The contribution of hypertension to stroke and dementia is now well established. 1 Hypertension‐induced hemorrhagic strokes killed the 3 allied leaders of World War II—Roosevelt, Stalin, and Churchill—a bit of historical trivia that reminds us how far we have come in terms of treatment. Despite antihypertensive drugs and dietary changes having dramatically reduced the incidence of strokes, 2 the devastating effects of hypertension on the brain remain a public health problem, and dementia is on the rise.
In addition to affecting large‐sized and middle‐sized cerebral arteries causing thrombotic and hemorrhagic strokes, hypertension also affects small vessels and capillaries in subcortical brain areas resulting in “silent” ischemia. In T2‐weighted brain magnetic resonance images, these silent strokes appear as hyperintensities in the deep white matter hyper intensities (WMH) in periventricular regions sparing subcortical U fibers, 3 and their presence is associated with an increased risk for cognitive impairment and dementia. 4 , 5
Although hypertension is the main cause of dementia in some patients, it can also be an accomplice in others. Namely, in patients with Alzheimer's disease (AD), coexistent cerebral small vessel disease and tissue inflammation likely accelerate brain injury. Postmortem studies in these patients show features of cerebral vascular damage in combination with β‐amyloid and tau neuropathology. 6 , 7 Hypertension‐associated WMH burden is associated with cognitive decline in AD, independent of the brain β‐amyloid burden. 4 Collectively, these pieces of evidence point to the notion that hypertension‐induced brain vascular disease contributes to the progressive functional deterioration of patients with AD. 8 Thus, treating hypertension could slow cognitive decline in AD. In the general population, treatment with antihypertensive agents significantly reduces the risk of all‐cause dementia and cognitive impairment. 9
Mounting evidence suggests that many individuals with synucleinopathies—including Parkinson's disease (PD), dementia with Lewy bodies, multiple system atrophy, and pure autonomic failure—also suffer vascular damage in their brain that may accelerate the neurodegenerative process, be responsible for specific deficits, and account for increased mortality. Interestingly, in patients with dementia with Lewy bodies, a synucleinopathy with dramatic cognitive impairment, the volume of WMH appears to be even higher than in patients with AD, 10 and several groups reported that higher WMH burden in patients with early PD predicts greater future cognitive decline and cortical atrophy compared with controls. 11 , 12 , 13 In this issue of Movement Disorders Clinical Practice, a meticulous study by Linortner and colleagues 14 expands our understanding of the link between WMH and cognition, showing that, in patients with PD, WMH severity is specifically associated with deficits in executive function, attention, and working memory.
Although these data suggest that we can reduce the incidence of dementia in the general population and the speed of cognitive deterioration in those with AD by treating hypertension, the blood pressure (BP) phenotype in patients with synucleinopathies is more complex, and the therapeutic decision‐making process much more challenging. Specifically, patients with synucleinopathies have baroreflex dysfunction resulting in marked BP variability with neurogenic orthostatic hypotension (nOH), causing syncope and falls, together with hypertension when supine. 15 What is the clinician to do? Treat hypertension and increase the risk of hypotension‐induced falls, or treat nOH and disregard supine hypertension? Of course, we must address both problems. However, to balance the risks and benefits of each approach we need to understand the impact of each abnormality.
In contrast to AD and vascular dementia, the link between hypertension, WMH, and cognition in PD has not been sufficiently emphasized, as the focus has been on the more evident and urgent nOH. Could nOH, similarly to hypertension, cause WMH? So far, only 3 studies looked into the association between cognitive impairment, nOH, and WMH in patients with PD. 16 , 17 , 18 The results were inconsistent, but nOH appeared to be associated with WMH and worse cognition. In this issue of Movement Disorders Clinical Practice, Dadar and colleagues look at the relationship between symptoms of autonomic dysfunction (Scales for Outcomes in Parkinson's Disease–Autonomic Dysfunction) and WMH at the time of diagnosis in patients with PD and controls and their impact on future (~4 years) cognitive decline as measured by the Montreal Cognitive Assessment score. 19 They found that the orthostatic diastolic BP drop—but not the systolic drop—at baseline was associated with higher rates of cognitive decline, but only when the WMH burden at baseline was taken into consideration.
Could nOH alone be the cause of WMH and cognitive impairment in the synucleinopathies? Difficult to know because most studies analyzing the relationship between nOH, WMH, and cognition did not correct their results for the presence of supine hypertension, which occurs in ~50% of patients with PD, even when not receiving antihypotensive drugs 20 , 21 ; and the more supine hypertension, the more nOH. Thus, it is unclear whether the findings are meaningful because nOH could be just a surrogate marker of supine hypertension.
Shedding some light on this question, we recently found that, in patients with PD and other synucleinopathies (multiple system atrophy, dementia with Lewy bodies, and pure autonomic failure), supine hypertension, particularly the average nocturnal BP but also the in‐office supine BP, was the best indicator of target organ damage and was independently associated with a higher burden of WMH, along with worse renal failure and higher prevalence of left ventricular hypertrophy. 22 Similarly, in the general population, reverse dipping (ie, nocturnal hypertension) is strongly associated with a higher burden of cerebral small vessel disease and cognitive impairment. 23 A prospective follow‐up of these patients showed that supine hypertension was independently associated with higher mortality when compared with those with nOH but without supine hypertension. 22 As it is the case in AD, it is likely that the combination of brain small vessel disease and abnormal protein accumulation (in this case, α‐synuclein) contributes to neurodegeneration and death in patients with synucleinopathies (Fig. 1).
FIG 1.
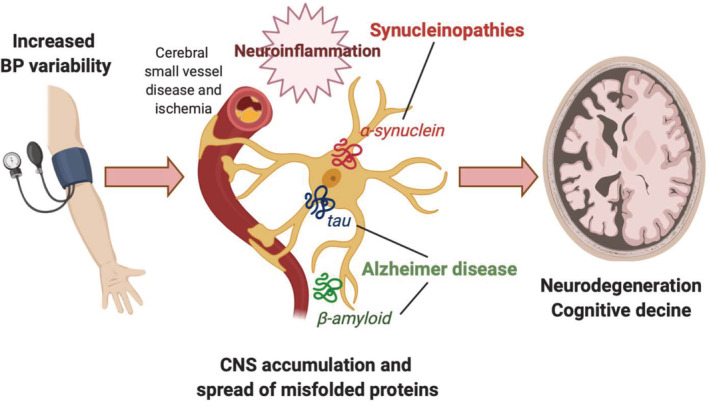
Labile BP, cerebral small vessel disease, and neurodegeneration. Mounting evidence suggest that high and abnormally labile BP causes cerebral small vessel disease with resulting ischemia and activation of neuroinflammatory mechanisms that contribute to the accumulation and spread of misfolded proteins (tau, β‐amyloid, α‐synuclein), accelerating neuronal loss and cognitive decline in Alzheimer's disease and in the synucleinopathies (Parkinson's disease, dementia with Lewy bodies, multiple system atrophy, pure autonomic failure). BP, blood pressure; CNS, central nervous system.
Most pertinent to patients with synucleinopathies, recent studies in hypertensive subjects indicate that the BP variability is a better predictor of target organ damage, including brain WMH, than the absolute BP values. Therefore, OH may contribute to target organ damage by markedly increasing BP variability. This is supported by studies showing that increased BP variability is a stronger predictor of cerebrovascular events, cognitive decline, and mortality than average BP. 24 , 25 , 26 Thus, treating BP variability, rather than absolute BP, may offer the best chance to reduce cardiovascular risk. 27
Prospective observational studies in patients with PD and other synucleinopathies to monitor daytime (standing and supine) and nocturnal BP are underway that should help define outcomes and therapeutic strategies. We need to determine the minimal BP values that are sufficient to maintain adequate organ perfusion in the standing position and the tolerable levels of high BP while supine. The stakes are high as cerebral small vessel disease is responsible for significant morbidity and mortality in patients with neurodegenerative disorders. Until disease‐modifying drugs are available, treating high and/or variable BP may be our best chance to reduce morbidity and mortality.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
H.K.: 1A, 1B, 1C, 2B
J.A.P.: 1A, 1B, 1C, 2A, 2B
Disclosures
Ethical Compliance Statement: The authors confirm that patient consent and institutional review board approval was not necessary for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This manuscript was funded in part by the National Institutes of Neurological Disorders and Stroke and the Familial Dysautonomia Foundation. J.A.P. reports funding from the National Institutes of Health, Michael J. Fox Foundation, MSA Coalition, Familial Dysautonomia Foundation, and US Food and Drug Administration. He is an advisory board member for Lundbeck, Biogen, Astellas, PTC Therapeutics, and Dr. Reddy's Laboratories; he is the managing editor of Clinical Autonomic Research and is the principal investigator in clinical studies funded by Theravance Biopharma, Biogen, and Biohaven. H.K. reports funding from the National Institutes of Health, Michael J. Fox Foundation, MSA Coalition, Familial Dysautonomia Foundation, and US Food and Drug Administration; is an advisory board Member for PTC Therapeutics, Ono, Lundbeck, Biogen, Theravance, Biohaven, Eli Lilly, Pfizer, and AstraZeneca; and is Editor‐in‐Chief of Clinical Autonomic Research.
Financial Disclosures for the Previous 12 Months: J.A.P. reports funding from the National Institutes of Health, Michael J. Fox Foundation, MSA Coalition, Familial Dysautonomia Foundation, and US Food and Drug Administration. He is an advisory board member for Lundbeck, Biogen, Astellas, PTC Therapeutics, and Dr. Reddy's Laboratories; he is the managing editor of Clinical Autonomic Research and is the principal investigator in clinical studies funded by Theravance Biopharma, Biogen, and Biohaven. H.K. reports funding from the National Institutes of Health, Michael J. Fox Foundation, MSA Coalition, Familial Dysautonomia Foundation, and US Food and Drug Administration; is advisory board member for PTC Therapeutics, Ono, Lundbeck, Biogen, Theravance, Biohaven, Eli Lilly, Pfizer, and AstraZeneca; and is Editor‐in‐Chief of Clinical Autonomic Research.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension 2013;62(5):810‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lackland DT, Roccella EJ, Deutsch AF, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke 2014;45(1):315‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marchant NL, Reed BR, DeCarli CS, et al. Cerebrovascular disease, β‐amyloid, and cognition in aging. Neurobiol Aging 2012;33(5):1006 e1025‐1006 e1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee S, Viqar F, Zimmerman ME, et al. White matter hyperintensities are a core feature of Alzheimer's disease: evidence from the dominantly inherited Alzheimer network. Ann Neurol 2016;79(6):929‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith EE, Greenberg SM. Beta‐amyloid, blood vessels, and brain function. Stroke 2009;40(7):2601‐2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu W, Wong A, Law AC, Mok VC. Cerebrovascular disease, amyloid plaques, and dementia. Stroke 2015;46(5):1402‐1407. [DOI] [PubMed] [Google Scholar]
- 8. Thorin E. Hypertension and Alzheimer disease: another brick in the wall of awareness. Hypertension 2015;65(1):36‐38. [DOI] [PubMed] [Google Scholar]
- 9. Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta‐analysis. JAMA 2020;323(19):1934‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarro L, Tosakulwong N, Schwarz CG, et al. An investigation of cerebrovascular lesions in dementia with Lewy bodies compared to Alzheimer's disease. Alzheimers Dement 2017;13(3):257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dadar M, Zeighami Y, Yau Y, et al. White matter hyperintensities are linked to future cognitive decline in de novo Parkinson's disease patients. Neuroimage Clin 2018;20:892‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chahine LM, Dos Santos C, Fullard M, et al. Modifiable vascular risk factors, white matter disease and cognition in early Parkinson's disease. Eur J Neurol 2019;26(2):246‐e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee SJ, Kim JS, Yoo JY, et al. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord 2010;24(3):227‐233. [DOI] [PubMed] [Google Scholar]
- 14. Linortner P, McDaniel C, Shahid M, Levine TF, Tian L, Cholerton B, Poston KL. White matter hyperintensities related to Parkinson's Disease executive function [published online ahead of print April 14, 2020]. Mov Disord Clin Pract . 10.1002/mdc3.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaufmann H, Norcliffe‐Kaufmann L, Palma JA. Baroreflex dysfunction. N Engl J Med 2020;382(2):163‐178. [DOI] [PubMed] [Google Scholar]
- 16. Kim JS, Oh YS, Lee KS, Kim YI, Yang DW, Goldstein DS. Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology 2012;79(13):1323‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pilleri M, Facchini S, Gasparoli E, et al. Cognitive and MRI correlates of orthostatic hypotension in Parkinson's disease. J Neurol 2013;260(1):253‐259. [DOI] [PubMed] [Google Scholar]
- 18. Oh YS, Kim JS, Lee KS. Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. J Mov Disord 2013;6(2):23‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dadar M, Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB, Collins DL. White matter hyperintensities mediate impact of dysautonomia on cognition in Parkinson's disease [published online ahead of print June 13, 2020]. Mov Disord Clin Pract. . 10.1002/mdc3.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fanciulli A, Gobel G, Ndayisaba JP, et al. Supine hypertension in Parkinson's disease and multiple system atrophy. Clin Auton Res 2016;26(2):97‐105. [DOI] [PubMed] [Google Scholar]
- 21. Fanciulli A, Jordan J, Biaggioni I, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res 2018;28(4):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palma JA, Redel‐Traub G, Porciuncula A, et al. The impact of supine hypertension on target organ damage and survival in patients with synucleinopathies and neurogenic orthostatic hypotension [published online ahead of print May 2020]. Parkinsonism Relat Disord 2020;75:97‐104. 10.1016/j.parkreldis.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chesebro AG, Melgarejo JD, Leendertz R, et al. White matter hyperintensities mediate the association of nocturnal blood pressure with cognition. Neurology 2020;94(17):e1803‐e1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Shi X, Ma C, et al. Visit‐to‐visit blood pressure variability is a risk factor for all‐cause mortality and cardiovascular disease: a systematic review and meta‐analysis. J Hypertens 2017;35(1):10‐17. [DOI] [PubMed] [Google Scholar]
- 25. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Heus RAA, Olde Rikkert MGM, Tully PJ, Lawlor BA, Claassen J, NILVAD Study Group . Blood pressure variability and progression of clinical Alzheimer disease. Hypertension 2019;74(5):1172‐1180. [DOI] [PubMed] [Google Scholar]
- 27. Kollias A, Stergiou GS, Kyriakoulis KG, Bilo G, Parati G. Treating visit‐to‐visit blood pressure variability to improve prognosis: is amlodipine the drug of choice? Hypertension 2017;70(5):862‐866. [DOI] [PubMed] [Google Scholar]


