exome reads were viewed using the Integrated Genomics Viewer software (Broad Institute). This analysis identified a single somatic c.1258T>C, p.420C>R mutation in PIK3CA with minor allele fraction of 4.7% and was confirmed by Sanger sequencing.
Discussion
In this patient with CLOVES syndrome, we identified the pathognomonic thoracic phlebectasia as well as an extremely rare upper extremity marginal vein. These are rare but well-described congenitally incompetent venous entities associated with phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA)-related overgrowth syndromes such as CLOVES and KTS.1,3 Inconsistent terminology—such as vein of Servelle, lateral embryonal vein, persistent embryonic vein, and Klippel-Trénaunay vein—has been used to describe these entities.3,4 We treated both anomalous venous structures by coil embolization of the venous outflow tract, followed by STS-based MOCA of the main trunk. This method is a slight modification of our previously described technique wherein transcatheter sclerosant delivery is performed from proximal to distal along the main venous trunk after coil occlusion of the venous outflow.9 We continue to recommend detachable coils to maximize precision of deployment and to allow retrieval and repositioning of the coil as needed while minimizing the risk of nontarget embolization.
The use of MOCA in treatment of congenitally incompetent anomalous vein has not been reported to date. One major limiting factor in its use to treat incompetent anomalous veins is the large diameter of these veins. The rotation centerline of the wire tip (6.5 mm in diameter) can be inadequate for outflow obstruction proximally and may increase the risk of nontarget embolization. In our experience, we have found MOCA most effective in ablating smaller caliber anomalous veins (<1 cm in diameter), such as those found in the lower extremity of patients with PIK3CA-related overgrowth syndromes. As demonstrated here, however, adjunctive embolotherapeutic maneuvers for outflow obstruction before MOCA can help minimize the risk of sclerosant escape in larger caliber anomalous veins.
Surgical excision of the main trunk of anomalous marginal veins has been associated with bleeding and wound-related complications.10,11 Moreover, certain anatomic features, such as extreme tortuosity of the marginal vein, presence of numerous venous tributaries, small secondary arteriovenous fistulas, and concomitant lymphedema and lymphatic malformations, can complicate excision or stripping.10,11 We therefore continue to recommend transcatheter embolization as first-line treatment.1 As seen in this particular case, prior embolization and thrombosis of the marginal vein minimize operative blood loss should surgical excision be needed in the future.
Complete thrombosis of the anomalous vein with an initial thrombophlebitic response is the desired outcome.1,9 Immediate postoperative symptoms and the severity thereof are variable, depending on the volume and location of the embolized vein. In general, larger, more voluminous venous trunks tend to have a more profound thrombophlebitic response after embolization, which warrants close postoperative surveillance. Moreover, the immediate subdermal location of the anomalous marginal veins and their characteristic lack of fascial encasement can render these entities more susceptible to symptomatic thrombophlebitis after embolization.1,9, 10, 11
Conclusions
This is the first ever report of exome sequencing performed on an anomalous extremity marginal vein. The p.C420R PIK3CA mutation is one of five recurrent oncogenic variants, found in up to 80% of the vascular malformations in KTS and CLOVES,12 and enhances binding to membrane phospholipids, triggering constitutive kinase activity.13 Identification of a somatic PIK3CA mutation questions the validity of terminology such as persistent embryonic vein in reference to these entities. This observation suggests that these veins are most likely anomalous because of the same underlying gene mutation that causes the malformations and related overgrowth syndrome, namely, a somatic gain of function in PIK3CA, rather than noninvoluting embryonic remnants.14 Therefore, we suggest the term anomalous marginal vein as more appropriate nomenclature for these subdermal venous entities.
Footnotes
This work was supported by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01AR071491 to K.C. Y.L. was supported by a Doris Duke Charitable Foundation Clinical Mentorship Award and the :Medical Scientist Training Program at Yale University (National Institutes of Health/National Institute of General Medical Sciences T32GM007205). A.F. was supported by a Howard Hughes Medical Institute Medical Research Fellowship, Society for Vascular Surgery Research Fellowship, and American Heart Association Fellowship.
Author conflict of interest: N.N. is a consultant for and on the speakers bureau of Penumbra Inc.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Fereydooni A., Nassiri N. Evaluation and management of the lateral marginal vein in Klippel-Trénaunay and other PIK3CA-related overgrowth syndromes. J Vasc Surg Venous Lymphat Disord. 2020;8:482–493. doi: 10.1016/j.jvsv.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Alomari A.I., Burrows P.E., Lee E.Y., Hedequist D.J., Mulliken J.B., Fishman S.J. CLOVES syndrome with thoracic and central phlebectasia: increased risk of pulmonary embolism. J Thorac Cardiovasc Surg. 2010;140:459–463. doi: 10.1016/j.jtcvs.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Mattassi R., Vaghi M. Management of the marginal vein: current issues. Phlebology. 2007;22:283–286. doi: 10.1177/026835550702200609. [DOI] [PubMed] [Google Scholar]
- 4.Servelle M. Klippel and Trénaunay's syndrome. 768 operated cases. Ann Surg. 1985;201:365–373. doi: 10.1097/00000658-198503000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H., Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cibulskis K., Lawrence M.S., Carter S.L., Sivachenko A., Jaffe D., Sougnez C. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Auwera G.A., Carneiro M.O., Hartl C., Poplin R., Del Angel G., Levy-Moonshine A. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43 doi: 10.1002/0471250953.bi1110s43. 11.10.1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nassiri N., Crystal D., Huntress L.A., Murphy S. Transcatheter embolization of persistent embryonic veins in venous malformation syndromes. J Vasc Surg Venous Lymphat Disord. 2017;5:749–755. doi: 10.1016/j.jvsv.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Roh Y.N., Do Y.S., Park K.B., Park H.S., Kim Y.W., Lee B.B. The results of surgical treatment for patients with venous malformations. Ann Vasc Surg. 2012;26:665–673. doi: 10.1016/j.avsg.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y.W., Lee B.B., Cho J.H., Do Y.S., Kim D.I., Kim E.S. Haemodynamic and clinical assessment of lateral marginal vein excision in patients with a predominantly venous malformation of the lower extremity. Eur J Vasc Endovasc Surg. 2007;33:122–127. doi: 10.1016/j.ejvs.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Blesinger H., Kaulfuss S., Aung T., Schwoch S., Prantl L., Rossler J. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations. PLoS One. 2018;13:e0200343. doi: 10.1371/journal.pone.0200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gymnopoulos M., Elsliger M.A., Vogt P.K. Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci U S A. 2007;104:5569–5574. doi: 10.1073/pnas.0701005104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fereydooni A., Dardik A., Nassiri N. Molecular changes associated with vascular malformations. J Vasc Surg. 2019;70:314–326.e1. doi: 10.1016/j.jvs.2018.12.033. [DOI] [PubMed] [Google Scholar]