Abstract
Background
People with Parkinson's disease (PD) can develop multidomain cognitive impairments; however, it is unclear whether different pathologies underlie domain‐specific cognitive dysfunction.
Objectives
We investigated the contribution of vascular copathology severity and location, as measured by MRI white matter hyperintensities (WMHs), to domain‐specific cognitive impairment in PD.
Methods
We studied 85 PD (66.6 ± 9.2 years) and 18 control (65.9 ± 6.6) participants. Using the Fazekas scale for rating the severity of WMH, we subdivided PD into 14 PD–WMH+ and 71 PD–WMH–. Participants underwent global, executive, visuospatial, episodic memory, and language testing. We performed nonparametric permutation testing to create WMH probability maps based on PD‐WMH group and cognitive test performance.
Results
The PD–WMH+ group showed worse global and executive cognitive performance than the PD–WMH– group. On individual tests, the PD–WMH+ group showed worse Montreal Cognitive Assessment (MoCA), Stroop, Symbol Digit Modalities Test (SDMT), and Digit Span scores. WMH probability maps showed that in the PD–WMH+ group, worse Stroop was associated with lesions centered around the corticospinal tract (CST), forceps major, inferior‐fronto‐occipital fasciculus, and superior longitudinal fasciculus; worse SDMT with lesions around the CST, forceps major, and posterior corona radiata; worse Digit Span with lesions around the posterior corona radiata; and worse MoCA with lesions around the CST.
Conclusions
We found that WMH severity was associated with PD executive dysfunction, including worse attention, working memory, and processing speed. Disruption of key white matter tracts in proximity to vascular lesions could contribute to these specific cognitive impairments. Early treatment of vascular disease might mitigate some executive dysfunction in a subset of patients with PD.
Keywords: cognitive impairment, executive function, lesion probability mapping, Parkinson's disease, white matter hyperintensities
Parkinson's disease (PD) is the second‐most common neurodegenerative disorder and affects 2‐3% of adults aged >65 years. Alongside the cardinal motor symptoms, many nonmotor symptoms contribute to reduced health‐related quality of life.1 Cognitive impairment and dementia are among the most devastating nonmotor symptoms, with deficits occurring in multiple cognitive domains, including executive function/attention, visuospatial ability, language, and memory.2
With increasing age, there is also an increased risk for incidental cerebral white matter hyperintensities (WMHs) to be found on routine MRI scans. As one form of small vessel disease, these WMHs are speculated to represent areas of incomplete infarcts,3, 4 tend to progress with time,5 and are associated with motor6 and cognitive dysfunction in older, healthy adults.7 Specifically, WMHs are primarily associated with decline in executive/attention abilities, but there are also reports of WMH‐associated global cognitive impairment as well as deficits in motor control and visuoconstructional abilities.8, 9
At autopsy, PD patients often show multiple copathologies in addition to Lewy bodies, with Alzheimer's and vascular copathology being the most common.10 Lewy‐body pathology with and without Alzheimer's copathology are strongly linked to PD‐associated cognitive impairments.11, 12 However, it is less clear whether vascular copathology contributes to PD‐associated cognitive impairments.13, 14 Some studies in PD patients who showed WMHs on MRI are associated with deficits in executive function, attention, memory, and visuospatial abilities,15, 16 whereas others did not find an association.17 Furthermore, it is unclear whether the location of the WMH is related to domain‐specific cognitive impairments.
We studied whether incidental WMHs observed on routine MRI scans could be associated with cognitive impairments in patients with PD. We hypothesized that the severity and location of WMH would contribute to domain‐specific cognitive dysfunction in PD. To test this hypothesis, we first studied the impact of WMH severity on cognitive performance in PD. Second, we determined the detrimental effect of WMH location by calculating the lesion probability map as a function of WMH severity and cognitive test performance.
Participants and Methods
Participants
We recruited 121 participants from the Stanford Movement Disorders Clinic and the surrounding community as previously described.18, 19, 20 PD was diagnosed using the UK Parkinson's Disease Society Brain Bank clinical diagnostic criteria.21 Healthy controls (HCs) were neurologically normal on exam and within 1.5 standard deviations (SDs) of normative values on comprehensive cognitive testing. The Stanford Institutional Review Board approved this study, and all study participants provided their informed written consent.
An experienced researcher, who was blinded to participant information, rated cerebral WMH using the Fazekas scale for deep and periventricular WMH.22 We excluded 2 participants because of WMH in the basal ganglia, 7 because of lacunar infarcts, and 8 because microbleeds were suspected on T1 and fluid‐attenuated inversion recovery (FLAIR) scans. PD participants were subcategorized into a WMH‐positive group (PD–WMH+) if they had deep WMH grades 2 or 3, or periventricular WMH grade 3, and a WMH‐negative group (PD–WMH–) if they had deep WMH grades 0 or 1, or periventricular WMH grades 0, 1, or 2.4 One HC was WMH+ according to these criteria.
Cognitive Testing
All participants underwent comprehensive cognitive testing while on their regularly prescribed dopaminergic medications, as previously published.18 We assessed global cognitive function using the Montreal Cognitive Assessment (MoCA) and domain‐specific cognitive function in four domains. For executive function, including attention and working memory, we used the Wechsler Memory Scale‐III Digit Span (Digit Span),23 Symbol Digit Modalities Test (SDMT),24 Controlled Oral Word Association Test‐letters F‐A‐S (FAS),25 Trail Making Test part B (TMT‐B),26 and Stroop Color and Word Test (Stroop).27 For visuospatial ability, we used the Hooper Visual Organization Test28 and the Benton Judgement of Line Orientation.29 For episodic memory, we used the California Verbal Learning Test30 and the Brief Visuospatial Memory Test‐Revised.31 For language, we used the Boston Naming Test32 and Semantic Fluency Test.25 We used standardized age‐ and education‐matched normative values to determine whether a participant had cognitive impairment, which was defined by scores >1.5 SDs below normative values on at least two tests, regardless of domain.33 We then determined whether cognitively impaired participants had domain‐specific impairment, if at least one test within the domain had a score > 1.5 SDs below the normative values.33 Dementia was defined as the Clinical Dementia Rating Scale ≥0.5 and impairment in activities of daily living attributed to cognition, as determined by the neurologist who was blinded to WMH ratings.34
Three PD participants did not perform the Stroop because of being colorblind, and 1 PD participant did not perform the TMT‐B because of fatigue; these 4 participants were excluded from executive domain‐level categorization and test‐level analysis.
MRI Data Acquisition
Participants were scanned on a 3 Tesla (T) General Electric SIGNA scanner (GE Healthcare, General Electric Company, Waukesha, WI) using an eight‐channel radiofrequency receive head coil contained within a quadrature transmit coil (Nova Medical, Inc., Wilmington, MA). We performed structural MRI (FLAIR and T1) sequences, similar to those performed during routine MRI. Specifically, we performed two‐dimensional FLAIR with axial slices covering the whole brain (repetition time [TR] = 8,000.0 ms, echo time [TE] = 120.0 ms, field of view [FOV] = 220 × 220 mm2, matrix size of 512 × 512, and spatial resolution of 0.43 × 0.43 × 5.00 mm3) and three‐dimensional inversion recovery spoiled gradient echo T1‐weighted MRI with 158 axial slices (TR = 6.0 ms, TE = 2.0 ms, FOV = 220 × 220 mm2, matrix size of 256 × 256, flip angle = 5 degrees, and spatial resolution of 0.86 × 0.86 × 1.00 mm3).
WMH Mask and Volume Assessments
We extracted WMH volumes using the FMRIB Software Library (FSL; v5.0; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL). First, we used the Brain Intensity AbNormality Classification Algorithm (BIANCA) to perform automatic WMH segmentation by applying a k‐nearest neighbor algorithm.35 As training data, we used WMH masks, manually segmented by a single researcher, from 15 FLAIR scans and FLAIR‐ and T1‐weighted MRI for intensity feature extraction. To make the training data more local, we set the spatial weighting value to 2 and defined the subject‐specific training data to use all lesion points available and an equal number of nonlesion points while excluding voxels close to the lesion's edge. We binarized the resulting WMH masks for further processing, and a single researcher applied manual corrections to masks with false positives (labeled incorrectly as WMH) and/or false negatives (missing WMH segmentation). Second, we registered the WMH masks from FLAIR‐native space to the T1‐weighted Montreal Neurological Institute (MNI‐152) 1‐mm standard‐space MRI template using FLIRT and FNIRT.36 We linearly registered each participant's FLAIR scan and lesion masks to the same participant's T1 image using FLIRT with 6 degrees of freedom (DOF). We linearly registered T1 images to MNI space with 12 DOF affine registration and subsequently refined with nonlinear registration using FNIRT. We used the resulting transformation matrices and warp fields to register the lesion masks to MNI space. To preserve binary values, we set the threshold for the resulting masks at 0.5 and binarized. In order to account for small variations caused by registration from native to MNI space,36 we then subsampled the resulting masks to a voxel size of 2 × 2 × 2 mm3 and rebinarized with a threshold of 0.5. Third, we extracted total WMH volumes in MNI‐152 (2‐mm) standard space (Table 1).
Table 1.
HC and PD group characteristics
| Characteristic | HC–WMH– | PD–WMH– | PD–WMH+ | P Value | Post Hoc |
|---|---|---|---|---|---|
| No. | 18 | 71 | 14 | ||
| Demographics | |||||
| Age, years |
65.9 ± 6.6 [57–78] |
65.1 ± 8.5 [42–83] |
74.4 ± 9.0 [55–85] |
0.001a | f , g |
| Female# | 10 (55.6) | 31 (43.7) | 4 (28.6) | 0.312b | |
| Education |
17.4 ± 1.7 [15–20] |
16.7 ± 2.4 [12–20] |
16.4 ± 2.8 [12–20] |
0.601c | |
| Disease duration | — |
5.3 ± 4.3 [0–22] |
5.8 ± 3.9 [0–15] |
0.426d | |
| Clinical features | |||||
| LEDD | — |
586.4 ± 349.4 [0–1,560] |
645.7 ± 416.7 [0–1,450] |
0.717d | |
| MDS‐UPDRS‐I | — |
11.7 ± 6.2 [2–29] |
13.5 ± 5.1 [4–23] |
0.182d | |
| MDS‐UPDRS‐II | — |
11.8 ± 7.1 [1–35] |
16.2 ± 7.2 [7–27] |
0.047d | |
| MDS‐UPDRS‐III On | — |
17.4 ± 9.7 [4–48] |
23.6 ± 12.4 [5–50] |
0.073d | |
| PIGD On | — |
2.2 ± 2.5 [0–13] |
3.9 ± 3.6 [0–14] |
0.036d | |
| Tremor On | — |
3.4 ± 3.4 [0–14] |
4.8 ± 3.1 [1–9] |
0.070d | |
| Bradykinesia‐Rigidity On | — |
10.3 ± 6.1 [0–28] |
12.7 ± 8.1 [2–27] |
0.381d | |
| H & Y On | — |
1.9 ± 0.7 [1–4] |
2.2 ± 0.6 [1–3] |
0.080d | |
| MDS‐UPDRS‐III Off | — |
31.4 ± 10.9 [6–59] |
39.2 ± 11.4 [19–59] |
0.022e | |
| PIGD Off | — |
3.0 ± 2.7 [0–15] |
5.2 ± 4.0 [0–14] |
0.020d | |
| Tremor Off | — |
6.9 ± 5.3 [0–20] |
8.9 ± 4.5 [3–15] |
0.127d | |
| Bradykinesia‐Rigidity Off | — |
17.8 ± 7.0 [5–37] |
20.9 ± 8.1 [9–32] |
0.148e | |
| H & Y Off | — |
2.1 ± 0.7 [1–5] |
2.4 ± 0.5 [2–3] |
0.020d | |
| MDS‐UPDRS‐IV | — |
3.9 ± 3.5 [0–15] |
5.5 ± 3.3 [0–11] |
0.123d | |
| Vascular risk factors | |||||
| Arterial hypertension# | 6 (35.3) | 22 (31.0) | 5 (38.5) | 0.842b | |
| Diabetes mellitus# | 1 (5.9) | 0 (0.0) | 1 (7.7) | 0.084b | |
| Smoking# | 2 (25.0) | 18 (31.6) | 3 (25.0) | 0.858b | |
| Hypercholesterolemia# | 7 (41.2) | 21 (29.6) | 7 (53.8) | 0.198b | |
| Body mass index(kg/m3) |
26.7 ± 4.9 [19–36] |
25.9 ± 5.2 [18–46] |
24.5 ± 5.5 [19–41] |
0.262c | |
| Total WMH volumes | |||||
| Native space (cm3) |
1.86 ± 2.16 [0.05–8.95] |
2.11 ± 1.62 [0.14–8.93] |
19.55 ± 16.90 [0.67–56.93] |
<0.001c | f , g |
|
Standard space (cm3) |
7.06 ± 6.81 [0.21–24.18] |
8.10 ± 5.51 [1.02–29.71] |
48.41 ± 33.72 [8.42–134.80] |
< 0.001c | f , g |
| Total brain volume | |||||
| Native space (cm3) |
1,071.85 ± 130.23 [837.99– 1,275.95] |
1,088.59 ± 112.11 [890.21–1,499.78] |
1,083.64 ± 141.81 [889.33–1,376.23] |
0.868a | |
| Standard space (cm3) |
1,357.10 ± 85.77 [1,205.94–1,510.70] |
1,384.61 ± 86.65 [1,201.86–1,604.67] |
1,327.31 ± 111.97 [1,162.45–1,601.17] |
0.075a | |
Table shows group comparisons after excluding the 1 HC–WMH+ participant, leaving a final cohort of 18 HC–WMH– participants.
All values shown as mean ± SD [range], except when designated with a pound sign (“#”) for number (percent within total group).
One‐way ANOVA, across all three groups.
Chi‐square, across all three groups.
Kruskal‐Wallis test, across all three groups.
Mann‐Whitney U test, between the two PD groups.
Student's t test, between the two PD groups.
Post‐hoc test (Bonferroni for all one‐way ANOVAs, Mann‐Whitney U test for all Kruskal‐Wallis tests) significant for:
HC‐WMH– vs. PD‐WMH–.
HC‐WMH– vs. PD‐WMH+.
PD‐WMH– vs. PD‐WMH+.
LEDD, levodopa equivalent daily dose.
Lesion Distribution and Lesion Probability Maps
Lesion Distribution Map
We generated a lesion distribution map showing lesion locations in the PD group by merging all standard‐space binary lesion masks.
Lesion Probability Maps
We performed nonparametric permutation testing using FSL randomise37 to create lesion probability maps, which identified the probability of lesion location, as predicted by worse cognitive test performance and the two PD subgroups (PD–WMH+ vs. PD–WMH–). We here tested for an interaction effect of worse cognitive performance on lesion location in the PD–WMH+ versus PD–WMH– groups. For all analyses, we concatenated standard‐space lesion masks into a four‐dimensional data matrix, then applied an MNI‐152 (2‐mm) standard‐space brain mask to mask out nonbrain voxels. In addition to modeling in information on the two PD‐WMH groups, the raw cognitive scores were first demeaned within the total group and then split into explanatory variables, based on the two PD‐WMH groups. Because age can influence both cognitive test scores and WMH lesion load, we used raw cognitive test scores and adjusted the general linear model for age, education, and lesion load (total WMH volume in standard space) as covariates of no interest to the model, which we demeaned within the total group. We used permutation testing, which is robust to unequal group variances, with 5,000 permutations randomly generated by reshuffling the labels of the design matrix to build up a null distribution to test against (for each voxel). To avoid overfitting, we performed four permutation tests using a different general linear model (GLM) for each covariate of interest (i.e., cognitive score). We thresholded the results using threshold‐free cluster enhancement,38 outputting only voxels with familywise error (FWE)‐corrected P values <0.05. We identified white matter tracts overlapping with significant lesion clusters using the Johns Hopkins University (JHU) White‐Matter Tractography Atlas and JHU ICBM‐DTI‐81 White‐Matter Labels atlas in MNI space, as part of the FSLeyes graphical user interface.
Statistical Analyses
We performed all statistical analyses using IBM SPSS Statistics software (version 25.0; SPSS Statistical Package for Social Science, IBM Corp; https://www.ibm.com). For all analyses, we used two‐tailed P values and defined P ≤ 0.05 as significant. We assessed between‐group differences using chi‐square tests for categorical variables, Mann‐Whitney U tests for non‐normally distributed variables (two groups), univariate one‐way analyses of variance (ANOVAs) for normally distributed variables (three groups), and Kruskal‐Wallis tests for non‐normally distributed variables (three groups), with post‐hoc Bonferroni or Mann‐Whitney U tests for multiple‐comparison correction, where appropriate.
For the lesion probability map analyses, we used GLMs with the lesion probability at each voxel as the dependent variable, PD‐WMH group and raw cognitive test score as the predictor variables, and age, education, and lesion load as covariates of no interest. In this analysis, we used the raw cognitive test score and adjusted for age in the model, rather than using age‐matched normative values, given that age can influence both cognitive test scores and WMH lesion load.
Results
Cohort Characteristics
Comparing all participants with and without WMH, there was no age difference between 85 PD and 19 HC participants (P = 0.972), and WMHs were just as frequent in PD as they were in HCs (P = 0.731). See Table 1 for detailed between‐group analyses after excluding the 1 HC–WMH+ participant. Within the PD group, PD–WMH+ participants were older (P = 0.001) and had worse International Parkinson and Movement Disorder Society UPDRS (MDS‐UPDRS) Part III Off (P = 0.022) compared to the PD–WMH– group.
PD Cognitive Impairment Related to WMH Severity and Location
The number of PD participants with and without cognitive impairment did not differ between WMH groups (Table 2). However, the PD–WMH+ group showed worse performance on the MoCA than the PD–WMH– group (P = 0.020).
Table 2.
Cognitive function in the HC and PD groups
| HC–WMH– | PD–WMH– | PD–WMH+ | P Value | Post Hoc | |
|---|---|---|---|---|---|
| No. | 18 | 71 | 14 | ||
| All cognitively impaired# | 0 (0.0) | 33 (46.5) | 10 (71.4) | < 0.001a | c , d |
| Dementia# | 0 (0.0) | 7 (9.9) | 5 (35.7) | 0.005a | d , e |
| Impaired cognitive domains | |||||
| Executive impairment# | 0 (0.0) | 28 (39.4) | 10 (76.9) | <0.001a | c , d , e |
| Visuospatial impairment# | 0 (0.0) | 7 (9.9) | 4 (28.6) | 0.032a | d |
| Episodic memory impairment# | 0 (0.0) | 28 (39.4) | 9 (64.3) | <0.001a | c , d |
| Language impairment# | 0 (0.0) | 10 (14.1) | 4 (28.6) | 0.063a | d |
| Cognitive tests | |||||
| MoCA | 27.8 ± 1.7 [24–30] |
25.4 ± 4.6 [24–29] |
22.8 ± 4.4 [19–26] |
0.002b | c , d , e |
| Digit Span* |
11.3 ± 2.1 [7–16] |
11.4 ± 3.5 [9–14] |
9.1 ± 2.2 [8–10] |
0.025b | d , e |
| SDMT* |
53.9 ± 8.4 [46–80] |
46.2 ± 14.8 [41–53] |
37.6 ± 15.3 [26–49] |
0.001b | c , d , e |
| Stroop* |
45.6 ± 6.4 [34–56] |
45.1 ± 8.1 [40–50] |
39.7 ± 5.9 [35–44] |
0.046b | d , e |
| TMT‐B* |
54.6 ± 4.4 [47–62] |
45.4 ± 16.2 [40–56] |
37.0 ± 17.0 [25–56] |
0.010b | c , d |
| FAS* |
52.1 ± 9.5 [30–70] |
50.0 ± 13.9 [40–62] |
46.1 ± 12.9 [36–56] |
0.391b |
Table shows group comparisons after excluding the 1 HC–WMH+ participant, leaving a final cohort of 18 HC–WMH– participants. Digit Span is the combined score, SDMT is the oral score, and Stroop is the interference score.
All values shown as mean ± SD [range], except when designated with a pound sign (“#”) for number (percent within total group). For those designated with an asterisk (“*”), we used standardized age‐ and education‐matched normative values.
Chi‐square, across all three groups.
Kruskal‐Wallis, across all three groups.
Significant post‐hoc tests (Mann‐Whitney U for Kruskal‐Wallis tests):
HC–WMH– versus PD–WMH–.
HC–WMH– versus PD–WMH+.
PD–WMH+ versus PD–WMH–.
Within the cognitive domains, more PD–WMH+ participants showed executive impairment (Table 1) compared to the PD–WMH– group, which maintained a trend relationship after Bonferroni's correction for multiple comparisons (P = 0.05/4 = 0.013). We then determined between‐group differences on individual executive tests using standardized age‐ and education‐matched normative values (Table 2). The PD–WMH+ group showed worse performance on Digit Span (P = 0.014), SDMT (P = 0.015), and Stroop (P = 0.026), compared to the PD–WMH– group.
As seen in the lesion distribution map (Fig. 1), the majority (94%) of PD participants showed lesions around the frontal horns of the lateral ventricles and almost half (47%) showed lesions around the posterior horns. This resembles the pattern in our HC group (data not shown) and is similar to studies in healthy older adults.39
Figure 1.
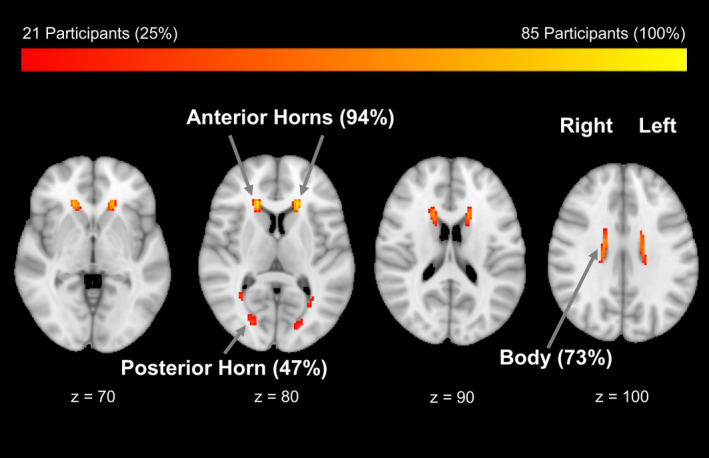
Topography of WMH in PD participants overlaid onto standard‐space brain mask. The majority showed WMH around the anterior horns of the lateral ventricles, with a maximum of 81 PD participants showing a lesion in a single voxel location.
As seen in the lesion probability map, for worse Stroop performance in the PD–WMH+ compared to the PD–WMH– group, we found significant lesion clusters (P < 0.05, FWE‐corrected) in the left corticospinal tract (CST), forceps major (FM), inferior fronto‐occipital fasciculus, and superior longitudinal fasciculus. For worse SDMT performance, we found significant lesion clusters in the left CST, left FM, right posterior corona radiata, and left corpus callosum. For worse Digit Span performance, we found significant lesion clusters in the right posterior thalamic radiation and left corpus callosum. Finally, for worse MoCA performance, we found a significant lesion cluster in the left CST (Fig. 2; Table 3).
Figure 2.
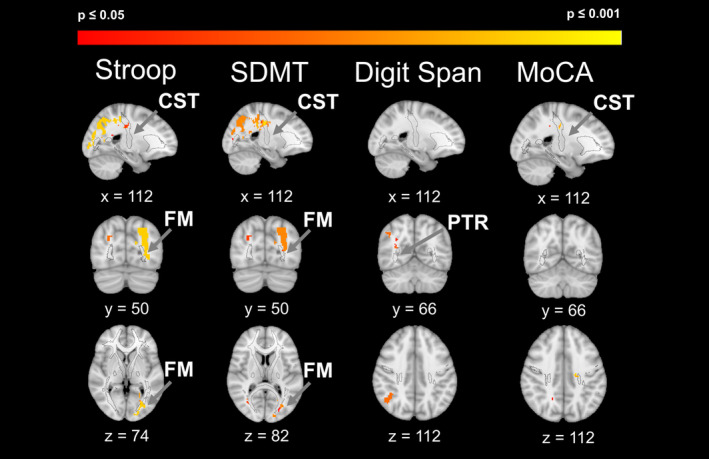
Lesion locations associated with worse cognitive test performance in the PD‐WMH+ versus the PD–WMH– group. Major fiber tracts are shown in black outlines. Clusters shown, FWE‐corrected P = 0.05 to 0.001. PTR, posterior thalamic radiation.
Table 3.
Fiber tracts associated with WMH lesion cluster
| Contrast and Fiber Tract(s) | Hemisphere | Voxels | MAX (Z) | X (Voxel) | Y (Voxel) | Z (Voxel) |
|---|---|---|---|---|---|---|
| Stroop | ||||||
| CST, forceps major, inferior fronto‐occipital fasciculus, superior longitudinal fasciculus | L | 1,704 | 0.99 | 116 | 54 | 88 |
| *Forceps major | R | 794 | 0.98 | 70 | 72 | 122 |
| SDMT | ||||||
| CST, Forceps major | L | 1,594 | 0.99 | 112 | 104 | 106 |
| *Posterior corona radiata | R | 1117 | 0.99 | 72 | 70 | 114 |
| Posterior corona radiata | R | 21 | 0.95 | 68 | 96 | 106 |
| Splenium of corpus callosum | L | 16 | 0.99 | 102 | 130 | 96 |
| — | — | 14 | 0.96 | 106 | 108 | 120 |
| Digit Span | ||||||
| *Posterior corona radiata | R | 153 | 0.98 | 50 | 64 | 106 |
| Body of corpus callosum | L | 11 | 0.97 | 102 | 130 | 96 |
| Posterior thalamic radiation | R | 11 | 0.96 | 58 | 68 | 102 |
| MoCA | ||||||
| CST | L | 13 | 0.99 | 112 | 104 | 106 |
Key(s): Voxels, number of voxel within cluster; MAX (Z), value of maximum z‐statistic; X/Y/Z (voxel), location of MAX (Z) voxel in MNI‐152 standard‐space coordinates (voxel).
Stroop, SDMT, Digit Span, and MoCA: lesion probability map results for the contrasts worse cognitive performance in PD–WMH+ versus worse cognitive performance in PD–WMH–. Major fiber tracts passing through or (*) near the significant WMH lesion cluster.
Only clusters with ≥10 voxels are reported.
Discussion
In this study, we showed an association between cerebral WMH and domain‐specific cognitive dysfunction in people with PD. We found that in PD patients, more severe WMHs of presumed vascular origin were associated with executive dysfunction, attention, and working memory. Finally, we identified several white matter tracts in PD patients with more severe WMHs, where lesions were associated with poorer cognitive performance. Our findings suggest that the severity and location of incidental WMH lesions observed on routine MRI might contribute to cognitive heterogeneity found in PD.
Vascular Brain Injury Could Contribute to the Heterogeneity of Cognitive Impairments in PD
PD patients commonly exhibit comorbidity beyond Lewy‐body pathology at autopsy.40, 41 Whereas Alzheimer's copathology is most common,13, 14 comorbid small‐vessel vascular brain injury is also frequent. One large retrospective study of 617 autopsy‐proven PD cases showed that almost 45% of patients had comorbid vascular brain injury,42 but their specific impact on early disease symptoms is still unclear. Studies investigating WMH on MRI, as a proxy for vascular brain injury, have sought to resolve this question.14
We studied the relationship between vascular injury and cognitive dysfunction in PD by comparing participants with and without WMH using MRI. Earlier studies in PD reported an association between WMH and performance on the Mini‐Mental State Examination.43 We found that PD participants with more severe WMH showed greater impairment on the MoCA, which is more commonly used clinically because of its greater sensitivity44 in detecting early PD cognitive impairments.18, 45
We then examined the relationship between WMH and domain‐specific cognitive impairments given that earlier studies show conflicting results.46 For example, two studies found WMH associated with executive function/attention and memory in PD,15, 47 but others did not find this association.48, 49 One of these studies limited the analysis to cholinergic white matter tracts,50 as has been used in the study of WMH in Alzheimer's disease patients. Our findings suggest that white matter tracts, in addition to the cholinergic system, should be studied with regard to executive dysfunctions in PD. Another used lower‐MRI‐field‐strength images,48, 49 which could account for the negative results. We used high‐field MRI (3T) and found that more PD with WMH showed executive impairment, compared to those without WMH.
We then found more impaired executive function (Stroop), processing speed (SDMT),51 and attention/working memory (Digit Span)52 in PD patients with more severe WMH. This is consistent with studies in healthy adults showing that WMHs are associated with worse executive function and information processing speed.53 Interestingly, processing speed also is abnormal in other neurological disorders, primarily affecting the white matter tracts, such as multiple sclerosis.54
WMH Locations Are Associated With the Specific Subtypes of PD Cognitive Impairment
For our lesion probability map analyses, we further studied the relationship between WMH severity and specific tests of executive function. We used lesion probability mapping to show that severe WMH in proximity to specific white matter tracts might explain some of the executive function deficits. We found most lesions centered around the CST, FM, inferior fronto‐occipital fasciculus, superior longitudinal fasciculus, and posterior corona radiata. Previous studies in people with PD have reported an association between executive function and anterior white matter tracts,55 which was independent of WMH load. In our study, we grouped people with PD based on WMH severity to study the mechanistic effects of severe vascular lesions on PD cognition (and with regard to presumed crossing fiber tracts). Similar to the Melzer et al. study, we here also added lesion load as a covariate of no interest to our model.
For the Stroop and SMDT, we found that the largest lesion cluster overlapped with regions of the left CST and FM. This is of interest, given that Zheng et al.56 used diffusion tensor imaging (DTI) to measure white matter integrity in relation to domain‐specific cognitive function in PD and found that the left CST was associated with attention. Bohnen and Albin14 speculated that periventricular WMH results in damage of both periventricular ascending thalamocortical and descending CST fibers, which would lead to impaired gait and postural control in participants with PD and WMH. Indeed, the CST travels from the cerebral cortex to the spinal cord and is typically associated with motor and sensory function. Studies have shown lower CST integrity to be associated with worse perceptual speed in persons with older age,57 but also higher neurite density in the CST to be associated with faster nondecision time in reaction time tasks, implying a more efficient network for voluntary actions.58 In persons with relapsing‐remitting multiple sclerosis, Riccitelli et al. determined that lesions in the CST and FM are associated with poor SDMT performance.59 Studies in patients with cerebral autosomal‐dominant arteriopathy with subcortical infarcts and leukoencephalopathy have shown that damage of the left CST is associated with poor processing speed.60, 61, 62 Whereas CST lesions have mostly been attributed to motor slowing on the written form of the SDMT, our study used the orally administered SDMT, thus minimizing the motor component of the test and highlighting the association with cognitive slowing.
Our lesion probability map findings are of particular interest with respect to the FM and posterior thalamic radiation. The FM white matter fiber bundle is one of the tracts that connects to the cingulate gyrus and posterior cingulate cortex, which are affected early in patients with Alzheimer's disease. The FM has been shown to be disrupted in early Alzheimer's disease patients with amnestic mild cognitive impairment, compared to persons with subjective cognitive decline63 and persons with subjective cognitive decline compared to HCs.64 The fiber pathways of the posterior thalamic radiation connect the thalamus with the parietal and occipital lobes and typically are associated with motor and sensory information transmission. However, greater microstructural integrity in the posterior thalamic radiation has been associated with better executive functioning performance in older adults.65
We also found that the SDMT and Digit Span associated lesion clusters in the bilateral corona radiata. WMHs in the posterior corona radiata have shown to accelerate the brain aging process in otherwise healthy elderly with WMHs.66 Studies using DTI have suggested that damage to these tracts is associated with executive function and/or attention capacity in participants with PD.56
Our goal was to study WMH specifically associated with cognitive impairments in patients with PD. In addition to the between‐group differences in cognition, we incidentally found that PD participants with WMH showed worse gait and balance compared to PD without WMH, as noted on the MDS‐UPDRS postural instability and gait disturbance (PIGD) subscale both On and Off dopaminergic medications. By contrast, we did not identify increased bradykinesia, rigidity, or tremor in PD with WMH. Numerous studies have found similar results, and a recent comprehensive review of the contributions of WMH to motor and gait symptoms in PD had similar conclusions67; namely, that WMH severity was significantly related to freezing of gait, but that the relations to bradykinesia and rigidity were inconsistent, and there was no association between WMH severity and tremor.
Methodological Considerations and Limitations
Our study has several methodological considerations. First, because the PD‐WMH+ group had a relatively small sample size with only 14 subjects, the conclusions from the present work may not be generalizable to the greater PD patient population and should be validated. Second, our HC group only included 1 participant defined as WMH+. We thus could not compare HC–WMH+ to PD–WMH+, which would have allowed us to determine whether the impact of WMH on cognition in PD is different from the impact on cognition in general aging. Third, our two PD‐WMH groups were not matched for age. To account for this, we used standardized age‐ and education‐matched normative values in the behavioral analyses and raw scores with age and education as covariates in the lesion probability map analyses. Fourth, our lesion probability map analyses tested for interaction effects between the PD‐WMH groups and cognitive test performance. This means that our significant clusters refer to a difference in the slope of the cognitive data between the PD‐WMH groups that varies as a function of cognitive performance.
In this study, we used WMH as a proxy for vascular brain injury. However, we did not find any differences between the PD‐WMH+/– groups in current and past vascular risk factors. A possible explanation for this could be our chosen grouping, where irregular periventricular WMH in the PD–WMH+ group might reflect increased periventricular water content or an intense venous network in this region rather than arteriosclerotic or periarteriorlar tissue damage.4 Another explanation could be that nonarteriosclerotic factors, such as orthostatic dysregulation18, 43 and watershed/border‐zone infarcts, contribute to the development of WMH on MRI in PD.
Previous studies showed mixed results when considering PD cognitive impairment based on WMH and cognitive category, such as mild cognitive impairments.48, 68, 69 We did find a between‐group difference in the number of participants with dementia, but this result should be interpreted with caution given the very low number of demented patients per WMH group. Furthermore, we determined groups based on WMH severity type, rather than cognitive function,69 and included punctate WMH in the PD–WMH– group. Studies on healthy elderly adults suggest that later‐stage early confluent WMHs are associated with vascular pathology and cognitive disturbances, whereas punctate WMHs are not.7 Thus, in the lesion probability analysis, we grouped the PD participants by WMH+ and WMH– with the aim of testing for specific location effects based on underlying pathology and associated cognitive effects. It is reassuring that despite these differences in approach, similar conclusions can be made. Namely, executive dysfunction in PD is related to the severity of WMH on MRI.
Finally, some studies have suggested that the incidental WMH observed on FLAIR could represent later‐stage, as opposed to early, vascular lesions. Longitudinal studies using DTI techniques, such as neurite orientation dispersion and density imaging,70 might be more sensitive for earlier‐stage vascular lesions. Therefore, longitudinal studies should consider a mixture of techniques to determine the relationship between visibly apparent WMH and the onset of PD executive impairments.
In conclusion, our study used lesion probability mapping to determine the relationship between domain‐specific PD cognitive impairments and WMH severity and location. Understanding whether vascular brain injury or other mechanisms, such as orthostatic hypotension or neuroinflammation, lead to the development of WMH in PD patients will be critical in guiding patient management given that early treatment targeting such mechanisms could mitigate some executive dysfunction in a subgroup of PD patients at risk.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
P.L.: 1A, 1B, 1C, 2A, 2B, 2C, 3A
C.M.: 1C, 2A, 2B, 2C, 3A, 3B
M.S.: 1C, 2B, 2C, 3A, 3B
T.F.L.: 1B, 1C, 3B
L.T.: 1C, 2A, 2C, 3B
B.C.: 1C, 2A, 2C, 3B
K.L.P.: 1A, 1B, 1C, 2A, 2C, 3A, 3B
Disclosures
Ethical Compliance Statement
This study was approved by the Stanford Institutional Review Board, and all study participants provided their informed written consent that was obtained in person. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
This work was supported by grants from the National Institutes of Health (NS115114 to K.L.P.; NS075097 to K.L.P.; NS062684 to K.L.P., B.C., and L.T.; and AG047366 to K.L.P. and L.T.) and the Michael J. Fox Foundation for Parkinson's Research (6440.0 and 6440.01 to K.L.P.). K.L.P. has received consulting fees from for Allergan and Curasen. The authors report no conflicts of interest.
Financial Disclosures for previous 12 months
L.T. has received research grants from the NIH. B.C. has received research grants from the NIH. K.L.P. has received consulting fees from Allergan and Curasen and research grants from Sanofi, the Michael J Fox Foundation for Parkinson's Research, and the NIH.
Acknowledgments
We thank Michelle Fenesy, BA, and Anisa Marshall, MSc, for their help with the cognitive data collection; Jaclyn Hwang and Loza Kebret for their help with gathering clinical information; and Jeehyun Kim for copyedit assistance.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386:896–912. [DOI] [PubMed] [Google Scholar]
- 2. Aarsland D, Creese B, Politis M, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol 2017;13:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 4. Schmidt R, Schmidt H, Haybaeck J, et al. Heterogeneity in age‐related white matter changes. Acta Neuropathol 2011;122:171–185. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt R, Enzinger C, Ropele S, Schmidt H, Fazekas F. Progression of cerebral white matter lesions: 6‐year results of the Austrian Stroke Prevention Study. Lancet 2003;361:2046–2048. [DOI] [PubMed] [Google Scholar]
- 6. Linortner P, Fazekas F, Schmidt R, et al. White matter hyperintensities alter functional organization of the motor system. Neurobiol Aging 2012;33:197.e1–e9. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke 2007;38:2619–2625. [DOI] [PubMed] [Google Scholar]
- 8. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 2015;11:157–165. [DOI] [PubMed] [Google Scholar]
- 9. Poggesi A, Pantoni L, Inzitari D, et al. 2001–2011: A decade of the LADIS (Leukoaraiosis And DISability) Study: what have we learned about white matter changes and small‐vessel disease? Cerebrovasc Dis 2011;32:577–588. [DOI] [PubMed] [Google Scholar]
- 10. Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age‐related and APOE4‐associated. Brain 2018;141:2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dickson DW, Heckman MG, Murray ME, et al. APOE epsilon4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology 2018;91:e1182–e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsuang D, Leverenz JB, Lopez OL, et al. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol 2013;70:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malek N, Lawton MA, Swallow DM, et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson's disease. Mov Disord 2016;31:1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nat Rev Neurol 2011;7:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SJ, Kim JS, Yoo JY, et al. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord 2010;24:227–233. [DOI] [PubMed] [Google Scholar]
- 16. Santangelo G, Vitale C, Trojano L, et al. Differential neuropsychological profiles in Parkinsonian patients with or without vascular lesions. Mov Disord 2010;25:50–56. [DOI] [PubMed] [Google Scholar]
- 17. Vesely B, Rektor I. The contribution of white matter lesions (WML) to Parkinson's disease cognitive impairment symptoms: a critical review of the literature. Parkinsonism Relat Disord 2016;22(Suppl. 1):S166–S170. [DOI] [PubMed] [Google Scholar]
- 18. Hendershott TR, Zhu D, Llanes S, Poston KL. Domain‐specific accuracy of the Montreal Cognitive Assessment subsections in Parkinson's disease. Parkinsonism Relat Disord 2017;38:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ng B, Varoquaux G, Poline JB, Thirion B, Greicius MD, Poston KL. Distinct alterations in Parkinson's medication‐state and disease‐state connectivity. Neuroimage Clin 2017;16:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hendershott TR, Zhu D, Llanes S, et al. Comparative sensitivity of the MoCA and Mattis Dementia Rating Scale‐2 in Parkinson's disease. Mov Disord 2019;34:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 2003;18:467–486. [DOI] [PubMed] [Google Scholar]
- 22. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 23. Wechsler D. Wechsler Adult Intelligence Scale—Fourth Edition Manual. San Antonio, TX: The Psychological Corporation; 2008. [Google Scholar]
- 24. Smith A. Symbol Digit Modalities Test Manual. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- 25. Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment 1999;6:147–178. [DOI] [PubMed] [Google Scholar]
- 26. Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol 1955;19:393–394. [DOI] [PubMed] [Google Scholar]
- 27. Golden CJ. Stroop Color Word Test: A Manual for Clinical and Experimental Use. Chicago, IL: Stoelting; 1978. [Google Scholar]
- 28. Hooper H. The Hooper Visual Organization Test Manual. Los Angeles, CA: Western Psychological Services; 1958. [Google Scholar]
- 29. Benton A. Contributions to Neuropsychological Assessment: A Clinical Manual (2nd. ed.). New York, NY: Oxford University Press; 1994. [Google Scholar]
- 30. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test—Second Edition Manual. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 31. Benedict R. Brief Visuospatial Memory Test: Revised—Professional Manual. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 32. Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test: Experimental Edition. Boston: Kaplan & Goodglass (second ed). Philadelphia, PA: Lea & Febinger; 1983. [Google Scholar]
- 33. Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 35. Griffanti L, Zamboni G, Khan A, et al. BIANCA (Brain Intensity AbNormality Classification Algorithm): a new tool for automated segmentation of white matter hyperintensities. Neuroimage 2016;141:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kincses ZT, Ropele S, Jenkinson M, et al. Lesion probability mapping to explain clinical deficits and cognitive performance in multiple sclerosis. Mult Scler 2011;17:681–689. [DOI] [PubMed] [Google Scholar]
- 37. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014;92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith SM, Nichols TE. Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 39. Rostrup E, Gouw AA, Vrenken H, et al. The spatial distribution of age‐related white matter changes as a function of vascular risk factors—results from the LADIS study. Neuroimage 2012;60:1597–1607. [DOI] [PubMed] [Google Scholar]
- 40. Choi SA, Evidente VG, Caviness JN, et al. Are there differences in cerebral white matter lesion burdens between Parkinson's disease patients with or without dementia? Acta Neuropathol 2010;119:147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 2012;72:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jellinger KA. Prevalence of cerebrovascular lesions in Parkinson's disease. A postmortem study. Acta Neuropathol 2003;105:415–419. [DOI] [PubMed] [Google Scholar]
- 43. Beyer MK, Aarsland D, Greve OJ, Larsen JP. Visual rating of white matter hyperintensities in Parkinson's disease. Mov Disord 2006;21:223–229. [DOI] [PubMed] [Google Scholar]
- 44. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009;73:1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shahid M, Kim J, Leaver K, et al. An increased rate of longitudinal cognitive decline is observed in Parkinson's disease patients with low CSF Ass42 and an APOE epsilon4 allele. Neurobiol Dis 2019;127:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson's disease: the dual syndrome hypothesis. Neurodegener Dis 2013;11:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kandiah N, Mak E, Ng A, et al. Cerebral white matter hyperintensity in Parkinson's disease: a major risk factor for mild cognitive impairment. Parkinsonism Relat Disord 2013;19:680–683. [DOI] [PubMed] [Google Scholar]
- 48. Dalaker TO , Larsen JP, Dwyer MG, et al. White matter hyperintensities do not impact cognitive function in patients with newly diagnosed Parkinson's disease. Neuroimage 2009;47:2083–2089. [DOI] [PubMed] [Google Scholar]
- 49. Slawek J, Wieczorek D, Derejko M, et al. The influence of vascular risk factors and white matter hyperintensities on the degree of cognitive impairment in Parkinson's disease. Neurol Neurochir Pol 2008;42:505–512. [PubMed] [Google Scholar]
- 50. Hanning U, Teuber A, Lang E, Trenkwalder C, Mollenhauer B, Minnerup H. White matter hyperintensities are not associated with cognitive decline in early Parkinson's disease—The DeNoPa cohort. Parkinsonism Relat Disord 2019;69:61–67. [DOI] [PubMed] [Google Scholar]
- 51. Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 2017;23:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scarpina F, Tagini S. The Stroop Color and Word Test. Front Psychol 2017;8:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small‐vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128(Pt. 9):2034–2041. [DOI] [PubMed] [Google Scholar]
- 54. D’Hooghe MB, Gielen J, Van Remoortel A, et al. Single MRI‐based volumetric assessment in clinical practice is associated with MS‐related disability. J Magn Reson Imaging 2019;49:1312–1321. [DOI] [PubMed] [Google Scholar]
- 55. Melzer TR, Watts R, MacAskill MR, et al. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology 2013;80:1841–1849. [DOI] [PubMed] [Google Scholar]
- 56. Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson's disease. Hum Brain Mapp 2014;35:1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lovden M, Kohncke Y, Laukka EJ, et al. Changes in perceptual speed and white matter microstructure in the corticospinal tract are associated in very old age. Neuroimage 2014;102(Pt. 2):520–530. [DOI] [PubMed] [Google Scholar]
- 58. Karahan E, Costigan AG, Graham KS, Lawrence AD, Zhang J. Cognitive and white‐matter compartment models reveal selective relations between corticospinal tract microstructure and simple reaction time. J Neurosci 2019;39:5910–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Riccitelli GC, Pagani E, Rodegher M, et al. Imaging patterns of gray and white matter abnormalities associated with PASAT and SDMT performance in relapsing‐remitting multiple sclerosis. Mult Scler 2019;25:204–216. [DOI] [PubMed] [Google Scholar]
- 60. Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci 2017;131:715–728. [DOI] [PubMed] [Google Scholar]
- 61. Duering M, Zieren N, Herve D, et al. Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel‐based lesion‐symptom mapping study in CADASIL. Brain 2011;134(Pt. 8):2366–2375. [DOI] [PubMed] [Google Scholar]
- 62. Duering M, Gonik M, Malik R, et al. Identification of a strategic brain network underlying processing speed deficits in vascular cognitive impairment. Neuroimage 2013;66:177–183. [DOI] [PubMed] [Google Scholar]
- 63. Luo C, Li M, Qin R, et al. White matter microstructural damage as an early sign of subjective cognitive decline. Front Aging Neurosci 2019;11:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ohlhauser L, Parker AF, Smart CM, Gawryluk JR; Alzheimer's Disease Neuroimaging Initiative. White matter and its relationship with cognition in subjective cognitive decline. Alzheimers Dement (Amst) 2019;11:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Halliday DWR, Gawryluk JR, Garcia‐Barrera MA, MacDonald SWS. White matter integrity is associated with intraindividual variability in neuropsychological test performance in healthy older adults. Front Hum Neurosci 2019;13:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Habes M, Erus G, Toledo JB, et al. Regional tract‐specific white matter hyperintensities are associated with patterns to aging‐related brain atrophy via vascular risk factors, but also independently. Alzheimers Dement 2018;10:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vesely B, Antonini A, Rektor I. The contribution of white matter lesions to Parkinson's disease motor and gait symptoms: a critical review of the literature. J Neural Transm (Vienna) 2016;123:241–250. [DOI] [PubMed] [Google Scholar]
- 68. Dadar M, Zeighami Y, Yau Y, et al. White matter hyperintensities are linked to future cognitive decline in de novo Parkinson's disease patients. Neuroimage Clin 2018;20:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mak E, Dwyer MG, Ramasamy DP, et al. White matter hyperintensities and mild cognitive impairment in Parkinson's disease. J Neuroimaging 2015;25:754–760. [DOI] [PubMed] [Google Scholar]
- 70. Edwards LJ, Pine KJ, Ellerbrock I, Weiskopf N, Mohammadi S. NODDI‐DTI: estimating neurite orientation and dispersion parameters from a diffusion tensor in healthy white matter. Front Neurosci 2017;11:720. [DOI] [PMC free article] [PubMed] [Google Scholar]


