ABSTRACT
Background
Patients with Parkinson's disease (PD) present with a broad spectrum of nonmotor features including autonomic disorders. More severe autonomic dysfunction in PD is associated with increased cognitive deficits. The presence of cerebral small‐vessel disease, measured by T2‐weighted magnetic resonance imaging white matter hyperintensity (WMH) burden, is also observed in patients with PD with faster cognitive decline.
Objective
To investigate whether baseline orthostatic hypotension and autonomic dysfunction in early‐stage PD affect later cognitive decline via mediation through cerebral small‐vessel disease.
Methods
De novo PD patients (N = 365) and age‐matched controls (N = 174) with baseline T2‐weighted/ fluid‐attenuated inversion recovery scans were selected from the Parkinson's Progression Markers Initiative. WMHs were automatically segmented. Mediation analysis was used to assess whether WMH load mediates the effect of orthostatic hypotension and autonomic dysfunction (measured by Scales for Outcomes in Parkinson's Disease–Autonomic) on future cognitive decline (measured by Montreal Cognitive Assessment) in an average of 4 years of follow‐up.
Results
Mediation analysis supported the existence of a full mediation of WMHs on the effect of diastolic orthostatic hypotension in patients with PD and future cognitive decline (average causal mediation effect: ab = −0.032, 95% confidence interval = −0.064 to −0.01, P = 0.01). There was also a partial mediation for overall autonomic dysfunction (ab = −0.027, 95% confidence interval = −0.054 to 0.00, P = 0.02).
Conclusions
WMHs fully mediate the effect of diastolic orthostatic hypotension and partially mediate the effect of autonomic dysregulation on future cognitive decline in patients with PD. Our findings support the hypothesis that autonomic dysfunction in early clinical stages predisposes the brain to WMHs through dysregulation of the blood flow in the small vessels. This in turn increases the risk of future cognitive impairment in early PD.
Keywords: Parkinson's disease, white matter hyperintensities, orthostatic hypotension, autonomic dysregulation
In addition to motor impairment, Parkinson's disease (PD) is characterized as a clinical spectrum of cognitive, neuropsychological, sleep, and a broad range of autonomic disorders including orofacial, gastrointestinal, urinary, cardiovascular, thermoregulatory, respiratory, pupillomotor, and sexual dysfunctions. 1 Dysautonomia has been found to manifest during the prodromal stage of parkinsonism, years before phenoconversion to PD. 2 , 3 , 4 The prevalence of autonomic features is associated with disease duration and severity as well as some PD medications. 5 , 6 More severe autonomic dysfunction has also been shown to be associated with increased cognitive impairment. 7 , 8 , 9
White matter hyperintensities (WMHs) are defined as areas of increased signal observed on T2‐weighted or fluid‐attenuated inversion recovery (FLAIR) magnetic resonance images. WMHs mainly result from chronic diffuse subclinical ischemia (ie, a restriction in blood supply to the brain tissue, creating oxygen and glucose shortage and consequently disturbing cellular metabolism) that primarily impacts periventricular regions but can affect all regions of the brain. 10 , 11 WMHs are commonly observed in the elderly population as well as individuals with Alzheimer's disease (AD) and reflect demyelination and axonal loss. 11 The presence of WMHs can impact cognitive function of otherwise healthy elderly individuals as well as patients with mild cognitive impairment and dementia. 12 , 13 The presence of WMHs may confound diagnosis for treating physicians and potentially hinder effective care for these patients.
In PD, WMHs have been associated with orthostatic hypotension, that is, a drop in blood pressure when going from the supine to upright position. 9 , 14 This is the most common cardiovascular autonomic dysfunction in patients with PD. 8 , 14 The association between WMH burden and postural blood pressure drop in patients with PD supports the vascular hypothesis that recurrent hypotension might lead to cerebral hypoperfusion, which might in turn cause anoxic damage to the vulnerable brain areas and potentially lead to cognitive deficits. 15 In addition to the vascular insufficiency, degenerative processes associated with aging and PD itself may also contribute to white matter changes. 14 Early WMHs have also been linked to cognitive deficits and a later increased risk of dementia in PD. 16 , 17 , 18
Here, using longitudinal data from a large multicenter cohort of de novo PD patients and age‐matched controls, we investigated the relationship between autonomic dysfunction and WMH at the time of diagnosis and future cognitive decline in PD. We also examined the hypothesis that WMHs mediate the effects of autonomic dysfunction on future cognitive decline in PD.
Methods
Patients
The Parkinson's Progression Markers Initiative (PPMI) is a longitudinal multicenter clinical study of de novo PD patients and age‐matched healthy controls (HC) (http://www.ppmi-info.org). 19 The study was approved by the institutional review board of all participating sites, and written informed consent was obtained from all participants before inclusion in the study. In the present study, all PPMI subjects who had either FLAIR or T2‐weighted magnetic resonance images at their baseline (enrolled within 7 months of the diagnosis) visit were included (NPD = 365, NHC = 174).
Clinical Assessments
HC and PD participants underwent comprehensive clinical and imaging assessments including Movement Disorders Society–Unified Parkinson's Disease Rating Scale 20 as well as additional clinical tests evaluating cognition, depression, anxiety, autonomic function, sleep, and olfaction. 19 The following are the clinical measures used:
(1) Montreal Cognitive Assessment (MoCA) measuring global cognition, short‐term memory recall (5 points), visuospatial ability (4 points), executive function (4 points), attention and working memory (6 points), language (5 points), and orientation to time and place (6 points). MoCA assessments were performed for all HC and PD subjects longitudinally (yearly follow‐ups). Higher scores indicate better cognitive performance. A cut‐off threshold of 26 (of 30) is generally used for detecting mild cognitive impairment. Decline in MoCA score during follow‐up visits after an average of 4.09 ± 1.14 years was considered as the main outcome variable in mediation analysis. Data from all available timepoints were used to estimate the rate of change in MoCA by performing a regression between the time from the baseline visit and the change in MoCA scores (from baseline value) at each timepoint. A total of 2587 of timepoints (a minimum of 3 timepoints, and a median of 5 timepoints per participant) were used to estimate decline in MoCA scores.
(2) Scales for Outcomes in Parkinson's Disease–Autonomic (SCOPA‐AUT) total score measuring global autonomic dysfunction and its subsections: orofacial (4 questions), gastrointestinal (3 questions), urinary (6 questions), cardiovascular (3 questions), thermoregulatory (4 questions), pupillomotor (1 question), and sexual (3 questions for men, 2 for women) symptoms. Higher scores indicate higher severity and frequency of symptoms of autonomic dysfunction. Baseline SCOPA‐AUT scores were used to assess the relationship between autonomic dysfunction and WMH loads. If the measurements were not obtained at the visit when scanning was performed, the closest (in terms of time) scores were used.
(3) Orthostatic blood pressure measurements: In PPMI, blood pressure was measured in both supine and standing positions at the first visit. Orthostatic changes in systolic and diastolic pressures after standing from supine position were recorded for each participant.
Image Processing
All T1‐weighted, T2‐weighted, and FLAIR images were preprocessed in 3 steps: noise reduction, intensity nonuniformity correction, and intensity normalization. 21 Using a 6‐parameter rigid registration, T2‐weighted and FLAIR images were linearly coregistered to the T1‐weighted images. The T1‐weighted images were first linearly 22 and then nonlinearly registered to a standard template of 152 healthy individuals (MNI‐ICBM‐152). 23 The WMHs were segmented using a previously validated automatic multimodality segmentation technique. 24 , 25 This technique combines a set of location and intensity features obtained from a library of manually segmented scans with a random forest classifier to detect the WMHs. The libraries used in this study were obtained from the Alzheimer's Disease Neuroimaging Initiative data set because the T2‐weighted and FLAIR sequences for the PPMI images follow the same acquisition protocol as the Alzheimer's Disease Neuroimaging Initiative. The quality of the registrations and segmentations was visually assessed and cases that did not pass this quality control were discarded (n = 43, mostly the result of acquisition artifacts such as motion). WMH load was defined as the volume of the voxels identified as WMH in the standard space (in cm3) and are thus normalized for head size. All magnetic resonance imaging (MRI) processing, segmentation, and quality control steps were blinded to clinical outcomes.
Statistical Analyses
Correlation analysis was used first to assess the relationships between WMH load, orthostatic change in blood pressure, and autonomic dysfunction, correcting for age, sex, and MRI modality (T2 or FLAIR) used for WMH segmentation. MoCA was used to assess global cognitive decline. Mixed effects models including age at baseline and time from baseline variables were used to investigate whether and how much the total MoCA score and its subdomains decline in the HC and patients with PD. In a second step, mediation analysis was used to test the hypothesis of whether vascular burden (as measured by WMH load) mediates the effect of baseline orthostatic hypotension (as measured by the drop in systolic or diastolic blood pressure between supine and standing postures) and baseline global autonomic dysfunction on the future rate of cognitive decline (as measured by the rate of change per year in MoCA scores in follow‐up visits). 26 A Sobel test 27 was used to determine if the relationship between the independent variables (orthostatic drops in blood pressure and global autonomic dysfunction) and dependent variable (cognition) has been significantly reduced after inclusion of the mediator variable (WMH load). Specifically, if WMH burden mediates much of the relationship between orthostatic hypotension or autonomic dysfunction and future cognitive decline, the relationship between orthostatic hypotension or autonomic dysfunction and future cognitive decline would be reduced (partial mediation) or eliminated (full mediation) when the model accounts for the effect of WMH burden, implying a causal relationship between the variables. Drop in systolic or diastolic blood pressure between supine and standing postures was used as a measure of orthostatic hypotension. The total SCOPA‐AUT score at baseline was used as a measure of global autonomic dysfunction, and the slope of change in MoCA was used as the rate of cognitive decline. The mediation analysis was performed using R Mediation package version 4.4.6 (freely available via the Comprehensive R Archive Network at https://cran.r-project.org/package=mediation). 28 All values were normalized prior to mediation analysis. A 95% bootstrap confidence interval based on 10,000 bootstrap samples was used to estimate significance. Mediation analysis was performed separately for the HC and patients with PD.
Results
Table 1 summarizes the demographics and clinical characteristics of the HC and PD subjects used in this study.
TABLE 1.
Descriptive statistics for the PPMI subjects enrolled in this study
| Measure | Control | De Novo PD | P Value |
|---|---|---|---|
| Participants (NTotal) | 174 | 365 | – |
| Female, n (%) | 57 (33) | 114 (32) | – |
| T1‐weighted and FLAIR scans, NBaseline, n (%) | 79 (45) | 167 (46) | – |
| T1‐weighted and T2‐weighted scans, NBaseline, n (%) | 95 (55) | 198 (54) | – |
| Age at baseline, y, mean ± SD | 60.07 ± 11.34 | 60.51 ± 9.86 | 0.414 |
| Education, y | 16.23 ± 2.81 | 15.66 ± 3.08 | 0.039 |
| MoCA at baseline, mean ± SD | 28.25 ± 1.12 | 27.24 ± 2.22 | <0.0001 |
| SCOPATotal at baseline, mean ± SD | 5.81 ± 3.77 | 9.29 ± 6.03 | <0.0001 |
| SCOPAOrofacial at baseline, mean ± SD | 0.31 ± 0.61 | 1.01 ± 1.27 | <0.0001 |
| SCOPAGastrointenstinal at baseline, mean ± SD | 0.34 ± 0.67 | 1.05 ± 1.28 | <0.0001 |
| SCOPAUrinary at baseline, mean ± SD | 3.07 ± 2.20 | 4.18 ± 2.99 | <0.0001 |
| SCOPACardiovascular at baseline, mean ± SD | 0.60 ± 0.95 | 0.87 ± 1.21 | 0.012 |
| SCOPAThermoregulatory at baseline, mean ± SD | 0.49 ± 0.74 | 0.73 ± 0.91 | 0.004 |
| SCOPAPupillomotor at baseline, mean ± SD | 0.29 ± 0.54 | 0.42 ± 0.65 | 0.039 |
| SCOPASexual dysfunction at baseline, mean ± SD | 0.71 ± 1.30 | 1.02 ± 1.54 | 0.026 |
| UPDRS III at baseline, mean ± SD | 1.17 ± 2.32 | 20.69 ± 8.91 | <0.0001 |
| Depression Scale at baseline, mean ± SD | 5.14 ± 1.43 | 5.21 ± 1.37 | 0.55 |
| State‐Train Anxiety Inventory at baseline, mean ± SD | 94.21 ± 7.17 | 93.55 ± 8.11 | 0.38 |
| WMH load at baseline, cm3, mean ± SD | 7.66 ± 10.38 | 6.93 ± 8.03 | 0.196 |
| Supine systolic blood pressure, mm Hg, mean ± SD | 132.01 ± 16.50 | 131.17 ± 15.65 | 0.58 |
| Standing systolic blood pressure, mm Hg, mean ± SD | 129.35 ± 17.71 | 126.34 ± 16.7 | 0.07 |
| Supine diastolic blood pressure, mm Hg, mean ± SD | 78.30 ± 9.10 | 77.88 ± 9.60 | 0.65 |
| Standing diastolic blood pressure, mm Hg, mean ± SD | 80.42 ± 10.04 | 78.88 ± 10.57 | 0.13 |
| BMI, kg/m2, mean ± SD | 27.37 ± 4.77 | 27.01 ± 4.38 | 0.415 |
| Excessive stroke risk factors, yes/no | 4/109 | 15/299 | 0.760 |
| Hypertension, yes/no | 43/116 | 95/239 | 0.83 |
| Hypercholesterolemia, yes/no | 19/140 | 26/308 | 0.18 |
| Hyperlipidemia, yes/no | 17/142 | 29/305 | 0.58 |
| Diabetes, yes/no | 8/151 | 16/318 | 0.91 |
Significant differences are displayed in bold. Data are number of participants in each category (N), percentage of the total population (%), and mean ± SD of key variables.
PPMI, Parkinson's Progression Marker Initiative; PD, Parkinson's disease; FLAIR, fluid attenuated inversion recovery; SD, standard deviation; MoCA, Montreal Cognitive Assessment; SCOPA‐AUT, Scales for Outcomes in Parkinson's Disease–Autonomic; WMH, white matter hyperintensity; UPDRS III, Unified Parkinson's Disease Rating Scale–Motor.
WMH load was significantly correlated with age in both the HC (moderate correlation, r = 0.474, P < 0.0001) and PD (moderate correlation, r = 0.455, P < 0.0001) cohorts, after correcting for sex and segmentation modality. Of 365 patients with PD, 25 had a pathological level of decrease in systolic blood pressure (>20 mm Hg), and 13 had a pathological level of decrease in diastolic blood pressure (>10 mm Hg). No control subjects had pathological levels of drop in either systolic or diastolic blood pressure. Controlling for overall blood pressure, age, sex, and segmentation modality, WMH load was significantly associated with orthostatic drop in diastolic blood pressure in the PD cohort (weak correlation, r = 0.12, P = 0.02), but not for systolic blood pressure (no correlation, r = 0.03, P = 0.57). There was no relationship between diastolic or systolic blood pressure drop and WMH load in the HC (no correlation, P > 0.23). In addition, controlling for age, sex, and segmentation modality, there was a significant correlation between body temperature and WMH load in the patients with PD (weak correlation, r = −0.15, P = 0.004), but not in HC (no correlation, r = 0.0379, P = 0.6235).
Table S1 summarizes the information on the use of cardiovascular medications. There was no significant difference between the HC and patients with PD in medication usage. Both the HC and patients with PD who were on cardiovascular prophylaxis medications had significantly lower WMH burden (P < 0.01). In addition, patients with PD who were taking cholesterol‐lowering medications had significantly lower WMH loads (P = 0.03). Including these medications as covariates in the correlation analyses between WMH load and orthostatic hypotension measures did not change the results. When controlling for age, sex, segmentation modality, and use of cardiovascular medications, there was no significant relationship between BMI, risk of stroke, hypertension, hypercholesterolemia, hyperlipidemia, diabetes, or WMH burden in either HC or patients with PD.
In both the HC and PD groups, MoCA scores decreased significantly with age at baseline (t Control = −5.06, t PD = −6.97, P < 0.00001) and time from the baseline visit (t Control = −2.99, t PD = −4.70, P < 0.001), equivalent to a yearly drop of 0.096 and 0.144 in MoCA scores for the HC and patients with PD, respectively. Table 2 shows the decline in MoCA subdomains for the HC and PD groups.
TABLE 2.
Decline in MoCA and its subdomains for controls and patients with PD
| Control | PD | |||||
|---|---|---|---|---|---|---|
| Domain | Age at Baseline | Time from Baseline | Yearly Drop | Age at Baseline | Time from Baseline | Yearly Drop |
| Orientation | −0.92 | −2.06 | 0.008 | −3.58*** | −4.77*** | 0.027 |
| Executive/visuospatial | −3.62** | −2.28 | 0.030 | −4.81*** | −5.78*** | 0.063 |
| Naming | −2.44* | −0.96 | 0.003 | −2.76* | −1.37 | 0.005 |
| Attention | −1.48 | −1.35 | 0.014 | −2.78* | −5.14*** | 0.046 |
| Language | −1.49 | −2.57* | 0.020 | −2.76* | −3.26** | 0.022 |
| Abstraction | 01.42 | −0.21 | 0.001 | −1.76 | −1.58 | 0.008 |
| Delayed recall | −4.40*** | −0.74 | 0.016 | −6.95*** | −0.87 | 0.015 |
| Total | −5.06*** | −2.99** | 0.096 | −6.97*** | −4.70*** | 0.144 |
Reported results are the t statistics from the mixed effects model: Score ∼ 1 + Age at Baseline + Time from Baseline + (1|ID) as well as the estimated average yearly drop in each score for each group.
P < 0.01.
P < 0.001.
P < 0.0001.
MoCA, Montreal Cognitive Assessment Score; PD, Parkinson's disease.
Autonomic Symptoms
The HC and PD cohorts were significantly different in all SCOPA‐AUT subscores (ranging from P < 0.0001 to P < 0.04; Table 1). Correcting for age, sex, segmentation sequence, and cardiovascular medications, WMH load was correlated with the cardiovascular subdomain in the PD cohort (weak correlation, r = 0.148, P = 0.007). There was no significant relationship between SCOPA‐AUT subscores and WMH load in the HC cohort (Table 3). Correcting for age, sex, segmentation modality, and cardiovascular medications, the annualized MoCA slope was significantly correlated with orofacial, gastrointestinal, cardiovascular, thermoregulatory, and pupillomotor subscores in the PD cohort, and with pupillomotor and sexual subscores in the HC cohort, reflecting significant cognitive decline in individuals with higher (worse) SCOPA‐AUT subscores (Table 3).
TABLE 3.
Correlation results of WMH load and MoCA slope with SCOPA‐AUT subscores, controlling for age, sex, and segmentation sequence
| WMH load | MoCA slope | |||
|---|---|---|---|---|
| Score | HC Cohort | PD Cohort | HC Cohort | PD Cohort |
| Orofacial | −0.014, P = 0.865 | 0.052, P = 0.348 | −0.121, P = 0.071 | −0.142, P = 0.006 |
| Gastrointestinal | 0.030, P = 0.292 | 0.076, P = 0.172 | 0.005, P = 0.941 | −0.143, P = 0.006 |
| Urinary | −0.092, P = 0.258 | 0.046, P = 0.411 | −0.074, P = 0.375 | −0.045, P = 0.234 |
| Cardiovascular | −0.071, P= 0.384 | 0.148, P = 0.007 | 0.011, P = 0.312 | −0.094, P = 0.049 |
| Thermoregulatory | 0.025, P = 0.760 | −0.004, P = 0.946 | −0.021, P = 0.796 | −0.099, P = 0.040 |
| Pupillomotor | 0.039, P = 0.639 | 0.005, P = 0.932 | −0.225, P = 0.007 | −0.189, P = 0.0004 |
| Sexual dysfunction | 0.018, P = 0.824 | 0.022, P = 0.655 | −0.126, P = 0.073 | 0.013, P = 0.824 |
Significant results after multiple comparison correction (false discovery rate) are shown in bold.
WMH, white matter hyperintensity; MoCA, Montreal Cognitive Assessment; SCOPA‐AUT, Scales for Outcomes in Parkinson's Disease–Autonomic; HC, healthy control; PD, Parkinson's disease.
Dysautonomia, WMHs, and Cognitive Decline
Linear regression analysis showed a significant effect of WMH load and total SCOPA‐AUT score on the slope of changes in MoCA score (during 4.09 ± 1.14 years of follow‐up) in the PD cohort (P = 0.02), but not in the HC cohort (Table 4).
TABLE 4.
Linear regression analysis assessing the relationship between the slope of changes in MoCA score after baseline visit and baseline WMH and SCOPA‐AUT scores, controlling for age, sex, and segmentation sequence
| Cohort | ||||
|---|---|---|---|---|
| HC | PD | |||
| Measure | Estimate | P‐Value | Estimate | P‐Value |
| Intercept | 0.046 | 0.764 | 0.027 | 0.812 |
| Age | −0.239 | 0.014 | −0.211 | 0.0006 |
| Sex | 0.314 | 0.060 | −0.186 | 0.114 |
| Modality | −0.248 | 0.128 | 0.003 | 0.976 |
| WMH load | −0.006 | 0.947 | −0.146 | 0.019 |
| SCOPA | 0.094 | 0.221 | −0.124 | 0.026 |
Model: MoCA slope ∼ WMH load + SCOPA + Age + Sex + Sequence + Cardiovascular Medications. Significant associations are displayed in bold.
MoCA, Montreal Cognitive Assessment; WMH, white matter hyperintensity; SCOPA‐AUT, Scales for Outcomes in Parkinson's Disease–Autonomic; HC, healthy control; PD, Parkinson's disease.
Mediation analysis supported the existence of a full mediation (ie, a direct effect of diastolic orthostatic hypotension on the rate of cognitive decline via WMH loads) in patients with PD (average causal mediation effect: ab = −0.032, 95% confidence interval [CI] = −0.064 to −0.01, P = 0.01; average direct effect: C′ = −0.08, 95% CI = −0.18 to 0.01, P = 0.08; proportion mediated = 0.281, 95% CI = 0.04–1.09, P = 0.03). Because there was no significant relationship between orthostatic hypotension and cognitive decline in the HC (ie, no direct effect), there was also no mediation effect in the HC (P = 0.14). Figure 1 summarizes the results of the mediation analysis for the PD cohort. Including cardiovascular medications as covariates in the mediation analysis did not change the results.
FIG. 1.
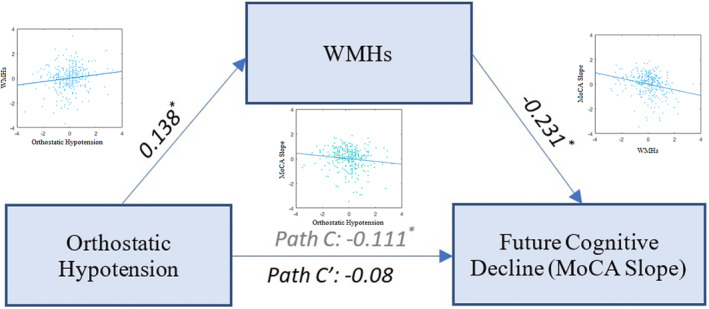
Mediation effect of WMH loads in the relationship between diastolic orthostatic hypotension (mm hg) and future cognitive decline. Path C shows the total effect of diastolic orthostatic hypotension associated with future cognitive decline as measured by the MoCA slope, and path C′ shows the same path after taking the WMH load into account as a mediator. Asterisks indicate significant paths (P < 0.05). MoCA, Montreal Cognitive Assessment Score; WMH, white matter hyperintensity.
Regarding the global dysautonomia, mediation analysis supported the existence of a partial mediation (ie, an indirect effect of autonomic dysfunction on the rate of cognitive decline via WMH loads) in patients with PD (average causal mediation effect: ab = −0.027, 95% CI = −0.054 to 0.00, P = 0.02; average direct effect: C′ = −0.167, 95% CI = −0.26 to −0.06, P = 0.001; proportion mediated = 0.139, 95% CI = 0.022–0.36, P = 0.02), but not in the HC (average causal mediation effect: ab = −0.005, 95% CI = −0.032 to 0.03, P = 0.77; average direct effect: C′ = −0.19, 95% CI = −0.36 to −0.04, P = 0.014; proportion mediated = 0.029, 95% CI = −0.18 to 0.23, P = 0.77). Figure 2 summarizes the results of the mediation analysis for the PD cohort. Including cardiovascular medications as covariates in the mediation analysis did not change the results.
FIG. 2.
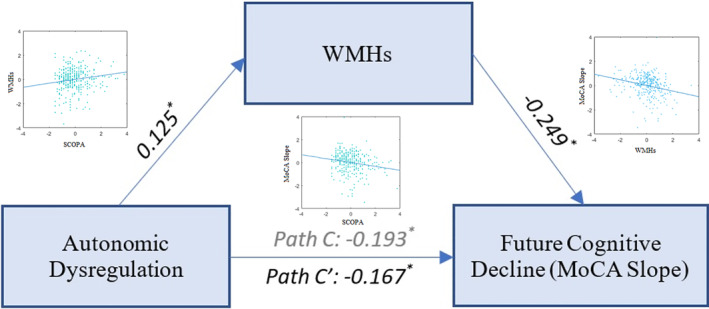
Mediation effect of WMH loads in the relationship between total SCOPA‐AUT score and future cognitive decline. Path C shows the total effect of SCOPA‐AUT associated with future cognitive decline as measured by the MoCA slope, and path C′ shows the same path after taking the WMH load into account as a mediator. Asterisks indicate significant paths (P < 0.05). MoCA, Montreal Cognitive Assessment Score; SCOPA‐AUT, Scales for Outcomes in Parkinson's Disease–Autonomic; WMH, white matter hyperintensity.
Discussion
Our findings demonstrate that WMH load (1) fully mediates the effect of diastolic orthostatic hypotension on future cognitive decline (for 4 years) and (2) partially mediates the effect of global autonomic dysfunction on future cognitive decline in patients with PD, but not in the HC. Direction of the mediation reveals that patients with PD with higher burden of dysautonomia or more severe orthostatic hypotention at the early stage present with greater WMH burden that later results in more rapid cognitive decline. The PPMI cohort comprises patients with PD who are at the early drug‐naïve stage at enrollment, ensuring that the findings of this study are not influenced by treatment effects or disease duration. In other words, we speculate that dysautonomia (which may have started during the prodromal stage, ie, potentially years prior to diagnosis of PD) predisposes the brain to subtle injuries (ie, demyelination and axonal loss), which in turn result in some cognitive impairment, even in the early PD stage. This is line with previous evidence estimating the onset of autonomic dysfunction as early as 11 to 20 years prior to diagnosis of PD, 2 , 29 years before subtle cognitive deficits become apparent.
Our findings are also in line with previous studies. Similar to previous studies, 14 we did not find significant associations between WMH burden in patients with PD and the presence of cerebrovascular risk factors, suggesting that other nonvascular factors might contribute to the white matter damage (as reflected by WMHs) in patients with PD. A retrospective cross‐sectional study has recently shown that neurogenic orthostatic hypotension correlates with the severity of WMHs in PD. 30 It has also been proposed to subtype individuals with simultaneous autonomic dysfunction, cognitive deficit, and rapid eye movement sleep behavior disorder as “diffuse malignant” PD from early stages, further emphasizing the association between dysautonomia and cognitive impairment in PD. 31 , 32
In the context of AD, accumulating evidence supports the hypothesis that an autonomic vascular event manifesting with variability in blood pressure during middle age associates with impaired cognition and functional decline in older ages. 33 , 34 A multifactorial data‐driven analysis has also demonstrated that vascular dysregulation plays an important role in initiating the pathological cascade of cognitive impairment in AD. 35 This suggests that dysautonomia, through dysregulation of cerebral blood flow, might also lead to WMHs that over time induce cognitive decline in PD, similar to the chain of events that occur in AD.
The image processing and WMH segmentation pipelines that have been used in this study were specifically designed to process data from multicenter studies, that is, they are able to deal with biases attributed to the differences in acquisition parameters across acquisition sites and have previously been applied to other multisite projects. 25 , 36 , 37 , 38 , 39 The robust WMH segmentation pipeline has been developed using data from multiple scanners with different acquisition parameters to ensure intersite and interscanner generalizability. 25
We acknowledge there are limitations to the present study. First, although their differences were accounted for, the segmentations were based on either T2‐weighted or FLAIR images, of which the latter has the better contrast for detecting WMHs. Second, participants had T2‐weighted and FLAIR scans only at their baseline visit; therefore, we were only able to investigate the impact of baseline orthostatic hypotension and autonomic dysfunction on baseline WMH burden. Future studies with longitudinal clinical and MRI assessments are necessary to further establish the longitudinal impact and interplay of orthostatic hypotension, autonomic dysfunction, WMHs, and cognition. Also, the population under study included only recently diagnosed de novo patients, which limited our ability in investigating WMH changes during the earlier prodromal stage or in the later stages of disease progression when the majority of patients with PD develop dementia. Longer follow‐ups might further increase the observed differences. Furthermore, clinical trials are warranted to investigate whether targeting dysautonomia in the prodromal or early stages can slow down or postpone cognitive decline in PD.
Controlling for overall blood pressure, age, sex, and segmentation modality, WMH load, and cognitive decline were significantly associated with orthostatic drop in diastolic blood pressure in the PD cohort, but not systolic blood pressure. This is in line with previous studies reporting a greater impact of diastolic orthostatic hypotension on WMH severity than systolic orthostatic hypotension. 14 Orthostatic hypotension leads to recurrent blood pressure drops, which could consequently result in cerebral hypoperfusion and neurocirculatory failures. Although systolic orthostatic hypotension is generally thought to be more closely related to cerebral hypoperfusion and orthostatic hypotension symptoms, and some studies in patients with multiple system atrophy report an impact of systolic blood pressure changes during orthostasis and WMHs, 40 , 41 our results and another similar study did not show this relationship in PD. 41 Further studies with a prospective design and more comprehensive and longitudinal measures are warranted to disentangle these relationships.
WMH load in different lobes as well as periventricular and deep WMHs are generally highly correlated with the overall WMH load (r Frontal = 0.96, r Parietal = 0.91, r Temporal = 0.85, r Occipital = 0.74, r PeriventricularWMHs = 0.97, r DeepWMHs = 0.92, all P < 0.00001), and to avoid increasing the number of assessments performed, we used total WMH load as the overall measure of WMH burden. We also observed similar relationships between regional WMHs, orthostatic hypotension, and cognitive decline.
MoCA score was used as a measure of global cognition. MoCA has been previously validated as a sensitive measure for detecting and monitoring cognitive change over time 42 in general and MCI or dementia in PD specifically. 43 An important follow‐up question would be to assess which specific cognitive domains are more affected in PD. However, controlling for age and sex, we did not find any direct effect of either orthostatic hypotension or SCOPA‐AUT on any of the other cognitive tasks acquired within the PPMI study (ie, the Hopkins Verbal Learning Test Revised, Benton Judgment of Line Orientation, Letter Number Sequencing, and Symbol Digit Modalities Test), which is a necessary condition before performing a mediation analysis. Further studies in patients with more advanced PD are required to establish whether such associations occur in later stages of the disease.
To ensure that other factors that might affect cognitive performance such as education level, motor performance, depression, and anxiety did not impact the findings, all the analyses were repeated including years of education, Unified Parkinson's Disease Rating Scale–Motor, Geriatric Depression Scale, and State–Trait Anxiety scores as covariates. As expected, a decline in global cognition was associated with education (P = 0.01) and Unified Parkinson's Disease Rating Scale–Motor (P = 0.01); however, other associations with WMH burden, orthostatic hypotension, and autonomic function remained similar. Another potential confounding factor might be PD medication. However, previous studies have found no significant difference between patients with PD on PD medications and patients with PD off medications in MoCA and several other cognitive tasks. 44 Similarly, we found no relationship between MoCA and medication dosage in patients with PD.
Understanding the temporal and causal relationships between dysautonomia, WMHs, and cognitive decline might elucidate some of the underlying mechanisms of these important nonmotor symptoms in PD. Our results suggest that dysautonomia and orthostatic hypotension (particularly in diastolic blood pressure), even at the early unmedicated stages of the disease, might predispose the brain to subtle white matter injuries that in turn result in increased cognitive impairment. In clinical practice, the screening and management of orthostatic hypotension in patients with PD should be considered as early as possible for both future prognostication and to potentially slow down cognitive impairment with timely interventions (after approval by future clinical trials).
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
M.D.: 1A, 1B, 1C, 2A, 2B, 3A
S.M.F.: 1A, 2C, 3B
Y.Z.: 2B, 2C, 3B
R.P.: 1A, 3B
A.D.: 1A, 3B
D.L.C.: 1A, 2A, 2C, 3B
Disclosures
Ethical Compliance Statement: The study was approved by the institutional review board of all participating sites and written informed consent was obtained from all participants before inclusion in the study (http://www.ppmi-info.org). We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: The authors declare that there are no conflicts of interest relevant to this work. There are no funding sources to report.
Financial Disclosures for the previous 12 months: R.P. receives funding from Fonds de la Recherche en Sante Quebec, Canadian Institute of Health Research, Parkinson Society of Canada, Weston‐Garfield Foundation, Webster Foundation, Michael J Fox Foundation, and Roche and has acted in a consultant or speaker capacity for Biotie, Roche, Biogen, Takeda, Jazz, Theranexus, GE, Abbvie, Jannsen, Otsuko, Boehringer, Novartis, Inception, and Phytopharmics. D.L. Collins receives funding from NSERC, CIHR, and the Weston‐Garfield Foundation and consulting for NeuroRx Inc. A.D., R.P., D.L.C., Y.Z., and S.M.F. receive funding from Healthy Brains, Healthy Lives.
Supporting information
Table S1. Medications used for concomitant co‐morbidities other than PD and their impact on WMH load. Significant associations and differences are displayed in bold font.
Acknowledgments
The data used in this article were obtained from the Parkinson's Progression Markers Initiative database (www.ppmi-info.org/data). For up‐to‐date information on the study, visit www.ppmi-info.org. The Parkinson's Progression Markers Initiative is sponsored and partially funded by the Michael J Fox Foundation for Parkinson's Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen, Bristol‐Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Eli Lilly and Company, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal Imaging, Roche, Servier, and UCB (www.ppmi-info.org/fundingpartners). M.D. and D.L.C. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson's disease: the SCOPA‐AUT. Mov Disord 2004;19:1306‐1312. [DOI] [PubMed] [Google Scholar]
- 2. Fereshtehnejad S‐M, Yao C, Pelletier A, Montplaisir JY, Gagnon J‐F, Postuma RB. Evolution of prodromal Parkinson's disease and dementia with Lewy bodies: a prospective study. Brain 2019;142:2051‐2067. [DOI] [PubMed] [Google Scholar]
- 3. Ferini‐Strambi L, Oertel W, Dauvilliers Y, et al. Autonomic symptoms in idiopathic REM behavior disorder: a multicentre case‐control study. J Neurol 2014;261:1112‐1118. [DOI] [PubMed] [Google Scholar]
- 4. Postuma RB, Lanfranchi PA, Blais H, Gagnon J‐F, Montplaisir JY. Cardiac autonomic dysfunction in idiopathic REM sleep behavior disorder. Mov Disord 2010;25:2304‐2310. [DOI] [PubMed] [Google Scholar]
- 5. Kujawa K, Leurgans S, Raman R, Blasucci L, Goetz CG. Acute orthostatic hypotension when starting dopamine agonists in Parkinson's disease. Arch Neurol 2000;57:1461‐1463. [DOI] [PubMed] [Google Scholar]
- 6. Turkka JT. Correlation of the severity of autonomic dysfunction to cardiovascular reflexes and to plasma noradrenaline levels in Parkinson's disease. Eur Neurol 1987;26:203‐210. [DOI] [PubMed] [Google Scholar]
- 7. Anang JB, Gagnon J‐F, Bertrand J‐A, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 2014;83:1253‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim J‐S, Oh YS, Lee KS, Kim Y, Goldstein DS . Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology 2012;79:1323‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verbaan D, Marinus J, Visser M, et al. Cognitive impairment in Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:1182‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McAleese KE, Alafuzoff I, Charidimou A, et al. Post‐mortem assessment in vascular dementia: advances and aspirations. BMC Med 2016;14:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 2015;11:157‐165. [DOI] [PubMed] [Google Scholar]
- 12. Dadar M, Maranzano J, Ducharme S, Collins DL. White matter in different regions evolves differently during progression to dementia. Neurobiol Aging 2019;76:71‐79. [DOI] [PubMed] [Google Scholar]
- 13. Gunning‐Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 2000;14:224‐232. [DOI] [PubMed] [Google Scholar]
- 14. Oh Y‐S, Kim J‐S, Lee K‐S. Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. Mov Disord 2013;6:23‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McDonald C, Newton JL, Burn DJ. Orthostatic hypotension and cognitive impairment in Parkinson's disease: causation or association? Mov Disord 2016;31:937‐946. [DOI] [PubMed] [Google Scholar]
- 16. Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nat Rev Neurol 2011;7:229‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dadar M, Zeighami Y, Yau Y, et al. White matter hyperintensities are linked to future cognitive decline in de novo Parkinson's disease patients. Neuroimage Clin 2018;20:892‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee S‐J, Kim J‐S, Yoo J‐Y, et al. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord 2010;24:227. [DOI] [PubMed] [Google Scholar]
- 19. Marek K, Jennings D, Lasch S, et al. The Parkinson progression marker initiative (PPMI). Prog Neurobiol 2011;95:629‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colosimo C, Morgante L, Antonini A, et al. Non‐motor symptoms in atypical and secondary parkinsonism: the PRIAMO study. J Neurol 2010;257:5‐14. [DOI] [PubMed] [Google Scholar]
- 21. Aubert‐Broche B, Fonov VS, García‐Lorenzo D, et al. A new method for structural volume analysis of longitudinal brain MRI data and its application in studying the growth trajectories of anatomical brain structures in childhood. Neuroimage 2013;82:393‐402. [DOI] [PubMed] [Google Scholar]
- 22. Dadar M, Fonov VS, Collins DL, Alzheimer's Disease Neuroimaging Initiative. A comparison of publicly available linear MRI stereotaxic registration techniques. Neuroimage 2018;174:191‐200. [DOI] [PubMed] [Google Scholar]
- 23. Collins DL, Evans AC. Animal: validation and applications of nonlinear registration‐based segmentation. Int J Pattern Recognit Artif Intell 1997;11:1271‐1294. [Google Scholar]
- 24. Dadar M, Pascoal T, Manitsirikul S, et al. Validation of a regression technique for segmentation of white matter hyperintensities in Alzheimer's disease. IEEE Trans Med Imaging 2017;36:1758‐1768. [DOI] [PubMed] [Google Scholar]
- 25. Dadar M, Maranzano J, Misquitta K, et al. Performance comparison of 10 different classification techniques in segmenting white matter hyperintensities in aging. Neuroimage 2017;157:233‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression‐Based Approach. New York: Guilford Publications; 2017. [Google Scholar]
- 27. Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol 1982;13:290‐312. [Google Scholar]
- 28. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw 2014;59:1‐38.26917999 [Google Scholar]
- 29. Postuma RB, Gagnon J‐F, Pelletier A, Montplaisir J. Prodromal autonomic symptoms and signs in Parkinson's disease and dementia with Lewy bodies. Mov Disord 2013;28:597‐604. [DOI] [PubMed] [Google Scholar]
- 30. Ten Harmsen BL, van Rumund A, Aerts MB, et al. Clinical correlates of cerebral white matter abnormalities in patients with Parkinson's disease. Parkinsonism Relat Disord 2018;49:28‐33. [DOI] [PubMed] [Google Scholar]
- 31. Fereshtehnejad S‐M, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson's disease: biomarkers and longitudinal progression. Brain 2017;140:1959‐1976. [DOI] [PubMed] [Google Scholar]
- 32. Fereshtehnejad S‐M, Romenets SR, Anang JB, Latreille V, Gagnon J‐F, Postuma RB. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol 2015;72:863‐873. [DOI] [PubMed] [Google Scholar]
- 33. Ogliari G, Smit RAJ, Westendorp RGJ, Jukema JW, de Craen AJM, Sabayan B. Visit‐to‐visit blood pressure variability and future functional decline in old age. J Hypertens 2016;34:1544‐1550. [DOI] [PubMed] [Google Scholar]
- 34. Sabayan B, Wijsman LW, Foster‐Dingley JC, et al. Association of visit‐to‐visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ 2013;347:f4600. [DOI] [PubMed] [Google Scholar]
- 35. Iturria‐Medina Y, Sotero RC, Toussaint PJ, Mateos‐Pérez JM, Evans AC. Early role of vascular dysregulation on late‐onset Alzheimer's disease based on multifactorial data‐driven analysis. Nat Commun 2016;7:11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boucetta S, Salimi A, Dadar M, Jones BE, Collins DL, Dang‐Vu TT. Structural brain alterations associated with rapid eye movement sleep behavior disorder in Parkinson's disease. Sci Rep 2016;6:26782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dadar M, Maranzano J, Ducharme S, Carmichael OT, Decarli C, Collins DL, Alzheimer's Disease Neuroimaging Initiative . Validation of T1w‐based segmentations of white matter hyperintensity volumes in large‐scale datasets of aging. Hum Brain Mapp 2018;39:1093‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeighami Y, Fereshtehnejad S‐M, Dadar M, et al. A clinical‐anatomical signature of Parkinson's disease identified with partial least squares and magnetic resonance imaging. Neuroimage 2019;190:69‐78. [DOI] [PubMed] [Google Scholar]
- 39. Zeighami Y, Ulla M, Iturria‐Medina Y, et al. Network structure of brain atrophy in de novo Parkinson's disease. Elife 2015;4:e08440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lim TS, Lee PH, Kim HS, Yong SW. White matter hyperintensities in patients with multiple system atrophy. J Neurol 2009;256:1663‐1670. [DOI] [PubMed] [Google Scholar]
- 41. Umoto M, Miwa H, Ando R, Kajimoto Y, Kondo T. White matter hyperintensities in patients with multiple system atrophy. Parkinsonism Relat Disord 2012;18:17‐20. [DOI] [PubMed] [Google Scholar]
- 42. Costa AS, Reich A, Fimm B, Ketteler ST, Schulz JB, Reetz K. Evidence of the sensitivity of the MoCA alternate forms in monitoring cognitive change in early Alzheimer's disease. Dement Geriatr Cogn Disord 2014;37:95‐103. [DOI] [PubMed] [Google Scholar]
- 43. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009;73:1738‐1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cools R, Altamirano L, D'Esposito M. Reversal learning in Parkinson's disease depends on medication status and outcome valence. Neuropsychologia 2006;44:1663‐1673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Medications used for concomitant co‐morbidities other than PD and their impact on WMH load. Significant associations and differences are displayed in bold font.


