Abstract
Background
The nine-valent human papillomavirus (9vHPV) vaccine protects against infection and disease related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The pivotal 36-month Phase III immunogenicity study of 9vHPV vaccine in 9- to 15-year-old girls and boys was extended to assess long-term immunogenicity and effectiveness through approximately 10 years after vaccination. We describe results of an interim analysis based on approximately 8 years of follow-up after vaccination.
Methods
Participants aged 9–15 years who received three doses of 9vHPV vaccine (at day 1, month 2, and month 6) in the base study and consented to follow-up were enrolled in the long-term follow-up study extension (N = 1272 [females, n = 971; males, n = 301]). Serum was collected at months 66 and 90 to assess antibody responses. For effectiveness analysis, genital swabs were collected (to assess HPV DNA by polymerase chain reaction [PCR]) and external genital examination was conducted (to detect external genital lesions) every 6 months starting when the participant reached 16 years of age. Cervical cytology tests were conducted annually when female participants reached 21 years of age; participants with cytological abnormalities were triaged to colposcopy based on a protocol-specified algorithm. External genital and cervical biopsies of abnormal lesions were performed, and histological diagnoses were adjudicated by a pathology panel. Specimens were tested by PCR to detect HPV DNA.
Results
Geometric mean titers for each 9vHPV vaccine HPV type peaked around month 7 and gradually decreased through month 90. Seropositivity rates remained >90% through month 90 for each of the 9vHPV vaccine types by HPV immunoglobulin Luminex Immunoassay. No cases of HPV6/11/16/18/31/33/45/52/58-related high-grade intraepithelial neoplasia or genital warts were observed in the per-protocol population (n = 1107) based on a maximum follow-up of 8.2 years (median 7.6 years) post-Dose 3. Incidence rates of HPV6/11/16/18/31/33/45/52/58-related 6-month persistent infection in females and males were 49.2 and 37.3 per 10,000 person-years, respectively, which were within ranges expected in vaccinated cohorts. There were no vaccine-related SAEs or deaths during the period covered by this interim analysis.
Conclusions
The 9vHPV vaccine provided sustained immunogenicity and durable effectiveness through approximately 7 and 8 years, respectively, following vaccination of girls and boys aged 9–15 years.
Keywords: Nine-valent human papillomavirus vaccine, Effectiveness, Immunogenicity, Long-term follow-up
Abbreviations: 9vHPV, nine-valent human papillomavirus; AIS, adenocarcinoma in situ; bHPV, bivalent human papillomavirus; BMI, body mass index; CI, confidence interval; CIN, cervical intraepithelial neoplasia; cLIA, competitive Luminex Immunoassay; EEC, endo-/ectocervical; GMT, geometric mean titer; HN-TS, HPV-naïve, type-specific; HPV, human papillomavirus; IgG-LIA, immunoglobulin G Luminex Immunoassay; LTFU, long-term follow-up; LVPP, labial/vulvar/perineal/perianal; PCR, polymerase chain reaction; PIN, penile intraepithelial neoplasia; PPE, per-protocol effectiveness; PPI, per-protocol immunogenicity; qHPV, quadrivalent human papillomavirus; SAE, serious adverse event; SD, standard deviation; VaIN, vaginal intraepithelial neoplasia; VIN, vulvar intraepithelial neoplasia; WHO, World Health Organization
Highlights
-
•
We report follow-up data up to 8 years after girls/boys received 9vHPV vaccine.
-
•
Durable effectiveness was observed through up to 8 years post-vaccination.
-
•
Sustained anti-HPV antibody responses were observed through at least 7 years.
1. Introduction
The nine-valent human papillomavirus (9vHPV) vaccine was developed to prevent infection with seven oncogenic HPV types (HPV16/18/31/33/45/52/58) that together account for approximately 90% of cervical cancers and HPV-related vulvar, vaginal, and anal cancers, and two HPV types (HPV6/11) that are responsible for approximately 90% of genital warts [[1], [2], [3], [4]].
In the pivotal efficacy trial in young women aged 16–26 years (Study V503-001; NCT00543543), the 9vHPV vaccine demonstrated efficacy in preventing persistent infection and disease related to those HPV types covered by the 9vHPV vaccine [[5], [6], [7]]. The vaccine also elicited robust and persistent antibody responses to all nine HPV types in young women through 5 years post-vaccination [5].
While adults remain at risk for HPV infection throughout their lives, HPV is often acquired soon after sexual debut [8]. As such, HPV vaccination should target individuals prior to sexual debut for maximal benefit. An immunogenicity and safety study (Study V503-002; NCT00943722) was conducted in girls and boys aged 9–15 years who were given three doses of the 9vHPV vaccine (at day 1 and months 2 and 6) [9,10]. At 4 weeks post-Dose 3, HPV antibody responses in girls and boys aged 9–15 years were non-inferior compared with those in young women aged 16–26 years; based on these results, vaccine efficacy previously established in young women [5,6] was inferred for the younger age groups [9]. In addition, the HPV antibody responses persisted through 2.5 years post-Dose 3 and the vaccine was generally well tolerated throughout the entire study [9].
Given that the risk of HPV infection is lifelong, the benefits of HPV vaccination will be fully realized if the protection is long-lasting. Therefore, the World Health Organization (WHO) has determined that long-term follow-up (LTFU) studies to assess long-term efficacy, safety, and immunogenicity should be an integral part of prophylactic HPV-vaccine development [11]. An LTFU study of the quadrivalent HPV (qHPV) vaccine has previously demonstrated durable effectiveness and sustained immunogenicity when given to girls and boys aged 9–15 years through 10 years post-vaccination [12]. Likewise, an LTFU study of the bivalent HPV (bHPV) vaccine demonstrated sustained immunogenicity through 10 years post-vaccination [13]. The immunogenicity and safety study of the 9vHPV vaccine in girls and boys 9 to 15 years of age was, therefore, extended to assess 9vHPV vaccine effectiveness, immunogenicity, and safety for approximately 10 years post-Dose 3. We describe results from an interim analysis of this LTFU study with up to approximately 8 years of follow-up post-Dose 3.
2. Methods
2.1. Study design and participants
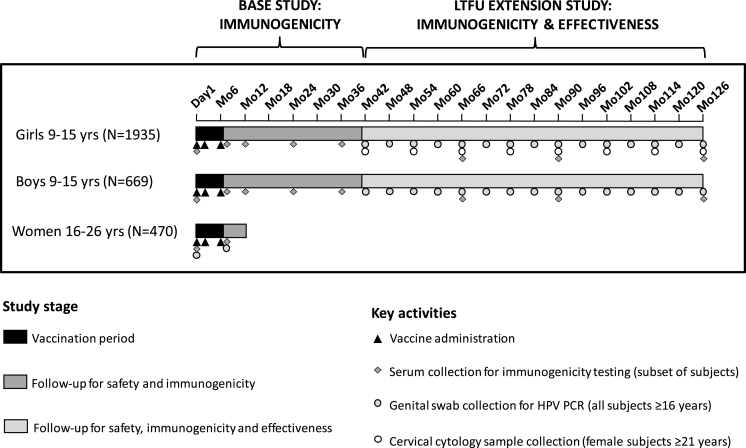
This study (Study V503-002; NCT00943722) assessed the immunogenicity and safety of the 9vHPV vaccine from day 1 through month 36 (base study), along with an LTFU extension study, which added an effectiveness and immunogenicity analysis through approximately 10 years post-Dose 3 (Fig. 1). The base study was initiated on August 27, 2009 and completed on May 2, 2013. The LTFU extension was initiated on March 7, 2013 and is ongoing.
Fig. 1.
Study design.
Abbreviations: HPV = human papillomavirus; LTFU = long-term follow-up; PCR, polymerase chain reaction.
The base study design and inclusion criteria have been described in detail previously [9,10]. Briefly, girls and boys (aged 9–15 years) and young women (aged 16–26 years) received three doses of the 9vHPV vaccine, administered at day 1, month 2, and month 6. Young women terminated the base study at month 12; however, girls and boys continued in the study through month 36 for assessment of antibody persistence [9].
Girls and boys who received all three vaccine doses during the base study and provided consent were eligible to participate in the LTFU study extension. All study sites from the base study were invited to join in the study extension. However, some sites could not commit to an additional 7.5 years of follow-up for various reasons, and therefore did not participate in the study extension. The study extension was carried out at 39 study sites located in 13 countries (Belgium, Brazil, Colombia, Costa Rica, Peru, Poland, South Africa, South Korea, Spain, Sweden, Taiwan, Thailand, United States).
The primary objective of the LTFU study is to evaluate HPV antibody responses to the 9vHPV vaccine through month 126 (10 years post-Dose 3). The long-term effectiveness of the 9vHPV vaccine was evaluated in two co-secondary endpoints. The first co-secondary endpoint was incidence of the composite endpoint of HPV6/11/16/18/31/33/45/52/58-related persistent infection of ≥6 months duration (referred to as “6-month persistent infection”) and cervical intraepithelial neoplasia (CIN), adenocarcinoma in situ (AIS), vulvar intraepithelial neoplasia (VIN), vaginal intraepithelial neoplasia (VaIN), genital warts, and cervical/vaginal/vulvar cancer in female participants. The second co-secondary endpoint was a composite of HPV6/11/16/18/31/33/45/52/58-related 6-month persistent infection and penile intraepithelial neoplasia (PIN), genital warts, and penile/perineal/perianal cancer in male participants. The pre-specified safety objective was to describe the incidence of deaths and vaccine- or procedure-related serious adverse events (SAEs) among LTFU-study participants.
The study was conducted in accordance with principles of Good Clinical Practice, and was approved by the respective institutional review boards and regulatory agencies. All participants (or parents/legal guardians for minors) provided written informed consent for the base study and for the LTFU study; participants coming of legal age were reconsented per local regulations.
2.2. Immunogenicity assessments
Serum samples were collected at day 1 and months 7, 12, 24, and 36 in the base study, and months 66 and 90 in the LTFU extension; a final serum sample is to be collected at month 126. HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 antibody responses were assessed at day 1 and month 7 for all study participants. Assessment of antibody persistence after month 7 was assessed in a subset of participants including all male participants and a random sample of 600 female participants [9]. For the primary immunogenicity analyses, antibody responses were detected by the HPV-9 competitive Luminex Immunoassay (cLIA) [14]. The more sensitive HPV-9 immunoglobulin G Luminex Immunoassay (IgG-LIA) was used in supportive analyses to assess antibody persistence [15]. Antibody responses to each of the nine vaccine HPV types are summarized as geometric mean titers (GMTs) and seropositivity rates.
As the current study did not include a control group, anti-HPV cLIA GMTs in young women who received 9vHPV vaccine or qHPV vaccine in the 9vHPV pivotal efficacy study, which was conducted in similar geographic regions, are provided for context; these data have been reported previously [5].
2.3. Effectiveness assessments
Upon reaching 16 years of age, visits occurred every 6 months and included collection of sexual history, genital examination, and genital clinical specimens. Genital samples were collected according to the participants' age, gender, and sexual activity. For females aged 16–20 years, external genital examinations for lesions were performed and labial/vulvar/perineal/perianal (LVPP) swabs were collected at each visit. In addition, for those who were sexually active (defined as having engaged in vaginal penetration), endo-/ectocervical (EEC) swabs were also collected at each visit. Cervical cytology tests were not required but could be performed annually, per the investigator's discretion and based on the local standard of care. Upon reaching 21 years of age, external genital examinations for lesions were performed and LVPP and EEC swabs were collected at each visit, and pelvic examinations and cervical cytology tests were performed annually. Procedures for collection and analysis of the cervical cytology samples were conducted as previously described [5]. For males over the age of 16 years, external genital examinations for lesions were performed and penile and glans penis swabs, scrotal swabs, and perineal/perianal swabs were collected at each visit using a nail file/swab system as previously described [16]. Chlamydia and gonorrhea testing in urine samples or cervical cytology test fluid were performed annually for both males and females. The requirement for EEC swabs, cervical cytology tests, and pelvic examination could be waived in sexually naïve participants at the investigator's discretion; chlamydia and gonorrhea testing was waived for a few study sites at the investigator's request to conform with the local standard of care.
All genital swabs were tested by polymerase chain reaction (PCR) for HPV types 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 using type-specific PCR assays [17,18].
Identified external genital lesions suspected to be HPV-related were biopsied. Female participants with abnormal cervical cytology findings were referred to colposcopy with collection of cervical samples (biopsy, definitive therapy) using a protocol-mandated triage algorithm, similar to that used for the pivotal efficacy study of the 9vHPV vaccine [5,6]. Female participants with histologically confirmed HPV-related external genital or vaginal lesions were also referred to colposcopy if the external genital or vaginal biopsy was not obtained during colposcopy, similar to what was done in the pivotal efficacy study of the 9vHPV vaccine [5,6].
Tissue from biopsy and cervical definitive therapy was analyzed by HPV Thinsection PCR assay, similar to the analysis conducted in previous clinical trials [19,20]. A consensus diagnosis from the HPV Vaccine Program Pathology Panel, consisting of four independent pathologists blinded to vaccination and HPV PCR status, was used to determine clinical disease efficacy endpoints, using a process similar to that used in the pivotal 9vHPV vaccine efficacy study [5].
An endpoint of CIN, AIS, VIN, VaIN, vulvar, cervical or vaginal cancer, PIN, penile/perineal/perianal cancer, or genital warts related to a given HPV type occurred if a participant developed a lesion with a relevant consensus diagnosis by the pathology panel, and PCR testing detected the relevant HPV type in an adjacent section from the same tissue block. Endpoints of persistent infection were defined as a participant who was positive by PCR for the same HPV type in genital swabs or tissue specimens collected at consecutive visits at least 6 months (±1-month visit windows) apart. At least two positive specimens were required to define a case of 6-month persistent infection, and at least three positive specimens were required to define a case of 12-month persistent infection.
As the current study did not include a control group, rates of 6-month persistent infection and markers of sexual activity from previous qHPV and 9vHPV vaccine efficacy trials which were generally conducted in the same geographic regions [5,21,22] are included to put the results in context.
2.4. Safety outcomes
Deaths (any causality) and SAEs judged by the study investigator to be related to prior administration of the 9vHPV vaccine or to a study procedure were reported during the LTFU study.
2.5. Statistical analysis
Immunogenicity analyses were conducted in the per-protocol immunogenicity (PPI) population, which included participants who were seronegative (by cLIA) to the appropriate HPV type at day 1, received all three vaccinations within acceptable day ranges, had a month 7 serology result within acceptable day ranges, and had no other protocol violations that could interfere with evaluation of immune response. To be included in the PPI population for HPV6 and 11, the participants must have been seronegative to both HPV6 and 11 at day 1. For all other HPV types, the participant needed to be seronegative for only the HPV type being analyzed. GMTs and seropositivity rates are summarized with associated 95% confidence intervals (CI) at each time point when serology samples were collected.
The primary effectiveness analysis population was the per-protocol effectiveness (PPE) population, which included participants who were seronegative (by cLIA) to the specific HPV type at the time of vaccine Dose 1 (seronegative for both types 6 and 11 for analysis of HPV6- and HPV11-related endpoints), received all three doses of 9vHPV vaccine within 1 year, and had no other protocol violations that could interfere with evaluation of vaccine effectiveness. Supportive analyses were conducted in the HPV-naïve, type-specific (HN-TS) population of participants who received three doses of 9vHPV vaccine within 1 year and were seronegative by cLIA to the specific HPV type at the time of first 9vHPV vaccination (seronegative to both HPV types 6 and 11 for analyses of HPV6- and HPV11-related endpoints). Summaries of the incidence rates (cases per 10,000 person-years) of the composite endpoint of HPV types 6/11/16/18/31/33/45/52/58-related 6-month persistent and disease are provided through month 96. Nominal 95% CI estimates of the incidence rates were calculated based on the Poisson distribution. Point and 95% CI estimates of incidence rates for the composite endpoint of 6-month persistent infection and disease related to non-vaccine HPV type (35, 39, 51, 56, and 59) were calculated similarly.
No formal hypothesis testing was conducted in the LTFU study. The sample size for the LTFU study was fixed based on the number of base-study participants who were eligible and willing to participate in the LTFU study.
All participants who received at least one study vaccination and had follow-up data were included in the safety summaries.
3. Results
3.1. Participants
Of the 2552 pre-adolescents and adolescents (girls, n = 1899; boys, n = 653) who received three doses of the 9vHPV vaccine in the base study, 1272 (girls, n = 971; boys, n = 301) were enrolled in the LTFU study after base-study completion (Supplementary Fig. S1). Approximately 18% of the LTFU-study participants discontinued the study between months 42 and 96, most commonly due to withdrawal of consent or loss to follow-up, and 82% were ongoing at the time of data cut-off (May 30, 2018). None of the participants discontinued the LTFU study due to an AE. Immunogenicity was assessed at the month 66 and 90 visits (Fig. 1). Participants were followed for effectiveness through the month 96 visit; this represents a maximum follow-up of 8.2 years post-Dose 3 (median 7.6 years).
Baseline characteristics (collected prior to the first vaccination) were generally similar between those participants who enrolled in the LTFU study (Table 1) compared with the overall population of girls and boys enrolled in the base study, as previously reported [9]. The median participant age at enrollment in the base study was 11.0 years (range, 9–15 years).
Table 1.
Participant characteristics for LTFU-study participants at the start (vaccination Dose 1) of the base study.
| Females | Males | |
|---|---|---|
| (N = 971) | (N = 301) | |
| Age, years | ||
| 9–12, n (%) | 653 (67.3) | 207 (68.8) |
| 13–15, n (%) | 318 (32.7) | 94 (31.2) |
| Mean (SD) | 11.6 (1.9) | 11.5 (1.8) |
| Median (range) | 11.0 (9–15) | 11.0 (9–15) |
| Race, n (%) | ||
| American Indian or Alaskan Native | 2 (0.2) | 1 (0.3) |
| Asian | 222 (22.9) | 74 (24.6) |
| Black or African American | 129 (13.3) | 31 (10.3) |
| Multi-racial | 204 (21.0) | 114 (37.9) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 3 (1.0) |
| White | 414 (42.6) | 78 (25.9) |
| Weight, kg | ||
| Mean (SD) | 45.0 (12.9) | 44.1 (13.3) |
| Median (range) | 44.0 (18.0–115.7) | 41.0 (15.4–87.0) |
| BMI | ||
| Participants with data, n | 970 | 301 |
| Mean (SD), kg/m2 | 19.8 (4.1) | 19.6 (4.0) |
| Median (range), kg/m2 | 19.1 (10.5–55.6) | 18.5 (11.1–43.0) |
| Region, n (%) | ||
| Africa | 79 (8.1) | 28 (9.3) |
| Asia-Pacific | 222 (22.9) | 74 (24.6) |
| Europe | 256 (26.4) | 27 (9.0) |
| Latin America | 297 (30.6) | 122 (40.5) |
| North America | 117 (12.0) | 50 (16.6) |
Abbreviations: BMI = body mass index; LTFU = long-term follow-up; SD = standard deviation.
3.2. Immunogenicity
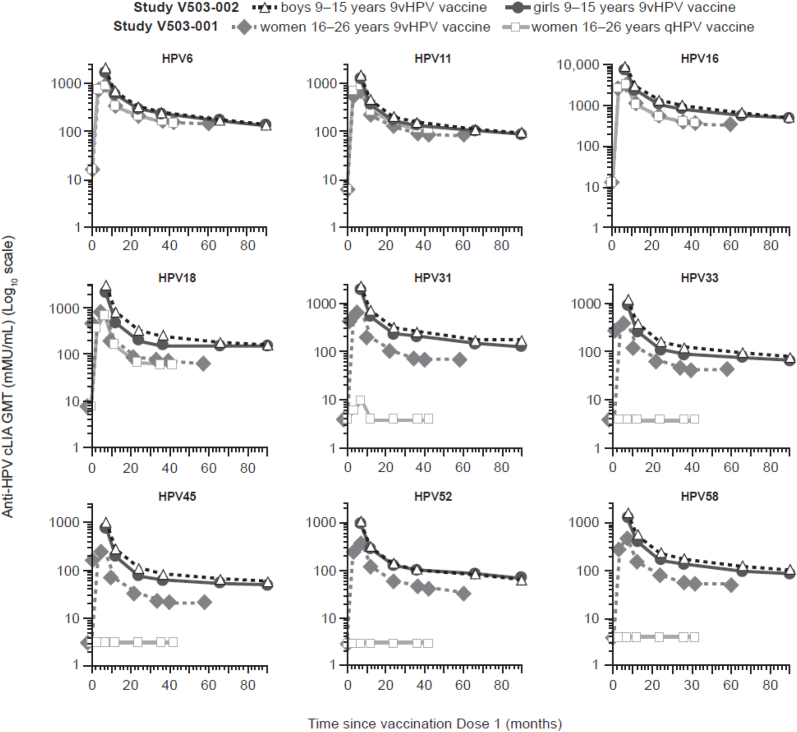
GMTs (assessed by cLIA) peaked at month 7, followed by a pronounced decrease between month 7 and month 12, with a further slight decrease between month 12 and month 90 (Fig. 2). This trend in GMTs was consistent with the immunogenicity profile observed in the pivotal 9vHPV vaccine efficacy study in young women 16–26 years of age through month 60 (year 5) after the first vaccine dose [5] (Fig. 2). GMTs in girls and boys during the long-term follow-up study are not directly comparable to GMTs in young women in the pivotal efficacy study, since the time of serum sample collection post-Dose 3 was different between the two studies. Nonetheless, the graphical representation of GMT indicates that the GMT trends were higher in girls and boys in the long-term follow-up study, compared with the corresponding trends in young women in the pivotal efficacy study. Since efficacy of 9vHPV vaccine was established in young women in that pivotal study, these results suggest that HPV antibody responses generated by the vaccine in girls and boys may be sufficient to induce high-level protective efficacy.
Fig. 2.
Anti-HPV cLIA GMTs over time since vaccine Dose 1 in female and male participants from the 9vHPV vaccine LTFU study and young women from the pivotal efficacy study [5].
Abbreviations: 9vHPV = nine-valent human papillomavirus; cLIA = competitive Luminex Immunoassay; GMT = geometric mean titer; HPV = human papillomavirus; LTFU = long-term follow-up; qHPV = quadrivalent human papillomavirus.
In supportive analysis using the HPV IgG-LIA, trends in GMTs were similar to those observed with the cLIA during the LTFU study (Supplementary Tables S1 and S2). The vast majority of female and male participants (>93% based on IgG-LIA) remained seropositive to each HPV type at month 90 (Supplementary Table S2).
3.3. Effectiveness
The numbers of participants who completed the scheduled effectiveness visits between month 42 and month 96 are reported in Supplementary Fig. S2. Most participants had reached age 16 years by the month 72 visit, and all had reached age 16 years by month 96. Most participants (i.e. 88.6% of females and 91.1% of males) who were at least 16 years of age have contributed effectiveness data as of month 96 (Supplementary Fig. S2).
Among females in the PPE population, there were 14 cases of the composite endpoint of HPV6/11/16/18/31/33/45/52/58-related 6-month persistent infection, CIN, AIS, condyloma, VIN, VaIN, and cervical/vulvar/vaginal cancers (Table 2). This analysis showed 14 cases of 6-month persistent infection, one case of CIN1 and no cases of high-grade cervical disease, condyloma, VIN, VaIN, or vulvar/vaginal cancer related to vaccine HPV types. The incidence rates of the endpoints of HPV6/11/16/18/31/33/45/52/58-related 6-month and 12-month persistent infection were 49.2 and 24.5 per 10,000 person-years, respectively.
Table 2.
Incidence of HPV6/11/16/18/31/33/45/52/58-related persistent infection and disease in vaccinated participants (PPE population).
| Females (N = 971) |
Males (N = 301) |
|||||
|---|---|---|---|---|---|---|
| Cases/n | Person-years follow-upa | Rate per 10,000 person-years (95% CI) | Cases/n | Person-years follow-upa | Rate per 10,000 person-years (95% CI) | |
| HPV6/11/16/18/31/33/45/52/58-related 6-month persistent infectionb or diseasec | 14/856 | 2843.8 | 49.2 (26.9–82.6) | 3/251 | 803.2 | 37.3 (7.7–109.1) |
| HPV6/11/16/18/31/33/45/52/58-related 6-month persistent infectionb | 14/856 | 2843.8 | 49.2 (26.9–82.6) | 3/251 | 803.2 | 37.3 (7.7–109.1) |
| By HPV type | ||||||
| HPV6/11/16/18 | 13/854 | 2842.2 | 45.7 (24.4–78.2) | 1/251 | 808.4 | 12.4 (0.3–68.9) |
| HPV6 | 1/831 | 2784.8 | 3.6 (0.1–20.0) | 0/245 | 790.6 | 0.0 (0.0–46.7) |
| HPV11 | 0/832 | 2789.2 | 0.0 (0.0–13.2) | 1/245 | 790.2 | 12.7 (0.3–70.5) |
| HPV16 | 11/843 | 2805.2 | 39.2 (19.6–70.2) | 0/250 | 805.2 | 0.0 (0.0–45.8) |
| HPV18 | 1/851 | 2847.1 | 3.5 (0.1–19.6) | 0/249 | 800.0 | 0.0 (0.0–46.1) |
| HPV31/33/45/52/58 | 1/856 | 2862.3 | 3.5 (0.1–19.5) | 2/251 | 803.6 | 24.9 (3.0–89.9) |
| HPV31 | 0/839 | 2804.7 | 0.0 (0.0–13.2) | 0/249 | 805.0 | 0.0 (0.0–45.8) |
| HPV33 | 1/850 | 2847.6 | 3.5 (0.1–19.6) | 0/249 | 806.3 | 0.0 (0.0–45.7) |
| HPV45 | 0/855 | 2863.7 | 0.0 (0.0–12.9) | 1/251 | 805.4 | 12.4 (0.3–69.2) |
| HPV52 | 0/854 | 2859.0 | 0.0 (0.0–12.9) | 2/251 | 803.6 | 24.9 (3.0–89.9) |
| HPV58 | 0/847 | 2837.8 | 0.0 (0.0–13.0) | 0/249 | 805.4 | 0.0 (0.0–45.8) |
| HPV6/11/16/18/31/33/45/52/58-related 12-month persistent infectiond | 7/856 | 2851.4 | 24.5 (9.9–50.6) | 1/251 | 807.4 | 12.4 (0.3–69.0) |
| By HPV type | ||||||
| HPV6/11/16/18 | 6/854 | 2849.7 | 21.1 (7.7–45.8) | 0/251 | 808.8 | 0.0 (0.0–45.6) |
| HPV6 | 1/832 | 2787.8 | 3.6 (0.1–20.0) | 0/245 | 790.6 | 0.0 (0.0–46.7) |
| HPV11 | 0/832 | 2789.2 | 0.0 (0.0–13.2) | 0/245 | 790.6 | 0.0 (0.0–46.7) |
| HPV16 | 4/844 | 2817.2 | 14.2 (3.9–36.4) | 0/250 | 805.2 | 0.0 (0.0–45.8) |
| HPV18 | 1/851 | 2847.1 | 3.5 (0.1–19.6) | 0/249 | 800.0 | 0.0 (0.0–46.1) |
| HPV31/33/45/52/58 | 1/856 | 2862.3 | 3.5 (0.1–19.5) | 1/251 | 807.4 | 12.4 (0.3–69.0) |
| HPV31 | 0/839 | 2804.7 | 0.0 (0.0–13.2) | 0/249 | 805.0 | 0.0 (0.0–45.8) |
| HPV33 | 1/850 | 2847.6 | 3.5 (0.1–19.6) | 0/249 | 806.3 | 0.0 (0.0–45.7) |
| HPV45 | 0/855 | 2863.7 | 0.0 (0.0–12.9) | 0/251 | 808.8 | 0.0 (0.0–45.6) |
| HPV52 | 0/854 | 2859.0 | 0.0 (0.0–12.9) | 1/251 | 807.4 | 12.4 (0.3–69.0) |
| HPV58 | 0/847 | 2837.8 | 0.0 (0.0–13.0) | 0/249 | 805.4 | 0.0 (0.0–45.8) |
| HPV6/11/16/18/31/33/45/52/58-related diseasec | 1/856e | 2865.0 | 3.5 (0.1–19.4) | 0/251 | 808.8 | 0.0 (0.0–45.6) |
| CIN1f | 1/856 | 2865.0 | 3.5 (0.1–19.4) | – | – | – |
| CIN2 or CIN3f | 0/856 | 2865.9 | 0.0 (0.0–12.9) | – | – | – |
| AISf | 0/856 | 2865.9 | 0.0 (0.0–12.9) | – | – | – |
| Cervical cancerf | 0/856 | 2865.9 | 0.0 (0.0–12.9) | – | – | – |
| Condyloma | 0/856 | 2865.9 | 0.0 (0.0–12.9) | 0/251 | 808.8 | 0.0 (0.0–45.6) |
| VIN1 or worsef | 0/856 | 2865.9 | 0.0 (0.0–12.9) | – | – | – |
| VaIN1 or worsef | 0/856 | 2865.9 | 0.0 (0.0–12.9) | – | – | – |
| PIN1 or worseg | – | – | – | 0/251 | 808.8 | 0.0 (0.0–45.6) |
Abbreviations: AIS = adenocarcinoma in situ; CI = confidence interval; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; PCR = polymerase chain reaction; PIN = penile intraepithelial neoplasia; PPE = per-protocol effectiveness; VaIN = vaginal intraepithelial neoplasia; VIN = vulvar intraepithelial neoplasia.
For each participant, person-years of follow-up was calculated starting from the beginning of the LTFU study (i.e. month 42 visit) or the date when the participant reached age 16 years, whichever came later.
A case of 6-month persistent infection is a participant who is positive to ≥1 common gene for the same HPV type in the HPV6/11/16/18/31/33/45/52/58 PCR assay in two or more cervicovaginal/external genital swab, biopsy, or definitive therapy samples obtained at two or more consecutive visits at least 6 months (±1 month) apart.
In females, disease includes condyloma, CIN, AIS, VIN, VaIN, and cervical/vulvar/vaginal cancer; in males, this includes condyloma, PIN, and penile/perineal/perianal cancer.
A case of 12-month persistent infection is a participant who is positive to ≥1 common gene for the same HPV type in the HPV6/11/16/18/31/33/45/52/58 PCR assay in two or more cervicovaginal/external genital swab, biopsy, or definitive therapy samples obtained at three or more consecutive visits at least 6 months (±1 month) apart.
HPV16-related CIN1.
In female participants.
In male participants.
The single case of CIN1 related to vaccine HPV types in the PPE analysis was reported at month 84. The participant had a diagnosis of CIN1 associated with HPV39 and HPV59 on a cervical biopsy and associated with HPV16, HPV39, and HPV59 on an endocervical curettage. Persistent infection with HPV39 and HPV59 was observed from month 84 to month 90, and a subsequent cervical biopsy with no pathological abnormality at month 90 tested positive for HPV39. This participant had no chronic or debilitating medical condition and received no immunosuppressive medication; she had two lifetime sexual partners and tested negative for gonorrhea at all study visits (from month 66 through month 114) and negative for chlamydia at all study visits except for the month 90 visit.
Among males in the PPE population, there were three cases of the composite endpoint of HPV6/11/16/18/31/33/45/52/58-related, 6-month persistent infection, condyloma, PIN, and penile/perineal/perianal cancers (Table 2). This analysis showed three cases of 6-month persistent infection and no cases of disease related to any of the vaccine HPV types. The incidence rates of the endpoints of HPV6/11/16/18/31/33/45/52/58-related 6-month and 12-month persistent infection were 37.3 and 12.4 per 10,000 person-years, respectively.
Rates of persistent infection and disease related to vaccine HPV types were similar in supportive analyses in the HN-TS population, with no additional cases of HPV6/11/16/18/31/33/45/52/58-related persistent infection or disease in male or female participants in this population compared with the PPE population (Supplementary Table S3).
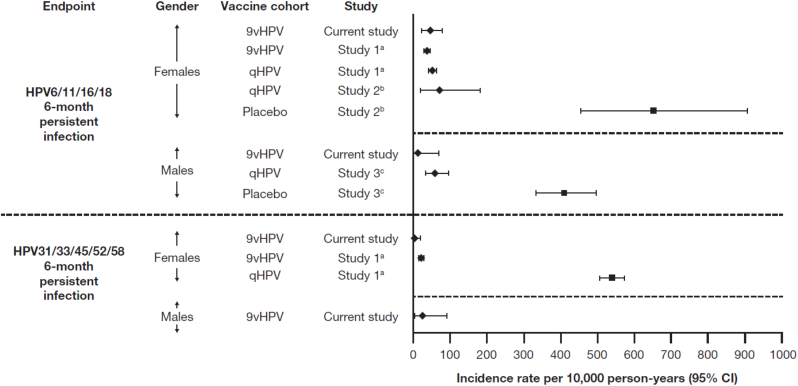
The incidence rates of 6-month persistent infection related to the nine vaccine types observed for male and female participants in the PPE population in this study were within the ranges expected in vaccinated cohorts, based on results from previous qHPV and 9vHPV vaccine trials [5,21,22] (Fig. 3).
Fig. 3.
Incidence rates of vaccine HPV-type-related persistent infection (at least 6 months duration) in qHPV and 9vHPV vaccine trials.
aNCT00543543 (see Huh et al., 2017 [5]).
bNCT00365716 (see Villa et al., 2006 [22]).
cNCT00090285 (see Giuliano et al., 2011 [21]).
Abbreviations: 9vHPV = nine-valent human papillomavirus; CI = confidence interval; HPV = human papillomavirus; qHPV = quadrivalent human papillomavirus.
Cases of the composite endpoint of 6-month persistent infection and disease related to non-vaccine HPV types (35/39/51/56/59) continued to accrue during the LTFU study. This analysis showed 189 cases of 6-month persistent infection and 11 cases of cervical, vulvar, and vaginal disease related to non-vaccine HPV types in females, and 20 cases of 6-month persistent infection and no cases of disease related to non-vaccine HPV types in males (Table 3). The incidence rates of the endpoint of HPV35/39/51/56/59-related 6-month persistent infection were 828.9 and 270.7 per 10,000 person-years in females and males, respectively. The incidence of this endpoint was approximately 800 per 10,000 person-years in the pivotal efficacy study of 9vHPV vaccine in females (unpublished results).
Table 3.
Incidence of non-9vHPV vaccine HPV type (35/39/51/56/59)-related persistent infection and disease in vaccinated participants (PPE populationa).
| Females (N = 971) |
Males (N = 301) |
|||||
|---|---|---|---|---|---|---|
| Cases/n | Person-years follow-upb | Rate per 10,000 person-years (95% CI) | Cases/n | Person-years follow-upb | Rate per 10,000 person-years (95% CI) | |
| HPV35/39/51/56/59-related 6-month persistent infectionc or diseased | 189/792 | 2280.2 | 828.9 (714.9–955.8) | 20/236 | 738.9 | 270.7 (165.3–418.0) |
| HPV35/39/51/56/59-related 6-month persistent infectionc | 189/792 | 2280.2 | 828.9 (714.9–955.8) | 20/236 | 738.9 | 270.7 (165.3–418.0) |
| By HPV type | ||||||
| HPV35 | 12/791 | 2635.4 | 45.5 (23.5–79.5) | 1/236 | 765.6 | 13.1 (0.3–72.8) |
| HPV39 | 59/782 | 2512.7 | 234.8 (178.7–302.9) | 3/236 | 767.1 | 39.1 (8.1–114.3) |
| HPV51 | 76/782 | 2485.1 | 305.8 (240.9–382.8) | 5/236 | 764.2 | 65.4 (21.2–152.7) |
| HPV56 | 101/782 | 2422.9 | 416.9 (339.5–506.5) | 11/235 | 744.8 | 147.7 (73.7–264.2) |
| HPV59 | 51/785 | 2539.8 | 200.8 (149.5–264.0) | 3/236 | 763.4 | 39.3 (8.1–114.9) |
| HPV35/39/51/56/59-related 12-month persistent infectione | 106/792 | 2410.7 | 439.7 (360.0–531.8) | 8/236 | 751.3 | 106.5 (46.0–209.8) |
| By HPV type | ||||||
| HPV35 | 7/791 | 2639.5 | 26.5 (10.7–54.6) | 1/236 | 765.6 | 13.1 (0.3–72.8) |
| HPV39 | 28/784 | 2554.4 | 109.6 (72.8–158.4) | 0/236 | 768.6 | 0.0 (0.0–48.0) |
| HPV51 | 29/789 | 2577.0 | 112.5 (75.4–161.6) | 1/236 | 767.1 | 13.0 (0.3–72.6) |
| HPV56 | 49/786 | 2523.2 | 194.2 (143.7–256.7) | 5/235 | 753.1 | 66.4 (21.6–154.9) |
| HPV59 | 27/789 | 2588.9 | 104.3 (68.7–151.7) | 2/236 | 765.1 | 26.1 (3.2–94.4) |
| HPV35/39/51/56/59-related diseased | 11/792 | 2639.2 | 41.7 (20.8–74.6) | 0/236 | 768.6 | 0.0 (0.0–48.0) |
| CIN1f | 9/792 | 2642.7 | 34.1 (15.6–64.6) | – | – | – |
| CIN2 or CIN3f | 2/792 | 2655.5 | 7.5 (0.9–27.2) | – | – | – |
| AISf | 0/792 | 2657.8 | 0.0 (0.0–13.9) | – | – | – |
| Cervical cancerf | 0/792 | 2657.8 | 0.0 (0.0–13.9) | – | – | – |
| Condyloma | 0/792 | 2657.8 | 0.0 (0.0–13.9) | 0/236 | 768.6 | 0.0 (0.0–48.0) |
| VIN1 or worsef,g | 1/792g | 2655.0 | 3.8 (0.1–21.0) | – | – | – |
| VaIN1 or worsef,h | 1/792h | 2655.0 | 3.8 (0.1–21.0) | – | – | – |
| PIN1 or worsei | – | – | – | 0/236 | 768.6 | 0.0 (0.0–48.0) |
Abbreviations: AIS = adenocarcinoma in situ; CI = confidence interval; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; PCR = polymerase chain reaction; PIN = penile intraepithelial neoplasia; PPE = per-protocol effectiveness; VaIN = vaginal intraepithelial neoplasia; VIN = vulvar intraepithelial neoplasia.
A baseline HPV-naïve population with respect to HPV types 35/39/51/56/59 cannot be defined in this study due to the absence of baseline sero- and PCR-status with respect to these non-vaccine HPV types. As such, a baseline HPV-naïve population with respect to these HPV types is approximated by the population of subjects who were naïve for all of HPV types 6/11/16/18/31/33/45/52/58. The PPE population for the non-vaccine HPV types 35/39/51/56/59 is comprised of subjects who were PPE-eligible for all of HPV types 6/11/16/18/31/33/45/52/58.
For each participant, person-years of follow-up was calculated starting from the beginning of the LTFU study (i.e. month 42 visit) or the date when the participant reached age 16 years, whichever came later.
A case of 6-month persistent infection is a participant who is positive to ≥1 common gene for the same HPV type in the HPV35/39/51/56/59 PCR assay in two or more cervicovaginal/external genital swab, biopsy, or definitive therapy samples obtained at two or more consecutive visits at least 6 months (±1 month) apart.
In females, disease includes condyloma, CIN, AIS, VIN, VaIN, and cervical/vulvar/vaginal cancer; in males, this includes condyloma, PIN, and penile/perineal/perianal cancer.
A case of 12-month persistent infection is a participant who is positive to ≥1 common gene for the same HPV type in the HPV35/39/51/56/59 PCR assay in two or more cervicovaginal/external genital swab, biopsy, or definitive therapy samples obtained at three or more consecutive visits at least 6 months (±1 month) apart.
In female participants.
Case of VIN 1.
Case of VaIN 1.
In male participants.
Males in the PPE population acquired new sexual partners at a rate of 1.05 per year of follow-up (95% CI 0.97–1.14), while females acquired new sexual partners at a rate of 0.73 per year of follow-up (95% CI 0.69–0.77) (Table 4). These rates of new sexual partner acquisition for males were slightly lower (with non-overlapping 95% CIs) than rates observed post-vaccination in a previous clinical study of qHPV vaccine; for females, these rates were higher (with non-overlapping 95% CIs) than rates observed post-vaccination in previous clinical studies of the 9vHPV and qHPV vaccines (Table 4). The incidence of chlamydia was 2.52 (95% CI 1.94–3.22) and 2.58 (1.55–4.03) per 100 person-years among females and males, respectively, and the corresponding rates of gonorrhea were 0.57 (95% CI 0.32–0.94) and 0.66 (95% CI 0.21–1.53), respectively (Table 4). These rates were similar to or higher than rates previously observed in clinical efficacy studies of the qHPV and 9vHPV vaccines in young women (Table 4).
Table 4.
Summary of incidence of new sexual partners and chlamydia or gonorrhea among male and female clinical trial participants in the current study and in other studies of HPV vaccines.
| Study | New sexual partners | Chlamydia | Gonorrhea | |||
|---|---|---|---|---|---|---|
| n | Rate per | n | Rate per | n | Rate per | |
| person-yeara | 100 person-years | 100 person-years | ||||
| (95% CI) | (95% CI) | (95% CI) | ||||
| Current study (PPE population)b | ||||||
| Females (N = 971) | 520 | 0.73c | 792 | 2.52d | 792 | 0.57d |
| (0.69–0.77) | (1.94–3.22) | (0.32–0.94) | ||||
| Males (N = 301) | 162 | 1.05c | 236 | 2.58d | 236 | 0.66d |
| (0.97–1.14) | (1.55–4.03) | (0.21–1.53) | ||||
| 9vHPV vaccine study in womene | ||||||
| 9vHPV vaccine arm (N = 7099) | 6738 | 0.38 | 6871 | 2.9 | 6871 | 0.2 |
| (0.37–0.39) | (2.7–3.2) | (0.1–0.2) | ||||
| qHPV vaccine arm (N = 7105) | 6750 | 0.38 | 6860 | 2.9 | 6860 | 0.2 |
| (0.37–0.39) | (2.7–3.2) | (0.1–0.2) | ||||
| qHPV vaccine efficacy study in womenf | ||||||
| qHPV vaccine arm (N = 276) | 235 | 0.37 | 232 | 1.2 | 237 | 0.0 |
| (0.33–0.43) | (0.5–2.6) | (0.0–0.6) | ||||
| Placebo arm (N = 275) | 233 | 0.47 | 234 | 1.6 | 237 | 0.7 |
| (0.42–0.53) | (0.7–3.1) | (0.2–1.8) | ||||
| qHPV vaccine efficacy study in meng | ||||||
| qHPV vaccine arm (N = 2025) | 1772 | 1.21 | – | – | ||
| (1.17–1.24) | ||||||
| Placebo arm (N = 2030) | 1760 | 1.26 | – | – | ||
| (1.22–1.29) | ||||||
Abbreviations: 9vHPV = nine-valent human papillomavirus; LTFU = long-term follow-up; n = participants included in the analysis of new sexual partners; PPE = per-protocol effectiveness; qHPV = quadrivalent human papillomavirus.
Rate estimates obtained from a Poisson regression model of the number of new male and female sexual partners post-month 7, adjusted for follow-up time.
Baseline HPV-naïve population was approximated by the population of participants who were naïve for all of HPV types 6/11/16/18/31/33/45/52/58. The HPV-naïve analysis population in this analysis is comprised of participants who were PPE analysis population-eligible for all of HPV types 6/11/16/18/31/33/45/52/58.
For each participant, person-years follow-up was calculated starting from the date of sexual debut through the last visit with assessment of sexual history.
For each participant, person-years follow-up was calculated starting from the beginning of the LTFU study (i.e. month 42 visit) or the date when the participant reached 16 years of age, whichever came later.
NCT00543543 (see Huh et al., 2017 [5]).
NCT00365716 (see Villa et al., 2006 [22]).
NCT00090285 (see Giuliano et al., 2011 [21]).
3.4. Safety
No vaccine- or procedure-related SAEs were observed during the LTFU study, and no participants died during the time period covered by this interim analysis. SAEs that occurred during the base study have been summarized previously [9].
4. Discussion
In girls and boys at 9–15 years of age, the 9vHPV vaccine elicited HPV antibodies that persisted through at least month 90 (7 years post-Dose 3). GMTs peaked at month 7 (1 month post-Dose 3), followed by a sharp decrease to month 12, then a slower decrease between months 24 and 90. The vast majority of participants remained seropositive to each HPV type in the 9vHPV vaccine at the last immunogenicity assessment. Anti-HPV cLIA GMTs at month 90 in the participants from the current study were comparable with or greater than those previously reported at month 60 in young women vaccinated with the 9vHPV vaccine in the pivotal efficacy study of the 9vHPV vaccine [5]; in that study, the 9vHPV vaccine provided durable protection against persistent infection and disease related to the 9vHPV vaccine HPV types through at least month 60 [5]. This cross-study comparison suggests that the antibody responses generated by a three-dose 9vHPV vaccination regimen in girls and boys at 9–15 years of age may be sufficient to induce protective efficacy through at least month 90 (i.e. 7 years post-Dose 1).
This supposition was further supported by analyses of long-term vaccine effectiveness. Most participants reached at least the age of 16 years during the LTFU study, allowing for evaluation of 9vHPV vaccine effectiveness against HPV6/11/16/18/31/33/45/52/58-related persistent infection and disease. The results indicate that the 9vHPV vaccine should provide durable protection from vaccine HPV type-related persistent infection and disease. During approximately 8 years post-vaccination, there were no cases of high-grade CIN, AIS, VIN, VaIN, or genital warts in females or PIN or genital warts in males related to the nine HPV vaccine types. The single case of HPV16-related CIN1 that was observed in the PPE population occurred in a participant who also had persistent infections with HPV39 and HPV59. Therefore, it is likely that the CIN1 lesion was caused by HPV39 and/or HPV59 and does not represent a breakthrough case, as these types are not covered by the 9vHPV vaccine. Moreover, the incidence rates of persistent infection related to the nine vaccine types observed in this study were within the ranges expected in vaccinated cohorts, based on results from previous qHPV and 9vHPV vaccine trials [5,21,22].
Previous studies of the qHPV vaccine have shown that seropositivity rates for HPV18 decrease with time based on HPV-4 cLIA, the primary immunoassay used to assess HPV antibody responses in the qHPV vaccine clinical program; however, seropositivity rates remained high when measured by IgG-LIA [23]. A concordance assessment between immunoassays has shown that the IgG-LIA is more sensitive than the HPV-4 cLIA for the detection of HPV16 and HPV18 neutralizing antibodies [24]. LTFU studies of up to 14 years did not show evidence of loss of protection against HPV18, indicating that the decrease in antibody levels detected by the cLIA does not appear to have clinical significance and may be linked to the lower sensitivity of the assay to detect HPV18 antibodies [25]. Although declines in GMTs through month 90 were observed in the current study, seropositivity rates to all nine HPV types in girls and boys remained >93% at month 90 based on the IgG-LIA.
Exposure to non-vaccine HPV types was substantial during this study. Numbers of new sexual partners observed in this study were higher than observations from previous qHPV and 9vHPV vaccine trials for females, and only slightly lower than observations from a previous qHPV vaccine efficacy trial for males. Incidence of sexually transmitted infections (including chlamydia and gonorrhea) were higher than or similar to observations from previous qHPV and 9vHPV vaccine trials. These data indicate that protection against infection and disease is not likely to be due to lack of sexual exposure to HPV. Taken together, these results indicate that the 9vHPV vaccine provides durable protection against infection and disease caused by vaccine HPV types through at least 8 years post-vaccination.
The 9vHPV vaccine continued to be generally well tolerated; there were no vaccine-related or procedure-related SAEs, and no participants died during the time period covered by this interim analysis.
The observed discontinuation rate (18% between month 42 and data cutoff, or approximately 4% per year) was within the expected range for this study. This discontinuation rate is similar to that seen in the pivotal efficacy study of the 9vHPV vaccine [5] and previous efficacy studies of the qHPV vaccine. Given the long study duration and that participants entered the study as adolescents, discontinuations from the study related to the life stage of the participants were expected (e.g., some participants were anticipated to move away to pursue college, careers, or family endeavors). Consistent with this expectation, the vast majority of discontinuations were due to loss to follow-up or participant decision.
The study design has a number of strengths that contribute to the robustness of the results presented here. The same rigorous methodology was used to assess study endpoints as in the pivotal efficacy studies of the 9vHPV and qHPV vaccines. The methodology is also similar to that used in studies that established long-term effectiveness of the qHPV vaccine in girls and boys aged 9–15 years [12] and women aged 16–23 years [25]. Thus, results of prior 9vHPV and qHPV vaccine studies can be used as benchmarks to interpret the results of this study. The study was conducted across multiple countries spread over five continents. The consistent results across this diverse population support the generalizability of these findings, which is important considering that HPV-related disease is a global health issue.
The study did not include a control group since all participants received 9vHPV vaccine during the open-label base study. Nonetheless, the study provides robust evidence of sustained vaccine effectiveness given (1) the absence of disease cases caused by vaccine HPV types (similar to observations in the 9vHPV or qHPV arms of previous 9vHPV or qHPV vaccine efficacy trials), (2) the consistency of rates of persistent infection associated with vaccine HPV types rates between this study and previous efficacy studies of 9vHPV and qHPV vaccine, and (3) the continued sexual activity and exposure to non-vaccine HPV types during the LTFU study involving >1200 participants.
The WHO recommends a two-dose vaccination regimen for individuals receiving the first vaccine dose between 9 and 14 years of age [26], and many countries have implemented two-dose vaccination regimens [27]. Previous studies have demonstrated that girls 9–13 years of age who received two doses of qHPV vaccine [[28], [29], [30]] and girls and boys 9–14 years of age who received two doses of 9vHPV vaccine [31,32] have anti-HPV antibody levels that are similar to or higher than those in young women 16–26 years of age who received three vaccine doses and, also, similar to or lower than those in girls the same age who received three vaccine doses. Persistent antibody responses to two-dose regimens have been demonstrated through 10 years for the qHPV vaccine [30] and 3 years for the 9vHPV vaccine [31].
As of October 2019, the qHPV vaccine has been licensed in over 130 countries and 9vHPV vaccine is licensed in over 80 countries. Since licensure, HPV vaccination has been implemented as part of the national vaccination programs in at least 80 countries [33]. Results from post-licensure studies – including assessments of vaccine effectiveness in vaccinated populations, post-marketing safety, and long-term effectiveness and immunogenicity trials – continue to support the concept that broad vaccination programs could help substantially decrease the incidence of HPV-related infection and disease. A dramatic, rapid decrease in genital warts burden (up to 90%) was observed in several countries following the introduction of the qHPV vaccine [34]. Similarly, the prevalence of HPV16/18 and related high-grade cervical lesions significantly decreased (up to 90% and 85%, respectively) following the introduction of the qHPV and bHPV vaccines, validating HPV vaccination as a means of primary prevention of HPV-related disease [[34], [35], [36]]. Active surveillance and large epidemiological studies continue to support the favorable safety profile of HPV vaccination observed in clinical trials [[37], [38], [39], [40]]. Real-world effectiveness and safety results, together with the demonstration of durable protection in LTFU studies, are important to inform implementation of HPV vaccination programs.
Funding statement
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. The authors and other employees of the sponsor were directly involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and the preparation and review of the manuscript. The opinions expressed in the manuscript represent the collective views of the authors and do not necessarily reflect the official position of the sponsor.
CRediT authorship contribution statement
Sven-Eric Olsson: Investigation, Data curation, Writing - review & editing. Jaime Alberto Restrepo: Investigation, Resources, Writing - review & editing. Julio Cesar Reina: Investigation, Resources, Writing - review & editing. Punnee Pitisuttithum: Data curation, Resources, Writing - review & editing. Angels Ulied: Investigation, Data curation, Resources, Writing - review & editing. Meera Varman: Investigation, Writing - review & editing. Pierre Van Damme: Investigation, Data curation, Resources, Writing - review & editing. Edson Duarte Moreira: Investigation, Data curation, Writing - original draft, Writing - review & editing. Daron Ferris: Investigation, Resources, Writing - review & editing. Stanley Block: Investigation, Formal analysis, Data curation, Writing - original draft, Writing - review & editing. Oliver Bautista: Formal analysis, Data curation, Writing - review & editing. Nancy Gallagher: Investigation, Writing - review & editing. Jennifer McCauley: Conceptualization, Writing - review & editing. Alain Luxembourg: Conceptualization, Formal analysis, Data curation, Writing - original draft.
Declaration of competing interest
Sven-Eric Olsson reports grants and non-financial support from Merck & Co., Inc., Kenilworth, NJ, USA during the conduct of this study.
Jaime Alberto Restrepo, Julio Cesar Reina, and Punnee Pitisuttithum have nothing to disclose.
Angels Ulied has received personal fees from Merck & Co., Inc., Kenilworth, NJ, USA for conducting this clinical trial and has also received fees from GSK (for conducting other clinical trials) and grants from Pfizer for lectures.
Meera Varman has received grants (for conducting HPV vaccine trials) and honorarium for speaking about vaccines from the study sponsor to Creighton University.
Pierre Van Damme has received a grant to his institution (University of Antwerp) from Merck & Co., Inc., Kenilworth, NJ, USA for the conduct of this vaccine trial; his institution also received grants from GSK Biologicals, Pfizer, SANOFI, Merck, Takeda, Baxter, CanSino China, Themis, Osivax, J&J, Abbott, The Bill & Melinda Gates Foundation, PATH, Flemish Government, and European Union, outside the submitted work.
Daron Ferris received grants from Merck & Co., Inc., Kenilworth, NJ, USA as a site for this study and has been a consultant for Merck & Co., Inc., Kenilworth, NJ, USA.
Stanley Block reports research grants from Merck & Co., Inc., Kenilworth, NJ, USA for enrollment and follow-up during this study and participation in an advisory board on vaccines for Merck & Co., Inc., Kenilworth, NJ, USA.
Edson D Moreira Jr has received research grants and financial compensation for consultation and advisory board work from Merck & Co., Inc., Kenilworth, NJ, USA, and his institution has received financial support for other HPV vaccine-related studies from Merck & Co., Inc., Kenilworth, NJ, USA.
Oliver Bautista, Nancy Gallagher, Jennifer McCauley, and Alain Luxembourg are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ USA.
Acknowledgements
The authors would like to thank the study participants and investigators.
Medical writing support, under the direction of the authors, was provided by Erin Bekes, PhD, of CMC AFFINITY, McCann Health Medical Communications, and funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, in accordance with Good Publication Practice (GPP3) guidelines.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pvr.2020.100203.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Alemany L., Saunier M., Alvarado-Cabrero I. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int. J. Cancer. 2015;136(1):98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Martel C., Plummer M., Vignat J., Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017;141(4):664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garland S.M., Steben M., Sings H.L. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 2009;199(6):805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 4.Serrano B., de Sanjosé S., Tous S. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur. J. Cancer. 2015;51(13):1732–1741. doi: 10.1016/j.ejca.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Huh W.K., Joura E.A., Giuliano A.R. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143–2159. doi: 10.1016/S0140-6736(17)31821-4. [DOI] [PubMed] [Google Scholar]
- 6.Joura E.A., Giuliano A.R., Iversen O.E. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano A.R., Joura E.A., Garland S.M. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: comparison with historic placebo population. Gynecol. Oncol. 2019;154(1):110–117. doi: 10.1016/j.ygyno.2019.03.253. [DOI] [PubMed] [Google Scholar]
- 8.Castellsagué X., Paavonen J., Jaisamrarn U. Risk of first cervical HPV infection and pre-cancerous lesions after onset of sexual activity: analysis of women in the control arm of the randomized, controlled PATRICIA trial. BMC Infect. Dis. 2014;14:551. doi: 10.1186/s12879-014-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Damme P., Olsson S.E., Block S. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136(1):e28–e39. doi: 10.1542/peds.2014-3745. [DOI] [PubMed] [Google Scholar]
- 10.Luxembourg A., Moreira E.D.J., Samakoses R. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum. Vaccin. Immunother. 2015;11(6):1306–1312. doi: 10.1080/21645515.2015.1009819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) Guidelines to assure the quality, safety and efficacy of recombinant HPV virus-like particle vaccines. 2006. http://screening.iarc.fr/doc/WHO_vaccine_guidelines_2006.pdf
- 12.Ferris D.G., Samakoses R., Block S.L. 4-Valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140(6) doi: 10.1542/peds.2016-3947. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz T.F., Galaj A., Spaczynski M. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15-55 years of age. Cancer Med. 2017;6(11):2723–2731. doi: 10.1002/cam4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts C., Green T., Hess E. Development of a human papillomavirus competitive luminex immunoassay for 9 HPV types. Hum. Vaccin. Immunother. 2014;10(8):2168–2174. doi: 10.4161/hv.29205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opalka D., Matys K., Bojczuk P. Multiplexed serologic assay for nine anogenital human papillomavirus types. Clin. Vaccine Immunol. 2010;17(5):818–827. doi: 10.1128/CVI.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstone S.E., Jessen H., Palefsky J.M. Quadrivalent HPV vaccine efficacy against disease related to vaccine and non-vaccine HPV types in males. Vaccine. 2013;31(37):3849–3855. doi: 10.1016/j.vaccine.2013.06.057. [DOI] [PubMed] [Google Scholar]
- 17.Else E.A., Swoyer R., Zhang Y. Comparison of real-time multiplex human papillomavirus (HPV) PCR assays with INNO-LiPA HPV genotyping extra assay. J. Clin. Microbiol. 2011;49(5):1907–1912. doi: 10.1128/JCM.00236-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts C.C., Swoyer R., Bryan J.T., Taddeo F.J. Comparison of real-time multiplex human papillomavirus (HPV) PCR assays with the linear array HPV genotyping PCR assay and influence of DNA extraction method on HPV detection. J. Clin. Microbiol. 2011;49(5):1899–1906. doi: 10.1128/JCM.00235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garland S.M., Hernandez-Avila M., Wheeler C.M. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 20.Luxembourg A., Bautista O., Moeller E., Ritter M., Chen J. Design of a large outcome trial for a multivalent human papillomavirus L1 virus-like particle vaccine. Contemp. Clin. Trials. 2015;42:18–25. doi: 10.1016/j.cct.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Giuliano A.R., Palefsky J.M., Goldstone S. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med. 2011;364(5):401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villa L.L., Costa R.L.R., Petta C.A. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br. J. Cancer. 2006;95(11):1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nygård M., Saah A., Munk C. Evaluation of the long-term anti-human papillomavirus 6 (HPV6), 11, 16, and 18 immune responses generated by the quadrivalent HPV vaccine. Clin. Vaccine Immunol. 2015;22(8):943–948. doi: 10.1128/CVI.00133-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown D., Müller M., Sehr P. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomaviruse types 16 and 18. Vaccine. 2014;32(44):5880–5887. doi: 10.1016/j.vaccine.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Kjaer S.K., Nygård M., Sundström K. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi: 10.1016/j.eclinm.2020.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization (WHO) Human papillomavirus vaccines: WHO position paper. 2017. https://apps.who.int/iris/bitstream/handle/10665/255353/WER9219.pdf;jsessionid=4200D7E8D81E0D2FDE619218CB9176E3?sequence=1 May 2017.
- 27.D'Addario M., Redmond S., Scott P. Two-dose schedules for human papillomavirus vaccine: systematic review and meta-analysis. Vaccine. 2017;35(22):2892–2901. doi: 10.1016/j.vaccine.2017.03.096. [DOI] [PubMed] [Google Scholar]
- 28.Dobson S.R., McNeil S., Dionne M. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–1802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 29.Ogilvie G., Sauvageau C., Dionne M. Immunogenicity of 2 vs 3 doses of the quadrivalent human papillomavirus vaccine in girls aged 9 to 13 Years after 60 months. JAMA. 2017;317(16):1687–1688. doi: 10.1001/jama.2017.1840. [DOI] [PubMed] [Google Scholar]
- 30.Donken R., Dobson S.R.M., Marty K.D. Immunogenicity of 2 and 3 doses of the quadrivalent human papillomavirus vaccine up to 120 Months postvaccination: follow-up of a randomized clinical trial. Clin. Infect. Dis. 2019 doi: 10.1093/cid/ciz887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bornstein J., Luxembourg A., , on behalf of the V503-010 study team Comparison of 2-dose and 3-dose regimens of 9-valent HPV vaccine: results from a 3-year randomized immunogenicity trial. European Research Organization on Genital Infection and Neoplasia. 2018 [Google Scholar]
- 32.Iversen O.E., Miranda M.J., Ulied A. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411–2421. doi: 10.1001/jama.2016.17615. [DOI] [PubMed] [Google Scholar]
- 33.Wigle J., Fontenot H.B., Zimet G.D. Global delivery of human papillomavirus vaccines, Pediatr. Clin. North Am. 2016;63(1):81–95. doi: 10.1016/j.pcl.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Garland S.M., Kjaer S.K., Munoz N. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin. Infect. Dis. 2016;63(4):519–527. doi: 10.1093/cid/ciw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markowitz L.E., Liu G., Hariri S., Steinau M., Dunne E.F., Unger E.R. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137(3) doi: 10.1542/peds.2015-1968. [DOI] [PubMed] [Google Scholar]
- 36.Drolet M., Bénard É., Pérez N., Brisson M., Group H.V.I.S. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497–509. doi: 10.1016/S0140-6736(19)30298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vichnin M., Bonanni P., Klein N.P. An overview of quadrivalent human papillomavirus vaccine safety: 2006 to 2015. Pediatr. Infect Dis. J. 2015;34(9):983–991. doi: 10.1097/INF.0000000000000793. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization (WHO) Meeting of the global advisory committee on vaccine safety. 2017. http://apps.who.int/iris/bitstream/10665/255870/1/WER9228.pdf?ua=1 June 2017. 7-8.
- 39.Shimabukuro T.T., Su J.R., Marquez P.L., Mba-Jonas A., Arana J.E., Cano M.V. Safety of the 9-valent human papillomavirus vaccine. Pediatrics. 2019;144(6) doi: 10.1542/peds.2019-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donahue J.G., Kieke B.A., Lewis E.M. Near real-time surveillance to assess the safety of the 9-valent human papillomavirus vaccine. Pediatrics. 2019;144(6) doi: 10.1542/peds.2019-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.