Abstract
Introduction
Recent studies have suggested that sex confers a differential risk in the incidence and prevalence of Alzheimer's disease (AD) thought to be the result of the increased lifespan of women compared to men. However, other factors may contribute to risk beyond the effect of increased lifespan.
Methods
This study examined the role of sex in hippocampal hyperactivity localized to the dentate gyrus (DG)/CA3 subregion of the hippocampus and associated episodic memory impairment, considered a characteristic feature of AD in patients with amnestic mild cognitive impairment (aMCI).
Results
While participants with aMCI showed decreased memory performance and increased activation in the DG/CA3 when compared to controls, no significant sex‐related differences in performance or activation were observed.
Discussion
Although other factors may contribute to sex differences in the prevalence of AD these findings show that no sex differences are observed in hippocampal dysfunction characteristic of the aMCI phase of AD.
Keywords: Alzheimer's disease, hippocampal hyperactivity, memory impairment, mild cognitive impairment, sex differences
1. INTRODUCTION
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by a decline of memory function in the prodromal phase of the disease and a progressive loss of cognition and functional impairment in the dementia phase. The incidence and prevalence of AD across the sexes in the aging population is an area of increasing interest, reflected by the growing body of literature addressing whether sex confers a differential risk in the development of AD. Several studies have suggested that women have an increased risk of developing AD while others have reported no sex‐related difference in risk (for a review refer to Munro 1 ). The reported increased risk in women is often thought to be the result of the increased lifespan of women compared to men. 2 However, some evidence suggests that other factors contribute to risk above and beyond the effect of increased lifespan (for a review refer to Fisher et al. 3 ). Overall, relatively few studies have addressed the role of sex across the AD trajectory (see Edland et al. 4 ), specifically with regard to the amnestic mild cognitive impairment (aMCI) phase of AD, which has been identified as a transitional state between normal memory function and AD dementia. aMCI is classified as a clinical condition defined by memory concerns as reported by the patient, informant, or clinician, with impairment in at least one cognitive domain (including memory), preserved independence in functional domains, but not meeting criteria for dementia. 5 , 6 Hippocampal dysfunction, specifically hippocampal hyperactivity, is considered a characteristic feature of the aMCI stage of AD 7 as studies have shown that neuronal hyperexcitability contributes to amyloid accumulation 8 , 9 and is correlated with disease progression (Huijbers et al. 14 ). This study aims to examine the role of sex in hippocampal hyperactivity and associated episodic memory functioning and assess whether sex plays a differentiating role in hippocampal dysfunction and memory impairment in this stage of the disease. The results show that there is no significant difference in activation localized to the dentate gyrus/CA3 (DG/CA3) subregion of the hippocampus and no difference in associated memory impairment between men and women. Both male and female aMCI patients show significantly increased activation in the DG/CA3 subregion of the hippocampus in the context of impaired memory function when compared to healthy controls. These findings suggest that while there may be sex differences in the prevalence of AD, no sex differences are observed in the expression of hippocampal hyperactivity in patients with aMCI and memory functions thought to critically rely on this network.
2. METHODS
The dataset used in this study was previously reported on in Tran et al., 10 which assessed the role of the apolipoprotein E‐4 (APOE) genetic variation in hippocampal dysfunction and associated memory impairment using detailed behavioral assessment and high‐resolution magnetic resonance imaging (MRI). Data from all patients with aMCI and cognitively normal control participants included in that report were used for analysis in this study to assess whether sex plays a differentiating role in hippocampal dysfunction underlying memory impairment in this stage of the disease. Detailed methods for the behavioral assessment and MRI data are provided in Tran et al. 10 and briefly described here as follows.
2.1. Participants and clinical characterization
Patients with aMCI had a global Clinical Dementia Rating scale (CDR) score of 0.5 and met criteria for aMCI as proposed by Petersen. 6 This includes a subjective memory complaint corroborated by an informant, impaired memory function on neuropsychological testing (eg, 1.5 standard deviations below the norm), and no impairment in activities of daily living. All control participants had a global CDR score of 0 and none of the aMCI or control participants met criteria for dementia. Participants were excluded from participation if they acknowledged either current or past major neurological or psychiatric conditions or general contraindications to having an MRI. Participation in hormone replacement therapy was not assessed in this study. The study protocol was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions. All participants in the study provided written informed consent. Participants were compensated for their participation.
RESEARCH IN CONTEXT
Systematic review: The authors have reviewed the English literature using traditional methods (eg, PubMed) and provide a brief overview of the literature on the effects of sex on the incidence and prevalence of Alzheimer's disease (AD). Very few studies have examined the differential risk of sex, particularly in patients with mild cognitive impairment (MCI). The current study examined the role of sex in hippocampal dysfunction and associated memory impairment in patients with amnestic mild cognitive impairment (aMCI).
Interpretation: While participants with aMCI showed decreased memory performance and increased activation localized to the dentate gyrus/CA3 subregion of the hippocampus, no significant differences in performance or activation were observed between men and women with aMCI.
Future directions: Additional studies are needed to assess the effects of sex on other characteristic features in both clinical and pre‐clinical phases of AD.
2.2. fMRI task
The functional MRI (fMRI) task, described in detail previously, 11 is a memory task designed to assess pattern separation, a process thought to critically depend on the DG/CA3 subregion of the hippocampus. Subjects were presented with a series of pictures of everyday objects and asked to determine for each object if the item was ''new,'' ''old,'' or ''similar.'' As in typical two‐judgment recognition memory tests, an item was correctly judged “new” if it was seen for the first time in the context of the task and ''old'' if the item was repeated. The third option of ''similar'' was the correct judgment when an object only resembled an item previously seen in the task. These ''similar lures'' were the critical trials for assessment of DG/CA3 contribution to memory performance. Correct identification of ''similar'' items is thought to depend on dentate gyrus mediated pattern separation, referring to the ability to encode inputs with some degree of overlapping information into distinctive representations.
2.3. fMRI procedures
Details regarding fMRI image collection and analysis are described in Tran et al. 10 Briefly, fMRI images were collected using a T2*‐weighted echo planar single shot pulse, a resolution of 1.5 × 1.5 × 1.5 mm, a TR of 1.5 seconds, and an acquisition matrix of 64 × 64. Functional volumes consisted of 19 slices oriented along the principal axis of the hippocampus covering the medial temporal lobe bilaterally. Structural images were acquired using T1‐weighted sequence with 231 oblique slices, a 0.65 mm isotropic resolution, and a field of view of 240 mm.
Functional runs were concatenated and six vectors were defined to model the different trial types using a deconvolution approach that was based on a general linear regression treating the single foil presentations that were correctly rejected as a non‐zero baseline against all other conditions. Alignment procedures were designed to focus the alignment power to the regions of interest using the segmentation of participant's anatomical image and are described in detail in Bakker et al. 12 , 13 and Tran et al. 10
3. RESULTS
Data from 35 control participants and 42 patients with aMCI were included in this study. No significant differences in age or education were observed between males and females in either the control or aMCI groups or between the control and aMCI groups. Female control participants also did not differ from male control participants in their proportion of correct “similar” responses to lure trials (t[32.10] = −0.08, P = .94) or task‐related activation localized to the left DG/CA3 (t[25.58] = −0.18, P = .91; Table 1). Because there were no differences between the male and female control participants, their data were combined into a single control group for all subsequent analyses to avoid interaction effects this study is not powered to detect.
TABLE 1.
Demographics and task performance of healthy controls and amnestic aMCI participants
| Controls | aMCI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||
| Mean | SD | Mean | SD | P | Mean | SD | Mean | SD | P | |
| Demographics | ||||||||||
| Subjects | 18 | 17 | ‐ | 19 | 23 | ‐ | ||||
| Age (years) | 70.2 | 7.9 | 68.2 | 7.2 | .44 | 71.1 | 9.13 | 71.83 | 6.24 | .80 |
| Education (years) | 17 | 2.4 | 15.24 | 2.54 | .05 | 16.4 | 2.22 | 14.91 | 3.1 | .08 |
| Task‐related measures | ||||||||||
| Proportion correct | 0.45 | 0.17 | 0.44 | 0.14 | .94 | 0.28 | 0.14 | 0.31 | 0.15 | .53 |
| Left DG/CA3 activation | −0.10 | 0.41 | −0.13 | 0.71 | .91 | 0.31 | 0.72 | 0.69 | 1.10 | .19 |
As reported in Tran et al. 10 patients with aMCI showed impaired memory performance by having a significantly lower proportion of correct “similar” responses to lure trials when compared to healthy control subjects. Female patients did not differ from male aMCI patients in their memory performance measured by the proportion of correct “similar” responses to lure trials (t[39.55] = 0.62, P = .53; Figure 1A). Critically, both male and female aMCI patients showed significantly impaired memory performance when compared to controls (control vs aMCI males: t[40.69] = 4.19, P < .001; control vs aMCI females: t[47.86] = 3.52, P < .001). As a signature characteristic of the aMCI phase of AD, increased activation in the hippocampus was examined. Previous work by Bakker et al. 12 , 13 and Tran et al. 10 have demonstrated that this increased activation is localized to the DG/CA3 subregion of the hippocampus and is correlated with memory impairment observed in these patients. As reported in Tran et al., 10 patients with aMCI showed significantly increased activation in the DG/CA3 subregion of the hippocampus during the performance of the critical lure trials when compared to controls. Female patients with aMCI did not differ from male patients with aMCI in activation in this subregion (t[38.26] = 1.34, P = .19; Figure 1B). Critically, both male and female aMCI patients showed significantly increased activation in the DG/CA3 subregion of the hippocampus when compared to control participants (controls vs aMCI males: t[30.37] = −2.19, P < .05; controls vs aMCI females: t[29.85] = −3.22, P < .01). Both male and female aMCI patients showed a moderate correlation between memory performance and increased activation in the DG/CA3 subregion of the hippocampus (aMCI females: r = −0.53, t[21] = −2.88, P < .01; aMCI males: r = −0.30, t[17] = −1.13, P = .21). Although this correlation was significant in female aMCI patients, this difference is largely due to a single data point and the correlation was no longer significant when this data point is removed (aMCI femalesout: r = −0.40, t[20] = −1.96, P = .06).
FIGURE 1.
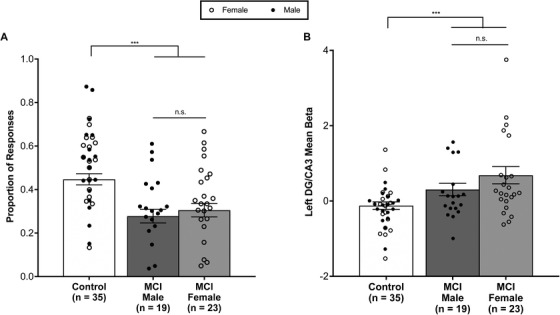
Hippocampal functioning does not differ between male and female aMCI patients. A, Female patients with aMCI did not significantly differ in the proportion of correct “similar” responses to the critical lure trials when compared to male patients with aMCI. Both female and male aMCI patients showed impaired memory performance by having a significantly lower proportion of correct “similar” responses to lure trials when compared to healthy control subjects. B, Female patients with aMCI did not differ from male aMCI patients in left hippocampal dentate gyrus (DG)/CA3 activation during critical lure trials. Increased left hippocampal DG/CA3 activation is observed in the context of impaired memory performance in female aMCI patients and male aMCI patients when compared to controls. Bars represent mean ± standard errof of the mean. ** P < .01, n.s., non‐significant
4. DISCUSSION
Although some studies have reported sex‐related differences in the prevalence of AD, the potential mechanism for such a difference remains unclear. Among a sample of patients with aMCI who are at increased risk for AD dementia, we observed no sex‐related differences in increased activation in the DG/CA3 subregion of the hippocampus or in the proportion of lure items correctly called “similar.” Both female and male patients with aMCI showed significantly increased hippocampal activation and a lower proportion of lure items correctly called “similar” when compared to control participants. Finally, both female and male patients with aMCI showed a similar correlation between memory performance and hippocampal activation. These findings are consistent with the absence of sex‐related differences in performance on delayed recall on the Buschke Selective Reminding Test, a commonly used neuropsychological assessment of episodic memory function, used in this study and are not the result of differences in APOE e4 status as male and female aMCI groups did not significantly differ in the number of APOE e4 carriers and non‐carriers. Although other factors may contribute to sex differences in the prevalence of AD that should be examined in larger future studies, the findings reported here show that no sex differences are observed in the expression of hippocampal hyperactivity and memory functions thought to critically rely on this network in the aMCI phase of AD.
ACKNOWLEDGMENTS
We would like to thank the staff of the F.M. Kirby Center for Functional Brain Imaging for their assistance with data collection. This work was supported by NIH grants RC2AG036419 and P50AG05146, and by an NIA T32 training grant and a National Defense Science and Engineering Graduate Fellowship (NDSEG) grant awarded by the DOD, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a to T.T.
CONFLICTS OF INTEREST
Michela Gallagher is the founder of AgeneBio. Michela Gallagher and Arnold Bakker are inventors on Johns Hopkins University intellectual property with patents pending and licensed to AgeneBio. Michela Gallagher consults for the company and owns company stock, which is subject to certain restrictions under university policy. Michela Gallagher and Arnold Bakker's role in the current study was in compliance with the conflict of interest policies of the Johns Hopkins School of Medicine. The other authors do not report any conflicts.
Corona‐Long CA, Tran TT, Chang E, Speck CL, Gallagher M, Bakker A. Comparison of male and female patients with amnestic mild cognitive impairment: Hippocampal hyperactivity and pattern separation memory performance. Alzheimer's Dement. 2020;12:e12043 10.1002/dad2.12043
REFERENCES
- 1. Munro CA. Sex differences in Alzheimer's disease risk: are we looking at the wrong hormones? International Psychogeriatrics.; 201426 10:1579–1584. [DOI] [PubMed] [Google Scholar]
- 2. Acar D, King CJ. Sex‐related differences in Alzheimer's disease In: M O'Neil, Neurology and Psychiatry of Women pp. 219‐225. Springer, Cham; 2019. [Google Scholar]
- 3. Fisher DW, Bennett DA, Dong H. Sexual dimorphism in predisposition to Alzheimer's disease. Neurobiology of Aging. : 201870: 308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol 2002;59(10):1589‐1593. [DOI] [PubMed] [Google Scholar]
- 5. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on. Alzheimers Dement. 2011;7(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256(3):183‐194. [DOI] [PubMed] [Google Scholar]
- 7. Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends in Neurosciences. 2011;34(8):430‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bero AW, Yan P, Hoon Roh J, et al. Neuronal activity regulates the regional vulnerability to amyloid‐β deposition. Nat Neurosci. 2011;14(6):750‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leal SL, Landau SM, Bell RK, Jagust WJ. Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. eLife. 2017;6(Mci):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tran TT, Speck CL, Pisupati A, Gallagher M, Bakker A. Increased hippocampal activation in ApoE‐4 carriers and non‐carriers with amnestic mild cognitive impairment. Neuroimage Clin.2017;13:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirwan CB, Stark CEL. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learn Mem. 2007;14(9):625‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bakker A, Krauss GL, Albert MS, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huijbers W, Schultz AP, Papp KV, et al. Tau Accumulation in clinically normal older adults is associated with increases in hippocampal fMRI activity. Alzheimers Dement. 2017;13(7):P1215. [Google Scholar]


