Paraneoplastic syndromes can be the presenting symptom of a new malignancy. Anti‐CV2/CRMP‐5‐IgG is a neuronal autoantibody most commonly associated with small cell lung cancer. 1 The associated paraneoplastic syndrome can include chorea (11% of patients), cranial neuropathy, peripheral and autonomic neuropathy, cerebellar ataxia, neuromuscular junction disorders, and subacute dementia. 1 Here, we present a case of CRMP‐5 associated paraneoplastic chorea with, to our knowledge, the first video report.
A 71‐year‐old female presented with several weeks of progressively worsening gait difficulty and abnormal uncontrolled movements, starting in the left arm. Movements were suppressible, stopped during sleep, and were worse in the head and left arm but also affected the other limbs and gait. She was a former smoker and reported a 30‐lb weight loss in the last year. Her past medical history was notable for non‐small cell bronchoalveolar lung adenocarcinoma treated with segmental resection but no chemotherapy or radiotherapy, in remission for the last 4 years.
Examination was notable for halting dysarthria, prominent choreiform movements of the head and neck, left greater than right arm, and to a lesser extent both legs (Video S1). The neurologic exam was otherwise normal, including normal vision, tone, and reflexes. She had a wide based mildly antalgic gait requiring support in part due to choreiform movements.
Initial laboratory workup was normal. Brain MRI demonstrated extensive T2 hyperintense signal affecting the bilateral caudate nuclei and putamina (Fig. 1).
Figure 1.
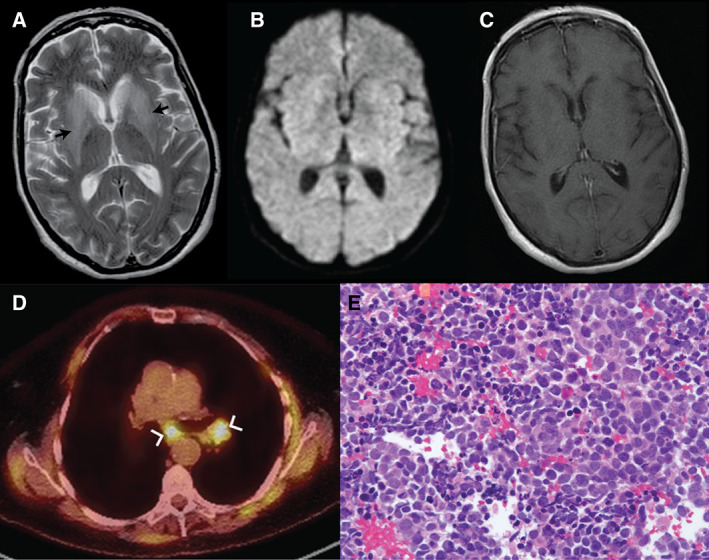
Axial brain MRI demonstrated (A) extensive T2 hyperintense signal (arrows) with mild mass effect affecting the bilateral caudate nuclei and putamina (B) without associated restricted diffusion on diffusion‐weighted sequences and (C) without enhancement on T1 post‐gadolinium sequences. (D) Axial chest CT (greyscale) with superimposed [F18] FDG PET (color) demonstrated avid mediastinal and hilar lymphadenopathy (arrows) (E) biopsy demonstrated small blue cells with sparse cytoplasm without distinct nucleoli on H&E, positive for keratin, TTF1, synaptophysin, and chromogranin (patchy), consistent with small cell carcinoma.
Cerebrospinal fluid (CSF) was obtained that demonstrated elevated while blood count cell count (22 cells/μL) with lymphocytic predominance, elevated protein (89 mg/dL), and normal CSF glucose. Cytologic evaluation of the CSF did not reveal malignant cells. [F18] FDG‐PET with diagnostic CT of the chest demonstrated avid mediastinal and hilar lymph nodes (Fig. 1C). Core needle biopsy of hilar lymph node demonstrated new primary small cell carcinoma of the lung (Fig. 1D). Collapsin response‐mediator protein‐5 (CRMP‐5) IgG antibodies were identified by western blot and immunofluorescence at high titer in the serum (titer 1:30,720) and CSF (titer 1:8,192). No other abnormal autoantibodies were identified in the serum or CSF, including Anti‐ANNA‐1, Anti‐ANNA‐2, Anti‐ANNA‐3, Anti‐PCA1, and Anti‐PCA2.
Treatment with intravenous methylprednisolone 1 g daily for 3 days was initiated with dramatic improvement in symptoms. Chorea recurred with taper of corticosteroids to prednisone 100 mg daily but improved significantly again with initiation of chemotherapy with cisplastin and etoposide. She had resolution of all hyperkinetic movements 3 weeks after initiation of chemotherapy with sustained improvement 3 months later.
There is a broad differential to the patient with subacute chorea with bilateral T2 basal ganglia hyperintensities. In this patient, weight loss and a history of smoking suggested a paraneoplastic etiology. The differential diagnosis also included nonketotic hyperglycemia in a patient with a history of diabetes. Anti‐CV2/CRMP‐5‐IgG is the most common paraneoplastic cause of chorea, followed by anti‐Hu/ANNA‐1. 2 MRI can demonstrate diffuse T2 white matter hyperintensity or basal ganglia T2 hyperintensity. 2 , 3 In many patients, chorea does not respond to immunomodulatory or oncologic therapy, 2 , 3 although our patient has had persistent symptomatic benefit.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
P.A.V.: 3A, 3B.
E.Y.K.: 3B.
A.Y.H.: 3B.
Disclosures
Ethical Compliance Statement: The authors confirm that the approval of an institutional review board was not required for this work. Oral and written informed consent was obtained from the patient for publication of this case. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for the Previous 12 Months: The authors declare that there are no additional disclosures to report.
Supporting information
Video S1. Segment 1: on initial examination, the patient demonstrated prominent choreiform movements of the head and neck, left greater than right arm, and to a lesser extent the bilateral legs. Hyperkinetic movements affected proximal and distal limbs, were more prominent at rest, transiently suppressible, but did interfere with activity. Cerebellar testing was limited by chorea; no superimposed cerebellar symptoms were noted. Segment 2: on subsequent evaluation after treatment with IV methylprednisolone, prednisone, and one dose of cisplatin and etoposide, choreiform movements were significantly improved.
Acknowledgments
We thank Dr. Amin Heidarian for providing pathology images.
References
- 1. Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP‐5 Neuronal Autoantibody: Marker of Lung Cancer and Thymoma‐Related Autoimmunity; 2001:146–154. . [DOI] [PubMed] [Google Scholar]
- 2. Vigliani MC, Honnorat J, Antoine JC, et al. Chorea and related movement disorders of paraneoplastic origin: the PNS EuroNetwork experience. J Neurol 2011;258(11):2058–2068. 10.1007/s00415-011-6074-1 [DOI] [PubMed] [Google Scholar]
- 3. Vernino S, Tuite P, Adler CH, et al. Paraneoplastic chorea associated with CRMP‐5 neuronal antibody and lung carcinoma. Ann Neurol 2002;51(5):625–630. 10.1002/ana.10178 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Segment 1: on initial examination, the patient demonstrated prominent choreiform movements of the head and neck, left greater than right arm, and to a lesser extent the bilateral legs. Hyperkinetic movements affected proximal and distal limbs, were more prominent at rest, transiently suppressible, but did interfere with activity. Cerebellar testing was limited by chorea; no superimposed cerebellar symptoms were noted. Segment 2: on subsequent evaluation after treatment with IV methylprednisolone, prednisone, and one dose of cisplatin and etoposide, choreiform movements were significantly improved.


