Abstract
Two of the key functions of arteries in the brain are (1) the well‐recognized supply of blood via the vascular lumen and (2) the emerging role for the arterial walls as routes for the elimination of interstitial fluid (ISF) and soluble metabolites, such as amyloid beta (Aβ), from the brain and retina. As the brain and retina possess no conventional lymphatic vessels, fluid drainage toward peripheral lymph nodes is mediated via transport along basement membranes in the walls of capillaries and arteries that form the intramural peri‐arterial drainage (IPAD) system. IPAD tends to fail as arteries age but the mechanisms underlying the failure are unclear. In some people this is reflected in the accumulation of Aβ plaques in the brain in Alzheimer's disease (AD) and deposition of Aβ within artery walls as cerebral amyloid angiopathy (CAA). Knowledge of the dynamics of IPAD and why it fails with age is essential for establishing diagnostic tests for the early stages of the disease and for devising therapies that promote the clearance of Aβ in the prevention and treatment of AD and CAA. This editorial is intended to introduce the rationale that has led to the establishment of the Clearance of Interstitial Fluid (ISF) and CSF (CLIC) group, within the Vascular Professional Interest Area of the Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment.
Keywords: cerebrospinal fluid, clearance, interstitial fluid, IPAD, ISTAART
1. FOCUS OF CLIC
Why is there a need for the CLIC Group within ISTAART's Vascular PIA? For 20 years, the focus of treatments to relieve the burden of amyloid in the Alzheimer's disease (AD) brain has been on immunotherapy, but this has not been a complete success. Although amyloid beta (Aβ) deposits are removed from the cerebral cortex, there is a significant increase in cerebral amyloid angiopathy (CAA) after Aβ immunotherapy; this indicates that Aβ removed from the cortex is deposited in artery walls due to blockage of the intramural peri‐arterial drainage (IPAD) system. 1 , 2 Shifting the focus from the brain tissue to IPAD may allow us to free a bottleneck and to increase the effectiveness of immunotherapy. A similar strategy for improving IPAD will help to prevent the initial age‐related accumulation of Aβ in the brain, thus preventing the development of AD.
What are the structure and aims of the group? This is an interdisciplinary assembly of scientists, clinicians, and drug developers who have already contributed significantly to the mechanisms of central nervous system (CNS) fluid balance and exchange, pathology of cerebrovascular disease, pathogenesis of Aβ accumulation in AD and cerebral amyloid angiopathy (CAA), development of AD therapeutics, and delivery of therapeutics to the brain via intrathecal dosing into the cerebrospinal fluid (CSF). The role of each member of CLIC will become apparent in the brief account below of the anatomy, physiology, and pathology of IPAD and CSF related to AD.
2. MISSION OF THE CLIC GROUP
In the short term, members of the group will establish contacts and familiarize themselves with the spectrum of research in IPAD and related fields. Virtual meetings hosting seminars for greater mutual understanding of respective research focuses are envisioned.
In the longer term, members of CLIC will form multidisciplinary groups to gain a greater understanding of the dynamics of IPAD and CSF circulation in AD and CAA. Each group will establish funding streams to facilitate collaborative multidisciplinary research.
The overarching objectives of the CLIC are to:
Understand the changes with age in the peripheral physiology that underly impaired IPAD.
Understand the cellular and molecular mechanisms underlying IPAD physiology in the brain.
Establish novel diagnostic tests for AD, CAA, and vasomotion based on our knowledge of IPAD and the fluid dynamics of CSF.
Establish novel therapies that facilitate IPAD for the elimination of Aβ from the aging brain to prevent or reduce established AD (and CAA?) pathology.
3. BACKGROUND TO IPAD AND ITS FAILURE WITH AGE, AD, AND CAA
Aβ and other soluble peptides such as cystatin C are present in the interstitial fluid (ISF) of the brain. Aβ is normally cleared from the brain by several mechanisms: it is eliminated across the vascular endothelium via a lipoprotein receptor LRP‐1, 3 or taken up by microglia, astrocytes, and perivascular macrophages. 4 , 5 , 6 Another major pathway for the elimination of Aβ is via ISF drainage. As there are no conventional lymphatic vessels in the brain, ISF is eliminated along the walls of cerebral blood vessels. Several anatomical routes have been proposed for elimination of ISF, including alongside venules (glymphatic system) and along basement membranes in the walls of capillaries and arteries (IPAD pathways; 7 , 8 Figure 1A,B). Although the exact roles of the observed drainage pathways have not been fully elucidated, the IPAD pathway corresponds more closely to neuropathological observations of vascular Aβ deposits in CAA, which are mainly found in the walls of capillaries and arteries. IPAD becomes less effective with age, in the presence of the apolipoprotein E (APOE)4 genotype, and with apparent transient overloading after immunotherapy for Aβ 7 and CAA‐related inflammation (CAA‐ri), 8 , 9 all leading to increased CAA. 10 , 11 , 12 , 13 , 14 IPAD is not a passive process and the motive force for IPAD is derived from vascular pulsations. Recently it has been suggested that the spontaneous low‐frequency contraction and relaxation of vascular smooth muscle cells (ie, vasomotion), and possibly pericytes, may be important drivers for IPAD. 15 , 16 , 17 The diversity of the vascular mural cells and their relative vulnerability during aging is likely to impact the efficiency of IPAD from different regions of the brain. 18 , 19 Components of the extracellular matrix within the IPAD pathways, in particular perlecan, appear to impede the clearance of Aβ. 20 Finally, the complex interactions between APOE and Aβ, although not fully understood, likely influence clearance of ISF and Aβ as well. 21 , 22
FIGURE 1.
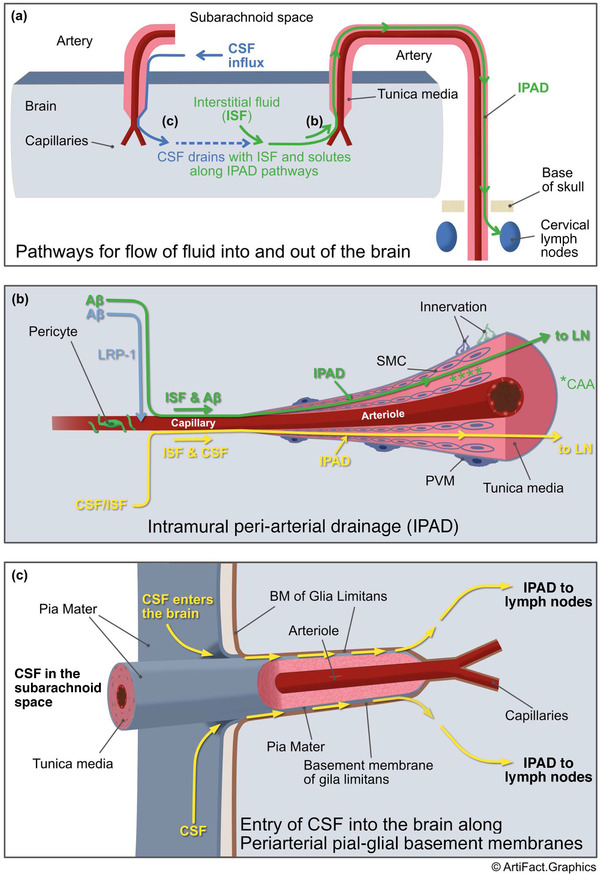
A, Intramural peri‐arterial drainage (IPAD) pathways for the lymphatic drainage of interstitial fluid (ISF) and soluble amyloid beta (Aβ) from the brain. Right side of diagram: ISF and Aβ (green line and arrows) pass from the extracellular spaces of the brain to drain along the walls of capillaries and arteries ultimately to the cervical lymph nodes adjacent to arteries under the base of the skull. Details of the IPAD pathway are shown in (B). Left side shows how cerebrospinal fluid (CSF) enters the brain along the outer aspects of penetrating arteries and passes into the ISF of the brain parenchyma (details in [C]) and then flows out of the brain along IPAD pathways (green line and arrows). B) Details of the IPAD pathway, cerebral amyloid angiopathy (CAA) and LRP‐1‐related absorption for Aβ. Soluble Aβ (light blue arrow), produced by cells in the brain, is absorbed into the blood involving LRP‐1 as one of the pathways for elimination of Aβ. Another major pathway is by IPAD (green line and arrows). Aβ in the ISF enters the basement membranes of endothelial cells in the walls of capillaries. Contractile pericytes surround capillaries and may supply the motive force for IPAD in capillaries. Aβ then rapidly passes into basement membranes (100 to 150 nm thick) surrounding smooth muscle cells (SMC)s in the tunica media of cerebral arteries. Changes occur in the walls of arteries as they age and IPAD is impaired resulting in the deposition of fibrillar Aβ in the IPAD pathways as CAA (green asterisks). As more Aβ is deposited and the severity of CAA increases, the wall of the artery is disrupted, SMCs are replaced by Aβ and IPAD is further impaired. The yellow line passing along the IPAD pathway shows how CSF that has entered the ISF of the brain also drains from the brain along IPAD pathways in artery walls. SMCs in the tunica media of arteries supply the motive force for IPAD and have both adrenergic and cholinergic innervations. C, Entry of CSF into the brain along periarterial pial–glial basement membranes. As arteries enter the surface of the cerebral cortex they are coated by a layer of pia mater that is firmly associated with the basement membranes of the glia limitans on the surface of the brain. There are no perivascular spaces around cortical arterioles so tracers injected into the CSF enter the brain along the periarterial pial–glial basement membranes and mix with the ISF in the brain parenchyma. CSF tracers are then eliminated from the brain along IPAD pathways. This route could be used to deliver drugs to increase the efficiency of elimination of Aβ along aging IPAD pathways. PVM, perivascular macophage; SAS, subarachnoid space
4. AMYLOID ANGIOPATHY IN RETINAL ARTERIES
Deposits of Aβ have recently been identified in the tunica media of retinal arteries in post‐mortem retinae of patients with AD. 23 , 24 Accumulation of Aβ in the retina has been closely linked to an early loss of retinal vascular smooth muscle cells (vSMC) and pericytes expressing platelet‐derived growth factor receptor (PDGFR)β. 25 Furthermore, the extent of loss of vascular PDGFRβ predicted retinal amyloid angiopathy scores. 25 Levels of amyloid angiopathy in the retinal vessels may prove to be a readily accessible and potentially valuable index of severity of CAA in the brain.
5. MODELS OF CAA OR SMALL VESSEL DISEASE
While CAA due to accumulation of Aβ in the IPAD pathway is a common feature of cerebral small vessel disease, CAA is also seen in rarer disorders involving IPAD such as Familial British dementia due to mutations in the BRI gene and Icelandic CAA due to mutations in the gene encoding the cystatin protein. 26 , 27 It is important to identify appropriate models to study therapeutic targets for CAA or other small vessel diseases. Different transgenic mouse models such as APP/PS1, TgSwDI, or Tg2576 are used to test individual hypotheses related to CAA, albeit with limitations. 28 Rodent models for the study of vascular dysfunction include hyperhomocysteinemia and hypoperfusion due to occlusion of a carotid artery. 29 , 30 , 31 , 32 In addition, non‐human primate models are known to develop extensive CAA thus offering potential preclinical avenues for investigation, especially in the context of white matter lesions. 33 , 34 Intramural accumulation of cystatin amyloid aggregates in Icelandic CAA are also observed in skin vessels, suggesting that in vitro models of this disorder could provide convenient platforms to study mechanistic properties of affected vessels. 35
6. DEVELOPING NOVEL THERAPEUTIC STRATEGIES
New therapeutic avenues for CAA (and AD) that act via IPAD include interventions modulating vasomotion, the postulated motive force of IPAD. This could potentially be accomplished by enhancing low‐frequency oscillations of the vascular smooth muscle cells through neurovascular coupling or during sleep, 36 or by noradrenergic innervation of cortical vSMCs. 37 Other potential therapeutic targets are intracellular mitochondrial systems, 38 chaperone molecules such as clusterin, 39 , 40 or combination therapies. 41 Pharmaceutical approaches may include vasoactive drugs that promote IPAD, resulting in maintenance of vascular integrity and reduction of Aβ deposits. 42
Other proposed mechanisms for vascular dysfunction in the brain include mitochondrial dysfunction, metabolic failure, autoimmunity, initiation of mechanisms of cell death and inflammation (with involvement of the neurovascular unit, including endothelial cells, vSMCs, pericytes, as well as glial cells). Each of these processes may contribute to impaired clearance of fluids, including soluble Aβ and hyperphosphorylated tau from the brain. 43 , 44 Multiple studies are currently evaluating strategies to ameliorate these pathways.
7. CEREBROSPINAL FLUID
CSF is produced by the choroid plexus and while some CSF may pass into venous blood via arachnoid villi and granulations in humans, CSF also drains along lymphatics located in the dural meninges and in cranial and spinal nerve sheaths en route to regional head, neck, and perispinal lymph nodes. 45 , 46 , 47 The route along channels that are adjacent to olfactory nerves entering the nasal mucosa is emerging as an important pathway for the diagnosis of AD. 48
The relative contribution of these drainage pathways to overall clearance of CSF and solutes as well as a specific point of anatomical confluence between vascular wall and lymphatic routes remains to be further elucidated. Measures of overall clearance of molecules via the CSF to the periphery or along each of these specific routes could emerge as important biomarkers for diagnosing failure of clearance of fluid from the CNS in diseases such as AD. 48 , 49 , 50 , 51
8. INTERCONNECTIONS BETWEEN CSF AND ISF IN THE BRAIN
In vivo imaging data from human studies show that molecules within the CSF are in direct communication with the ISF. 52 , 53 In vivo imaging studies showed that tracers administered into CSF enter the brain along the periarterial pial‐glial basement membranes between pia mater coating the arteries and the glia limitans of the cerebral cortex. 54 , 55 These boundaries give rise to a periarterial compartment filled with extracellular matrix around arteries (observed as “perivascular spaces” on in vivo imaging 56 ), facilitating the transport of CSF into the brain. 8 After tracers have entered the parenchyma from the CSF they mix with ISF, 55 prior to leaving the brain by IPAD 55 (Figure 1a‐c). As suggested by the glymphatic system, an alternative clearance route for ISF may be alongside the walls of venules. Further experimental studies are needed to fully elucidate the relative contribution of venules and arteries to the clearance of ISF from the parenchyma. There is an increased incidence of AD pathology, including CAA, in patients with idiopathic normal pressure hydrocephalus (iNPH), most likely as a result of disturbances in the dynamics of CSF‐ISF. 57 Patients with iNPH could therefore be a valuable model for the study of interactions CSF–ISF.
As CSF enters the brain along basement membranes surrounding the walls of cortical arteries, this represents an important pathway for drug delivery, including novel antisense oligonucleotides. 49 Drugs injected into the CSF may also have unique access to influence the dynamics of ISF clearance along IPAD pathways.
There are meningeal lymphatics in the dural lining of the skull, but their role in clearing ISF is unclear. 58 Tracers injected into the CSF drain into parasaggital lymphatics in the dura and reach cervical lymph nodes. 53
9. CAA‐RI AND ARIA: CLINICAL–RADIOLOGICAL ABNORMALITIES POTENTIALLY RELATED TO THE FAILURE OF DRAINAGE OF FLUID FROM THE BRAIN
It is widely accepted that amyloid‐related imaging abnormalities (ARIA) represent a major unwanted effect of Aβ immunotherapy for AD. The features of both ARIA‐E, in which there is evidence of vasogenic edema and inflammation, and ARIA‐H, in which there is evidence of hemosiderin deposits, microhemorrhages, and cortical superficial siderosis suggest the disruption of the normal interactions among ISF, CSF, and walls of blood vessels. 7 , 59 Both ARIA‐E and ARIA‐H phenomena have been demonstrated spontaneously in CAA‐ri, a rare autoimmune encephalopathy mediated by autoantibody targeting cerebrovascular Aβ. 8
Like immunotherapy‐induced ARIA, it is hypothesized that increased anti‐Aβ auto antibodies in the CSF promote the clearance of Aβ plaques from the CNS as evidenced by the increased amount of soluble Aβ40 and Aβ42 and reduced Aβ‐PET uptake. According to the “ARIA Paradox” pathogenic model 9 the initiating factors and immune‐mediated mechanisms of CAA‐ri and ARIA are thought to be a complicated mixture of genetic, vascular, and immunological risk factors closely related to the Aβ burden and the dose‐ and time‐related effects of anti‐Aβ antibodies. 59 It is thus likely that the severity of CAA and the CAA‐related impairment of neurovascular coupling functions, including the complex interplay among microglia, astrocytes, endothelial, and vSMC are the game‐changers in determining vascular dysfunction and impairment of clearance of Aβ and hyperphosphorylated tau from the brain. 60 , 61
To this end, anti‐Aβ autoantibodies and CAA‐ri could offer a unique possibility to explore the relationships between pathways of Aβ clearance and enable development of innovative therapies, representing a human spontaneous model of Aβ immunotherapy.
10. CONCLUSION
Our interdisciplinary group aims to further the understanding of how ISF and CSF are involved in the pathology of AD and related dementias and how ISF and CSF may be harnessed for diagnostic tests and for disease‐modifying therapies.
CONFLICTS OF INTEREST
Multiple authors are members of the ISTAART Vascular Cognitive Disorders PIA. The authors declare no conflicts of interest.
Carare RO, Aldea R, Agarwal N, et al. Clearance of interstitial fluid (ISF) and CSF (CLIC) group—part of Vascular Professional Interest Area (PIA). Alzheimer's Dement. 2020;12:e12053 10.1002/dad2.12053
REFERENCES
- 1. Boche D, Zotova E, Weller RO, et al. Consequence of Abeta immunization on the vasculature of human Alzheimer's disease brain. Brain. 2008;131:3299‐3310. [DOI] [PubMed] [Google Scholar]
- 2. Nicoll JAR, Buckland GR, Harrison CH, et al. Persistent neuropathological effects 14 years following amyloid‐beta immunization in Alzheimer's disease. Brain. 2019;142:2113‐2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deane R, Sagare A, Hamm K, et al. apoE isoform‐specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002‐4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta‐amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci. 2009;106:1261‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilcock DM, Munireddy SK, Rosenthal A, Ugen KE, Gordon MN, Morgan D. Microglial activation facilitates Abeta plaque removal following intracranial anti‐Abeta antibody administration. NeurobiolDis. 2004;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 6. Bechmann I, Priller J, Kovac A, et al. Immune surveillance of mouse brain perivascular spaces by blood‐borne macrophages. Eur J Neurosci. 2001;14:1651‐1658. [DOI] [PubMed] [Google Scholar]
- 7. Sperling RA, Jack CR, Jr , Black SE, et al. Amyloid‐related imaging abnormalities in amyloid‐modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piazza F, Greenberg SM, Savoiardo M, et al. Anti‐amyloid beta autoantibodies in cerebral amyloid angiopathy‐related inflammation: implications for amyloid‐modifying therapies. Ann Neurol. 2013;73(4):449‐458. [DOI] [PubMed] [Google Scholar]
- 9. Piazza F, Winblad B. Amyloid‐related imaging abnormalities (ARIA) in immunotherapy trials for Alzheimer's disease: need for prognostic biomarkers?. J Alzheimers Dis. 2016;52:417‐420. [DOI] [PubMed] [Google Scholar]
- 10. Hawkes CA, Sullivan PM, Hands S, Weller RO, Nicoll JA, Carare RO. Disruption of arterial perivascular drainage of amyloid‐beta from the brains of mice expressing the human APOE epsilon4 allele. PLoS One. 2012;7:e41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arbel‐Ornath M, Hudry E, Eikermann‐Haerter K, et al. Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer's disease mouse models. Acta Neuropathol. 2013;126(3):353‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO. Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol Appl Neurobiol. 2013;39:593‐611. [DOI] [PubMed] [Google Scholar]
- 13. Carare RO, Teeling JL, Hawkes CA, et al. Immune complex formation impairs the elimination of solutes from the brain: implications for immunotherapy in Alzheimer's disease. Acta Neuropathol Commun. 2013;1:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hawkes CA, Gatherer M, Sharp MM, et al. Regional differences in the morphological and functional effects of aging on cerebral basement membranes and perivascular drainage of amyloid‐beta from the mouse brain. Aging Cell. 2013;12:224‐236. [DOI] [PubMed] [Google Scholar]
- 15. Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G. Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front Aging Neurosci. 2019;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carare RO, Aldea R, Bulters D, et al. Vasomotion drives periarterial drainage of abeta from the brain. Neuron. 2020;105:400‐401. [DOI] [PubMed] [Google Scholar]
- 17. van Veluw SJ, Hou SS, Calvo‐Rodriguez M, et al. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron. 2019;105(3):549‐561.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grant RI, Hartmann DA, Underly RG, Berthiaume AA, Bhat NR, Shih AY. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J Cereb Blood Flow Metab. 2019;39:411‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watson AN, Berthiaume AA, Faino AV, et al. Mild pericyte deficiency is associated with aberrant brain microvascular flow in aged PDGFRbeta(+/‐) mice. J Cereb Blood Flow Metab. 2020;271678×19900543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu CC, Zhao N, Yamaguchi Y, et al. Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid‐beta clearance and aggregation in Alzheimer's disease. Sci Transl Med. 2016;8:332ra344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu CC, Zhao N, Fu Y, et al. ApoE4 accelerates early seeding of amyloid pathology. Neuron. 2017;96:1024‐1032.e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martel CL, Mackic JB, Matsubara E, et al. Isoform‐specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood‐brain barrier transport of circulating Alzheimer's amyloid beta. J Neurochem. 1997;69:1995‐2004. [DOI] [PubMed] [Google Scholar]
- 23. Koronyo‐Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer's patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54(suppl 1):S204‐S217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koronyo Y, Salumbides BC, Black KL, Koronyo‐Hamaoui M. Alzheimer's disease in the retina: imaging retinal abeta plaques for early diagnosis and therapy assessment. Neurodegener Dis. 2012;10:285‐293. [DOI] [PubMed] [Google Scholar]
- 25. Shi H, Koronyo Y, Rentsendorj A, et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer's disease retina. Acta Neuropathol. 2020;139(5):813‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lashley T, Holton JL, Verbeek MM, et al. Molecular chaperons, amyloid and preamyloid lesions in the BRI2 gene‐related dementias: a morphological study. Neuropathol Appl Neurobiol. 2006;32:492‐504. [DOI] [PubMed] [Google Scholar]
- 27. Snorradottir AO, Isaksson HJ, Kaeser SA, et al. Deposition of collagen IV and aggrecan in leptomeningeal arteries of hereditary brain haemorrhage with amyloidosis. Brain Res. 2013;1535:106‐114. [DOI] [PubMed] [Google Scholar]
- 28. Jakel L, Van Nostrand WE, Nicoll JAR, Werring DJ, Verbeek MM. Animal models of cerebral amyloid angiopathy. Clin Sci. 2017;131:2469‐2488. [DOI] [PubMed] [Google Scholar]
- 29. Sudduth TL, Weekman EM, Brothers HM, Braun K, Wilcock DM. Beta‐amyloid deposition is shifted to the vasculature and memory impairment is exacerbated when hyperhomocysteinemia is induced in APP/PS1 transgenic mice. Alzheimers Res Ther. 2014;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gooch J, Wilcock DM. Animal models of vascular cognitive impairment and dementia (VCID). Cell Mol Neurobiol. 2016;36:233‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hainsworth AH, Yeo NE, Weekman EM, Wilcock DM. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta. 2016;1862:1008‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okamoto Y, Yamamoto T, Kalaria RN, et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 2012;123:381‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alber J, Alladi S, Bae HJ, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dement. 2019;5:107‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nehete PN, Williams LE, Chitta S, et al. Class C CpG Oligodeoxynucleotide immunomodulatory response in aged squirrel monkey (Saimiri Boliviensis Boliviensis). Front Aging Neurosci. 2020;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Snorradottir AO, Isaksson HJ, Ingthorsson S, Olafsson E, Palsdottir A, Bragason BT. Pathological changes in basement membranes and dermal connective tissue of skin from patients with hereditary cystatin C amyloid angiopathy. Lab Invest. 2017;97(4):383‐394. [DOI] [PubMed] [Google Scholar]
- 36. Meghdadi AH, Popovic D, Rupp G, Smith S, Berka C, Verma A. Transcranial impedance changes during sleep: a rheoencephalography study. IEEE J Transl Eng Health Med. 2019;7:2700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kelly SC, McKay EC, Beck JS, Collier TJ, Dorrance AM, Counts SE. Locus coeruleus degeneration induces forebrain vascular pathology in a transgenic rat model of Alzheimer's disease. J Alzheimers Dis. 2019;70:371‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Provensi G, Carta F, Nocentini A, et al. A new kid on the block? Carbonic anhydrases as possible new targets in Alzheimer's disease. Int J Mol Sci. 2019;20(19):4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilson MR, Zoubeidi A. Clusterin as a therapeutic target. Expert Opin Ther Targets. 2017;21:201‐213. [DOI] [PubMed] [Google Scholar]
- 40. Wojtas AM, Kang SS, Olley BM, et al. Loss of clusterin shifts amyloid deposition to the cerebrovasculature via disruption of perivascular drainage pathways. Proc Natl Acad Sci. 2017;114:E6962‐E6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saito S, Ihara M. New therapeutic approaches for Alzheimer's disease and cerebral amyloid angiopathy. Front Aging Neurosci. 2014;6:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maki T, Okamoto Y, Carare RO, et al. Phosphodiesterase III inhibitor promotes drainage of cerebrovascular beta‐amyloid. Ann Clin Transl Neurol. 2014;1:519‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fossati S, Giannoni P, Solesio ME, et al. The carbonic anhydrase inhibitor methazolamide prevents amyloid beta‐induced mitochondrial dysfunction and caspase activation protecting neuronal and glial cells in vitro and in the mouse brain. Neurobiol Dis. 2016;86:29‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Solesio ME, Peixoto PM, Debure L, et al. Carbonic anhydrase inhibition selectively prevents amyloid beta neurovascular mitochondrial toxicity. Aging Cell. 2018;17:e12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow‐Robin) spaces in the human cerebrum. J Anat. 1990;170:111‐123. [PMC free article] [PubMed] [Google Scholar]
- 46. Weller RO, Kida S, Zhang ET. Pathways of fluid drainage from the brain–morphological aspects and immunological significance in rat and man 64. Brain Pathol. 1992;2:277‐284. [DOI] [PubMed] [Google Scholar]
- 47. Zhang ET, Richards HK, Kida S, Weller RO. Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropathol. 1992;83:233‐239. [DOI] [PubMed] [Google Scholar]
- 48. de Leon MJ, Li Y, Okamura N, et al. Cerebrospinal fluid clearance in Alzheimer disease measured with dynamic PET. J Nucl Med. 2017;58:1471‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mazur C, Powers B, Zasadny K, et al. Brain pharmacology of intrathecal antisense oligonucleotides revealed through multimodal imaging. JCI Insight. 2019;4(20):e129240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tangen K, Nestorov I, Verma A, Sullivan J, Holt RW, Linninger AA. In vivo intrathecal tracer dispersion in cynomolgus monkey validates wide biodistribution along neuraxis. IEEE Trans Biomed Eng. 2020;67:1122‐1132. [DOI] [PubMed] [Google Scholar]
- 51. Verma A, Hesterman JY, Chazen JL, et al. Intrathecal (99m)Tc‐DTPA imaging of molecular passage from lumbar cerebrospinal fluid to brain and periphery in humans. Alzheimers Dement. 2020;12:e12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eide PK, Ringstad G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol Open. 2015;4:2058460115609635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ringstad G, Eide PK. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun. 2020;11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morris AW, Sharp MM, Albargothy NJ, et al. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016;131:725‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Albargothy NJ, Johnston DA, MacGregor‐Sharp M, et al. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 2018;136(1):139‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16:137‐153. [DOI] [PubMed] [Google Scholar]
- 57. Luikku AJ, Hall A, Nerg O, et al. Predicting development of Alzheimer's disease in patients with shunted idiopathic normal pressure hydrocephalus. J Alzheimers Dis. 2019;71:1233‐1243. [DOI] [PubMed] [Google Scholar]
- 58. Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018;560:185‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sperling R, Salloway S, Brooks DJ, et al. Amyloid‐related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Greenberg SM, Bacskai BJ, Hernandez‐Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways. Nat Rev Neurol. 2020;16:30‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction‐The disregarded partner of Alzheimer's disease. Alzheimers Dement. 2019;15:158‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]


